- 1Department of Rheumatology and Immunology, The First Hospital of China Medical University, China Medical University, Shen Yang, China
- 2Department of Thyroid Surgery, The First Hospital of China Medical University, China Medical University, Shen Yang, China
Meteorin-like, also known as Metrnl, Meteorin-β, Subfatin, and Cometin, is a novel secreted protein exerting pleiotropic effects on inflammation, immunology, and metabolism. Earlier research on this hormone focused on regulating energy expenditure and glucose homeostasis. Consequently, several studies attempted to characterize the molecule mechanism of Metrnl in glucose metabolism and obesity-related disorders but reported contradictory clinical results. Recent studies gradually noticed its multiple protective functions in inflammatory immune regulations and cardiometabolic diseases, such as inducing macrophage activation, angiogenesis, tissue remodeling, bone formation, and preventing dyslipidemias. A comprehensive understanding of this novel protein is essential to identify its significance as a potential therapeutic drug or a biomarker of certain diseases. In this review, we present the current knowledge on the physiology of Metrnl and its roles in inflammation, immunology, and metabolism, including animal/cell interventional preclinical studies and human clinical studies. We also describe controversies regarding the data of circulation Metrnl in different disease states to determine its clinical application better.
Introduction
Secreted proteins play an important role in certain physiological or pathological processes to reflect and regulate the organism’s state at the molecular level. Exploring the secreted protein’s mechanism, which may be applied as a targeted agent or a predictive biomarker, would help diagnose and treat clinical diseases. Metrnl, also known as Meteorin-like, Meteorin-β, Subfatin, and Cometin, is a novel secretory protein that shares 46% amino acid sequence homology with a neurotrophic factor named Meteorin. Unlike Meteorin’s brain-specific expression in the central nervous system, Metrnl is abundant in metabolism-related organs and barrier tissues (1). Besides, its secretion and regulation depend not on the fixed model of cell types but on the concrete physiological and pathological context.
When Metrnl was described in early studies as an identity of adipokine, exerting pleiotropic effects on glucose homeostasis, such as improving insulin sensitivity, facilitating adipose tissue browning, and increasing energy expenditure (2). While recent research results suggested that Metrnl may play protective roles in several cardio-metabolic and other inflammatory immune diseases. Different physiological activities could also regulate Metrnl expression, including exercise, temperature variation, bariatric surgery, and high-fat diets. A comprehensive understanding of Metrnl is essential to determine its significance as a potential therapeutic agent or biomarker for certain diseases. As such, this present review would offer an overview of the valid evidence on the effects of Metrnl in inflammation, immunology, metabolism, and related diseases, including animal/cell interventional preclinical studies and human clinical studies.
Physiology of Metrnl
Metrnl is located on regions of human chromosome 17q25.3 and mouse chromosome 11qE2, consisting of 311 amino acids encoded by 936 base pairs (3). There is a 45 amino acid sequence at the amino terminus, but Metrnl does not have a membrane-spanning region. Since Metrnl was the only gene on the q-arm terminal end of chromosome 17, it may regulate a mild clinical phenotype of a rare disorder named Mild Ring 17 Syndrome (4). This phenotype presented as growth delay and intellectual disability and was presumed to be associated with abnormal expression of genes (including Metrnl) related to proximal telomere side neurogenesis. Interestingly, 17q25.3 was a susceptibility locus of cardiovascular disease (5), psoriasis (6), and atopic dermatitis (7), where its fragment deletion could result in cardiovascular deficiencies or cardiac phenotype changes. Notably, Metrnl has been studied preliminarily in these diseases, thus providing a genomic rationale for involvement in pathogenesis development. However, its intracellular localization and distribution still need to be clarified. Its molecular structure and family ligands are also poorly studied, which poses an obstacle to exploring the relationship between Metrnl structure and function.
Metrnl was highly conservative in its evolution and 40% homologous to the neurotrophic factor Meteorin (8). Unlike Meteorin’s concentrated expression in the central nervous system (CNS), Metrnl was highly expressed in adipose, skin, and mucosal barrier tissue, whereas less in CNS (1, 9). Nevertheless, several studies still attempted to explore Metrnl functions in CNS. Based on original bioinformatics, Ramialison et al. (10) detected Metrnl as one of the direct downstream targets of PAX2/5/8 genes involved in inner ear growth. A similar study was reported by Jørgensen et al. (3) that Metrnl exclusively in dorsal root ganglions and inner ear during early mouse development but not in the adult CNS. These studies suggest Metrnl may be involved in inner ear development.
As a new neurotrophic factor, Metrnl could promote neurite outgrowth, migration, and neuroprotection (3, 11). Of note, Metrnl was able to cross the human blood-brain barrier to migrate into the CNS, as well as Metrnl levels in human cerebrospinal fluid were significantly correlated with its serum levels and albumin CSF/serum ratios (12). Considering Metrnl could induce significantly upon exercise and muscle contraction, prompting that Metrnl may migrate from the periphery tissues into the CNS under these activities. However, to address the effect of de novo innervation on human skeletal muscle cells, Jan et al. (13) found that co-culture myotubes with the embryonic rat spinal cord explants did not affect Metrnl mRNA expression. It may be interesting to explore whether Metrnl directly influences the muscle-brain axis or whether Metrnl involves in neuromuscular transmission. Recently, Hong et al. (14) reported that Metrnl could regulate cognitive dysfunction in D-galactose-induced aging models as Metrnl deficiency significantly aggravated the cognitive impairment and decreased hippocampal BDNF, TrkB, and GFAP levels. It would be promising that exploring Metrnl as a candidate treatment and alleviation of aging-related cognitive dysfunction. But further studies are required to clarify the specific mechanisms among Metrnl, neurons and other glial cells.
Metrnl and immunology
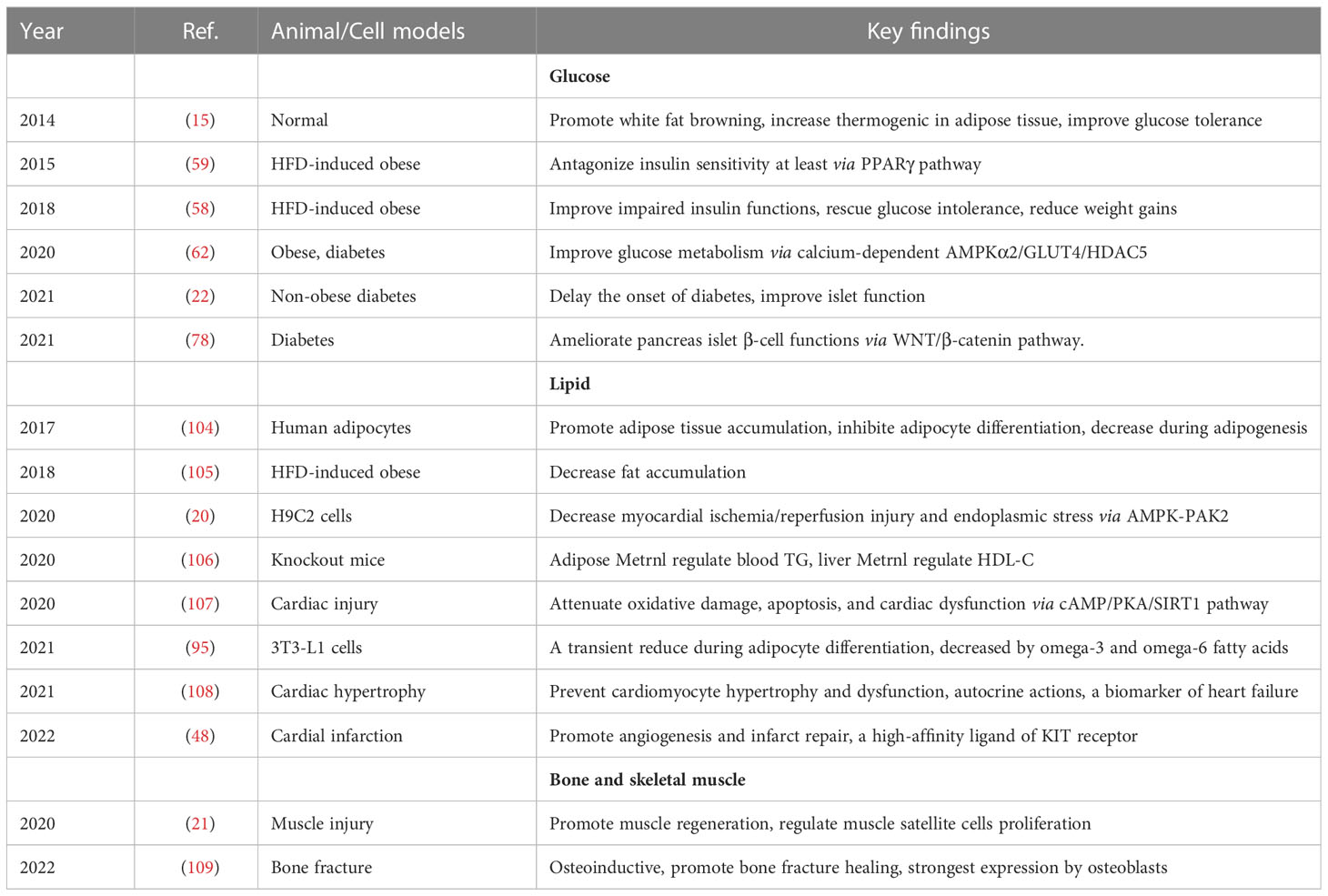
Considering Metrnl is involved in modulating the immune response, several studies have explored its implications in abnormal immune conditions, detected the expression of Metrnl in autoimmune diseases, and analyzed its relationship with these diseases. In this part, we described current knowledge of Metrnl’s role in immunology, including regulations of innate or adaptive immunity and involvement in autoimmune diseases. We also summarized main findings of Metrnl-related preclinical (Table 1) and clinical studies (Table 2) on immunology to understand better.
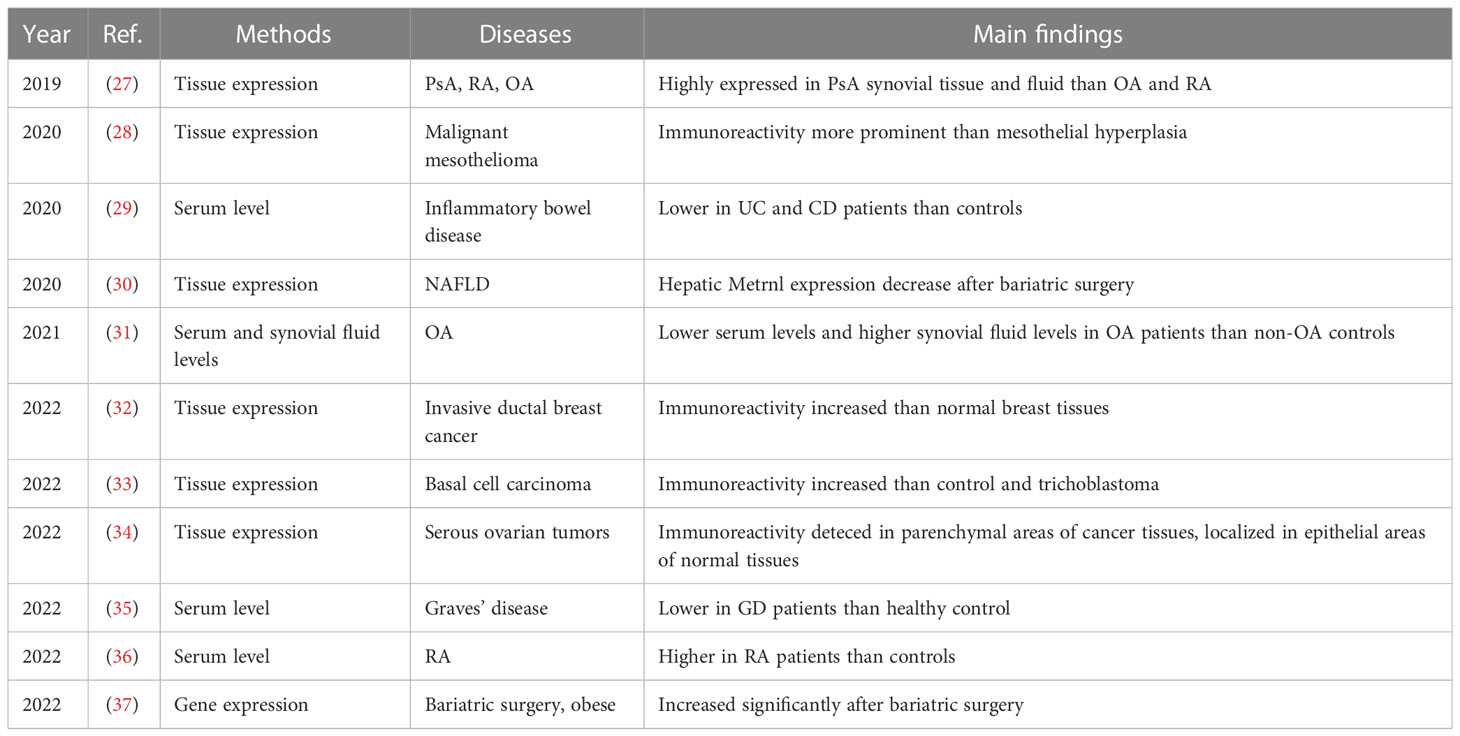
Table 2 Metrnl-related circulation levels and tissue expressions of human clinical studies on inflammation and immunology.
