- 1Institute of Basic Research in Clinical Medicine, College of Basic Medical Science, Zhejiang Chinese Medical University, Hangzhou, China
- 2Department of Breast Surgery, The Second Affiliated Hospital, Zhejiang University, College of Medicine, Hangzhou, China
- 3Department of Radiation Oncology, The Second Affiliated Hospital and Cancer Institute (National Ministry of Education Key Laboratory of Cancer Prevention and Intervention), Zhejiang University School of Medicine, Hangzhou, China
Fusobacterium nucleatum (F. nucleatum) is originally an oral opportunistic pathogen and accumulating evidence links the presence of F. nucleatum with the pathogenicity, development, and prognosis of colorectal cancer (CRC). However, only limited preliminary data is available dealing with the role of F. nucleatum in other malignancies except for CRC. The present review aims to update and systematize the latest information about the mechanisms of F. nucleatum-mediating carcinogenesis, together with the detection rates, clinicopathological, and molecular features in F. nucleatum-associated malignancies. Comparing with adjacent non-tumorous tissue, previous studies have shown an overabundance of intratumoural F. nucleatum. Although the prognostic role of F. nucleatum is still controversial, a higher prevalence of F. nucleatum was usually associated with a more advanced tumor stage and a worse overall survival. Preliminary evidence have shown that epithelial-to-mesenchymal transition (EMT) and relevant inflammation and immune response aroused by F. nucleatum may be the probable link between F. nucleatum infection and the initiation of oral/head and neck cancer. Further studies are needed to elucidate the etiologic role of the specific microbiota and the connection between the extent of periodontitis and carcinogenesis in different tumor types. The mechanisms of how the antibiotics exerts the critical role in the carcinogenesis and antitumor effects in malignancies other than CRC need to be further explored.
Introduction
Over 100 trillion bacteria inhabit the human body (1) and the composition of human microbiome is closely associated with the risk of cancer (2). Approximately 20% of total cancer incidences are attributable to bacterial and viral infections, with the primary focus being on the carcinogenic role of Helicobacter pylori (Hp) in gastric cancer and the human papilloma virus (HPV) in cervical cancer (3). A better understanding of the mechanisms and contribution of the pathogenic microbiota to chronic inflammation and initiation of malignancy may aid the development of novel approaches to the prevention and treatment of cancer.
Fusobacterium nucleatum (F. nucleatum) is a non-spore-forming, anaerobic, gram-negative, pro-inflammatory bacterium, and primarily inhabits the human oral cavity (4). It has been recognized that F. nucleatum acts as an opportunistic pathogen in multiple inflammatory diseases, including periodontitis (5), inflammatory bowel disease (6), liver abscesses (7), rheumatoid arthritis (8), and chorioamnionitis (9). Its involvement in multiple systemic conditions has also been found, such as Alzheimer’s disease (10), ruptured cerebral aneurysm (11), atherosclerosis (12), cardiovascular diseases, adverse pregnancy outcomes, gastro-intestinal disorders, and diabetes (13). Meanwhile, F. nucleatum has been initially considered as a bridging organism in the assembly and architecture of polymicrobial biofilms, which has prompted the hypothese that F. nucleatum contributes to cancer development (14).
Landmark publication from two independent groups reported an overabundance of intratumoural F. nucleatum in colorectal cancer (CRC) tissues, comparing with adjacent non-tumorous mucosa by metagenomic analysis (15, 16). A meta-analysis indicated that the DNA of F. nucleatum was higher in CRC tissue, as well as in colorectal polyp tissue compared with adjacent healthy tissue from controls (17). F. nucleatum was also more abundant in fecal samples from CRC patients in comparison with healthy controls or individuals with premalignant lesions of the colorectum (17).
Interestingly, Komiya et al. identified the identical strains of F. nucleatum in the oral cavity and the CRC tissues from the patients, thus suggesting the oral dissemination of the F. nucleatum and its potential role in CRC carcinogenesis (18). Furthermore, F. nucleatum could be detected from stages 0 to IV, and no significant differences in the detection rate of F. nucleatum could be found among each CRC lesion site from 8 patients, indicating that F. nucleatum might adhere to CRC tissue from an early stage of tumorigenesis (19). The elevated level of F. nucleatum DNA in CRC tissues has been linked to certain molecules and cell functions, including the microsatellite instability, CpG island methylator phenotype, hMLH1 methylation, and genetic mutations in BRAF and TP53 (20, 21).
Several studies have explored the mechanisms by which F. nucleatum exerted its pathogenic roles in CRC. F. nucleatum can express a novel bacterial cell surface adhesin protein (FadA), which binds to E-cadherin, triggers β-catenin signaling pathway, consequently stimulates the production of inflammatory cytokines and chemokines and finally drives CRC cell proliferation in in vitro and in vivo models (16, 22, 23). Another possible etiological factor is that F. nucleatum can modulate the tumor-immune microenvironment and exert immunosuppressive activity by impairing natural killer cell and T cell functions (16, 24). F. nucleatum is also capable of inducing apoptosis cell death in the peripheral mononuclear blood cells and the polymorphonuclear neutrophils (25). F. nucleatum has been associated with a lower density of CD3+ T cell, and the secretion of immune cytokines in CRC (16, 26, 27). Furthermore, hydrogen sulfide, a metabolite of F. nucleatum, can generate reactive oxygen species, induce DNA damage, and cause single-nucleotide mutations. Hence, F. nucleatum may promote oncogenesis by acting as a DNA-damaging agent (28). F. nucleatum can also trigger the production of matrix metalloproteinase-9 (MMP-9) and MMP-12, which are important factors for the tumor proliferation, invasion and metastasis in epithelial cells (29). Collectively, the potential of F. nucleatum to act as a carcinogen is credible, as it has been shown to activate cell proliferation, induce chronic inflammation and suppress local immune responses.
Strikingly, a high prevalence of F. nucleatum in CRC was associated with the poor overall survival (30). Meanwhile, intratumoral F. nucleatum was more abundant in CRC tissues of patients who experienced recurrence post chemotherapy compared with those who did not exhibit tumor recurrence (31). However, the relationship between Fusobacterium’s abundance and the clinical outcome of CRC has been inconclusive. Two other studies found no relevance between Fusobacterium and the prognosis of CRC patients (15, 32). This discrepancy may attribute to the differences in patient cohorts, the methods used for assessing F. nucleatum, and chance variations between independent researches.
Overall, there has been extensive research demonstrated that the presence of F. nucleatum is associated with the pathogenicity, development, and prognosis of CRC. Epidemiological evidence and molecular mechanism studies have proved a positive association between preexisting inflammatory lesions, such as periodontitis and cancer risk (33). Given that F. nucleatum is originally an oral opportunistic pathogen, however, only limited preliminary data is available dealing with the role of F. nucleatum in other types of cancer, including esophageal, gastric, or pancreatic cancer, etc. It remains unclear whether F. nucleatum exerts a similar oncogenic effect on malignant tumor types except for CRC. The present review aims to update and systematize the latest information about the potential involvement of F. nucleatum in carcinogenesis and the significance of F. nucleatum as a prognostic and predictive biomarker to anti-tumor therapy in other types of malignancies.
F. nucleatum in oral and head and neck cancer
Human Oral Microbiome Database (www.homd.org) reported the existence of over 700 bacterial strains in human oral cavity (34). The oral bacterial plays an essential role in maintaining the healthy physiological environment in oral cavity and the periodontitis has been identified as an independent risk factor of oral cancer development (35). It has been concerning whether the infection of F. nucleatum, a well-known oral bacterium involved in the formation of typical dental plaque on human teeth, may cause oral cancer.
F. nucleatum is overabundant in oral and head and neck cancer
In 1998, the first association study by Nagy et al. found that the level of Fusobacterium were significantly higher in oral squamous cell carcinoma (OSCC) than in healthy mucosa (36). The DNA of saliva microbiome isolated from oropharyngeal squamous cell carcinoma (OPSCC), OSCC patients and normal epithelium controls were compared using 16S rRNA amplicon sequencing, to characterize the compositions of saliva microbiota and examine their abundance before and after surgical treatment. The analyses identified a total of 13 assigned phyla present, with 5 of these dominating across all of the samples: Fusobacteria, Proteobacteria, Bacteroidetes, Actinobacteria, and Firmicutes (37). In another study, cancer lesion samples and anatomically matched normal samples were obtained from the same patients to unravel the connections underlying oral bacterial dysbiosis and OSCC. Bacterial dysbiosis was observed within OSCC surface lesion samples, and drastic changes in surface bacterial communities of OSCC was observed. In particular, Fusobacterium, which belongs to periodontitis-correlated taxa, was found to be significantly enriched in OSCC samples. Additionally, several operational taxonomic units belonging to Fusobacterium were inferred to be highly involved in OSCC and demonstrated good diagnostic power (38). Consistently, two other studies (39, 40) also confirmed the significantly higher abundance of F. nucleatum in swabs of OSCC lesion surface compared to those of normal mucosa from the same patients (Table 1).
