- 1Department of Thoracic Surgery, Lanzhou University Second Hospital, Lanzhou University, Lanzhou, China
- 2Laboratory of Extracorporeal Life Support, Lanzhou University Second Hospital, Lanzhou University, Lanzhou, China
- 3Department of Cardiac Surgery, Lanzhou University Second Hospital, Lanzhou University, Lanzhou, China
- 4Department of Anesthesiology, Lanzhou University Second Hospital, Lanzhou University, Lanzhou, China
- 5Department of Extracorporeal Circulation, Lanzhou University Second Hospital, Lanzhou University, Lanzhou, China
Background: After its approval by the European Union in 2011, CytoSorb therapy has been applied to control cytokine storm and lower the increased levels of cytokines and other inflammatory mediators in blood. However, the efficiency of this CytoSorb treatment in patients with coronavirus disease (COVID-19) still remains unclear. To elucidate the Cytosorb efficiency, we conducted a systematic review and single-arm proportion meta-analysis to combine all evidence available in the published literature to date, so that this comprehensive knowledge can guide clinical decision-making and future research.
Methods: The literature published within the period 1 December 2019 to 31 December 2021 and stored in the Cochrane Library, Embase, PubMed, and International Clinical Trials Registry Platform (ICTRP) was searched for all relevant studies including the cases where COVID-19 patients were treated with CytoSorb. We performed random-effects meta-analyses by R software (3.6.1) and used the Joanna Briggs Institute checklist to assess the risk of bias. Both categorical and continuous variables were presented with 95% confidence intervals (CIs) as pooled proportions for categorical variables and pooled means for continuous outcomes.
Results: We included 14 studies with 241 COVID-19 patients treated with CytoSorb hemadsorption. Our findings reveal that for COVID-19 patients receiving CytoSorb treatment, the combined in-hospital mortality was 42.1% (95% CI 29.5–54.6%, I2 = 74%). The pooled incidence of adjunctive extracorporeal membrane oxygenation (ECMO) support was 73.2%. Both the C-reactive protein (CRP) and interleukin-6 (IL-6) levels decreased after CytoSorb treatment. The pooled mean of the CRP level decreased from 147.55 (95% CI 91.14–203.96) to 92.36 mg/L (95% CI 46.74–137.98), while that of IL-6 decreased from 339.49 (95% CI 164.35–514.63) to 168.83 pg/mL (95% CI 82.22–255.45).
Conclusions: The majority of the COVID-19 patients treated with CytoSorb received ECMO support. In-hospital mortality was 42.1% for the COVID-19 patients who had CytoSorb treatment. Both CRP and IL-6 levels decreased after Cytosorb treatment.
Introduction
After a new disease caused by the novel coronavirus broke out in Wuhan, China, millions of confirmed cases of coronavirus disease 2019 (COVID-19), were reported. The disease led to numerous deaths (1). Because of its high mortality rate, COVID-19-induced acute respiratory syndrome has put considerable strain on healthcare systems (2). Although it has previously been reported that during outbreaks of emerging infections, acute respiratory distress syndrome (ARDS) could be treated with extracorporeal membrane oxygenation (ECMO) (3, 4), the clinical outcome varies for COVID-19 patients with ECMO support (5–7). Hence, the same strategy cannot be adopted for COVID-19 cases.
ECMO support could provide stable hemodynamics and oxygen saturation, which is very recommended for patients with severe ARDS (8). However, COVID-19 is additionally associated with the excessive release of pro-inflammatory cytokines, such as granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-6 (IL-6), interleukin-1 (IL-1), monocyte chemoattractant protein-1 (MCP-1), and tumor necrosis factor (TNF). This excessive release of the pro-inflammatory cytokines initiates an extreme inflammatory process and results in a cytokine storm (9). COVID-19 patients in the intensive care unit (ICU) had levels of interleukin-10 (IL-10), interleukin-2 (IL-2), interleukin-7 (IL-7), and TNF-α that were considerably higher than those of the non-ICU patients (10). This observation suggests that cytokines may be crucial in the pathophysiology of COVID-19 (11, 12). As an adjuvant therapy for systemic inflammation, CytoSorb therapy is an adsorptive blood purification procedure that attempts to lower the increased levels of cytokines and other inflammatory mediators in the blood and regulate the cytokine storm (13). Following its approval by the European Union (EU) in 2011, CytoSorb has been used to treat over 130,000 patients globally. It has been mainly used to treat systemic hyperinflammation and refractory shock (14). By controlling the cytokine storm, CytoSorb might be a promising choice for COVID-19 patients.
Owing to the complex pathophysiological environment of COVID-19, involving multiple mediators and highly redundant, overlapping feedback mechanisms, cytokine adsorption therapy may benefit from removing a wide range of inflammatory substances and other cytokines, at least in theory (15). Nevertheless, very few studies reported the outcomes after CytoSorb treatment in COVID-19 patients. Reaching a credible conclusion with such a small number of studies is difficult, and hence, we need some strong evidence to guide the choices for clinical treatment. The purpose of this study is to combine all the evidence in the published literature related to cytokine adsorption therapy, especially the use of the CytoSorb adsorption column for COVID-19 patients, and provide credible guidance for clinical practice.
Methods
Search strategy
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement was followed when conducting this study (16). Using the Cochrane Library, PubMed, Embase, and the International Clinical Trials Registry Platform (ICTRP), we performed a comprehensive online search from 1 December 2019 to 31 December 2021, using the following: “COVID-19”, “2019 novel coronavirus”, “coronavirus disease 2019”, “2019-nCoV”, “SARS-CoV-2”, “severe acute respiratory syndrome coronavirus 2”, “CytoSorb”, “CytoSorb cartridge”, “CytoSorb hemadsorption”, “cytokine hemadsorption”, “cytokine adsorber”, and “cytokine adsorption” as MeSH and EMTREE keywords. To find and include relevant studies, we evaluated all related studies and their citations.
Study eligibility
We identified and included all relevant studies published regarding CytoSorb treatment in adult patients with COVID-19. We excluded any animal studies, review articles, case reports of a single patient, or pediatric studies (< 18 years). The latest publication was used when multiple articles relating to a single study had been published, to avoid the possible overlapping of patients. Literature searches, study eligibility assessments, and data extraction was conducted by two independent investigators (S.L.W and Y.C.Z). By consensus and consultation with experienced reviewers (Z.Z.L and Y.N.L), discrepancies were resolved.
