- State Key Laboratory of Molecular Oncology, Department of Medical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Objectives: Malignant pleural mesothelioma (MPM) is characterized as an incredibly aggressive form of cancer with a dismal diagnosis and a dearth of specific biomarkers and therapeutic options. For MPM patients, the effectiveness of immunotherapy may be influenced by damage-associated molecular pattern (DAMP)-induced immunogenic cell death (ICD).The objective of this work is to create a molecular profile associated with DAMPs to categorize MPM patients and predict their prognosis and response to immunotherapy.
Methods: The RNA-seq of 397 patients (263 patients with clinical data, 57.2% male, 73.0% over 60 yrs.) were gathered from eight public datasets as a training cohort to identify the DAMPs-associated subgroups of MPMs using K-means analysis. Three validation cohorts of patients or murine were established from TCGA and GEO databases. Comparisons were made across each subtype’s immune status, gene mutations, survival prognosis, and predicted response to therapy.
Results: Based on the DAMPs gene expression, MPMs were categorized into two subtypes: the nuclear DAMPs subtype, which is classified by the upregulation of immune-suppressed pathways, and the inflammatory DAMPs subtype, which is distinguished by the enrichment of proinflammatory cytokine signaling. The inflammatory DAMPs subgroup had a better prognosis, while the nuclear DAMPs subgroup exhibited a worse outcome. In validation cohorts, the subtyping system was effectively verified. We further identified the genetic differences between the two DAMPs subtypes. It was projected that the inflammatory DAMPs subtype will respond to immunotherapy more favorably, suggesting that the developed clustering method may be implemented to predict the effectiveness of immunotherapy.
Conclusion: We constructed a subtyping model based on ICD-associated DAMPs in MPM, which might serve as a signature to gauge the outcomes of immune checkpoint blockades. Our research may aid in the development of innovative immunomodulators as well as the advancement of precision immunotherapy for MPM.
1 Introduction
Mesothelioma is an unusual malignancy that originates from the mesothelial cells of the pleural or other regions. About 81% of the tumors originate from the pleura. The prevalence of malignant mesothelioma is increasing, but the mortality remains unchanged. In China, the incidence rate of malignant mesothelioma was only 1.50/106 whereas the fatality rate was 1.22/106 (1). MPM is mainly seen in older men exposed to asbestos. Compared with European and American countries, the onset age of mesothelioma is younger in China. The prevalence and fatality of malignant mesothelioma in China increase rapidly after the age of 35 or 40, reaching a peak at the age of 80 or 85 (1). Malignant pleural mesothelioma (MPM) is difficult to treat and has a dismal prognosis because most patients are at advanced stages when first diagnosed and are with early onset of evident clinical manifestations. Due to its resistance to conventional therapies and the absence of effective alternative regimens, MPM presents a highly difficult challenge. Despite the prompt advancement of immunotherapy and the fairly encouraging outcomes of ICIs in treating MPM, it remains high mortality on a global scale. The 5-year survival rate is around 10%, and the median overall survival is roughly one year.
Based on multiple studies conducted in MPM with immunotherapy alone or combined applied, the median PFS of 4~7 months does not seem particularly impressive. However, the increased median OS is mainly driven by a small portion of patients with long-lasting responses and deserves more explorations (2). CheckMate-743, a phase 3 randomized controlled trial, recently showed that MPM could benefit from PD-1 inhibitors combined with CTLA-4 inhibitors (3). Subgroup analyses revealed that the response rate to ICIs in MPM is somewhat but not entirely related to histology. Coupled with the fact that ICIs are more expensive and not covered by health insurance, it will result in a low cost-benefit ratio if the treatment is not effective. Therefore, there is an urgent need for identifying the subtypes of MPM patients who would potentially benefit from immunotherapy (4).
Immunogenic cell death (ICD) is a form of regulated cell death (RCD), acting as a major initiator of adaptive immune response in the context of malignant neoplasms (5). The promotion of ICD sensitizes MPM to ICIs treatments, as demonstrated by in-vitro experiments, preclinical models, and preliminary trials (6–11). which raises the possibility that ICD-associated biomarkers could serve as prospective predictive indicators for immunotherapy. An increased amount of work has discovered that induction of adaptive immune responses by cancer cells undergoing ICD is dependent on the emission and detection of a particular panel of DAMPs, including cell surface-exposed calreticulin (CALR), high mobility group box 1 (HMGB1) and extracellular adenosine triphosphate (ATP) (12, 13). In addition, previous studies also have demonstrated that ICD-associated DAMPs produced by chemotherapy or radiotherapy activate the cytotoxic CD8+ T cell and alleviate the immunosuppressive tumor microenvironment (TME), thus suggesting an essential role of DAMPs in immunotherapy (10). Pattern recognition receptors (PRRs) bound by DAMPs present adjuvanticity by activating transcription factors, eliciting APC cell activation, differentiation, and maturation, promoting the release of type 1 interferons and chemokines, resulting in the recruitment of APCs and T cells, and ultimately modulating intrinsic and adaptive immunity (14). Whether there is a pre-existing anti-tumor immune response is essential for effective immune checkpoint blockade. Effector T cells release interferon-γ (IFN-γ) by recognizing tumor neoantigens, which activates the Janus kinase (JAK)– signal transducer and activator of transcription (STAT) signaling pathway. The expression of programmed cell death ligand 1 (PD-L1) on the surface of tumor cells is mediated by the subsequent stimulation of the transcription factor interferon regulatory factor 1 (IRF1), which negatively regulates the effector T cell response in turn (15, 16). Immune checkpoint inhibitors (ICIs) disrupt this negative feedback loop as one of the primary mechanisms to restore anti-tumor immunity and exert anti-tumor efficiency. Hence, sensitizing tumors to ICIs by maneuvering ICD-associated DAMPs hinges on the inflammatory tumor immune microenvironment of MPM. Thus, we plan to assess the distinctive TiME to analyze the immune profiles of distinct MPM subtypes, which is crucial to interpret varied prognoses and efficacy of immunotherapy.
Despite the fact that an increasing amount of predictive models related to immunotherapy have been constructed to elaborate subtypes of MPM, ICD-associated DAMPs and their receptors were barely based upon to construct a predictive classification model. In this research, we performed consensus clustering analysis based on the ICD-associated DAMPs gene set and investigated the impact of DAMPs and their sensing receptors on the immune status from a variety of perspectives and on the survival expectancy of MPM patients. Additionally, the DAMPs-based classification we established was assessed for its predictive value of immune checkpoint inhibitors (ICIs) applied to mesothelioma (the flow chart of analysis is demonstrated in Figure 1). Our research offers novel information to discover the potential molecular mechanisms in different subtypes of MPM, which may fulfill the demand for precision immunotherapy of MPM.
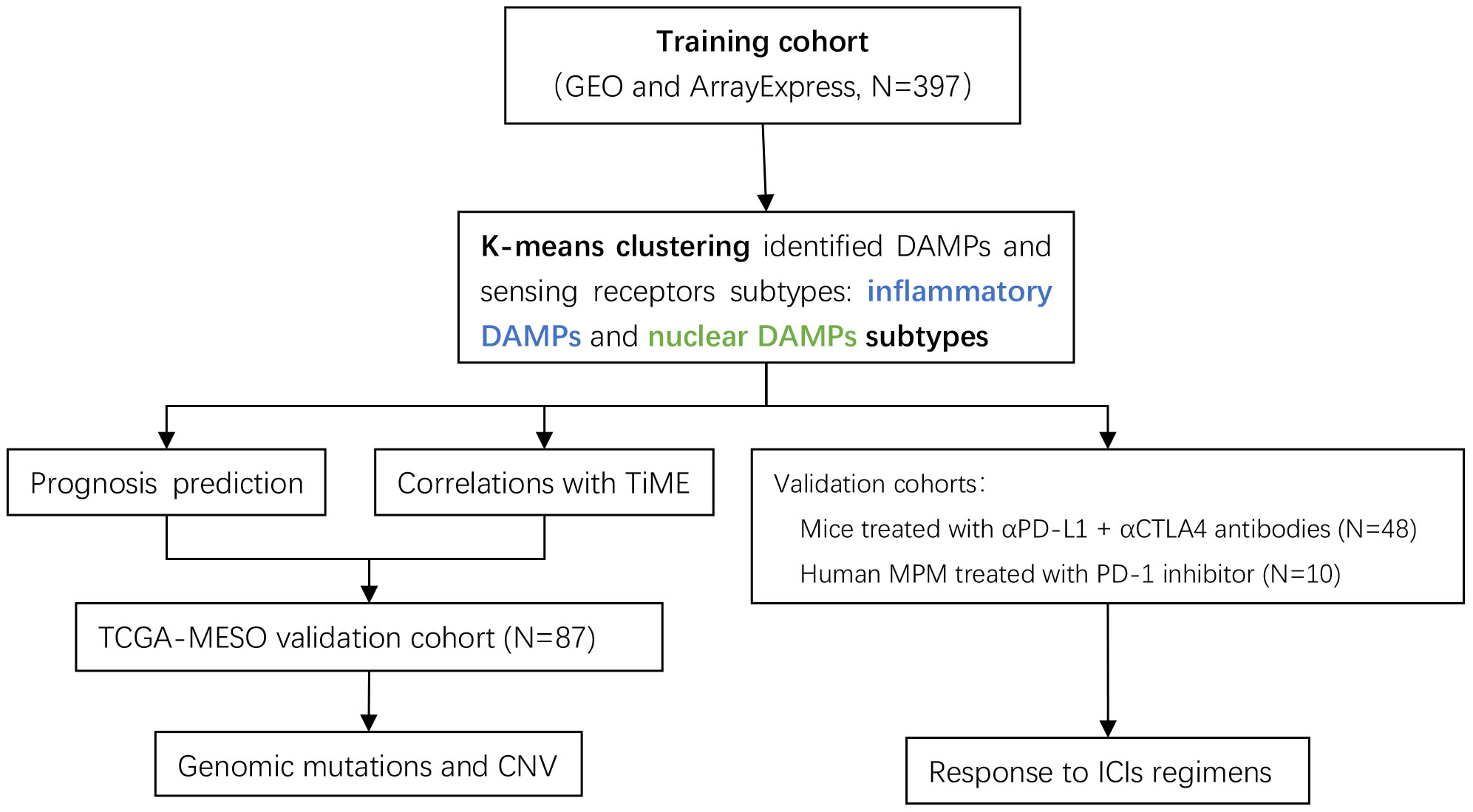
Figure 1 Flow chart of the data analysis process. The DAMPs-associated subtypes were established based on 397 TNBCs from the training cohort and validated in the TCGA cohort. DAMPs, damage-associated molecular patterns; TCGA, The Cancer Genome Atlas.
2 Materials and methods
2.1 Data collection
Normalized microarray gene expression data and clinical information of GSE42977, GSE2549, GSE12345, GSE51024, GSE163720, GSE163721, GSE29354, and GSE99070 were obtained from the GEO database (https://www.ncbi.nlm.nih.gov/geo/). Raw microarray gene expression data and follow-up data were downloaded from the ArrayExpress repository under accession code E-MTAB-6877 (https://www.ebi.ac.uk/biostudies/arrayexpress). TCGA sequencing data (including mRNA and genomic data) and clinical data of MPM patients were collected from Genomic Data Commons Data Portal (https://portal.gdc.cancer.gov/). Raw Next-Generation Sequencing (NGS) data of GSE117358 and GSE153941 were obtained from GEO database (https://www.ncbi.nlm.nih.gov/geo/).
Our study established one training cohort from malignant pleural mesothelioma patients from GEO and ArrayExpress datasets, including GSE42977, GSE2549, GSE12345, GSE51024, GSE163720, GSE163721, GSE29354 and E-MTAB-6877 (a total of 397 MPM patients, 57.2% male, 63 patients with overall survival data) and three validation cohorts consist of the TCGA-MESO datasets (a total of 86 MPM patients with CNV and WES data, 82.5% male, 86 patients with overall survival data), two murine GEO datasets (including GSE117358 and GSE153941), and one GEO dataset(GSE99070), respectively.
The raw data were normalized by using the RMA algorithm provided by “limma” package of R software (http://bioconductor.org/packages/limma/). Furthermore, the batch effect across datasets was subtracted using the “removeBatchEffect” function implemented in the “limma” package.
The demographic information and clinical characteristics of the training cohort are displayed in Table 1.
