
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 17 January 2023
Sec. Cancer Immunity and Immunotherapy
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1084843
This article is part of the Research TopicAdenosine Pathways in Cancer Immunity and ImmunotherapyView all 14 articles
 Yunchao Wang†
Yunchao Wang† Nan Zhang†
Nan Zhang† Jingnan Xue†
Jingnan Xue† Chengpei Zhu†
Chengpei Zhu† Yanyu Wang
Yanyu Wang Longhao Zhang
Longhao Zhang Xu Yang
Xu Yang Hao Wang
Hao Wang Shanshan Wang
Shanshan Wang Jiashuo Chao
Jiashuo Chao Xiaobo Yang*
Xiaobo Yang* Haitao Zhao*
Haitao Zhao*Background: Toripalimab shows antitumor efficacy in cholangiocarcinoma. Radiotherapy (RT) may enhance systemic responses of PD-1 inhibitors and lenvatinib. This study was designed to assess the safety and feasibility of toripalimab plus lenvatinib with or without RT in advanced BTC.
Methods: This study involved 88 patients with advanced BTC receiving toripalimab plus lenvatinib with or without RT from the clinical trials (NCT03892577). Propensity score matching (PSM) (1:1) analysis was used to balance potential bias. The overall survival (OS), progression-free survival (PFS), objective response rate (ORR), and adverse events (AEs) were evaluated.
Results: After PSM, the final analysis included 40 patients: 20 receiving toripalimab plus lenvatinib without RT (NRT); 20 receiving toripalimab plus lenvatinib with RT. The AEs were more frequent in the RT group than in the NRT group without treatment-associated mortality. The addition of RT did not cause specific AEs. The median PFS was significantly longer with RT (10.8 versus 4.6 months, p<0.001). The median OS was 13.7 months with RT versus 9.2 months in the NRT group (p=0.008). The ORR was 35% (95% CI: 12.1-57.9) in the RT group versus 20% (95% CI: 0.8-39.2) in the NRT group.
Conclusions: The addition of RT may enhance the efficacy of toripalimab plus lenvatinib. Toripalimab plus lenvatinib with RT have a good safety profile without an increase in specific toxicities in advanced BTC patients.
Biliary tract carcinoma (BTC), including intrahepatic cholangiocarcinoma (ICC), extrahepatic cholangiocarcinoma (ECC), and gallbladder cancer (GBC), are aggressive malignancies (1). Most patients are diagnosed at an advanced stage with a poor prognosis (2, 3). Chemotherapy has been the mainstay of treatment for patients with advanced BTC (2, 4). However, conventional chemotherapy is often accompanied by side effects and the limited survival benefit, necessitating an evaluation of alternative drug combinations (5).
PD-1/PD-L1 inhibitors have exhibited encouraging therapeutic effects. However, the response rates of either PD-1/PD-L1 inhibitors alone or PD-1/PD-L1 inhibitors with targeted therapies remain less than ideal in BTC (6, 7). Continuous exploration has been made to improve the response of PD-1/PD-L1 inhibitors, including PD-1/PD-L1 inhibitors combined with chemotherapy (8) or locoregional treatment approaches (9–11). The phase III TOPAZ-1 study showed that the combination of durvalumab plus gemcitabine and cisplatin significantly improved the survival of patients with advanced BTC (12). Recently, durvalumab plus gemcitabine and cisplatin proved as first-line treatment by FDA and NCCN guidelines. New data have emerged that radiotherapy work in synergy with immunotherapies to increase patient response (13, 14). A study showed that adding RT into the combination of PD-1/PD-L1 inhibitors and targeted therapy was feasible and could improve treatment outcomes (15). However, combination of immunotherapy plus radiotherapy may lead to more AEs. Data on immunomodulatory effects of RT in BTC remains limited.
Toripalimab, a humanized programmed death-1 (PD-1) antibody, has shown a manageable safety profile and has promising antitumor activity in patients with advanced gastric cancer and metastatic mucosal melanoma (16, 17). Toripalimab shows antitumor efficacy in cholangiocarcinoma (18).
Considering the different anti-malignancy mechanisms of lenvatinib, toripalimab, and RT, combining these three modalities may show a potential synergic effect and promising preliminary efficacy results in advanced BTC. In this study, we assessed the safety and feasibility of RT plus toripalimab and lenvatinib in patients with advanced BTC.
This retrospective study assessed the safety and feasibility of non-first-line toripalimab plus lenvatinib with RT in advanced BTC. Advanced BTC was defined as initially diagnosed unresectable BTC (histologically confirmed ECC, ICC, or GBC by biopsy or surgical specimen). Other eligibility criteria included a good physical status with an Eastern Co-operative Oncology Group (ECOG) performance status score of 0–1, Child-Pugh A or B liver function status, at least one measurable or evaluable tumor lesion according to the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1). The study protocol was compliant with the Declaration of Helsinki and was approved by the Institutional Review Board and Ethics Committee at Peking Union Medical College Hospital.
