
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol. , 27 October 2022
Sec. Alloimmunity and Transplantation
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.997808
This article is part of the Research Topic Immunobiology of Allogeneic Stem Cell Transplantation and Acute Graft-Versus-Host-Disease View all 11 articles
 Yanjing Huang1
Yanjing Huang1 Mengdi Zhu1
Mengdi Zhu1 Zhuo Liu1
Zhuo Liu1 Runan Hu1
Runan Hu1 Fan Li1
Fan Li1 Yufan Song1
Yufan Song1 Yuli Geng1
Yuli Geng1 Wenwen Ma2
Wenwen Ma2 Kunkun Song2*
Kunkun Song2* Mingmin Zhang2*
Mingmin Zhang2*Premature ovarian failure (POF) is a common female reproductive disorder and characterized by menopause, increased gonadotropin levels and estrogen deficiency before the age of 40 years old. The etiologies and pathogenesis of POF are not fully clear. At present, hormone replacement therapy (HRT) is the main treatment options for POF. It helps to ameliorate perimenopausal symptoms and related health risks, but can’t restore ovarian function and fertility fundamentally. With the development of regenerative medicine, bone marrow mesenchymal stem cells (BMSCs) have shown great potential for the recovery of ovarian function and fertility based on the advantages of abundant sources, high capacity for self-renewal and differentiation, low immunogenicity and less ethical considerations. This systematic review aims to summarize the possible therapeutic mechanisms of BMSCs for POF. A detailed search strategy of preclinical studies and clinical trials on BMSCs and POF was performed on PubMed, MEDLINE, Web of Science and Embase database. A total of 21 studies were included in this review. Although the standardization of BMSCs need more explorations, there is no doubt that BMSCs transplantation may represent a prospective therapy for POF. It is hope to provide a theoretical basis for further research and treatment for POF.
Premature ovarian failure (POF) refers to the decline of the ovarian function that occurs before the age of 40 in female. It is a clinical syndrome defined by oligomenorrhea or amenorrhea, increased gonadotropin levels, and decreased estradiol levels, and it is often accompanied by a variety of perimenopausal symptoms such as hot flashes, night sweats, alopecia, dry skin and mucous membranes, decreased libido, sleep disorders, irritability (1, 2). The diagnosis of POF in clinical is usually based on FSH >40 IU/L, oligo/amenorrhea for 4-6 months in female under 40 years old, and hypoestrogenemia (3, 4). In addition, the reduction of anti-Mullerian hormone is also an important auxiliary diagnostic criterion.
Women with POF have an increased risk of psychological disorders, cardiovascular diseases, osteoporosis, autoimmune diseases, cognitive dysfunction, urinary and reproductive system infections and other diseases compared with normal people. In addition, low fertility and even infertility are also major problems for POF patients (5, 6). Statistics showed that the incidence of POF was about 1% in female before the age of 40. The incidence of POF is on the rise due to the younger age of cancer onset, environmental pollution, lifestyle changes and other factors. Nevertheless, the etiology of POF is complex and not fully understood. Current studies show that the pathogenic factors of POF include iatrogenic factors (chemotherapy, radiotherapy, pelvic surgery, etc.), X chromosome abnormality, genetic syndrome, single gene mutation, congenital enzyme deficiency, autoimmune diseases, infection, HPV vaccination, environmental influence, etc. (5, 7). Complex clinical symptoms and adverse consequences caused by POF have greatly affected the quality of life of patients. Exploring effective treatment of POF has been a goal of clinical and scientific researchers.
Currently, there is no effective treatment for POF. HRT is the main therapeutic schemes for POF, which can effectively improve the menopause symptoms and reduce the risk of osteoporosis and cardiovascular diseases, as well as improve the quality of life of patients. However, HRT can’t fully restore ovarian function, such as hormone secretion, follicular growth or ovulation (8). Moreover, it is not entirely clear that whether or not HRT increases the risk of breast cancer (9). Ovarian tissue cryopreservation is a novel treatment for POF. Nonetheless, there are many problems with ovarian tissue after cryopreservation such as low survival rate and difficulty in natural conception (10). The common treatments for POF include psychological support, melatonin, androgen or dehydroepiandrosterone supplementation, traditional Chinese medicine therapy, diet and exercise conditioning, immune regulation, etc., but none of them can fundamentally improve ovarian function and meet the fertility needs of patients (7, 11). To protect POF patients from the disease, researchers have been exploring new treatments in recent years, such as perfusing platelet-rich plasma into the ovaries, ovarian tissue transplantation, building artificial ovary, artificial gametes and mitochondria replacement therapy, etc., which provide a new way for treating POF. However, they are limited by high cost, poor practical application and ethics (12).
Stem cell therapy has made great strides in regenerative medicine over the past two decades. “Stem cells” refer to the specific cell types that are insufficiently differentiated and immature, capable of self-renewal, which can proliferate and differentiate into various tissues and organs. Based on the therapeutic potential of stem cells in multiple systems, exploring the potential role of stem cells in treating female reproductive system diseases has become the focus of cutting-edge research. Recent years, a number of studies have also confirmed that many stem cells are effective in treating POF, including mesenchymal stem cells (MSCs), ovarian germ stem cells (OGSCs), embryonic stem cells (ESCs). Among them, BMSCs have shown great potential in repairing ovarian damage and restoring ovarian function in POF animal models or patients (13, 14). Due to their advantage of self-renewal capacity, multipotency, low immunogenicity, injury chemotaxis and less ethical controversy (15–18). BMSCs show a great therapeutic prospect in POF. Therefore, the mechanism and research progress of BMSCs in the treatment of POF are reviewed below.
The study was carried out following the PRISMA guidelines (19). Keywords and their combinations included: (Mesenchymal Stem Cell) OR (Stem Cell, Mesenchymal) OR (Bone Marrow Mesenchymal Stem Cell) OR (Bone Marrow Stromal Cell) OR (Mesenchymal Stromal Cell) OR (Stromal Cell, Mesenchymal) OR (Multipotent Mesenchymal Stromal Cell) OR (Mesenchymal Progenitor Cell) AND (Primary Ovarian Insufficiency) OR (Premature Ovarian Failure) OR (menopause, premature). The search strategy was applied to PubMed, MEDLINE, Web of Science and Embase database. The filters included: full text and female and the publications in the English language and year=“2005-Current”. The abstracts of the articles were included as following criteria (1): BMSCs transplantation in treating premature ovarian failure (2). Only original research articles were included, but not reviews.
A total of 1202 articles were retrieved after the initial search. 154 duplicate records were removed after importing into endnote software. After screening the title and abstract, 1014 articles were excluded mainly because they were not relevance with the current analysis, or they were reviews or meta-analysis, or other sources of MSCs but not bone marrow, or duplicate reports. Among the 34 potentially relevant studies, 14 were further excluded after reviewing full texts due to 5 studies were unrelated to the treatment of POF, 4 studies were unrelated bone marrow derived MSCs, 3 studies were related to bone marrow derived acellular therapy and one paper was meeting abstract (Figure 1).
MSCs are a kind of pluripotent stem cells originating from the early mesoderm, which can be isolated from bone marrow, adipose, dental pulp, placenta, umbilical cord, amniotic membrane, amniotic fluid and other tissues (20). Among them, bone marrow is the most important source of MSCs (21).
In the mid-1960s, Friedenstein et al. first identified BMSCs in mouse bone marrow, which were characterized by fibroblast-like cells with clonal potential (22). In recent decades, BMSCs have attracted plenty of attention for their potential in regenerative medicine and tissue engineering in replacing, repairing or restoring the function of damaged tissues or organs. Among the 1102 studies on “bone marrow stem cell” in the U.S. National Library of Medicine database, there are 95 studies of Phase III and 20 studies of Phase IV showing higher potency of BMSCs in clinical application than other stem cell types (23). Although MSCs are widely used in regenerative medicine, tissue engineering and immune regulation, defining for MSCs basing on surface markers and differentiation potential has so far been fragmentary (24). Depending on the minimum criteria of define MSCs published by the International Society for Cellular Therapy and recent studies, MSCs are defined as follows: first, the growth of cells in vitro will adhere to the substrate; second, the cells are characterized by expressing CD105, CD90, CD73, CD44 and Sca1 surface antigens, while lack of CD34, CD45, CD14 or CD19, CD79α, CD11b and HLA-DR; Meanwhile, these cells must have the ability of differentiating towards osteoblasts, chondroblasts and adipocytes in vitro (25–27). In addition to the surface markers mentioned above, the following antigens, including CD9, CD10, CD13, CD29, CD49, CD51, CD54, CD117, CD146, CD166 and Stro-1 are also expressed on the surface of MSCs (28). However, the specific combination expression of these markers varies with different host tissues (28). As mentioned above, a variety of positive markers of MSCs have been identified, but the specific markers of MSCs have not been found yet. Even though the cells meet the minimum standards of defining MSCs, there are great differences in their transcription patterns and differentiation potential in vitro (29). For example, human BMSCs express CD29, CD44, CD73, CD90, CD105 and Sca1, while lack of expression CD14, CD34, CD45, CD19, CD11b, CD31, CD86, Ia and HLA-DR, but the human adipose-derived MSCs (ADMSCs) are not completely identical with BMSCs, which express CD29, CD44, CD73, CD90, CD105, CD146, CD166 and MHC-I but not CD31, CD45 and HLA-DR (30). Single-cell experiments showed that there are differences in metabolic pattern, stress response and immunogenicity between BMSCs and adipose MSCs, and moreover, BMSCs were more heterogeneous (29). Andrzejewska and Chu et al. also found that the characteristics of BMSCs are tightly related to the age or pathological status of the donor (31). With the increase of age, the number of BMSCs and their potential of adipogenic, chondrogenic and osteogenic differentiation will decrease, meanwhile, the marker phenotype or stress level markers will also change (23, 31). Hass R et al. confirmed that compared with adult-MSCs, neonatal tissue-derived MSCs may have notable biological properties, such as higher multiplication capacity, differentiation potential and life span (32). In addition, depending on the source, the immunophenotypic and secretome vary from different MSCs that accounts for some differences in their responses (33, 34). BMSCs have been widely used due to their great potential of proliferation and multidirectional differentiation as well as stable genetic background (35–38). Recent studies also confirmed that BMSCs have shown remarkable therapeutic effects in many diseases including hematopoietic diseases, musculoskeletal diseases, immune disorders, neurodegenerative diseases, cardiovascular diseases, sports injuries, gastrointestinal and cutaneous diseases as well as POF (39–44). The therapeutic mechanisms of BMSCs are diverse. First, they can secrete a variety of soluble factors, including cytokines, growth factors and chemokines as well as immunomodulatory molecules, which participate in regulating proliferation, apoptosis, fibrosis and immune regulation of damaged tissues. In addition, BMSCs can also home to the damaged tissue and differentiate into specific cells to reconstruct the damaged local microenvironment, so as to maintain the integrity of tissue morphology and function stability (35, 45–47).
Studies have shown that BMSCs can improve ovarian reserve function in POF patients through various mechanisms, including homing, paracrine, regulation of ovarian angiogenesis, anti-fibrosis, anti-inflammatory and immune regulation, anti-apoptosis, mitochondrial transfer, autophagy regulation (Figure 2, Table 1).
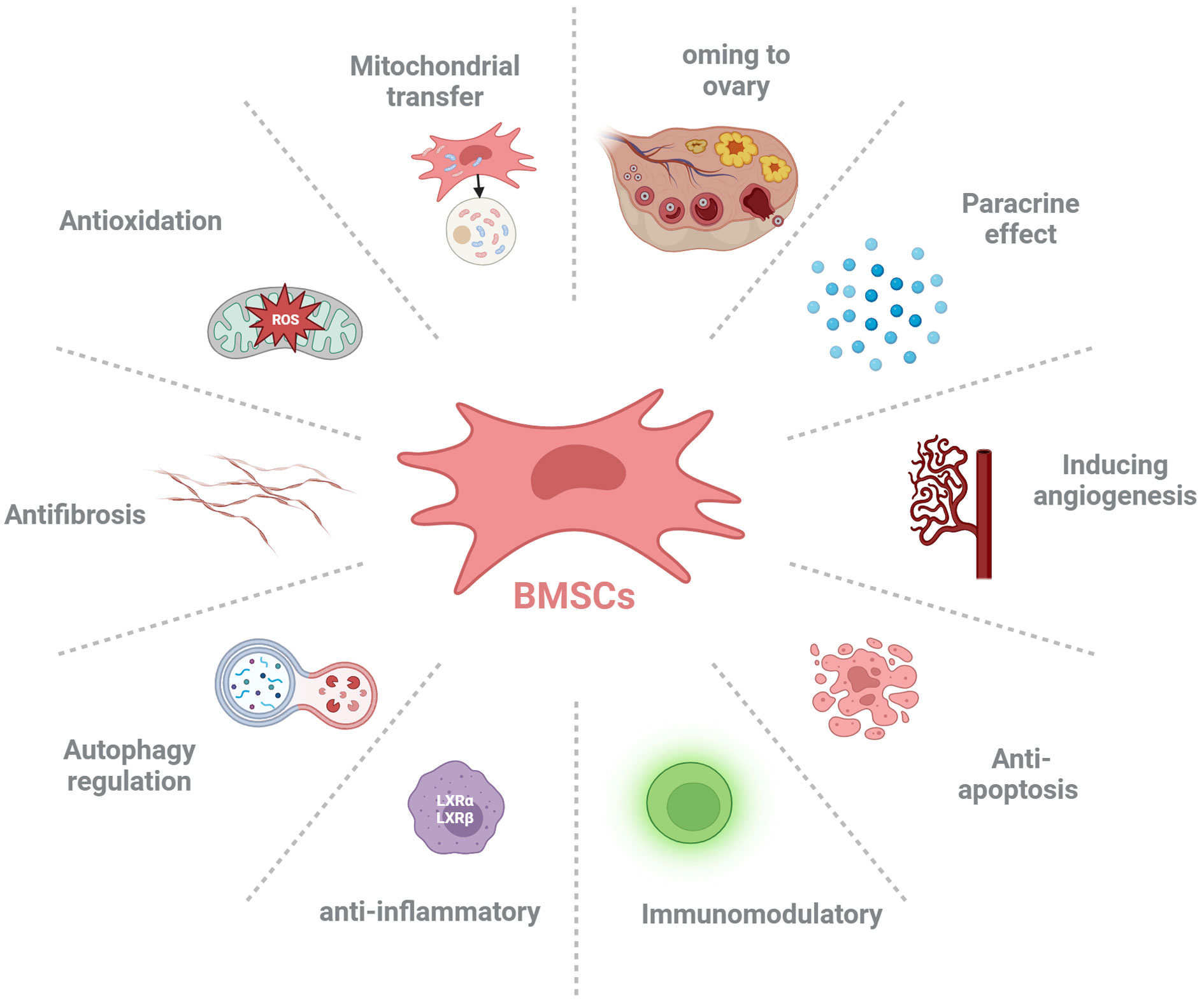
Figure 2 The possible mechanisms of bone marrow-derived mesenchymal stem cells (BMSCs) ameliorate premature ovarian failure (POF). BMSCs ameliorate ovarian function of POF through homing to injured ovary, paracrine effect, inducing angiogenesis, anti-apoptosis, anti-inflammatory, immunoregulation, autophagy regulation, antifibrosis, anti-oxidative stress and mitochondrial transfer.
MSCs homing is the process that self-derived or exogenous MSCs are captured in the vasculature of the target tissue and then migrate to the target tissue across vascular endothelial cells actuated by a variety of factors, which will undergo the process of selectin thrombus, cytokine activation, integrin block, transvascular endothelial cell and extravascular migration towards chemokine gradient (71, 72). Lu J et al. indicated that the therapeutic effect of BMSCs was evaluated through the amount of BMSCs migration to the site of lesion (73). Therefore, it’s crucial to understand the detailed mechanism about the homing of BMSCs (Figure 3).
