- 1Department of Laboratory Medicine, Shanxi Provincial People’s Hospital, Taiyuan, China
- 2Department of Internal Medicine, Shanxi Children’s Hospital, Shanxi Maternal and Child Health Hospital, Taiyuan, China
- 3Department of Pathology, Stanford University School of Medicine, Palo Alto, CA, United States
Introduction: Recent studies have shown much progress in the research of neutrophil extracellular traps (NETs) in autoimmune diseases (AIDs). However, there is no bibliometric analysis in this research field. This study aimed to provide a bibliometrics review of the knowledge structure and research hotspots of NETs in AIDs.
Methods: Articles relevant to NETs in AIDs from 2010 to 2022 were retrieved through the Web of Science Core Collection (WoSCC) database. This bibliometric analysis was performed by VOSview, CiteSpace, and Scimago Graphica.
Results: A total of 289 papers analyzed in this research were from 493 organizations in 47 countries by 1537 authors. They were published in 133 journals and cited 20,180 citations from 2,465 journals. The number of annual publications in this field is growing steadily and rapidly, with the United States, China and Germany leading the research effort. Frontiers in Immunology and Journal of Immunology have significantly impacted research in this field. Kaplan, Mariana J, from the National Institutes of Health (The United States), has the most published articles, and Brinkmann, v, from Max Planck Institute for Infection Biology (Germany), is the most co-cited author. Systemic lupus erythematosus and rheumatoid arthritis are the leading topics in this field. The trend of clinical application in the future is the development of new therapies by controlling NETs in the progression of AIDs.
Conclusions: Our study summarized the research trends and developments of NETs in AIDs in recent years and would provide a reference for scholars in this field.
1. Introduction
Neutrophils play an essential role in innate immunity by initiating phagocytosis, degranulation and releasing neutrophil extracellular traps (NETs) after recognizing specific pathogens (1, 2). NETs, first reported in 2004, are large reticular structures assembled on a scaffold of decondensed chromatin composed of histones and granular proteins, such as proteinase 3 (PR3), myeloperoxidase (MPO), high mobility group protein B1 (HMGB1), and neutrophil elastase (NE) (3, 4). NETs extrude into the extracellular space primarily through a unique form of cell death known as “NET formation” or NETosis, which allows the neutrophil to effectively capture and kill pathogens at sites of inflammation (5). During infection, NETs can persist for some time, and then be decomposed by the secreted plasma nuclease DNase I or DNase-like protein, thus establishing a delicate balance between the formation and elimination of NETs (6–8). However, tissue damage or autoimmunity may occur when excess NETs yield and dysregulation of suppressive mechanisms (9, 10). Therefore, NETs may be a double-edged sword and are closely associated with the pathogenesis of various diseases (11–18).
Autoimmune diseases (AIDs) are a group of diseases that result in self-tissue damage due to the failure of autoimmune tolerance. The estimated prevalence of AIDs is 7.6–9.4%, including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), Sjogren’s syndrome (SS), primary biliary cirrhosis (PBC), antiphospholipid syndrome (APS), type 1 diabetes (T1D), autoimmune thyroid disease (AITD) and other nearly 100 different diseases (19–21). In recent years, more and more studies have found the presence of NETs in AIDs. When NETs are overproduced or fail to be cleared, they can deliver multiple autoantigens to the host immune system, inducing autoimmune responses. Therefore, it has been widely accepted that NETs play a crucial role in the pathogenesis and progression of AIDs (9, 11, 12, 22–25).
The bibliometric analysis focuses on studying the publications in a specific research field from the quantitative and qualitative perspectives. Using visualization, the structure, rules and distribution of the knowledge graphs in this research field are presented, and the research status, hotspots and development trends in this field are obtained (26, 27). Currently, the study of NETs in AIDs has not been analyzed by bibliometric methods. To fill this knowledge gap, our study aimed to conduct a bibliometric analysis of NETs in AIDs from 2010 to 2022 to identify the current research status and principal contributors, and to prospect the future research trends in this field.
2. Methods
2.1. Search strategy
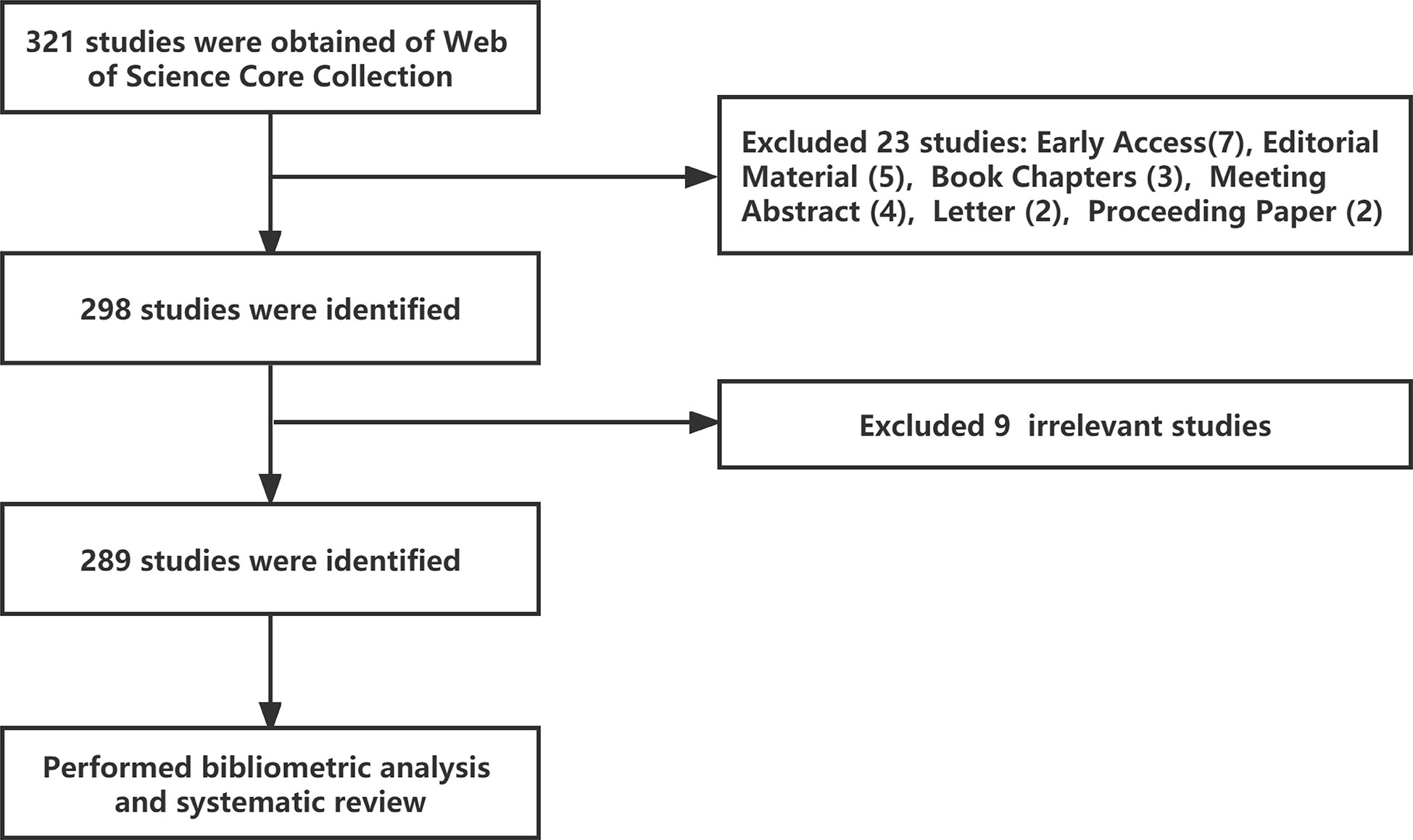
The Web of Science Core Collection (WoSCC) database (https://www.webofscience.com/wos/woscc/basic-search) was selected as the data source. Bibliometric analyses were conducted on December 5, 2022. The chosen search formula was TS= ((“Autoimmune Diseases” or “Rheumatic” or “Rheumatological Diseases”) and (“Neutrophil Extracellular Traps” or “NETs” or “NETosis”)), with a time from 2010-01-01 to 2022-10-31, and 321 literature records were retrieved. Then, literature types were selected as “Article” and “Review Article, and 298 literature records were retrieved in English. Finally, nine irrelevant articles were excluded after the researchers screened the documents, and 289 valid articles were obtained (Figure 1).
2.2. Data analysis
Bibliometric analysis software VOSviewer (version 1.6.18) and CiteSpace (version 6.1 R2) were used to process and analyze the data obtained. VOSviewer was used to extract key information from publications, and the network view can intuitively reflect the weight of the analyzed data and the relationship between the analyzed data. The different nodes in the map generated by VOSviewer represent items such as countries, organizations, authors, keywords, and the size of the nodes indicate the number of these elements. The lines between nodes reflect the degree of co-operation or co-reference of the project, and are represented by the same color. CiteSpace was used to map the figures for top-level reference bursts, keyword bursts, and keyword time zone maps (26–28). Scimago Graphica (Version 1.0.25) was used to generate a geographic distribution map of publications on NETs in AIDS (29). Microsoft Office Excel 2021 was used to conduct a quantitative analysis of publications.