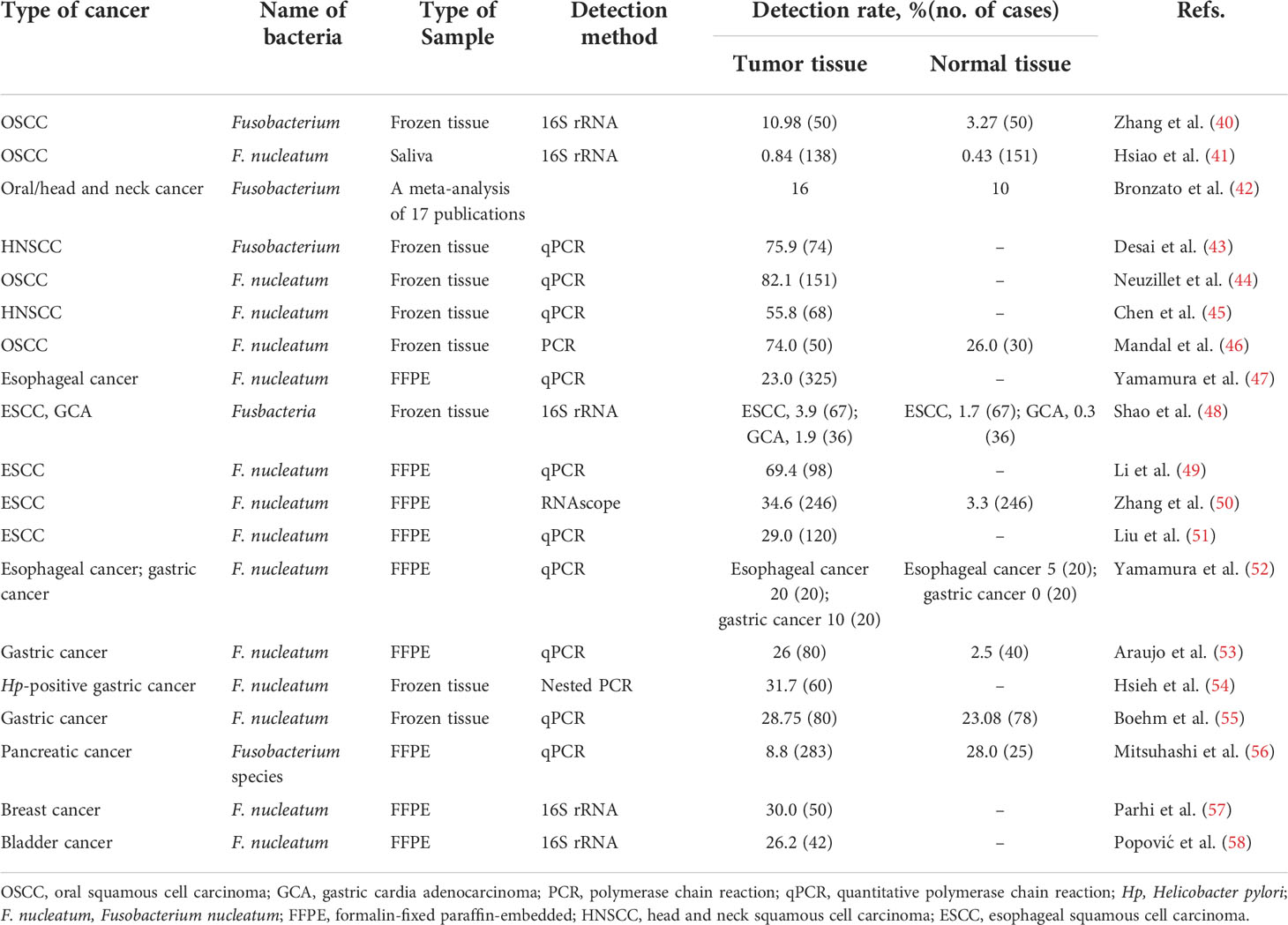
Table 1 Detection rates of Fusobacterium spp. in malignancies except for colorectal cancer from previous studies.
Since F. nucleatum is a bridge organism in the dental plaque and can influence other microorganisms in the oral cancer environment, some studies tried to investigate the correlation between F. nucleatum and other microorganisms in oral and head and neck cancer (38, 59–61). The oral bacterial DNA obtained from 20 fresh OSCC biopsies (cases) and 20 deep-epithelium swabs (matched control subjects) was sequenced to characterize the composition of bacterial species as well as the potential function of the bacteriome associated with OSCC. At the species level, this study provided the first epidemiological evidence ever for the association of F. nucleatum and Pseudomonas aeruginosa with OSCC, substantiating existing evidence on its carcinogenicity. At the subspecies level, some variations in the carcinogenicity of F. nucleatum were also suggested (60). F. nucleatum and Porphyromonas gingivalis existed at higher levels in tumor tissues than in normal tissues, while Streptococcus sanguinis was more frequent in normal tissues (61). The association between oral bacterial profile and the risk of OSCC was investigated in another case–control study based on 138 OSCC cases and 151 controls. Three species of periodontopathogenic bacteria, Prevotella tannerae, F. nucleatum, and Prevotella intermedia, were positively associated with the risk of OSCC. Every 1% increase in the total percentage of the above three oral periodontopathogenic bacteria was associated with the 28% increase in the risk of OSCC (OR = 1.28, 95% CI: 1.13–1.44) (41). These results were in accordance with a meta-analysis, which showed an increased prevalence of 6% of Fusobacterium in tumor lesions than in non-tumor lesions and a 2.93-fold higher chance of Fusobacterium being present in tumor lesion (95% CI, 1.47-5.81) (42).
The clinicopathologic characteristics associate with F. nucleatum
In addition, the abundance of F. nucleatum significantly varied by the tumor stage and was related with the prognosis of oral/head and neck cancer patients. An increased oral relative abundance of F. nucleatum was observed in the stage III disease of HPV+ OPSCC patients (p < 0.05) (62). Another pilot study analyzed the relationship between microbial diversity and the different OSCC stages. At the genus level, Acinetobacter and Fusobacterium were found being predominant in the late stage of OSCC (63).
A recently published study identified a significant prevalence of Fusobacterium in head and neck cancer comparable to CRC, from the genomic dataset of Indian origin. It was worth noting that F. nucleatum was related with the presence of extracapsular spread, the potential of invasion and metastasis, and poor survival in early-stage HPV-negative tongue cancer. Furthermore, a genomic landscape of pathogens identified the mutual exclusivity between F. nucleatum and HPV in head and squamous cell carcinoma (SCC). Fusobacterium-high subgroup of head and neck tumors was associated with an inflamed and pro-tumorigenic microenvironment (43). Hsueh et al. also verified that the high abundance of F. nucleatum in laryngeal squamous cell cancer (LSCC) was associated with poor prognosis (64). In contrast, Neuzillet et al. (44) and Chen et al. (45) found that the enrichment of F. nucleatum in oral/head and neck SCC cohort was significantly associated with a lower tumor stage, improved overall survival, relapse-free survival, and metastasis-free survival. F. nucleatum-positivity was also more frequent in older patients lacking the traditional risk factors of alcohol and smoking. Although these findings were unexpected given its association with poor prognosis in other cancer types, particularly in CRC (65), it was further verified that tumors with high F. nucleatum loads displayed low RNA levels of OX40 ligand (TNFSF4) and fibroblasts (PDGFRβ) (44), which predicted the favorable prognosis (66).
In conclusion, F. nucleatum is more abundant in oral/head and neck cancer samples than non-cancer samples, highlighting the importance of further research on the possible contribution of F. nucleatum to the development of oral/head and neck cancer (Table 2). The correlation between the abundance of F. nucleatum and the clinical stage of oral/head and neck cancer is conflicting. In contrast with the previous studies, F. nucleatum was suggested to be associated with “permissive” tumor microenvironment with low Toll-like receptor 4 (TLR4) signaling and M2 macrophage infiltration in OSCC (44). Further prospective explorations of unique microbial signature in oral/head and neck cancer may facilitate the use of oral bacteria as the biomarker for disease prevention, screening and response evaluation.
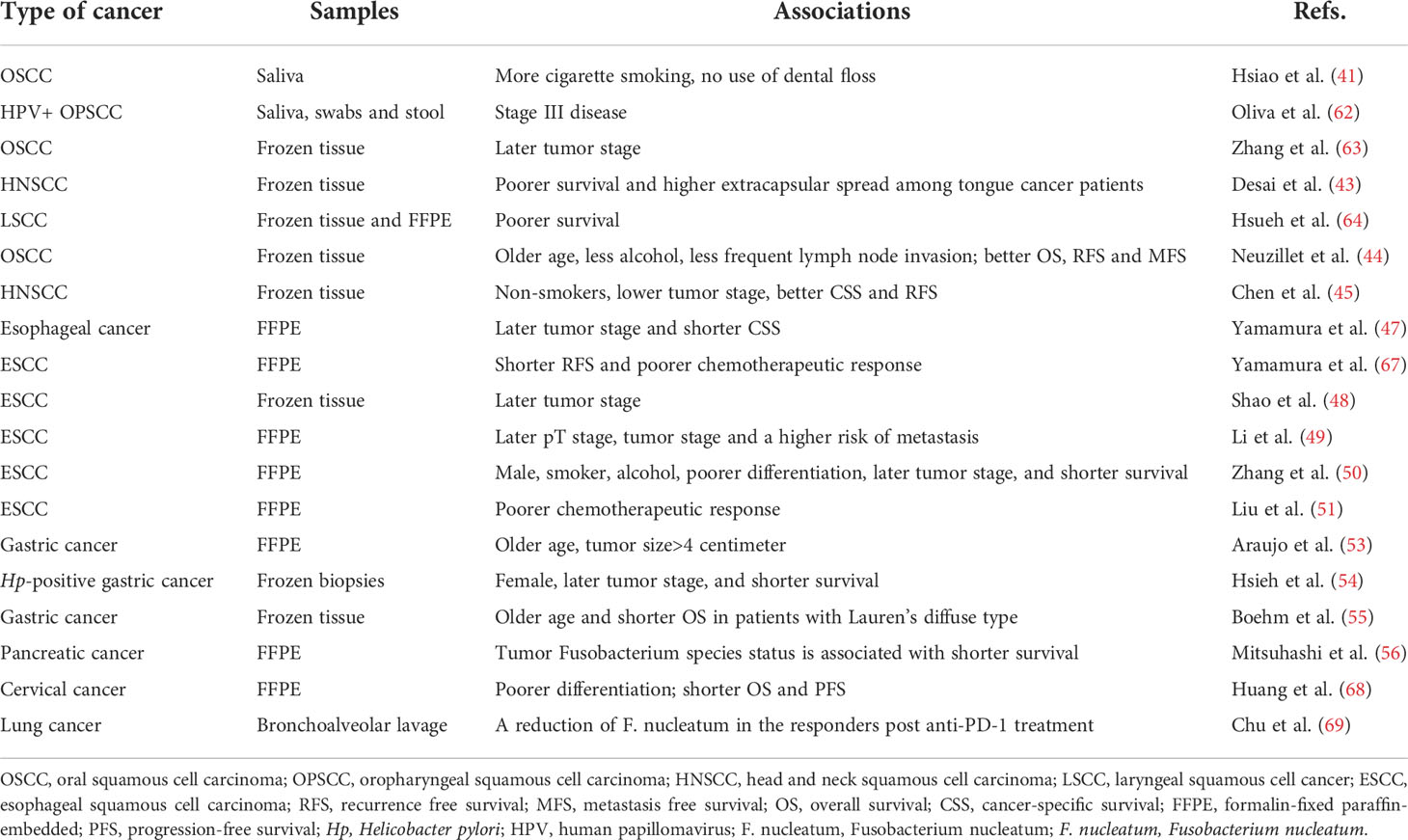
Table 2 Summary of publications showing the associations between clinicopathological features and F. nucleatum positivity in malignancies except for colorectal cancer.
