
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Immunol., 03 March 2023
Sec. Multiple Sclerosis and Neuroimmunology
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1090548
This article is part of the Research TopicBiomarkers in Autoimmune Diseases of the Central Nervous SystemView all 22 articles
 Katsuichi Miyamoto1,2
Katsuichi Miyamoto1,2 Mai Minamino1
Mai Minamino1 Motoi Kuwahara2
Motoi Kuwahara2 Hiroshi Tsujimoto3
Hiroshi Tsujimoto3 Katsuki Ohtani4
Katsuki Ohtani4 Nobutaka Wakamiya5
Nobutaka Wakamiya5 Kei-ichi Katayama3
Kei-ichi Katayama3 Norimitsu Inoue3*
Norimitsu Inoue3* Hidefumi Ito1
Hidefumi Ito1Complement is involved in the pathogenesis of neuroimmune disease, but the detailed pathological roles of the complement pathway remain incompletely understood. Recently, eculizumab, a humanized anti-C5 monoclonal antibody, has been clinically applied against neuroimmune diseases such as myasthenia gravis and neuromyelitis optica spectrum disorders (NMOSD). Clinical application of eculizumab is also being investigated for another neuroimmune disease, Guillain-Barré syndrome (GBS). However, while the effectiveness of eculizumab for NMOSD is extremely high in many cases, there are some cases of myasthenia gravis and GBS in which eculizumab has little or no efficacy. Development of effective biomarkers that reflect complement activation in these diseases is therefore important. To identify biomarkers that could predict disease status, we retrospectively analyzed serum levels of complement factors in 21 patients with NMOSD and 25 patients with GBS. Ba, an activation marker of the alternative complement pathway, was elevated in the acute phases of both NMOSD and GBS. Meanwhile, sC5b-9, an activation marker generated by the terminal complement pathway, was elevated in NMOSD but not in GBS. Complement factor H (CFH), a complement regulatory factor, was decreased in the acute phase as well as in the remission phase of NMOSD, but not in any phases of GBS. Together, these findings suggest that complement biomarkers, such as Ba, sC5b-9 and CFH in peripheral blood, have potential utility in understanding the pathological status of NMOSD.
The complement system plays important roles in the innate immune system, which protects the body from foreign pathogens (1). However, when the regulatory mechanisms of complement activation are disrupted, dysregulated complement activation damages autologous cells and causes diseases such as paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS) (2, 3). Eculizumab, a humanized anti-C5 monoclonal antibody, is effective against PNH and aHUS (3). It specifically inhibits production of anaphylatoxin C5a and subsequent formation of the membrane attack complex (MAC), suppressing pathological complement activation. Its effectivity has been shown against neuroimmune diseases such as myasthenia gravis (MG) and neuromyelitis optica spectrum disorders (NMOSD) (4).
In MG (5) and NMOSD (6, 7), autoantibodies against acetylcholine receptor and aquaporin-4 (AQP4), respectively, activate the complement system, causing neurological symptoms due to destruction of the nervous system by the terminal complement pathway. Eculizumab is effective against these diseases and has been clinically applied (8, 9). Guillain-Barré syndrome (GBS) is also a neuroimmune disease, in which anti-ganglioside autoantibodies are produced after infection with Campylobacter jejuni or other organisms, and damage to the myelin sheath causes peripheral neuropathy (10). Clinical application of eculizumab for GBS is currently under investigation (11). Although eculizumab is effective in MG and GBS, some cases are non-responders, and the basis for non-response is unknown (4).