A total of 113 patients were initially enrolled. Twenty-five patients have excluded: 2 patients received other target therapy; 14 patients received other PD-1/L1 inhibitors; 9 patients had no measurable lesion. Finally, 37 patients who received toripalimab plus lenvatinib with RT and 51 patients who received toripalimab plus lenvatinib without RT remained. Consecutive PSM was conducted by 1:1 matching with a caliper of 0.05 to balance potential bias. Finally, 40 patients with advanced BTC who received toripalimab plus lenvatinib with RT (RT group) or without RT (NRT group) were included for statistical analysis as a matched cohort (Figure 1).
In the NRT group, lenvatinib was administered at a dosage of 12 mg (for patients with a body weight≥60 kg) or 8 mg (for patients with a body weight <60 kg) orally once a day. The PD-1 dose included a fixed dosage of 200 mg (240 mg for toripalimab) every three weeks or 3 mg/kg every three weeks.
In the RT group, patients received intensity-modulated radiation therapy (IMRT) plus lenvatinib and toripalimab. Lenvatinib plus toripalimab was not discontinued before or after each RT session. The radiation dose was prescribed to the isocenter or 95% planning target volume as 24.0–60.0 Gy in 6-25 fractions, a single dose between 1.8 and 6.0 Gy for tumor sites at the physician’s discretion, no more than five times a week. RT was given during PD-1 inhibitors no later than six weeks (19).
The overall response was assessed using enhanced computed tomography (CT) or magnetic resonance imaging (MRI) according to RECIST 1.1 after the patient’s treatment. Professional radiologists evaluated the imaging examinations.
The therapeutic efficacy assessment included the objective response rate (ORR) [the percentage of patients with a confirmed complete/partial response (CR/PR)], progression-free survival (PFS) (the time from receiving toripalimab to disease progression at any site or death), the overall survival (OS) (the time from receiving toripalimab to the date of death), the disease control rate (DCR) (the proportion of patients who achieved an objective response or SD), and the safety. The adverse events (AEs) were collected and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0 (CTCAE 4.0).
The Data cut-off was June 1, 2022. We performed propensity score matching (PSM) in a 1:1 fashion to further reduce selection bias. We used a caliper (i.e., the maximum distance that two cases can be apart from each other based on their estimated propensity scores) of 0.05 to prevent matches with very dissimilar estimated propensity scores. Variables used for PSM include age, sex, ECOG, subtype, and tumor stage. The Kaplan–Meier and bilateral log-rank tests were used to generate PFS and OS curves. The two treatment groups’ baseline characteristics, efficacy, and AEs were compared using the chi-square test or Fisher’s exact test. The hazard ratios of each clinicopathological feature for the OS were estimated by Cox proportional hazard modeling. All statistical analyses were undertaken using SPSS 22 (vision 22.0, SPSS, Inc., Chicago, IL) and R (version 4.0.3).
From March 19, 2019, to June 1, 2022, 40 patients with advanced BTC were included in this study: 20 in the NRT group and 20 in the RT group. The median duration of follow-up was 21.3 months. The demographics and baseline characteristics of the two groups are summarized in Table 1.
The two groups were well-balanced regarding demographics and characteristics. The median age of the patients was 61.5 years. Cholangiocarcinoma, including ICC and ECC, is the primary tumor type (75%). Most patients had a better ECOG performance status. The two groups did not differ significantly concerning differentiated histology, previous antitumor therapy, TNM stage, tumor diameter, or sites of metastases. The pathological differentiation types of 18 patients were unknown due to a lack of further pathological tissue analyses. The liver and lymph nodes were the common metastatic sites, and other metastatic lesions included uterine metastasis (one patient) and adrenal metastases (one patient).
The radiotherapy sites were mainly distributed in the liver (70%) and soft tissue or lymph nodes (60%). The median radiation dose delivered was 45 Gy (range 24 to 60 Gy) in 6–25 fractions with IMRT. 13 (65%) patients received one course, and 7 (35%) two courses.
At the time of analysis, 17 patients had disease progression, and 17 patients had died in the NRT group, while 12 patients had disease progression and 10 patients had died in the RT group. The median PFS was 10.8 months (95% CI: 6.2-15.4) in the RT group versus 4.6 months (95% CI: 3.3-5.8) in the NRT group (HR 0.21 [95% CI: 0.09-0.49], p<0.01, Figure 2A). Likewise, the median OS was significantly longer in the RT group (13.7 months, 95% CI: 7.8-19.6) than that in the NRT group (9.2 months, 95% CI: 6.5-11.8) (HR 0.36 [95% CI: 0.16-0.80]; p=0.008, Figure 2B).