Metrnl and immune system
Metrnl was first described in the immune field when Albert et al. (9) sought to discover a novel or uncharacterized gene associated with the immune system through bioinformatics analyses of the BIGE database (a comprehensive human gene expression database) (38, 39). Therefore, Metrnl was identified, and it is strongly produced by various macrophages. Importantly, Metrnl−/− mice exhibited B-cell immune system defects, including lower serum IgG levels (mainly because of IgG2b and IgG3 low levels) (17). Besides, its splenocytes showed an inability to secret some chemokines like CCL3 and CCL4, as well as a significant reduction in IFN levels. This study also reported that Metrnl could inhibit the expression of class MHC-II in peritoneal macrophages during IFN-γ induction (17). According to the ImmGen Datasets (https://www.immgen.org/), Metrnl could be produced by thymic medullary epithelial cells, suggesting Metrnl may also affect T-cell development.
While exploring specific mechanisms, Metrnl seems to be associated with Type 2 immune responses. Rao et al. (15) observed that Metrnl overexpression could trigger Type 2 immune cascade activation and secret cytokines IL-4/IL-13, promoting M2 macrophage activation to produce catecholamines (40). In an allergic asthma mice study, Metrnl could impair dendritic cells (DCs) maturation and function of antigen presentation both in vitro and vivo, thereby reducing Type 2 inflammatory responses (25). This study provided a novel treatment strategy for targeting Metrnl in allergic asthma. Besides, Legaki et al. (41) have recently identified Metrnl as one differentially methylated loci biomarker in asthma or rhinitis, which provides epigenetics evidence of Metrnl’s involvement in asthma molecularly mechanisms. It is noteworthy that injecting recombinant Metrnl could not affect B-cell class switching or production of IgE but significantly decreased T-cell proliferation if coculturing splenic CD4+ T cells with bone marrow-derived DCs (25). Interestingly, human helminth infections shared similar pathways with allergic asthma, a change in Type 2 phenotype differentiation (42, 43). In this state, Th2 cells develop a PD-1/PD-L2-dependent intrinsically hypo-responsive phenotype, denoted by parasite killing or impaired functionality. Furthermore, microarray data analysis revealed that Metrnl was identified as one immune regulatory gene associated with Th2 cell-intrinsic hypo-responsiveness transcriptional changes (44).
Given the immunomodulatory properties of Metrnl, it may influence tumorigenesis. Actually, Metrnl exerting pro-tumor and pro-apoptotic effects has been observed in pancreatic cancer (45, 46). But studies on Metrnl and tumor immune mechanisms are fewer, while other studies only preliminarily investigated Metrnl immunoreactivity and its relation with tumor prognosis. Metrnl immunoreactivity was increased significantly in invasive ductal breast cancer tissue compared with normal breast tissues, but no difference among the breast cancer grades (32). Similar elevation was also observed in tumor and stromal tissues of basal cell carcinoma and trichoblastoma (33), malignant mesothelioma (28), and serous ovarian tumors (34). Besides, a recent study on bladder cancer identified Metrnl as a target gene in epigenetic synergistic interactions between miRNA and DNA methylation, which was associated with the survival of potential prognostic markers in bladder cancer (47). However, this study did not provide any clinical data supporting it. Interestingly, tumor growth and metastasis depend on angiogenesis, while Metrnl has been identified as a driver of heart postinfarction angiogenesis (48). It would be a promising research orientation to explore Metrnl’s roles in tumor growth, angiogenesis, invasion, and metastasis, thus determining whether Metrnl might be a target in tumor therapy.
Metrnl and autoimmune diseases
Metrnl was involved in multiple autoimmune disorders. As Type 1 diabetes (T1DM) is occurred by autoimmune progressive attacking pancreatic beta cells, a recent study by Zhina et al. (22) observed that intravenous administration of Metrnl could postpone the onset of diabetes in non-obese diabetic mice. This study further explored the mechanism and showed that Metrnl treatment could decrease islet lymphocyte infiltration and modulate immune cell responses. It would be prospective to combine Metrnl-related research results in T1DM patients to determine the possibility as a drug agent. Recently, Gong et al. (35) detected Metrnl levels in thyroid autoimmune diseases and found that Metrnl was significantly lower in Graves’ disease (GD) patients than in healthy controls, positively correlated with CRP and WBC. Besides, Ushach et al. (9) calculated that Metrnl expression was up-regulated in rheumatoid arthritis (RA) synovial membranes by using a gene expression database of RA they constructed before (49). Further investigation proved this finding as Metrnl was significantly increased in the synovial fluid of psoriatic arthritis (PsA) and RA compared to osteoarthritis (OA) patients, and synovial biopsies also mirrored this expression (27, 50). In contrast, Metrnl levels reduced in OA serum (31). Importantly, our academic team recently reported that Metrnl serum level was higher in RA patients than in OA and healthy controls, positively correlated with disease activity indices (36). These results suggested the close relationship between Metrnl and autoimmune-associated arthritis, but the specific mechanism mediating this role remains to be found.
Using tissue microarray, Li et al. (16) found Metrnl highly expressed in the gastrointestinal tract and the intestinal epithelium. Interestingly, a study by Gholamrezayi et al. (29) reported that Metrnl circulation concentration was lower in both Crohn’s disease (CD) and Ulcerative Colitis (UC) patients and inversely related with TNF-α and IL-6. To further figure out the mechanism, scholars generate the intestinal epithelial cell-specific Metrnl-KO mice, where Metrnl levels in the gut fluid and antimicrobial peptides release decreased significantly but did not influence colon length, intestinal permeability, or mucus content. However, UC was more prone to be induced in intestinal Metrnl−/− mice than in WT mice (19). In this study, intestinal Metrnl deficiency was related to reduced autophagy in epithelial cells via the AMPK-mTOR-p70S6K pathway. Similar to this study, Zuo et al. (18) showed that Metrnl was increased in CD mesenteric adipose tissue and that injecting Metrnl into IL-10−/− mice (CD-like model) could decrease the disease activity index and inflammatory score, indicating Metrnl mediated protective effects. In this study, Metrnl activated STAT5/PPAR-γ signaling and promoted adipocyte function, thereby ameliorating chronic colitis. Collectively, maybe different tissues produce Metrnl with different environmental regulatory mechanisms, and Metrnl may be a therapeutic target for UC and CD.
Metrnl and inflammation
Similar to inflammary factor, Metrnl also play roles in immune inflammatory response. In this part, we first overviewed Metrnl developments with inflammatory factor network in multiple diseases. Then we discussed its role of inflammation regulation in different tissues. We also summarized main findings of preclinical studies and clinical studies in inflammation (Tables 1, 2). Finally, considering that Metrnl regulated several functions with the help of macrophages, we drew relationships among these relevant cells to better understand (Figure 1).
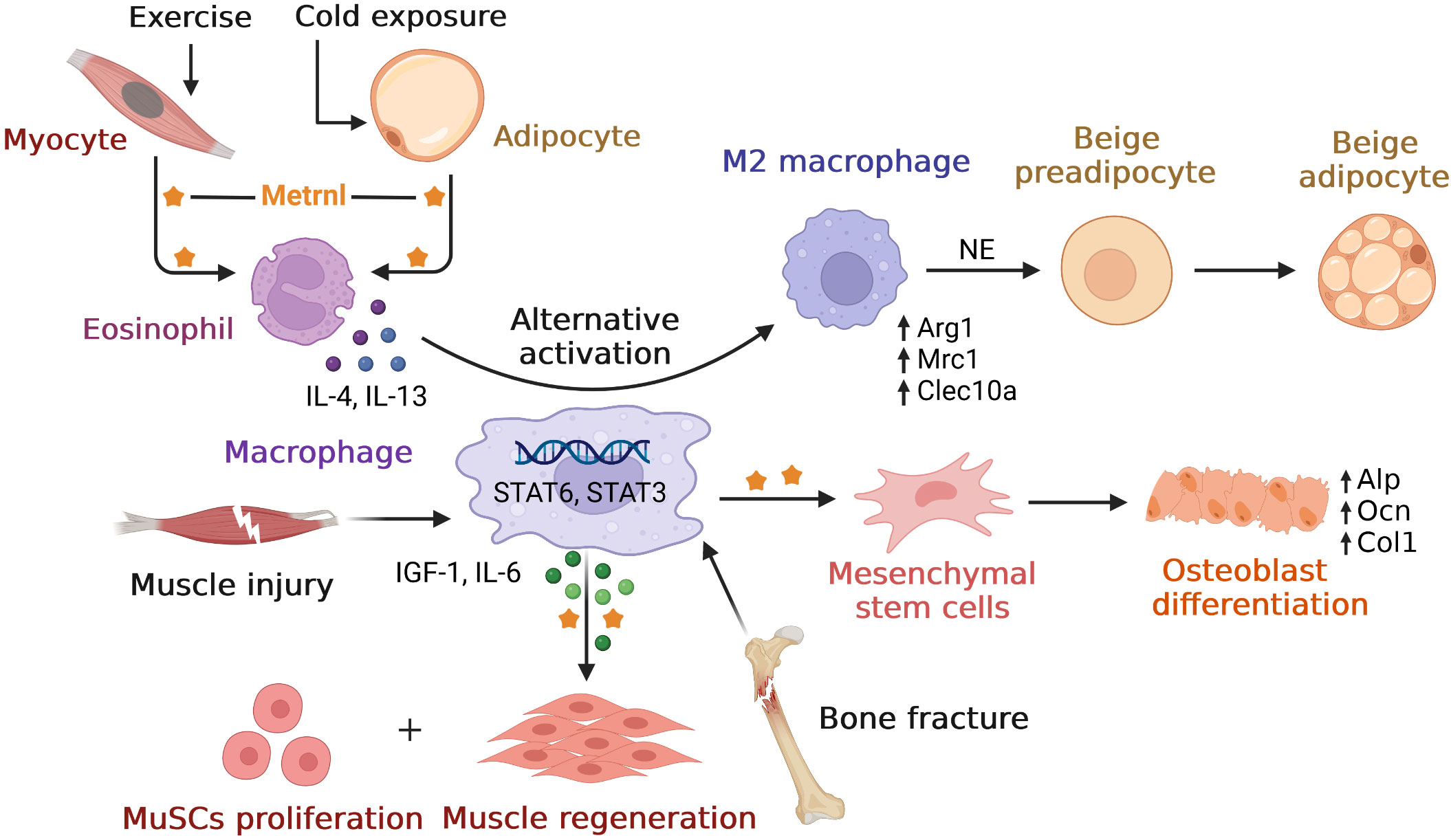
Figure 1 Overview of relationships between Metrnl and macrophages. Metrnl could induce IL-4 and IL-13 secretions by eosinophil upon exercise in myocytes and cold exposure in adipocytes. These cytokines stimulated macrophages’ alternative activation to M2 type via STAT6 signaling, which released norepinephrine (NE) and promoted adipocyte browning to increase thermogenesis. After muscle injury, macrophage-derived Metrnl activated STAT3 signal to induce IGF-1 and IL-6 secretions, thus promoting muscle stem cells (MuSCs) proliferation and skeletal muscle regeneration. Metrnl was highly expressed at regions of osteoblast active and bone depositions during bone fracture healing. Besides, Metrnl could promote mesenchymal stem cells differentiating into osteoblasts with increasing osteogenic transcripts.
Metrnl and inflammatory factor network
Many studies presented so far indicate that Metrnl is associated with inflammation. Metrnl−/− mice, generated by Ushach et al. (17), were prone to develop inflammatory lesions (including uterus unilateral horn, kidneys, and liver) and endotoxin shock, suggesting Metrnl deficiency promotes inflammation development. It may play an anti-inflammatory role during sepsis. Metrnl was diffusely in activated macrophages (1, 9), as well as its production can be induced by several cytokines and inversely regulate other chemokines and cytokine production. Bone marrow macrophages can produce Metrnl by IL-4, IL-12, IL-17α, and TNF-α stimulating, of which TNF-α is the most potent inducer and can inhibit by IFN-γ and TGF-β (17). Incubating macrophages with Metrnl can also increase IL-6, IL-10, CXCL1, and CCL2 expression. Another study with similar findings by Junya et al. (26) reported that Metrnl homologues upregulated pro-inflammatory cytokines (TNF-α, IL-17A, IL-1β, IL-6, and IL-8) and promoted Type 1 immune response (IFN-γ and IL-2) via NF-κB dependent pathway in grass carp head kidney leukocytes. In addition, Metrnl overexpression inhibited inflammation and alleviated endoplasmic reticulum stress in injured H9C2 cells by activating AMPK-PAK2 signaling, playing an anti-inflammation role (20).
Several studies have reported that Metrnl serum levels were negatively associated with inflammatory cytokines like TNF-α and IL-6 in many metabolic diseases (20, 29, 51–53). A study on human umbilical vein endothelial cells (HUVECs) with Metrnl deficiency reported that pro-inflammatory cytokines IL-6, IL-1β, and MCP-1 were increased under normal conditions, and IL-6, IL-1β, TNF-α were elevated under ox-LDL stimulation (24). In this study, Metrnl could ameliorate LPS-induced endothelial cell inflammation through AMPK and PPAR-γ dependent pathways as endothelial injury inducing many metabolic diseases. Besides, disease activity index CRP and hs-CRP were also negatively correlated with Metrnl circulation in polycystic ovarian syndrome (PCOS), CAD, impaired glucose tolerance (IGT), GD, and type 2 diabetes Mellitus (T2DM) (35, 52, 54, 55). In contrast to these findings, two studies found that Metrnl serum levels were positively correlated with inflammation factors in chronic obstructive pulmonary diseases (COPD) and neurological disease patients (12, 56). Thus, larger participants should be involved in determining the correlation between Metrnl and the network of inflammation factors.