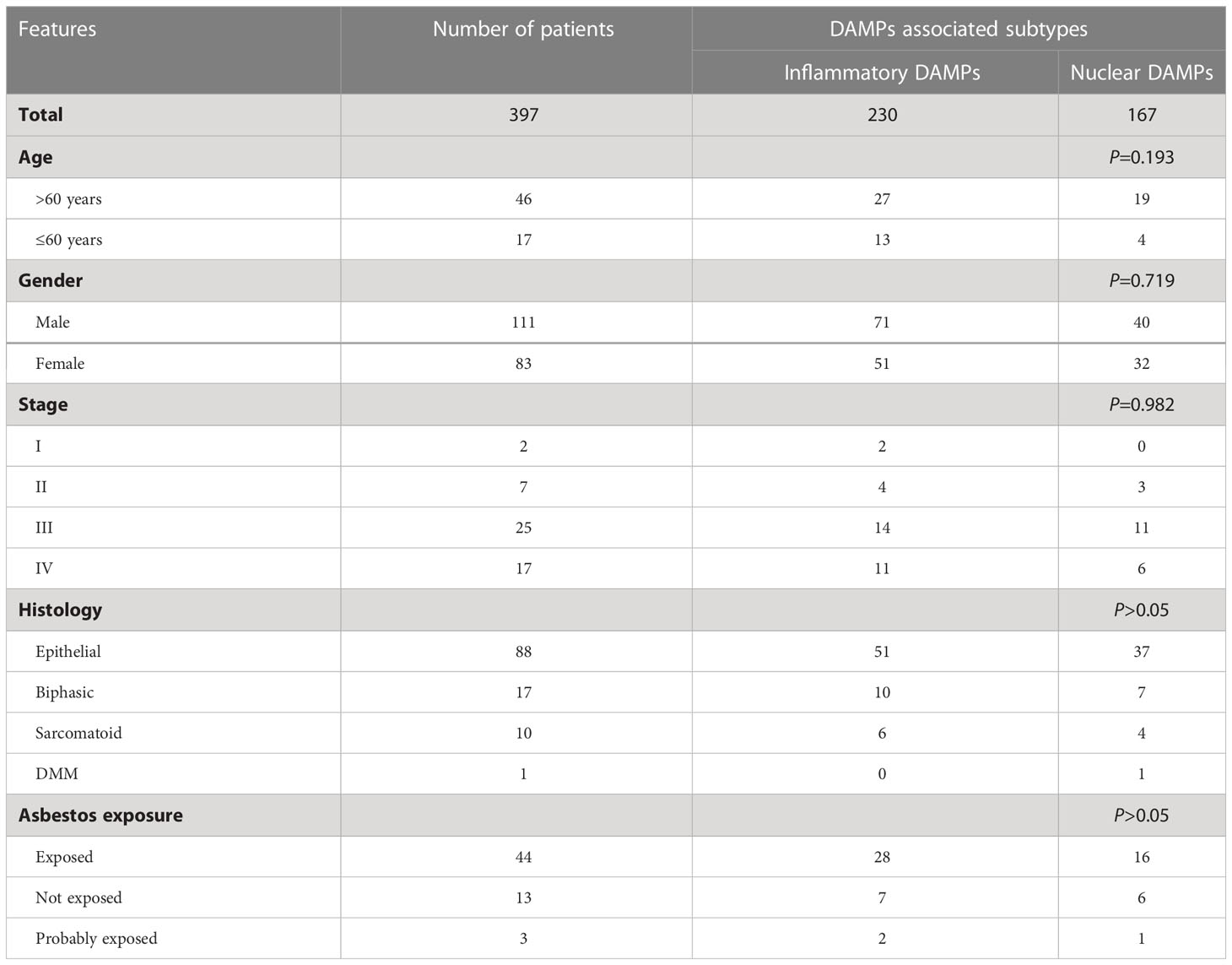
Table 1 Correlation between clinical characteristics and pathological features and DAMPs associated subtypes.
2.2 Identification of DAMPs subgroups by K-means analysis
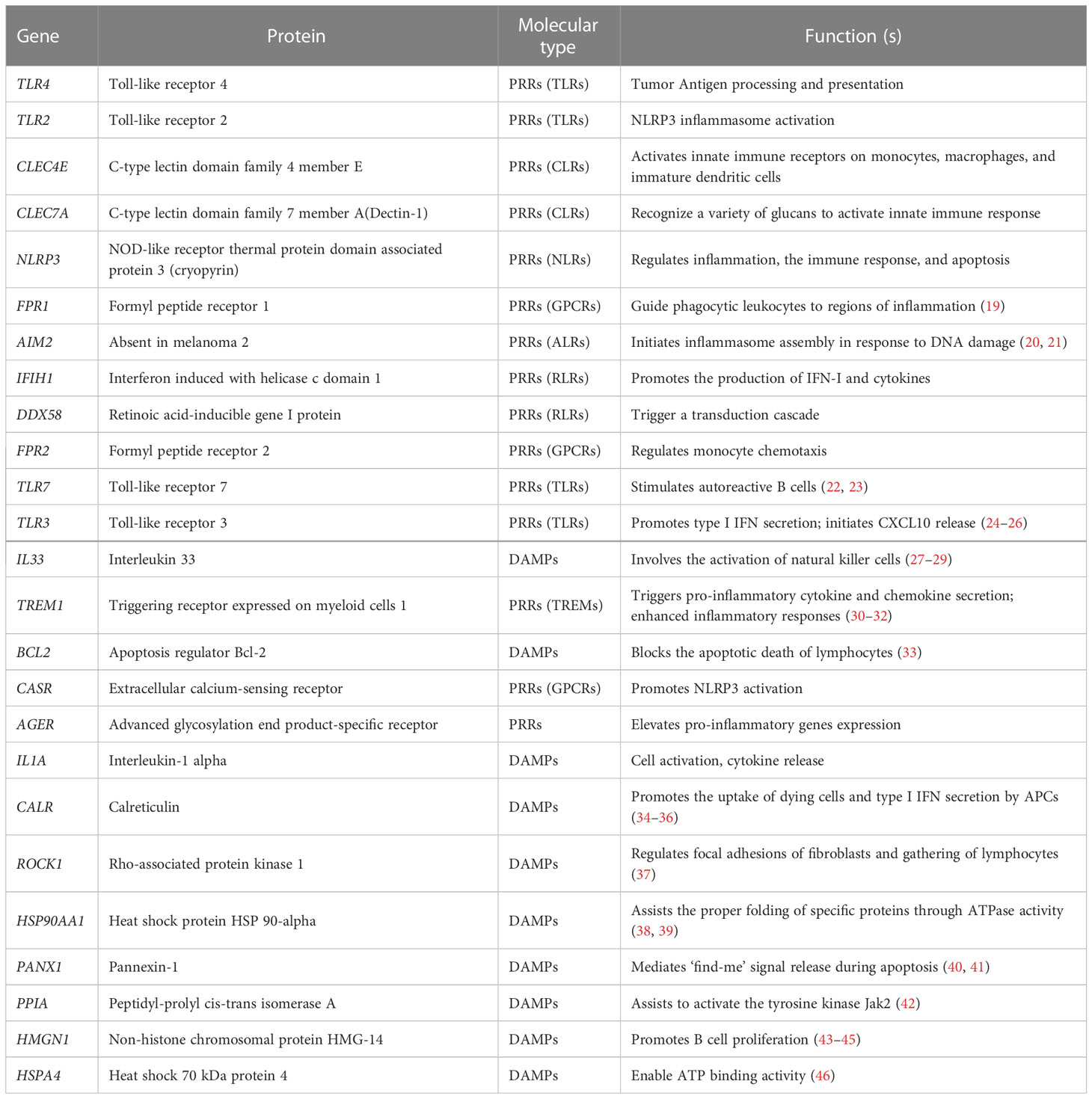
32 DAMPs-related genes were collected according to previous research (5, 17, 18). 25 DAMPs-related genes were contained in the training and validation cohorts and their information is shown in Table 2.
R package “ConsensusClusterPlus” based on the DAMPs-related gene list expressed in cohorts were employed to conduct unsupervised clustering. K-means clustering (the “kmeans” algorithm in R) was performed to define stable DAMPs-associated subtypes of MPM.
2.3 Signaling pathways analyses
Differentially expressed genes (DEGs) between two groups were defined as genes whose false discovery rate (FDR) value was < 0.05 and |Log2 (Fold Change (FC))|> 1.
Metascape database was used for Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis (47).
Furthermore, gene set enrichment analysis (GSEA) and gene set variation analysis (GSVA) were employed in two subtypes to exploit the differences in mechanisms (48–50). An adjusted P-value < 0.05 was deemed as statistically significant.
The gene sets utilized for GSEA and GSVA were downloaded from the MSigDB database.
2.4 Immune status analyses
CIBERSORT was applied to characterize immune infiltrating cell type proportions in expression profiles using a validated leukocyte gene signature matrix (LM22) (51).
The R package “estimate” contains the Estimation of Stromal and Immune cells in Malignant Tumor tissues using Expression data (ESTIMATE) program that derived the immune score (52).
2.5 Genomic analyses
Copy number variations (CNV) and genomic mutations were analyzed using GISTIC2.0 in TCGA-MESO cohort (53). We depicted the variances in gene amplification or deletion events and genomic mutations between DAMPs-associated MPM subtypes.
The “ComplexHeatmap” package in R was implemented to visualize the waterfall plot of CNV and genomic mutation data (54).
2.6 Statistical analyses
Statistical analyses were conducted by R (version 4.1.1, https://www.r-project.org).
The Kaplan–Meier algorithm included in the “survival” R package was used to perform the survival analysis.
For the comparison of the two groups, one-way analysis of variance (ANOVA), Chi-square test, or Fisher exact test was performed.
Mantel-Haenszel test was used to analyze the rates of occurrence of death over time.
A P<0.05 was deemed statistically significant.
3 Results
3.1 Consensus clustering identified two DAMPs-associated subtypes
We included malignant pleural mesothelioma samples (n=397) from GEO and ArrayExpress datasets as the training cohort. Based on the gene expression related to DAMPs, they were divided into two subtypes via K-means clustering selecting 2 as the ideal and meaningful value of K (Figures 2A–C).
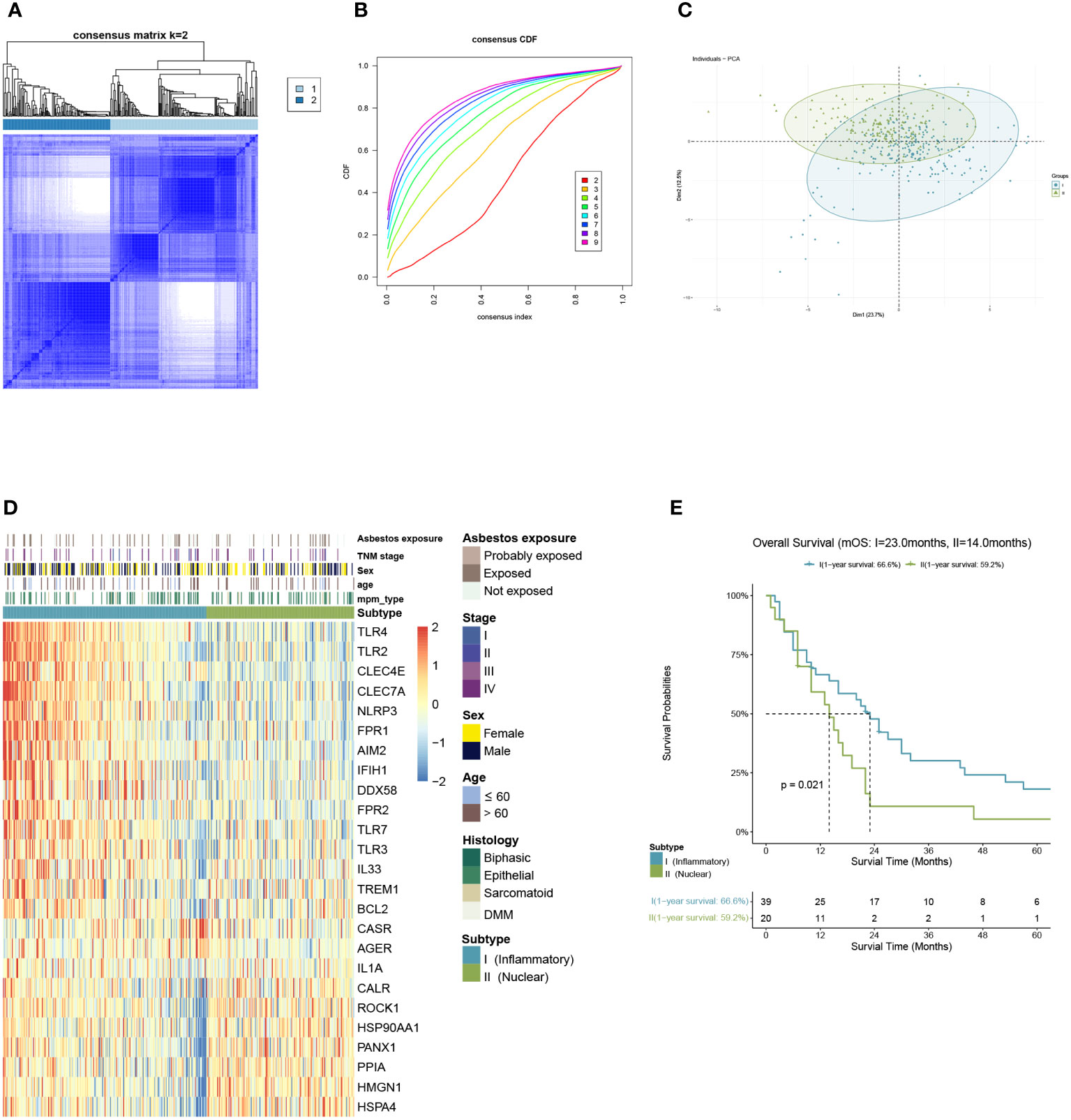
Figure 2 Identification of DAMPs-associated subtypes by K-means analysis. (A–C) K=2 was identified as the optimum value for consensus clustering. (D) DAMPs-associated subtyping of MPM patients (n = 397) from the training cohort. Heatmap displays normalized enrichment scores of the two subtypes. (E) Kaplan-Meier curves of overall survival (OS) between the two subtypes in the training cohort.
