
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 20 January 2023
Sec. Cancer Immunity and Immunotherapy
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1100079
This article is part of the Research Topic Immunomodulatory Factors, Conversion, and Postoperative Adjuvant Therapy for Hepatobiliary Tumors Based on Immunotherapy View all 5 articles
Immune checkpoint inhibitors (ICIs) have revolutionized cancer treatment in recent years and provide new opportunities to treat hepatocellular carcinoma (HCC). To date, several ICIs have been approved by the FDA for advanced HCC in first-line or second-line therapy. Downstaging conversion therapy for potentially resectable HCC to provide opportunities for surgical intervention is challenging. ICIs have become a hot spot in this field due to their high response rate. However, HCC has various etiologies and can evade the immune system through multiple mechanisms, which limit the efficacy of ICI monotherapy and demand novel combination strategies. Radiation therapy (RT) is also a candidate for conversion therapy in HCC and is currently gaining increasing attention as a good combination partner with ICIs due to its ability to modulate the tumor microenvironment. In this review, we illustrate the current indications for ICIs and RT in HCC, the rationale for their synergistic combination, and the current clinical trials in combination therapy. We also speculate on predictive biomarkers and novel future strategies to further enhance the efficacy of this combination. This review aims to provide references for future research on radiation and immunotherapy to arrive at a promising new era of HCC treatment.
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer-related death globally (1). According to clinical practice guidelines and consensus (2–7), including the EASL and ESMO guidelines (2, 3), the standard treatments for early tumors include resection, liver transplantation, and local ablation, and those for intermediate-stage tumors include transarterial chemoembolization (TACE) and systemic drugs. Although several breakthroughs have occurred, the median overall survival is only 20~30 months for intermediate stages and 10~19 months for advanced-stage HCC (8). At the time of diagnosis, approximately 64% of HCC patients are identified with stage B or C disease [Barcelona Clinic Liver Cancer (BCLC)], losing the opportunity for radical hepatectomy (9, 10). Therefore, novel treatment approaches, especially conversion therapy for unresectable HCC patients, are desperately needed in the clinic.
Downstaging conversion therapy for potentially resectable HCC to provide opportunities for surgical intervention is challenging. HCC was proven to be an immunosuppressive microenvironment and related to inflammation (11). The expression of programmed death-ligand 1 (PD-L1) correlates with prognosis in resected HCC patients (12). The upregulation of PD-1 and PD-L1 is associated with more advanced stages and higher recurrence risks (13), which suggests the benefit of immunotherapy in HCC patients. Currently, several immune checkpoint inhibitors (ICIs), including anti-PD1 (nivolumab, pembrolizumab, and nivolumab plus ipilimumab) and anti-PD-L1 (atezolizumab, in combination with bevacizumab) therapies, have received FDA approval and provided new opportunities to treat advanced HCC (14).
Radiotherapy is a promising treatment for unresectable HCC, with a local control rate of 60%~100% at 2 years (15–17). Radiation can induce immunogenic cell death, promote the immune system (18), and reprogram the tumor microenvironment (19). The combination of radiation and immunotherapy offers better local tumor regression and systemic control than single treatments (20, 21), as reported in multiple tumor types (22, 23).
In this review, we will illustrate the current indications for immunotherapy and radiation, especially in the field of neoadjuvant or conversion therapy. Second, we will briefly summarize the current clinical trials in combination therapy and the combination scheme. Finally, we will speculate on predictive biomarkers and future research directions for immunotherapy.
The international multicenter phase III IMbrave150 study showed that atezolizumab plus bevacizumab significantly improved overall survival (19.2 vs. 13.4 months) and progression-free survival (6.9 months vs. 4.3 months) compared with sorafenib (24). Based on this research, atezolizumab plus bevacizumab was approved by the FDA as a first-line systematic treatment for unresectable HCC. The ORIENT-32 study was similar to research in China, in which sintilimab plus a bevacizumab biosimilar (IBI305) was approved as the first-line treatment for unresectable HCC by the National Medical Products Administration (NMPA) in 2021 (25). Several phase I/II clinical studies, such as the RESCUE study of camrelizumab plus apatinib (26) and KEYNOTE−524 study of pembrolizumab combined with lenvatinib, also demonstrated the efficacy and controllable safety of combined immunotherapy. The phase III study CheckMate 459 of single ICI nivolumab (NIVO) compared with sorafenib (SOR) did not achieve the primary endpoint OS but demonstrated improved safety, which also provided a choice for patients with contraindications for antiangiogenic targeted therapy.
At present, a phase III clinical study of combined immunotherapy results for HCC second-line therapy is lacking. However, multiple phase II studies have shown that the combination of ICIs has some advantages over monotherapy. The main evidence included (1) single ICI treatment: nivolumab (CheckMate 040 study) (27), pembrolizumab (KEYNOTE 224 study) (27), camrelizumab (28), and terelizumab (RATIONALE 208) (2); ICIs combined with antiangiogenic targeted therapy: camrelizumab combined with apatinib (RESCUE study) (26); and (3) ICI combination therapy: nivolumab (NIVO) + ipilimumab (CheckMate 040 study) (29).
Conversion therapy for HCC includes the conversion of surgically unresectable lesions, such as insufficient volume of the future liver remnant (FLR), to resectable lesions, and it supports the conversion of R1 and R2 resection to R0 resection. Specifically, conversion therapy is characterized by active treatment regimens with a relatively high response rate. Previous studies have shown that radiotherapy, interventional therapy, and targeted therapy can convert 11%~27% of advanced HCC with portal vein tumor thrombus (PVTT) into surgical resection (30–32). ICIs combined with antiangiogenic therapy improved response rates by approximately 24% to 46% with good conversion therapy potential (24, 26, 33). In particular, ICI-based therapy combined with radiotherapy for PVTT has become an important conversion strategy for advanced HCC.
The 5-year recurrence rate after surgery for HCC is as high as 50%~70%, but a standard adjuvant treatment is lacking. ICIs used as adjuvant therapy for patients with high-risk recurrence of HCC are currently a research hotspot, and some phase III clinical studies are underway, including nivolumab (NCT03383458), pembrolizumab (NCT03867084), toripalimab (NCT03859128), atezolizumab combined with bevacizumab (NCT04102098), durvalumab alone or in combination with bevacizumab (NCT03847428), and camrelizumab combined with apatinib (NCT04639180).