In these diseases, autoantibody titers do not correlate with disease pathology, and accurate biomarkers for complement activation could be useful not only in determining disease severity, but also in determining the potential utility of anti-complement drugs. However, biomarkers that accurately reflect complement activation in the pathogenesis of neurological diseases have not yet been identified. NMOSD and GBS are characterized by activation of the classical complement pathway. In the present retrospective cohort study, however, we measured serum levels of complement-activated markers and complement regulators involved in the alternative or terminal complement pathway in NMOSD and GBS for three reasons. First, eculizumab, which blocks the C5 cleavage involved in the initiation of the terminal complement pathway, is effective in these diseases, so activation of the alternative complement pathway and the formation of MAC in the terminal complement pathway would be expected to cause development of these diseases. Second, although the autoantibodies in NMOSD constantly exist in blood and may always activate the classical complement pathway, symptoms of NMOSD appear suddenly and recurrently, suggesting that the appearance of symptoms requires further complement activation by the alternative complement pathway in addition to the classical complement pathway. Third, in transplant-associated thrombotic microangiopathy (TA-TMA), which is thought to be a disease involving the classical and lectin complement pathways, our group previously demonstrated that abnormally high levels of plasma complement factor Ba fragment (Ba), a biomarker of activation of the alternative pathway, can be used to predict TA-TMA development and non-relapse mortality (12). We examined whether biomarkers that predict activation of the alternative and terminal complement pathways could therefore also be associated with disease pathogenesis, prognosis, and status.
Patients with NMOSD and GBS treated at Wakayama Medical University Hospital or Kindai University Hospital between 2016 and 2021 were included, and cases with both acute- and remission-paired sera archived were retrospectively selected and enrolled. Medical information was collected from medical charts. Diagnostic criteria were the 2015 international diagnostic criteria for NMOSD (13) and the Asberry diagnostic criteria for GBS (14). Seventy healthy Japanese adults, consisting of 35 males (age, mean ± SD: 45.7± 10.3 years; range: 26-68 years) and 35 females (age, mean ± SD: 44.7± 12.3 years; range: 27-75 years) were enrolled as healthy controls (15).
NMOSD relapse was defined based on criteria from previous clinical studies (8). Briefly, new onset or worsening neurologic symptoms must persist >24 hours and should not be attributable to confounding clinical factors. Remission was defined as a period when neurologic symptoms were stable for at least one month, and no new lesions shown on MRI imaging.
The acute phase of GBS was defined as the peak of symptoms prior to treatment. The stable phase was defined as a time when symptoms became mild and stable following treatment. Disabilities were evaluated using the Hughes functional grade scale (11).
Anti-AQP4 antibodies titers were analyzed using a cell-based assay with live human embryonic kidney 293 cells stably transfected with the M23 isoform of AQP4. Goat anti-human IgG Fc labelled with DyLight 488 (Thermo Fisher Scientific, Waltham, MA) was used as a secondary antibody after the transfected cells were exposed to the patients’ diluted sera. Anti-ganglioside antibodies were examined by ELISA. Serum IgG antibodies to 11 glycolipid antigens (GM1, GM2, GM3, GD1a, GD1b, GD3, GT1b, GQ1b, GT1a, Gal-C, and GalNAc-GD1a) were analyzed.
Serum samples obtained from patients and healthy controls were stocked until analysis at -80°C. Serum levels of sC5b-9 and Ba were measured using MicroVue SC5b-9 Plus EIA and MicroVue Ba EIA, respectively (Quidel, San Diego, CA). Serum levels of complement factor H (CFH) and complement factor I (CFI) were measured using ELISA kits (Abnova, Taipei, Taiwan). Complement data from 70 healthy Japanese volunteers (age: 26–75 years) were used as healthy controls, and reference ranges of complement markers (average levels ± 2 S.D.) in their serum were defined as previously described (15). The normal ranges of serum for sC5b-9 and Ba have been found to be greater than that of EDTA plasma, but the ranges in serum stored at -80 °C until analysis confirmed stability, even after five freeze-thaw cycles. In the present study, we compared the patients data with previous data of 70 healthy Japanese adults as controls.
Statistically significant differences were evaluated between three groups (healthy controls, patients with NMOSD and patients with GBS) using a one-way analysis of variance (ANOVA) and a Tukey-Kramer test as a post hoc test, and between two groups (the acute and remission phases) using a paired t-test. P < 0.05 (two-tailed) was considered significant for all results. Pearson correlation analysis was performed using JMP pro 16.0 software.
