- Key Laboratory of Molecular Biology for Infectious Diseases (Ministry of Education), Institute for Viral Hepatitis, Department of Infectious Diseases, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China
Background: The neutralizing antibodies (NAbs) response after COVID-19 vaccination after liver dysfunction is unclear. In this study, we evaluated the NAbs response after COVID-19 vaccination in hospitalized patients suffering from liver dysfunction.
Methods: In this cross-sectional study with longitudinal follow-up, we enrolled eligible patients with liver dysfunction and healthy volunteers with full-course COVID-19 vaccination. Blood samples were collected for the NAbs testing at the time of admission and after treatment. Multiple regression analysis to assess independent risk factors affecting NAbs response.
Results: A total of 137 patients and 134 healthy controls (HC) were enrolled. Both seropositivity (65.7% vs 80.6%, p<0.01) and titer (3.95 vs 4.94 log2 AU/ml, p<0.001) of NAbs in patients were significantly lower than that in HC. The decrease of antibody titer in patients was significantly faster than that in HC. After adjusting for potential confounding factors, males (odds ratio [OR]: 0.17; 95% confidence interval [CI]: 0.06, 0.46; p<0.001) and severe liver damage (OR: 0.30; 95% CI: 0.12, 0.71; p<0.01) were significantly associated with reduction of the probability of NAbs seropositivity in the multiple regression analysis. Males (β =-1.18; 95% CI: -1.73,-0.64) and chronic liver diseases (β =-1.45; 95% CI: -2.13, -0.76) were significantly associated with lower NAbs titers. In 26 patients with liver failure, both antibody seropositivity (53.8% vs 84.6%, p<0.05) and titer (3.55 vs 4.32 log2 AU/ml, p<0.001) did not decrease but increased after artificial liver plasmapheresis.
Conclusions: NAbs response to COVID-19 inactivated or subunit recombinant vaccines was waning in patients with liver dysfunction. Moreover, patients with male sex, severe liver injury and chronic liver diseases have an increased risk of poor antibody responses.
1. Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, has been a life-threatening global health burden (1, 2). As of 23 October 2022, over 624 million confirmed cases and over 6.5 million deaths have been reported globally. Numerous studies have confirmed that SARS-CoV-2 vaccines can effectively reduce the morbidity and mortality of COVID-19 (3, 4). Vaccination against COVID-19 is currently considered the most effective, safe, and cost-effective way to prevent and control COVID-19 worldwide.
Previous studies have shown that patients with chronic liver disease are highly susceptible to COVID-19 and often have poor prognosis (5, 6). Therefore, some guidelines and expert consensus have been developed, and patients with chronic liver disease are encouraged to receive full-course SARS-CoV-2 vaccination (7, 8). Many previous studies have evaluated the safety and immunogenicity of SARS-CoV-2 vaccination in various chronic liver disease populations, including nonalcoholic fatty liver disease (9), post-liver transplant population (10), chronic hepatitis B (11), cirrhosis (12), and autoimmune liver disease (13, 14). These studies all showed the safety of SARS-CoV-2 vaccine in patients with liver disease, but the immune response to the vaccine was weaker than that in healthy people. The time after vaccination in these studies was too short for long-term observational data. In addition, some patients with chronic liver disease may experience acute liver damage due to various reasons. It is urgent for doctors to evaluate the antibody levels produced by prior COVID-19 immunizations after liver damage-caused by various reasons.
COVID-19 inactivated and receptor-binding domain (RBD) subunit recombinant vaccines are two common types of vaccines in China. However, whether the antibody responses following COVID-19 immunization is affected by liver injury is still unclear. In this study, we evaluated antibody responses in hospitalized patients with various causes of liver dysfunction after immunization with COVID-19 inactivated and RBD-subunit recombinant vaccines.
2. Patients and methods
2.1. Study design and participants
We did a cross-sectional study with longitudinal follow-up at the Second Affiliated Hospital of Chongqing Medical University from October 1, 2021, to January 30, 2022. The hospitalized patients with liver dysfunction were enrolled in the Center for Liver and Infectious diseases. At the same time, an equal number of healthy volunteers (healthy controls) who underwent annual physical examinations at the health management center were enrolled as controls. Inclusion criteria of participants were age 18 years or older, completed full-course of COVID-19 vaccination, no SARS-CoV-2 infection before receipt of the first vaccine dose (determined based on either a negative anti-SARS-CoV-2 immunoglobulin (Ig)-M/IgG test or the absence of a positive polymerase chain reaction (PCR) assay result for SARS-CoV-2, with no history of suspected clinical SARS-CoV-2 infection), and ability to understand and complete questionnaires in Chongqing, China. For healthy controls, no underlying disease history was also required. Participants with HIV co-infection, sepsis, cancers, severe extra-hepatic organ dysfunction, pregnancy during study entry, those who did not complete the full-course of vaccination, and those who provided incomplete vaccination information (including the date of their first vaccine dose and complete vaccination, and vaccine manufacturer [due to different vaccine types by manufacturers]) were excluded. All participants in this study provided about 5-10 microliters (mL) of peripheral blood samples for serological assays.
This study was approved by the Ethics Committee of the Second Affiliated Hospital of Chongqing Medical University. All participants signed an informed consent form.
2.2. Variable collection
We collected demographic characteristics (age and sex), history of SARS-CoV-2 infection (yes or no, results of anti-SARS-CoV-2 IgM/IgG and PCR assay for SARS-CoV-2 nucleic acid, and whether had clinical manifestations of SARS-CoV-2 infection), history of diseases, and vaccination-related information (the date of their first vaccine dose and complete vaccination, and vaccine types) in all participants utilizing a standardized questionnaire. Dates of vaccination and the types of vaccine were further confirmed by viewing the individual’s health code which contains complete vaccination-related information. Clinical parameters including laboratory test results and comorbidities were also collected from the electronic patient files in the hospital.