The purpose of radiotherapy can be divided into radical, palliative, consolidation or translational, and adjuvant (preoperative or postoperative) radiotherapy. Stereotactic radiotherapy for small HCC is aimed at radical treatment, while palliative radiation therapy is recommended for symptomatic primary HCC and/or tumor thrombus. For unresectable HCC, consolidative radiation is mostly after systemic therapy. Adjuvant radiation is recommended for resected IHC with high-risk features (34).
Intrahepatic stereotactic body radiation therapy (SBRT) for liver cancer mainly targets small hepatocellular carcinomas (microtumors). A study comparing SBRT with radiofrequency showed that the 3-year overall survival rates were similar (70.4% versus 69.1%), but the 3-year recurrence rates were 5.3% and 12.9%, showing the local control advantage of SBRT (35).
SBRT is a safe and effective bridging therapy in the waiting period prior to transplantation for HCC patients. An analysis comparing SBRT and transcatheter arterial chemoembolization (TACE) and radiofrequency ablation used as bridging therapy before liver transplantation showed similar safety and efficacy (36).
For HCC lesions larger than 5cm, with the double blood supply of the hepatic artery and portal vein, the portal venous blood supply remains intact after single TACE therapy, resulting in a residual tumor. Radiotherapy after TACE can compensate for the deficiency of TACE. A meta-analysis showed that the 3-year survival rates ranged from 24% to 44% for TACE combined with external radiotherapy, an increase of 10%~28% compared to TACE alone (37).
For patients with intermediate or advanced HCC, surgery following neoadjuvant or conversion therapy improved treatment efficacy (32, 38, 39). A meta-analysis that enrolled a total of 2577 patients with unresectable HCC showed that patients who received interventional therapy combined with radiotherapy had improved long-term survival compared with interventional therapy alone. The survival pooled odds ratios at 2, 3, 4, and 5 years were 1.55, 1.91, 3.01, and 3.98, respectively, showing that radiotherapy can significantly improve overall survival, especially long-term survival (40).
Radiotherapy is recommended as neoadjuvant treatment with surgical resection for stage IIIA HCC with PVTT, and it also improved the local tumor control rate and prolonged survival in combination with TACE. A multicenter randomized controlled study enrolled resectable HCC patients with PVTT who were randomly divided into a preoperative neoadjuvant radiotherapy group (82 cases) and a single surgery group (82 cases). The preoperative radiotherapy dose was 18 Gy/6 F for the tumor and PVTT, and surgery was performed 4 weeks after radiotherapy. The results showed that the preoperative radiotherapy group had 1- and 2-year survival rates (75.2% and 27.4%, respectively) that were significantly higher than those in the simple surgical resection group (43.1% and 9.4%, respectively) (41). Another study compared TACE plus radiotherapy with sorafenib in HCC patients with tumor thrombus, the median overall survival was longer in the TACE + radiotherapy group than in the sorafenib group (55 weeks vs. 43 weeks, P=0.04), and PFS (30 weeks vs. 11.3 weeks, P<0.01) (31).
For the majority of central HCC and a small number of peripheral HCC cases, the requirement of a surgical safety margin >1 cm is difficult to meet due to tumor proximity or the involvement of the hilar vascular trunk, even after surgical resection of the tumor. Some patients even have a positive margin, which limits the curative effect of surgery. The Chinese Academy of Medical Sciences reported the results of adjuvant radiotherapy after narrow margin resection of HCC for the first time (42). A total of 181 patients were enrolled, including 33 patients in the narrow-margin surgery combined with postoperative radiotherapy group (Group A), 83 patients in the narrow-margin surgery group who did not receive radiotherapy (Group B), and 65 patients in the wide-margin surgery group (Group C). The 3-year survival rates were 89.1%, 67.7% and 86.0%, respectively, and the 3-year disease-free survival rates were 64.2%, 52.5% and 60.1%, respectively. Furthermore, patients in Groups A and C experienced significantly fewer early recurrences (P = 0.002) and substantially fewer intrahepatic margins (P = 0.048), diffuse recurrences (P = 0.018), and extrahepatic metastases (P = 0.038) than patients in Group B, with no patient developing radiation-induced liver disease. This study preliminarily suggests that postoperative adjuvant radiotherapy can compensate for the lack of narrow-margin surgery.
External radiotherapy for HCC liver lymph node metastasis is safe and effective (43, 44). For adrenal gland metastasis (45), bone or soft tissue metastasis (46), lung metastasis (47), and brain metastasis (48), radiotherapy can also reduce the size of metastases and relieve symptoms, which has clinical benefits.
Most cases of HCC are due to chronic hepatitis infection, which is characterized by a chronic inflammatory state (49). Chronic HBV infection is the main risk factor for HCC in Southeast Asia and Africa, whereas chronic infection with hepatitis C is the main risk factor for HCC in Western countries and Japan (50–54). Among patients with HCC in the United States, approximately 50% to 60% are infected with HCV, 10% to 15% are infected with HBV, approximately 20% to 25% have alcoholic liver disease, and approximately 20% to 30% of patients with HCC have some features of metabolic syndrome (55). Chronic hepatitis infection perturbs the liver microenvironment and tips the scales in favor of carcinogenesis (49). The tumor microenvironment (TME) of HCC is a complex mixture of hepatic nonparenchymal resident cells, tumor cells, immune cells, and tumor-associated fibroblasts. Cytokines and chemokines secreted in the liver can promote angiogenesis, anti-apoptotic responses, and immune evasion, which facilitates an immunosuppressive microenvironment and promotes tumor growth (56, 57). Some studies suggested that the objective response rate (ORR) for PD-1/PD-L1 inhibitors did not significantly differ between virally infected and uninfected HCC patients, the tumor mutational burden was similar between the two groups of patients, and a difference was not observed in the ORR between HBV-HCC and HCV-HCC patients (58, 59).
ICIs have recently revolutionized cancer treatment, while the overall response rates of single ICI therapy are only approximately 20%, which needs further improvement. Several rationales support RT as one of the most encouraging combination strategies. Specifically, RT can augment both antigenicity and adjuvanticity in addition to altering the local TME, which are critical for the immune response. First, radiation induces cell death, which results in cytosolic DNA accumulation, and enhances antigen release in the tumor to activate the production of type I interferon (IFN) genes via the cGAS/STING pathway (60–62). Second, RT promotes the release of tumor antigens and danger-associated molecular patterns (DAMPs), activates antigen-presenting cells (APCs), such as dendritic cells (DCs), and primes T cells within draining lymph nodes (63, 64). Third, radiation helps tumor-infiltrating lymphocytes (TILs) overcome the physical barriers of a tumor and facilitates the adaptive immune response (20, 65, 66). Fourth, reactive oxygen species (ROS) generated during radiation can modify macromolecules, such as proteins and DNA, which increases antigenicity. The presence of oxygen and generation of ROS are critical for direct DNA damage and radiation-induced tissue injury (67). At the same time, RT could change the immunogenicity of tumors from low to high through the increased expression of MHC class I and FAS on tumor cells (66, 68, 69). In addition to the cGAS-STING pathway, the release of DAMPs and cytokines can enhance adjuvanticity, elicit the migration of the pro-anticancer immune subpopulation, alter the TME and tilt the immune response to cancer cell killing. Overall, radiation converts cancer cells into an in situ vaccine to elicit anticancer immunity (70).