Metrnl and adipose tissue inflammation
Adipose tissue eosinophils (ATEs) distribution and function are important in controlling age-related and obesity-related inflammation (57). Metrnl could stimulate an eosinophil-dependent increase in IL-4/IL-13 secreting, which promoted alternate activation of M2 macrophages to act as an anti-inflammatory function (15). Meanwhile, overexpression in Metrnl mice increases eosinophils in adipose tissue, and injecting an antibody against Metrnl prevents eosinophil accumulation (15, 40). Because Metrnl could not directly activate macrophages or adipocytes, eosinophils are indeed required for this mechanism (15). Interestingly, it is reported that transferring young mice eosinophils into an aged host can inhibit adipose tissue age-related inflammation and improve immune fitness, partially mediated by eosinophil-derived IL-4 (57). Many studies proved Metrnl can attenuate inflammation in adipose tissue (17, 18, 23, 24, 58, 59), and adipose tissue function was crucial in contributing to age-related metabolic diseases (60). Importantly, Ushach et al. (17) observed that older Metrnl-KO mice were prone to develop inflammatory lesions, especially unilateral inflammatory lesions of one uterus horn, while white adipose tissue developed no lesions. Furthermore, Metrnl played essential roles in body inflammation regulation and adipose tissue inflammation homeostasis. Beyond that, Metrnl may also slow adipose tissue inflammation associated with aging by affecting eosinophils.
Metrnl and skeletal muscle inflammation
Increasing evidence suggests that skeletal muscle inflammation is accompanied by obesity and metabolic disorders development, which is manifested by proinflammatory activation and immune cell infiltration in intramyocellular and perimuscular adipose tissue (61). Exercise has many beneficial effects on metabolic disorders partly attributed to its anti-inflammatory effect. Metrnl, one of the myokines, can be induced in the skeletal muscle upon exercise (15), especially during muscle contractions (62). Javaid et al. (23) showed that exercise could significantly enhance Metrnl expression in various muscle depots, inhibiting NLRP3 inflammasome activation and downregulating IL-1β and IL-18 in adipose tissue. Further in vitro macrophage experiments also confirmed this anti-inflammation role. Moreover, Jung et al. (58) found that treating high-fat-diet (HFD) mice with Metrnl can suppress inflammatory markers and attenuate the impaired insulin response in mouse skeletal muscle and differentiated C2C12 cells.
Of note, Metrnl also played a role in regenerating damaged muscle, as it was increased sharply (30-fold) after muscle injury 24h in murine models of muscle regeneration (21). Subsequently, single-cell RNA-seq demonstrated that the most robust Metrnl transcript was by macrophage clusters in the injured muscle. Meanwhile, the LysM Cre mouse and macrophage-specific Metrnl-KO mice experiments confirmed the essential role of macrophage-secreted Metrnl in successful muscle regeneration. Moreover, Metrnl can directly signal to macrophages through Stat3 to result in differentiation to an anti-inflammatory phenotype and indirectly to primary muscle satellite cells by IGF-1 and IL-6 secretion to promote muscle stem cell expansion (21, 63). Besides, we summarized main signal pathways of Metrnl in myocytes. Collectively, Metrnl has exerted a healing and anti-inflammation effect on skeletal muscle damage, indicating that Metrnl may be a novel therapeutic target for treating aging or other inflammatory myopathies.
Metrnl regulations in glucose metabolism
In humans, secretory proteins such as adipokines could modulate glucose uptake and release to regulate glucose homeostasis directly. Once a long-term lack of physical activities or a chronic high-fat diet induces obesity may result in disturbances of glucose metabolism, which leads to the development of insulin resistance and diabetes. In this section, we described the roles of Metrnl in glucose homeostasis, including glucose uptake, thermogenesis, and adipose browning. We also summarized regulations of Metrnl in related metabolic diseases, including insulin sensitivity, polycystic ovarian syndrome, and diabetes. Regarding controversial data in clinical studies, we analyzed it from multiple angles and summarized it in Supplementary Table 1.
Metrnl and glucose homeostasis
The human body can adapt to external and internal environment variations by adjusting metabolism in peripheral tissues, such as cold exposure could induce skeletal muscle and adipose tissue a switch of glucose and fat metabolism (64). This adaptation to acute environment change might be a protective mechanism to ensure enough glucose priority for the central nervous system, whereas fatty acids and amino acids are allocated to other peripheral metabolic organs (65). Metrnl can be strongly induced in the adipose tissue upon acute cold exposure and muscle upon exercise, leading to increased energy expenditure by stimulating the browning of white adipose tissue. Unlike most adipokines identified in obesity models, Metrnl was first described in the PGC-1α4 transgenics mice model (15). PGC-1α4 is an exercise-induced splice isoform of PPARγ coactivator 1a, and skeletal muscle-specific PGC-1α4-KO transgenics mice were lean and showed an increase in basal energy expenditure (66). Given that adipose thermogenesis can augment whole-body energy expenditure, this study analyzed adipose tissues for expression of genes related to thermogenesis or involved in imparting adipose tissue browning, so METRNL was the candidate gene (15). Besides, Metrnl may promote thermogenesis function through activations of the UCP family in brown adipose tissue (67). Recently, Şekerci et al. (68) reported that central administrating of Metrnl could activate hypothalamus-pituitary-thyroid axis hormones via peripheral UCPs. UCPs and PGC-1α were typical thermogenic genes in the nucleus, so Metrnl played roles in body energy homeostasis regulation.
Metrnl’s ability to stimulate the browning of white adipose tissue seemed not to act directly on adipocytes but was dependent on the eosinophils-mediated IL-4/IL-13 signaling cascade of M2 alternately activated macrophages (15). This academic team subsequently demonstrated that eosinophils are indeed required for Metrnl-induced brownin. Owing to this mechanism, Metrnl has been distinguished from other adipokines with similar functions, such as irisin (69), asprosin (70), and Fgf-21 (71), as they directly stimulated adipocytes to thermogenesis. Furthermore, Zhiyong et al. (59) used inguinal subcutaneous white adipose tissue in adipocyte-specific Metrnl-KO mice to verify these effects. However, there existed no Metrnl-induced adipose browning or no thermogenesis-associated gene change. Notably, the former report showed that Metrnl-induced adipose browning occurred briefly and disappeared in one week (15). So maybe using different experimental models (relatively acute and chronic models) caused this contradiction.
Both modest cold exposure and exercise could promote the conversion of white to brown adipose tissue, increasing the body’s metabolism and helping weight loss (72). Metrnl could adapt to cold temperatures by regulating immune-adipose interactions to increase thermogenesis. Interestingly, a recent study based on Zebrafish using CRISPR/Cas9 system knockout gluk2, a protein that perceived cold in the periphery sensory neuron, revealed Metrnl was one of the differentially expressed genes (73). Although cold exposure induces no-shivering thermogenesis, long-term periods of negative caloric balance would diminish physiological resilience. In a recent study focusing on athletes in extreme cold during continuous physical activity, Coker et al. (74) observed that Metrnl serum levels remained stable throughout the 430-mile distance in temperature -45°C. In contrast to this finding, Saghebjoo et al. (75) investigated serum Metrnl changes in exercises with different temperature conditions on overweight young women. They observed that Metrnl was increased when exercising in temperate and warm water and decreased in cold water. Taking effects that the duration of exposure temperatures, the intensity of exercise, and the physical fitness of participants also affect the response of Metrnl, further investigations are required to clear appropriate temperature to enhance Metrnl expression.
Metrnl and insulin sensitivity
Insulin resistance is a risk factor for many metabolic disorders such as obesity and diabetes. Adipose tissue regulates insulin function via secreting adipokines and sequestering lipids, which or else would accumulate in other tissues and produce bad effects (76). Actually, the major participant to insulin resistance is exact the excessive deposition of lipids in other organs (77). Therefore, adipokines play crucial roles in lipid-associated insulin resistance. Researchers have established many animal models to explore the relationship between Metrnl and insulin resistance. In specific-adipocyte Metrnl-KO mice, though the plasma concentration of Metrnl remained unchanged, Metrnl expression was increased in adipose tissue with its phenotypes of insulin resistance induced by high-fat-diet exacerbated a lot (59). On the contrary, overexpression Metrnl of adipocyte-specific mice could antagonize insulin resistance induced by HFD or hyperphagia (leptin knockout). These results supported that Metrnl was associated with mice’s overall insulin resistance.
Recently, in an injecting recombinant Metrnl to treat HFD-fed mice study, Metrnl ameliorated the impaired insulin response in skeletal muscle and C2C12 myoblast cells via AMPK/PPARδ-mediated signaling (58). Similar studies also concentrated on T2D mice; injection of Metrnl protein can reduce the high glucose-induced insulin secretion and promote the islet β-cell function recovery by activating pancreatic islet β-cell proliferation in mice and inhibiting β-cell apoptosis (78). This function was achieved via the WNT/β-catenin pathway, where β-catenin could protect apoptosis of islet microvascular endothelium role (79), which also pointed to the importance of the WNT/β-catenin pathway in maintaining β-cell function. In addition, Lee et al. (62) found that Metrnl treatment with C2C12 myoblasts could increase glucose uptake via the calcium-dependent AMPKα2 and p38 MAPK pathways, as well as in an AMPKα2-dependent manner to regulate the binding of HDAC5 to the GLUT4 promoter. In this study, Metrnl treatment could not elevate glucose tolerance in AMPK β1β2-KO mice, demonstrating the necessity of the AMPK pathway. Notably, Wang et al. (80) reported that serum Metrnl level was correlated with insulin resistance merely, but not with β-cell function (Figure 2). These animal studies suggested that Metrnl has strong insulin sensitization and antagonizes insulin resistance in vitro; its specific dosage, duration, effects, and mechanism need further study.
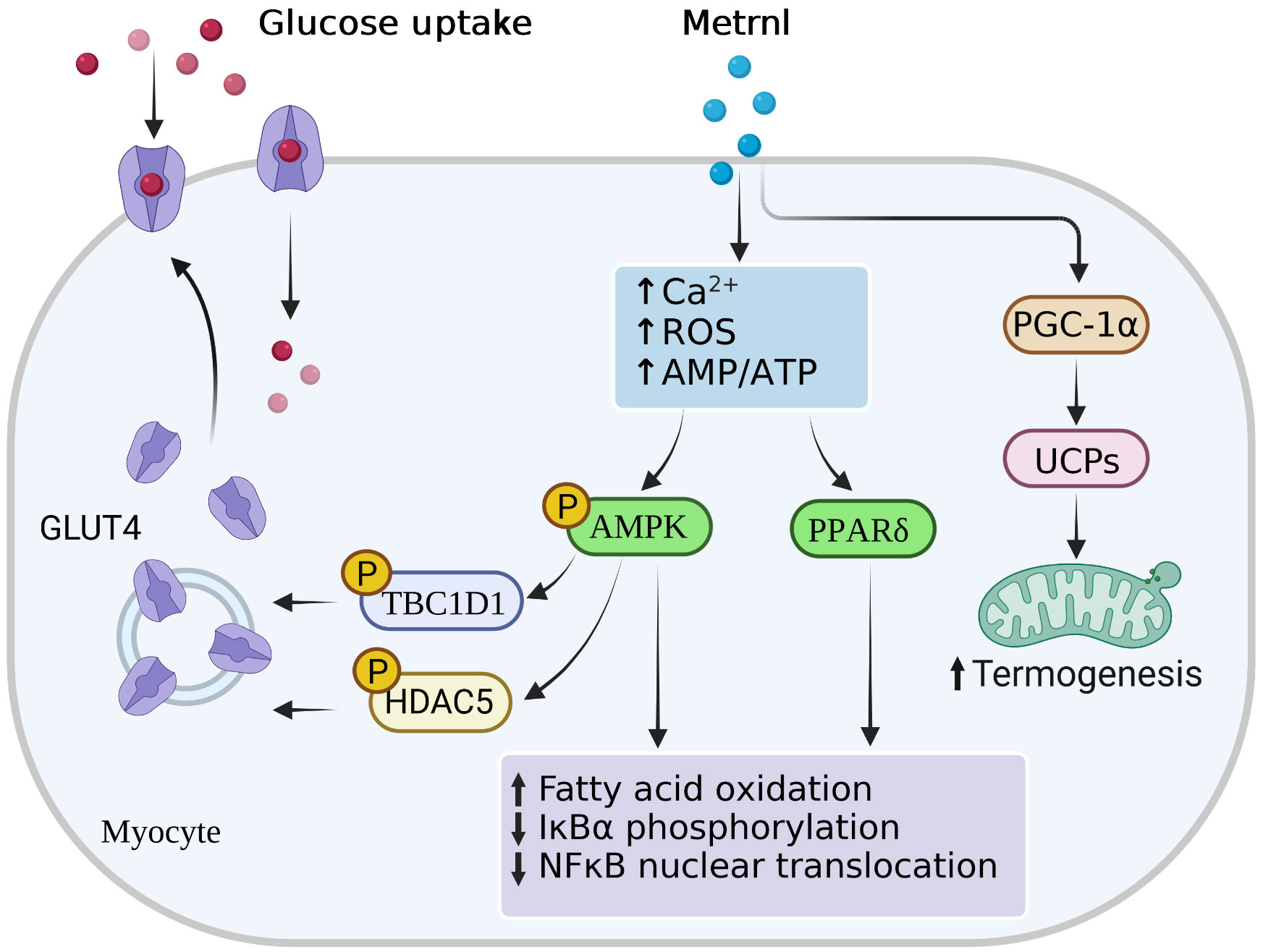
Figure 2 Main signal pathways of Metrnl in myocytes. Metrnl could activate AMP-activated protein kinase (AMPK) and peroxisome proliferator-activated receptor-δ (PPAR-γ) signaling by increasing intracellular calcium ion, reactive oxygen species (ROS), or AMP/ATP ratio levels in skeletal muscle cells. Activation of AMPK phosphorylation stimulated phosphorylation of HDAC5 and TBC1DI, both of which resulted in the GLUT4 transcription activation and translocation from the cytoplasm to the membrane. Highly PPAR-γ expressions and AMPK phosphorylation increased fatty acid oxidation, IκBα phosphorylation, and NFκB nuclear translocation. Besides, Metrnl also increased intramuscular PGC-1α and UCPs expressions, thus promoting mitochondria thermogenesis.
