- 1Department of Comprehensive Chemotherapy/Head and Neck Cancer, Hunan Cancer Hospital, The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, China
- 2Department of Pain Management and Anesthesiology, The Second Xiangya Hospital, Central South University, Changsha, China
- 3Center for Experimental Medicine, Third Xiangya Hospital of Central South University, Changsha, China
- 4Department of Oncologic Chemotheraphy, Affiliated Haikou Hospital of Xiangya Medical College, Central South University, Haikou, China
Head and neck cancer has high heterogeneity with poor prognosis, and emerging researches have been focusing on the prognostic markers of head and neck cancer. PD-L1 expression is an important basis for strategies of immunosuppressive treatment, but whether it has prognostic value is still controversial. Although meta-analysis on PD-L1 expression versus head and neck cancer prognosis has been performed, the conclusions are controversial. Since PD-L1 and PD-L2 are two receptors for PD-1, here we summarize and analyze the different prognostic values of PD-1, PD-L1, and PD-L2 in head and neck cancer in the context of different cell types, tissue localization and protein forms. We propose that for head and neck cancer, the risk warning value of PD-1/PD-L1 expression in precancerous lesions is worthy of attention, and the prognostic value of PD-L1 expression at different subcellular levels as well as the judgment convenience of prognostic value of PD-1, PD-L1, PD-L2 should be fully considered. The PD-L1 evaluation systems established based on immune checkpoint inhibitors (ICIs) are not fully suitable for the evaluation of PD-L1 prognosis in head and neck cancer. It is necessary to establish a new PD-L1 evaluation system based on the prognosis for further explorations. The prognostic value of PD-L1, PD-L2 expression in head and neck cancer may be different for early-stage and late-stage samples, and further stratification is required.
1 Introduction
Head and neck cancer (HNC) refers to tumors that occur in the lips, oral cavity, pharynx, larynx, and paranasal sinuses; occult primary cancers, salivary gland cancers and mucosal melanomas also deserve attention (1). About 90% head and neck cancers originating in the oral cavity, larynx, pharynx (hypopharynx, nasopharynx, or oropharynx) and sinus tract are squamous cell carcinoma (SCC) in pathological type (2, 3). There are approximately 880,000 new cases of head and neck squamous cell carcinoma (HNSCC) and more than 440,000 deaths worldwide annually (4). Therefore, prognostic judgement of HNC is helpful to disease prevention and treatment.
The PD-1/PD-L1 axis plays an important role in HNC therapy (5, 6). Among them, PD-L1 is not only a guidance for the use of immune checkpoint inhibitors (ICIs) (7), but also a potential prognostic indicator for head and neck cancer. Although studies on the prognosis of PD-L1 expression in head and neck cancer emerge one after another, the conclusions are controversial, and even the conclusions of some meta-analyses including large sample data are inconsistent (8–14). Meanwhile, PD-L2 is another important receptor of PD-1, and its binding affinity to PD-1 is about 2-6 times higher than that of PD-L1 (15). We have noticed a gradual increase in research on the prognostic value of PD-1 (CD279) and PD-L2 in HNC in recent years.
In terms of tumor development process, PD-1 and PD-L1 are also expressed in HNC precancerous lesions (16). From the perspective of subcellular localization of PD-L1 in tumor cells, there are membrane PD-L1, cytoplasmic PD-L1, and nuclear PD-L1 respectively (17). In terms of the distribution of tumor microenvironment at the cellular level, PD-1, PD-L1 and PD-L2 are not only expressed in tumor cells, but also in immune cells (18–21). In terms of protein forms, there are soluble PD-1 and PD-L1, and exosomal PD-L1, etc. (17). With the deepening of research, the prognostic value of PD-1, PD-L1 and PD-L2 indifferent localization and forms in head and neck cancer is not completely consistent. In the present review, we will comprehensively summarize the prognostic value of PD-1, PD-L1 and PD-L2 expression in HNC from different cellular localizations and forms mentioned above.
2 Precancerous lesions
In the precancerous lesions of laryngeal cancer, such as respiratory papilloma (22), actinic cheilitis (AC) (23), and oral leukoplakia (24), the expressions of both PD-1 and PD-L1 are up-regulated. There were differences in PD-1 and PD-L1 expression in the epithelium (E) and sub-epithelial (S) of oral lichen planus (OLP) with malignant transformation within 5 years, and increased PD-L1 levels were significantly associated with malignant transformation within 5 years. Studies have suggested that immune regulation through the PD-L1/PD-1 pathway occurs before the malignant transformation of oral precancerous lesions (16, 25). In a systematic review and meta-analysis of PD-L1 expression in head and neck precancerous lesions, PD-L1 appeared to be more frequently expressed in precancerous lesions than in normal mucosa, but to be less frequently expressed than in cancer lesions of invasive squamous cell carcinoma (26).
3 Subcellular level localization
The commonly used systems for detecting PD-L1 are 22C3, SP263, 28-8, SP142 and 73-10. Although these detection systems use different cut-off values in the indications for ICI use (27), the staining targets are basically the same in both the cell membrane and endomembrane system of tumor cell (TC) and immune cell (IC) (28). PD-L1 positivity was defined as any partial or complete membrane staining for tumor cells, and both membrane and cytoplasmic staining for mononuclear inflammatory cells (lymphocytes and macrophages) (28). In both radioresistant (RR) and radiosensitive (RS) HNSCC cell lines, strong PD-L1 expression was found in the nuclear and cytoplasmic fractions of RR cancer cell lines, and PD-L1 was decreased in the nuclear fraction after irradiation but increased in the cytoplasmic fraction (29). This suggests that the nuclear localization of PD-L1 may reflect the disease prognosis by affecting the radiosensitivity.
4 Cell and tissue-level localization
4.1 Cell-level localization
In EBV-positive nasopharyngeal carcinoma (NPC), high PD-L1 expression on IC and TC is an independent favorable prognostic factor for overall survival (30). Also, PD-L1 expression in TCs is a favorable prognostic factor in NPC patients with pre-existing TILs (31). Among young patients with oral cavity squamous cell carcinoma (OCSCC), those with higher membrane PD-L1 positivity and the presence of TIL had a reduced risk of recurrence and improved survival (32).
4.1.1 Tumor cells
In NPC, the patients with positive PD-1 staining on TC have a longer OS and progression-free survival (PFS), which is an independent prognostic factor for PFS (33). PD-1 mRNA over-expression in tumor tissue is associated with good prognosis in HNSCC patients treated with primary surgery, and low PD-1 mRNA levels are associated with the high risk of recurrence (34). In addition, in laryngeal squamous cell carcinoma (LSCC), the higher the expression of PD-1 in tumor tissue, the larger the tumor diameter (35).
In NPC patients, the uptake of 18F-FDG (18F-fluorodeoxyglucose) into NPC lesions was positively correlated with PD-L1 expression in TCs (36), the expression of PD-L1 in TC is positively correlated with T staging (37), and high PD-L1 expression is significantly associated with poor OS (38, 39) and DFS (40–42). The patients with concurrent chemoradiotherapy with high PD-L1 expression are more prone to complete response (CR) (43), allowing these patients to have a longer local-regional failure-free survival (LRFFS) time and slightly longer progression-free survival (PFS) (44). But it has also been suggested that increased PD-L1 expression is significantly associated with local failure after radiotherapy (45). Overall, PD-L1 over-expression in TCs of NPC is generally considered to be a poor prognostic factor. In oral squamous cell carcinoma (OSCC), PD-L1 expression in more than 10% of TCs was associated with tumor recurrence and lower disease-specific survival (46), and when the PD-L1 staining threshold was not considered, PD-L1 expression was positively correlated with the tumor size (47), lymph node metastasis at diagnosis, and overall tumor-related death (48). In the subgroup with oral submucous fibrosis (OSF), high PD-L1 expression resulted in a worse prognosis (49). Studies suggest that the prognostic significance of TC PD-L1 expression in oral cancer depends on the specificity of tumor site (50); PD-L1 upregulation in tongue squamous cell carcinoma (TSCC) is associated with a higher recurrence rate after tongue cancer surgery (51), later TNM staging and shorter PFS (52); but it was not correlated with OS (53). In SGCs, PD-L1 expression of TCs is positively correlated with tumor staging (54), and correlated with lymph node (LN) metastasis (55), postoperative recurrence and metastasis (56), and poor DFS (57). Compared with the PD-L1 low-expressing group, the OS of PD-L1 high-expressing cases was significantly shortened (56, 58), however, there was also opposite conclusion reported (59). In oral mucoepidermoid carcinomas (MECs), except that PD-L1 expression was positively correlated with histological grade, no relationship was observed between immunosuppressive proteins and other clinic pathological parameters (60). When PD-L1/PD-1 is co-expressed on TC, it can lead to NPC (33, 61), OSCC (62), TSCC (63), and hypopharyngeal carcinoma with poor prognosis (64), but there are still opposite observation (65).
