
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 24 October 2022
Sec. Cancer Immunity and Immunotherapy
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.992232
This article is part of the Research Topic A year in review: Discussions in Cancer Immunity and Immunotherapy View all 11 articles
The development of chimeric antigen receptor T (CAR-T) cell therapy, a specific type of immunotherapy, in recent decades was a fantastic breakthrough for the treatment of hematological malignancies. However, difficulties in collecting normal T cells from patients and the time cost of manufacturing CAR-T cells have limited the application of CAR-T-cell therapy. In addition, the termination of related clinical trials on universal CAR-T cell therapy has made further research more difficult. Natural killer (NK) cells have drawn great attention in recent years. Chimeric antigen receptor-NK (CAR-NK) cell therapy is a promising strategy in the treatment of malignant tumors because of its lack of potential for causing graft-versus-host disease (GVHD). In this review, we will address the advances in and achievements of CAR-NK cell therapy.
In recent decades, CAR-T-cell therapy was a research focus and was thought to be a promising targeted immunotherapy, especially in the treatment of relapsed and refractory B-cell malignant tumors. To date, two CD19-CAR-T-cell therapies have been approved for the treatment of acute lymphocytic leukemia (ALL) and diffuse large B-cell lymphoma (DLBCL) (1). Studies of CAR-T cells targeting CD38 and BCMA for the treatment of multiple myeloma (MM) have been implemented in clinical trials (2). However, CAR-T cell therapy is still facing several problems. The FDA has terminated all clinical trials concerning universal CAR-T-cell therapy due to safety consideration and related increased attention on gene editing. It is also difficult to collect sufficient numbers of T lymphocytes from patients who have been heavily pretreated. Furthermore, several weeks of CAR-T-cell preparation time hinder the use of this therapy to patients with rapid disease progression (3). In addition, cytokine release syndrome (CRS) and neurological toxicity (NT), the most common adverse events of CAR-T-cell therapy, are life-threatening (4). All of these factors may restrict further clinical applications of CAR-T-cell therapy.
In recent years, NK cells have been regarded as an alternative to T cells due to their accessibility and safety (5). Considering the short duration in vivo, the cytotoxicity and adverse events of CAR-NK-cell therapy are better manageable than those of CAR-T cell therapy. Moreover, the lower incidence of GVHD induced by NK cells makes them a promising immunotherapy for allogenic cell transplantation (6). CAR-NK-cell therapy has thus become a research hotspot and new strategy for malignancies.
In this review, we will discuss the similarities and differences between CAR-T cells and CAR-NK cells and focus on recent advances and preclinical studies of CAR-NK cells.
NK cells are innate immune effectors and are found mainly in the bone marrow, peripheral blood, spleen and liver (7). NK cells possess cytotoxic features similar to those of CD8+ T cells and play important roles in tumor immunology. CD8+ T-cell-mediated cytotoxicity relies on the combination of the T-cell receptor (TCR) and an antigen presented by major histocompatibility complex-I (MHC-I). NK cells can recognize MHC-I expressed on healthy cells and avoid attacking them (8, 9). Tumor cells can down-modulate MHC-I to escape CD8+ T-cell-mediated cytotoxicity, while NK cells can be activated through the loss of MHC-I and control the proliferation and metastasis of tumors (8, 10). Thus, NK cells have more specific anti-tumor effects and are associated with fewer off-target complications (9, 11).
The activation of NK cells can be mediated through different pathways, including signals from Toll-like receptors (TLRs) recognizing pathogen-associated molecular patterns (PAMPs), cytokines such as interleukin (IL)-2 or IL-15, and interplay between activating and inhibitory receptors (7, 12, 13). Activating NK-cell receptors include members of the natural cytotoxicity receptor (NCR) family (NKp30, NKp44 and NKp46), C-type lectin-like activating receptors (NKG2C and NKG2D), activating killer immunoglobulin receptors (KIR2DS1, KIR2DS4 and KIR2DL4) and costimulatory receptor DNAX accessory molecule 1 (DNAM-1) (14). While killer cell immunoglobulin-like receptors (KIRs) and the heterodimeric C-type lectin receptor NKG2A are inhibitory receptors associated with the tolerance of NK cells to normal cells (14).
NK cells for preclinical studies and clinical therapy may be derived from a wide range of sources, such as peripheral blood (PB), cord blood (CB), hematopoietic stem cells (HSCs), induced pluripotent stem cells (iPSCs) and NK-cell lines (15–19).
The most accessible source of NK cells is peripheral blood. However, a number of issues limit the use of NK cells from peripheral blood, including the high monetary and time costs, low cell proliferation capacity and short survival time (20). The expression of genes related to the cell cycle and cell proliferation is higher in NK cells from umbilical cord blood (UCB) than in those from peripheral blood (21). Furthermore, the advantages of UCB-derived NK cells, including the convenience of collection and low associated incidence of GVHD, make UCB a better source of NK cells than PB (22, 23). In addition, human stem and progenitor cells (HSPCs) isolated from cord blood can also be derived into NK cells with the stimulation of various growth factors and cytokines, including IL-2, IL-7 and IL-15 (24). Similarly, NK cells can also be derived from iPSCs in the presence of these stimulators (25).
NK-cell lines, mostly derived from NK/T-cell lymphoma (NKTCL) patients, such as the NK-92 and KHYG-1 cell lines, may be a potential rapid and abundant source for NK cells for immunotherapy (26, 27). These cell lines are easily transduced and maintain cytotoxicity during expansion. The NK-92 cell line, obtained from a good manufacturing practice (GMP)-compliant master cell bank and treated in a GMP-compliant procedure, is the only cell line approved by the FDA for clinical use (28, 29). Since the first report of the transfusion of irradiated NK-92 cells for adoptive immunotherapy of malignancies (30) and the first CAR-NK-92 cells targeting HER-2 (31), NK-92 cells has been applied in several clinical trials, and some encouraging results have been achieved in the treatment of refractory lymphoma, multiple myeloma and other solid tumors. Several patients even achieved a complete response (CR) (32–34). NK-cell lines must be irradiated before infusion due to the risk of tumor engraftment and tumorigenicity. The short lifespan of irradiated cells may result in treatment failure or a short duration of disease remission, thus limiting their clinical application (32, 33, 35).
CARs consist of an extracellular domain (a single-chain variable antibody fragment (scFv) or a functional domain of a specific ligand) for the identification of target antigens, a transmembrane region and an intracellular domain (36). The intracellular domain of CAR-T cells is composed of CD3ζ activation signaling (first generation of CARs) and costimulatory molecules (CD28, 4-1BB or CD134) (second or third generation of CARs) (Figure 1A). Based on NK-cell characteristics, several CAR-NK cells contain DNAX-activation protein (DAP) 10 or DAP12 as an intracellular domain (Figure 1C). DAP12 and NKG2D are expressed on NK cells and participate in the activation of downstream signals, while DAP10 is necessary for NKG2D costimulatory signaling. These CAR-NK cells were mainly designed for the treatment of both leukemia and solid tumors and showed strong anti-tumor effects (37, 38). A lack of cytokines such as IL-2 or IL-15 may lead to the short in vivo lifetime of NK cells. NK cells can be engineered to both express CARs and autonomously produce IL-2 or IL-15 (fourth generation of CARs), thus enhancing their persistence and proliferation (Figure 1B) (39, 40).
