
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 09 September 2022
Sec. Vaccines and Molecular Therapeutics
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.967051
 Fuzhen Wang1†
Fuzhen Wang1† Baoying Huang2†
Baoying Huang2† Huakun Lv3†
Huakun Lv3† Lizhong Feng4†
Lizhong Feng4† Weihong Ren5†
Weihong Ren5† Xiaoqi Wang1
Xiaoqi Wang1 Lin Tang1
Lin Tang1 Qianqian Liu1
Qianqian Liu1 Dan Wu1
Dan Wu1 Hui Zheng1
Hui Zheng1 Zhijie An1
Zhijie An1 Yao Deng2
Yao Deng2 Li Zhao2
Li Zhao2 Fei Ye2
Fei Ye2 Wenling Wang2
Wenling Wang2 Hangjie Zhang3
Hangjie Zhang3 Shaoying Chang4
Shaoying Chang4 Yuting Liao6
Yuting Liao6 Fengyang Chen5
Fengyang Chen5 Lance E. Rodewald1
Lance E. Rodewald1 George F. Gao7
George F. Gao7 Zundong Yin1*
Zundong Yin1* Wenjie Tan2*
Wenjie Tan2*Background: BBIBP-CorV and CoronaVac inactivated COVID-19 vaccines are widely-used, World Health Organization-emergency-listed vaccines. Understanding antibody level changes over time after vaccination is important for booster dose policies. We evaluated neutralizing antibody (nAb) titers and associated factors for the first 12 months after primary-series vaccination with BBIBP-CorV and CoronaVac.
Methods: Our study consisted of a set of cross-sectional sero-surveys in Zhejiang and Shanxi provinces, China. In 2021, we enrolled 1,527 consenting 18-59-year-olds who received two doses of BBIBP-CorV or CoronaVac 1, 3, 6, 9, or 12 months earlier and obtained blood samples and demographic and medical data. We obtained 6-month convalescent sera from 62 individuals in Hebei province. Serum nAb titers were measured by standard micro-neutralization cytopathic effect assay in Vero cells with ancestral SARS-CoV-2 strain HB01. We used the first WHO International Standard (IS) for anti-SARS-CoV-2 immunoglobulin (NIBSC code 20/136) to standardized geometric mean concentrations (IU/mL) derived from the nAb geometric mean titers (GMT over 1:4 was considered seropositive). We analyzed nAb titer trends using Chi-square and factors related to nAb titers with logistic regression and linear models.
Results: Numbers of subjects in each of the five month-groupings ranged from 100 to 200 for each vaccine and met group-specific target sample sizes. Seropositivity rates from BBIBP-CorV were 98.0% at 1 month and 53.5% at 12 months, and GMTs were 25.0 and 4.0. Respective seropositivity rates from CoronaVac were 90.0% and 62.5%, and GMTs were 20.2 and 4.1. One-, three-, six-, nine-, and twelve-month GMCs were 217.2, 84.1, 85.7, 44.6, and 10.9 IU/mL in BBIBP-CorV recipients and 195.7, 94.6, 51.7, 27.6, and 13.4 IU/mL in CoronaVac recipients. Six-month convalescent seropositivity was 95.2%; GMC was 108.9 IU/mL. Seropositivity and GMCs were associated with age, sex, and time since vaccination.
Conclusions: Neutralizing Ab levels against ancestral SARS-CoV-2 from BBIBP-CorV or CoronaVac vaccination were similar and decreased with increasing time since vaccination; over half of 12-month post-vaccination subjects were seropositive. Seropositivity and GMCs from BBIBP-CorV and CoronaVac six and nine months after vaccination were similar to or slightly lower than in six-month convalescent sera. These real-world data suggest necessity of six-month booster doses.
Vaccination is indispensable for reducing suffering and death from the COVID-19 pandemic. To date, there are five COVID-19 vaccines that have received conditional approval for market authorization in China by the National Medical Products Administration (NMPA). The vast majority of COVID-19 vaccine doses administered are Vero cell grown, whole virus, alum adjuvanted inactivated vaccine produced by Beijing Institute of Biological Products CO., LTD, SINOPHARM (BBIBP-CorV vaccine) and Sinovac Research & Development Co., Ltd (CoronaVac vaccine) (1, 2). To date, the World Health Organization (WHO) has listed eleven vaccines based on four technical platforms for Emergency Use (EUL), including inactivated virus vaccines, mRNA based vaccines, protein subunit vaccines, and viral vectored vaccines. WHO listed the two China-produced inactivated vaccines for emergency use in May (BBIBP-CorV) and June (CoronaVac) of 2021 (3, 4). By October 2021, BBIBP-CorV and CoronaVac accounted for nearly half of COVID-19 vaccines doses administered globally (5).
Phase 1, 2, and 3 clinical trials demonstrated acceptable immunogenicity and safety of both vaccines. Studies of antibody response kinetics and immune persistence showed that neutralizing antibody (nAb) levels from all COVID-19 vaccines wane, regardless of technological platform (6–9), and that waning is associated with decreased protection. Before and after large-scale use in populations, kinetics and persistence of nAb from inactivated vaccines showed immune attenuation (10, 11), but data are limited, especially in China. Some immune persistence studies are challenged by non-standardized laboratory methods and high background rates of natural infection (12–14).
There are no published population-based real-world studies that have explored antibody response kinetics and immune persistence more than 6 months after China’s large-scale vaccination campaign, despite the administration of over 2.8 billion doses of COVID-19 vaccines by the end of 2021 (15). No data have been reported on the influence of population factors on immune persistence. It is important to document immunity by time since vaccination with real world data in China to provide evidence supporting vaccination strategy refinement in this infection-naïve population where immunity against SARS-CoV-2 comes almost solely from vaccines.
We conducted a real-world, observational, cross-sectional study of healthy adults aged 18-59 years to assess immune marker persistence during the first year after full-series vaccination with BBIBP-CorV and CoronaVac COVID-19 vaccines in China using standardized laboratory testing methods. We report results of our study.
The study was set in two provinces in the south and north of mainland China (Zhejiang and Shanxi) and was initiated after nationwide containment of SARS-CoV-2 in April 2020 and completed during sustained implementation Dynamic COVID-Zero policy that prevents local transmission. COVID-19 vaccination started in the summer of 2020 during a period of emergency use authorization, and a nationwide campaign started in December 2020. During the study period, March 2021 to December 2021, there was almost no transmission of SARS-CoV-2 in China other than small, rapidly contained import-related outbreaks, none of which were in the study areas. Lack of local transmission allowed observation of immune persistence following vaccination in the absence of exogenous boosting by SARS-CoV-2 exposure.