PD-L2 is expressed in both the TC and stroma of HNSCC (66), and in a cohort of operable HNSCC patients, 62.7% of HNSCC tumors show positive staining of PD-L2, revealing PD-L2 an independent predictor of shorter OS (67). OSCC patients with higher PD-L2 score had a significantly worse prognosis (47, 68). PD-L2 over-expression is common in SGC, and it is associated with a decrease in disease-specific survival (DSS) and disease-free survival (DFS) (69), but there are also opposite conclusions reported (70).
4.1.2 Immune cells
High percentage of PD-1-positivetumor-associated immune cells (TAICs) is an independent positive prognostic marker in oral OSCC (71). In oropharyngeal and hypopharyngeal carcinomas, Expression of PD-1 in tumor infiltrating lymphocyte (TIL) is significantly correlated with better overall survival (OS) and disease-free survival (DFS) (72). Nevertheless, in salivary gland carcinomas (SGC), conclusions of the prognosis judgment by the expression of PD-1 on TILs remains consistent (73, 74).
In NPC, the uptake of 18F-FDG (18F-fluorodeoxyglucose) into NPC lesions was negatively correlated with PD-L1 expression in tumor infiltrating immune cells (TIICs) (36), PD-L1 expression on TIL was negatively correlated with plasma EBV (Epstein-Barr virus) DNA load, N staging, M staging and clinical staging (37); It was also associated with significantly improved 1 and 2-year survival rates (75), and was positively correlated with the 5-year PFS rate (76). In EBV-positive NPCs, low PD-L1 expression in IC is an independent poor prognostic factor for DFS (30). However, in NPC patients with different ethnic backgrounds, PD-L1 expression in TILs had diametrically opposite effects on DFS (41, 42).
Further large-scale researches are needed to explore both the prognostic value of PD-L1 expression of TIL in OSCC (50, 77), and prognostic value of PD-L1 expression in tumor infiltrating mononuclear cells (TIMC) in salivary duct carcinoma (SDC) (56, 58). In TSCC, patients with high expression of PD-L1 in lymphocytes had better OS and RFS (78). PD-L1 expression in IC indicates better prognosis in LSCC and HPV-negative HNC (79).
4.2 Tissue-level localization
Approximately 19-46% of HNSCC patients have lymph node metastases at diagnosis (80, 81). In specimens of HNSCC primary cancer and corresponding lymph node metastases, PD-L1 expression in lymph node metastases was found to be significantly associated with decreased OS and DFS during oral chemotherapy treatment (72). With the development of separation technology of circulating tumor cells (CTCs) (82), the acquisition of CTCs has become more convenient. Although the expression of PD-L1 in tumor tissues is not completely consistent with the expression in CTCs (83), there are still studies suggesting that PD-L1 expression in CTCs has prognostic value. In a prospective study of locally advanced HNSCC patients treated with curative intent, the researchers have found that at the end of induction chemotherapy and concurrent chemotherapy, patients with PD-L1 mRNA over-expression in EpCAM(+) CTCs had shorter PFS and OS, so PD-L1 expression in EpCAM(+) CTCs is an independent prognostic factor for PFS and OS (84). In OSCC, high expression of PD-L1 in the CTC cytoplasm correlates with tumor size and LN metastasis and is an independent positive prognostic factor (85). Additionally, PD-L1 mRNA expression in peripheral blood may be an indicator of the presence of metastatic disease (N+) in OSCC (86) and has shown poor survival rate (87).
4.3 The prognostic value of PD-L1 evaluation systems
Currently, commonly used PD-L1 evaluation systems include tumor cell positive proportion score (i.e. tumor proportion score, TPS), combined positive score (CPS), and immune cell positive proportion score (IPS, IC score, etc.). Among the SGCs of unlimited pathological types, TPS and CPS were associated with histological grade, TPS-positive patients had poorer PFS and OS, while TPS was an independent prognostic factor (88); but CPS and IC scores had no effect on DFS or OS (54). In specific pathological types of SGC, IC score was associated with poor prognosis of PFS and OS (89). In LSCC, compared with CPS ≤ 1, CPS≥1 was correlated with lower postoperative recurrence rate (90) and longer DFS (91).
5 Different protein forms
5.1 Soluble protein
Soluble proteins are circulating proteins produced from alternative splicing of membrane proteins (92). In ICI-unresponsive patients, serum concentrations of soluble PD-L1 were significantly higher than ICI-responsive ones (93). In NPC, sPD-L1 expression is positively correlated with clinical staging (94), patients with high sPD-1 have a longer survival than those with low sPD-1 (95), but some scholars have suggested that sPD-1is not associated with prognosis (96). Soluble PD-1 and PD-L1 in peripheral blood of patients with recurrent/metastatic head and neck cell carcinoma (R/M HNSCC) did not affect prognosis (92). In OSCC, sPD-L1 expression correlates with clinical staging, tumor cell differentiation and lymph node status (97).
5.2 Exosomal proteins
With the rapid development of exosome and PD-L1 detection technologies (98), in animal models of HNSCC, exosomal PD-L1 levels may reflect the efficacy of antitumor drugs to a certain extent (99). In a phase I clinical trial of cetuximab, ipilimumab and radiation therapy, PD-L1(+) exosomes increased from baseline in patients with disease relapse (100). In a study by the International Union for Cancer Control (UICC), exosomal PD-L1 and COX-2 levels were higher in HNSCC stage III/IV patients than in stage I/II patients (101).
6 Combination indicators
The heterogeneity of HNC is strong, and the combination of PD-L1 with different localization and forms with other indicators helps to improve its strength as a prognostic indicator (102), as summarized in Table 1.
7 Summary and outlook
In conclusion, it is of great significance to further expand the knowledge of the prognostic value of PD-1 and its receptors PD-L1 and PD-L2 in head and neck cancer. We summarize and analyze the different localizations and forms of PD-1, PD-L1 and PD-L2 with prognostic value in head and neck cancer, as shown in Figure 1. Interestingly, PD-1/PD-L1 expression in precancerous lesions may be a prognostic indicator for assessing the risk of malignant progression in precancerous lesions. Considering the effect of PD-L1 localization at different subcellular levels on radiosensitivity, the prognostic value of PD-L1 expression at the subcellular level deserves further exploration. At the same time, in terms of the convenience of judging the prognostic value of PD-1/PD-L1, it is necessary to expand the sample size to explore the possibility of prognostic value of PD-1/PD-L1/PD-L2 associated exosomes and related indicators. So far, the commonly used PD-L1evaluation systems (TPS, CPS, etc.) are based on the needs of ICI treatment. Considering the intratumor heterogeneity of PD-L1 expression (126), the sensitivity of the evaluation system (127, 128), the influence of factors such as specimens of different sources (27) on the expression of PD-L1, and that the expression pattern of specific immune checkpoint in HNSCC patients may also promote the development and efficacy of immunotherapy (129), all these urge us to pay attention to the importance of the expression pattern of PD-1/PD-L1 rather than to singly explore the expression level of PD-1/PD-L1. Some researchers reported that in OSCC, PD-L1 was expressed in a patchy or diffuse pattern in TCs by immunohistochemical method, and proposed that a mottled pattern was an independent risk factor for overall survival (130). Referring to this idea, if the prognostic value of PD-1/PD-L1 is to be more accurately described, new advanced prognostic evaluation criteria for PD-1/PD-L1 will be established.