3. Results
3.1. Quantitative analysis of publication
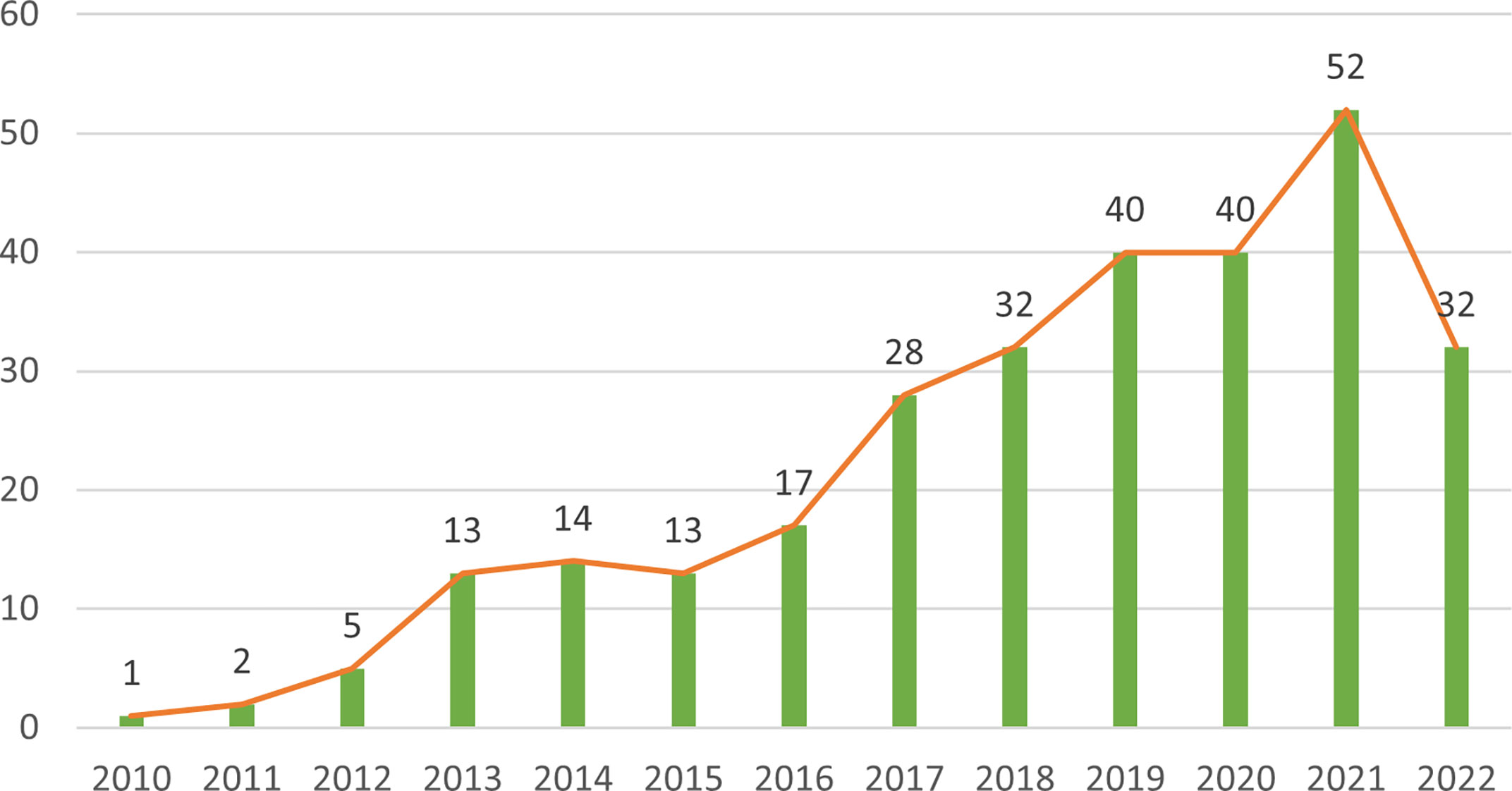
The 289 papers used in this research were from 493 organizations in 47 countries by 1537 authors, published in 133 journals, and cited 20,180 citations from 2,465 journals. Figure 2 shows the annual number of articles on the research of NETs in AIDs from 2010 to 2022. Overall, annual publications in this field have grown steadily and rapidly. Before 2010, research on NETs in AIDs had not been carried out. From 2010 to 2015, the number of publications was relatively small, and the study was at an early stage. After 2016, the number of publications began to increase significantly. In 2019 and 2020, the number of publications was stable at around 40 per year, and the number in 2021 continued to rise, indicating that NETs have received increasing attention from scholars in recent years and have become a new focus of AIDs research.
3.2. Analysis of countries and institutional
To study which countries have made the most outstanding contributions to the NETs research in AIDs, 493 organizations in 47 countries were analyzed. The geographical distribution and visualization of countries are shown in Figure 3. The top ten countries with the highest number of publications are distributed in North America, Asia and Europe. The top five countries are the United States (n=70), China (n=43), Germany (n=40), Italy (n=33) and France (n=17). In addition, the average citations/publications are higher in North American and Europe countries and slightly lower in Asia countries (China and Japan) (Table 1).
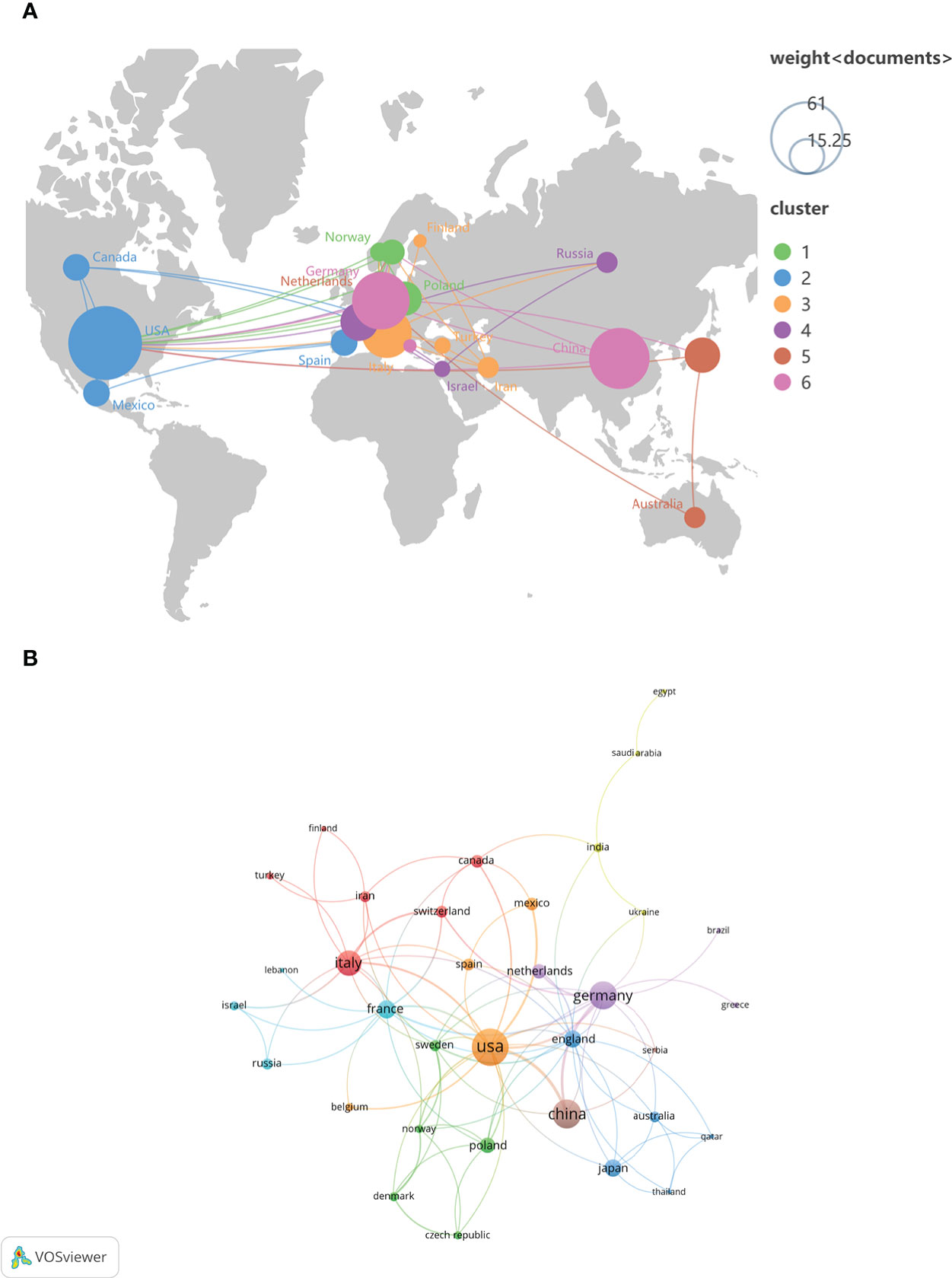
Figure 3 The geographical distribution (A) and visualization of countries (B) on the research of NETs in AIDs.
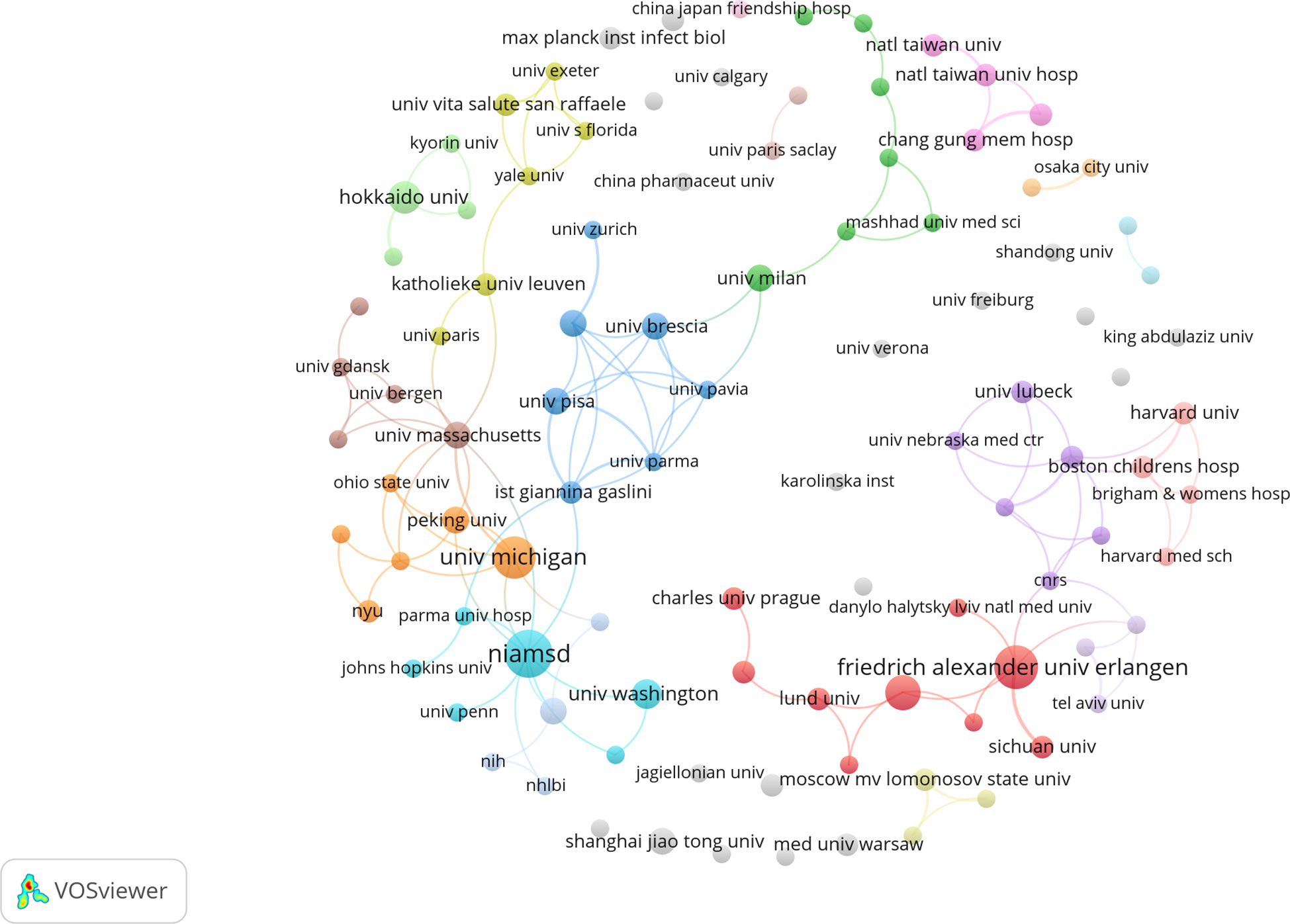
As shown in Table 1, the top ten organizations are in the United States, Germany, Netherlands, Japan, China and Mexico. Subsequently, 102 institutions were selected for a visualization based on a minimum number of publications equal to 2, and a collaborative network was constructed based on each institution’s number and relationship of publications. As shown in Figure 4, collaborations mainly occurred among North American and Europe organizations.
