
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Immunol., 08 September 2022
Sec. Autoimmune and Autoinflammatory Disorders
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.970371
Psoriasis (PsO) and psoriatic arthritis (PsA), together known as psoriatic disease (PsD), are immune-mediated diseases with a chronic and relapsing course that affect the skin, the joints or both. The pathophysiology of PsO is complex and involves abnormal expression of keratinocytes and infiltration of the skin with dendritic cells, macrophages, neutrophils and T lymphocytes. Around 30% of patients with PsO develop arthritis with axial and/or peripheral manifestations. Both PsO and PsA share similar Th1- and Th17-driven inflammation, with increased production of inflammatory cytokines, including TNFα, IFN-γ, IL-17, IL-22, IL-23 in the skin and the synovial membrane. PsD is associated with a high burden of cardiometabolic diseases such as hypertension, diabetes, dyslipidemia, obesity, metabolic syndrome and cardiovascular (CV) complications as compared to the general population. These comorbidities share common immunopathogenic pathways linked to systemic inflammation, and are associated with the extent and severity of the disease. Morever, they can influence treatment outcomes in PsD. In this short review, we summarize the available evidence on the epidemiology, clinical aspects and mechanisms of cardiometabolic conditions in patients with PsD. We also discuss the impact of targeted treatments such as methotrexate and biological agents on these cardiometabolic conditions.
Psoriasis (PsO) is a chronic skin disease affecting around 3% of the worldwide population. It is an immune-mediated disease characterized by abnormal expression of keratinocytes and infiltration of the dermis with dendritic cells, macrophages, neutrophils and T lymphocytes (1). Around 30% of patients with PsO develop psoriatic arthritis (PsA), a condition that is included in the spondyloarthritis group. Indeed, PsA may involve the axial skeleton and/or the peripheral joints, but also entheseal structures, overall leading to joint damage, physical limitation and disability (2). Both PsO and PsA share similar Th1- and Th17-driven inflammation, with increased production of inflammatory cytokines including TNFα, IFN-γ, IL-6, IL-8, IL-17, IL-23 in the skin and synovial membrane. Due to common pathophysiological mechanisms and complications, PsOs and PsA are collectively grouped under the term psoriatic disease (PsD) and are associated with specific extra-cutaneous/articular manifestations and comorbidities. Collaborative and multidisplinary approaches with dermatologists and rheumatologists have been developed in different countries in order to better diagnose and manage patients with PsD (3). There is compelling evidence that PsD carries a greater of developing cardiometabolic diseases as compared to the general population (4, 5). Higher cardiovascular (CV) risk is well documented in immune-mediated diseases such as rheumatoid arthritis (RA) or systemic lupus erythematosus (6). In parallel, there is a growing interest in CV and metabolic diseases in patients with PsD (4, 5, 7).
In this narrative review, we aim to analyse the cardiometabolic conditions of patients with PsD, with a focus on the underlying mechanisms. We also examine the influences that these comorbidities may have on treatment response in PsD patients, as well as the impact of conventional and targeted drugs on these conditions.
The association between CV mortality and morbidity is well described in patients with RA, and RA itself is considered to be an independant CV risk factor similar to the risk induced by type 2 diabetes (8). CV risk is also enhanced in PsA, and the prevalence of CV diseases in PsA is reportedly comparable to that observed in RA (9). A meta-analysis based on 11 studies found a 43% increase in CV diseases in PsA compared to the general population. In addition, the risk of myocardial infarction, cerebrovascular disease and heart failure was increased by 68%, 22% and 31% respectively (10). The relationship between inflammation in patients with PsA and the subsequent development of CV diseases has been examined in specific cohort studies. The conclusions indicate that markers of disease activity and/or severity (polyarthritis, dactylitis, extent of PsO, elevation of acute phase reactants) in PsA were associated with future CV events (11, 12). In addition, the extent of atherosclerotic plaque was associated with disease activity and inflammation in patients with PsA (13). In a meta-analysis including 31 studies involving 665,009 patients with PsO and 17,902,757 non-psoriatic control subjects, pooled analyses revealed that PsO patients, especially severe PsO, had a higher risk of ischemic heart disease, myocardial infarction, stroke, thromboembolism, arrhythmia and cardiovascular death. Psoriasis remained an independent risk factor for adverse CV outcomes (14). The association between venous thromboembolism (VTE) and PsD has also been reported: a systematic review and meta-analysis based on 13 cohort studies reported an increased risk of incident VTE in patients with PsO (pooled Hazard ratio (HR)[95% confidence interval (CI)]: 1.26 [1.08-1.48] but also in patients with PsA (pooled HR: 1.24 [1.01- 1.53]) (15). Furthermore, it is considered that the CV burden is higher in patients with PsA compared to patients with PsO alone: in a population-based study from Taiwan, patients with PsA had a higher incidence of cerebrovascular diseases compared to psoriatic patients without arthritis (HR: 1.83 [:1.17-2.82]) (16). A comparative study by Husted et al. found significantly higher prevalence of hypertension, obesity, dyslipidemia, type 2 diabetes and CV diseases in patients with PsA compared to those with PsO alone (unadjusted odds ratio (OR) for CV diseases : 2.59 [1.43-4.67]) (17). Finally, the risk of major adverse CV events (MACE) in patients with PsA not using a disease-modifying antirheumatic drug (DMARD) was similar to the risk observed in RA after adjustment for traditional CV risk factors (HR for PsA : 1.24 [1.03-1.49], HR for RA : 1.39 [1.28-1.5]) (18).
Overweight and obesity are more common in patients with PsO or PsA compared to the general population, and compared to patients with RA (19, 20). In a Canadian case-control study comparing the body mass index (BMI) of patients with PsA, PsO, RA and normal controls, the proportion of individuals with obesity was 37%, 29%, 27% and 18% respectively. The odds of obesity were 61% higher for PsA than PsO (21). This was confirmed in a cohort study from a UK database, in which the prevalence of obesity was higher in PsA compared to PsO alone (18). Some studies suggest that being overweight or obese could be a risk factor for developing PsO and/or PsA. In the prospective Nurse’s Health Study, there was a link between weight gain and incident PsO (22). The prevalence of obesity is higher in psoriatic patients than in the general population and the BMI of psoriatic patients tends to increase over time. Furthermore, being overweight with abdominal obesity and being obese is more common in children with PsO than in controls (23). Using Mendelian randomization, it was established that a higher BMI increase the odds of PsO (by 9% per 1 unit increase in BMI) (24). Moreover, it was demonstrated that obesity could be a factor for the transition from skin disease to joint involvement. Indeed, in an electronic database of medical records from the UK, the incidence of PsA increased in parallel with BMI both in patients with PsO and in the general population (25). In a prospective study performed in the USA (US Nurse’s Health Study II), BMI, weight changes and measures of central obesity were recorded over a 14-year period. It was found that BMI was monotonically associated with an increased incidence of PsA (26). In contrast, there were limited data on body composition in PsD. Using dual-energy X ray absorptiometry (DXA) measurements, Pedreira et al. found an increased total fat percentage in patients with PsA, but no changes in lean mass (27). Our group evaluated body composition in patients with PsA or PsO alone and matched controls. We found no significant differences in body composition measurements between PsA patients and their matched controls, while patients with PsO had higher visceral fat compared to their controls (28).