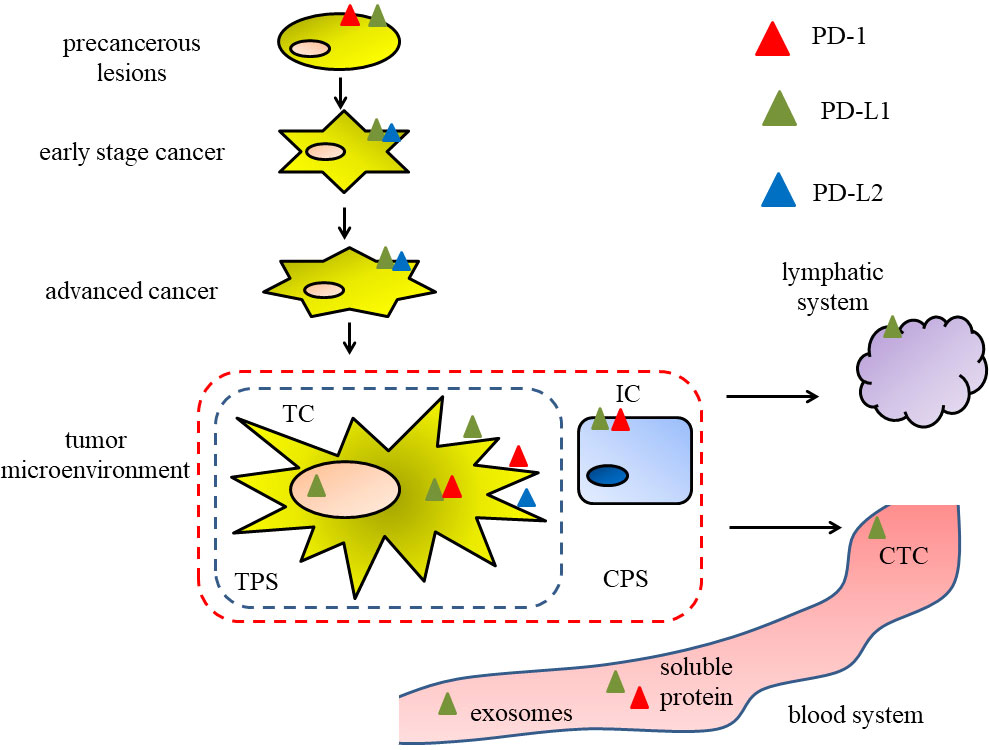
Figure 1 Figure 1 The different localizations and forms of PD-1, PD-L1 and PD-L2 in different stages of head and neck cancer, associating with the prognostic value.
We also note that although PD-L1 expression on TCs is an unfavorable prognostic factor in OSCC, in locally advanced oral squamous cell carcinoma (LAOSCC), positive PD-L1 expression is significantly associated with higher disease-free survival and overall survival in patients (131). In operable HNSCC patients, PD-L1 positivity in TC was an independent factor for poor PFS in young patients (<45 years) (132), and is also an independent risk factor for OS and DFS (133, 134). However, in R/M HNSCC treated with standard-of-care chemotherapy, PD-L1 expression in TC was not considered to predict the patient’s OS (135). Similarly, although PD-L2 expression in TC was an independent prognostic factor for poor OS in operable HNSCC, in a small clinical study of R/M HNSCC, the PFS and the median time for overall survival in patients with positive PD-L2 expression were significantly longer than PD-L2-negative patients (20). The difference between the above conclusions suggests that the prognostic value of specimens obtained at different disease stages is different even if the same indicators are detected.
In conclusion, PD-1, PD-L1 and PD-L2 may have different prognostic values in head and neck cancer considering their expression in different cell types, their tissue localization and their different protein forms. The clinical data, association and mechanisms of PD-1, PD-L1 and PD-L2 prognostic values in head and neck cancer deserve further studies.
Author contributions
SJ wrote the article, XL provided drawing support, LH provided language editing assistance, ZX revised the article, and JL provided the publication fee. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pfister DG, Spencer S, Adelstein D, Adkins D, Anzai Y, Brizel DM, et al. Head and neck cancers, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl ComprCancNetw (2020) 18(7):873–98. doi: 10.6004/jnccn.2020.0031
2. Cohen N, Fedewa S, Chen AY. Epidemiology and demographics of the head and neck cancer population. Oral MaxillofacSurgClin North Am (2018) 30(4):381–95. doi: 10.1016/j.coms.2018.06.001
3. Johnson DE, Burtness B, Leemans CR, Leemans CR, Lui V, Bauman JE, et al. Head and neck squamous cell carcinoma. Nat Rev Dis Primers (2020) 6(1):92. doi: 10.1038/s41572-020-00224-3
4. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
5. Siu LL, Even C, Mesia R, Remenar E, Daste A, Delord JP, et al. Safety and efficacy of durvalumab with or without tremelimumab in patients with PD-L1-Low/Negative recurrent or metastatic HNSCC: The phase 2 CONDOR randomized clinical trial. JAMA Oncol (2019) 5(2):195–203. doi: 10.1001/jamaoncol.2018.4628
6. Powell SF, Gold KA, Gitau MM, Sumey CJ, Lohr MM, McGraw SC, et al. Safety and efficacy of pembrolizumab with chemoradiotherapy in locally advanced head and neck squamous cell carcinoma: A phase IB study. J ClinOncol (2020) 38(21):2427–37. doi: 10.1200/JCO.19.03156
7. Burtness B, Rischin D, Greil R, Soulieres D, Tahara M, de Castro GJ, et al. Pembrolizumab alone or with chemotherapy for Recurrent/Metastatic head and neck squamous cell carcinoma in KEYNOTE-048: Subgroup analysis by programmed death ligand-1 combined positive score. J ClinOncol (2022) 40 (21):2321–32, O2102198. doi: 10.1200/JCO.21.02198
8. He J, Chen XF, Xu MG, Zhao J. Relationship of programmed death ligand-1 expression with clinicopathological features and prognosis in patients with oral squamous cell carcinoma: A meta-analysis. Arch Oral Biol (2020) 114:104717. doi: 10.1016/j.archoralbio.2020.104717
9. Cui YX, Su XS. Clinicopathological features of programmed cell death-ligand 1 expression in patients with oral squamous cell carcinoma. Open Med (Wars) (2020) 15:292–301. doi: 10.1515/med-2020-0041
10. Tang H, Zhou X, Ye Y, Zhou Y, Wu C, et al. The different role of PD-L1 in head and neck squamous cell carcinomas: A meta-analysis. Pathol Res Pract (2020) 216(1):152768. doi: 10.1016/j.prp.2019.152768
11. Polesel J, Menegaldo A, Tirelli G, Giacomarra V, Guerrieri R, Baboci L, et al. Prognostic significance of PD-L1 expression in patients with primary oropharyngeal squamous cell carcinoma: A meta-analysis. Front Oncol (2021) 11:787864. doi: 10.3389/fonc.2021.787864
12. Huang Z, Zheng S, Ding S, Wei Y, Chen C, Liu X, et al. Prognostic role of programmed cell death ligand-1 expression in head and neck cancer treated with programmed cell death protein-1/programmed cell death ligand-1 inhibitors: A meta-analysis based on clinical trials. J Cancer Res Ther (2021) 17(3):676–87. doi: 10.4103/jcrt.JCRT_1606_20
13. Kujan O, van Schaijik B, Farah CS. Immune checkpoint inhibitors in oral cavity squamous cell carcinoma and oral potentially malignant disorders: A systematic review. Cancers (Basel) (2020) 12(7):1937. doi: 10.3390/cancers12071937
14. Patel JJ, Levy DA, Nguyen SA, Knochelmann HM, Day T. Impact of PD-L1 expression and human papillomavirus status in anti-PD1/PDL1 immunotherapy for head and neck squamous cell carcinoma-systematic review and meta-analysis. Head Neck (2020) 42(4):774–86. doi: 10.1002/hed.26036
15. Youngnak P, Kozono Y, Kozono H, Iwai H, Otsuki N, Jin H, et al. Differential binding properties of B7-H1 and B7-DC to programmed death-1. BiochemBiophys Res Commun (2003) 307(3):672–7. doi: 10.1016/S0006-291X(03)01257-9
16. Dave K, Ali A, Magalhaes M. Increased expression of PD-1 and PD-L1 in oral lesions progressing to oral squamous cell carcinoma: a pilot study. Sci Rep (2020) 10(1):9705. doi: 10.1038/s41598-020-66257-6
17. Ying H, Zhang X, Duan Y, Lao M, Xu J, Yang H, et al. Non-cytomembrane PD-L1: An atypical target for cancer. Pharmacol Res (2021) 170:105741. doi: 10.1016/j.phrs.2021.105741
18. Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J (1992) 11(11):3887–95. doi: 10.1002/j.1460-2075.1992.tb05481.x
19. Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol (2018) 18(3):153–67. doi: 10.1038/nri.2017.108