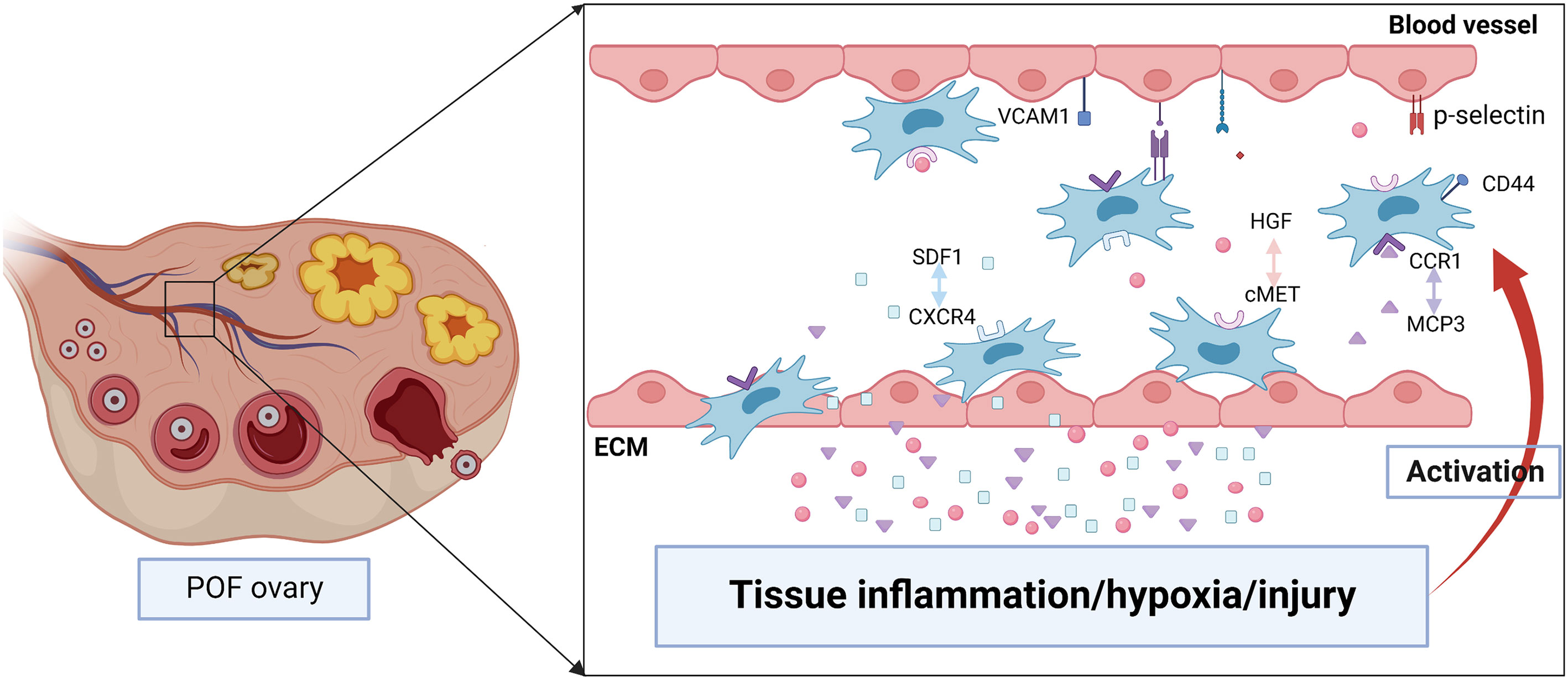
Figure 3 The possible mechanisms of BMSCs homing to injured ovary. Tissue inflammation, hypoxia or injury may induce the high level of chemokines including stromal cell derived factor 1 (SDF1), hepatocyte growth factor (HGF), monocyte chemotactic protein-3 (MCP3). They are released into the bloodstream and promote BMSCs proliferation as well as express specific receptors such as CXC chemokine receptor 4 (CXCR4), cMET, CC chemokine receptor 1(CCR1). After binding with the ligand, BMSCs migrate to the injured tissue along the gradient of chemokines concentration. In addition, various adhesion molecules including CD44, p-selectin, vascular cell adhesion molecule 1 (VCAM1) participate in regulating the homing of BMSCs.
The homing of stem cells is regulated by “stem cell niches microenvironment” of target tissues, which regulates the proliferation, migration and differentiation of stem cells through different signaling pathways. A large number of signaling molecules including stromal cell derived factor1(SDF1), hepatocyte growth factor (HGF), monocyte chemotactic protein (MCP)3, platelet-derived growth factor are released in injured tissues, while these factors stimulate high expression of specific receptors (CXC chemokine receptor 4 (CXCR4), cMET, CC chemokine receptor 1, platelet-derived growth factor receptor, respectively) on the surface of BMSCs, thereby promoting the homing of BMSCs (74, 75). Chemokines are closely relevant to activation and migration of cell, and at the same time, play a key role in many diseases including hematopoiesis, immune monitoring and inflammation, morphogenesis and neovascularization, as well as autoimmune diseases and cancer. Chemokine CXCL12, also known as SDF1, is one of the important molecules that induce homing of stem cells (76). The subtypes of CXCL12 are various, among which SDF1α and SDF1β are the main forms. SDF1 is widely expressed in many tissues including brain, thymus, heart, lung, liver, kidney, spleen and bone marrow, and maintains low secretion physiologically (77). It can activate phosphatidylinositol-3-kinase (PI3K)/Akt or mitogen-activated protein kinase (MAPK)/extracellular signal-related kinas (ERK) signaling pathway by binding to specific receptors and then regulate cell migration, proliferation, apoptosis, tube formation and chemotaxis (78). The receptors of SDF1 are various, among which CXCR4 is the typical one. CXCR4 is a G-protein-coupled receptor. SDF1/CXCR4 is involved in cell migration in early embryonic development and may influence stem cell migration from bone marrow or niche to damaged tissue during the whole life, especially in adulthood (79). Studies have shown that SDF1/CXCR4 axis plays a key role in promoting MSCs homing and survival (80). First, when contacting with CXCR4 at the extracellular domain, SDF1 will induce conformational change of the receptor, and which will strengthen SDF1 binding with the receptor pocket (81). Then, CXCR4 experiences a second conformational change, which activates the intracellular trimeric G protein by dissociating of Gα subunit from the Gβ/Gγ dimer (81). Activated G proteins can activate multiple signal pathways, and then participate in increasing intracellular calcium, modifying cellular proteins, altering transcription factor binding and gene expression, thereby regulating cell proliferation, migration, survival and senescence (81). The expression of SDF1 is greatly increased in damaged tissues, which promotes the homing and survival of stem cells in the damaged tissue respectively by binding with CXCR4 and CXC chemokine receptor 7 on the surface of MSCs (82). Studies have shown that BMSCs mobilized from bone marrow to peripheral blood and then migrated to injured tissue, possibly along with the gradient of SDF1 concentration (83). Tamari et al. found that adding SDF-1 in standard medium could promote the migration of MSCs, while the MSCs migration was significantly reduced after the intervention of SDF1 receptor antagonist (84). Similarly, after transducing lentivirus carrying SDF1α into mouse BMSCs, overexpression of SDF1α can promote the proliferation, migration, and osteogenic differentiation of BMSCs, and which partly by activating the Wnt pathway (85). In addition, when lentivirus carrying CXCR4 are transduced into human BMSCs, the migration ability of CXCR4-BMSCs toward SDF1 is significantly increased due to the overexpression of CXCR4 (86). Ling L et al. also indicated that the levels of SDF1 in ovaries and serum were remarkably increased in rats with cyclophosphamide-induced POF, and ovaries with POF induced the homing of MSCs expressing CXCR4 (64), which further confirmed that SDF1/CXCR4 axis partially regulated the migration and homing of transplanted MSCs to the ovaries of POF.
HGF is a growth factor consisting of α and β chains, which contains four cyclic domains and one serine protease-like domain, respectively. Evidences demonstrate that HGF plays an important role in growth stimulation, tissue regeneration, migration, angiogenesis, morphogenesis, tumorigenesis, and tumor invasion (87). In addition, Han P et al. found that HGF could promote the proliferation and differentiation of pluripotent stem cells, ESCs and BMSCs (88). The ovulation process is thought to be hormone-induced tissue injury (89). HGF is activated through the process of ovulation injury-tissue factor–thrombin–HGF activator–HGF cleavage and promote repair of tissue injury after ovulation (90), which indicates that ovarian injury increases the level of HGF. Han P et al. also confirmed that the expression of HGF in injured tissues was significantly increased (88). The receptor tyrosine kinase cMET is the specific receptor of HGF, which is a member of the transmembrane tyrosine kinase receptor superfamily and has independent phosphorylation activity (91). The high-affinity binding of HGF to cMET induces homodimerization and autophosphorylation of the cytoplasmic domain of cMET, and then activates HGF/cMET and downstream pathways such as the MAPK/ERK, PI3K, p-38, and the Akt/protein kinase B pathways, thereby promoting cell proliferation, invasion, survival, motility and angiogenesis (88, 92, 93). The HGF/cMET pathway plays an important role in the BMSCs homing. Studies have shown that the high level of HGF in injured tissues can upregulate the expression of cMET in stem cells (88). And the overexpression of cMET promotes the homing of BMSCs significantly (94).
MCP is a member of the CC chemokine family, which mediates cell chemotactic. Previous studies demonstrated that significant upregulation of the stem cell homing cytokine MCP-3 in urethral and vaginal tissues following simulated birth trauma (95–97). Yamada et al. found that MCP-1 and MCP-3 were the homing factors of MSCs, which could recruit MSCs to the injured tissues (98, 99). Cui L et al. indicated that the level of TGF-β1 in ovarian tissue of POF rats was increased (100). It can promote BMSCs migrate to lesion sites in vitro and in vivo though histone demethylase KDM6B mediated inhibition of methylation marker H3K27me3 (101). Clinical studies also showed that the level of chemokines and growth factors of POF patients in follicular fluid significantly increased comparing with control group, including interferon-γ-inducible protein 10, macrophage inflammatory protein-1α, C-X-C motif chemokine ligand 8, eosinophil chemokine factor-1 and leukaemia inhibitory factor as well as brain-derived neurotrophic factor, vascular endothelial growth factors (VEGF)‐D and basic fibroblast growth factor (bFGF) (102), which may be tightly related to enhance the homing efficiency of BMSCs.
In the process of chemotaxis to damaged tissue, apart from the chemokines mentioned above, the rolling and adhesion process of MSCs are also regulated by various adhesion molecules including CD44, vascular cell adhesion molecule 1, intercellular adhesion molecule 1, p-selective protein, integrin and α4β1 (103). Finally, MSCs cross vascular endothelial cells and basement membrane under the mediation of matrix metalloproteinases and follow the gradient of chemokine concentration homing to the target tissues (104).
Recent studies have confirmed that BMSCs can migrate to damaged parts and ameliorate ovarian structure and function via inhibiting apoptosis, promoting proliferation and improving folliculogenesis in POF mice (13, 49, 50). Moreover, BMSCs regulates the function of local cells after homing through intercellular contact (105). Previous studies have shown that BMSCs can differentiate into specific cells to replace damaged cells and then repair damaged tissues (51, 106, 107). On the contrary, recent studies indicated that the transplanted BMSCs were located in the interstitial area rather than in follicles of ovary, which suggested that BMSCs could homing to the injured ovary and may enhance the ovarian function by regulating the microenvironment around ovarian follicles rather than differentiating into oocytes or GCs (49, 52). Park HS et al. also confirmed that the engrafted BMSCs to the POF ovary didn’t differentiate into ovarian cells, but restored ovarian GCs via secreting paracrine factors (53). Moreover, the number of engrafted BMSCs decreased gradually within 2 weeks and disappeared entirely in majority animals within 4 weeks after transplantation (53). It is remarkable that the number of transplanted stem cells in injured tissues isn’t always correlated with the healing rate, because even though stem cells can’t exist in the target tissues for a long time, their paracrine and autocrine roles may help to heal and activate local stem cells of stationary stage, which may promote the recovery of damaged tissue (54). Zahra et al. also indicated that there were small number of OGSCs in ovarian surface epithelium and cortical tissues, which could express ovarian germline markers and differentiate into cells of all three embryonic germ layers (108). And in addition, these cells could generate new GCs and primary follicles (108). Therefore, it can be concluded that BMSCs may restore the structure and function of damaged ovary by activating OGSCs (109), which can differentiate into new GCs and primary follicles, but the specific mechanism still needs to be further studied.
It isn’t completely clear that whether or not BMSCs can differentiate into ovarian cells after homing to injured ovary. Currently, it is widely believed that the key of BMSCs restoring POF is based on paracrine effect of stem cells rather than differentiation. However, it is remarkable that these secretory factors are not the only mechanism that BMSCs improve ovarian function. Ling et al. demonstrated that MSCs transplantation was more effective in alleviating ovarian damage and restoring ovarian function in POF rats compared with injection of MSCs conditioned media (CM) (65). Therefore, further studies are needed to clarify the mechanism of interaction between BMSCs and ovaries, which is greatly significant to the clinical development of BMSCs transplantation.
BMSCs can synthesize and secrete a variety of chemokines, growth factors and hormones, including VEGF, insulin-like growth factor-1 (IGF-1), HGF, bFGF (50, 55). These molecules play important roles in angiogenesis, anti-fibrosis, anti-inflammatory and immunomodulatory, anti-apoptotic, thereby improving the local microenvironment and promoting the recovery of damaged tissues (Table 2). Many studies have shown that BMSCs restore ovarian structure and function possibly through paracrine effect (49, 53, 56). Among all the BMSCs paracrine factors, brain-derived neurotrophic factor is a member of the neurotrophic factor growth factor family, which promotes oocyte maturation and embryo development (102). bFGF and VEGF are involved in ovarian angiogenesis, which help to provide nutrition for GCs (102). Meanwhile, VEGF and its receptors play an important role in inhibiting apoptosis of GCs, and promoting the development of follicles (51, 110, 111). IGF-1 is a growth hormone that stimulates GCs proliferation by regulating DNA replication in theca cells and GCs, and helps to enhance the function of gonadotropin, regulate the activity of aromatase and promote the formation of follicular cavity as well as inhibit cell apoptosis (48). HGF has significant anti-apoptosis effect in ovarian GCs and oocytes, which helps to promote blood vessel growth and improve ovarian function (113). bFGF serves as an initiator of folliculogenesis via inducing primordial follicle development (117). Although BMSCs transplantation has been widely used in repairing the damaged tissues, it has been reported that there are some risks in stem cells transplantation including tumor formation, pulmonary embolism (122, 123).
In order to find the safer treatments, the researcher study on whether the CM of BMSCs is a feasible treatment. BMSCs-CM contains a variety of cytokines, such as VEGF, HGF, IGF-1 etc., which can inhibit apoptosis and promote proliferation of GCs in vivo or in vitro. These results indicate that the secretion of these factors plays an important role in BMSCs improving ovarian function (124). Khanmohammadi N.et al. indicated that injecting the BMSCs-derived CM or BMSCs into the damaged ovaries had almost the same effect on repairing the damaged ovaries (57). Similarly, human BMSCs-derived CM plays a similar role to human BMSCs in reducing apoptosis of human GCs, promoting cell proliferation, improving the viability of ovarian GCs and restoring ovarian structure as well as stimulating estrogen production (58). Above studies indicated that paracrine effect plays a major role in BMSCs therapy. However, Ling et al. indicated that the therapeutic effect of MSCs medium on POF was not as good as MSCs (65). Thus, whether the efficiency of BMSCs-CM is equal to BMSCs still warrants further study.
Angiogenesis plays an important role in repairing damaged ovaries. Previous studies have shown that MSCs may participate in angiogenesis through the following two mechanisms. Firstly, MSCs have the potential to differentiate into endothelial cells, vascular smooth muscle cells and other types of cells. Secondly, MSCs can secrete a variety of bioactive factors that can promote angiogenesis (125). Comparing with other tissue-derived MSCs, BMSCs have higher angiogenic activity, which can differentiate into endothelial cells, pericytes, and vascular wall, and then promote angiogenesis (59). Moreover, BMSCs contributing to angiogenesis at least partly depending on secreting various angiogenic factors including VEGF, MCP1, interleukin-6, SDF-1α, macrophage colony-stimulating factor, IL-1 receptor, IGF-1, interleukin-8, metalloproteinase 3 (114). The composition and concentration of angiogenic factors will also ultimately affect the functional responses of BMSCs (114). VEGF is a powerful angiogenic factor and significantly affects ovarian angiogenesis, which is closely related to follicular formation and development (110, 111). After binding to the receptor, VEGF activates endogenous VEGF signaling through the PI3K/Akt and GSK3β/β-catenin pathways, which results in ovarian vascular remodeling and ultimately enhances follicular formation (66). IGF-1 is also an effective angiogenic factor, which is highly expressed in the damaged vessels (52). After binding to its receptors, IGF-1 can activate the PI3K/Akt signaling pathway, which induces endothelial cell proliferation, differentiation and migration, and then possibly improves the structure and function of damaged ovaries by promoting angiogenesis (52, 112). SDF1 secreting by BMSCs can directly promote the differentiation of BMSCs into vascular endothelial cells through binding to CXCR4 on BMSCs (116). In addition, SDF1 indirectly promotes the proliferation of vascular endothelial cells by promoting the secretion of VEGF from BMSCs (116). BMSCs-derived CM treatment can activate human ovarian vascular endothelial cells, and then in which the expression of angiogenic marker genes, transforming growth factor-α, C-C motif chemokine ligand 11 are increased, which inducing endothelial cell proliferation and promoting angiogenesis as well as increasing the density of new blood vessels (115). Moreover, studies have shown that the key mechanism of BMSCs CM enhancing angiogenesis is highly correlated with the PI3K/Akt pathway (115). Zhang et al. also found that BMSCs may control angiogenesis and follicular survival of xenografted human ovarian tissues by angiotensin (126) (Figure 4).