Several studies have shown that patients with PsD are at increased risk of diabetes (4). In a systematic review and meta-analysis of cohort studies, the incidence of type 2 diabetes was 13.4 and 7.8/1000 patient-years in patients with PsA and non-rheumatic control subjects, respectively (29). In a systematic review of CV comorbidities in PsA, the prevalence of diabetes was found to be increased in patients with PsA compared with controls (30). In addition, patients with PsA are more likely to have higher fasting glycemia compared to patients with RA (20). The prevalence of diabetes and insulin resistance were higher in 102 patients with PsA compared to 82 control subjects, after adjusting for BMI (31). In a cohort of 60 children with PsO aged 3-10 years, 27% had insulin resistance (32). Compared to mild disease, the severity of PsA (defined by joint erosive changes, osteolysis and sacroiliitis) was linked to insulin resistance in an Irish cohort study of 283 patients (OR: 3.49 [1.08- 11.2]) (33). The risk of diabetes seems to be higher in women and in patients with active disease (34). Abnormal lipid profile has been reported in PsA: indeed, patients were characterized by an unfavorable atherogenic ratio with a reduction in HDL cholesterol and elevated circulating triglyceride levels (20, 30). Systemic inflammation, as estimated by C-reactive protein (CRP) has been linked to low HDL cholesterol and high total/HDL cholesterol ratio (13). Some studies have also shown that patients with PsA had more lipid abnormalities compared to patients with PsO alone (28% versus 13.5%, OR 2.5 [95%CI 1.7- 3.3]) (35). In addition, the lipid profile is more altered in PsA compared to RA (35). Hypertension is also a well-recognized comorbidity of PsA. In the study by Husted et al, hypertension was the most frequent comorbidity of PsA (37.1%) and was more prevalent than in patients with PsO alone (20%) (17). Systemic inflammation seems to influence hypertension in PsA (31). Accumulating evidence suggests that there is a relationship between PsD and an increased risk of metabolic syndrome (MetS) (36). Metabolic syndrome and its components (central obesity, hypertension, insulin resistance and dyslipidemia) are strongly represented in PsA, ranging from 24% to 58% (37). MetS has consistently been reported in several series of patients with PsA, and is associated with the severity of the disease (30, 33), more consistently than in cases of PsO (38). Again, the prevalence of MetS is higher in PsA than in PsO alone (16) and higher than in RA (20). In a systematic review and meta-analysis, the pooled prevalence of MetS was found to be higher in PsA (0.46 ± 0.06 [0.40- 0.51] than in PsO (0.34 ± 0.03 [0.32-0.37]) (39).
It is now well established that inflammation is a major determinant of atherosclerosis, playing a role in plaque formation and progression (40, 41). The relationships between inflammation and atherosclerosis have been well demonstrated in RA: proinflammatory cytokines such as TNFα, IL-1 and IL-6 produced by various activated cells (T lymphocytes, monocytes, mastocytes, adipocytes) are released into the circulation and have potential effects on different tissues, including the blood vessels, leading to endothelial activation, vascular dysfunction as well as altered lipid profile and prothrombotic effects (6). The ultimate consequence is to promote atherogenesis. In PsD, several cytokines may play a role in atherosclerosis. Shared chronic inflammatory pathways such as Th1 and Th17 activation lead to the production of proinflammatory cytokines (TNFα, IFN-γ following Th1 differentiation, and IL-17A, IL-17F, IL-22 after Th17 activation). These inflammatory mediators are increased in the skin, the joint and the circulation in patients with PsO and/or PsA compared to subjects from the general population. They have various effects on the endothelium, leading to a proatherogenic phenotype (42). Circulating TNFα alone or in combination with IL-17 have been associated with endothelial dysfunction in PsA. It is well established that IL-17 is a key cytokine driving inflammation in PsD, and IL-17 is considered to be a solid candidate linking PsD to the developement of CV diseases (43, 44). As it was observed in RA, IL-6 is involved in joint inflammation and degradation of patients with PsA. Specific polymorphisms of IL-6 gene have been linked to CVD in PsA (45). The development of MetS in PsD is related to the systemic and chronic inflammation. In addition, obesity, type 2 diabetes and insulin resistance share common inflammatory pathways with the involvement of specific inflammatory cytokines such as TNFα and IL-6 (44). Moreover, adipose tissue is a reservoir of proinflammatory cytokines, mainly IL-6 and TNFα, and obesity may promote expansion of Th17 cells (46). The links between obesity and inflammation may also be substantiated by the involvement of specific adipokines, such as leptin and adiponectin, in the metabolic disturbances observed in PsD (47, 48). Smoking is a risk factor for CV disease that has been found significantly associated with PsO (4) and PsA (49). In parallel, gout augments the risk of CV diseases in PsO: in a population-based cohort study from Taiwan, patients with PsO and gout had a significantly higher risk for CV disease compared to patients with PsO alone (50) (Figure 1). Finally, the concept of “psoriatic march” has been proposed in order to explain the link between inflammation and the development of atherosclerosis in PsD: systemic inflammation, in conjunction with obesity and metabolic abnormalities, promotes the developement of insulin resistance, endothelial dysfuction, atherosclerosis and ultimately CV diseases (51).