20. Yearley JH, Gibson C, Yu N, Moon C, Murphy E. PD-L2 expression in human tumors: Relevance to anti-PD-1 therapy in cancer. Clin Cancer Res (2017) 23(12):3158–67. doi: 10.1158/1078-0432.CCR-16-1761
21. Zhang Y, Xu J, Hua J, Liu J, Liang C, Meng Q, et al. A PD-L2-based immune marker signature helps to predict survival in resected pancreatic ductal adenocarcinoma. J Immunother Cancer (2019) 7(1):233. doi: 10.1186/s40425-019-0703-0
22. Hatam LJ, Devoti JA, Rosenthal DW, Lam F, Abramson AL, Steinberg BM, et al. Immune suppression in premalignant respiratory papillomas: enriched functional CD4+Foxp3+ regulatory T cells and PD-1/PD-L1/L2 expression. Clin Cancer Res (2012) 18(7):1925–35. doi: 10.1158/1078-0432.CCR-11-2941
23. Malaspina TS, Gasparoto TH, Costa MR, de Melo EJ, Ikoma MR, Damante JH, et al. Enhanced programmed death 1 (PD-1) and PD-1 ligand (PD-L1) expression in patients with actinic cheilitis and oral squamous cell carcinoma. Cancer ImmunolImmunother (2011) 60(7):965–74. doi: 10.1007/s00262-011-1007-5
24. Das D, Maitra A, Panda CK, Ghose S, Roy B, Sarin R, et al. Genes and pathways monotonically dysregulated during progression from normal through leukoplakia to gingivo-buccal oral cancer. NPJ Genom Med (2021) 6(1):32. doi: 10.1038/s41525-021-00195-8
25. Kujan O, Agag M, Smaga M, Vaishnaw Y, Idrees M, Shearston K, et al. PD-1/PD-L1, treg-related proteins, and tumour-infiltrating lymphocytes are associated with the development of oral squamous cell carcinoma. Pathology (2021) 54(4):409–16. doi: 10.1016/j.pathol.2021.09.013
26. Girolami I, Pantanowitz L, Munari E, Martini M, Nocini R, Bisi N, et al. Prevalence of PD-L1 expression in head and neck squamous precancerous lesions: a systematic review and meta-analysis. Head Neck (2020) 42(10):3018–30. doi: 10.1002/hed.26339
27. Paolino G, Pantanowitz L, Barresi V, et al. PD-L1 evaluation in head and neck squamous cell carcinoma: Insights regarding specimens, heterogeneity and therapy. Pathol Res Pract (2021) 226:153605. doi: 10.1016/j.prp.2021.153605
28. Heidarian A, Wenig BM, Hernandez-Prera JC. Evaluation of programmed death ligand 1 immunohistochemistry in cytology specimens of head and neck squamous cell carcinoma. Cancer Cytopathol (2022) 130(2):91–5. doi: 10.1002/cncy.22500
29. Schulz D, Streller M, Piendl G, Brockhoff G, Reichert TE, Menevse AN, et al. Differential localization of PD-L1 and akt-1 involvement in radioresistant and radiosensitive cell lines of head and neck squamous cell carcinoma. Carcinogenesis (2020) 41(7):984–92. doi: 10.1093/carcin/bgz177
30. Liu YJ, Tsang NM, Hsueh C, Yeh CJ, Ueng SH, Wang TH, et al. Low PD-L1 expression strongly correlates with local recurrence in Epstein-Barr virus-positive nasopharyngeal carcinoma after radiation-based therapy. Cancers (Basel) (2018) 10(10):374. doi: 10.3390/cancers10100374
31. Zhu Q, Cai MY, Chen CL, Hu H, Lin HX, Li M, et al. Tumor cells PD-L1 expression as a favorable prognosis factor in nasopharyngeal carcinoma patients with pre-existing intratumor-infiltrating lymphocytes. Oncoimmunology (2017) 6(5):e1312240. doi: 10.1080/2162402X.2017.1312240
32. Hanna GJ, Woo SB, Li YY, Barletta JA, Hammerman PS, et al. Tumor PD-L1 expression is associated with improved survival and lower recurrence risk in young women with oral cavity squamous cell carcinoma. Int J Oral MaxillofacSurg (2018) 47(5):568–77. doi: 10.1016/j.ijom.2017.09.006
33. Cao C, Wei Q, Tang X, Jia Y, Sun X, Li W, et al. PD-1 and PD-L1 in locoregionally advanced nasopharyngeal carcinoma: Substudy of a randomized phase III trial. Head Neck (2019) 41(5):1427–33. doi: 10.1002/hed.25601
34. Lecerf C, Kamal M, Vacher S, Chemlali W, Schnitzler A, Morel C, et al. Immune gene expression in head and neck squamous cell carcinoma patients. Eur J Cancer (2019) 121:210–23. doi: 10.1016/j.ejca.2019.08.028
35. Kowalski A, Malinowska K, Olszewski J, Zielinska-Blizniewska H, et al. Expression of programmed death receptor 1 (PD-1) gene and its ligand (PD-L1) in patients with laryngeal cancer. Biomolecules (2021) 11(7):970. doi: 10.3390/biom11070970
36. Zhao L, Zhuang Y, Fu K, Chen P, Wang Y, Zhuo J, et al. Usefulness of[(18)F]fluorodeoxyglucose PET/CT for evaluating the PD-L1 status in nasopharyngeal carcinoma. Eur J Nucl Med Mol Imaging (2020) 47(5):1065–74. doi: 10.1007/s00259-019-04654-4
37. Zhao L, Liao X, Hong G, Zhuang Y, Fu K, Chen P, et al. Mismatch repair status and high expression of PD-L1 in nasopharyngeal carcinoma. Cancer Manag Res (2019) 11:1631–40. doi: 10.2147/CMAR.S193878
38. Ngamphaiboon N, Chureemas T, Siripoon T, Arsa L, Trachu N, Jiarpinitnun C, et al. Characteristics and impact of programmed death-ligand 1 expression, CD8+ tumor-infiltrating lymphocytes, and p16 status in head and neck squamous cell carcinoma. Med Oncol (2019) 36(2):21. doi: 10.1007/s12032-018-1241-1
39. Zheng L, Cao C, Cheng G, Hu Q, Chen X, et al. Cytomembranic PD-L1 expression in locoregionally advanced nasopharyngeal carcinoma. Onco Targets Ther (2017) 10:5483–7. doi: 10.2147/OTT.S152007
40. Li YF, Ding JW, Liao LM, Zhang ZL, Liao SS, Wu Y, et al. Expression of programmed death ligand-1 predicts poor outcome in nasopharyngeal carcinoma. MolClinOncol (2017) 7(3):378–82. doi: 10.3892/mco.2017.1318
41. Luo F, Cao J, Lu F, Zeng K, Ma W, Huang Y, et al. Lymphocyte activating gene 3 protein expression in nasopharyngeal carcinoma is correlated with programmed cell death-1 and programmed cell death ligand-1, tumor-infiltrating lymphocytes. Cancer Cell Int (2021) 21(1):458. doi: 10.1186/s12935-021-02162-w
42. Minichsdorfer C, Oberndorfer F, Krall C, Kornek G, Mullauer L, Wagner C, et al. PD-L1 expression on tumor cells is associated with a poor outcome in a cohort of Caucasian nasopharyngeal carcinoma patients. Front Oncol (2019) 9:1334. doi: 10.3389/fonc.2019.01334
43. Long G, Li X, Yang L, Zhao J, Lu X, Wang H, et al. The clinical prognostic value of PD-L1 after concurrent chemoradiotherapy in Chinese nasopharyngeal carcinoma patients. Ann Transl Med (2021) 9(22):1650. doi: 10.21037/atm-21-5175
44. Lee VH, Lo AW, Leung CY, Shek WH, Kwong DL, Lam KO, et al. Correlation of PD-L1 expression of tumor cells with survival outcomes after radical intensity-modulated radiation therapy for non-metastatic nasopharyngeal carcinoma. PLoS One (2016) 11(6):e157969. doi: 10.1371/journal.pone.0157969