We retrospectively analyzed 21 patients with NMOSD (19 females and 2 males) and 25 patients with GBS (14 females and 11 males) (Table 1). The mean age at the time of blood collection in the acute phase of NMOSD was 48.0 years, mean duration of illness was 5.1 years, and mean expanded disability status scale (EDSS) was 5.3. Mean EDSS during NMOSD remission was 4.5. The mean age at GBS onset was 50.8 years, and the mean severity of illness was Hughes functional grade scale 3.4. Mean Hughes functional grade scale during the remission phase of GBS (at discharge) was 1.7. Anti-AQP4 and anti-glycolipid autoantibodies were positive in 81% patients with NMOSD and 88% patients with GBS, respectively.
sC5b-9, an activation marker generated by the terminal complement pathway, was significantly higher in the acute phase of NMOSD compared with in the acute phase of GBS (Figure 1A). Activation of the complement system was thus indicated to have progressed to the terminal complement pathway in the acute phase of NMOSD. Serum Ba, an activation marker of the alternative complement pathway, was also higher in the acute phases of both NMOSD and GBS compared with healthy controls (Figure 1A).
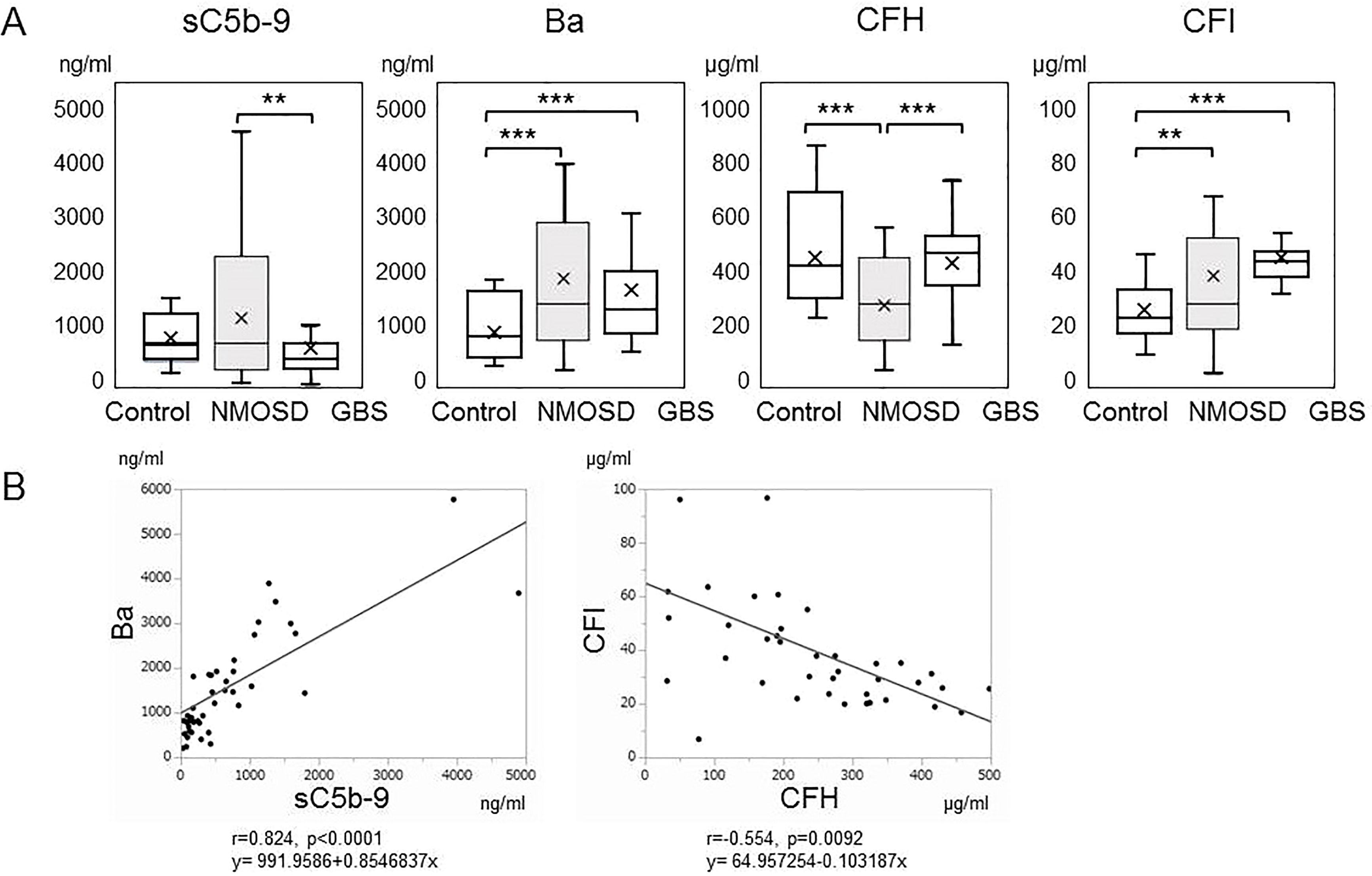
Figure 1 (A) Serum levels of complement markers in the acute phases of neuromyelitis optica spectrum disorders and Guillain-Barré syndrome. Serum levels of sC5b-9, Ba, complement factor H, and complement factor I in the acute phases of neuromyelitis optica spectrum disorders and Guillain-Barré syndrome, together with those of healthy controls, are shown by box plots. **p < 0.01, and ***p < 0.001, ANOVA and Tukey-Kramer test as a post hoc test. (B) Correlation analysis of complement markers in the acute phase of neuromyelitis optica spectrum disorders. The relevance of serum levels of sC5b-9, Ba, complement factor H and complement factor I in the acute phase of neuromyelitis optica spectrum disorders were analyzed. Ba and sC5b-9 (r=0.824, p<0.00010), and complement factor H and complement factor I (r=-0.554, p=0.0092) showed positive and negative correlations, respectively.
