- Department of Plastic Surgery, The Third Xiangya Hospital, Central South University, Changsha, China
Hyperpigmentation is a common complication in patients with burn injuries during wound healing; however, the mechanisms underlying its occurrence and development remain unclear. Recently, postinflammatory hyperpigmentation (PIH) was found to result from overproduction of melanin. Local or systemic inflammatory responses are often observed in patients who develop hyperpigmentation. However, we lack studies on the relationship between PIH and burn injury. Therefore, we comprehensively reviewed the existing literature on the melanogenesis of the skin, inflammatory mechanisms in pigmentation, and local or systemic alteration in inflammatory cytokines in patients suffering from burn trauma to elucidate the relationship between PIH and burn injury. We believe that this review will guide further research on regulating melanin production in the burn management process.
1 Introduction
An estimated 11 million burn injury cases are reported annually worldwide (1). Approximately 50%–60% of the affected people, especially those with a dark complexion, develop hyperpigmentation at the burn injury site (2). Hyperpigmentation is a common condition that increases the economic burden of patients and reduces their quality of life by leaving an irreversible dermatological abrasion and severely affecting their psychological health. Postinflammatory hyperpigmentation (PIH) is a reactive and acquired hyperpigmentation of the epidermis and dermis, with variable pathogeneses and etiologies, including dermatoses, burn injury, and cosmetic procedures (3–5). Among these, burn trauma, especially severe burns, should never be ignored, as it is associated with pigmentation disorders (6, 7) as well as increased systemic and local inflammatory activities (8, 9). However, further studies are required to establish the relationship between PIH and burn injury-related inflammatory responses. Therefore, we have presented an overview of melanogenesis, mechanisms of PIH, and inflammation induced by burn injury. We have discussed their potential relationships, the limitations of the present research, and an orientation for future research.
2 Melanogenesis
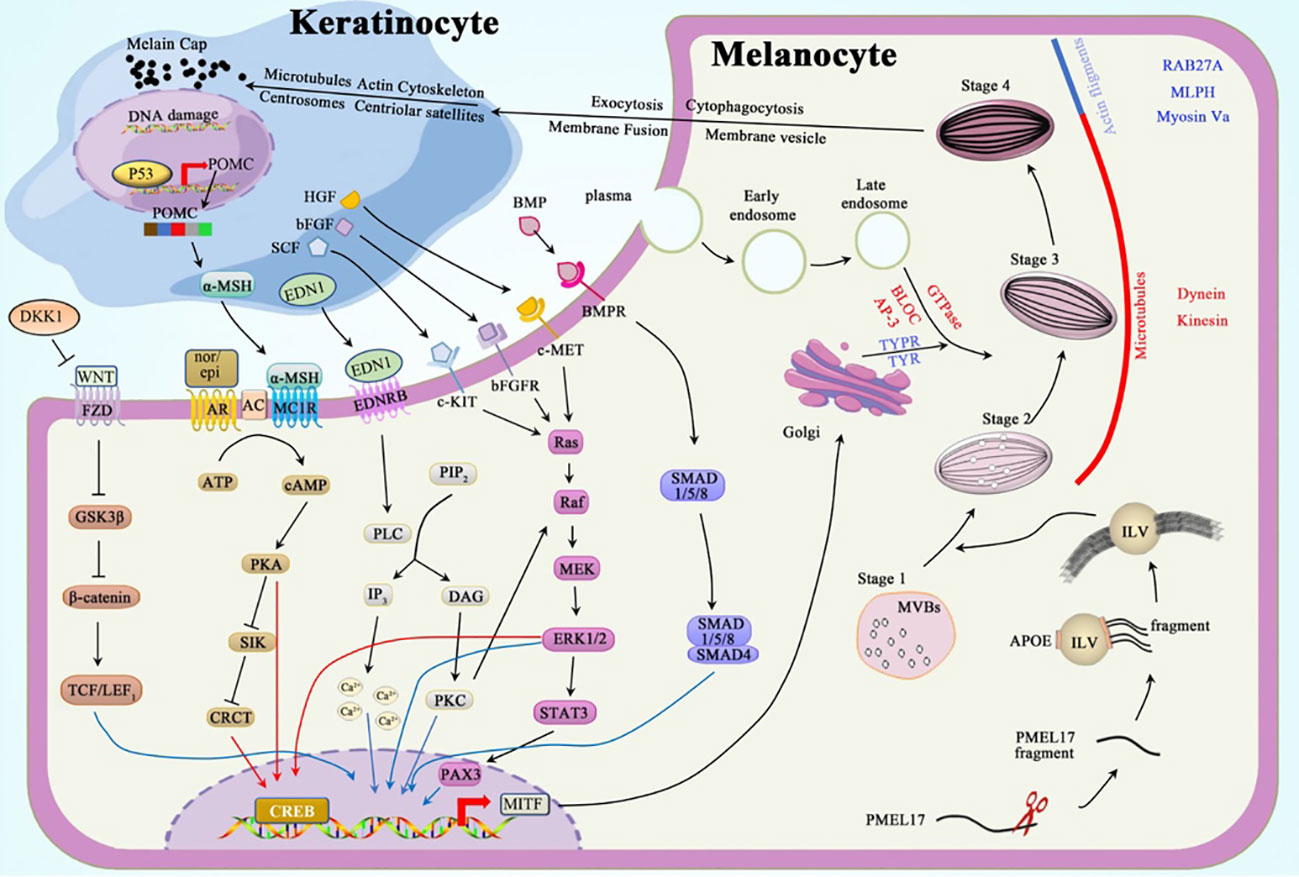
Skin phenotype in individuals is mediated by the deposition of melanin granules in keratinocytes. These granules are transported from epidermal melanocytes through melanosomes (10, 11). Each melanocyte can interact with 40 viable keratinocytes adjacent to its dendrites, forming an epidermal melanin unit (12). The mature melanosomes carrying melanin granules are transferred from the dendrites of melanocytes into the cytoplasm of keratinocytes through exocytosis, cytophagocytosis, plasma membrane fusion, and membrane vesicle transfer (11, 13–15). The movement of microtubules, actin cytoskeleton, centrosomes, and centriolar satellites in keratinocytes carries the melanin-laden melanosomes to the supranuclear region to form microparasols, thereby protecting the epidermal DNA from UV-induced stimuli or damage (16). Conversely, keratinocytes, fibroblasts, and immune cells regulate pigmentation through hormones and cytokines (17).
Neural crest cells, migrating from the dorsolateral and ventral route, differentiate into melanoblasts by wingless-related integration site (WNT) signaling and subsequently form melanocytes in the hair follicles and epidermis (18, 19). Melanosomes, the tissue-specific lysosome-related organelles (LROs) located in the melanocytes, are the factories synthesizing and packing melanin.
Melanosomes undergo maturation through four stages (Figure 1): Stages I–II include premelanosomes, which cannot synthesize melanin until they mature to stages III–IV. Stage I melanosomes originate as multivesicular bodies (MVBs) containing intraluminal vesicles (ILVs) and melanocyte protein (PMEL17) (20). PMEL17 fragments are cleaved from the pre-melanosomal membrane and bound to the ILV surface. This process is modulated by apolipoprotein E (ApoE); this explains the elliptical shape of eumelanosomes since stage II, whereas pheomelanosomes are spherical owing to the suppression of PMEL17 expression by agouti signaling (21). Concordantly, the shape of melanosomes depends entirely on the form of PMEL17 fragments, and the reduced levels of these protein fibrils lead to the morphological disruption of melanosomes (22, 23). In stage III, melanogenic enzymes, tyrosinase (TYR) and tyrosinase-related protein-1 (TYRP1), are transported from the Golgi apparatus into melanosomes to produce melanin to be deposited on the amyloid fibrils of PMEL17 (24). In stage IV, the melanin accumulation on these fibrils is complete, contributing to the heavy pigmentation of melanosomes observed under the electron microscope (EM) (25).
2.1 Melanin biogenesis and transport within melanosomes
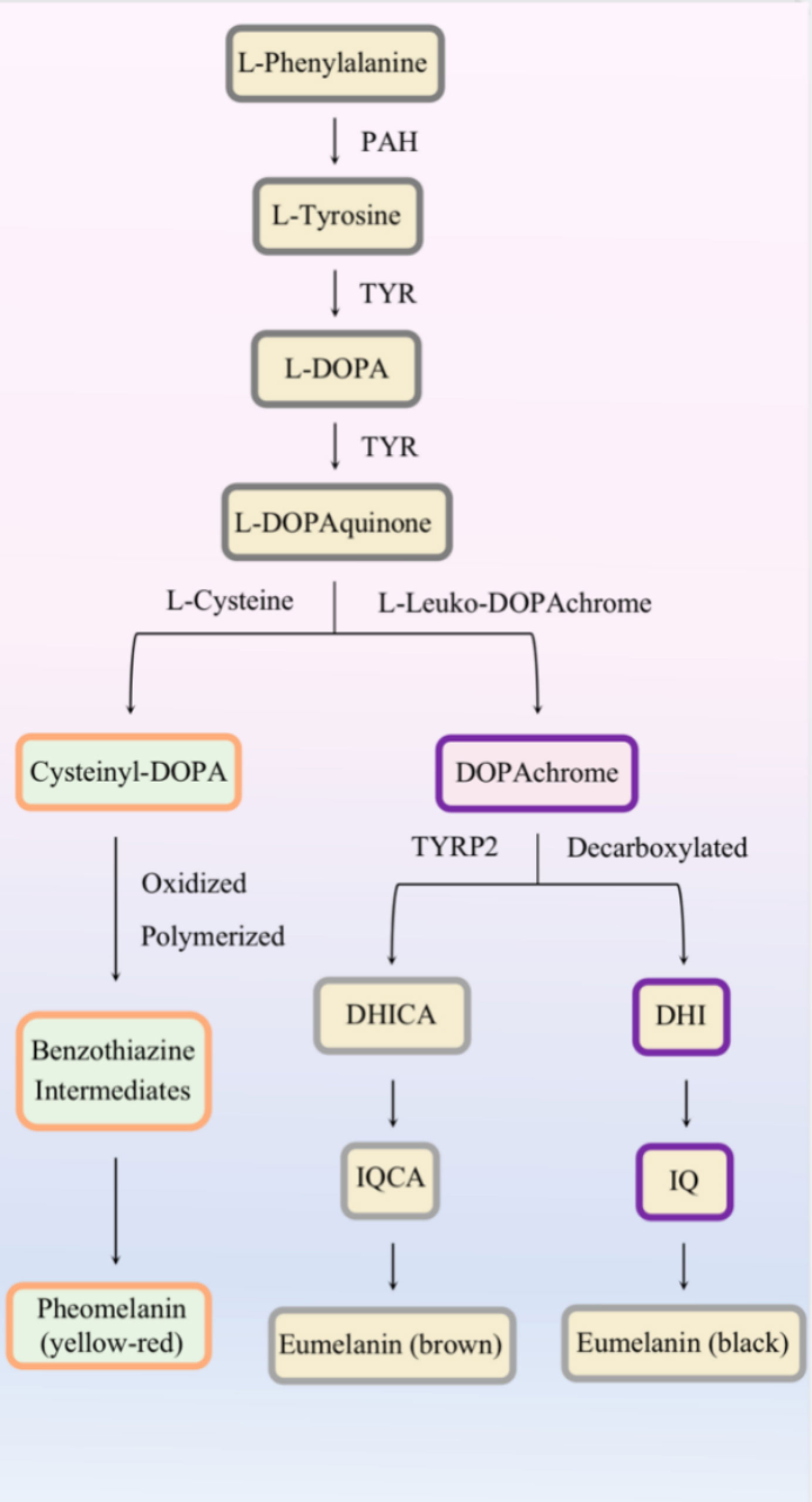
Eumelanin and pheomelanin are synthesized in stages III–IV under the regulation of melanogenic enzymes (Figure 2). Except for the presence or absence of L-cysteine, altered pH in melanosomes can influence the balance between pheomelanogenesis and eumelanogenesis (26, 27).