Moreover, the addition of ICIs to radiation could help overcome radiation-induced immunosuppressive effects, and ICIs also enhance the RT-induced abscopal effect, referring to the regression of unirradiated tumors (71). Therefore, the synergistic effects of radiotherapy and ICI combinations are diversified (Figure 1).
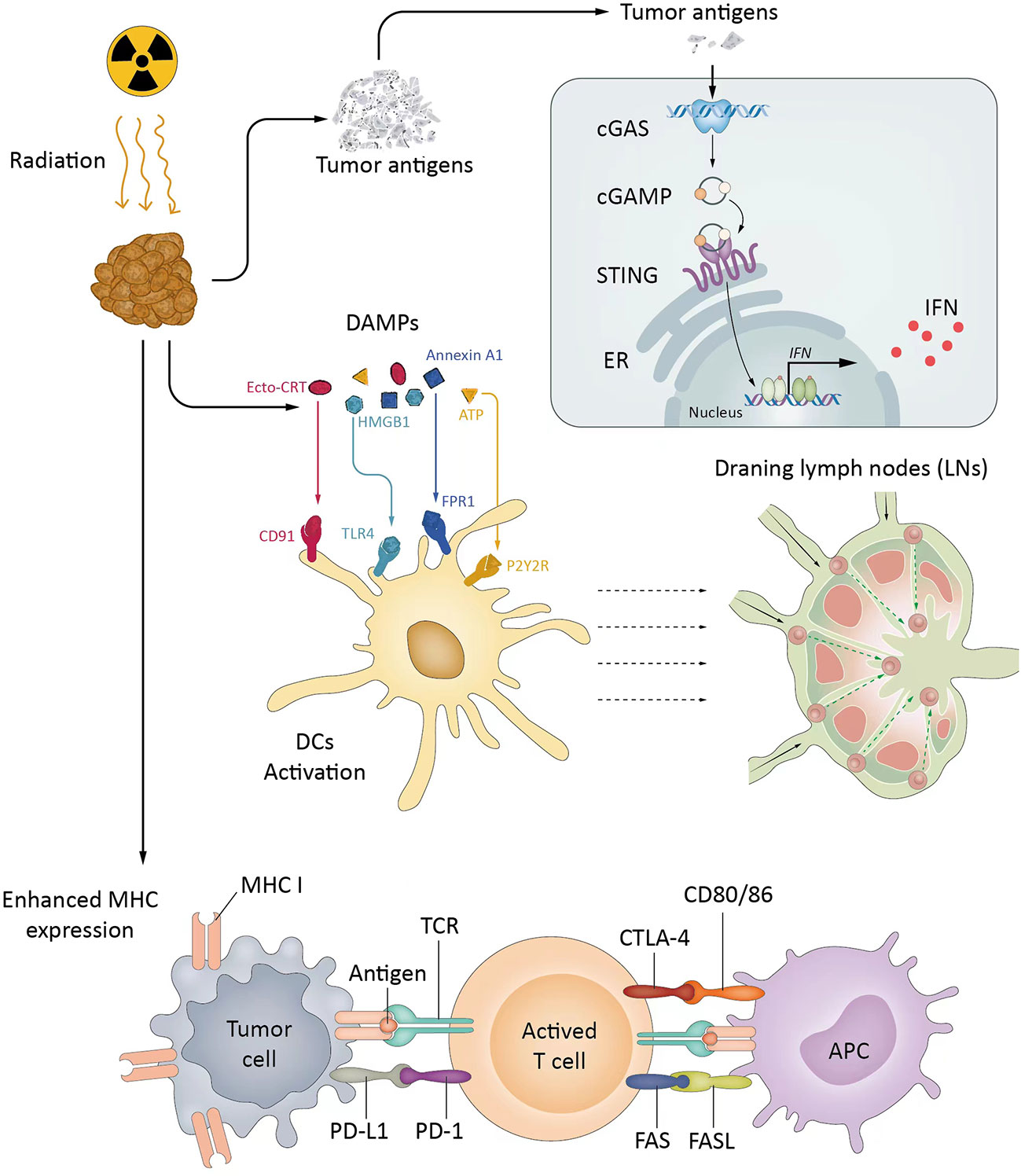
Figure 1 The synergistic effects of radiotherapy and ICIs. RT activates the cGAS/STING pathway, promotes APCs, and primes Tils into TME (cold to hot). RT increases MHC expression and changes the immunogenicity of tumors. ICIs reverse inhibitory signals and exhaustion pathways mediated by RT. cGAS, Cyclic guanosine monophosphate-adenosine monophosphate synthase; CTLA-4, Cytotoxic T lymphocyte-associated protein 4; IFN, Interferon; LN, Lymph node; MHC, Major histocompatibility complex; PD-1, Programmed death 1; PD-L1, Programmed death-ligand 1; STING, Stimulator of interferon genes; TAA, Tumor-associated antigen; TCR, T-cell receptor; Trex1, Three prime repair exonuclease 1.