The design was a set of cross-sectional sero-surveys of people who had been vaccinated in pre-specified times before study enrollment. Participants were individuals aged 18-59 years who were fully vaccinated with two doses of an inactivated vaccine, with inter-dose intervals of 3 to 4 weeks and the second dose administered 1, 3, 6, 9, or 12 months prior to enrollment and obtaining blood samples. This was not a longitudinal survey, as the subjects were different at each evaluation month. Exclusion criteria were history of infection with SARS-CoV-2, improper inter-dose interval, receipt of vaccine other than BBIBP-CorV or CoronaVac, history of using blood products or immunosuppressive drugs, and history of severe adverse reaction following immunization.
The study vaccines were BBIBP-CorV and CoronaVac; both are ancestral strain, whole-virus, β-propiolactone-inactivated, aluminum hydroxide adjuvanted, liquid COVID-19 vaccines. These two inactivated vaccines have been conditionally approved for individuals 18 years and older by the National Medical Products Administration (NMPA), the vaccine regulatory authority of China, prior to the start of the study. The recommended immunization schedule for both vaccines is two doses via intramuscular injection with an interval of 3-8 weeks.
In addition to sera from the vaccinated subjects, we obtained convalescent sera following a COVID-19 outbreak in Hebei province. On January 3, 2021, Nangong City, part of Xingtai City, Hebei Province, reported its first symptomatic case of COVID-19, leading to an outbreak in Xingtai, representing local transmission caused by an imported case (11). All cases were infected the SARS-CoV-2 D614G variant; 10.5% of infections were asymptomatic and the 89.5% of cases that were symptomatic were of mild or moderate severity. Convalescent sera were obtained from cases in this outbreak for comparison with sera from vaccinated subjects in our survey.
Provincial centers of diseases control and prevention (CDCs) recruited subjects using identification and contact data from their immunization information systems. CDCs listed potentially-eligible subjects whose vaccinations were managed in the jurisdiction of the CDC. Trained staff called potentially-eligible subjects and explained the study purpose, protocol, implementation process, information and specimen collection procedures, and risks and benefits over the phone. Individuals willing and eligible to participate became study subjects, representing convenience samples of individuals who had been vaccinated at just short of one of the five pre-determined intervals prior to study participation.
We made separate estimates of sample sizes for each of the two vaccines in two age groups (18-39 years and 40-59 years), and for five discrete times since administration of the second dose (1, 3, 6, 9, and 12 months) – a total of 20 groups. We based sample sizes using α=0.05, β=0.20, two-sided tests, with expert-opinion estimates of expected seropositive by time since vaccination of 98% at 1 month, 90% at 3 months, 80% at 6 months, 70% at 9 months, and 60% at 12 months and effect sizes of no more than 10 percentage point differences from expected values. The required sample sizes for each vaccine were 95, 108, 153, 182, and 194 subjects, respectively; these required sample sizes were adjusted upward to target sample sizes of 100, 110, 160, 190, and 200 subjects for each vaccine – a total target sample size of 1,520 subjects.
Participants had been vaccinated over a range of times prior to enrollment. Blood draws were scheduled for the next discrete time since vaccination: 1, 3, 6, 9 or 12 months after the administration of a subject’s second dose. We administered a questionnaire survey at blood drawing visits to gather demographic information.
We obtained six-month convalescent sera and demographic information from individuals, 18-59-years-of-age, who had been infected in a COVID-19 outbreak in Xingtai city of Hebei province (Figure 1).
Neutralization assays were conducted in a BSL-3 laboratory. Serum nAb responses were assessed by reduction of cytopathic effect (CPE) in Vero cells with infectious ancestral SARS-CoV-2 strain 19nCoV-CDC-Tan-HB01 (HB01). Briefly, serum was inactivated at 56°C for 30 minutes and successively diluted from 1:4 to the required concentration in 2-fold series. An equal volume of challenge virus solution containing 100 CCID50 virus was added. After neutralization in a 37°C incubator for 2 hours, a 1.5-2.5×105/mL cell suspension was added to the wells; cytopathic effect was assessed 4 days after infection. Neutralization titers (NT50) were expressed as the reciprocal of the highest dilution protecting 50% of the cells from the virus challenge.
To facilitate comparison of SARS-CoV-2 neutralization assay data from multiple assay formats and vaccines, we used WHO international standard (IS) and an internal neutralization standard. The WHO 1st IS for antiserum to SARS-CoV-2 (NIBSC code 20/136) was obtained from National Institute for Biological Standards and Control (NIBSC). The internal neutralization standard ‘R1’ was generated in-house by Beijing Minhai Biotechnology Co., Ltd. by pooling a selection of SARS-CoV-2 RBD protein immunized goat sera. All neutralization standards were run in triplicate on the SARS-CoV-2 neutralizing assay described above. The internal reference was calibrated according to the WHO 1st IS for anti-SARS-CoV-2 immunoglobulin; test samples compared to the IS can be expressed in IU/mL by calculation: GMT of test samples/(GMT of SARS-CoV-2 IS/1000) = IU/mL. The calibrated potency of internal standard ‘R1’ was 16,734 IU/mL. The internal reference was included in every experiment and used for correction of tested sample results. To convert sample neutralization titers into international units, the neutralization dilution values of the sample were divided by the neutralization of the internal standard run during the same experiment and then multiplied by the calibrated potency of the respective internal standards in international units. Use of the reference standard sera allowed neutralization assay outputs to be converted to international units per milliliter (IU/mL).
We used mean ± standard deviation (SD) for age, medians, and interquartile ranges (IQR) for other continuous variables, and numbers (percentages) for categorical variables. Outcome variables were immune markers and seropositivity (GMT over 1:4). Immunogenicity was expressed by nAb seroconversion percentage, geometric mean titers (GMT), and geometric mean concentrations (GMC, IU/mL) referenced to WHO, with associated 95% confidence intervals (CI). Antibody titers were log-transformed to calculate GMT per group. We assessed trends over time with the Cochran-Armitage trend test, and used the Kruskal Wallis test to compare GMCs of convalescent sera with GMCs of sera from the vaccinated subjects. To determine factors related to nAb titers, we used logistic regression analysis and a linear model with gender, age group, inter-dose interval, and manufacturer as covariates. All analyses were done using R (version 4.1.0). Statistical tests were two-sided, and we considered P values of 0.05 or less as statistically significant.