Full-course COVID-19 vaccination was defined as having had two vaccinations of inactivated vaccines or three vaccinations for the RBD-subunit recombinant vaccine. The interval time was defined as the days between the final dose and the first blood sample collection for serological assays. In the longitudinal analysis, we divided the time post-vaccination into three points, M1-2 (30-60 days), M3-4 (61-120 days), and >M4 (more than 120 days), respectively.
In patients receiving artificial liver therapy for liver failure, antibody testing was performed before and after treatment to assess the effect of artificial liver therapy on antibody levels. The treatment mode of artificial liver was plasma exchange combined with plasma adsorption.
2.3. SARS-CoV-2 antibody testing
Neutralizing antibodies (NAbs) testing was performed using the commercially available Novel Coronavirus Neutralizing Antibody Detection Kit (Chemiluminescence) (Shenzhen Yahuilong Biological Technology Co., LTD, Shenzhen, China) according to the manufacturer’s instructions in use. The cut-off value was determined to be 5.00 AU/mL.
2.4. Statistical analysis
Continuous variables are presented as means and standard deviations (SD) or as medians and interquartile ranges (IQR) for normally and nonnormally distributed data, respectively. Categorical variables are presented as percentages (%). NAbs titers were log2-transformed due to skewed distribution. To compare the distribution of NAbs seropositivity and titers, we used analysis of chi-square tests and Student t-test tests. We used paired t-tests to analyze NAbs titers before and after artificial liver treatment.
We used multiple regression analysis to characterize the association between variables and NAbs response. We adjusted for covariates using the crude model and full adjustment models. The crude model was unadjusted. The full adjustment model was adjusted for age (continuous variable), total bilirubin (continuous variable), cirrhosis (binary variable), ACLF (binary variable), vaccine types (binary variable), and interval time (continuous variable). Data are presented as point estimates and corresponding 95% confidence intervals (CI) of the effect size estimates.
All statistical analysis was performed using SPSS (version 24.0) and R 3.0 (http://www.R-project.org, the R Foundation). All statistical tests were two-sided, and p<0.05 was considered statistically significant.
3. Results
3.1. Participant characteristics
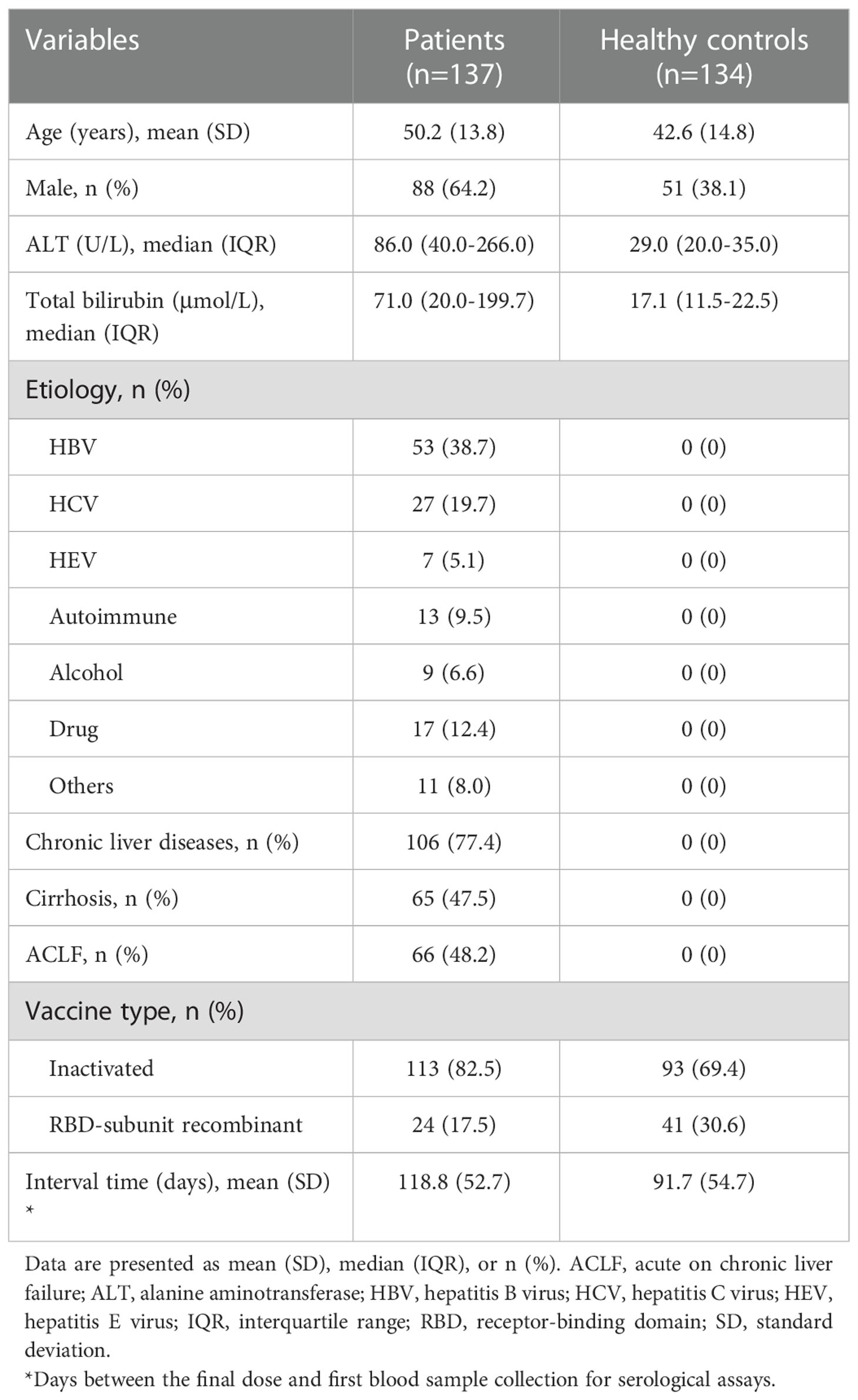
A total of 137 patients and 134 healthy controls (HC) were enrolled. Table 1 presents the demographic characteristics, laboratory test results, comorbidities, and vaccination information of these participants. The mean age was 50.2 years (SD: 13.8 years) in patients and 42.6 years (SD:14.8 years) in HC. The proportions of males were 64.2% (88/137) and 38.1% (51/134) in patients and HC groups, respectively. The etiology of liver injury included 38.7% (53/137) of hepatitis B virus (HBV), 19.7% (27/137) of hepatitis C virus (HCV), 5.1% of hepatitis E virus (HEV), 9.5% (13/137) of autoimmune hepatitis, 6.6% (9/137) of alcohol, and 12.4% (17/137) of drugs. Of the 137 patients, 77.4% (106/137) had chronic liver diseases, 47.5% (65/137) had decompensated cirrhosis, and 48.2% (66/137) were diagnosed with acute-on-chronic liver failure (ACLF). The majority of patients and HC received the COVID-19 inactivated vaccine (82.5% vs 69.4%, respectively).