Of the 397 MPM patients included in the training cohort, 167 patients were classified into the nuclear DAMPs subgroup, and 230 were clustered in the inflammatory DAMPs subgroup. The heatmap reveals significant differences between the two subtypes in normalized enrichment scores of genes associated with DAMPs (Figure 2D). Cluster I is classified as the inflammatory DAMPs subtype distinguished by increased expression of PRRs regulating activities of inflammasome or immune cells, such as TLRs, FPR1, CLEC4E, NLRP3, etc. Cluster II is defined as the nuclear DAMPs subtype, with nuclear-associated DAMPs, such as HSP90AA1, HSPA4, CALR and high-mobility group nucleosome binding protein 1 (HMGN1) generally overexpressed, but the receptors being expressed at low levels. According to Kaplan–Meier survival analysis revealed that MPM patients enjoyed a better overall survival (OS) in the inflammatory DAMPs subtype, whereas patients in the nuclear DAMPs subtype had a worse prognosis (median overall survival 23.0months vs. 14.0months, P=0.021; Figure 2E).
3.2 Identification of differentially expressed genes and enrichment of signal pathways in different DAMPs-associated subtypes
We detected DEGs between tumor tissues belonging to two subtypes and normal pleural tissues respectively and then conducted GSEA analysis to investigate their putative signaling pathways. A total of 327 DEGs were identified, among which 311 were belonged to the nuclear DAMPs subtype, and 219 were resided in the inflammatory DAMPs subtype.
For the inflammatory DAMPs subtype, pro-inflammatory pathways were primarily enriched. As shown by KEGG enrichment analysis, the DEGs were intensely enriched in the toll-like receptor (TLR) signaling pathway. The DEGs were observed to be enriched in immune-related signaling pathways by GO enrichment analysis, including MHC protein binding, tumor necrosis factor receptor (TNFR) binding, and regulation of type I interferon-mediated signaling. GSEA similarly revealed that the subtype of inflammatory DAMPs exhibited strong upregulation of the ATM pathway, TNFR1 pathway, FGF pathway, inflammatory response pathway, CD28-dependent PI3K-AKT signaling pathway, and integrin-A4B1 pathway (Figure 3A).
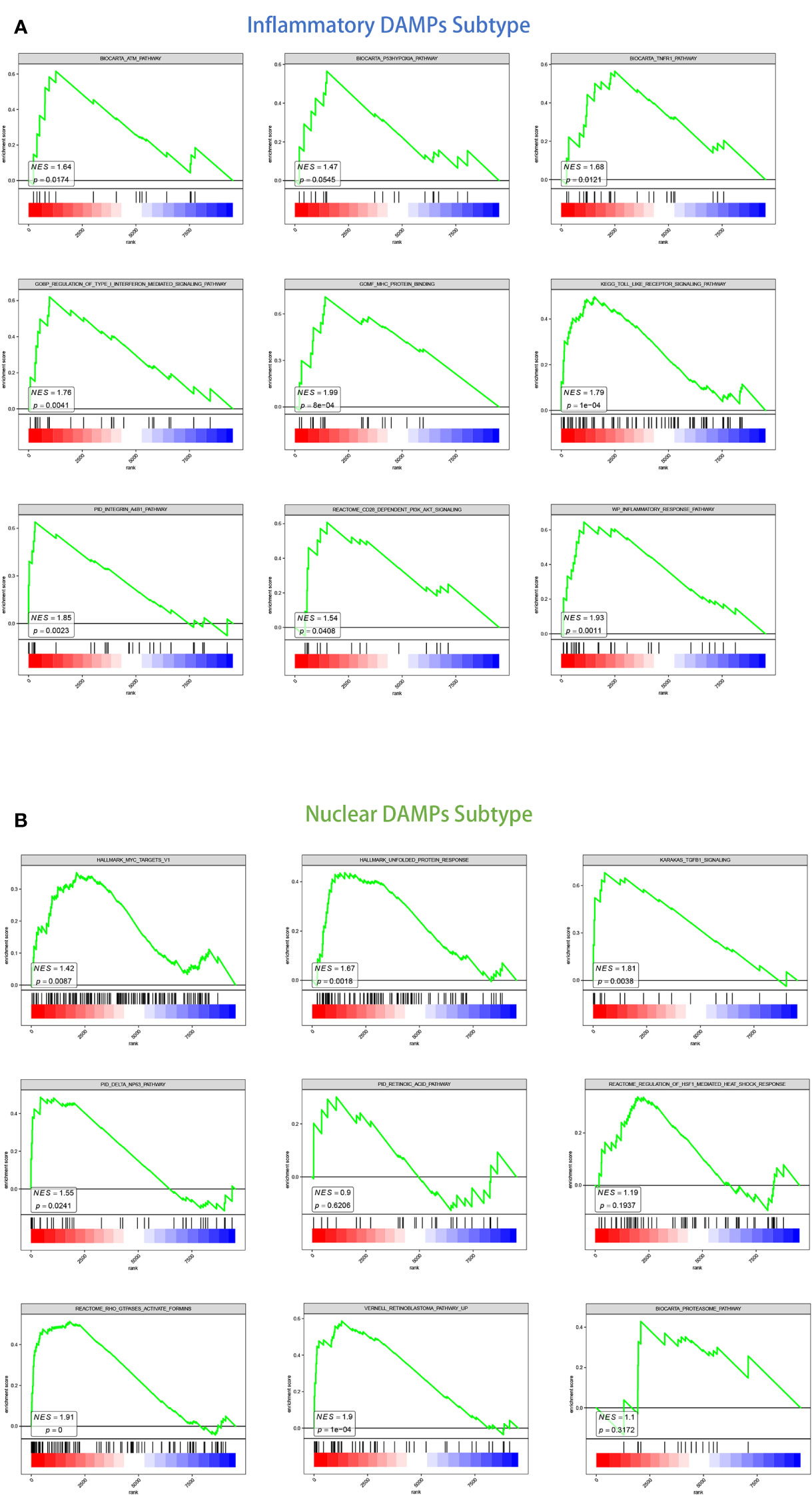
Figure 3 GSEA analysis of DEGs in two subtypes. (A) GSEA analysis of canonical pathways, gene ontology, hallmark gene sets for patients in the inflammatory DAMPs subtype. (B) GSEA analysis of canonical pathways, gene ontology, hallmark gene sets for patients in the nuclear DAMPs subtype.
Based on GSEA enrichment analysis, on the other hand, the DEGs were comparatively elevated in heat shock protein (HSP)-related signaling and immune-suppressed pathways for the nuclear DAMPs subtype, including proteasome pathway, unfolded protein response (UPR), TGFB1 signaling, Rho GTPases activate formins and regulation of HSF1-mediated heat shock response. The retinoblastoma pathway, delta NP63 pathway, MYC targets, defective intrinsic pathway for apoptosis, and retinoic acid pathway were also enriched significantly indicating that the nuclear DAMPs subtype proliferates fiercely (Figure 3B).
We then identified DEGs between two subtypes, and GSVA was performed to compare the significantly differential pathway (Figures 4A–C). GSVA analysis of cancer hallmarks, canonical pathways, and gene ontology revealed that natural killer T cell (NKT), DC, and T cell activation, complement, inflammatory response, adaptive immune response, antigen binding, cytokine receptor, IL-2 family, IL-12, IFN-γ, TNF superfamily, TLR, chemokine signaling, IL6-JAK-STAT signaling pathways were triggered in the inflammatory DAMPs subtype. In contrast, the DAMPs in the nuclear DAMPs subtype triggered cancer hallmark MYC targets, NOTCH signaling, TGF-β signaling, unfolded protein response (UPR), MTORC1 signaling, and IL-8 production.
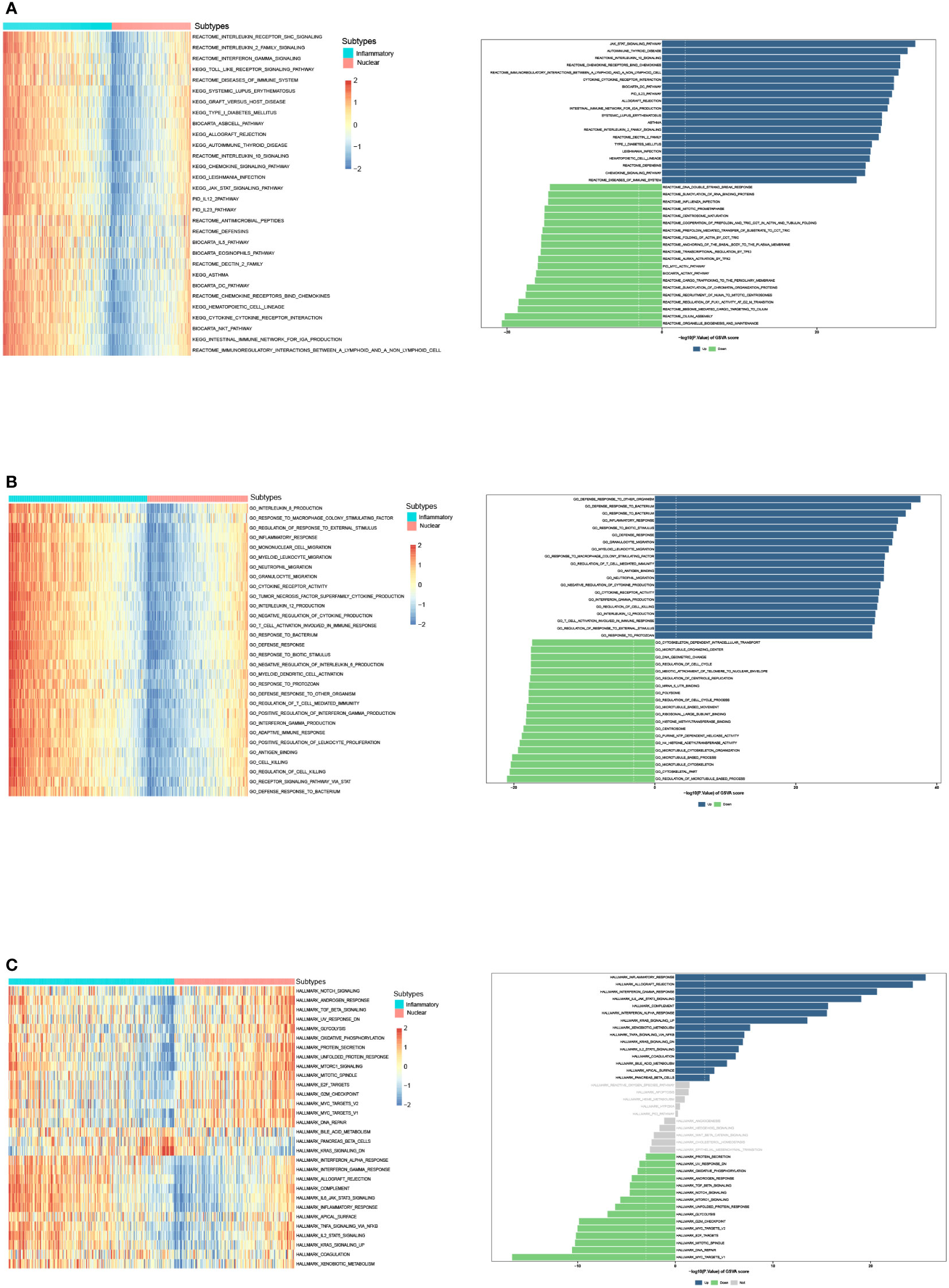
Figure 4 GSVA analysis of DEGs in the inflammatory DAMPs and nuclear DAMPs subtype. (A) GSVA analysis of canonical pathways (BIOCARTA, KEGG, PID, REACTOME and WIKIPATHWAYS) for patients in the inflammatory DAMPs and nuclear DAMPs subtype. (B) GSVA analysis of gene ontology (GO) for patients in the inflammatory DAMPs and nuclear DAMPs subtype. (C) GSVA analysis of hallmark gene sets for patients in the inflammatory DAMPs and nuclear DAMPs subtype.
3.3 Immune statuses of the patients in the two molecular subtypes varied
The immune variances between the two subtypes were investigated by immune analysis. The infiltration ratio of 22 immune cell types was analyzed in the training cohort using CIBERSORT and compared between the two groups. As demonstrated in Figure 5, the predominant infiltrating immune cells in the inflammatory DAMPs subtype were memory CD4+ T cells, while M1-like macrophages and activated DCs had a tendency to infiltration more, whereas Treg cells increased significantly with M2-like macrophages showed a tendency of higher infiltration in the nuclear DAMPs subtype (Figure 5A). Moreover, ESTIMATE indicated that patients within the inflammatory DAMPs subtype had considerably greater stromal, immune, and ESTIMATE scores compared to the others (Figures 5B–D). Additionally, the expression of CD8A (P=0.0000), PD-1 (P=0.0000), PD-L1 (P= 0.0010), and CTLA4 (P=0.0000) in the inflammatory DAMPs subtype were also notably greater than in the nuclear DAMPs subtype (Figure 5E), implying that PD-1/PD-L1 and CTLA4 may be the biomarkers of immune checkpoint inhibitors efficacy in MPM.