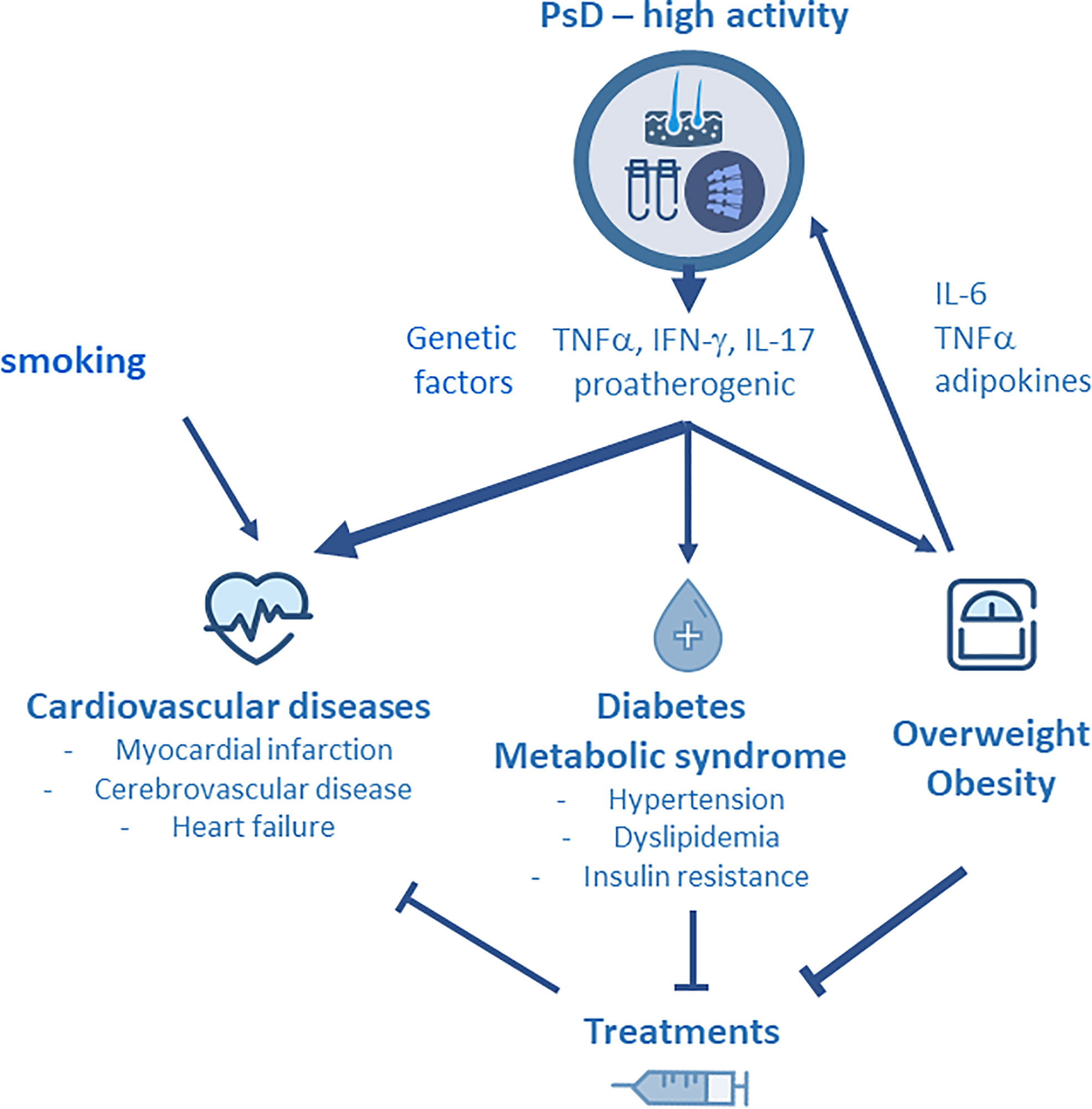
Figure 1 Due to disease activity, psoriatic disease (PsD) is associated with the production of Th1 (TNFα, IFNγ) and Th17 (IL-17, IL-22) derived cytokines that have potential effects on the blood vessels. They lead to endothelial activation, vascular dysfunction, altered lipid profile and prothrombotic effects, and ultimately, they promote atherosclerosis and cardiovascular (CV) disease. PsD is strongly associated with specific comorbidities including obesity and metabolic syndrome (MetS). Smoking and genetic factors may contribute to the development of CV diseases in PsD. Obesity is a predisposing factor for the developement of PsD and contributes to disease activity by the release of adipokines. Specific comorbidities, such as MetS and obesity, and therapeutic response are interrelated in PsD : on the one hand, MetS and obesity impair the therapeutic effectiveness of TNF inhibitors (TNFi), while on the other hand, methotrexate and TNFi have been shown to limit the CV burden of PsD.
The European Alliance of Associations for Rheumatology (EULAR) group has published recommendations for the management of the CV risk in inflammatory rheumatic diseases (IRD) including PsA (52). It is considered that the different tools assessing CV risk in IRD, such as the Framingham risk score, the European systemic coronary risk evaluation SCORE or the Reynold’s CV risk score, all underestimate the real risk in IRD, especially in RA, but also in PsA (53). Thus, the EULAR has proposed the use of a correcting factor, by multiplying the score obtained in patients with RA by 1.5. In PsD, this correcting factor has not yet been validated. Despite the substantial evidence in support of an increased CV risk in PsD, the different CV risk factors remain undertreated (54). In a population-based study from the UK, the management of CV risk factors in patients PsA was similar to that of subjects from the general population, despite an increased prevalence of hypertension, hyperlipidemia, diabetes mellitus and obesity (35). Despite a vast body of literature showing an overall increased risk of CV disease in patients with PsO, it has been found, that patients with psoriasis and CV risk factors receive less cardio-protective medical therapy than controls without PsO, or no such treatment at all (55). The Joint American Academy of Dermatology — National Psoriasis Foundation guidelines (56) advocate that dermatologists inform psoriatic patients of their elevated CV risk and ensure engagement with their primary care doctor or cardiologist. The US (56) and European Academy of Dermatology and Venerology’s (57) guidelines recommend screening patients with PsO upon systemic treatment for CV disease risk factors every twelve months. Furthermore, the British (58) and French (59) guideline recommends CVD risk assessment in adults with severe PsO at presentation and further CV assessment every five years (58). The European Society of Cardiology (60) recommends CV screening and similar therapeutic interventions as in the general high-risk population.