Carcinogenesis mechanisms of F. nucleatum in oral and head and neck cancer
Although the detailed carcinogenesis mechanism of F. nucleatum is still unclear, F. nucleatum have been suggested to be associated with DNA damage, cell adhesion, epithelial-to-mesenchymal transition (EMT), inflammatory host response, and cell cycle in oral cancer (70, 71). F. nucleatum could cause cellular DNA damage by increasing upregulation of γH2AX, and promote cell proliferation via the Ku70/p53 pathway in oral cancer cells (70). In addition, by using immunohistochemistry, Mandal et al. provided the first study to propose that the carcinogenesis of F. nucleatum in OSCC may be CCL20-related, since there was a significant difference in the prevalence of CCL20-positive cell population between F. nucleatum-positive and negative OSCC (P=0.034) (46). The component of F. nucleatum cell wall extract (FnCW), iso-electric focusing (IEF) and beta-defensin inducer peptide (FAD-I), have been shown to stimulate human oral epithelial cells to secrete CCL20 (72). In addition, CCL20 has been shown to contribute to the oral immune response to bacterial infections and might be involved in the initiation and progression of OSCC (73). Meanwhile, Hsiao et al. suggested that periodontopathogenic bacteria might promote the OSCC oncogenesis by inducing inflammation, and the percentage of periodontopathogenic bacteria were positively related with the level of salivary cytokines, interleukin-1β (IL-1β) and IL-2 (41). Infection of gingival epithelial cells with F. nucleatum resulted in the translocation of NF-κB into the nucleus and activated NLRP3 inflammasome and caspase-1, following the expression of IL-1β (74). IL-1β has been shown to promote the OSCC carcinogenesis by increasing the proliferation of dysplastic oral cells and stimulating the oncogenic cytokines (75). Meanwhile, bacterial infections could effectively stimulate the dendritic cells to produce IL-2, which would exert its immuno-regulatory functions (76). Functional prediction based on OSCC biopsies and control subjects also showed that the “inflammatory bacteriome” was enriched in OSCC and genes involved in bacterial mobility, flagellar assembly, bacterial chemotaxis and lipopolysaccharides (LPS) synthesis were enriched in the tumors (60).
Gallimidi et al. provided the first demonstration of a mechanistic role for F. nucleatum and P. gingivalis in the chemically induced-OSCC tumorigenesis. Co-incubation with F. nucleatum and/or P. gingivalis profoundly promoted the proliferation of 4-nitroquinoline-1-oxide (4NQO) induced OSCC. Moreover, periodontal pathogens might stimulate tumorigenesis via direct interaction with oral epithelial cells, through activating epithelial TLR2 and augmenting signal transducer of IL-6 and activator of transcription-3 (STAT3) axis (77) (Figure 1). In the similar 4NQO-induced oral tumor murine model, it was consistent that the mice infected with F. nucleatum developed significantly larger and more numerous lesions compared to uninfected controls. Infected oral cancer cells had upregulated expression levels of MMP1, MMP9, and IL-8, the expression of cell survival markers MYC, JAK1, and STAT3, which are implicated in pathways that promote tumorigenesis (80), and EMT markers, ZEB1 and TGF-β were also significantly elevated. Interestingly, Fusobacterial culture supernatant, primarily LPS, was sufficient to induce the expression of IL-8 and MMP, demonstrating that direct contact of the bacteria with cancer cells might not be required to promote carcinogenesis (78).
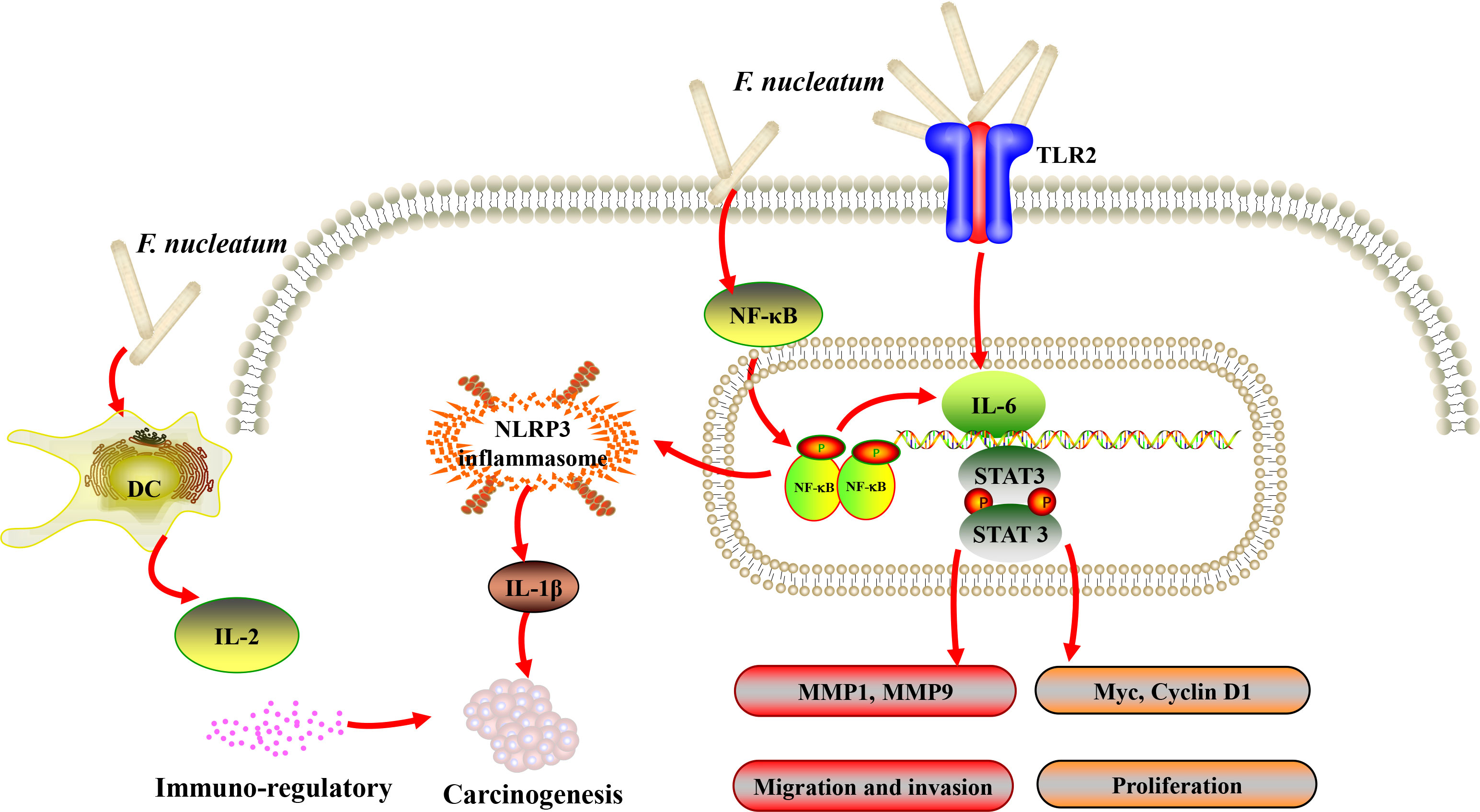
Figure 1 A schematic diagram of the proposed inflammation and immune response aroused by Fusobacterium nucleatum (F. nucleatum) in oral and head and neck cancer based on the literature review (41, 74, 76, 77, 80).
It is noteworthy that EMT process has been well described in various carcinomas of epithelial origin and regarded to be associated with initiation and metastasis of tumor (81). EMT was likely to be induced in OSCC cells in vitro in response to the stimulation of periodontal pathogens, including F. nucleatum and P. gingivalis (82). In addition, Zhang et al. analyzed the transcriptome profile of human immortalized oral epithelial cell in response to F. nucleatum infection. Tumor-associated genes were integrated, and top 10 potential hub genes (FYN, RAF1, ATM, FOS, CREB, NCOA3, VEGFA, JAK2, CREM and ATF3) were revealed by protein-protein interaction (PPI) network, and LncRNA-hub genes co-expression network comprising 67 dysregulated lncRNAs were generated (83). The same group further reported that F. nucleatum infection could eventually trigger EMT in both normal and cancerous oral epithelial cells via lncRNA MIR4435-2HG/miR-296-5p/Akt2/SNAI1 signaling pathway (79). A recent study showed that F. nucleatum increased the expression of miR-155-5p and miR-205-5p though MYD88-dependent TLR4 signaling, resulting in ethanol metabolism reprogramming via suppression of transforming growth factor β receptor 2 (TGFBR2) expression and subsequent suppression of alcohol dehydrogenase 1B (ADH1B) expression and promotion of EMT in LSCC. The positive feed-forward loop between F. nucleatum and ethanol metabolism reprogramming finally exacerbated the uncontrolled progression and metastasis of LSCC (64). Taken together, recent data provided preliminary evidence that EMT could be a probable link between F. nucleatum infection and the initiation of oral/head and neck epithelial carcinomas (Figure 2).