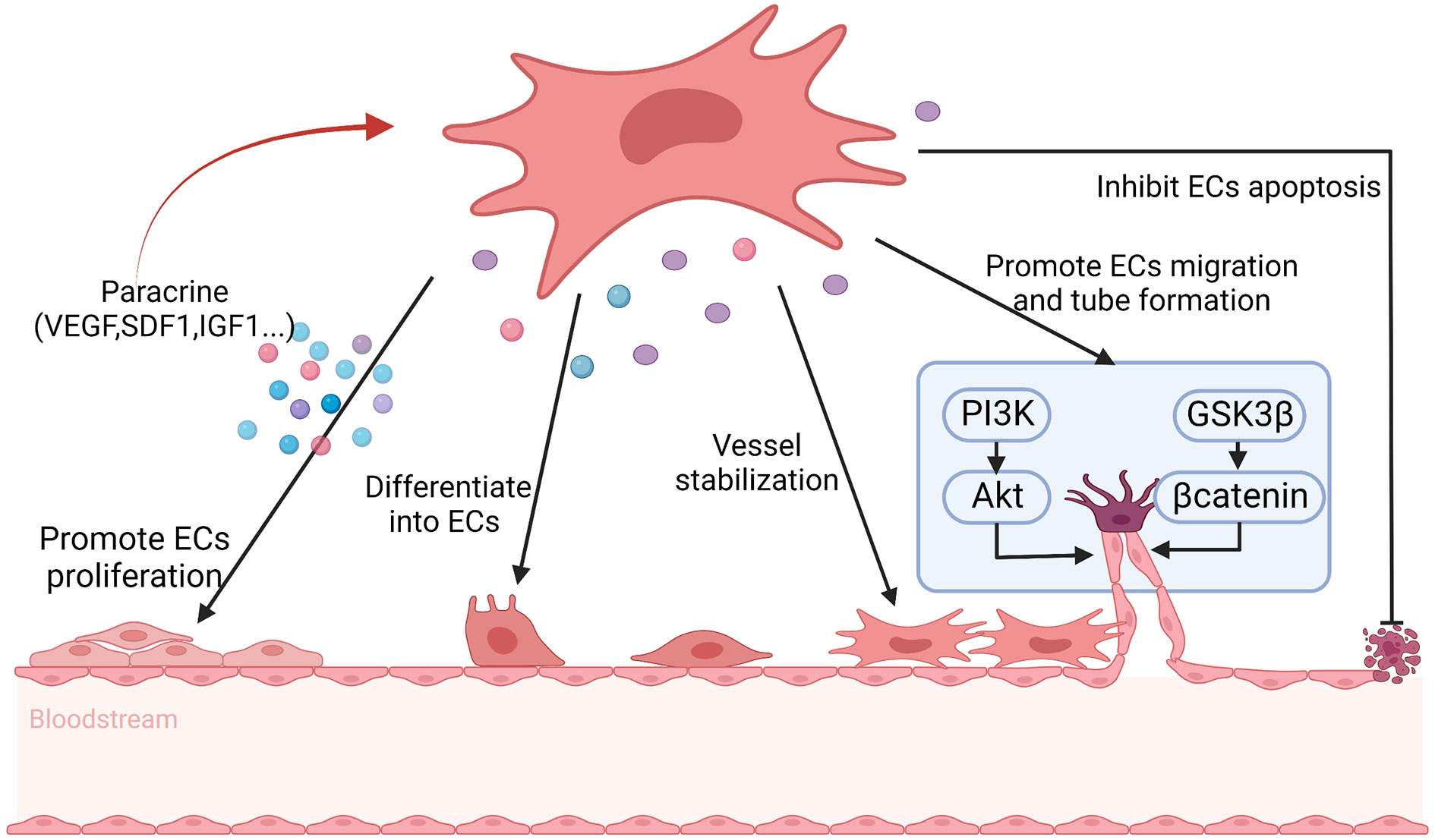
Figure 4 The possible mechanisms of BMSCs promote angiogenesis in POF. BMSCs help to promote angiogenesis through differentiating into endothelial cells (ECs), maintaining vascular stability, inhibiting ECs apoptosis and secreting angiogenic factors, including vascular endothelial growth factor (VEGF), stromal cell derived factor 1 (SDF1) and insulin-like growth factor 1 (IGF1) et. to promote ECs proliferation, as well as promoting ECs migration and tube formation via PI3K/Akt and GSK3β/βcatenin signal pathway. At the same time, the secrete factors reinforce the angiogenesis of BMSCs in turn.
In ovarian tissue, stromal cells can proliferate and differentiate into endometrial cells or myofibroblasts. The myofibroblasts synthesize and secrete extracellular matrix including type I and III collagen fibers. The excessive accumulation of extracellular matrix will lead to organ fibrosis, which is the basic pathological change of POF (100). Ovarian fibrogenesis is associated with various cytokines, including matrix metalloproteinases, tissue inhibitors of metalloproteinases, transforming growth factor-β1 (TGF-β1), connective tissue growth factor, peroxisome proliferator-activated receptor γ, VEGF, endothelin -1 (127). TGF-β1 is a key mediator of tissue fibrosis, which is involved in fibrosis of multiple organs by activating its downstream small mother against decapentaplegic (Smad) signaling and triggering pre-fibrotic gene overexpression (128). Moreover, Inagaki Y et al. also indicated that TGF-β participated in inducing transcription of alpha-smooth muscle actin and other extracellular matrix proteins (129), which would promote the development of tissue fibrosis. Studies confirmed that the level of TGF-β1 in ovarian tissue of POF rats was increased notably (100). And after BMSCs transplantation, the level of TGF-β1 in the POF ovary was down-regulated prominently (13). Moreover, Cui et al. found that human umbilical cord-derived MSCs (hUMSCs) inhibited ovarian fibrosis by regulating stromal cell differentiation through TGF-β1/Smad3 signaling pathway, thereby promoting the recovery of ovarian function in POF rats (100). And MSCs derived from menstrual blood help to improve ovarian function by reducing ovarian interstitial fibrosis and apoptosis in GCs, which may be partially mediated by secreting fibroblast growth factor 2 (69). Therefore, we guess that BMSCs may also inhibit ovarian fibrosis by the same mechanism. However, due to the reactivity of different sources of MSCs is diverse, further studies are needed to confirm whether this hypothesis is reliable. In addition, Wang PP et al. indicated that the anti-fibrosis effect of BMSCs in liver fibrosis induced by lipopolysaccharide was through secreting HGF and cell-cell contact, which was closely bound up with the inhibition of Toll-like receptor 4/Myeloid differentiation primary response gene 88/nuclear factor kappa-B signaling pathway (130). In view of this, whether BMSCs protect ovarian fibrosis in POF model (131) by the same mechanism is worthy of further exploration.
Chronic inflammation is one of the main factors driving the process of fibrosis, which can change the normal structure of tissues and result in functional deterioration. BMSCs exhibit significant anti-inflammatory bioactivity, which may be another mechanism of BMSCs inhibiting ovarian fibrosis. BMSCs promote the secretion of anti-inflammatory cytokine IL-10 and inhibit the expression of pro-inflammatory cytokines tumor necrosis factor α and interleukin-6, thereby inhibiting the inflammatory response, which plays an important role in anti-fibrosis effect (118). In addition, angiogenesis and reperfusion are also critical to repair damaged tissue and prevent fibrosis.
Abnormally elevated levels of chemokines and cytokines in follicular fluid of POF patients will induce intracellular inflammatory in follicular niche by cellular and paracrine interactions, which adversely affects oocyte quality and the function of GCs or theca cell (102). Moreover, it can attract a large number of white blood cells to migrate to the ovaries or activate plenty of immune cells, which leads to chronic low-grade inflammatory state, all of which will further aggravate follicular atresia and apoptosis, and ultimately affect the quality and quantity of oocytes (102). The concentrations of pro-inflammatory cytokines interleukin-6, interleukin-8 and tumor necrosis factor α in serum of chemotherapy-induced POF mice were significantly increased, while the levels of anti-inflammatory cytokines IL-10 were markedly decreased (132). The abnormalities of inflammation-related factors may result in apoptosis of GCs, which tightly contributed to the development of ovarian damage in POF mice (132). The signaling molecules from inflammatory sites promote the homing of BMSCs, which can help to inhibit the production of inflammatory cytokines and proliferation of lymphocytes, thereby inhibiting local inflammation (133). The immunomodulatory function of MSCs is mainly through intercellular contact, paracrine activity and interaction with T cells, B cells, natural killer cells, macrophages, monocytes, dendritic cells and neutrophils (134) (Figure 5). For example, the direct contact betwixt the proinflammatory macrophages and BMSCs promotes not only the production of tumor necrosis factor-stimulated gene-6, but also the expression of CD200 on BMSCs (105). The elevated tumor necrosis factor-stimulated gene-6 helps to suppress the proliferation of T cells and promote the transform between proinflammatory macrophages and anti-inflammatory phenotype, while the increased CD200 participates in mediating the interaction betwixt BMSCs and proinflammatory macrophages (105). Moreover, BMSCs secrete a variety of immunomodulatory mediators, such as indoleamine 2,3 dioxygenase, nitric oxide, prostaglandin E2, TGF-β, heme oxygenase 1, HGF, which play an important role in anti-inflammatory and immunomodulatory (119). Pro-inflammatory cytokine interferon-γ has a synergistic immunosuppressive effect with BMSCs, which mediates immune regulation by upregulating the expression of prostaglandin E2, HGF, TGF-β1 in BMSCs and inducing the expression of indoleamine 2,3 dioxygenase as well as participating in tryptophan catabolism (135). Regulatory T cells deficiency is associated with the pathogenesis of POF via mediating apoptosis and steroidogenesis dysfunction of GCs (136). Luz-Crawford P et al. found that BMSCs were capable to induce functional regulatory T cells during the differentiation process of Th1 and Th17 cells, which was related to the increase of IL-10 production by BMSCs (137). Moreover, BMSCs participate in regulating the number and function of T cells (138). Therefore, we surmise that maybe BMSCs regulate immune system in POF through procedure mentioned above.
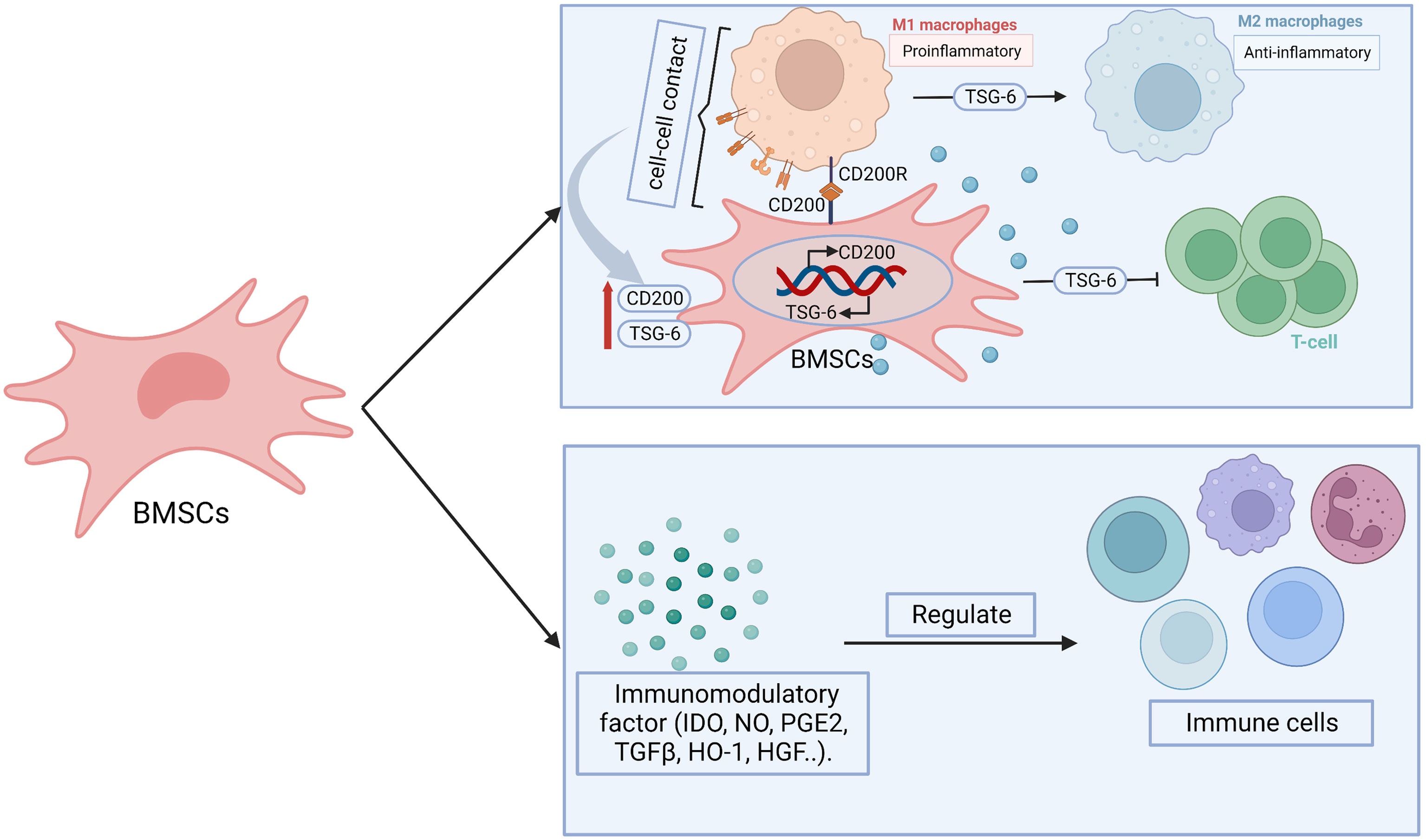
Figure 5 The possible mechanisms of BMSCs in immunoregulation. BMSCs display immunomodulatory effect through paracrine effect and cell-cell contact. BMSCs secrete immunomodulatory related factors, including indoleamine 2,3 dioxygenase (IDO), nitric oxide (NO), prostaglandin E2 (PGE2), transforming growth factor-β (TGF-β), heme oxygenase 1 (HO1), hepatocyte growth factor (HGF), which participate in regulating the number or function of immune cells. BMSCs-derived tumor necrosis factor-stimulated gene-6 (TSG-6) promote the transform from pro-inflammatory macrophages (M1) to anti-inflammatory (M2) phenotype as well as inhibit the proliferation and inflammatory response of T cells. The paracrine effect is heightened after BMSCs- M1 contact, which enhance the expression of TSG-6. Moreover, CD200R on M1 bind with CD200 on BMSCs promote the transition of M1 to M2. At the same time, which contribute to promote the expression of CD200 on BMSCs.
Apoptosis of GCs and theca cells increase in the ovaries of cyclophosphamide (CTX)-induced POF mice (55). BMSCs transplantation can help to inhibit the apoptosis of ovarian cells by regulating the levels of apoptosis-related genes such as Bax, p53, caspase-3 and Bcl-2. Meanwhile, it can regulate the expression of cyclinD2 and p21 to promote the proliferation of residual ovarian cells, so as to repair the structure and function of damaged ovary (37, 55). Kilic S et al. revealed that BMSCs could protect germ cells from CTX-induced cell apoptosis and DNA damage (60). In addition, BMSCs regulate apoptosis, proliferation, and differentiation of ovarian follicles via genetic and epigenetic regulation of the integrated TGF-β, Wnt/β-catenin and Hippo signaling pathways, which are associated with ovarian follicles growth and maturation (13). Transplanting BMSCs with overexpressing miR-21 into the rat ovaries damaged by chemotherapy can more efficiently inhibit the apoptosis of GCs and improve the ovarian structure and function through targeting to PDCD4 and PTEN (the target genes of miR-21) compared with the transplantation of BMSCs or miR-21 alone (139). In addition, BMSCs transplantation may inhibit GCs apoptosis and ameliorate ovarian function through releasing VEGF, HGF, IGF-1 and IGF-2, while upregulating the expression of Bcl-2 (61, 62). Endoplasmic reticulum stress plays a crucial role in promoting autophagy and apoptosis in GCs, which results in excessive follicle loss and endocrine disorders (67, 140). Hongxing Li et al. found that human placenta MSCs (hPMSCs) transplantation inhibited the activation of inositol-requiring enzyme 1α pathway of endoplasmic reticulum stress in ovaries and reduced the up-regulation of XBP1, GRP78, caspase-12 in zona pellucida glycoprotein 3 peptides-induced POF mice, which subsequently decreased GCs apoptosis (67). Hence, it’s worth to explore whether BMSCs restore ovarian function through regulating endoplasmic reticulum stress as hPMSCs (Figure 6).
