- 1Department of Respiratory Medicine, National Key Clinical Specialty, Branch of National Clinical Research Center for Respiratory Disease, Xiangya Hospital, Central South University, Changsha, Hunan, China
- 2Center of Respiratory Medicine, Xiangya Hospital, Central South University, Changsha, Hunan, China
- 3Department of Respiratory Medicine, Clinical Research Center for Respiratory Diseases in Hunan Province, Changsha, Hunan, China
- 4Department of Respiratory Medicine, Hunan Engineering Research Center for Intelligent Diagnosis and Treatment of Respiratory Disease, Changsha, Hunan, China
- 5National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Changsha, Hunan, China
- 6Department of Radiology, Xiangya Hospital, Central South University, Changsha, Hunan, China
Background: Krebs von den Lungen 6 (KL-6) is a potential biomarker for determining the severity of interstitial lung disease (ILD) in patients with connective tissue disease (CTD). Whether KL-6 levels can be affected by potential confounders such as underlying CTD patterns, patient-associated demographics, and comorbidities needs further investigation.
Methods: From the database created by Xiangya Hospital, 524 patients with CTD, with or without ILD, were recruited for this retrospective analysis. Recorded data included demographic information, comorbidities, inflammatory biomarkers, autoimmune antibodies, and the KL-6 level at admission. Results of CT and pulmonary function tests were collected one week before or after KL-6 measurements. The percent of predicted diffusing capacity of the lung for carbon monoxide (DLCO%) and computed tomography (CT) scans were used to determine the severity of ILD.
Results: Univariate linear regression analysis showed that BMI, lung cancer, TB, lung infections, underlying CTD type, white blood cell (WBC) counts, neutrophil (Neu) counts, and hemoglobin (Hb) were related to KL-6 levels. Multiple linear regression confirmed that Hb and lung infections could affect KL-6 levels independently; the β were 9.64 and 315.93, and the P values were 0.015 and 0.039, respectively. CTD-ILD patients had higher levels of KL-6 (864.9 vs 463.9, P < 0.001) than those without ILD. KL-6 levels were closely correlated to the severity of ILD assessed both by CT and DLCO%. Additionally, we found that KL-6 level was an independent predictive factor for the presence of ILD and further constructed a decision tree model to rapidly determine the risk of developing ILD among CTD patients.
Conclusion: KL-6 is a potential biomarker for gauging the incidence and severity of ILD in CTD patients. To use this typical value of KL-6, however, doctors should take Hb and the presence of lung infections into account.
1 Introduction
Connective tissue diseases (CTDs), including systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), systemic sclerosis (SSc), inflammatory myositis (IM), Sjogren’s syndrome (SS), and mixed connective tissue disease (MCTD), are commonly complicated by the involvement of many organ systems, such as the lung and the kidney (1, 2). CTD-related pulmonary lesions are mainly manifested as interstitial changes, which are named CTD-interstitial lung disease (CTD-ILD). CTD-ILD is one of the main causes of high morbidity and even mortality among CTD patients. High-resolution computed tomography (HRCT) is an important means of differentiating early ILD. However, it is challenging to perform routine screening due to the limitations of cost, radiation, and other considerations; as such, the identification of biomarkers able to recognize ILD could decrease economic costs and increase the timeliness of therapy to improve patient outcomes.
It is generally accepted that alveolar epithelial cell (AEC) injury is the key event for the occurrence of ILD. The published studies have shown that damaged AEC can secrete a large number of pro-fibrotic factors, provoking the migration, proliferation, activation, and myofibroblast differentiation of fibroblasts and causing the accumulation of extracellular matrix, leading to irreversible lung fibrosis (3–5). Therefore, measuring the level of molecules secreted by damaged epithelial cells is a potential means to assess the severity of the injury and to predict the incidence of ILD.
Krebs von den Lungen-6 (KL-6) is a circulating high-molecular-weight mucin-like glycoprotein, also categorized as MUC1, that is expressed primarily on alveolar type II pneumocytes and bronchial epithelial cells. Accumulation of KL-6 can further disrupt alveolar capillaries and the regeneration of AEC2 (6, 7). Meanwhile, patients with ILD often also have systemic inflammatory response syndrome, leading to more severe AEC2 damage and more KL-6 release (8). Additionally, KL-6 is reported to be one of the key molecules involved in epithelial-mesenchymal interactions by regulating myofibroblast differentiation (9). Because there are no epitopes in animals other than apes, there have been few animal studies on KL-6. However, KL-6 has been reported to be detectable in mice expressing human MUC1(hMUC1-exp) mice and can reflect the severity of bleomycin-induced lung fibrosis (10). All of the above factors may be the underlying pathological mechanisms of KL-6 to predict the incidence of ILD and to predict its prognosis.
The available studies have shown that elevated serum levels of KL6 are related to disease severity (11–14). However, there are drawbacks to these previous studies: the greatest issue is the exclusion of patients with comorbidities. Patients with CTD were more likely to also have cancer (15, 16), lung infections (17), and tuberculosis(TB) (18, 19). These comorbidities may also result in abnormal KL-6 levels, which may cause some confusion for clinicians attempting to judge the presence or severity of ILD. Additionally, the conclusions are not convincing enough because the sample size is typically low-less than 100 cases. Moreover, KL-6 levels at baseline may vary depending on the different underlying types of CTD. In addition, whether patient-related demographic characteristics such as age, gender, and BMI, among others, can affect KL-6 levels also needs further investigation, and these potential confounders may have an influence on the real value of KL-6 in assessing the presence or severity of ILD among patients with CTD.
As such, we performed this retrospective study to determine the potential confounding factors related to KL-6 levels and to re-identify the role of KL-6 among patients with CTD-ILD after adjusting for confounders. Most importantly, we constructed a decision tree model to predict the incidence of ILD.
2 Methods
This research was approved by the local Ethics Committee of the Xiangya Hospital of Central South University and was conducted in accordance with the Declaration of Helsinki and its amendments. The Ethics number is 202104005, and it is approved on May 6th, 2021”. The detail information was shown on (https://ethics.tonoinfo.com/#/home/zndxxyyy). Informed consent was waived because of the retrospective nature of the study, and the analysis used anonymous clinical data.
2.1 Study design and subjects
This was a retrospective study. Data were collected from the database setup by the Xiangya Hospital of Central South University (Hunan, China). This database recruited patients diagnosed with ILD, COPD, or lung cancer in both the outpatient and inpatient departments of Xiangya Hospital for 20 years. We screened the inpatients with KL-6 measurements in the entire database. For patients with multiple KL-6 tests, we collected the first test on admission. Then, we searched for the discharge diagnosis in the medical records with the following keywords: ‘RA’, ‘SLE’, ‘SSc’, ‘polymyositis (PM)’, ‘dermatomyositis (DM)’, ‘anti-synthetase antibody syndrome’, ‘inflammatory myopathy’, ‘SS’, ‘undetermined CTD’, and ‘mixed CTD’. Then, two pulmonologists and radiologists double checked the CT images of these recruited patients. We excluded patients without CT data. Finally, we divided the recruited patients into a CTD-NILD group and a CTD-ILD group based on the presence of ILD. Professional radiologists and pulmonologists double checked the diagnosis of ILD. The recorded data comprised basic demographic information, including age, gender, BMI, blood type, occupation, smoking history, dust exposure, and atopy history. In addition, regular blood biochemical tests, KL-6 levels, CT scans, and lung function parameters were also collected. All the data were collected at admission. CT and pulmonary function results were collected within 1 week of the KL-6 data.