We recruited 1,527 individuals who met all inclusion criteria and no exclusion criteria and who had received two doses of inactivated COVID-19 vaccine in October 2020 or later. Among these individuals, 768 had been vaccinated with BBIBP-CorV and 759 had been vaccinated with CoronaVac. Participants ranged in age from 18 to 59 years, with a mean age of 39.3 (sd 10.7 years); 52.5% of participants were female; 9.0% had a BMI ≥28.0 kg/m2; 8.9% had ≥1 underlying comorbidities (most commonly hypertension and diabetes); 480 (31.4%) were vaccinated with an inter-dose interval of 21-27 days and 1,047 (68.6%) with an inter-dose interval of 28-35 days; no participants had been diagnosed with COVID-19 before the study (Table 1).
Among participants vaccinated with BBIBP-CorV, 100 had blood samples collected at 1 month after the second dose (median 34 days, IQR 32-34 days); 111 at 3 months (92 days, IQR 90.5-94); 162 at 6 months (186 days, IQR 183-186); 195 at 9 months (273 days, IQR 273-274); and 200 at 12 months (366 days, IQR 362-366). Among participants vaccinated with CoronaVac, 100 had blood samples drawn at 1 month (30 days, IQR 30-32); 110 at 3 months (93 days, IQR 92-96); 159 at 6 months (184 days, IQR 183-184); 190 at 9 months (275 days, IQR 274-275); and 200 at 12 months (364 days, IQR 362-366 days).
We obtained 62 blood samples from recovered COVID-19 patients who had been infected with the SARS-CoV-2 D614G variant six months previously. These survivors ranged in age from 18 to 58 years, with a mean age of 37.5 (sd 12.1 years); 37 (59.7%) were male (Table 2).
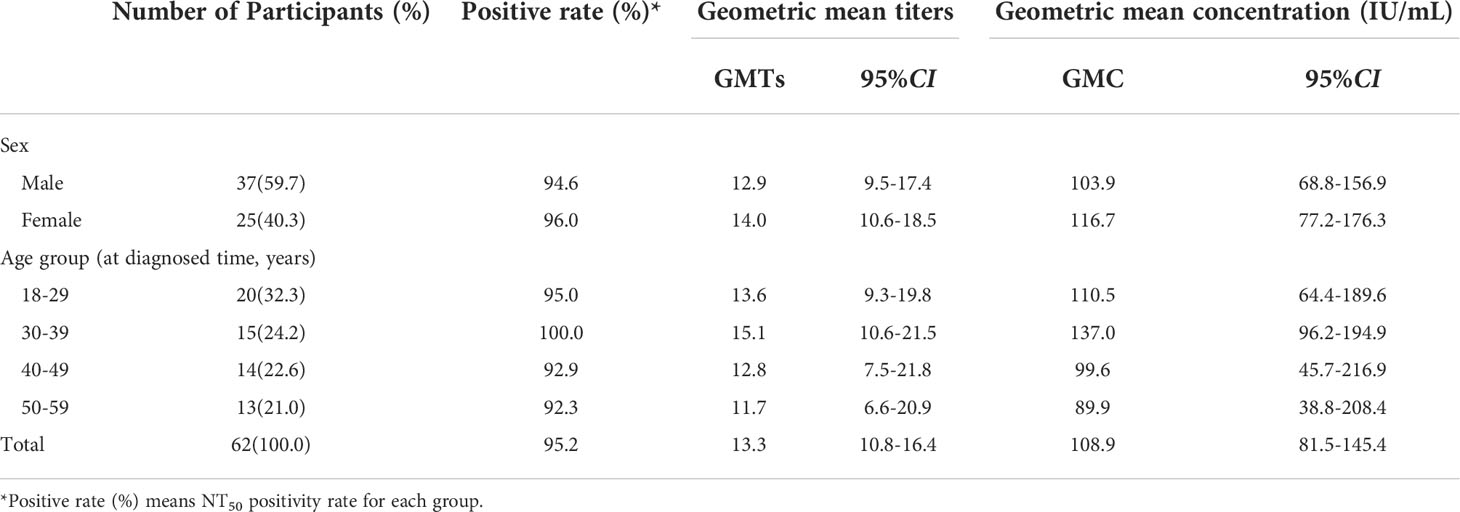
Table 2 Participant characteristics and nAb responses to COVID-19 convalescent sera obtained 6 months after diagnosis.
The primary immunogenicity outcomes were NT50 positivity rates, geometric mean titers (GMTs), and geometric mean concentrations (GMC, IU/mL) of neutralizing antibodies. NT50 seropositivity was defined as a neutralizing antibody titer of at least 1:4. A seropositivity threshold of 1:4 has been used as a measure of seropositivity in live SARS-CoV-2 virus neutralization tests in published clinical trials of COVID-19 vaccines (16–18).
Table 1 shows NT50 positivity rates and geometric mean titers (GMTs) and geometric mean concentrations (GMC, IU/mL) of neutralizing antibodies by selected characteristics of participants. NT50 positive rates for BBIBP-CorV declined from 98.0% at 1 month to 53.5% at 12 months (trend χ2 = 109.0, P<0.001), with corresponding nAb titers declining from 25.0 [95%CI: 21.3-29.4] to 4.0 [95%CI: 3.5-4.4], an 84.0% decrease; nAb concentrations declined from 217.2 [95%CI: 178.5-264.3] to 10.9 [95%CI: 8.7-13.8] (IU/mL). NT50 positivity rates for CoronaVac declined from 90.0% at 1 month to 62.5% at 12 months (trend χ2 = 56.3, P<0.001) with corresponding nAb titers declining from 20.2 [95%CI: 16.3-24.9] to 4.1 [95%CI: 3.7-4.5], a 79.7% decrease; nAb concentration declined from 195.7 [95%CI: 139.5-274.5] to 13.4 [95%CI: 10.8-16.6] (IU/mL).