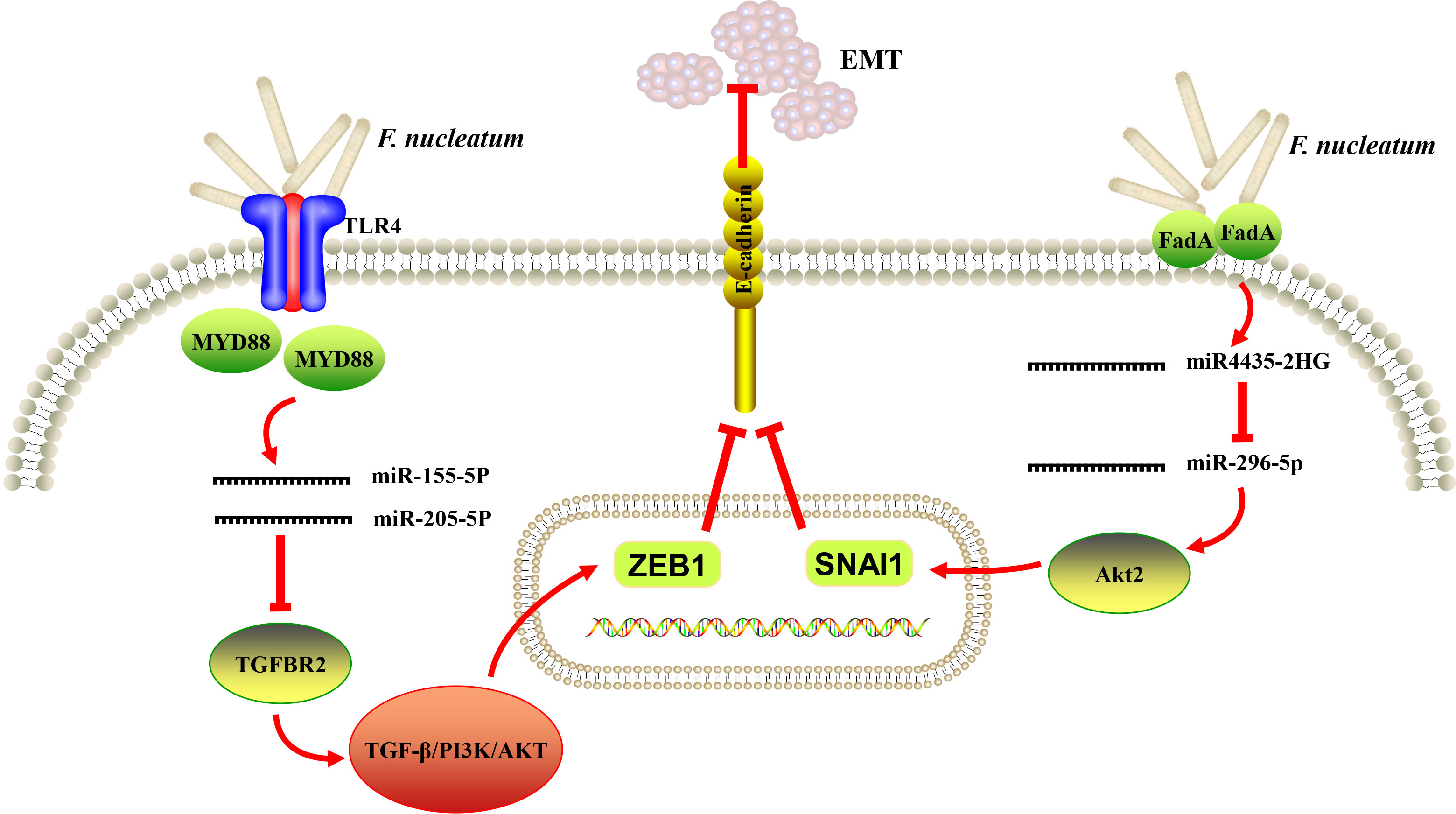
Figure 2 A schematic diagram of the proposed epithelial-to-mesenchymal transition (EMT) mechanism of Fusobacterium nucleatum (F. nucleatum) in oral and head and neck cancer based on the literature reviews (64, 78, 79).
F. nucleatum in esophageal cancer
Several literatures analyzed the relative abundance of F. nucleatum in esophageal cancer. F. nucleatum DNA was detected in 74 of 325 (23%) resected esophageal cancer specimens, which was significantly more than the matched normal esophageal mucosa. The positive rate of F. nucleatum DNA was verified to be significantly associated with tumor stage and shorter cancer-specific survival of esophageal cancer (47). Similarly, high intratumoral F. nucleatum burden was also found significantly associated with the poor response to neoadjuvant chemotherapy and had a prognostic significance for predicting poor recurrence-free survival in esophageal squamous cell carcinoma (ESCC) patients (67). Consistent with the previous studies, a positive association between the relative abundance of Fusobacterium and the more advanced tumor stage was found in ESCC tissues, by characterizing the microbial communities of paired tumor and normal samples from 67 patients with ESCC in Henan, China (48). In another study from China, the abundance of Fusobacterium was increased in tumor tissues and the relative abundance of F. nucleatum was closely related to the pT stage and clinical stage of ESCC. The abundance of F. nucleatum and tumor mutation burden might be used in combination as a method to predict the potential of metastasis in ESCC, since both a higher mutational burden and F. nucleatum-positive was observed in tumors with metastasis than without metastasis (49). However, F. nucleatum in the samples of subgingival dental plaque and unstimulated saliva was found not being different between esophageal cancer patients and matched healthy individuals (84). Consequently, the relationship between the abundance of F. nucleatum and esophageal cancer is still controversial and the underlying mechanism remains unclear. Clarifying the characteristics of the oral bacteria have potential implications for the early diagnosis in the context of esophageal cancer.
The mechanisms of the involvement of F. nucleatum in the esophageal cancer carcinogenesis and chemoresistance were preliminary explored. The top-ranked KEGG pathway in F. nucleatum-positive esophageal cancer was the “cytokine–cytokine receptor interaction”, thereby supporting the possible mechanism that F. nucleatum might contribute to the aggressive tumor behavior through the activation of chemokines, such as CCL20 (47). The infection and colonization of F. nucleatum might also facilitate the immune escape of tumor cells and weaken the antitumor immune response through enriching Treg cells, assisting the long-term self-colonization, and promoting the malignant progression of ESCC (50). In addition, 13 samples of F. nucleatum-positive ESCC were analyzed by the whole-exome sequencing and the results showed that the function of the mutant gene was mainly concentrated in the pathways regulating apoptosis and the epidermal growth factor-like protein domain (49). Further analysis revealed that F. nucleatum could mediate chemoresistance of ESCC cells by modulating autophagy. In addition, F. nucleatum could induce LC3 and ATG7, as well as autophagosome formation to cause chemoresistance against 5-fluorouracil, cisplatin, and Docetaxel. Immunohistochemical studies also confirmed the correlation between F. nucleatum infection and ATG7 expression in 284 ESCC specimens (51).
F. nucleatum in gastric cancer
The composition of gastric microbiota is unique and subject to rapid changes caused by the food consumption. The gastric microbiota includes various passenger bacteria undergoing transit from the oral cavity to the lower gut, besides the normal resident bacteria (85). Hp was the predominant pathogen in gastric and responsible for the development of gastric cancer (86). According to several preliminary reports, with new sequencing tools, it is increasingly appreciated that not Hp alone, but rather the microbiome in whole complexity may trigger a more aggressive oncogenesis (52, 87).
DNA was extracted from 120 gastric fragment samples embedded in paraffin (80 gastric cancer patients; 40 cancer-free patients). F. nucleatum was found to be positive in 19 samples (26.0%) of gastric cancer patients, while 1 sample (2.5%) was found to be positive in cancer-free patients. The prevalence of F. nucleatum was associated with 11-fold increase in the risk of developing gastric cancer, and was related with advanced age (P=0.030) and large tumor size (P=0.053) (53). According to the profiles of gastric epithelium-associated microbiota in patients with gastritis, intestinal metaplasia, and gastric cancer, it was indicated that gastric microenvironment was frequently enriched with Clostridium and Fusobacterium in gastric cancer patients. Furthermore, a receiver operating characteristic curve analysis showed that Clostridium colicanis, together with F. nucleatum, were potentially considered as the viable diagnostic markers for early diagnosis and positively identified gastric cancer with 100% sensitivity. It also raised the possibility that those bacteria might participate in gastric oncogenesis and the progression of gastric cancer (85). According to another study from southwestern region of Taiwan, F. nucleatum was frequently enriched in gastric cancer tissues and positive in 4 samples among the 11 gastric cancer biopsies. The colonization of F. nucleatum was able to alter actin filament dynamics to promote the mobility and invasiveness of gastric cancer cells. Additionally, cohort analysis demonstrated that the combined infection of F. nucleatum with Hp led to a poorer prognosis in gastric cancer patients, indicating the two pathogens acted synergistically to promote the aggressiveness of gastric cancer. Overall, F. nucleatum could increase the invasiveness and metastasis of gastric cancer and negatively impact the prognosis of gastric cancer patients (54). F. nucleatum was found substantially higher in CRC compared than gastric cancer among a well-characterized cohort of gastric cancer patients. The frequency and bacterial load of F. nucleatum were higher in tumorous tissues of CRC and gastric cancer than non-tumorous tissues. Meanwhile, the positivity of F. nucleatum was associated with a significantly worse prognosis in Lauren’s diffuse type gastric cancer, but not in the intestinal type gastric cancer patients. As mentioned, F. nucleatum may promote carcinogenesis via FadA adhesin, which binds to E-cadherin, activates β-catenin signaling and various inflammatory and oncogenic properties of the cells (23). Diffuse type gastric cancer was strongly related with E-cadherin deregulation, which might account for potential molecular mimicry and specific prognostic relevance of F. nucleatum to diffuse type of gastric cancer. Further studies are urgently needed to evaluate the possible molecular alterations and therapeutic implications responsible for Lauren’s diffuse type gastric cancer (55).
F. nucleatum in pancreatic cancer
The detection rate of Fusobacterium species in 283 patients with pancreatic ductal adenocarcinoma who underwent surgical treatment was 8.8%. Moreover, Fusobacterium species status of pancreatic cancer tissue specimens was independently associated with a worse prognosis, suggesting that the presence of Fusobacterium species might be related to the malignant potential of pancreatic cancer. Fusobacterium species were detected in 28% (7/25) of the paired specimens of normal tissues, using the tumor Fusobacterium species-positive cases. Hence, Fusobacterium spp. may play a role in the pathogenesis of pancreatic cancer. Further studies are needed to elucidate the roles of these bacteria in the development of pancreatic cancer, which can lead to the development of new diagnostic and therapeutic methods (i.e., eradication) for pancreatic cancer patients (56). Furthermore, circulating plasma and salivary antibodies to F. nucleatum were correlated with the severity of intraductal papillary mucinous neoplasms. It was demonstrated that humoral reactivities against F. nucleatum were associated with cystic pancreatic neoplasm malignancy (88). Apart from biopsy, a non-invasive method for bacterial analysis may be applied as a diagnostic tool in the future.
F. nucleatum in other non-digestive malignancies
Taken together, except for CRC, F. nucleatum has been previously isolated from cancers at other sites along the digestive tract, including the oral, esophagus, stomach, and pancreas. Furthermore, F. nucleatum has also recently been implicated in the carcinogenesis and progression of other non-digestive malignancies.