Data collection
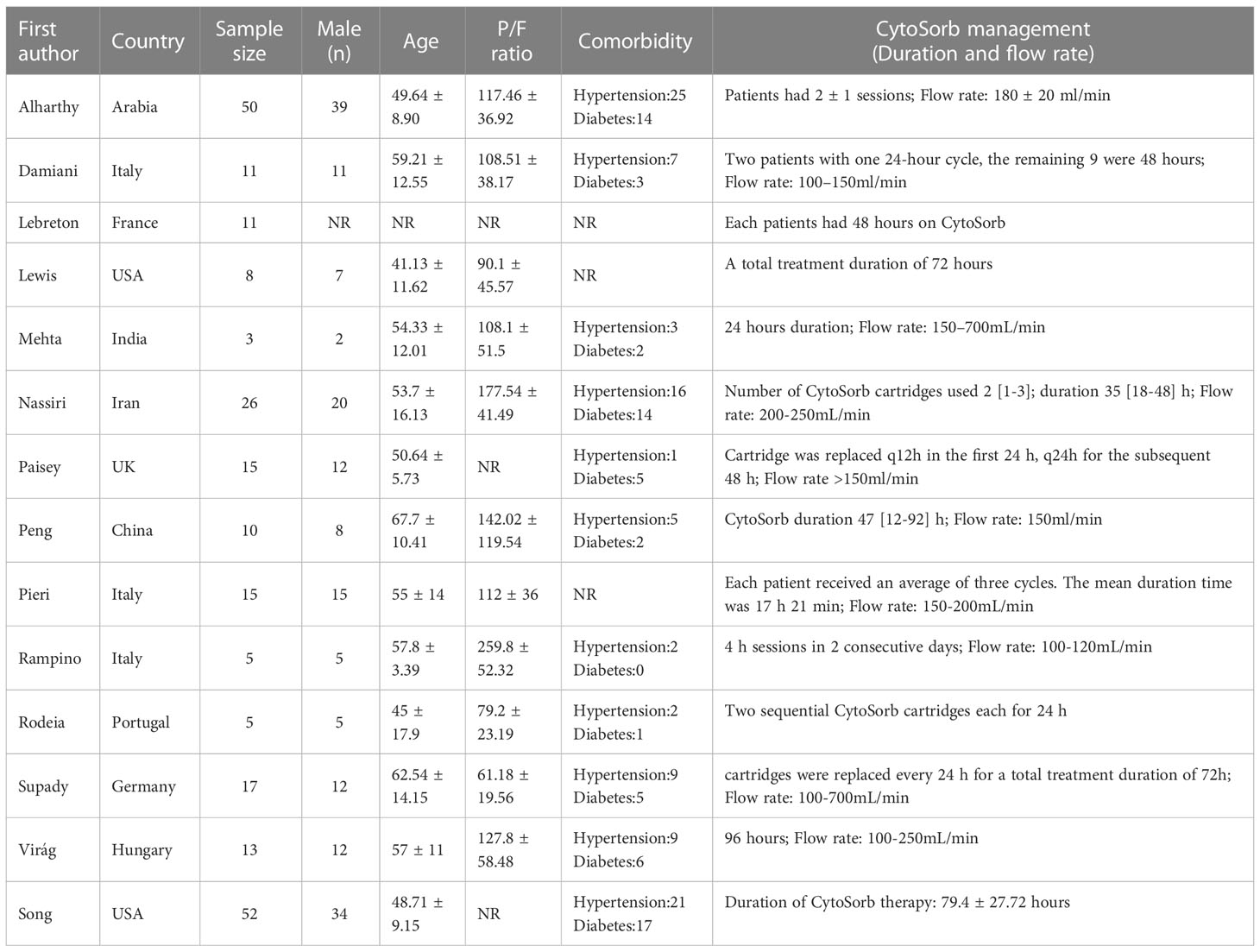
Two independent investigators (S.L.W and Y.C.Z) abstracted data using a predefined standardized protocol and tool. WebPlotDigitizer 4.5 Software (https://automeris.io/WebPlotDigitizer) was used to extract data from graphics. Two experienced reviewers (Z.Z.L and Y.N.L) consulted with each other and settled any disagreements. The data collection included study characteristics (first author, publication year, country); patient demographics (numbers of patients, age, body mass index [BMI], the proportion of male patients, comorbidities including hypertension and diabetes); pre-CytoSorb characteristics (Acute Physiology and Chronic Health Evaluation II [APCHE II] score, Sequential Organ Failure Assessment [SOFA] score, partial pressure of arterial oxygen to fraction of inspired oxygen ratio [PaO2/FiO2], serum C-reactive protein [CRP] and interleukin-6 [IL-6] levels); post-CytoSorb characteristics (serum CRP and IL-6 levels); mortality (both in-hospital and substitution of the nearest common mortality time point); and length of stay in ICU.
Assessment of risk of bias
Joanna Briggs Institute (JBI) checklists were used to assess the study quality of case series and cohort studies. Egger’s test was employed to estimate publication bias. I2 statistics, Chi-squared tests, and visual inspections of forest plots were used to assess statistical heterogeneity.
Outcomes of interest
In-hospital mortality was the primary outcome of interest. The secondary outcomes were serum IL-6 and CRP levels before and after CytoSorb treatment, length of ICU stay, duration of mechanical ventilation, adjunctive ECMO therapy, and mortality in patients with or without ECMO.
Statistical analysis
The continuous variables were presented as mean (± standard deviation) or median (interquartile range). We used the formulas available in the literature to convert the median (interquartile range) into a mean (± standard deviation) (17, 18). We reported the categorical variables as numbers and percentages (%). To calculate summary effects, we used R 3.6.1 software. The summary effects were presented with 95% confidence intervals (CIs). Both odds ratios (OR) and mean differences (MD) were used as summary statistics for dichotomous data and continuous data, respectively. The heterogeneity of the studies was assessed by the I2. A random effects model was applied if the I2 value was greater than 50%, otherwise, a fixed effects model was used.
Results
Study identification
We identified 215 potentially relevant studies, removed 103 duplicates, and excluded 64 studies after carefully reviewing the titles and abstracts. A full-text review of the remaining 34 studies led to their exclusion, details can be found in Figure 1. Following a strict appraisal, 14 studies were selected, including 10 case series and 4 cohort studies. These studies included a total of 241 COVID-19 patients treated with CytoSorb hemadsorption (19–32).