3.2. Antibody responses after COVID-19 vaccination
In the cross-sectional study, the seropositivity for NAbs in patients was significantly lower than that in the HC group (65.7% vs 80.6%, p<0.01) (Figure 1A). The mean NAbs titer in patients was also lower than in the HC group (3.95 vs 4.94 log2AU/ml, p<0.001) (Figure 1B). During longitudinal follow-up, both seropositivity and titers of NAbs decreased over time in the patient and HC groups (Figures 1C, D). Between 3 and 4 months, the NAbs seropositivity in the patients decreased from 95.0% to 66.1%, while that in the HC group decreased from 96.0% to 85.7%. Therefore, the NAbs seropositivity in the patients was significantly lower than that in the HC group at M3-4 (p<0.05). Over time, only half of the participants were positive for NAbs, either in patients or in HC (55.7%, and 51.4% respectively) (Figure 1C). As for NAbs titers, the rate of decrease in patients was significantly faster than that in the HC group (-2.64 vs -0.97 AU/ml per day, p<0.01) (Figure 1E). Our results showed that the NAbs responses were impaired in patients with liver dysfunction. In addition, these patients have less persistence of antibody response than the HC group within 3-4 months after vaccination.
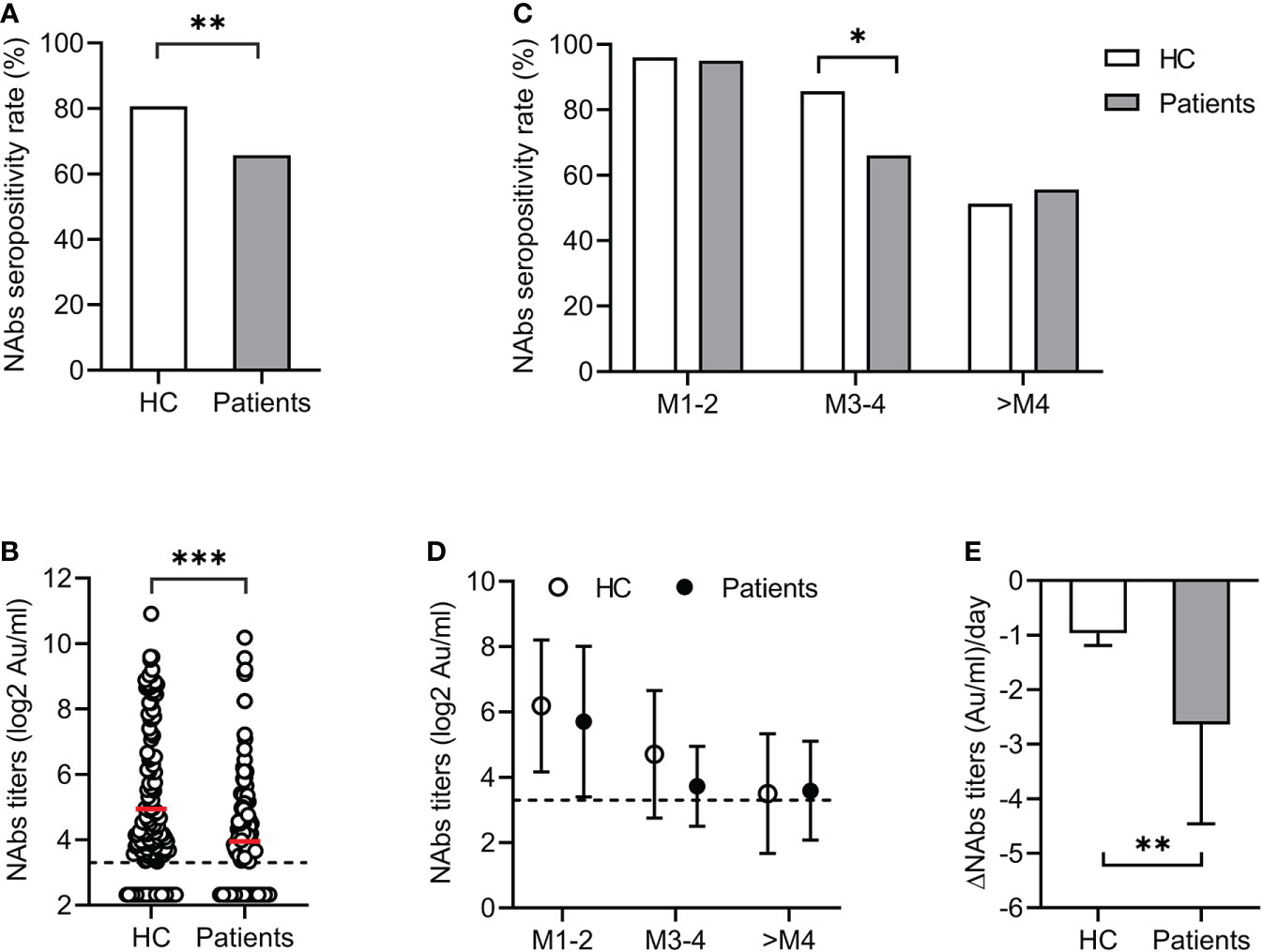
Figure 1 Antibody responses after COVID-19 vaccination. The seropositivity (A) and titers (B) of NAbs in patients with liver dysfunction and healthy controls (HC). Dynamic changes of NAbs seropositivity (C) and titers (D) over time during longitudinal follow-up. (E) Comparison of decline rate of NAbs titers in patients with liver dysfunction and HC groups. *<0.05; **<0.01; ***<0.001. AU, arbitrary units; COVID-19, coronavirus disease 2019; HC, healthy controls; NAbs, neutralizing antibodies.
