- 1Department of Respiratory Diseases, Shenzhen Children’s Hospital Affiliated to Shantou University Medical College, Shenzhen, China
- 2Department of Research and Development, Shenzhen Nuclear Gene Technology Co., Ltd., Shenzhen, China
- 3Department of Pharmacy, Shenzhen Children’s Hospital Affiliated to Shantou University Medical College, Shenzhen, China
- 4Clinical Laboratory, Shenzhen Children’s Hospital Affiliated to Shantou University Medical College, Shenzhen, China
- 5Department of Radiology, Shenzhen Children’s Hospital Affiliated to Shantou University Medical College, Shenzhen, China
Talaromyces marneffei and Pneumocystis jirovecii are the common opportunistic pathogens in immunodeficient patients. There have been no reports of T. marneffei and P. jirovecii coinfection in immunodeficient children. Signal transducer and activator of transcription 1 (STAT1) is a key transcription factor in immune responses. STAT1 mutations are predominately associated with chronic mucocutaneous candidiasis and invasive mycosis. We report a 1-year-2-month-old boy diagnosed with severe laryngitis and pneumonia caused by T. marneffei and P. jirovecii coinfection, which was confirmed by smear, culture, polymerase chain reaction and metagenome next-generation sequencing of bronchoalveolar lavage fluid. He has a known STAT1 mutation at amino acid 274 in the coiled-coil domain of STAT1 according to whole exome sequencing. Based on the pathogen results, itraconazole and trimethoprim-sulfamethoxazole were administered. This patient’s condition improved, and he was discharged after two weeks of targeted therapy. In the one-year follow-up, the boy remained symptom-free without recurrence.
Introduction
Talaromyces marneffei is one of the common opportunistic pathogens prevalent in southeast Asia (1). Pneumocystis jirovecii most commonly affects immunocompromised individuals worldwide (2). Signal transducer and activator of transcription 1 (STAT1) is the primary transcription factor downstream of interferons and cytokines, so it plays a major role in normal immune responses, particularly to viral, bacterial, and fungal pathogens (3). STAT1 mutations have been identified worldwide since their discovery in 2003. The clinical manifestations associated with STAT1 mutations are unexpectedly broad, including chronic mucocutaneous candidiasis, and susceptibility to various viruses, bacteria, and invasive fungi (4). T. marneffei and P. jirovecii infection have been reported separately in individuals carrying STAT1 mutations (5, 6). Here, we present a boy carrying a known STAT1 mutation, with complicated and repeated infections characterized by rare T. marneffei and P. jirovecii coinfection. To the best of our knowledge, this is the first case of such mixed infection in immunodeficient children.
Case presentation
A 1-year-2-month-old boy was admitted to our hospital because of a cough and wheezing for half a month. On admission, the child had dyspnea, wheezing, and moist rales can be heard in the lungs. Laboratory data revealed the white blood cell (WBC) count of 17.89×109/L and the C-reactive protein (CRP) concentration of 8.65 mg/L. Electronic bronchoscope showed endobronchial inflammation (Figure 1A). Electronic fiber laryngoscope indicated laryngitis. Chest computed tomography (CT) revealed inflammatory lesions, nodules, and swelling lymph nodes. The bronchoalveolar lavage fluid (BALF) polymerase chain reaction (PCR) test of Mycoplasma pneumoniae was weakly positive. The BALF culture showed Streptococcus pneumoniae (amoxicillin sensitive). After admission, the patient was given amoxicillin sulbactam (on days 2-6) and azithromycin (on days 5-7) for anti-infective therapy (Figure 2). He was discharged on day 8 with amoxicillin-clavulanate potassium (on days 8-14) and azithromycin (on days 12-14). He returned on day 15 for cough, wheezing, and trachyphonia, with a temperature of 37.0°C. The throat swab PCR tests showed positive Rhinovirus (RHV), Adenovirus, and Epstein-Barr virus (EBV). He was diagnosed with acute laryngitis. Anti-infective therapy was switched to methylprednisolone (on day 15), followed by prednisone (on days 16-20) (Figure 2). He was discharged home on day 18 with intermittent coughing.