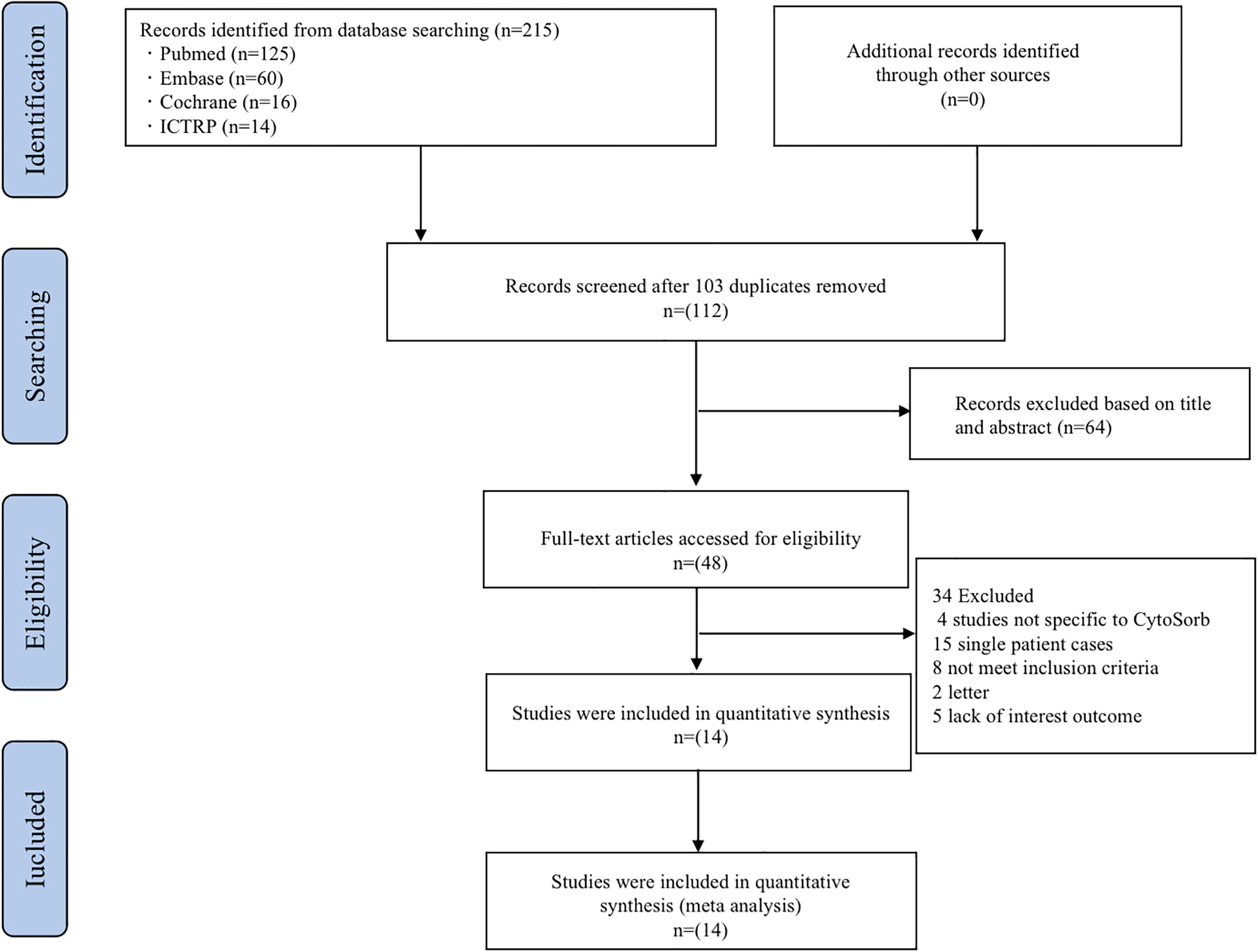
Figure 1 Flow diagram of the study selection process. ICTRP, the International Clinical Trials Registry Platform.
Baseline characteristics of the included studies
241 patients from 11 different countries were enrolled in the study, characteristics were summarized in Table 1. All of the included studies dealt with patients who were infected with the 2019 novel coronavirus. Sex was reported in 13 studies and 75.52% of patients were male. In 4 studies, all included patients were male. Age was reported in 13 studies including 178 patients, the pooled mean age was 54.29 (95% CI 50.54–58.05). Notably, hypertension and diabetes were the most common comorbidities in this population. The pooled incidence of hypertension is 48.2% (95% CI 33–63.4%) and that of diabetes is 31% (95% CI 22.1–39.9%).
Assessment of study quality
To appraise study quality for cohort studies and case series, we used the Joanna Briggs Institute (JBI) checklist. The results showed that all case series studies had scores above 8/10 and cohort studies had scores above 9/11 (Supplementary Table 1). This indicated that high quality was strictly maintained for our study.
Pre−CytoSorb variables
BMI was reported in 8 studies and the pooled mean BMI was found to be 29.54 (95% CI 27.36–31.72). 11 studies reported PaO2/FiO2 before CytoSorb initiation. The pooled mean PaO2/FiO2 was 123.81 (95% CI 92.46–155.16). Pre-CytoSorb SOFA score before CytoSorb initiation was reported in 9 studies with a pooled mean SOFA score of 9.6 (95% CI 6.81–12.4). Pre-CytoSorb APACHE2 score before CytoSorb initiation was reported in 4 studies with a pooled mean APACHE2 score of 22.75 (95% CI 16.63–28.87). Serum CRP before CytoSorb initiation was reported in 10 studies with a pooled mean CRP of 147.55 mg/L (95% CI 91.14–203.96). Serum IL-6 before CytoSorb initiation was reported in 10 studies with a pooled mean IL-6 of 339.49 pg/mL (95% CI 164.35–514.63).
Primary outcomes
In hospital mortality
The pooled in-hospital mortality of COVID-19 patients receiving Cytosorb (12 studies, 230 patients) was 42.1% (95% CI 29.5–54.6%, I2 = 74%) (Figure 2) However, the in-hospital mortality reported varied from 18.2–76.9%. Visual inspection of the funnel plot indicated a low risk of publication bias (Supplementary Figure 1). In addition, Egger’s tests confirmed the absence of any overt risk of publication bias in the studies included (Pegger = 0.2194) (Supplementary Figure 2). A sensitive analysis (using the single-study-removed method) was also performed, which demonstrated decent stability in the primary outcome of in-hospital mortality (Supplementary Figure 3).