3.3. Risk factors for poor antibody response after COVID-19 vaccination in patients with liver injury
To investigate risk factors affecting NAbs response, we compared the distribution of seropositivity and titers according to demographics, clinical parameters, and vaccine types. There were no significantly different in seropositivity and titers of NAbs between age (≤50 vs >50 years), total bilirubin level (≤5 times the upper limits of normal [ULN] vs >5×ULN), albumin level (<35 vs ≥35 g/L), cirrhosis (vs noncirrhotic), international normalized ratio (INR) value (≤1.5 vs >1.5), ACLF (vs non-ACLF), and vaccine types (inactivated vs subunit recombinant) (all p>0.05) (Figure 2). However, both seropositivity (54.5% vs 85.7%, p<0.05) and titers (3.46 vs 4.76 log2AU/ml, p<0.001) of NAbs in males were significantly lower than those in females (Figure 2B). Compared with the ALT ≤ 5×ULN group, the seropositivity of NAbs was significantly lower in the ALT > 5×ULN group (46.2% vs 73.5%, p<0.05), but there was no difference in the mean titers (3.63 vs 4.06 log2AU/ml, p>0.05) between two groups (Figure 2C). In addition, patients with the chronic liver disease also had lower NAbs titers compared to those with acute liver disease (3.59 vs 4.78 log2AU/ml, p<0.001) (Figure 2F).
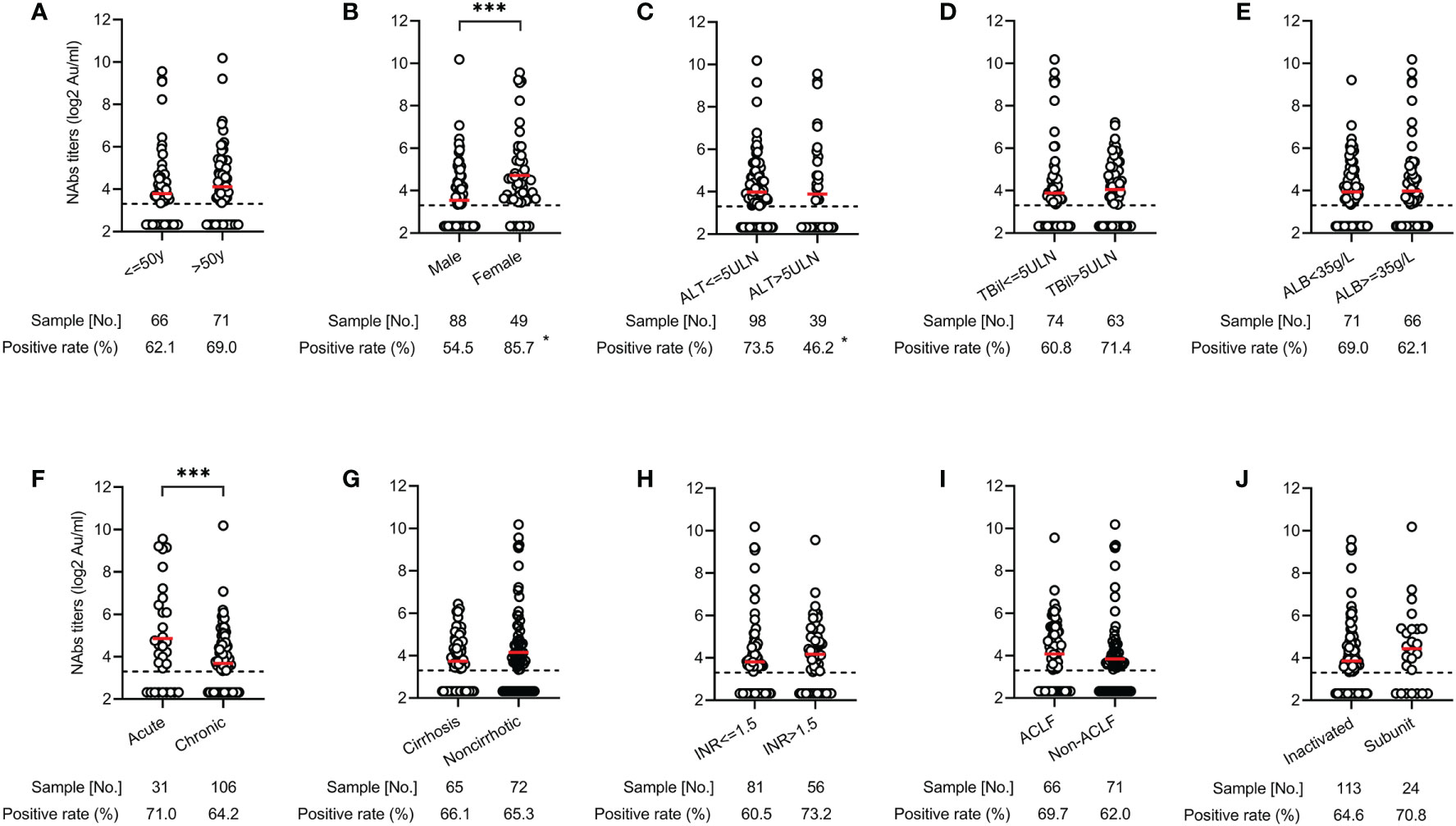
Figure 2 Distribution of antibody responses after COVID-19 vaccination across various clinical parameters in patients with liver dysfunction. Comparison of NAbs seropositivity and titers in the different age (≤50 vs >50 years) (A), gender (male vs