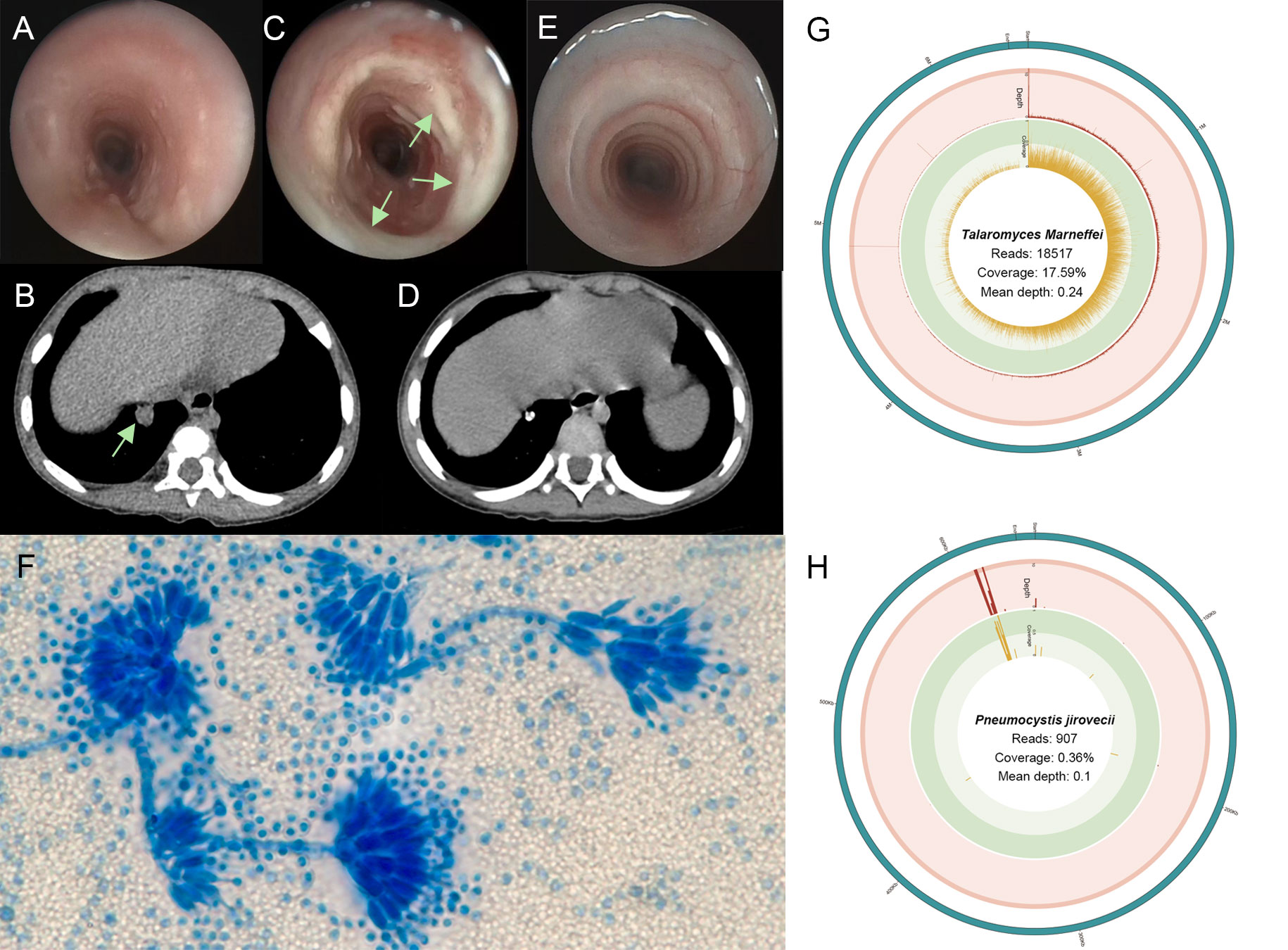
Figure 1 (A) The first bronchoscope showing a little mucus in the airway. (B) Three-dimensional computed tomography reconstruction of lung window showing a round and high-density shadow in the basal segment (arrows). (C) The bronchoscopic image showing plenty of white secretion in the tracheal inner membrane (arrows). (D) After one year, chest computed tomography showed the nodule shadows was smaller than before, and the calcification was obvious. (E) One year after treatment, tracheoscopy showed no secretion adhesion in the trachea. (F) The lactophenol cotton blue of lavage fluid-stained slide on day 33 showing Talaromyces marneffei with broom-like branches (oil immersion lens, 1000× magnification). (G) T. marneffei coverage and depth in BALF metagenome next-generation sequencing (mNGS). (H) Pneumocystis jirovecii coverage and depth in BALF mNGS.