2.2 Pulmonary function data
The pulmonary function test was performed by professional technicians with a spirometer (MasterScreen-Body/Diff, CareFusion, Germany) according to the American Thoracic Society guidelines. The severity of diffusion impairment was assessed by DLCO%, (grade 1, DLCO% ≥80%; grade 2, 60 ≤DLCO% <80%; grade 3, 40 ≤DLCO% <60%, grade 4, DLCO% <40%).
2.3 Severity assessment of ILD by CT scan
One professional radiologist, who was blinded to the clinical information, graded the ILD severity of CT scans semi-quantitatively (grade 1, 0–25%; grade 2, 26%–50%; grade 3, 51%–75%; grade 4, 76%–100%) (11).
2.4 KL-6 measurements
Serum KL-6 concentrations (µg/mL) were measured through the KL-6 assay using the latex-enhanced immunoturbidimetric assay method by qualified laboratory physicians. 500µg/ml was the cutoff point for differentiating normal and abnormal.
2.5 Statistical analysis
Continuous variables are presented as the mean and standard deviation (if the data were normally distributed) and the median and interquartile range (IQR) values (if the data were not normally distributed). Categorical variables are described as frequency rates and percentages. We compared the means for continuous variables with the t-test or analysis of variance (ANOVA) if the data were normally distributed. We used a non-parametric test for non-normally distributed data. We analysed proportions of categorical variables with a χ2 test. We used Pearson’s and Spearman’s rank correlation coefficients to analyse the relationship between KL-6 and other parameters. We used a receiver operating characteristic (ROC) curve to determine the cut-off point of KL-6 for predicting the incidence of CTD-ILD. We calculated the results shown in this paper according to the censoring method. We used R Statistical Software (http://www.R-project.org, The R Foundation) and the Free Statistics analysis platform for the statistical analyses. We considered P < 0.05 to be statistically significant.
3 Results
3.1 Clinical characteristics of the study population
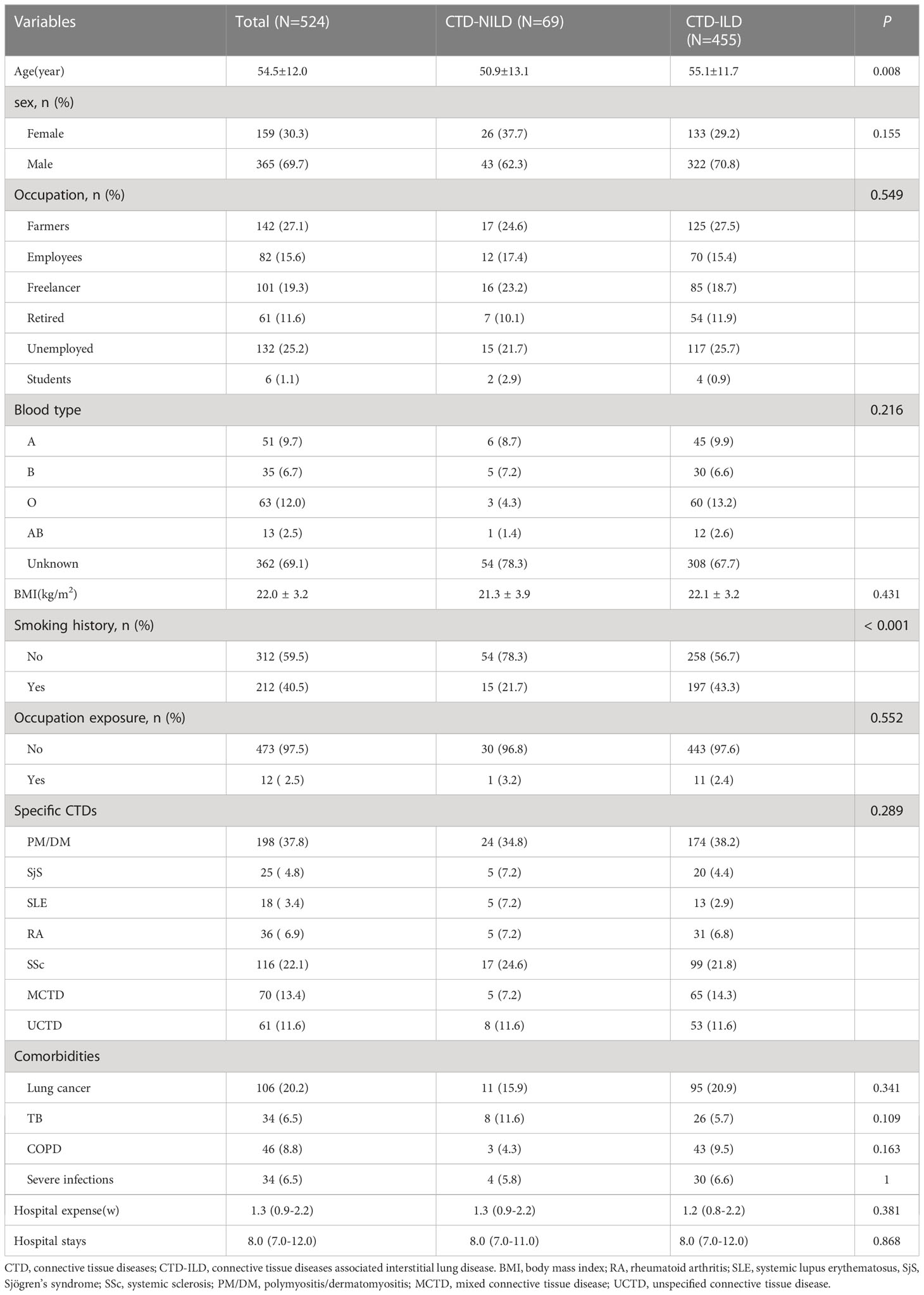
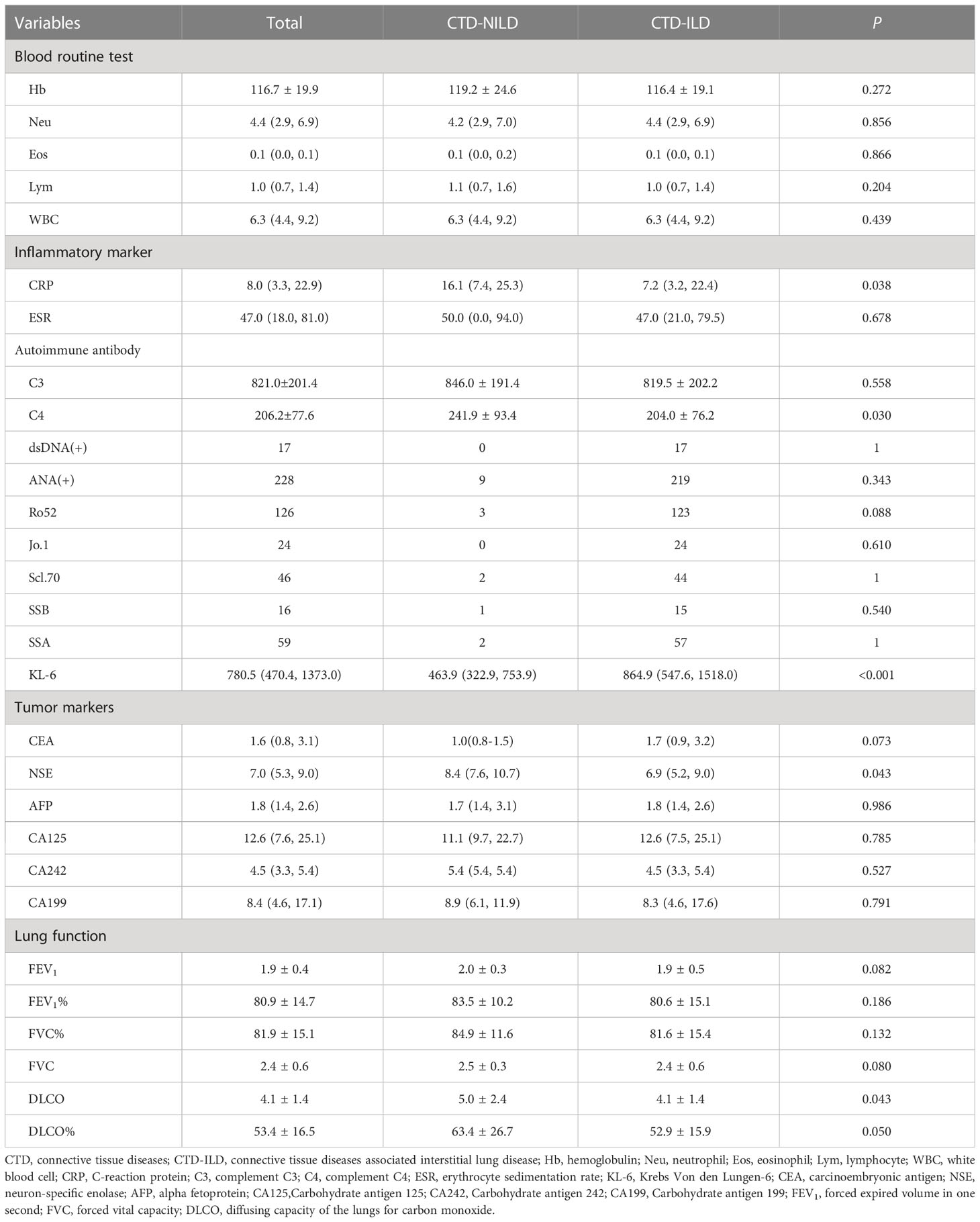