The melanosomes are trafficked through a centrifugal route in the melanocytes; melanosomes are transferred from the perinuclear area to the dendrites through complementary routes mediated by microtubules (MTs) and actin filaments (AFs) (28). These trafficking mechanisms are independent: maturing melanosomes move to the periphery of melanocytes through (driven by kinesin/dynein motors) during long-distance and bidirectional transport. Once at the periphery, these pigment-deposited organelles are transferred by actin-based Rab27a/Melanophilin/Myosin-Va complex to undergo short-distance dispersion, thereby preventing reverse transport along MTs (29, 30).
The mechanism by which melanosome transfers into the keratinocytes remains elusive; however, four classic models have been proposed to explain this process: 1) exocytosis, 2) cytophagocytosis, 3) fusion of plasma membrane, and 4) membrane vesicle. Among them, exocytosis may be the most plausible mechanism, as the melanocytes in the extracellular space and melanin in keratinocytes are only surrounded by a single membrane lacking TYRP1. After entering the keratinocytes, the microtubules, actin cytoskeleton, centrosomes, and centriolar satellites likely facilitate melanin granule distribution in keratinocytes (16).
2.2 Melanogenesis regulation
Intrinsic and extrinsic factors regulate melanogenesis by various signal pathways, including protein kinase A (PKA), mitogen-activated protein kinase (MAPK), protein kinase C (PKC), WNT/β-catenin, and bone morphogenetic protein (BMP)/Smad cascades. Microphthalmia-associated transcription factor (MITF) in its phosphorylated active form plays a vital role in regulating these cascades; it enhances the expression of melanogenic enzymes, Rab27a protein, and the melanosomal matrix protein PMEL17 (31).
2.2.1 PKA cascade
The activation of adenylate cyclase (AC) catalyzes the conversion of abundant ATP into the second messenger cyclic AMP (cAMP), which attaches to the R-subunit of PKA, thereby activating PKA. PKA subsequently phosphorylates the cAMP response element-binding protein (CREB) and salt-inducible kinase (SIK). CREB can directly enhance the MITF overexpression (32). SIK, which is suppressed after phosphorylation, releases more unphosphorylated CREB-regulated transcription coactivator (CRCT), which shuttles into the nucleus and binds to the already activated CREB. This complex cooperatively activates the promotor of MITF (33–35).
Alpha-melanocyte-stimulating hormone (α-MSH) and catecholamines can regulate melanogenesis via the cAMP-PKA pathway. Impairment of the keratinocyte DNA results in the upregulation of p53 and, subsequently, the pro-opiomelanocortin (POMC) gene (36). Proteolytic cleavage of the POMC protein results in the formation of adrenocorticotropic hormone (ACTH) and α-MSH. α-MSHs produced from keratinocytes act as agonists to melanocortin 1 receptor (MC1R) on melanocytes, thereby increasing cAMP levels. Epinephrine and norepinephrine, which are catecholamines, act on their G protein-coupled receptors (GPCRs); the binding of these two first messengers to GPCRs separates Gαs subunit and stimulates the production of AC (32).
2.2.2 MAPK cascade
MAPK signaling is achieved through the following process: mitogen-activated protein kinase kinase kinase (Raf or MAPKKK) activates mitogen-activated protein kinase kinase (MEK or MAPKK) and, consequently, extracellular signal-regulated kinase (ERK or MAPK). The activation of downstream Raf-MEK-ERK boosts the transcription of CREB and MITF (37).
Stem cell factor (SCF) (38), basic fibroblast growth factor (bFGF) (39), and hepatocyte growth factor (HGF) (40) can bind to their tyrosine kinase receptors, c-Kit, bFGFR, and c-MET, respectively. Once bound, these receptors dimerize to boost the activity of tyrosine kinases in the intracellular juxtamembrane region to control autophosphorylation. Phosphorylated tyrosine residues conscript Src homology 2 (SH2) and pTyr-binding (PTB) domains (41). This alteration converts Ras GDP into Ras GTP-binding proteins, which are essential for the activation of Raf-1, which consequently activates the MAPK cascade.
2.2.3 PKC cascade
The PKC pathway, which regulates melanogenesis, can be induced by the binding of endothelin 1 (EDN1) to its GPCR. After the complex formation, the Gαq unit activates phospholipase Cβ (PLCβ), which hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) to inositol triphosphate (IP3) and diacylglycerol (DAG). IP3 elicits a strong cytosolic Ca2+ response in melanocyte dendrites (42, 43), whereas DAG activates PKC, which can enhance the expression of MITF directly or indirectly via the MAPK cascade (44).
2.2.4 WNT/β-catenin cascade
Frizzled receptors (FZD) bind to the transmembrane molecule LRP5/6 to form the LRP-FZD dimer complex that modulates cell differentiation and proliferation (45). As a ligand of the LRP-FZD, wingless-type MMTV integration site family members (Wnt), whose expression can be upregulated by exposure to a high dose of UV rays (46). This excessively expressed growth factor phosphorylates and inactivates glycogen synthase kinase 3β (GSK3β) after binding to the LRP-FZD complex. In the absence of active GSK3β, the accumulated β‐catenin protein in the cytoplasm is then translocated into the nucleus, where it interacts with T-cell Factor (TCF)/Lymphoid Enhancing Factor 1 (LEF1) to increase MITF levels (47, 48).
2.2.5 BMP/Smad cascade
BMP regulates dorsoventral and anterior/posterior axis formation, particularly in the neural crest cells, which require the expression of BMP2 and BMP4 (49). Signals generated by the assembly of BMP-BMPR and SMAD1/5/8 are recruited in the cytoplasm to be phosphorylated and to form a complex with co-SMAD4. This complex subsequently relocates into the nucleus to regulate MITF expression (50).