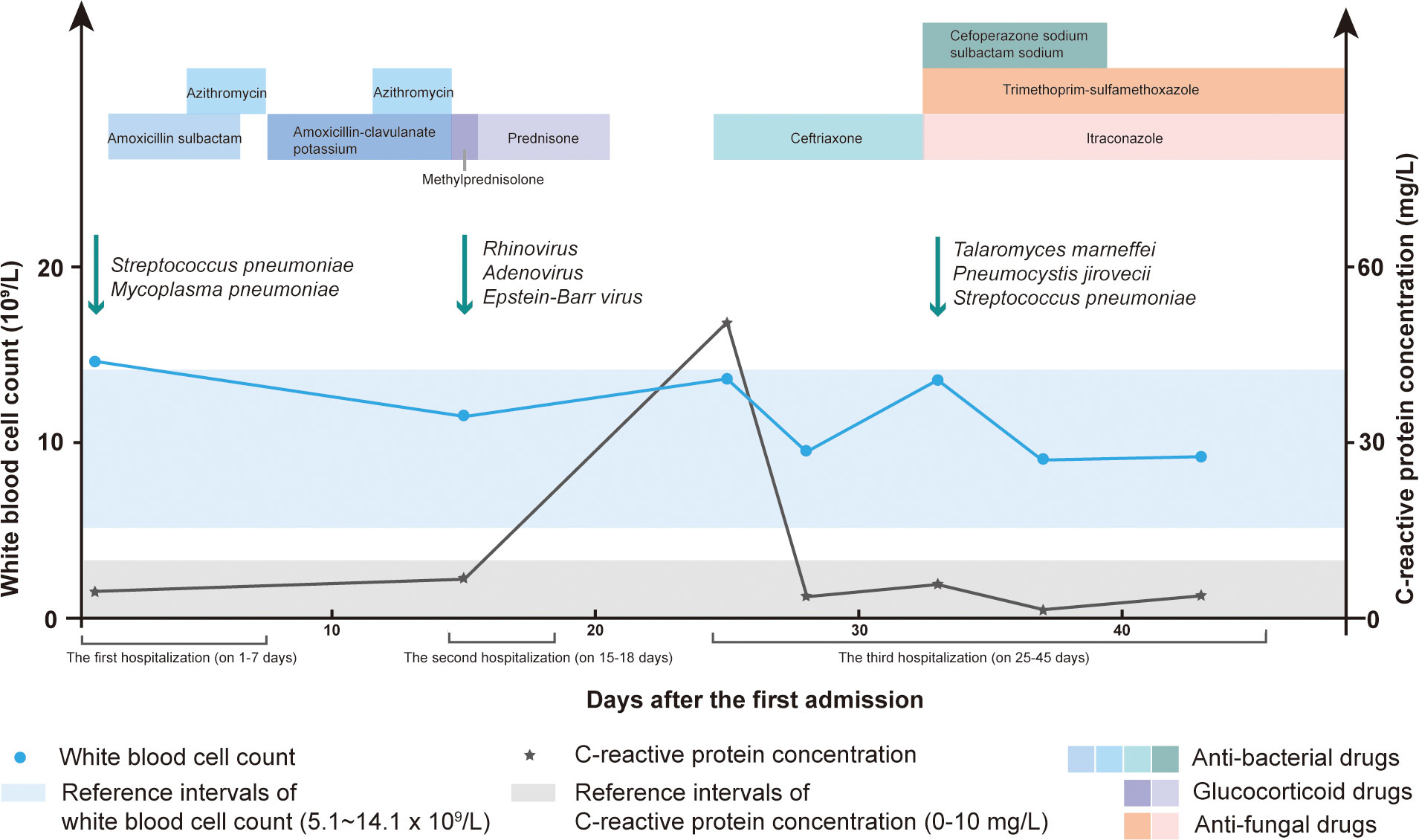
Figure 2 The white blood cell (WBC) count, C-reactive protein (CRP) concentration, detected pathogens and therapy methods during the hospital stay.
One week later, he returned because of shortness of breath, aggravated trachyphonia, and fever. Upon admission, CRP concentration was elevated (50.84mg/L) (Figure 2). On day 26, Chest CT showed multiple enlarged necrotic lymph nodes in the hilus and mediastinum and a high-density round shadow in the basal segment in the right lung inferior lobe (Figure 1B). He was given ceftriaxone (on days 25-32) as an antibacterial treatment. But the symptoms did not improve. On day 32, the second bronchoscope observed plenty of mucus in the inner tracheal membrane (Figure 1C). Various test methods were executed immediately to identify the pathogens. The BALF smear and culture revealed T. marneffei (Figure 1F). By the same BALF token, PCR tests for targeted pathogen detection and metagenome next-generation sequencing (mNGS) for unbiased pathogen detection were performed. The PCR results revealed P. jirovecii. BALF mNGS identified 1515121 microbial sequence reads, of which 18517 reads and 907 reads mapped to T. marneffei (Figure 1G) and P. jirovecii (Figure 1H), respectively. 158 reads aligned to S. pneumoniae. Following the pathogen results, cefoperazone sodium sulbactam sodium (on days 33-39), itraconazole (on days 33-45), and trimethoprim-sulfamethoxazole (on days 33-45) were commenced as the targeted antimicrobial therapy (Figure 2).
He had no history of exposure to wild bamboo rats, and his HIV test result was negative. His humoral immunity of IgG, IgA, IgM, IgE, C3, and C4 was normal. The fine immunoassay of lymphocytes showed impaired B cell differentiation, and the number of CD4 T cells and natural killer (NK) cells were 2365.88 and 63.80 cells/ul, respectively (Supplementary Table 1). Considering that T. marneffei and P. jirovecii are the main opportunistic pathogens in patients with immune deficiency, genetic test was recommended to clarify the genetic risk of immunodeficiency. Whole-exome sequencing (WES) results identified a missense variant c.820C>T (p.R274W) in the STAT1 gene. According to the American College of Medical Genetics and Genomics standard, this mutation should be categorized as pathogenic, with proofs of PS4+PM1+PM2+PM5+PM6+PP3. Verification of this variant site using sanger sequencing showed negative results in his family, and it was a de novo variant in this patient (Figure 3). STAT1 mutation can inhibit the differentiation of T cells into T-helper 17 (Th17) cells, resulting in a decrease in IL-17 secretion, which is closely related to chronic mucocutaneous candidiasis and invasive mycosis. This boy’s evident decline in Th17 cells through flow cytometry confirmed the consistency between gene mutation and phenotype (Supplementary Table 1).