These data strongly underline the need to carefully screen for and manage the different CV risk factors in PsD. In parallel to CV risk factors, obesity, diabetes and MetS require specific attention in patients with PsD to be adequately managed (7, 37, 43). Optimal lifestyle modification in PsO including hypocaloric and Mediterranean diet and smoking cessation if required, is a cornerstone of strategies to reduce CV disease (61). Clinical trials evaluating traditional CV risk factor treatment thresholds and goals in the psoriasis population are lacking. However, given the pattern of dyslipidemia, lipid-lowering, i.e. statins, play a key role in CV risk reduction strategies in PsO (62). While it is also reasonable to promote aggressive blood pressure and hemoglobin A1c goals, in-line with ACC/AHA recommendations in patients at elevated risk of CVD, clinical studies evaluating this approach in PsO are still needed (63). Whether statin therapy in PsO confers additional anti-inflammatory benefit beyond lipid-lowering is not yet known. Aspirin in the primary prevention of CVD is controversial even in the non–psoriatic patient (64).
Obesity and MetS are associated with impaired response to the treatments that are used in PsD. In contrast, weight loss has a positive impact on the severity of PsO (65). Overweight and obesity have been reported to be associated with a reduced probability of achieving a sustained minimal disease activity (MDA) state in PsA, irrespective of the treatment used (–) (66). In an Italian prospective study, being obese was an independent risk factor for not acheiving MDA (HR: 4.9 [3.04- 7.87]) and for relapse over 24 months (67). The response to TNF inhibitors (TNFi) is particularly influenced by BMI: in a meta-analysis including 22 cohorts with PsD, obesity was associated with poor response to TNFi in obese patients (OR for failing to respond to TNFi: 1.57 [1.30-1.89]) (68). MetS was also associated with poor response to TNFi: in an Italian cohort of patients with PsA receiving their first TNFi, the presence of MetS was associated with a lower probablility of achieving MDA at 24 months (OR: 0.56) (69). Lastly, in the Danish (DANBIO) and Icelandic (ICEBIO) biologics registries, obesity was associated with an increased risk of TNFi withdrawal (HR: 1.6 [1.3-2.0]) and reduced EULAR good or moderate responses (OR: 0.47 [0.29- 0.72]) (70). For the IL-12/23 inhibitor ustekinumab, data showed that obesity impared the skin response (71). For secukinumab, BMI does not seem to influence the therapeutic response in PsA according to recent data (72). Tofacitinib is a pan-JAK inhibitor (JAKi) licensed for the treatment of PsA. In a post hoc analysis of 3 randomized controlled trials, American College of Rheumatology (ACR) response rate to tofacitinib was reduced in patients with BMI ≥ 35 kg/m² (73).
Taking into account the link between systemic inflammation and CV diseases, it is conceivable that controlling inflammation by systemic agents may positively impact the CV risk in PsD (73, 74). It is now well demonstrated that conventional synthetic agents such as methotrexate (MTX), but also TNFi, improve the CV burden of patients with RA (75). In PsD, MTX has been associated with a reduction in CV risk (76). A meta-analysis examined the effects of MTX on CV risk in patients with RA, PsO or PsA. The conclusion was that MTX was associated with a 21% reduction in overall CV risk and an 18% reduction in the risk of myocardial infarction (77). In a population-based cohort study in the USA, Ogdie et al. concluded that the risk of MACE was higher in patients with PsA or PsO not receiving a conventional synthetic DMARD (csDMARD) or in patients with severe PsO (18). A larger body of data is available regarding the specific impact of TNFi on CV events. Their beneficial effects are well demonstrated in RA. The meta-analysis by Roubille et al. based on 6 studies in PsD showed a significant reduction in CV events under csDMARD or TNFi in PsO and PsA (relative risk [RR] : 0.72 [0.57-0.91] and 0.7 [0.54- 0.9], respectively) (75). A second meta-analysis came to the same conclusion, namely that compared to MTX, the risk of CV events was markedly decreased under TNFi (RR: 0.67 [0.52-0.88]) (78). Data regarding other bDMARDs are more limited. Conversely, the impact of PsD treatment on CV risk must be discussed. Indeed, blockade of the IL-23/IL-17 axis, however, warrants caution as a cardiovascular intervention. A case-time-control analysis based on data from 9290 patients with records in the French national health insurance database from 2010 to 2016 suggested that the initiation of ustekinumab treatment is associated with an increased risk of acute coronary syndrome or stroke in patients with a high baseline cardiovascular risk (79). IL-17A appears to be a differential regulator of atherosclerosis, and its effects in mouse models suggest that its modulation may have contradictory effects on plaque size and possibly stability. Targeting this pathway has improved PsO, but may augment CV risk in certain patients (80, 81). However, it has been suggested in an observational study of Pso patients that secukinumab, an IL-17 inhibitor, improved left venricular function and coronary flow reserve compared with MTX and cyclosporine (82). In the same way, concerns have been raised about the risk of MACE under briakinumab, another anti-IL-23 agent. However, a meta-analysis of 38 randomized clinical trials in PsO concluded that there was no increase in MACE with the use of different biological agents, including ustekinumab but also TNFi and anti IL-17 agents (83). A large Korean PsO cohort study assessed MACE risk according to the treatment modalities of phototherapy, biologic and conventional systemic agents. Phototherapy and biologic groups showed a lower incidence of MACE than the control cohort, and the difference in the cumulative incidence remained significant over the 36-month follow-up period. Cyclosporine and mixed conventional systemic treatments were significantly associated with an increased MACE risk, whereas MTX was not associated with MACE (84). Lastly, concerns have recently emerged regarding the risk of thrombosis under the JAKi tofacitinib, leading to specific warnings. The ORAL Surveillance randomized trial analyzed the safety of tofacitinib (5 and 10 mg twice daily) versus a TNFi in subjects with RA aged 50 years or older who had at least one additional CV risk factor (85). The study concluded that there was a higher risk of MACE and malignancies with tofacitinib as compared to TNFi in patients with RA (HR for MACE: 1.33 [0.91-1.94]; HR for malignancies: 1.48 [1.04- 2.09]). Following these results, healthcare professionals were advised to consider the benefits and risks of tofacitinib, but also other JAKi, when deciding to prescribe and continue patients on the drug. However, in the interim analysis of tofacitinib in PsA patients in the OPAL Balance trial (3 year, open-label extension study of tofacitinib in PsA), there was no evidence of an increased risk of CV events (86). Similarly, in an integrated analysis of 2 randomized, placebo-controlled phase 3 trials with upadacitinib in PsA, including one trial with adalimumab, rates of MACE were similar across treatment groups (87). It has been reported that TNFi induce body composition changes in IRD with weight and fat mass gain, especially in the central abdominal region (88). In contrast, there are some data showing that TNFi may improve different components of MetS (89) and diabetes (90) in PsD (91).