female) (B), ALT level (≤ 5×ULN vs >5×ULN) (C), total bilirubin level (≤5×ULN vs >5×ULN) (D), albumin level (<35 vs ≥35 g/L) (E), liver disease status (acute vs chronic) (F), cirrhosis (yes vs no) (G), INR value (≤1.5 vs >1.5) (H), ACLF (yes vs no) (I), and vaccine types (inactivated vs subunit recombinant) (J). *<0.05; ***<0.001. ACLF, acute on chronic liver failure; ALT, alanine aminotransferase; COVID-19, coronavirus disease 2019; INR, international normalized ratio; NAbs, neutralizing antibodies; ULN, upper limits of normal.
Next, we performed multiple regression analysis to assess the association of sex, ALT levels, and liver disease status with NAbs response. After adjusting for potential confounding factors including age, total bilirubin, cirrhosis, ACLF, vaccine types, and interval time, male sex (OR: 0.17; 95% CI: 0.06, 0.46; p<0.001) and more severe liver damage (ALT > 5×ULN) (OR: 0.30; 95% CI: 0.12, 0.71; p<0.01) were significantly associated with reduction of the probability of NAbs seropositivity (Table 2). Regarding NAbs titers, male sex (β =-1.18; 95% CI: -1.73, -0.64; p<0.001) and chronic liver diseases (β =-1.45; 95% CI: -2.13, -0.76; p<0.001) were significantly associated with lower NAbs titers (Table 2). Our findings showed that male sex, severe liver injury, and chronic liver diseases have increased risk of poor antibody responses in patients with liver damage.
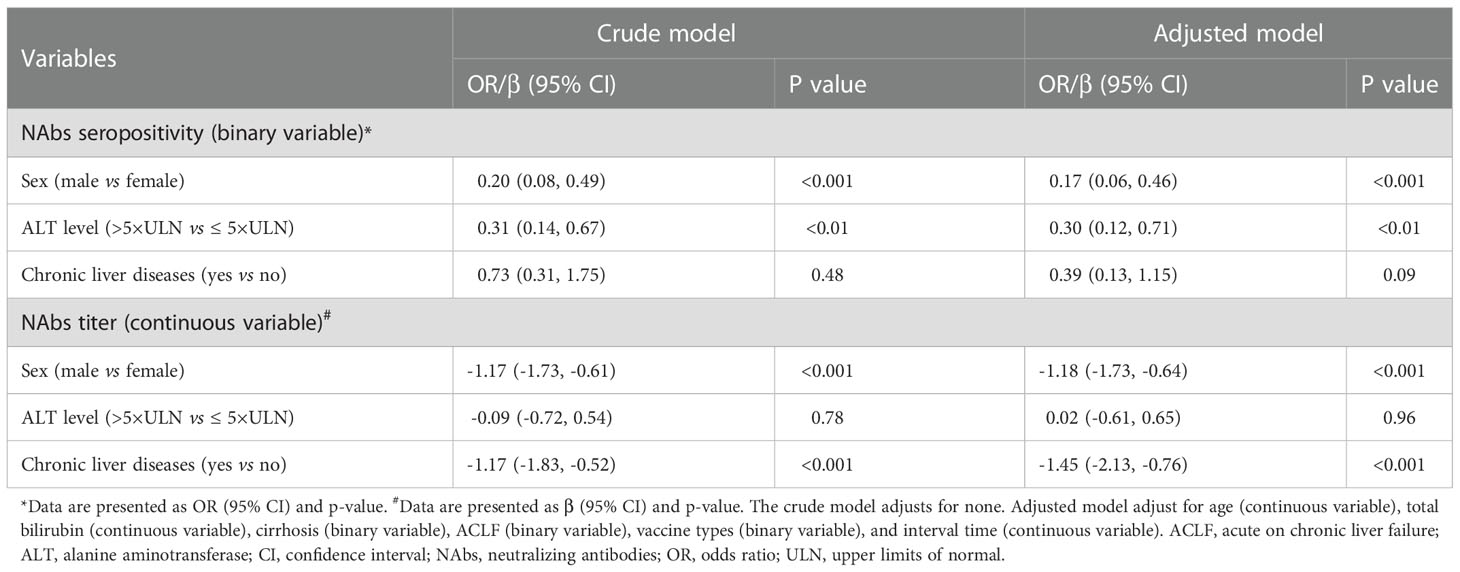
Table 2 Multiple regression analysis of risk factors associated with NAbs response to COVID-19 vaccines in patients with liver dysfunction.