HOMA-IR (Homeostatic Model Assessment of Insulin Resistance) can quantitatively assess how much insulin your pancreas needs to keep your blood sugar levels in check (81). Although HOMA-IR is an indirect measure by calculating body fasting glucose and fasting insulin levels, it is still the most popular used model in clinical research as a direct measure is impractical (82). Many clinical researchers have analyzed the correlation between Metrnl and insulin resistance in diabetes or prediabetes via calculating this index, but the results seemed a little controversial. A study on healthy Iranian adults found plasma Metrnl in recreational athletes increased significantly compared to sedentary subjects after correcting the insulin resistance degree (83). In contrast, baseline plasma Metrnl remained no different. Corresponding to the former study, another report on T2DM patients observed that after adjusting for HOMA-IR, Metrnl did not correlate with increased OR for T2DM. Besides, this study concluded an increase in Metrnl levels in T2DM compared to the control (84). However, most recent clinical studies reported that serum Metrnl demonstrated a negative correlation with fasting insulin and HOMA-IR, as well as T2DM patients with lower Metrnl levels (51, 85–87).
Metrnl and polycystic ovarian syndrome
Polycystic ovarian syndrome (PCOS) is a women’s endocrine condition with more susceptibility to insulin resistance and T2DM, of which one common treatment is insulin sensitizers (88). Considering Metrnl may play roles in insulin resistance and insulin resistance is a common finding in PCOS, two independent research teams analyzed the plasma level of Metrnl in PCOS. They both report that Metrnl level was decreased in PCOS and showed a negative correlation with insulin resistance, in contrast to changes in insulin resistance markers and FSH (54, 89, 90). Moreover, in studies of patients with gestational diabetes mellitus in women of a similarly child-bearing period, Metrnl levels were significantly elevated between 24 and 28 weeks of pregnancy than normal gestation (91). Similar results were also confirmed in maternal and cord blood samples in the later stage of pregnancy; nevertheless, these increased Metrnl effects disappeared sharply after delivery (92). Taken together, a deeper understanding of the glucoregulatory mechanism of Metrnl may reveal novel strategies to treat insulin resistance and other metabolic disorders.
Metrnl and diabetes
In line with Metrnl ameliorating insulin resistance both at animal and cellular levels, many clinical studies have also reported correlations between Metrnl circulation levels and diabetes participants with contradictory results (Supplementary Table 1) and regarded Metrnl as an independent risk factor of T2DM. In newly diagnosed T2DM individuals, several studies reported that serum Metrnl showed lower levels than normal glucose tolerance (NGT). Besides, Metrnl was negatively correlated with a glycemic index (including HbA1c, HOMA-IR, FBG, fasting C-peptide) and positively correlated with HOMA-β (52, 85, 93–98). Another study reported this negative correlation in long-standing diagnosed T2DM patients (85). While after 12 weeks of metformin treatment, though Metrnl remained no changes, HbA1c and FBG reduced a lot (99). Interestingly, statins medicine therapy positively correlated with Metrnl level (94). It seems the duration of diabetes has little effect on Metrnl; thus, Metrnl may not directly reflect blood glucose fluctuations in T2DM. Besides, different drug therapy may influence Metrnl secretion.
However, Wang et al. (84) found Metrnl was elevated in prediabetes and was highest in T2DM compared to IGT and NGT. Similar findings were also reported by Chung et al. (100), Wang et al. (80), and Cherian et al. (101). Notably, part of these reports lacked indicator data concerning insulin resistance and insulin sensitivity, which was challenging to explain the negative correlation between blood Metrnl and FBG. Furthermore, a study on healthy Iranian adults also concerned with FBG showed a negative correlation with baseline plasma Metrnl (83). It is worth noting that Metrnl was found to be lower in COPD patients with comorbidities such as T2DM and CAD, instead, higher Metrnl level in protopathic exacerbations (56). Dadmanesh et al. (51) noticed conflicting data regarding Metrnl circulating levels in T2DM and put to serious evaluations concluding results that Metrnl was lower and showed an independent association with the risk of T2DM presence. More recently, Wu et al. (102) used a meta-analysis indicating no significant difference between T2DM and NGT individuals but found circulation Metrnl level was lower in subgroups with HOMA-IR ≥ four and age ≤ 50 years, while higher in subgroups with BMI < 25 kg/m2. Collectively, this result may interfere with glucose-lowering drugs, duration of disease, sample size, age, BMI, and HOMA-IR; therefore, further longitudinal studies are required to clarify the relationship.
As diabetic nephropathy (DN) with impaired kidney function is the leading cause of end-stage renal disease, a study by Wang et al. (103) compared serum Metrnl levels in healthy controls and T2DM patients with normoalbuminuria, microalbuminuria, or macroalbuminuria, showing that all T2DM subgroups were significantly decreased, with even lower levels in macroalbuminuria group. This study reported negative associations between circulating Metrnl and BUN, Cr, GFR, ACR, CCB treatment, and ACEI/ARB treatment. Similar negative correlations with Metrnl and indicators of renal function were also reported in diabetes by Chung et al. (100). These data suggested that circulation Metrnl levels may be involved in the progression of renal disease in T2DM; however, further studies are required to figure out such potential associations.
Metrnl regulations in lipid metabolism
Regulating the distribution of lipoprotein particles is crucial in maintaining body lipid metabolism, manifested by the liver producing and clearing lipids. Imbalances of lipoprotein would accelerate the accumulation of cholesterol in blood peripheral tissues, which leads to cardiometabolic disorders. In this part, we first concluded the direct effects of Metrnl on adipose tissue and adipocytes. Then we described the roles of Metrnl in lipoprotein homeostasis and lipid-associated diseases (Table 3). Finally, we introduced new findings of Metrnl therapeutical effect on cardiac diseases.
Direct effects on adipose tissue and adipocytes
Considering adipocytes represent the most crucial lipid-storing cell type, and Metrnl was expressed highly in subcutaneous adipose tissue (1); therefore, investigating Metrnl’s direct effects on adipose tissue and adipocytes is essential. Metrnl can regulate adipocyte differentiation (59, 95, 104), and its expression could reduce during human adipogenesis (104). In mice of 3T3-L1 adipocytes and human SGBS cells model, overexpression of Metrnl inhibited pre-adipocytes in differentiation to mature adipocytes and promoted cell proliferation. In contrast, adipocyte-specific knockout of Metrnl upregulated adipocyte mature-specific markers and promoted lipid accumulation but had no significance on proliferation (59, 104). Notably, silencing adipocyte PPARγ or using PPARγ inhibitors demonstrated a decrease in Metrnl expression. This study suggested PPARγ was one of Metrnl signal pathways regulating adipocyte phenotypes, but the specific mechanism needed further exploration.
Although Metrnl was upregulated in pre-adipocytes and mature adipocytes with a transient decrease during adipocyte differentiation, active substances, including glucose, insulin, and fatty acids, stimulated adipocyte Metrnl expression with no effects, whereas omega-3 and omega-6 fatty acids inhibit Metrnl expression (95). In mice studies, overexpression of Metrnl increased the abundance of beige adipocytes and revealed a robust increase in thermogenic and β-oxidation gene programs (15). Similarly, the administration of recombinant Metrnl to HFD-induced mice model also stimulated the expression of broad beige thermogenic gene programs with accompanying weight loss (15, 40). Combining Metrnl-induced browning with these results indicated that Metrnl could be regarded as a classical adipokine to investigate its physiological mechanism further.
Metrnl and lipoprotein homeostasis
The distribution of lipoprotein particles is essential for maintaining human lipid homeostasis, whereas breaking this balance would lead to cholesterol imbalance and even a variety of diseases. Some clinical studies have reported that Metrnl was independently associated with adverse blood lipid parameters in metabolic diseases, including negatively correlating with triglyceride (TG), total cholesterol (TC), and low-density lipoprotein cholesterol (LDL-C) and positively correlating with high-density lipoprotein cholesterol (HDL-C) (55, 99, 100, 110, 111). In order to figure out the overall effects of Metrnl on clinical blood lipids, Qi et al. (106) generated global and tissue-specific knockout of Metrnl mice models. In this study, global-KO Metrnl didn’t alter any lipid parameters under a normal chow diet but increased blood TG by 14%, TC by 16%, and HDL-C by 24% upon a high-fat diet. Consistent with these findings, Li et al. (59) have demonstrated adipose Metrnl could participate in TG metabolism as its deficiency deteriorated HFD-induced acute hypertriglyceridemia. In contrast, its overexpression in adipose tissue improved TG tolerance. Interestingly, liver-specific knockout of Metrnl reduced HDL and TC but didn’t decrease VLDL release, similar to deficiency of total Metrnl (106). Moreover, intestine-specific knockout of Metrnl did not influence HDL or TC. These results imply that blood TG was at least partly modulated by adipose Metrnl rather than liver or intestine, as well tissue-specific Metrnl may control different blood lipid components.
Metrnl and atherosclerosis
Lipid metabolic abnormality is a significant risk factor for atherosclerosis (AS), closely associated with endothelial cell damage and dysfunction. In AS mice models, Metrnl mRNA was significantly decreased in the aorta while still highly expressed in healthy controls (112). Some studies found circulation Metrnl levels were reduced in AS and coronary artery disease (CAD) patients and negatively correlated with endothelial parameters, including baPWV, ICAM-1, VCAM-1, and E-selectin (51, 52, 94, 113). Moreover, circulating Metrnl was lower in elderly patients with chronic heart failure (CHF) and negatively correlated with cardiovascular mortality, CHF rehospitalization, and combined major adverse cardiac events (MACEs) (113). Of note, a previous study has demonstrated that Metrnl could ameliorate LPS-induced endothelial cells’ inflammatory response (24). Consistent with these findings, based on a large-scale vascular adhesion molecules Genome-Wide Association Study (GWAS), Metrnl was identified as a candidate gene associated with carotid intima-media thickness (cIMT) in whole blood gene expression (114). cIMT is a new biomarker of subclinical AS and a predictor of impending cardiovascular events, where indicating Metrnl may also involve in AS development (115). It seemed endothelial impairment factors could facilitate Metrnl secretions by endothelial cells, and circulation Metrnl levels decreased significantly with the AS progression. Moreover, areas of aortic plaques, necrotic injuries, and lipid accumulations of aortic root were heavier in aorta plaque of endothelial Metrnl deficiency mice models than those in control, with more severe spontaneous atherosclerosis (112). These results suggested that the AS mechanism is related to Metrnl deficiency in endothelial cells.
Many studies have reported that Metrnl was a novel myokine with protective effects on cardiovascular diseases. Two previous studies have detected Metrnl abundantly expressed in cardiac muscle (15, 59), as well following Hu et al. (107) found it significantly decreased upon doxorubicin (DOX)-induced cardiotoxicity exposure. This study further found Metrnl could exert cardioprotective effects via activating the cAMP/PKA/SIRT1 pathway as cardiac-specific overexpression of Metrnl markedly improved mice cardiac dysfunction and survival status, while Metrnl deficiency aggravated cardiac injury. Of note, Metrnl could not affect the tumor-killing capacity of DOX (107). Rupérez et al. (108) also confirmed a similar conclusion: they constructed Metrnl-KO mice exhibiting asymmetrical cardiac hypertrophy, fibrosis, and aging. Conversely, overexpression of heart Metrnl could prevent cardiac remodeling. In addition, this study used cardiomyocytes in vitro, demonstrating that Metrnl could inhibit cardiac hypertrophy development, indicating a direct effect on cardiac cells.
Metrnl and heart regenerative
Although the human heart has limited regenerative capability after damage, stimulation of new blood vessel formation is an essential part of the reparative process and might be a potential approach to ameliorate heart function. When recognizing that cell-cell communications between cardiac myeloid cells and other nonmyocytes might participate in heart injury (116), Reboll et al. (48) found that Metrnl protein may serve as a potential mediator aspect of this cellular cross-talk. Notably, previous studies have noticed Metrnl expression was prominently increased in mouse infarcts and patients’ tissue with acute myocardial infarction, but circulating Metrnl level was decreased (117). In mice with a cardiac attack, monocytes and macrophages migrated to the heart, producing Metrnl to stimulate the expansion of vascular endothelial cells, resulting in an angiogenic response that limited damage (118). Besides, delivering Metrnl protein with an infusion pump into the mouse could promote angiogenesis after acute myocardial infarction. More importantly, Metrnl was found as a ligand of KIT receptor tyrosine kinase and could respond to the secreted protein stem cell factor (SCF) (119). The activation of KIT is necessary for the normal angiogenic response after cardiac infarction, and SCF is the function of maintaining KIT-expressing hematopoietic stem cells during development, both of which could stimulate the expansion of KIT-expressing mice endothelial cells (120). Accordingly, the role of METRNL-KIT signaling would open new avenues for developing therapies for cardiac disease.
Metrnl regulations in bone metabolism
Metrnl and osteoblasts
Many studies have reported that Metrnl was involved in skeletal development, remodeling, and some bone-related diseases. After constructing a human osteoblast full-length cDNA library, Gong et al. (121) screened unreported genes closely related AP-1 transcription complex and identified Metrnl as the only candidate gene. In multiple parts of mouse bones, RNA-seq demonstrated the strongest expression of Metrnl transcript by osteoblast. Meanwhile, Metrnl deficiency would decrease mice’s osteogenic capacity in bone marrow stromal cells, suggesting that Metrnl is abundant in bone deposition areas where osteoblasts differentiate and activate (109). Besides, Huang et al. (109) found that Metrnl could increase osteoblast differentiation and mineralization in vitro and promote bone fracture healing. Notably, knockdown of the Metrnl gene did not affect overall skeletal development, fracture healing, or osteoblasts in the animals in this study. Nevertheless, it seemed overexpression of Metrnl would inhibit mineralized nodule formation (121). Recently, Cherian et al. (101) observed a strong positive association between serum Metrnl levels and various molecules with osteogenic properties in obesity and diabetes patients, suggesting Metrnl may potentially affect bone-related development complications.