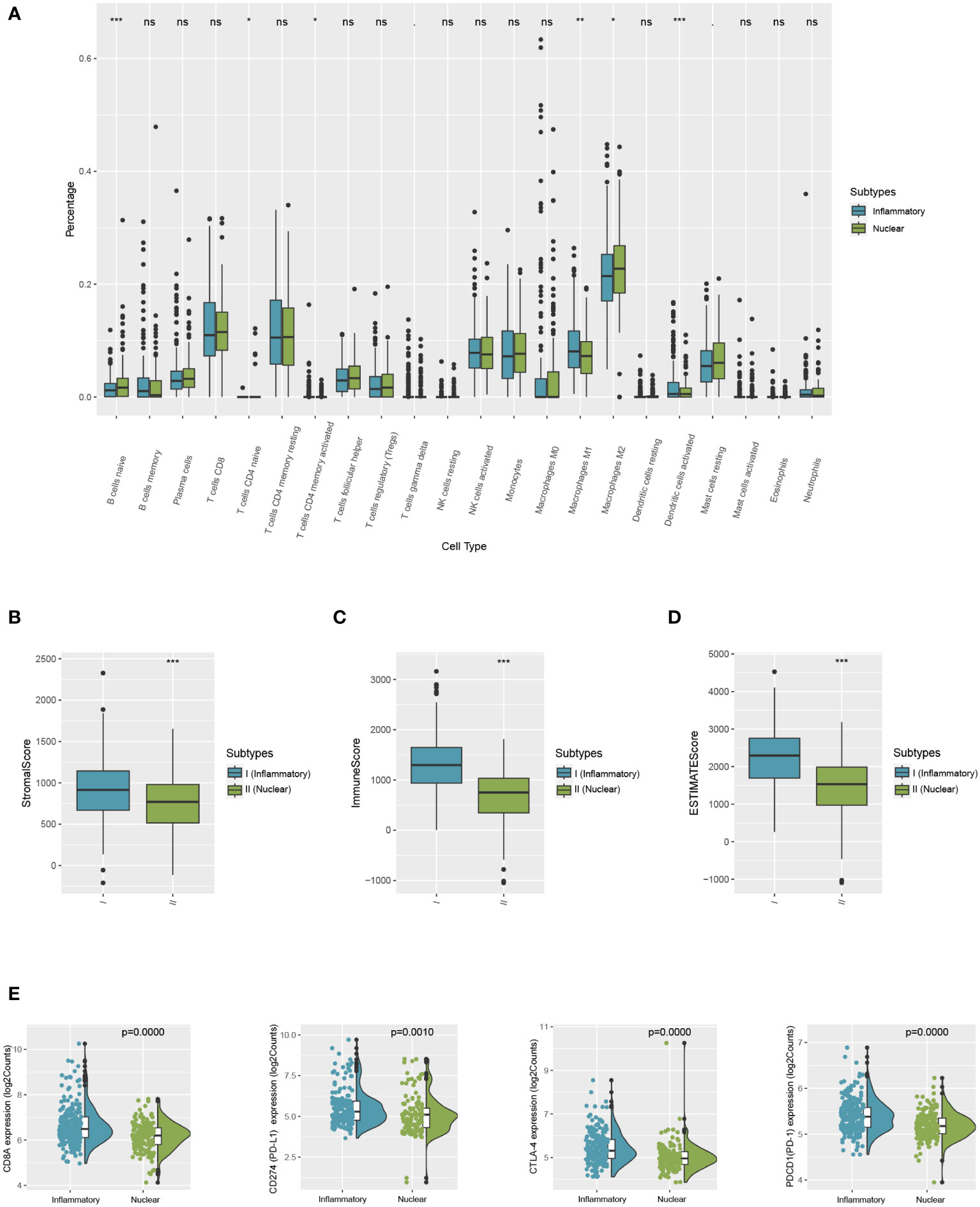
Figure 5 Comparison of differences between two subtypes in immune microenvironment status and immune signature expression levels. (A) The differential estimated proportion of 22 CIBERSORT immune cell types in DAMPs-associated subtypes. The central line represents the median value. The bottom and top of the boxes are the 25th and 75th percentiles (interquartile range). (B) Stromal score in DAMPs-associated subtypes. (C) Immune score in DAMPs-associated subtypes. (D) ESTIMATE score difference between the two subtypes in the training cohort. (E) Expression differences in CD8A, PD-1, PD-L1 and CTLA4 between two subtypes. *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant.
3.4 Solid validation of the immune characteristics in the TCGA-MESO cohort
Patients of MPM were selected from the TCGA dataset as a validation cohort and they were segregated into two DAMPs-associated subgroups based on the processed algorithm in order to further test whether the features we outlined in the two subtypes of the training cohort could be generalized. By applying CIBERSORT as above, we inferred that M2-like macrophages in the nuclear DAMPs subtype infiltrated substantially and that activated memory CD4+ T cells, M1-like macrophages, and activated DCs infiltrated in the inflammatory DAMPs subtype increased significantly (Figure 6A). These findings are comparable to those of the training cohort. Additionally, patients of the inflammatory DAMPs subtype also exhibited better immune, stromal, and ESTIMATE scores (Figures 6C–E).
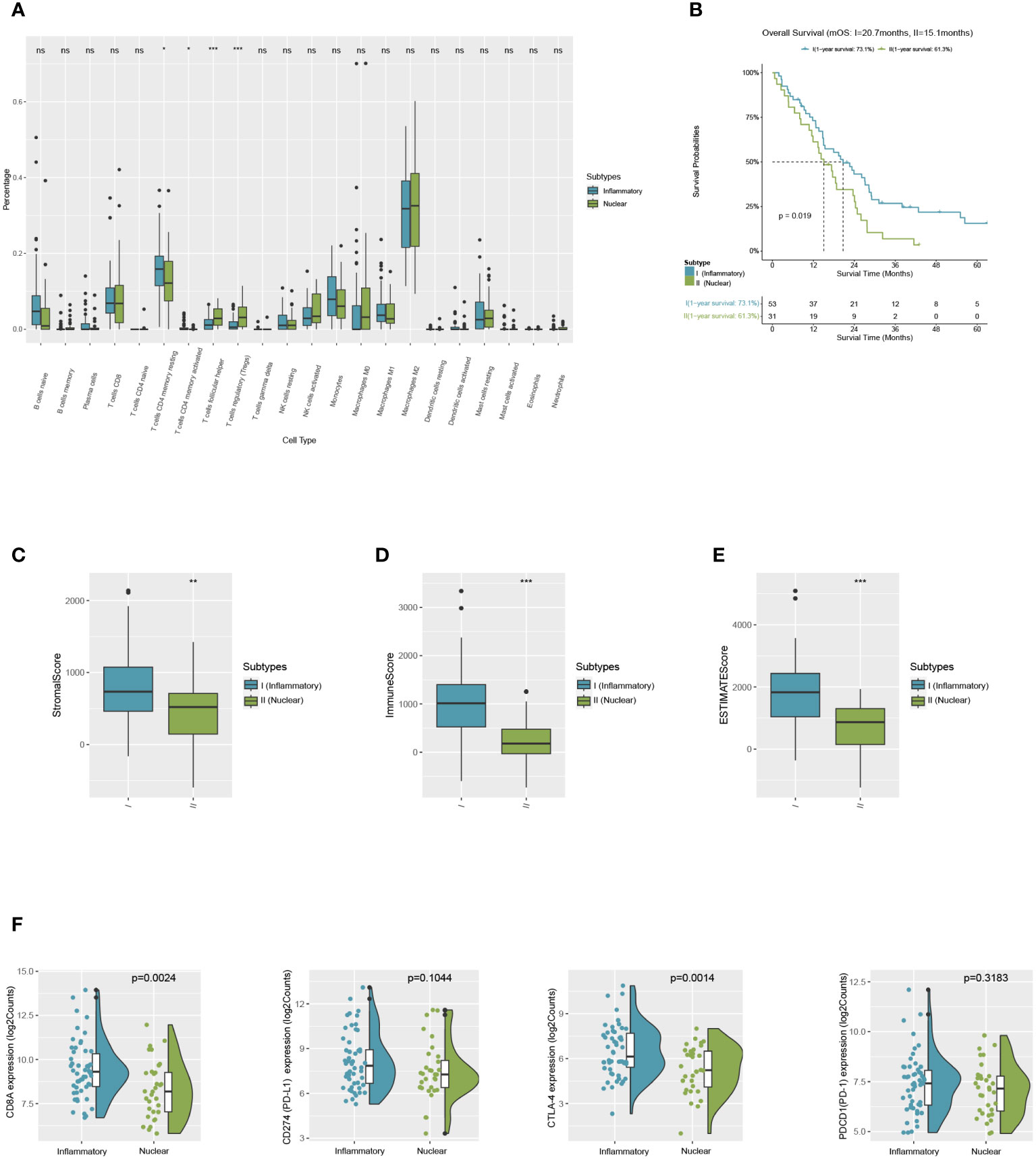
Figure 6 Successful validation of DAMPs-associated subtypes in the validation cohort of TCGA. (A) The differential estimated proportion of 22 CIBERSORT immune cell types in DAMPs-associated subtypes. The central line represents the median value. The bottom and top of the boxes are the 25th and 75th percentiles (interquartile range). (B) Kaplan-Meier curves of overall survival (OS) between the two subtypes in the validation cohort of TCGA. (C) Stromal score in DAMPs-associated subtypes. (D) Immune score in DAMPs-associated subtypes. (E) ESTIMATE score difference between the two subtypes in the validation cohort of TCGA. (F) Expression differences in CD8A, PD-1, PD-L1 and CTLA4 between two subtypes. *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant.
In terms of survival analysis, patients of the inflammatory DAMPs subtype exhibited a superior OS than those in the nuclear DAMPs subtype (median overall survival 20.7months vs. 15.1months, P=0.019; Figure 6B). Additionally, the expression of CD8A (P=0.0024) and CTLA-4(P=0.0014) was notably higher in the inflammatory DAMPs subtype than in the nuclear DAMPs subtypes of TCGA cohort, while no statistical significance was found in terms of PDCD1 (PD-1) and CD274 (PD-L1) (Figure 6F), indicating that PD-L1 is possibly not the optimal indicators for ICIs effectiveness in MPM.
3.5 Contrast of genomic variations in two subgroups in the TCGA-MESO cohort
We employed waterfall plots in order to detect genomic mutations and copy number variations between the two subgroups in the TCGA (Figures 7A, B). Although TTN mutations were more frequently detected in the inflammatory DAMPs subtype with genetic alterations (21.6% vs 14.3%, P=0.73; Figure 7C) and TP53 mutations in nuclear DAMPs subtypes (21.6% vs 28.6%, P=0.55; Figure 7C), it did not achieve statistical significance, which may still be a hint to the significant prolonged OS of inflammatory DAMPs subtypes.
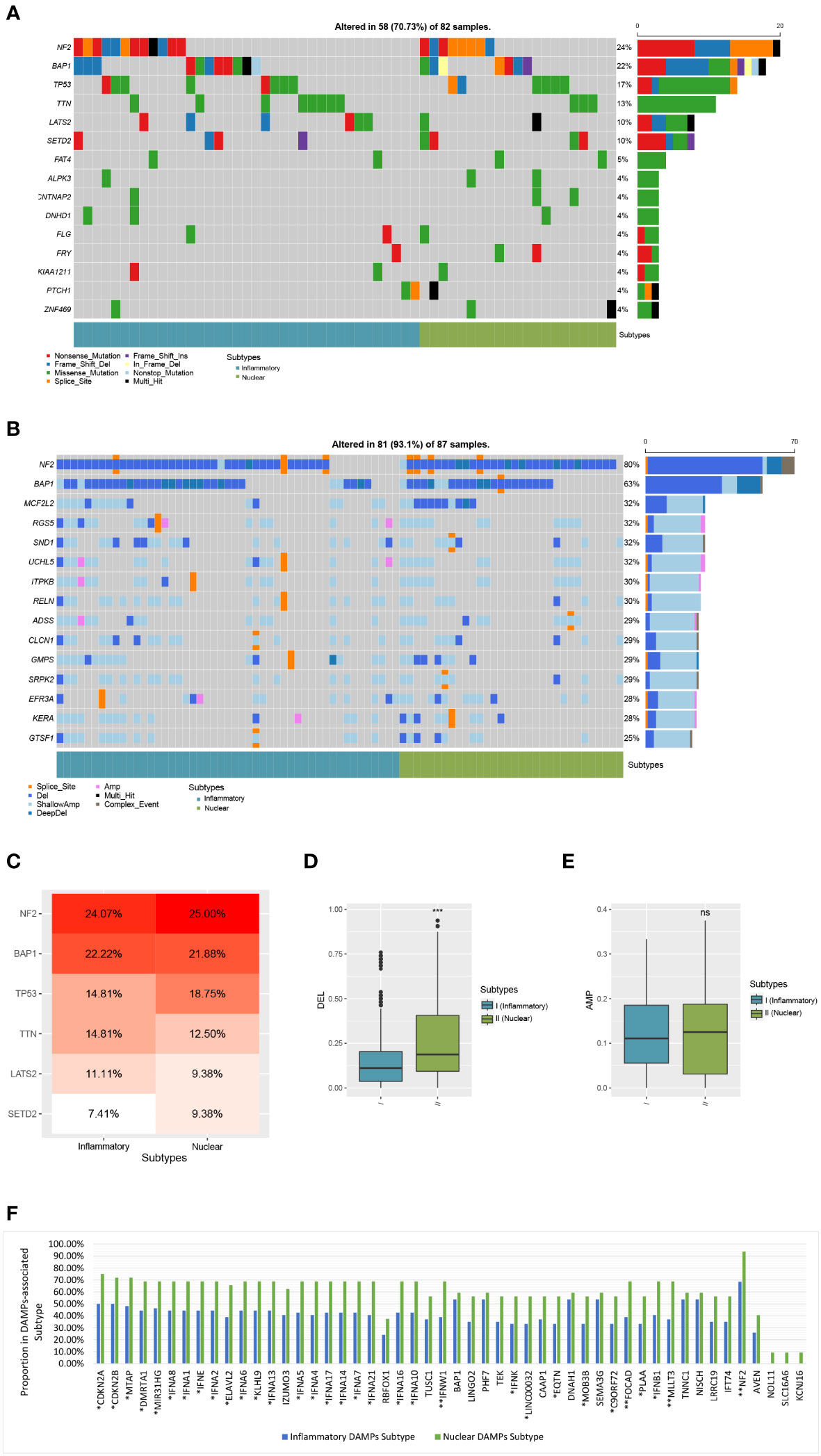
Figure 7 Comparison of genomic alterations of DAMPs-associated subtypes in the validation cohort of TCGA. (A) Differential somatic mutation analysis between the two subgroups. (B) Differential CNV analysis between the two subgroups. (C) Somatic mutation percentage of mostly mutated genes. (D) Arm-level copy number deletion in DAMPs-associated subtypes. (E) Arm-level copy number amplification in DAMPs-associated subtypes. (F) Comparison of Genes with copy number deletion in DAMPs-associated subtypes. *P < 0.05; **P < 0.01; ***P<0.001; ns, not significant.