PsD is associated with a higher prevalence of cardiometabolic diseases compared to the general population. This prevalence is higher in PsA compared to PsO alone, and also higher compared to other IRD, such as RA. Obesity and MetS are strongly represented in PsD, and obesity is a known risk factor for the development of PsO and PsA. These metabolic comorbidities must be adequately screened for and managed by the physicians caring for patients with PsD. Obesity influences the treatment response to specific biologic agents such as TNFi. In parallel, MTX and TNFi have been shown to have a positive impact on CV risk and on MetS.
ET performed the bibliographic search and wrote the first version of the manuscript. FA completed the bibliographic search and specific sections of the manuscript. IG-S made the figure. All the authors reviewed and edited the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med (2009) 361(5):496–509. doi: 10.1056/NEJMra0804595
2. Mease PJ. Psoriatic arthritis: Update on pathophysiology, assessment and management. Ann Rheum Dis (2011) 70 Suppl 1:i77–84. doi: 10.1136/ard.2010.140582
3. Hong C, Fang S, Yeo YW, Koh HY, Lee HY, Low AH, et al. Patient and learner experience in a new set up of a multidisciplinary dermatology-rheumatology clinic care model for psoriatic arthritis. Int J Rheum Dis (2022) 25:861–868. doi: 10.1111/1756-185X.14359
4. Puig L. Cardiometabolic comorbidities in psoriasis and psoriatic arthritis. Int J Mol Sci (2017) 19(1):58. doi: 10.3390/ijms19010058
5. Sobchak C, Eder L. Cardiometabolic disorders in psoriatic disease. Curr Rheumatol Rep (2017) 19(10):63. doi: 10.1007/s11926-017-0692-2
6. Castaneda S, Nurmohamed MT, Gonzalez-Gay MA. Cardiovascular disease in inflammatory rheumatic diseases. Best Pract Res Clin Rheumatol (2016) 30(5):851–69. doi: 10.1016/j.berh.2016.10.006
7. Ramirez J, Azuaga-Pinango AB, Celis R, Canete JD. Update on cardiovascular risk and obesity in psoriatic arthritis. Front Med (Lausanne) (2021) 8:742713. doi: 10.3389/fmed.2021.742713
8. Avina-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum (2008) 59(12):1690–7. doi: 10.1002/art.24092
9. Jamnitski A, Visman IM, Peters MJ, Boers M, Dijkmans BA, Nurmohamed MT. Prevalence of cardiovascular diseases in psoriatic arthritis resembles that of rheumatoid arthritis. Ann Rheum Dis (2011) 70(5):875–6. doi: 10.1136/ard.2010.136499
10. Polachek A, Touma Z, Anderson M, Eder L. Risk of cardiovascular morbidity in patients with psoriatic arthritis: A meta-analysis of observational studies. Arthritis Care Res (Hoboken) (2017) 69(1):67–74. doi: 10.1002/acr.22926
11. Gladman DD, Ang M, Su L, Tom BD, Schentag CT, Farewell VT. Cardiovascular morbidity in psoriatic arthritis. Ann Rheum Dis (2009) 68(7):1131–5. doi: 10.1136/ard.2008.094839
12. Eder L, Wu Y, Chandran V, Cook R, Gladman DD. Incidence and predictors for cardiovascular events in patients with psoriatic arthritis. Ann Rheum Dis (2016) 75(9):1680– 6. doi: 10.1136/annrheumdis-2015-207980
13. Eder L, Thavaneswaran A, Chandran V, Cook R, Gladman DD. Increased burden of inflammation over time is associated with the extent of atherosclerotic plaques in patients with psoriatic arthritis. Ann Rheum Dis (2015) 74(10):1830–5. doi: 10.1136/annrheumdis-2014-205267
14. Liu L, Cui S, Liu M, Huo X, Zhang G, Wang N. Psoriasis increased the risk of adverse cardiovascular outcomes: A new systematic review and meta-analysis of cohort study. Front Cardiovasc Med (2022) 9:829709. doi: 10.3389/fcvm.2022.829709
15. Chen TL, Lee LL, Huang HK, Wang JH, Chen LY, Tsai HR, et al. Association of psoriasis with incident venous thromboembolism and peripheral vascular disease: A systematic review and meta-analysis. JAMA Dermatol (2022) 158:59–67. doi: 10.1001/jamadermatol.2021.4918
16. Chin YY, Yu HS, Li WC, Ko YC, Chen GS, Wu CS, et al. Arthritis as an important determinant for psoriatic patients to develop severe vascular events in Taiwan: A nation- wide study. J Eur Acad Dermatol Venereol (2013) 27(10):1262–8. doi: 10.1111/j.1468-3083.2012.04706.x
17. Husted JA, Thavaneswaran A, Chandran V, Eder L, Rosen CF, Cook RJ, et al. Cardiovascular and other comorbidities in patients with psoriatic arthritis: A comparison with patients with psoriasis. Arthritis Care Res (Hoboken) (2011) 63(12):1729–35. doi: 10.1002/acr.20627
18. Ogdie A, Yu Y, Haynes K, Love TJ, Maliha S, Jiang Y, et al. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: A population-based cohort study. Ann Rheum Dis (2015) 74(2):326–32. doi: 10.1136/annrheumdis-2014-205675
19. Toussirot E, Aubin F, Dumoulin G. Relationships between adipose tissue and psoriasis, with or without arthritis. Front Immunol (2014) 5:368. doi: 10.3389/fimmu.2014.00368
20. Mok CC, Ko GT, Ho LY, Yu KL, Chan PT, To CH. Prevalence of atherosclerotic risk factors and the metabolic syndrome in patients with chronic inflammatory arthritis. Arthritis Care Res (Hoboken) (2011) 63(2):195–202. doi: 10.1002/acr.20363
21. Bhole VM, Choi HK, Burns LC, Vera Kellet C, Lacaille DV, Gladman DD, et al. Differences in body mass index among individuals with psa, psoriasis, Ra and the general population. Rheumatol (Oxford) (2012) 51(3):552–6. doi: 10.1093/rheumatology/ker349
22. Setty AR, Curhan G, Choi HK. Obesity, waist circumference, weight change, and the risk of psoriasis in women: Nurses’ health study ii. Arch Intern Med (2007) 167(15):1670–5. doi: 10.1001/archinte.167.15.1670