3 Postinflammatory hyperpigmentation
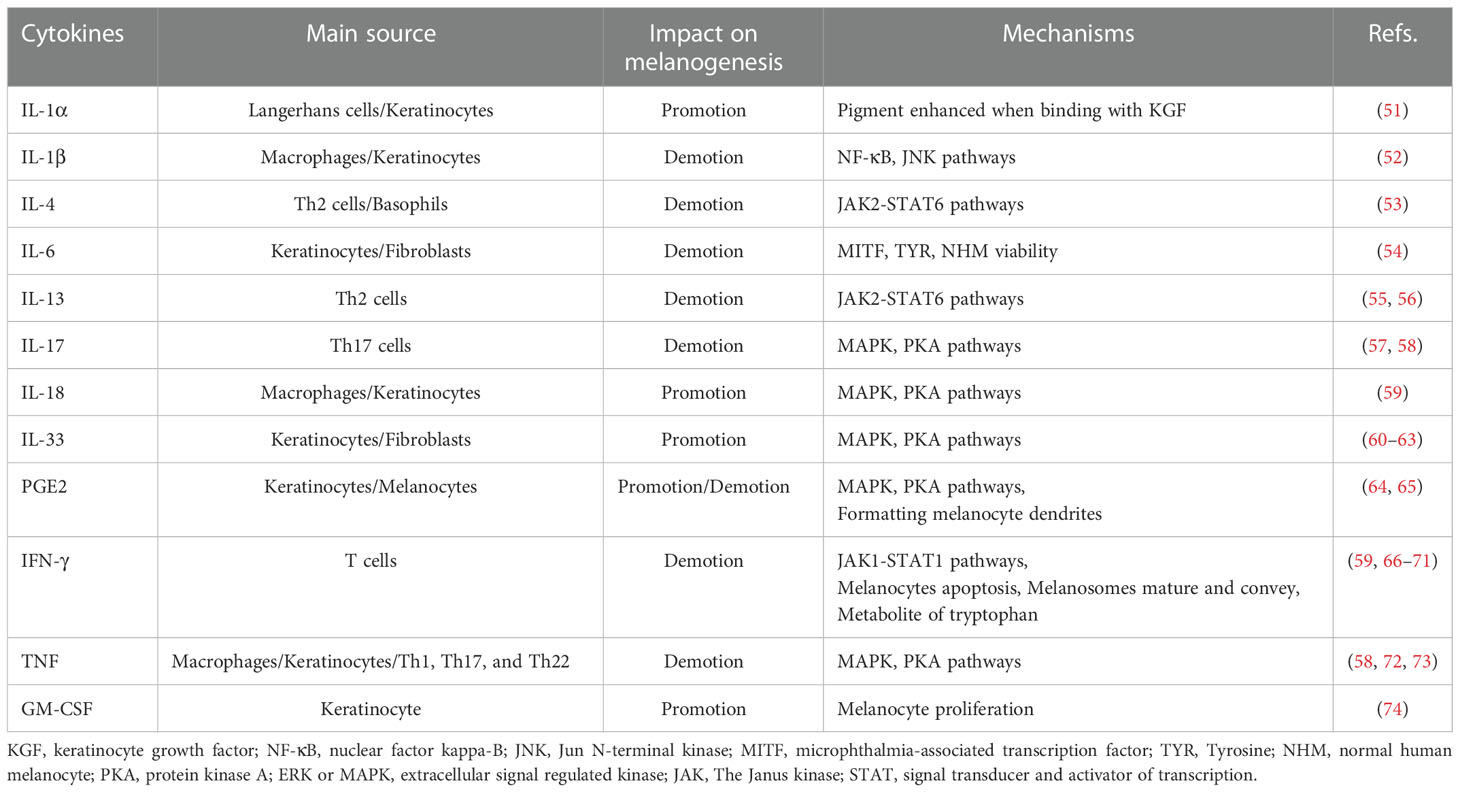
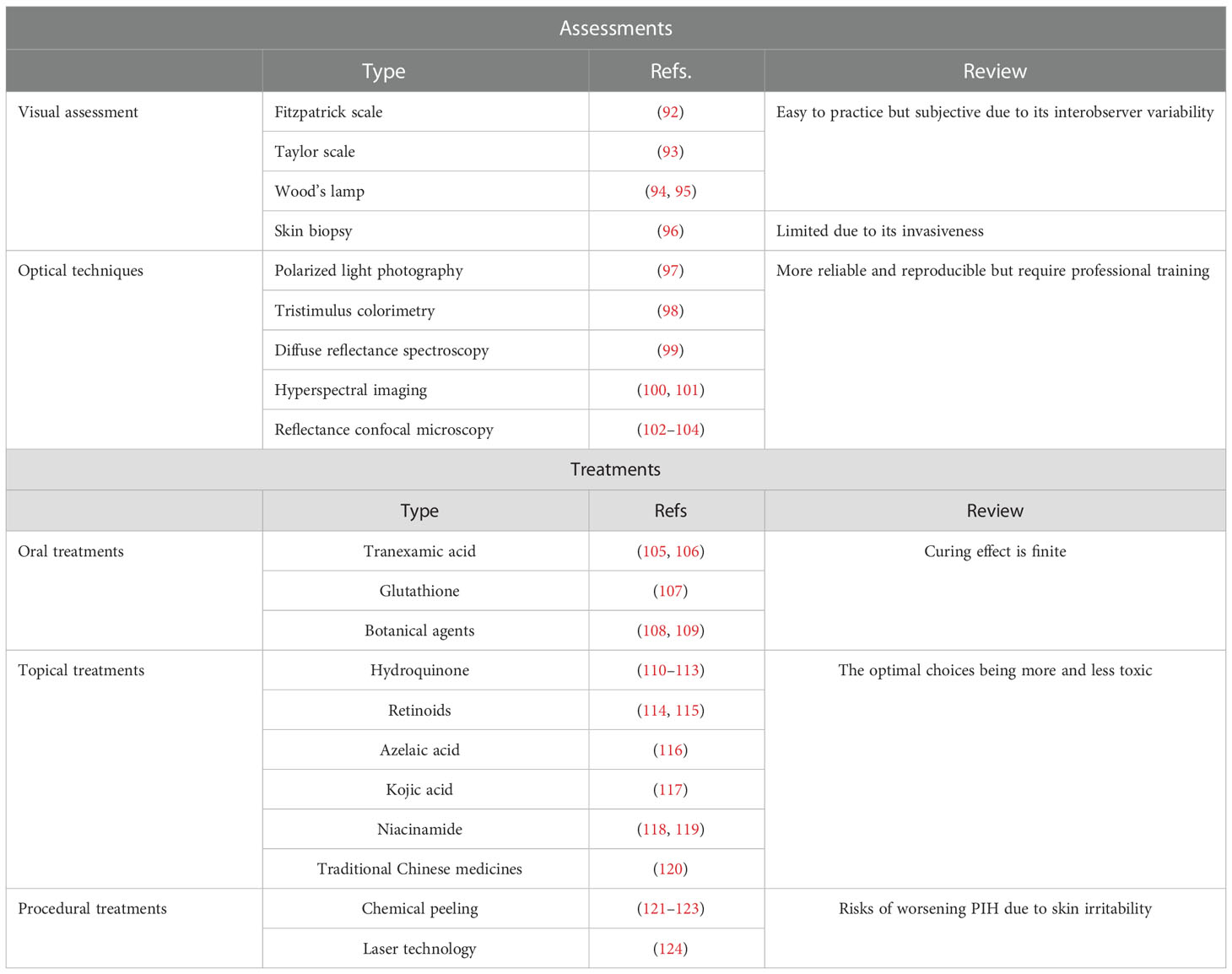
PIH is an acquired pigmentary disorder that primarily affects patients with darker skin types (Fitzpatrick types III–VI). Nonetheless, all skin types suffer from PIH owing to endogenous and exogenous injuries. Several experiments exploring inflammatory mediators have been conducted based on the specific mechanism of inflammatory responses (Table 1).
The advent of genomic medicine has confirmed the indispensable role of PKA and MAPK signaling pathways in various biological processes, especially those related to oncogenesis or its progression. Similarly, most inflammatory cytokines regulate melanogenesis via these two cascades. Interleukin (IL)-18 and IL-33 contribute to the expression of MITF and other related enzymatic expressions by stimulating the PKA and MAPK pathways (59, 60). IL-33 expression, influenced by IL-17 and interferon (IFN)-γ, establishes a negative feedback loop resulting in pigmentation (61, 62). However, IL-33 is also speculated to have a positive feedback loop with tumor necrosis factor alpha (TNF-α), which induces melanocyte death resulting in vitiligo (63).
IL-17 adversely affects melanogenesis because the expression of MITF and its downstream genes increases on blocking with anti-IL-17RA (57). IL-17 and TNF synergistically inhibit melanin production (58). Neutralization of TNF and IL-17 with monoclonal antibodies (mAbs) increased the levels of c-KIT, MITF, and TYRP2 (58, 72). This suggests that TNF induces IL-17 to exert a negative effect on melanin synthesis through MAPK and PKA signaling pathways (72, 73).
Under the control of phospholipase A2 (PLA2), cyclooxygenase (COX), and prostaglandin E synthase (PGES), PGE2 is released by keratinocytes and epidermal melanocytes (64). In response to PGE2, its receptor EP3 suppresses cAMP production, thereby preventing pigmentation. In contrast, EP4 receptor activation may increase basal cAMP levels, stimulating tyrosinase and the formation of dendrites in melanocytes (65).
Janus kinase (JAK) and signal transducer and activator of transcription (STAT) have been known as rapid membrane-to-nucleus signaling modules. They have been associated with cancer and inflammation for the past two decades. However, compared with PKA and MAPK pathways, the regulation of melanogenesis by the JAK-STAT pathway is relatively unknown. However, several cell cytokines have been reported to alter melanocyte function through the JAK-STAT pathway.
Studies on normal human melanocyte (NHM) cultures confirmed that IL-4 produces melanin by downregulating MITF and dopachrome tautomerase expression through the JAK2-STAT6 signaling pathway (53). IL-13, which shares JAK2-STAT6 signaling pathways with IL-4 (55), can inhibit the mRNA and protein expression levels of both tyrosinase and Dopachrome Tautomerase (DCT), thus impacting melanin synthesis (56).
By upgrading the phosphorylation of the JAK1-STAT1 cascade, IFN-γ mediates reversible and independent MITF dyspigmentation through Recombinant Interferon Regulatory Factor 1 (IRF1) binding and DCT promoter repression in a dose-independent manner (66). By associating CREB -binding protein (CBP) with elevated STAT1, IFN-γ can also inhibit the binding between CBP and CREB. In this manner, by not affecting CREB phosphorylation, α-MSH-induced melanogenesis exhibits inhibition (67).
IFN-γ can induce hypopigmentation via other mechanisms, including apoptosis in melanocytes (68), arresting melanosome maturation and transportation (66), and metabolism of tryptophan (69–71). Moreover, it is also known that IFN-γ shares crosstalk with IL-18, wherein IFN-γ inhibits IL-18-induced melanogenesis indirectly (59).
Other than the cascades referred to in this section, some cytokines can also influence pigmentary deposition via other mechanisms. IL-1α, a subtype of IL-1, initiates IL-1 receptor type I (IL-1RI), increasing little pigment deposition. However, this effect is in combination with the keratinocyte growth factor (KGF) (51). Conversely, IL-1β, another form of IL-1, is presumed to inhibit MITF through the nuclear factor kappa-B (NF-κB) and Jun N-terminal kinase (JNK) pathways (52). IL-6 treatment, analyzed in vitro and in vivo, decreases the viability of NHM, MITF, and TYR in a dose-dependent manner (54). Anti-granulocyte-macrophage colony-stimulating factor (GM-CSF) treatment led to the nulling of the keratinocyte regulates Melan-A melanocyte proliferation, indicating that the proliferation of melanocytes is augmented by GM-CSF (74).
4 Inflammation after burn injury
Pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) are the two main mechanisms that trigger inflammatory responses. PAMPs are caused by diverse microbial molecules, especially lipopolysaccharides (LPSs) discovered in Gram-negative bacteria. In contrast, DAMPs require environmental alterations, such as trauma, thermal stimuli, or other damage to cause sterile inflammation (75). Toll-like receptors (TLRs), members of pattern recognition receptors (PRRs), stimulated and shared by both DAMPs and PAMPs, can activate intracellular signals to regulate inflammation and ensure similarity of inflammatory responses even under different stimuli (76). The activation of TLRs releases many inflammatory cytokines from the damaged tissue into circulation through myeloid differentiation primary response protein-88 (MyD88), leading to the transcription of activator protein 1 (AP-1) and NF-κB (77). The increase in cytokines, locally and systemically, causes infection, impaired healing, pigmentation disorders, and systemic inflammatory response syndrome.