3.4. Impact of artificial liver therapy on seropositivity and antibody titer
The artificial liver blood purification system is an important treatment for patients with liver failure. It is unclear whether artificial liver therapy will reduce the seropositivity and titer of SARS-CoV-2 antibodies. We conducted pairwise comparisons of the NAbs seropositivity and antibody titers in 26 ACLF patients before and after artificial liver plasmapheresis. The characteristics of these patients are listed in Supplementary Table 1. Interestingly, the seropositivity and titers of NAbs in ACLF patients did not decrease but increased significantly after artificial liver therapy (before vs after: 53.8% vs 84.6%, p<0.05; 3.55 vs 4.32 log2AU/ml, p<0.001) (Figure 3).
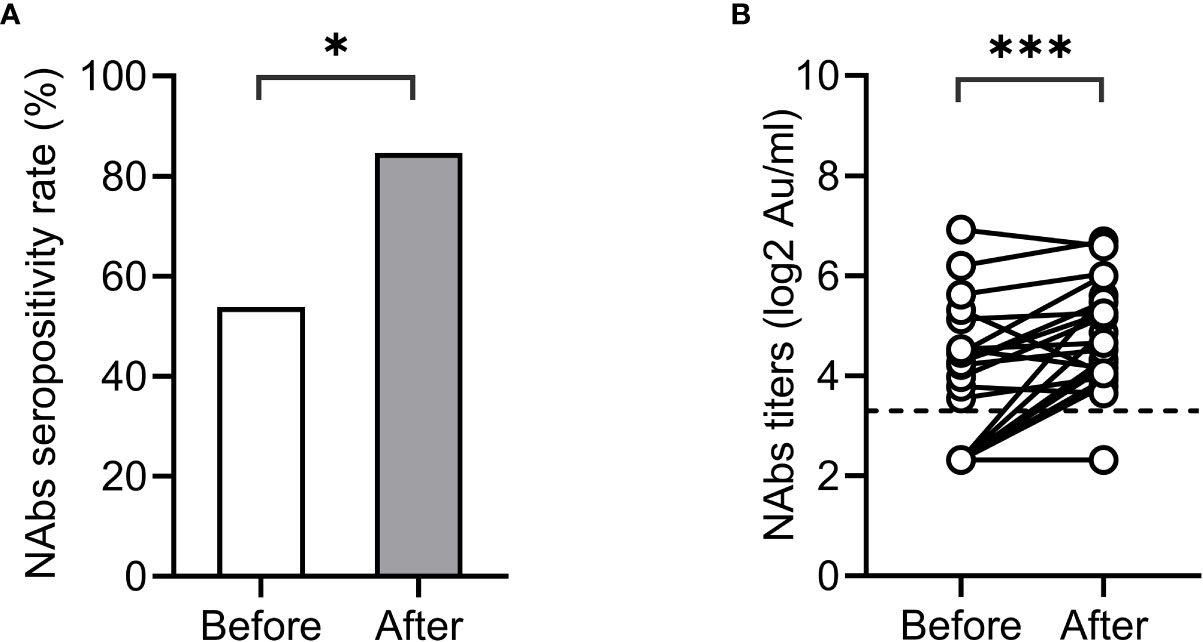
Figure 3 Impact of artificial liver therapy on NAbs levels. NAbs seropositivity (A) and titers (B) before and after artificial liver therapy in patients with liver failure.*<0.001.
3.5. Sensitivity analysis
To eliminate the potential bias that autoimmune liver disease might introduce to investigating risk factors for NAbs response, we performed a sensitivity analysis. After excluding patients with autoimmune hepatitis, male sex (OR=0.16; 95% CI 0.06, 0.44; p<0.001) and severe liver damage (OR=0.30; 95% CI 0.12, 0.73; p<0.01) were still significantly associated with lower NAbs seropositivity. Male sex (β=-1.21; 95% CI -1.75, -0.66; p<0.001) and chronic liver disease (β=-1.40; 95% CI -2.08, -0.72; p<0.001) also remained associated with lower NAbs titers (Supplementary Table 2).
4. Discussion
In this study, we investigated the effect of liver injury on antibody responses following prior COVID-19 vaccinations and reported three key findings (1): compared with healthy people, the seropositivity and titer of NAbs in patients with liver dysfunction were significantly lower and the titer decreased faster, (2) male sex, severe liver injury and chronic liver diseases have increased risk of poor antibody responses, and (3) artificial liver treatment does not reduce NAbs titers in patients with liver failure.
Most of the patients with liver damage in this study had chronic liver disease, and the study found that the seropositivity and titer of NAbs after COVID-19 vaccination in patients with liver damage were significantly lower than those in healthy subjects. Similar to the results of previous studies (9–12), our research also suggested that the antibody response rate and titer after COVID-19 vaccination in patients with chronic liver disease were reduced, which might be related to the basis of immune impairment in patients with chronic liver disease. This view is also supported by the significantly increased rates of antibody non-response in patients with immunosuppressive liver transplantation, autoimmune liver disease, and patients with systemic diseases mediated by immune disorders (13–16).
In this study, male sex, severe liver injury, and chronic liver diseases were found to be risk factors for poor antibody response. In the phase 3 randomized controlled trial of an inactivated vaccine, antibody response rates were lower in healthy male volunteers than in women (17). Zheng (18) and other studies (19) also found that female participants developed higher titers of NAbs compared to the male sex. In a study of a population with cirrhosis, Zhang and his colleagues (12) found that Child-Pugh score of B and C levels is associated with hyporesponsive to COVID-19 vaccination. However, in this study, the presence of cirrhosis was not found to be associated with either seropositivity or titer of NAbs, possibly due to the relatively small number of patients with cirrhosis observed in our study.