Numerous preclinical studies and clinical settings have proven the synergistic effects of the combination of ICI and RT (iRT) in various cancer types (72–76). Several clinical studies have shown clinical benefits from iRT therapy. A meta-analysis including 20 clinical trials and 2,027 NSCLC patients showed that iRT therapy was associated with a significantly improved ORR and OS (77). Durvalumab has already been approved as maintenance therapy after chemoradiation therapy for stage III NSCLC patients based on the Phase III PACIFIC trial (78, 79). However, studies on the synergistic effect of HCC are still ongoing. Several preclinical studies have investigated the efficacy and mechanism of iRT in murine HCC models. Kim et al. (80) showed that the combination of anti-PD-L1 and 10 Gy RT significantly suppressed HCC tumor cell growth and improved the survival of tumor-bearing mice compared to those with anti-PD-L1 alone or RT alone, which occurred via IFN-γ/STAT3 signaling. Another preclinical study (81) supported the synergistic effect of the SBRT and anti-PD-1 combination in an orthotopic murine HCC model. The combination of SBRT (30 Gy in 3 fractions) and anti-PD-1 antibodies markedly suppressed tumor growth and improved survival with increased infiltration of CD8+ cytotoxic T cells within the tumor. Another study showed that the ionizing radiation (IR)-induced DNA damage repair (DDR) inhibitor AZD6738 in combination with RT and ICIs in HCC led to stronger immunologic memory, lasting antitumor immunity than radioimmunotherapy, and the prevention of tumor recurrence in mouse models, which relied on the activation of the cyclic GMP-AMP synthase/stimulator of interferon genes (cGAS/STING) signaling pathway (82). These data showed that the tumor response to radiation can be augmented by concurrent treatment with ICIs. These findings are promising and indicate the potential mechanisms by which combination therapy overcomes immune resistance to generate lasting immunity and protect against tumor recurrence.
In addition to encouraging data in preclinical studies, some published clinical data and case reports have shown promising results regarding the combination treatment of RT and ICIs in HCC. Chiang et al. reported a 100% ORR with SBRT followed by nivolumab for 5 patients with unresectable HCC (83). A phase I trial (NCT02239900) that evaluated liver/lung SBRT with ipilimumab reported that 23% of patients had clinical benefits (84). A retrospective cohort study showed that SBRT combined with a PD-1 inhibitor improves PFS and OS in TACE-refractory patients with intermediate-stage HCC (85). Wonmo Sung’s mechanistic mathematical model showed that for the ICI-RT treatment regimen, the irradiated tumor fraction is the most important parameter for the efficacy, and adding RT to ICI yields an increase in clinical benefit from 33% to 71% in nonirradiated tumor sites for 90% of the tumor cells being irradiated (86). One retrospective study showed that albumin-bilirubin (ALBI) scores and age were independent prognostic factors for PFS and OS in unresectable HCC treated with combined ICIs and RT (87). Radiation-induced liver disease (RILD) is the main adverse effect after liver radiation due to overexposure (88) and typically occurs 4–8 weeks after the termination of RT (89). The key point for preventing RILD is to maintain the liver within the tolerance range limit when designing the RT plan (89). The liver tolerance dose (average dose of the liver) is 23 Gy for Child−Pugh A patients and only 6 Gy for Child−Pugh B patients (90). The ASTRO Clinical Practice Guideline suggests that the selection of dose-fractionation regimen and technique should be based on disease extent, disease location, underlying liver function, and available technologies (34). These trials showed that the most common grade 3/4 treatment-related adverse reactions were elevated AST and ALT levels, colitis, or hand-foot skin reactions attributable to either radiation or immunotherapy, but no patient experienced grade 4 or 5 treatment-related toxicity. Most episodes of toxicity were transient and could be managed (83–85). These studies provide preliminary evidence to show that iRT is safe and efficacious, and they show that systemic immune activation was greater after liver irradiation. Several prospective clinical trials registered at www.ClinicalTrials.gov to evalute the combination of RT and ICIs in HCC are ongoing (Table 1).
Although previous studies have demonstrated the efficacy and tolerable toxicity of RT and ICI combination therapy in HCC patients, an optimal RT dose, fractionation scheme, and RT/ICI sequencing have not been specified, and these parameters may depend on the treatment purpose, choice of ICIs, mutational burden and patient performance status (91).
ICIs can be administered before, after or even concurrently with RT. Several studies have been performed to determine the optimal sequences of RT and ICIs. The PACIFIC trial showed that durvalumab started within 14 days improved PFS after completing RT (79). One report showed that administering ICIs 7 days after RT was less effective in enhancing OS than concurrent administration (92). Further studies are required to determine the optimal timing of RT to maximize benefits in HCC patients when combined with ICIs.
SBRT or hypofractionated RT is widely accepted to possibly be more immunogenic than conventional fractionated RT with 2 Gy per day. Dewan et al. reported that an 8 Gy × 3 regimen plus anti-CTLA-4 mAb showed better local tumor control and a systemic abscopal effect than two other regimens, 20 Gy × 1 and 6 Gy × 5 (20). A retrospective cohort observation even showed that proton beam radiotherapy (PBT) combined with anti-PD1/PD-L1 provides a sustained and high rate of local tumor control in the irradiation field and an excellent systemic therapeutic effect, resulting in overall tumor control and survival (93). Gaining an understanding of the effect of different fractionations on the immune response is important to improve the combination of RT and ICIs to increase their efficacy and administer personalized ICIs (63).
Finally, appropriate candidates for iRT need to be selected to improve outcomes for HCC patients. PD-L1 expression, tumor mutation burden, and the infiltration of effector T lymphocytes are selectable predictive biomarkers for ICIs in some tumor types but have not been evaluated in HCC (94). The next-generation sequencing-based profiling of immune gene expression signatures, T-cell receptor repertoire, T-cell-inflamed gene expression, and the microbiome is on the way to help identify patients most likely to derive a therapeutic response with HCC (95), not only for predicting susceptibility to ICIs but also individual radiation sensitivity (96–98). Therefore, further efforts to identify appropriate biomarkers to better select patients with HCC who are suitable for iRT are needed.
Although the introduction of ICIs in HCC has been notably behind that in other tumors, progress in HCC immunotherapy has still advanced. In the near future, ICI treatments might be found to increase the efficacy of locoregional and radical treatments for HCC. In particular, neoadjuvant or conversion therapy for resectable or nonresectable HCC patients will have the chance of paving the way for a significant drop in mortality rates in this fatal disease.
Ideally, to achieve this goal, efforts should be made to combine ICIs, including radiation, antiangiogenic agents, and tyrosine kinase inhibitors (TKIs). These efforts should also include the development of new immunotherapy agents, such as agonist immunostimulatory monoclonal antibodies, bispecific antibodies, and neoantigen vaccination. The identification of useful biomarkers for classifying sensitivity and resistance to individual agents or combinations is important for advanced personalized therapy.
Within the last decade, immunotherapy research has grown remarkably and changed the treatment paradigm for HCC. In addition to neoadjuvant or conversion therapy, ICIs have a well-established role in the advanced stage. Improvements in radiation have boosted the efficacy of RT in HCC. RT can remodel the “cold” TME to an immune-reactive “hot” TME, synergistically improving the effectiveness of ICIs. The development of synergistic combinations will probably be an important direction in the future. Along the way, the quest for accurate, user-friendly biomarkers will probably also be necessary for personalized immunotherapies.