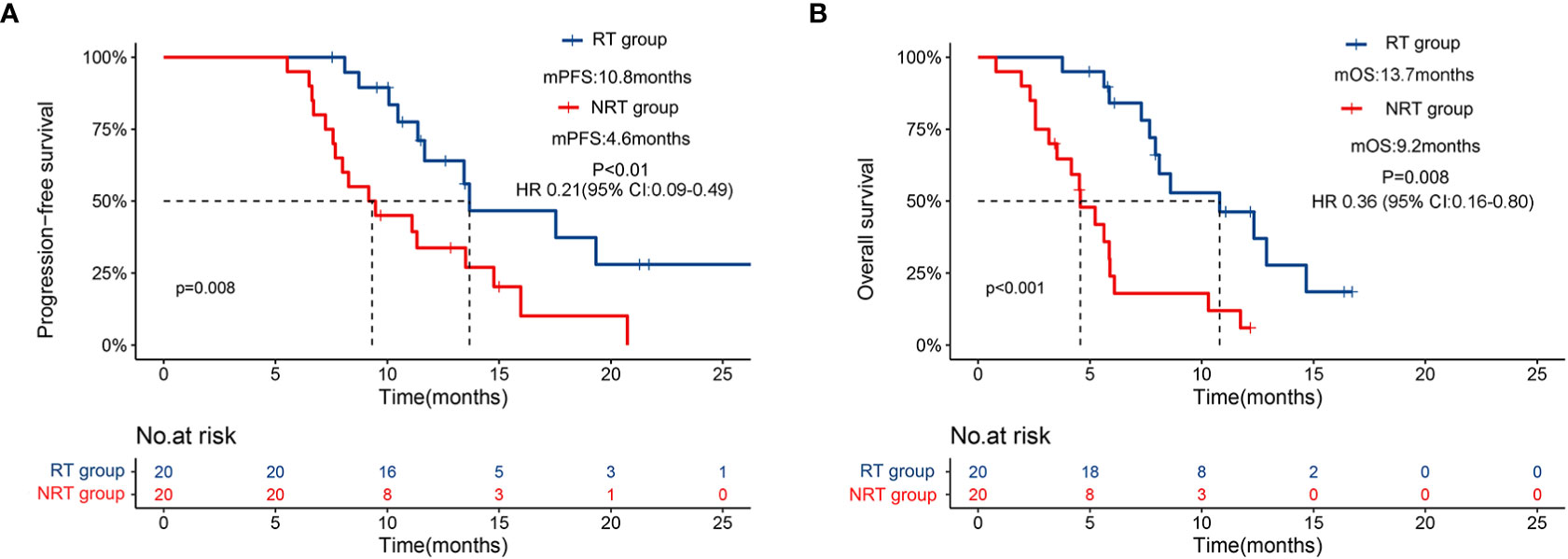
Figure 2 Kaplan–Meier curves for progression-free survival (A) and overall survival (B) for patients receiving PD-1 inhibitors plus lenvatinib with or without RT.
No patient achieved a complete response (CR) in the two groups. In the RT group, 4 patients achieved a partial response (PR), 11 patients had SD, and 5 patients exhibited progressive disease (PD) (Table 2). The ORR was 20% (4/20; 95% CI: 0.8-39.2), and the DCR was 75% (15/20; 95% CI: 54.2-95.8) in the NRT group. However, in the RT group, 7 patients achieved a partial response (PR), 10 patients had SD, and 3 patients exhibited progressive disease (PD), the ORR was 35% (7/20; 95% CI: 12.1-57.9), and the DCR was 85% (17/20; 95% CI: 67.9-102.1). The survival benefits in the RT group were observed. Among the two cohorts, the RT group showed a higher DCR than the NRT group but did not find a significant difference.
Univariate and multivariate analyses were performed to identify independent prognostic factors associated with OS. Potential predictors include age, sex, ECOG, method of treatment, and metastasis. Univariate and multivariate analyses found ECOG and treatment methods were associated with OS (Figure 3). Figure 4A shows a waterfall plot of the target lesions from baseline in the RT group: 13 of the 20 (65%) patients exhibited a decrease. In comparison, 7 of the 20 (35%) patients showed a decrease in the NRT group (Figure 4B). Three patients exhibited a decrease in tumor size from baseline after analysis of nine measurable non-target lesions in the RT group (Figure 4C).