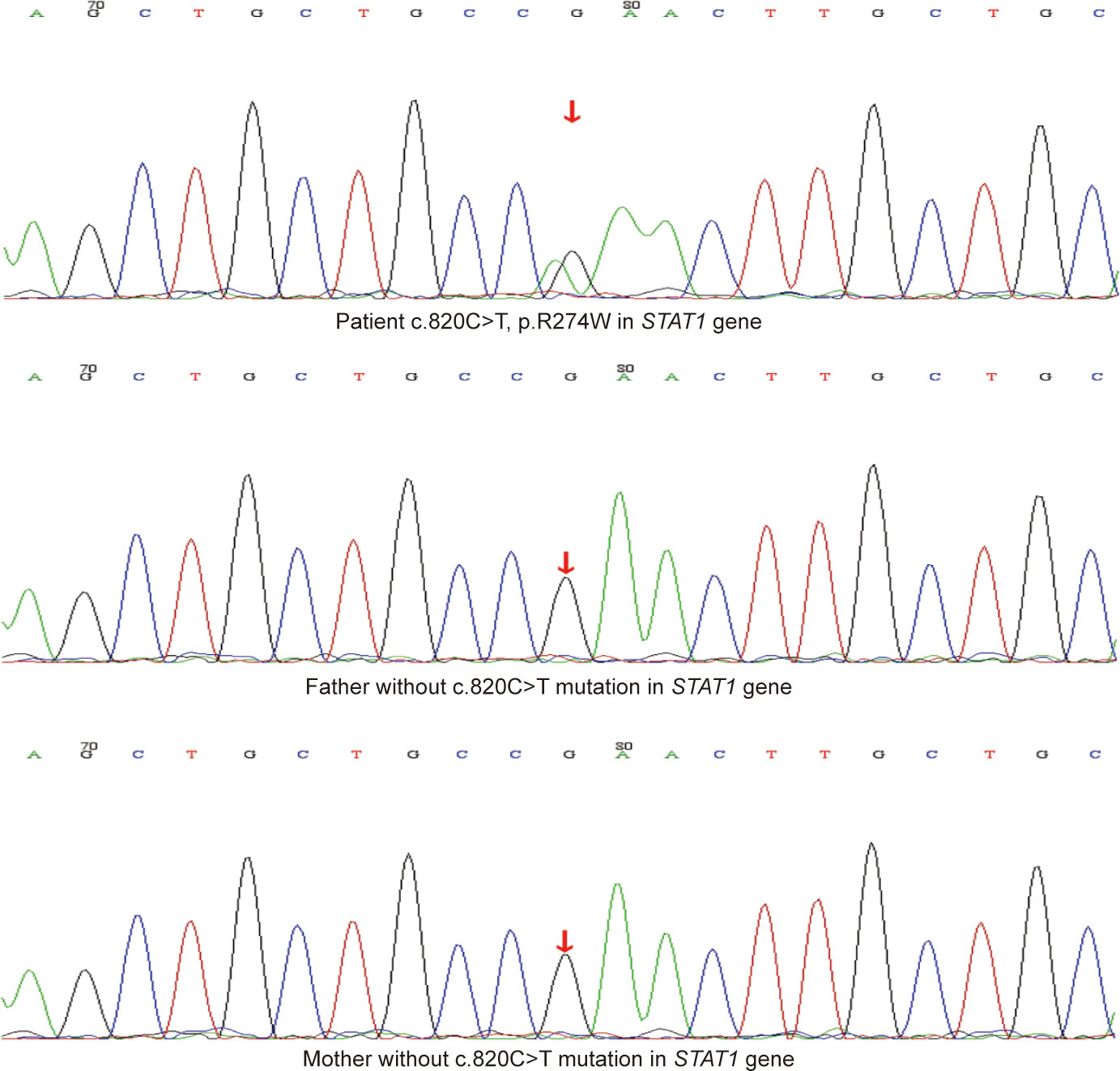
Figure 3 The Sanger sequencing results of the mutation site (c.820C>T, p.R274W) in STAT1 gene of the patient and his family.
On day 45, his symptoms improved significantly. The patient was discharged with itraconazole and trimethoprim-sulfamethoxazole until now. One year after discharge, the chest CT image was improved, indicating calcification of the primary lesion (Figure 1D). The bronchoscope showed that the white mucus in the tracheal membrane disappeared totally (Figure 1E).
Discussion
To the best of our knowledge, this is the first coinfection case with T.marneffei and P. jirovecii in immunodeficient children. The mixed infection cases related to T.marneffei or P. jirovecii in HIV-negative children are listed in Table 1. 11 cases (68.8%) and 9 cases (56.3%) with T.marneffei infection (16 cases) showed bacteria and virus mixed infection, respectively. The reported bacteria mainly contained S. pneumoniae (n=3, 18.8%), Klebsiella pneumoniae (n=2, 12.5%), Moraxella catarrhalis (n=2, 12.5%), Mycobacterium Tuberculosis (n=2, 12.5%), and M. pneumoniae (n=2, 12.5%). Cytomegalovirus (CMV, n=2, 12.5%), EBV (n=3, 18.8%), Hepatitis B virus (n=2, 12.5%), and RHV (n=2, 12.5%) were more common in the mixed virus infection. 6 cases (40%) with fungi coinfection of T.marneffei all belong to Candida spp. More than half of P. jirovecii mixed infection cases (n=16) showed coinfection with bacteria (62.5%, n=10) or virus (62.5%, n=10). The most frequent bacterium was Haemophilus influenzae (n=3, 18.8%), followed by Pseudomonas aeruginosa (n=2, 12.5%) and S. pneumoniae (n=2, 12.5%). The virus included in P. jirovecii cases were CMV (n=4, 25%) and RHV (n=4, 25%). Only two children (13.3%) showed mixed fungi infection, caused by Aspergillus fumigatus. In this case, testing results of BALF identified bacterial infection of S. pneumoniae and fungi infection of T.marneffei and P. jirovecii. The rare coinfection of T.marneffei and P. jirovecii provided a reference for higher awareness of mixed fungi infections.
