- 1Centre for Cardiovascular Biology and Disease Research, Department of Microbiology, Anatomy Physiology and Pharmacology, School of Agriculture, Biomedicine and Environment, La Trobe University, Bundoora, VIC, Australia
- 2Department of Microbiology, Anatomy Physiology and Pharmacology, School of Agriculture, Biomedicine and Environment, La Trobe University, Bundoora, VIC, Australia
Obesity is defined as the excessive accumulation of body fat and is associated with an increased risk of developing major health problems such as cardiovascular disease, diabetes and stroke. There are clear sexual dimorphisms in the epidemiology, pathophysiology and sequelae of obesity and its accompanying metabolic disorders, with females often better protected compared to males. This protection has predominantly been attributed to the female sex hormone estrogen and differences in fat distribution. More recently, the sexual dimorphisms of obesity have also been attributed to the differences in the composition and function of the gut microbiota, and the intestinal immune system. This review will comprehensively summarize the pre-clinical and clinical evidence for these sexual dimorphisms and discuss the interplay between sex hormones, intestinal inflammation and the gut microbiome in obesity. Major gaps and limitations of this rapidly growing area of research will also be highlighted in this review.
Introduction
Obesity is a globally increasing pandemic affecting all ages, ethnicities, sexes, and socio-economic groups. The prevalence of obesity has tripled in the last forty years now affecting ~30% of adults worldwide (1). Obesity is the excessive accumulation of body fat and is associated with an increased risk of developing major health problems such as cardiovascular disease, diabetes and stroke (2). The most used standard in identifying overweight and obesity is a body mass index (BMI; body weight (kg)/height (m) 2) > 25 kg/m2 classified as overweight and > 30 as obese (3). It is important to note, that while these are the most widely reported BMI cutoffs, they are only relevant to Caucasians. The BMI cutoffs for obesity for other racial and ethnic categories vary to these values (4). For example, the cutoffs for South Asian populations are slightly lower with a BMI > 23 are classified as overweight and > 25 as obese (3). Concomitant metabolic disturbances of obesity include low-grade chronic inflammation, metabolic endotoxemia, hypertension, dyslipidemia, hyperglycemia, and insulin resistance (5). Interestingly, there are clear sexual dimorphisms in the epidemiology and pathophysiology of obesity and its accompanying metabolic disorders. Generally, females are better protected compared to males – this phenomenon will be discussed in much more detail throughout this review (6). Protection in females has been attributed to various biological processes, that will be the focus of this review, such as the influence of adipose distribution, sex hormones, sex chromosomes, the gut microbiota and the intestinal immune system (7–10).
Adipose tissue biology in obesity
Obesity is instigated by a chronic imbalance of increased energy intake and/or reduced energy expenditure (1). This increases adiposity, a key driver in the development of obesity and the consequential inflammatory state (11). Adipocytes are the predominant cell type in adipose tissue. However, a variety of other cell types also reside in fat beds including leukocytes, endothelial cells and fibroblasts (12). Adipose is a major source of both inflammatory and hormonal signals, and thus is becoming recognized as an endocrine organ in its own right (12). Adipocytes are traditionally classified as either white or brown (12). White adipocytes are particularly important in the storage of energy, whereas brown adipocytes are primarily involved in thermoregulation (via non-shivering thermogenesis) (12). In obesity, where there is a persistent excess of energy, white adipocytes undergo hypertrophy and proliferate to adapt to the accumulation of triglycerides (13). As a result, white adipocytes promote a chronic inflammatory response by secreting pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), and interleukin-1 beta (IL-1β) (14). This pro-inflammatory phenotype is further compounded by a reduction in the release of anti-inflammatory molecules by obesogenic adipose (15). Ultimately, these changes aid the infiltration of pro-inflammatory immune cells into the adipose tissue and surrounding organs (16). Unsurprisingly, in obesity, white adipose tissue provokes dyslipidemia, insulin resistance and hyperglycemia further exacerbating the dysregulation of whole-body energy homeostasis (16) (Figure 1). Importantly, the changes in adipocyte biology and the subsequent downstream metabolic processes in obesity significantly differ between the sexes and therefore, serve as a major source for the sexual dimorphism of obesity (17).
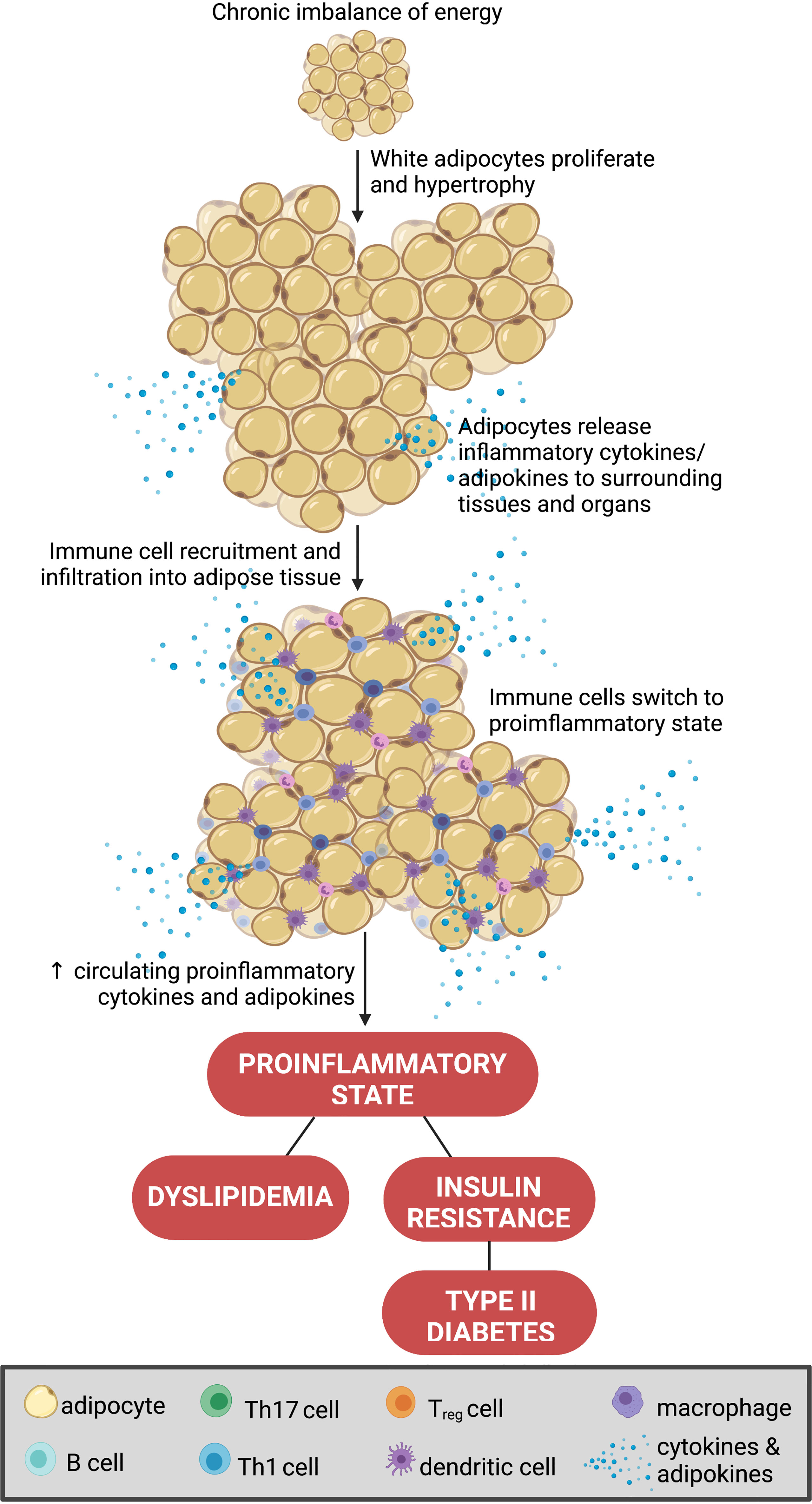
Figure 1 Adipocyte biology in obesity. A chronic imbalance of increased energy intake and or reduced energy expenditure increases adiposity, via hypertrophy and proliferation of white adipocytes. This promotes the secretion of pro-inflammatory cytokines (i.e., tumor necrosis factor alpha (TNF-α), interleukin 6 (IL-6), IL-1β, and IL-10) to aid the infiltration of pro-inflammatory immune cells into the adipose tissue and surrounding organs (16). This process promotes dyslipidemia, insulin resistance and hyperglycemia further exacerbating the dysregulation of whole-body energy homeostasis. Created with BioRender.com.
Sexual dimorphisms in adipose tissue distribution, sex hormones and sex chromosomes
Historically, females have been grossly underrepresented in clinical trials and pre-clinical research. Part of this sex bias in research is the result of an early misconception that men and women are the same. We now know that men and women are unique on a cellular level, and in the setting of obesity there are major sexual dimorphisms. Obesity is slightly more common in females. However, compared to males, females are protected from many of the metabolic disturbances and sequalae that are associated with disease progression in obesity (18, 19). These sexual dimorphisms are also reflected in experimental animal models of diet-induced obesity (19). Male rodents experience an earlier onset and greater degree of obesity, as well as more prevalent concomitant risk factors compared to their female counterparts (such as hyperglycemia, hyperinsulinemia and hypertension) (20, 21). Interestingly, older female animals, or those which model a post-menopause stage (i.e., ovariectomized) are less protected than young females with intact ovaries (22). This correlates with human epidemiology of obesity, whereby men and post-menopausal women are at the greatest risk of developing complications of obesity (23). Collectively, this supports the notion that sex hormones in pre-menopausal women are protective in the setting of obesity. Indeed, sex hormones, such as estrogen, testosterone and androgens are related to the regulation of energy metabolism, food intake and body weight in humans (22, 24). Estrogen is of particular importance and well-established to be protective against cardiometabolic disorders such as obesity, hypertension, and diabetes (25).
The correlation between adipose tissue distribution, sex hormones and the concomitant metabolic disturbances of obesity are well defined (Figure 2), and visceral adiposity is a known driver in the progression of disease in obesity (26). The distribution of adipose tissue throughout the body differs between men and women (27–29). Women have a greater degree of subcutaneous fat (‘gynoid’ pattern), primarily in the gluteofemoral region. Whereas adipose tissue in men is predominantly seen in the abdominal area (‘android’ pattern) as visceral fat (30, 31). The sexual bias of these effects has been reported in both rodent models of obesity and in a clinical setting. Male mice on a high fat diet are at a higher risk of developing a pro-inflammatory profile (visceral inflammation, glucose intolerance, insulin resistance and hyperinsulinemia) when compared to their female counterparts (32, 33). Increased visceral adiposity in men exacerbates the secretion of pro-inflammatory molecules into systemic circulation which produces a knock-on effect whereby the risk of cardiovascular events is markedly increased. This was observed in the European Health Examination Survey in Luxembourg (34). Interestingly, the Netherlands Epidemiology of Obesity Study reported that visceral adipose tissue distribution was more strongly associated with cardiometabolic risk factors in obese females than in obese males (35). The differences observed in these two studies may be due to the Netherlands study including only obese participants, whereas in the Luxembourg study the BMI of participants ranged from <20 to >35 kg/m2.