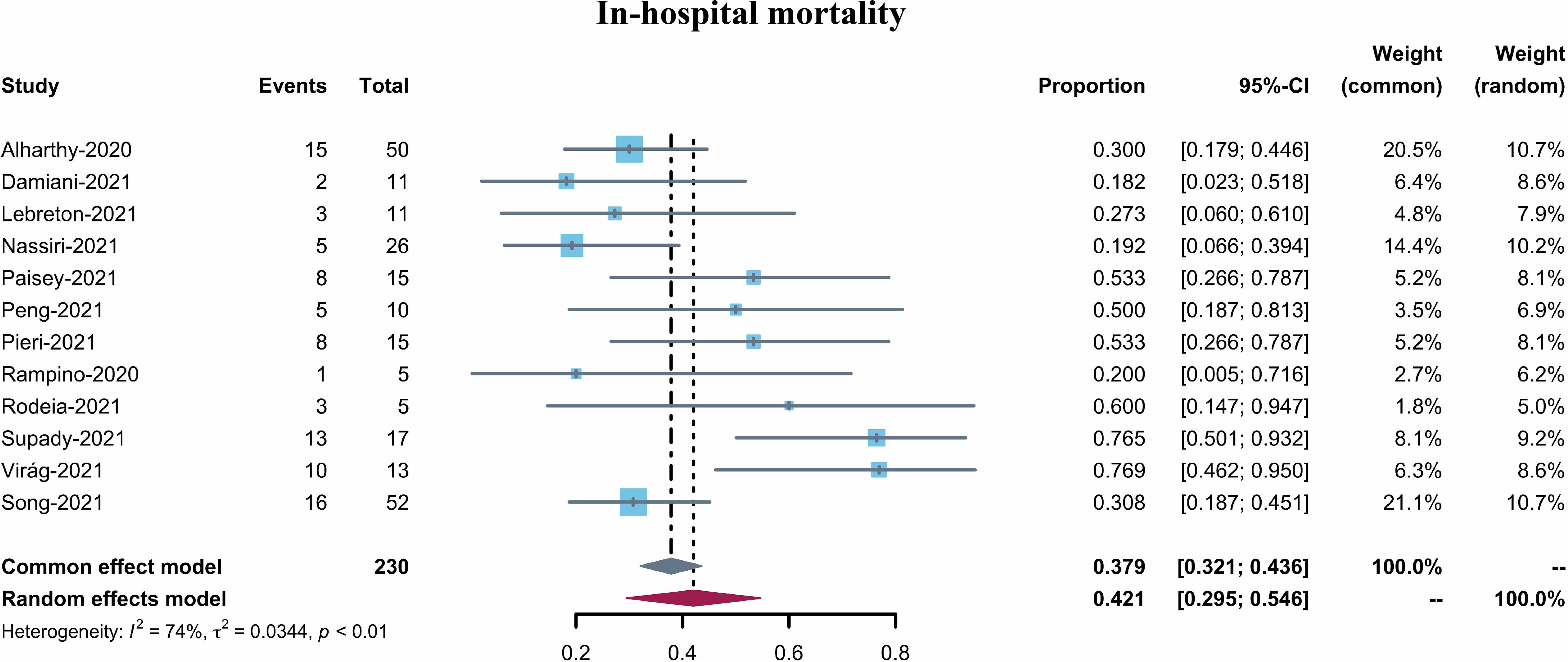
Figure 2 Forest plot of in-hospital mortality for COVID-19 patients with CytoSorb treatment. CI: confidence interval.
Secondary outcomes
Serum IL-6 and CRP
Ten studies reported serum IL-6 levels in COVID-19 patients after CytoSorb hemadsorption treatment. The mean serum IL-6 level varied from 37.44 to 442.23 pg/mL, with a pooled mean of 168.83 pg/mL (95% CI 82.22–255.45, I2 = 96%) (Figure 3). It is to be noted that the pooled mean IL-6 level before CytoSorb initiation was 339.49 pg/mL (95% CI 164.35–514.63). Nine studies reported post-CytoSorb hemadsorption treatment serum CRP in COVID-19 patients. The mean serum CRP varied from 18.9 to 220.40, with a pooled mean of 92.36 (95% CI 46.74–137.98) (Figure 4).
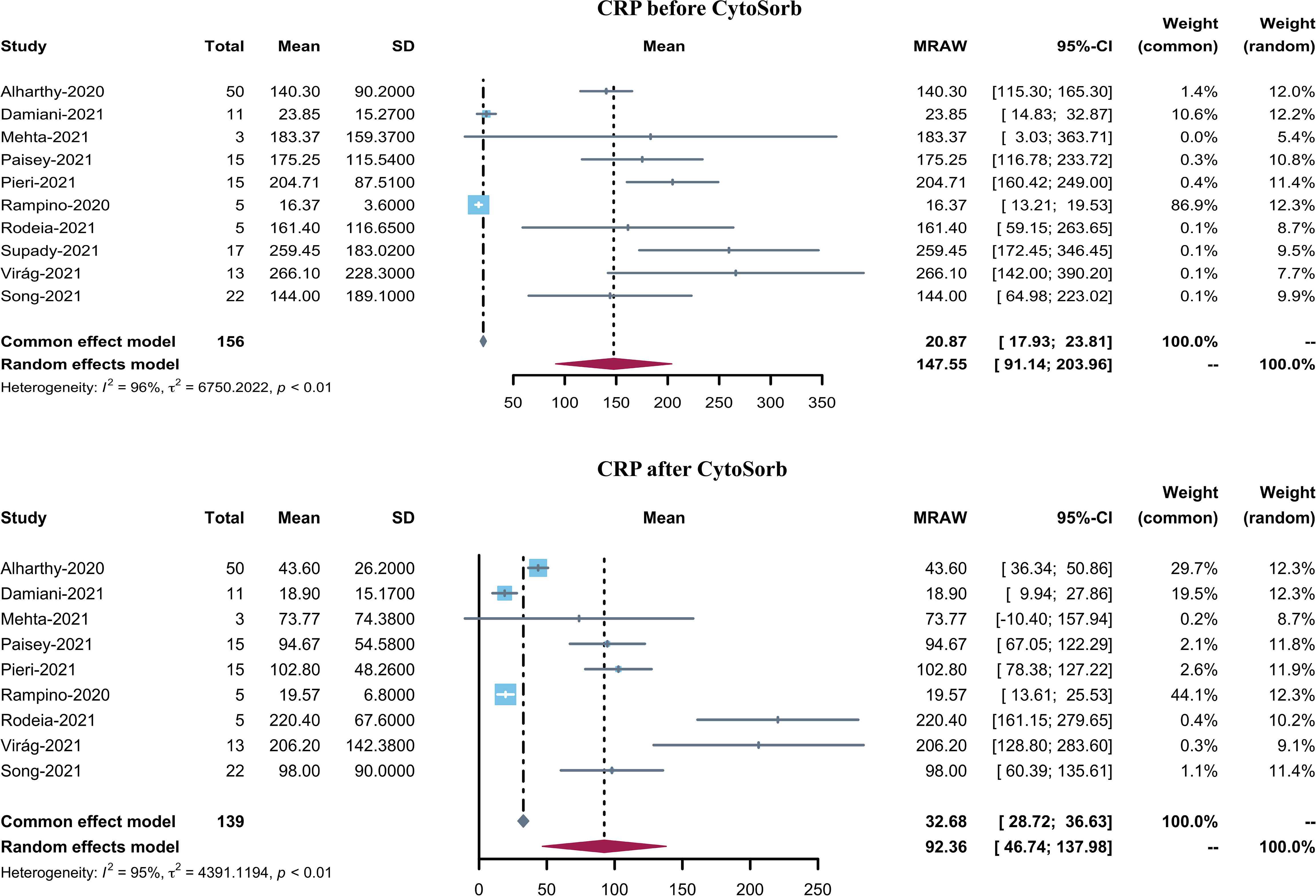
Figure 3 Forest plot of serum CRP before and after CytoSorb treatment for COVID-19 patients. CI: confidence interval; SD: standard deviation; CRP: C-reactive protein.