Although Metrnl could affect osteoblasts both in vitro and vivo, considering the effects of Metrnl on inflammation, some studies regarded Metrnl play roles after skeletal injury precisely with the help of macrophages (17). Similar results were also observed in studies of skeletal muscle adjacent to skeletal anatomy, where Metrnl was highly expressed in the early stages after muscle damage while knocking out the Metrnl gene did not result in changes in various muscle properties either (21). Interestingly, many investigators noticed that numerous factors from local circulation were able to alter bone regeneration (122–124). Consequently, this academic team proposed a compensatory mechanism where Metrnl may be a compensating molecule lacking in vitro culture models. This would explain why Metrnl was dispensable for both bone and muscle development or healing in vivo but served as an osteoinductive molecule and an effector of skeletal muscle regeneration in vitro (63, 109). Taken together, supplementing Metrnl at cellular levels may potentially improve osteoblast function. Besides, further investigations are needed to understand specific mechanisms of Metrnl in osteogenic differentiation and how the body delivers Metrnl into sites of active bone formation.
Metrnl and skeletal-related diseases
Some studies have reported that Metrnl was expressed abnormally in the cartilage tissue and synovium of skeletal-related diseases. In patients with OA, serum Metrnl levels were significantly lower than normal healthy controls but elevated in synovial fluid (31). This confirmed a hypothesis that Metrnl in different tissues might have different environmental mechanisms. Similarly, in two gene expression profiles of cartilage tissue, compared with healthy cartilage from patients with traumatic femoral neck fractures alone, Metrnl was strongly downregulated in OA patients (ratio = 0.34) but upregulated in non-traumatic osteonecrosis of femoral head (NOFH) (ratio = 11.77) (125, 126). These revealed the Metrnl gene involved in pathogenetic differences in NOFH and OA cartilage damage, and it would be interesting to explore the mechanism further. Besides, as we mentioned in the previous section of Metrnl and autoimmune diseases, Metrnl was elevated in synovial fluid of PsA and RA compared to OA patients, and our team also found serum Metrnl levels were higher in RA with a positively correlated with disease activity indices (27, 36, 50). Collectively, the mechanism of Metrnl in skeletal-related diseases merits further research, especially bone-related autoimmune diseases, because immune function interacts with the homeostasis of the skeletal system’s internal environment closely.
Metrnl regulations in obesity and exercise
Obesity is a worldwide health problem with an increased prevalence of metabolic-related disorders. Exercise is an effective strategy to prevent and treat obesity and its related metabolic syndromes. It is well-documented that changes in circulating cytokines could occur after exercise but with unclear mechanisms. Here we concluded circulation Metrnl levels in obese merged with or without metabolic diseases and discussed Metrnl involvements in bariatric surgery (Supplementary Table 1). We also discussed the roles of Metrnl after exercise and compared circulating Metrnl changes upon different exercises.
Metrnl and obesity
Obesity is an important promoter of chronic and low-grade inflammation states with lipid accumulation, which results in many metabolic syndromes (61, 127). Previously, Li et al. (1) discovered that Metrnl was upregulated in circulation and adipose tissue of HFD-induced obese mice. Similarly, Löffler et al. (104) reported that Metrnl was highly expressed in adipocytes of obese compared to lean children. These interactions with adipose tissue dynamics indicated the association between Metrnl and obesity. However, correlations between serum Metrnl level and obesity indexes were contradictory. Some studies reported that simply obese individuals (29, 85, 103, 128) or obese patients with metabolic syndrome have been shown lower Metrnl levels and were negatively related to body mass index (BMI) (51, 54, 55, 85, 92, 94, 103, 129). Notably, this negative association was prone to arise in T2DM patients compared with other diseases. Actually, it seems more studies figured out that there is no relationship between Metrnl and BMI in healthy or metabolic syndrome (31, 52, 80, 83, 87, 89, 95, 99, 100). Apart from total height and body weight, the waist-to-hip ratio (WHR) was further measured but still no correlation (52). Considering visceral fat obesity (VFO) is close to obesity and insulin resistance, there were few studies on the correlations between Metrnl and VFO, as most obesity evaluations focused on measurements of waist circumference or BMI. Du et al. (111) used Dual Energy X-ray Absorptiometry (DXA) to quantify the visceral fat area and found that Metrnl level was independently inversely associated with visceral fat deposition. This finding was the first and only report demonstrating the association between Metrnl and VFO, which provided a possibility that Metrnl concentration may be a useful noninvasive, cost-effective marker for assessing VFO.
Moreover, some results also found a positive correlation between Metrnl levels and BMI. An Arab survey stratified T2DM and healthy controls based on obesity and found Metrnl plasma levels elevated more in obese T2DM (BMI > 30 kg/m2) than in non-obese T2DM (20 kg/m2 ≤ BMI ≤ 30 kg/m2) (130). Wang et al. (84) and Cherian et al. (101) reported similar findings. This increase was probably one of the physiological regulation mechanisms to restore glucose tolerance or defense response to counteract metabolic stress. Of note, these studies have not excluded clinical confounding factors’ effects, such as diabetes-related drugs, physical activity, sex, and age. Besides, obesity or overweight could accelerate the loss of muscle mass, as Metrnl was also secreted from muscle, where different Metrnl levels might result from adipose tissue dysfunction and sarcopenia. Collectively, larger sample sizes for clinical studies and more rigorous experimental designs are needed to identify the correlation between circulating Metrnl levels and obesity, thus determining whether Metrnl could be used as a biomarker of obesity.
Bariatric surgery (BS) has been currently the most effective therapy for morbid obesity and associated complications. A recent mice model study reported that Metrnl and UCPs were changed in favor of increased thermogenesis through fat browning to induce weight loss comparing laparoscopic sleeve gastrectomy (LSG) mice group with sham surgery and pair-fed groups (131, 132). They showed a positive correlation between Metrnl and weight loss after LSG-induced weight loss in adipose tissue, muscle, and plasma. Of note, this scholarly team recently reported the same results in the human interventional study (37). A similar clinical context study was also reported by Grander et al. (30), which found Metrnl expression was decreased in hepatic-and adipose tissues after BS in non-alcoholic fatty liver disease (NAFLD). Interestingly, Metrnl has been identified as a target protein with Iah1, a candidate gene for diet-induced NAFLD (133). Maybe Metrnl as one of the hepatokines play role in NAFLD progress but further investigations are needed to clarify the mechanism. However, another study by Pellitero et al. (128) showed the opposite result, which reported that Metrnl circulation levels were increased after LSG 6 and 12 months in obese patients, whereas at baseline patients with obesity showed lower. Schmid et al. (95) also observed serum Metrnl concentrations in patients undergoing BS and a low-calorie diet were increased after 3 months and back to baseline levels after 12 months, which also exerted no gender-specific effect. Taken together, these clinical studies showed contradicting results and this difference may attribute to co-morbidities, obesity, smaller sample size, or observation time as confounding factors. Further prospective studies with better design are required to better explain the relationship between Metrnl and weight loss or obesity.
Metrnl and exercise
Exercise-inducible soluble factors, such as adipokines, cytokines, myokines, and osteokines are regarded to play important roles in the body’s response to exercise (134, 135). Metrnl was first described by Rao et al. (15) who investigated Metrnl’s ability to moderate energy thermogenesis originally not in specific-METRNL transgenic mice but in PGC-1α4 transgenic mice. PGC-1α4 is an exercise-induced splice isoform of PPARγ coactivator 1a, where Metrnl was regarded as its downstream effector protein. A recent study reported by Amano et al. (136) supported this effector pathway of PGC-1α along with Metrnl. After they applied 4-week electrical stimulation to rats’ legs, which aimed to simulate chronic resistance exercise, an apparent positive correlation was found between the expression of PGC-1α in brown adipose tissues and plasma Metrnl levels. Similar results have been confirmed by Bae et al. (137) who exerted an 8-week training on HFD-induced obese mice increasing muscle proteins including AMPK, PGC-1α, and plasma Metrnl. Further studies proved Metrnl can be significantly induced in various muscle depots and adipose tissue by exercise (15, 23, 62). Besides, up-regulation of intramuscular Metrnl induced by regular exercise would be a pathway to suppress obesity and metabolic syndromes (105, 138, 139). Jung et al. (58) reported that administration of Metrnl in obese mice could reduce body weight gain and rescue glucose intolerance, whereas not affect calorie intake. Moreover, results from Hafiz et al. (23) found that exercise exerted anti-inflammatory function by NLRP3 inflammasome activation of HFD-induced obese mice, and this program was associated with Metrnl anti-inflammatory effects in macrophages in muscle.
A number of clinical studies have reported higher circulation Metrnl levels in response to exercise (140–146). Interestingly, exercise with different intensity, type, or duration may also influence Metrnl activity. Some studies have investigated the effects of regular exercises on obese mice, such as undergoing treadmill training, with obtaining similar conclusion that Metrnl levels increases (137). Of note, a previous study didn’t observe any changes in resting Metrnl level upon endurance training in mice (15). Given that recent research has reported beneficial effects of high-intensity interval training (HIIT) with metabolic diseases, they recently examined the impact of high-intensity interval training and short-term interval training on the Metrnl mRNA of skeletal muscle biopsy samples in 9 healthy males (147). An increase in Metrnl expression at 3-hour recovery compared to rest was reported after a single bout of high-intensity interval exercise before training, yet no statistical significance of post-training increases. It would be interesting to determine whether increased muscle Metrnl expression was a common adaptive response to certain types of exercise.
However, a number of earlier studies were based on mRNA levels, while accompanied by different commercial ELISA coming into service (93) more results were prone to support that circulation Metrnl levels can be strongly induced by both acute and chronic exercise (148). More recently, a similar study on serum Metrnl levels to investigate the different types of exercise effect (including aerobic exercise, HIIT, and resistance exercise), all exercise groups significantly elevated Metrnl levels, whereas no assessment changes within the group (149) Additionally, Amano et al. (136) figured out the chronic resistance exercise training also up-regulated Mertnl levels in obese mice. As such, findings from Alizadeh et al. (150) suggested that human downhill running exercise, regarded as a muscle-damaging exercise protocol, significantly elevated Metrnl levels as well as positively correlated with eosinophils’ number. Moreover, combined training (CT) for its metabolic benefits has been recommended by diabetes guidelines, Bonfante et al. (151) evaluated effects of 16-week-CT period on serum pro-thermogenic/anti-inflammatory inducers in overweight T2DM individuals and found Metrnl was positively correlated with brown adipose tissue (BAT) thermogenic activity. Interestingly, fed and fasting states in the pre-training or post-training also influence Metrnl secretion reported, with Metrnl was increased both in two states (152). Taken together, although some important questions remain unanswered, it would be interesting to evaluate Metrnl’s roles in the beneficial effects of exercise with metabolic diseases.
Future perspectives and conclusions
Overall, well-documented evidence has suggested that this novel secreted protein exerts pleiotropic effects on inflammation, immunology, and metabolism. However, Metrnl is not secreted by the fixed model of cell types but depends on the concrete physiological and pathological context. Based on different tissue homeostasis, more preclinical studies are needed to clarify the molecular mechanisms that control the selective expression of Metrnl by different cell types. Meanwhile, contradictoriness in reported results highlights the necessity for more-accurate methods to measure Metrnl and for clinical studies to be better designed, revealing the role of Metrnl in different diseases. Whether Metrnl is used as a predictive biomarker or disease activity indicator for cardio-metabolic syndromes including morbid obesity, insulin resistance, T2DM, CAD, and PCOS, or other inflammatory immune diseases such as asthma, RA, OA, PsA, UC, CD, T1DM, and GD, depending on more rigorous prospective studies. Finally, based on current knowledge, trials to assess the application of Metrnl as a therapeutic agent in humans is premature but should be one of the research goals for the near future.
Author contributions
ZL conceived this article. ZL and ZG drafted the manuscript. TS and SZ drew the illustrations and tables. SY and MZ revised the article. ZL, ZG, TS, SZ, SY, and MZ checked and edited the article. HS supervised the article. All authors have read and agreed to the final version of the manuscript.
Funding
This review was supported by grants from the National Natural Science Foundation of China (No. 81373219) and the Liaoning Education Department (No. JC2019009).