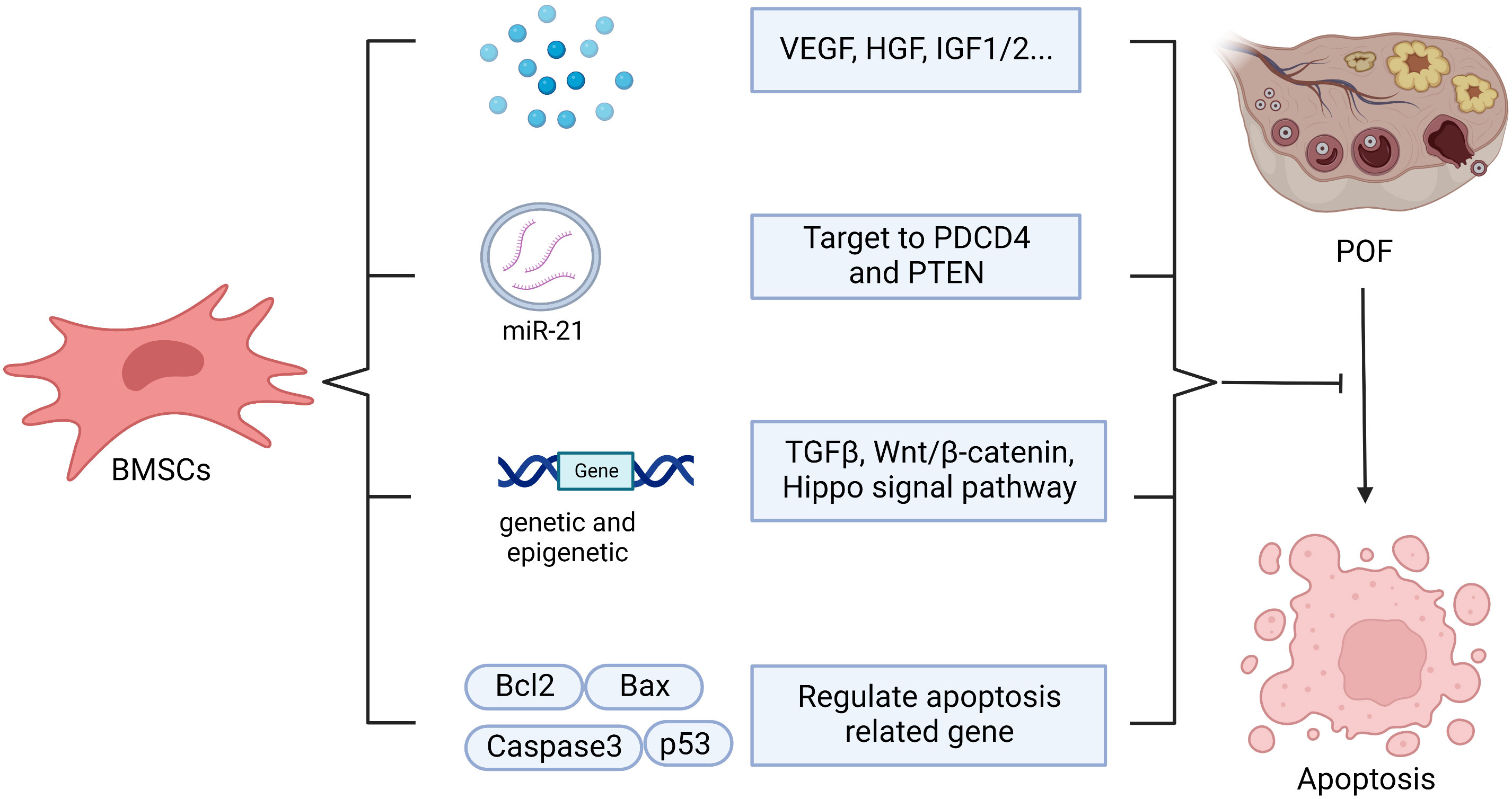
Figure 6 The possible mechanisms of BMSCs in anti-apoptosis. BMSCs help to inhibit apoptosis through secreting vesicle containing microRNA-21(miR-210), which targeting to PDCD4 and PTEN, or secreting vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), insulin-like growth factor 1/2 (IGF1/2) or and regulating apoptosis related gene, including Bcl2, Bax, Caspase3, p53, as well as regulating genetic and epigenetic via TGFβ, Wnt/β-catenin, Hippo signal pathway.
Oxidative stress participates in inducing lipid peroxidation functionally and structurally, and changing protein and DNA as well as promoting apoptosis, which plays an important role in the pathogenesis of POF (17, 141). Ağaçayak E et al. found that the total oxidation status and oxidative stress index levels were increased in POF patients (17). Superoxide dismutase (SOD), which is an antioxidant enzyme, can help to partially restore ovarian function by inhibiting ROS production (141). However, the levels of SOD and nuclear factor erythroid 2-related factor (Nrf2) were decreased in the POF mice ovary, leading to the accumulation of ROS (142). ROS accumulation may impair to ovarian function and oocyte quality (141). Several studies have found that BMSCs play an important role in antioxidant stress. Proteomic analysis revealed that various antioxidant mediators such as cyclophilin A, cyclophilin B, thioredoxin, DJ-1, heat shock protein 27, peroxiredoxin-1 secreted by BMSCs showed significant antioxidant stress effects (120). IGF1 releasing from BMSCs also possesses effective anti-oxidative abilities (52). In addition, BMSCs transplantation impacts the activity of SOD and the content of malondialdehyde through reducing the expression of cyclin-dependent kinase inhibitor 2A (P16) and increasing proliferating cell nuclear antigen, thus improving the morphology and function of ovary (63). Seok J et al. indicated that the transplanted hPMSCs reduced oxidative stress and apoptosis in ovariectomized rat model via changing the expression of HO-1/HO-2 and enhancing catalase and SOD1 gene expression (68). Furthermore, hUMSCs improve cisplatin-induced autophagy in injured ovarian tissue via reducing the levels of ROS and regulating AMPK/mTOR signaling pathway (70). From this, we conjecture that maybe the mechanism of BMSCs in anti-oxidative stress is consistent with aforesaid MSCs. Currently, there are few studies about the mechanism of BMSCs regulating oxidative stress to improve POF ovarian function, but they have been widely studied in other diseases. Niu Y et al. revealed that BMSCs-CM could alleviate oxidative stress injury of neural stem cells through decreasing the expression of lactate dehydrogenase and malondialdehyde, increasing the expression of SOD and inhibiting the Notch1 signaling pathway (143). Moreover, BMSCs help to reduce oxidative stress and inflammation via down-regulating NF-kB signaling pathway, thereby reducing doxorubicin-induced nephropathy (144). Whether the effect of BMSCs on antioxidant stress in POF is consistent with others or not still needs further research.
Gomzikova et al. have found that MSCs can transfer mitochondria to injured cells through various methods such as tunneling nanotubes, extracellular vesicle and cell fusion, thereby restoring aerobic respiration and mitochondrial function of cells and inhibiting apoptosis (145). Wang L et al. revealed that BMSCs transplantation could resist to mitochondrial dysfunction in age-associated ovarian hypofunction mice through enhancing mitochondrial membrane potential, increasing mitochondrial DNA copies, and improving mitochondrial cristae alignment and vacuolation, as well as regulating expression of mitochondrial dynamics-related proteins (146). Whether it is related to mitochondrial transfer still needs further investigations. Tseng N et al. indicated that when co-cultured with oxidant-damaged neurons, BMSCs could transfer complete mitochondria to injured neurons via instantaneous tunneling nanotubes, which may contribute to the preservation and functional recovery of neurons after stroke (147). Human BMSCs can transfer mitochondria to injured human umbilical vein endothelial cells via tunneling nanotubes, which helps to promote cell proliferation, reduce cell apoptosis, and enhance their capacity of transmembrane migration and angiogenesis, thereby improving endothelial cells function and hematopoietic system regeneration (148). Furthermore, the mitochondrial transfer induced by BMSC-derived extracellular vesicle helps to enhance phagocytic capacity, decrease secretion of proinflammatory cytokine, and upregulate expression of the M2 phenotype marker CD206 of human macrophages, thereby ameliorating lung injury in the acute respiratory distress syndrome environment (149). In conclusion, mitochondria transfer of BMSCs plays an important role in the treatment of various diseases. Whether BMSCs can recover damaged ovarian function through mitochondrial transfer in POF treatment still needs further investigation.
Autophagy is a crucial molecular pathway for maintaining cellular and organismal homeostasis, which can remove damaged or excess proteins, organelles, and foreign pathogens in the cells (150). It has been proved that autophagy is involved in the preservation of primordial follicles pools in young mice and the elimination of inferior follicles during follicular development (151). However, Xie QE et al. suggested that excessive autophagy was connected with the pathogenesis of POF (152). Therefore, regulating the activities of autophagy may be an effective method to improve ovarian function of POF (153). Yin et al. indicated that heme oxygenase-1 gene expressed in UMSCs is crucial in restoring the ovarian function of POF mice with UMSCs transplantation by activating JNK/Bcl-2 signaling pathway-regulated autophagy and upregulating the number of CD8+CD28− T cells in the circulation (121). Moreover, Lu X et al. suggested that hUMSCs transplantation could alleviate ovarian function in POF rats via inhibiting the theca-interstitial cells apoptosis through reducing autophagy, which achieving in part through regulating ROS levels and inhibiting the AMPK/mTOR signaling pathway (70). From this, we can speculate that maybe BMSCs regulate autophagy to ameliorate ovarian function. Presently, the fact that BMSCs influence autophagy to restore ovarian function in POF has not been reported, but BMSCs do have an effect on autophagy in other diseases, such as ischemia/reperfusion injury (154). Therefore, exploring the mechanism of BMSCs in modulating autophagy may provide a feasible therapeutic strategy for POF.
Recent years, lots of studies have proved that BMSCs transplantation improves ovarian damage caused by chemotherapy or other factors (13, 49, 50, 53, 56), which provides promising treatment options for POF. However, chronic inflammatory response, hypoxia, oxidative stress and other microenvironmental changes in the damaged tissue area often leads to apoptosis or low homing efficiency of transplanted MSCs (155, 156). Saberi K et al. illustrated that more than 80% of the transplanted cells underwent apoptosis after transplantation to the target organ (157). The low mobility and survival rate of BMSCs transplantation often limit their therapeutic potential. Therefore, enhancing the homing and survival rate of transplanted cells is critical to improve the therapeutic effect of BMSCs (Table 3).
To enhance repairing capabilities of transplanted cells, gene modification is worth to take into consideration before BMSCs transplantation. Previous studies indicated that overexpression of homing related factors, such as SDF1, CXCR4, CMET, can help to promote the homing of BMSCs significantly (85, 86, 94). After dual genetic modification of BMSCs via transducing CXCR4 and IL-35, the migration capability and immunoregulation effects of BMSCs are remarkably improved compared to their natural counterparts (158), which implies that dual CXCR4/IL-35 overexpression-BMSCs may be a promising and attractive treatment in autoimmune POF. Fu X et al. revealed that miR-21-overexpression BMSCs showed less apoptosis and more vitality after transplanting and stronger repairing effect in chemotherapy-induced POF compared with the transplantation of BMSCs injection alone (139). Ni X et al. found that co-overexpression of VEGF and Bcl-2 protected BMSCs from a hostile environment through inhibiting apoptosis, suppressing autophagy and enhancing paracrine signaling (159). In addition, Parkinson’s disease protein 7 (PARK7) is an antioxidant protein that enhances cellular resistance to oxidative stress and stress-induced apoptosis (180, 181). PARK7 overexpression enhances antioxidative‐stress capacity in BMSCs via activating the ERK1/2 signal pathway, which effectively decreases the level of ROS/malondialdehyde and protects the mitochondrial membrane potential as well as inhibits apoptosis of BMSCs subjected to oxidative stress (160). Moreover, PARK7 can also promote the disintegration of Nrf2/Kelch-like echinacoside associated protein 1 complex, thereby activating Nrf2. And then the activated Nrf2 will enter the nucleus to activate the expression of manganese superoxide dismutase, catalase, glutathione peroxidase, and other antioxidant enzymes. This cascade helps to remove excessive cellular ROS and protect BMSCs from stress-induced apoptosis (161). Therefore, BMSCs overexpressing PARK7 before transplantation may help to reduce BMSCs apoptosis induced by oxidative stress and increase the homing efficiency of BMSCs to the damaged microenvironment, thereby enhancing their repairing effect. From this, gene‐modified BMSCs transplantation may be a promising method to enhance the therapeutic effect of BMSCs.
Studies have reported that when the transplanted cells are trapped in an undesirable environment, such as free radicals, inflammation or hypoxia, they may suffer early death, which may limit the beneficial effects of these cells (157). Thus, it will be beneficial to develop a special pre-treatment system or co-transplantation to enhance the proliferation, homing and survival ability of BMSCs to improve their performance. Zhu X et al. revealed that shikonin pretreatment significantly inhibited apoptosis in hUMSCs under hypoxic-ischemic conditions in vitro or vivo studies through regulation of autophagy via regulating AMPK/mTOR signal pathway, thereby improving hUMSCs survival surrounding injured tissues (163). In addition, the immunoregulation capability of human ADMSCs can be changed when cultivated under serum starvation to adapt to the different culture conditions in vitro (164), which suggests that serum starvation pretreatment may be beneficial to enhance the therapeutic effect of transplanted BMSCs. Cunningham CJ et al. revealed that hypoxic pretreatment of BMSCs could increase the secretion of VEGF, FGF2, HGF, IGF1 (167). Erythropoietin (EPO) is a glycoprotein hormone with the effect of antioxidant and anti-inflammatory. Vitro studies showed that the proliferation rate and mobility of BMSCs were significantly increased after 48h pretreatment with 500 IU/mL EPO, which may be achieved by regulating BMSCs cytoskeletal rearrangement and upregulating the expression of CXCR4 (165). In vivo studies also confirmed that pretreatment with EPO before transplantation significantly increased the homing and therapeutic capacity of BMSCs (165). In addition, EPO is related to the activation of SIRT1 signal in BMSCs, and then regulates the expression of P53 and Bcl-2, which shows an anti-apoptotic effect (166). Colony-stimulating factors, which are hematopoietic growth factors, participate in regulating the proliferation, migration and differentiation of bone marrow cells. BMSCs pretreated with granulocyte colony-stimulating factor help to enhance the homing efficiency of BMSCs to injured tissues by upregulating the CXCR4 expression, which significantly increases repairing effects of BMSCs (162). Sameni HR et al. also confirmed that the recovery of ovarian function in POF rats was more favorable in coadministration of BMSCs with granulocyte colony-stimulating factor compared with the administration of either of them individually (48).
Low intensity pulsed ultrasound (LIPUS) is a pulse emission with low intensity and low thermal effect. Studies have found that LIPUS exposure can activate MAPK/ERK and PI3K/Akt signal pathways by up-regulating CyclinD1 and C-MYC genes, thus promoting the proliferation of MSCs (168, 169). Ling L et al. also indicated that the activation of ERK1/2 and PI3K/Akt signal pathways may be one of the potential mechanisms of LIPUS promoting the proliferation of MSCs (170). Animal studies also found that compared with human ADMSCs transplantation, LIPUS-pretreated human ADMSCs transplantation could not only repair chemotherapy-induced ovarian damage and improve ovarian function in POF rats, but also show greater advantages in alleviating ovarian tissue inflammation, improving local microenvironment and inhibiting chemotherapy-induced GCs apoptosis (171). Heat shock pretreatment (HSP) is an effective way to protect cells before and after transplantation. Recent studies have shown that HSP can inhibit apoptosis and improve the survival of BMSCs in the chemotherapy environment. The mechanism may be related to the elevated expression of HSP90 and HSP70 and the reduction of autophagy (172). In addition, HSP can also enhance the immunomodulatory ability of MSCs (173), which may enhance the therapeutic potential of BMSCs. Studies indicated that melatonin, L-carnitine, apigenin pretreatment could remarkably improve the homing and survival of BMSCs and improve the beneficial effects of MSCs therapy (50, 157, 174, 175, 182). Wang J et al. found that human cord blood platelet-rich plasma could promote proliferation and reduce apoptosis of hUMSCs. Co-transplantation hUMSCs with human cord blood platelet-rich plasma could increase the number of MSCs homing to the ovary of POF rats, which more effectively restored the estrous cycle and repaired damaged follicles of POF rats (176). In addition, Xu H et al. indicated that co-transplantation of BMSCs and endothelial progenitor cells could promote angiogenesis in the site of osteonecrosis of the femoral head (183). From this, we speculate that maybe co-transplantation BMSCs with endothelial progenitor cells can significantly ameliorate ovarian function in POF in the same way, which deserves further exploration.
Biomaterials take the advantages of promoting cell interactions, excellent stability and biodegradability, good passive and active targeting and show great potential in various applications including regenerative medicine (184). Su et al. reported that ADMSCs transplantation by collagen scaffold increased the retention of MSCs in ovaries and contributed to long time restoration of ovarian function in the POF rat model (177). However, transplanting cells directly into the core of the ovaries may lead to ovarian injury resulting from needle puncture. Shin EY et al. effectively avoid the disadvantage through subcutaneous transplanted MSCs using hyaluronic acid gel scaffold, and at the same time, the method effectively prolongs the survival rate of transplanted cells and significantly recovers ovarian functions in POF rats, too (178). Moreover, Mao AS et al. found that biomaterial encapsulation of BMSCs into programmable microencapsulation using a microfluidic device could partly reduce donor rejection and efficiently increase retention in vivo after intravenous injection, which substantially sustained BMSCs survival and enhanced overall immunomodulatory capacity of BMSCs in a model of allogeneic transplantation (179).
Although numerous studies have shown that BMSCs transplantation can effectively restored the ovarian structure and function of POF, there are still some limitations, such as invasive operation, uncontrollable preparation quality, immunological rejection, post-transplantation infection, secondary injury, descendant safety, chromosomal aberration, potential tumorigenicity (185–187). Chen S et al. demonstrated that the repairing effects of BMSCs and their exosomes were consistent in reducing senescent and apoptotic of GCs after phosphoramide mustard injury (21). From it, it’s worth to explore whether BMSCs-derived exosomes can provide a new strategy and direction for restoring POF ovarian function and at the same time avoid the disadvantages of direct BMSCs transplantation.