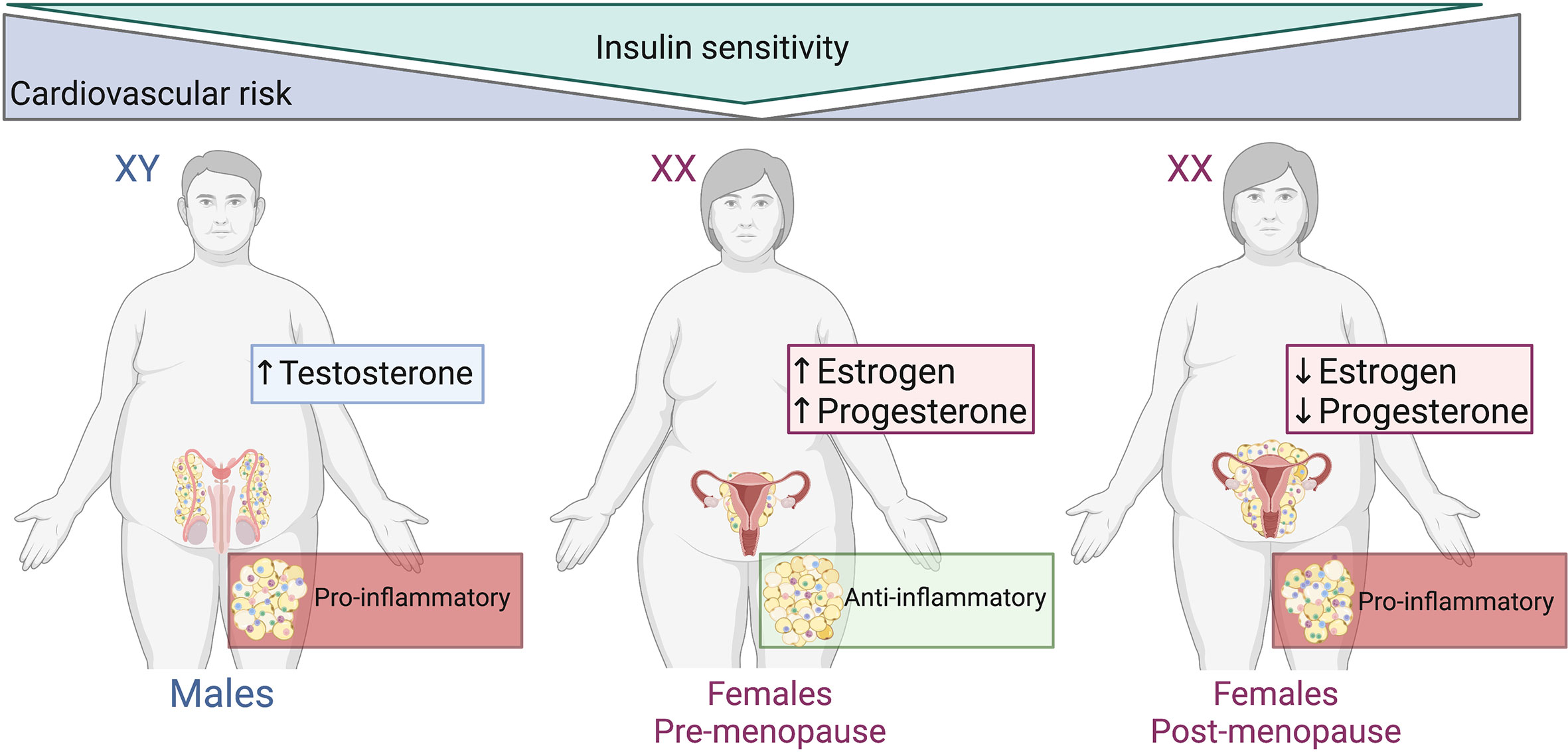
Figure 2 Adipose tissue distribution, sex hormones and metabolic disturbances of obesity. Males and post-menopausal females have increased cardiovascular risk, abdominal/visceral obesity and reduced insulin subcutaneous adipose distribution compared to pre-menopausal females. The adipose tissue within males and post-menopausal females is more pro-inflammatory than that of pre-menopausal females. Created with BioRender.com.
Pre- and post-menopausal studies in women emphasize the role of estrogen in the distribution of adipose tissue by which intra-abdominal visceral fat is increased in post-menopausal women (25, 36–38). With this shift in fat distribution, post-menopausal women undergo metabolic alterations. Lipoprotein lipase activity increases and lipolysis decreases with the fall of estrogen and increased androgenicity is induced during the transition to menopause (36). Ovariectomy in rodents is commonly used as a model of estrogen depletion that occurs in humans. White adipose inflammation is increased and comparable to a male-like phenotype of inflammatory gene expression in ovariectomized mice (39). Despite this pro-inflammatory profile, there were no differences in adipocyte size and total adiposity between ovariectomized and sham mice. This suggests that ovarian hormones are not important in the expansion or apoptosis of adipocytes (39).
In addition to the detrimental effects of visceral adipose, studies also report striking protective effects of gluteofemoral subcutaneous adipose tissue (40). Specifically, increased gluteofemoral mass is associated with lower arterial calcification, arterial stiffness, improved blood lipid levels and atherosclerotic protection (41). While the precise protective mechanisms remain unclear, gluteofemoral adipose has an active role in fatty acid uptake and release by ‘trapping’ excessive fatty acids, preventing lipid accumulation and lipotoxicity (41–43). Lipolysis relative to energy expenditure is therefore higher in women. Other studies link the protective effects of gluteofemoral adipose with the secretion of anti-inflammatory molecules such as adipokines (41).
Sex chromosomes are another crucial contributing factor to the sexual dimorphisms of adipose tissue distribution and the subsequent metabolic complications of obesity. Female gonads typically occur in individuals with XX chromosomes and male gonads in those with XY chromosomes (44). In a unique mouse model, gonadectomized male and female mice carrying XX chromosome complements developed worse obesity disease outcomes than gonadectomized mice carrying the XY chromosome complements (i.e. increased adiposity, increased satiety, and elevated lipid and insulin levels) (45). Gonadectomized mice carrying XO and XXY chromosome complements revealed that the differences between the XX and XY mice due to the additional X chromosome (or “X chromosome dosage”) rather than the lack of a Y chromosome. Indeed, several genes that escape X chromosome inactivation are highly expressed in adipose and liver tissues – both of which are key regulators of metabolism. Thus, the X chromosome may be an important factor in addition to gonads/sex hormones that causes sex differences in obesity and metabolism (45).
Human sex chromosome anomalies also exist such as Klinefelter syndrome (XXY) and Turner syndrome (XO) (46). In Klinefelter syndrome, the most common sex chromosome disorder in men, patients typically present with hypergonadotropic hypogonadism and infertility, with a 5-fold higher incidence in metabolic syndrome, stemming from hypogonadism and low testosterone levels, affecting adiposity and different metabolic traits (47). Turner syndrome patients (the most common sex disorder observed in females, whereby one of the X chromosomes are partially or completely missing) have dramatically reduced gonadal hormone levels. These patients also lack protection against abdominal obesity and have a 4-fold increase in risk for type 2 diabetes (48). Notably, the presence of XX and XY chromosomes influence the developmental path between sexes and gonadal hormones. This ultimately affects the gene expression that may underpin the differences in obesity and metabolism observed between males and females. Although largely attributed to sex hormones and sex chromosomes, the sexual dimorphism of obesity has also been partially credited to sex differences in the microflora residing in our intestines.
The gut microbiota: A key player in health and disease
The gut microbiota is made up of trillions of complex and dynamic microorganisms living within the intestines and working symbiotically with their host for essential metabolic functions (49). Dietary carbohydrates are fermented by the gut microbiota generating short chain fatty acids (SCFA) as by-products, primarily acetate, butyrate and propionate (50). A higher abundance of SCFA, particularly butyrate, is associated with reduced intestinal inflammation and offers protection against the development of insulin resistance and obesity (51, 52). Additionally, there are certain beneficial, anti-inflammatory bacterial species that respond well to fiber rich diets such as Akkermansia muciniphila, Bifidobacterium spp., Prevotella spp., and Veillonella spp. forming a favored environment in terms of functionality and immunity (53). Other by-products of the gut microbiota include energy metabolites including pyruvic, citric, fumaric and malic acid (54, 55). These organic acids aid in digestion, immunity, and specifically in preventing the growth of pathogenic bacteria and thus, offer further protection for their host (56, 57).
In addition to aiding in the digestion of foods to produce favorable by-products, the gut microbiota also has an important role in stimulating and regulating hormone production (58). Previous studies show significant correlations between sex steroid levels (i.e., estrogen, progesterone, and testosterone) and gut microbiota composition (7, 59–61). These studies of the interactions between sex hormones and the gut microbiota revealed sexual dimorphisms in the composition of the gut microbiota which will be discussed later. Another crucial function of the gut microbiota is the maintenance of the intestinal immune system response and its tolerance to the bacterial community (62). Due to their close proximity, it is essential that the gut microbiota and intestinal immune system tolerate one another (62). The interaction between the immune system and gut microbiota is a recognized key player in the development of cardiometabolic diseases and will be discussed in detail later in this review. The next section of the review will focus on the role of the gut microbiota in regulating metabolic functions, particularly in the context of diseases such as obesity and other cardiometabolic diseases (63).
The gut microbiota clearly influences the health of its host and various disease states are associated with “dysbiosis” of the gut microbiota (i.e. an altered composition or functionality). However, dysbiosis is often disease-specific and not consistent between different studies. This is likely due to environmental factors such as diet, lifestyle and drugs being major determinants of gut microbiome composition. Consequently, the gut microbiome is highly individualized which makes it difficult to define what constitutes a healthy microbiome (64). Thus, both clinical and experimental studies should be replicated in independent locations to maximize reproducibility and translatability of findings (65). Moreover, it is largely unclear if gut dysbiosis is a cause or the consequence of disease, highlighting the need for further studies defining the molecular mechanisms by which altered microbiomes cause disease. A recent study built a machine learning model that included both human variables and gut microbiota to try to infer gut microbiota and disease associations more accurately (66).
Despite the striking variations in findings between studies, one of the most consistent findings of intestinal dysbiosis in the setting of disease is the loss of microbiota diversity (67, 68). A highly diverse microbiota is thought to be crucial to good gut health as it is more resilient against pathogens, has a greater functionally complex community and builds a stronger and more stable immune system (69–71). Therefore, reduced gut microbiome diversity is most likely detrimental in disease due to a subsequent loss of microbial community function. Many studies have highlighted that microbial community composition is less important than microbial community function. Therefore, increased microbial diversity can be both beneficial or detrimental, more context is often required for accurate interpretation. For example, germ-free mice lacking a microbiome (and thus lack microbiome diversity), but are protected diet-induced obesity, compared to mice with a gut microbiota (72). Ultimately, making conclusions based on microbiota diversity alone has limited value, and should be avoided.
Major shifts in gut microbiota in obesity
Dysbiosis is a particularly common consequence of a poor diet – a common factor in obesity (73). Diets have a marked influence on the gut microbiota, for example, diets low in fiber and rich in bad fats can modify the bacterial population in as little as 24 hours (74, 75). Diet-induced obesity in animal models is often used to mimic metabolic disturbances and the concomitant gut dysbiosis seen in humans (76, 77). Typically, obesogenic diets include high fat and/or high sugar contents with variations in the types of fat and sugar as well as differences in the duration of diet regimes (77, 78). Importantly, the gut microbiome also influences the concomitant metabolic disturbances of obesity. Oral antibiotic treatment (ampicillin) improves glucose tolerance in high fat diet-fed obese mice. These ‘protective’ effects of antibiotics in obesity are only effective in early life, suggesting that the plasticity of the gut microbiome reduces with age (79, 80).