The artificial liver system is an important treatment method for severe liver function injury, especially liver failure, among which plasma exchange and plasma adsorption are the most commonly used modes (20). In some autoimmune diseases, such as systemic lupus erythematosus, plasma exchange, and plasma adsorption can reduce the titer of circulating antibodies in patients to achieve the purpose of disease control (21–23). It is still unclear whether artificial liver therapy will reduce COVID-19 antibody titers in patients with chronic liver disease who have received the COVID-19 vaccine when liver function is severely impaired and liver failure occurs due to disease progression. In this study, in 26 patients with liver failure, the titer of NAbs in patients with artificial liver treatment did not decrease compared with that before treatment. Interestingly, most patients had significantly higher titers of NAbs than before treatment. This phenomenon may reveal that these patients received a large amount of serum from healthy volunteers, while healthy adults in China have extremely high rates of COVID-19 vaccination and antibody response. The duration of changes in antibody titers associated with artificial livers requires further follow-up studies.
Several previous studies have suggested that patients with diseases mediated by immune disorders have significantly reduced antibody responses to COVID-19 vaccines (15, 16). In a prospective observational study including patients with autoimmune liver disease, the use of immunosuppressive agents increases the risk of poor antibody response to inactivated COVID-19 vaccine in AILD patients (14). Considering the potentially negative effects of immunosuppressive agents using in patients with autoimmune hepatitis, we performed a sensitivity analysis after excluding these patients. The results showed that male sex, severe liver damage, and chronic liver disease were still associated with poor antibody responses to the COVID-19 vaccines. These results indicated that our conclusions are robust and reliable.
There are some shortcomings in this study. First, the number and duration of longitudinal follow-up of participants were relatively insufficient. Second, the effects of medications on patients were not analyzed, such as statins. Previous studies have shown that statins can reduce influenza vaccine response (24). Finally, although we adjusted for some important confounding variables (eg, age, vaccine types, interval times, etc.) to make the results reliable, it is undeniable that there are still some confounding factors that were not included.
In conclusion, this study provides evidence for poor response to the COVID-19 vaccines in patients with liver dysfunction. Especially in males, those with chronic liver disease and severe liver injury, the NAbs response will be significantly reduced, resulting in less protective effect.
Author contributions
HL, SL, YL and SZ contributed to the conception and design, analysis, interpretation of the data, and critical revision of important intellectual content. PX, XW and HD collected the data and did the analysis. All authors approved the final version and agreed to be accountable for all aspects of the work. All authors contributed to the article and approved the submitted version.
Funding
This work is supported by the National Natural Science Foundation of China (82173237, 81902068); Senior Medical Talents Program of Chongqing for Young and Middle-aged (No. [2022]15); Program for Youth Innovation in Future Medicine, Chongqing Medical University (W0082); Kuanren Talents Program of the Second Affiliated Hospital of Chongqing Medical University (SZ, HL).
Acknowledgments
We wish to acknowledge Professor Zhi Zhou of Chongqing Medical University for his full support of our team in completing this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1084646/full#supplementary-material
Abbreviations
ACLF, acute on chronic liver failure; ALT, alanine aminotransferase; ALP, alkaline phosphatase; AST, aspartate aminotransferase; CI, confidence interval; COVID-19, coronavirus disease 2019; GGT, gamma-glutamyl transferase; HBV, hepatitis B virus; HC, healthy controls; HCV, hepatitis C virus; HEV, hepatitis E virus; INR, international normalized ratio; IQR, interquartile range; NAbs, neutralizing antibodies; OR, odds ratio; RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation; ULN, upper limits of normal.
References
1. Whittaker C, Walker PGT, Alhaffar M, Hamlet A, Djaafara BA, Ghani A, et al. Under-reporting of deaths limits our understanding of true burden of covid-19. BMJ (2021) 12:375:n2239. doi: 10.1136/bmj.n2239
2. Shang W, Wang Y, Yuan J, Guo Z, Liu J, Liu M. Global excess mortality during COVID-19 pandemic: A systematic review and meta-analysis. vaccines. (Basel) (2022) 12;10(10):1702. doi: 10.3390/vaccines10101702
3. Tulimilli SV, Dallavalasa S, Basavaraju CG, Rao VK, Chikkahonnaiah P, Madhunapantula SV, et al. Variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and vaccine effectiveness. vaccines. (Basel) (2022) 19;10(10):1751. doi: 10.3390/vaccines10101751
4. Steele MK, Couture A, Reed C, Iuliano D, Whitaker M, Fast H, et al. Estimated number of COVID-19 infections, hospitalizations, and deaths prevented among vaccinated persons in the US, December 2020 to September 2021. JAMA Netw Open (2022) 5(7):e2220385. doi: 10.1001/jamanetworkopen.2022.20385