Moreover, the ratio of patients which have a copy number deletion in the nuclear DAMPs subtype was greater than in the inflammatory DAMPs subtype (P<0.001; Figure 7D) while the frequency of amplification between the two subtypes has no significant difference (P>0.05; Figure 7E). Deletions in genes were more frequently observed in the nuclear DAMPs subtype than in the inflammatory DAMPs subtype significantly, among which copy number loss in NF2 presented at the highest frequency (Figure 7F). Deletion was observed in MTAP, FOCAD, MLLT3 and type I IFN (e.g., IFNA1/2/4-10/13/14/16/17/21, IFNB1, IFNE, IFNK, IFNW1, and etc.) but at lower frequencies. Deletions on other genes were also analyzed as displayed in Figure 7F.
3.6 Prediction of immunotherapy efficacy in two subtypes
Furthermore, we evaluated the predictive value of immunotherapy efficacy between two subtypes. The expression of major histocompatibility complex (MHC), and cytokines and their receptors in two subtypes exhibited by heatmap (Figures 8A, B). It notably demonstrated that MHC molecules, immunostimulatory and immunoinhibitory molecules, and cytokines and their receptors are unevenly expressed in distinct subtypes, with the higher expressions, especially of CXCL10 and its receptor CXCR3, in the inflammatory DAMPs subtype, both in the training cohort and the validation cohort of TCGA. In the contrast, the expression of TGFB and TGFBR elevated in the nuclear DAMPs subtype, consistent with the GSEA and GSVA results.
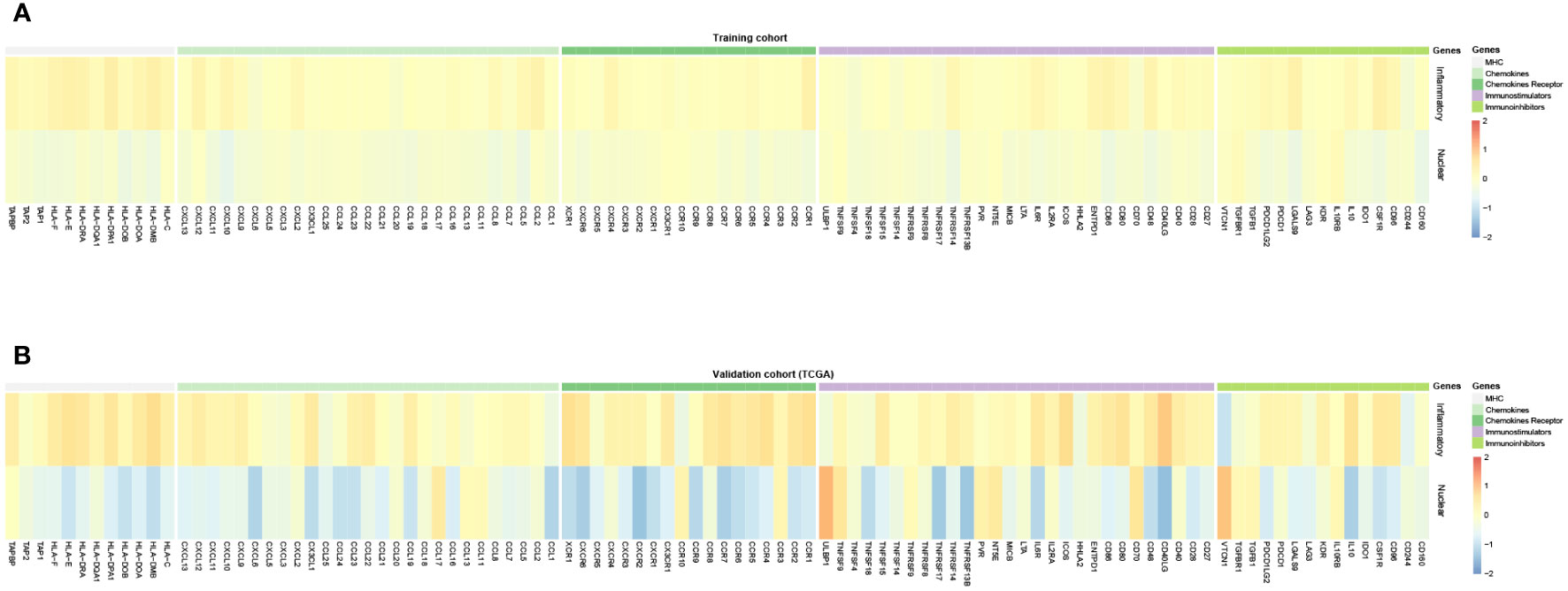
Figure 8 (A) Expression of MHC, chemokines and receptors, and immunomodulatory molecules for DAMPs-associated subtypes in the training cohort. (B) Expression of MHC, chemokines and receptors, and immunomodulatory molecules for DAMPs-associated subtypes in the TCGA cohort.
Then the efficacy of ICIs treatment was validated in a murine cohort and a MPM patient cohort treated with ICIs. The inflammatory DAMPs subtype showed a greater response rate than the nuclear DAMPs subtype in the validation cohort of 48 MPM mice receiving PD-L1 inhibitor combined with CTLA-4 inhibitor (100% vs. 7.7%, P<0.001; Figure 9A). The response rate of the two subtypes from the validation cohort of 10 MPM patients receiving PD-1 inhibitor as a single agent suggested a similar trend to the results of the murine cohort but failed to achieve statistical significance (57.1% vs. 0%, P=0.2; Figure 9B), while the disease control rate of the two subtypes was deemed to be statistically significant (85.7% vs. 0%, P=0.033; Figure 9C). These findings indicated that the developed DAMPs-associated clustering is capable of forecasting the efficacy of ICBs in MPM.
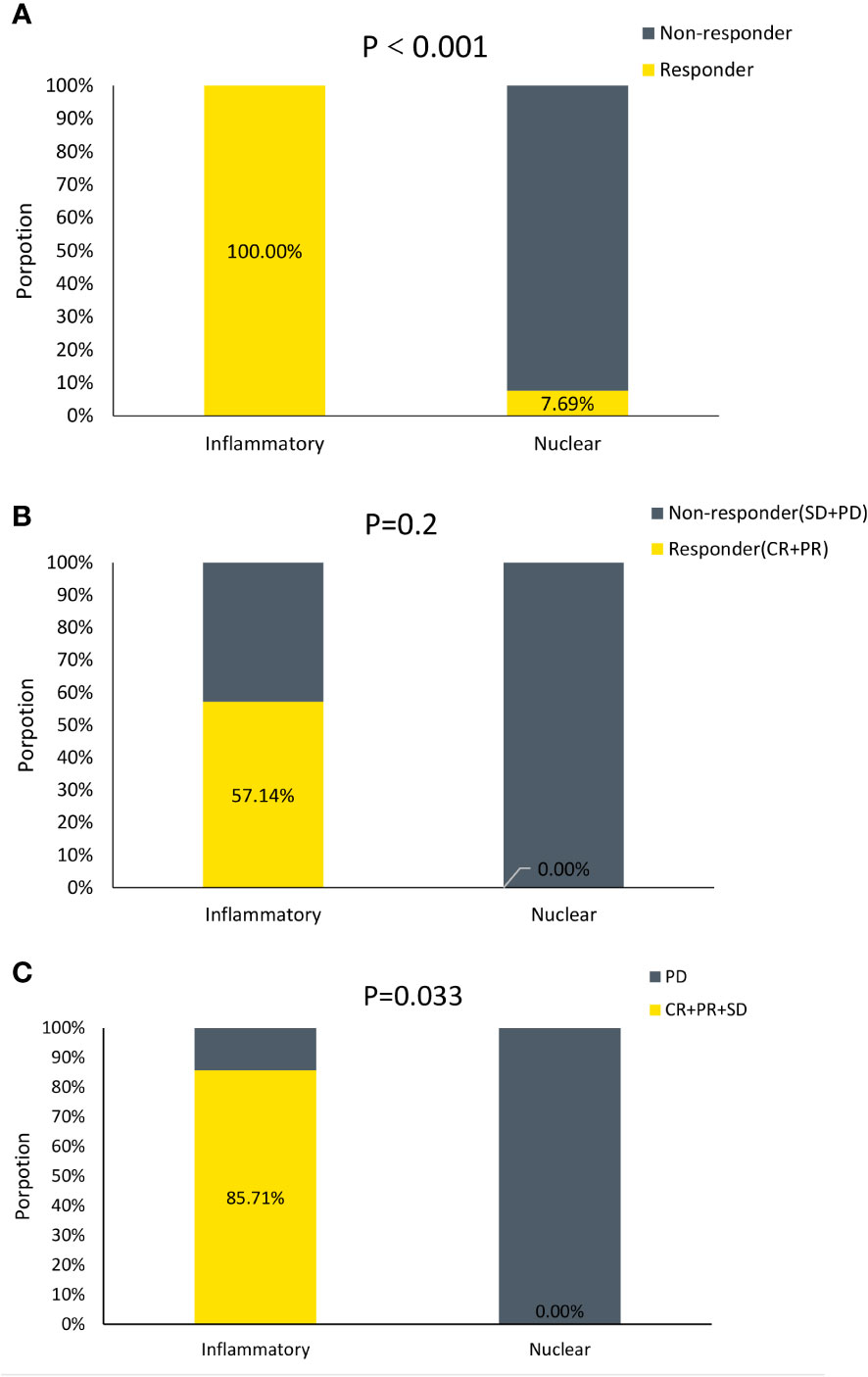
Figure 9 DAMPs-associated clusters can predict immunotherapy outcome. (A) Proportion of Responder and Non-responder to ICIs in mice. (B) Proportion of Responder and Non−responder to ICIs in MPM patients. (C) Proportion of Disease Control (CR+PR+SD) and Disease Progression (PD) to ICIs in MPM patients.
4 Discussion
Our study categorized MPM patients into two subgroups based on DAMPs and PRRs. In the nuclear DAMPs subtype, nuclear-associated HSP90AA1, HSPA4, CALR and HMGN1 were strongly expressed in the nuclear DAMPs subtype, whereas PRRs modulating activities of inflammasome or immune cells, such as TLRs, AIM2 and NLRP3, were primarily expressed in the inflammatory DAMPs subtype.
HMGN1(also known as alarmin), as a member of the high-mobility group protein family, is activated in undifferentiated cells which proliferate continuously. Extracellular HMGN1 functions as an innate danger-associated inflammatory mediator directly inducing the generation of cytokine and DC maturation. Upon translocation to the cytoplasm, it binds to PRRs to initiate proinflammatory signaling. Increasing expression of HMGN1 might provoke chronic inflammation contributing to carcinogenesis and indicating a poorer prognosis (55). The intracellular functions of CALR as a crucial regulator of Ca2+ homeostasis and the integrin-dependent signaling is probably required for tumor progression (56) which therefore implies that CALR expression is robustly related to prompt tumor progression and poor prognosis (57, 58) in the nuclear DAMPs subtype. HSPs in cells are essential in protein folding. The enriched Notch signaling positively regulate the activity of the mTOR pathway (59), and mTOR complex 1 (mTORC1) activates MYC-induced protein synthesis (60). Extreme endoplasmic reticulum (ER) stress and UPR are triggered by the uncontrolled buildup of misfolded proteins in the ER, which induce biological effects via upregulation of molecular chaperones such as HSP (e.g., HSP90AA1 and HSPA4). Elevated expression of HSPA4 and HSP90AA1 is related with poor clinical outcomes in cancer patients (61).