Polymicrobial infections are important features of immunocompromised hosts and affect prognosis. Early and accurate pathogen diagnosis is particularly crucial in these patients. As the methods listed in Table 1, smear, culture, PCR, and mNGS are commonly used for pathogen detection. T. marneffei is usually diagnosed by microscopy and cultivation based on its morphological and dimorphic characteristics (19). Our patient was diagnosed with T. marneffei infection because of positive BALF smear, culture, and mNGS. Since P. jirovecii is hard to be cultured, definitive diagnosis requires detection and identification of the organism mainly by dye staining or PCR (2, 17). In this case, the P. jirovecii infection was diagnosed by PCR and mNGS assays of BALF. T. marneffei and P. jirovecii were identified in one test of mNGS, but not accomplished in one assay of culture, smear, or PCR. Considering the high risk of mixed infection in immunocompromised individuals, timely use of mNGS could play a positive role in avoiding missed diagnoses and improving prognosis (20).
T.marneffei mainly causes upper or lower respiratory infection, especially pulmonary infection, in immunocompromised individuals with HIV infection or functional impairments of cellular immunity (21). The dimorphic ability of T.marneffei to switch from environmental mycelium to parasitic yeast form is recognized as a challenging virulence factor to host immune defenses (1). P. jirovecii most commonly affects the respiratory function of immunocompromised patients, possibly with nonspecific signs of fever, cough, and dyspnea (2). Adherence of P. jirovecii to alveoli and the host’s inflammatory response are the main reasons causing significant lung injury, hypoxia, or even respiratory failure (2). Except for the common symptoms of fever and pneumonia in fungi infection, our patient manifested trachyphonia. The inner tracheal membrane was the rare infection site for these two pathogens, thus, accumulating experience of the infection sites and manifestations is beneficial for promoting early diagnosis and timely therapy.
T.marneffei and P. jirovecii are opportunistic pathogenic fungi that have a major impact on immunocompromised patients. This boy was diagnosed with primary immunodeficiency caused by STAT1 R274W mutation, with proofs of WES and sanger sequencing. Among the mutation regions in STAT1, the 274th amino acid of arginine (R274), which is in the coiled-coil domain, is one of the most common mutation sites found in more than 70 patients (4, 22–24). The STAT family members can be activated through phosphorylation. Briefly, they are phosphorylated by the receptor-associated kinases, then form homodimers or heterodimers that translocate from the cytoplasm to the nucleus and bind to the specific DNA consensus sequences to induce target gene transcription. Additionally, STAT1 influences the transcription of STAT3-inducible genes, as STAT1 and STAT3 compete for the DNA-binding sites (25). STAT1 R274W mutation leads to an increased phosphorylated STAT1, thus, called gain-of-function (GOF) mutation (26). In line with the abundant downstream genes regulated by the STAT family, the clinical spectrum associated with immunodeficient patients carrying STAT1 mutation was unexpectedly broad (4, 27). In statistics of more than 250 STAT1 GOF patients, most STAT1 patients had normal total T (75.6%) and CD4+ T (68.1%) lymphocytes, only a few patients showed increased total T (1.4%) and CD4+ T (1.1%) lymphocytes (28). Leiding analyzed one STAT1 R274W case, diagnosed with chronic mucocutaneous candidiasis, mycotic cerebral aneurysms, and pneumonia (caused by H. influenzae, P. aeruginosa, S. pneumoniae), showing T cell lymphopenia (24). Different from the observations of Leiding, our patient had normal T lymphocyte counts but increased CD4+ T cells. In a case review, 87.8% of the 90 patients with STAT1 GOF mutation showed Th17 cytopenia, and the remaining 12.2% of patients presented normal levels of Th17 cells (28). Similar to most cases, the boy had decreased Th17 of CD3+. The GOF mutation can decrease IL-17 secretion through two mechanisms, 1) directly inhibits the differentiation of T cells into Th17 cells; 2) impairs the pathway that IL-6, IL-21, and IL-23 induce Th17 cell differentiation through STAT3 (29). The decreased Th17 differentiation impairs IL-17 function in the defense against extracellular pathogens like fungi, which might explain the susceptibility of our patient to T.marneffei and P. jirovecii (29, 30). Interestingly, the CD4+ subset analysis was also performed in our patient, and the decreased CD4+ effector memory (EM) was observed, which might be following one of the differentiation models that CD4+ EM are generated from Th17 (31). However, the roles and biology of memory CD4+ cells are complex and less well understood. There are 32.1% of 209 STAT1 GOF patients with a reduced percentage of NK cells and 1.4% with increased NK cells, while most cases showed normal NK cells (28). In this study, the declined NK cells were consistent with a few cases. The impaired NK cell proliferation was associated with increased STAT1 phosphorylation and reduced STAT5 activation in NK cells of STAT1 GOF patients (32). NK lymphocytes confer a primary immune response against intracellular pathogens and virally infected cells. Therefore, our patient’s severely reduced NK cells indicated an impaired defense against intracellular T.marneffei (1, 32). In the 264 STAT1 GOF patients summarized by Zhang, 74.2% had normal B lymphocytes (28). Consistently, our patient presented normal B lymphocytes. Among the 63 STAT1 GOF patients for whom memory B cell data were available, 50.8% had a reduced memory B lymphocyte subset (28). Our patient presented lower memory B lymphocytes and impaired B-cell differentiation, common with a STAT1 R274W patient with disseminated Cryptococcosis (22). Since the activation of STAT1, STAT3, and STAT5 is fundamental for the differentiation of human B cells into memory cells, the B cell differentiation might be impaired by the higher level of STAT1 phosphorylation in STAT1 GOF patients (33, 34). Although reported STAT1 cases are increasing, there have been no reports of T.marneffei and P. jirovecii coinfection. The immune responses of our STAT1 GOF patient illustrated the complexity of STAT1- associated immunodeficiency, which needs additional research.