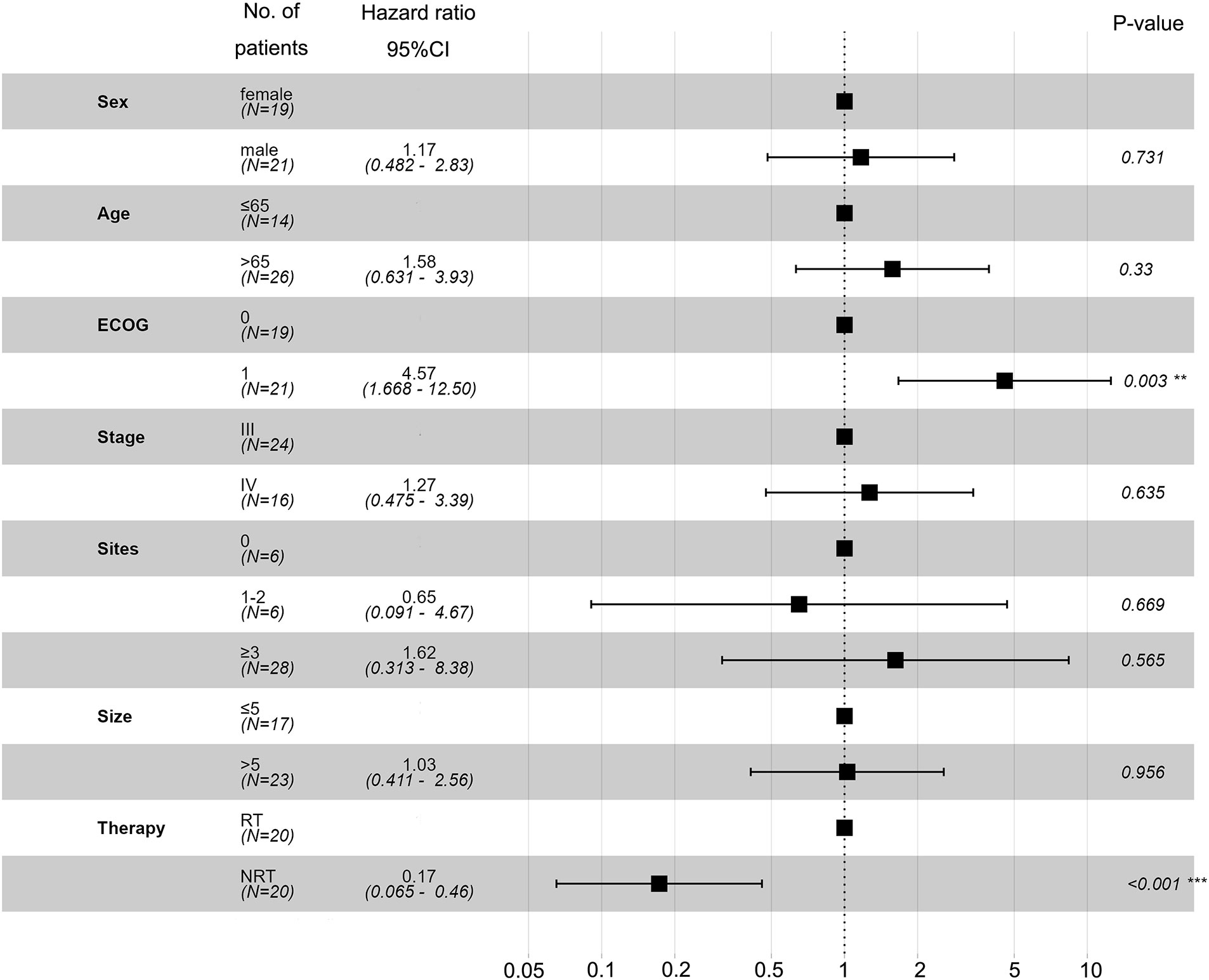
Figure 3 Univariate and multivariate analyses based on the Cox regression model were performed to identify independent prognostic factors associated with OS.
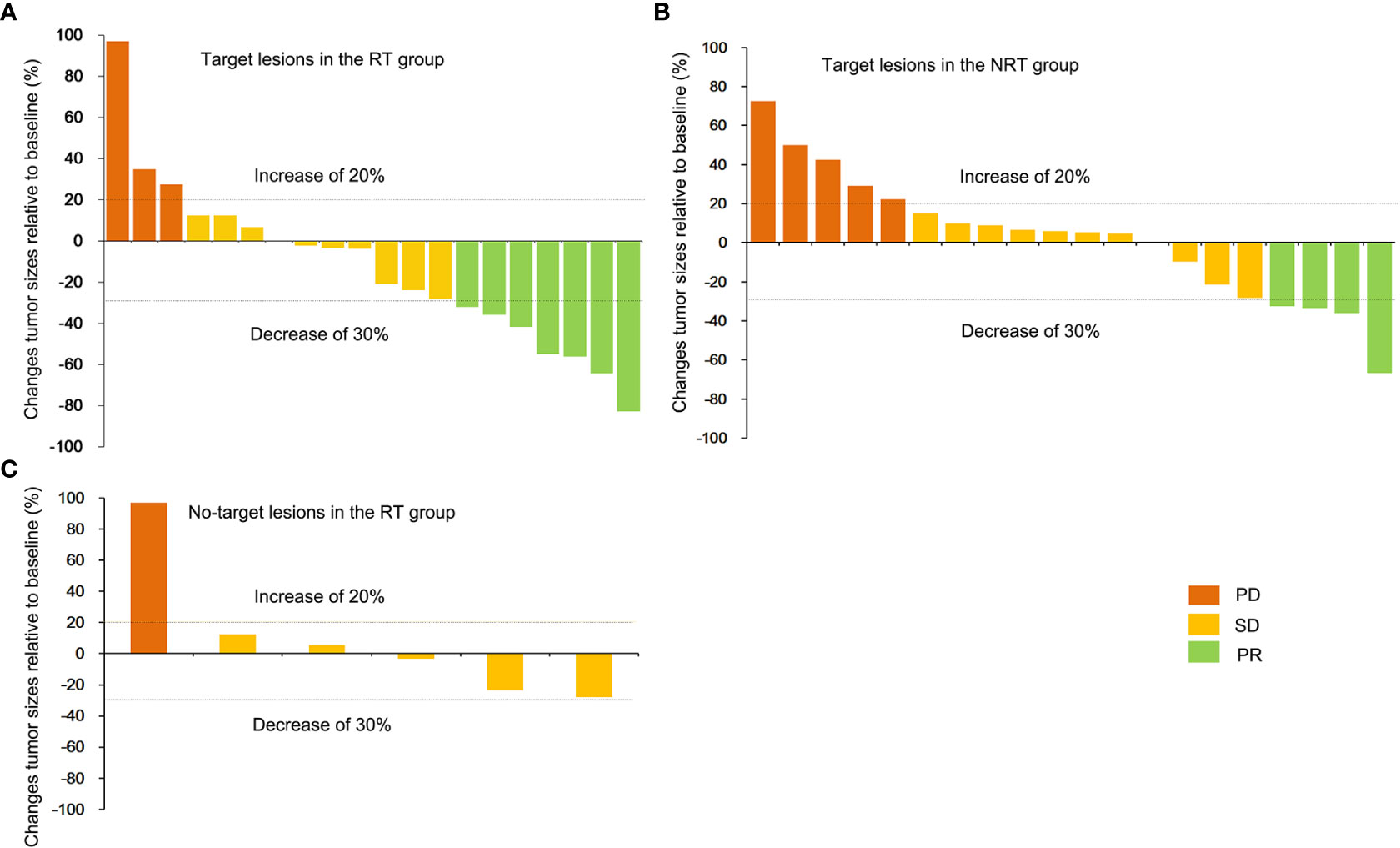
Figure 4 Best percentage change in the RT group. The best percentage change in the sum of the diameters of the target lesions from baseline (A) in the RT group and non-target lesions from baseline for nine patients in the RT group (C). (B) shows the maximum percentage change in the sum of the diameters of the target lesions from baseline in the NRT group.
In the RT group, one patient achieved a PR, who had been PD before radiotherapy; two patients had achieved PR, who had been SD before radiotherapy; five patients achieved SD, who was PD before radiotherapy.
All patients experienced ≥1 adverse event (AE), and no treatment-related deaths occurred in this study (Table 3). The adverse events were more frequent in the RT group than in the NRT group, especially hypothyroidism [8 (5.6%) versus 1, p = 0.008]. The most common AEs (any grade) in the RT group were fatigue (70%), ALT or AST elevation (60%), and bilirubin elevation (50%), while fatigue (65%), AST or ALT increased (50%) in NRT group. The RT group had a higher incidence of grade 3–4 AEs than the NRT group. The most frequent grade 3 AEs were rash, with an incidence of 20%. One patient experienced grade 4 severe AEs (SAEs) (gastrointestinal hemorrhage). All the recorded any-grade AEs were reversible.