3.3. Analysis of top journals and co-cited journals
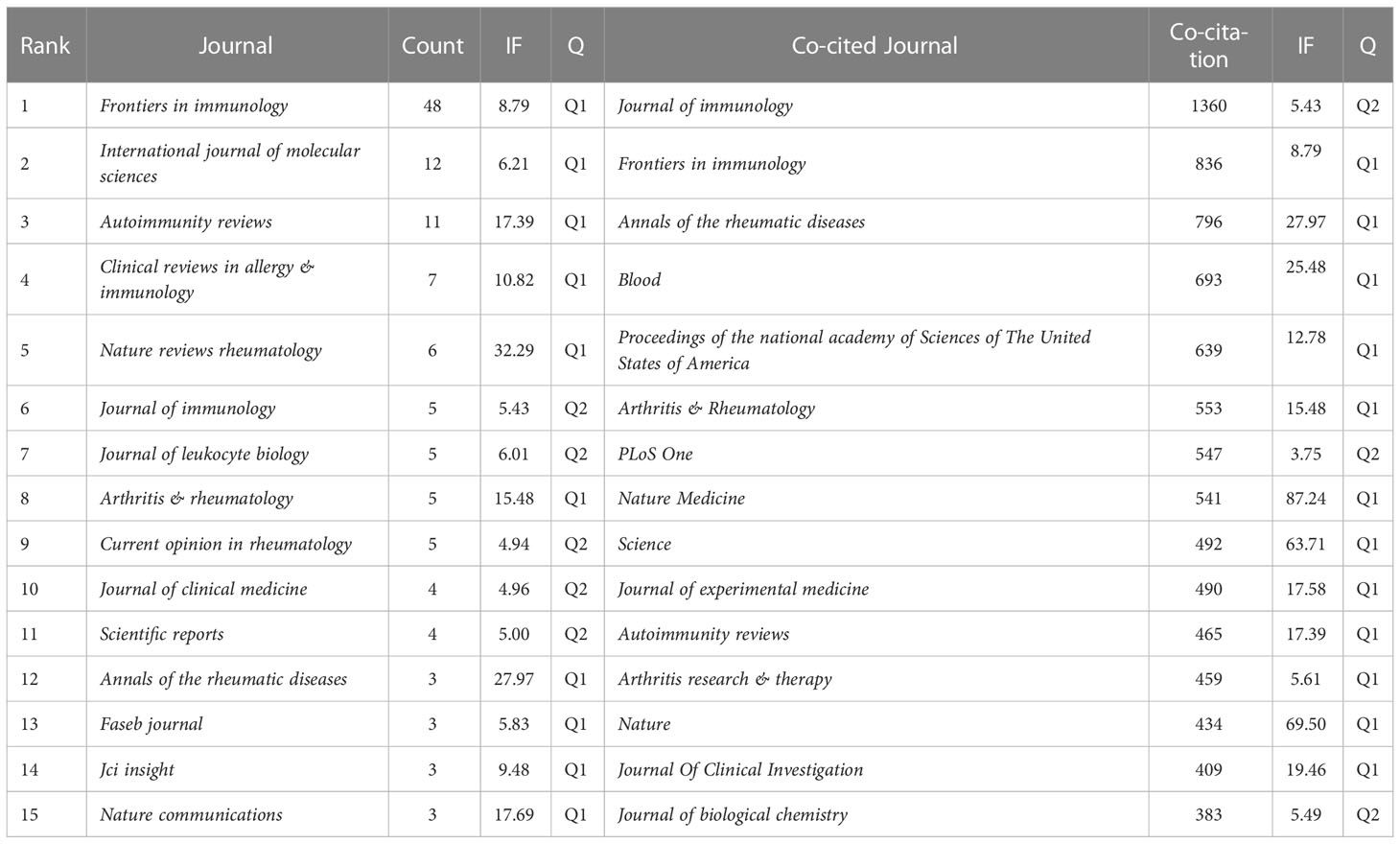
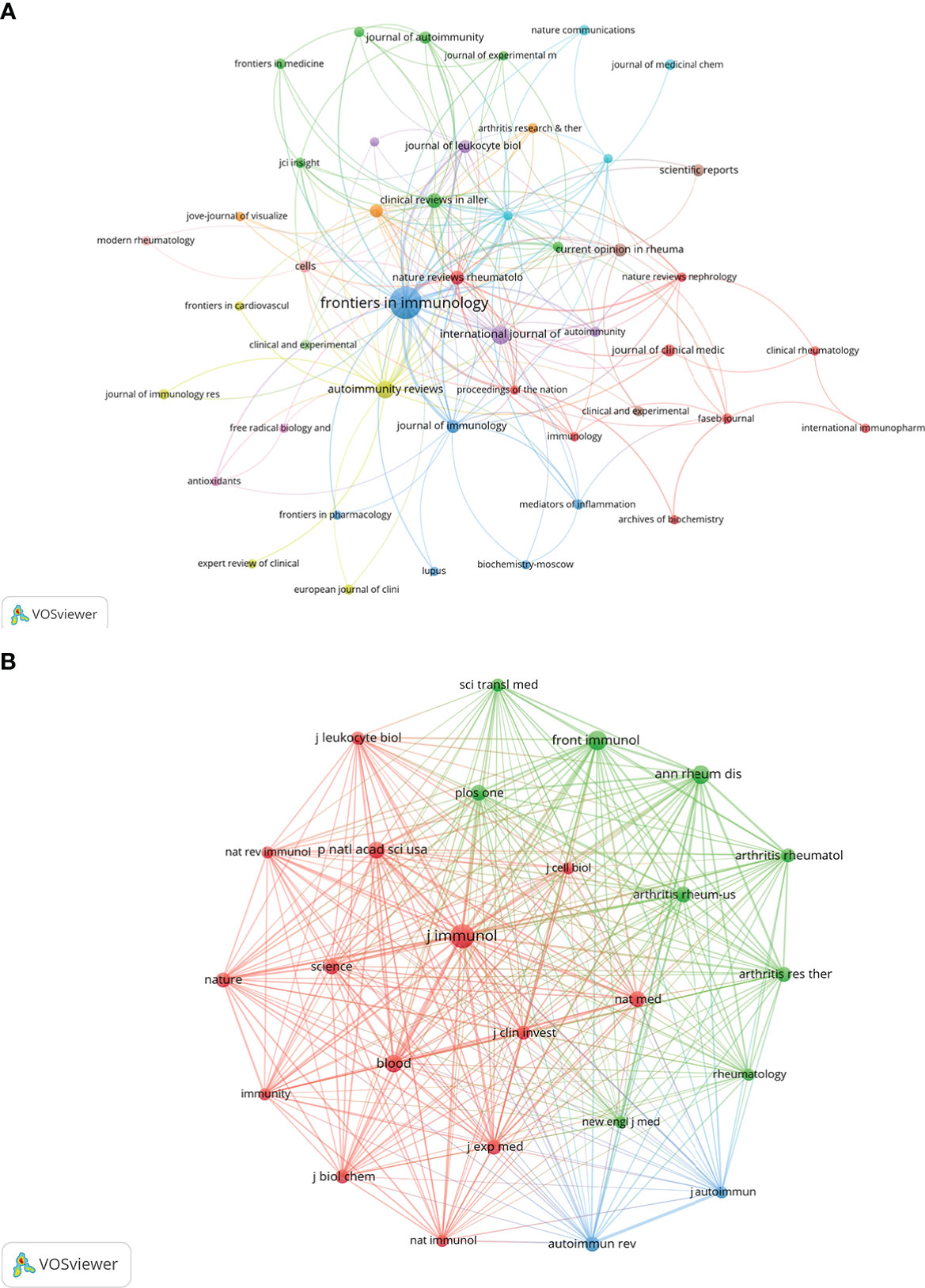
Research related to NETs in AIDs was published in 133 journals. The top 15 journals are shown in Table 2, with the most published papers being Frontiers in Immunology (n=48), followed by International Journal of Molecular Sciences (n=12), Autoimmunity reviews (n=11), Clinical reviews in allergy & immunology (n=7), and Nature reviews rheumatology (n=6). In the top 15 journals, the journal with the highest impact factor is Nature reviews rheumatology (IF=32.29), followed by Annals of the rheumatic diseases (IF=27.97). Then, based on the principle that the minimum number of relevant literatures equals 2, 48 journals were selected and the journal network was drawn (Figure 5A).
On the top 15 co-cited journals, five were cited more than 600 times, with Journal of Immunology (Co-citation=1360) cited the most, followed by Frontiers in immunology (Co-citation=836), Annals of the rheumatic diseases (Co-citation=796), Blood (Co-citation=693) and Proceedings of the national academy of Sciences of The United States of America (Co-citation=639) (Table 2). More than 250 co-citation journals (25 journals) were filtered to produce the top co-cited network (Figure 5B). From the analysis results, it can be inferred that Journal of immunology and Frontiers in immunology have a great influence on the research of NETs in AIDs, and this field is concerned with blood, skin, arthritis and immunology research.