We retrospectively reviewed 965 inpatients with KL-6 measurements in the database. Overall, 534 patients had a diagnosis of CTD, but we excluded 10 patients due to a lack of CT data. Finally, 524 patients were recruited. Among these patients, 455 were diagnosed with CTD-ILD (Supplementary Figure 1). The mean age (55.1 vs 50.9 years, P = 0.008) was significantly higher in the ILD group. Other variables, including sex, blood type, the underlying CTD group, and comorbidities, were similar between these two groups. In the CTD-ILD group, there were 31 cases of RA, 13 cases of SLE, 99 cases of SSc, 20 cases of primary SjS, 174 cases of DM, and 65 patients with MCTD and 53 with UCTD (Table 1). The lung diffuse function including DLCO (5.0 vs 4.1, P = 0.043) and DLCO% (63.4 vs 52.9, P = 0.050) were much higher in patients with CTD but without ILD than in patients with CTD-ILD (Table 2). Patients with CTD-ILD had lower CRP (7.2 vs 16.1 mg/L, P = 0.038), NSE (6.9 vs 8.4 ng/ml, P = 0.043) and C4 levels (204.0 vs 241.9 mg/L, P = 0.03) than CTD patients without ILD (n = 69). While CTD-ILD patients had higher levels of KL-6 (864.9 vs 463.9 µg/ml, P < 0.001) (Table 2). When we stratified the data by the type of underlying CTD, we found that PM had the highest KL-6 level (995.7 µg/ml) of all types of CTD (Figure 1A). Then, we further analyzed the KL-6 level between CTD and CTD-ILD based on underlying CTD subgroups. We observed that serum KL-6 values were significantly higher in patients with specific CTD-ILD, including PM (1097.0 vs 454.7 µg/ml, P < 0.001), SSc (823.7 vs 485.2 µg/ml, P = 0.0028), and UCTD (690.6 vs 330.5 µg/ml, P = 0.038) (Figure 1B). In addition, we found that patients with PM showed the highest KL-6 relative to other CTD patterns in the CTD-ILD group (P < 0.001) but presented no difference in the CTD group (P = 0.246) (Figure 1C).
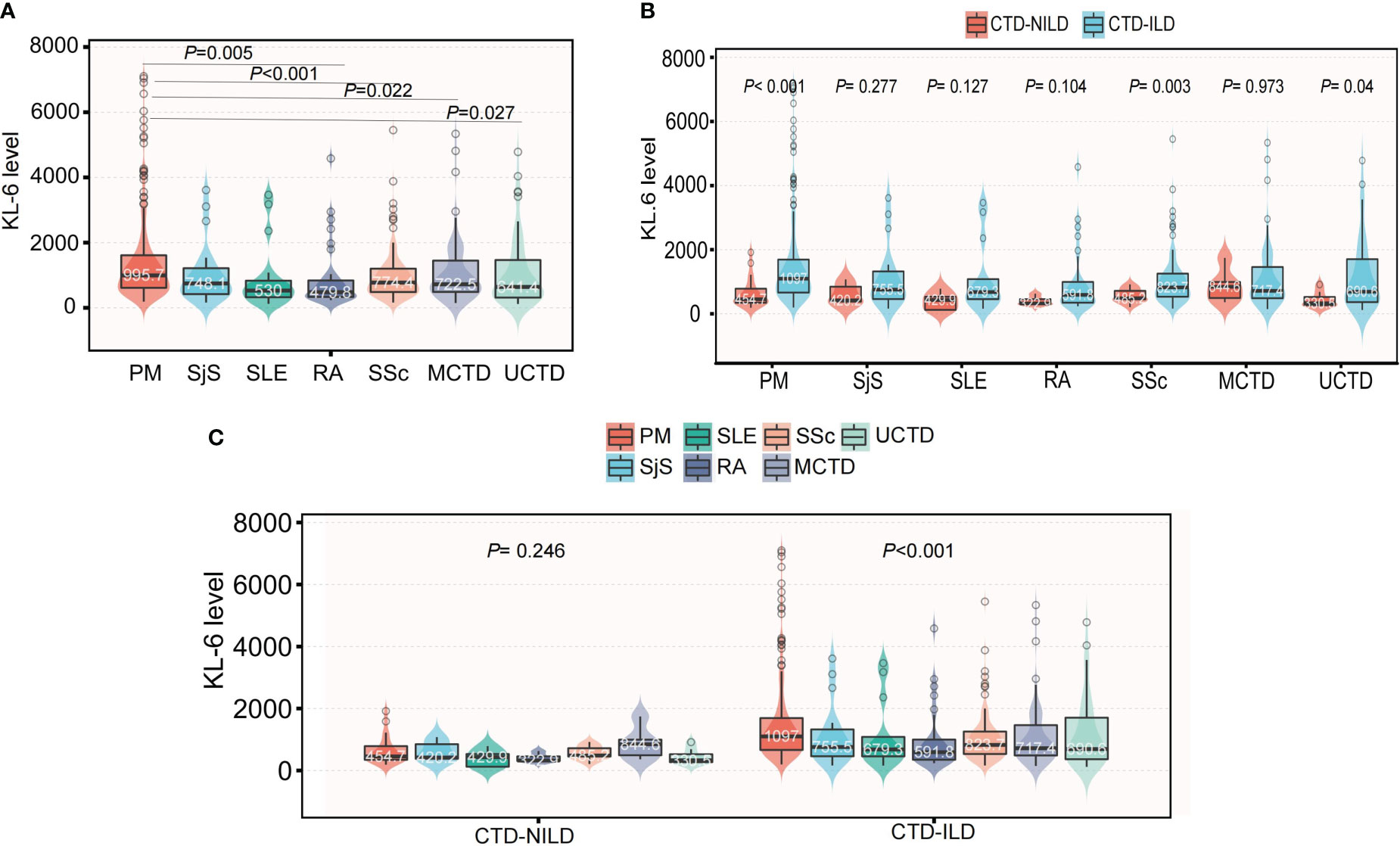
Figure 1 The differences of KL-6 level among specific CTD patterns. (A) The differences of KL-6 levels among specific CTD patterns in the whole population. (B) Subgroup analysis of the differences of KL-6 level between CTD-NILD and CTD-ILD groups based on underlying CTD pattern. (C) Subgroup analysis of the differences of KL-6 level among different CTD patterns based on the presence of ILD.