45. Skinner HD, Giri U, Yang LP, Kumar M, Liu Y, Story MD, et al. Integrative analysis identifies a novel AXL-PI3 kinase-PD-L1 signaling axis associated with radiation resistance in head and neck cancer. Clin Cancer Res (2017) 23(11):2713–22. doi: 10.1158/1078-0432.CCR-16-2586
46. de Vicente JC, Rodriguez-Santamarta T, Rodrigo J P, Blanco-Lorenzo V, Allonca E, et al. PD-L1 expression in tumor cells is an independent unfavorable prognostic factor in oral squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev (2019) 28(3):546–54. doi: 10.1158/1055-9965.EPI-18-0779
47. Moratin J, Metzger K, Safaltin A, Herpel E, Hoffmann J, Freier K, et al. Upregulation of PD-L1 and PD-L2 in neck node metastases of head and neck squamous cell carcinoma. Head Neck (2019) 41(8):2484–91. doi: 10.1002/hed.25713
48. Straub M, Drecoll E, Pfarr N, Weichert W, Langer R, Hapfelmeier A, et al. CD274/PD-L1 gene amplification and PD-L1 protein expression are common events in squamous cell carcinoma of the oral cavity. Oncotarget (2016) 7(11):12024–34. doi: 10.18632/oncotarget.7593
49. Quan H, Liu S, Shan Z, Liu Z, Chen T, Hu Y, et al. Differential expression of programmed death-1 and its ligand, programmed death ligand-1 in oral squamous cell carcinoma with and without oral submucousfibrosis. Arch Oral Biol (2020) 119:104916. doi: 10.1016/j.archoralbio.2020.104916
50. Adamski LJ, Starzynska A, Adamska P, Kunc M, Sakowicz-Burkiewicz M, Marvaso G, et al. High PD-L1 expression on tumor cells indicates worse overall survival in advanced oral squamous cell carcinomas of the tongue and the floor of the mouth but not in other oral compartments. Biomedicines (2021) 9(9):1132. doi: 10.3390/biomedicines9091132
51. Meehan K, Leslie C, Lucas M, Jacques A, Mirzai B, Lim J, et al. Characterization of the immune profile of oral tongue squamous cell carcinomas with advancing disease. Cancer Med (2020) 9(13):4791–807. doi: 10.1002/cam4.3106
52. Akisada N, Nishimoto K, Takao S, Gion Y, Marunaka H, Tachibana T, et al. PD-L1 expression in tongue squamous cell carcinoma. Med MolMorphol (2021) 54(1):52–9. doi: 10.1007/s00795-020-00261-7
53. Yoshida S, Nagatsuka H, Nakano K, Kogashiwa Y, Ebihara Y, Yano M, et al. Significance of PD-L1 expression in tongue cancer development. Int J Med Sci (2018) 15(14):1723–30. doi: 10.7150/ijms.27860
54. Fang Q, Wu Y, Du W, Zhang X, Chen D, et al. Incidence and prognostic significance of PD-L1 expression in high-grade salivary gland carcinoma. Front Oncol (2021) 11:701181. doi: 10.3389/fonc.2021.701181
55. Kuchar M, Strizova Z, Capkova L, Komarc M, Skrivan J, Bartunkova J, et al. The periphery of salivary gland carcinoma tumors reveals a PD-L1/PD-1 biomarker niche for the evaluation of disease severity and tumor-immune system interplay. Biomedicines (2021) 9(2):97. doi: 10.3390/biomedicines9020097
56. Harada K, Ferdous T, Ueyama Y. PD-L1 expression in malignant salivary gland tumors. BMC Cancer (2018) 18(1):156. doi: 10.1186/s12885-018-4069-3
57. Mukaigawa T, Hayashi R, Hashimoto K, Ugumori T, Hato N, et al. Programmed death ligand-1 expression is associated with poor disease free survival in salivary gland carcinomas. J SurgOncol (2016) 114(1):36–43. doi: 10.1002/jso.24266
58. Sato F, Akiba J, Kawahara A, Naito Y, Ono T, Takase Y, et al. The expression of programed death ligand-1 could be related with unfavorable prognosis in salivary duct carcinoma. J Oral Pathol Med (2018) 47(7):683–90. doi: 10.1111/jop.12722
59. Higashino M, Kawata R, Nishikawa S, Terada T, Haginomori SI, Kurisu Y, et al. Programmed death ligand-1 expression is associated with stage and histological grade of parotid carcinoma. ActaOtolaryngol (2020) 140(2):175–80. doi: 10.1080/00016489.2019.1683604
60. Mosconi C, Arantes D, Goncalves AS, Alencar R, Oliveira JC, Silva TA, et al. Immunohistochemical investigations on the expression of programmed cell death ligand 1, human leukocyte antigens G and e, and granzyme b in intraoral mucoepidermoid carcinoma. Arch Oral Biol (2017) 83:55–62. doi: 10.1016/j.archoralbio.2017.07.004
61. Zhang Y, Chen X, Zheng H, Zhan Y, Luo J, Yang Y, et al. Expression of cancer cell-intrinsic PD-1 associates with PD-L1 and p-S6 and predicts a good prognosis in nasopharyngeal carcinoma. J Cancer (2021) 12(20):6118–25. doi: 10.7150/jca.60739
62. Maruse Y, Kawano S, Jinno T, Matsubara R, Goto Y, Kaneko N, et al. Significant association of increased PD-L1 and PD-1 expression with nodal metastasis and a poor prognosis in oral squamous cell carcinoma. Int J Oral MaxillofacSurg (2018) 47(7):836–45. doi: 10.1016/j.ijom.2018.01.004
63. Naruse T, Yanamoto S, Okuyama K, Ohmori K, Tsuchihashi H, Furukawa K, et al. Immunohistochemical study of PD-1/PD-L1 axis expression in oral tongue squamous cell carcinomas: Effect of neoadjuvant chemotherapy on local recurrence. PatholOncol Res (2020) 26(2):735–42. doi: 10.1007/s12253-019-00606-3
64. Gao Q, Liu HT, Xu YQ, Zhang L, Liu Y R, Ren Q, et al. Serum-derived exosomes promote CD8+ T cells to overexpress PD-1, affecting the prognosis of hypopharyngeal carcinoma. Cancer Cell Int (2021) 21(1):584. doi: 10.1186/s12935-021-02294-z
65. Chen SW, Li SH, Shi DB, Jiang WM, Song M, Yang AK, et al. Expression of PD-1/PD-L1 in head and neck squamous cell carcinoma and its clinical significance. Int J Biol Markers (2019) 34(4):398–405. doi: 10.1177/1724600819884722
66. Baruah P, Bullenkamp J, Wilson P, Lee M, Kaski JC, et al. TLR9 mediated tumor-stroma interactions in human papilloma virus (HPV)-positive head and neck squamous cell carcinoma up-regulate PD-L1 and PD-L2. Front Immunol (2019) 10:1644. doi: 10.3389/fimmu.2019.01644
67. Qiao Y, Liu C, Zhang X, Zhou Q, Li Y, Xu Y, et al. PD-L2 based immune signature confers poor prognosis in HNSCC. Oncoimmunology (2021) 10(1):1947569. doi: 10.1080/2162402X.2021.1947569
68. Furukawa K, Kawasaki G, Yoshida T, Umeda M. Clinicopathological and prognostic analysis of PD-L1 and PD-L2 expression in surgically resected primary tongue squamous cell carcinoma. Anticancer Res (2021) 41(1):101–11. doi: 10.21873/anticanres.14755