Exosome is a vesicle with a diameter of 40-100 nm secreting by cells and contains various proteins, mRNA and microRNAs, which is involved in cell communication and migration, angiogenesis and growth of tumor cells (37, 187). Yang M et al. revealed that BMSC-derived exosome miR-144-5p-mediated PTEN inhibition resulted in increasing PI3K/AKT signal activation, which was conducive to decrease GCs apoptosis and increase ovarian reserve in chemotherapy-induced POF (37). Similarly, the delivery of BMSC-derived exosome miR-644-5p to GCs plays a key role in regulating p53 expression of cells and thereby inhibiting GCs apoptosis and restoring ovarian function in cisplatin-induced POF mice model (188). MiR-126 is an important regulator for the function of endothelial cells and angiogenesis (189). BMSCs-derived miR-126 can enhance the survival and angiogenic function of injured endothelial cells by activating the PI3K/Akt/eNOS pathway and reducing cleaved caspase-3 expression, while increasing the expression of VEGF, epidermal growth factor, platelet derived growth factor, bFGF and other angiogenesis and growth factors (190). In addition, human BMSCs-derived exosomes can induce tubule formation in vitro and promote angiogenesis through NF-kB signaling pathway (191). Related studies have shown that exosomes secreted by MSCs from different tissues such as amniotic membrane and umbilical cord also play an important role in POF treatment. hUMSCs-derived exosome helps to rescue POF and reduces ROS accumulation by downregulating the expression of SIRT7 and its downstream target genes via delivering exosome miR-320a (192). Similarly, hUMSCs-derived exosome can recover ovarian structure and function, promote GCs proliferation and inhibit ROS accumulation in CTX -induced POF mouse model by down-regulating the expression of SIRT7 and its downstream target genes (PARP1, γH2AX and XRCC6) via delivering miR-17-5p (193). Xin Mi et al. also found that the secretome of hUMSCs helped effectively to activate primordial follicle both in vivo and vitro. And furthermore, hUMSCs-derived HGF upregulated the expression of KIT ligand in GCs, thereby promoting the activation of PI3K/Akt signaling pathway in dormant oocytes (194). Li Z et al. revealed that hUMSCs-derived exosome significantly ameliorated ovarian function and reproductive ability of POF mice models through promoting GCs proliferation via the Hippo signaling pathway (195). Cai et al. also found that hUMSCs-derived miR-21 could inhibit the expression of LATS1, so as to reduce phosphorylated LOXL2 and YAP, and ultimately promote E2 secretion in ovarian GCs (196). Moreover, hUMSCs-derived miR-126-3p promote proliferation while inhibit the apoptosis of GCs through PIK3R2/PI3K/AKT/mTOR pathway (197). Similarly, Yang et al. demonstrated that hUMSCs exosome transplantation could ameliorate ovarian function through promoting angiogenesis by activating PI3K/AKT signaling pathway (198).
In a word, the above studies have proved that exosomes derived from MSCs effectively ameliorate ovarian function in POF by promoting angiogenesis, inhibiting oxidative stress, suppressing cell apoptosis, and exerting many beneficial effects, which may represent a prospective cell-free therapy for developing therapeutic regimen for POF (Table 4). But there are still some limitations. Firstly, there is no standardized methods to produce enough exosomes. Moreover, the exosomes transplanted into the body are quickly degraded and lost. Finally, whether exosome therapy alone is equivalent to stem cell treatment is still uncertain (199). All in all, how to avoid the drawbacks of BMSCs transplantation effectively or find a better alternative method still needs further research.
POF is a common endocrine disorder that causes infertility in women, which affecting about 0.1% and 1% of women under 30 and 40 years old, respectively. POF is irreversible and currently incurable. Currently, HRT is the preferred treatment for POF, but it is unclear whether HRT increases the risk of breast cancer and venous thromboembolism (200). Therefore, it is crucial to find a better treatment for POF patients. Studies reveal that BMSCs have great potential for alleviating POF in laboratory-based investigations and pre-clinical as well as clinical studies in the last decade (64, 201, 202). BMSCs transplantation is a terrific option for POF treatment due to their low immunogenicity, availability and broad sources (35, 37). They can improve POF ovarian function through various mechanisms including paracrine, angiogenesis, anti-fibrosis, anti-inflammatory and immune regulation, anti-oxidative stress, inhibition of apoptosis, mitochondrial transfer and autophagy regulation after homing to the damaged ovary. At the same time, more and more researchers focus on optimizing the efficacy and safety of MSCs through genetic modification, pretreatment or co-transplantation and biomaterials processing. Moreover, exosomes secreted by MSCs may be a prospective cell-free therapy for developing therapeutic option for POF.
BMSCs have great therapeutic potential in many diseases, however, there are still some limitations. First and foremost, the specific markers of MSCs are unclear, so it is difficult to purify MSCs. Next, the mechanism of MSCs homing is still not fully understood, especially the mobilizing mechanism of MSCs, therefore targeted transplantation of BMSCs to injured ovaries remains a challenge (203). Thirdly, the existence of certain biosafety and biological efficacy concerns of MSCs may restrict their clinical applications, including tumor formation, chromosomal aberration, and immunological rejection (37, 186). Moreover, failure of homing to the target damaged tissue precisely and efficiently may lead to some fatal complications such as pulmonary embolism and induced thromboembolism (204–206). More than that, cell senescence is still the bottleneck for clinical applications of BMSCs. Last but not least, there is still no unified standard for BMSCs treatment. For instance, the dosage of stem cell transplantation and the choice of transplantation method, intravenous, orthotopic or intraperitoneal injection? Libing Shi et al. found that intraovarian injection may effectively improve the utilization of MSCs in the ovaries and reduce the adverse effect to other organs, nevertheless, the data also found that intraovarian hUMSCs injections may be toxic to the ovaries and oviduct, which manifested as ovarian lesions and inflammatory cell infiltration in the ovaries (206). In addition, although MSCs have demonstrated obvious therapeutic efficacy in animal models of POF, it is not sufficient to ensure that the majority of patients with POF regain their ovarian reserve in clinical (207).
In view of the problems existing in BMSCs transplantation, it is urgent to seek positive solutions. Firstly, improving standardization of BMSCs including manufacturing protocols, therapeutic targets, dosage, delivery strategy, number, and treatment protocols may partly avoid transplanted relevant risks such as thromboembolism and immunological rejection. Secondly, the tumorigenicity of BMSCs transplantation has long been a concern. Park HS et al. indicated that the number of transplanted cells gradually decreased and almost disappeared after 4 weeks in POF ovaries (53), therefore, the transient residence time and low survival of exogenous BMSCs after transplantation in vivo decrease the risk of tumorigenesis (186). Not only that, previous studies also revealed that BMSCs was safe at least six months after transplantation and without significant found of tumorigenicity (146). In addition, Zhang S et al. revealed that ovarian regenerative patch that composed by clinically relevant hydrolysable scaffolds and synthetic MSCs, which encapsulated the secretome of MSCs, restored ovarian function and rescued fertility in POF rats efficiently. The strategy could efficiently avoid the drawback of direct implantation of live cells that increased the risk of developing tumor and immunological rejection, and at the same time, it was able to maintain secretome of MSCs sustainable release to play a more effective therapy (208), which may provide a clinically feasible treatment for POF.
In summary, BMSCs have undergone a long process of scientific research and exploration from initial discovery to gradual application in the treatment of clinical diseases. Long-term studies found that BMSCs show great potential in ameliorating POF ovarian function through homing, paracrine, angiogenesis, anti-fibrosis, anti-inflammatory and immune regulation, anti-oxidative stress, inhibition of apoptosis, mitochondrial transfer and autophagy regulation. And genetic modification, pretreatment, co-transplantation and biomaterials processing effectively enhance the therapeutic effects of BMSCs. In addition, exosome is a prospective cell-free therapy in POF. Up to now, more and more clinical trials of MSCs in the treatment of POF have been gradually carried out (59, 209) (Table 5), but there are still many details which need to be further improved and explored. Formulating systematic standards of BMSCs from culture to application can help to increase the safety of BMSCs-based applications and avoid the side effects. The application of gene modification, biomaterial and exosomes may bring good news to the treatment of POF. It is believed that BMSCs-mediated therapy has broad application prospect for fundamental restoration of ovarian function in POF patients.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
YH performed the literature search and writing. MZ contributed to draft modification. MMZ, KS and YS contributed to suggestions for revision. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China, No. 81904008.
Figures were created with BioRender software (https://biorender.com/).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Lee DH, Pei CZ, Song JY, Lee KJ, Yun BS, Kwack KB, et al. Identification of serum biomarkers for premature ovarian failure. Biochim Biophys Acta Proteins Proteom (2019) 1867(3):219–26. doi: 10.1016/j.bbapap.2018.12.007
2. Jankowska K. Premature ovarian failure. Prz Menopauzalny (2017) 16(2):51–6. doi: 10.5114/pm.2017.68592
3. Pastore LM, Christianson MS, Stelling J, Kearns WG, Segars JH. Reproductive ovarian testing and the alphabet soup of diagnoses: Dor, poi, pof, por, and for. J Assist Reprod Genet (2018) 35(1):17–23. doi: 10.1007/s10815-017-1058-4
4. Wang S, Sun M, Yu L, Wang Y, Yao Y, Wang D. Niacin inhibits apoptosis and rescues premature ovarian failure. Cell Physiol Biochem (2018) 50(6):2060–70. doi: 10.1159/000495051
5. Wesevich V, Kellen AN, Pal L. Recent advances in understanding primary ovarian insufficiency. F1000Res (2020) 9::F1000 Faculty Rev-1101. doi: 10.12688/f1000research.26423.1
6. Machura P, Grymowicz M, Rudnicka E, Pieta W, Calik-Ksepka A, Skorska J, et al. Premature ovarian insufficiency - hormone replacement therapy and management of long-term consequences. Prz Menopauzalny (2018) 17(3):135–8. doi: 10.5114/pm.2018.78559
7. Sheikhansari G, Aghebati-Maleki L, Nouri M, Jadidi-Niaragh F, Yousefi M. Current approaches for the treatment of premature ovarian failure with stem cell therapy. BioMed Pharmacother (2018) 102:254–62. doi: 10.1016/j.biopha.2018.03.056
8. Ishizuka B. Current understanding of the etiology, symptomatology, and treatment options in premature ovarian insufficiency (Poi). Front Endocrinol (Lausanne) (2021) 12:626924. doi: 10.3389/fendo.2021.626924
9. Manson JE, Aragaki AK, Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: The women's health initiative randomized trials. JAMA (2017) 318(10):927–38. doi: 10.1001/jama.2017.11217
10. Silber S. Ovarian tissue cryopreservation and transplantation: Scientific implications. J Assist Reprod Genet (2016) 33(12):1595–603. doi: 10.1007/s10815-016-0814-1
11. Kou MJ, Ding XF, Chen JX, Liu Y, Liu YY. Traditional Chinese medicine combined with hormone therapy to treat premature ovarian failure: A meta-analysis of randomized controlled trials. Afr J Tradit Complement Altern Med (2016) 13(5):160–9. doi: 10.21010/ajtcam.v13i5.21
12. Sfakianoudis K, Rapani A, Grigoriadis S, Retsina D, Maziotis E, Tsioulou P, et al. Novel approaches in addressing ovarian insufficiency in 2019: Are we there yet? Cell Transplant (2020) 29:963689720926154. doi: 10.1177/0963689720926154
13. El-Derany MO, Said RS, El-Demerdash E. Bone marrow-derived mesenchymal stem cells reverse radiotherapy-induced premature ovarian failure: Emphasis on signal integration of tgf-beta, Wnt/Beta-catenin and hippo pathways. Stem Cell Rev Rep (2021) 17(4):1429–45. doi: 10.1007/s12015-021-10135-9
14. Badawy A, Sobh MA, Ahdy M, Abdelhafez MS. Bone marrow mesenchymal stem cell repair of cyclophosphamide-induced ovarian insufficiency in a mouse model. Int J Womens Health (2017) 9:441–7. doi: 10.2147/IJWH.S134074
15. Wang LT, Ting CH, Yen ML, Liu KJ, Sytwu HK, Wu KK, et al. Human mesenchymal stem cells (Mscs) for treatment towards immune- and inflammation-mediated diseases: Review of current clinical trials. J BioMed Sci (2016) 23(1):76. doi: 10.1186/s12929-016-0289-5
16. Samiec M, Opiela J, Lipinski D, Romanek J. Trichostatin a-mediated epigenetic transformation of adult bone marrow-derived mesenchymal stem cells biases the in vitro developmental capability, quality, and pluripotency extent of porcine cloned embryos. BioMed Res Int (2015) 2015:814686. doi: 10.1155/2015/814686
17. Agacayak E, Yaman Goruk N, Kusen H, Yaman Tunc S, Basaranoglu S, Icen MS, et al. Role of inflammation and oxidative stress in the etiology of primary ovarian insufficiency. Turk J Obstet Gynecol (2016) 13(3):109–15. doi: 10.4274/tjod.00334
18. Kisiel AH, McDuffee LA, Masaoud E, Bailey TR, Esparza Gonzalez BP, Nino-Fong R. Isolation, characterization, and in vitro proliferation of canine mesenchymal stem cells derived from bone marrow, adipose tissue, muscle, and periosteum. Am J Vet Res (2012) 73(8):1305–17. doi: 10.2460/ajvr.73.8.1305
19. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The prisma 2020 statement: An updated guideline for reporting systematic reviews. BMJ (2021) 372:n71. doi: 10.1136/bmj.n71
20. Lin Y, Ren X, Chen Y, Chen D. Interaction between mesenchymal stem cells and retinal degenerative microenvironment. Front Neurosci (2020) 14:617377. doi: 10.3389/fnins.2020.617377
21. Chen S, Wang Y, Liao L, Meng L, Li J, Shi C, et al. Similar repair effects of human placenta, bone marrow mesenchymal stem cells, and their exosomes for damaged svog ovarian granulosa cells. Stem Cells Int (2020) 2020:8861557. doi: 10.1155/2020/8861557
22. Friedenstein AJ, Piatetzky S II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol (1966) 16(3):381–90.
23. Chu DT, Phuong TNT, Tien NLB, Tran DK, Thanh VV, Quang TL, et al. An update on the progress of isolation, culture, storage, and clinical application of human bone marrow mesenchymal Stem/Stromal cells. Int J Mol Sci (2020) 21(3):708. doi: 10.3390/ijms21030708
24. Samsonraj RM, Raghunath M, Nurcombe V, Hui JH, van Wijnen AJ, Cool SM. Concise review: Multifaceted characterization of human mesenchymal stem cells for use in regenerative medicine. Stem Cells Transl Med (2017) 6(12):2173–85. doi: 10.1002/sctm.17-0129
25. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. Int Soc Cell Ther Position Statement. Cytotherapy (2006) 8(4):315–7. doi: 10.1080/14653240600855905
26. Cakiroglu F, Osbahr JW, Kramer J, Rohwedel J. Differences of cell surface marker expression between bone marrow- and kidney-derived murine mesenchymal stromal cells and fibroblasts. Cell Mol Biol (Noisy-le-grand) (2016) 62(12):11–7. doi: 10.14715/cmb/2016.62.12.3
27. Chen X, Wang Q, Li X, Wang Q, Xie J, Fu X. Heat shock pretreatment of mesenchymal stem cells for inhibiting the apoptosis of ovarian granulosa cells enhanced the repair effect on chemotherapy-induced premature ovarian failure. Stem Cell Res Ther (2018) 9(1):240. doi: 10.1186/s13287-018-0964-4
28. Musial-Wysocka A, Kot M, Majka M. The pros and cons of mesenchymal stem cell-based therapies. Cell Transplant (2019) 28(7):801–12. doi: 10.1177/0963689719837897
29. Zhou W, Lin J, Zhao K, Jin K, He Q, Hu Y, et al. Single-cell profiles and clinically useful properties of human mesenchymal stem cells of adipose and bone marrow origin. Am J Sports Med (2019) 47(7):1722–33. doi: 10.1177/0363546519848678
30. Han Y, Li X, Zhang Y, Han Y, Chang F, Ding J. Mesenchymal stem cells for regenerative medicine. Cells (2019) 8(8):886. doi: 10.3390/cells8080886
31. Andrzejewska A, Lukomska B, Janowski M. Concise review: Mesenchymal stem cells: From roots to boost. Stem Cells (2019) 37(7):855–64. doi: 10.1002/stem.3016
32. Hass R, Kasper C, Bohm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (Msc): A comparison of adult and neonatal tissue-derived msc. Cell Commun Signal (2011) 9:12. doi: 10.1186/1478-811X-9-12
33. Rodriguez-Fuentes DE, Fernandez-Garza LE, Samia-Meza JA, Barrera-Barrera SA, Caplan AI, Barrera-Saldana HA. Mesenchymal stem cells current clinical applications: A systematic review. Arch Med Res (2021) 52(1):93–101. doi: 10.1016/j.arcmed.2020.08.006
34. Cunningham CJ, Redondo-Castro E, Allan SM. The therapeutic potential of the mesenchymal stem cell secretome in ischaemic stroke. J Cereb Blood Flow Metab (2018) 38(8):1276–92. doi: 10.1177/0271678X18776802
35. Lau F, Dalisson B, Zhang YL, Zhao J, Eliopoulos N, Barralet JE. Effects of oxygen and glucose on bone marrow mesenchymal stem cell culture. Adv Biosyst (2020) 4(11):e2000094. doi: 10.1002/adbi.202000094
36. Heo JS, Choi Y, Kim HS, Kim HO. Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue. Int J Mol Med (2016) 37(1):115–25. doi: 10.3892/ijmm.2015.2413
37. Yang M, Lin L, Sha C, Li T, Zhao D, Wei H, et al. Bone marrow mesenchymal stem cell-derived exosomal mir-144-5p improves rat ovarian function after chemotherapy-induced ovarian failure by targeting pten. Lab Invest (2020) 100(3):342–52. doi: 10.1038/s41374-019-0321-y
38. Xia X, Wang T, Yin T, Yan L, Yan J, Lu C, et al. Mesenchymal stem cells facilitate in vitro development of human preantral follicle. Reprod Sci (2015) 22(11):1367–76. doi: 10.1177/1933719115578922
39. Zou W, Zhao J, Li Y, Wang Z, Yan H, Liu Y, et al. Rat bone marrow-derived mesenchymal stem cells promote the migration and invasion of colorectal cancer stem cells. Onco Targets Ther (2020) 13:6617–28. doi: 10.2147/OTT.S249353
40. Zorina TD. New insights on the role of the mesenchymal-hematopoietic stem cell axis in autologous and allogeneic hematopoiesis. Stem Cells Dev (2021) 30(1):2–16. doi: 10.1089/scd.2020.0148
41. Liu H, Li R, Liu T, Yang L, Yin G, Xie Q. Immunomodulatory effects of mesenchymal stem cells and mesenchymal stem cell-derived extracellular vesicles in rheumatoid arthritis. Front Immunol (2020) 11:1912. doi: 10.3389/fimmu.2020.01912
42. Galipeau J, Sensebe L. Mesenchymal stromal cells: Clinical challenges and therapeutic opportunities. Cell Stem Cell (2018) 22(6):824–33. doi: 10.1016/j.stem.2018.05.004
43. Sylakowski K, Bradshaw A, Wells A. Mesenchymal stem Cell/Multipotent stromal cell augmentation of wound healing: Lessons from the physiology of matrix and hypoxia support. Am J Pathol (2020) 190(7):1370–81. doi: 10.1016/j.ajpath.2020.03.017
44. Fu YX, Ji J, Shan F, Li J, Hu R. Human mesenchymal stem cell treatment of premature ovarian failure: New challenges and opportunities. Stem Cell Res Ther (2021) 12(1):161. doi: 10.1186/s13287-021-02212-0
45. Chen L, Luo W, Wang Y, Song X, Li S, Wu J, et al. Directional homing of glycosylation-modified bone marrow mesenchymal stem cells for bone defect repair. J Nanobiotechnol (2021) 19(1):228. doi: 10.1186/s12951-021-00969-3
46. Dadheech N, Srivastava A, Vakani M, Shrimali P, Bhonde R, Gupta S. Direct lineage tracing reveals activin-a potential for improved pancreatic homing of bone marrow mesenchymal stem cells and efficient ss-cell regeneration in vivo. Stem Cell Res Ther (2020) 11(1):327. doi: 10.1186/s13287-020-01843-z
47. Wei X, Ma W, Gu H, Liu D, Luo W, Bai Y, et al. Transamniotic mesenchymal stem cell therapy for neural tube defects preserves neural function through lesion-specific engraftment and regeneration. Cell Death Dis (2020) 11(7):523. doi: 10.1038/s41419-020-2734-3
48. Sameni HR, Seiri M, Safari M, Tabrizi Amjad MH, Khanmohammadi N, Zarbakhsh S. Bone marrow stromal cells with the granulocyte colony-stimulating factor in the management of chemotherapy-induced ovarian failure in a rat model. Iran J Med Sci (2019) 44(2):135–45.