The treatment for mixed infection was challenging and lacked a standard. Amphotericin B is highly effective as induction therapy for T.marneffei infection, but can cause serious adverse effects, such as liver and kidney damage and severe hypokalemia (35). Voriconazole and itraconazole are more frequently used in children for anti-fungal therapy and have been confirmed to be safe and effective (36, 37). The first-line treatment choice for P. jirovecii pneumonia is trimethoprim-sulfamethoxazole (2). Considering the severely mixed fungi infection and the persistent fungal susceptibility in primary immunodeficient patients, the boy was given long-term itraconazole and trimethoprim-sulfamethoxazole as the dominating treatments for therapy and precaution (38). The subsequent anti-bacterial therapy was short-term due to the low copy numbers of S. pneumoniae and the anti-bacterial treatments administered before. The child improved significantly and showed no recurrent infections in the one-year follow-up, which suggested a successful therapy for unusual mixed fungi infection.
Conclusion
When anti-infective treatment is ineffective, pathogens are hard to be detected by conventional methods. It is necessary to consider opportunistic pathogen infections. mNGS can rapidly and accurately identify the pathogen, especially for the mixed infections, helping clinical decision-making. When T. marneffei and P. jirovecii co-infection occurs, a genetic test should be taken to discover underlying immunodeficiency disease, achieve an early diagnosis, and improve the patient’s prognosis.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Shenzhen Children’s Hospital. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
QY and CY analyzed data and wrote the paper. YW, KC, XL and WC collected patients’ clinical data and modified the paper. LC, YB and SZ made the figures and tables. WW, YZ and HZ supervised the whole writing process. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Shenzhen Key Medical Discipline Construction Fund (No. SZXK032) and Shenzhen Fund for Guangdong Provincial High-level Clinical Key Specialties (No. SZGSP012).
Acknowledgments
We are very appreciative to the child and his families.
Conflict of interest
Authors CY, LC, SZ, YB and HZ are employed by Shenzhen Nuclear Gene Technology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1103184/full#supplementary-material
Supplementary Table 1 | The results of flow cytometry for immune cells.
References
1. Pruksaphon K, Nosanchuk JD, Ratanabanangkoon K, Youngchim S. Talaromyces marneffei infection: Virulence, intracellular lifestyle and host defense mechanisms. J fungi (Basel Switzerland) (2022) 8(2):200. doi: 10.3390/jof8020200
2. Truong J, Ashurst JV. Pneumocystis jirovecii pneumonia. In: StatPearls. (Florida, United States of America: StatPearls Publishing) (2022).
3. Fleisher TA, Oliveira JB, Torgerson TR. Congenital immune dysregulation disorders. In: Pediatric allergy: Principles and practice. Amsterdam, The Netherlands: Elsevier (2016). p. 124–32.