3.4. Analysis of top authors and co-cited authors
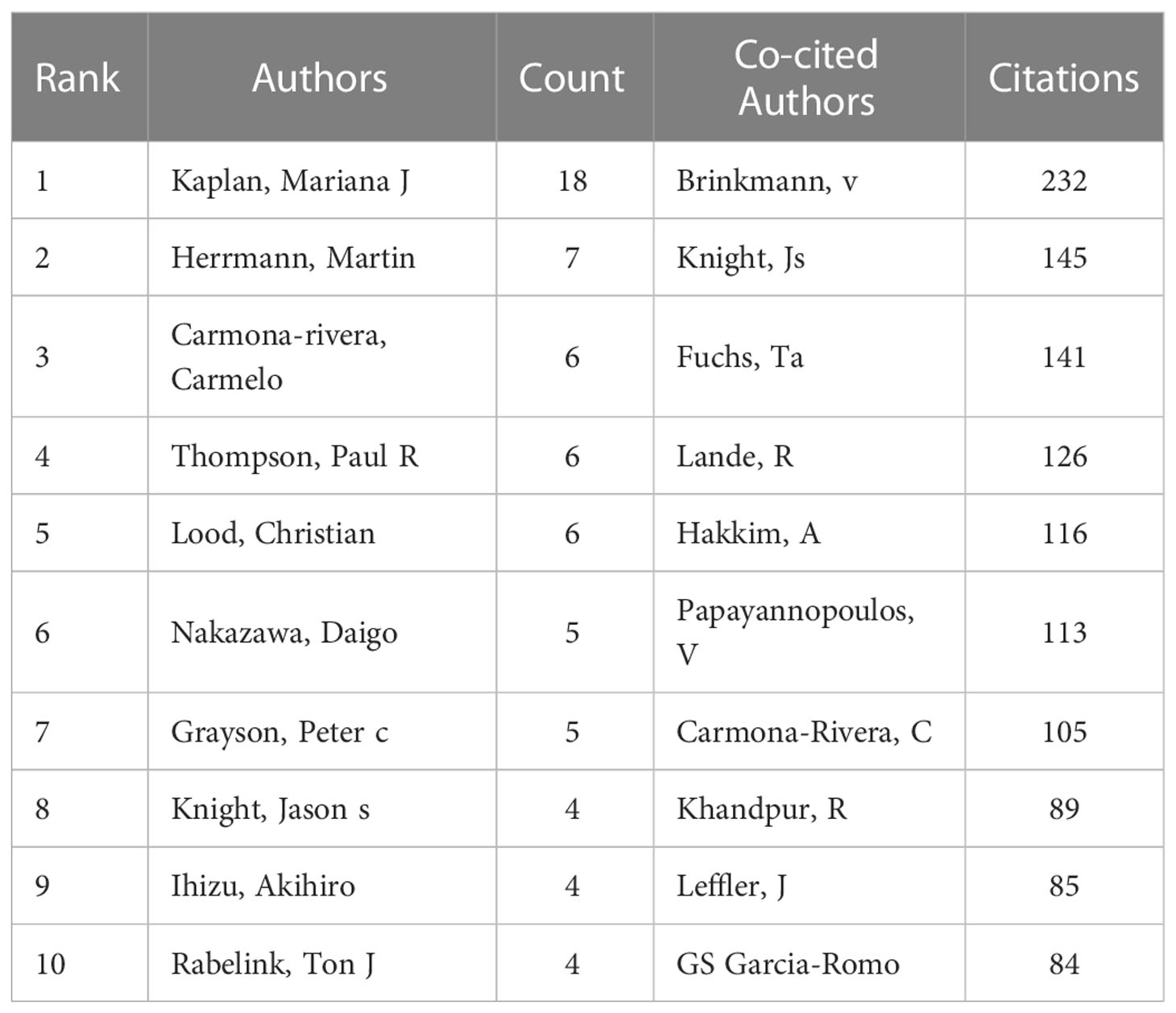
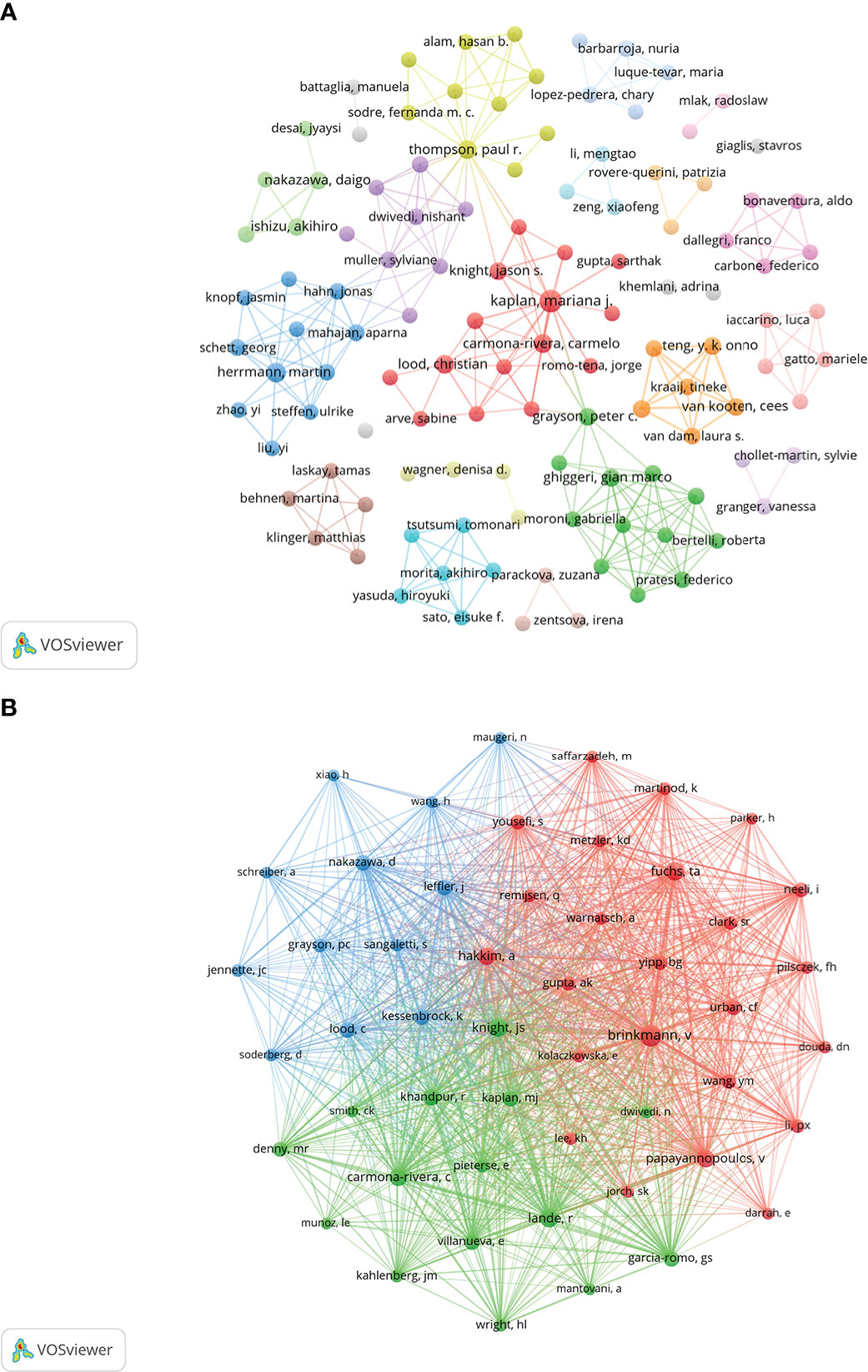
One thousand five hundred thirty-seven authors participated in the published study on NETs in AIDs. The top 10 authors are shown in Table 3, and a collaborative network was drawn based on the authors’ published papers (Figure 6A). The top five authors with the largest nodes in Figure 6A: Kaplan, Mariana J and Carmona-Rivera, Carmelo, from the National Institutes of Health (The United States), focused on identifying mechanisms of immune dysregulation, organ damage, and premature vascular disease in systemic autoimmunity (23, 25, 30–36); Herrmann, Martin, from the University of Erlangen–Nuremberg (Germany), focused on investigating neutrophil-driven inflammation and the role of NETs in this process (6, 37); Thompson, Paul R from UMASS Medical School (The United States) focused on the target-based design of novel anti-cancer and anti-rheumatoid arthritis chemotherapeutics (38, 39).
The top 10 co-cited authors were shown in Table 3, and 51 (minimum co-citations equal to 30) were filtered to plot the co-citation network (Figure 6B). The comprehensive analysis showed that Brinkmann, v, Knight, Js and Fuchs, Ta et al. had an important influence in this field. Brinkmann, v, from Max Planck Institute for Infection Biology (Germany), first reported in 2004 that NETs are an essential step in killing bacteria by neutrophils (3). Knight, Js from the University of Michigan (The United States) was studying the triggers and effectors of autoimmunity in lupus and related antiphospholipid syndrome (APS) (23, 40). The research goal of Fuchs, Ta from University Medical Center Hamburg-Eppendorf (Germany) was to understand how NETs control the progression and outcome of inflammation in different disease categories and to use NETs as a target for developing novel diagnostic assays and therapies for cardiovascular and thromboembolic diseases (8, 41, 42).
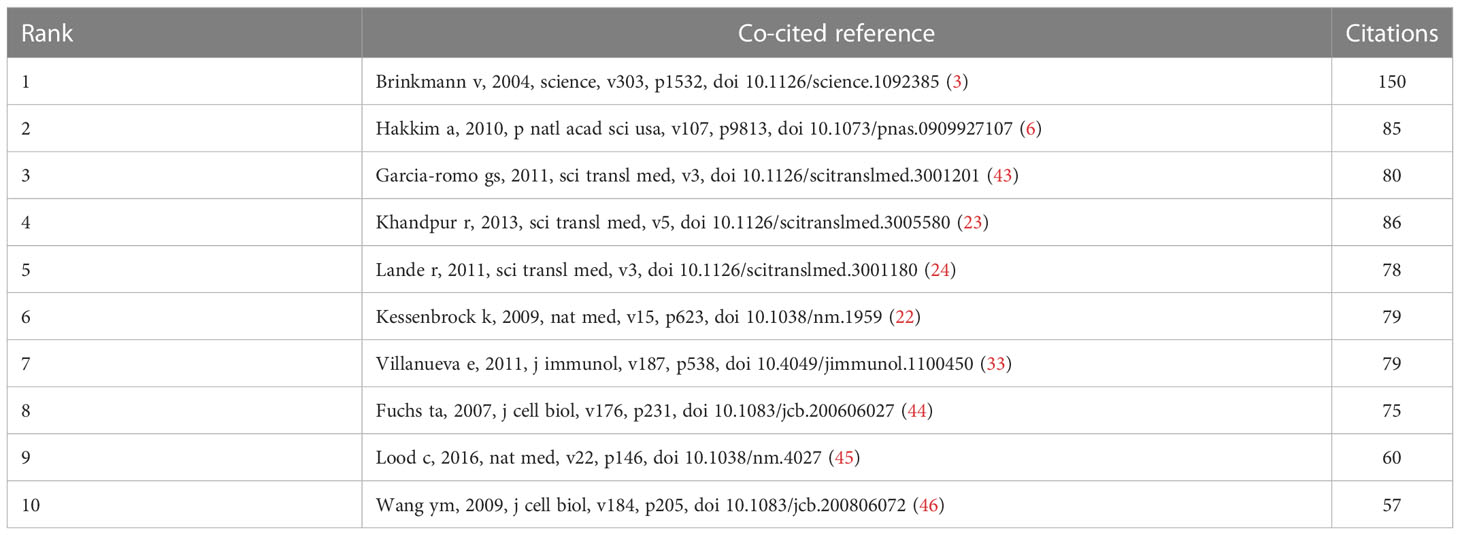
3.5. Analysis of top co-cited references
Co-cited literature refers to literature co-cited by multiple other publications, so that co-cited literature can be regarded as the basis of research in a field (28). From 2010 to 2022, 20,180 references on NETs in AIDs were cited. Table 4 lists the top 10 references that were co-cited, with all references cited at least 57 times. After filtering out 33 references (the minimum number of co-citations is 30), the co-citation network diagram was drawn (Figure 7). The reference “Brinkmann v, 2004, science, v303, p1532, doi 10.1126/science.1092385”, the first reported NETs, was co-cited up to 150 times (3).