However, DAMPs alone are insufficient to elicit an ICD, and a corresponding receptor is required to generate biological effects. Toll-like receptors (TLRs), an evolutionarily conserved transmembrane protein expressed in epithelial cells and immune cells, serve as an important receptor to identify the DAMPs. TLRs bind with ligands such as HMGN1, CALR, HSPA4 and HSP90 to generate the affiliated biological effects via activation of MyD88-dependent and independent pathways. AIM2 and NLRP3 are sensors of DNA released from necrotic cells or increased Ca2+ release that initiate the inflammasome assembly (62, 63). Interleukin (IL)-1β and IL-18 are the inflammasome effector cytokines released as a result of signaling pathways that are regulated by inflammasomes. These cytokines assure an optimum inflammatory immune response against cancer cells (64). Therefore, MPM belonging to nuclear DAMPs subtype with higher expression of nuclear-associated DAMPs genes and lower corresponding PRRs expression are more likely to exhibit more aggressive biological behaviors. Nuclear-associated DAMPs are needed to regulate the progression of nuclear DAMPs subtype of MPM, which in consequence leads to poor outcomes. Under the circumstances of PRRs deficiency, the downstream signaling pathway cannot be activated, even with the presence or overexpression of DAMPs, causing effector cells suppressed and adapted immunity muted, which probably accounts for the suppressed immune microenvironment of the nuclear DAMPs subtype and primary resistance to immunotherapy, consistent with the predicted results of our survival analysis and exploration of immune status and ICIs efficacy.
The underlying biological pathways were then explored with functional analyses. Based on the DEGs, GSEA and GSVA identified inflammatory DAMPs subtype considerably enriched in the pro-inflammatory pathways that enhance adaptive immune responses. Apart from the toll-like receptor signaling pathway in line with higher expression of TLRs, TNFR binding and regulation of type I interferon-mediated signaling generates the antiproliferative effects of type I IFNs and TNFs (65) via activating the proinflammatory NF-κb pathway (66). Cytokine/chemokines pathways (such as IL-2 family, IL-12, IFN-γ, TNF superfamily, chemokine signaling, and IL6-JAK-STAT signaling) and effector immune cell pathways (such as NKT, DC, and T cell activation) were also significantly overexpressed. Upregulated IFN-γ signaling amplifies the antitumor response by mediating induced effects of IL-12 (67) and produces chemokines that attract immune effector cells, effectively changing the TME (68). The release of IFN-γ also leads to increasing antigen presentation of cancer and noncancer cells. Inflammatory chemokines induce recruitment of monocytes and help to support and regulate activated T cells (69). IL-2 mainly produced by CD4+ T cells activates effector T cells and innate lymphoid cells (ILCs) (70). The cytokines aforementioned regulate pro-inflammatory immunity by linking intrinsic and adaptive immune responses. Furthermore, ATM pathway, integrin A4B1 pathway and FGF pathway are enriched in this subtype. ATM (Ataxia-telangiectasia mutated proteins) is a key regulator of the DNA damage response (DDR) and contributes to cell cycle checkpoint maintenance, DNA damage repair and telomere maintenance in DNA double-strand breaks (DSB). The ATM pathway also regulates the suppression of anti-tumor immune cancer-associated fibroblasts (CAFs) differentiation (71). Advanced desmoplasia and stromal changes caused by CAFs have been identified as substantial factors in the progression of MPM (72). Accordingly, inhibition of ATM has the potential to overcome immune resistance in combination with ICIs in a minor subset of MPM patients of inflammatory DAMPs subtype. Fibroblast growth factors (FGFs) and integrin-α4β1 pathways are involved in oncogenic behaviors such as metastasis, angiogenesis, and activation of CAFs (73–75). Thereby, inhibitors targeting FGFs or integrin A4B1, or administrating anti-angiogenic agents may introduce promising directions for the management of this subtype. For the nuclear DAMPs subtype, GSEA revealed that expression of cancer hallmarks of MYC targets, NOTCH signaling, TGF-β signaling, unfolded protein response (UPR), MTORC1 signaling, and IL-8 production was higher in the nuclear DAMPs subtype. MM cells are dependent on Notch signaling, leading to activation of the phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) signaling pathway (76). NOTCH signaling facilitates immune escape by up-regulating PD-L1 and is associated with the expansion of exhausted CD8+ T cells (76). Expression profiles of malignant mesotheliomas revealed that 46% displayed altered expression of RPTOR (mTORC1 component) that activates the mechanistic target of rapamycin complex 1 (mTORC1) to enhance MM cell growth (77) suggesting the worse outcome of nuclear DAMPs subtype. On the other hand, studies have demonstrated the potential role of IL-8 as a driver of resistance to ICIs and that IL-8 has an essential role in reinforcing the immunosuppressive microenvironment and triggering EMT by determining the types and quantity of myeloid cells infiltrating tumors (78, 79). TGF-β is correlated with suppressing T cell proliferation and activation, impairing DC and NK cell function, encouraging Treg cell differentiation, and boosting CAF activities, ultimately gives rise to resistance to the immunotherapy (80) in the nuclear DAMPs subtype. Thus, targeting the aforementioned pathways is a plausible way to modify the suppressive immune microenvironment and provides new therapeutic options for this subtype.
The components in the tumor immune microenvironment (TiME) provide clues to predict MPM patient outcomes and ICIs responses (81). Activated memory CD4+ T cells, M1-like macrophages, and activated DCs were more prevalent in the inflammatory DAMPs subtype according to the CIBERSORT, while the ESTIMATE revealed that a significantly higher immune score, which have positive relations with better outcomes and more benefits from ICIs. Comparatively, M2-like macrophages of the nuclear DAMPs subtype were massively recruited, which have recently been discovered to participate in promoting resistance to ICIs therapy and predicting a poor outcome (82, 83). Furthermore, the improved OS and response rate to ICIs in the inflammatory DAMPs subtype may be associated with the dramatically elevated expression of CD8A and PD-1. These findings support the previous report that MPM patients with increased expression of CD8A and PD-1 enjoy a favorable prognosis and potentially benefit from ICIs in preceding clinical trials (84) (85). The expression of MHC class I and class II protein, particularly human leukocyte antigen class I (HLA-I) alleles, and proinflammatory chemokines and immunomodulators, especially CXCL10 and CXCR3, is more robust in the inflammatory DAMPs subtypes than the other. MHC expression on tumor cells from treatment-naive patients positively correlates with the clinical outcome and response to anti-CTLA-4, anti-PD-1, or their combination by recognizing tumor-specific antigens (86). In respect of CXCL10 and CXCR3 as downstream adjuvant effectors of type I IFNs, their signaling boosts the efficacy of immunotherapy by increasing immune infiltration of cytotoxic lymphocytes (CTLs), natural killer cells (NKs) and DCs (87). Therefore, upregulated CXCL10 and CXCR3 are positively linked with the efficacy of immunotherapy (88, 89). The above results laid further foundations for our reasonably predicting the better prognosis and response rate of ICIs treatment in the inflammatory DAMPs subtype than the other.
In the TCGA cohort, mutations of TTN were found more frequently in the inflammatory DAMPs subtypes and mutations of TP53 in the nuclear DAMPs subtypes were clinical outcome and efficacy on the trend. Previous studies showed that patients with mutated TTN are associated with longer progression-free survival or overall survival (90). TTN expression was favorably associated with the infiltration levels of effector T cells owning an inflammatory TiME and TMB in numerous tumor types, and therefore is linked with susceptibility to immune checkpoint inhibitors (91–93). We can infer that TTN may have a connection with clinical outcomes and efficacy of immunotherapy as our model projected (94). As for TP53 mutation implying a more malignant nature, the nuclear DAMPs subtype is indicated to require more intense management (95).
Copy number deletion in MPM is a characteristic genetic alteration that may result from altered methylation caused by external factors such as asbestos. Copy number deletions of cancer suppressor genes (including CDKN2A/B, FOCAD, NF2, etc.) are always accompanied by adjacent functional genes (including MTAP, MLLT3, etc.) deletion that synergistically contributes to oncogenesis. Studies have shown that copy number deletion is associated with loss of tumor neoantigens and reduced gene expression of immune-related pathways (96), which predicts dismal immune efficacy in this subtype as our subtyping model does (97). Copy number loss of Cyclin-dependent kinase (CDK) inhibitor 2A (CDKN2A) occurs at a significantly higher frequency in the nuclear DAMPs subtype. CDKN2A copy number loss suggests dismal outcomes and predicts immunotherapy resistance (98). CDKN2A encodes p16INK4a which regulates cell-cycle by inhibition of CDK4/6. Emerging clinical data demonstrates selective CDK4/6 inhibitors widely used in clinical practice contribute to PD-L1 up-regulation and immune surveillance enhancement. Hence the combination of ICIs and CDK4/6 inhibitors is a worthwhile strategy for improving outcomes in the immunotherapy-tolerant nuclear DAMPs subtype of MPM. Fifty-seven percent of patients with CDKN2A copy number loss had methylthioadenosine phosphorylase (MTAP) co-deletion in the TCGA validation cohort, since both genes reside in the same cluster of the 9p21 region (99). Co-deletion occurs more frequently indicating poorer prognosis in nuclear DAMPs subtype than the other (72% vs 48%, P=0.032). Studies show that methionine adenosyltransferase 2A (MAT2A) inhibitors induce synthetic lethality of MTAP-deleted cancer, especially in combination with taxanes and gemcitabine (100), which demonstrate a potential to treat MPM of the nuclear DAMPs subtype. Accordingly, distinctive copy number deletion also offers novel approaches to the management of the immune-resistant subtype of MPM.
The antigenicity and adjuvanticity determine the immunogenicity of cell death. Despite MPM is characterized by genetic alterations in tumor suppressor genes (101, 102) suggesting a lack of tumor-associated antigens (TAAs) and a low level of tumor mutation burden (TMB) (103), studies showed that the lowest antigenicity associated with tumorigenesis might be minor but is sufficient to support immunogenicity (12). In this regard, the adjuvanticity of DAMPs and their PRRs in immunogenic cell death may constitute a promising target for activating the immune response, since it is in a superior position to preserve homeostasis of immune microenvironment. Counteracting the inhibition of DAMPs and PRRs (e.g., TLRs stimulators) may suppress tumor growth as well as balance the production of cytokines within the TiME, and further, suppress the immunosuppressive cells while activating the immunostimulatory or effector cells (12), and thus the efficacy of immunotherapy can be enhanced by intervening with the immunomodulatory effects of DAMPs and its downstream signaling. According to the perspectives above, pathways and genomic alterations more peculiar in the nuclear DAMPs subtype of MPM which is primarily resistant to ICIs can be manipulated to modulate the immune status to some extent.
However, there are drawbacks to our study. Firstly, a few genes in the established gene list were excluded due to limitations of expression sequencing by microarray, yet the modified gene set was representative of the concerned genes in the process of ICD. Secondly, there is insufficient sequencing data on patients treated with ICIs since mesothelioma is a rare tumor. Models constructed of mice of the same strain (BALB/c) with identical genetic backgrounds inoculated subcutaneously with the same cell lines (AB1-HA cells) and then treated with anti-CTLA-4 and anti-PD-L1 were selected for additional validation. Moreover, the ORR of PD-1 inhibitors applied to MPM patients of two separate subtypes in the validation cohort did not reach statistical significance, although there was a trend that implied patients belonging to the inflammatory subtype benefit more from ICIs and the DCR, probably on account of small sample size or limited efficacy of ICIs as a single agent in MPM. Additional research is expected to confirm the validity and clinical practicability in larger cohorts or elaborately designed clinical trials.
5 Conclusion
In conclusion, we identified two molecular subtypes via K-means analysis based on the expression of ICD-associated DAMPs and their corresponding receptors in MPM. Characteristic signaling pathways and different immune statuses in these two subtypes result in disparate prognoses and efficacy of immune checkpoint inhibitors.
Our research offers a novel method to predict the prognoses and identify the MPM patients with a potential to benefit from ICIs and provides a new perspective to enhance the efficacy of immunotherapy for MPM patients with primary resistance to ICIs. Our work has made a step forward in the process of development of precision therapy in MPM.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found within the article/supplementary materials.