69. Nakano T, Takizawa K, Uezato A, Taguchi K, Toh S, et al. Prognostic value of programed death ligand-1 and ligand-2 co-expression in salivary gland carcinomas. Oral Oncol (2019) 90:30–7. doi: 10.1016/j.oraloncology.2019.01.015
70. Chang H, Kim JS, Choi YJ, Cho JG, Woo JS, Kim A, et al. Overexpression of PD-L2 is associated with shorter relapse-free survival in patients with malignant salivary gland tumors. Onco Targets Ther (2017) 10:2983–92. doi: 10.2147/OTT.S134589
71. Kikuchi M, Yamashita D, Hara S, Takebayashi S, Hamaguchi K, Mizuno K, et al. Clinical significance of tumor-associated immune cells in patients with oral squamous cell carcinoma. Head Neck (2021) 43(2):534–43. doi: 10.1002/hed.26498
72. Schneider S, Kadletz L, Wiebringhaus R, Kenner L, Selzer E, Fureder T, et al. PD-1 and PD-L1 expression in HNSCC primary cancer and related lymph node metastasis - impact on clinical outcome. Histopathology (2018) 73(4):573–84. doi: 10.1111/his.13646
73. Xu B, Jungbluth AA, Frosina D, Alzumaili B, Aleynick N, Slodkowska E, et al. The immune microenvironment and expression of PD-L1, PD-1, PRAME and MHC I in salivary duct carcinoma. Histopathology (2019) 75(5):672–82. doi: 10.1111/his.13944
74. Kesar N, Winkelmann R, Oppermann J, Ghanaati S, Martin D, Neumayer, et al. Prognostic impact of CD8-positive tumour-infiltrating lymphocytes and PD-L1 expression in salivary gland cancer. Oral Oncol (2020) 111:104931. doi: 10.1016/j.oraloncology.2020.104931
75. Sahinli H, Akyurek N, Yilmaz M, Kandemir O, Duran A O, Kulacoglu S, et al. PD-L1 expression in immune cells is a favorable prognostic factor for nasopharyngeal carcinoma. Indian J Cancer (2021) 58(4):561–6. doi: 10.4103/ijc.IJC_459_19
76. Hu B, Sun M, Wang Z, Zheng Y, Cai W, Shi HH, et al. Prognostic value of programmed cell death-ligand 1 expression in tumor-infiltrating lymphocytes and viral load in peripheral blood mononuclear cells for Epstein-Barr virus-positive nasopharyngeal carcinoma. Clin Chem (2020) 66(9):1219–27. doi: 10.1093/clinchem/hvaa170
77. Quan H, Shan Z, Liu Z, Liu S, Yang L, Fang X, et al. The repertoire of tumor-infiltrating lymphocytes within the microenvironment of oral squamous cell carcinoma reveals immune dysfunction. Cancer ImmunolImmunother (2020) 69(3):465–76. doi: 10.1007/s00262-020-02479-x
78. Huang W, Zhou X, Liao Q, Tang Y, Zuo L, Wang H, et al. Clinicopathological and prognostic significance of PD-1/PD-L1 axis expression in patients with tongue squamous cell carcinoma. J Cell Physiol (2020) 235(10):6942–53. doi: 10.1002/jcp.29590
79. Birtalan E, Danos K, Gurbi B, Brauswetter D, Halasz J, Kalocsane PV, et al. Expression of PD-L1 on immune cells shows better prognosis in laryngeal, oropharygeal, and hypopharyngeal cancer. ApplImmunohistochemMolMorphol (2018) 26(7):e79–85. doi: 10.1097/PAI.0000000000000590
80. Li Y, Liu K, Ke Y, Zeng Y, Chen M, Li W, et al. Risk factors analysis of pathologically confirmed cervical lymph nodes metastasis in oral squamous cell carcinoma patients with clinically negative cervical lymph node: Results from a cancer center of central China. J Cancer (2019) 10(13):3062–9. doi: 10.7150/jca.30502
81. Ho AS, Kim S, Tighiouart M, Gudino C, Mita A, Scher K, et al. Metastatic lymph node burden and survival in oral cavity cancer. J ClinOncol (2017) 35(31):3601–9. doi: 10.1200/JCO.2016.71.1176
82. Zavridou M, Mastoraki S, Strati A, Koutsodontis G, Klinakis A, Psyrri A, et al. Direct comparison of size-dependent versus EpCAM-dependent CTC enrichment at the gene expression and DNA methylation level in head and neck squamous cell carcinoma. Sci Rep (2020) 10(1):6551. doi: 10.1038/s41598-020-63055-y
83. Chikamatsu K, Tada H, Takahashi H, Kuwabara-Yokobori Y, Ishii H, Ida S, et al. Expression of immune-regulatory molecules in circulating tumor cells derived from patients with head and neck squamous cell carcinoma. Oral Oncol (2019) 89:34–9. doi: 10.1016/j.oraloncology.2018.12.002
84. Strati A, Koutsodontis G, Papaxoinis G, Angelidis I, Zavridou M, Economopoulou P, et al. Prognostic significance of PD-L1 expression on circulating tumor cells in patients with head and neck squamous cell carcinoma. Ann Oncol (2017) 28(8):1923–33. doi: 10.1093/annonc/mdx206
85. Oliveira-Costa JP, de Carvalho AF, Da SDG, Amaya P, Wu Y, Park K J, et al. Gene expression patterns through oral squamous cell carcinoma development: PD-L1 expression in primary tumor and circulating tumor cells. Oncotarget (2015) 6(25):20902–20. doi: 10.18632/oncotarget.3939
86. Weber M, Wehrhan F, Baran C, Agaimy A, Buttner-Herold M, Preidl R, et al. PD-L1 expression in tumor tissue and peripheral blood of patients with oral squamous cell carcinoma. Oncotarget (2017) 8(68):112584–97. doi: 10.18632/oncotarget.22576
87. Weber M, Wehrhan F, Baran C, Agaimy A, Buttner-Herold M, Kesting M, et al. Prognostic significance of PD-L2 expression in patients with oral squamous cell carcinoma-a comparison to the PD-L1 expression profile. Cancer Med (2019) 8(3):1124–34. doi: 10.1002/cam4.1929
88. Sato F, Ono T, Kawahara A, Matsuo K, Kondo R, Sato K, et al. Prognostic value of tumor proportion score in salivary gland carcinoma. Laryngoscope (2021) 131(5):E1481–8. doi: 10.1002/lary.29120
89. Witte HM, Gebauer N, Lappohn D, Umathum VG, Riecke A, Arndt A, et al. Prognostic impact of PD-L1 expression in malignant salivary gland tumors as assessed by established scoring criteria: Tumor proportion score (TPS), combined positivity score (CPS), and immune cell (IC) infiltrate. Cancers (Basel) (2020) 12(4):873. doi: 10.3390/cancers12040873
90. Alessandrini L, Franz L, Ottaviano G, Ghi MG, Lanza C, Blandamura S, et al. Prognostic role of programmed death ligand 1 (PD-L1) and the immune microenvironment in laryngeal carcinoma. Oral Oncol (2020) 108:104836. doi: 10.1016/j.oraloncology.2020.104836
91. Franz L, Alessandrini L, Ottaviano G, di Carlo R, Fasanaro E, Ramacciotti G, et al. Postoperative radiotherapy for laryngeal cancer. the prognostic role of programmed death-ligand 1: An immune microenvironment-based cluster analysis. Pathol Res Pract (2020) 216(9):153120. doi: 10.1016/j.prp.2020.153120