LC was involved in the writing and editing of the manuscript. RZ, ZL and QT participated in data and literature review. ZH and BL were responsible for the study conception and design. All authors contributed to the article and approved the submitted version.
This work was supported by the Bethune-Cancer Radiotherapy Translational Medicine Research Fund of China (Grant No. flzh202117) and the Beijing Kechuang Medical Development Foundation Fund of China (Grant No. KC2021-JX-0186-31).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. Aasld guidelines for the treatment of hepatocellular carcinoma. Hepatology (2018) 67(1):358–80. doi: 10.1002/hep.29086
2. Vogel A, Cervantes A, Chau I, Daniele B, Llovet JM, Meyer T, et al. Hepatocellular carcinoma: Esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2018) 29(Suppl 4):iv238–iv55. doi: 10.1093/annonc/mdy308
3. European Association for the Study of the Liver. Electronic address eee, European association for the study of the l. easl clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol (2018) 69(1):182–236. doi: 10.1016/j.jhep.2018.03.019
4. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology (2018) 68(2):723–50. doi: 10.1002/hep.29913
5. Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol (2018) 15(10):599–616. doi: 10.1038/s41571-018-0073-4
6. Greten TF, Lai CW, Li G, Staveley-O'Carroll KF. Targeted and immune-based therapies for hepatocellular carcinoma. Gastroenterology (2019) 156(2):510–24. doi: 10.1053/j.gastro.2018.09.051
7. Chen LT, Martinelli E, Cheng AL, Pentheroudakis G, Qin S, Bhattacharyya GS, et al. Pan-Asian adapted esmo clinical practice guidelines for the management of patients with intermediate and Advanced/Relapsed hepatocellular carcinoma: A tos-esmo initiative endorsed by csco, ismpo, jsmo, ksmo, mos and sso. Ann Oncol (2020) 31(3):334–51. doi: 10.1016/j.annonc.2019.12.001
8. Nault JC, Cheng AL, Sangro B, Llovet JM. Milestones in the pathogenesis and management of primary liver cancer. J Hepatol (2020) 72(2):209–14. doi: 10.1016/j.jhep.2019.11.006
9. Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Pineros M, Znaor A, et al. Cancer statistics for the year 2020: An overview. Int J Cancer (2021). doi: 10.1002/ijc.33588
10. Bruix J, Sherman M. Practice guidelines committee AASLD. management of hepatocellular carcinoma. Hepatology (2005) 42(5):1208–36. doi: 10.1002/hep.20933
11. Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol (2015) 12(12):681–700. doi: 10.1038/nrgastro.2015.173
12. Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, et al. Overexpression of pd-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res (2009) 15(3):971–9. doi: 10.1158/1078-0432.CCR-08-1608
13. Shi F, Shi M, Zeng Z, Qi RZ, Liu ZW, Zhang JY, et al. Pd-1 and pd-L1 upregulation promotes Cd8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int J Cancer (2011) 128(4):887–96. doi: 10.1002/ijc.25397
14. Llovet JM, Villanueva A, Marrero JA, Schwartz M, Meyer T, Galle PR, et al. Trial design and endpoints in hepatocellular carcinoma: Aasld consensus conference. Hepatology (2021) 73(Suppl 1):158–91. doi: 10.1002/hep.31327
15. Bujold A, Massey CA, Kim JJ, Brierley J, Cho C, Wong RK, et al. Sequential phase I and ii trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol (2013) 31(13):1631–9. doi: 10.1200/JCO.2012.44.1659
16. Andolino DL, Johnson CS, Maluccio M, Kwo P, Tector AJ, Zook J, et al. Stereotactic body radiotherapy for primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys (2011) 81(4):e447–53. doi: 10.1016/j.ijrobp.2011.04.011
17. Cardenes HR, Price TR, Perkins SM, Maluccio M, Kwo P, Breen TE, et al. Phase I feasibility trial of stereotactic body radiation therapy for primary hepatocellular carcinoma. Clin Transl Oncol (2010) 12(3):218–25. doi: 10.1007/s12094-010-0492-x
18. Bernstein MB, Krishnan S, Hodge JW, Chang JY. Immunotherapy and stereotactic ablative radiotherapy (Isabr): A curative approach? Nat Rev Clin Oncol (2016) 13(8):516–24. doi: 10.1038/nrclinonc.2016.30
19. Menon H, Ramapriyan R, Cushman TR, Verma V, Kim HH, Schoenhals JE, et al. Role of radiation therapy in modulation of the tumor stroma and microenvironment. Front Immunol (2019) 10:193. doi: 10.3389/fimmu.2019.00193
20. Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-Ctla-4 antibody. Clin Cancer Res (2009) 15(17):5379–88. doi: 10.1158/1078-0432.CCR-09-0265
21. Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, et al. Immune-mediated inhibition of metastases after treatment with local radiation and ctla-4 blockade in a mouse model of breast cancer. Clin Cancer Res (2005) 11(2 Pt 1):728–34. doi: 10.1158/1078-0432.728.11.2
22. Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res (2013) 1(6):365–72. doi: 10.1158/2326-6066.CIR-13-0115
23. Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med (2012) 366(10):925–31. doi: 10.1056/NEJMoa1112824
24. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med (2020) 382(20):1894–905. doi: 10.1056/NEJMoa1915745
25. Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): A randomised, open-label, phase 2-3 study. Lancet Oncol (2021) 22(7):977–90. doi: 10.1016/S1470-2045(21)00252-7
26. Xu J, Shen J, Gu S, Zhang Y, Wu L, Wu J, et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (Rescue): A nonrandomized, open-label, phase ii trial. Clin Cancer Res (2021) 27(4):1003–11. doi: 10.1158/1078-0432.CCR-20-2571
27. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (Checkmate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet (2017) 389(10088):2492–502. doi: 10.1016/S0140-6736(17)31046-2