Author contributions
ZL: conceptualization, methodology, software, data curation, investigation, validation, visualization, writing- original draft preparation, writing- reviewing and editing. HB: supervision, funding acquisition. RW: writing- reviewing and editing. JW: conceptualization, supervision, writing- reviewing and editing, funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the National key R&D program of China (2022YFC2505000); NSFC general program (82272796); CAMS Innovation Fund for Medical Sciences(CIFMS)2022-I2M-1-009; CAMS Key Laboratory of Translational Research on Lung Cancer (2018PT31035); Aiyou foundation (KY201701).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhao J, Zuo T, Zheng R, Zhang S, Zeng H, Xia C, et al. Epidemiology and trend analysis on malignant mesothelioma in China. Chin J Cancer Res (2017) 29(4):361–8. doi: 10.21147/j.issn.1000-9604.2017.04.09
2. Fennell DA, Dulloo S, Harber J. Immunotherapy approaches for malignant pleural mesothelioma. Nat Rev Clin Oncol (2022) 19(9):573–84. doi: 10.1038/s41571-022-00649-7
3. Peters S, Scherpereel A, Cornelissen R, Oulkhouir Y, Greillier L, Kaplan MA, et al. First-line nivolumab plus ipilimumab versus chemotherapy in patients with unresectable malignant pleural mesothelioma: 3-year outcomes from CheckMate 743. Ann Oncol (2022) 33(5):488–99. doi: 10.1016/j.annonc.2022.01.074
4. Quispel-Janssen J, van der Noort V, de Vries JF, Zimmerman M, Lalezari F, Thunnissen E, et al. Programmed death 1 blockade with nivolumab in patients with recurrent malignant pleural mesothelioma. J Thorac Oncol (2018) 13(10):1569–76. doi: 10.1016/j.jtho.2018.05.038
5. Galluzzi L, van der Noort V, de Vries JF, Zimmerman M, Lalezari F, Thunnissen E, et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J Immunother Cancer (2020) 8(1):e000337. doi: 10.1136/jitc-2019-000337corr1
6. Di Somma S, Iannuzzi CA, Passaro C, Forte IM, Iannone R, Gigantino V, et al. The oncolytic virus dl922-947 triggers immunogenic cell death in mesothelioma and reduces xenograft growth. Front Oncol (2019) 9:564. doi: 10.3389/fonc.2019.00564
7. Riganti C, Lingua MF, Salaroglio IC, Falcomatà C, Righi L, Morena D, et al. Bromodomain inhibition exerts its therapeutic potential in malignant pleural mesothelioma by promoting immunogenic cell death and changing the tumor immune-environment. Oncoimmunology (2018) 7(3):e1398874. doi: 10.1080/2162402X.2017.1398874
8. Leclercq S, Gueugnon F, Boutin B, Guillot F, Blanquart C, Rogel A, et al. A 5-aza-2’-deoxycytidine/valproate combination induces cytotoxic T-cell response against mesothelioma. Eur Respir J (2011) 38(5):1105–16. doi: 10.1183/09031936.00081310
9. Salas-Benito D, Pérez-Gracia JL, Ponz-Sarvisé M, Rodriguez-Ruiz ME, Martínez-Forero I, Castañón E, et al. Paradigms on immunotherapy combinations with chemotherapy. Cancer Discovery (2021) 11(6):1353–67. doi: 10.1158/2159-8290.CD-20-1312
10. Jeong SD, Jung BK, Ahn HM, Lee D, Ha J, Noh I, et al. Immunogenic cell death inducing fluorinated mitochondria-disrupting helical polypeptide synergizes with PD-L1 immune checkpoint blockade. Adv Sci (Weinh) (2021) 8(7):2001308. doi: 10.1002/advs.202001308
11. Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol (2013) 31:51–72. doi: 10.1146/annurev-immunol-032712-100008
12. Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol (2017) 17(2):97–111. doi: 10.1038/nri.2016.107
13. Krysko O, Løve Aaes T, Bachert C, Vandenabeele P, Krysko DV. Many faces of DAMPs in cancer therapy. Cell Death Dis (2013) 4(5):e631. doi: 10.1038/cddis.2013.156
14. Man SM, Jenkins BJ. Context-dependent functions of pattern recognition receptors in cancer. Nat Rev Cancer (2022) 22(7):397–413. doi: 10.1038/s41568-022-00462-5
15. Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature (2014) 515(7528):568–71. doi: 10.1038/nature13954
16. Kalbasi A, Ribas A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat Rev Immunol (2020) 20(1):25–39. doi: 10.1038/s41577-019-0218-4
17. Gong T, Liu L, Jiang W, Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol (2020) 20(2):95–112. doi: 10.1038/s41577-019-0215-7
18. Hernandez C, Huebener P, Schwabe RF. Damage-associated molecular patterns in cancer: a double-edged sword. Oncogene (2016) 35(46):5931–41. doi: 10.1038/onc.2016.104
19. Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature (2010) 464(7285):104–7. doi: 10.1038/nature08780
20. Komada T, Chung H, Lau A, Platnich JM, Beck PL, Benediktsson H, et al. Macrophage uptake of necrotic cell DNA activates the AIM2 inflammasome to regulate a proinflammatory phenotype in CKD. J Am Soc Nephrol (2018) 29(4):1165–81. doi: 10.1681/ASN.2017080863
21. Li T, Chen ZJ. The cGAS-cGAMP-STING pathway connects DNA damage to inflammation, senescence, and cancer. J Exp Med (2018) 215(5):1287–99. doi: 10.1084/jem.20180139
22. Viglianti GA, Lau CM, Hanley TM, Miko BA, Shlomchik MJ, Marshak-Rothstein A. Activation of autoreactive b cells by CpG dsDNA. Immunity (2003) 19(6):837–47. doi: 10.1016/S1074-7613(03)00323-6
23. Lau CM, Broughton C, Tabor AS, Akira S, Flavell RA, Mamula MJ, et al. RNA-Associated autoantigens activate b cells by combined b cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med (2005) 202(9):1171–7. doi: 10.1084/jem.20050630
24. Sistigu A, Yamazaki T, Vacchelli E, Chaba K, Enot DP, Adam J, et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med (2014) 20(11):1301–9. doi: 10.1038/nm.3708
25. Chiba S, Baghdadi M, Akiba H, Yoshiyama H, Kinoshita I, Dosaka-Akita H, et al. Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat Immunol (2012) 13(9):832–42. doi: 10.1038/ni.2376
26. Cavassani KA, Ishii M, Wen H, Schaller MA, Lincoln PM, Lukacs NW, et al. TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. J Exp Med (2008) 205(11):2609–21. doi: 10.1084/jem.20081370
27. Chen L, Song Z, Cao X, Fan M, Zhou Y, Zhang G. Interleukin-33 regulates the endoplasmic reticulum stress of human myometrium via an influx of calcium during initiation of labor. Elife (2022) 11:e75072. doi: 10.7554/eLife.75072.sa2
28. Vasanthakumar A, Kallies A. Interleukin (IL)-33 and the IL-1 family of cytokines-regulators of inflammation and tissue homeostasis. Cold Spring Harb Perspect Biol (2019) 11(3):a028506. doi: 10.1101/cshperspect.a028506
29. Brunner SM, Rubner C, Kesselring R, Martin M, Griesshammer E, Ruemmele P, et al. Tumor-infiltrating, interleukin-33-producing effector-memory CD8(+) T cells in resected hepatocellular carcinoma prolong patient survival. Hepatology (2015) 61(6):1957–67. doi: 10.1002/hep.27728
30. Tammaro A, Derive M, Gibot S, Leemans JC, Florquin S, Dessing MC. TREM-1 and its potential ligands in non-infectious diseases: from biology to clinical perspectives. Pharmacol Ther (2017) 177:81–95. doi: 10.1016/j.pharmthera.2017.02.043
31. Bouchon A, Dietrich J, Colonna M. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol (2000) 164(10):4991–5. doi: 10.4049/jimmunol.164.10.4991
32. Bouchon A, Facchetti F, Weigand MA, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature (2001) 410(6832):1103–7. doi: 10.1038/35074114
33. Iwata A, Morgan-Stevenson V, Schwartz B, Liu L, Tupper J, Zhu X, et al. Extracellular BCL2 proteins are danger-associated molecular patterns that reduce tissue damage in murine models of ischemia-reperfusion injury. PloS One (2010) 5(2):e9103. doi: 10.1371/journal.pone.0009103
34. Garg AD, Krysko DV, Verfaillie T, Kaczmarek A, Ferreira GB, Marysael T, et al. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. EMBO J (2012) 31(5):1062–79. doi: 10.1038/emboj.2011.497
35. Panaretakis T, Joza N, Modjtahedi N, Tesniere A, Vitale I, Durchschlag M, et al. The co-translocation of ERp57 and calreticulin determines the immunogenicity of cell death. Cell Death Differ (2008) 15(9):1499–509. doi: 10.1038/cdd.2008.67
36. Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med (2007) 13(1):54–61. doi: 10.1038/nm1523
37. Patel RA, Forinash KD, Pireddu R, Sun Y, Sun N, Martin MP, et al. RKI-1447 is a potent inhibitor of the rho-associated ROCK kinases with anti-invasive and antitumor activities in breast cancer. Cancer Res (2012) 72(19):5025–34. doi: 10.1158/0008-5472.CAN-12-0954
38. Song KH, Oh SJ, Kim S, Cho H, Lee HJ, Song JS, et al. HSP90A inhibition promotes anti-tumor immunity by reversing multi-modal resistance and stem-like property of immune-refractory tumors. Nat Commun (2020) 11(1):562. doi: 10.1038/s41467-019-14259-y
39. Xu Q, Tu J, Dou C, Zhang J, Yang L, Liu X, et al. HSP90 promotes cell glycolysis, proliferation and inhibits apoptosis by regulating PKM2 abundance via thr-328 phosphorylation in hepatocellular carcinoma. Mol Cancer (2017) 16(1):178. doi: 10.1186/s12943-017-0748-y
40. Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, et al. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature (2010) 467(7317):863–7. doi: 10.1038/nature09413
41. Ruan Z, Orozco IJ, Du J, Lü W. Structures of human pannexin 1 reveal ion pathways and mechanism of gating. Nature (2020) 584(7822):646–51. doi: 10.1038/s41586-020-2357-y
42. Hakim S, Craig JM, Koblinski JE, Clevenger CV. Inhibition of the activity of cyclophilin a impedes prolactin receptor-mediated signaling, mammary tumorigenesis, and metastases. iScience (2020) 23(10):101581. doi: 10.1016/j.isci.2020.101581
43. Lane AA, Chapuy B, Lin CY, Tivey T, Li H, Townsend EC, et al. Triplication of a 21q22 region contributes to b cell transformation through HMGN1 overexpression and loss of histone H3 Lys27 trimethylation. Nat Genet (2014) 46(6):618–23. doi: 10.1038/ng.2949
44. Rattner BP, Yusufzai T, Kadonaga JT. HMGN proteins act in opposition to ATP-dependent chromatin remodeling factors to restrict nucleosome mobility. Mol Cell (2009) 34(5):620–6. doi: 10.1016/j.molcel.2009.04.014
45. Lim JH, Catez F, Birger Y, West KL, Prymakowska-Bosak M, Postnikov YV, et al. Chromosomal protein HMGN1 modulates histone H3 phosphorylation. Mol Cell (2004) 15(4):573–84. doi: 10.1016/j.molcel.2004.08.006
46. Wu HY, Trevino JG, Fang BL, Riner AN, Vudatha V, Zhang GH, et al. Patient-derived pancreatic cancer cells induce C2C12 myotube atrophy by releasing Hsp70 and Hsp90. Cells (2022) 11(17):2756. doi: 10.3390/cells11172756
47. Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun (2019) 10(1):1523. doi: 10.1038/s41467-019-09234-6
48. Mootha VK, Lindgren CM, Eriksson K-F, Subramanian A, Sihag S, Lehar J, et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet (2003) 34(3):267–73. doi: 10.1038/ng1180
49. Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A (2005) 102(43):15545–50. doi: 10.1073/pnas.0506580102
50. Hänzelmann S, Castelo R, Guinney J. GSVA: Gene set variation analysis for microarray and RNA-seq data. BMC Bioinf (2013) 14:7. doi: 10.1186/1471-2105-14-7
51. Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods (2015) 12(5):453–7. doi: 10.1038/nmeth.3337
52. Yoshihara K, Shahmoradgoli M, Martínez E, Vegesna R, Kim H, Torres-Garcia W, et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat Commun (2013) 4:2612. doi: 10.1038/ncomms3612
53. Mermel CH, Schumacher SE, Hill B, Meyerson ML, Beroukhim R, Getz G. GISTIC2.0 facilitates sensitive and confident localization of the targets of focal somatic copy-number alteration in human cancers. Genome Biol (2011) 12(4):R41.
54. Gu Z, Eils R, Schlesner M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics (2016) 32(18):2847–9. doi: 10.1093/bioinformatics/btw313
55. Wei F, Yang F, Jiang X, Yu W, Ren X. High-mobility group nucleosome-binding protein 1 is a novel clinical biomarker in non-small cell lung cancer. Tumor Biol (2015) 36(12):9405–10. doi: 10.1007/s13277-015-3693-7
56. Fucikova J, Spisek R, Kroemer G, Galluzzi L. Calreticulin and cancer. Cell Res (2021) 31(1):5–16. doi: 10.1038/s41422-020-0383-9
57. Lwin ZM, Guo C, Salim A, Yip GW, Chew FT, Nan J, et al. Clinicopathological significance of calreticulin in breast invasive ductal carcinoma. Mod Pathol (2010) 23(12):1559–66. doi: 10.1038/modpathol.2010.173
58. Chen CN, Chang CC, Su TE, Hsu WM, Jeng YM, Ho MC, et al. Identification of calreticulin as a prognosis marker and angiogenic regulator in human gastric cancer. Ann Surg Oncol (2009) 16(2):524–33. doi: 10.1245/s10434-008-0243-1
59. Chan SM, Weng AP, Tibshirani R, Aster JC, Utz PJ. Notch signals positively regulate activity of the mTOR pathway in T-cell acute lymphoblastic leukemia. Blood (2007) 110(1):278–86. doi: 10.1182/blood-2006-08-039883
60. Mossmann D, Park S, Hall MN. mTOR signalling and cellular metabolism are mutual determinants in cancer. Nat Rev Cancer (2018) 18(12):744–57. doi: 10.1038/s41568-018-0074-8
61. Düzgün MB, Theofilatos K, Georgakilas AG, Pavlopoulou A. A bioinformatic approach for the identification of molecular determinants of Resistance/Sensitivity to cancer thermotherapy. Oxid Med Cell Longev 2019 (2019) p:4606219.