Gut microbiota dysbiosis describes the imbalance of microorganisms within the gut resulting in metabolic disturbances in the body and contributing to the development of obesity (81, 82). Overall, dysbiosis can be identified by the loss of beneficial bacteria, the increased abundance of harmful bacteria and a loss of compositional and functional diversity (83). Notably, an emphasis has been placed on the status of the Firmicutes: Bacteroidetes ratio, two dominant phyla in the gut microbiota, and how these phyla alter with disease (84). Many studies conclude that disease states such as obesity are associated with an increase in the abundance of the Firmicutes phyla and a decrease in the abundance of the Bacteroidetes phyla (85–87). Moreover, this phenomenon has proven to be reversible with weight loss (88). While the majority of studies report increased Firmicutes : Bacteroidetes ratios in obesity, it is important to highlight that this is not always the case, and contrasting findings have become more common in recent years. For example, in a recent small cohort study of Beijing volunteers the ratio of Firmicutes/Bacteroidetes decreased significantly in people with obesity (89). Larger studies have also reported similar findings (90). Unfortunately, the Firmicutes: Bacteroidetes ratio is not a robust marker of obesity-related microbiome dysbiosis and many of the studies interpreting changes to the Firmicutes: Bacteroidetes ratio are drastically underpowered (91).
A more accurate approach may be to detect obesity-related changes to the genus, family and species levels within the gut microbiome (54). Beneficial bacteria such as Akkermansia muciniphila and members of the Bifidobacterium genus have a negative correlation in the development of obesity (92, 93). The beneficial effects of A. muciniphila on the intestinal epithelial barrier have long been reported, as it a highly effective mucin-degrading bacterium, with the ability to use various enzyme combinations to hydrolyze up to 85% of mucin structures within the gut (94). A reduction in A. muciniphila is associated with increased intestinal permeability or a “leaky gut” – a hallmark of gut dysbiosis in obesity (95). Intestinal permeability allows leakage of water, proteins and other endotoxic molecules such as lipopolysaccharide (LPS) into systemic circulation with the ability to reach other organs and tissues (96). High circulating levels of LPS, termed metabolic endotoxemia, promotes further inflammation, weight gain and diabetes in experimental animals and humans (97). Recent studies have explored the possibility of using A. muciniphila-associated therapies as a next-generation treatment for obesity (98). Opposingly, harmful bacteria such as the those from the Desulfovibrio, Fusobacterium and Bilophila genera are positively correlated with obesity (92, 93, 99).
The metabolic and hormonal consequences of gut dysbiosis in obesity
Harmful bacteria within the gut have specified mechanisms that can be destructive to the host. For example, members of the Desulfovibrio genus and other sulphate-reducing bacteria induce apoptosis of cells on the intestinal epithelial barrier allowing barrier degradation (100). Additionally, the abundance of gram-negative bacteria increases, with endotoxic lipopolysaccharide (LPS) in their outer membrane (101). LPS then gains access into systemic circulation due to the increased permeability of the epithelial barrier (102). The combination of an increase in harmful bacteria, the decrease in beneficial bacteria, and an increased concentration of pro-inflammatory cytokines within the intestines causes degradation of tight junction proteins between cells allowing LPS and other molecules into underlying tissues and thus, increasing intestinal inflammation (100, 103, 104). Some studies have explored the therapeutic potential of targeting this increase in harmful bacteria in obesity. Alteration of the gut microbiota via antibiotics in mice with diet-induced obesity inhibits weight gain, increases lipid oxidation, thermogenesis, and adiponectin gene expression in epididymal adipose tissue. Increases in these molecular pathways likely inhibit fat synthesis and promote a “leaner” phenotype (105). Advances in metagenomics and metabolomics revealed new associations between microbial-derived metabolites (i.e. LPS, short chain fatty acids (SCFAs), ethanol, trimethylamine (TMA), and bile acids) and obesity.
Bile acids are a class of amphipathic steroids synthesized in the liver from cholesterol and metabolized by the gut microbiota. Bile acids facilitate intestinal fat absorption but also modulate glucose, lipid and energy metabolism, intestinal integrity and immunity (106). While there are some discrepancies between studies, circulating bile acid levels are generally positively correlated with obesity. Importantly, microbiome-derived bile acid species have different signaling functions to liver-derived species. There is a growing body of evidence suggesting a link between the microbiome – an important player in bile acid metabolism – and bile acid levels/composition in obesity (106). Fecal microbiota transplantation (FMT; from a single lean donor) in obese, metabolically uncompromised patients had sustained shifts in microbiomes and bile acid levels toward those of the donor (107). Like much of the microbiome research to date, further studies are still needed to establish whether there is causality, as these “beneficial” changes were not associated with weight loss or changes to glucose metabolism.
To date, numerous studies suggest that gut microbiomes influence eating behavior in humans and animals. Appetite-related hormones such leptin (inhibits appetite) and ghrelin (promotes appetite) are produced by peripheral organs, including gut and adipose tissue. Changes to specific microbial compositions have reported effects on these hormones, and vice versa. For example, in obese and non-obese humans, higher circulating leptin concentrations are associated with reduced gut microbiome diversity (108). Moreover, in vivo and in vitro studies showed that the translocation of living gut microbiota to adipose tissues in obese patients with increased intestinal permeability inhibits leptin signaling (109). Alternatively, the gut microbiota may modulate appetite via grehlin. In another study, treatment with SCFAs, lactate, or bacterial supernatants to promote gut microbiome health attenuated ghrelin-mediated signaling (110).
Clearly, whilst gut dysbiosis has been consistently reported in obesity, the severity, and subsequent consequences of dysbiosis vary among obese individuals depend on many factors, which likely explains inconsistent findings between studies (111, 112). One such factor that has more recently been recognized to influence the gut microbiota is an individual’s sex.
Sexual dimorphisms and gut microbiota
The impact of the gut microbiota and its influence on the development of obesity has been well documented. However, one aspect that was overlooked in earlier research is the effect of sex. Many studies have investigated the impact of the gut microbiota by altering variables such as diet, lifestyle, and drugs but it is important to recognize that the gut microbiota is different for males and females prior to any manipulations (113). Sequencing the microbial community of prepubescent male and female mice does not show any separation between sexes indicating that sex differences are influenced by gonadal-derived sex hormones and puberty (59, 114). Typically, in mice, the female gut microbiota more closely resembles that of prepubescent males, or castrated males, rather than age-matched males (59, 115). Furthermore, the diversity differs between sexes, with males having a lower species richness and evenness compared to females of the same age in mice models (116). As well as differences in diversity, both animal and human studies show clear variance in the abundance of specific bacteria being higher in one sex compared to the other (113, 117–119). The distinct differences in the male and female gut microbiota, for both animal and human models, inevitably generate differences in metabolic processes and therefore, differences in dysbiosis and the protection or susceptibility to metabolic diseases including obesity (56, 115, 120).
It is well-established that sex steroid hormones are the major drivers of sexual dimorphisms in males and females however, whether there is the strong interaction between sex hormones and gut microbiota is still unclear (59). An observational study that compared the microbiota of men and women with higher serum hormone levels to those with low hormone levels suggests that sex hormones do indeed influence the gut microbiota (121). Higher levels of hormones were associated with a greater diversity in the gut community compared to those with lower hormone levels in both sexes. Moreover, bacteria such as those from the Acinetobacter, Ruminococcus and Megamonas genus were significantly associated with testosterone levels in men and Slackia and Butyricimonas were significantly associated with estradiol levels in women (121). Gut microbial transplants to the opposite sex have also been used to determine the hormonal association (10). In these studies females receiving male donor gut microbiota, not only showed higher levels of gut inflammation (a common sign of obesity and cardiometabolic disease) but also resulted in raised testosterone levels (10, 115).
The gut microbiota has also been shown to directly influence sex hormone levels in animal studies using microbial transplants between germ-free mice and mice of opposite sex (7, 10, 122). Colonizing germ-free mice with gut microbiota increases the levels of circulating androgens and begins the development of immune and protective pathways (7, 59). However, there is also evidence that sex hormones can also influence the gut microbiome. Inoculating germ-free mice with human male donor gut microbiota results in males and females harboring these microorganisms differently (122). In female mice, the gut microbiota significantly differed from the matched males and donor, with a higher bacterial diversity (122). Collectively, these studies indicate that there is likely a two-way communication between systemic sex hormones and the gut microbiome, whereby both factors impact one another.
The interactions between sex hormones and gut microbiota have also been studied in animal models using hormone and gonadectomy treatments (61, 101). Estradiol, the most common form of estrogen, is used in hormone treatments to remedy the loss of ovarian estrogen typically seen in menopausal women (123). High fat diet-fed female mice treated with estradiol are protected from cardiometabolic disease (reduced weight gain, improved glucose tolerance and insulin sensitivity) when compared to untreated high fat diet-fed female mice (61). Moreover, estradiol alters the gut microbiota by slowing the increase in Firmicutes: Bacteroidetes ratio that is usually seen in high fat diet-fed mice (61). Interestingly, sequencing of the gut microbiome of these mice revealed that bacteria from the S24-7 and Ruminococcaceae families, known to generate beneficial SCFA, were in higher abundance in estradiol-treated mice, compared to untreated mice (61). The benefits of estradiol treatment are not limited to just females. Male mice treated with estradiol have a reduced susceptibility to gut epithelial permeability, inflammation and weight gain compared to untreated males (101, 124).
Sexual dimorphisms of the gut microbiota in obesity
As previously discussed, the female sex is also protected from the development of metabolic disturbances in obesity, and the gut microbiota responds differently to diet based on sex (summarized in Table 1). This was demonstrated in overweight and obese adults undergoing either a high protein or low-fat weight loss intervention diet (125). Changes in the gut microbiota occurred not only in diet-specific manner but also differed based on sex (125). Additionally, in animal models of obesity, high fat/high sugar diet-fed mice, demonstrated that females respond slower to the biological adverse effects of the diet as well as differentiating in the composition of the gut microbiota, compared to males (126). The increased Firmicutes: Bacteroidetes ratio typically seen in the development of obesity and metabolic disease is significantly slower in female mice (127). Moreover, differences in the abundance of specific genera are also observed in metabolic syndrome patients. Higher abundances of Veillonella, Methanobrevibacter, Acidaminococcus, Clostridium, Roseburia and Faecalibacterium genera in males, whereas genera such as Bilophila, Ruminococcus and Bacteroides were greater in females (68, 82). In the male gut microbiota for example, Veillonella genera are found in higher abundance in children with type 1 diabetes however, Roseburia genera is found to improve metabolic alterations brought on by high fat diets (128, 129). Likewise, for females, Bilophila genera aggravates metabolic dysfunction however, Bacteroides genera has numerous metabolic benefits on the host (130, 131). These findings suggest that it is not simply the abundance of specific bacteria in the gut microbiota that determine health and disease within the host.