28. Qin S, Ren Z, Meng Z, Chen Z, Chai X, Xiong J, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: A multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol (2020) 21(4):571–80. doi: 10.1016/S1470-2045(20)30011-5
29. Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: The checkmate 040 randomized clinical trial. JAMA Oncol (2020) 6(11):e204564. doi: 10.1001/jamaoncol.2020.4564
30. He M, Li Q, Zou R, Shen J, Fang W, Tan G, et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: A randomized clinical trial. JAMA Oncol (2019) 5(7):953–60. doi: 10.1001/jamaoncol.2019.0250
31. Yoon SM, Ryoo BY, Lee SJ, Kim JH, Shin JH, An JH, et al. Efficacy and safety of transarterial chemoembolization plus external beam radiotherapy vs sorafenib in hepatocellular carcinoma with macroscopic vascular invasion: A randomized clinical trial. JAMA Oncol (2018) 4(5):661–9. doi: 10.1001/jamaoncol.2017.5847
32. Hamaoka M, Kobayashi T, Kuroda S, Iwako H, Okimoto S, Kimura T, et al. Hepatectomy after down-staging of hepatocellular carcinoma with portal vein tumor thrombus using chemoradiotherapy: A retrospective cohort study. Int J Surg (2017) 44:223–8. doi: 10.1016/j.ijsu.2017.06.082
33. Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, et al. Sintilimab plus a bevacizumab biosimilar (Ibi305) versus sorafenib in unresectable hepatocellular carcinoma (Orient-32): A randomised, open-label, phase 2-3 study. Lancet Oncol (2021) 22(7):977–90. doi: 10.1016/S1470-2045(21)00252-7
34. Apisarnthanarax S, Barry A, Cao M, Czito B, DeMatteo R, Drinane M, et al. External beam radiation therapy for primary liver cancers: An astro clinical practice guideline. Pract Radiat Oncol (2022) 12(1):28–51. doi: 10.1016/j.prro.2021.09.004
35. Hara K, Takeda A, Tsurugai Y, Saigusa Y, Sanuki N, Eriguchi T, et al. Radiotherapy for hepatocellular carcinoma results in comparable survival to radiofrequency ablation: A propensity score analysis. Hepatology (2019) 69(6):2533–45. doi: 10.1002/hep.30591
36. Sapisochin G, Barry A, Doherty M, Fischer S, Goldaracena N, Rosales R, et al. Stereotactic body radiotherapy vs. tace or rfa as a bridge to transplant in patients with hepatocellular carcinoma. an intention-to-Treat analysis. J Hepatol (2017) 67(1):92–9. doi: 10.1016/j.jhep.2017.02.022
37. Meng MB, Cui YL, Lu Y, She B, Chen Y, Guan YS, et al. Transcatheter arterial chemoembolization in combination with radiotherapy for unresectable hepatocellular carcinoma: A systematic review and meta-analysis. Radiother Oncol (2009) 92(2):184–94. doi: 10.1016/j.radonc.2008.11.002
38. Sun HC, Zhou J, Wang Z, Liu X, Xie Q, Jia W, et al. Chinese Expert consensus on conversion therapy for hepatocellular carcinoma (2021 edition). Hepatobiliary Surg Nutr (2022) 11(2):227–52. doi: 10.21037/hbsn-21-328
39. Chong JU, Choi GH, Han DH, Kim KS, Seong J, Han KH, et al. Downstaging with localized concurrent chemoradiotherapy can identify optimal surgical candidates in hepatocellular carcinoma with portal vein tumor thrombus. Ann Surg Oncol (2018) 25(11):3308–15. doi: 10.1245/s10434-018-6653-9
40. Huo YR, Eslick GD. Transcatheter arterial chemoembolization plus radiotherapy compared with chemoembolization alone for hepatocellular carcinoma: A systematic review and meta-analysis. JAMA Oncol (2015) 1(6):756–65. doi: 10.1001/jamaoncol.2015.2189
41. Wei X, Jiang Y, Zhang X, Feng S, Zhou B, Ye X, et al. Neoadjuvant three-dimensional conformal radiotherapy for resectable hepatocellular carcinoma with portal vein tumor thrombus: A randomized, open-label, multicenter controlled study. J Clin Oncol (2019) 37(24):2141–51. doi: 10.1200/JCO.18.02184
42. Wang WH, Wang Z, Wu JX, Zhang T, Rong WQ, Wang LM, et al. Survival benefit with imrt following narrow-margin hepatectomy in patients with hepatocellular carcinoma close to major vessels. Liver Int (2015) 35(12):2603–10. doi: 10.1111/liv.12857
43. Kim K, Chie EK, Kim W, Kim YJ, Yoon JH, Lee HS, et al. Absence of symptom and intact liver function are positive prognosticators for patients undergoing radiotherapy for lymph node metastasis from hepatocellular carcinoma. Int J Radiat Oncol Biol Phys (2010) 78(3):729–34. doi: 10.1016/j.ijrobp.2009.08.047
44. Yamashita H, Nakagawa K, Shiraishi K, Tago M, Igaki H, Nakamura N, et al. Radiotherapy for lymph node metastases in patients with hepatocellular carcinoma: Retrospective study. J Gastroenterol Hepatol (2007) 22(4):523–7. doi: 10.1111/j.1440-1746.2006.04450.x
45. Zhou LY, Zeng ZC, Fan J, Chen B, Rao SX, He J, et al. Radiotherapy treatment of adrenal gland metastases from hepatocellular carcinoma: Clinical features and prognostic factors. BMC Cancer (2014) 14:878. doi: 10.1186/1471-2407-14-878
46. He J, Zeng ZC, Tang ZY, Fan J, Zhou J, Zeng MS, et al. Clinical features and prognostic factors in patients with bone metastases from hepatocellular carcinoma receiving external beam radiotherapy. Cancer (2009) 115(12):2710–20. doi: 10.1002/cncr.24300
47. Jiang W, Zeng ZC, Zhang JY, Fan J, Zeng MS, Zhou J. Palliative radiation therapy for pulmonary metastases from hepatocellular carcinoma. Clin Exp Metastasis (2012) 29(3):197–205. doi: 10.1007/s10585-011-9442-4
48. Park Y, Kim KS, Kim K, Chie EK, Kim JH, Kim JS, et al. Nomogram prediction of survival in patients with brain metastases from hepatocellular carcinoma treated with whole-brain radiotherapy: A multicenter retrospective study. J Neurooncol (2015) 125(2):377–83. doi: 10.1007/s11060-015-1926-7
49. Medzhitov R. Origin and physiological roles of inflammation. Nature (2008) 454(7203):428–35. doi: 10.1038/nature07201