Subsequently, we measured complement regulatory protein levels in NMOSD and GBS. CFH was within the reference range but significantly lower in patients with NMOSD than in healthy controls or in patients with GBS (Figure 1A). However, CFI, another complement regulatory protein, was higher in patients with NMOSD and in patients with GBS than in healthy controls.
To determine the correlations of these biomarkers with each other in NMOSD, we performed a correlation analysis (Figure 1B). Ba and sC5b-9 levels (r=0.824, p<0.00010), and CFH and CFI levels (r=-0.554, p=0.0092) showed positive and negative correlations, respectively. However, no other correlations were detected in samples obtained from patients in the acute phase of NMOSD.
The above-mentioned complement factors examined in the acute phase were also analyzed for changes in the remission phase. The main laboratory data did not change between the acute and remission phases (Table 2). The sC5b-9 and Ba markers, which were elevated in the acute phase of NMOSD, decreased significantly in the remission phase (Figure 2). Although CFH levels were increased in the remission phase of 12 patients with NMOSD, the average levels of CFH still remained lower than the healthy control level during the remission phase as well as during the acute phase. Moreover, in some patients, CFH levels were markedly reduced in the remission phase. The levels of CFI were decreased in 10 patients in the remission phase of NMOSD, but the average levels of CFI were still higher than those of healthy controls during the remission phase. To rule out these changes of complement markers being due to previously-received treatments, we analyzed complement markers in 10 patients that had not received any treatment at the time of the first-episode of NMOSD and obtained similar results (Supplementary Table 1, Supplementary Figures 1, 2). However, in patients with GBS, sC5b-9, Ba, CFH, and CFI did not change between the acute and remission phases, and Ba and CFI in the remission phase remained higher than those in the healthy controls (Figure 2).