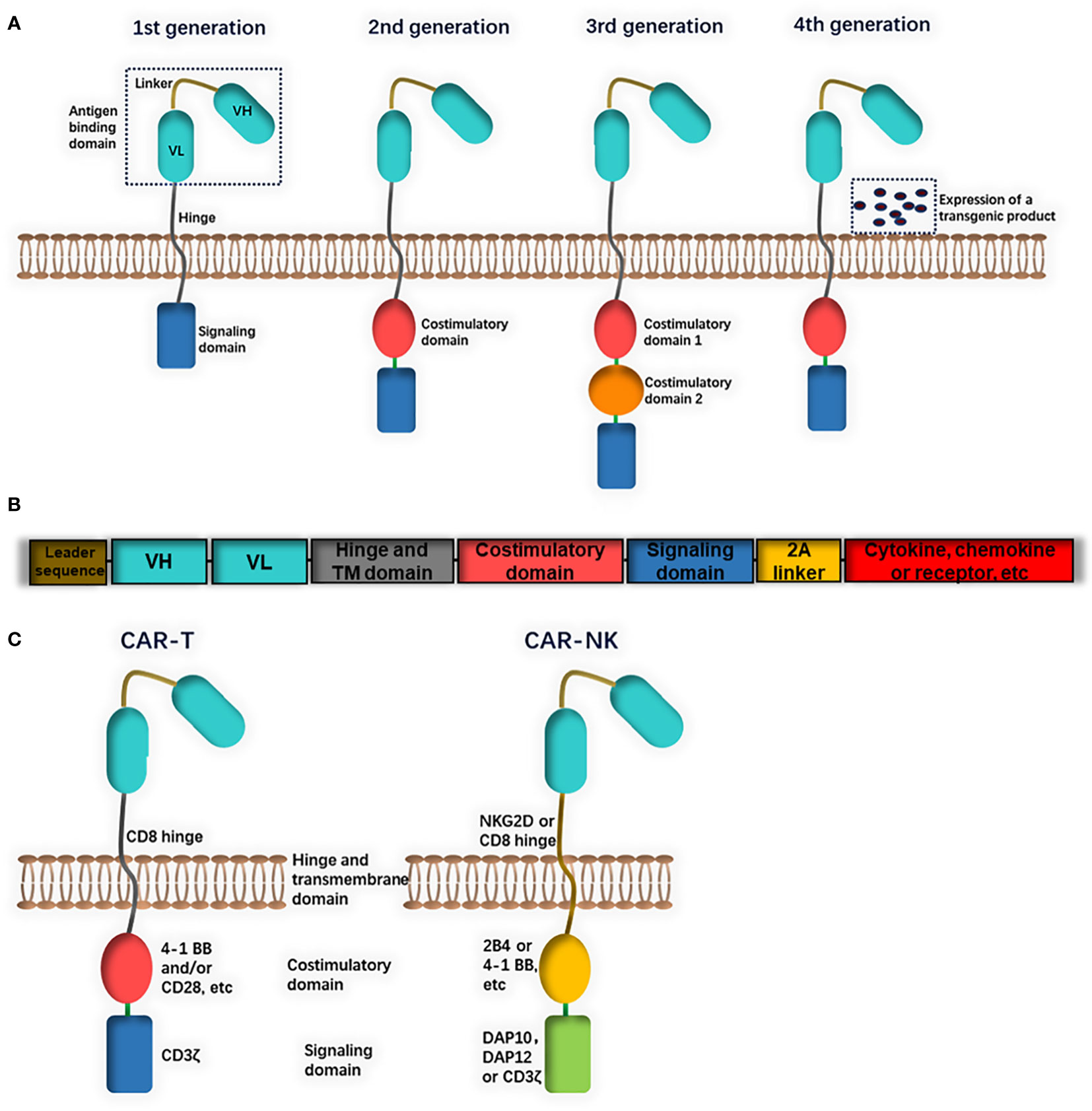
Figure 1 The structure of chimeric antigen receptors (CAR). (A) CAR consist of an extracellular antigen binding domain, a transmembrane hinge and intracellular domain. The extracellular domain could be a single chain fragment of variable region (ScFv) antibody or a functional domain of specific ligand. The intracellular domain is composed of a signaling domain (first generation) and one costimulatory domain (second generation) or two (third generation). (B) Fourth generation CARs include a constitutive or inducible expression of a transgenic product (cytokine, chemokine or receptor, etc.). (C) Differences in CAR constructs between CAR-T and CAR-NK: CAR-T cells usually contains a CD8 transmembrane domain, CD3ζ signaling domain and 4-1BB and/or CD28 costimulatory domain. CAR-NK cells may be with different domains (for example, NKG2D transmembrane domain, DAP10 or DAP12 signaling domain and 2B4 costimulatory domain).
Lentivirus-based vectors have been extensively used in CAR gene transduction of T cells. Compared with T cells, NK cells showed resistance to viral transfection and lower transduction efficiency, which may be due to the natural capacity of NK cells to defend against viral infection (41, 42). Other approaches, including retroviral vectors, transposon vectors and the electroporation of DNA or mRNA plasmids, are alternative ways to transfer the CAR gene into NK cells (43–48).
CAR-T cells can kill tumor cells with specific target antigens through active cell lysis and the production of cytokines, including IL-1α, IL-2, IL-6, IL-8, IL-10, and tumor necrosis factor-α (TNF-α) (6, 49). However, these cytokines are also highly associated with CRS and severe neurotoxicity (49). CAR-NK cells secrete a different cytokine profile, such as IFN-γ and GM-CSF, which are associated with a lower risk of CRS and neurotoxicity (50). In addition, CAR-NK cells can lyse tumor cells directly by releasing cytoplasmic granules containing perforin and granzyme or inducing tumor cell apoptosis by expression of Fas ligand or TNF-related apoptosis-inducing ligand (TRAIL) (51). NK cells also participate in antibody-dependent cellular cytotoxicity (ADCC) (52). NK cells can activate and interact with other immune cells, such as T cells, dendritic cells and macrophages (53). All these features enable them to exert anti-tumor activity in pathways other than the CAR-specific pathway and reduce the risk of relapse or resistance mediated by target antigen escape (54–56).
NK cells have been engineered to express CARs to redirect their activity against B-cell malignancies. To date, CD19 is the most common target in both preclinical and clinical studies of CAR-T-cell therapy. Similarly, a number of preclinical studies of CAR-NK therapy have focused on this target. NK-92 cells engineered with CARs recognizing CD19 showed increased cytotoxicity against B-cell malignancies (57, 58). CD19-CAR-NK cells from other cell sources, including PB, iPSCs and CB, also showed activity against B-cell malignancies in vitro (40, 59, 60). Other molecules, including CD20 and Flt3, were also developed as specific targets for CAR-NK immunotherapy against B-cell tumors (61, 62).