62. Lian Q, Xu J, Yan S, Huang M, Ding H, Sun X, et al. Chemotherapy-induced intestinal inflammatory responses are mediated by exosome secretion of double-strand DNA via AIM2 inflammasome activation. Cell Res (2017) 27(6):784–800. doi: 10.1038/cr.2017.54
63. Mangan MSJ, Olhava EJ, Roush WR, Seidel HM, Glick GD, Latz E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discovery (2018) 17(8):588–606. doi: 10.1038/nrd.2018.97
64. Sharma BR, Kanneganti TD. Inflammasome signaling in colorectal cancer. Transl Res (2022) 252:45–52. doi: 10.1016/j.trsl.2022.09.002
65. MacEwan DJ. TNF ligands and receptors–a matter of life and death. Br J Pharmacol (2002) 135(4):855–75. doi: 10.1038/sj.bjp.0704549
66. Dostert C, Grusdat M, Letellier E, Brenner D. The TNF family of ligands and receptors: Communication modules in the immune system and beyond. Physiol Rev (2019) 99(1):115–60. doi: 10.1152/physrev.00045.2017
67. Tugues S, Burkhard SH, Ohs I, Vrohlings M, Nussbaum K, Vom Berg J, et al. New insights into IL-12-mediated tumor suppression. Cell Death Differ (2015) 22(2):237–46. doi: 10.1038/cdd.2014.134
68. Ribas A, Haining WN, Schumacher TNM. When cancer cells become the enablers of an antitumor immune response. Cancer Discovery (2022) 12(10):2244–8. doi: 10.1158/2159-8290.CD-22-0706
69. Palomino DC, Marti LC. Chemokines and immunity. Einstein (Sao Paulo) (2015) 13(3):469–73. doi: 10.1590/S1679-45082015RB3438
70. Raeber ME, Sahin D, Boyman O. Interleukin-2-based therapies in cancer. Sci Transl Med (2022) 14(670):eabo5409. doi: 10.1126/scitranslmed.abo5409
71. Mellone M, Piotrowska K, Venturi G, James L, Bzura A, Lopez MA, et al. ATM Regulates differentiation of myofibroblastic cancer-associated fibroblasts and can be targeted to overcome immunotherapy resistance. Cancer Res (2022) 82(24):4571–85. doi: 10.1158/0008-5472.CAN-22-0435
72. Mathilakathu A, Wessolly M, Mairinger E, Uebner H, Kreidt D, Brcic L, et al. Cancer-associated fibroblasts regulate kinase activity in mesothelioma cell lines via paracrine signaling and thereby dictate cell faith and behavior. Int J Mol Sci (2022) 23(6):3278. doi: 10.3390/ijms23063278
73. Bordignon P, Bottoni G, Xu X, Popescu AS, Truan Z, Guenova E, et al. Dualism of FGF and TGF-β signaling in heterogeneous cancer-associated fibroblast activation with ETV1 as a critical determinant. Cell Rep (2019) 28(9):2358–2372.e6. doi: 10.1016/j.celrep.2019.07.092
74. Turner N, Grose R. Fibroblast growth factor signalling: From development to cancer. Nat Rev Cancer (2010) 10(2):116–29. doi: 10.1038/nrc2780
75. Baiula M, Spampinato S, Gentilucci L, Tolomelli A. Novel ligands targeting α(4)β(1) integrin: Therapeutic applications and perspectives. Front Chem (2019) 7:489. doi: 10.3389/fchem.2019.00489
76. Graziani I, Eliasz S, De Marco MA, Chen Y, Pass HI, De May RM, et al. Opposite effects of notch-1 and notch-2 on mesothelioma cell survival under hypoxia are exerted through the akt pathway. Cancer Res (2008) 68(23):9678–85. doi: 10.1158/0008-5472.CAN-08-0969
77. Sato T, Mukai S, Ikeda H, Mishiro-Sato E, Akao K, Kobayashi T, et al. Silencing of SmgGDS, a novel mTORC1 inducer that binds to RHEBs, inhibits malignant mesothelioma cell proliferation. Mol Cancer Res (2021) 19(5):921–31. doi: 10.1158/1541-7786.MCR-20-0637
78. Bakouny Z, Choueiri TK. IL-8 and cancer prognosis on immunotherapy. Nat Med (2020) 26(5):650–1. doi: 10.1038/s41591-020-0873-9
79. David JM, Dominguez C, Hamilton DH, Palena C. The IL-8/IL-8R axis: A double agent in tumor immune resistance. Vaccines (Basel) (2016) 4(3):22. doi: 10.3390/vaccines4030022
80. Yi M, Niu M, Wu Y, Ge H, Jiao D, Zhu S, et al. Combination of oral STING agonist MSA-2 and anti-TGF-β/PD-L1 bispecific antibody YM101: a novel immune cocktail therapy for non-inflamed tumors. J Hematol Oncol (2022) 15(1):142. doi: 10.1186/s13045-022-01363-8
81. Harber J, Kamata T, Pritchard C, Fennell D. Matter of TIME: The tumor-immune microenvironment of mesothelioma and implications for checkpoint blockade efficacy. J Immunother Cancer (2021) 9(9):e003032. doi: 10.1136/jitc-2021-003032
82. Zheng X, Weigert A, Reu S, Guenther S, Mansouri S, Bassaly B, et al. Spatial density and distribution of tumor-associated macrophages predict survival in non-small cell lung carcinoma. Cancer Res (2020) 80(20):4414–25. doi: 10.1158/0008-5472.CAN-20-0069
83. Chen SMY, Popolizio V, Woolaver RA, Ge H, Krinsky AL, John J, et al. Differential responses to immune checkpoint inhibitor dictated by pre-existing differential immune profiles in squamous cell carcinomas caused by same initial oncogenic drivers. J Exp Clin Cancer Res (2022) 41(1):123. doi: 10.1186/s13046-022-02337-x
84. Alcala N, Mangiante L, Le-Stang N, Gustafson CE, Boyault S, Damiola F, et al. Redefining malignant pleural mesothelioma types as a continuum uncovers immune-vascular interactions. EBioMedicine (2019) 48:191–202. doi: 10.1016/j.ebiom.2019.09.003
85. Popat S, Scherpereel A, Antonia S, Oulkhouir Y, Bautista Y, Cornelissen R, et al. First-line nivolumab plus ipilimumab versus chemotherapy in unresectable malignant pleural mesothelioma (MPM) in CheckMate 743. J Oncol Pharm Pract (2022) 28(2 SUPPL):5.
86. Rodig SJ, Gusenleitner D, Jackson DG, Gjini E, Giobbie-Hurder A, Jin C, et al. MHC proteins confer differential sensitivity to CTLA-4 and PD-1 blockade in untreated metastatic melanoma. Sci Transl Med (2018) 10(450):eaar3342. doi: 10.1126/scitranslmed.aar3342
87. Li X, Lu M, Yuan M, Ye J, Zhang W, Xu L, et al. CXCL10-armed oncolytic adenovirus promotes tumor-infiltrating T-cell chemotaxis to enhance anti-PD-1 therapy. Oncoimmunology (2022) 11(1):2118210. doi: 10.1080/2162402X.2022.2118210
88. Shi Z, Shen J, Qiu J, Zhao Q, Hua K, Wang H. CXCL10 potentiates immune checkpoint blockade therapy in homologous recombination-deficient tumors. Theranostics (2021) 11(15):7175–87. doi: 10.7150/thno.59056
89. Janji B, Hasmim M, Parpal S, De Milito A, Berchem G, Noman MZ. Lighting up the fire in cold tumors to improve cancer immunotherapy by blocking the activity of the autophagy-related protein PIK3C3/VPS34. Autophagy (2020) 16(11):2110–1. doi: 10.1080/15548627.2020.1815439
90. Wang Z, Wang C, Lin S, Yu X. Effect of TTN mutations on immune microenvironment and efficacy of immunotherapy in lung adenocarcinoma patients. Front Oncol (2021) 11:725292. doi: 10.3389/fonc.2021.725292
91. Oh J-H, Jang SJ, Kim J, Sohn I, Lee J-Y, Cho EJ, et al. Spontaneous mutations in the single TTN gene represent high tumor mutation burden. NPJ Genomic Med (2020) 5(1):33. doi: 10.1038/s41525-019-0107-6
92. Chen J, Wen Y, Su H, Yu X, Hong R, Chen C, et al. Deciphering prognostic value of TTN and its correlation with immune infiltration in lung adenocarcinoma. Front Oncol (2022) 12:877878. doi: 10.3389/fonc.2022.877878
93. Jia Q, Wang J, He N, He J, Zhu B. Titin mutation associated with responsiveness to checkpoint blockades in solid tumors. JCI Insight (2019) 4(10):e127901. doi: 10.1172/jci.insight.127901
94. Su C, Wang X, Zhou J, Zhao J, Zhou F, Zhao G, et al. Titin mutation in circulatory tumor DNA is associated with efficacy to immune checkpoint blockade in advanced non-small cell lung cancer. Transl Lung Cancer Res (2021) 10(3):1256–65. doi: 10.21037/tlcr-20-1118
95. Baslan T, Morris JPT, Zhao Z, Reyes J, Ho YJ, Tsanov KM, et al. Ordered and deterministic cancer genome evolution after p53 loss. Nature (2022) 608(7924):795–802. doi: 10.1038/s41586-022-05082-5
96. Jhunjhunwala S, Hammer C, Delamarre L. Antigen presentation in cancer: insights into tumour immunogenicity and immune evasion. Nat Rev Cancer (2021) 21(5):298–312. doi: 10.1038/s41568-021-00339-z
97. Roh W, Chen PL, Reuben A, Spencer CN, Prieto PA, Miller JP, et al. Integrated molecular analysis of tumor biopsies on sequential CTLA-4 and PD-1 blockade reveals markers of response and resistance. Sci Transl Med (2017) 9(379):eaah3560. doi: 10.1126/scitranslmed.aah3560
98. Gutiontov SI, Turchan WT, Spurr LF, Rouhani SJ, Chervin CS, Steinhardt G, et al. CDKN2A loss-of-function predicts immunotherapy resistance in non-small cell lung cancer. Sci Rep (2021) 11(1):20059. doi: 10.1038/s41598-021-99524-1
99. Krasinskas AM, Bartlett DL, Cieply K, Dacic S. CDKN2A and MTAP deletions in peritoneal mesotheliomas are correlated with loss of p16 protein expression and poor survival. Modern Pathol (2010) 23(4):531–8. doi: 10.1038/modpathol.2009.186
100. Kalev P, Hyer ML, Gross S, Konteatis Z, Chen CC, Fletcher M, et al. MAT2A inhibition blocks the growth of MTAP-deleted cancer cells by reducing PRMT5-dependent mRNA splicing and inducing DNA damage. Cancer Cell (2021) 39(2):209–224.e11. doi: 10.1016/j.ccell.2020.12.010
101. Shao C, Li G, Huang L, Pruitt S, Castellanos E, Frampton G, et al. Prevalence of high tumor mutational burden and association with survival in patients with less common solid tumors. JAMA Netw Open (2020) 3(10):e2025109. doi: 10.1001/jamanetworkopen.2020.25109
102. Frampton GM, Fichtenholtz A, Otto GA, Wang K, Downing SR, He J, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol (2013) 31(11):1023–31. doi: 10.1038/nbt.2696
Keywords: malignant mesothelioma, damage-associated molecular patterns, immunogenic cell death, immunotherapy, tumor microenvironment
Citation: Liu Z, Wan R, Bai H and Wang J (2023) Damage-associated molecular patterns and sensing receptors based molecular subtypes in malignant pleural mesothelioma and implications for immunotherapy. Front. Immunol. 14:1104560. doi: 10.3389/fimmu.2023.1104560
Received: 21 November 2022; Accepted: 10 March 2023;
Published: 24 March 2023.
Edited by:
Chiara Porta, University of Eastern Piedmont, ItalyReviewed by:
Rebecca Kesselring, University of Freiburg Medical Center, GermanySabahattin Cömertpay, Kahramanmaras Sütçü Imam University, Türkiye
Paul Baas, The Netherlands Cancer Institute (NKI), Netherlands
Copyright © 2023 Liu, Wan, Bai and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Wang, emxodXhpQDE2My5jb20=
 Zheng Liu
Zheng Liu Rui Wan
Rui Wan Hua Bai
Hua Bai Jie Wang
Jie Wang