49. Mohamed SA, Shalaby SM, Abdelaziz M, Brakta S, Hill WD, Ismail N, et al. Human mesenchymal stem cells partially reverse infertility in chemotherapy-induced ovarian failure. Reprod Sci (2018) 25(1):51–63. doi: 10.1177/1933719117699705
50. Zarbakhsh S, Safari R, Sameni HR, Yousefi B, Safari M, Khanmohammadi N, et al. Effects of Co-administration of bone marrow stromal cells and l-carnitine on the recovery of damaged ovaries by performing chemotherapy model in rat. Int J Fertil Steril (2019) 13(3):196–202. doi: 10.22074/ijfs.2019.5725
51. Abd-Allah SH, Shalaby SM, Pasha HF, El-Shal AS, Raafat N, Shabrawy SM, et al. Mechanistic action of mesenchymal stem cell injection in the treatment of chemically induced ovarian failure in rabbits. Cytotherapy (2013) 15(1):64–75. doi: 10.1016/j.jcyt.2012.08.001
52. Gabr H, Rateb MA, El Sissy MH, Ahmed Seddiek H, Ali Abdelhameed Gouda S. The effect of bone marrow-derived mesenchymal stem cells on chemotherapy induced ovarian failure in albino rats. Microsc Res Tech (2016) 79(10):938–47. doi: 10.1002/jemt.22725
53. Park HS, Chugh RM, Elsharoud A, Ulin M, Esfandyari S, Aboalsoud A, et al. Safety of intraovarian injection of human mesenchymal stem cells in a premature ovarian insufficiency mouse model. Cell Transplant (2021) 30:963689720988502. doi: 10.1177/0963689720988502
54. Besikcioglu HE, Saribas GS, Ozogul C, Tiryaki M, Kilic S, Pinarli FA, et al. Determination of the effects of bone marrow derived mesenchymal stem cells and ovarian stromal stem cells on follicular maturation in cyclophosphamide induced ovarian failure in rats. Taiwan J Obstet Gynecol (2019) 58(1):53–9. doi: 10.1016/j.tjog.2018.11.010
55. Bao R, Xu P, Wang Y, Wang J, Xiao L, Li G, et al. Bone marrow derived mesenchymal stem cells transplantation rescues premature ovarian insufficiency induced by chemotherapy. Gynecol Endocrinol (2018) 34(4):320–6. doi: 10.1080/09513590.2017.1393661
56. Liu J, Zhang H, Zhang Y, Li N, Wen Y, Cao F, et al. Homing and restorative effects of bone marrow-derived mesenchymal stem cells on cisplatin injured ovaries in rats. Mol Cells (2014) 37(12):865–72. doi: 10.14348/molcells.2014.0145
57. Khanmohammadi N, Sameni HR, Mohammadi M, Pakdel A, Mirmohammadkhani M, Parsaie H, et al. Effect of transplantation of bone marrow stromal cell- conditioned medium on ovarian function, morphology and cell death in cyclophosphamide-treated rats. Cell J (2018) 20(1):10–8. doi: 10.22074/cellj.2018.4919
58. Park HS, Chugh RM, El Andaloussi A, Hobeika E, Esfandyari S, Elsharoud A, et al. Human bm-msc secretome enhances human granulosa cell proliferation and steroidogenesis and restores ovarian function in primary ovarian insufficiency mouse model. Sci Rep (2021) 11(1):4525. doi: 10.1038/s41598-021-84216-7
59. Igboeli P, El Andaloussi A, Sheikh U, Takala H, ElSharoud A, McHugh A, et al. Intraovarian injection of autologous human mesenchymal stem cells increases estrogen production and reduces menopausal symptoms in women with premature ovarian failure: Two case reports and a review of the literature. J Med Case Rep (2020) 14(1):108. doi: 10.1186/s13256-020-02426-5
60. Kilic S, Pinarli F, Ozogul C, Tasdemir N, Naz Sarac G, Delibasi T. Protection from cyclophosphamide-induced ovarian damage with bone marrow-derived mesenchymal stem cells during puberty. Gynecol Endocrinol (2014) 30(2):135–40. doi: 10.3109/09513590.2013.860127
61. Fu X, He Y, Xie C, Liu W. Bone marrow mesenchymal stem cell transplantation improves ovarian function and structure in rats with chemotherapy-induced ovarian damage. Cytotherapy (2008) 10(4):353–63. doi: 10.1080/14653240802035926
62. Bahrehbar K, Rezazadeh Valojerdi M, Esfandiari F, Fathi R, Hassani SN, Baharvand H. Human embryonic stem cell-derived mesenchymal stem cells improved premature ovarian failure. World J Stem Cells (2020) 12(8):857–78. doi: 10.4252/wjsc.v12.i8.857
63. Wang Z, Yang T, Liu S, Chen Y. Effects of bone marrow mesenchymal stem cells on ovarian and testicular function in aging sprague-dawley rats induced by d-galactose. Cell Cycle (2020) 19(18):2340–50. doi: 10.1080/15384101.2020.1806434
64. Ling L, Hou J, Liu D, Tang D, Zhang Y, Zeng Q, et al. Important role of the sdf-1/Cxcr4 axis in the homing of systemically transplanted human amnion-derived mesenchymal stem cells (Had-mscs) to ovaries in rats with chemotherapy-induced premature ovarian insufficiency (Poi). Stem Cell Res Ther (2022) 13(1):79. doi: 10.1186/s13287-022-02759-6
65. Ling L, Feng X, Wei T, Wang Y, Wang Y, Wang Z, et al. Human amnion-derived mesenchymal stem cell (Had-msc) transplantation improves ovarian function in rats with premature ovarian insufficiency (Poi) at least partly through a paracrine mechanism. Stem Cell Res Ther (2019) 10(1):46. doi: 10.1186/s13287-019-1136-x
66. Cho J, Kim TH, Seok J, Jun JH, Park H, Kweon M, et al. Vascular remodeling by placenta-derived mesenchymal stem cells restores ovarian function in ovariectomized rat model Via the vegf pathway. Lab Invest (2021) 101(3):304–17. doi: 10.1038/s41374-020-00513-1
67. Li H, Zhao W, Wang L, Luo Q, Yin N, Lu X, et al. Human placenta-derived mesenchymal stem cells inhibit apoptosis of granulosa cells induced by Ire1alpha pathway in autoimmune pof mice. Cell Biol Int (2019) 43(8):899–909. doi: 10.1002/cbin.11165
68. Seok J, Park H, Choi JH, Lim JY, Kim KG, Kim GJ. Placenta-derived mesenchymal stem cells restore the ovary function in an ovariectomized rat model via an antioxidant effect. Antioxidants (Basel) (2020) 9(7):591. doi: 10.3390/antiox9070591
69. Wang Z, Wang Y, Yang T, Li J, Yang X. Study of the reparative effects of menstrual-derived stem cells on premature ovarian failure in mice. Stem Cell Res Ther (2017) 8(1):11. doi: 10.1186/s13287-016-0458-1
70. Lu X, Bao H, Cui L, Zhu W, Zhang L, Xu Z, et al. Humsc transplantation restores ovarian function in poi rats by inhibiting autophagy of theca-interstitial cells Via the Ampk/Mtor signaling pathway. Stem Cell Res Ther (2020) 11(1):268. doi: 10.1186/s13287-020-01784-7
71. Liesveld JL, Sharma N, Aljitawi OS. Stem cell homing: From physiology to therapeutics. Stem Cells (2020) 38(10):1241–53. doi: 10.1002/stem.3242
72. Garcia-Bernal D, Garcia-Arranz M, Yanez RM, Hervas-Salcedo R, Cortes A, Fernandez-Garcia M, et al. The current status of mesenchymal stromal cells: Controversies, unresolved issues and some promising solutions to improve their therapeutic efficacy. Front Cell Dev Biol (2021) 9:650664. doi: 10.3389/fcell.2021.650664
73. Lu J, Shen X, Sun X, Yin H, Yang S, Lu C, et al. Increased recruitment of endogenous stem cells and chondrogenic differentiation by a composite scaffold containing bone marrow homing peptide for cartilage regeneration. Theranostics (2018) 8(18):5039–58. doi: 10.7150/thno.26981
74. Fu X, Liu G, Halim A, Ju Y, Luo Q, Song AG. Mesenchymal stem cell migration and tissue repair. Cells (2019) 8(8):784. doi: 10.3390/cells8080784
75. Bang OY, Moon GJ, Kim DH, Lee JH, Kim S, Son JP, et al. Stroke induces mesenchymal stem cell migration to infarcted brain areas Via Cxcr4 and c-met signaling. Transl Stroke Res (2017). doi: 10.1007/s12975-017-0538-2
76. Janssens R, Struyf S, Proost P. Pathological roles of the homeostatic chemokine Cxcl12. Cytokine Growth Factor Rev (2018) 44:51–68. doi: 10.1016/j.cytogfr.2018.10.004
77. Britton C, Poznansky MC, Reeves P. Polyfunctionality of the Cxcr4/Cxcl12 axis in health and disease: Implications for therapeutic interventions in cancer and immune-mediated diseases. FASEB J (2021) 35(4):e21260. doi: 10.1096/fj.202001273R
78. Cun Y, Diao B, Zhang Z, Wang G, Yu J, Ma L, et al. Role of the stromal cell derived factor-1 in the biological functions of endothelial progenitor cells and its underlying mechanisms. Exp Ther Med (2021) 21(1):39. doi: 10.3892/etm.2020.9471
79. Kawaguchi N, Zhang TT, Nakanishi T. Involvement of Cxcr4 in normal and abnormal development. Cells (2019) 8(2):185. doi: 10.3390/cells8020185
80. Zheng XB, He XW, Zhang LJ, Qin HB, Lin XT, Liu XH, et al. Bone marrow-derived Cxcr4-overexpressing mscs display increased homing to intestine and ameliorate colitis-associated tumorigenesis in mice. Gastroenterol Rep (Oxf) (2019) 7(2):127–38. doi: 10.1093/gastro/goy017
81. Pozzobon T, Goldoni G, Viola A, Molon B. Cxcr4 signaling in health and disease. Immunol Lett (2016) 177:6–15. doi: 10.1016/j.imlet.2016.06.006
82. Guo L, Du J, Yuan DF, Zhang Y, Zhang S, Zhang HC, et al. Optimal H2o2 preconditioning to improve bone marrow mesenchymal stem cells' engraftment in wound healing. Stem Cell Res Ther (2020) 11(1):434. doi: 10.1186/s13287-020-01910-5
83. Xiao Ling K, Peng L, Jian Feng Z, Wei C, Wei Yan Y, Nan S, et al. Stromal derived factor-1/Cxcr4 axis involved in bone marrow mesenchymal stem cells recruitment to injured liver. Stem Cells Int (2016) 2016:8906945. doi: 10.1155/2016/8906945
84. Tamari T, Kawar-Jaraisy R, Doppelt O, Giladi B, Sabbah N, Zigdon-Giladi H. The paracrine role of endothelial cells in bone formation Via Cxcr4/Sdf-1 pathway. Cells (2020) 9(6):1325. doi: 10.3390/cells9061325
85. Meng Z, Feng G, Hu X, Yang L, Yang X, Jin Q. Sdf factor-1alpha promotes the migration, proliferation, and osteogenic differentiation of mouse bone marrow mesenchymal stem cells through the Wnt/Beta-catenin pathway. Stem Cells Dev (2021) 30(2):106–17. doi: 10.1089/scd.2020.0165
86. Chen L, Li Y, Chen W, Han N, Li K, Guo R, et al. Enhanced recruitment and hematopoietic reconstitution of bone marrow-derived mesenchymal stem cells in bone marrow failure by the sdf-1/Cxcr4. J Tissue Eng Regener Med (2020) 14(9):1250–60. doi: 10.1002/term.3096
87. Wang L, Li S, Wang HY, Zeng J, Zhang ZZ, Lv DY, et al. In a rat model of acute liver failure, icaritin improved the therapeutic effect of mesenchymal stem cells by activation of the hepatocyte growth Factor/C-met pathway. Evid Based Complement Alternat Med (2019) 2019:4253846. doi: 10.1155/2019/4253846
88. Han P, Cui Q, Lu W, Yang S, Shi M, Li Z, et al. Hepatocyte growth factor plays a dual role in tendon-derived stem cell proliferation, migration, and differentiation. J Cell Physiol (2019) 234(10):17382–91. doi: 10.1002/jcp.28360
89. Kwon Y, Godwin AK. Regulation of hgf and c-met interaction in normal ovary and ovarian cancer. Reprod Sci (2017) 24(4):494–501. doi: 10.1177/1933719116648212
90. Huang HS, Chen PC, Chu SC, Lee MH, Huang CY, Chu TY. Ovulation sources coagulation protease cascade and hepatocyte growth factor to support physiological growth and malignant transformation. Neoplasia (2021) 23(11):1123–36. doi: 10.1016/j.neo.2021.09.006
91. Song P, Han T, Xiang X, Wang Y, Fang H, Niu Y, et al. The role of hepatocyte growth factor in mesenchymal stem cell-induced recovery in spinal cord injured rats. Stem Cell Res Ther (2020) 11(1):178. doi: 10.1186/s13287-020-01691-x
92. Wang H, Rao B, Lou J, Li J, Liu Z, Li A, et al. The function of the Hgf/C-met axis in hepatocellular carcinoma. Front Cell Dev Biol (2020) 8:55. doi: 10.3389/fcell.2020.00055
93. Hu X, Tang F, Liu P, Zhong T, Yuan F, He Q, et al. Structural and functional insight into the glycosylation impact upon the Hgf/C-met signaling pathway. Front Cell Dev Biol (2020) 8:490. doi: 10.3389/fcell.2020.00490
94. Wang K, Li Y, Zhu T, Zhang Y, Li W, Lin W, et al. Overexpression of c-met in bone marrow mesenchymal stem cells improves their effectiveness in homing and repair of acute liver failure. Stem Cell Res Ther (2017) 8(1):162. doi: 10.1186/s13287-017-0614-2
95. Woo LL, Hijaz A, Pan HQ, Kuang M, Rackley RR, Damaser MS. Simulated childbirth injuries in an inbred rat strain. Neurourol Urodyn (2009) 28(4):356–61. doi: 10.1002/nau.20644
96. Woo LL, Hijaz A, Kuang M, Penn MS, Damaser MS, Rackley RR. Over expression of stem cell homing cytokines in urogenital organs following vaginal distention. J Urol (2007) 177(4):1568–72. doi: 10.1016/j.juro.2006.11.047
97. Vricella GJ, Tao M, Altuntas CZ, Liu G, Kavran M, Daneshgari F, et al. Expression of monocyte chemotactic protein 3 following simulated birth trauma in a murine model of obesity. Urology (2010) 76(6):1517 e12–7. doi: 10.1016/j.urology.2010.07.466
98. Yamada Y, Nakamura-Yamada S, Umemura-Kubota E, Baba S. Diagnostic cytokines and comparative analysis secreted from exfoliated deciduous teeth, dental pulp, and bone marrow derived mesenchymal stem cells for functional cell-based therapy. Int J Mol Sci (2019) 20(23):5900. doi: 10.3390/ijms20235900
99. Schenk S, Mal N, Finan A, Zhang M, Kiedrowski M, Popovic Z, et al. Monocyte chemotactic protein-3 is a myocardial mesenchymal stem cell homing factor. Stem Cells (2007) 25(1):245–51. doi: 10.1634/stemcells.2006-0293
100. Cui L, Bao H, Liu Z, Man X, Liu H, Hou Y, et al. Humscs regulate the differentiation of ovarian stromal cells Via tgf-Beta1/Smad3 signaling pathway to inhibit ovarian fibrosis to repair ovarian function in poi rats. Stem Cell Res Ther (2020) 11(1):386. doi: 10.1186/s13287-020-01904-3
101. He Q, Shi J, Liu W, Zhao W, Wang Z, Liu K, et al. Tgf-Beta1-Induced bone marrow mesenchymal stem cells (Bmscs) migration Via histone demethylase Kdm6b mediated inhibition of methylation marker H3k27me3. Cell Death Discovery (2022) 8(1):339. doi: 10.1038/s41420-022-01132-z
102. Liu P, Zhang X, Hu J, Cui L, Zhao S, Jiao X, et al. Dysregulated cytokine profile associated with biochemical premature ovarian insufficiency. Am J Reprod Immunol (2020) 84(4):e13292. doi: 10.1111/aji.13292
103. Ullah M, Liu DD, Thakor AS. Mesenchymal stromal cell homing: Mechanisms and strategies for improvement. iScience (2019) 15:421–38. doi: 10.1016/j.isci.2019.05.004
104. De Becker A, Riet IV. Homing and migration of mesenchymal stromal cells: How to improve the efficacy of cell therapy? World J Stem Cells (2016) 8(3):73–87. doi: 10.4252/wjsc.v8.i3.73