3.2 Factors associated with KL-6 levels
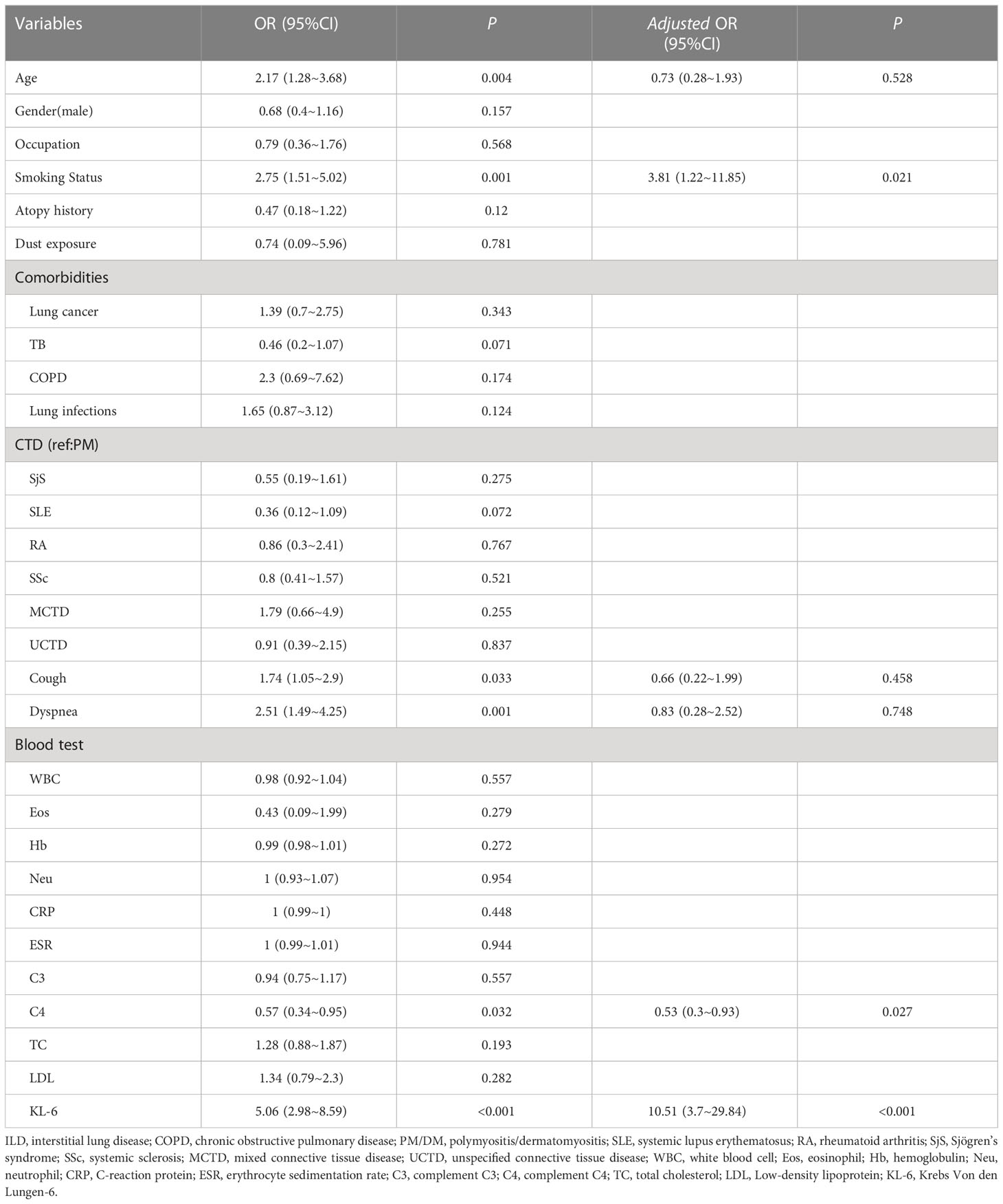
Univariate linear regression analysis showed that BMI, lung cancer, TB, pulmonary infections, white blood cell (WBC) counts, hemoglobin (Hb), neutrophil counts and underlying CTD type were related to the KL-6 level. Age, gender, smoking index, atopy history, dust exposure, and combination with COPD had no effect on the KL-6 level. Patients with high Hb were more likely to present higher KL-6 levels (β = 6.03, P = 0.017). Subjects diagnosed with pulmonary infections had higher KL-6 levels than those with only CTD-ILD but no infections. Multiple linear regression confirmed that Hb and pulmonary infections could affect the KL-6 level independently; the β were 9.64 and 315.93, and the P values were 0.015 and 0.039, respectively (Table 3). The formula for correcting KL6 is Y=-422.24+315.93*1 (when lung infection exists) +9.64 *Hb.
3.3 The role of KL-6 in assessing the severity of CTD-ILD
Patients with severe (grade 4) diffusion function impairment presented the highest KL-6 level of all three grades; the mean values of KL-6 in different diffusion impairment grades were 1054.7, 780.7, 614.2, and 542.6(µg/ml), respectively (Figure 2A). Additionally, semiquantitative grades of ILD on the CT scan were significantly correlated to the KL-6 level (Rho = 0.426, P < 0.001). Serum KL-6 levels successfully differentiated grades 1 and 2 (P < 0.001), as well as grades 2 and 3 (P < 0.001) in patients with CTD-ILD (Figure 2B). Grade 3 and grade 4 showed similar KL-6 levels. Therefore, serum KL-6 can be used to reflect the current status of CTD-ILD defined by CT scans. To utilize the serum KL-6 level in clinical practice, the cut-off points of KL-6 values to predict the presence of ILD in CTD patients were analyzed by ROC. The analysis showed that the KL-6 level at 532.75 U/mL was the best cut-off point for differentiating ILD among CTD patients. The AUC value was 0.736, with a 95% CI of 0.680–0.792. The sensitivity and specificity were 75.4% and 65.2%, respectively (Figure 3A). Then, we further explored the cut-off point of KL-6 to assess the severity of ILD among patients with CTD-ILD quantified by semiquantitative grades on CT scans and lung function stratified by DLCO%. The cut-off point for KL-6 was 643.15 µg/ml, the AUC values of KL-6 levels to differentiate grade 3 and 4 defined by DLCO% was 0.625, and the sensitivity and specificity were 69.4% and 58.5%, respectively. In addition, the positive and negative predictive values were 79.1% and 46.8%, respectively (Figure 3B). Furthermore, the AUC values of KL-6 levels to differentiate grade 3 and 4 defined by CT scan were 0.762 (95% CI: 0.706–0.818), the negative predictive value was 92.0%, and the cut-off point of KL-6 was 1060.75 µg/ml (Figure 3C).

Figure 2 Comparison of KL-6 level based on the severity of ILD assessed by both CT scan and DLCO%; (A) Comparison of KL-6 level based on the severity of ILD assessed by DLCO%; (B) Comparison of KL-6 level based on the severity of ILD assessed by CT scan.