This is the first reported study that assessed the efficacy and safety of toripalimab plus lenvatinib with or without RT in advanced BTC patients and represents a potentially shifting approach to improve immunotherapy response. The combination of PD-1 inhibitor plus lenvatinib with RT was promising. Patients who received toripalimab plus lenvatinib with RT have significantly longer OS (13.7 versus 9.2 months, p=0.008) and PFS (10.8 versus 4.6 months, p<0.01) than patients who received toripalimab plus lenvatinib without RT. The risk of death was reduced by 64% in the RT group compared with the NRT group. Importantly, we found that toripalimab plus lenvatinib with RT were well tolerated.
In this study, patients accepting toripalimab plus lenvatinib with RT achieved approximately 35% ORR and 85% DCR, which were higher than the toripalimab plus lenvatinib regimen in our study and previous studies (7, 20, 21). The response rates of toripalimab with targeted therapies in BTC are not satisfactory. Previous studies showed that lenvatinib plus pembrolizumab has an ORR of 10% to 25% in advanced BTC (7, 20). Recently, a retrospective study of 74 patients who received PD-1 inhibitor plus lenvatinib revealed that the ORR was 20.27% (95% CI: 10.89%–29.65%), and the DCR was 71.62% (21). A pool analysis showed that pembrolizumab plus RT significantly increased responses and outcomes in patients with metastatic non-small-cell lung cancer (22). A growing body of evidence suggests that the addition of RT to PD-1 inhibitor may improve the efficacy of immune checkpoint inhibitors (ICIs) (23, 24), where RT is administered before ICIs or concurrently with ICIs (25).