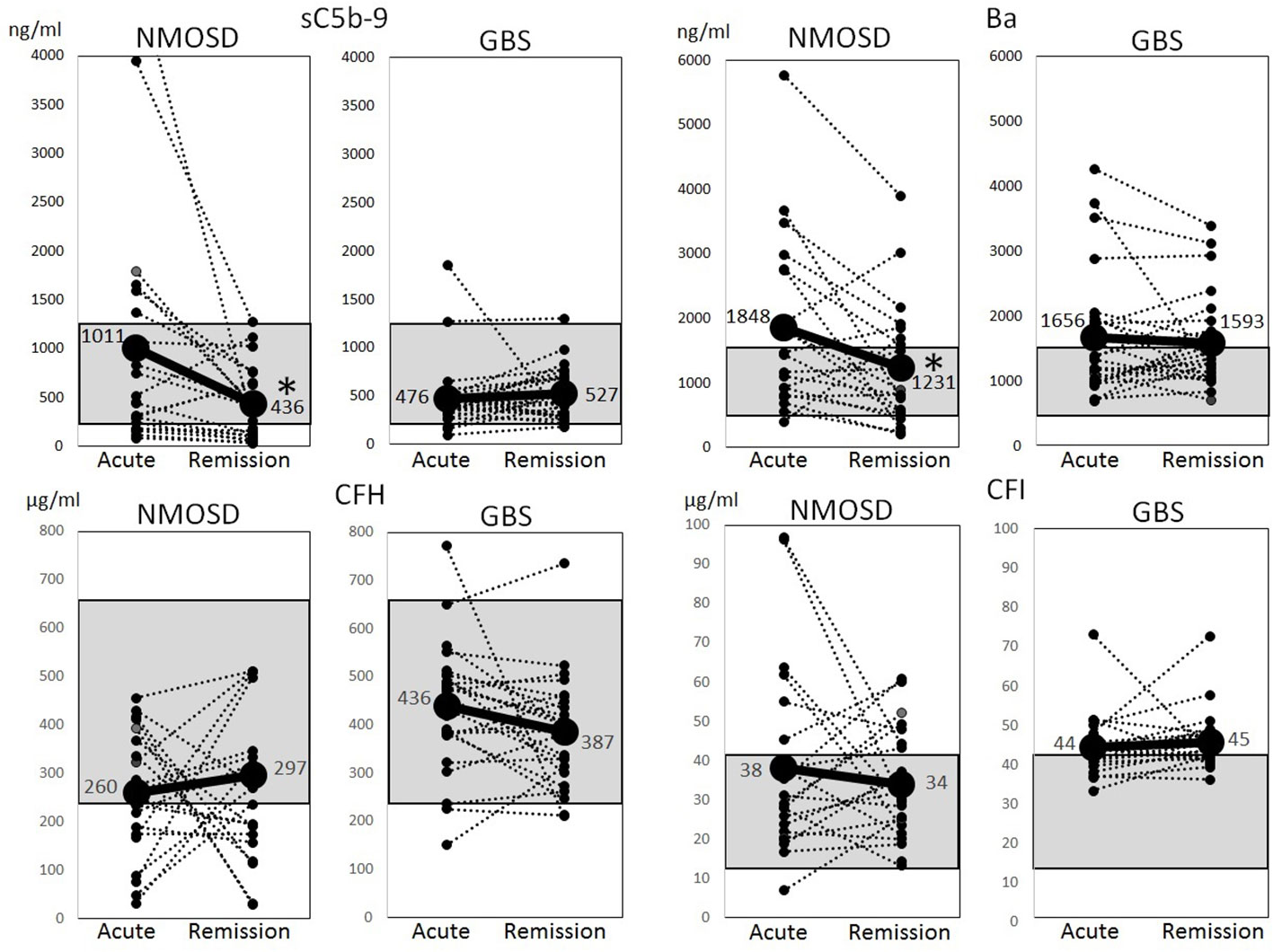
Figure 2 Changes of complement markers during the acute and remission phases of neuromyelitis optica spectrum disorders and Guillain-Barré syndrome. Changes of serum levels of sC5b-9, Ba, complement factor H and complement factor I during the acute and remission phases of neuromyelitis optica spectrum disorders and Guillain-Barré syndrome were analyzed. The dotted lines indicate changes in individual cases, and solid lines indicate changes in average levels. The gray shadow indicates reference ranges in healthy Japanese adults (sC5b-9: 181–1266 ng/ml, Ba: 438–1546 ng/ml, complement factor H: 238–663 µg/ml, complement factor I: 11–42 µg/ml) (15). *p < 0.01, paired t-test.