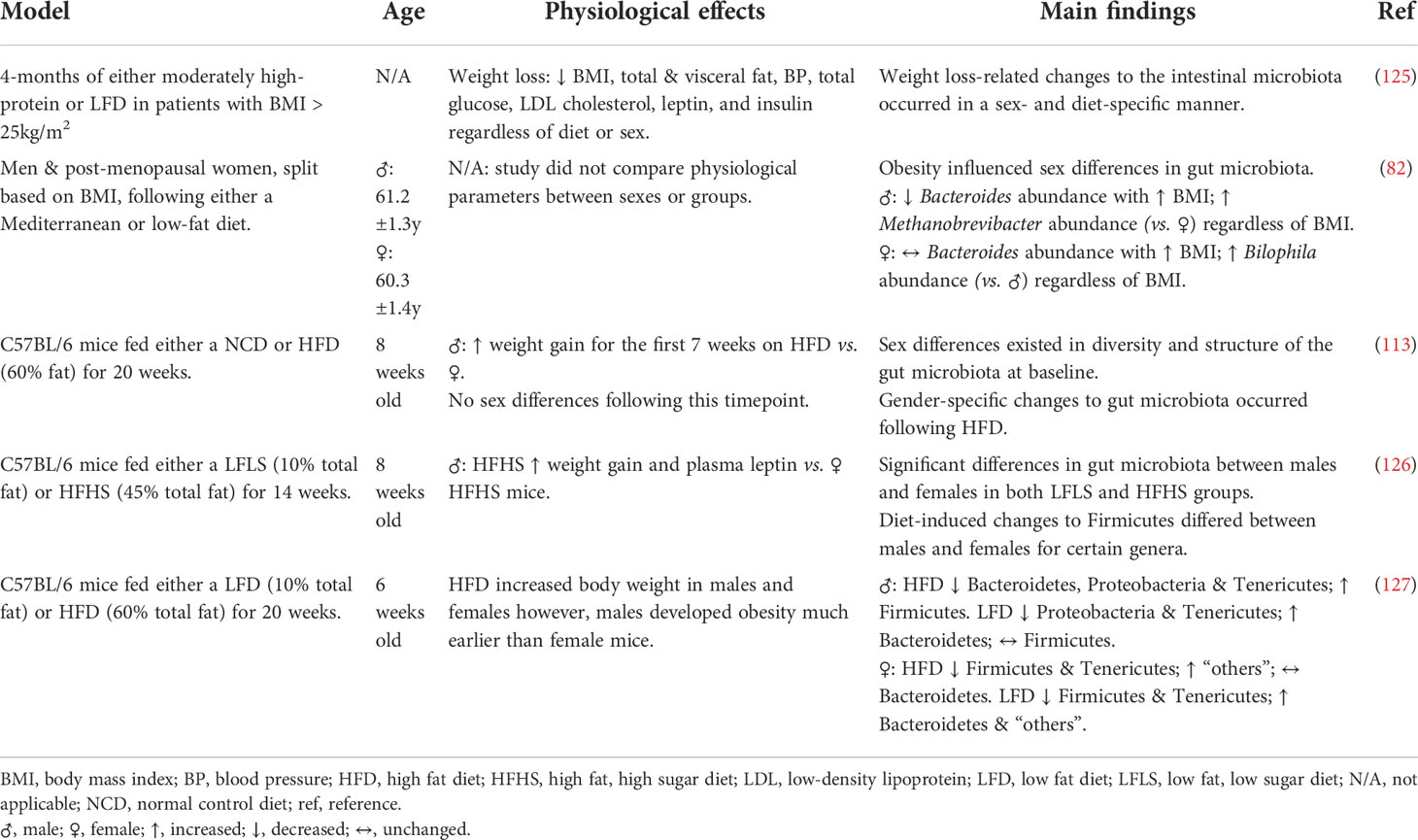
Table 1 Summary of human and mouse studies investigating the sexual dimorphisms of intestinal microbiota in obesity.
In addition to the sexual dimorphism in response to poor “Western-style” diets, the sex-specific response to beneficial diet supplementations have also been studied (132, 133). Beneficial fiber compounds, including pre- and probiotics, can attenuate the unfavorable effects of the diet by shaping the gut microbiota (93). The addition of prebiotic fibers, such as oligofructose, significantly increases beneficial gut bacteria in healthy and gnotobiotic female, but not male mice, such as Bacteroides and Bifidobacterium genera and A. muciniphila (122, 134). Furthermore, probiotic treatments also adjust the gut microbiota differently for males and females (135). Administration of Lactobacillus reuteri increased the abundance of the Bacteroidetes phylum and decreased Firmicutes in healthy female mice, but showed opposite effects in healthy male mice (135). Given the differences at the phylum level, this also incurred significant differences at the genus level. Females had a significantly greater abundance of Bacteroides, Prevotella and Lactobacillus, but males had a higher abundance of Clostridium (135). These findings not only illustrate that the female gut microbiota has a stronger protection against the adversities of poor diet, but also that the male and female gut microbiota respond differently to the effect of beneficial supplements and how they harbor their microbial communities. The explanation for the sexual dimorphisms in gut microbial composition and function, in both healthy and metabolically disturbed subjects, comes full circle and back to differences in sex steroid hormones and inflammatory responses.
Gonadectomy surgery can be used to eliminate sex steroid hormones and therefore, also be used to study the interactions between sex hormones and the gut microbiota in obesity. In ovariectomized obese female mice, the Firmicutes phyla dominated the gut microbiota community which is commonly seen in obese and high fat diet-fed mice and the sequenced gut community of ovariectomized female mice more closely resembles that of male mice (101, 136). Furthermore, when treating ovariectomized female mice and male mice with estrogen the microbial composition resembles that of non-ovariectomized female mice (101). Similar to ovariectomized mice and the reduction in estrogen, is the changes occurring to the gut microbiota with menopause (137). Studies have shown that the gut microbiota of post-menopausal women reveal higher abundances of Firmicutes compared to both pre-menopausal women and age-matched males (137). These findings reveal that sex and sex hormones, specifically in the presence of obesity, strongly guide the shape of the gut microbiota. In addition to the sexual dimorphism existing within the gut microbiota and obesity, research has revealed that sex-based differences of obesity are also associated with the immune system specifically within the gut (Figure 3).
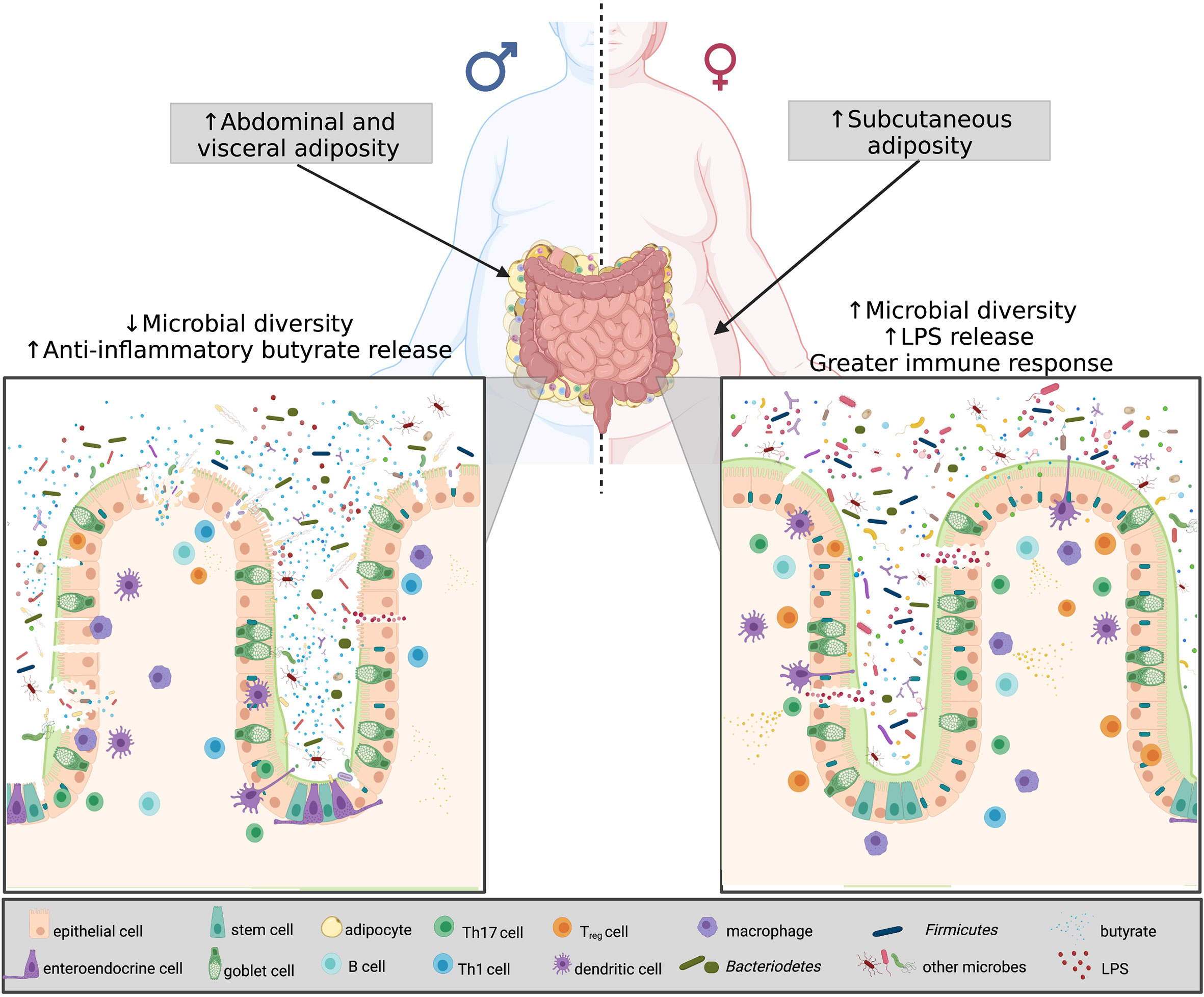
Figure 3 Microbial diversity, sex hormones and chromosomes in obesity. Differences in sex-based characteristics are modulated by a variety of factors. Women have a greater degree of subcutaneous fat, whereas males predominantly accumulate visceral fat. In obesity, the shift in the Firmicutes: Bacteroidetes determines disease severity. Obese males have less species richness, and testosterone was found to be associated with increased Firmicutes, thus more anti-inflammatory butyrate release. Obese females, on the other hand, despite having greater microbial diversity, have an increase estradiol and Bacteroidetes, resulting in greater LPS release, thus eliciting a greater immune response. Created with BioRender.com.
Sexual dimorphism of intestinal inflammation
Obesity is commonly accompanied with low-grade systemic inflammation which is a key driver of the subsequent comorbidities of obesity due to higher concentrations of endotoxic molecules (i.e., LPS from bacteria) and in circulation increased adiposity increasing cytokines such as TNF-α, IL-1, and IL-6 (138, 139). Many studies in obesity have concentrated on visceral adipose tissue as the driving force of inflammation however, inflammation within the intestinal tract precedes both adipose tissue inflammation and obese characteristics such as weight gain (138). This finding is of particular importance as a significant proportion of the systemic innate and adaptive immune cells within the body (70%) reside within the intestinal tract (140). To our knowledge, the sexual dimorphisms of the intestinal immune system in the setting of obesity has not been researched in a preclinical setting. However, studies have identified sex differences in healthy individuals (141, 142). For example, in the lamina propria layer of the intestines female have higher immune activation and higher CD4+ and CD8+ T cell counts in compared to males (141).