4. Toubiana J, Okada S, Hiller J, Oleastro M, Lagos Gomez M, Becerra A, et al. Heterozygous STAT1 gain-of-function mutations underlie an unexpectedly broad clinical phenotype. Blood (2016) 127(25):3154–64. doi: 10.1182/blood-2015-11-679902
5. Chen K, Tan J, Qian S, Wu S, Chen Q. Case report: Disseminated Talaromyces marneffei infection in a patient with chronic mucocutaneous candidiasis and a novel STAT1 gain-of-Function mutation. Front Immunol (2021) 12:682350. doi: 10.3389/fimmu.2021.682350
6. van de Veerdonk FL, Plantinga TS, Hoischen A, Smeekens SP, Joosten LA, Gilissen C, et al. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. New Engl J Med (2011) 365(1):54–61. doi: 10.1056/NEJMoa1100102
7. Wang L, Luo Y, Li X, Li Y, Xia Y, He T, et al. Talaromyces marneffei infections in 8 Chinese children with inborn errors of immunity. Mycopathologia (2022) 187(5–6):455–467. doi: 10.1007/s11046-022-00659-0
8. Guo J, Li BK, Li TM, Wei FL, Fu YJ, Zheng YQ, et al. Characteristics and prognosis of Talaromyces marneffei infection in non-HIV-Infected children in southern China. Mycopathologia (2019) 184(6):735–45. doi: 10.1007/s11046-019-00373-4
9. Chen X, Xu Q, Li X, Wang L, Yang L, Chen Z, et al. Molecular and phenotypic characterization of nine patients with STAT1 GOF mutations in China. J Clin Immunol (2020) 40(1):82–95. doi: 10.1007/s10875-019-00688-3
10. Fan JH, Luo HY, Yang LG, Wang MY, Xiao ZH. Penicilliosis marneffei in HIV negative children: three case reports. Ann palliative Med (2021) 10(7):8437–47. doi: 10.21037/apm-20-2056
11. Tang W, Zhang Y, Luo C, Zhou L, Zhang Z, Tang X, et al. Clinical application of metagenomic next-generation sequencing for suspected infections in patients with primary immunodeficiency disease. Front Immunol (2021) 12:696403. doi: 10.3389/fimmu.2021.696403
12. Li J, Miao H, Wu L, Fang Y. Interstitial pneumonia as the initial presentation in an infant with a novel mutation of CD40 ligand-associated X-linked hyper-IgM syndrome: A case report. Medicine (2020) 99(24):e20505. doi: 10.1097/MD.0000000000020505
13. Lyu J, Deng Q, Li R, Tian B, Zhao Y, Hu X, et al. Pneumonia caused by coinfection with Cytomegalovirus and Pneumocystis jirovecii in an HIV-negative infant diagnosed by metagenomic next-generation sequencing. Infect Drug resistance (2022) 15:3417–25. doi: 10.2147/IDR.S364241
14. Varadaraju S, Khandelwal P, Sankar J, Hari P. Multiple opportunistic infection-associated hemophagocytic lymphohistiocytosis in nephrotic syndrome: A case report. J Pediatr Crit Care (2021) 8(6):295. doi: 10.4103/jpcc.jpcc_64_21
15. Sedighi P, Esfahani H. Pneumothorax and acute kidney injury in the early phase of acute lymphoblastic leukemia induction therapy due to Aspergillus fumigatus and pneumocystis jirovecii co-infection: A case report. Iranian J Blood Cancer (2019) 11(4):139–42.
16. Menu E, Driouich JS, Luciani L, Morand A, Ranque S, L'Ollivier C. Detection of Pneumocystis jirovecii in hospitalized children less than 3 years of age. J fungi (Basel Switzerland) (2021) 7(7):546. doi: 10.3390/jof7070546
17. Chen Y, Ai L, Zhou Y, Zhao Y, Huang J, Tang W, et al. Rapid and precise diagnosis of pneumonia coinfected by Pneumocystis jirovecii and Aspergillus fumigatus assisted by next-generation sequencing in a patient with systemic lupus erythematosus: a case report. Ann Clin Microbiol antimicrob (2021) 20(1):47. doi: 10.1186/s12941-021-00448-5
18. Musallam N, Bamberger E, Srugo I, Dabbah H, Glikman D, Zonis Z, et al. Legionella pneumophila and Pneumocystis jirovecii coinfection in an infant treated with adrenocorticotropic hormone for infantile spasm: case report and literature review. J Child Neurol (2014) 29(2):240–2. doi: 10.1177/0883073813511148
19. Ning C, Lai J, Wei W, Zhou B, Huang J, Jiang J, et al. Accuracy of rapid diagnosis of Talaromyces marneffei: A systematic review and meta-analysis. PloS One (2018) 13(4):e0195569. doi: 10.1371/journal.pone.0195569
20. Zheng Y, Qiu X, Wang T, Zhang J. The diagnostic value of metagenomic next-generation sequencing in lower respiratory tract infection. Front Cell infect Microbiol (2021) 11:694756. doi: 10.3389/fcimb.2021.694756
21. Narayanasamy S, Dougherty J, van Doorn HR, Le T. Pulmonary talaromycosis: A window into the immunopathogenesis of an endemic mycosis. Mycopathologia (2021) 186(5):707–15. doi: 10.1007/s11046-021-00570-0
22. Nemoto K, Kawanami T, Hoshina T, Ishimura M, Yamasaki K, Okada S, et al. Impaired b-cell differentiation in a patient with STAT1 gain-of-Function mutation. Front Immunol (2020) 11:557521. doi: 10.3389/fimmu.2020.557521