CD38 and CD138 are classic markers of plasma cells and are highly expressed in multiple myeloma (MM). Although CD38-CAR-T-cell therapy for MM and CD38-CAR-NK-cell therapy for acute myeloid leukemia (AML) have been reported in several studies (63, 64), CD38-CAR-NK cells have not been evaluated for the treatment of multiple myeloma. Jiang et al. developed CD138-targeting CAR-NK cells and demonstrated enhanced anti-tumor activity in vitro and in xenograft mouse models (65). B-cell maturation antigen (BCMA) is another ideal target for CAR cell therapy due to its restricted expression in B-cell lineage cells. BCMA-CAR-NK cells modified with CXCR4 significantly reduced the tumor burden and extended the survival of tumor-bearing mice (66). Signaling lymphocytic activation molecule family member 7 (SLAMF7 or CS1) is another potential target for its high expression in plasma cells and MM. Second-generation CS1-specific CAR-NK-92 cells were established by Chu et al. and showed cytotoxicity against CS1-positive MM cells and xenograft models (67).
To date, T-cell malignancies, including peripheral T-cell lymphoma and T-cell acute lymphoblastic leukemia (T-ALL), remains a refractory disease. Three CAR-NK cell therapies targeting CD3, CD5 and CD7 have been investigated for the treatment of T-cell malignancies. These modified CAR-NK-92 cells showed significant anti-tumor cytotoxicity against T-cell lymphomas and T-ALL both in vitro and in vivo (68–70).
In addition to specific tumor markers, antigens that are widely expressed in multiple malignancies have been developed as immunotherapy targets. For example, NKG2D ligands are expressed on a variety of tumor cells. MHC class I chain-related protein A (MICA), an NKG2D ligand, has been identified on some leukemia cells and solid tumor cells, such as lung, breast, ovary and colon cancer cells (71–73). NKG2D ligands have also been detected on MM cells and glioma cells (74, 75). Leivas et al. developed engineered NK cells targeting NKG2D ligands in MM (76). Data from in vitro tests and mouse models showed enhanced anti-tumor activity of NKG2D-CAR-NK cells compared with memory CAR-T cells (76). Du et al. generated peripheral blood-derived NK cells coexpressing NKG2D-specific CAR and IL-15 and demonstrated their activity in lysing tumor cells both in vitro and in a xenograft AML model (77).
Although CAR-T-cell therapies have achieved great progress in the treatment of hematological malignancies, their effect on solid malignancies has been poor. This poor efficacy may be due to the insufficient homing capacity and the immunosuppressive tumor microenvironment (78). Thus, CAR-NK cell therapies for solid tumors have become a promising immunotherapy strategy. Glioblastoma, breast cancer and ovarian cancer are the most widely researched solid tumors to determine the potential of CAR-NK-cell therapy (summarized in Table 1).
Glioblastoma is the most common malignant primary cerebral tumor in adults. Even though patients undergo surgical resection and receive radio- and/or chemotherapy, the median survival time is approximately 15 months (98). Interleukin-13 receptor α2 (IL-13Rα2), epidermal growth factor receptor (EGFR), EGFR variant III (EGFRvIII) and growth factor receptor tyrosine kinase Erb2 (HER2) have been explored as immunotherapy targets for glioblastoma. They are overexpressed in 40-60% of glioblastoma patients, while these antigens are undetectable or only minimally expressed in normal brain tissue (99–102). IL-13Rα2 can enhance the invasiveness of glioblastoma (103). EGFRvIII drives tumorigenicity and mediates resistance to radiotherapy and chemotherapy (104, 105). Together, IL-13Rα2 and EGFRvIII can promote the proliferation of glioblastoma cells (103), while overexpression of HER2 contributes to malignant transformation (106).
There have been several preclinical studies of IL-13Rα2-specific CAR-T-cell therapy in the treatment of glioblastoma (107–110). Other studies demonstrated the significant cytotoxicity of CAR-T cells against EGFRvIII- or HER2-positive glioblastoma both in vitro and in vivo (111–114).
Until now, most preclinical studies of CAR-NK-cell therapy for glioblastoma were targeting EGFR, EGFRvIII and HER2. Different NK cells, including NK-92, NKL, KHYG-1 and YTS cells, engineered to target EGFR and/or EGFRvIII, showed enhanced cytotoxicity against glioblastoma both in vitro and in vivo (80–83). CAR-NK cells recognizing both EGFR and EGFRvIII showed stronger anti-tumor effects than single targeted NK cells (84). NK-92/5.28z cells, engineered HER2-specific NK cells with CD28 and CD3ζ signaling domains, have been demonstrated to have the ability to lyse HER2-positive glioblastoma cells in vitro and in orthotopic glioblastoma xenograft NSG mouse models (79).
As a very common malignancy in female patients, breast cancer is another solid tumor that is studied for CAR-NK-cell immunotherapy. Similar to glioblastoma, HER2, EGFR and EGFRvIII are also targets for breast cancer.
The anti-tumor activity of NK-92/5.28z cells was also evaluated in HER-2-positive breast cancer. Data revealed that tumor cells expressing HER-2 enhanced the proliferation and cytokine release (such as granzyme B, IFN-γ, IL-8 and IL-10) of NK-92/5.28z cells [87]. The modified NK-92 cells displayed significant cytotoxicity in vitro and in xenograft mouse models (85). NK-92 cells engineered to target HER2 developed by Liu et al. also demonstrated similar anti-tumor effects (86).
A second-generation CAR that can recognize both EGFR and EGFRvIII was constructed by Chen et al. (87). NK-92 cells transduced with this CAR showed enhanced cytotoxicity and production of IFN-γ against breast cancer cells. Xenograft mouse models of breast cancer brain metastasis were used for in vivo evaluation of anti-tumor activity. CAR-NK-92 cell infusion significantly suppressed tumor growth. Similarly, two EGFR-targeted CAR-NK cells were developed (87). Cytokine release and cytotoxicity assays were performed and revealed that EGFR-CAR NK cells specifically lysed triple-negative breast cancer cells in vitro and suppressed breast cancer cell line-derived xenograft and patient-derived xenograft (PDX) tumors in mouse models (87).
Epithelial cell adhesion molecule (EpCAM), tissue factor (TF) and B7-H6 have also been reported as targets for the treatment of breast cancer. Studies have shown the increased tumor killing ability of these CAR-NK-92 cells against breast cancer cells (88–90).
Ovarian cancer is a highly malignant tumor with a 5-year survival rate lower than 40% (115). Several studies have focused on CAR-NK immunotherapies for the treatment of ovarian cancer.
Human leukocyte antigen G (HLA-G) is a tumor-associated antigen (TAA) that is expressed on 40-100% of solid tumors and a limited subset of immune-privileged tissues and adult tissues, such as erythroid precursors and pancreatic islets (116, 117). Jan et al. developed CAR-NK cells targeting HLA-G and evaluated the synergy of CAR-NK cells combined with low-dose chemotherapy (118). Jan et al. developed CAR-NK cells targeting HLA-G and evaluated the synergy of CAR-NK cells combined with low-dose chemotherapy (116). Their study showed that pretreatment with low-dose chemotherapy can induce the overexpression of HLA-G, thus enhancing the anti-tumor cytotoxicity of HLA-G-CAR-NK cells (91).
Since cancer stem cells (CSC) play an important role in metastatic spread and chemoresistance in solid tumors, CSC markers such as CD24, CD44 and CD133 have been explored as specific targets for ovarian cancer immunotherapy (92–94). CAR-NK-92 cells targeting CD24, CD44 or CD133 have shown significant anti-tumor effects in preclinical studies (92–94).