23. Mahe E, Beauchet A, Bodemer C, Phan A, Bursztejn AC, Boralevi F, et al. Psoriasis and obesity in French children: A case-control, multicentre study. Br J Dermatol (2015) 172(6):1593–600. doi: 10.1111/bjd.13507
24. Budu-Aggrey A, Brumpton B, Tyrrell J, Watkins S, Modalsli EH, Celis-Morales C, et al. Evidence of a causal relationship between body mass index and psoriasis: A mendelian randomization study. PloS Med (2019) 16(1):e1002739. doi: 10.1371/journal.pmed.1002739
25. Love TJ, Zhu Y, Zhang Y, Wall-Burns L, Ogdie A, Gelfand JM, et al. Obesity and the risk of psoriatic arthritis: A population-based study. Ann Rheum Dis (2012) 71(8):1273–7. doi: 10.1136/annrheumdis-2012-201299
26. Li W, Han J, Qureshi AA. Obesity and risk of incident psoriatic arthritis in us women. Ann Rheum Dis (2012) 71(8):1267–72. doi: 10.1136/annrheumdis-2011-201273
27. Pedreira PG, Pinheiro MM, Szejnfeld VL. Bone mineral density and body composition in postmenopausal women with psoriasis and psoriatic arthritis. Arthritis Res Ther (2011) 13(1):R16. doi: 10.1186/ar3240
28. Toussirot E, Aubin F, Desmarets M, Wendling D, Auge B, Gillard J, et al. Visceral adiposity in patients with psoriatic arthritis and psoriasis alone and its relationship with metabolic and cardiovascular risk. Rheumatol (Oxford) (2021) 60(6):2816–25. doi: 10.1093/rheumatology/keaa720
29. Yuan Z, Guo Y. Risk of incident type 2 diabetes in patients with psoriatic arthritis: A systematic review and meta-analysis of cohort studies. Int J Rheum Dis (2022). doi: 10.1111/1756-185X.14375
30. Jamnitski A, Symmons D, Peters MJ, Sattar N, McInnes I, Nurmohamed MT. Cardiovascular comorbidities in patients with psoriatic arthritis: A systematic review. Ann Rheum Dis (2013) 72(2):211–6. doi: 10.1136/annrheumdis-2011-201194
31. Tam LS, Tomlinson B, Chu TT, Li M, Leung YY, Kwok LW, et al. Cardiovascular risk profile of patients with psoriatic arthritis compared to controls–the role of inflammation. Rheumatol (Oxford) (2008) 47(5):718–23. doi: 10.1093/rheumatology/ken090
32. Caroppo F, Galderisi A, Ventura L, Belloni Fortina A. Metabolic syndrome and insulin resistance in pre-pubertal children with psoriasis. Eur J Pediatr (2021) 180(6):1739–45. doi: 10.1007/s00431-020-03924-w
33. Haroon M, Gallagher P, Heffernan E, FitzGerald O. High prevalence of metabolic syndrome and of insulin resistance in psoriatic arthritis is associated with the severity of underlying disease. J Rheumatol (2014) 41(7):1357–65. doi: 10.3899/jrheum.140021
34. Dreiher J, Freud T, Cohen AD. Psoriatic arthritis and diabetes: A population-based cross-sectional study. Dermatol Res Pract (2013) 2013:580404. doi: 10.1155/2013/580404
35. Jafri K, Bartels CM, Shin D, Gelfand JM, Ogdie A. Incidence and management of cardiovascular risk factors in psoriatic arthritis and rheumatoid arthritis: A population- based study. Arthritis Care Res (Hoboken) (2017) 69(1):51–7. doi: 10.1002/acr.23094
36. Hao Y, Zhu YJ, Zou S, Zhou P, Hu YW, Zhao QX, et al. Metabolic syndrome and psoriasis: Mechanisms and future directions. Front Immunol (2021) 12:711060. doi: 10.3389/fimmu.2021.711060
37. Karmacharya P, Ogdie A, Eder L. Psoriatic arthritis and the association with cardiometabolic disease: A narrative review. Ther Adv Musculoskelet Dis (2021) 13:1759720X21998279. doi: 10.1177/1759720X21998279
38. Lin IC, Heck JE, Chen L, Feldman SR. Psoriasis severity and cardiometabolic risk factors in a representative us national study. Am J Clin Dermatol (2021) 22(5):719–30. doi: 10.1007/s40257-021-00600-z
39. Loganathan A, Kamalaraj N, El-Haddad C, Pile K. Systematic review and meta-analysis on prevalence of metabolic syndrome in psoriatic arthritis, rheumatoid arthritis and psoriasis. Int J Rheum Dis (2021) 24:1112–20. doi: 10.1111/1756-185X.14147
40. Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation (2004) 109(21 Suppl 1):II2–10. doi: 10.1161/01.CIR.0000129535.04194.38
41. Teklu M, Parel PM, Mehta NN. Psoriasis and cardiometabolic diseases: The impact of inflammation on vascular health. Psoriasis (Auckl) (2021) 11:99–108. doi: 10.2147/PTT.S320016
42. Ferguson LD, Siebert S, McInnes IB, Sattar N. Cardiometabolic comorbidities in Ra and psa: Lessons learned and future directions. Nat Rev Rheumatol (2019) 15(8):461–74. doi: 10.1038/s41584-019-0256-0
43. Armstrong EJ, Krueger JG. Lipoprotein metabolism and inflammation in patients with psoriasis. Am J Cardiol (2016) 118(4):603–9. doi: 10.1016/j.amjcard.2016.05.060
44. Hotamisligil GS. Inflammation and metabolic disorders. Nature (2006) 444(7121):860– 7. doi: 10.1038/nature05485
45. IL6R Genetics Consortium Emerging Risk Factors Collaboration, Sarwar N, Butterworth AS, Freitag DF, Gregson J, Willeit P, et al. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet (2012) 379:1205–13. doi: 10.1016/S0140-6736(11)61931-4
46. Chehimi M, Vidal H, Eljaafari A. Pathogenic role of il-17-Producing immune cells in obesity, and related inflammatory diseases. J Clin Med (2017). doi: 10.3390/jcm6070068
47. Gerdes S, Rostami-Yazdi M, Mrowietz U. Adipokines and psoriasis. Exp Dermatol (2011) 20(2):81–7. doi: 10.1111/j.1600-0625.2010.01210.x
48. Toussirot E. Mini-review: The contribution of adipokines to joint inflammation in inflammatory rheumatic diseases. Front Endocrinol (Lausanne) (2020) 11:606560. doi: 10.3389/fendo.2020.606560
49. Pezzolo E, Naldi L. The relationship between smoking, psoriasis and psoriatic arthritis. Expert Rev Clin Immunol (2019) 15:41–8. doi: 10.1080/1744666X.2019.1543591