23. Okada S, Asano T, Moriya K, Boisson-Dupuis S, Kobayashi M, Casanova JL, et al. Human STAT1 gain-of-Function heterozygous mutations: Chronic mucocutaneous candidiasis and type I interferonopathy. J Clin Immunol (2020) 40(8):1065–81. doi: 10.1007/s10875-020-00847-x
24. Leiding JW, Okada S, Hagin D, Abinun M, Shcherbina A, Balashov DN, et al. Hematopoietic stem cell transplantation in patients with gain-of-function signal transducer and activator of transcription 1 mutations. J Allergy Clin Immunol (2018) 141(2):704–717.e5. doi: 10.1016/j.jaci.2017.03.049
25. Bloomfield M, Zentsova I, Milota T, Sediva A, Parackova Z. Immunoprofiling of monocytes in STAT1 gain-of-function chronic mucocutaneous candidiasis. Front Immunol (2022) 13:983977. doi: 10.3389/fimmu.2022.983977
26. Zimmerman O, Olbrich P, Freeman AF, Rosen LB, Uzel G, Zerbe CS, et al. STAT1 gain-of-Function mutations cause high total STAT1 levels with normal dephosphorylation. Front Immunol (2019) 10:1433. doi: 10.3389/fimmu.2019.01433
27. Tolomeo M, Cavalli A, Cascio A. STAT1 and its crucial role in the control of viral infections. Int J Mol Sci (2022) 23(8):4095. doi: 10.3390/ijms23084095
28. Zhang W, Chen X, Gao G, Xing S, Zhou L, Tang X, et al. Clinical relevance of gain- and loss-of-Function germline mutations in STAT1: A systematic review. Front Immunol (2021) 12:654406. doi: 10.3389/fimmu.2021.654406
29. Liu L, Okada S, Kong XF, Kreins AY, Cypowyj S, Abhyankar A, et al. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J Exp Med (2011) 208(8):1635–48. doi: 10.1084/jem.20110958
30. Lionakis MS, Drummond RA, Hohl TM. Immune responses to human fungal pathogens and therapeutic prospects. nature reviews. Immunology (2023) 41:1–20. doi: 10.1038/s41577-022-00826-w
31. Raphael I, Joern RR, Forsthuber TG. Memory CD4+ T cells in immunity and autoimmune diseases. Cells (2020) 9(3):531. doi: 10.3390/cells9030531
32. Tabellini G, Vairo D, Scomodon O, Tamassia N, Ferraro RM, Patrizi O, et al. Impaired natural killer cell functions in patients with signal transducer and activator of transcription 1 (STAT1) gain-of-function mutations. J Allergy Clin Immunol (2017) 140(2):553–564.e4. doi: 10.1016/j.jaci.2016.10.051
33. Pelham SJ, Caldirola MS, Avery DT, Mackie J, Rao G, Gothe F, et al. STAT5B restrains human b-cell differentiation to maintain humoral immune homeostasis. J Allergy Clin Immunol (2022) 150(4):931–46. doi: 10.1016/j.jaci.2022.04.011
34. Deenick EK, Avery DT, Chan A, Berglund LJ, Ives ML, Moens L, et al. Naive and memory human b cells have distinct requirements for STAT3 activation to differentiate into antibody-secreting plasma cells. J Exp Med (2013) 210(12):2739–53. doi: 10.1084/jem.20130323
35. Hamill RJ. Amphotericin b formulations: a comparative review of efficacy and toxicity. Drugs (2013) 73(9):919–34. doi: 10.1007/s40265-013-0069-4
36. Zeng Q, Jin Y, Yin G, Yang D, Li W, Shi T, et al. Peripheral immune profile of children with talaromyces marneffei infections: a retrospective analysis of 21 cases. BMC Infect Dis (2021) 21(1):287. doi: 10.1186/s12879-021-05978-z
37. Lee PP, Mao H, Yang W, Chan KW, Ho MH, Lee TL, et al. Penicillium marneffei infection and impaired IFN-γ immunity in humans with autosomal-dominant gain-of-phosphorylation STAT1 mutations. J Allergy Clin Immunol (2014) 133(3):894–6.e5. doi: 10.1016/j.jaci.2013.08.051
Keywords: Talaromyces marneffei, Pneumocystis jirovecii, coinfection, STAT1, metagenome next-generation sequencing
Citation: Yang Q, Yu C, Wu Y, Cao K, Li X, Cao W, Cao L, Zhang S, Ba Y, Zheng Y, Zhang H and Wang W (2023) Unusual Talaromyces marneffei and Pneumocystis jirovecii coinfection in a child with a STAT1 mutation: A case report and literature review. Front. Immunol. 14:1103184. doi: 10.3389/fimmu.2023.1103184
Received: 20 November 2022; Accepted: 08 February 2023;
Published: 20 February 2023.
Edited by:
John Bernard Ziegler, Sydney Children’s Hospital, AustraliaReviewed by:
Amy P. Hsu, National Institute of Allergy and Infectious Diseases (NIH), United StatesYae-Jean Kim, Sungkyunkwan University, Republic of Korea
Copyright © 2023 Yang, Yu, Wu, Cao, Li, Cao, Cao, Zhang, Ba, Zheng, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenjian Wang, d3dqeHhAMTI2LmNvbQ==; Hezi Zhang, aGV6aXpoYW5nMjAyMEAxNjMuY29t
 Qin Yang1
Qin Yang1 Chendi Yu
Chendi Yu Yue Wu
Yue Wu Ke Cao
Ke Cao Lichao Cao
Lichao Cao Yuejie Zheng
Yuejie Zheng Hezi Zhang
Hezi Zhang Wenjian Wang
Wenjian Wang