Burn injury, a unique DAMP (78), affects the skin, which is the barrier protecting the human body from pathogens and environmental stimuli. The impacted skin forms debris of tissues and eschar, which serve as a reservoir for diverse bacteria and PAMPs, especially around hair follicles with a higher bacterial load (79). Additionally, TLR expression on dendritic cells is upregulated in severe burn wounds (80).
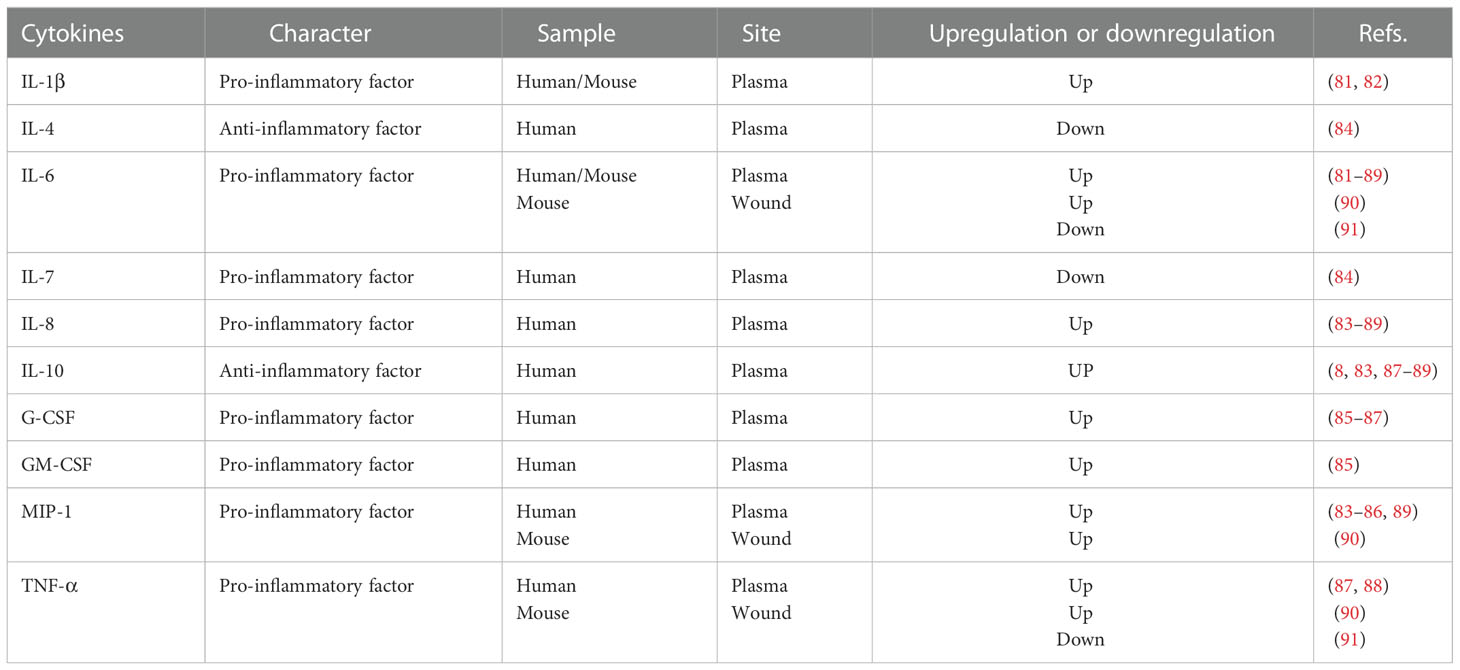
The downstream nucleus, called cytokines in circulation, has been described for appraising and monitoring patients with burns. IL-1β has been reported for its poor outcome correlation, such as systemic inflammatory response syndrome or even death (81, 82). IL-6 specifically induces remote organ inflammation (81–89) (Table 2). IL-8 and IL-10 expression levels are upregulated (8, 83–89), whereas IL-4 and IL-7 levels are downregulated (84). Meanwhile, the plasma levels of other cytokines, such as granulocyte colony-stimulating factor (G-CSF), GM-CSF, macrophage inflammatory protein-1 (MIP-1), and TNF-α, were also observed to increase in patients with burn wounds (83–89). Similarly, changes in cytokine levels in local burn wounds have also been observed. In the burn trauma mouse model, levels of IL-6, TNF-α, and MCP-1 showed a significant increase (90). However, Schwacha et al. (91) reported contradictory results based on their burn wound mouse models.
5 Assessments and treatments
Currently, only limited assessments and treatments specifically target burn trauma-induced PIH. We have summarized and evaluated the currently available therapies for PIH to facilitate further research on developing treatments against burn injury-induced pigmentation disorders (Table 3).
5.1 Visual assessment
The use and application of the visual hyperpigmentation scale in clinical practice are straightforward. The Fitzpatrick scale was created to classify human skin into six phototypes (I–VI) based on tanning response to UVR (92). The Taylor hyperpigmentation scale with 150 gradations for hyperpigmentation was proposed to obtain further information; this scale comprises plastic cards in 15 different colors (A–J) and 10 pigmentary gradations for each color card (93).
Wood’s lamp (340–400 nm, with maximum output at 365 nm) emits UV and visible light (94). Hence, epidermal PIH appears darker in contrast to unaffected healthy skin. However, PIH that occurs in the dermis cannot be distinguished as well as that in the epidermis under the Wood’s lamp examination. The accuracy of Wood’s lamps in differentiating between dermal and mixed melasma can be improved with the assistance of dermoscopy (95).
Skin biopsy is a standard method to support PIH diagnosis, excluding certain hyperpigmentation disorders such as melasma and drug-induced hyperpigmentation. Histopathologically, PIH presents perivascular or perifollicular lymphocytic inflammation, dermal melanophages, and epidermal melanin without basal cell vacuolization (96). Using skin biopsy as a criterion for diagnosing PIH and other similar disorders is limited owing to its invasive nature.
5.2 Optical techniques
Unlike subjective clinical assessments, noninvasive technologies supplement more credible and repeatable outcomes for PIH. These consist of polarized light photography, colorimetry, diffuse reflectance spectroscopy (DRS), hyperspectral imaging (HSI), and reflectance confocal microscopy (RCM).
Polarized light photography is sensitive to detecting dermal changes, especially vascular changes. When using parallel polarizing filters, melanosis on the skin surface can be visualized using the incident light source at a certain angle to the camera. Cross-polarized photography can be used to visualize hyperpigmentation and subsurface features such as vascularity, whereas parallel-polarized photography is used for skin texture (97).
Tristimulus colorimetry is analogous to human eyes in perceiving color. There are three axes, including L* (lightness-darkness) axis, a* (red-green) axis, and b* (blue-yellow) axis, which describe and plot color in a three-dimensional space. L* and b* values are used to measure pigmentation (98).
DRS, an in vivo measurement, can compute and quantify the biochemical concentrations of skin melanin, oxyhemoglobin, and deoxyhemoglobin, according to their absorbance characteristics (99). It is also used to estimate PIH because of its function of quantifying melanin.
HSI measures spectral bands and provides sensitive measurements. In reflectance spectra, it can capture subtle alterations and quantify optical properties of the skin, containing information about hemoglobin and melanin (100). Recently, this method was integrated with machine learning—an established structure-adaptive normalized convolution algorithm (101).
RCM, a great advance in dermatology, provides high-contrast images of melanin granules. By emitting and detecting near-infrared wavelength laser beam, RCM can visualize the layers between the epidermis to the upper reticular dermis to a depth of 250–300 μm (102). Owing to the relative differences in refractive indices and sizes of cell organelles and keratin, signal contrast of melanin can be obtained (103). Therefore, RCM has been used in evaluating the PIH model dynamically (104).
5.3 Oral treatments
Tranexamic acid (TA), widely used to fix abnormal fibrinolysis, effectively inhibits melanogenesis. TA likely blocks UV-induced plasmin activity, which decreases the levels of raw material of tyrosinase— arachidonic acid and prostaglandins (105). Oral TA effectively decreases the mean melanin index in TA-treated keratinocyte-conditioned medium (KCM) (106).
Glutathione, an antioxidant with skin-lightening activity, can switch eumelanin production into pheomelanin. The reaction of the glutathione thiol with dopaquinone forms a sulfhydryl–dopa conjugate that results in the formation of pheomelanin instead of eumelanin (107).
Other botanical agents proposed for their lightening effects include ginseng and grape seed. Ginsenosides, the major active compounds of ginseng, have a synergistic effect on bFGF-induced antiproliferation of melanocytes via ERK cascades (108). Proanthocyanidins extracted from grape seeds can potentially regulate the NHM cell cycle and inhibit the production of melanogenic enzymes (109).
5.4 Topical treatments
Hydroquinone, the most common ingredient of skin-lightening agents, can prevent the conversion of Dihydroxyphenylalanine (DOPA) to melanin and restrict the differentiation of melanocytes from neural crest cells (110). Hydroquinone is the most effective in the concentration of 2%–5%. To guarantee its efficiency and minimize its defects, many combination formulae have been approved by the US Food and Drug Administration (FDA) (111). However, hydroquinone topical creams reportedly cause skin toxicity and other side effects (112). Therefore, mequinol (4-hydroxyanisole) may be used as a substitute for the parent compound to reduce skin irritation (113).
Retinoids, which are vitamin A analogs, facilitate epidermal turnover, thus removing melanin. Isotretinoin, adapalene, and tazarotene are three mainstream retinoids that have been proven to be useful in treating hyperpigmentation (114). Although its irritant activity is not as high as the other two forms, isotretinoin should be prescribed in high concentrations (115).
Azelaic acid (AzA) is used as a depigmenting drug in acne owing to its anti-inflammatory, antibacterial, and antioxidant properties. In acne-related PIH, alleviation and clearance of pigmentation were observed at the end of the study (116). In addition, AzA is safer and was assigned pregnancy category B by the FDA for its mild side effects.
Kojic acid (KA) inactivates tyrosinase by chelating copper, which is the prosthetic group for this enzyme; it is effective in concentrations ranging from 1% to 4% (117). The maximum potential human systemic exposure dose (SED) of KA is 0.028 mg/kg/day. KA reduces the synthesis of melanin and is less toxic to melanocytes (117).