92. Botticelli A, Zizzari IG, Scagnoli S, Pomati G, Strigari L, Cirillo A, et al. The role of soluble LAG3 and soluble immune checkpoints profile in advanced head and neck cancer: A pilot study. J Pers Med (2021) 11(7):651. doi: 10.3390/jpm11070651
93. Boschert V, Teusch J, Aljasem A, Schmucker P, Klenk N, Straub A, et al. HGF-induced PD-L1 expression in head and neck cancer: Preclinical and clinical findings. Int J MolSci (2020) 21(22):8770. doi: 10.3390/ijms21228770
94. Yang J, Hu M, Bai X, Ding X, Xie L, Ma J, et al. Plasma levels of soluble programmed death ligand 1 (sPD-L1) in WHO II/III nasopharyngeal carcinoma (NPC): A preliminary study. Med (Baltimore) (2019) 98(39):e17231. doi: 10.1097/MD.0000000000017231
95. Ruan Y, Hu W, Li W, Lu H, Gu H, Zhang Y, et al. Analysis of plasma EBV-DNA and soluble checkpoint proteins in nasopharyngeal carcinoma patients after definitive intensity-modulated radiotherapy. BioMed Res Int (2019) 2019:3939720. doi: 10.1155/2019/3939720
96. Kase K, Kondo S, Wakisaka N, Dochi H, Mizokami H, Kobayashi E, et al. Epstein-Barr Virus LMP1 induces soluble PD-L1 in nasopharyngeal carcinoma. Microorganisms (2021) 9(3):603. doi: 10.3390/microorganisms9030603
97. Zhang P, Ouyang S, Wang J, Huang Z, Wang J, et al. [Levels of programmed death-1 and programmed death ligand-1 in the peripheral blood of patients with oral squamous cell carcinoma and its clinical implications]. Hua Xi Kou Qiang Yi XueZaZhi (2015) 33(5):529–33.
98. Xing S, Lu Z, Huang Q, Li H, Wang Y, Lai Y, et al. An ultrasensitive hybridization chain reaction-amplified CRISPR-Cas12a aptasensor for extracellular vesicle surface protein quantification. Theranostics (2020) 10(22):10262–73. doi: 10.7150/thno.49047
99. Lee JC, Wu A, Chen JH, Huang WY, Lawal B, Mokgautsi N, et al. HNC0014, a multi-targeted small-molecule, inhibits head and neck squamous cell carcinoma by suppressing c-Met/STAT3/CD44/PD-L1 oncoimmune signature and eliciting antitumor immune responses. Cancers (Basel) (2020) 12(12):3759. doi: 10.3390/cancers12123759
100. Theodoraki MN, Yerneni S, Gooding WE, Ohr J, Clump DA, Bauman JE, et al. Circulating exosomes measure responses to therapy in head and neck cancer patients treated with cetuximab, ipilimumab, and IMRT. Oncoimmunology (2019) 8(7):1593805. doi: 10.1080/2162402X.2019.1593805
101. Theodoraki MN, Hoffmann TK, Whiteside TL. Separation of plasma-derived exosomes into CD3(+) and CD3(-) fractions allows for association of immune cell and tumour cell markers with disease activity in HNSCC patients. ClinExpImmunol (2018) 192(3):271–83. doi: 10.1111/cei.13113
102. Jiang W, Song Y, Zhong Z, Gao J, Meng X, et al. Ferroptosis-related long non-coding RNA signature contributes to the prediction of prognosis outcomes in head and neck squamous cell carcinomas. Front Genet (2021) 12:785839. doi: 10.3389/fgene.2021.785839
103. Deng R, Lu J, Liu X, Peng XH, Wang J, et al. PD-L1 expression is highly associated with tumor-associated macrophage infiltration in nasopharyngeal carcinoma. Cancer Manag Res (2020) 12:11585–96. doi: 10.2147/CMAR.S274913
104. Ono T, Azuma K, Kawahara A, Sasada T, Matsuo N, Kakuma T, et al. Prognostic stratification of patients with nasopharyngeal carcinoma based on tumor immune microenvironment. Head Neck (2018) 40(9):2007–19. doi: 10.1002/hed.25189
105. Al-Rajhi N, Soudy H, Ahmed SA, Elhassan T, Mohammed SF, Khoja HA, et al. CD3+T-lymphocyte infiltration is an independent prognostic factor for advanced nasopharyngeal carcinoma. BMC Cancer (2020) 20(1):240. doi: 10.1186/s12885-020-06757-w
106. Cao Y, Chan KI, Xiao G, Chen Y, Qiu X, Hao H, et al. Expression and clinical significance of PD-L1 and BRAF expression in nasopharyngeal carcinoma. BMC Cancer (2019) 19(1):1022. doi: 10.1186/s12885-019-6276-y
107. Wilms T, Gu X, Boldrup L, Coates PJ, Fahraeus R, Wang L, et al. PD-L1 in squamous cell carcinoma of the oral tongue shows gender-specific association with prognosis. Oral Dis (2020) 26(7):1414–23. doi: 10.1111/odi.13414
108. Lin YM, Sung WW, Hsieh MJ, Tsai S C, Lai HW, Yang SM, et al. High PD-L1 expression correlates with metastasis and poor prognosis in oral squamous cell carcinoma. PLoS One (2015) 10(11):e142656. doi: 10.1371/journal.pone.0142656
109. Chen TC, Wu CT, Wang CP, Hsu WL, Yang TL, Lou PJ, et al. Associations among pretreatment tumor necrosis and the expression of HIF-1alpha and PD-L1 in advanced oral squamous cell carcinoma and the prognostic impact thereof. Oral Oncol (2015) 51(11):1004–10. doi: 10.1016/j.oraloncology.2015.08.011
110. Kawaguchi T, Ono T, Sato F, Kawahara A, Kakuma T, Akiba J, et al. CD8+ T cell infiltration predicts chemoradiosensitivity in nasopharyngeal or oropharyngeal cancer. Laryngoscope (2021) 131(4):E1179–89. doi: 10.1002/lary.29097
111. Takahashi H, Sakakura K, Arisaka Y, Tokue A, Kaira K, Tada H, et al. Clinical and biological significance of PD-L1 expression within the tumor microenvironment of oral squamous cell carcinoma. Anticancer Res (2019) 39(6):3039–46. doi: 10.21873/anticanres.13437
112. Vassilakopoulou M, Avgeris M, Velcheti V, Kotoula V, Rampias T, Chatzopoulos K, et al. Evaluation of PD-L1 expression and associated tumor-infiltrating lymphocytes in laryngeal squamous cell carcinoma. Clin Cancer Res (2016) 22(3):704–13. doi: 10.1158/1078-0432.CCR-15-1543
113. Franz L, Alessandrini L, Fasanaro E, Gaudioso P, Carli A, Nicolai P, et al. Prognostic impact of neutrophils-to-lymphocytes ratio (NLR), PD-L1 expression, and tumor immune microenvironment in laryngeal cancer. Ann Diagn Pathol (2021) 50:151657. doi: 10.1016/j.anndiagpath.2020.151657
114. Ono T, Azuma K, Kawahara A, Kakuma T, Sato F, Akiba J, et al. Predictive value of CD8/FOXP3 ratio combined with PD-L1 expression for radiosensitivity in patients with squamous cell carcinoma of the larynx receiving definitive radiation therapy. Head Neck (2020) 42(12):3518–30. doi: 10.1002/hed.26416
115. Yang SM, Wu M, Han FY, Sun YM, Yang JQ, et al. Role of HPV status and PD-L1 expression in prognosis of laryngeal squamous cell carcinoma. Int J Clin Exp Pathol (2021) 14(1):107–15.