50. Chen Z, Xu Y, Chen M, Cui R, Wang YH, Dai SM, et al. Gout augments the risk of cardiovascular disease in patients with psoriasis: A population-based cohort study. Front Immunol (2021) 12:703119. doi: 10.3389/fimmu.2021.703119
51. Boehncke WH, Boehncke S, Tobin AM, Kirby B. The ‘psoriatic march’: A concept of how severe psoriasis may drive cardiovascular comorbidity. Exp Dermatol (2011) 20(4):303–7. doi: 10.1111/j.1600-0625.2011.01261.x
52. Agca R, Heslinga SC, Rollefstad S, Heslinga M, McInnes IB, Peters MJ, et al. Eular recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis (2017) 76(1):17–28. doi: 10.1136/annrheumdis-2016-209775
53. Eder L, Chandran V, Gladman DD. The framingham risk score underestimates the extent of subclinical atherosclerosis in patients with psoriatic disease. Ann Rheum Dis (2014) 73(11):1990–6. doi: 10.1136/annrheumdis-2013-203433
54. Kimball AB, Szapary P, Mrowietz U, Reich K, Langley RG, You Y, et al. Underdiagnosis and undertreatment of cardiovascular risk factors in patients with moderate to severe psoriasis. J Am Acad Dermatol (2012) 67(1):76–85. doi: 10.1016/j.jaad.2011.06.035
55. Ahlehoff O, Skov L, Gislason G, Lindhardsen J, Kristensen SL, Iversen L, et al. Pharmacological undertreatment of coronary risk factors in patients with psoriasis: observational study of the Danish nationwide registries. PloS One (2012) 7(4):e36342. doi: 10.1371/journal.pone.0036342
56. Elmets CA, Leonardi CL, Davis DMR, Gelfand JM, Lichten J, Mehta NN, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol (2019) 80(4):1073–113. doi: 10.1016/j.jaad.2018.11.058
57. Dauden E, Blasco AJ, Bonanad C, Botella R, Carrascosa JM, González-Parra E, et al. Position statement for the management of comorbidities in psoriasis. J Eur Acad Dermatol Venereol (2018) 32(12):2058–73. doi: 10.1111/jdv.15177
58. Psoriasis - assessment and management of psoriasis. Clinical guideline, in: Methods, evidence and recommendations (2017). Available at: https://www.nice.org.uk/guidance/cg153/evidence/full-guideline-pdf-188351533 (Accessed March 25, 2022).
59. Amatore F, Villani AP, Tauber M, Viguier M, Guillot B. Psoriasis research group of the French society of dermatology (Groupe de recherche sur le psoriasis de la société française de dermatologie). French guidelines on the use of systemic treatments for moderate-to-severe psoriasis in adults. J Eur Acad Dermatol Venereol (2019) 33(3):464–83. doi: 10.1111/jdv.15340
60. Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur J Prev Cardiol (2022) 29(1):5–115. doi: 10.1093/eurjpc/zwab154
61. Ford AR, Siegel M, Bagel J, Cordoro KM, Garg A, Gottlieb A, et al. Dietary recommendations for adults with psoriasis or psoriatic arthritis from the medical board of the national psoriasis foundation: A systematic review. JAMA Dermatol (2018) 154(8):934–50. doi: 10.1001/jamadermatol.2018.1412
62. Ports WC, Fayyad R, DeMicco DA, Laskey R, Wolk R. Effectiveness of lipid-lowering statin therapy in patients with and without psoriasis. Clin Drug Investig (2017) 37:775–85. doi: 10.1007/s40261-017-0533-0
63. Garshick MS, Ward NL, Krueger JG, Berger JS. Cardiovascular risk in patients with psoriasis: JACC review topic of the week. J Am Coll Cardiol (2021) 77(13):1670–80. doi: 10.1016/j.jacc.2021.02.009
64. US Preventive Services Task Force, Davidson KW, Barry MJ, Mangione CM, Cabana M, Chelmow D, et al. Aspirin use to prevent cardiovascular disease: US preventive services task force recommendation statement. JAMA (2022) 327(16):1577–84. doi: 10.1001/jama.2022.4983
65. Alotaibi HA. Effects of weight loss on psoriasis: A review of clinical trials. Cureus (2018) 10(10):e3491. doi: 10.7759/cureus.3491
66. Eder L, Thavaneswaran A, Chandran V, Cook RJ, Gladman DD. Obesity is associated with a lower probability of achieving sustained minimal disease activity state among patients with psoriatic arthritis. Ann Rheum Dis (2015) 74(5):813–7. doi: 10.1136/annrheumdis-2013-204448
67. di Minno MN, Peluso R, Iervolino S, Lupoli R, Russolillo A, Scarpa R, et al. Obesity and the prediction of minimal disease activity: A prospective study in psoriatic arthritis. Arthritis Care Res (Hoboken) (2013) 65(1):141–7. doi: 10.1002/acr.21711
68. Singh S, Facciorusso A, Singh AG, Vande Casteele N, Zarrinpar A, Prokop LJ, et al. Obesity and response to anti-tumor necrosis factor-alpha agents in patients with select immune-mediated inflammatory diseases: A systematic review and meta-analysis. PloS One (2018) 13(5):e0195123. doi: 10.1371/journal.pone.0195123
69. Costa L, Caso F, Ramonda R, Del Puente A, Cantarini L, Darda MA, et al. Metabolic syndrome and its relationship with the achievement of minimal disease activity state in psoriatic arthritis patients: An observational study. Immunol Res (2015) 61(1-2):147–53. doi: 10.1007/s12026-014-8595-z
70. Hojgaard P, Glintborg B, Kristensen LE, Gudbjornsson B, Love TJ, Dreyer L. The influence of obesity on response to tumour necrosis factor-alpha inhibitors in psoriatic arthritis: Results from the danbio and icebio registries. Rheumatol (Oxford) (2016) 55(12):2191–9. doi: 10.1093/rheumatology/kew326
71. Del Alcazar E, Ferran M, Lopez-Ferrer A, Notario J, Vidal D, Riera J, et al. Effectiveness and safety of ustekinumab 90 mg in patients weighing 100 kg or less: A retrospective, observational, multicenter study. J Dermatolog Treat (2020) 31(3):222–6. doi: 10.1080/09546634.2019.1597245