F. nucleatum in breast cancer
Utilizing a permutation test to assess differential taxa of the breast tissue in malignant and benign states demonstrated that the genus Fusobacterium was significantly enriched in the breast tissue samples from patients with invasive malignant disease (89). Genomic DNA of F. nucleatum was overabundant in human breast cancer. Using two different murine orthotropic models, F. nucleatum was found to contribute to breast tumor growth and metastatic progression, most likely through suppressing the accumulation of tumor infiltrating T cells in the tumor microenvironment. F. nucleatum could colonize in mammary tumors via D-galactose–β(1–3)-N-acetyl-D-galactosamine (Gal-GalNAc), which was overdisplayed on breast cancer cells. Fap2, which was the surface-exposed lectin of F. nucleatum, could bind through Gal-GalNAc and mediate breast cancer colonization. Furthermore, antibiotic treatment with metronidazole could counteract F. nucleatum-induced breast tumor exacerbation, suggesting that targeting F. nucleatum might benefit the treatment of breast cancer (57). F. nucleatum promotes CRC progression by activating the TLR4/MyD88 pathway and exhibiting immunomodulatory effects. Whether F. nucleatum promote breast cancer progression in a TLR4 dependent manner and through immunomodulation need further exploration (90).
F. nucleatum in cervical cancer
Phylum Fusobacteria was predominant in the vaginal microbiota and associated with a high risk of cervical intraepithelial neoplasia in Korea (91). The association between cervical microbiota diversity and the histopathological diagnosis of each stage of cervical cancer was also assessed. Remarkably, Fusobacterium spp. was significantly more abundant in the late stages of cervical cancer than in the early stages (HPV-negative or HPV-positive non-cervical lesions) (92). Similarly, there was a distinct high levels of F. nucleatum in cervical cancer, especially in relapsed disease. The increased burden of intratumoral F. nucleatum predicted correspondingly poorer prognosis in locally advanced stage cervical cancer. Notably, the level of F. nucleatum was positively correlated with tumor differentiation, and high burden of intratumoral F. nucleatum possessed the characteristic of cancer stem cells. It was proposed that F. nucleatum might be one potential cervical cancer diagnostic and prognostic biomarker, and these findings would help to provide a sound rationale and merit for further study of this bacterium (68).
F. nucleatum in bladder cancer and lung cancer
Bacterial communities present in urine samples collected from 12 male patients diagnosed with bladder cancer, and from 11 healthy, age-matched individuals were analyzed using 16S sequencing. Genus Fusobacterium was significantly enriched in the bladder cancer group. In an independent sample of 42 bladder cancer tissues, 11 (26%) cases were positive for F. nucleatum, detecting by PCR (58).
A metagenomic sequencing analysis on microbial compositions was performed from bronchoalveolar lavage of lung cancer patients who were treated with anti-PD-1 immunotherapy, including 21 non-responders and 19 responders. The relationship between bacterial load and diversity with the clinical response to anti-PD-1 therapy was further analyzed. Airway enriched Fusobacterium prior to anti-PD-1 monotherapy was associated with resistance to anti-PD-1 response, providing potential implication in treatment resistance in the immunotherapy of lung cancer (69).
Discussion
Periodontitis plays an active role in the pathogenesis of human CRC and the rate of new diagnosis of CRC in persons with a positive history of periodontal disease was 1.45 times higher than in those with the negative history after adjusting for a number of potential confounders (93, 94). Upon growing evidences, F. nucleatum was shown to be highly abundant in CRC and could be a causative agent of CRC. However, its effects on the development of cancer in other parts of the body have been little studied. We tried to review and enrich almost all the known mechanisms in F. nucleatum-mediated carcinogenesis in malignancies except for CRC (Figures 1, 2). The relationships between F. nucleatum status and clinicopathological and molecular features in tumor types except for CRC were also reviewed and summarized in Tables 2, 3. Further studies are warranted to fully unravel the intricate regulatory networks of molecular and cellular events underlying the action of F. nucleatum in tumorigenesis.

Table 3 Reported genes involved in F. nucleatum-associated malignancies except for colorectal cancer.
F. nucleatum has been primarily characterized as an implicated factor in multi-species biofilms of dental plaque and F. nucleatum was enriched in biofilms of OSCC patients (95). By forming bridges between the early and late colonizers, F. nucleatum tended to co-adhere with other species in the periodontal biofilms (96). Hence, F. nucleatum played a critical role in increasing bacterial diversity of OSCC. Oral hygiene management was beneficial for the reduction of the periodontal biofilm and consequently the amount of F. nucleatum, which might contribute to the prevention of OSCC. Although emerging studies suggested that gingivitis and periodontitis, usually caused by microorganisms, could be risk factors for oral/head and neck cancer, it was still difficult to discriminate the concomitant effect or association effect among different pathogenic microorganisms (59). Meanwhile, the extent and severity of periodontitis acted as risk indicators for oral/head and neck cancer even after the adjustments for traditional confound factors (97). Further studies are needed to elucidate the etiologic role of the specific microbiota and the connection between the extent of periodontitis and carcinogenesis in different tumor types.
In addition, the development of biofilm-like structure in the tumor spheroid microenvironment by F. nucleatum was also observed in the three-dimensional CRC spheroid model (98). The property of F. nucleatum as a bridging organism was demonstrated to be dependent on the host microenvironment in response to alkaline pH (99). Thus, the pathogenicity of F. nucleatum in cancer might dependent on pH value influenced by the interplay between the unique microenvironment and microbiome. A low abundance of F. nucleatum was found in stomach, which might due to the protective properties of acidic milieu preventing the bacteria dissemination. However, F. nucleatum was also not enriched in atrophic gastritis/intestinal metaplasia, where higher pH due to mucosa atrophy was expected. Therefore, further studies will be necessary to address the mechanisms and the appropriate microenvironment of the biofilm formation and development. Clinical studies should also be proposed to explore the microbial profiles and the composition of multiple biofilms during the different stages of tumor evolvement.
In addition to the relevance of F. nucleatum with chronic inflammation, F. nucleatum has also been suggested to be linked to carcinogenesis and antitumor effect of chemoradiotherapy. F. nucleatum enhanced CRC chemoresistance through modulating of TLR4 and MYD88 innate immune signaling, along with specific microRNAs that subsequently activated the autophagy pathway and promoted chemoresistance (31). Furthermore, the colonization of Fusobacterium has been traced from primary tumors to liver distal metastases in CRC patients. Treatment of mice bearing a colon cancer xenograft with antibiotic therapy of metronidazole led to a significant decrease in Fusobacterium load, cancer cell proliferation and overall tumor exacerbation (100). Similarly, metronidazole could counteract F. nucleatum-induced accumulation of tumor infiltrating T cells and growth of breast cancer cells (57). F. nucleatum was verified to migrate and locate at the CRC site and metronidazole treatment could cause a lower relative abundance of F. nucleatum in both the oral cavity and CRC locus. CRC mice treated with radiotherapy combined with metronidazole had a significantly reduced tumor burden and radiation enteritis (101). High intratumoral F. nucleatum burden predicted a poor response to neoadjuvant chemotherapy in ESCC and airway enriched Fusobacterium prior to anti-PD-1 monotherapy was associated with resistance to immunotherapy in lung cancer (47, 67, 69). The mechanisms of how the antibiotics exerts the critical role in the carcinogenesis and antitumor effects in malignancies other than CRC need to be further explored.
Furthermore, previous results have been inconsistent regarding the prevalence and prognostic roles of intratumoral F. nucleatum, and the biological interplay between this bacterium and the different tumor immune microenvironment. Inconsistent results among different studies might be due to different ethnic groups (102), methodological variations in terms of technology for detecting F. nucleatum (culture, real-time PCR, 16S rRNA metagenomics), the types of samples (biopsy, surface swab or saliva), and the selection of controls (healthy controls or non-cancerous tissues as controls) (103). In addition, the relatively small sample size in the retrospective cohort and case-control studies might also interpret the controversial results. Furthermore, the immune-related gene analysis was only based on selected genes, which were not fully specific of each immune cell subtypes. Overall, further exploration on the mechanisms linking the colonization of F. nucleatum with immune microenvironment in different tumor types are urgently needed. The above controversial results also motivate the randomized clinical trials from larger prospective cohorts to verify these findings.
The host polysaccharide Gal-GalNAc, which is overexpressed in CRC and recognized by a microbial protein, Fap2, could trigger F. nucleatum binding to the tumor tissues (96). Besides CRC, increasing Gal-GalNAc level was also found in additional various adenocarcinomas of the stomach, prostate, pancreas, ovary, uterus, breast, esophagus, and lung (104). It was demonstrated that oral F. nucleatum might probably translocate to the colon via the hematogenous route during transient bacteremia, which was frequent in periodontal disease, instead of the gastrointestinal route (57). Its selectivity for Gal-GalNAc-displaying tumors, suggested that additional tumors might be colonized by F. nucleatum (57, 96, 104). Therefore, a potential fusobacterial elimination-based cancer therapy may be engineered as a platform for treating high Gal-GaNAc displaying tumors individually in the future (104).
Conclusions
The current studies shed light on the potential application of F. nucleatum as a diagnostic and prognostic biomarker in the context of multiple tumor types. The early periodontal screening, detection and prognosis judgement yield valuable insights into clinical management, which lead to a reduced morbidity and mortality rate and improve the oncologic outcome of cancer patients. In addition, the involvement of periodontal disease in the evolution of some types of cancers through the action of F. nucleatum has been proven. Hematogenous F. nucleatum can bind and/or invade diverse cell types including oral, colonic epithelial cells, T-cells, keratinocytes and macrophages through its lectin Fap2 (105), which also plays a putative role in carcinogenesis (106). In mice, intravascularly inoculated Fap2-expressing F. nucleatum ATCC 23726 specifically colonized mammary tumors, whereas Fap2-deficient bacteria were impaired in tumor colonization. Thus, targeting F. nucleatum or Fap2 might be efficient during the treatment of breast cancer (57). There have been multiple studies concerning about anti-cancer therapies through targeting the bacteria, such as vaccination, the change of diet, and the use of probiotics (107–109). This would motivate future research on the mechanisms of the F. nucleatum-initiated cancers for the development of novel approaches to prevent or treat F. nucleatum-related diseases.