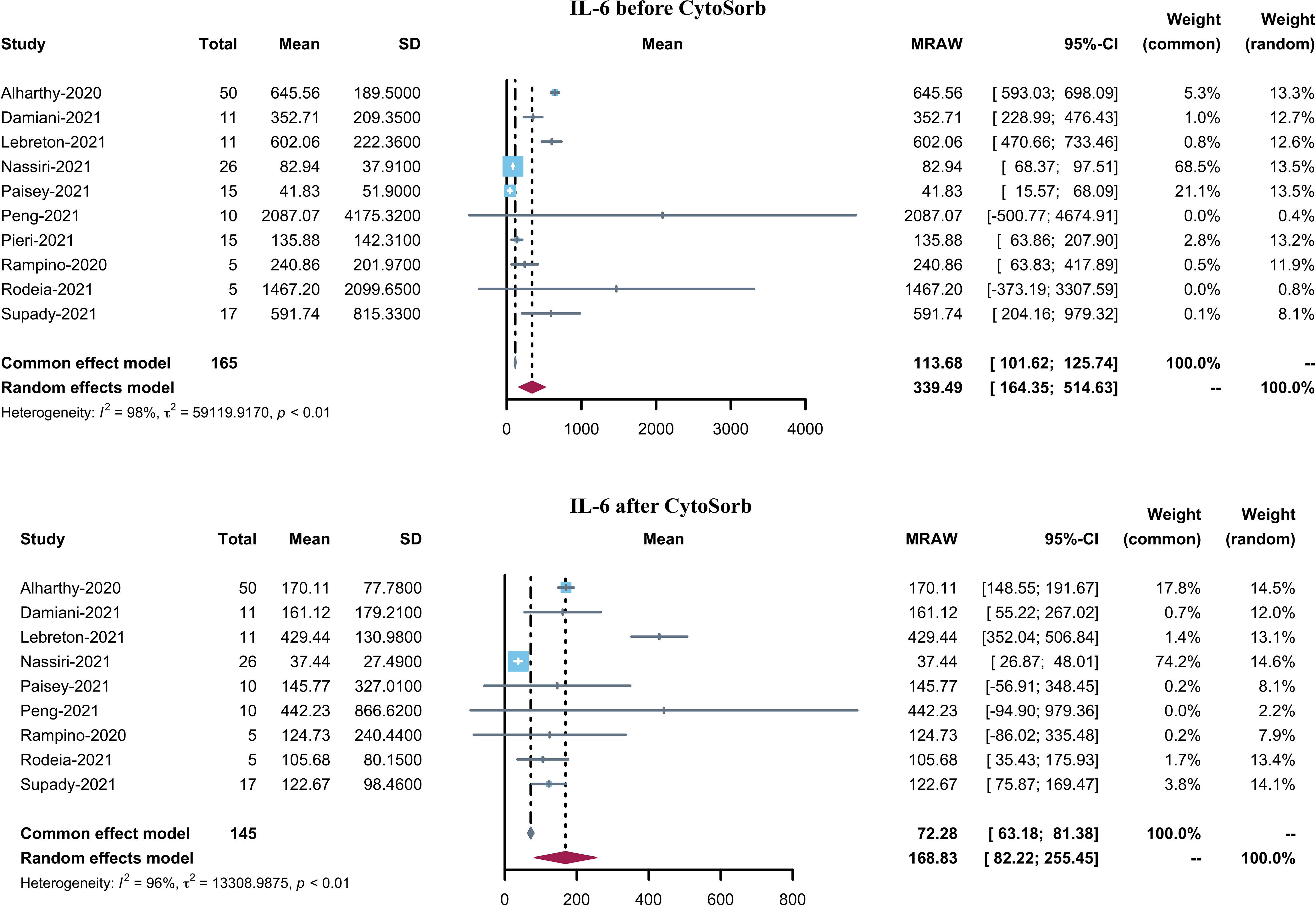
Figure 4 Forest plot of serum IL-6 before and after CytoSorb treatment for COVID-19 patients. CI: confidence interval; SD: standard deviation; IL-6: interleukin-6.
Duration of mechanical ventilation, duration of ICU stay, and incidence of ECMO support
Four studies reported the duration of mechanical ventilation (MV) after CytoSorb hemadsorption treatment with a pooled mean of 14.37 days (95% CI 7.96–20.78, I2 = 94%). Six studies reported length of stay in the ICU with a pooled mean of 19.74 days (95% CI 12.92–26.56, I2 = 96%). Except for CytoSorb hemadsorption treatment, 9 studies reported COVID-19 patients with ECMO support, with a pooled incidence of ECMO support of 73.2% (95% CI 48.7–97.7%).
Mortality for patients with or without ECMO
There are eight studies reported the in-hospital mortality for patients received CytoSorb therapy with ECMO support. The in-hospital mortality reported varied from 27.3%-82.4%, and the pooled in-hospital mortality of COVID-19 patients receiving CytoSorb therapy with ECMO support was 54% (95% CI 36.9%-71.1%, I2 = 74%) (Supplementary Figure 4). For patients without ECMO support, it was reported in eight studies, the mortality varied from 18.2%-76.9%, and the pooled in-hospital mortality of COVID-19 patients receiving CytoSorb therapy without ECMO support was 37.6% (95% CI 21.2%-54.1%, I2 = 70%) (Supplementary Figure 5).