Mesothelin and folate receptor alpha (αFR) are alternative targets that are overexpressed in ovarian cancer. Both iPSC-derived CAR-NK cells and NK-92 cell line-derived CAR-NK cells targeting mesothelin showed robust specific anti-tumor activity both in vitro and in vivo (95, 96). Ao et al. developed αFR-targeted CAR-NK-92 cells and demonstrated not only their antigen-specific cytotoxicity and proliferation in vitro but also their ability to eliminate cancer cells in mouse models (97).
Since the first CAR-NK-cell clinical trials (NCT00995137, clinicaltrials.gov) started in 2009, there have been 39 studies registered in clinicaltrials.gov evaluating the feasibility, safety and efficacy of CAR-NK cells in the treatment of malignancies. Eight clinical trials sponsored by PersonGen BioTherapeutics and Asclepius Technology Company Group, including NCT02742727, NCT02839954, NCT02892695, NCT02944162, NCT03941457, NCT03931720, NCT03940820 and NCT03940833, which were estimated to be completed in 2018-2019, have been stopped updating for 3 years. It’s a pity that no data of these trials were reported till now. The rest of 31 trials were summarized in Table 2.
Similar to CAR-T-cell therapies, most CAR-NK-cell trials target markers on hematopoietic malignancies, such as CD19, CD20, CD22 and BCMA. Notably, there have been eight CAR-NK-cell clinical studies have focused on solid malignancies, which are thought to poorly responsive to CAR-T cells. These CAR-NK cells may target markers such as HER2, NKG2D, mesothelin and PSMA expressed on malignancies, including brain, prostate, ovarian, pancreatic and lung cancers (Table 2).
Studies in recent years suggest that CAR-NK-cell therapies may be equally effective as CAR-T-cell therapies. Compared with CAR-T cells, CAR-NK cells have multiple advantages for the treatment of malignancies. CAR-NK-cell therapy seldom causes severe CRS or neurotoxicity. The low associated risk of GVHD and the safety of allogeneic NK-cell infusion shorten the time of cell preparation, which greatly benefits patients with lymphopenia or rapid progression. However, several nonnegligible problems still exist. The best source of NK cells and their in vitro expansion strategy, and the most effective signaling domain for CAR activation still need to be elaborated. Antigen escape and tumor heterogeneity, the most common difficulties in immunotherapies, as well as in vivo duration, are also problems to be considered. CAR-NK-cell immunotherapy is still in its early stages. Strategies to improve the efficacy and safety of CAR-NK-cell immunotherapy must be further explored in the future.
HL: conceptualization and writing original draft. WS: writing review and editing. ZL: writing review and editing. MZ: conceptualization, supervision, and writing – review and editing. All authors contributed to the article and approved the submitted version.
The work was supported by the Oncology Department, State Key Laboratory of Esophageal Cancer Prevention & Treatment and Henan Key Laboratory for Esophageal Cancer Research, and the Medical Sciences Academy and Research Institute of Nephrology of Zhengzhou University. This work was supported by the National Natural Science Foundation of China (81970184; 82170183; U1904139; 82070209).
I would like to show great gratitude to them all.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Wang W, Jiang J, Wu C. CAR-NK for tumor immunotherapy: Clinical transformation and future prospects. Cancer Lett (2020) 472:175–80. doi: 10.1016/j.canlet.2019.11.033
2. Feng Y, Liu X, Li X, Zhou Y, Song Z, Zhang J, et al. Novel BCMA-OR-CD38 tandem-dual chimeric antigen receptor T cells robustly control multiple myeloma. Oncoimmunology (2021) 10:1959102. doi: 10.1080/2162402X.2021.1959102
3. Rezvani K, Rouce R, Liu E, Shpall E. Engineering natural killer cells for cancer immunotherapy. Mol Ther (2017) 25:1769–81. doi: 10.1016/j.ymthe.2017.06.012
4. Grigor EJM, Fergusson D, Kekre N, Montroy J, Atkins H, Seftel MD, et al. Risks and benefits of chimeric antigen receptor T-cell (CAR-T) therapy in cancer: A systematic review and meta-analysis. Transfus Med Rev (2019) 33:98–110. doi: 10.1016/j.tmrv.2019.01.005
5. Zhang C, Oberoi P, Oelsner S, Waldmann A, Lindner A, Tonn T, et al. Chimeric antigen receptor-engineered NK-92 cells: An off-the-Shelf cellular therapeutic for targeted elimination of cancer cells and induction of protective antitumor immunity. Front Immunol (2017) 8:533. doi: 10.3389/fimmu.2017.00533
6. Shifrin N, Raulet DH, Ardolino M. NK cell self tolerance, responsiveness and missing self recognition. Semin Immunol (2014) 26:138–44. doi: 10.1016/j.smim.2014.02.007
7. Rafei H, Daher M, Rezvani K. Chimeric antigen receptor (CAR) natural killer (NK)-cell therapy: leveraging the power of innate immunity. Br J Haematol (2021) 193:216–30. doi: 10.1111/bjh.17186
8. Chiossone L, Dumas PY, Vienne M, Vivier E. Natural killer cells and other innate lymphoid cells in cancer. Nat Rev Immunol (2018) 18:671–88. doi: 10.1038/s41577-018-0061-z
9. Guillerey C, Huntington ND, Smyth MJ. Targeting natural killer cells in cancer immunotherapy. Nat Immunol (2016) 17:1025–36. doi: 10.1038/ni.3518
10. Wu SY, Fu T, Jiang YZ, Shao ZM. Natural killer cells in cancer biology and therapy. Mol Cancer (2020) 19:120. doi: 10.1186/s12943-020-01238-x
11. Vivier E, Ugolini S, Blaise D, Chabannon C, Brossay L. Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol (2012) 12:239–52. doi: 10.1038/nri3174
12. Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on toll-like receptors. Nat Immunol (2010) 11:373–84. doi: 10.1038/ni.1863
13. Chan CJ, Smyth MJ, Martinet L. Molecular mechanisms of natural killer cell activation in response to cellular stress. Cell Death Differ (2014) 21:5–14. doi: 10.1038/cdd.2013.26
14. Marofi F, Saleh MM, Rahman HS, Suksatan W, Al-Gazally ME, Abdelbasset WK, et al. CAR-engineered NK cells; a promising therapeutic option for treatment of hematological malignancies. Stem Cell Res Ther (2021) 12:374. doi: 10.1186/s13287-021-02462-y
15. Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, et al. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood (2001) 97:3146–51. doi: 10.1182/blood.V97.10.3146