Table 2 shows NT50 positivity rates and GMTs and GMC (IU/mL) of neutralizing antibodies in people who recovered from SARS-CoV-2 infection. Six months after infection, the NT50 positive rate was 95.2%, the GMT was 13.3 [95%CI: 10.8-16.4], and the GMC was 108.9 [95%CI: 81.5-145.4] IU/mL. Kruskal Wallis testing showed that GMCs of these survivors were comparable to the GMCs of BBIBP-CorV-vaccinated subjects at 6 months (P=0.39), and were slightly greater than GMCs for CoronaVac at 6 months (P<0.001) and comparable to CoronaVac at 3 months (P=0.07) (Figures 2, 3).
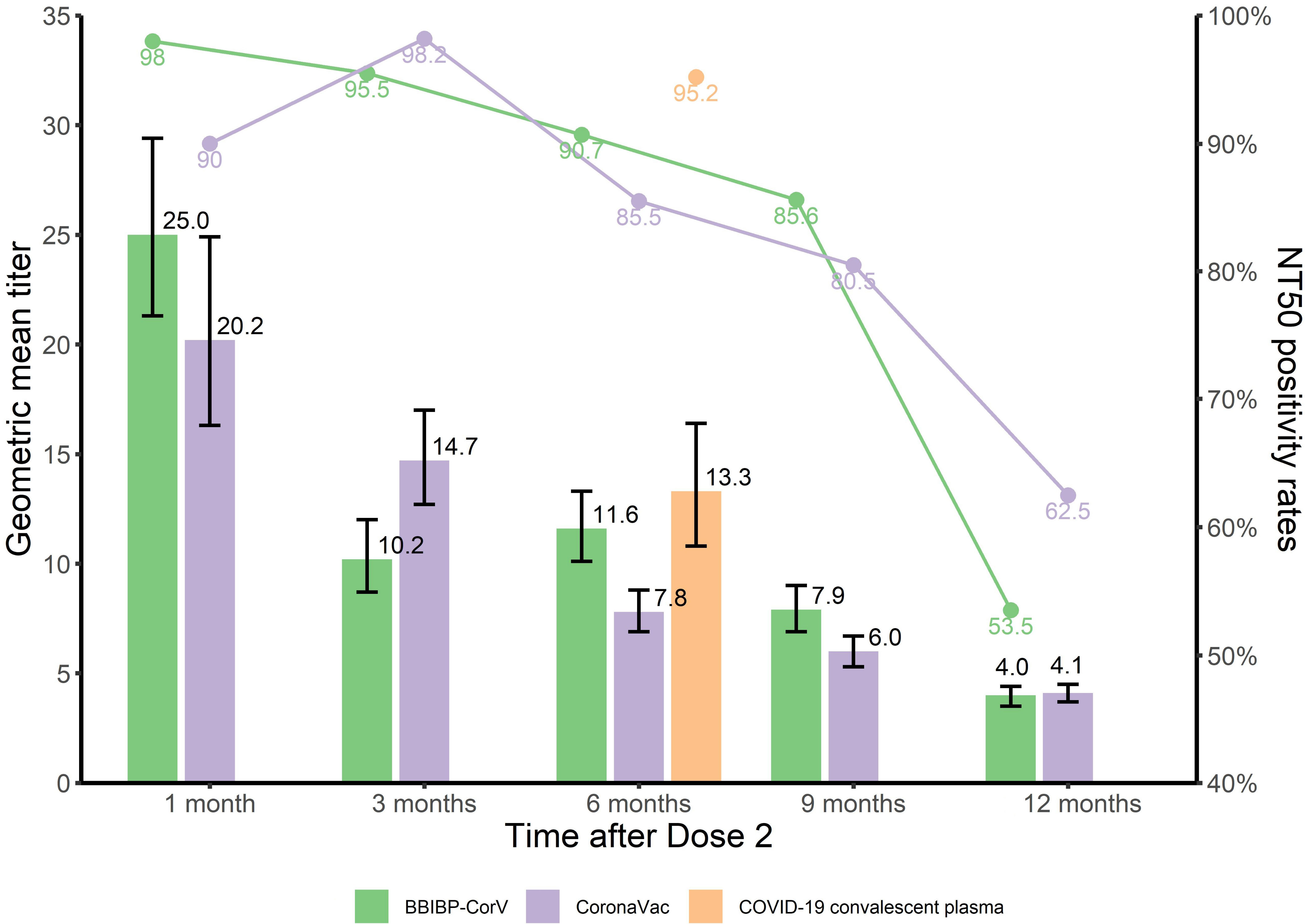
Figure 2 NT50 positivity rates and GMTs of nAb at different time points after immunization with inactivated COVID-19 vaccine and COVID-19 convalescent sera.
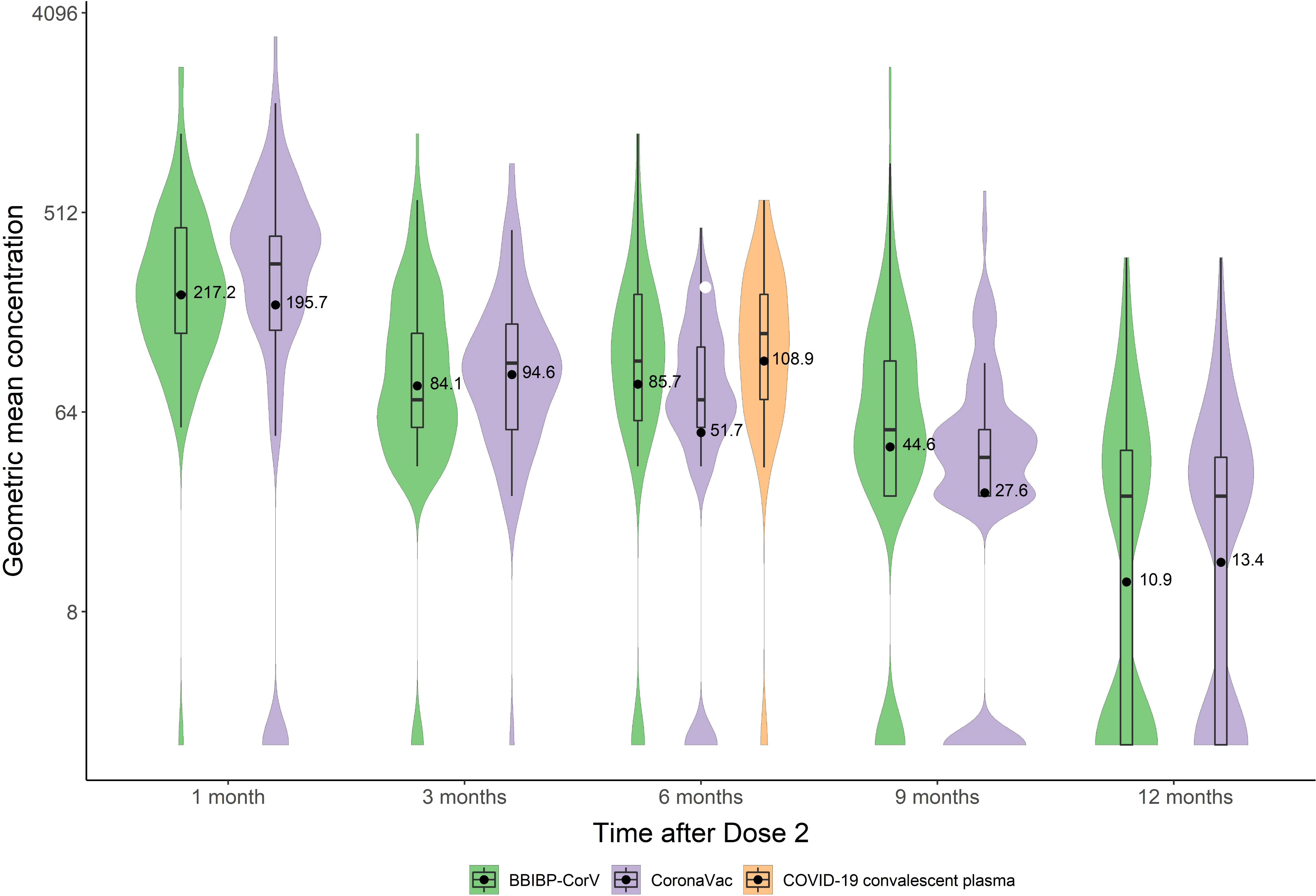
Figure 3 GMCs of nAb at different time points after immunization with inactivated COVID-19 vaccine and COVID-19 convalescent sera.