3.6. Analysis of reference with citation bursts
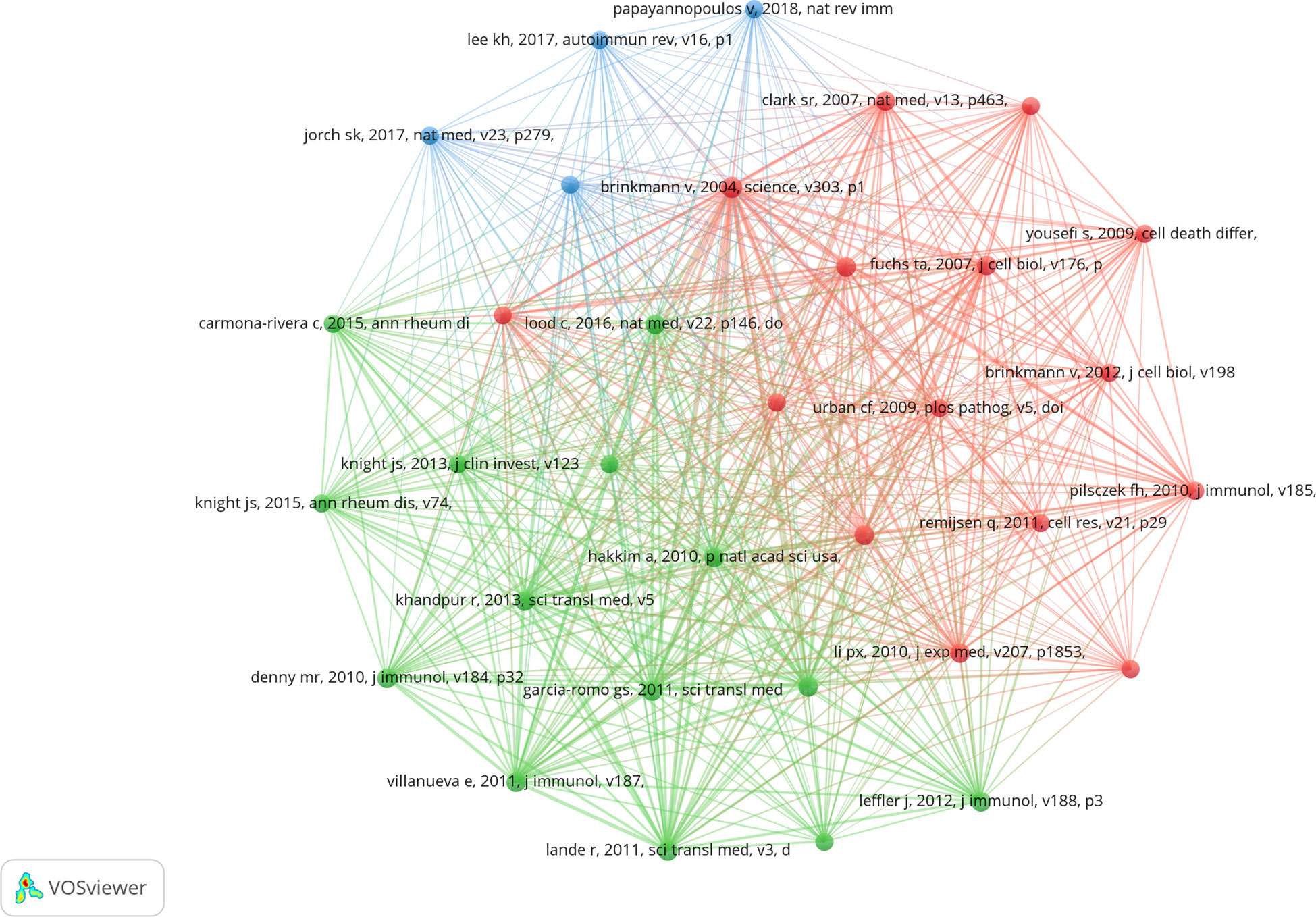
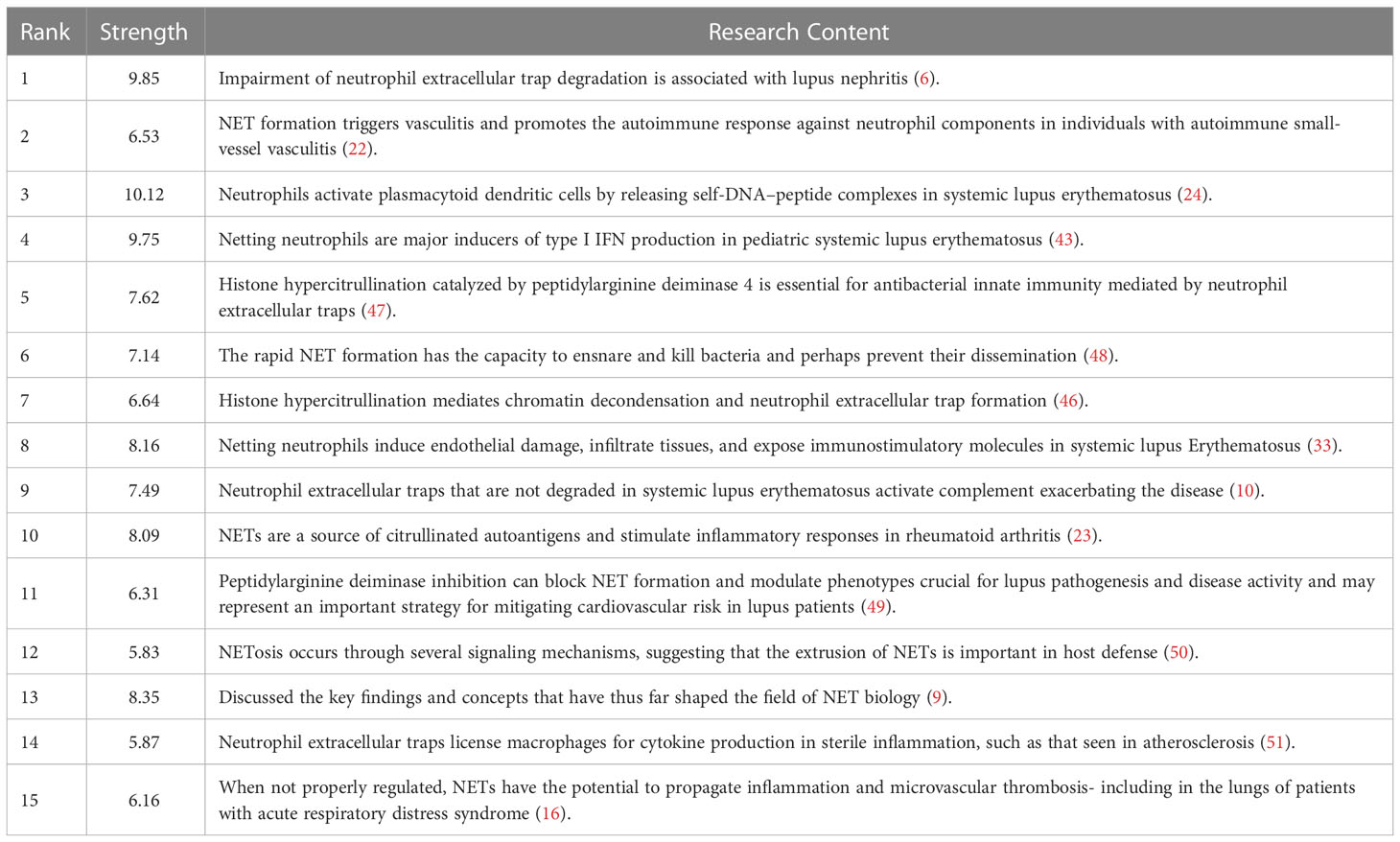
Reference with Citation Bursts refers to the literature frequently cited by scholars in a particular field over some time. Figure 8 shows the top 15 references with strong citation bursts (indicated by red bars), and the main research is summarized in Table 5.
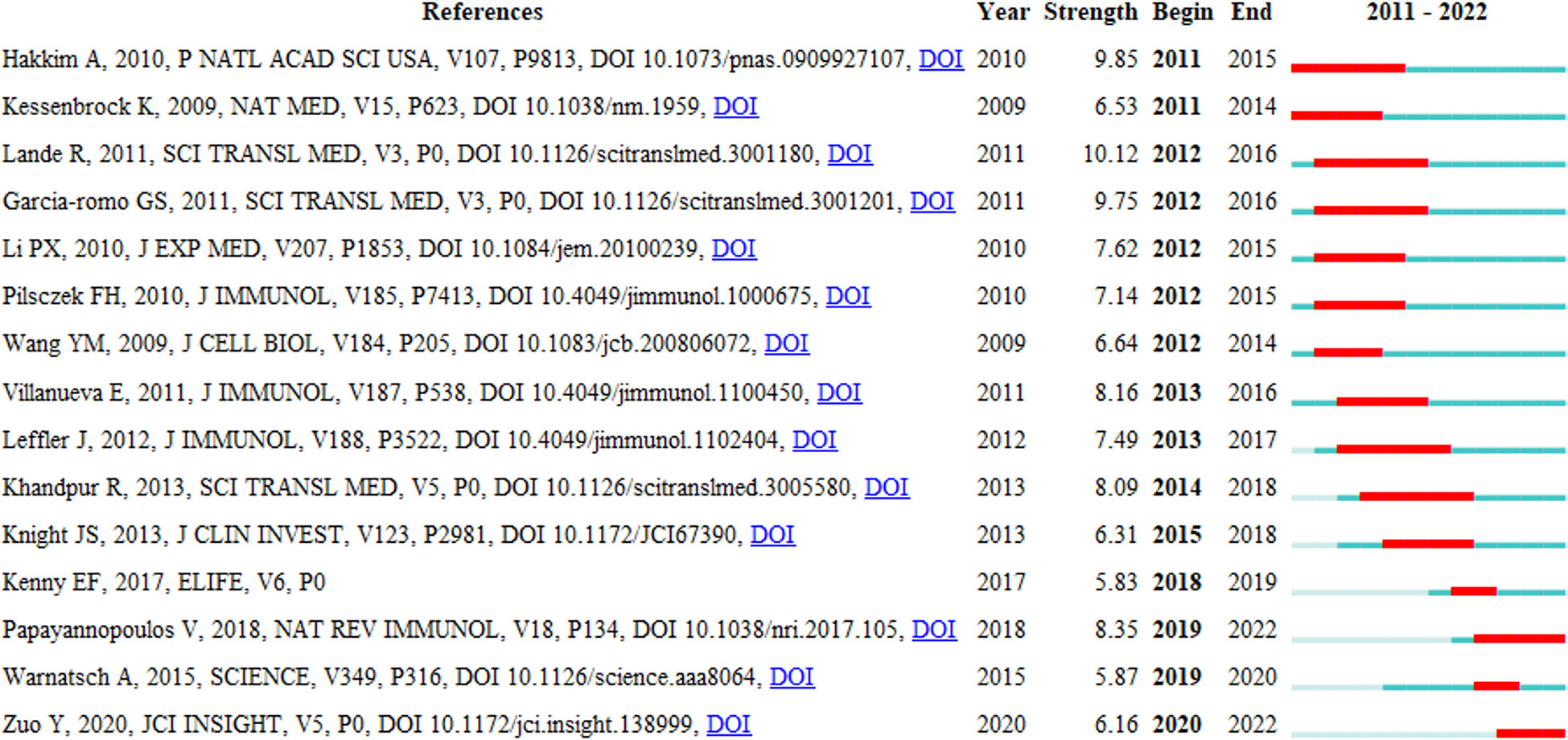
Figure 8 Top 15 references with strong citation bursts. A red bar indicates high citations in that year and the references are ranked by the beginning year of burst.
The citation bursts of references appeared in 2011 at the earliest and 2020 at the latest. Between 2011 and 2018, the citation bursts were mainly related to NETs in SLE, autoimmune small-vessel vasculitis, rheumatoid arthritis and innate immunity. “NET inhibition” was burst cited from 2015 to 2018, and “NETs associated with sterile inflammation and microvascular thrombosis (COVID-19)” were burst cited from 2019 to 2022.