72. Pantano I, Iacono D, Favalli EG, Scalise G, Costa L, Caso F, et al. Secukinumab efficacy in patients with psa is not dependent on patients’ body mass index. Ann Rheum Dis (2022) 81(3):e42. doi: 10.1136/annrheumdis-2020-217251
73. Giles JT, Ogdie A, Gomez Reino JJ, Helliwell P, Germino R, Stockert L, et al. Impact of baseline body mass index on the efficacy and safety of tofacitinib in patients with psoriatic arthritis. RMD Open (2021) 7(1). doi: 10.1136/rmdopen-2020-001486
74. Cai J, Cui L, Wang Y, Li Y, Zhang X, Shi Y. Cardiometabolic comorbidities in patients with psoriasis: Focusing on risk, biological therapy, and pathogenesis. Front Pharmacol (2021) 12:774808. doi: 10.3389/fphar.2021.774808
75. Roubille C, Richer V, Starnino T, McCourt C, McFarlane A, Fleming P, et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: A systematic review and meta-analysis. Ann Rheum Dis (2015) 74(3):480–9. doi: 10.1136/annrheumdis-2014-206624
76. Prodanovich S, Ma F, Taylor JR, Pezon C, Fasihi T, Kirsner RS. Methotrexate reduces incidence of vascular diseases in veterans with psoriasis or rheumatoid arthritis. J Am Acad Dermatol (2005) 52(2):262–7. doi: 10.1016/j.jaad.2004.06.017
77. Micha R, Imamura F, Wyler von Ballmoos M, Solomon DH, Hernan MA, Ridker PM, et al. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol (2011) 108(9):1362–70. doi: 10.1016/j.amjcard.2011.06.054
78. Yang ZS, Lin NN, Li L, Li Y. The effect of tnf inhibitors on cardiovascular events in psoriasis and psoriatic arthritis: An updated meta-analysis. Clin Rev Allergy Immunol (2016) 51(2):240–7. doi: 10.1007/s12016-016-8560-9
79. Poizeau F, Nowak E, Kerbrat S, Le Nautout B, Droitcourt C, Drici MD, et al. Association between early severe cardiovascular events and the initiation of treatment with the anti-interleukin 12/23p40 antibody ustekinumab. JAMA Dermatol (2020) 156(11):1208– 15. doi: 10.1001/jamadermatol.2020.2977
80. Ait-Oufella H, Libby P, Tedgui A. Anticytokine immune therapy and atherothrombotic cardiovascular risk. Arterioscler Thromb Vasc Biol (2019) 39(8):1510–9. doi: 10.1161/ATVBAHA.119.311998
81. Nordlohne J, von Vietinghoff S. Interleukin 17a in atherosclerosis - regulation and pathophysiologic effector function. Cytokine (2019) 122:154089. doi: 10.1016/j.cyto.2017.06.016
82. Makavos G, Ikonomidis I, Andreadou I, Varoudi M, Kapniari I, Loukeri E, et al. Effects of interleukin 17A inhibition on myocardial deformation and vascular function in psoriasis. Can J Cardiol (2020) 36(1):100–11. doi: 10.1016/j.cjca.2019.06.021
83. Langley RG, Papp K, Gottlieb AB, Krueger GG, Gordon KB, Williams D, et al. Safety results from a pooled analysis of randomized, controlled phase ii and iii clinical trials and interim data from an open-label extension trial of the interleukin-12/23 monoclonal antibody, briakinumab, in moderate to severe psoriasis. J Eur Acad Dermatol Venereol (2013) 27(10):1252–61. doi: 10.1111/j.1468-3083.2012.04705.x
84. Hong JR, Jeong H, Kim H, Yang HS, Hong JY, Kim SM, et al. The potential impact of systemic anti-inflammatory therapies in psoriasis on major adverse cardiovascular events: A Korean nationwide cohort study. Sci Rep (2021) 11(1):8588. doi: 10.1038/s41598-021-87766-y
85. Ytterberg SR, Bhatt DL, Mikuls TR, Koch GG, Fleischmann R, Rivas JL, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med (2022) 386(4):316–26. doi: 10.1056/NEJMoa2109927
86. Nash P, Coates LC, Kivitz AJ, Mease PJ, Gladman DD, Covarrubias-Cobos JA, et al. Safety and efficacy of tofacitinib in patients with active psoriatic arthritis: Interim analysis of opal balance, an open-label, long-term extension study. Rheumatol Ther (2020) 7(3):553–80. doi: 10.1007/s40744-020-00209-4
87. Burmester GR, Winthrop K, Blanco R, Nash P, Goupille P, Azevedo VF, et al. Safety profile of upadacitinib up to 3 years in psoriatic arthritis: An integrated analysis of two pivotal phase 3 trials. Rheumatol Ther (2022) 9(2):521–39. doi: 10.1007/s40744-021-00410-z
88. Toussirot E, Mourot L, Dehecq B, Wendling D, Grandclement E, Dumoulin G, et al. Tnfalpha blockade for inflammatory rheumatic diseases is associated with a significant gain in android fat mass and has varying effects on adipokines: A 2-year prospective study. Eur J Nutr (2014) 53(3):951–61. doi: 10.1007/s00394-013-0599-2
89. Costa L, Caso F, Atteno M, Del Puente A, Darda MA, Caso P, et al. Impact of 24- month treatment with etanercept, adalimumab, or methotrexate on metabolic syndrome components in a cohort of 210 psoriatic arthritis patients. Clin Rheumatol (2014) 33(6):833– 9. doi: 10.1007/s10067-013-2369-1
90. Solomon DH, Love TJ, Canning C, Schneeweiss S. Risk of diabetes among patients with rheumatoid arthritis, psoriatic arthritis and psoriasis. Ann Rheum Dis (2010) 69(12):2114–7. doi: 10.1136/ard.2009.125476
Keywords: psoriatic arthritis, cardiovascular risk, metabolic syndrome, psoriasis, obesity
Citation: Toussirot E, Gallais-Sérézal I and Aubin F (2022) The cardiometabolic conditions of psoriatic disease. Front. Immunol. 13:970371. doi: 10.3389/fimmu.2022.970371
Received: 15 June 2022; Accepted: 22 August 2022;
Published: 08 September 2022.
Edited by:
Anja Saalbach, Leipzig University, GermanyReviewed by:
James Cheng-Chung Wei, Chung Shan Medical University Hospital, TaiwanCopyright © 2022 Toussirot, Gallais-Sérézal and Aubin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eric Toussirot, ZXRvdXNzaXJvdEBjaHUtYmVzYW5jb24uZnI=
†ORCID: Eric Toussirot, orcid.org/0000-0002-6228-1464
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.