The addition of RT represented an encouraging response: one patient converted from PD to PR, two patients achieved PR from SD, and five from PD to SD. In addition, we observed that both target and non-target lesions in three patients were reduced, indicating that RT may have a synergistic effect with PD-1 inhibitors and lenvatinib. Evidence has revealed that radiation can exert potent immunomodulatory effects (26). Previous studies have demonstrated that radiation could induce immunogenic cell death (ICD), release tumor antigens and promote T-cell-mediated immune response against antigens derived from dying cells (23, 27–29).
The optimal radiotherapy dose, fractionation, timing, and target selection currently lack a consensus (30, 31). To choose the optimal radiation dose and fractionated dose, on the one hand, it is necessary to ensure that antitumor immunity is fully activated. On the other hand, the occurrence of adverse reactions should be minimized. Likewise, there is no clear framework for whether RT should be performed before or after PD-1/PD-L1 inhibitors (32). The sequence of radiotherapy and immunotherapy still needs further study and comparison.
Although the incorporation of RT into immunotherapy caused more AEs, they were generally manageable. The adverse events in the RT group were consistent with previous reports: fatigue was the most common all-grade adverse event (33). One patient experienced grade 4 severe AEs (SAEs) (gastrointestinal hemorrhage). Gastrointestinal hemorrhage was controlled after drug discontinuation and active management. No death-related adverse effects occurred. The combination of RT plus non-first-line toripalimab and lenvatinib could have a good safety profile.
We acknowledge that this study has some limitations. First, as a single-center retrospective study, the interpretation of the efficacy and safety of the combination of RT plus toripalimab and lenvatinib must be very cautious. Prospective studies are needed to validate the findings further. Second, some selection biases, including recall, observation, and selection biases, arose from the limited sample size and a retrospective study. A heterogeneous population of patients cannot be ruled out. Third, this study lacks evidence of synergy between radiation and immunotherapy, such as immune cell infiltration and transcriptional changes in tumor cells before and after radiotherapy. Although the study has certain limitations, these “real” data are still helpful for prospective follow-up studies.
Toripalimab plus lenvatinib with RT are safe and well tolerated in advanced BTC. Toripalimab plus lenvatinib with RT may prolong the survival of patients with previously treated advanced BTC. The addition of RT may enhance the efficacy of toripalimab and lenvatinib. Further research on prospective larger cohorts is needed.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the institutional review board and ethics committee at Peking Union Medical College Hospital. The patients/participants provided their written informed consent to participate in this study.
YCW, NZ, and JX collected the data and wrote the manuscript. XBY and HZ designed and examined the study. YYW, LZ, and JC helped to collect the literature and participated in discussions. CZ, JX, and YYW performed the statistical analyses. All authors contributed to the article and approved the submitted version.
This work was supported by CAMS Innovation Fund for Medical Sciences (CIFMS) (2021-1-I2M-003 and 2021-I2M-1-061), CSCO-hengrui Cancer Research Fund (Y-HR2019-0239), CSCO-MSD Cancer Research Fund (Y-MSDZD2021-0213) and National Ten-thousand Talent Program.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1084843/full#supplementary-material
PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; BTCs, biliary tract cancers; ECC, extrahepatic cholangiocarcinoma; ICC, intrahepatic cholangiocarcinoma; GBC, gallbladder cancer; Lenvatinib, tyrosine kinase inhibitors; OS, overall survival; PFS, progression-free survival; ORR, objective response rate; DCR, disease control rate; SD, stable disease; PD, progressive disease; CR, complete response; PR, partial response; HR, hazard rate; RECIST, response evaluation criteria in solid tumors; AEs, adverse events; CTCAE, Common Terminology Criteria Coastocellular Group; RT, radiotherapy.