Another crucial mediator of the sexual dimorphisms in intestinal immunity is the gut microbiota. Due to their close proximity, the interplay between the gut microbiota and intestinal immune system is well-established as shaping and developing one another (143). This is highlighted in studies using germ-free mice, which lack a gut microbiota. The consequence of this is poorly developed intestinal lymphatic tissue (Peyer’s patches) and immune cell populations (144). Moreover, the sex differences of intestinal immunity in autoimmune disease settings are abolished in germ-free mice, suggesting that the sex bias in immunity is driven by sex differences in the microbiome rather than sex hormones (59, 115).
As mentioned previously, females have a stronger intestinal immune response compared to males and this influence of the gut microbiota on this must also be considered (145). Therefore, the sexual dimorphisms of the gut microbiota and in particular, the difference in biomarkers of obesity such as the Firmicutes: Bacteroidetes phyla and taxa abundance difference likely drive the discrepancies in the intestinal immune system of males and females (127). For example, the Firmicutes phyla are the predominant producers of butyrate, a known anti-inflammatory molecular metabolite (146). Therefore, the increased Firmicutes abundance typical of obese males (compared to obese females), elevates butyrate production, which could suppress the intestinal immune response in males. Alternatively, the Bacteroidetes phyla, generally seen in higher abundance in obese females compared to obese males, are gram-negative bacteria (147). Gram-negative bacteria contain LPS in their outer membrane thus, an increased abundance of these taxa, and subsequent increased circulating LPS, correlates with a stronger intestinal immune response (147).
Although a stronger immune response is associated with an increased inflammatory profile, this may be beneficial in the context of obesity and intestinal inflammation. For example, females are superior in eliminating pathogenic and opportunistic bacteria (possibly obesity-related bacteria) present in the gut, which might be a by-product of their enhanced immune response. The enhanced immune response in females may very well be the factor that protects or delays the development of obesity-related metabolic disturbances in females (148). In the opposing manner, the intestinal immune response is relatively smaller in males, thus allowing the manifestation of deleterious microorganisms and thus, possibly exacerbating the disease development of obesity.
Conclusion
The sexual dimorphisms in the epidemiology and pathophysiology of obesity put males and post-menopausal women at the greatest risk of metabolic disturbances and end-organ damage. Although several factors such as sex hormones, sex chromosomes and fat distribution serve as a basis for these sexual dimorphisms, they can also be attributed to differences in the composition and function of the gut microbiota and the intestinal immune response. Both the gut microbiota and immune system are well-documented influencers of the development of obesity however, the important role that sex plays in this relationship is often overlooked. The “give-and-take” relationship each of these three factors have on one another is an important consideration for future studies (Figure 4). Moreover, the vast majority of studies to date are purely associative. More studies that assess causality are needed to unequivocally identify which harmful gut bacteria and or specific gut microbiome imbalances cause obesity. Importantly, it is crucial that these causal studies firstly consider the sex differences in the gut microbiota prior to commencing the study; and secondly, assess the role that sex plays throughout the treatment that will influence the study outcomes. In addition to this, the sex differences in the intestinal immune response in obesity must also be considered in future studies. Very few studies examine both the microbiome and intestinal immune response. Finally, due to the sexual dimorphisms that exist in both the gut microbiota and intestinal immune response, it is crucial that females – both pre- and post-menopausal – are represented in research studies to the same extent as males for findings and future treatments to be valid in both sexes.
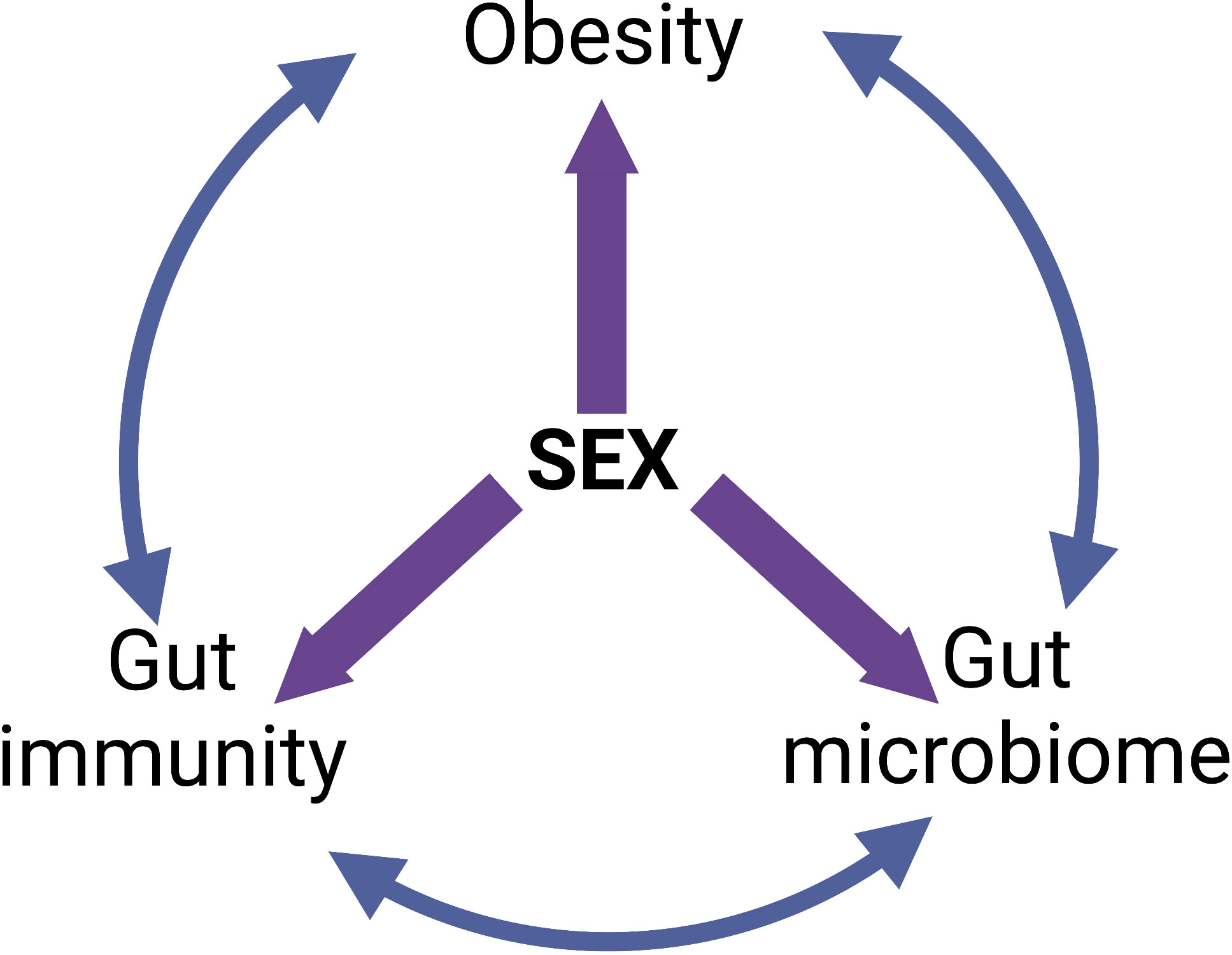
Figure 4 The “give-and-take” relationships between obesity, intestinal immunity, gut microbiome and sex. Created with BioRender.com.
Author contributions
HB wrote the first draft of the manuscript. VT, AV and MJ wrote sections of the manuscript. HB, VT and MJ created the figures. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
HB and VT were funded by Australian Research Training Scholarships. MJ was funded by a joint NHMRC and National Heart Foundation Early Career Fellowship (GNT1146314 and 101943, respectively).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. World Health Organization. Overweight and Obesity. (2021). Available at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
2. Heymsfield SB, Longo DL, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med (2017) 376:254–66. doi: 10.1056/NEJMra1514009
3. Smith KB, Smith MS. Obesity statistics. Prim Care Clin Office Pract (2016) 43:121–35. doi: 10.1016/j.pop.2015.10.001
4. Caleyachetty R, Barber TM, Mohammed NI, Cappuccio FP, Hardy R, Mathur R, et al. Ethnicity-specific BMI cutoffs for obesity based on type 2 diabetes risk in England: A population-based cohort study. Lancet Diabetes Endocrinol (2021) 9:419–26. doi: 10.1016/S2213-8587(21)00088-7
5. Chrostowska M, Szyndler A, Hoffmann M, Narkiewicz K. Impact of obesity on cardiovascular health. Best Pract Res Clin Endocrinol Metab (2013) 27:147–56. doi: 10.1016/j.beem.2013.01.004
6. Palmer BF, Clegg DJ. The sexual dimorphism of obesity. Mol Cell Endocrinol (2015) 402:113–9. doi: 10.1016/j.mce.2014.11.029
7. Org E, Mehrabian M, Parks BW, Shipkova P, Liu X, Drake TA, et al. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes (2016) 7:313–22. doi: 10.1080/19490976.2016.1203502
8. Dean R, Mank JE. The role of sex chromosomes in sexual dimorphism: discordance between molecular and phenotypic data. J Evol Biol (2014) 27:1443–53. doi: 10.1111/jeb.12345
9. Elderman M, van Beek A, Brandsma E, de Haan B, Savelkoul H, de Vos P, et al. Sex impacts Th1 cells, tregs, and DCs in both intestinal and systemic immunity in a mouse strain and location-dependent manner. Biol Sex Differ (2016) 7:21. doi: 10.1186/s13293-016-0075-9
10. Fransen F, van Beek AA, Borghuis T, Meijer B, Hugenholtz F, van der Gaast-de Jongh C, et al. The impact of gut microbiota on gender-specific differences in immunity. Front Immunol (2017) 8:754. doi: 10.3389/fimmu.2017.00754
11. Zheng C, Yang Q, Cao J, Xie N, Liu K, Shou P, et al. Local proliferation initiates macrophage accumulation in adipose tissue during obesity. Cell Death Dis (2016) 7:e2167. doi: 10.1038/cddis.2016.54
12. Chakraborty A, Barajas S, Lammoglia GM, Reyna AJ, Morley TS, Johnson JA, et al. Vascular endothelial growth factor-d (VEGF-d) overexpression and lymphatic expansion in murine adipose tissue improves metabolism in obesity. Am J Pathol (2019) 189:924–39. doi: 10.1016/j.ajpath.2018.12.008
13. Muir LA, Neeley CK, Meyer KA, Baker NA, Brosius AM, Washabaugh AR, et al. Adipose tissue fibrosis, hypertrophy, and hyperplasia: Correlations with diabetes in human obesity. Obes (Silver Spring) (2016) 24:597–605. doi: 10.1002/oby.21377
14. Kang YE, Kim JM, Joung KH, Lee JH, You BR, Choi MJ, et al. The roles of adipokines, proinflammatory cytokines, and adipose tissue macrophages in obesity-associated insulin resistance in modest obesity and early metabolic dysfunction. PLoS One (2016) 11:e0154003. doi: 10.1371/journal.pone.0154003
15. Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Immunol (2011) 11:85–97. doi: 10.1038/nri2921
16. Brestoff JR, Artis D. Immune regulation of metabolic homeostasis in health and disease. Cell (2015) 161:146–60. doi: 10.1016/j.cell.2015.02.022
17. Link JC, Reue K. Genetic basis for sex differences in obesity and lipid metabolism. Annu Rev Nutr (2017) 37:225–45. doi: 10.1146/annurev-nutr-071816-064827
18. Kanter R, Caballero B. Global gender disparities in obesity: A review. Adv Nutr (2012) 3:491–8. doi: 10.3945/an.112.002063
19. Hong J, Stubbins RE, Smith RR, Harvey AE, Nunez NP. Differential susceptibility to obesity between male, female and ovariectomized female mice. Nutr J (2009) 8:11. doi: 10.1186/1475-2891-8-11
20. Arcones AC, Cruces-Sande M, Ramos P, Mayor F Jr., Murga C. Sex differences in high fat diet-induced metabolic alterations correlate with changes in the modulation of GRK2 levels. Cells (2019) 8(11):1464. doi: 10.3390/cells8111464
21. Hwang LL, Wang CH, Li TL, Chang SD, Lin LC, Chen CP, et al. Sex differences in high-fat diet-induced obesity, metabolic alterations and learning, and synaptic plasticity deficits in mice. Obes (Silver Spring) (2010) 18:463–9. doi: 10.1038/oby.2009.273
22. Sharma G, Mauvais-Jarvis F, Prossnitz ER. Roles of G protein-coupled estrogen receptor GPER in metabolic regulation. J Steroid Biochem Mol Biol (2018) 176:31–7. doi: 10.1016/j.jsbmb.2017.02.012
23. Pradhan AD. Sex differences in the metabolic syndrome: implications for cardiovascular health in women. Clin Chem (2014) 60:44–52. doi: 10.1373/clinchem.2013.202549