Discussion
This prompt review of the relevant evidence and subsequent meta-analysis studied the efficiency of CytoSorb hemadsorption in adult COVID-19 patients. The pooled in-hospital mortality in these COVID-19 patients was 42.1%, and this estimate was supported by highly credible evidence. However, a study reported in-hospital mortality of 37% for COVID-19 patients with ECMO support (33). This implies that mortality was slightly higher (42.1%) in patients receiving CytoSorb hemadsorption treatment. The pooled mean serum IL-6 and CRP were 168.83 and 92.41 mg/L, respectively. The values indicate that compared with the baseline levels before the application of CytoSorb, both IL-6 and CRP levels have decreased after hemadsorption treatment. The pooled mean duration of MV was 14.37 days and the pooled mean length of ICU stay was 19.74 days. The majority of patients (73.2%) received adjunctive ECMO support.
COVID-19 triggers cytokine storm, a systemic inflammatory reaction that increases immune cell activation and pro-inflammatory cytokine production (34). Furthermore, a postmortem examination of the lung of a person who died of COVID-19 revealed the presence of acute respiratory distress syndrome (ARDS) and T-cell overactivity (35). Consequent to infection with SARS-CoV-2, innate and adaptive immune responses were triggered, resulting in uncontrolled inflammatory responses and, eventually, the cytokine storm (36). Several studies found that seriously sick COVID-19 patients had greater levels of pro-inflammatory cytokines, particularly IL-6, than moderately unwell individuals (37, 38). The increase of IL-6 and IL-10 in the COVID-19 patients was constant. IL-6 targets the IL-6 receptor, and the latter recruits JAK, which activates the signal transducer and activator of transcription 3 through a cascade signal (39). The transcriptome sequencing of bronchoalveolar lavage fluid (BALF) cells indicated that SARS-CoV-2 infection causes increased chemokine releases, such as C-X-C motif chemokine ligand 10 (CXCL-10) and chemokine C-C motif ligand 2 (CCL-2) (40). The excessive release of cytokines in COVID-19 patients also suggests a bad prognosis. The cytokine storm may cause epithelial and endothelial cell apoptosis and vascular leakage, which can lead to ARDS, other severe symptoms, and eventually to death.
Serum IL-6 concentrations can be reduced from a median value of 5000 to 289 pg/mL after 24 h of cytokine adsorption in patients with severe infections (41). IL-6 levels have been associated with increased severity and mortality in COVID-19 patients. Herein, the pooled results indicated that the mean serum IL-6 level decreased from 339.49 to 168.83 pg/mL. The RECOVERY trial showed benefits in COVID-19 patients with low-dose dexamethasone (42). Although CytoSorb treatment demonstrated effective suppression of inflammation in COVID-19 patients, the in-hospital mortality remains higher (42.1%) compared with ECMO-support patients (36.9%) (43). One possible explanation could be these patients were severely ill with high SOFA and APACHE2 scores.
Although CytoSorb device has neither been cleared nor approved by the Food and Drug Administration (FDA) for the indication of treating patients with COVID-19. In April 2020, CytoSorb therapy was granted by Emergency Use Authorizations (EUA) to adsorb inflammatory cytokines in adult COVID-19 patients admitted to the ICU with imminent or confirmed respiratory failure (44). Therefore, early acute lung injury, early acute respiratory distress syndrome, septic shock, and multiple organ dysfunction are indications to initiate CytoSorb therapy in adult COVID-19 patients. However, exact criteria for hyperinflammation did not defined by EUA, the decision to initiate CytoSorb therapy is at the discretion of the treating doctor. What’s more, The CytoSorb device can be easily integrated into extracorporeal circuits including continuous renal replacement therapy (CRRT) and ECMO. In this way, CytoSorb is also suitable for patients with renal dysfunction or cardiopulmonary failure as adjuvant therapy.
Our study concludes that when conventional treatment fails to provide adequate clinical stability to the patient, CytoSorb therapy should be explored as an additional therapy. Although patients included in our study were critically ill with 73.2% of them being supported with ECMO, the in-hospital mortality was lower. It is to be noted that CRP is a very sensitive marker of inflammation and tissue damage. Our study showed that after CytoSorb treatment, the CRP level decreased. The removal spectrum of CytoSorb seems to encompass other inflammation-related trigger substances such as damage-associated molecular patterns, which could provoke and maintain a generalized inflammatory host response.
Despite certain important insights obtained, we recognize several limitations of the current study. First, the research was primarily a case series study, except for the inclusion of one randomized controlled trial (RCT). Although publication bias was absent and JBI critical appraisal indicated most of the articles as high quality and suitable for inclusion, a relatively small sample size of these studies may induce increased heterogeneity in our results. Including the primary outcome of in-hospital mortality, other pooled results also indicated high heterogeneity. Second, the application of CytoSorb is a new technique that has been widely used in systemic hyperinflammation patients. However, there is no experience in applying this blood purification technique to COVID-19 patients. Hence, any standard protocol for CytoSorb usage is absent to date and the pharmacological treatments and application procedures of CytoSorb may be diverse for different countries and centers. Therefore, these confounding factors inevitably affected outcomes. Third, as the advantages of CytoSorb in COVID-19 patients have not yet been fully investigated, only a small number of studies each with a very small sample size reported Cytosorb treatment for COVID-19 patients. Hence, the credibility of the conclusion from our meta-analysis may not be sufficient. Therefore, more RCTs with larger sample sizes will be needed to validate the conclusion.
In summary, this single-arm proportion meta-analysis demonstrated that the majority of COVID-19 patients treated with CytoSorb also received ECMO support. Furthermore, both CRP and IL-6 levels decreased after Cytosorb treatment, and the pooled in-hospital mortality was 42.1%. However, more RCTs are required to confirm the efficiency of Cytosorb treatment in COVID-19 patients.
Author contributions
YL proposed the view, designed and participated in the whole research process. Literature searching, screening, and data extracting was carried out by SW, YZ, KZ, JL, and ML. The data was checked and graphs and tables were generated by SW and YZ. The data results were analyzed by JY and RZ. YL and ZL provided guidance on the research and contributed to the final version of the paper. SW and YZ contributed equally to the overall research. A decision was made between YL and ZL regarding the specifics of publication of the article. All authors contributed to the article and approved the submitted version.