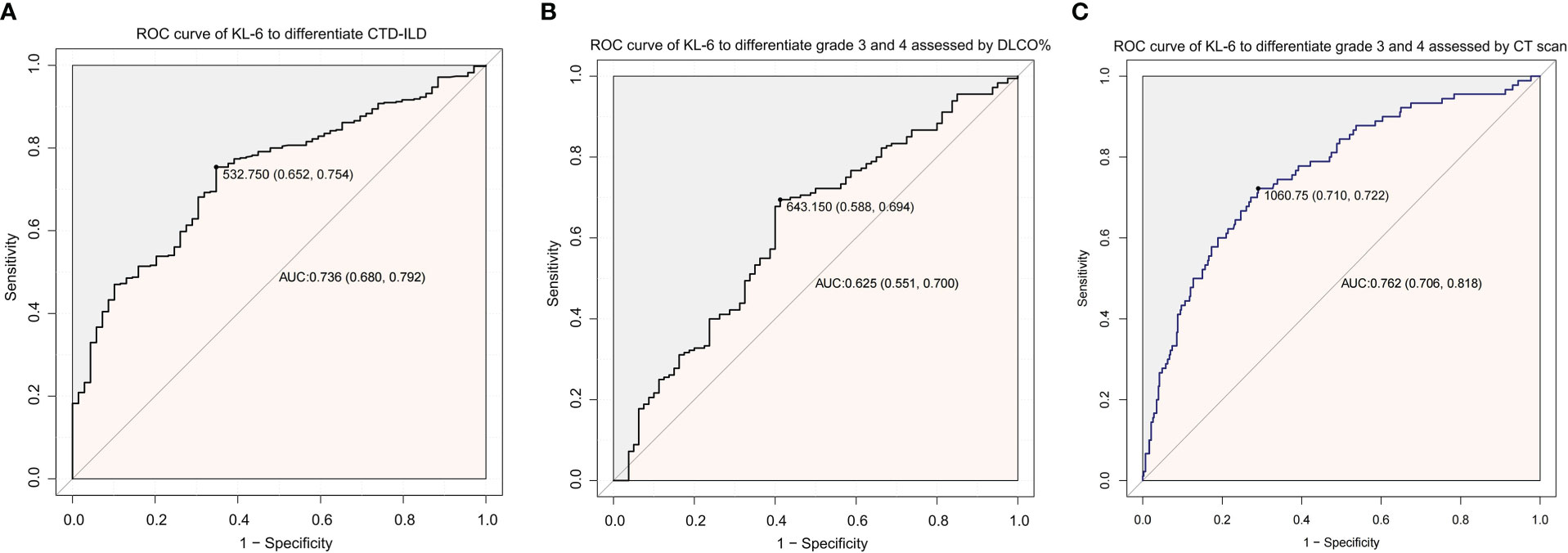
Figure 3 ROC curve analysis to utilize the role of KL-6 in CTD patients. (A) ROC curve of KL-6 levels to differentiate the presence of ILD among CTD patients. (B) ROC curve of KL-6 levels to differentiate Grade 3 and Grade 4 assessed by DLCO%. (C) ROC curve of KL-6 levels to differentiate Grade 3 and Grade 4 assessed by CT scan.
3.4 The relationship between KL-6 and lung function, CT scan hints
The KL-6 level showed a mild, negative relationship with FEV1%, FVC%, DLCO, and DLCO%. The R values were –0.18, –0.21, –0.20, and –0.24, respectively (Supplementary Figure 2).Then, we compared the predictive value of KL6 combined with DLCO and KL6 combined with FVC with that of KL-6 alone to diagnose ILD. We found that KL6, whether combined with DLCO or FVC, had a similar predictive value to ILD as KL-6 alone to diagnose ILD (Supplementary Figure 3).
We analysed the association between different CT signs with KL-6 levels and found that patients with CT signs related to ILD, – including ground-glass opacity, honeycomb, and reticular shadow – presented higher levels of KL-6 than patients without these corresponding signs(918.3 vs 703.3µg/ml, P=0.003). Other signs, such as emphysema, consolidation, nodules, or tumous, had no relation to the KL-6 level. After adjustment for significant signs, we found that only ILD-related signs had an effect on the KL-6 level β = 415.26, P = 0.001 (Supplementary Figure 4 and Supplementary Table 1).
3.5 Serum KL-6 levels are associated with the presence of ILD
We performed a logistic regression analysis of factors related to ILD and found that age, cough, dyspnoea, complement C4, smoking status and KL-6 may be related to ILD. Multivariable regression analysis revealed that KL-6 levels were independently associated with ILD (Table 4). The likelihood of ILD incidence increased 10.51 times in patients with abnormal levels compared with patients with normal KL-6 levels (OR 10.51, 95%CI 3.7~29.84, P <0.001; Table 4).To further analyse the stability of the adjusted model, we stratified patients by gender, age, smoking status, cough, dyspnoea, and complement C4. The forest plot revealed that there were no significant interactions between the aforementioned subgroups(P >0.05, Figure 4).
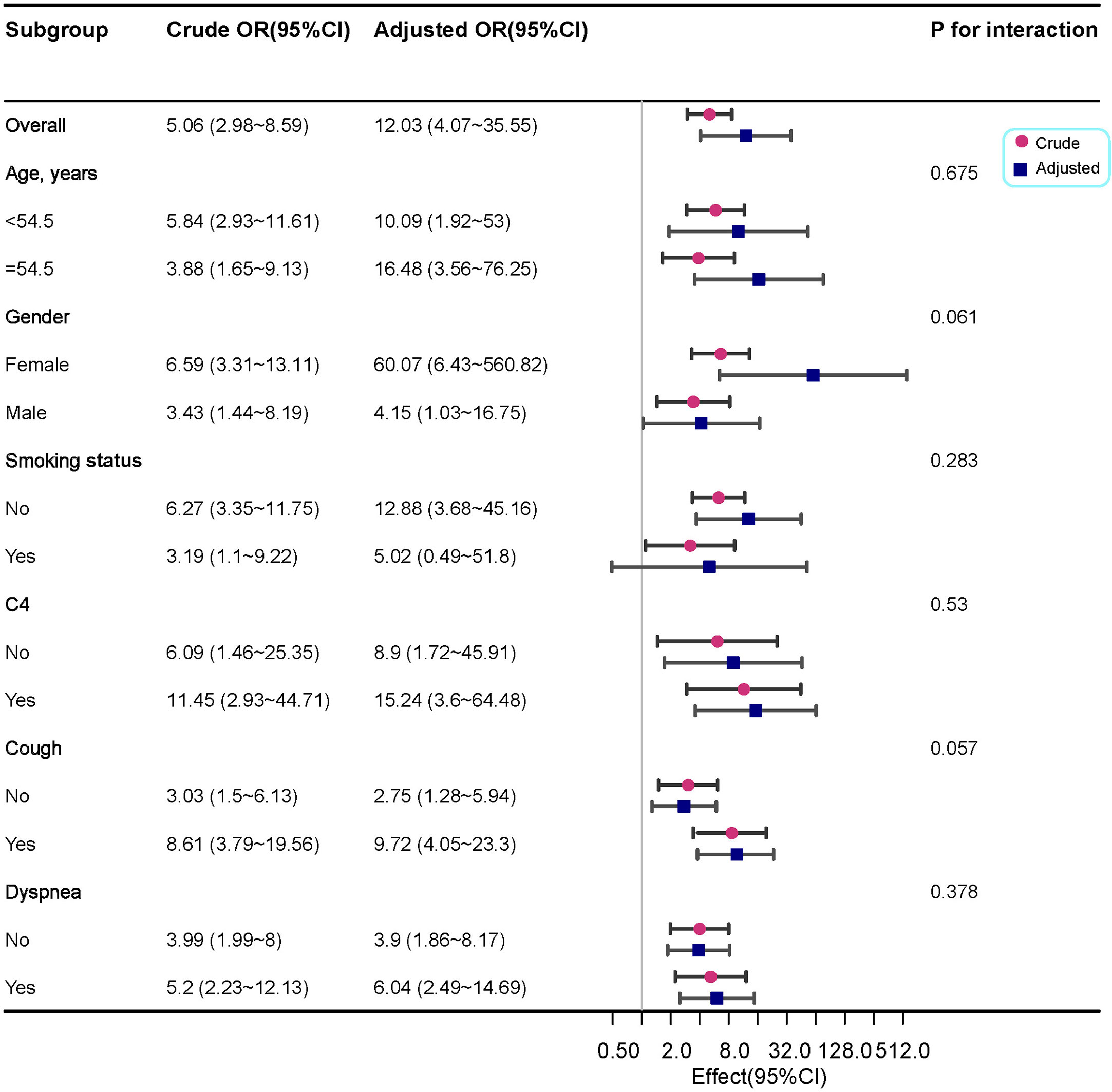
Figure 4 Forest plot for the subgroup analysis of the presence of ILD according to KL-6 levels. For each group of interest, the gray horizontal lines represent the 95% confidence interval (CI).