Author contributions
ZH, WT, and JX researched data for the article, designed the figures, and wrote the manuscript. QW reviewed/edited the manuscript before submission. All authors approved the final version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (82074217).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature (2007) 449(7164):804–10. doi: 10.1038/nature06244
2. Routy B, Gopalakrishnan V, Daillere R, Zitvogel L, Wargo JA, Kroemer G. The gut microbiota influences anticancer immunosurveillance and general health. Nat Rev Clin Oncol (2018) 15(6):382–96. doi: 10.1038/s41571-018-0006-2
3. Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health (2016) 4(9):e609–16. doi: 10.1016/S2214-109X(16)30143-7
4. Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet (2012) 13(4):260–70. doi: 10.1038/nrg3182
5. Engevik MA, Danhof HA, Auchtung J, Endres BT, Ruan W, Basseres E, et al. Fusobacteriumnucleatum adheres to clostridioides difficile via the RadD adhesin to enhance biofilm formation in intestinal mucus. Gastroenterology (2021) 160(4):1301–14 e8. doi: 10.1053/j.gastro.2020.11.034
6. Liu L, Liang L, Yang C, Zhou Y, Chen Y. Extracellular vesicles of fusobacterium nucleatum compromise intestinal barrier through targeting RIPK1-mediated cell death pathway. Gut Microbes (2021) 13(1):1–20. doi: 10.1080/19490976.2021.1902718
7. Song YG, Shim SG, Kim KM, Lee DH, Kim DS, Choi SH, et al. Profiling of the bacteria responsible for pyogenic liver abscess by 16S rRNA gene pyrosequencing. J Microbiol (2014) 52(6):504–9. doi: 10.1007/s12275-014-4241-7
8. Eriksson K, Lundmark A, Delgado LF, Hu YOO, Fei G, Lee L, et al. Salivary microbiota and host-inflammatory responses in periodontitis affected individuals with and without rheumatoid arthritis. Front Cell Infect Microbiol (2022) 12:841139. doi: 10.3389/fcimb.2022.841139
9. Vander Haar EL, So J, Gyamfi-Bannerman C, Han YW. Fusobacterium nucleatum and adverse pregnancy outcomes: Epidemiological and mechanistic evidence. Anaerobe (2018) 50:55–9. doi: 10.1016/j.anaerobe.2018.01.008
10. Piekut T, Hurla M, Banaszek N, Szejn P, Dorszewska J, Kozubski W, et al. Infectious agents and alzheimer’s disease. J Integr Neurosci (2022) 21(2):73. doi: 10.31083/j.jin2102073.
11. Pyysalo MJ, Pyysalo LM, Pessi T, Karhunen PJ, Ohman JE. The connection between ruptured cerebral aneurysms and odontogenic bacteria. J Neurol Neurosurg Psychiatry (2013) 84(11):1214–8. doi: 10.1136/jnnp-2012-304635
12. Zhou J, Liu L, Wu P, Zhao L, Wu Y. Fusobacterium nucleatum accelerates atherosclerosis via macrophage-driven aberrant proinflammatory response and lipid metabolism. Front Microbiol (2022) 13:798685. doi: 10.3389/fmicb.2022.798685
13. Han YW. Fusobacterium nucleatum: a commensal-turned pathogen. Curr Opin Microbiol (2015) 23:141–7. doi: 10.1016/j.mib.2014.11.013
14. Sun CH, Li BB, Wang B, Zhao J, Zhang XY, Li TT, et al. The role of fusobacterium nucleatum in colorectal cancer: from carcinogenesis to clinical management. Chronic Dis Transl Med (2019) 5(3):178–87. doi: 10.1016/j.cdtm.2019.09.001
15. Castellarin M, Warren RL, Freeman JD, Dreolini L, Krzywinski M, Strauss J, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res (2012) 22(2):299–306. doi: 10.1101/gr.126516.111
16. Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe (2013) 14(2):207–15. doi: 10.1016/j.chom.2013.07.007
17. Gethings-Behncke C, Coleman HG, Jordao HWT, Longley DB, Crawford N, Murray LJ, et al. Fusobacterium nucleatum in the colorectum and its association with cancer risk and survival: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev (2020) 29(3):539–48. doi: 10.1158/1055-9965.EPI-18-1295
18. Komiya Y, Shimomura Y, Higurashi T, Sugi Y, Arimoto J, Umezawa S, et al. Patients with colorectal cancer have identical strains of fusobacterium nucleatum in their colorectal cancer and oral cavity. Gut (2019) 68(7):1335–7. doi: 10.1136/gutjnl-2018-316661
19. Flemer B, Warren RD, Barrett MP, Cisek K, Das A, Jeffery IB, et al. The oral microbiota in colorectal cancer is distinctive and predictive. Gut (2018) 67(8):1454–63. doi: 10.1136/gutjnl-2017-314814
20. Tahara T, Yamamoto E, Suzuki H, Maruyama R, Chung W, Garriga J, et al. Fusobacterium in colonic flora and molecular features of colorectal carcinoma. Cancer Res (2014) 74(5):1311–8. doi: 10.1158/0008-5472.CAN-13-1865
21. Hamada T, Zhang X, Mima K, Bullman S, Sukawa Y, Nowak JA, et al. Fusobacterium nucleatum in colorectal cancer relates to immune response differentially by tumor microsatellite instability status. Cancer Immunol Res (2018) 6(11):1327–36. doi: 10.1158/2326-6066.CIR-18-0174
22. Rubinstein MR, Baik JE, Lagana SM, Han RP, Raab WJ, Sahoo D, et al. Fusobacterium nucleatum promotes colorectal cancer by inducing wnt/beta-catenin modulator annexin A1. EMBO Rep (2019) 20(4):e47638. doi: 10.15252/embr.201847638
23. Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating e-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe (2013) 14(2):195–206. doi: 10.1016/j.chom.2013.07.012
24. Wu J, Li Q, Fu X. Fusobacterium nucleatum contributes to the carcinogenesis of colorectal cancer by inducing inflammation and suppressing host immunity. Transl Oncol (2019) 12(6):846–51. doi: 10.1016/j.tranon.2019.03.003
25. Jewett A, Hume WR, Le H, Huynh TN, Han YW, Cheng G, et al. Induction of apoptotic cell death in peripheral blood mononuclear and polymorphonuclear cells by an oral bacterium, fusobacterium nucleatum. Infect Immun (2000) 68(4):1893–8. doi: 10.1128/IAI.68.4.1893-1898.2000
26. Mima K, Sukawa Y, Nishihara R, Qian ZR, Yamauchi M, Inamura K, et al. Fusobacterium nucleatum and T cells in colorectal carcinoma. JAMA Oncol (2015) 1(5):653–61. doi: 10.1001/jamaoncol.2015.1377
27. Dharmani P, Strauss J, Ambrose C, Allen-Vercoe E, Chadee K. Fusobacterium nucleatum infection of colonic cells stimulates MUC2 mucin and tumor necrosis factor alpha. Infect Immun (2011) 79(7):2597–607. doi: 10.1128/IAI.05118-11
28. Yoshida Y, Suwabe K, Nagano K, Kezuka Y, Kato H, Yoshimura F. Identification and enzymic analysis of a novel protein associated with production of hydrogen sulfide and l-serine from l-cysteine in fusobacterium nucleatum subsp. nucleatum ATCC 25586. Microbiol (Reading) (2011) 157(Pt 7):2164–71. doi: 10.1099/mic.0.048934-0
29. Uitto VJ, Baillie D, Wu Q, Gendron R, Grenier D, Putnins EE, et al. Fusobacterium nucleatum increases collagenase 3 production and migration of epithelial cells. Infect Immun (2005) 73(2):1171–9. doi: 10.1128/IAI.73.2.1171-1179.2005
30. Mima K, Nishihara R, Qian ZR, Cao Y, Sukawa Y, Nowak JA, et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut (2016) 65(12):1973–80. doi: 10.1136/gutjnl-2015-310101
31. Yu T, Guo F, Yu Y, Sun T, Ma D, Han J, et al. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell (2017) 170(3):548–63.e16. doi: 10.1016/j.cell.2017.07.008
32. Ito M, Kanno S, Nosho K, Sukawa Y, Mitsuhashi K, Kurihara H, et al. Association of fusobacterium nucleatum with clinical and molecular features in colorectal serrated pathway. Int J Cancer (2015) 137(6):1258–68. doi: 10.1002/ijc.29488
33. Nwizu N, Wactawski-Wende J, Genco RJ. Periodontal disease and cancer: Epidemiologic studies and possible mechanisms. Periodontol 2000 (2020) 83(1):213–33. doi: 10.1111/prd.12329
34. Chattopadhyay I, Verma M, Panda M. Role of oral microbiome signatures in diagnosis and prognosis of oral cancer. Technol Cancer Res Treat (2019) 18:1533033819867354. doi: 10.1177/1533033819867354
35. Hajishengallis G, Chavakis T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat Rev Immunol (2021) 21(7):426–40. doi: 10.1038/s41577-020-00488-6
36. Nagy KN, Sonkodi I, Szoke I, Nagy E, Newman HN. The microflora associated with human oral carcinomas. Oral Oncol (1998) 34(4):304–8. doi: 10.1016/S1368-8375(98)80012-2
37. Guerrero-Preston R, Godoy-Vitorino F, Jedlicka A, Rodriguez-Hilario A, Gonzalez H, Bondy J, et al. 16S rRNA amplicon sequencing identifies microbiota associated with oral cancer, human papilloma virus infection and surgical treatment. Oncotarget (2016) 7(32):51320–34. doi: 10.18632/oncotarget.9710
38. Zhao H, Chu M, Huang Z, Yang X, Ran S, Hu B, et al. Variations in oral microbiota associated with oral cancer. Sci Rep (2017) 7(1):11773. doi: 10.1038/s41598-017-11779-9
39. Schmidt BL, Kuczynski J, Bhattacharya A, Huey B, Corby PM, Queiroz EL, et al. Changes in abundance of oral microbiota associated with oral cancer. PloS One (2014) 9(6):e98741. doi: 10.1371/journal.pone.0098741