105. Li Y, Zhang D, Xu L, Dong L, Zheng J, Lin Y, et al. Cell-cell contact with proinflammatory macrophages enhances the immunotherapeutic effect of mesenchymal stem cells in two abortion models. Cell Mol Immunol (2019) 16(12):908–20. doi: 10.1038/s41423-019-0204-6
106. Satue M, Schuler C, Ginner N, Erben RG. Intra-articularly injected mesenchymal stem cells promote cartilage regeneration, but do not permanently engraft in distant organs. Sci Rep (2019) 9(1):10153. doi: 10.1038/s41598-019-46554-5
107. Kim N, Cho SG. Clinical applications of mesenchymal stem cells. Korean J Intern Med (2013) 28(4):387–402. doi: 10.3904/kjim.2013.28.4.387
108. Fazeli Z, Abedindo A, Omrani MD, Ghaderian SMH. Mesenchymal stem cells (Mscs) therapy for recovery of fertility: A systematic review. Stem Cell Rev Rep (2018) 14(1):1–12. doi: 10.1007/s12015-017-9765-x
109. Almasry SM, Elfayomy AK, El-Sherbiny MH. Regeneration of the fallopian tube mucosa using bone marrow mesenchymal stem cell transplantation after induced chemical injury in a rat model. Reprod Sci (2018) 25(5):773–81. doi: 10.1177/1933719117725824
110. Yao X, Guo Y, Wang Q, Xu M, Zhang Q, Li T, et al. The paracrine effect of transplanted human amniotic epithelial cells on ovarian function improvement in a mouse model of chemotherapy-induced primary ovarian insufficiency. Stem Cells Int (2016) 2016:4148923. doi: 10.1155/2016/4148923
111. Ruhle A, Lopez Perez R, Zou B, Grosu AL, Huber PE, Nicolay NH. The therapeutic potential of mesenchymal stromal cells in the treatment of chemotherapy-induced tissue damage. Stem Cell Rev Rep (2019) 15(3):356–73. doi: 10.1007/s12015-019-09886-3
112. Lin S, Zhang Q, Shao X, Zhang T, Xue C, Shi S, et al. Igf-1 promotes angiogenesis in endothelial Cells/Adipose-derived stem cells Co-culture system with activation of Pi3k/Akt signal pathway. Cell Prolif (2017) 50(6):e12390. doi: 10.1111/cpr.12390
113. Zhang S, Zhu D, Mei X, Li Z, Li J, Xie M, et al. Advances in biomaterials and regenerative medicine for primary ovarian insufficiency therapy. Bioact Mater (2021) 6(7):1957–72. doi: 10.1016/j.bioactmat.2020.12.008
114. Maacha S, Sidahmed H, Jacob S, Gentilcore G, Calzone R, Grivel JC, et al. Paracrine mechanisms of mesenchymal stromal cells in angiogenesis. Stem Cells Int (2020) 2020:4356359. doi: 10.1155/2020/4356359
115. Park HS, Ashour D, Elsharoud A, Chugh RM, Ismail N, El Andaloussi A, et al. Towards cell free therapy of premature ovarian insufficiency: Human bone marrow mesenchymal stem cells secretome enhances angiogenesis in human ovarian microvascular endothelial cells. HSOA J Stem Cells Res Dev Ther (2019) 5(2):019. doi: 10.24966/srdt-2060/100019
116. Fang J, Xu J, Zhang Y, Chen H, Ma Z, Huang Z, et al. Stromal cell-derived factor-1 may play pivotal role in distraction-stimulated neovascularization of diabetic foot ulcer. Med Hypotheses (2021) 149:110548. doi: 10.1016/j.mehy.2021.110548
117. Wang L, Ying YF, Ouyang YL, Wang JF, Xu J. Vegf and bfgf increase survival of xenografted human ovarian tissue in an experimental rabbit model. J Assist Reprod Genet (2013) 30(10):1301–11. doi: 10.1007/s10815-013-0043-9
118. Zhang L, Zhou D, Li J, Yan X, Zhu J, Xiao P, et al. Effects of bone marrow-derived mesenchymal stem cells on hypoxia and the transforming growth factor beta 1 (Tgfbeta-1) and smads pathway in a mouse model of cirrhosis. Med Sci Monit (2019) 25:7182–90. doi: 10.12659/MSM.916428
119. Forsberg MH, Kink JA, Hematti P, Capitini CM. Mesenchymal stromal cells and exosomes: Progress and challenges. Front Cell Dev Biol (2020) 8:665. doi: 10.3389/fcell.2020.00665
120. Pires AO, Mendes-Pinheiro B, Teixeira FG, Anjo SI, Ribeiro-Samy S, Gomes ED, et al. Unveiling the differences of secretome of human bone marrow mesenchymal stem cells, adipose tissue-derived stem cells, and human umbilical cord perivascular cells: A proteomic analysis. Stem Cells Dev (2016) 25(14):1073–83. doi: 10.1089/scd.2016.0048
121. Yin N, Wu C, Qiu J, Zhang Y, Bo L, Xu Y, et al. Protective properties of heme oxygenase-1 expressed in umbilical cord mesenchymal stem cells help restore the ovarian function of premature ovarian failure mice through activating the Jnk/Bcl-2 signal pathway-regulated autophagy and upregulating the circulating of Cd8(+)Cd28(-) T cells. Stem Cell Res Ther (2020) 11(1):49. doi: 10.1186/s13287-019-1537-x
122. Bagley RG, Weber W, Rouleau C, Yao M, Honma N, Kataoka S, et al. Human mesenchymal stem cells from bone marrow express tumor endothelial and stromal markers. Int J Oncol (2009) 34(3):619–27. doi: 10.3892/ijo_00000187
123. Coppin L, Sokal E, Stephenne X. Thrombogenic risk induced by intravascular mesenchymal stem cell therapy: Current status and future perspectives. Cells (2019) 8(10):1160. doi: 10.3390/cells8101160
124. Polonio AM, Garcia-Velasco JA, Herraiz S. Stem cell paracrine signaling for treatment of premature ovarian insufficiency. Front Endocrinol (Lausanne) (2020) 11:626322. doi: 10.3389/fendo.2020.626322
125. Gu W, Hong X, Potter C, Qu A, Xu Q. Mesenchymal stem cells and vascular regeneration. Microcirculation (2017) 24(1). doi: 10.1111/micc.12324
126. Zhang Y, Xia X, Yan J, Yan L, Lu C, Zhu X, et al. Mesenchymal stem cell-derived angiogenin promotes primodial follicle survival and angiogenesis in transplanted human ovarian tissue. Reprod Biol Endocrinol (2017) 15(1):18. doi: 10.1186/s12958-017-0235-8
127. Zhou F, Shi LB, Zhang SY. Ovarian fibrosis: A phenomenon of concern. Chin Med J (Engl) (2017) 130(3):365–71. doi: 10.4103/0366-6999.198931
128. Hu HH, Chen DQ, Wang YN, Feng YL, Cao G, Vaziri ND, et al. New insights into tgf-Beta/Smad signaling in tissue fibrosis. Chem Biol Interact (2018) 292:76–83. doi: 10.1016/j.cbi.2018.07.008
129. Inagaki Y, Okazaki I. Emerging insights into transforming growth factor beta smad signal in hepatic fibrogenesis. Gut (2007) 56(2):284–92. doi: 10.1136/gut.2005.088690
130. Wang PP, Xie DY, Liang XJ, Peng L, Zhang GL, Ye YN, et al. Hgf and direct mesenchymal stem cells contact synergize to inhibit hepatic stellate cells activation through Tlr4/Nf-kb pathway. PloS One (2012) 7(8):e43408. doi: 10.1371/journal.pone.0043408
131. Lv SJ, Hou SH, Gan L, Sun J. Establishment and mechanism study of a primary ovarian insufficiency mouse model using lipopolysaccharide. Anal Cell Pathol (Amst) (2021) 2021:1781532. doi: 10.1155/2021/1781532
132. Luo Q, Yin N, Zhang L, Yuan W, Zhao W, Luan X, et al. Role of sdf-1/Cxcr4 and cytokines in the development of ovary injury in chemotherapy drug induced premature ovarian failure mice. Life Sci (2017) 179:103–9. doi: 10.1016/j.lfs.2017.05.001
133. Kalhori Z, Azadbakht M, Soleimani Mehranjani M, Shariatzadeh MA. Improvement of the folliculogenesis by transplantation of bone marrow mesenchymal stromal cells in mice with induced polycystic ovary syndrome. Cytotherapy (2018) 20(12):1445–58. doi: 10.1016/j.jcyt.2018.09.005
134. Song N, Scholtemeijer M, Shah K. Mesenchymal stem cell immunomodulation: Mechanisms and therapeutic potential. Trends Pharmacol Sci (2020) 41(9):653–64. doi: 10.1016/j.tips.2020.06.009
135. Liang C, Jiang E, Yao J, Wang M, Chen S, Zhou Z, et al. Interferon-gamma mediates the immunosuppression of bone marrow mesenchymal stem cells on T-lymphocytes in vitro. Hematology (2018) 23(1):44–9. doi: 10.1080/10245332.2017.1333245
136. Jiao X, Zhang X, Li N, Zhang D, Zhao S, Dang Y, et al. Treg deficiency-mediated Th 1 response causes human premature ovarian insufficiency through apoptosis and steroidogenesis dysfunction of granulosa cells. Clin Transl Med (2021) 11(6):e448. doi: 10.1002/ctm2.448
137. Luz-Crawford P, Kurte M, Bravo-Alegria J, Contreras R, Nova-Lamperti E, Tejedor G, et al. Mesenchymal stem cells generate a Cd4+Cd25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res Ther (2013) 4(3):65. doi: 10.1186/scrt216
138. Li H, Guan Y, Sun B, Dou X, Liu X, Xue F, et al. Role of bone marrow-derived mesenchymal stem cell defects in Cd8(+) Cd28(-) suppressor T-lymphocyte induction in patients with immune thrombocytopenia and associated mechanisms. Br J Haematol (2020) 191(5):852–62. doi: 10.1111/bjh.16953
139. Fu X, He Y, Wang X, Peng D, Chen X, Li X, et al. Overexpression of mir-21 in stem cells improves ovarian structure and function in rats with chemotherapy-induced ovarian damage by targeting Pdcd4 and pten to inhibit granulosa cell apoptosis. Stem Cell Res Ther (2017) 8(1):187. doi: 10.1186/s13287-017-0641-z
140. Wu Y, Ma C, Zhao H, Zhou Y, Chen Z, Wang L. Alleviation of endoplasmic reticulum stress protects against cisplatin-induced ovarian damage. Reprod Biol Endocrinol (2018) 16(1):85. doi: 10.1186/s12958-018-0404-4
141. Nie X, Dai Y, Zheng Y, Bao D, Chen Q, Yin Y, et al. Establishment of a mouse model of premature ovarian failure using consecutive superovulation. Cell Physiol Biochem (2018) 51(5):2341–58. doi: 10.1159/000495895
142. Chen C, Li S, Hu C, Cao W, Fu Q, Li J, et al. Protective effects of puerarin on premature ovarian failure Via regulation of Wnt/Beta-catenin signaling pathway and oxidative stress. Reprod Sci (2021) 28(4):982–90. doi: 10.1007/s43032-020-00325-0
143. Niu Y, Xia X, Song P, Fang H, Dong F, Tao H, et al. Bone mesenchymal stem cell-conditioned medium attenuates the effect of oxidative stress injury on nscs by inhibiting the Notch1 signaling pathway. Cell Biol Int (2019) 43(11):1267–75. doi: 10.1002/cbin.11126
144. Song IH, Jung KJ, Lee TJ, Kim JY, Sung EG, Bae YC, et al. Mesenchymal stem cells attenuate adriamycin-induced nephropathy by diminishing oxidative stress and inflammation Via downregulation of the nf-kb. Nephrol (Carlton) (2018) 23(5):483–92. doi: 10.1111/nep.13047
145. Gomzikova MO, James V, Rizvanov AA. Mitochondria donation by mesenchymal stem cells: Current understanding and mitochondria transplantation strategies. Front Cell Dev Biol (2021) 9:653322. doi: 10.3389/fcell.2021.653322
146. Wang L, Mei Q, Xie Q, Li H, Su P, Zhang L, et al. A comparative study of mesenchymal stem cells transplantation approach to antagonize age-associated ovarian hypofunction with consideration of safety and efficiency. J Adv Res (2022) 38:245–59. doi: 10.1016/j.jare.2021.09.001
147. Tseng N, Lambie SC, Huynh CQ, Sanford B, Patel M, Herson PS, et al. Mitochondrial transfer from mesenchymal stem cells improves neuronal metabolism after oxidant injury in vitro: The role of Miro1. J Cereb Blood Flow Metab (2021) 41(4):761–70. doi: 10.1177/0271678X20928147
148. Feng Y, Zhu R, Shen J, Wu J, Lu W, Zhang J, et al. Human bone marrow mesenchymal stem cells rescue endothelial cells experiencing chemotherapy stress by mitochondrial transfer Via tunneling nanotubes. Stem Cells Dev (2019) 28(10):674–82. doi: 10.1089/scd.2018.0248
149. Morrison TJ, Jackson MV, Cunningham EK, Kissenpfennig A, McAuley DF, O'Kane CM, et al. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am J Respir Crit Care Med (2017) 196(10):1275–86. doi: 10.1164/rccm.201701-0170OC
150. Ceccariglia S, Cargnoni A, Silini AR, Parolini O. Autophagy: A potential key contributor to the therapeutic action of mesenchymal stem cells. Autophagy (2020) 16(1):28–37. doi: 10.1080/15548627.2019.1630223
151. Hulas-Stasiak M, Gawron A. Follicular atresia in the prepubertal spiny mouse (Acomys cahirinus) ovary. Apoptosis (2011) 16(10):967–75. doi: 10.1007/s10495-011-0626-9
152. Xie QE, Wang MY, Cao ZP, Du X, Ji DM, Liang D, et al. Melatonin protects against excessive autophagy-induced mitochondrial and ovarian reserve function deficiency though erk signaling pathway in Chinese hamster ovary (Cho) cells. Mitochondrion (2021) 61:44–53. doi: 10.1016/j.mito.2021.09.009
153. Liu XH, Zhao ZH, Zhou Y, Wang CL, Han YJ, Zhou W. [Effect of ginsenoside Rg_1 in delaying premature ovarian failure induced by d-gal in mice through Pi3k/Akt/Mtor autophagy pathway]. Zhongguo Zhong Yao Za Zhi (2020) 45(24):6036–42. doi: 10.19540/j.cnki.cjcmm.20200901.405
154. He H, Zeng Q, Huang G, Lin Y, Lin H, Liu W, et al. Bone marrow mesenchymal stem cell transplantation exerts neuroprotective effects following cerebral Ischemia/Reperfusion injury by inhibiting autophagy Via the Pi3k/Akt pathway. Brain Res (2019) 1707:124–32. doi: 10.1016/j.brainres.2018.11.018
155. Hu C, Wu Z, Li L. Pre-treatments enhance the therapeutic effects of mesenchymal stem cells in liver diseases. J Cell Mol Med (2020) 24(1):40–9. doi: 10.1111/jcmm.14788
156. Haider H, Ashraf M. Strategies to promote donor cell survival: Combining preconditioning approach with stem cell transplantation. J Mol Cell Cardiol (2008) 45(4):554–66. doi: 10.1016/j.yjmcc.2008.05.004
157. Saberi K, Pasbakhsh P, Omidi A, Borhani-Haghighi M, Nekoonam S, Omidi N, et al. Melatonin preconditioning of bone marrow-derived mesenchymal stem cells promotes their engraftment and improves renal regeneration in a rat model of chronic kidney disease. J Mol Histol (2019) 50(2):129–40. doi: 10.1007/s10735-019-09812-4
158. Tan C, Tan S, Zhang H, Zhang M, Fan H, Nan Z, et al. Enhanced migration and immunoregulatory capacity of bmscs mediated by overexpression of Cxcr4 and il-35. Mol Immunol (2022) 150:1–8. doi: 10.1016/j.molimm.2022.07.005
159. Ni X, Ou C, Guo J, Liu B, Zhang J, Wu Z, et al. Lentiviral vector-mediated Co-overexpression of vegf and bcl-2 improves mesenchymal stem cell survival and enhances paracrine effects in vitro. Int J Mol Med (2017) 40(2):418–26. doi: 10.3892/ijmm.2017.3019
160. Zhang F, Peng W, Zhang J, Wang L, Dong W, Zheng Y, et al. Park7 enhances antioxidative-stress processes of bmscs Via the Erk1/2 pathway. J Cell Biochem (2021) 122(2):222–34. doi: 10.1002/jcb.29845