3.6 A decision tree based on KL-6 levels to assess ILD
Based on the above factors obtained by logistic regression analysis, we constructed decision tree models (Figure 5). When the KL-6 level is > 533 µg/ml, the probability of the patient having ILD is 70%. If the KL-6 level is < 233 µg/ml and there is no smoking history, then the probability of patients having ILD is only 2%. Based on the decision tree, KL-6 is an important factor for evaluating ILD. In the crude model, the weight of KL-6 is 61.9%. After matching various confounding factors, the weight of KL-6 is 75.4%, as shown in Supplementary Table 2.
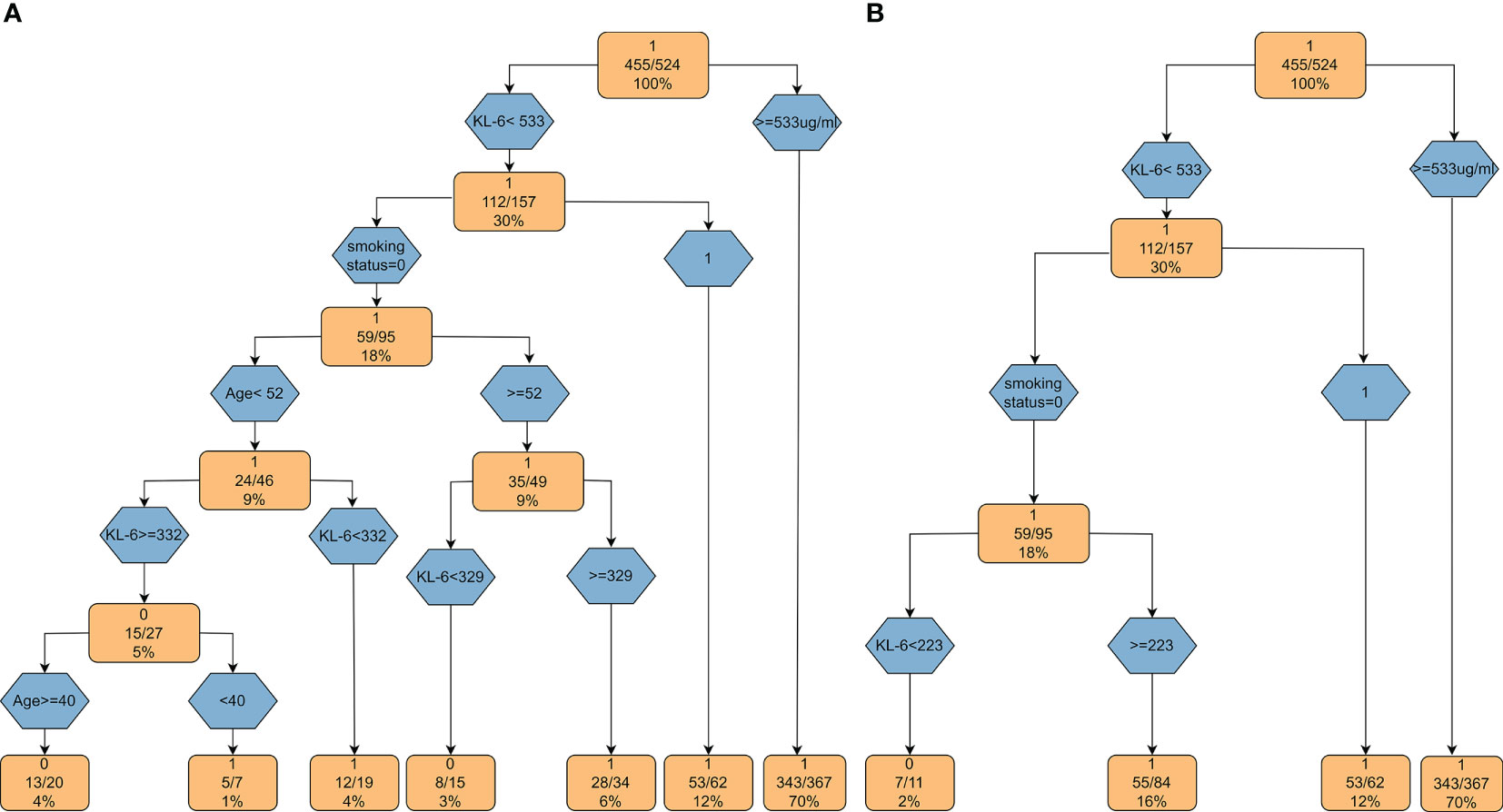
Figure 5 Decision tree models based on KL-6. (A) The decision tree model based on the factors obtained by univariate regression analysis; (B) The decision tree model based on the variables from multiple regression analysis.
4 Discussion
In this study, we comprehensively analysed the factors affecting KL-6 and investigated its clinical significance among patients with CTD. We found that Hb and lung infections could affect the KL-6 level independently. KL-6 presented a significant correlation with the severity of CTD-ILD not only measured with CT scan but also stratified by DLCO%. More importantly, we constructed a decision tree model to determine the presence of ILD, which would be beneficial for future clinical work. To our knowledge, this is the first study to systematically investigate the factors affecting KL-6 levels; additionally, we developed a decision tree model to determine the presence of ILD among patients with CTD in the real world without the exclusion of patients with comorbidities.
KL-6 as a biomarker of lung epithelial cell injury has been widely used among ILD patients. However, whether patients’ self-reported characteristics, including age, BMI, and gender, would have an effect on KL-6 levels remains seldom investigated. In addition, some of the patients have more than one pulmonary disease; as ILD is likely to be combined with lung cancer, COPD, and TB, patients with these diseases would have abnormal levels of KL-6, and most of them were excluded in clinical trials (15, 18, 20), the clinical use of KL-6 levels among these overlap patients should be comprehensively assessed. In this study, we found that Hb and lung infections could affect KL-6 levels independently, suggesting that KL-6 should be better adjusted by Hb and further corrected if patients were complicated by lung infections. The relationship between KL-6 and Hb is seldom reported; a recent study showed that KL-6 and Hb can both be used to assess bone marrow fibrosis (21), but the interaction between KL-6 and Hb is still unknown, and the mechanism through which Hb is correlated to KL-6 needs further investigation. Several studies have shown that lung infections could elevate the expression of KL-6 (22–24). In this study, we confirmed that lung infections could increase the serum KL-6 level after adjusting for other confounders, indicating that clinicians should check CTD patients for anemia or accompanying lung infections once they receive the results of KL-6 levels.