We detected no correlations between levels of complement markers and most of the clinical manifestations, disease severity, or cerebrospinal fluid test values in the acute phase of NMOSD. There was, however, a moderate positive correlation between levels of CFI and disease duration (r=0.520) (Supplementary Table 2).
In the present study, we measured serum levels of Ba, sC5b-9, CFH, and CFI in the acute and remission phases of NMOSD and GBS. In NMOSD, we identified that sC5b-9 and Ba levels correlated significantly with clinical stage, suggesting that activation of the alternative and terminal complement pathways contributes to exacerbation of NMOSD. The levels of sC5b-9 and Ba may be influenced by the types of treatments and whether they were obtained at the time of the first-episode or after some treatments, but similar results were also obtained in the 10 patients who had not received any treatment at the time of the first-episode. Furthermore, the increased levels of Ba and sC5b-9 were strongly correlated, suggesting that activation of the classical complement pathway by autoantibodies in the periphery led to activation of the alternative and terminal complement pathway. In addition to increased levels of C5a in cerebrospinal fluid that were previously reported as a biomarker of NMOSD (16), the present findings suggest that sC5b-9 and Ba levels in peripheral blood could be useful markers in determining whether NMOSD is in the active stage. NMOSD is known to be caused by injury to astrocytes which express AQP4 (17). Circulating anti-AQP4 antibodies must destroy the brain-blood barrier (BBB) in order to reach astrocytes. IL-6 (18), anti-glucose-regulated protein 78 autoantibodies (19), and polymorphonuclear leukocytes (20) have been reported to be involved in the disruption of BBB. Complement activation in the periphery may also contribute to the destruction. The involvement of peripheral complement activation in the pathogenesis of NMOSD using animal models should be clarified in future studies.
CFH was decreased during both acute and remission phases of NMOSD. There are three possible reasons for these decreased levels. First, AQP4 is expressed not only in astrocytes, but also in muscle and renal tubules, and anti-AQP4 antibodies react with them to activate complement in the periphery. CFH may therefore be consumed and reduced in NMOSD to control activation of the complement system. In the present study, CFH levels in 12 patients were increased in the remission phase. A second possible reason for the decreased levels is that NMOSD could be originally caused in individuals with low CFH levels and activation of alternative and terminal complement pathways initiated by anti-AQP4 autoantibodies might not be adequately suppressed by low CFH levels. Eculizumab, which blocks the C5 cleavage involved in the initiation of the terminal complement pathway, is an effective treatment for almost all NMOSD cases with anti-AQP4 autoantibodies (8). In patients with NMOSD, low CFH levels may be a significant cause of complement activation in the periphery. A third possible reason for the decreased levels could be that CFH production may be suppressed by steroid or immunosuppressive therapies. In some patients, remarkably decreased levels of CFH were observed in the remission phase.
In NMOSD, modest increase of CFI levels was also observed, and the levels of CFH and CFI had negative correlation.We detected a moderate positive correlation between CFI levels and disease duration, so CFI may increase by inflammation induced in the acute phase to block activation of the complement system in the periphery.
In GBS, there were no significant differences in sC5b-9, Ba, CFH, or CFI levels between the acute and remission phases. In addition, in the acute phase of GBS, Ba was increased but sC5b-9 was unchanged, suggesting that activation of classical complement pathway by autoantibodies led to activation of the alternative pathway in the periphery, but did not progress to the terminal complement pathway. The levels of CFH and CFI remained high in both acute and remission phases of GBS, suggesting that their regulatory functions would be maintained. Therapies targeting complement pathways other than the terminal complement pathway could therefore be effective in cases of GBS without elevated sC5b-9 levels. Alternatively, anti-C5 antibodies could be effective in cases of GBS with elevated sC5b-9.