Logistic regression was used to investigate the association of sociodemographic and immunization-related factors with NT50 positivity rates. Age and time since the second dose were associated with NT50 positivity for both BBIBP-CorV and CoronaVac. NT50 positivity rates were higher for females vaccinated with BBIBP-CorV (OR=1.669, P=0.020) but not for females vaccinated with CoronaVac. Comorbidities were associated with a decline of NT50 positivity for CoronaVac (OR=0.453, P=0.018) but not for BBIBP-CorV. There were no statistically significant associations between NT50 positivity and obesity or inter-dose interval. A logistic regression model that included these two vaccines showed no statistically significant association between NT50 positivity and the brand of vaccine (OR=0.909, P=0.512).
Multiple linear regression on log-transformed GMCs was used to investigate associations of sociodemographic and immunization-related variables with GMCs. Time since the second dose was associated with lower GMCs for both BBIBP-CorV and CoronaVac. For BBIBP-CorV, GMCs were lower among 40-49-year-olds (P=0.007) and 50-59-year-olds (P<0.001) than 18-29-year-olds. For CoronaVac, only 50-59-year-olds had lower GMCs than 18-29-year-olds (P=0.018). Being female was associated with a slower GMCs decline for BBIBP-CorV but not for CoronaVac. Presence of comorbidity was associated with more rapid decline of GMCs; longer inter-dose interval was associated with slower GMC decline for CoronaVac but not for BBIBP-CorV. Obesity was not statistically significantly associated with GMCs for either vaccine. A multiple linear regression model that included these two vaccines showed slighter lower GMCs with CoronaVac than BBIBP-CorV 6 months after completion of the primary series (P=0.010). Full regression model results are shown in Tables 3, 4.
Our study of post-vaccination neutralizing antibody levels showed that under real-world conditions with no exogenous boosting by SARS-CoV-2 infection, BBIBP-CorV and CoronaVac inactivated COVID-19 vaccines were immunogenic, inducing seropositivity against ancestral SARS-CoV-2 in more than 90% of adults and maintaining seropositivity for 12 months for more than half of the 12-month post-vaccination subjects. Neutralizing antibody titers and geometric mean concentrations were highest among one- to three-month post-vaccination subjects and were similar to 6-month post-infection convalescent nAb levels among the three- to six-month post-vaccination subjects. Among 12-month post-vaccination subjects, nAb levels were low. Declining neutralizing antibody levels is consistent with a need for booster vaccination approximately six months after primary series vaccination.
Many vaccines have been developed for controlling the epidemic (19, 20) and several are in large-scale global use (21, 22). As of the end of 2021, more than 9 billion doses of COVID-19 vaccines have been administered globally, with inactivated vaccine CoronaVac and BBIBP-CorV comprising 41% of the doses administered (5, 23). Full-series coverage of COVID-19 vaccines in China was 80% by the end of 2021, and the vast majority (86%) of doses administered have been of these two inactivated vaccines (15). With widespread use of these vaccines, it is essential to understand changes in seropositivity and nAb levels over 12 months.
We found that BBIBP-CorV and CoronaVac had similar nAb kinetics and persistence. Zeng and colleagues found that CoronaVac immunity endures for 6 months, with nAb GMT 6 months after two doses of 6.8 [95%CI: 5.2-8.8] and seropositivity of 35% (19/54, with 1:8 as positive) (10). Jingxin Li and colleagues showed that GMT was 2.2-2.5 at 3-6 months after 2 doses of CoronaVac (24). Results from their pre-enhanced neutralization antibody response levels were slightly lower than what we found. Differences may be due to differences in test methods or conditions, as standardization of nAb testing is challenging and has not yet been achieved globally.
Previous studies have shown that nAb levels decrease over time after initial vaccination with COVID-19 vaccines, regardless of vaccine type, and showing substantial decrease by 6 to 8 months. The rate and magnitude of decline varies significantly by technical vaccine platform (7, 8, 25). For example, nAb from Moderna’s mRNA-1273 vaccine persists 3-6 months after second dose with all participants sustaining detectable activity and with ID50 GMTs of 775 [95% CI: 560-1071] at 3 months and 361 [95% CI: 258-504] at 6 months among 18-to-55-year-olds (8, 26, 27). Following vaccination with Pfizer BNT162b2 vaccine, neutralizing antibodies declined similarly (28). A study from Israel among health care workers showed that GMTs decreased from 557.1 to 119.4 in 6 months (7, 29). In our study, seropositivity remained above 85% and GMTs remained 8 or above (1:4 considered as positive) with either BBIBP-CorV or CoronaVac for 6 months after completion of full-series vaccination – lower GMTs than other types of COVID-19 vaccines, especially mRNA vaccines.