16. Campbell JJ, Qin S, Unutmaz D, Soler D, Murphy KE, Hodge MR, et al. Unique subpopulations of CD56+ NK and NK-T peripheral blood lymphocytes identified by chemokine receptor expression repertoire. J Immunol (2001) 166:6477–82. doi: 10.4049/jimmunol.166.11.6477
17. Chabannon C, Mfarrej B, Guia S, Ugolini S, Devillier R, Blaise D, et al. Manufacturing natural killer cells as medicinal products. Front Immunol (2016) 7:504. doi: 10.3389/fimmu.2016.00504
18. Geiger TL, Sun JC. Development and maturation of natural killer cells. Curr Opin Immunol (2016) 39:82–9. doi: 10.1016/j.coi.2016.01.007
19. Mehta RS, Shpall EJ, Rezvani K. Cord blood as a source of natural killer cells. Front Med (Lausanne) (2015) 2:93. doi: 10.3389/fmed.2015.00093
20. Martin-Antonio B, Sune G, Perez-Amill L, Castella M, Urbano-Ispizua A. Natural killer cells: Angels and devils for immunotherapy. Int J Mol Sci (2017) 18:1868-87. doi: 10.3390/ijms18091868
21. Li L, Chen H, Marin D, Xi Y, Miao Q, Lv J, et al. A novel immature natural killer cell subpopulation predicts relapse after cord blood transplantation. Blood Adv (2019) 3:4117–30. doi: 10.1182/bloodadvances.2019000835
22. Oran B, Shpall E. Umbilical cord blood transplantation: a maturing technology. Hematol Am Soc Hematol Educ Program (2012) 2012:215–22. doi: 10.1182/asheducation.V2012.1.215.3798291
23. Nomura A, Takada H, Jin CH, Tanaka T, Ohga S, Hara T. Functional analyses of cord blood natural killer cells and T cells: a distinctive interleukin-18 response. Exp Hematol (2001) 29:1169–76. doi: 10.1016/S0301-472X(01)00689-0
24. Spanholtz J, Preijers F, Tordoir M, Trilsbeek C, Paardekooper J, de Witte T, et al. Clinical-grade generation of active NK cells from cord blood hematopoietic progenitor cells for immunotherapy using a closed-system culture process. PLoS One (2011) 6:e20740. doi: 10.1371/journal.pone.0020740
25. Miller JS, Alley KA, McGlave P. Differentiation of natural killer (NK) cells from human primitive marrow progenitors in a stroma-based long-term culture system: identification of a CD34+7+ NK progenitor. Blood (1994) 83:2594–601. doi: 10.1182/blood.V83.9.2594.2594
26. Maki G, Klingemann HG, Martinson JA, Tam YK. Factors regulating the cytotoxic activity of the human natural killer cell line, NK-92. J Hematother Stem Cell Res (2001) 10:369–83. doi: 10.1089/152581601750288975
27. Cheng M, Zhang J, Jiang W, Chen Y, Tian Z. Natural killer cell lines in tumor immunotherapy. Front Med (2012) 6:56–66. doi: 10.1007/s11684-012-0177-7
28. Suck G, Odendahl M, Nowakowska P, Seidl C, Wels WS, Klingemann HG, et al. NK-92: an 'off-the-shelf therapeutic' for adoptive natural killer cell-based cancer immunotherapy. Cancer Immunol Immunother (2016) 65:485–92. doi: 10.1007/s00262-015-1761-x
29. Nowakowska P, Romanski A, Miller N, Odendahl M, Bonig H, Zhang C, et al. Clinical grade manufacturing of genetically modified, CAR-expressing NK-92 cells for the treatment of ErbB2-positive malignancies. Cancer Immunol Immunother (2018) 67:25–38. doi: 10.1007/s00262-017-2055-2
30. Tonn T, Becker S, Esser R, Schwabe D, Seifried E. Cellular immunotherapy of malignancies using the clonal natural killer cell line NK-92. J Hematother Stem Cell Res (2001) 10:535–44. doi: 10.1089/15258160152509145
31. Uherek C, Tonn T, Uherek B, Becker S, Schnierle B, Klingemann HG, et al. Retargeting of natural killer-cell cytolytic activity to ErbB2-expressing cancer cells results in efficient and selective tumor cell destruction. Blood (2002) 100:1265–73. doi: 10.1182/blood.V100.4.1265.h81602001265_1265_1273
32. Arai S, Meagher R, Swearingen M, Myint H, Rich E, Martinson J, et al. Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: a phase I trial. Cytotherapy (2008) 10:625–32. doi: 10.1080/14653240802301872
33. Tonn T, Schwabe D, Klingemann HG, Becker S, Esser R, Koehl U, et al. Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy (2013) 15:1563–70. doi: 10.1016/j.jcyt.2013.06.017
34. Williams BA, Law AD, Routy B, denHollander N, Gupta V, Wang XH, et al. A phase I trial of NK-92 cells for refractory hematological malignancies relapsing after autologous hematopoietic cell transplantation shows safety and evidence of efficacy. Oncotarget (2017) 8:89256–68. doi: 10.18632/oncotarget.19204
35. Boyiadzis M, Agha M, Redner RL, Sehgal A, Im A, Hou JZ, et al. Phase 1 clinical trial of adoptive immunotherapy using "off-the-shelf" activated natural killer cells in patients with refractory and relapsed acute myeloid leukemia. Cytotherapy (2017) 19:1225–32. doi: 10.1016/j.jcyt.2017.07.008
36. Sadelain M. Chimeric antigen receptors: driving immunology towards synthetic biology. Curr Opin Immunol (2016) 41:68–76. doi: 10.1016/j.coi.2016.06.004
37. Chang YH, Connolly J, Shimasaki N, Mimura K, Kono K, Campana D. A chimeric receptor with NKG2D specificity enhances natural killer cell activation and killing of tumor cells. Cancer Res (2013) 73:1777–86. doi: 10.1158/0008-5472.CAN-12-3558
38. Topfer K, Cartellieri M, Michen S, Wiedemuth R, Muller N, Lindemann D, et al. DAP12-based activating chimeric antigen receptor for NK cell tumor immunotherapy. J Immunol (2015) 194:3201–12. doi: 10.4049/jimmunol.1400330
39. Imamura M, Shook D, Kamiya T, Shimasaki N, Chai SM, Coustan-Smith E, et al. Autonomous growth and increased cytotoxicity of natural killer cells expressing membrane-bound interleukin-15. Blood (2014) 124:1081–8. doi: 10.1182/blood-2014-02-556837
40. Liu E, Tong Y, Dotti G, Shaim H, Savoldo B, Mukherjee M, et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia (2018) 32:520–31. doi: 10.1038/leu.2017.226
41. Schmidt P, Raftery MJ, Pecher G. Engineering NK cells for CAR therapy-recent advances in gene transfer methodology. Front Immunol (2020) 11:611163. doi: 10.3389/fimmu.2020.611163
42. Littwitz E, Francois S, Dittmer U, Gibbert K. Distinct roles of NK cells in viral immunity during different phases of acute friend retrovirus infection. Retrovirology (2013) 10:127. doi: 10.1186/1742-4690-10-127
43. Matosevic S. Viral and nonviral engineering of natural killer cells as emerging adoptive cancer immunotherapies. J Immunol Res (2018) 2018:4054815. doi: 10.1155/2018/4054815
44. Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood (2005) 106:376–83. doi: 10.1182/blood-2004-12-4797
45. Zayed H, Izsvak Z, Walisko O, Ivics Z. Development of hyperactive sleeping beauty transposon vectors by mutational analysis. Mol Ther (2004) 9:292–304. doi: 10.1016/j.ymthe.2003.11.024
46. Rostovskaya M, Fu J, Obst M, Baer I, Weidlich S, Wang H, et al. Transposon-mediated BAC transgenesis in human ES cells. Nucleic Acids Res (2012) 40:e150. doi: 10.1093/nar/gks643
47. Xiao L, Cen D, Gan H, Sun Y, Huang N, Xiong H, et al. Adoptive transfer of NKG2D CAR mRNA-engineered natural killer cells in colorectal cancer patients. Mol Ther (2019) 27:1114–25. doi: 10.1016/j.ymthe.2019.03.011
48. Heintz N, Gong S. Two-step bacterial artificial chromosome (BAC) engineering: Electroporation of competent BAC host cells with the recombinant shuttle vector. Cold Spring Harb Protoc (2020) 2020:098079. doi: 10.1101/pdb.prot098079
49. Hunter BD, Jacobson CA. CAR T-cell associated neurotoxicity: Mechanisms, clinicopathologic correlates, and future directions. J Natl Cancer Inst (2019) 111:646–54. doi: 10.1093/jnci/djz017
50. Klingemann H. Are natural killer cells superior CAR drivers? Oncoimmunology (2014) 3:e28147. doi: 10.4161/onci.28147
51. Screpanti V, Wallin RP, Ljunggren HG, Grandien A. A central role for death receptor-mediated apoptosis in the rejection of tumors by NK cells. J Immunol (2001) 167:2068–73. doi: 10.4049/jimmunol.167.4.2068
52. Wu J, Mishra HK, Walcheck B. Role of ADAM17 as a regulatory checkpoint of CD16A in NK cells and as a potential target for cancer immunotherapy. J Leukoc Biol (2019) 105:1297–303. doi: 10.1002/JLB.2MR1218-501R
53. Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, et al. Innate or adaptive immunity? the example of natural killer cells. Science (2011) 331:44–9. doi: 10.1126/science.1198687
54. Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T Cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet (2015) 385:517–28. doi: 10.1016/S0140-6736(14)61403-3
55. Sotillo E, Barrett DM, Black KL, Bagashev A, Oldridge D, Wu G, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discovery (2015) 5:1282–95. doi: 10.1158/2159-8290.CD-15-1020
56. Farag SS, Caligiuri MA. Human natural killer cell development and biology. Blood Rev (2006) 20:123–37. doi: 10.1016/j.blre.2005.10.001
57. Romanski A, Uherek C, Bug G, Seifried E, Klingemann H, Wels WS, et al. CD19-CAR engineered NK-92 cells are sufficient to overcome NK cell resistance in b-cell malignancies. J Cell Mol Med (2016) 20:1287–94. doi: 10.1111/jcmm.12810
58. Boissel L, Betancur M, Wels WS, Tuncer H, Klingemann H. Transfection with mRNA for CD19 specific chimeric antigen receptor restores NK cell mediated killing of CLL cells. Leuk Res (2009) 33:1255–9. doi: 10.1016/j.leukres.2008.11.024
59. Quintarelli C, Sivori S, Caruso S, Carlomagno S, Falco M, Boffa I, et al. Efficacy of third-party chimeric antigen receptor modified peripheral blood natural killer cells for adoptive cell therapy of b-cell precursor acute lymphoblastic leukemia. Leukemia (2020) 34:1102–15. doi: 10.1038/s41375-019-0613-7
60. Muller S, Bexte T, Gebel V, Kalensee F, Stolzenberg E, Hartmann J, et al. High cytotoxic efficiency of lentivirally and alpharetrovirally engineered CD19-specific chimeric antigen receptor natural killer cells against acute lymphoblastic leukemia. Front Immunol (2019) 10:3123. doi: 10.3389/fimmu.2019.03123
61. Muller T, Uherek C, Maki G, Chow KU, Schimpf A, Klingemann HG, et al. Expression of a CD20-specific chimeric antigen receptor enhances cytotoxic activity of NK cells and overcomes NK-resistance of lymphoma and leukemia cells. Cancer Immunol Immunother (2008) 57:411–23. doi: 10.1007/s00262-007-0383-3
62. Oelsner S, Waldmann A, Billmeier A, Roder J, Lindner A, Ullrich E, et al. Genetically engineered CAR NK cells display selective cytotoxicity against FLT3-positive b-ALL and inhibit in vivo leukemia growth. Int J Cancer (2019) 145:1935–45. doi: 10.1002/ijc.32269
63. Drent E, Groen RW, Noort WA, Themeli M, Lammerts van Bueren JJ, Parren PW, et al. Pre-clinical evaluation of CD38 chimeric antigen receptor engineered T cells for the treatment of multiple myeloma. Haematologica (2016) 101:616–25. doi: 10.3324/haematol.2015.137620
64. Gurney M, Stikvoort A, Nolan E, Kirkham-McCarthy L, Khoruzhenko S, Shivakumar R, et al. CD38 knockout natural killer cells expressing an affinity optimized CD38 chimeric antigen receptor successfully target acute myeloid leukemia with reduced effector cell fratricide. Haematologica (2022) 107:437–45. doi: 10.3324/haematol.2020.271908
65. Jiang H, Zhang W, Shang P, Zhang H, Fu W, Ye F, et al. Transfection of chimeric anti-CD138 gene enhances natural killer cell activation and killing of multiple myeloma cells. Mol Oncol (2014) 8:297–310. doi: 10.1016/j.molonc.2013.12.001
66. Ng YY, Du Z, Zhang X, Chng WJ, Wang S. CXCR4 and anti-BCMA CAR co-modified natural killer cells suppress multiple myeloma progression in a xenograft mouse model. Cancer Gene Ther (2022) 29:475–83. doi: 10.1038/s41417-021-00365-x
67. Chu J, Deng Y, Benson DM, He S, Hughes T, Zhang J, et al. CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia (2014) 28:917–27. doi: 10.1038/leu.2013.279
68. Chen KH, Wada M, Firor AE, Pinz KG, Jares A, Liu H, et al. Novel anti-CD3 chimeric antigen receptor targeting of aggressive T cell malignancies. Oncotarget (2016) 7:56219–32. doi: 10.18632/oncotarget.11019
69. Chen KH, Wada M, Pinz KG, Liu H, Lin KW, Jares A, et al. Preclinical targeting of aggressive T-cell malignancies using anti-CD5 chimeric antigen receptor. Leukemia (2017) 31:2151–60. doi: 10.1038/leu.2017.8
70. You F, Wang Y, Jiang L, Zhu X, Chen D, Yuan L, et al. A novel CD7 chimeric antigen receptor-modified NK-92MI cell line targeting T-cell acute lymphoblastic leukemia. Am J Cancer Res (2019) 9:64–78.
71. Pende D, Rivera P, Marcenaro S, Chang CC, Biassoni R, Conte R, et al. Major histocompatibility complex class I-related chain a and UL16-binding protein expression on tumor cell lines of different histotypes: analysis of tumor susceptibility to NKG2D-dependent natural killer cell cytotoxicity. Cancer Res (2002) 62:6178–86.