Funding
The study was funded by the Natural Sciences Foundation of Gansu (No. 20JR10RA760); the Talent Introduction Plan of the Lanzhou University Second Hospital (No. YJRCKYQDJ-2021-02); the Scientific Research Projects of Colleges in Gansu Province (No. 2020B-028, No. 2020B-037); the Cuiying Scientific and Technological Innovation Program of Lanzhou University Second Hospital (No. CY2019-QN01, CY2021-QN-B11, CY2021-QN-B04).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1067214/full#supplementary-material
References
1. Ismail SA, Saliba V, Lopez Bernal J, Ramsay ME, Ladhani SN. Sars-Cov-2 infection and transmission in educational settings: A prospective, cross-sectional analysis of infection clusters and outbreaks in England. Lancet Infect Dis (2021) 21(3):344–53. doi: 10.1016/s1473-3099(20)30882-3
2. Pérez-Fernández XL, Sabater-Riera J, Fuset-Cabanes M. Covid-19 Ards: Getting ventilation right. Lancet (2022) 399(10319):22. doi: 10.1016/s0140-6736(21)02439-9
3. Davies A, Jones D, Bailey M, Beca J, Bellomo R, Blackwell N, et al. Extracorporeal membrane oxygenation for 2009 influenza a(H1n1) acute respiratory distress syndrome. Jama (2009) 302(17):1888–95. doi: 10.1001/jama.2009.1535
4. Forrest P, Ratchford J, Burns B, Herkes R, Jackson A, Plunkett B, et al. Retrieval of critically ill adults using extracorporeal membrane oxygenation: An Australian experience. Intensive Care Med (2011) 37(5):824–30. doi: 10.1007/s00134-011-2158-8
5. Schmidt M, Hajage D, Lebreton G, Monsel A, Voiriot G, Levy D, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with covid-19: A retrospective cohort study. Lancet Respir Med (2020) 8(11):1121–31. doi: 10.1016/s2213-2600(20)30328-3
6. Barbaro RP, MacLaren G, Boonstra PS, Iwashyna TJ, Slutsky AS, Fan E, et al. Extracorporeal membrane oxygenation support in covid-19: An international cohort study of the extracorporeal life support organization registry. Lancet (2020) 396(10257):1071–8. doi: 10.1016/s0140-6736(20)32008-0
7. Zeng Y, Cai Z, Xianyu Y, Yang BX, Song T, Yan Q. Prognosis when using extracorporeal membrane oxygenation (Ecmo) for critically ill covid-19 patients in China: A retrospective case series. Crit Care (2020) 24(1):148. doi: 10.1186/s13054-020-2840-8
8. Goligher EC, Tomlinson G, Hajage D, Wijeysundera DN, Fan E, Jüni P, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome and posterior probability of mortality benefit in a Post hoc Bayesian analysis of a randomized clinical trial. Jama (2018) 320(21):2251–9. doi: 10.1001/jama.2018.14276
9. Cron RQ, Caricchio R, Chatham WW. Calming the cytokine storm in covid-19. Nat Med (2021) 27(10):1674–5. doi: 10.1038/s41591-021-01500-9
10. Mortaz E, Tabarsi P, Jamaati H, Dalil Roofchayee N, Dezfuli NK, Hashemian SM, et al. Increased serum levels of soluble tnf-α receptor is associated with icu mortality in covid-19 patients. Front Immunol (2021) 12:592727. doi: 10.3389/fimmu.2021.592727
11. Fonseca W, Asai N, Yagi K, Malinczak CA, Savickas G, Johnson CC, et al. Covid-19 modulates inflammatory and renal markers that may predict hospital outcomes among African American males. Viruses (2021) 13(12):2415. doi: 10.3390/v13122415
12. Perreau M, Suffiotti M, Marques-Vidal P, Wiedemann A, Levy Y, Laouénan C, et al. The cytokines hgf and Cxcl13 predict the severity and the mortality in covid-19 patients. Nat Commun (2021) 12(1):4888. doi: 10.1038/s41467-021-25191-5
13. Köhler T, Schwier E, Praxenthaler J, Kirchner C, Henzler D, Eickmeyer C. Therapeutic modulation of the host defense by hemoadsorption with cytosorb(®)-basics, indications and perspectives-a scoping review. Int J Mol Sci (2021) 22(23):12786. doi: 10.3390/ijms222312786
14. Datzmann T, Träger K. Extracorporeal membrane oxygenation and cytokine adsorption. J Thorac Dis (2018) 10(Suppl 5):S653–s60. doi: 10.21037/jtd.2017.10.128
15. Ruiz-Rodríguez JC, Molnar Z, Deliargyris EN, Ferrer R. The use of cytosorb therapy in critically ill covid-19 patients: Review of the rationale and current clinical experiences. Crit Care Res Pract (2021) 2021:7769516. doi: 10.1155/2021/7769516
16. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. Bmj (2009) 339:b2700. doi: 10.1136/bmj.b2700
17. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res (2018) 27(6):1785–805. doi: 10.1177/0962280216669183
18. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol (2014) 14:135. doi: 10.1186/1471-2288-14-135
19. Alharthy A, Faqihi F, Memish ZA, Balhamar A, Nasim N, Shahzad A, et al. Continuous renal replacement therapy with the addition of cytosorb cartridge in critically ill patients with covid-19 plus acute kidney injury: A case-series. Artif Organs (2021) 45(5):E101–e12. doi: 10.1111/aor.13864
20. Damiani M, Gandini L, Landi F, Borleri G, Fabretti F, Gritti G, et al. Extracorporeal cytokine hemadsorption in severe covid-19 respiratory failure. Respir Med (2021) 185:106477. doi: 10.1016/j.rmed.2021.106477
21. Lebreton G, Dorgham K, Quentric P, Combes A, Gorochov G, Schmidt M. Longitudinal cytokine profiling in patients with severe covid-19 on extracorporeal membrane oxygenation and hemoadsorption. Am J Respir Crit Care Med (2021) 203(11):1433–5. doi: 10.1164/rccm.202011-4140LE
22. Lewis TC, Merchan C, Toy B, Goldenberg RM, Geraci TC, Chang SH, et al. Impact of cytosorb hemoadsorption on sedation requirements in patients with severe covid-19 on venovenous extracorporeal membrane oxygenation. Asaio J (2021) 67(8):856–61. doi: 10.1097/mat.0000000000001513
23. Mehta Y, Mehta C, Nanda S, Kochar G, George JV, Singh MK. Use of cytosorb therapy to treat critically ill coronavirus disease 2019 patients: A case series. J Med Case Rep (2021) 15(1):476. doi: 10.1186/s13256-021-03021-y