24. Bianchi VE, Locatelli V. Testosterone a key factor in gender related metabolic syndrome. Obes Rev (2018) 19:557–75. doi: 10.1111/obr.12633
25. Shi H, Clegg DJ. Sex differences in the regulation of body weight. Physiol Behav (2009) 97:199–204. doi: 10.1016/j.physbeh.2009.02.017
26. Hetemaki N, Savolainen-Peltonen H, Tikkanen MJ, Wang F, Paatela H, Hamalainen E, et al. Estrogen metabolism in abdominal subcutaneous and visceral adipose tissue in postmenopausal women. J Clin Endocrinol Metab (2017) 102:4588–95. doi: 10.1210/jc.2017-01474
27. Bjorntrop P. Body fat distribution, insulin resistance, and metabolic diseases. J Nutr (1997) 13:795–803. doi: 10.1016/S0899-9007(97)00191-3
28. Gruzdeva O, Borodkina D, Uchasova E, Dyleva Y, Barbarash O. Localization of fat depots and cardiovascular risk. Lipids Health Dis (2018) 17:218. doi: 10.1186/s12944-018-0856-8
29. Rask-Andersen M, Karlsson T, Ek WE, Johansson A. Genome-wide association study of body fat distribution identifies adiposity loci and sex-specific genetic effects. Nat Commun (2019) 10:339. doi: 10.1038/s41467-018-08000-4
30. Jelavic MM, Babic Z, Pintaric H. The importance of two metabolic syndrome diagnostic criteria and body fat distribution in predicting clinical severity and prognosis of acute myocardial infarction. Arch Med Sci (2017) 13:795–806. doi: 10.5114/aoms.2016.59703
31. Ladeiras-Lopes R, Sampaio F, Bettencourt N, Fontes-Carvalho R, Ferreira N, Leite-Moreira A, et al. The ratio between visceral and subcutaneous abdominal fat assessed by computed tomography is an independent predictor of mortality and cardiac events. Rev Esp Cardiol (2017) 70:331–7. doi: 10.1016/j.recesp.2016.09.006
32. Pettersson US, Walden TB, Carlsson PO, Jansson L, Phillipson M. Female mice are protected against high-fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PloS One (2012) 7:e46057. doi: 10.1371/journal.pone.0046057
33. Christensen RH, von Scholten BJ, Hansen CS, Jensen MT, Vilsboll T, Rossing P, et al. Epicardial adipose tissue predicts incident cardiovascular disease and mortality in patients with type 2 diabetes. Cardiovasc Diabetol (2019) 18:114. doi: 10.1186/s12933-019-0917-y
34. Ruiz-Castell M, Samouda H, Bocquet V, Fagherazzi G, Stranges S, Huiart L. Estimated visceral adiposity is associated with risk of cardiometabolic conditions in a population based study. Sci Rep (2021) 11:9121. doi: 10.1038/s41598-021-88587-9
35. Elffers TW, de Mutsert R, Lamb HJ, de Roos A, Willems van Dijk K, Rosendaal FR, et al. Body fat distribution, in particular visceral fat, is associated with cardiometabolic risk factors in obese women. PLoS One (2017) 12:e0185403. doi: 10.1371/journal.pone.0185403
36. Toth MJ, Tchernof A, Sites CK, Poehlman ET. Effect of menopausal status on body composition and abdominal fat distribution. Int J Obes (2000) 24:226–31. doi: 10.1038/sj.ijo.0801118
37. Chen GC, Arthur R, Iyengar NM, Kamensky V, Xue X, Wassertheil-Smoller S, et al. Association between regional body fat and cardiovascular disease risk among postmenopausal women with normal body mass index. Eur Heart J (2019) 40:2849–55. doi: 10.1093/eurheartj/ehz391
38. Karvonen-Gutierrez C, Kim C. Association of mid-life changes in body size, body composition and obesity status with the menopausal transition. Healthcare (Basel) (2016) 4(3):42. doi: 10.3390/healthcare4030042
39. Vieira Potter VJ, Strissel KJ, Xie C, Chang E, Bennett G, Defuria J, et al. Adipose tissue inflammation and reduced insulin sensitivity in ovariectomized mice occurs in the absence of increased adiposity. Endocrinology (2012) 153:4266–77. doi: 10.1210/en.2011-2006
40. Giannini A, Montt-Guevara M, Shortrede JE, Palla G, Chedraui P, Genazzani AR, et al. Metabolic syndrome and excessive body weight in peri- and postmenopausal women. In: Postmenopausal diseases and disorders. Cham: Springer (2019).
41. Manolopoulos KN, Karpe F, Frayn KN. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes (Lond) (2010) 34:949–59. doi: 10.1038/ijo.2009.286
42. Lee JW, Kim SY, Lee HJ, Han SW, Lee JE, Lee SM. Prognostic significance of abdominal-to-Gluteofemoral adipose tissue distribution in patients with breast cancer. J Clin Med (2019) 8(9):1358. doi: 10.3390/jcm8091358
43. Lotta LA, Wittemans LBL, Zuber V, Stewart ID, Sharp SJ, Luan J, et al. Association of genetic variants related to gluteofemoral vs abdominal fat distribution with type 2 diabetes, coronary disease, and cardiovascular risk factors. JAMA (2018) 320:2553–63. doi: 10.1001/jama.2018.19329
44. Link JC, Chen X, Arnold AP, Reue K. Metabolic impact of sex chromosomes. Adipocyte (2013) 2:74–9. doi: 10.4161/adip.23320
45. Chen X, McClusky R, Chen J, Beaven SW, Tontonoz P, Arnold AP, et al. The number of x chromosomes causes sex differences in adiposity in mice. PLoS Genet (2012) 8:e1002709. doi: 10.1371/journal.pgen.1002709
46. Zore T, Palafox M, Reue K. Sex differences in obesity, lipid metabolism, and inflammation-a role for the sex chromosomes? Mol Metab (2018) 15:35–44. doi: 10.1016/j.molmet.2018.04.003
47. Bojesen A, Host C, Gravholt CH. Klinefelter's syndrome, type 2 diabetes and the metabolic syndrome: the impact of body composition. Mol Hum Reprod (2010) 16:396–401. doi: 10.1093/molehr/gaq016
48. Baldin AD, Siviero-Miachon AA, Fabbri T, de Lemos-Marini SH, Spinola-Castro AM, Baptista MT, et al. Turner syndrome and metabolic derangements: another example of fetal programming. Early Hum Dev (2012) 88:99–102. doi: 10.1016/j.earlhumdev.2011.07.014
49. Lloyd-Price J, Abu-Ali G, Huttenhower C. The healthy human microbiome. Genome Med (2016) 8:51. doi: 10.1186/s13073-016-0307-y
50. Chen Y, Chang SKC, Zhang Y, Hsu CY, Nannapaneni R. Gut microbiota and short chain fatty acid composition as affected by legume type and processing methods as assessed by simulated in vitro digestion assays. Food Chem (2020) 312:126040. doi: 10.1016/j.foodchem.2019.126040
51. Jiminez JA, Uwiera TC, Abbott DW, Uwiera RRE, Inglis GD. Butyrate supplementation at high concentrations alters enteric bacterial communities and reduces intestinal inflammation in mice infected with citrobacter rodentium. mSphere (2017) 2(4):e00243-17. doi: 10.1128/mSphere.00243-17
52. Mollica MP, Mattace Raso G, Cavaliere G, Trinchese G, De Filippo C, Aceto S, et al. Butyrate regulates liver mitochondrial function, efficiency, and dynamics in insulin-resistant obese mice. Diabetes (2017) 66:1405–18. doi: 10.2337/db16-0924
53. Velikonja A, Lipoglavsek L, Zorec M, Orel R, Avgustin G. Alterations in gut microbiota composition and metabolic parameters after dietary intervention with barley beta glucans in patients with high risk for metabolic syndrome development. Anaerobe (2019) 55:67–77. doi: 10.1016/j.anaerobe.2018.11.002
54. Khan MJ, Gerasimidis K, Edwards CA, Shaikh MG. Role of gut microbiota in the aetiology of obesity: Proposed mechanisms and review of the literature. J Obes (2016) 2016:7353642. doi: 10.1155/2016/7353642
55. Yadav S, Jha R. Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J Anim Sci Biotechnol (2019) 10:2. doi: 10.1186/s40104-018-0310-9
56. Elderman M, de Vos P, Faas M. Role of microbiota in sexually dimorphic immunity. Front Immunol (2018) 9:1018. doi: 10.3389/fimmu.2018.01018
57. Shimizu K, Yamada T, Ogura H, Mohri T, Kiguchi T, Fujimi S, et al. Synbiotics modulate gut microbiota and reduce enteritis and ventilator-associated pneumonia in patients with sepsis: A randomized controlled trial. J Crit Care (2018) 22:239. doi: 10.1186/s13054-018-2167-x
58. Li JY, Chassaing B, Tyagi AM, Vaccaro C, Luo T, Adams J, et al. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest (2016) 126:2049–63. doi: 10.1172/JCI86062
59. Yurkovetskiy L, Burrows M, Khan AA, Graham L, Volchkov P, Becker L, et al. Gender bias in autoimmunity is influenced by microbiota. Immunity (2013) 39:400–12. doi: 10.1016/j.immuni.2013.08.013
60. Cross TL, Kasahara K, Rey FE. Sexual dimorphism of cardiometabolic dysfunction: Gut microbiome in the play? Mol Metab (2018) 15:70–81. doi: 10.1016/j.molmet.2018.05.016
61. Acharya KD, Gao X, Bless EP, Chen J, Tetel MJ. Estradiol and high fat diet associate with changes in gut microbiota in female ob/ob mice. Sci Rep (2019) 9:20192. doi: 10.1038/s41598-019-56723-1
62. Ottman N, Ruokolainen L, Suomalainen A, Sinkko H, Karisola P, Lehtimaki J, et al. Soil exposure modifies the gut microbiota and supports immune tolerance in a mouse model. J Allergy Clin Immunol (2019) 143:1198–206.e12. doi: 10.1016/j.jaci.2018.06.024
63. Li X, Watanabe K, Kimura I. Gut microbiota dysbiosis drives and implies novel therapeutic strategies for diabetes mellitus and related metabolic diseases. Front Immunol (2017) 8:1882. doi: 10.3389/fimmu.2017.01882
64. McBurney MI, Davis C, Fraser CM, Schneeman BO, Huttenhower C, Verbeke K, et al. Establishing what constitutes a healthy human gut microbiome: State of the science, regulatory considerations, and future directions. J Nutr (2019) 149:1882–95. doi: 10.1093/jn/nxz154
65. Marques FZ, Jama HA, Tsyganov K, Gill PA, Rhys-Jones D, Muralitharan RR, et al. Guidelines for transparency on gut microbiome studies in essential and experimental hypertension. Hypertension (2019) 74:1279–93. doi: 10.1161/HYPERTENSIONAHA.119.13079