3.7. Analysis of hotspots and frontiers
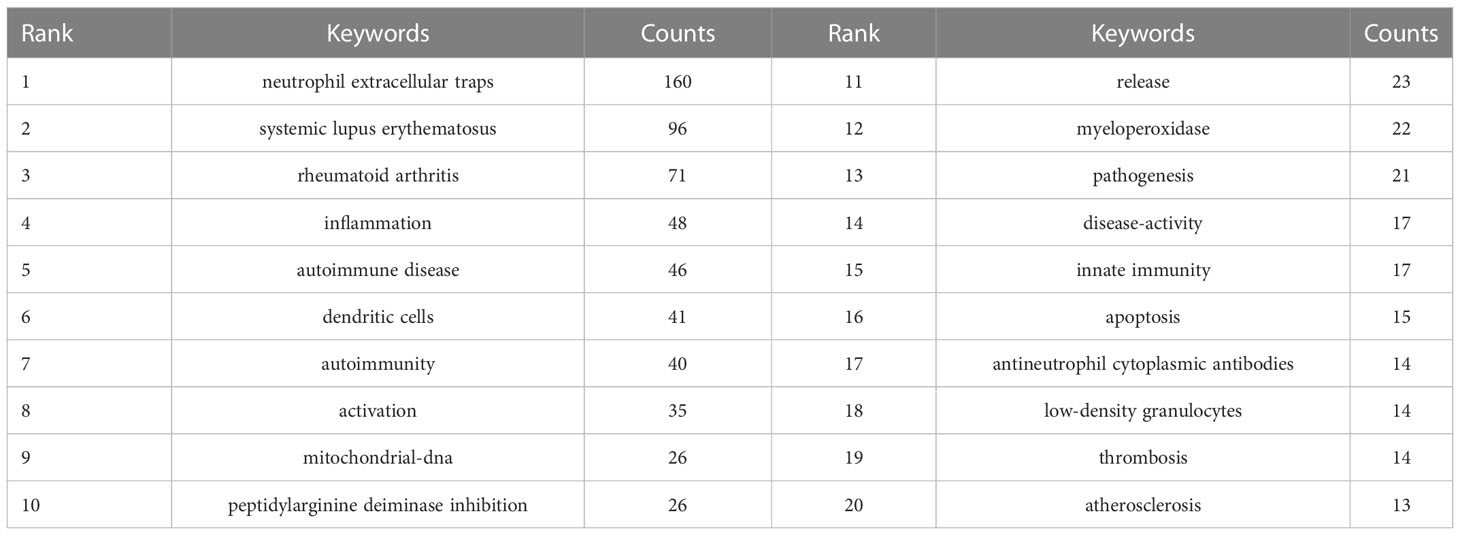
The research hotspots and frontiers of NETs in AIDs could quickly be captured through the co-occurrence analysis of keywords (Figure 9A). The top 20 high-frequency keywords are shown in Table 6, and the time zone view of keywords was drawn in Figure 9B.

Figure 9 Cluster analysis and trend topic analysis for top 20 keywords (A) and Timezone view for keywords (B).
Among these keywords, “systemic lupus erythematosus” and “rheumatoid arthritis” appeared more than 70 times, and have always been the high-frequency keywords from 2010 to 2022, representing the main research direction of NETs in AIDs. The keywords “dendritic cells,” “activation,” “mitochondrial-DNA,” and “myeloperoxidase” are related to the role of NETs in pathogenesis. As shown in Figure 9A, the keywords in green clusters consist of systemic lupus erythematosus-related and rheumatoid arthritis-related studies, and in red clusters consist of pathogenesis-related studies. Additionally, the keyword “peptidylarginine deiminase inhibition” may be associated with controlling NETs in inflammation progression and uses NETs as a target for developing novel diagnostic assays and therapies. The keyword “atherosclerosis” may be related to the association of NETs with atherosclerosis in patients with autoimmunity, and the keyword “low−density granulocytes (LDGs)” has been implicated in neutrophil subsets of patients with some autoimmune diseases who are more prone to spontaneous release of NETs.
4. Discussion
4.1. General information
A large number of high-quality studies of NETs in AIDs have been conducted. From 2010 to 2015, research in this area was still in its infancy; after 2016, the publications began to increase significantly. In 2019 and 2020, the number of articles published was stable at about 40 per year, and continued to rise in 2021. In recent years, NETs have become a new focus of research in AIDs, and annual publications in this field are growing steadily and rapidly. The United States, China, Germany, Italy and France are the leading countries to carry out NETs research in AIDS. The top ten organizations are in the United States, Germany, Netherlands, Japan, China and Mexico. In addition, the average citations/publications are higher in North America and Europe, which may be related to the earlier initiation of research in this field in the relevant countries.
The journals Journal of immunology (IF=5.43) and Frontiers in immunology (IF=8.79) have a significant influence on the research of NETs in AIDs. Frontier in immunology published the most articles in the field of NETs in AIDs (n=48), and Journal of Immunology (Co-citation=1360) was cited the most. Moreover, the journal with the highest impact factor is Nature reviews rheumatology (IF=32.29). From the authors’ perspective, Kaplan, Mariana J and Carmona-Rivera, Carmelo from the National Institutes of Health published the most articles. They focused on the mechanisms of immune dysregulation, organ damage, and early-onset vascular disease in AIDs, and have proposed that NET formation and its subsequent responses play an essential role in lupus pathogenesis by damaging endothelial cells and synthesizing elevated levels of proinflammatory cytokines and type I IFNs (33–35). Furthermore, they propose that NETosis in RA pathogenesis is accelerated by the externalization of citrullinated self-antigens and immunostimulatory molecules, which may promote aberrant adaptive and innate immune responses in joints and the periphery, and perpetuate the pathogenic mechanism of the disease (23, 36). In terms of co-cited authors, Brinkmann first reported in 2004 that NETs are an important step in neutrophil bactericidal action with essential implications in this field (2).
4.2. Research basis
Through co-citation analysis, we can understand the basis of research in the field of NETs in AIDs (28). NETs, first reported in 2004, extruded into the extracellular space primarily through a unique form of cell death known as NETosis, enabling the neutrophil to potently capture and kill pathogens at sites of inflammation (3, 5). In recent years, several different NETs formation pathways have been revealed. The most common pathway is the traditional suicidal NETosis, also known as the reactive oxygen species (ROS)-dependent pathway. NETosis is initiated when neutrophils are exposed to various stimuli (extracellular microbes or ribonucleoprotein immune complexes), activating the Raf/MERK/ERK signal transduction pathway via protein kinase C. Subsequently, the levels of reactive oxygen species (ROS) are elevated by activated NADPH oxidase complex (NOX), promoting the degradation of neutrophil elastase (NE) and cytoplasmic granules containing myeloperoxidase (MPO). NE and MPO, together with peptidyl arginine deiminase 4 (PAD4), induce the citrullination of histone H3 (CitH3), further leading to chromatin decondensation. NETs are formed by the combination of chromatin and granule proteins and are released into the extracellular space once the cell membrane ruptures, ultimately leading to neutrophil death (5, 9, 48, 52–54). Another pathway is the ROS-independent pathway, in which neutrophils release NETs without disrupting the nuclear or plasma membrane, and retain several conventional functions. After microbial products or activated platelets are recognized by Toll-like receptors or C3 complement receptors on neutrophil cell membranes, the resulting cytosolic calcium peak activates PAD4, triggering chromatin decondensation, and nuclear DNA is then packaged into vesicles and released in the form of budding. Neutrophils released by NETs in this way can still perform cellular functions, such as cell phagocytosis and migration (4, 46, 55). Another ROS-dependent form of mitochondrial NETosis, which releases mitochondrial DNA rather than nuclear DNA, has also been reported. After stimulation with lipopolysaccharide (LPS) or C5a, mitochondria in neutrophils serve as a ROS generator and facilitate the innate immune function of neutrophils via NADPH oxidase-independent NET formation, which is not accompanied by neutrophil death (45, 56, 57). Notably, different stimuli appear to activate different pathways of NET formation, and the role of these pathways in neutrophil dysregulation and autoimmunity has not been determined systematically.
4.3. Hotspots and frontiers
Analysis of references with citation bursts and keywords can quickly grasp the hotspots of distribution and evolution in the field of NETs in AIDs. At present, systemic lupus erythematosus and rheumatoid arthritis are the main research topics on NETs in AIDs. In addition, developing new therapies by controlling the progress of NETs is the trend of clinical application in the future.
4.3.1. Neutrophil extracellular traps in systemic lupus erythematosus
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease affecting multiple organs, such as the skin, kidneys, and blood vessels. It is characterized by the overproduction of autoantibodies against nuclear antigens (nucleic acids and associated proteins). The autoantibodies and immune complexes can induce NET formation, and subsequently activate plasmacytoid dendritic cells (pDCs) to produce type I interferon (24, 43, 45). In addition, a neutrophil population called low-density granulocytes (LDGs) is elevated in SLE and shows an increased capacity to release NETs (34). Moreover, owing to the presence of DNase I inhibitors or autoantibodies against NETs that block the access of DNase I into NETs, patients with SLE exhibit a reduced ability to degrade NETs (6, 58).
Excessive NET formation and impaired clearance contribute to the persistence and prolongation of NETs in SLE patients. The elevated autoantigens and immunostimulatory proteins, such as IL−17 and IL−37 in NETs, can promote the secretion of type I IFN by activating TLR9 in plasmacytoid dendritic cells. And it can also facilitate the activation of NLRP3 inflammasome in macrophages, thus improving the levels of active IL−1β and IL−18 to promote the NETs release, further amplifying the inflammatory responses. In turn, the active IL-18 can induce NETosis, creating a proinflammatory feedback loop (24, 30). In addition, increasing the expression level of matrix metalloproteinase-9 (MMP-9) in NETs induces endothelium apoptosis, thereby activating endothelial MMP-2 (25). Moreover, the components of NETs can mediate the oxidation of high−density lipoprotein, resulting in SLE-related atherosclerotic cardiovascular disease (59).
4.3.2. Neutrophil extracellular traps in rheumatoid arthritis
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disease that affects joints and connective tissue, and is characterized by the production of autoantibodies against citrullinated protein antigens (ACPAs). Like SLE, NETs are closely associated with the pathogenesis of RA, and the formation of NETs is increased in synovial and peripheral blood neutrophils (60). Concomitant with NETs formation in RA patients, histones and other NET-associated proteins can be citrullinated by PAD enzymes (in particular PAD4), and citrullinated NETs proteins are important autoantigens in RA (61).
NETs released by neutrophils in RA patients are more numerous and faster than in healthy subjects. NETs can promote the production of ACPAs, release immune stimulatory molecules such as IL-6 and IL-8, and stimulate autoimmune responses. In turn, ACPAs can also stimulate the production of NETs, thus forming a vicious cycle. NETs levels in RA patients were positively correlated with ACPAs levels (22, 58). IL6 may induce B cells to mature plasma cells and indirectly promote the formation of NETs by generating ACPAs. NETs also release carbamylated proteins (CarP), leading to the production of CarP antibodies and carbamylated NETs protein antibodies, which activate macrophages to release proinflammatory cytokines and stimulate fibroblast-like synoviocyte (FLS) to release RANKL to promote osteoclast formation and activation (62). Moreover, neutrophil elastase (NE) in NETs can directly degrade cartilage components, and amplified FLS and macrophage can further contribute to joint injury (63).
4.3.3. Neutrophil extracellular traps in other autoimmune diseases
In addition to SLE and RA, NETs have also been revealed in other AIDs. Antiphospholipid antibodies (aPLs) purified from the patients of antiphospholipid syndrome (APS) can induce NETs formation in neutrophils of healthy volunteers, and therefore the induction of NETs by aPLs may be one of the reasons for APS (40). On the other hand, the degradation of NETs in the serum of APS patients is impaired, which is closely related to the specific clinical manifestations and antibodies against NETs in patients with secondary APS (64). In the patients of anti-neutrophil cytoplasmic antibody (ANCA) associated vasculitis (AAV), the serum DNase1 activity and the ability to degrade NETs are significantly lower compared to healthy individuals, and excessive NETosis can lead to complement generation and endothelial damage (65, 66). In the patients of Behcet’s disease (BD), circulating neutrophils are more likely to release NETs in vitro and express higher levels of PAD4 than healthy control. In addition, in biopsies of vasculitis from BD patients, NETosis is found mainly around the affected blood vessels. These results suggest that NETosis plays a role in the pathophysiology of BD, especially in the induction of vasculitis (67, 68). Together, these findings further clarify the critical role of NETs in the pathogenesis and development of AIDs.