161. Zhang F, Yan Y, Peng W, Wang L, Wang T, Xie Z, et al. Park7 promotes repair in early steroid-induced osteonecrosis of the femoral head by enhancing resistance to stress-induced apoptosis in bone marrow mesenchymal stem cells Via regulation of the Nrf2 signaling pathway. Cell Death Dis (2021) 12(10):940. doi: 10.1038/s41419-021-04226-1
162. Zhao F, Liu W, Yue S, Yang L, Hua Q, Zhou Y, et al. Pretreatment with G-csf could enhance the antifibrotic effect of bm-mscs on pulmonary fibrosis. Stem Cells Int (2019) 2019:1726743. doi: 10.1155/2019/1726743
163. Zhu X, Huang L, Wu K, Sun Z, Wang K, Ru J, et al. Shikonin regulates autophagy Via the Ampk/Mtor pathway and reduces apoptosis of human umbilical cord mesenchymal stem cells to improve survival in tissues surrounding brain contusion. Exp Ther Med (2021) 22(6):1475. doi: 10.3892/etm.2021.10910
164. Vu BT, Le HT, Nguyen KN, Van Pham P. Hypoxia, serum starvation, and tnf-alpha can modify the immunomodulation potency of human adipose-derived stem cells. Adv Exp Med Biol (2021). doi: 10.1007/5584_2021_672
165. Zhou S, Liu YG, Zhang Y, Hu JM, Liu D, Chen H, et al. Bone mesenchymal stem cells pretreated with erythropoietin enhance the effect to ameliorate cyclosporine a-induced nephrotoxicity in rats. J Cell Biochem (2018) 119(10):8220–32. doi: 10.1002/jcb.26833
166. Zhou S, Qiao YM, Liu YG, Liu D, Hu JM, Liao J, et al. Bone marrow derived mesenchymal stem cells pretreated with erythropoietin accelerate the repair of acute kidney injury. Cell Biosci (2020) 10(1):130. doi: 10.1186/s13578-020-00492-2
167. Crisostomo PR, Wang Y, Markel TA, Wang M, Lahm T, Meldrum DR. Human mesenchymal stem cells stimulated by tnf-alpha, lps, or hypoxia produce growth factors by an nf kappa b- but not jnk-dependent mechanism. Am J Physiol Cell Physiol (2008) 294(3):C675–82. doi: 10.1152/ajpcell.00437.2007
168. Huang D, Gao Y, Wang S, Zhang W, Cao H, Zheng L, et al. Impact of low-intensity pulsed ultrasound on transcription and metabolite compositions in proliferation and functionalization of human adipose-derived mesenchymal stromal cells. Sci Rep (2020) 10(1):13690. doi: 10.1038/s41598-020-69430-z
169. Xie S, Jiang X, Wang R, Xie S, Hua Y, Zhou S, et al. Low-intensity pulsed ultrasound promotes the proliferation of human bone mesenchymal stem cells by activating Pi3k/Akt signaling pathways. J Cell Biochem (2019) 120(9):15823–33. doi: 10.1002/jcb.28853
170. Ling L, Wei T, He L, Wang Y, Wang Y, Feng X, et al. Low-intensity pulsed ultrasound activates Erk1/2 and Pi3k-akt signalling pathways and promotes the proliferation of human amnion-derived mesenchymal stem cells. Cell Prolif (2017) 50(6):15823–33. doi: 10.1111/cpr.12383
171. Ling L, Feng X, Wei T, Wang Y, Wang Y, Zhang W, et al. Effects of low-intensity pulsed ultrasound (Lipus)-pretreated human amnion-derived mesenchymal stem cell (Had-msc) transplantation on primary ovarian insufficiency in rats. Stem Cell Res Ther (2017) 8(1):283. doi: 10.1186/s13287-017-0739-3
172. Wang Q, Li X, Wang Q, Xie J, Xie C, Fu X. Heat shock pretreatment improves mesenchymal stem cell viability by heat shock proteins and autophagy to prevent cisplatin-induced granulosa cell apoptosis. Stem Cell Res Ther (2019) 10(1):348. doi: 10.1186/s13287-019-1425-4
173. Lv H, Yuan X, Zhang J, Lu T, Yao J, Zheng J, et al. Heat shock preconditioning mesenchymal stem cells attenuate acute lung injury Via reducing Nlrp3 inflammasome activation in macrophages. Stem Cell Res Ther (2021) 12(1):290. doi: 10.1186/s13287-021-02328-3
174. Fujisawa K, Takami T, Fukui Y, Quintanilha LF, Matsumoto T, Yamamoto N, et al. Evaluating effects of l-carnitine on human bone-Marrow-Derived mesenchymal stem cells. Cell Tissue Res (2017) 368(2):301–10. doi: 10.1007/s00441-017-2569-0
175. Talebi A, Hayati Roodbari N, Reza Sameni H, Zarbakhsh S. Impact of coadministration of apigenin and bone marrow stromal cells on damaged ovaries due to chemotherapy in rat: An experimental study. Int J Reprod BioMed (2020) 18(7):551–60. doi: 10.18502/ijrm.v13i7.7372
176. Wang J, Zhao Y, Zheng F, Ma N, Qin R, Qin W, et al. Activated human umbilical cord blood platelet-rich plasma enhances the beneficial effects of human umbilical cord mesenchymal stem cells in chemotherapy-induced pof rats. Stem Cells Int (2021) 2021:8293699. doi: 10.1155/2021/8293699
177. Su J, Ding L, Cheng J, Yang J, Li X, Yan G, et al. Transplantation of adipose-derived stem cells combined with collagen scaffolds restores ovarian function in a rat model of premature ovarian insufficiency. Hum Reprod (2016) 31(5):1075–86. doi: 10.1093/humrep/dew041
178. Shin EY, Kim DS, Lee MJ, Lee AR, Shim SH, Baek SW, et al. Prevention of chemotherapy-induced premature ovarian insufficiency in mice by scaffold-based local delivery of human embryonic stem cell-derived mesenchymal progenitor cells. Stem Cell Res Ther (2021) 12(1):431. doi: 10.1186/s13287-021-02479-3
179. Mao AS, Ozkale B, Shah NJ, Vining KH, Descombes T, Zhang L, et al. Programmable microencapsulation for enhanced mesenchymal stem cell persistence and immunomodulation. Proc Natl Acad Sci U.S.A. (2019) 116(31):15392–7. doi: 10.1073/pnas.1819415116
180. Burbulla LF, Song P, Mazzulli JR, Zampese E, Wong YC, Jeon S, et al. Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in parkinson's disease. Science (2017) 357(6357):1255–61. doi: 10.1126/science.aam9080
181. Amatullah H, Shan Y, Beauchamp BL, Gali PL, Gupta S, Maron-Gutierrez T, et al. Dj-1/Park7 impairs bacterial clearance in sepsis. Am J Respir Crit Care Med (2017) 195(7):889–905. doi: 10.1164/rccm.201604-0730OC
182. Zhang Q, Wang SM, Yao PB, Zhang L, Zhang YJ, Chen RX, et al. Effects of l-carnitine on follicular survival and graft function following autotransplantation of cryopreserved-thawed ovarian tissues. Cryobiology (2015) 71(1):135–40. doi: 10.1016/j.cryobiol.2015.04.008
183. Xu H, Wang C, Liu C, Peng Z, Li J, Jin Y, et al. Cotransplantation of mesenchymal stem cells and endothelial progenitor cells for treating steroid-induced osteonecrosis of the femoral head. Stem Cells Transl Med (2021) 10(5):781–96. doi: 10.1002/sctm.20-0346
184. Wu M, Guo Y, Wei S, Xue L, Tang W, Chen D, et al. Biomaterials and advanced technologies for the evaluation and treatment of ovarian aging. J Nanobiotechnol (2022) 20(1):374. doi: 10.1186/s12951-022-01566-8
185. Berebichez-Fridman R, Gomez-Garcia R, Granados-Montiel J, Berebichez-Fastlicht E, Olivos-Meza A, Granados J, et al. The holy grail of orthopedic surgery: Mesenchymal stem cells-their current uses and potential applications. Stem Cells Int (2017) 2017:2638305. doi: 10.1155/2017/2638305
186. Marodin MSJ, Godoy JA, Alves-Paiva RM, Alvarez K, Mitsugi TG, Krepischi ACV, et al. Preclinical evaluation of the tumorigenic and immunomodulatory properties of human bone marrow mesenchymal stromal cell populations with clonal trisomy 5. Stem Cells Int (2022) 2022:1613636. doi: 10.1155/2022/1613636
187. Marquardt LM, Heilshorn SC. Design of injectable materials to improve stem cell transplantation. Curr Stem Cell Rep (2016) 2(3):207–20. doi: 10.1007/s40778-016-0058-0
188. Sun B, Ma Y, Wang F, Hu L, Sun Y. Mir-644-5p carried by bone mesenchymal stem cell-derived exosomes targets regulation of P53 to inhibit ovarian granulosa cell apoptosis. Stem Cell Res Ther (2019) 10(1):360. doi: 10.1186/s13287-019-1442-3
189. Venkat P, Cui C, Chopp M, Zacharek A, Wang F, Landschoot-Ward J, et al. Mir-126 mediates brain endothelial cell exosome treatment-induced neurorestorative effects after stroke in type 2 diabetes mellitus mice. Stroke (2019) 50(10):2865–74. doi: 10.1161/STROKEAHA.119.025371
190. Pan Q, Wang Y, Lan Q, Wu W, Li Z, Ma X, et al. Exosomes derived from mesenchymal stem cells ameliorate Hypoxia/Reoxygenation-injured ecs Via transferring microrna-126. Stem Cells Int (2019) 2019:2831756. doi: 10.1155/2019/2831756
191. Anderson JD, Johansson HJ, Graham CS, Vesterlund M, Pham MT, Bramlett CS, et al. Comprehensive proteomic analysis of mesenchymal stem cell exosomes reveals modulation of angiogenesis Via nuclear factor-kappab signaling. Stem Cells (2016) 34(3):601–13. doi: 10.1002/stem.2298
192. Ding C, Qian C, Hou S, Lu J, Zou Q, Li H, et al. Exosomal mirna-320a is released from hamscs and regulates Sirt4 to prevent reactive oxygen species generation in poi. Mol Ther Nucleic Acids (2020) 21:37–50. doi: 10.1016/j.omtn.2020.05.013
193. Ding C, Zhu L, Shen H, Lu J, Zou Q, Huang C, et al. Exosomal mirna-17-5p derived from human umbilical cord mesenchymal stem cells improves ovarian function in premature ovarian insufficiency by regulating Sirt7. Stem Cells (2020) 38(9):1137–48. doi: 10.1002/stem.3204
194. Mi X, Jiao W, Yang Y, Qin Y, Chen ZJ, Zhao S. Hgf secreted by mesenchymal stromal cells promotes primordial follicle activation by increasing the activity of the Pi3k-akt signaling pathway. Stem Cell Rev Rep (2022) 18(5):1834–50. doi: 10.1007/s12015-022-10335-x
195. Li Z, Zhang M, Zheng J, Tian Y, Zhang H, Tan Y, et al. Human umbilical cord mesenchymal stem cell-derived exosomes improve ovarian function and proliferation of premature ovarian insufficiency by regulating the hippo signaling pathway. Front Endocrinol (Lausanne) (2021) 12:711902. doi: 10.3389/fendo.2021.711902
196. Cai JH, Sun YT, Bao S. Hucmscs-exosomes containing mir-21 promoted estrogen production in ovarian granulosa cells via Lats1-mediated phosphorylation of Loxl2 and yap. Gen Comp Endocrinol (2022) 321-322:114015. doi: 10.1016/j.ygcen.2022.114015
197. Qu Q, Liu L, Cui Y, Liu H, Yi J, Bing W, et al. Mir-126-3p containing exosomes derived from human umbilical cord mesenchymal stem cells promote angiogenesis and attenuate ovarian granulosa cell apoptosis in a preclinical rat model of premature ovarian failure. Stem Cell Res Ther (2022) 13(1):352. doi: 10.1186/s13287-022-03056-y
198. Yang Z, Du X, Wang C, Zhang J, Liu C, Li Y, et al. Therapeutic effects of human umbilical cord mesenchymal stem cell-derived microvesicles on premature ovarian insufficiency in mice. Stem Cell Res Ther (2019) 10(1):250. doi: 10.1186/s13287-019-1327-5
199. Watanabe Y, Tsuchiya A, Terai S. The development of mesenchymal stem cell therapy in the present, and the perspective of cell-free therapy in the future. Clin Mol Hepatol (2021) 27(1):70–80. doi: 10.3350/cmh.2020.0194
200. Hamoda H, British Menopause S, Women's Health C. The British menopause society and women's health concern recommendations on the management of women with premature ovarian insufficiency. Post Reprod Health (2017) 23(1):22–35. doi: 10.1177/2053369117699358
201. Salvatore G, De Felici M, Dolci S, Tudisco C, Cicconi R, Campagnolo L, et al. Human adipose-derived stromal cells transplantation prolongs reproductive lifespan on mouse models of mild and severe premature ovarian insufficiency. Stem Cell Res Ther (2021) 12(1):537. doi: 10.1186/s13287-021-02590-5
202. Lv X, Guan C, Li Y, Su X, Zhang L, Wang X, et al. Effects of single and multiple transplantations of human umbilical cord mesenchymal stem cells on the recovery of ovarian function in the treatment of premature ovarian failure in mice. J Ovarian Res (2021) 14(1):119. doi: 10.1186/s13048-021-00871-4
203. Bahrehbar K, Khanjarpoor Malakhond M, Gholami S. Tracking of human embryonic stem cell-derived mesenchymal stem cells in premature ovarian failure model mice. Biochem Biophys Res Commun (2021) 577:6–11. doi: 10.1016/j.bbrc.2021.08.063
204. Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: The pulmonary first-pass effect. Stem Cells Dev (2009) 18(5):683–92. doi: 10.1089/scd.2008.0253
205. Yong KW, Choi JR, Dolbashid AS, Wan Safwani WKZ. Biosafety and bioefficacy assessment of human mesenchymal stem cells: What do we know so far? Regener Med (2018) 13(2):219–32. doi: 10.2217/rme-2017-0078
206. Shi L, Zhang Y, Dong X, Pan Y, Ying H, Chen J, et al. Toxicity from a single injection of human umbilical cord mesenchymal stem cells into rat ovaries. Reprod Toxicol (2022) 110:9–18. doi: 10.1016/j.reprotox.2022.03.006
207. Herraiz S, Romeu M, Buigues A, Martinez S, Diaz-Garcia C, Gomez-Segui I, et al. Autologous stem cell ovarian transplantation to increase reproductive potential in patients who are poor responders. Fertil Steril (2018) 110(3):496–505.e1. doi: 10.1016/j.fertnstert.2018.04.025
208. Zhang S, Zhu D, Li Z, Huang K, Hu S, Lutz H, et al. A stem cell-derived ovarian regenerative patch restores ovarian function and rescues fertility in rats with primary ovarian insufficiency. Theranostics (2021) 11(18):8894–908. doi: 10.7150/thno.61690
209. Mashayekhi M, Mirzadeh E, Chekini Z, Ahmadi F, Eftekhari-Yazdi P, Vesali S, et al. Evaluation of safety, feasibility and efficacy of intra-ovarian transplantation of autologous adipose derived mesenchymal stromal cells in idiopathic premature ovarian failure patients: Non-randomized clinical trial, phase I, first in human. J Ovarian Res (2021) 14(1):5. doi: 10.1186/s13048-020-00743-3
Keywords: premature ovarian failure, mesenchymal stem cells, ovary, ovarian function, bone marrow
Citation: Huang Y, Zhu M, Liu Z, Hu R, Li F, Song Y, Geng Y, Ma W, Song K and Zhang M (2022) Bone marrow mesenchymal stem cells in premature ovarian failure: Mechanisms and prospects. Front. Immunol. 13:997808. doi: 10.3389/fimmu.2022.997808
Received: 19 July 2022; Accepted: 17 October 2022;
Published: 27 October 2022.
Edited by:
Christian M Capitini, University of Wisconsin-Madison, United StatesReviewed by:
Sara SHAMDANI, Hôpital Paul Brousse, FranceCopyright © 2022 Huang, Zhu, Liu, Hu, Li, Song, Geng, Ma, Song and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingmin Zhang, bW16aGFuZ2VpbnNAMTYzLmNvbQ==; Kunkun Song, MTM5MzEzNzM2OEBxcS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.