Niacinamide, an amide form of vitamin B3, was observed to impede the transfer of melanin to epidermal keratinocytes in a keratinocyte–melanocyte cocultured system (118). The level of nicotinamide nucleotide transhydrogenase (NNT) is low in individuals with PIH (119). Thus, the effect of nicotinamide on NNT activity and skin pigmentation alteration can be explored in the future.
Many traditional Chinese medicines (TCMs) with skin-whitening functions that are recorded in prescriptions, such as Fructus Ligustri Lucidi, Hedysarum multijugum Maxim., Ampelopsis japonica, Pseudobulbus Cremastrae seu Pleiones, and Paeoniae Radix Alba, have also been observed to inhibit TYR expression and activity (120).
5.5 Procedural treatment
Chemical peeling, which includes the removal of the superficial and deeper skin layers to regenerate the epidermis and part of the dermis, causes reversible damage to the skin and redistributes melanin. The superficial peeling strips the stratum corneum, whereas medium to deeper peelings penetrate the papillary and reticular dermis. Superficial peeling agents contain glycolic acid (GA), salicylic acid (SA), trichloroacetic acid (TCA), and tretinoin. In a study, repeated use of 5% GA improved brightness and reduced redness with respect to the melanin and erythema in the fourth week (121). SA modifies skin indices, such as melanin, pores, and texture; this alteration is also enhanced by oral administration of isotretinoin (122). TCA peels improve signs of photoaging such as hyperpigmentation, erythema, and fine lines with repeated treatment (123). Therefore, superficial peeling is the most viable treatment for removing melanin. In contrast, deeper peeling agents may result in PIH owing to irritation.
According to the principles of photothermolysis, current laser technologies selectively and specifically destroy targeted tissue. Modern melanin-directing laser machines consist of a Q-switched (QS) laser and a picosecond (PS) laser. The wavelengths used in these two systems are Nd : YAG (532 nm), Nd : YAG (1,064 nm), and alexandrite (755 nm) (124). All these new generations of lasers reflect patient selection with safer technologies and less postoperative recovery time. However, although it is anticipatory and largely transient, care and attention must be provided to avoid laser irradiation-induced PIH.
6 Discussion
In the field of burns, more attention has been paid to the patient’s systemic status and the maintenance of all vital organs, and little research has been done on changes in skin color after burns. Although not well documented, it is clinically possible to observe that some burn patients with a severe inflammatory response have a greater proportion of hyperpigmentation than less severe patients. Therefore, this suggests a possible relationship between burns, inflammation, and melanogenesis. Few scholars have focused on this phenomenon, and there is no definitive evidence for the relationship between these three. Therefore, to fill this research gap, this review discusses the relationship between the three as much as possible in relation to the existing literature.
It summarizes pigment formation and its transport, regulation of melanogenesis, mechanisms of PIH, and changes in inflammatory cytokines induced by burns. It bridges the gap between PIH and burn wounds, thereby providing insights that would aid in its treatment and management. Pigmentation is mainly regulated through various pathways, which, in turn, are regulated by various inflammatory factors, resulting in PIH. Burns, as a stimulus to DAMP, often cause cytokine storms. Burn wounds often appear hyperpigmented. This suggests that the pigmentary changes in burn wounds may be caused by changes in the levels of inflammatory mediators. Collectively, controlling inflammation in burn wounds may help reduce hyperpigmentation.
The mechanism of PIH in the current study mainly focuses on the PKA and MAPK pathways. We possess limited information regarding the other mechanisms of pigmentation, such as JAK-STAT, and the interaction among the various pathways. In addition, most PIH models are based on vitiligo and acne, with few studies on burn models. Changes in inflammatory mediators after burns are mainly observed in the plasma, with few studies focusing on their profiles in wounds. Therefore, much effort should be paid to developing effective burn models for studying PIH and to excavating more unknown mechanisms, especially pathways of PIH, and how these mechanisms promote or inhibit it.
Few assessments and therapies exist for PIH, specifically those induced by burns, and these mainstream approaches treating PIH are mainly directed toward melanogenesis as a phenotype to address hyperpigmentation. Their mechanisms for reducing melanogenesis are not solely, e.g., anti-inflammatory, antioxidant properties, direct reduction of melanocytes, accelerated exfoliation of the stratum corneum, etc. Some treatments, such as systemic and topical drugs and laser therapy, have been used to relieve pigmentation disorders in regular clinical practice. Procedural treatments might worsen PIH. Despite their toxicity, topical treatments are the current optimal choices owing to the limited number of oral drugs to cure PIH.
Since the inflammatory phase partially overlaps the pigmentation phase, further research is required to shed light on the alterations in inflammatory cytokines in the burn wound site, especially in humans. Additionally, the direct or indirect action of these alterations on the pigmentation must be elucidated. If the direct action is confirmed, anti-inflammatory and pigment-inhibiting drugs and treatments would be potential and superior choices for alleviating PIH in patients suffering from burn wounds. Finally, persistent effort is required to identify nontoxic and reliable whitening agents.
Author contributions
CZ collated the topics for review, retrieved the relevant literature, and wrote this review. DX, GL, PL, KS, FL, and JZ edited this review. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
PIH, postinflammatory pigmentation; WNT, wingless-related integration site; LRO, lysosome-related organelle; ApoE, apolipoprotein E; PMEL17, melanocyte protein; TYR, tyrosinase; TYRP1, tyrosinase-related protein-1; AP-3, adaptor protein complex-3; BLOC, biogenesis of lysosome-related organelle complex; PKA, protein kinase A; MITF, microphthalmia-associated transcription factor; CREB, cAMP response element-binding protein; PKA, protein kinase A; α-MSH, alpha-melanocyte-stimulating hormone; TA, tranexamic acid; AzA, azelaic acid; KA, kojic acid.
References
1. Jeschke MG, van Baar ME, Choudhry MA, Chung KK, Gibran NS, Logsetty S. Burn injury. Nat Rev Dis Primers (2020) 6(1):11. doi: 10.1038/s41572-020-0145-5
2. Dai NT, Chang HI, Wang YW, Fu KY, Huang TC, Huang NC, et al. Restoration of skin pigmentation after deep partial or full-thickness burn injury. Adv Drug Delivery Rev (2018) 123:155–64. doi: 10.1016/j.addr.2017.10.010
3. Kaufman BP, Aman T, Alexis AF. Postinflammatory hyperpigmentation: Epidemiology, clinical presentation, pathogenesis and treatment. Am J Clin Dermatol (2018) 19(4):489–503. doi: 10.1007/s40257-017-0333-6
4. Silpa-Archa N, Kohli I, Chaowattanapanit S, Lim HW, Hamzavi I. Postinflammatory hyperpigmentation: A comprehensive overview: Epidemiology, pathogenesis, clinical presentation, and noninvasive assessment technique. J Am Acad Dermatol (2017) 77(4):591–605. doi: 10.1016/j.jaad.2017.01.035
5. Chaowattanapanit S, Silpa-Archa N, Kohli I, Lim HW, Hamzavi I. Postinflammatory hyperpigmentation: A comprehensive overview: Treatment options and prevention. J Am Acad Dermatol (2017) 77(4):607–21. doi: 10.1016/j.jaad.2017.01.036
6. Wang Y, Beekman J, Hew J, Jackson S, Issler-Fisher AC, Parungao R, et al. Burn injury: Challenges and advances in burn wound healing, infection, pain and scarring. Adv Drug Delivery Rev (2018) 123:3–17. doi: 10.1016/j.addr.2017.09.018
7. Finnerty CC, Jeschke MG, Branski LK, Barret JP, Dziewulski P, Herndon DN. Hypertrophic scarring: the greatest unmet challenge after burn injury. Lancet (2016) 388(10052):1427–36. doi: 10.1016/S0140-6736(16)31406-4
8. Stanojcic M, Abdullahi A, Rehou S, Parousis A, Jeschke MG. Pathophysiological response to burn injury in adults. Ann Surg (2018) 267(3):576–84. doi: 10.1097/SLA.0000000000002097
9. Jeschke MG, Mlcak RP, Finnerty CC, Norbury WB, Gauglitz GG, Kulp GA, et al. Burn size determines the inflammatory and hypermetabolic response. Crit Care (2007) 11(4):R90. doi: 10.1186/cc6102
10. Xiong XX, Ding GZ, Zhao WE, Li X, Ling YT, Sun L, et al. Differences in the melanosome distribution within the epidermal melanin units and its association with the impairing background of leukoderma in vitiligo and halo nevi: A retrospective study. Arch Dermatol Res (2017) 309(5):323–33. doi: 10.1007/s00403-017-1730-7
11. Singh SK, Baker R, Sikkink SK, Nizard C, Schnebert S, Kurfurst R, et al. E-cadherin mediates ultraviolet radiation- and calcium-induced melanin transfer in human skin cells. Exp Dermatol (2017) 26(11):1125–33. doi: 10.1111/exd.13395
12. Videira IF, Moura DF, Magina S. Mechanisms regulating melanogenesis. Bras Dermatol (2013) 88(1):76–83. doi: 10.1590/S0365-05962013000100009
13. Tadokoro R, Takahashi Y. Intercellular transfer of organelles during body pigmentation. Curr Opin Genet Dev (2017) 45:132–8. doi: 10.1016/j.gde.2017.05.001
14. Noh S, Choi H, Kim JS, Kim IH, Mun JY. Study of hyperpigmentation in human skin disorder using different electron microscopy techniques. Microsc Res Tech (2019) 82(1):18–24. doi: 10.1002/jemt.23052
15. Moreiras H, Pereira FJC, Neto MV, Bento-Lopes L, Festas TC, Seabra MC, et al. The exocyst is required for melanin exocytosis from melanocytes and transfer to keratinocytes. Pigment Cell Melanoma Res (2020) 33(2):366–71. doi: 10.1111/pcmr.12840
16. Castellano-Pellicena I, Morrison CG, Bell M, O’Connor C, Tobin DJ. Melanin distribution in human skin: Influence of cytoskeletal, polarity, and centrosome-related machinery of stratum basale keratinocytes. Int J Mol Sci (2021) 22(6). doi: 10.3390/ijms22063143
17. Yuan XH, Jin ZH. Paracrine regulation of melanogenesis. Br J Dermatol (2018) 178(3):632–9. doi: 10.1111/bjd.15651
18. Vandamme N, Berx G. From neural crest cells to melanocytes: Cellular plasticity during development and beyond. Cell Mol Life Sci (2019) 76(10):1919–34. doi: 10.1007/s00018-019-03049-w
19. Sinnberg T, Levesque MP, Krochmann J, Cheng PF, Ikenberg K, Meraz-Torres F, et al. Wnt-signaling enhances neural crest migration of melanoma cells and induces an invasive phenotype. Mol Cancer (2018) 17(1):59. doi: 10.1186/s12943-018-0773-5
20. Burgoyne T, Jolly R, Martin-Martin B, Seabra MC, Piccirillo R, Schiaffino MV, et al. Expression of OA1 limits the fusion of a subset of MVBs with lysosomes - a mechanism potentially involved in the initial biogenesis of melanosomes. J Cell Sci (2013) 126(Pt 22):5143–52. doi: 10.1242/jcs.128561.