Comprehensive measurement of complement biomarkers such as Ba, sC5b-9, and CFH could contribute to delineating the pathogenesis and pathological status of NMOSD. The complement biomarkers in cerebrospinal fluid should also be measured to clarify the contribution to the pathogenesis of NMSD. We will also analyze the complement biomarkers in patients treated with eculizumab in a future study to determine whether these could be predictive biomarkers for response to eculizumab treatment. The present study was performed retrospectively using previously collected serum samples, so the reference ranges were too broad to determine valid cut-off values of Ba and sC5b-9 for prediction of acute and remission phases. However, this study suggests that the results should be validated in a future prospective study using plasma treated with ethylenediaminetetraacetic acid-disodium salt.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by The study was approved by Research Ethics Committee of Wakayama Medical University (approval number: G154 and 3278) and Kindai University (approval number: 2021-164). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
KM, NI, and HI contributed to the conception and design of the study. KM, MM, KO, NW, and MK contributed to acquisition and analysis of data. KM, KK and NI contributed to drafting of the text and preparation of the figures. HT contributed to statistical analyses of data. All authors contributed to the article and approved the submitted version.
This work was supported by JSPS KAKENHI Grant Numbers JP17H04108, JP20H03580, JP20K07894, and JP22K07498, and a grant on Priority Areas from Wakayama Medical University Research.
We thank the patients for their participation in the study and Kazuko Watase and Fumiko Saito for their technical assistance. We acknowledge proofreading and editing by Benjamin Phillis at the Clinical Study Support Center at Wakayama Medical University.
KM received speaker honoraria from Alexion Pharmaceuticals, Inc., Chugai Pharmaceutical Co. Ltd., Mitsubishi Tanabe Pharma Corporation, and Teijin Pharma Ltd. KO received research support from Asahikasei Pharma. NI received speaker honoraria from Alexion Pharma Corporation, Novartis Pharmaceutical Corporation, UCB Japan Co. Ltd., Sanofi, Chugai Pharmaceutical Co. Ltd. and Japan Blood Products Organization and research support from Alexion Pharmaceuticals, Inc. HI received from Alexion Pharmaceuticals, Inc. and Teijin Pharma Ltd. KO, NW, and NI are councilors of The Japanese Association for Complement Research. NW is a councilor of the International Complement Society.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1090548/full#supplementary-material
1. Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol (2010) 11(9):785–97. doi: 10.1038/ni.1923
2. Ricklin D, Reis ES, Lambris JD. Complement in disease: a defence system turning offensive. Nat Rev Nephrol (2016) 12(7):383–401. doi: 10.1038/nrneph.2016.70
3. Garred P, Tenner AJ, Mollnes TE. Therapeutic targeting of the complement system: From rare diseases to pandemics. Pharmacol Rev (2021) 73(2):792–827. doi: 10.1124/pharmrev.120.000072
4. Dalakas MC, Alexopoulos H, Spaeth PJ. Complement in neurological disorders and emerging complement-targeted therapeutics. Nat Rev Neurol (2020) 16(11):601–17. doi: 10.1038/s41582-020-0400-0
5. Masi G, O’Connor KC. Novel pathophysiological insights in autoimmune myasthenia gravis. Curr Opin Neurol (2022) 35(5):586–96. doi: 10.1097/WCO.0000000000001088
6. Weinshenker BG, Wingerchuk DM. Neuromyelitis spectrum disorders. Mayo Clin Proc (2017) 92(4):663–79. doi: 10.1016/j.mayocp.2016.12.014