72. Watson NF, Spendlove I, Madjd Z, McGilvray R, Green AR, Ellis IO, et al. Expression of the stress-related MHC class I chain-related protein MICA is an indicator of good prognosis in colorectal cancer patients. Int J Cancer (2006) 118:1445–52. doi: 10.1002/ijc.21510
73. Carlsten M, Bjorkstrom NK, Norell H, Bryceson Y, van Hall T, Baumann BC, et al. DNAX accessory molecule-1 mediated recognition of freshly isolated ovarian carcinoma by resting natural killer cells. Cancer Res (2007) 67:1317–25. doi: 10.1158/0008-5472.CAN-06-2264
74. Friese MA, Platten M, Lutz SZ, Naumann U, Aulwurm S, Bischof F, et al. MICA/NKG2D-mediated immunogene therapy of experimental gliomas. Cancer Res (2003) 63:8996–9006.
75. Carbone E, Neri P, Mesuraca M, Fulciniti MT, Otsuki T, Pende D, et al. HLA class I, NKG2D, and natural cytotoxicity receptors regulate multiple myeloma cell recognition by natural killer cells. Blood (2005) 105:251–8. doi: 10.1182/blood-2004-04-1422
76. Leivas A, Valeri A, Cordoba L, Garcia-Ortiz A, Ortiz A, Sanchez-Vega L, et al. NKG2D-CAR-transduced natural killer cells efficiently target multiple myeloma. Blood Cancer J (2021) 11:146. doi: 10.1038/s41408-021-00537-w
77. Du Z, Ng YY, Zha S, Wang S. piggyBac system to co-express NKG2D CAR and IL-15 to augment the in vivo persistence and anti-AML activity of human peripheral blood NK cells. Mol Ther Methods Clin Dev (2021) 23:582–96. doi: 10.1016/j.omtm.2021.10.014
78. Daher M, Melo Garcia L, Li Y, Rezvani K. CAR-NK cells: the next wave of cellular therapy for cancer. Clin Transl Immunol (2021) 10:e1274. doi: 10.1002/cti2.1274
79. Zhang C, Burger MC, Jennewein L, Genssler S, Schonfeld K, Zeiner P, et al. ErbB2/HER2-specific NK cells for targeted therapy of glioblastoma. J Natl Cancer Inst (2016) 108:djv375. doi: 10.1093/jnci/djv375
80. Han J, Chu J, Keung Chan W, Zhang J, Wang Y, Cohen JB, et al. CAR-engineered NK cells targeting wild-type EGFR and EGFRvIII enhance killing of glioblastoma and patient-derived glioblastoma stem cells. Sci Rep (2015) 5:11483. doi: 10.1038/srep11483
81. Murakami T, Nakazawa T, Natsume A, Nishimura F, Nakamura M, Matsuda R, et al. Novel human NK cell line carrying CAR targeting EGFRvIII induces antitumor effects in glioblastoma cells. Anticancer Res (2018) 38:5049–56. doi: 10.21873/anticanres.12824
82. Nakazawa T, Murakami T, Natsume A, Nishimura F, Morimoto T, Matsuda R, et al. KHYG-1 cells with EGFRvIII-specific CAR induced a pseudoprogression-like feature in subcutaneous tumors derived from glioblastoma-like cells. Anticancer Res (2020) 40:3231–7. doi: 10.21873/anticanres.14304
83. Muller N, Michen S, Tietze S, Topfer K, Schulte A, Lamszus K, et al. Engineering NK cells modified with an EGFRvIII-specific chimeric antigen receptor to overexpress CXCR4 improves immunotherapy of CXCL12/SDF-1alpha-secreting glioblastoma. J Immunother (2015) 38:197–210. doi: 10.1097/CJI.0000000000000082
84. Genssler S, Burger MC, Zhang C, Oelsner S, Mildenberger I, Wagner M, et al. Dual targeting of glioblastoma with chimeric antigen receptor-engineered natural killer cells overcomes heterogeneity of target antigen expression and enhances antitumor activity and survival. Oncoimmunology (2016) 5:e1119354. doi: 10.1080/2162402X.2015.1119354
85. Schonfeld K, Sahm C, Zhang C, Naundorf S, Brendel C, Odendahl M, et al. Selective inhibition of tumor growth by clonal NK cells expressing an ErbB2/HER2-specific chimeric antigen receptor. Mol Ther (2015) 23:330–8. doi: 10.1038/mt.2014.219
86. Liu H, Yang B, Sun T, Lin L, Hu Y, Deng M, et al. Specific growth inhibition of ErbB2expressing human breast cancer cells by genetically modified NK92 cells. Oncol Rep (2015) 33:95–102. doi: 10.3892/or.2014.3548
87. Liu Y, Zhou Y, Huang KH, Fang X, Li Y, Wang F, et al. Targeting epidermal growth factor-overexpressing triple-negative breast cancer by natural killer cells expressing a specific chimeric antigen receptor. Cell Prolif (2020) 53:e12858. doi: 10.1111/cpr.12858
88. Sahm C, Schonfeld K, Wels WS. Expression of IL-15 in NK cells results in rapid enrichment and selective cytotoxicity of gene-modified effectors that carry a tumor-specific antigen receptor. Cancer Immunol Immunother (2012) 61:1451–61. doi: 10.1007/s00262-012-1212-x
89. Hu Z. Tissue factor as a new target for CAR-NK cell immunotherapy of triple-negative breast cancer. Sci Rep (2020) 10:2815. doi: 10.1038/s41598-020-59736-3
90. Lin YZ, Lee CC, Cho DY, Wang YL, Chen CY, Weng CY, et al. Suppression of breast cancer cells resistant to a pure anti-estrogen with CAR-transduced natural killer cells. Am J Cancer Res (2021) 11:4455–69.
91. Jan CI, Huang SW, Canoll P, Bruce JN, Lin YC, Pan CM, et al. Targeting human leukocyte antigen G with chimeric antigen receptors of natural killer cells convert immunosuppression to ablate solid tumors. J Immunother Cancer (2021) 9:e003050. doi: 10.1136/jitc-2021-003050
92. Klapdor R, Wang S, Morgan M, Dork T, Hacker U, Hillemanns P, et al. Characterization of a novel third-generation anti-CD24-CAR against ovarian cancer. Int J Mol Sci (2019) 20:660-74. doi: 10.3390/ijms20030660
93. Klapdor R, Wang S, Morgan MA, Zimmermann K, Hachenberg J, Buning H, et al. NK cell-mediated eradication of ovarian cancer cells with a novel chimeric antigen receptor directed against CD44. Biomedicines (2021) 9:1339-52. doi: 10.3390/biomedicines9101339
94. Klapdor R, Wang S, Hacker U, Buning H, Morgan M, Dork T, et al. Improved killing of ovarian cancer stem cells by combining a novel chimeric antigen receptor-based immunotherapy and chemotherapy. Hum Gene Ther (2017) 28:886–96. doi: 10.1089/hum.2017.168
95. Li Y, Hermanson DL, Moriarity BS, Kaufman DS. Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell (2018) 23:181–92.e5. doi: 10.1016/j.stem.2018.06.002
96. Cao B, Liu M, Wang L, Liang B, Feng Y, Chen X, et al. Use of chimeric antigen receptor NK-92 cells to target mesothelin in ovarian cancer. Biochem Biophys Res Commun (2020) 524:96–102. doi: 10.1016/j.bbrc.2020.01.053
97. Ao X, Yang Y, Li W, Tan Y, Guo W, Ao L, et al. Anti-alphaFR CAR-engineered NK-92 cells display potent cytotoxicity against alphaFR-positive ovarian cancer. J Immunother (2019) 42:284–96. doi: 10.1097/CJI.0000000000000286
98. Bahr O, Herrlinger U, Weller M, Steinbach JP. Very late relapses in glioblastoma long-term survivors. J Neurol (2009) 256:1756–8. doi: 10.1007/s00415-009-5167-6
99. Debinski W, Gibo DM, Hulet SW, Connor JR, Gillespie GY. Receptor for interleukin 13 is a marker and therapeutic target for human high-grade gliomas. Clin Cancer Res (1999) 5:985–90.