24. Nassiri AA, Hakemi MS, Miri MM, Shahrami R, Koomleh AA, Sabaghian T. Blood purification with cytosorb in critically ill covid-19 patients: A case series of 26 patients. Artif Organs (2021) 45(11):1338–47. doi: 10.1111/aor.14024
25. Paisey C, Patvardhan C, Mackay M, Vuylsteke A, Bhagra SK. Continuous hemadsorption with cytokine adsorber for severe covid-19: A case series of 15 patients. Int J Artif Organs (2021) 44(10):664–74. doi: 10.1177/03913988211023782
26. Peng JY, Li L, Zhao X, Ding F, Hou X, Peng Z. Hemoperfusion with cytosorb® in critically ill covid-19 patients. Blood Purif (2021) 51(5):1–7. doi: 10.1159/000517721
27. Pieri M, Fominskiy E, Nardelli P, Bonizzoni MA, Scandroglio AM. Cytosorb purification in critically ill sars-Cov-2 patients. Int J Artif Organs (2022) 45(2):216–20. doi: 10.1177/03913988211052572
28. Rampino T, Gregorini M, Perotti L, Ferrari F, Pattonieri EF, Grignano MA, et al. Hemoperfusion with cytosorb as adjuvant therapy in critically ill patients with sars-Cov2 pneumonia. Blood Purif (2021) 50(4-5):566–71. doi: 10.1159/000511725
29. Rodeia SC, Martins FL, Fortuna P, Bento L. Cytokine adsorption therapy during extracorporeal membrane oxygenation in adult patients with covid-19. Blood Purif (2021) 51(9):1–7. doi: 10.1159/000518712
30. Supady A, Weber E, Rieder M, Lother A, Niklaus T, Zahn T, et al. Cytokine adsorption in patients with severe covid-19 pneumonia requiring extracorporeal membrane oxygenation (Cycov): A single centre, open-label, randomised, controlled trial. Lancet Respir Med (2021) 9(7):755–62. doi: 10.1016/s2213-2600(21)00177-6
31. Virág M, Rottler M, Ocskay K, Leiner T, Horváth B, Blanco DA, et al. Extracorporeal cytokine removal in critically ill covid-19 patients: A case series. Front Med (Lausanne) (2021) 8:760435. doi: 10.3389/fmed.2021.760435
32. Song T, Hayanga J, Durham L, Garrison L, McCarthy P, Barksdale A, et al. Cytosorb therapy in covid-19 (Ctc) patients requiring extracorporeal membrane oxygenation: A multicenter, retrospective registry. Front Med (Lausanne) (2021) 8:773461. doi: 10.3389/fmed.2021.773461
33. Ramanathan K, Shekar K, Ling RR, Barbaro RP, Wong SN, Tan CS, et al. Extracorporeal membrane oxygenation for covid-19: A systematic review and meta-analysis. Crit Care (2021) 25(1):211. doi: 10.1186/s13054-021-03634-1
34. Zhang JJ, Dong X, Liu GH, Gao YD. Risk and protective factors for covid-19 morbidity, severity, and mortality. Clin Rev Allergy Immunol (2022) 19:1–18. doi: 10.1007/s12016-022-08921-5
35. Tsukamoto T, Nakajima N, Sakurai A, Nakajima M, Sakurai E, Sato Y, et al. Lung pathology of mutually exclusive Co-infection with sars-Cov-2 and streptococcus pneumoniae. Emerg Infect Dis (2021) 27(3):919–23. doi: 10.3201/eid2703.204024
36. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. Covid-19: Consider cytokine storm syndromes and immunosuppression. Lancet (2020) 395(10229):1033–4. doi: 10.1016/s0140-6736(20)30628-0
37. Masotti L, Grifoni E, Pelagalli G, Cioni E, Mattaliano C, Cioffi E, et al. Prognostic role of interleukin-6/Lymphocytes ratio in sars-Cov2 related pneumonia. Int Immunopharmacol (2022) 103:108435. doi: 10.1016/j.intimp.2021.108435
38. Kox M, Waalders NJB, Kooistra EJ, Gerretsen J, Pickkers P. Cytokine levels in critically ill patients with covid-19 and other conditions. Jama (2020) 324(15):1565–7. doi: 10.1001/jama.2020.17052
39. Dzobo K, Chiririwa H, Dandara C, Dzobo W. Coronavirus disease-2019 treatment strategies targeting interleukin-6 signaling and herbal medicine. Omics (2021) 25(1):13–22. doi: 10.1089/omi.2020.0122
40. Mast AE, Wolberg AS, Gailani D, Garvin MR, Alvarez C, Miller JI, et al. Sars-Cov-2 suppresses anticoagulant and fibrinolytic gene expression in the lung. Elife (2021) 10:e64330. doi: 10.7554/eLife.64330
41. Friesecke S, Träger K, Schittek GA, Molnar Z, Bach F, Kogelmann K, et al. International registry on the use of the cytosorb® adsorber in icu patients : Study protocol and preliminary results. Med Klin Intensivmed Notfmed (2019) 114(8):699–707. doi: 10.1007/s00063-017-0342-5
42. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in hospitalized patients with covid-19. N Engl J Med (2021) 384(8):693–704. doi: 10.1056/NEJMoa2021436
43. Barbaro RP, MacLaren G, Boonstra PS, Combes A, Agerstrand C, Annich G, et al. Extracorporeal membrane oxygenation for covid-19: Evolving outcomes from the international extracorporeal life support organization registry. Lancet (2021) 398(10307):1230–8. doi: 10.1016/s0140-6736(21)01960-7
44. CytoSorb 300mL device: Authorized by FDA for emergency treatment of COVID-19(2020). Available at: https://www.fda.gov/media/136866/download (Accessed September 03, 2021).
Keywords: CytoSorb, coronavirus disease 2019, cytokines, cytokine hemadsorption, meta-analysis
Citation: Wei S, Zhang Y, Zhai K, Li J, Li M, Yang J, Zhang R, Li Y and Li Z (2023) CytoSorb in patients with coronavirus disease 2019: A rapid evidence review and meta-analysis. Front. Immunol. 14:1067214. doi: 10.3389/fimmu.2023.1067214
Received: 11 October 2022; Accepted: 17 January 2023;
Published: 31 January 2023.
Edited by:
Congshan Jiang, Xi’an Children’s Hospital, ChinaReviewed by:
JW Hayanga, West Virginia University, United StatesSamuele Ceruti, Clinica Luganese Moncucco, Switzerland
Copyright © 2023 Wei, Zhang, Zhai, Li, Li, Yang, Zhang, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenzhen Li, NTY5MjgxNDcwQHFxLmNvbQ==; Yongnan Li, bHluZ3lxMjAwNkBmb3htYWlsLmNvbQ==
†These authors have contributed equally to this work
 Shilin Wei1,2†
Shilin Wei1,2† Jian Li
Jian Li Yongnan Li
Yongnan Li