21. D’Alba L, Shawkey MD. Melanosomes: Biogenesis, properties, and evolution of an ancient organelle. Physiol Rev (2019) 99(1):1–19. doi: 10.1152/physrev.00059.2017.
22. Rok J, Rzepka Z, Kowalska J, Banach K, Beberok A, Wrześniok D. Molecular and biochemical basis of minocycline-induced hyperpigmentation-the study on normal human melanocytes exposed to UVA and UVB radiation. Int J Mol Sci (2021) 22(7). doi: 10.3390/ijms22073755
23. O’Neill BT, Beck EM, Butler CR, Nolan CE, Gonzales C, Zhang L, et al. Design and synthesis of clinical candidate PF-06751979: A potent, brain penetrant, β-site amyloid precursor protein cleaving enzyme 1 (BACE1) inhibitor lacking hypopigmentation. J Med Chem (2018) 61(10):4476–504. doi: 10.1021/acs.jmedchem.8b00246
24. Ho T, Watt B, Spruce LA, Seeholzer SH, Marks MS. The kringle-like domain facilitates post-endoplasmic reticulum changes to premelanosome protein (PMEL) oligomerization and disulfide bond configuration and promotes amyloid formation. J Biol Chem (2016) 291(7):3595–612. doi: 10.1074/jbc.M115.692442
25. Fukuda M. Rab GTPases: Key players in melanosome biogenesis, transport, and transfer. Pigment Cell Melanoma Res (2021) 34(2):222–35. doi: 10.1111/pcmr.12931
26. Wakamatsu K, Zippin JH, Ito S. Chemical and biochemical control of skin pigmentation with special emphasis on mixed melanogenesis. Pigment Cell Melanoma Res (2021) 34(4):730–47. doi: 10.1111/pcmr.12970
27. Wakamatsu K, Nagao A, Watanabe M, Nakao K, Ito S. Pheomelanogenesis is promoted at a weakly acidic pH. Pigment Cell Melanoma Res (2017) 30(3):372–7. doi: 10.1111/pcmr.12587
28. Robinson CL, Evans RD, Briggs DA, Ramalho JS, Hume AN. Inefficient recruitment of kinesin-1 to melanosomes precludes it from facilitating their transport. J Cell Sci (2017) 130(12):2056–65. doi: 10.1242/jcs.186064
29. Jiang M, Paniagua AE, Volland S, Wang H, Balaji A, Li DG, et al. Microtubule motor transport in the delivery of melanosomes to the actin-rich apical domain of the retinal pigment epithelium. J Cell Sci (2020) 133(15). doi: 10.1242/jcs.242214
30. Oberhofer A, Spieler P, Rosenfeld Y, Stepp WL, Cleetus A, Hume AN, et al. Myosin va’s adaptor protein melanophilin enforces track selection on the microtubule and actin networks in vitroin vitro. Proc Natl Acad Sci U.S.A. (2017) 114(24):E4714–e23. doi: 10.1073/pnas.1619473114
31. Lim D, Lee KJ, Kim Y, Kim M, Ju HM, Kim MJ, et al. A basic domain-derived tripeptide inhibits MITF activity by reducing its binding to the promoter of target genes. J Invest Dermatol (2021) 141(10):2459–69. doi: 10.1016/j.jid.2021.01.037
32. Shin H, Hong SD, Roh E, Jung SH, Cho WJ, Park SH, et al. cAMP-dependent activation of protein kinase a as a therapeutic target of skin hyperpigmentation by diphenylmethylene hydrazinecarbothioamide. Br J Pharmacol (2015) 172(13):3434–45. doi: 10.1111/bph.13134
33. Mujahid N, Liang Y, Murakami R, Choi HG, Dobry AS, Wang J, et al. A UV-independent topical small-molecule approach for melanin production in human skin. Cell Rep (2017) 19(11):2177–84. doi: 10.1016/j.celrep.2017.05.042
34. Yoo H, Lee HR, Kim KH, Kim MA, Bang S, Kang YH, et al. CRTC3, a sensor and key regulator for melanogenesis, as a tunable therapeutic target for pigmentary disorders. Theranostics (2021) 11(20):9918–36. doi: 10.7150/thno.66378
35. Yun CY, Hong SD, Lee YH, Lee J, Jung DE, Kim GH, et al. Nuclear entry of CRTC1 as druggable target of acquired pigmentary disorder. Theranostics (2019) 9(3):646–60. doi: 10.7150/thno.30276
36. Cui R, Widlund HR, Feige E, Lin JY, Wilensky DL, Igras VE, et al. Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell (2007) 128(5):853–64. doi: 10.1016/j.cell.2006.12.045
37. Estrada C, Mirabal-Ortega L, Méry L, Dingli F, Besse L, Messaoudi C, et al. MITF activity is regulated by a direct interaction with RAF proteins in melanoma cells. Commun Biol (2022) 5(1):101. doi: 10.1038/s42003-022-03049-w
38. Lee SJ, Jeong D, Park WK, Kong JY, Choi G, Kim H, et al. Screening of kit inhibitors: Suppression of kit signaling and melanogenesis by emodin. Phytother Res (2010) 24(2):308–12. doi: 10.1002/ptr.2928
39. Wang Y, Viennet C, Robin S, Berthon JY, He L, Humbert P. Precise role of dermal fibroblasts on melanocyte pigmentation. J Dermatol Sci (2017) 88(2):159–66. doi: 10.1016/j.jdermsci.2017.06.018
40. Czyz M. HGF/c-MET signaling in melanocytes and melanoma. Int J Mol Sci (2018) 19(12). doi: 10.3390/ijms19123844
41. Wagner MJ, Stacey MM, Liu BA, Pawson T. Molecular mechanisms of SH2- and PTB-domain-containing proteins in receptor tyrosine kinase signaling. Cold Spring Harb Perspect Biol (2013) 5(12):a008987. doi: 10.1101/cshperspect.a008987
42. Belote RL, Simon SM. Ca2+ transients in melanocyte dendrites and dendritic spine-like structures evoked by cell-to-cell signaling. J Cell Biol (2020) 219(1). doi: 10.1083/jcb.201902014
43. Stanisz H, Stark A, Kilch T, Schwarz EC, Müller CS, Peinelt C, et al. ORAI1 Ca(2+) channels control endothelin-1-induced mitogenesis and melanogenesis in primary human melanocytes. J Invest Dermatol (2012) 132(5):1443–51. doi: 10.1038/jid.2011.478
44. Serre C, Busuttil V, Botto JM. Intrinsic and extrinsic regulation of human skin melanogenesis and pigmentation. Int J Cosmet Sci (2018) 40(4):328–47. doi: 10.1111/ics.12466
45. Nusse R, Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell (2017) 169(6):985–99. doi: 10.1016/j.cell.2017.05.016
46. Yamada T, Hasegawa S, Iwata Y, Arima M, Kobayashi T, Numata S, et al. UV Irradiation-induced DNA hypomethylation around WNT1 gene: Implications for solar lentigines. Exp Dermatol (2019) 28(6):723–9. doi: 10.1111/exd.13949
47. Birlea SA, Costin GE, Roop DR, Norris DA. Trends in regenerative medicine: Repigmentation in vitiligo through melanocyte stem cell mobilization. Med Res Rev (2017) 37(4):907–35. doi: 10.1002/med.21426
48. Zou DP, Chen YM, Zhang LZ, Yuan XH, Zhang YJ, Inggawati A, et al. SFRP5 inhibits melanin synthesis of melanocytes in vitiligo by suppressing the wnt/β-catenin signaling. Genes Dis (2021) 8(5):677–88. doi: 10.1016/j.gendis.2020.06.003
49. Kraj P. Bone morphogenic protein signaling and melanoma. Curr Treat Options Oncol (2021) 22(6):48. doi: 10.1007/s11864-021-00849-w
50. Gramann AK, Venkatesan AM, Guerin M, Ceol CJ. Regulation of zebrafish melanocyte development by ligand-dependent BMP signaling. Elife (2019) 8. doi: 10.7554/eLife.50047
51. Chen N, Hu Y, Li WH, Eisinger M, Seiberg M, Lin CB. The role of keratinocyte growth factor in melanogenesis: A possible mechanism for the initiation of solar lentigines. Exp Dermatol (2010) 19(10):865–72. doi: 10.1111/j.1600-0625.2009.00957.x
52. Kholmanskikh O, van Baren N, Brasseur F, Ottaviani S, Vanacker J, Arts N, et al. Interleukins 1alpha and 1beta secreted by some melanoma cell lines strongly reduce expression of MITF-m and melanocyte differentiation antigens. Int J Cancer (2010) 127(7):1625–36. doi: 10.1002/ijc.25182
53. Choi H, Choi H, Han J, Jin SH, Park JY, Shin DW, et al. IL-4 inhibits the melanogenesis of normal human melanocytes through the JAK2-STAT6 signaling pathway. J Invest Dermatol (2013) 133(2):528–36. doi: 10.1038/jid.2012.331
54. Singh M, Jadeja SD, Vaishnav J, Mansuri MS, Shah C, Mayatra JM, et al. Investigation of the role of interleukin 6 in vitiligo pathogenesis. Immunol Invest (2022) 51(1):120–37. doi: 10.1080/08820139.2020.1813756
55. Iwaszko M, Biały S, Bogunia-Kubik K. Significance of interleukin (IL)-4 and IL-13 in inflammatory arthritis. Cells (2021) 10(11). doi: 10.3390/cells10113000
56. Han J, Lee E, Kim E, Yeom MH, Kwon O, Yoon TH, et al. Role of epidermal γδ T-cell-derived interleukin 13 in the skin-whitening effect of ginsenoside F1. Exp Dermatol (2014) 23(11):860–2. doi: 10.1111/exd.12531
57. Bhardwaj S, Bhatia A, Kumaran MS, Parsad D. Role of IL-17A receptor blocking in melanocyte survival: A strategic intervention against vitiligo. Exp Dermatol (2019) 28(6):682–9. doi: 10.1111/exd.13773