1. Brindley PJ, Bachini M, Ilyas SI, Khan SA, Loukas A, Sirica AE, et al. Cholangiocarcinoma. Nat Rev Dis Primers (2021) 7(1):65. doi: 10.1038/s41572-021-00300-2
2. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med (2010) 362(14):1273–81. doi: 10.1056/NEJMoa0908721
3. Lamarca A, Palmer DH, Wasan HS, Ross PJ, Ma YT, Arora A, et al. Second-line folfox chemotherapy versus active symptom control for advanced biliary tract cancer (Abc-06): A phase 3, open-label, randomised, controlled trial. Lancet Oncol (2021) 22(5):690–701. doi: 10.1016/s1470-2045(21)00027-9
4. Kam AE, Masood A, Shroff RT. Current and emerging therapies for advanced biliary tract cancers. Lancet Gastroenterol Hepatol (2021) 6(11):956–69. doi: 10.1016/s2468-1253(21)00171-0
5. Wang Y, Yang X, Wang D, Yang X, Wang Y, Long J, et al. Lenvatinib beyond first-line therapy in patients with advanced biliary tract carcinoma. Front Oncol (2022) 12:785535. doi: 10.3389/fonc.2022.785535
6. Piha-Paul SA, Oh DY, Ueno M, Malka D, Chung HC, Nagrial A, et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: Results from the KEYNOTE-158 and KEYNOTE-028 studies. Int J Cancer (2020) 147(8):2190–8. doi: 10.1002/ijc.33013
7. Villanueva L, Lwin Z, Chung HCC, Gomez-Roca CA, Graham DM. Lenvatinib plus pembrolizumab for patients with previously treated biliary tract cancers in the multicohort phase 2 LEAP-005 study. J Clin Oncol (2021) 39(15_suppl):4080–0. doi: 10.1200/JCO.2021.39.15_suppl.4080
8. Jian Z, Fan J, Shi G-M, Huang X-Y, Wu D, Yang G-H, et al. Gemox chemotherapy in combination with anti-PD1 antibody toripalimab and lenvatinib as first-line treatment for advanced intrahepatic cholangiocarcinoma: A phase 2 clinical trial. J Clin Oncol (2021) 39(15_suppl):4094–4. doi: 10.1200/JCO.2021.39.15_suppl.4094
9. Yang X, Xu H, Zuo B, Yang X, Bian J, Long J, et al. Downstaging and resection of hepatocellular carcinoma in patients with extrahepatic metastases after stereotactic therapy. Hepatobil Surg Nutr (2021) 10(4):434–42. doi: 10.21037/hbsn-21-188
10. Franzese C, Bonu ML, Comito T, Clerici E, Loi M, Navarria P, et al. Stereotactic body radiotherapy in the management of oligometastatic and recurrent biliary tract cancer: single-institution analysis of outcome and toxicity. J Cancer Res Clin Oncol (2020) 146(9):2289–97. doi: 10.1007/s00432-020-03285-9
11. Taggar AS, Mann P, Folkert MR, Aliakbari S, Myrehaug SD, Dawson LA. A systematic review of intraluminal high dose rate brachytherapy in the management of malignant biliary tract obstruction and cholangiocarcinoma. Radiother Oncol (2021) 165:60–74. doi: 10.1016/j.radonc.2021.10.011
12. Oh D, He A, Qin S, Chen L, Okusaka T, Vogel A, et al. A phase 3 randomized, double-blind, placebo-controlled study of durvalumab in combination with gemcitabine plus cisplatin (Gemcis) in patients (Pts) with advanced biliary tract cancer (Btc): Topaz-1. J Clin Oncol (2022) 40(4_suppl):378–8. doi: 10.1200/JCO.2022.40.4_suppl.378
13. Chen D, Barsoumian HB, Fischer G, Yang L, Verma V, Younes AI, et al. Combination treatment with radiotherapy and a novel oxidative phosphorylation inhibitor overcomes PD-1 resistance and enhances antitumor immunity. J Immunother Cancer (2020) 8(1):e000289. doi: 10.1136/jitc-2019-000289
14. Geng Y, Zhang Q, Feng S, Li C, Wang L, Zhao X, et al. Safety and efficacy of PD-1/PD-L1 inhibitors combined with radiotherapy in patients with non-small-cell lung cancer: a systematic review and meta-analysis. Cancer Med (2021) 10(4):1222–39. doi: 10.1002/cam4.3718
15. Zhong L, Wu D, Peng W, Sheng H, Xiao Y, Zhang X, et al. Safety of PD-1/PD-L1 inhibitors combined with palliative radiotherapy and anti-angiogenic therapy in advanced hepatocellular carcinoma. Front Oncol (2021) 11:686621. doi: 10.3389/fonc.2021.686621
16. Sheng X, Yan X, Chi Z, Si L, Cui C, Tang B, et al. Axitinib in combination with toripalimab, a humanized IgG4 mAb against programmed death-1 (PD-1) in patients with metastatic mucosal melanoma: A non-randomized, open-label, dose-finding, and cohort-expansion phase 1b trial. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology (2019) 37(32):2987–99. doi: 10.1200/jco.19.00210
17. Wang F, Wei XL, Wang FH, Xu N, Shen L, Dai GH, et al. Safety, efficacy and tumor mutational burden as a biomarker of overall survival benefit in chemo-refractory gastric cancer treated with toripalimab, a PD-1 antibody in phase Ib/II clinical trial NCT02915432. Ann Oncol (2019) 30(9):1479–86. doi: 10.1093/annonc/mdz197