50. Somboon K, Siramolpiwat S, Vilaichone RK. Epidemiology and survival of hepatocellular carcinoma in the central region of Thailand. Asian Pac J Cancer Prev (2014) 15(8):3567–70. doi: 10.7314/apjcp.2014.15.8.3567
51. Norsa'adah B, Nurhazalini-Zayani CG. Epidemiology and survival of hepatocellular carcinoma in north-East peninsular Malaysia. Asian Pac J Cancer Prev (2013) 14(11):6955–9. doi: 10.7314/apjcp.2013.14.11.6955
52. Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: Consider the population. J Clin Gastroenterol (2013) 47(Suppl 0):S2–6. doi: 10.1097/MCG.0b013e3182872f29
53. Kew MC. Epidemiology of hepatocellular carcinoma in Sub-Saharan Africa. Ann Hepatol (2013) 12(2):173–82. doi: 10.1016/S1665-2681(19)31354-7
54. Umemura T, Ichijo T, Yoshizawa K, Tanaka E, Kiyosawa K. Epidemiology of hepatocellular carcinoma in Japan. J Gastroenterol (2009) 44(Suppl 19):102–7. doi: 10.1007/s00535-008-2251-0
55. El-Serag HB, Kanwal F. Epidemiology of hepatocellular carcinoma in the united states: Where are we? Where do we go? Hepatology (2014) 60(5):1767–75. doi: 10.1002/hep.27222
56. Zhu AX, Duda DG, Sahani DV, Jain RK. Hcc and angiogenesis: Possible targets and future directions. Nat Rev Clin Oncol (2011) 8(5):292–301. doi: 10.1038/nrclinonc.2011.30
57. Balkwill F, Mantovani A. Inflammation and cancer: Back to virchow? Lancet (2001) 357(9255):539–45. doi: 10.1016/S0140-6736(00)04046-0
58. Ding Z, Dong Z, Chen Z, Hong J, Yan L, Li H, et al. Viral status and efficacy of immunotherapy in hepatocellular carcinoma: A systematic review with meta-analysis. Front Immunol (2021) 12:733530. doi: 10.3389/fimmu.2021.733530
59. Ho WJ, Danilova L, Lim SJ, Verma R, Xavier S, Leatherman JM, et al. Viral status, immune microenvironment and immunological response to checkpoint inhibitors in hepatocellular carcinoma. J Immunother Cancer (2020) 8(1):e000394. doi: 10.1136/jitc-2019-000394
60. Vanpouille-Box C, Formenti SC, Demaria S. Trex1 dictates the immune fate of irradiated cancer cells. Oncoimmunology (2017) 6(9):e1339857. doi: 10.1080/2162402X.2017.1339857
61. Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, et al. Sting-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity (2014) 41(5):843–52. doi: 10.1016/j.immuni.2014.10.019
62. Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN, Weichselbaum RR, et al. The efficacy of radiotherapy relies upon induction of type I interferon-dependent innate and adaptive immunity. Cancer Res (2011) 71(7):2488–96. doi: 10.1158/0008-5472.CAN-10-2820
63. Arnold KM, Flynn NJ, Raben A, Romak L, Yu Y, Dicker AP, et al. The impact of radiation on the tumor microenvironment: Effect of dose and fractionation schedules. Cancer Growth Metastasis (2018) 11:1179064418761639. doi: 10.1177/1179064418761639
64. Sharabi AB, Nirschl CJ, Kochel CM, Nirschl TR, Francica BJ, Velarde E, et al. Stereotactic radiation therapy augments antigen-specific pd-1-Mediated antitumor immune responses Via cross-presentation of tumor antigen. Cancer Immunol Res (2015) 3(4):345–55. doi: 10.1158/2326-6066.CIR-14-0196
65. Zeng J, See AP, Phallen J, Jackson CM, Belcaid Z, Ruzevick J, et al. Anti-Pd-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys (2013) 86(2):343–9. doi: 10.1016/j.ijrobp.2012.12.025
66. Chakraborty M, Abrams SI, Camphausen K, Liu K, Scott T, Coleman CN, et al. Irradiation of tumor cells up-regulates fas and enhances ctl lytic activity and ctl adoptive immunotherapy. J Immunol (2003) 170(12):6338–47. doi: 10.4049/jimmunol.170.12.6338
67. Spitz DR, Azzam EI, Li JJ, Gius D. Metabolic Oxidation/Reduction reactions and cellular responses to ionizing radiation: A unifying concept in stress response biology. Cancer Metastasis Rev (2004) 23(3-4):311–22. doi: 10.1023/B:CANC.0000031769.14728.bc
68. Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol (2005) 174(12):7516–23. doi: 10.4049/jimmunol.174.12.7516
69. Garnett CT, Palena C, Chakraborty M, Tsang KY, Schlom J, Hodge JW. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res (2004) 64(21):7985–94. doi: 10.1158/0008-5472.CAN-04-1525
70. Zhu S, Zhang T, Zheng L, Liu H, Song W, Liu D, et al. Combination strategies to maximize the benefits of cancer immunotherapy. J Hematol Oncol (2021) 14(1):156. doi: 10.1186/s13045-021-01164-5
71. Liu Y, Dong Y, Kong L, Shi F, Zhu H, Yu J. Abscopal effect of radiotherapy combined with immune checkpoint inhibitors. J Hematol Oncol (2018) 11(1):104. doi: 10.1186/s13045-018-0647-8
72. Wang SJ, Haffty B. Radiotherapy as a new player in immuno-oncology. Cancers (Basel) (2018) 10(12):515. doi: 10.3390/cancers10120515
73. Wang Y, Deng W, Li N, Neri S, Sharma A, Jiang W, et al. Combining immunotherapy and radiotherapy for cancer treatment: Current challenges and future directions. Front Pharmacol (2018) 9:185. doi: 10.3389/fphar.2018.00185
74. Weichselbaum RR, Liang H, Deng L, Fu YX. Radiotherapy and immunotherapy: A beneficial liaison? Nat Rev Clin Oncol (2017) 14(6):365–79. doi: 10.1038/nrclinonc.2016.211
75. Herrera FG, Bourhis J, Coukos G. Radiotherapy combination opportunities leveraging immunity for the next oncology practice. CA Cancer J Clin (2017) 67(1):65–85. doi: 10.3322/caac.21358
76. Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: Radiosensitisation and potential mechanisms of synergy. Lancet Oncol (2015) 16(13):e498–509. doi: 10.1016/S1470-2045(15)00007-8
77. Geng Y, Zhang Q, Feng S, Li C, Wang L, Zhao X, et al. Safety and efficacy of pd-1/Pd-L1 inhibitors combined with radiotherapy in patients with non-Small-Cell lung cancer: A systematic review and meta-analysis. Cancer Med (2021) 10(4):1222–39. doi: 10.1002/cam4.3718
78. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall survival with durvalumab after chemoradiotherapy in stage iii nsclc. N Engl J Med (2018) 379(24):2342–50. doi: 10.1056/NEJMoa1809697
79. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage iii non-Small-Cell lung cancer. N Engl J Med (2017) 377(20):1919–29. doi: 10.1056/NEJMoa1709937
80. Kim KJ, Kim JH, Lee SJ, Lee EJ, Shin EC, Seong J. Radiation improves antitumor effect of immune checkpoint inhibitor in murine hepatocellular carcinoma model. Oncotarget (2017) 8(25):41242–55. doi: 10.18632/oncotarget.17168
81. Friedman D, Baird JR, Young KH, Cottam B, Crittenden MR, Friedman S, et al. Programmed cell death-1 blockade enhances response to stereotactic radiation in an orthotopic murine model of hepatocellular carcinoma. Hepatol Res (2017) 47(7):702–14. doi: 10.1111/hepr.12789
82. Sheng H, Huang Y, Xiao Y, Zhu Z, Shen M, Zhou P, et al. Atr inhibitor Azd6738 enhances the antitumor activity of radiotherapy and immune checkpoint inhibitors by potentiating the tumor immune microenvironment in hepatocellular carcinoma. J Immunother Cancer (2020) 8(1):e000340. doi: 10.1136/jitc-2019-000340
83. Chiang CL, Chan ACY, Chiu KWH, Kong FS. Combined stereotactic body radiotherapy and checkpoint inhibition in unresectable hepatocellular carcinoma: A potential synergistic treatment strategy. Front Oncol (2019) 9:1157. doi: 10.3389/fonc.2019.01157
84. Tang C, Welsh JW, de Groot P, Massarelli E, Chang JY, Hess KR, et al. Ipilimumab with stereotactic ablative radiation therapy: Phase I results and immunologic correlates from peripheral T cells. Clin Cancer Res (2017) 23(6):1388–96. doi: 10.1158/1078-0432.CCR-16-1432
85. Xiang YJ, Wang K, Zheng YT, Feng S, Yu HM, Li XW, et al. Effects of stereotactic body radiation therapy plus pd-1 inhibitors for patients with transarterial chemoembolization refractory. Front Oncol (2022) 12:839605. doi: 10.3389/fonc.2022.839605
86. Sung W, Hong TS, Poznansky MC, Paganetti H, Grassberger C. Mathematical modeling to simulate the effect of adding radiation therapy to immunotherapy and application to hepatocellular carcinoma. Int J Radiat Oncol Biol Phys (2022) 112(4):1055–62. doi: 10.1016/j.ijrobp.2021.11.008
87. Dong D, Zhu X, Wang H, Li L, Wan M, Li S, et al. Prognostic significance of albumin-bilirubin score in patients with unresectable hepatocellular carcinoma undergoing combined immunotherapy and radiotherapy. J Med Imaging Radiat Oncol (2022) 66(5):662–70. doi: 10.1111/1754-9485.13398
88. Tang QH, Li AJ, Yang GM, Lai EC, Zhou WP, Jiang ZH, et al. Surgical resection versus conformal radiotherapy combined with tace for resectable hepatocellular carcinoma with portal vein tumor thrombus: A comparative study. World J Surg (2013) 37(6):1362–70. doi: 10.1007/s00268-013-1969-x
89. Benson R, Madan R, Kilambi R, Chander S. Radiation induced liver disease: A clinical update. J Egypt Natl Canc Inst (2016) 28(1):7–11. doi: 10.1016/j.jnci.2015.08.001
90. Liang SX, Zhu XD, Xu ZY, Zhu J, Zhao JD, Lu HJ, et al. Radiation-induced liver disease in three-dimensional conformal radiation therapy for primary liver carcinoma: The risk factors and hepatic radiation tolerance. Int J Radiat Oncol Biol Phys (2006) 65(2):426–34. doi: 10.1016/j.ijrobp.2005.12.031
91. Buchwald ZS, Wynne J, Nasti TH, Zhu S, Mourad WF, Yan W, et al. Radiation, immune checkpoint blockade and the abscopal effect: A critical review on timing, dose and fractionation. Front Oncol (2018) 8:612. doi: 10.3389/fonc.2018.00612
92. Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent pd-L1 blockade. Cancer Res (2014) 74(19):5458–68. doi: 10.1158/0008-5472.CAN-14-1258
93. Su CW, Hou MM, Huang PW, Chou YC, Huang BS, Tseng JH, et al. Proton beam radiotherapy combined with anti-Pd1/Pdl1 immune checkpoint inhibitors for advanced hepatocellular carcinoma. Am J Cancer Res (2022) 12(4):1606–20.
94. Chan TA, Yarchoan M, Jaffee E, Swanton C, Quezada SA, Stenzinger A, et al. Development of tumor mutation burden as an immunotherapy biomarker: Utility for the oncology clinic. Ann Oncol (2019) 30(1):44–56. doi: 10.1093/annonc/mdy495
95. Bernicker E. Next-generation sequencing and immunotherapy biomarkers: A medical oncology perspective. Arch Pathol Lab Med (2016) 140(3):245–8. doi: 10.5858/arpa.2015-0287-SA
96. Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, et al. Sustained efficacy of adjuvant immunotherapy with cytokine-induced killer cells for hepatocellular carcinoma: An extended 5-year follow-up. Cancer Immunol Immunother (2019) 68(1):23–32. doi: 10.1007/s00262-018-2247-4
97. Cui Y, Li B, Pollom EL, Horst KC, Li R. Integrating radiosensitivity and immune gene signatures for predicting benefit of radiotherapy in breast cancer. Clin Cancer Res (2018) 24(19):4754–62. doi: 10.1158/1078-0432.CCR-18-0825
Keywords: radiation therapy, immune checkpoint inhibitor (ICI), combination therapy, tumor microenevironment, hepatocellula carcinoma
Citation: Chen L, Zhang R, Lin Z, Tan Q, Huang Z and Liang B (2023) Radiation therapy in the era of immune treatment for hepatocellular carcinoma. Front. Immunol. 14:1100079. doi: 10.3389/fimmu.2023.1100079
Received: 16 November 2022; Accepted: 06 January 2023;
Published: 20 January 2023.
Edited by:
Zong Sheng Guo, University at Buffalo, United StatesReviewed by:
Dandan Hu, Sun Yat-sen University Cancer Center (SYSUCC), ChinaCopyright © 2023 Chen, Zhang, Lin, Tan, Huang and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Binyong Liang, YnlsaWFuZy10amhAaG90bWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.