There are breakthrough infections from the current SARS-CoV-2 variants for all COVID-19 vaccines, regardless of platform (30–32). As has been the case with other COVID-19 vaccines, inactivated vaccines have been shown to be effective for reducing severe illness and death. Evaluation in China showed no statistically significant difference in effectiveness between BBIBP-CorV and CoronaVac (22, 33). However, more evidence is needed on levels of neutralizing antibody necessary for protection and the role of immune responses other than humoral immunity for protection.
Khoury and colleagues analyzed data from seven mainstream vaccines and convalescent cohorts, and found that the higher the ratio of neutralizing antibodies generated after vaccination compared with convalescent levels, the higher the protective rate of the vaccine. She and her colleagues estimated that a neutralization level for 50% protection against detectable SARS-CoV-2 infection is 20.2% of the mean convalescent level (95% CI: 14.4–28.4%) (34). Previous reports have shown that inactivated vaccines were less immunogenic than natural infection (35), while we found that neutralizing immunity induced by BBIBP-CorV and CoronaVac at 6 months and 9 months after vaccination were similar to or slightly lower than convalescent sera 6 months after natural infection based on seropositivity and GMCs. Our finding is consistent with findings by Wang and colleagues using wild-type pseudovirus assays (36), perhaps because most of the recovered serum used in our study came from patients with mild or asymptomatic infection.
Using regression models, we found that nAb seropositivity and GMCs of both inactivated vaccines decreased with age, especially in the 50-59 age group, similar to findings in other reports (7, 37, 38). Antibody levels following BBIBP-CorV vaccine were higher in women than in men, which is similar to results with BNT162b2 mRNA vaccine (7). Obesity had no significant effect on nAb levels in our study, however, several studies have showed that SARS-CoV-2 neutralizing antibodies are positively associated with BMI (39), and obesity increases risk for hospitalization, ICU admission, and death among patients with COVID-19 (40). In our study, only CoronaVac was found to have an association between comorbidities and immunogenicity, a finding that was also observed in a study from Chile (41). It is not clear why there are differences in the immune responses to these two vaccines by gender and comorbidity and whether these differences are sustained in other studies and with other variants. Differences may be related to the survey population, the sample size, or differences the vaccines, and will require further study to evaluate.
Although neutralizing antibodies are known to be potential correlates of protection against COVID-19 (42), comparison of immunological data is often challenged by differences in assays, target antigens, numerical readouts, and endpoints. The WHO international standard for anti-SARS-CoV-2 immunoglobulin is expected to standardize measurements for clinical trial results of neutralization assays from different laboratories (34). In our study, we used the first WHO IS, NIBSC code 20/136 to facilitate comparison of SARS-CoV-2 neutralization assay data from multiple assay formats and vaccine candidates. The WHO IS 20/136 standard was established by the WHO Expert Committee on Biological Standardization in December, 2020. Wider use of the WHO IS will facilitate vaccine evaluations.
A recent study conducted by the Oxford COVID vaccine trial group reported potential correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection, using WHO international standard units for all assays (43). This study demonstrated that the AZD1222 vaccine efficacy of 80% against symptomatic infection with B.1.1.7 variant of SARS-CoV-2 was achieved with 26 (95% CI: NC, NC) international unit IU/mL and 247 (95% CI: 101, NC) normalized neutralization titers (NF50) for pseudovirus and live-virus neutralization, respectively. With live virus neutralization assays, vaccine efficacy of 80% against symptomatic was achieved with 120 IU/mL (95% CI: 56 to 298), which was lower than GMCs at 1 month for both the inactivated vaccines in our study (BBIBP-CorV 217.2 IU/mL, and CoronaVac 195.7 IU/mL), but higher than that at 3 months (BBIBP-CorV 84.1 IU/mL, and CoronaVac 94.6 IU/mL). To our knowledge, our study is the first to report neutralization titer results post BBIBP-CorV and CoronaVac vaccination in IU/mL, facilitating assay-to-assay comparisons for future studies.
One of the strengths of our study is providing a measure of pure vaccine induced immunity, without exogenous booster by natural infection. This was possible because there were no large COVID-19 outbreaks in mainland China during the study period and no participants had history of infection with SARS-CoV-2. A second strength is that we had sufficient sample sizes to provide immunity estimates for 12 months separately for both BBIBP-CorV and CoronaVac, and with reasonably narrow CIs. Third, our study used WHO 1st international standard (NIBSC code 20/136) for assay immunogenicity, allowing comparison with other studies.
Our study has several limitations. First, we used a cross-sectional observation study design, rather than a longitudinal study of the same cohort. Although this limits the precision of results, a cross-sectional study, especially one conducted in an environment without exogenous boosting, represents a real-world study of pure vaccine-induced population immunity over time. Second, our study analyzed neutralizing antibodies and not individual antigens. We did not evaluate cellular immunity, which has been provided by other studies. Third, due to the limited sample size, our study could not conduct analyses of subgroups with specific comorbidities and we used a small number of covariates in our analyses. Fourth, our study did not include children or subjects over 60 years, limiting generalizability. Fifth, neutralizing antibody levels were tested against ancestral SARS-CoV-2 only, precluding results and conclusions related to variants of concern such as Omicron.
Our study has implication for immunization strategy. Over 85% of people in China have completed their primary vaccination series, and the vast majority (86%) of doses administered have been these two inactivated vaccines. Real-world evidence of waning markers of immunity supports booster dose programs to enhance and maintain immunity. We should continue monitoring population immunity and assess protection effectiveness of China’s vaccines during outbreaks to provide information that will be useful for updating immunization strategy.
In conclusion, we found similar kinetics of post-vaccination nAb levels of BBIBP-CorV and CoronaVac along with prominent immune persistence (seropositivity persisted for at least 12 months). Although nAb levels decreased rapidly, neutralizing immunity induced by BBIBP-CorV six months and CoronaVac three months postvaccination were comparable with six-month convalescent sera from individuals recovering from COVID-19. Future research will focus on immunity of inactivated vaccine booster doses and optimization of population immunity in China.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This study was reviewed and approved by Medical Ethics Committee of The Chinese Center for Disease Control and Prevention (Approval notice: 202101). The patients/participants provided their written informed consent to participate in this study.