100. Bigner SH, Humphrey PA, Wong AJ, Vogelstein B, Mark J, Friedman HS, et al. Characterization of the epidermal growth factor receptor in human glioma cell lines and xenografts. Cancer Res (1990) 50:8017–22.
101. Ekstrand AJ, Sugawa N, James CD, Collins VP. Amplified and rearranged epidermal growth factor receptor genes in human glioblastomas reveal deletions of sequences encoding portions of the n- and/or c-terminal tails. Proc Natl Acad Sci U.S.A. (1992) 89:4309–13. doi: 10.1073/pnas.89.10.4309
102. Koka V, Potti A, Forseen SE, Pervez H, Fraiman GN, Koch M, et al. Role of her-2/neu overexpression and clinical determinants of early mortality in glioblastoma multiforme. Am J Clin Oncol (2003) 26:332–5. doi: 10.1097/01.COC.0000020922.66984.E7
103. Newman JP, Wang GY, Arima K, Guan SP, Waters MR, Cavenee WK, et al. Interleukin-13 receptor alpha 2 cooperates with EGFRvIII signaling to promote glioblastoma multiforme. Nat Commun (2017) 8:1913. doi: 10.1038/s41467-017-01392-9
104. Nishikawa R, Ji XD, Harmon RC, Lazar CS, Gill GN, Cavenee WK, et al. A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity. Proc Natl Acad Sci U.S.A. (1994) 91:7727–31. doi: 10.1073/pnas.91.16.7727
105. Nagane M, Levitzki A, Gazit A, Cavenee WK, Huang HJ. Drug resistance of human glioblastoma cells conferred by a tumor-specific mutant epidermal growth factor receptor through modulation of bcl-XL and caspase-3-like proteases. Proc Natl Acad Sci U.S.A. (1998) 95:5724–9. doi: 10.1073/pnas.95.10.5724
106. Hynes NE, MacDonald G. ErbB receptors and signaling pathways in cancer. Curr Opin Cell Biol (2009) 21:177–84. doi: 10.1016/j.ceb.2008.12.010
107. Krenciute G, Prinzing BL, Yi Z, Wu MF, Liu H, Dotti G, et al. Transgenic expression of IL15 improves antiglioma activity of IL13Ralpha2-CAR T cells but results in antigen loss variants. Cancer Immunol Res (2017) 5:571–81. doi: 10.1158/2326-6066.CIR-16-0376
108. Pituch KC, Miska J, Krenciute G, Panek WK, Li G, Rodriguez-Cruz T, et al. Adoptive transfer of IL13Ralpha2-specific chimeric antigen receptor T cells creates a pro-inflammatory environment in glioblastoma. Mol Ther (2018) 26:986–95. doi: 10.1016/j.ymthe.2018.02.001
109. Yin Y, Boesteanu AC, Binder ZA, Xu C, Reid RA, Rodriguez JL, et al. Checkpoint blockade reverses anergy in IL-13Ralpha2 humanized scFv-based CAR T cells to treat murine and canine gliomas. Mol Ther Oncolytics (2018) 11:20–38. doi: 10.1016/j.omto.2018.08.002
110. Xu C, Bai Y, An Z, Hu Y, Zhang C, Zhong X. IL-13Ralpha2 humanized scFv-based CAR-T cells exhibit therapeutic activity against glioblastoma. Mol Ther Oncolytics (2022) 24:443–51. doi: 10.1016/j.omto.2022.01.002
111. Thokala R, Binder ZA, Yin Y, Zhang L, Zhang JV, Zhang DY, et al. High-affinity chimeric antigen receptor with cross-reactive scFv to clinically relevant EGFR oncogenic isoforms. Front Oncol (2021) 11:664236. doi: 10.3389/fonc.2021.664236
112. Abbott RC, Verdon DJ, Gracey FM, Hughes-Parry HE, Iliopoulos M, Watson KA, et al. Novel high-affinity EGFRvIII-specific chimeric antigen receptor T cells effectively eliminate human glioblastoma. Clin Transl Immunol (2021) 10:e1283. doi: 10.1002/cti2.1283
113. Agliardi G, Liuzzi AR, Hotblack A, De Feo D, Nunez N, Stowe CL, et al. Intratumoral IL-12 delivery empowers CAR-T cell immunotherapy in a pre-clinical model of glioblastoma. Nat Commun (2021) 12:444. doi: 10.1038/s41467-020-20599-x
114. Ahmed N, Salsman VS, Kew Y, Shaffer D, Powell S, Zhang YJ, et al. HER2-specific T cells target primary glioblastoma stem cells and induce regression of autologous experimental tumors. Clin Cancer Res (2010) 16:474–85. doi: 10.1158/1078-0432.CCR-09-1322
115. Huang X, Qiu M, Wang T, Li B, Zhang S, Zhang T, et al. Carrier-free multifunctional nanomedicine for intraperitoneal disseminated ovarian cancer therapy. J Nanobiotechnol (2022) 20:93. doi: 10.1186/s12951-022-01300-4
116. Menier C, Rabreau M, Challier JC, Le Discorde M, Carosella ED, Rouas-Freiss N. Erythroblasts secrete the nonclassical HLA-G molecule from primitive to definitive hematopoiesis. Blood (2004) 104:3153–60. doi: 10.1182/blood-2004-03-0809
117. Cirulli V, Zalatan J, McMaster M, Prinsen R, Salomon DR, Ricordi C, et al. The class I HLA repertoire of pancreatic islets comprises the nonclassical class ib antigen HLA-G. Diabetes (2006) 55:1214–22. doi: 10.2337/db05-0731
118. Anna F, Bole-Richard E, LeMaoult J, Escande M, Lecomte M, Certoux JM, et al. First immunotherapeutic CAR-T cells against the immune checkpoint protein HLA-G. J Immunother Cancer (2021) 9:e001998. doi: 10.1136/jitc-2020-001998
Keywords: chimeric antigen receptor, T cells, natural killer cells, immunotherapy, malignancies
Citation: Li H, Song W, Li Z and Zhang M (2022) Preclinical and clinical studies of CAR-NK-cell therapies for malignancies. Front. Immunol. 13:992232. doi: 10.3389/fimmu.2022.992232
Received: 12 July 2022; Accepted: 13 October 2022;
Published: 24 October 2022.
Edited by:
Catherine Sautes-Fridman, INSERM U1138 Centre de Recherche des Cordeliers (CRC), FranceReviewed by:
Torsten Tonn, Technical University Dresden, GermanyCopyright © 2022 Li, Song, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingzhi Zhang, bWluZ3poaV96aGFuZzFAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.