58. Prinz JC. The woronoff ring in psoriasis and the mechanisms of postinflammatory hypopigmentation. Acta Derm Venereol (2020) 100(3):adv00031. doi: 10.2340/00015555-3385
59. Zhou J, Ling J, Wang Y, Shang J, Ping F. Cross-talk between interferon-gamma and interleukin-18 in melanogenesis. J Photochem Photobiol B (2016) 163:133–43. doi: 10.1016/j.jphotobiol.2016.08.024
60. Zhou J, Song J, Ping F, Shang J. Enhancement of the p38 MAPK and PKA signaling pathways is associated with the pro-melanogenic activity of interleukin 33 in primary melanocytes. J Dermatol Sci (2014) 73(2):110–6. doi: 10.1016/j.jdermsci.2013.09.005
61. Meephansan J, Komine M, Tsuda H, Karakawa M, Tominaga S, Ohtsuki M. Expression of IL-33 in the epidermis: The mechanism of induction by IL-17. J Dermatol Sci (2013) 71(2):107–14. doi: 10.1016/j.jdermsci.2013.04.014
62. Meephansan J, Tsuda H, Komine M, Tominaga S, Ohtsuki M. Regulation of IL-33 expression by IFN-γ and tumor necrosis factor-α in normal human epidermal keratinocytes. J Invest Dermatol (2012) 132(11):2593–600. doi: 10.1038/jid.2012.185
63. Li P, Ma H, Han D, Mou K. Interleukin-33 affects cytokine production by keratinocytes in vitiligo. Clin Exp Dermatol (2015) 40(2):163–70. doi: 10.1111/ced.12464
64. Gledhill K, Rhodes LE, Brownrigg M, Haylett AK, Masoodi M, Thody AJ, et al. Prostaglandin-E2 is produced by adult human epidermal melanocytes in response to UVB in a melanogenesis-independent manner. Pigment Cell Melanoma Res (2010) 23(3):394–403. doi: 10.1111/j.1755-148X.2010.00696.x
65. Starner RJ, McClelland L, Abdel-Malek Z, Fricke A, Scott G. PGE(2) is a UVR-inducible autocrine factor for human melanocytes that stimulates tyrosinase activation. Exp Dermatol (2010) 19(7):682–4. doi: 10.1111/j.1600-0625.2010.01074.x
66. Natarajan VT, Ganju P, Singh A, Vijayan V, Kirty K, Yadav S, et al. IFN-γ signaling maintains skin pigmentation homeostasis through regulation of melanosome maturation. Proc Natl Acad Sci U S A (2014) 111(6):2301–6. doi: 10.1073/pnas.1304988111
67. Son J, Kim M, Jou I, Park KC, Kang HY. IFN-γ inhibits basal and α-MSH-induced melanogenesis. Pigment Cell Melanoma Res (2014) 27(2):201–8. doi: 10.1111/pcmr.12190
68. Yang L, Wei Y, Sun Y, Shi W, Yang J, Zhu L, et al. Interferon-gamma inhibits melanogenesis and induces apoptosis in melanocytes: A pivotal role of CD8+ cytotoxic T lymphocytes in vitiligo. Acta Derm Venereol (2015) 95(6):664–70. doi: 10.2340/00015555-2080
69. Zhou J, Ling J, Ping F. Interferon-γ attenuates 5-Hydroxytryptamine-Induced melanogenesis in primary melanocyte. Biol Pharm Bull (2016) 39(7):1091–9. doi: 10.1248/bpb.b15-00914
70. Cai M, Zhou L, Liao J, Huang Q, Xia Z, Shang J. IFN-γ inhibits 5-HT-induced melanin biosynthesis via downregulation of 5-HT receptors in vivo/in vitro. J Pharmacol Sci (2019) 141(1):1–8. doi: 10.1016/j.jphs.2019.05.005
71. Ferreira Branquinho MS, Silva MBB, Castilho GA, Cavalcante J, Barros SBM, Clara RO, et al. Kynurenine inhibits melanogenesis in human melanocyte-keratinocyte co-cultures and in a reconstructed 3D skin model. Exp Dermatol (2022) 31(3):427–32. doi: 10.1111/exd.14486
72. Wang CQF, Akalu YT, Suarez-Farinas M, Gonzalez J, Mitsui H, Lowes MA, et al. IL-17 and TNF synergistically modulate cytokine expression while suppressing melanogenesis: Potential relevance to psoriasis. J Invest Dermatol (2013) 133(12):2741–52. doi: 10.1038/jid.2013.237
73. Grine L, Dejager L, Libert C, Vandenbroucke RE. An inflammatory triangle in psoriasis: TNF, type I IFNs and IL-17. Cytokine Growth Factor Rev (2015) 26(1):25–33. doi: 10.1016/j.cytogfr.2014.10.009
74. Oh CT, Park JI, Jung YR, Joo YA, Shin DH, Cho HJ, et al. Inhibitory effect of Korean red ginseng on melanocyte proliferation and its possible implication in GM-CSF mediated signaling. J Ginseng Res (2013) 37(4):389–400. doi: 10.5142/jgr.2013.37.389
75. Zindel J, Kubes P. DAMPs, PAMPs, and LAMPs in immunity and sterile inflammation. Annu Rev Pathol (2020) 15:493–518. doi: 10.1146/annurev-pathmechdis-012419-032847
76. Duan T, Du Y, Xing C, Wang HY, Wang RF. Toll-like receptor signaling and its role in cell-mediated immunity. Front Immunol (2022) 13:812774. doi: 10.3389/fimmu.2022.812774
77. Cheng Z, Taylor B, Ourthiague DR, Hoffmann A. Distinct single-cell signaling characteristics are conferred by the MyD88 and TRIF pathways during TLR4 activation. Sci Signal (2015) 8(385):ra69. doi: 10.1126/scisignal.aaa5208
78. Rani M, Nicholson SE, Zhang Q, Schwacha MG. Damage-associated molecular patterns (DAMPs) released after burn are associated with inflammation and monocyte activation. Burns (2017) 43(2):297–303. doi: 10.1016/j.burns.2016.10.001
79. Lange-Asschenfeldt B, Marenbach D, Lang C, Patzelt A, Ulrich M, Maltusch A, et al. Distribution of bacteria in the epidermal layers and hair follicles of the human skin. Skin Pharmacol Physiol (2011) 24(6):305–11. doi: 10.1159/000328728
80. Zhang X, Li N, Meng Y, Zhang R, Bian J, Yao Y, et al. High-level expression of toll-like receptors on dendritic cells in adult patients with burns on ≥90% of total body surface area (TBSA). Med Sci Monit (2016) 22:3493–9. doi: 10.12659/msm.897433
81. Gibson BHY, Wollenman CC, Moore-Lotridge SN, Keller PR, Summitt JB, Revenko AR, et al. Plasmin drives burn-induced systemic inflammatory response syndrome. JCI Insight (2021) 6(23). doi: 10.1172/jci.insight.154439
82. Yang J, Ma K, Zhang C, Liu Y, Liang F, Hu W, et al. Burns impair blood-brain barrier and mesenchymal stem cells can reverse the process in mice. Front Immunol (2020) 11:578879. doi: 10.3389/fimmu.2020.578879
83. Bergquist M, Hästbacka J, Glaumann C, Freden F, Huss F, Lipcsey M. The time-course of the inflammatory response to major burn injury and its relation to organ failure and outcome. Burns (2019) 45(2):354–63. doi: 10.1016/j.burns.2018.09.001
84. Davis CS, Janus SE, Mosier MJ, Carter SR, Gibbs JT, Ramirez L, et al. Inhalation injury severity and systemic immune perturbations in burned adults. Ann Surg (2013) 257(6):1137–46. doi: 10.1097/SLA.0b013e318275f424
85. Hur J, Yang HT, Chun W, Kim JH, Shin SH, Kang HJ, et al. Inflammatory cytokines and their prognostic ability in cases of major burn injury. Ann Lab Med (2015) 35(1):105–10. doi: 10.3343/alm.2015.35.1.105
86. Jeschke MG, Gauglitz GG, Finnerty CC, Kraft R, Mlcak RP, Herndon DN. Survivors versus nonsurvivors postburn: differences in inflammatory and hypermetabolic trajectories. Ann Surg (2014) 259(4):814–23. doi: 10.1097/SLA.0b013e31828dfbf1
87. Kim HS, Kim JH, Yim H, Kim D. Changes in the levels of interleukins 6, 8, and 10, tumor necrosis factor alpha, and granulocyte-colony stimulating factor in Korean burn patients: Relation to burn size and postburn time. Ann Lab Med (2012) 32(5):339–44. doi: 10.3343/alm.2012.32.5.339
88. Sobouti B, Ghavami Y, Asadifar B, Jafarzadeh M, Ghelman M, Vaghardoost R. Determination of serum levels of interleukin-6, interleukin-8, interleukin-10, and tumor necrosis-alpha and their relationship with the total body surface area in children. J Burn Care Res (2020) 41(3):539–43. doi: 10.1093/jbcr/irz180
89. Matsuura H, Matsumoto H, Osuka A, Ogura H, Shimizu K, Kang S, et al. Clinical importance of a cytokine network in major burns. Shock (2019) 51(2):185–93. doi: 10.1097/SHK.0000000000001152
90. Schwacha MG, Thobe BM, Daniel T, Hubbard WJ. Impact of thermal injury on wound infiltration and the dermal inflammatory response. J Surg Res (2010) 158(1):112–20. doi: 10.1016/j.jss.2008.07.034
91. Schwacha MG, Nickel E, Daniel T. Burn injury-induced alterations in wound inflammation and healing are associated with suppressed hypoxia inducible factor-1alpha expression. Mol Med (2008) 14(9-10):628–33. doi: 10.2119/2008-00069.Schwacha
92. Moreiras H, O’Connor C, Bell M, Tobin DJ. Visible light and human skin pigmentation: The importance of skin phototype. Exp Dermatol (2021) 30(9):1324–31. doi: 10.1111/exd.14400
93. Taylor SC, Arsonnaud S, Czernielewski J. The Taylor hyperpigmentation scale: a new visual assessment tool for the evaluation of skin color and pigmentation. Cutis (2005) 76(4):270–4.