116. Hu C, Tian S, Lin L, Zhang J, Ding H, et al. Prognostic and clinicopathological significance of PD-L1 and tumor infiltrating lymphocytes in hypopharyngeal squamous cell carcinoma. Oral Oncol (2020) 102:104560. doi: 10.1016/j.oraloncology.2019.104560
117. Shen LF, Zhou SH, Guo Y. Role of GLUT-1 in the upregulation of PD-L1 expression after radiotherapy and association of PD-L1 with favourable overall survival in hypopharyngeal cancer. Onco Targets Ther (2020) 13:11221–35. doi: 10.2147/OTT.S269767
118. Sun J, Lian M, Ma H, Wang R, Ma Z, Wang H, et al. Competing endogenous RNA network analysis of CD274, IL10 and FOXP3 coexpression in laryngeal squamous cell carcinoma. Mol Med Rep (2018) 17(3):3859–69. doi: 10.3892/mmr.2017.8307
119. Ruhle A, Grosu AL, Wiedenmann N, Mix M, Stoian R, Niedermann, et al. Hypoxia dynamics on FMISO-PET in combination with PD-1/PD-L1 expression has an impact on the clinical outcome of patients with head-and-neck squamous cell carcinoma undergoing chemoradiation. Theranostics (2020) 10(20):9395–406. doi: 10.7150/thno.48392
120. Yoo SH, Keam B, Ock CY, Kim S, Han B, Kim JW, et al. Prognostic value of the association between MHC class I downregulation and PD-L1 upregulation in head and neck squamous cell carcinoma patients. Sci Rep (2019) 9(1):7680. doi: 10.1038/s41598-019-44206-2
121. Ou D, Adam J, Garberis I, Blanchard P, Nguyen F, Levy A, et al. Clinical relevance of tumor infiltrating lymphocytes, PD-L1 expression and correlation with HPV/p16 in head and neck cancer treated with bio- or chemo-radiotherapy. Oncoimmunology (2017) 6(9):e1341030. doi: 10.1080/2162402X.2017.1341030
122. Ock CY, Kim S, Keam B, Kim M, Kim TM, Kim JH, et al. PD-L1 expression is associated with epithelial-mesenchymal transition in head and neck squamous cell carcinoma. Oncotarget (2016) 7(13):15901–14. doi: 10.18632/oncotarget.7431
123. Prat A, Navarro A, Pare L, Reguart N, Galvan P, Pascual T, et al. Immune-related gene expression profiling after PD-1 blockade in non-small cell lung carcinoma, head and neck squamous cell carcinoma, and melanoma. Cancer Res (2017) 77(13):3540–50. doi: 10.1158/0008-5472.CAN-16-3556
124. Zhao Y, Zhang Z, Lei W, Wei Y, Ma R, Wen Y, et al. IL-21 is an accomplice of PD-L1 in the induction of PD-1-Dependent treg generation in head and neck cancer. Front Oncol (2021) 11:648293. doi: 10.3389/fonc.2021.648293
125. Dai D, Guo Y, Shui Y, Li J, Jiang B, et al. Combination of radiosensitivity gene signature and PD-L1 status predicts clinical outcome of patients with locally advanced head and neck squamous cell carcinoma: A study based on the cancer genome atlas dataset. Front MolBiosci (2021) 8:775562. doi: 10.3389/fmolb.2021.775562
126. Rasmussen JH, Lelkaitis G, Hakansson K, Vogelius IR, Johannesen HH, Fischer BM, et al. Intratumor heterogeneity of PD-L1 expression in head and neck squamous cell carcinoma. Br J Cancer (2019) 120(10):1003–6. doi: 10.1038/s41416-019-0449-y
127. Emancipator K, Huang L, Aurora-Garg D, Bal T, Cohen E, Harrington K, et al. Comparing programmed death ligand 1 scores for predicting pembrolizumab efficacy in head and neck cancer. Mod Pathol (2021) 34(3):532–41. doi: 10.1038/s41379-020-00710-9
128. de Ruiter EJ, de Roest RH, Brakenhoff RH, Leemans CR, de Bree R, Terhaard C, et al. Digital pathology-aided assessment of tumor-infiltrating T lymphocytes in advanced stage, HPV-negative head and neck tumors. Cancer ImmunolImmunother (2020) 69(4):581–91. doi: 10.1007/s00262-020-02481-3
129. Puntigam LK, Jeske SS, Gotz M, Greiner J, Laban S, Theodoraki MN, et al. Immune checkpoint expression on immune cells of HNSCC patients and modulation by chemo- and immunotherapy. Int J MolSci (2020) 21(15):5181. doi: 10.3390/ijms21155181
130. Miranda-Galvis M, Rumayor PA, Sales DSR, Almeida LA, Agustin VP, Calsavara VF, et al. PD-L1 expression patterns in oral cancer as an integrated approach for further prognostic classification. Oral Dis (2021) 27(7):1699–710. doi: 10.1111/odi.13714
131. Kogashiwa Y, Yasuda M, Sakurai H, Nakahira M, Sano Y, Gonda K, et al. PD-L1 expression confers better prognosis in locally advanced oral squamous cell carcinoma. Anticancer Res (2017) 37(3):1417–24. doi: 10.21873/anticanres.11465
132. Ryu HJ, Kim EK, Cho BC, Cho BC, Yoon SO, et al. Characterization of head and neck squamous cell carcinoma arising in young patients: Particular focus on molecular alteration and tumor immunity. Head Neck (2019) 41(1):198–207. doi: 10.1002/hed.25507
133. Yang F, Zeng Z, Li J, Zheng Y, Wei F, et al. PD-1/PD-L1 axis, rather than high-mobility group alarmins or CD8+ tumor-infiltrating lymphocytes, is associated with survival in head and neck squamous cell carcinoma patients who received surgical resection. Front Oncol (2018) 8:604. doi: 10.3389/fonc.2018.00604
134. Schweizer C, Schubert P, Rutzner S, Eckstein M, Haderlein M, Lettmaier S, et al. Prospective evaluation of the prognostic value of immune-related adverse events in patients with non-melanoma solid tumour treated with PD-1/PD-L1 inhibitors alone and in combination with radiotherapy. Eur J Cancer (2020) 140:55–62. doi: 10.1016/j.ejca.2020.09.001
135. Pai SI, Cohen E, Lin D, Fountzilas G, Kim ES, Mehlhorn H, et al. SUPREME-HN: a retrospective biomarker study assessing the prognostic value of PD-L1 expression in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. J Transl Med (2019) 17(1):429. doi: 10.1186/s12967-019-02182-1
Keywords: PD-1, PD-L1, PD-L2, prognostic value, head and neck cancer, HNSCC, spatiotemporal heterogeneity
Citation: Jiang S, Li X, Huang L, Xu Z and Lin J (2022) Prognostic value of PD-1, PD-L1 and PD-L2 deserves attention in head and neck cancer. Front. Immunol. 13:988416. doi: 10.3389/fimmu.2022.988416
Received: 07 July 2022; Accepted: 08 August 2022;
Published: 02 September 2022.
Edited by:
Afsheen Raza, Hamad Medical Corporation, QatarCopyright © 2022 Jiang, Li, Huang, Xu and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhensheng Xu, MjI0NzMxNkBxcS5jb20=; Jinguan Lin, bGluamluZ2d1YW5AaG5jYS5vcmcuY24=
 Siqing Jiang
Siqing Jiang Xin Li
Xin Li Lihua Huang
Lihua Huang Zhensheng Xu4*
Zhensheng Xu4*