Acknowledgments
We acknowledged that this article illustrations were created with BioRender Figures 1, 2.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1098570/full#supplementary-material
References
1. Li ZY, Zheng SL, Wang P, Xu TY, Guan YF, Zhang YJ, et al. Subfatin is a novel adipokine and unlike meteorin in adipose and brain expression. CNS Neurosci Ther (2014) 20(4):344–54. doi: 10.1111/cns.12219
2. Zheng SL, Li ZY, Song J, Liu JM, Miao CY. Metrnl: A secreted protein with new emerging functions. Acta Pharmacol Sin (2016) 37(5):571–9. doi: 10.1038/aps.2016.9
3. Jørgensen JR, Fransson A, Fjord-Larsen L, Thompson LH, Houchins JP, Andrade N, et al. Cometin is a novel neurotrophic factor that promotes neurite outgrowth and neuroblast migration in vitro and supports survival of spiral ganglion neurons in vivo. Exp Neurol (2012) 233(1):172–81. doi: 10.1016/j.expneurol.2011.09.027
4. Surace C, Piazzolla S, Sirleto P, Digilio MC, Roberti MC, Lombardo A, et al. Mild ring 17 syndrome shares common phenotypic features irrespective of the chromosomal breakpoints location. Clin Genet (2009) 76(3):256–62. doi: 10.1111/j.1399-0004.2009.01203.x
5. Song Y, Choi JE, Kwon YJ, Chang HJ, Kim JO, Park DH, et al. Identification of susceptibility loci for cardiovascular disease in adults with hypertension, diabetes, and dyslipidemia. J Transl Med (2021) 19(1):85. doi: 10.1186/s12967-021-02751-3
6. Cookson WO, Ubhi B, Lawrence R, Abecasis GR, Walley AJ, Cox HE, et al. Genetic linkage of childhood atopic dermatitis to psoriasis susceptibility loci. Nat Genet (2001) 27(4):372–3. doi: 10.1038/86867
7. Morar N, Bowcock AM, Harper JI, Cookson WO, Moffatt MF. Investigation of the chromosome 17q25 PSORS2 locus in atopic dermatitis. J Invest Dermatol (2006) 126(3):603–6. doi: 10.1038/sj.jid.5700108
8. Wen D, Xiao Y, Vecchi MM, Gong BJ, Dolnikova J, Pepinsky RB. Determination of the disulfide structure of murine meteorin, a neurotrophic factor, by LC-MS and electron transfer dissociation-High-Energy collisional dissociation analysis of proteolytic fragments. Anal Chem (2017) 89(7):4021–30. doi: 10.1021/acs.analchem.6b04600
9. Ushach I, Burkhardt AM, Martinez C, Hevezi PA, Gerber PA, Buhren BA, et al. METEORIN-LIKE is a cytokine associated with barrier tissues and alternatively activated macrophages. Clin Immunol (2015) 156(2):119–27. doi: 10.1016/j.clim.2014.11.006
10. Ramialison M, Bajoghli B, Aghaallaei N, Ettwiller L, Gaudan S, Wittbrodt B, et al. Rapid identification of PAX2/5/8 direct downstream targets in the otic vesicle by combinatorial use of bioinformatics tools. Genome Biol (2008) 9(10):R145. doi: 10.1186/gb-2008-9-10-r145
11. Watanabe K, Akimoto Y, Yugi K, Uda S, Chung J, Nakamuta S, et al. Latent process genes for cell differentiation are common decoders of neurite extension length. J Cell Sci (2012) 125(Pt 9):2198–211. doi: 10.1242/jcs.097709
12. Berghoff M, Höpfinger A, Rajendran R, Karrasch T, Schmid A, Schäffler A. Evidence of a muscle-brain axis by quantification of the neurotrophic myokine METRNL (Meteorin-like protein) in human cerebrospinal fluid and serum. J Clin Med (2021) 10(15):3271. doi: 10.3390/jcm10153271
13. Jan V, Miš K, Nikolic N, Dolinar K, Petrič M, Bone A, et al. Effect of differentiation, de novo innervation, and electrical pulse stimulation on mRNA and protein expression of Na+,K+-ATPase, FXYD1, and FXYD5 in cultured human skeletal muscle cells. PloS One (2021) 16(2):e0247377. doi: 10.1371/journal.pone.0247377
14. Hong C, Wang Z, Zheng SL, Hu WJ, Wang SN, Zhao Y, et al. Metrnl regulates cognitive dysfunction and hippocampal BDNF levels in d-galactose-induced aging mice. Acta Pharmacol Sin (2022). doi: 10.1038/s41401-022-01009-y
15. Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell (2014) 157(6):1279–91. doi: 10.1016/j.cell.2014.03.065
16. Li ZY, Fan MB, Zhang SL, Qu Y, Zheng SL, Song J, et al. Intestinal metrnl released into the gut lumen acts as a local regulator for gut antimicrobial peptides. Acta Pharmacol Sin (2016) 37(11):1458–66. doi: 10.1038/aps.2016.70
17. Ushach I, Arrevillaga-Boni G, Heller GN, Pone E, Hernandez-Ruiz M, Catalan-Dibene J, et al. Meteorin-like/Meteorin-β is a novel immunoregulatory cytokine associated with inflammation. J Immunol (2018) 201(12):3669–76. doi: 10.4049/jimmunol.1800435
18. Zuo L, Ge S, Ge Y, Li J, Zhu B, Zhang Z, et al. The adipokine metrnl ameliorates chronic colitis in il-10-/- mice by attenuating mesenteric adipose tissue lesions during spontaneous colitis. J Crohns Colitis (2019) 13(7):931–41. doi: 10.1093/ecco-jcc/jjz001
19. Zhang SL, Li ZY, Wang DS, Xu TY, Fan MB, Cheng MH, et al. Aggravated ulcerative colitis caused by intestinal metrnl deficiency is associated with reduced autophagy in epithelial cells. Acta Pharmacol Sin (2020) 41(6):763–70. doi: 10.1038/s41401-019-0343-4
20. Xu L, Cai Y, Wang Y, Xu C. Meteorin-like (METRNL) attenuates myocardial Ischemia/Reperfusion injury-induced cardiomyocytes apoptosis by alleviating endoplasmic reticulum stress via activation of AMPK-PAK2 signaling in H9C2 cells. Med Sci Monit (2020) 26:e924564. doi: 10.12659/MSM.924564
21. Baht GS, Bareja A, Lee DE, Rao RR, Huang R, Huebner JL, et al. Meteorin-like facilitates skeletal muscle repair through a Stat3/IGF-1 mechanism. Nat Metab (2020) 2(3):278–89. doi: 10.1038/s42255-020-0184-y
22. Yao Z, Lin P, Wang C, Wang K, Sun Y. Administration of metrnl delays the onset of diabetes in non-obese diabetic mice. Endocr J (2021) 68(2):179–88. doi: 10.1507/endocrj.EJ20-0351
23. Javaid HMA, Sahar NE, ZhuGe DL, Huh JY. Exercise inhibits NLRP3 inflammasome activation in obese mice via the anti-inflammatory effect of meteorin-like. Cells (2021) 10(12):3480. doi: 10.3390/cells10123480
24. Jung TW, Pyun DH, Kim TJ, Lee HJ, Park ES, Abd El-Aty AM, et al. Meteorin-like protein (METRNL)/IL-41 improves LPS-induced inflammatory responses via AMPK or PPARδ-mediated signaling pathways. Adv Med Sci (2021) 66(1):155–61. doi: 10.1016/j.advms.2021.01.007
25. Gao X, Leung TF, Wong GW, Ko WH, Cai M, He EJ, et al. Meteorin-β/Meteorin like/IL-41 attenuates airway inflammation in house dust mite-induced allergic asthma. Cell Mol Immunol (2022) 19(2):245–59. doi: 10.1038/s41423-021-00803-8
26. Wang J, Jia Z, Dang H, Zou J. Meteorin-like/Meteorin-β upregulates proinflammatory cytokines via NF-κB pathway in grass carp ctenopharyngodon idella. Dev Comp Immunol (2022) 127:104289. doi: 10.1016/j.dci.2021.104289
27. Bridgewood C, Russell T, Weedon H, Baboolal T, Watad A, Sharif K, et al. The novel cytokine Metrnl/IL-41 is elevated in psoriatic arthritis synovium and inducible from both entheseal and synovial fibroblasts. Clin Immunol (2019) 208:108253. doi: 10.1016/j.clim.2019.108253
28. Kocaman N, Artaş G. Can novel adipokines, asprosin and meteorin-like, be biomarkers for malignant mesothelioma? Biotech Histochem (2020) 95(3):171–5. doi: 10.1080/10520295.2019.1656344
29. Gholamrezayi A, Mohamadinarab M, Rahbarinejad P, Fallah S, Barez SR, Setayesh L, et al. Characterization of the serum levels of meteorin-like in patients with inflammatory bowel disease and its association with inflammatory cytokines. Lipids Health Dis (2020) 19(1):230. doi: 10.1186/s12944-020-01404-6
30. Grander C, Grabherr F, Enrich B, Meyer M, Mayr L, Schwärzler J, et al. Hepatic meteorin-like and krüppel-like factor 3 are associated with weight loss and liver injury. Exp Clin Endocrinol Diabetes (2021). doi: 10.1055/a-1537-8950
31. Sobieh BH, Kassem DH, Zakaria ZM, El-Mesallamy HO. Potential emerging roles of the novel adipokines adipolin/CTRP12 and meteorin-like/METRNL in obesity-osteoarthritis interplay. Cytokine (2021) 138:155368. doi: 10.1016/j.cyto.2020.155368
32. Akkus G, Koyuturk LC, Yilmaz M, Hancer S, Ozercan IH, Kuloglu T. Asprosin and meteorin-like protein immunoreactivity in invasive ductal breast carcinoma stages. Tissue Cell (2022) 77:101855. doi: 10.1016/j.tice.2022.101855
33. Kocaman N, Yuksel EI, Demir B, Calik I, Cicek D. Two novel biomarker candidates for differentiating basal cell carcinoma from trichoblastoma; asprosin and meteorine like peptide. Tissue Cell (2022) 76:101752. doi: 10.1016/j.tice.2022.101752
34. Mirzaoglu M, Yavuzkir S, Mirzaoglu C, Yurt N, Dagli AF, Ozcan Yildirim S, et al. Use of asprosin and subfatin for differential diagnosis of serous ovarian tumors. Biotech Histochem (2023) 98(2):140–146. doi: 10.1080/10520295.2022.2135763
35. Gong L, Huang G, Weng L, Xu J, Li Y, Cui W, et al. Decreased serum interleukin-41/Metrnl levels in patients with graves’ disease. J Clin Lab Anal (2022) 36(10):e24676. doi: 10.1002/jcla.24676
36. Zhang S, Lei Y, Sun T, Gao Z, Li Z, Shen H. Elevated levels of metrnl in rheumatoid arthritis: Association with disease activity. Cytokine (2022) 159:156026. doi: 10.1016/j.cyto.2022.156026
37. Jamal MH, AlOtaibi F, Dsouza C, Al-Sabah S, Al-Khaledi G, Al-Ali W, et al. Changes in the expression of meteorin-like (METRNL), irisin (FNDC5), and uncoupling proteins (UCPs) after bariatric surgery. Obes (Silver Spring) (2022) 30(8):1629–38. doi: 10.1002/oby.23473
38. Lee J, Hever A, Willhite D, Zlotnik A, Hevezi P. Effects of RNA degradation on gene expression analysis of human postmortem tissues. FASEB J (2005) 19(10):1356–8. doi: 10.1096/fj.04-3552fje
39. Roth RB, Hevezi P, Lee J, Willhite D, Lechner SM, Foster AC, et al. Gene expression analyses reveal molecular relationships among 20 regions of the human CNS. Neurogenetics (2006) 7(2):67–80. doi: 10.1007/s10048-006-0032-6
40. Lee SD, Tontonoz P. Eosinophils in fat: Pink is the new brown. Cell (2014) 157(6):1249–50. doi: 10.1016/j.cell.2014.05.025
41. Legaki E, Arsenis C, Taka S, Papadopoulos NG. DNA Methylation biomarkers in asthma and rhinitis: Are we there yet? Clin Transl Allergy (2022) 12(3):e12131. doi: 10.1002/clt2.12131
42. Allen JE, Maizels RM. Diversity and dialogue in immunity to helminths. Nat Rev Immunol (2011) 11(6):375–88. doi: 10.1038/nri2992
43. Platts-Mills T, Vaughan J, Squillace S, Woodfolk J, Sporik R. Sensitisation, asthma, and a modified Th2 response in children exposed to cat allergen: A population-based cross-sectional study. Lancet (2001) 357(9258):752–6. doi: 10.1016/S0140-6736(00)04168-4
44. Knipper JA, Ivens A, Taylor MD. Helminth-induced Th2 cell dysfunction is distinct from exhaustion and is maintained in the absence of antigen. PloS Negl Trop Dis (2019) 13(12):e0007908. doi: 10.1371/journal.pntd.0007908
45. Manning AA, Zhao L, Zhu Z, Xiao H, Redington CG, Ding VA, et al. IL-39 acts as a friend to pancreatic cancer. Med Oncol (2018) 36(1):12. doi: 10.1007/s12032-018-1236-y
46. Manning AA, Zhao L, Zhu Z, Xiao H, Redington CG, Ding VA, et al. Correction to: IL-39 acts as a friend to pancreatic cancer. Med Oncol (2019) 36(2):22. doi: 10.1007/s12032-018-1244-y
47. Shivakumar M, Lee Y, Bang L, Garg T, Sohn KA, Kim D. Identification of epigenetic interactions between miRNA and DNA methylation associated with gene expression as potential prognostic markers in bladder cancer. BMC Med Genomics (2017) 10(Suppl 1):30. doi: 10.1186/s12920-017-0269-y
48. Reboll MR, Klede S, Taft MH, Cai CL, Field LJ, Lavine KJ, et al. Meteorin-like promotes heart repair through endothelial KIT receptor tyrosine kinase. Science (2022) 376(6599):1343–7. doi: 10.1126/science.abn3027
49. Soto H, Hevezi P, Roth RB, Pahuja A, Alleva D, Acosta HM, et al. Gene array analysis comparison between rat collagen-induced arthritis and human rheumatoid arthritis. Scand J Immunol (2008) 68(1):43–57. doi: 10.1111/j.1365-3083.2008.02117.x