KL-6 has been reported to have a role in evaluating ILD severity among CTD patients. We found that SSc or IM had a relatively high prevalence of ILD compared with RA, SLE, and SjS, which was in close agreement with another study (11), indicating that patients who tend to have SSc, IM, or PM should be screened for ILD more frequently. Patients with PM showed the highest KL-6 relative to other CTD patterns in the CTD-ILD group but presented no difference in the CTD group, suggesting that the underlying CTD type had no effect on the KL-6 level, but patients with PM were more likely to have worse lung conditions.
In previous studies, researchers have reported significant inverse correlations between serum KL-6 levels and DLCO% in patients with polymyositis and dermatomyositis (25, 26). We also found that KL-6 presented a significant correlation with the severity of CTD-ILD not only measured with CT, but also stratified by DLCO%. We further analysed the relationship between CT signs and the KL-6 level and found that the KL-6 level was correlated with ILD signs, including ground-glass opacity, honeycomb, and reticular shadow, but was not related to COPD signs. Doishita et al. (27) also reported positive correlations between KL-6 levels and both the presence and activity of ILD. KL-6, a mucin-associated glycoprotein, may be a trigger for transforming growth factor beta(TGF-β) signalling and fibrosis (28, 29), giving it the potential to be a biomarker not only of the presence of ILD but also of disease activity. A higher KL-6 level indicates greater ground-glass opacity, honeycomb, or reticular shadow lesions, suggesting that KL-6 should be an alternative method to screen ILD among patients with CTD without the radiology-associated risk. More importantly, it may also have the potential to predict the prognosis of patients with CTD-ILD. Multiple logistic regression analysis revealed that KL-6 is an independent predictive factor for the presence of ILD among patients with CTD. Based on this result, we constructed a decision tree model to predict the possibility of ILD based on a set of decision rules.
There are some limitations to the current study. First, we collected the data from our database, patients who had KL6 measurements were more likely to have ILD. Therefore, the sample size of patients with CTD-NILD was much smaller than that of patients with CTD-ILD, but this did not affect our main findings that KL-6 levels should be corrected for Hb and lung infections. Second, not all the patients underwent high-resolution CT. However, in our hospital the scanning thickness is 1.0 mm for conventional CT and 0.6 mm for high-resolution CT, both of which can identify interstitial lesions. Hence, the absence of high-resolution CT would not affect the identification of ILD lesions. Additionally, a small number of patients in CTD-NILD group do not have lung fuction data, but the correlation analysis between KL-6 and the severity of ILD were from the CTD-ILD group, the absence of lung function in the CTD-NILD group had no effect on the main findings. Finally, due to the small sample size, we could not verify the accuracy of the decision tree. Thus, longitudinal cohort studies will be essential in the future.
5 Conclusion
Hb and lung infections are independent factors affecting KL-6 levels among CTD-ILD patients. KL-6 is a potential biomarker to predict ILD and assess the ILD severity in the real world.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the ethics committee of Xiangya Hospital, Central South University (No. 202104005). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
AZ, RL, and PP designed the study. All authors contributed toward recruiting subjects, statistical analysis and drafting the paper, and each of the authors agreed to be accountable for all aspects of the work. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by grants from The Youth Science Foundation of Xiangya Hospital (2022Q06 to AZ), the Natural Science Foundation of Hunan Province, China (No. 2023JJ41025 to AZ), the Scientific Research Project of Hunan Health Commission(Grant No.D202303029041), Project Program of National Clinical Research Center for Geriatric Disorders (Xiangya Hospital, Grant No. 2020LNJJ05), The National Key Clinical Specialist Construction Program of China (Grant Number z047-02), and Key R & D Program of Hunan Province (No.2022SK2038).
Acknowledgments
We wish to thank Dr. David R Price (Division of Pulmonary and Critical Care Medicine, Weill Cornell Medical College) for proofreading the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1098602/full#supplementary-material
Abbreviations
AEC, alveolar epithelial cell; AFP, alpha fetoprotein; ANOVA, analysis of variance; AUC, the area under the curve; BMI, body mass index; CA125,Carbohydrate antigen 125; CA242, Carbohydrate antigen 242; CA199, Carbohydrate antigen 199; CEA, carcinoembryonic antigen; CRP, C-reaction protein; CTD, connective tissue diseases; CTD-ILD, connective tissue diseases associated interstitial lung disease; C3, complement C3; C4, complement C4; HRCT, high resolution computed tomography; COPD, chronic obstructive pulmonary disease; DLCO, diffusing capacity of the lungs for carbon monoxide; Eos, eosinophil; ESR, erythrocyte sedimentation rate; FEV1, forced expired volume in one second; FVC, forced vital capacity; Hb, hemoglobulin; KL-6, Krebs Von den Lungen-6; LDL, Low-density lipoprotein; Lym, lymphocyte; CTD, mixed connective tissue disease; Neu, neutrophil; NSE, neuron-specific enolase; PM/DM, polymyositis/dermatomyositis; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus, SjS, Sjögren’s syndrome; SSc, systemic sclerosis; ROC: receiver operating characteristic curve; TB, tuberculosis; TC, total cholesterol; TGF-β, transforming growth factor beta; UCTD, unspecified connective tissue disease; WBC, white blood cell.
References
1. Yu H, Nagafuchi Y, Fujio K. Clinical and immunological biomarkers for systemic lupus erythematosus. Biomolecules (2021) 11(7):928. doi: 10.3390/biom11070928
2. Khanna D, Tashkin DP, Denton CP, Renzoni EA, Desai SR, Varga J. Etiology, risk factors, and biomarkers in systemic sclerosis with interstitial lung disease. Am J Respir Crit Care Med (2020) 201(6):650–60. doi: 10.1164/rccm.201903-0563CI
3. Yao C, Guan X, Carraro G, Parimon T, Liu X, Huang G, et al. Senescence of alveolar type 2 cells drives progressive pulmonary fibrosis. Am J Respir Crit Care Med (2021) 203(6):707–17. doi: 10.1164/rccm.202004-1274OC
4. Olajuyin AM, Zhang X, Ji HL. Alveolar type 2 progenitor cells for lung injury repair. Cell Death Discovery (2019) 5:63. doi: 10.1038/s41420-019-0147-9
5. Lopez-Rodriguez E, Gay-Jordi G, Knudsen L, Ochs M, Serrano-Mollar A. Improved alveolar dynamics and structure after alveolar epithelial type II cell transplantation in bleomycin induced lung fibrosis. Front Med (Lausanne) (2021) 8:640020. doi: 10.3389/fmed.2021.640020
6. Hant FN, Ludwicka-Bradley A, Wang HJ, Li N, Elashoff R, Tashkin DP, et al. Scleroderma lung study research G surfactant protein d and KL-6 as serum biomarkers of interstitial lung disease in patients with scleroderma. J Rheumatol (2009) 36(4):773–80. doi: 10.3899/jrheum.080633
7. Zhang T, Shen P, Duan C, Gao L. KL-6 as an immunological biomarker predicts the severity, progression, acute exacerbation, and poor outcomes of interstitial lung disease: a systematic review and meta-analysis. Front Immunol (2021) 12:745233. doi: 10.3389/fimmu.2021.745233
8. Elhai M, Hoffmann-Vold AM, Avouac J, Pezet S, Cauvet A, Leblond A, et al. Performance of candidate serum biomarkers for systemic sclerosis-associated interstitial lung disease. Arthritis Rheumatol (2019) 71(6):972–82. doi: 10.1002/art.40815
9. Xu L, Yan DR, Zhu SL, Gu J, Bian W, Rong ZH, et al. KL-6 regulated the expression of HGF, collagen and myofibroblast differentiation. Eur Rev Med Pharmacol Sci (2013) 17(22):3073–7.