4.3.4. NETs as a target to develop therapies
Given the importance of NETs in AIDs, developing novel therapies targeting the formation or clearance of NETs might be a potential clinical strategy (54). DNAse is most commonly used to dismantle DNA, the main framework of NETs, which could degrade NETs and apoptotic cell microparticles and suppresses autoreactivity, thus having therapeutic effects in SLE and lupus nephritis (69, 70). Some stimuli can bind to neutrophil receptors, such as TLRs and complement receptors, to activate neutrophils and trigger NETosis. Disruption of these interactions can inhibit the NETosis process and disease progression. Hydroxychloroquine (HDQ) is an antimalarial drug that could reduce NETosis by inhibiting the expression of PAD4 and Rac2, as well as inhibiting TLR9. And TAK-242 is also a TLR4 inhibitor that could reduce NET formation, suggesting that it may be helpful in treating AIDS (69, 71). NETosis requires calcium mobilization. Therefore, calcineurin inhibitors such as cyclosporine A or tacrolimus would be potential therapeutic agents to inhibit the calcineurin pathway, and reduce the activation of neutrophils in AIDs. In fact, they have been used to treat patients with SLE and have been very productive (69, 72). ROS is essential for forming NETs, and a series of ROS scavengers have therapeutic effects on AIDs. For example, ROS scavengers such as N−acetylcysteine (NAC) could suppress ROS production to reduce NETosis, and the application of NAC can improve disease outcomes in SLE patients (73). Citrullination of histones is associated with chromatin decondensation during the formation of NETs, and thus PAD inhibitors are thought to prevent NETs formation and have also been studied in AIDs. PAD inhibitors were observed to reduce NETs formation effectively and disease severity in mouse models of SLE and RA (74, 75). Vitamin D can inhibit the adaptive immune system (76), and it has been reported that Vitamin D 1,25(OH)2D3 can inhibit NETs-induced endothelial damage in SLE patients by inhibiting NE externalization (77).
Although it is feasible to target NETs for the therapeutic benefit of AIDs in clinical practice, some scholars believe that neutrophilic phagocytosis plays a key role in host defense, and any therapy targeting NETs formation must avoid impairing the physiological function of these cells. Meraj A et al. have suppressed both types of NETosis by inhibiting transcription, while maintaining the production of ROS, so that the function of physiological neutrophils would not be lost (78). Therefore, it is a promising approach to use a NETs formation inhibitor that selectively impinges NETs production while retaining phagocytosis and degranulation in the future.
4.4. Advantages and shortcomings
Our visualization analysis could provide more insight into hotspots and frontiers than traditional reviews. We comprehensively analyzed the research trends and developments of NETs in AIDs by bibliometrics for the first time, and would provide a reference for scholars in this field.
Limited by the data extracted only from the WoSCC database, some relevant studies might be missed. Second, we filtered data published in English, and the trends of non-English writing papers were ignored. Moreover, bias could also come from the data extraction protocol.
4.5. Conclusion
NETs played a crucial role in the pathogenesis and development of AIDs, and annual publications in this field are growing steadily and rapidly. The United States, China and Germany are major countries to carry out the related research, and Frontiers in Immunology and Journal of Immunology have a significant influence on the research. Systemic lupus erythematosus and rheumatoid arthritis are the current major topics in this field, and the development of new treatment methods via control of NETs in the progression of AIDs is the trend of future clinical application. Drugs targeting NETs formation have shown promise in clinical models and are an exciting possibility for treating AIDs.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
WW and XZ contributed to conception and design, and initial draft of the manuscript. XZ, MY, and JS contributed to literature search, analysis and drew pictures. JP contributed to edited drafts of the manuscript. JS reviewed and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Youth Foundation of Science and Technology Department of Shanxi Province (20210302123448).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Manfredi AA, Ramirez GA, Rovere-Querini P, Maugeri N. The neutrophil’s choice: phagocytose vs make neutrophil extracellular traps. Front Immunol (2018) 9:288. doi: 10.3389/fimmu.2018.00288
2. Ravindran M, Khan MA, Palaniyar N. Neutrophil extracellular trap formation: Physiology, pathology, and pharmacology. Biomolecules (2019) 9(8):365. doi: 10.3390/biom9080365
3. Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Sci (New York NY) (2004) 303(5663):1532–5. doi: 10.1126/science.1092385
4. Bonaventura A, Liberale L, Carbone F, Vecchié A, Diaz-Cañestro C, Camici GG, et al. The pathophysiological role of neutrophil extracellular traps in inflammatory diseases. Thromb haemostasis (2018) 118(01):006–27. doi: 10.1160/TH17-09-0630
5. Steinberg BE, Grinstein S. Unconventional roles of the NADPH oxidase: signaling, ion homeostasis, and cell death. Science's STKE (2007) 2007(379):pe11–1. doi: 10.1126/stke.3792007pe11
6. Hakkim A, Fürnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci United States America (2010) 107(21):9813–8. doi: 10.1073/pnas.0909927107
7. Zonta YR, Dezen ALO, Della Coletta AM, Yu KST, Carvalho L, Santos L, et al. Paracoccidioides brasiliensis releases a DNase-like protein that degrades NETs and allows for fungal escape. Front Cell Infect Microbiol (2021) 10. doi: 10.3389/fcimb.2020.592022
8. Jiménez-Alcázar M, Rangaswamy C, Panda R, Bitterling J, Simsek YJ, Long AT, et al. Host DNases prevent vascular occlusion by neutrophil extracellular traps. Sci (New York NY) (2017) 358(6367):1202–6. doi: 10.1126/science.aam8897
9. Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol (2018) 18(2):134–47. doi: 10.1038/nri.2017.105
10. Leffler J, Martin M, Gullstrand B, Tydén H, Lood C, Truedsson L, et al. Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease. J Immunol (2012) 188(7):3522–31. doi: 10.4049/jimmunol.1102404
11. Moore S, Juo H-H, Nielsen CT, Tyden H, Bengtsson AA, Lood C. Role of neutrophil extracellular traps regarding patients at risk of increased disease activity and cardiovascular comorbidity in systemic lupus erythematosus. J Rheumatol (2020) 47(11):1652–60. doi: 10.3899/jrheum.190875
12. Song W, Ye J, Pan N, Tan C, Herrmann M. Neutrophil extracellular traps tied to rheumatoid arthritis: points to ponder. Front Immunol (2021) 11:578129. doi: 10.3389/fimmu.2020.578129
13. Laridan E, Martinod K, De Meyer SF. Neutrophil extracellular traps in arterial and venous thrombosis. In: Seminars in thrombosis and hemostasis. Thieme Medical Publishers (2019). p. 086–93.
14. Ramos-Kichik V, Mondragón-Flores R, Mondragón-Castelán M, Gonzalez-Pozos S, Muñiz-Hernandez S, Rojas-Espinosa O, et al. Neutrophil extracellular traps are induced by mycobacterium tuberculosis. Tuberculosis (2009) 89(1):29–37. doi: 10.1016/j.tube.2008.09.009
15. Berezin A. Neutrophil extracellular traps: The core player in vascular complications of diabetes mellitus. Diabetes Metab Syndr: Clin Res Rev (2019) 13(5):3017–23. doi: 10.1016/j.dsx.2018.07.010
16. Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA, et al. Neutrophil extracellular traps in COVID-19. JCI Insight (2020) 5(11):e138999. doi: 10.1172/jci.insight.138999
17. Zuo Y, Zuo M, Yalavarthi S, Gockman K, Madison JA, Shi H, et al. Neutrophil extracellular traps and thrombosis in COVID-19. J Thromb Thrombolysis (2021) 51(2):446–53. doi: 10.1007/s11239-020-02324-z
18. Trivedi A, Khan MA, Bade G, Talwar A. Orchestration of neutrophil extracellular traps (Nets), a unique innate immune function during chronic obstructive pulmonary disease (COPD) development. Biomedicines (2021) 9(1):53. doi: 10.3390/biomedicines9010053
19. Cooper GS, Bynum ML, Somers EC. Recent insights in the epidemiology of autoimmune diseases: improved prevalence estimates and understanding of clustering of diseases. J Autoimmun (2009) 33(3-4):197–207. doi: 10.1016/j.jaut.2009.09.008
20. Lerner A, Jeremias P, Matthias T. The world incidence and prevalence of autoimmune diseases is increasing. Int J Celiac Dis (2015) 3(4):151–5. doi: 10.12691/ijcd-3-4-8
21. Wang L, Wang FS, Gershwin ME. Human autoimmune diseases: a comprehensive update. J Internal Med (2015) 278(4):369–95. doi: 10.1111/joim.12395
22. Kessenbrock K, Krumbholz M, Schönermarck U, Back W, Gross WL, Werb Z, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med (2009) 15(6):623–5. doi: 10.1038/nm.1959
23. Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Trans Med (2013) 5(178):178ra140–178ra140. doi: 10.1126/scitranslmed.3005580
24. Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA–peptide complexes in systemic lupus erythematosus. Sci Trans Med (2011) 3(73):73ra19–9. doi: 10.1126/scitranslmed.3001180
25. Carmona-Rivera C, Zhao W, Yalavarthi S, Kaplan MJ. Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase-2. Ann Rheum Dis (2015) 74(7):1417–24. doi: 10.1136/annrheumdis-2013-204837
26. Pan X, Yan E, Cui M, Hua W. Examining the usage, citation, and diffusion patterns of bibliometric mapping software: A comparative study of three tools. J informetrics (2018) 12(2):481–93. doi: 10.1016/j.joi.2018.03.005
27. Chen C, Song M. Visualizing a field of research: A methodology of systematic scientometric reviews. PloS One (2019) 14(10):e0223994. doi: 10.1371/journal.pone.0223994
28. Chen C. CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. J Am Soc Inf Sci Technol (2006) 57(3):359–77. doi: 10.1002/asi.20317
29. Hassan-Montero Y, De-Moya-Anegón F, Guerrero-Bote VP. SCImago graphica: a new tool for exploring and visually communicating data. Prof la inform (2022) 31(5):e310502. doi: 10.3145/epi.2022.sep.02
30. Kahlenberg JM, Carmona-Rivera C, Smith CK, Kaplan MJ. Neutrophil extracellular trap–associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages. J Immunol (2013) 190(3):1217–26. doi: 10.4049/jimmunol.1202388
31. Carmona-Rivera C, Khaznadar SS, Shwin KW, Irizarry-Caro JA, O’Neil LJ, Liu Y, et al. Deficiency of adenosine deaminase 2 triggers adenosine-mediated NETosis and TNF production in patients with DADA2. Blood (2019) 134(4):395–406. doi: 10.1182/blood.2018892752