50. Onuora S. Novel cytokine, IL-41, linked with PsA. Nat Rev Rheumatol (2019) 15(11):636. doi: 10.1038/s41584-019-0314-7
51. Dadmanesh M, Aghajani H, Fadaei R, Ghorban K. Lower serum levels of meteorin-like/Subfatin in patients with coronary artery disease and type 2 diabetes mellitus are negatively associated with insulin resistance and inflammatory cytokines. PloS One (2018) 13(9):e0204180. doi: 10.1371/journal.pone.0204180
52. El-Ashmawy HM, Selim FO, Hosny TAM, Almassry HN. Association of low serum meteorin like (Metrnl) concentrations with worsening of glucose tolerance, impaired endothelial function and atherosclerosis. Diabetes Res Clin Pract (2019) 150:57–63. doi: 10.1016/j.diabres.2019.02.026
53. Sun H, Zhang Y, Wang J, Kong J. Correlation of serum meteorin-like concentration with the presence and severity of obstructive sleep apnoea syndrome. Ann Clin Biochem (2019) 56(5):593–7. doi: 10.1177/0004563219854115
54. Fouani FZ, Fadaei R, Moradi N, Zandieh Z, Ansaripour S, Yekaninejad MS, et al. Circulating levels of meteorin-like protein in polycystic ovary syndrome: A case-control study. PloS One (2020) 15(4):e0231943. doi: 10.1371/journal.pone.0231943
55. Liu ZX, Ji HH, Yao MP, Wang L, Wang Y, Zhou P, et al. Serum metrnl is associated with the presence and severity of coronary artery disease. J Cell Mol Med (2019) 23(1):271–80. doi: 10.1111/jcmm.13915
56. Kerget B, Afşin DE, Kerget F, Aşkın S, Akgün M. Is metrnl an adipokine İnvolved in the anti-inflammatory response to acute exacerbations of COPD? Lung (2020) 198(2):307–14. doi: 10.1007/s00408-020-00327-4
57. Brigger D, Riether C, van Brummelen R, Mosher KI, Shiu A, Ding Z, et al. Eosinophils regulate adipose tissue inflammation and sustain physical and immunological fitness in old age. Nat Metab (2020) 2(8):688–702. doi: 10.1038/s42255-020-0228-3
58. Jung TW, Lee SH, Kim HC, Bang JS, Abd El-Aty AM, Hacımüftüoğlu A, et al. METRNL attenuates lipid-induced inflammation and insulin resistance via AMPK or PPARδ-dependent pathways in skeletal muscle of mice. Exp Mol Med (2018) 50(9):1–11. doi: 10.1038/s12276-018-0147-5
59. Li ZY, Song J, Zheng SL, Fan MB, Guan YF, Qu Y, et al. Adipocyte metrnl antagonizes insulin resistance through PPARγ signaling. Diabetes (2015) 64(12):4011–22. doi: 10.2337/db15-0274
60. Varghese M, Song J, Singer K. Age and sex: Impact on adipose tissue metabolism and inflammation. Mech Ageing Dev (2021) 199:111563. doi: 10.1016/j.mad.2021.111563
61. Wu H, Ballantyne CM. Skeletal muscle inflammation and insulin resistance in obesity. J Clin Invest (2017) 127(1):43–54. doi: 10.1172/JCI88880
62. Lee JO, Byun WS, Kang MJ, Han JA, Moon J, Shin MJ, et al. The myokine meteorin-like (metrnl) improves glucose tolerance in both skeletal muscle cells and mice by targeting AMPKα2. FEBS J (2020) 287(10):2087–104. doi: 10.1111/febs.15301
63. Chazaud B. A macrophage-derived adipokine supports skeletal muscle regeneration. Nat Metab (2020) 2(3):213–4. doi: 10.1038/s42255-020-0186-9
64. Kajimura S, Spiegelman BM, Seale P. Brown and beige fat: Physiological roles beyond heat generation. Cell Metab (2015) 22(4):546–59. doi: 10.1016/j.cmet.2015.09.007
65. Chouchani ET, Kajimura S. Metabolic adaptation and maladaptation in adipose tissue. Nat Metab (2019) 1(2):189–200. doi: 10.1038/s42255-018-0021-8
66. Ruas JL, White JP, Rao RR, Kleiner S, Brannan KT, Harrison BC, et al. A PGC-1α isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell (2012) 151(6):1319–31. doi: 10.1016/j.cell.2012.10.050
67. Townsend LK, Wright DC. Looking on the “brite” side exercise-induced browning of white adipose tissue. Pflugers Arch (2019) 471(3):455–65. doi: 10.1007/s00424-018-2177-1
68. Şekerci G, Erden Y, Tekin S. Effects of meteorin-like hormone on endocrine function of hypothalamo-hypophysial system and peripheral uncoupling proteins in rats. Mol Biol Rep (2022). doi: 10.1007/s11033-022-07374-5
69. Colaianni G, Cinti S, Colucci S, Grano M. Irisin and musculoskeletal health. Ann N Y Acad Sci (2017) 1402(1):5–9. doi: 10.1111/nyas.13345
70. Miao Y, Qin H, Zhong Y, Huang K, Rao C. Novel adipokine asprosin modulates browning and adipogenesis in white adipose tissue. J Endocrinol (2021) 249(2):83–93. doi: 10.1530/JOE-20-0503
71. BonDurant LD, Ameka M, Naber MC, Markan KR, Idiga SO, Acevedo MR, et al. FGF21 regulates metabolism through adipose-dependent and -independent mechanisms. Cell Metab (2017) 25(4):935–44.e4. doi: 10.1016/j.cmet.2017.03.005
72. Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science (2011) 332(6026):243–7. doi: 10.1126/science.1201475
73. Yan Q, Li W, Gong X, Hu R, Chen L. Transcriptomic and phenotypic analysis of CRISPR/Cas9-mediated gluk2 knockout in zebrafish. Genes (Basel) (2022) 13(8). doi: 10.3390/genes13081441
74. Coker RH, Weaver AN, Coker MS, Murphy CJ, Gunga HC, Steinach M. Metabolic responses to the Yukon Arctic ultra: Longest and coldest in the world. Med Sci Sports Exerc. (2017) 49(2):357–62. doi: 10.1249/MSS.0000000000001095
75. Saghebjoo M, Einaloo A, Mogharnasi M, Ahmadabadi F. The response of meteorin-like hormone and interleukin-4 in overweight women during exercise in temperate, warm and cold water. Horm Mol Biol Clin Investig (2018) 36(3). doi: 10.1515/hmbci-2018-0027
76. Sakers A, De Siqueira MK, Seale P, Villanueva CJ. Adipose-tissue plasticity in health and disease. Cell (2022) 185(3):419–46. doi: 10.1016/j.cell.2021.12.016
77. Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev (2018) 98(4):2133–223. doi: 10.1152/physrev.00063.2017
78. Hu W, Wang R, Sun B. Meteorin-like ameliorates β cell function by inhibiting β cell apoptosis of and promoting β cell proliferation via activating the WNT/β-catenin pathway. Front Pharmacol (2021) 12:627147. doi: 10.3389/fphar.2021.627147
79. Yao DD, Yang L, Wang Y, Liu C, Wei YJ, Jia XB, et al. Geniposide promotes beta-cell regeneration and survival through regulating β-catenin/TCF7L2 pathway. Cell Death Dis (2015) 6(5):e1746. doi: 10.1038/cddis.2015.107
80. Wang C, Pan Y, Song J, Sun Y, Li H, Chen L, et al. Serum metrnl level is correlated with insulin resistance, but not with β-cell function in type 2 diabetics. Med Sci Monit (2019) 25:8968–74. doi: 10.12659/MSM.920222
81. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia (1985) 28(7):412–9. doi: 10.1007/BF00280883
82. Sinaiko AR, Caprio S. Insulin resistance. J Pediatr (2012) 161(1):11–5. doi: 10.1016/j.jpeds.2012.01.012
83. Alizadeh H, Alizadeh A. Association of meteorin-like hormone with insulin resistance and body composition in healthy Iranian adults. Diabetes Metab Syndr (2020) 14(5):881–5. doi: 10.1016/j.dsx.2020.05.031
84. Wang K, Li F, Wang C, Deng Y, Cao Z, Cui Y, et al. Serum levels of meteorin-like (Metrnl) are increased in patients with newly diagnosed type 2 diabetes mellitus and are associated with insulin resistance. Med Sci Monit (2019) 25:2337–43. doi: 10.12659/MSM.915331
85. Onalan E, Cavlı C, Dogan Y, Onalan E, Gozel N, Buran I, et al. Low serum levels of meteorin-like/subfatin: An indicator of diabetes mellitus and insulin resistance? Endokrynol Pol (2020) 71(5):397–403. doi: 10.5603/EP.a2020.0038
86. Timurkaan M, Timurkaan ES. Two important players for type 2 diabetes mellitus: Metrnl and asprosin. Clin Lab (2022) 68(9). doi: 10.7754/Clin.Lab.2021.211015
87. Ugur K, Erman F, Turkoglu S, Aydin Y, Aksoy A, Lale A, et al. Asprosin, visfatin and subfatin as new biomarkers of obesity and metabolic syndrome. Eur Rev Med Pharmacol Sci (2022) 26(6):2124–33. doi: 10.26355/eurrev_202203_28360
88. Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev (2012) 33(6):981–1030. doi: 10.1210/er.2011-1034
89. Deniz R, Yavuzkir S, Ugur K, Ustebay DU, Baykus Y, Ustebay S, et al. Subfatin and asprosin, two new metabolic players of polycystic ovary syndrome. J Obstetrics Gynaecol (2021) 41(2):279–84. doi: 10.1080/01443615.2020.1758926
90. Chen P, Jia R, Liu Y, Cao M, Zhou L, Zhao Z. Progress of adipokines in the female reproductive system: A focus on polycystic ovary syndrome. Front Endocrinol (Lausanne) (2022) 13:881684. doi: 10.3389/fendo.2022.881684
91. Yavuzkir S, Ugur K, Deniz R, Ustebay DU, Mirzaoglu M, Yardim M, et al. Maternal and umbilical cord blood subfatin and spexin levels in patients with gestational diabetes mellitus. Peptides (2020) 126:170277. doi: 10.1016/j.peptides.2020.170277
92. Lappas M. Maternal obesity and gestational diabetes decrease metrnl concentrations in cord plasma. J Matern Fetal Neonatal Med (2021) 34(18):2991–5. doi: 10.1080/14767058.2019.1676713
93. Zheng SL, Li ZY, Zhang Z, Wang DS, Xu J, Miao CY. Evaluation of two commercial enzyme-linked immunosorbent assay kits for the detection of human circulating metrnl. Chem Pharm Bull (Tokyo) (2018) 66(4):391–8. doi: 10.1248/cpb.c17-00846
94. Fadaei R, Dadmanesh M, Moradi N, Ahmadi R, Shokoohi Nahrkhalaji A, Aghajani H, et al. Serum levels of subfatin in patients with type 2 diabetes mellitus and its association with vascular adhesion molecules. Arch Physiol Biochem (2020) 126(4):335–40. doi: 10.1080/13813455.2018.1538248
95. Schmid A, Karrasch T, Schäffler A. Meteorin-like protein (Metrnl) in obesity, during weight loss and in adipocyte differentiation. J Clin Med (2021) 10(19):4338. doi: 10.3390/jcm10194338
96. Khajebishak Y, Faghfouri AH, Soleimani A, Madani S, Payahoo L. Exploration of meteorin-like peptide (metrnl) predictors in type 2 diabetic patients: The potential role of irisin, and other biochemical parameters. Horm Mol Biol Clin Investig (2022). doi: 10.1515/hmbci-2022-0037
97. Cheng JX, Yu K. New discovered adipokines associated with the pathogenesis of obesity and type 2 diabetes. Diabetes Metab Syndr Obes (2022) 15:2381–9. doi: 10.2147/DMSO.S376163
98. Tuncer Kara K, Kizil M, Çakmak E, Yildirim TT, Kilinc F, Kuloğlu T, et al. Comparison of plasma and salivary meteorin-like protein levels in patients with newly diagnosed type-2 diabetes and treated with metformin. Eur Rev Med Pharmacol Sci (2022) 26(19):7145–50. doi: 10.26355/eurrev_202210_29900
99. Lee JH, Kang YE, Kim JM, Choung S, Joung KH, Kim HJ, et al. Serum meteorin-like protein levels decreased in patients newly diagnosed with type 2 diabetes. Diabetes Res Clin Pract (2018) 135:7–10. doi: 10.1016/j.diabres.2017.10.005
100. Chung HS, Hwang SY, Choi JH, Lee HJ, Kim NH, Yoo HJ, et al. Implications of circulating meteorin-like (Metrnl) level in human subjects with type 2 diabetes. Diabetes Res Clin Pract (2018) 136:100–7. doi: 10.1016/j.diabres.2017.11.031
101. Cherian P, Al-Khairi I, Jamal M, Al-Sabah S, Ali H, Dsouza C, et al. Association between factors involved in bone remodeling (Osteoactivin and OPG) with plasma levels of irisin and meteorin-like protein in people with T2D and obesity. Front Endocrinol (Lausanne) (2021) 12:752892. doi: 10.3389/fendo.2021.752892
102. Wu Q, Dan YL, He YS, Xiang K, Hu YQ, Zhao CN, et al. Circulating meteorin-like levels in patients with type 2 diabetes mellitus: A meta-analysis. Curr Pharm Des (2020) 26(44):5732–8. doi: 10.2174/1381612826666201007163930
103. Wang R, Hu D, Zhao X, Hu W. Correlation of serum meteorin-like concentrations with diabetic nephropathy. Diabetes Res Clin Pract (2020) 169:108443. doi: 10.1016/j.diabres.2020.108443
104. Löffler D, Landgraf K, Rockstroh D, Schwartze JT, Dunzendorfer H, Kiess W, et al. METRNL decreases during adipogenesis and inhibits adipocyte differentiation leading to adipocyte hypertrophy in humans. Int J Obes (Lond) (2017) 41(1):112–9. doi: 10.1038/ijo.2016.180
105. Bae JY. Aerobic exercise increases meteorin-like protein in muscle and adipose tissue of chronic high-fat diet-induced obese mice. BioMed Res Int (2018) 2018:6283932. doi: 10.1155/2018/6283932