7. Pittock SJ, Zekeridou A, Weinshenker BG. Hope for patients with neuromyelitis optica spectrum disorders - from mechanisms to trials. Nat Rev Neurol (2021) 17(12):759–73. doi: 10.1038/s41582-021-00568-8
8. Pittock SJ, Berthele A, Fujihara K, Kim HJ, Levy M, Palace J, et al. Eculizumab in aquaporin-4-Positive neuromyelitis optica spectrum disorder. N Engl J Med (2019) 381(7):614–25. doi: 10.1056/NEJMoa1900866
9. Schneider-Gold C, Gilhus NE. Advances and challenges in the treatment of myasthenia gravis. Ther Adv Neurol Disord (2021) 14:17562864211065406. doi: 10.1177/17562864211065406
10. Kusunoki S, Willison HJ, Jacobs BC. Antiglycolipid antibodies in Guillain-barre and Fisher syndromes: discovery, current status and future perspective. J Neurol Neurosurg Psychiatry (2021) 92(3):311–8. doi: 10.1136/jnnp-2020-325053
11. Misawa S, Kuwabara S, Sato Y, Yamaguchi N, Nagashima K, Katayama K, et al. Safety and efficacy of eculizumab in Guillain-barre syndrome: a multicentre, double-blind, randomised phase 2 trial. Lancet Neurol (2018) 17(6):519–29. doi: 10.1016/S1474-4422(18)30114-5
12. Okamura H, Nakamae H, Shindo T, Ohtani K, Hidaka Y, Ohtsuka Y, et al. Early elevation of complement factor ba is a predictive biomarker for transplant-associated thrombotic microangiopathy. Front Immunol (2021) 12:695037. doi: 10.3389/fimmu.2021.695037
13. Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology (2015) 85(2):177–89. doi: 10.1212/WNL.0000000000001729
14. Asbury AK, Cornblath DR. Assessment of current diagnostic criteria for Guillain-barre syndrome. Ann Neurol (1990) 27 Suppl:S21–4. doi: 10.1002/ana.410270707
15. Ohtani K, Inoue N, Hidaka Y, Wakamiya N. The analysis of complement factors and activation markers in normal Japanese individuals. J Jpn Assoc Comple Res (2019) 56(2):13–22.
16. Piatek P, Domowicz M, Lewkowicz N, Przygodzka P, Matysiak M, Dzitko K, et al. C5a-preactivated neutrophils are critical for autoimmune-induced astrocyte dysregulation in neuromyelitis optica spectrum disorder. Front Immunol (2018) 9:1694. doi: 10.3389/fimmu.2018.01694
17. Papadopoulos MC, Verkman AS. Aquaporin 4 and neuromyelitis optica. Lancet Neurol (2012) 11(6):535–44. doi: 10.1016/S1474-4422(12)70133-3
18. Takeshita Y, Obermeier B, Cotleur AC, Spampinato SF, Shimizu F, Yamamoto E, et al. Effects of neuromyelitis optica-IgG at the blood-brain barrier. vitro Neurol Neuroimmunol Neuroinflamm (2017) 4(1):e311. doi: 10.1212/NXI.0000000000000311
19. Shimizu F, Schaller KL, Owens GP, Cotleur AC, Kellner D, Takeshita Y, et al. Glucose-regulated protein 78 autoantibody associates with blood-brain barrier disruption in neuromyelitis optica. Sci Transl Med (2017) 9(397):eaai9111. doi: 10.1126/scitranslmed.aai9111
Keywords: neuromyelitis optica spectrum disorders, Guillain-Barré syndrome, complement, alternative pathway, sC5b-9, CFH, Ba
Citation: Miyamoto K, Minamino M, Kuwahara M, Tsujimoto H, Ohtani K, Wakamiya N, Katayama K-i, Inoue N and Ito H (2023) Complement biomarkers reflect the pathological status of neuromyelitis optica spectrum disorders. Front. Immunol. 14:1090548. doi: 10.3389/fimmu.2023.1090548
Received: 05 November 2022; Accepted: 20 February 2023;
Published: 03 March 2023.
Edited by:
Shougang Guo, Shandong Provincial Hospital, ChinaReviewed by:
Wioleta Zelek, Cardiff University, United KingdomCopyright © 2023 Miyamoto, Minamino, Kuwahara, Tsujimoto, Ohtani, Wakamiya, Katayama, Inoue and Ito. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Norimitsu Inoue, aW5vdWUtbm9Ad2FrYXlhbWEtbWVkLmFjLmpw
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.