10. Sakai M, Kubota T, Ohnishi H, Yokoyama A. A novel lung injury animal model using KL-6-measurable human MUC1-expressing mice. Biochem Biophys Res Commun (2013) 432(3):460–5. doi: 10.1016/j.bbrc.2013.01.123
11. Lee JS, Lee EY, Ha YJ, Kang EH, Lee YJ, Song YW. Serum KL-6 levels reflect the severity of interstitial lung disease associated with connective tissue disease. Arthritis Res Ther (2019) 21(1):58. doi: 10.1186/s13075-019-1835-9
12. Zheng M, Lou A, Zhang H, Zhu S, Yang M, Lai W. Serum KL-6, CA19-9, CA125 and CEA are diagnostic biomarkers for rheumatoid arthritis-associated interstitial lung disease in the Chinese population. Rheumatol Ther (2021) 8(1):517–27. doi: 10.1007/s40744-021-00288-x
13. Wu X, Wu LJ, Luo CN, Shi YM, Zou JM, Meng XY. The diagnostic value of serum KL-6 in connective tissue disease associated interstitial lung disease. Zhonghua Yi Xue Za Zhi (2019) 99(40):3172–5. doi: 10.1136/annrheumdis-2019-eular.7673
14. He Q, Tang Y, Huang J, Rao Y, Lu Y. The value of KL-6 in the diagnosis and assessment of interstitial lung disease. Am J Transl Res (2021) 13(8):9216–23.
15. Watanabe S, Saeki K, Waseda Y, Murata A, Takato H, Ichikawa Y, et al. Lung cancer in connective tissue disease-associated interstitial lung disease: clinical features and impact on outcomes. J Thorac Dis (2018) 10(2):799–807. doi: 10.21037/jtd.2017.12.134
16. D'alessandro M, Bergantini L, Cameli P, Pieroni M, Refini RM, Sestini P, et al. Serum concentrations of KL-6 in patients with IPF and lung cancer and serial measurements of KL-6 in IPF patients treated with antifibrotic therapy. Cancers (Basel) (2021) 13(4):689. doi: 10.3390/cancers13040689
17. Simon TA, Boers M, Hochberg M, Baker N, Skovron ML, Ray N, et al. Comparative risk of malignancies and infections in patients with rheumatoid arthritis initiating abatacept versus other biologics: a multi-database real-world study. Arthritis Res Ther (2019) 21(1):228. doi: 10.1186/s13075-019-1992-x
18. Zafari P, Golpour M, Hafezi N, Bashash D, Esmaeili SA, Tavakolinia N, et al. Tuberculosis comorbidity with rheumatoid arthritis: gene signatures, associated biomarkers, and screening. IUBMB Life (2021) 73(1):26–39. doi: 10.1002/iub.2413
19. Miwa S, Suzuki Y, Shirai M, Ohba H, Kanai M, Eifuku T, et al. Assessment of serum KL-6 as a prognostic marker in pulmonary tuberculosis patients. Int J Tuberc Lung Dis (2013) 17(2):240–2. doi: 10.5588/ijtld.12.0498
20. Ishikawa N, Hattori N, Tanaka S, Horimasu Y, Haruta Y, Yokoyama A, et al. Levels of surfactant proteins a and d and KL-6 are elevated in the induced sputum of chronic obstructive pulmonary disease patients: a sequential sputum analysis. Respiration (2011) 82(1):10–8. doi: 10.1159/000324539
21. Nam M, Hur M, Park M, Kim H. Novel usefulness of Krebs von den lungen 6 (KL-6) with hemoglobin and lactate dehydrogenase for assessing bone marrow fibrosis. Diagnostics (Basel) (2022) 12(3):628. doi: 10.3390/diagnostics12030628
22. Frix AN, Schoneveld L, Ladang A, Henket M, Duysinx B, Vaillant F, et al. And guiot J could KL-6 levels in COVID-19 help to predict lung disease? Respir Res (2020) 21(1):309. doi: 10.1186/s12931-020-01560-4
23. D'alessandro M, Bergantini L, Cameli P, Curatola G, Remediani L, Bennett D, et al. Serial KL-6 measurements in COVID-19 patients. Intern Emerg Med (2021) 16(6):1541–5. doi: 10.1007/s11739-020-02614-7
24. Kubota M, Haruta T. The role of serum KL-6 measurement in common pediatric respiratory infections. J Infect Chemother (2006) 12(1):22–4. doi: 10.1007/s10156-005-0416-9
25. Hu C, Wu C, Yang E, Huang H, Xu D, Hou Y, et al. Serum KL-6 is associated with the severity of interstitial lung disease in Chinese patients with polymyositis and dermatomyositis. Clin Rheumatol (2019) 38(8):2181–7. doi: 10.1007/s10067-019-04501-9
26. Wang Y, Chen S, Lin J, Xie X, Hu S, Lin Q, et al. Lung ultrasound b-lines and serum KL-6 correlate with the severity of idiopathic inflammatory myositis-associated interstitial lung disease. Rheumatol (Oxford) (2020) 59(8):2024–9. doi: 10.1093/rheumatology/kez571
27. Doishita S, Inokuma S, Asashima H, Nakachi S, Matsuo Y, Rokutanda R, et al. Serum KL-6 level as an indicator of active or inactive interstitial pneumonitis associated with connective tissue diseases. Intern Med (2011) 50(23):2889–92. doi: 10.2169/internalmedicine.50.5866
28. Majewski S, Szewczyk K, Zal A, Bialas AJ, Milkowska-Dymanowska J, Piotrowski WJ. Serial measurements of circulating KL-6, SP-d, MMP-7, CA19-9, CA-125, CCL18, and periostin in patients with idiopathic pulmonary fibrosis receiving antifibrotic therapy: an exploratory study. J Clin Med (2021) 10(17):3864. doi: 10.3390/jcm10173864
Keywords: CTD, ILD, KL-6, CTD-ILD, CTD-NILD
Citation: Zhou A, Tang H, Peng W, Wang Y, Tang X, Yang H, Lu R and Pan P (2023) KL-6 levels in the connective tissue disease population: typical values and potential confounders–a retrospective, real-world study. Front. Immunol. 14:1098602. doi: 10.3389/fimmu.2023.1098602
Received: 15 November 2022; Accepted: 24 May 2023;
Published: 20 June 2023.
Edited by:
Yujing Zhang, Nanjing University, ChinaReviewed by:
Ya-fei Qin, Tianjin Medical University General Hospital, ChinaYongbo Huang, Guangzhou Medical University, China
Copyright © 2023 Zhou, Tang, Peng, Wang, Tang, Yang, Lu and Pan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rongli Lu, bHVyb25nbGlAY3N1LmVkdS5jbg==; Pinhua Pan, cGluaHVhcGFuNjY4QGNzdS5lZHUuY24=
 Aiyuan Zhou
Aiyuan Zhou Haiyun Tang6
Haiyun Tang6 Pinhua Pan
Pinhua Pan