18. Jian Z, Fan J, Shi GM, Huang XY, Wu D, Liang F, et al. Lenvatinib plus toripalimab as first-line treatment for advanced intrahepatic cholangiocarcinoma: A single-arm, phase 2 trial. (2021) 39(15_suppl):4099–9. doi: 10.1200/JCO.2021.39.15_suppl.4099
19. Liniker E, Menzies AM, Kong BY, Cooper A, Ramanujam S, Lo S, et al. Activity and safety of radiotherapy with anti-PD-1 drug therapy in patients with metastatic melanoma. Oncoimmunology (2016) 5(9):e1214788. doi: 10.1080/2162402x.2016.1214788
20. Lin J, Yang X, Long J, Zhao S, Mao J, Wang D, et al. Pembrolizumab combined with lenvatinib as non-first-line therapy in patients with refractory biliary tract carcinoma. Hepatobil Surg Nutr (2020) 9(4):414–24. doi: 10.21037/hbsn-20-338
21. Shi C, Li Y, Yang C, Qiao L, Tang L, Zheng Y, et al. Lenvatinib plus programmed cell death protein-1 inhibitor beyond first-line systemic therapy in refractory advanced biliary tract cancer: A real-world retrospective study in China. Front Immunol (2022) 13:946861. doi: 10.3389/fimmu.2022.946861
22. Theelen W, Chen D, Verma V, Hobbs BP, Peulen HMU, Aerts J, et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Respir Med (2021) 9(5):467–75. doi: 10.1016/s2213-2600(20)30391-x
23. Mondini M, Levy A, Meziani L, Milliat F, Deutsch E. Radiotherapy-immunotherapy combinations - perspectives and challenges. Mol Oncol (2020) 14(7):1529–37. doi: 10.1002/1878-0261.12658
24. Lan Y, Moustafa M, Knoll M, Xu C, Furkel J, Lazorchak A, et al. Simultaneous targeting of TGF-β/PD-L1 synergizes with radiotherapy by reprogramming the tumor microenvironment to overcome immune evasion. Cancer Cell (2021) 39(10):1388–1403.e1310. doi: 10.1016/j.ccell.2021.08.008
25. Mody K, Starr J, Saul M, Poorman K, Shields AF. Patterns and genomic correlates of PD-L1 expression in patients with biliary tract cancers. J Gastrointest Oncol (2019) 10(6):1099–109. doi: 10.21037/jgo.2019.08.08
26. Yu WD, Sun G, Li J, Xu J, Wang X. Mechanisms and therapeutic potentials of cancer immunotherapy in combination with radiotherapy and/or chemotherapy. Cancer Lett (2019) 452:66–70. doi: 10.1016/j.canlet.2019.02.048
27. Wennerberg E, Vanpouille-Box C, Bornstein S, Yamazaki T, Demaria S, Galluzzi L. Immune recognition of irradiated cancer cells. Immunol Rev (2017) 280(1):220–30. doi: 10.1111/imr.12568
28. Cox MC, Lapenta C, Santini SM. Advances and perspectives of dendritic cell-based active immunotherapies in follicular lymphoma. Cancer Immunol Immunother (2020) 69(6):913–25. doi: 10.1007/s00262-020-02577-w
29. Procureur A, Simonaggio A, Bibault JE, Oudard S, Vano YA. Enhance the immune checkpoint inhibitors efficacy with radiotherapy induced immunogenic cell death: A comprehensive review and latest developments. Cancers (Basel) (2021) 13(4):678. doi: 10.3390/cancers13040678
30. Pitroda SP, Chmura SJ, Weichselbaum RR. Integration of radiotherapy and immunotherapy for treatment of oligometastases. Lancet Oncol (2019) 20(8):e434–42. doi: 10.1016/s1470-2045(19)30157-3
31. Arina A, Gutiontov SI, Weichselbaum RR. Radiotherapy and immunotherapy for cancer: From “Systemic” to “Multisite”. Clin Cancer Res (2020) 26(12):2777–82. doi: 10.1158/1078-0432.Ccr-19-2034
32. Chen Y, Gao M, Huang Z, Yu J, Meng X. SBRT combined with PD-1/PD-L1 inhibitors in NSCLC treatment: a focus on the mechanisms, advances, and future challenges. J Hematol Oncol (2020) 13(1):105. doi: 10.1186/s13045-020-00940-z
Keywords: advanced biliary tract cancer, PD-1 inhibitor, lenvatinib, radiotherapy, synergic effect
Citation: Wang Y, Zhang N, Xue J, Zhu C, Wang Y, Zhang L, Yang X, Wang H, Wang S, Chao J, Yang X and Zhao H (2023) Safety and feasibility of toripalimab plus lenvatinib with or without radiotherapy in advanced BTC. Front. Immunol. 14:1084843. doi: 10.3389/fimmu.2023.1084843
Received: 31 October 2022; Accepted: 02 January 2023;
Published: 17 January 2023.
Edited by:
Junjiang Fu, Southwest Medical University, ChinaReviewed by:
Khalil Saleh, Gustave Roussy Cancer Campus, FranceCopyright © 2023 Wang, Zhang, Xue, Zhu, Wang, Zhang, Yang, Wang, Wang, Chao, Yang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaobo Yang, eWFuZ3hpYW9ibzY3QHB1bWNoLmNu; Haitao Zhao, emhhb2h0QHB1bWNoLmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.