The study was conceived and designed by WT and ZY. Data analysis were performed by FW, XW, LT, QL and BH, YD, LZ, FY, WW made laboratory testing. HL, LF, WR, HJZ, SC, YL, FC were responsible for data collection, FW, XW, LT, QL, HZ and DW wrote the first draft, ZA, FG and LR contributed to the final version of the paper. All authors contributed to the article and approved the submitted version.
This work was supported by the Chinese Center for Disease Control and Prevention Research Founding (JY21-3-01), the National Key Research and Development Program of China (2021YFC2301600), and the National Natural Science Foundation of China (Grant 82041021 and Grant 82061138008).
We gratefully thank the fellow from Zhejiang CDC, Shanxi CDC, for participants recruitment and blood sample collection.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. N.M.P. Administration. China Grants conditional approval for first COVID vaccine. (2020). Available at: http://subsites.chinadaily.com.cn/nmpa/2020-12/31/c_579192.htm.
2. N.M.P. Administration. Sinovac COVID-19 vaccine granted conditional market approval in China. (2021). Available at: http://subsites.chinadaily.com.cn/nmpa/2021-02/07/c_588422.htm.
3. W.H. Organization. WHO lists additional COVID-19 vaccine for emergency use and issues interim policy recommendations. (2021). Available at: https://www.who.int/news/item/07-05-2021-who-lists-additional-covid-19-vaccine-foremergency-use-and-issues-interim-policy-recommendations.
4. W.H. Organization. WHO validates sinovac COVID-19 vaccine for emergency use and issues interim policy recommendations. (2021). Available at: https://www.who.int/news/item/01-06-2021-who-validates-sinovac-covid-19-vaccine-for-emergency-use-and-issues-interim-policy-recommendations.
5. Mallapaty S. China’s COVID vaccines have been crucial — now immunity is waning. Nature (2021) 598:398–9. doi: 10.1038/d41586-021-02796-w
6. Shrotri M, Navaratnam AMD, Nguyen V, Byrne T, Geismar C, Fragaszy E, et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet (2021) 398:385–7. doi: 10.1016/S0140-6736(21)01642-1
7. Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit S, et al. Waning immune humoral response to BNT162b2 covid-19 vaccine over 6 months. New Engl J Med (2021) 385:e84. doi: 10.1056/NEJMoa2114583
8. Pegu A, O’Connell S, Schmidt SD, O'Dell S, Talana CA, Lai L, et al. Durability of mRNA-1273 vaccine-induced antibodies against SARS-CoV-2 variants. Science (2021) 373(6561):1372–7. doi: 10.1126/science.abj4176
9. Tartof SY, Slezak JM, Fischer H, Hong V, Ackerson BK, Ranasinghe ON, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: A retrospective cohort study. Lancet (2021) 398:1407–16. doi: 10.1016/S0140-6736(21)02183-8
10. Zeng G, Wu Q, Pan H, Li M, Yang J, Wang L, et al. Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two-dose schedule, in healthy adults: Interim results from two single-centre, double-blind, randomised, placebo-controlled phase 2 clinical trials. Lancet Infect Dis (2021) 22(4):483–95. doi: 10.1016/S1473-3099(21)00681-2
11. Liu S, Yuan S, Sun Y, Zhang B, Wang H, Lu J, et al. A COVID-19 outbreak — nangong city, hebei province, China, January 2021. China CDC Weekly (2021) 3:401–4. doi: 10.46234/ccdcw2021.077
12. Wang Y. Standardised neutralising antibody assays are needed for evaluating COVID-19 vaccines. EBioMedicine (2021) 73:103677. doi: 10.1016/j.ebiom.2021.103677
13. Nam M, Seo JD, Moon H-W, Kim H, Hur M, Yun Y-M. Evaluation of humoral immune response after SARS-CoV-2 vaccination using two binding antibody assays and a neutralizing antibody assay. Microbiol Spectr (2021) 9:e0120221–e0120221. doi: 10.1128/Spectrum.01202-21
14. Lopera TJ, Chvatal-Medina M, Flórez-Álvarez L, Zapata-Cardona MI, Taborda NA, Rugeles MT, et al. Humoral response to BNT162b2 vaccine against SARS-CoV-2 variants decays after six months. Front Immunol (2022) 13:879036–6. doi: 10.3389/fimmu.2022.879036
15. O.W.i. Data. Total number of COVID-19 vaccinations administered. (2021). Available at: https://ourworldindata.org/covid-vaccinations.
16. Liu J, Huang B, Li G, Chang X, Liu Y, Chu K, et al. Immunogenicity and safety of a 3-dose regimen of a SARS-CoV-2 inactivated vaccine in adults: A randomized, double-blind, placebo-controlled phase 2 trial. J Infect Dis (2022) 225(10):1701–9. doi: 10.1093/infdis/jiab627
17. Pan HX, Liu JK, Huang BY, et al. Immunogenicity and safety of a severe acute respiratory syndrome coronavirus 2 inactivated vaccine in healthy adults: Randomized, double-blind, and placebo-controlled phase 1 and phase 2 clinical trials. Chin Med J (Engl) (2021) 134(11):1289–98. doi: 10.1097/CM9.0000000000001573
18. Li JX, Wu SP, Guo XL, Tang R, Huang BY, Chen XQ, et al. Safety and immunogenicity of heterologous boost immunisation with an orally administered aerosolised Ad5-nCoV after two-dose priming with an inactivated SARS-CoV-2 vaccine in Chinese adults: A randomised, open-label, single-centre trial. Lancet Respir Med (2022) 10(8):739–48. doi: 10.1016/S2213-2600(22)00087-X
19. Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis (2021) 21:39–51. doi: 10.1016/S1473-3099(20)30831-8
20. Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis (2021) 21:181–92. doi: 10.1016/S1473-3099(20)30843-4
21. Al Kaabi N, Zhang Y, Xia S, Yang Y, Al Qahtani MM, Abdulrazzaq N, et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: A randomized clinical trial. JAMA (2021) 326:35–45. doi: 10.1001/jama.2021.8565
22. Jara A, Undurraga EA, González C, Paredes F, Fontecilla T, Jara G, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. New Engl J Med (2021) 385:875–84. doi: 10.1056/NEJMoa2107715