32. Mistry P, Nakabo S, O’Neil L, Goel RR, Jiang K, Carmona-Rivera C, et al. Transcriptomic, epigenetic, and functional analyses implicate neutrophil diversity in the pathogenesis of systemic lupus erythematosus. Proc Natl Acad Sci (2019) 116(50):25222–8. doi: 10.1073/pnas.1908576116
33. Villanueva E, Yalavarthi S, Berthier CC, Hodgin JB, Khandpur R, Lin AM, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol (2011) 187(1):538–52. doi: 10.4049/jimmunol.1100450
34. Denny MF, Yalavarthi S, Zhao W, Thacker SG, Anderson M, Sandy AR, et al. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J Immunol (2010) 184(6):3284–97. doi: 10.4049/jimmunol.0902199
35. Kaplan MJ, Radic M. Neutrophil extracellular traps: Double-edged swords of innate immunity. J Immunol (2012) 189(6):2689–95. doi: 10.4049/jimmunol.1201719
36. Kahlenberg JM, Kaplan MJ. Mechanisms of premature atherosclerosis in rheumatoid arthritis and lupus. Annu Rev Med (2013) 64:249–63. doi: 10.1146/annurev-med-060911-090007
37. Schauer C, Janko C, Munoz LE, Zhao Y, Kienhöfer D, Frey B, et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med (2014) 20(5):511–7. doi: 10.1038/nm.3547
38. Mondal S, Thompson PR. Protein arginine deiminases (PADs): biochemistry and chemical biology of protein citrullination. Acc Chem Res (2019) 52(3):818–32. doi: 10.1021/acs.accounts.9b00024
39. Subramanian V, Knight JS, Parelkar S, Anguish L, Coonrod SA, Kaplan MJ, et al. Design, synthesis, and biological evaluation of tetrazole analogs of cl-amidine as protein arginine deiminase inhibitors. J med Chem (2015) 58(3):1337–44. doi: 10.1021/jm501636x
40. Yalavarthi S, Gould TJ, Rao AN, Mazza LF, Morris AE, Núñez-Álvarez C, et al. Release of neutrophil extracellular traps by neutrophils stimulated with antiphospholipid antibodies: a newly identified mechanism of thrombosis in the antiphospholipid syndrome. Arthritis Rheumatol (2015) 67(11):2990–3003. doi: 10.1002/art.39247
41. Nickel KF, Long AT, Fuchs TA, Butler LM, Renné T. Factor XII as a therapeutic target in thromboembolic and inflammatory diseases. Arterioscler thromb Vasc Biol (2017) 37(1):13–20. doi: 10.1161/ATVBAHA.116.308595
42. Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD Jr., et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci (2010) 107(36):15880–5. doi: 10.1073/pnas.1005743107
43. Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Trans Med (2011) 3(73):73ra20–0. doi: 10.1126/scitranslmed.3001201
44. Bager P, Nielsen NM, Bihrmann K, Frisch M, Hjalgrim H, Wohlfart J, et al. Childhood infections and risk of multiple sclerosis. Brain (2004) 127(11):2491–7. doi: 10.1093/brain/awh283
45. Lood C, Blanco LP, Purmalek MM, Carmona-Rivera C, De Ravin SS, Smith CK, et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med (2016) 22(2):146–53. doi: 10.1038/nm.4027
46. Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol (2009) 184(2):205–13. doi: 10.1083/jcb.200806072
47. Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med (2010) 207(9):1853–62. doi: 10.1084/jem.20100239
48. Pilsczek FH, Salina D, Poon KKH, Fahey C, Yipp BG, Sibley CD, et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to staphylococcus aureus. J Immunol (2010) 185(12):7413–25. doi: 10.4049/jimmunol.1000675
49. Knight JS, Zhao W, Luo W, Subramanian V, O’Dell AA, Yalavarthi S, et al. Peptidylarginine deiminase inhibition is immunomodulatory and vasculoprotective in murine lupus. J Clin Invest (2013) 123(7):2981–93. doi: 10.1172/JCI67390
50. Kenny EF, Herzig A, Krüger R, Muth A, Mondal S, Thompson PR, et al. Diverse stimuli engage different neutrophil extracellular trap pathways. Elife (2017) 6:e24437. doi: 10.7554/eLife.24437
51. Warnatsch A, Ioannou M, Wang Q, Papayannopoulos V. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Sci (New York NY) (2015) 349(6245):316–20. doi: 10.1126/science.aaa8064
52. Sørensen OE, Borregaard N. Neutrophil extracellular traps–the dark side of neutrophils. J Clin Invest (2016) 126(5):1612–20. doi: 10.1172/JCI84538
53. Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol (2010) 191(3):677–91. doi: 10.1083/jcb.201006052
54. Huang J, Hong W, Wan M, Zheng L. Molecular mechanisms and therapeutic target of NETosis in diseases. MedComm (2022) 3(3):e162. doi: 10.1002/mco2.162
55. Chamilos G, Bassett RL, Liu W, Gilliet M. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med (2011) 3 (73) :73ra19. doi: 10.1126/scitranslmed.3001180
56. Yousefi S, Mihalache C, Kozlowski E, Schmid I, Simon H-U. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ (2009) 16(11):1438–44. doi: 10.1038/cdd.2009.96
57. Douda DN, Khan MA, Grasemann H, Palaniyar N. SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc Natl Acad Sci United States America (2015) 112(9):2817–22. doi: 10.1073/pnas.1414055112
58. Leffler J, Gullstrand B, Jönsen A, Nilsson J-Å, Martin M, Blom AM, et al. Degradation of neutrophil extracellular traps co-varies with disease activity in patients with systemic lupus erythematosus. Arthritis Res Ther (2013) 15(4):1–9. doi: 10.1186/ar4264
59. Smith CK, Vivekanandan-Giri A, Tang C, Knight JS, Mathew A, Padilla RL, et al. Neutrophil extracellular trap–derived enzymes oxidize high-density lipoprotein: an additional proatherogenic mechanism in systemic lupus erythematosus. Arthritis Rheumatol (2014) 66(9):2532–44. doi: 10.1002/art.38703
60. Chang HH, Dwivedi N, Nicholas AP, Ho IC. The W620 polymorphism in PTPN22 disrupts its interaction with peptidylarginine deiminase type 4 and enhances citrullination and NETosis. Arthritis Rheumatol (2015) 67(9):2323–34. doi: 10.1002/art.39215
61. Spengler J, Lugonja B, Jimmy Ytterberg A, Zubarev RA, Creese AJ, Pearson MJ, et al. Release of active peptidyl arginine deiminases by neutrophils can explain production of extracellular citrullinated autoantigens in rheumatoid arthritis synovial fluid. Arthritis Rheumatol (2015) 67(12):3135–45. doi: 10.1002/art.39313
62. O’Neil LJ, Barrera-Vargas A, Sandoval-Heglund D, Merayo-Chalico J, Aguirre-Aguilar E, Aponte AM, et al. Neutrophil-mediated carbamylation promotes articular damage in rheumatoid arthritis. Sci Adv (2020) 6(44):eabd2688. doi: 10.1126/sciadv.abd2688
63. Carmona-Rivera C, Carlucci PM, Goel RR, James E, Brooks SR, Rims C, et al. Neutrophil extracellular traps mediate articular cartilage damage and enhance cartilage component immunogenicity in rheumatoid arthritis. JCI Insight (2020) 5(13):e139388. doi: 10.1172/jci.insight.139388
64. Leffler J, Stojanovich L, Shoenfeld Y, Bogdanovic G, Hesselstrand R, Blom AM. Degradation of neutrophil extracellular traps is decreased in patients with antiphospholipid syndrome. Clin Exp Rheumatol (2014) 32(1):66–70.
65. Nakazawa D, Shida H, Tomaru U, Yoshida M, Nishio S, Atsumi T, et al. Enhanced formation and disordered regulation of NETs in myeloperoxidase-ANCA-associated microscopic polyangiitis. J Am Soc Nephrol (2014) 25(5):990–7. doi: 10.1681/ASN.2013060606
66. Li SS, Wang SL, Zhang HJ, He SH, Liang X, Li TF. The pathogenesis and treatment in antineutrophil cytoplasmic antibody associated vasculitis. Am J Trans Res (2020) 12(8):4094–107.
67. Safi R, Kallas R, Bardawil T, Mehanna CJ, Abbas O, Hamam R, et al. Neutrophils contribute to vasculitis by increased release of neutrophil extracellular traps in behçet's disease. J Dermatol Sci (2018) 92(2):143–50. doi: 10.1016/j.jdermsci.2018.08.010
68. Perazzio SF, Soeiro-Pereira PV, Dos Santos VC, de Brito MV, Salu B, Oliva MLV, et al. Soluble CD40L is associated with increased oxidative burst and neutrophil extracellular trap release in behçet’s disease. Arthritis Res Ther (2017) 19(1):1–15. doi: 10.1186/s13075-017-1443-5
69. Gupta S, Kaplan MJ. The role of neutrophils and NETosis in autoimmune and renal diseases. Nat Rev Nephrol (2016) 12(7):402–13. doi: 10.1038/nrneph.2016.71
70. Sisirak V, Sally B, d’Agati V, Martinez-Ortiz W, Özçakar ZB, David J, et al. Digestion of chromatin in apoptotic cell microparticles prevents autoimmunity. Cell (2016) 166(1):88–101. doi: 10.1016/j.cell.2016.05.034
71. Zhang S, Zhang Q, Wang F, Guo X, Liu T, Zhao Y, et al. Hydroxychloroquine inhibiting neutrophil extracellular trap formation alleviates hepatic ischemia/reperfusion injury by blocking TLR9 in mice. Clin Immunol (2020) 216:108461. doi: 10.1016/j.clim.2020.108461
72. Gupta AK, Giaglis S, Hasler P, Hahn S. Efficient neutrophil extracellular trap induction requires mobilization of both intracellular and extracellular calcium pools and is modulated by cyclosporine a. PloS One (2014) 9(5):e97088. doi: 10.1371/journal.pone.0097088
73. Zawrotniak M, Kozik A, Rapala-Kozik M. Selected mucolytic, anti-inflammatory and cardiovascular drugs change the ability of neutrophils to form extracellular traps (NETs). Acta Biochim Polonica (2015) 62(3):465-73. doi: 10.18388/abp.2015_1055
74. Knight JS, Subramanian V, O'Dell AA, Yalavarthi S, Zhao W, Smith CK, et al. Peptidylarginine deiminase inhibition disrupts NET formation and protects against kidney, skin and vascular disease in lupus-prone MRL/lpr mice. Ann rheum Dis (2015) 74(12):2199–206. doi: 10.1136/annrheumdis-2014-205365
75. Willis V, Banda N, Cordova K, Chandra P, Robinson W, Cooper D, et al. Protein arginine deiminase 4 inhibition is sufficient for the amelioration of collagen-induced arthritis. Clin Exp Immunol (2017) 188(2):263–74. doi: 10.1111/cei.12932
76. Aranow C. Vitamin d and the immune system. J Invest Med (2011) 59(6):881–6. doi: 10.2310/JIM.0b013e31821b8755
77. Handono K, Sidarta YO, Pradana BA, Nugroho RA, Hartono IA, Kalim H, et al. Vitamin d prevents endothelial damage induced by increased neutrophil extracellular traps formation in patients with systemic lupus erythematosus. Acta Med Indonesiana (2016) 46(3):189-98.
Keywords: bibliometric analysis, neutrophil extracellular traps, autoimmune disease, systemic lupus erythematosus, rheumatoid arthritis
Citation: Wang W, Su J, Kang W, Yan M, Pan J and Zhang X (2023) Neutrophil extracellular traps in autoimmune diseases: Analysis of the knowledge map. Front. Immunol. 14:1095421. doi: 10.3389/fimmu.2023.1095421
Received: 11 November 2022; Accepted: 16 January 2023;
Published: 27 January 2023.
Edited by:
Meraj Alam Khan, University of Toronto, CanadaReviewed by:
Manoj Tembhre, All India Institute of Medical Sciences, IndiaAkhilesh Srivastava, Tulane University, United States
Copyright © 2023 Wang, Su, Kang, Yan, Pan and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xianhui Zhang, emhhbmd4aWFuaHVpNzhAMTYzLmNvbQ==; Jing Su, MTEwMjk4NDQ0OEBxcS5jb20=
†These authors have contributed equally to this work
 Wei Wang
Wei Wang Jing Su2*†
Jing Su2*† Jie Pan
Jie Pan Xianhui Zhang
Xianhui Zhang