40. Zhang L, Liu Y, Zheng HJ, Zhang CP. The oral microbiota may have influence on oral cancer. Front Cell Infect Microbiol (2019) 9:476. doi: 10.3389/fcimb.2019.00476
41. Hsiao JR, Chang CC, Lee WT, Huang CC, Ou CY, Tsai ST, et al. The interplay between oral microbiome, lifestyle factors and genetic polymorphisms in the risk of oral squamous cell carcinoma. Carcinogenesis (2018) 39(6):778–87. doi: 10.1093/carcin/bgy053
42. Bronzato JD, Bomfim RA, Edwards DH, Crouch D, Hector MP, Gomes B. Detection of fusobacterium in oral and head and neck cancer samples: A systematic review and meta-analysis. Arch Oral Biol (2020) 112:104669. doi: 10.1016/j.archoralbio.2020.104669
43. Desai S, Dharavath B, Manavalan S, Rane A, Redhu AK, Sunder R, et al. Fusobacterium nucleatum is associated with inflammation and poor survival in early-stage HPV-negative tongue cancer. NAR Cancer (2022) 4(1):zcac006. doi: 10.1093/narcan/zcac006
44. Neuzillet C, Marchais M, Vacher S, Hilmi M, Schnitzler A, Meseure D, et al. Prognostic value of intratumoral fusobacterium nucleatum and association with immune-related gene expression in oral squamous cell carcinoma patients. Sci Rep (2021) 11(1):7870. doi: 10.1038/s41598-021-86816-9
45. Chen Z, Wong PY, Ng CWK, Lan L, Fung S, Li JW, et al. The intersection between oral microbiota, host gene methylation and patient outcomes in head and neck squamous cell carcinoma. Cancers (Basel) (2020) 12(11):3425. doi: 10.3390/cancers12113425
46. Mandal DP, Mohanty N, Behera PK, Gopinath D, Panda S, Al-Kheraif AA, et al. A plausible proposition of ccl20-related mechanism in fusobacterium nucleatum-associated oral carcinogenesis. Life (Basel) (2021) 11(11):1218. doi: 10.3390/life11111218
47. Yamamura K, Baba Y, Nakagawa S, Mima K, Miyake K, Nakamura K, et al. Human microbiome fusobacterium nucleatum in esophageal cancer tissue is associated with prognosis. Clin Cancer Res (2016) 22(22):5574–81. doi: 10.1158/1078-0432.CCR-16-1786
48. Shao D, Vogtmann E, Liu A, Qin J, Chen W, Abnet CC, et al. Microbial characterization of esophageal squamous cell carcinoma and gastric cardia adenocarcinoma from a high-risk region of China. Cancer (2019) 125(22):3993–4002. doi: 10.1002/cncr.32403
49. Li Z, Shi C, Zheng J, Guo Y, Fan T, Zhao H, et al. Fusobacterium nucleatum predicts a high risk of metastasis for esophageal squamous cell carcinoma. BMC Microbiol (2021) 21(1):301. doi: 10.1186/s12866-021-02352-6
50. Zhang N, Liu Y, Yang H, Liang M, Wang X, Wang M, et al. Clinical significance of fusobacterium nucleatum infection and regulatory t cell enrichment in esophageal squamous cell carcinoma. Pathol Oncol Res (2021) 27:1609846. doi: 10.3389/pore.2021.1609846
51. Liu Y, Baba Y, Ishimoto T, Tsutsuki H, Zhang T, Nomoto D, et al. Fusobacterium nucleatum confers chemoresistance by modulating autophagy in oesophageal squamous cell carcinoma. Br J Cancer (2021) 124(5):963–74. doi: 10.1038/s41416-020-01198-5
52. Yamamura K, Baba Y, Miyake K, Nakamura K, Shigaki H, Mima K, et al. Fusobacterium nucleatum in gastroenterological cancer: Evaluation of measurement methods using quantitative polymerase chain reaction and a literature review. Oncol Lett (2017) 14(6):6373–8. doi: 10.3892/ol.2017.7001
53. Nascimento Araujo CD, Amorim AT, Barbosa MS, Alexandre J, Campos GB, Macedo CL, et al. Evaluating the presence of mycoplasma hyorhinis, fusobacterium nucleatum, and helicobacter pylori in biopsies of patients with gastric cancer. Infect Agent Cancer (2021) 16(1):70. doi: 10.1186/s13027-021-00410-2
54. Hsieh YY, Tung SY, Pan HY, Chang TS, Wei KL, Chen WM, et al. Fusobacterium nucleatum colonization is associated with decreased survival of helicobacter pylori-positive gastric cancer patients. World J Gastroenterol (2021) 27(42):7311–23. doi: 10.3748/wjg.v27.i42.7311
55. Boehm ET, Thon C, Kupcinskas J, Steponaitiene R, Skieceviciene J, Canbay A, et al. Fusobacterium nucleatum is associated with worse prognosis in lauren’s diffuse type gastric cancer patients. Sci Rep (2020) 10(1):16240. doi: 10.1038/s41598-020-73448-8
56. Mitsuhashi K, Nosho K, Sukawa Y, Matsunaga Y, Ito M, Kurihara H, et al. Association of fusobacterium species in pancreatic cancer tissues with molecular features and prognosis. Oncotarget (2015) 6(9):7209–20. doi: 10.18632/oncotarget.3109
57. Parhi L, Alon-Maimon T, Sol A, Nejman D, Shhadeh A, Fainsod-Levi T, et al. Breast cancer colonization by fusobacterium nucleatum accelerates tumor growth and metastatic progression. Nat Commun (2020) 11(1):3259. doi: 10.1038/s41467-020-16967-2
58. Bucevic Popovic V, Situm M, Chow CT, Chan LS, Roje B, Terzic J. The urinary microbiome associated with bladder cancer. Sci Rep (2018) 8(1):12157. doi: 10.1038/s41598-018-29054-w
59. Shin JM, Luo T, Kamarajan P, Fenno JC, Rickard AH, Kapila YL. Microbial communities associated with primary and metastatic head and neck squamous cell carcinoma - a high fusobacterial and low streptococcal signature. Sci Rep (2017) 7(1):9934. doi: 10.1038/s41598-017-09786-x
60. Al-Hebshi NN, Nasher AT, Maryoud MY, Homeida HE, Chen T, Idris AM, et al. Inflammatory bacteriome featuring fusobacterium nucleatum and pseudomonas aeruginosa identified in association with oral squamous cell carcinoma. Sci Rep (2017) 7(1):1834. doi: 10.1038/s41598-017-02079-3
61. Chang C, Geng F, Shi X, Li Y, Zhang X, Zhao X, et al. The prevalence rate of periodontal pathogens and its association with oral squamous cell carcinoma. Appl Microbiol Biotechnol (2019) 103(3):1393–404. doi: 10.1007/s00253-018-9475-6
62. Oliva M, Schneeberger PHH, Rey V, Cho M, Taylor R, Hansen AR, et al. Transitions in oral and gut microbiome of HPV+ oropharyngeal squamous cell carcinoma following definitive chemoradiotherapy (ROMA LA-OPSCC study). Br J Cancer (2021) 124(9):1543–51. doi: 10.1038/s41416-020-01253-1
63. Zhang Z, Yang J, Feng Q, Chen B, Li M, Liang C, et al. Compositional and functional analysis of the microbiome in tissue and saliva of oral squamous cell carcinoma. Front Microbiol (2019) 10:1439. doi: 10.3389/fmicb.2019.01439
64. Hsueh CY, Huang Q, Gong H, Shen Y, Sun J, Lau HC, et al. A positive feed-forward loop between fusobacterium nucleatum and ethanol metabolism reprogramming drives laryngeal cancer progression and metastasis. iScience (2022) 25(2):103829. doi: 10.1016/j.isci.2022.103829
65. Lee SA, Liu F, Riordan SM, Lee CS, Zhang L. Global investigations of fusobacterium nucleatum in human colorectal cancer. Front Oncol (2019) 9:566. doi: 10.3389/fonc.2019.00566
66. Lecerf C, Kamal M, Vacher S, Chemlali W, Schnitzler A, Morel C, et al. Immune gene expression in head and neck squamous cell carcinoma patients. Eur J Cancer (2019) 121:210–23. doi: 10.1016/j.ejca.2019.08.028
67. Yamamura K, Izumi D, Kandimalla R, Sonohara F, Baba Y, Yoshida N, et al. Intratumoral fusobacterium nucleatum levels predict therapeutic response to neoadjuvant chemotherapy in esophageal squamous cell carcinoma. Clin Cancer Res (2019) 25(20):6170–9. doi: 10.1158/1078-0432.CCR-19-0318
68. Huang ST, Chen J, Lian LY, Cai HH, Zeng HS, Zheng M, et al. Intratumoral levels and prognostic significance of fusobacterium nucleatum in cervical carcinoma. Aging (Albany NY) (2020) 12(22):23337–50. doi: 10.18632/aging.104188
69. Chu S, Cheng Z, Yin Z, Xu J, Wu F, Jin Y, et al. Airway fusobacterium is associated with poor response to immunotherapy in lung cancer. Onco Targets Ther (2022) 15:201–13. doi: 10.2147/OTT.S348382
70. Geng F, Zhang Y, Lu Z, Zhang S, Pan Y. Fusobacterium nucleatum caused DNA damage and promoted cell proliferation by the Ku70/p53 pathway in oral cancer cells. DNA Cell Biol (2020) 39(1):144–51. doi: 10.1089/dna.2019.5064
71. Fujiwara N, Kitamura N, Yoshida K, Yamamoto T, Ozaki K, Kudo Y. Involvement of fusobacterium species in oral cancer progression: a literature review including other types of cancer. Int J Mol Sci (2020) 21(17):6207. doi: 10.3390/ijms21176207
72. Ghosh SK, Gupta S, Jiang B, Weinberg A. Fusobacterium nucleatum and human beta-defensins modulate the release of antimicrobial chemokine CCL20/macrophage inflammatory protein 3alpha. Infect Immun (2011) 79(11):4578–87. doi: 10.1128/IAI.05586-11
73. Abiko Y, Nishimura M, Kusano K, Nakashima K, Okumura K, Arakawa T, et al. Expression of MIP-3alpha/CCL20, a macrophage inflammatory protein in oral squamous cell carcinoma. Arch Oral Biol (2003) 48(2):171–5. doi: 10.1016/S0003-9969(02)00167-X
74. Bui FQ, Johnson L, Roberts J, Hung SC, Lee J, Atanasova KR, et al. Fusobacterium nucleatum infection of gingival epithelial cells leads to NLRP3 inflammasome-dependent secretion of IL-1beta and the danger signals ASC and HMGB1. Cell Microbiol (2016) 18(7):970–81. doi: 10.1111/cmi.12560