94. Gilchrest BA, Fitzpatrick TB, Anderson RR, Parrish JA. Localization of malanin pigmentation in the skin with wood’s lamp. Br J Dermatol (1977) 96(3):245–8. doi: 10.1111/j.1365-2133.1977.tb06132.x
95. Amatya B. Evaluation of dermoscopic features in facial melanosis with wood lamp examination. Dermatol Pract Concept (2022) 12(1):e2022030. doi: 10.5826/dpc.1201a30
96. Isedeh P, Kohli I, Al-Jamal M, Agbai ON, Chaffins M, Devpura S, et al. An in vivoin vivo model for postinflammatory hyperpigmentation: An analysis of histological, spectroscopic, colorimetric and clinical traits. Br J Dermatol (2016) 174(4):862–8. doi: 10.1111/bjd.14184
97. Marcum KK, Goldman ND, Sandoval LF. Comparison of photographic methods. J Drugs Dermatol (2015) 14(2):134–9.
98. Ly BCK, Dyer EB, Feig JL, Chien AL, Del Bino S. Research techniques made simple: Cutaneous colorimetry: A reliable technique for objective skin color measurement. J Invest Dermatol (2020) 140(1):3–12.e1. doi: 10.1016/j.jid.2019.11.003
99. Stamatas GN, Zmudzka BZ, Kollias N, Beer JZ. In vivo measurement of skin erythema and pigmentation: New means of implementation of diffuse reflectance spectroscopy with a commercial instrument. Br J Dermatol (2008) 159(3):683–90. doi: 10.1111/j.1365-2133.2008.08642.x
100. He Q, Wang R. Hyperspectral imaging enabled by an unmodified smartphone for analyzing skin morphological features and monitoring hemodynamics. BioMed Opt Express (2020) 11(2):895–910. doi: 10.1364/BOE.378470
101. He Q, Liu T, Wang RK. Enhanced spatial resolution for snapshot hyperspectral imaging of blood perfusion and melanin information within human tissue. J Biophotonics (2020) 13(5):e202000019. doi: 10.1002/jbio.202000019
102. Fuchs CSK, Andersen AJB, Ardigo M, Philipsen PA, Haedersdal M, Mogensen M. Acne vulgaris severity graded by in vivo reflectance confocal microscopy and optical coherence tomography. Lasers Surg Med (2019) 51(1):104–13. doi: 10.1002/lsm.23008
103. Rajadhyaksha M, Marghoob A, Rossi A, Halpern AC, Nehal KS. Reflectance confocal microscopy of skin in vivo: From bench to bedside. Lasers Surg Med (2017) 49(1):7–19. doi: 10.1002/lsm.22600
104. Zhao J, Liu Z, Zhang C, Wu J, Huang N, Du Y, et al. Dynamic evaluation of an in vivo postinflammatory hyperpigmentation model using reflectance confocal microscopy and spectrophotometry. J Cosmet Dermatol (2021) 20(9):2950–62. doi: 10.1111/jocd.13932
105. Perper M, Eber AE, Fayne R, Verne SH, Magno RJ, Cervantes J, et al. Tranexamic acid in the treatment of melasma: A review of the literature. Am J Clin Dermatol (2017) 18(3):373–81. doi: 10.1007/s40257-017-0263-3
106. Xing X, Xu Z, Chen L, Jin S, Zhang C, Xiang L. Tranexamic acid inhibits melanogenesis partially via stimulation of TGF-β1 expression in human epidermal keratinocytes. Exp Dermatol (2022) 31(4):633–40. doi: 10.1111/exd.14509
107. Lu Y, Tonissen KF, Di Trapani G. Modulating skin colour: Role of the thioredoxin and glutathione systems in regulating melanogenesis. Biosci Rep (2021) 41(5). doi: 10.1042/BSR20210427
108. Lee JE, Park JI, Myung CH, Hwang JS. Inhibitory effects of ginsenosides on basic fibroblast growth factor-induced melanocyte proliferation. J Ginseng Res (2017) 41(3):268–76. doi: 10.1016/j.jgr.2016.05.001
109. Zi SX, Ma HJ, Li Y, Liu W, Yang QQ, Zhao G, et al. Oligomeric proanthocyanidins from grape seeds effectively inhibit ultraviolet-induced melanogenesis of human melanocytes in vitro. Int J Mol Med (2009) 23(2):197–204.
110. Inoue Y, Hasegawa S, Yamada T, Date Y, Mizutani H, Nakata S, et al. Analysis of the effects of hydroquinone and arbutin on the differentiation of melanocytes. Biol Pharm Bull (2013) 36(11):1722–30. doi: 10.1248/bpb.b13-00206
111. Ahmad Nasrollahi S, Sabet Nematzadeh M, Samadi A, Ayatollahi A, Yadangi S, Abels C, et al. Evaluation of the safety and efficacy of a triple combination cream (hydroquinone, tretinoin, and fluocinolone) for treatment of melasma in middle Eastern skin. Clin Cosmet Investig Dermatol (2019) 12:437–44. doi: 10.2147/CCID.S202285
112. Das A, Ghosh A, Kumar P. Chemical leukoderma due to hydroquinone: An unusual phenomenon. Indian J Dermatol Venereol Leprol (2019) 85(5):567. doi: 10.4103/ijdvl.IJDVL_209_17
113. Keeling J, Cardona L, Benitez A, Epstein R, Rendon M. Mequinol 2%/tretinoin 0.01% topical solution for the treatment of melasma in men: A case series and review of the literature. Cutis (2008) 81(2):179–83.
114. Callender VD, Baldwin H, Cook-Bolden FE, Alexis AF, Stein Gold L, Guenin E. Effects of topical retinoids on acne and post-inflammatory hyperpigmentation in patients with skin of color: A clinical review and implications for practice. Am J Clin Dermatol (2022) 23(1):69–81. doi: 10.1007/s40257-021-00643-2
115. Kaplan R, Meehan SA, Leger M. A case of isotretinoin-induced purpura annularis telangiectodes of majocchi and review of substance-induced pigmented purpuric dermatosis. JAMA Dermatol (2014) 150(2):182–4. doi: 10.1001/jamadermatol.2013.7371
116. Kircik LH. Efficacy and safety of azelaic acid (AzA) gel 15% in the treatment of post-inflammatory hyperpigmentation and acne: A 16-week, baseline-controlled study. J Drugs Dermatol (2011) 10(6):586–90.
117. Saeedi M, Eslamifar M, Khezri K. Kojic acid applications in cosmetic and pharmaceutical preparations. BioMed Pharmacother (2019) 110:582–93. doi: 10.1016/j.biopha.2018.12.006
118. Kim B, Hwang JS, Kim HS. N-nicotinoyl dopamine inhibits skin pigmentation by suppressing of melanosome transfer. Eur J Pharmacol (2015) 769:250–6. doi: 10.1016/j.ejphar.2015.11.025
119. Allouche J, Rachmin I, Adhikari K, Pardo LM, Lee JH, McConnell AM, et al. NNT mediates redox-dependent pigmentation via a UVB- and MITF-independent mechanism. Cell (2021) 184(16):4268–83.e20. doi: 10.1016/j.cell.2021.06.022
120. Guo H, Zeng H, Fu C, Huang J, Lu J, Hu Y, et al. Identification of sitogluside as a potential skin-Pigmentation-Reducing agent through network pharmacology. Oxid Med Cell Longev (2021) 2021:4883398. doi: 10.1155/2021/4883398
121. Chen L, Lu L, Tu S, Zhang T, Du X, Chen L, et al. Efficacy and safety of 5% glycolic acid-based gel essence in the treatment of mild to moderate acne. J Cosmet Dermatol (2022). doi: 10.1111/jocd.14865
122. Ye D, Xue H, Huang S, He S, Li Y, Liu J, et al. A prospective, randomized, split-face study of concomitant administration of low-dose oral isotretinoin with 30% salicylic acid chemical peeling for the treatment of acne vulgaris in Asian population. Int J Dermatol (2022). doi: 10.1111/ijd.16127
123. Kubiak M, Mucha P, Rotsztejn H. Comparative study of 15% trichloroacetic acid peel combined with 70% glycolic acid and 35% trichloroacetic acid peel for the treatment of photodamaged facial skin in aging women. J Cosmet Dermatol (2020) 19(1):137–46. doi: 10.1111/jocd.13171
Keywords: inflammatory response, hyperpigmentation, burn wound, treatment, cytokines, postinflammatory hyperpigmentation
Citation: Zhong C, Liang G, Li P, Shi K, Li F, Zhou J and Xu D (2023) Inflammatory response: The target for treating hyperpigmentation during the repair of a burn wound. Front. Immunol. 14:1009137. doi: 10.3389/fimmu.2023.1009137
Received: 01 August 2022; Accepted: 04 January 2023;
Published: 01 February 2023.
Edited by:
Nanlin Li, Air Force Military Medical University, ChinaReviewed by:
Zhang Jianglin, Jinan University, ChinaYing Zou, Tongji University School of Medicine, China
Copyright © 2023 Zhong, Liang, Li, Shi, Li, Zhou and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dan Xu, NDQ2NTQyNjZAcXEuY29t
 Chi Zhong
Chi Zhong Geao Liang
Geao Liang Peiting Li
Peiting Li Jianda Zhou
Jianda Zhou