23. W.H. Organization. The sinopharm COVID-19 vaccine: What you need to know. (2021). Available at: https://www.who.int/news-room/feature-stories/detail/the-sinopharm-covid-19-vaccine-what-you-need-to-know
24. Li J, Hou L, Guo X, Jin P, Wu S, Zhu J, et al. Heterologous AD5-nCOV plus CoronaVac versus homologous CoronaVac vaccination: A randomized phase 4 trial. Nat Med (2022) 28(2):401–9. doi: 10.1038/s41591-021-01677-z
25. Barouch DH, Stephenson KE, Sadoff J, Yu J, Chang A, Gebre M, et al. Durable humoral and cellular immune responses 8 months after Ad26.COV2.S vaccination. New Engl J Med (2021) 385:951–3. doi: 10.1056/NEJMc2108829
26. Widge AT, Rouphael NG, Jackson LA, Anderson EJ, Roberts PC, Makhene M, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. New Engl J Med (2021) 384:80–2. doi: 10.1056/NEJMc2032195
27. Doria-Rose N, Suthar MS, Makowski M, O’Connell S, McDermott AB, Flach B, et al. Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for covid-19. New Engl J Med (2021) 384:2259–61. doi: 10.1056/NEJMc2103916
28. Naaber P, Tserel L, Kangro K, Sepp E, Jürjenson V, Adamson A, et al. Dynamics of antibody response to BNT162b2 vaccine after six months: A longitudinal prospective study. Lancet Reg Health Eur (2021) 10:100208–8. doi: 10.1016/j.lanepe.2021.100208
29. Thomas SJ, Moreira ED Jr., Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine through 6 months. New Engl J Med (2021) 385:1761–73. doi: 10.1056/NEJMoa2110345
30. Juthani PV, Gupta A, Borges KA, Price CC, Lee AI, Won CH, et al. Hospitalisation among vaccine breakthrough COVID-19 infections. Lancet Infect Dis (2021) 21:1485–6. doi: 10.1016/S1473-3099(21)00558-2
31. Alqahtani M, Bhattacharyya S, Alawadi A, Mahmeed HA, Sayed JA, Justman J, et al. Morbidity and mortality from COVID-19 post-vaccination breakthrough infections in association with vaccines and the emergence of variants in Bahrain. (2021). Research Square Platform LLC. doi: 10.21203/rs.3.rs-828021/v1
32. Bergwerk M, Gonen T, Lustig Y, Amit S, Lipsitch M, Cohen C, et al. Covid-19 breakthrough infections in vaccinated health care workers. New Engl J Med (2021) 385:1474–84. doi: 10.1056/NEJMoa2109072
33. Dan W, Yanyang Z, Lin T, Fuzhen W, Ying Y, Chao M, et al. Effectiveness of inactivated COVID-19 vaccines against symptomatic, pneumonia, and severe disease caused by the delta variant: Real world study and evidence — China, 2021. China CDC Weekly (2022) 4:57–65. doi: 10.46234/ccdcw2022.009
34. Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med (2021) 27:1205–11. doi: 10.1038/s41591-021-01377-8
35. de Souza WM, Amorim MR, et al. Levels of SARS-CoV-2 lineage P.1 neutralization by antibodies elicited after natural infection and vaccination (2021). Available at: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3793486.
36. Wang G-L, Wang Z-Y, Duan L-J, Meng Q-C, Jiang M-D, Cao J, et al. Susceptibility of circulating SARS-CoV-2 variants to neutralization. New Engl J Med (2021) 384:2354–6. doi: 10.1056/NEJMc2103022
37. Lustig Y, Sapir E, Regev-Yochay G, Cohen C, Fluss R, Olmer L, et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: A prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir Med (2021) 9:999–1009. doi: 10.1016/S2213-2600(21)00220-4
38. Bates TA, Leier HC, Lyski ZL, Goodman JR, Curlin ME, Messer WB, et al. Age-dependent neutralization of SARS-CoV-2 and P.1 variant by vaccine immune serum samples. JAMA (2021) 326:868. doi: 10.1001/jama.2021.11656
39. Soffer S, Glicksberg BS, Zimlichman E, Efros O, Levin MA, Freeman R, et al. The association between obesity and peak antibody titer response in COVID-19 infection. Obesity (2021) 29:1547–53. doi: 10.1002/oby.23208
40. Huang Y, Lu Y, Huang YM, Wang M, Ling W, Sui Y, et al. Obesity in patients with COVID-19: A systematic review and meta-analysis. Metabolism (2020) 113:154378. doi: 10.1016/j.metabol.2020.154378
41. Sauré D, O'Ryan M, Torres JP, Zuniga M, Santelices E, Basso LJ. Dynamic IgG seropositivity after rollout of CoronaVac and BNT162b2 COVID-19 vaccines in Chile: A sentinel surveillance study. Lancet Infect Dis (2022) 22:56–63. doi: 10.1016/S1473-3099(21)00479-5
42. Knezevic I, Mattiuzzo G, Page M, Minor P, Griffiths E, Nuebling M, et al. WHO international standard for evaluation of the antibody response to COVID-19 vaccines: Call for urgent action by the scientific community. Lancet Microbe (2021) 3(3):e235–e240. doi: 10.1016/S2666-5247(21)00266-4
Keywords: SARS-CoV-2, COVID-19 vaccine, immunogenicity, immune persistence, influencing factors
Citation: Wang F, Huang B, Lv H, Feng L, Ren W, Wang X, Tang L, Liu Q, Wu D, Zheng H, An Z, Deng Y, Zhao L, Ye F, Wang W, Zhang H, Chang S, Liao Y, Chen F, Rodewald LE, Gao GF, Yin Z and Tan W (2022) Factors associated with neutralizing antibody levels induced by two inactivated COVID-19 vaccines for 12 months after primary series vaccination. Front. Immunol. 13:967051. doi: 10.3389/fimmu.2022.967051
Received: 12 June 2022; Accepted: 22 August 2022;
Published: 09 September 2022.
Edited by:
Giorgio Fedele, National Institute of Health (ISS), ItalyReviewed by:
Ahmet Cagkan Inkaya, Hacettepe University, TurkeyCopyright © 2022 Wang, Huang, Lv, Feng, Ren, Wang, Tang, Liu, Wu, Zheng, An, Deng, Zhao, Ye, Wang, Zhang, Chang, Liao, Chen, Rodewald, Gao, Yin and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zundong Yin, eWluemRAY2hpbmFjZGMuY24=; Wenjie Tan, dGFud2pAaXZkYy5jaGluYWNkYy5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.