5. Alqahtani SA, Aljumah AA, Hashim A, Alenazi TH, AlJawad M, Al Hamoudi WK, et al. Principles of care for patients with liver disease during the coronavirus disease 2019 (COVID-19) pandemic: Position statement of the Saudi association for the study of liver disease and transplantation. Ann Saudi Med (2020) 40(4):273–80. doi: 10.5144/0256-4947.2020.273
6. Boettler T, Marjot T, Newsome PN, Mondelli MU, Maticic M, Cordero E, et al. Impact of COVID-19 on the care of patients with liver disease: EASL-ESCMID position paper after 6 months of the pandemic. JHEP Rep (2020) 2(5):100169. doi: 10.1016/j.jhepr.2020.100169
7. C.M.A. Chinese Society of Hepatology. Rapid guideline for COVID-19 vaccination in patients with chronic liver diseases, hepatolbiliary malignancy and liver transplant. Zhonghua Gan Zang Bing Za Zhi (2021) 29:523–6. doi: 10.3760/cma.j.cn501113-20210612-00278
8. Cornberg M, Buti M, Eberhardt CS, Grossi PA, Shouval D. EASL position paper on the use of COVID-19 vaccines in patients with chronic liver diseases, hepatobiliary cancer and liver transplant recipients. J Hepatol (2021) 74(4):944–51. doi: 10.1016/j.jhep.2021.01.032
9. Wang J, Hou Z, Liu J, Gu Ye, Wu Y, Chen Z, et al. Safety and immunogenicity of COVID-19 vaccination in patients with non-alcoholic fatty liver disease (CHESS2101): A multicenter study. J Hepatol (2021) 75(2):439–41. doi: 10.1016/j.jhep.2021.04.026
10. Sripongpun P, Pinpathomrat N, Bruminhent J, Kaewdech A. Coronavirus disease 2019 vaccinations in patients with chronic liver disease and liver transplant recipients: An update. Front Med (2022) 9:924454. doi: 10.3389/fmed.2022.924454
11. He T, Zhou Y, Xu P, Ling N, Chen M, Huang T, et al. Safety and antibody response to inactivated COVID-19 vaccine in patients with chronic hepatitis b virus infection. Liver Int (2022) 42(6):1287–96. doi: 10.1111/liv.15173
12. Wang J, Zhang Q, Ai J, Liu D, Liu C, Xiang H, et al. Safety and immunogenicity of SARS-CoV-2 vaccines in Chinese patients with cirrhosis: a prospective multicenter study. Hepatol Int (2022) 16(3):691–701. doi: 10.1007/s12072-022-10332-9
13. Efe C, Taşçılar K, Gerussi A, Bolis F, Lammert C, Ebik B, et al. SARS-CoV-2 vaccination and risk of severe COVID-19 outcomes in patients with autoimmune hepatitis. J Autoimmun (2022) 7:132:102906. doi: 10.1016/j.jaut.2022.102906
14. Li Hu, Wang Y, Ao L, Ke M, Chen Z, Chen M, et al. Association between immunosuppressants and poor antibody responses to SARS-CoV-2 vaccines in patients with autoimmune liver diseases. Front Immunol (2022) 5:988004(13). doi: 10.3389/fimmu.2022.988004
15. Boekel L, Steenhuis M, Hooijberg F, Hooijberg F, van Dam KPJ, Besten YR, et al. Antibody development after COVID-19 vaccination in patients with autoimmune diseases in the Netherlands: A substudy of data from two prospective cohort studies. Lancet Rheumatol (2021) 3(11):e778–88. doi: 10.1016/S2665-9913(21)00222-8
16. Furer V, Eviatar T, Zisman D, Peleg H, Paran D, Levartovsky D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: A multicentre study. Ann Rheum Dis (2021) 10:1–9. doi: 10.1136/annrheumdis-2021-220647
17. Tanriover MD, Doğanay HL, Akova M, Güner HR, Azap A, Akhan Sıla, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet (2021) 398:213–22. doi: 10.1016/S0140-6736(21)01429-X
18. Xiang T, Liang B, Wang H, Quan X, He S, Zhou H, et al. Safety and immunogenicity of a SARS-CoV-2 inactivated vaccine in patients with chronic hepatitis b virus infection. Cell Mol Immunol (2021) 18(12):2679–81. doi: 10.1038/s41423-021-00795-5
19. Li Z, Xiang T, Liang B, Deng H, Wang H, Fenget X, et al. Characterization of SARS-CoV-2-Specific humoral and cellular immune responses induced by inactivated COVID-19 vaccines in a real-world setting. Front Immunol (2021) 12:802858. doi: 10.3389/fimmu.2021.802858
20. Li LJ, Zhang YM, Liu XL, Du WB, Huang JR, Yang Q, et al. Artificial liver support system in China: a review over the last 30 years. Ther Apher Dial (2006) 10(2):160–7. doi: 10.1111/j.1744-9987.2006.00358.x
21. Kronbichler A, Brezina B, Quintana LF, Jayne DRW. Efficacy of plasma exchange and immunoadsorption in systemic lupus erythematosus and antiphospholipid syndrome: A systematic review. Autoimmun Rev (2016) 15(1):38–49. doi: 10.1016/j.autrev.2015.08.010
22. Zanatta E, Cozzi M, Marson P, Cozzi F. The role of plasma exchange in the management of autoimmune disorders. Br J Haematol (2019) 186(2):207–19. doi: 10.1111/bjh.15903
23. Hohenstein B, Bornstein SR, Aringer M. Immunoadsorption for connective tissue disease. Atheroscler Suppl (2013) 14(1):185–9. doi: 10.1016/j.atherosclerosissup.2012.10.034
Keywords: COVID-19, SARS-CoV-2, vaccine, neutralizing antibodies, liver dysfunction, artificial liver
Citation: Li H, Li S, Xu P, Wang X, Deng H, Lei Y and Zhong S (2023) Analysis of neutralizing antibodies to COVID-19 inactivated or subunit recombinant vaccines in hospitalized patients with liver dysfunction. Front. Immunol. 14:1084646. doi: 10.3389/fimmu.2023.1084646
Received: 30 October 2022; Accepted: 04 January 2023;
Published: 18 January 2023.
Edited by:
E. Marion Schneider, University of Ulm, GermanyReviewed by:
Lok Bahadur Shrestha, University of New South Wales, AustraliaWill Hudson, Baylor College of Medicine, United States
Copyright © 2023 Li, Li, Xu, Wang, Deng, Lei and Zhong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shan Zhong, emhvbmdzaGFuQGNxbXUuZWR1LmNu; Yu Lei, bGVpeXVfd3dAYWxpeXVuLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Hu Li
Hu Li Shiyin Li†
Shiyin Li† Shan Zhong
Shan Zhong