106. Qi Q, Hu WJ, Zheng SL, Zhang SL, Le YY, Li ZY, et al. Metrnl deficiency decreases blood HDL cholesterol and increases blood triglyceride. Acta Pharmacol Sin (2020) 41(12):1568–75. doi: 10.1038/s41401-020-0368-8
107. Hu C, Zhang X, Song P, Yuan YP, Kong CY, Wu HM, et al. Meteorin-like protein attenuates doxorubicin-induced cardiotoxicity via activating cAMP/PKA/SIRT1 pathway. Redox Biol (2020) 37:101747. doi: 10.1016/j.redox.2020.101747
108. Rupérez C, Ferrer-Curriu G, Cervera-Barea A, Florit L, Guitart-Mampel M, Garrabou G, et al. Meteorin-like/Meteorin-β protects heart against cardiac dysfunction. J Exp Med (2021) 218(5):e20201206. doi: 10.1084/jem.20201206
109. Huang R, Balu AR, Molitoris KH, White JP, Robling AG, Ayturk UM, et al. The role of meteorin-like in skeletal development and bone fracture healing. J Orthop Res (2022) 40(11):2510–21. doi: 10.1002/jor.25286
110. Ding X, Chang X, Wang J, Bian N, An Y, Wang G, et al. Serum metrnl levels are decreased in subjects with overweight or obesity and are independently associated with adverse lipid profile. Front Endocrinol (Lausanne) (2022) 13:938341. doi: 10.3389/fendo.2022.938341
111. Du Y, Ye X, Lu A, Zhao D, Liu J, Cheng J, et al. Inverse relationship between serum metrnl levels and visceral fat obesity (VFO) in patients with type 2 diabetes. Diabetes Res Clin Pract (2020) 161:108068. doi: 10.1016/j.diabres.2020.108068
112. Kong YY, Li GQ, Zhang WJ, Hua X, Zhou CC, Xu TY, et al. Nicotinamide phosphoribosyltransferase aggravates inflammation and promotes atherosclerosis in ApoE knockout mice. Acta Pharmacol Sin (2019) 40(9):1184–92. doi: 10.1038/s41401-018-0207-3
113. Cai J, Wang QM, Li JW, Xu F, Bu YL, Wang M, et al. Serum meteorin-like is associated with weight loss in the elderly patients with chronic heart failure. J Cachexia Sarcopenia Muscle (2022) 13(1):409–17. doi: 10.1002/jcsm.12865
114. Castaneda AB, Petty LE, Scholz M, Jansen R, Weiss S, Zhang X, et al. Associations of carotid intima media thickness with gene expression in whole blood and genetically predicted gene expression across 48 tissues. Hum Mol Genet (2021) 31(7):1171–82. doi: 10.1093/hmg/ddab236
115. Naqvi TZ, Lee MS. Carotid intima-media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging (2014) 7(10):1025–38. doi: 10.1016/j.jcmg.2013.11.014
116. Swirski FK, Nahrendorf M. Cardioimmunology: The immune system in cardiac homeostasis and disease. Nat Rev Immunol (2018) 18(12):733–44. doi: 10.1038/s41577-018-0065-8
117. Yilmaz M, Cagri Goktekin M, Ilhan N. Subfatin concentration decreases in acute coronary syndrome. Biochem Med (Zagreb) (2022) 32(2):020704. doi: 10.11613/BM.2022.020704
118. Srivastava D. Cellular cross-talk in heart repair. Science (2022) 376(6599):1271–2. doi: 10.1126/science.adc8698
119. Huynh K. Meteorin-like protein repairs the ischaemic heart via receptor KIT in endothelial cells. Nat Rev Cardiol (2022) 19(9):575. doi: 10.1038/s41569-022-00752-3
120. Majithia A, Bhatt DL. Novel antiplatelet therapies for atherothrombotic diseases. Arterioscler Thromb Vasc Biol (2019) 39(4):546–57. doi: 10.1161/ATVBAHA.118.310955
121. Gong W, Liu Y, Wu Z, Wang S, Qiu G, Lin S. Meteorin-like shows unique expression pattern in bone and its overexpression inhibits osteoblast differentiation. PloS One (2016) 11(10):e0164446. doi: 10.1371/journal.pone.0164446
122. Vi L, Baht GS, Whetstone H, Ng A, Wei Q, Poon R, et al. Macrophages promote osteoblastic differentiation in-vivo: Implications in fracture repair and bone homeostasis. J Bone Miner Res (2015) 30(6):1090–102. doi: 10.1002/jbmr.2422
123. Zeng Y, Shih YV, Baht GS, Varghese S. In vivo sequestration of innate small molecules to promote bone healing. Adv Mater (2020) 32(8):e1906022. doi: 10.1002/adma.201906022
124. Baht GS, Silkstone D, Vi L, Nadesan P, Amani Y, Whetstone H, et al. Exposure to a youthful circulaton rejuvenates bone repair through modulation of β-catenin. Nat Commun (2015) 6:7131. doi: 10.1038/ncomms8131
125. Xu Y, Barter MJ, Swan DC, Rankin KS, Rowan AD, Santibanez-Koref M, et al. Identification of the pathogenic pathways in osteoarthritic hip cartilage: commonality and discord between hip and knee OA. Osteoarthritis Cartilage (2012) 20(9):1029–38. doi: 10.1016/j.joca.2012.05.006
126. Wang W, Liu Y, Hao J, Zheng S, Wen Y, Xiao X, et al. Comparative analysis of gene expression profiles of hip articular cartilage between non-traumatic necrosis and osteoarthritis. Gene (2016) 591(1):43–7. doi: 10.1016/j.gene.2016.06.058
127. Wu H, Ballantyne CM. Metabolic inflammation and insulin resistance in obesity. Circ Res (2020) 126(11):1549–64. doi: 10.1161/CIRCRESAHA.119.315896
128. Pellitero S, Piquer-Garcia I, Ferrer-Curriu G, Puig R, Martínez E, Moreno P, et al. Opposite changes in meteorin-like and oncostatin m levels are associated with metabolic improvements after bariatric surgery. Int J Obes (Lond) (2018) 42(4):919–22. doi: 10.1038/ijo.2017.268
129. Ferns GA, Fekri K, Shahini Shams Abadi M, Banitalebi Dehkordi M, Arjmand MH. A meta-analysis of the relationship between serums metrnl-like protein/subfatin and risk of type 2 diabetes mellitus and coronary artery disease. Arch Physiol Biochem (2021), 1–7. doi: 10.1080/13813455.2021.1899239
130. AlKhairi I, Cherian P, Abu-Farha M, Madhoun AA, Nizam R, Melhem M, et al. Increased expression of meteorin-like hormone in type 2 diabetes and obesity and its association with irisin. Cells (2019) 8(10). doi: 10.3390/cells8101283
131. Jamal MH, Abu-Farha M, Al-Khaledi G, Al-Sabah S, Ali H, Cherian P, et al. Effect of sleeve gastrectomy on the expression of meteorin-like (METRNL) and irisin (FNDC5) in muscle and brown adipose tissue and its impact on uncoupling proteins in diet-induced obesity rats. Surg Obes Relat Dis (2020) 16(12):1910–8. doi: 10.1016/j.soard.2020.07.022
132. Aleassa EM. Comment on: Effect of sleeve gastrectomy on the expression of meteorin-like (METRNL) and irisin (FNDC5) in muscle and brown adipose tissue and its impact on (UCPs) in diet-induced obesity rats. Surg Obes Relat Dis (2020) 16(12):1918–9. doi: 10.1016/j.soard.2020.08.027
133. Masuya T, Suzuki M, Tsujimura J, Kanamori S, Miyasaka Y, Ohno T, et al. Ablation of Iah1, a candidate gene for diet-induced fatty liver, does not affect liver lipid accumulation in mice. PloS One (2020) 15(5):e0233087. doi: 10.1371/journal.pone.0233087
134. Ost M, Coleman V, Kasch J, Klaus S. Regulation of myokine expression: Role of exercise and cellular stress. Free Radic Biol Med (2016) 98:78–89. doi: 10.1016/j.freeradbiomed.2016.02.018
135. Szabó MR, Pipicz M, Csont T, Csonka C. Modulatory effect of myokines on reactive oxygen species in Ischemia/Reperfusion. Int J Mol Sci (2020) 21(24):9382. doi: 10.3390/ijms21249382
136. Amano Y, Nonaka Y, Takeda R, Kano Y, Hoshino D. Effects of electrical stimulation-induced resistance exercise training on white and brown adipose tissues and plasma meteorin-like concentration in rats. Physiol Rep (2020) 8(16):e14540. doi: 10.14814/phy2.14540
137. Bae JY, Woo J, Kang S, Shin KO. Effects of detraining and retraining on muscle energy-sensing network and meteorin-like levels in obese mice. Lipids Health Dis (2018) 17(1):97. doi: 10.1186/s12944-018-0751-3
138. Saeidi A, Tayebi SM, Khosravi A, Malekian F, Khodamoradi A, Sellami M, et al. Effects of exercise training on type 2-diabetes: The role of meteorin-like protein. Health Promot Perspect (2019) 9(2):89–91. doi: 10.15171/hpp.2019.12
139. Martínez-Gayo A, Félix-Soriano E, Sáinz N, González-Muniesa P, Moreno-Aliaga MJ. Changes induced by aging and long-term exercise and/or DHA supplementation in muscle of obese female mice. Nutrients (2022) 14(20):4240. doi: 10.3390/nu14204240
140. Huang S, Cao L, Cheng H, Li D, Li Y, Wu Z. The blooming intersection of subfatin and metabolic syndrome. Rev Cardiovasc Med (2021) 22(3):799–805. doi: 10.31083/j.rcm2203086
141. Alizadeh H. Meteorin-like protein (Metrnl): A metabolic syndrome biomarker and an exercise mediator. Cytokine (2022) 157:155952. doi: 10.1016/j.cyto.2022.155952
142. Miao ZW, Hu WJ, Li ZY, Miao CY. Involvement of the secreted protein metrnl in human diseases. Acta Pharmacol Sin (2020) 41(12):1525–30. doi: 10.1038/s41401-020-00529-9
143. Alizadeh H. Myokine-mediated exercise effects: The role of myokine meteorin-like hormone (Metrnl). Growth Factors (2021) 39(1-6):71–78. doi: 10.1080/08977194.2022.2032689
144. Gonzalez-Gil AM, Elizondo-Montemayor L. The role of exercise in the interplay between myokines, hepatokines, osteokines, adipokines, and modulation of inflammation for energy substrate redistribution and fat mass loss: A review. Nutrients (2020) 12(6):1899. doi: 10.3390/nu12061899
145. Gries KJ, Zysik VS, Jobe TK, Griffin N, Leeds BP, Lowery JW. Muscle-derived factors influencing bone metabolism. Semin Cell Dev Biol (2022) 123:57–63. doi: 10.1016/j.semcdb.2021.10.009
146. Huh JY. The role of exercise-induced myokines in regulating metabolism. Arch Pharm Res (2018) 41(1):14–29. doi: 10.1007/s12272-017-0994-y
147. Eaton M, Granata C, Barry J, Safdar A, Bishop D, Little JP. Impact of a single bout of high-intensity interval exercise and short-term interval training on interleukin-6, FNDC5, and METRNL mRNA expression in human skeletal muscle. J Sport Health Sci (2018) 7(2):191–6. doi: 10.1016/j.jshs.2017.01.003
148. Tok Ö, Kişioğlu SV, Ersöz H, Kahveci B, Göktaş Z. Effects of increased physical activity and/or weight loss diet on serum myokine and adipokine levels in overweight adults with impaired glucose metabolism. J Diabetes Complications (2021) 35(5):107892. doi: 10.1016/j.jdiacomp.2021.107892
149. Akbulut T, Cinar V, Ugur K, Yardim M, Karagoz ZK, Aydin S. Effect of regular exercise on the levels of subfatin and asprosin: A trial with different types of exercise. Eur Rev Med Pharmacol Sci (2022) 26(8):2683–91. doi: 10.26355/eurrev_202204_28598
150. Alizadeh A, Alizadeh H. Downhill running exercise increases circulating level of myokine meteorin-like hormone in humans. J Sports Med Phys Fitness (2021) 62(5):700–4. doi: 10.23736/S0022-4707.21.12246-7
151. Bonfante ILP, Monfort-Pires M, Duft RG, da Silva Mateus KC, de Lima Júnior JC, Dos Santos Trombeta JC, et al. Combined training increases thermogenic fat activity in patients with overweight and type 2 diabetes. Int J Obes (Lond) (2022) 46(6):1145–54. doi: 10.1038/s41366-022-01086-3
152. Bonfante ILP, Duft RG, Mateus K, Trombeta J, Finardi EAR, Ramkrapes APB, et al. Acute/Chronic responses of combined training on serum pro-thermogenic/Anti-inflammatory inducers and its relation with fed and fasting state in overweight type 2 diabetic individuals. Front Physiol (2021) 12:736244. doi: 10.3389/fphys.2021.736244
Keywords: Metrnl, inflammation, metabolism, immunity, bone, heart, obesity, diabetes
Citation: Li Z, Gao Z, Sun T, Zhang S, Yang S, Zheng M and Shen H (2023) Meteorin-like/Metrnl, a novel secreted protein implicated in inflammation, immunology, and metabolism: A comprehensive review of preclinical and clinical studies. Front. Immunol. 14:1098570. doi: 10.3389/fimmu.2023.1098570
Received: 15 November 2022; Accepted: 10 February 2023;
Published: 24 February 2023.
Edited by:
Yujing Zhang, Nanjing University, ChinaCopyright © 2023 Li, Gao, Sun, Zhang, Yang, Zheng and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Shen, c2hlbmh1aWNhbUBzb2h1LmNvbQ==
†These authors have contributed equally to this work
 Zhuoqi Li
Zhuoqi Li Ziyu Gao2†
Ziyu Gao2† Tao Sun
Tao Sun