66. Zhu C, Wang X, Li J, Jiang R, Chen H, Chen T, et al. Determine independent gut microbiota-diseases association by eliminating the effects of human lifestyle factors. BMC Microbiol (2022) 22:4. doi: 10.1186/s12866-021-02414-9
67. Brüssow H. Problems with the concept of gut microbiota dysbiosis. Microbial Biotechnol (2020) 13:423–34. doi: 10.1111/1751-7915.13479
68. Santos-Marcos JA, Perez-Jimenez F, Camargo A. The role of diet and intestinal microbiota in the development of metabolic syndrome. J Nutr Biochem (2019) 70:1–27. doi: 10.1016/j.jnutbio.2019.03.017
69. Linninge C, Xu J, Bahl MI, Ahrne S, Molin G. Lactobacillus fermentum and lactobacillus plantarum increased gut microbiota diversity and functionality, and mitigated enterobacteriaceae, in a mouse model. Benef Microbes (2019) 10:413–24. doi: 10.3920/BM2018.0074
70. Jakobsson HE, Abrahamsson TR, Jenmalm MC, Harris K, Quince C, Jernberg C, et al. Decreased gut microbiota diversity, delayed bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut (2014) 63:559–66. doi: 10.1136/gutjnl-2012-303249
71. Wang H, Hong T, Li N, Zang B, Wu X. Soluble dietary fiber improves energy homeostasis in obese mice by remodeling the gut microbiota. Biochem Biophys Res Commun (2018) 498:146–51. doi: 10.1016/j.bbrc.2018.02.017
72. Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci (2007) 104:979–84. doi: 10.1073/pnas.0605374104
73. McFadyen T, Chai LK, Wyse R, Kingsland M, Yoong SL, Clinton-McHarg T, et al. Strategies to improve the implementation of policies, practices or programmes in sporting organisations targeting poor diet, physical inactivity, obesity, risky alcohol use or tobacco use: A systematic review. BMJ Open (2018) 8:e019151. doi: 10.1136/bmjopen-2017-019151
74. Kulecka M, Paziewska A, Zeber-Lubecka N, Ambrozkiewicz F, Kopczynski M, Kuklinska U, et al. Prolonged transfer of feces from the lean mice modulates gut microbiota in obese mice. Nutr Metab (2016) 13:57. doi: 10.1186/s12986-016-0116-8
75. Razavi AC, Potts KS, Kelly TN, Bazzano LA. Sex, gut microbiome, and cardiovascular disease risk. Biol Sex Differ (2019) 10:29. doi: 10.1186/s13293-019-0240-z
76. Pindjakova J, Sartini C, Lo Re O, Rappa F, Coupe B, Lelouvier B, et al. Gut dysbiosis and adaptive immune response in diet-induced obesity vs. systemic inflammation. Front Microbiol (2017) 8:1157. doi: 10.3389/fmicb.2017.01157
77. Panchal SK, Brown L. Rodent models for metabolic syndrome research. J BioMed Biotechnol (2011) 2011:351982. doi: 10.1155/2011/351982
78. Gilbert ER, Fu Z, Liu D. Development of a nongenetic mouse model of type 2 diabetes. Exp Diabetes Res (2011) 2011:416254. doi: 10.1155/2011/416254
79. Rune I, Hansen CH, Ellekilde M, Nielsen DS, Skovgaard K, Rolin BC, et al. Ampicillin-improved glucose tolerance in diet-induced obese C57BL/6NTac mice is age dependent. J Diabetes Res (2013) 2013:319321. doi: 10.1155/2013/319321
80. Cho I, Yamanishi S, Cox L, Methe BA, Zavadil J, Li K, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature (2012) 488:621–6. doi: 10.1038/nature11400
81. Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E. Dysbiosis and the immune system. Nat Rev Immunol (2017) 17:219–32. doi: 10.1038/nri.2017.7
82. Haro C, Rangel-Zuniga OA, Alcala-Diaz JF, Gomez-Delgado F, Perez-Martinez P, Delgado-Lista J, et al. Intestinal microbiota is influenced by gender and body mass index. PloS One (2016) 11:e0154090. doi: 10.1371/journal.pone.0154090
83. DeGruttola AK, Low D, Mizoguchi A, Mizoguchi E. Current understanding of dysbiosis in disease in human and animal models. Inflammation Bowel Dis (2016) 22:1137–50. doi: 10.1097/MIB.0000000000000750
84. Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature (2012) 486:207–14. doi: 10.1038/nature11234
85. Patrone V, Vajana E, Minuti A, Callegari ML, Federico A, Loguercio C, et al. Postoperative changes in fecal bacterial communities and fermentation products in obese patients undergoing bilio-intestinal bypass. Front Microbiol (2016) 7:200. doi: 10.3389/fmicb.2016.00200
86. Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Human gut microbes associated with obesity. Nature (2006) 444:1022–3. doi: 10.1038/4441022a
87. Palmas V, Pisanu S, Madau V, Casula E, Deledda A, Cusano R, et al. Gut microbiota markers associated with obesity and overweight in Italian adults. Sci Rep (2021) 11:5532. doi: 10.1038/s41598-021-84928-w
88. Ulker I, Yildiran H. The effects of bariatric surgery on gut microbiota in patients with obesity: A review of the literature. Biosci Microb Food Health (2019) 38:3–9. doi: 10.12938/bmfh.18-018
89. Duan M, Wang Y, Zhang Q, Zou R, Guo M, Zheng H. Characteristics of gut microbiota in people with obesity. PloS One (2021) 16:e0255446. doi: 10.1371/journal.pone.0255446
90. Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, Donus C, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obes (Silver Spring) (2010) 18:190–5. doi: 10.1038/oby.2009.167
91. Magne F, Gotteland M, Gauthier L, Zazueta A, Pesoa S, Navarrete P, et al. The Firmicutes/Bacteroidetes ratio: A relevant marker of gut dysbiosis in obese patients? Nutrients (2020) 12(5):1474. doi: 10.3390/nu12051474
92. Schneeberger M, Everard A, Gomez-Valades AG, Matamoros S, Ramirez S, Delzenne NM, et al. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci Rep (2015) 5:16643. doi: 10.1038/srep16643
93. Wang J, Tang H, Zhang C, Zhao Y, Derrien M, Rocher E, et al. Modulation of gut microbiota during probiotic-mediated attenuation of metabolic syndrome in high fat diet-fed mice. ISME J (2015) 9:1–15. doi: 10.1038/ismej.2014.99
94. Trastoy B, Naegeli A, Anso I, Sjögren J, Guerin ME. Structural basis of mammalian mucin processing by the human gut O-glycopeptidase OgpA from akkermansia muciniphila. Nat Commun (2020) 11:4844. doi: 10.1038/s41467-020-18696-y
95. Chelakkot C, Choi Y, Kim DK, Park HT, Ghim J, Kwon Y, et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp Mol Med (2018) 50:e450. doi: 10.1038/emm.2017.282
96. Stenman LK, Burcelin R, Lahtinen S. Establishing a causal link between gut microbes, body weight gain and glucose metabolism in humans - towards treatment with probiotics. Benef Microbes (2016) 7:11–22. doi: 10.3920/BM2015.0069
97. Gruber L, Kisling S, Lichti P, Martin FP, May S, Klingenspor M, et al. High fat diet accelerates pathogenesis of murine crohn's disease-like ileitis independently of obesity. PloS One (2013) 8:e71661. doi: 10.1371/journal.pone.0071661
98. Xu Y, Wang N, Tan HY, Li S, Zhang C, Feng Y. Function of akkermansia muciniphila in obesity: Interactions with lipid metabolism, immune response and gut systems. Front Microbiol (2020) 11:219. doi: 10.3389/fmicb.2020.00219
99. Si X, Shang W, Zhou Z, Strappe P, Wang B, Bird A, et al. Gut microbiome-induced shift of acetate to butyrate positively manages dysbiosis in high fat diet. Mol Nutr Food Res (2018) 62:1700670. doi: 10.1002/mnfr.201700670
100. Coutinho C, Coutinho-Silva R, Zinkevich V, Pearce CB, Ojcius DM, Beech I. Sulphate-reducing bacteria from ulcerative colitis patients induce apoptosis of gastrointestinal epithelial cells. Microb Pathog (2017) 112:126–34. doi: 10.1016/j.micpath.2017.09.054
101. Kaliannan K, Robertson RC, Murphy K, Stanton C, Kang C, Wang B, et al. Estrogen-mediated gut microbiome alterations influence sexual dimorphism in metabolic syndrome in mice. Microbiome (2018) 6:205. doi: 10.1186/s40168-018-0587-0
102. Nighot M, Al-Sadi R, Guo S, Rawat M, Nighot P, Watterson MD, et al. Lipopolysaccharide-induced increase in intestinal epithelial tight permeability is mediated by toll-like receptor 4/myeloid differentiation primary response 88 (MyD88) activation of myosin light chain kinase expression. Am J Pathol (2017) 187:2698–710. doi: 10.1016/j.ajpath.2017.08.005
103. Shin J, Noh JR, Chang DH, Kim YH, Kim MH, Lee ES, et al. Elucidation of akkermansia muciniphila probiotic traits driven by mucin depletion. Front Microbiol (2019) 10:1137. doi: 10.3389/fmicb.2019.01137
104. Kawano M, Miyoshi M, Ogawa A, Sakai F, Kadooka Y. Lactobacillus gasseri SBT2055 inhibits adipose tissue inflammation and intestinal permeability in mice fed a high-fat diet. J Nutr Sci (2016) 5:e23. doi: 10.1017/jns.2016.12
105. Yao H, Fan C, Lu Y, Fan X, Xia L, Li P, et al. Alteration of gut microbiota affects expression of adiponectin and resistin through modifying DNA methylation in high-fat diet-induced obese mice. Genes Nutr (2020) 15:12. doi: 10.1186/s12263-020-00671-3
106. Li R, Andreu-Sánchez S, Kuipers F, Fu J. Gut microbiome and bile acids in obesity-related diseases. Best Pract Res Clin Endocrinol Metab (2021) 35:101493. doi: 10.1016/j.beem.2021.101493
107. Allegretti JR, Kassam Z, Mullish BH, Chiang A, Carrellas M, Hurtado J, et al. Effects of fecal microbiota transplantation with oral capsules in obese patients. Clin Gastroenterol Hepatol (2020) 18:855–863.e2. doi: 10.1016/j.cgh.2019.07.006
108. Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, et al. Richness of human gut microbiome correlates with metabolic markers. Nature (2013) 500:541–6. doi: 10.1038/nature12506
109. Massier L, Chakaroun R, Tabei S, Crane A, Didt KD, Fallmann J, et al. Adipose tissue derived bacteria are associated with inflammation in obesity and type 2 diabetes. Gut (2020) 69:1796–806. doi: 10.1136/gutjnl-2019-320118
110. Torres-Fuentes C, Golubeva AV, Zhdanov AV, Wallace S, Arboleya S, Papkovsky DB, et al. Short-chain fatty acids and microbiota metabolites attenuate ghrelin receptor signaling. FASEB J (2019) 33:13546–59. doi: 10.1096/fj.201901433R
111. Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D, et al. Environment dominates over host genetics in shaping human gut microbiota. Nature (2018) 555:210–5. doi: 10.1038/nature25973
112. Davenport ER. Genetic variation shapes murine gut microbiota via immunity. Trends Immunol (2020) 41:1–3. doi: 10.1016/j.it.2019.11.009
113. Bridgewater LC, Zhang C, Wu Y, Hu W, Zhang Q, Wang J, et al. Gender-based differences in host behavior and gut microbiota composition in response to high fat diet and stress in a mouse model. Sci Rep (2017) 7:10776. doi: 10.1038/s41598-017-11069-4
114. Steegenga WT, Mischke M, Lute C, Boekschoten MV, Pruis MGM, Lendvai A, et al. Sexually dimorphic characteristics of the small intestine and colon of prepubescent C57BL/6 mice. Biol Sex Differ (2014) 5:1–17. doi: 10.1186/s13293-014-0011-9
115. Markle J, Frank D, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science (2013) 339:1084–8. doi: 10.1126/science.1233521
116. Kozik AJ, Nakatsu CH, Chun H, Jones-Hall YL. Age, sex, and TNF associated differences in the gut microbiota of mice and their impact on acute TNBS colitis. Exp Mol Pathol (2017) 103:311–9. doi: 10.1016/j.yexmp.2017.11.014
117. Suzuki Y, Ikeda K, Sakuma K, Kawai S, Sawaki K, Asahara T, et al. Association between yogurt consumption and intestinal microbiota in healthy young adults differs by host gender. Front Microbiol (2017) 8:847. doi: 10.3389/fmicb.2017.00847
118. Mueller S, Saunier K, Hanisch C, Norin E, Alm L, Midtvedt T, et al. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: A cross-sectional study. Appl Environ Microbiol (2006) 72:1027–33. doi: 10.1128/AEM.72.2.1027-1033.2006
119. Takagi T, Naito Y, Inoue R, Kashiwagi S, Uchiyama K, Mizushima K, et al. Differences in gut microbiota associated with age, sex, and stool consistency in healthy Japanese subjects. J Gastroenterol (2019) 54:53–63. doi: 10.1007/s00535-018-1488-5
120. Faith JJ, McNulty NP, Rey FE, Gordon JI. Predicting a human gut microbiota's response to diet in gnotobiotic mice. Science (2011) 333:101–4. doi: 10.1126/science.1206025
121. Shin JH, Park YH, Sim M, Kim SA, Joung H, Shin DM. Serum level of sex steroid hormone is associated with diversity and profiles of human gut microbiome. Res Microbiol (2019) 170:192–201. doi: 10.1016/j.resmic.2019.03.003
122. Wang JJ, Wang J, Pang XY, Zhao LP, Tian L, Wang XP. Sex differences in colonization of gut microbiota from a man with short-term vegetarian and inulin-supplemented diet in germ-free mice. Sci Rep (2016) 6:36137. doi: 10.1038/srep36137
123. Baber RJ, Panay N, Fenton A, I.M.S.W. Group. 2016 IMS recommendations on women's midlife health and menopause hormone therapy. Climacteric (2016) 19:109–50. doi: 10.3109/13697137.2015.1129166
124. Garratt M, Lagerborg KA, Tsai YM, Galecki A, Jain M, Miller RA. Male Lifespan extension with 17-alpha estradiol is linked to a sex-specific metabolomic response modulated by gonadal hormones in mice. Aging Cell (2018) 17:e12786. doi: 10.1111/acel.12786
125. Cuevas-Sierra A, Romo-Hualde A, Aranaz P, Goni L, Cuervo M, Martinez JA, et al. Diet- and sex-related changes of gut microbiota composition and functional profiles after 4 months of weight loss intervention. Eur J Nutr (2021) 60:3279–301. doi: 10.1007/s00394-021-02508-0
126. Daly CM, Saxena J, Singh J, Bullard MR, Bondy EO, Saxena A, et al. Sex differences in response to a high fat, high sucrose diet in both the gut microbiome and hypothalamic astrocytes and microglia. Nutr Neurosci (2020)25(2):321–35. doi: 10.1080/1028415X.2020.1752996
127. Qin Y, Roberts JD, Grimm SA, Lih FB, Deterding LJ, Li R, et al. An obesity-associated gut microbiome reprograms the intestinal epigenome and leads to altered colonic gene expression. Genome Biol (2018) 19:7. doi: 10.1186/s13059-018-1389-1
128. Vesth T, Ozen A, Andersen SC, Kaas RS, Lukjancenko O, Bohlin J, et al. Veillonella, firmicutes: Microbes disguised as gram negatives. Stand Genom Sci (2013) 9:431–48. doi: 10.4056/sigs.2981345
129. Neyrinck AM, Possemiers S, Verstraete W, De Backer F, Cani PD, Delzenne NM. Dietary modulation of clostridial cluster XIVa gut bacteria (Roseburia spp.) by chitin-glucan fiber improves host metabolic alterations induced by high-fat diet in mice. J Nutr Biochem (2012) 23:51–9. doi: 10.1016/j.jnutbio.2010.10.008
130. Natividad JM, Lamas B, Pham HP, Michel ML, Rainteau D, Bridonneau C, et al. Bilophila wadsworthia aggravates high fat diet induced metabolic dysfunctions in mice. Nat Commun (2018) 9:2802. doi: 10.1038/s41467-018-05249-7
131. Wexler AG, Goodman AL. An insider's perspective: Bacteroides as a window into the microbiome. Nat Microbiol (2017) 2:17026. doi: 10.1038/nmicrobiol.2017.26
132. Roopchand DE, Carmody RN, Kuhn P, Moskal K, Rojas-Silva P, Turnbaugh PJ, et al. Dietary polyphenols promote growth of the gut bacterium akkermansia muciniphila and attenuate high-fat diet–induced metabolic syndrome. Diabetes (2015) 64:2847–58. doi: 10.2337/db14-1916
133. Zhu G, Ma F, Wang G, Wang Y, Zhao J, Zhang H, et al. Bifidobacteria attenuate the development of metabolic disorders, with inter- and intra-species differences. Food Funct (2018) 9:3509–22. doi: 10.1039/C8FO00100F
134. Shastri P, McCarville J, Kalmokoff M, Brooks SP, Green-Johnson JM. Sex differences in gut fermentation and immune parameters in rats fed an oligofructose-supplemented diet. Biol Sex Differ (2015) 6:13. doi: 10.1186/s13293-015-0031-0
135. He J, Wang W, Wu Z, Pan D, Guo Y, Cai Z, et al. Effect of lactobacillus reuteri on intestinal microbiota and immune parameters: Involvement of sex differences. J Funct Foods (2019) 53:36–43. doi: 10.1016/j.jff.2018.12.010
136. Choi S, Hwang YJ, Shin MJ, Yi H. Difference in the gut microbiome between ovariectomy-induced obesity and diet-induced obesity. J Microbiol Biotechnol (2017) 27:2228–36. doi: 10.4014/jmb.1710.10001
137. Santos-Marcos JA, Rangel-Zuniga OA, Jimenez-Lucena R, Quintana-Navarro GM, Garcia-Carpintero S, Malagon MM, et al. Influence of gender and menopausal status on gut microbiota. Maturitas (2018) 116:43–53. doi: 10.1016/j.maturitas.2018.07.008
138. Ding S, Lund PK. Role of intestinal inflammation as an early event in obesity and insulin resistance. Curr Opin Clin Nutr Metab Care (2011) 14:328–33. doi: 10.1097/MCO.0b013e3283478727
139. Winer DA, Luck H, Tsai S, Winer S. The intestinal immune system in obesity and insulin resistance. Cell Metab (2016) 23:413–26. doi: 10.1016/j.cmet.2016.01.003
140. Vancamelbeke M, Vermeire S. The intestinal barrier: A fundamental role in health and disease. Expert Rev Gastroenterol Hepatol (2017) 11:821–34. doi: 10.1080/17474124.2017.1343143
141. Sankaran-Walters S, Macal M, Grishina I, Nagy L, Goulart L, Coolidge K, et al. Sex differences matter in the gut: Effect on mucosal immune activation and inflammation. Biol Sex Differ (2013) 4:1–12. doi: 10.1186/2042-6410-4-10
142. Houghton LA, Heitkemper M, Crowell M, Emmanuel A, Halpert A, McRoberts JA, et al. Age, gender and women's health and the patient. Gastroenterology (2016) S0016-5085(16)00183-9. doi: 10.1053/j.gastro.2016.02.017
143. Ciampolillo A. Metabolic syndrome and gut microbiota: There is a gender difference? Ital J Gend-Specif Med (2019) 5:21–6. doi: 10.1723/3148.31295
144. Fiebiger U, Bereswill S, Heimesaat MM. Dissecting the interplay between intestinal microbiota and host immunity in health and disease: Lessons learned from germfree and gnotobiotic animal models. Eur J Microbiol Immunol (2016) 6:253–71. doi: 10.1556/1886.2016.00036
145. Vemuri R, Sylvia KE, Klein SL, Forster SC, Plebanski M, Eri R, et al. The microgenderome revealed: Sex differences in bidirectional interactions between the microbiota, hormones, immunity and disease susceptibility. Semin Immunopathol (2019) 41:265–75. doi: 10.1007/s00281-018-0716-7
146. Postler TS, Ghosh S. Understanding the holobiont: How microbial metabolites affect human health and shape the immune system. Cell Metab (2017) 26:110–30. doi: 10.1016/j.cmet.2017.05.008
147. Wu S, Pan L, Liao H, Yao W, Shen N, Chen C, et al. High-fat diet increased NADPH-oxidase-related oxidative stress and aggravated LPS-induced intestine injury. Life Sci (2020) 253:117539. doi: 10.1016/j.lfs.2020.117539
Keywords: leukocytes, obesity, gut microbiota, estrogen (17β-estradiol), testosterone
Citation: Brettle H, Tran V, Drummond GR, Franks AE, Petrovski S, Vinh A and Jelinic M (2022) Sex hormones, intestinal inflammation, and the gut microbiome: Major influencers of the sexual dimorphisms in obesity. Front. Immunol. 13:971048. doi: 10.3389/fimmu.2022.971048
Received: 16 June 2022; Accepted: 16 August 2022;
Published: 27 September 2022.
Edited by:
Mario M. D’Elios, University of Florence, ItalyReviewed by:
Guojun Wu, Rutgers, The State University of New Jersey, United StatesChiara Della Bella, University of Florence, Italy
Nagaja Capitani, University of Siena, Italy
Copyright © 2022 Brettle, Tran, Drummond, Franks, Petrovski, Vinh and Jelinic. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Jelinic, bS5qZWxpbmljQGxhdHJvYmUuZWR1LmF1
†These authors share first authorship
 Holly Brettle
Holly Brettle Vivian Tran1†
Vivian Tran1† Grant R. Drummond
Grant R. Drummond Ashley E. Franks
Ashley E. Franks Antony Vinh
Antony Vinh Maria Jelinic
Maria Jelinic