75. Lee CH, Chang JS, Syu SH, Wong TS, Chan JY, Tang YC, et al. IL-1beta promotes malignant transformation and tumor aggressiveness in oral cancer. J Cell Physiol (2015) 230(4):875–84. doi: 10.1002/jcp.24816
76. Zelante T, Fric J, Wong AY, Ricciardi-Castagnoli P. Interleukin-2 production by dendritic cells and its immuno-regulatory functions. Front Immunol (2012) 3:161. doi: 10.3389/fimmu.2012.00161
77. Binder Gallimidi A, Fischman S, Revach B, Bulvik R, Maliutina A, Rubinstein AM, et al. Periodontal pathogens porphyromonas gingivalis and fusobacterium nucleatum promote tumor progression in an oral-specific chemical carcinogenesis model. Oncotarget (2015) 6(26):22613–23. doi: 10.18632/oncotarget.4209
78. Harrandah AM, Chukkapalli SS, Bhattacharyya I, Progulske-Fox A, Chan EKL. Fusobacteria modulate oral carcinogenesis and promote cancer progression. J Oral Microbiol (2020) 13(1):1849493. doi: 10.1080/20002297.2020.1849493
79. Zhang S, Li C, Liu J, Geng F, Shi X, Li Q, et al. Fusobacterium nucleatum promotes epithelial-mesenchymal transiton through regulation of the lncRNA MIR4435-2HG/miR-296-5p/Akt2/SNAI1 signaling pathway. FEBS J (2020) 287(18):4032–47. doi: 10.1111/febs.15233
80. Chen BR, Deshpande A, Barbosa K, Kleppe M, Lei X, Yeddula N, et al. A JAK/STAT-mediated inflammatory signaling cascade drives oncogenesis in AF10-rearranged AML. Blood (2021) 137(24):3403–15. doi: 10.1182/blood.2020009023
81. Zhang N, Ng AS, Cai S, Li Q, Yang L, Kerr D. Novel therapeutic strategies: targeting epithelial-mesenchymal transition in colorectal cancer. Lancet Oncol (2021) 22(8):e358–e68. doi: 10.1016/S1470-2045(21)00343-0
82. Abdulkareem AA, Shelton RM, Landini G, Cooper PR, Milward MR. Periodontal pathogens promote epithelial-mesenchymal transition in oral squamous carcinoma cells in vitro. Cell Adh Migr (2018) 12(2):127–37. doi: 10.1080/19336918.2017.1322253
83. Zhang S, Li C, Zhang Z, Li Y, Li Q, Geng F, et al. Analysis of differentially expressed genes in oral epithelial cells infected with fusobacterium nucleatum for revealing genes associated with oral cancer. J Cell Mol Med (2021) 25(2):892–904. doi: 10.1111/jcmm.16142
84. Kawasaki M, Ikeda Y, Ikeda E, Takahashi M, Tanaka D, Nakajima Y, et al. Oral infectious bacteria in dental plaque and saliva as risk factors in patients with esophageal cancer. Cancer (2021) 127(4):512–9. doi: 10.1002/cncr.33316
85. Hsieh YY, Tung SY, Pan HY, Yen CW, Xu HW, Lin YJ, et al. Increased abundance of clostridium and fusobacterium in gastric microbiota of patients with gastric cancer in taiwan. Sci Rep (2018) 8(1):158. doi: 10.1038/s41598-017-18596-0
86. Yang L, Kartsonaki C, Yao P, de Martel C, Plummer M, Chapman D, et al. The relative and attributable risks of cardia and non-cardia gastric cancer associated with helicobacter pylori infection in China: a case-cohort study. Lancet Public Health (2021) 6(12):e888–e96. doi: 10.1016/S2468-2667(21)00164-X
87. Coker OO, Dai Z, Nie Y, Zhao G, Cao L, Nakatsu G, et al. Mucosal microbiome dysbiosis in gastric carcinogenesis. Gut (2018) 67(6):1024–32. doi: 10.1136/gutjnl-2017-314281
88. Alkharaan H, Lu L, Gabarrini G, Halimi A, Ateeb Z, Sobkowiak MJ, et al. Circulating and salivary antibodies to fusobacterium nucleatum are associated with cystic pancreatic neoplasm malignancy. Front Immunol (2020) 11:2003. doi: 10.3389/fimmu.2020.02003
89. Hieken TJ, Chen J, Hoskin TL, Walther-Antonio M, Johnson S, Ramaker S, et al. The microbiome of aseptically collected human breast tissue in benign and malignant disease. Sci Rep (2016) 6:30751. doi: 10.1038/srep30751
90. Van der Merwe M, Van Niekerk G, Botha A, Engelbrecht AM. The onco-immunological implications of fusobacterium nucleatum in breast cancer. Immunol Lett (2021) 232:60–6. doi: 10.1016/j.imlet.2021.02.007
91. Oh HY, Kim BS, Seo SS, Kong JS, Lee JK, Park SY, et al. The association of uterine cervical microbiota with an increased risk for cervical intraepithelial neoplasia in Korea. Clin Microbiol Infect (2015) 21(7):674 e1–9. doi: 10.1016/j.cmi.2015.02.026
92. Audirac-Chalifour A, Torres-Poveda K, Bahena-Roman M, Tellez-Sosa J, Martinez-Barnetche J, Cortina-Ceballos B, et al. Cervical microbiome and cytokine profile at various stages of cervical cancer: a pilot study. PloS One (2016) 11(4):e0153274. doi: 10.1371/journal.pone.0153274
93. Di Spirito F, Toti P, Pilone V, Carinci F, Lauritano D, Sbordone L. The association between periodontitis and human colorectal cancer: genetic and pathogenic linkage. Life (Basel) (2020) 10(9):211. doi: 10.3390/life10090211
94. Idrissi Janati A, Karp I, Latulippe JF, Charlebois P, Emami E. Periodontal disease as a risk factor for sporadic colorectal cancer: results from COLDENT study. Cancer Causes Control (2022) 33(3):463–72. doi: 10.1007/s10552-021-01541-y
95. Kononen E, Gursoy M, Gursoy UK. Periodontitis: A multifaceted disease of tooth-supporting tissues. J Clin Med (2019) 8(8):1135. doi: 10.3390/jcm8081135
96. Abed J, Emgard JE, Zamir G, Faroja M, Almogy G, Grenov A, et al. Fap2 mediates fusobacterium nucleatum colorectal adenocarcinoma enrichment by binding to tumor-expressed gal-galnac. Cell Host Microbe (2016) 20(2):215–25. doi: 10.1016/j.chom.2016.07.006
97. Moraes RC, Dias FL, Figueredo CM, Fischer RG. Association between chronic periodontitis and oral/oropharyngeal cancer. Braz Dent J (2016) 27(3):261–6. doi: 10.1590/0103-6440201600754
98. Kasper SH, Morell-Perez C, Wyche TP, Sana TR, Lieberman LA, Hett EC. Colorectal cancer-associated anaerobic bacteria proliferate in tumor spheroids and alter the microenvironment. Sci Rep (2020) 10(1):5321. doi: 10.1038/s41598-020-62139-z
99. Chew J, Zilm PS, Fuss JM, Gully NJ. A proteomic investigation of fusobacterium nucleatum alkaline-induced biofilms. BMC Microbiol (2012) 12:189. doi: 10.1186/1471-2180-12-189
100. Bullman S, Pedamallu CS, Sicinska E, Clancy TE, Zhang X, Cai D, et al. Analysis of fusobacterium persistence and antibiotic response in colorectal cancer. Science (2017) 358(6369):1443–8. doi: 10.1126/science.aal5240
101. Dong J, Li Y, Xiao H, Zhang S, Wang B, Wang H, et al. Oral microbiota affects the efficacy and prognosis of radiotherapy for colorectal cancer in mouse models. Cell Rep (2021) 37(4):109886. doi: 10.1016/j.celrep.2021.109886
102. Gupta VK, Paul S, Dutta C. Geography, ethnicity or subsistence-specific variations in human microbiome composition and diversity. Front Microbiol (2017) 8:1162. doi: 10.3389/fmicb.2017.01162
103. Perera M, Al-Hebshi NN, Speicher DJ, Perera I, Johnson NW. Emerging role of bacteria in oral carcinogenesis: a review with special reference to perio-pathogenic bacteria. J Oral Microbiol (2016) 8:32762. doi: 10.3402/jom.v8.32762
104. Abed J, Maalouf N, Parhi L, Chaushu S, Mandelboim O, Bachrach G. Tumor targeting by fusobacterium nucleatum: A pilot study and future perspectives. Front Cell Infect Microbiol (2017) 7:295. doi: 10.3389/fcimb.2017.00295
105. Coppenhagen-Glazer S, Sol A, Abed J, Naor R, Zhang X, Han YW, et al. Fap2 of fusobacterium nucleatum is a galactose-inhibitable adhesin involved in coaggregation, cell adhesion, and preterm birth. Infect Immun (2015) 83(3):1104–13. doi: 10.1128/IAI.02838-14
106. Brennan CA, Garrett WS. Fusobacterium nucleatum - symbiont, opportunist and oncobacterium. Nat Rev Microbiol (2019) 17(3):156–66. doi: 10.1038/s41579-018-0129-6
107. Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science (2018) 359(6371):97–103. doi: 10.1126/science.aan4236
108. David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature (2014) 505(7484):559–63. doi: 10.1038/nature12820
Keywords: Fusobacterium nucleatum, cancer, Gal-GalNAc, prognosis, oral and head and neck cancer
Citation: He Z, Tian W, Wei Q and Xu J (2022) Involvement of Fusobacterium nucleatum in malignancies except for colorectal cancer: A literature review. Front. Immunol. 13:968649. doi: 10.3389/fimmu.2022.968649
Received: 14 June 2022; Accepted: 16 August 2022;
Published: 17 August 2022.
Edited by:
Hiroaki Inaba, Okayama University, JapanReviewed by:
Catalina Lunca, Grigore T. Popa University of Medicine and Pharmacy, RomaniaJiiang-Huei Jeng, National Taiwan University, Taiwan
Copyright © 2022 He, Tian, Wei and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Xu, amluZ194dUB6anUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Zhixing He
Zhixing He Wei Tian
Wei Tian Qichun Wei
Qichun Wei Jing Xu
Jing Xu