- 1Maharaj Nakorn Chiang Mai Hospital, Chiang Mai, Thailand
- 2Division of Dermatology, Department of Internal Medicine, Chiang Mai University, Chiang Mai, Thailand
- 3Division of Oncology, Department of Internal Medicine, Chiang Mai University, Chiang Mai, Thailand
- 4Pharmacoepidemiology and Statistics Research Center, Faculty of Pharmacy, Chiang Mai University, Chiang Mai, Thailand
- 5Department of Pharmaceutical Care, Faculty of Pharmacy, Chiang Mai University, Chiang Mai, Thailand
Background: Although immune checkpoint inhibitors (ICIs) have become the frontline treatment option for patients with various advanced cancers due to improved survival, they can be associated with a spectrum of cutaneous immune-related adverse events (cirAEs). However, little is known regarding the occurrence and patterns of cirAE-related ICI therapy in patients of different races other than white populations. Therefore, we investigated the incidence and associated factors of cirAEs among cancer patients in northern Thailand.
Methods: A referral-center-based ambispective cohort study was conducted from January 1, 2017, to March 31, 2021. Based on a linked database and merged patient-level data, adult patients with pathologically confirmed cancer who were diagnosed and received ICI therapy regardless of cancer type and followed up through August 31, 2021, were included. All cirAE-related ICI therapy was based on clinical evaluation and ascertainment by a board-certified dermatologist. The incidence of cirAE-related ICI therapy with confidence intervals (CIs) across cancer- and ICI therapy-specific groups was estimated. Factors associated with cirAEs were evaluated using multivariable modified Poisson regression to estimate risk ratios (RRs) and 95% CIs.
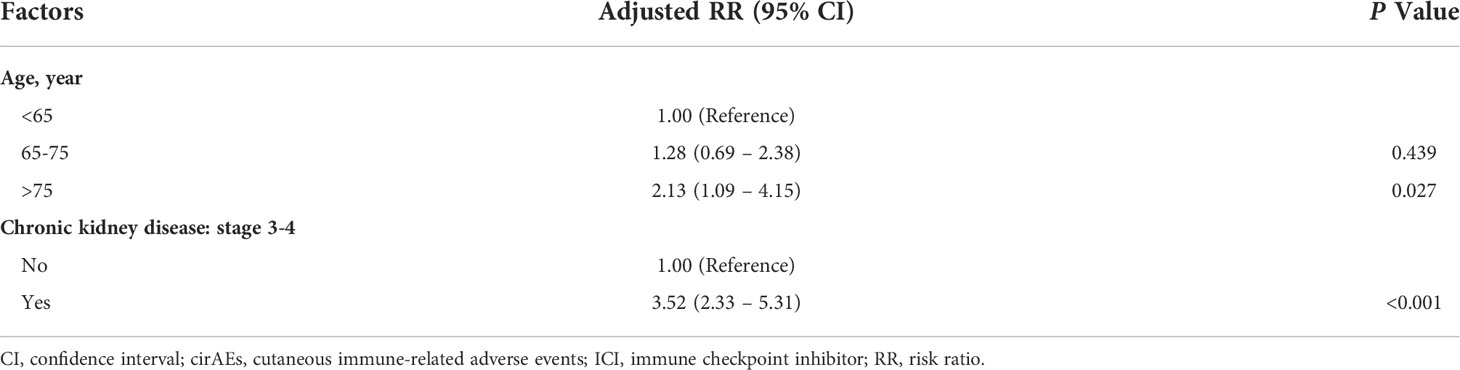
Results: The study included 112 patients (67 men [59.8%]; mean age, 65.0 [range, 31.0-88.0] years), who were mainly diagnosed with lung cancer (56.3%), followed by liver cancer (19.6%). The overall incidence of cirAE-related ICI therapy was 32.1% (95% CI, 24.1-41.4); however, there was no substantial difference in sex, cancer type, or individual ICI therapy. The two identified prognostic risk factors of cirAE-related ICI therapy were age >75 years (adjusted RR, 2.13; 95% CI, 1.09-4.15; P=0.027) and pre-existing chronic kidney disease stages 3-4 (adjusted RR, 3.52; 95% CI, 2.33-5.31; P<0.001).
Conclusions: The incidence of cirAE-related ICI therapy among Thai cancer patients was comparable to that in white populations. Early identification, particularly in elderly patients and those with CKD, should be implemented in clinical practice to help optimize therapeutic decision-making and patient health outcomes.
Introduction
Over the past decade, immune checkpoint inhibitors (ICIs) have become the frontline treatment option and have revolutionized the management of various advanced cancers (1, 2). ICIs target immune system activation against cancer cells; specifically, these monoclonal antibodies are cell surface proteins (i.e., cytotoxic T-lymphocyte antigen-4 [CTLA-4]) and programmed cell death-1 (PD-1)/programmed cell death ligand-1 (PD-L1) (1). In practice, anti-CTLA-4 (e.g., ipilimumab), anti-PD-1 (e.g., nivolumab or pembrolizumab), and anti-PD-L1 (e.g., atezolizumab or durvalumab) agents have been widely used either as monotherapy or in combination for the treatment of advanced solid tumors (e.g., breast, lung, head and neck, renal and bladder, skin cancers, cervical cancer, colorectal cancer, and hepatocellular carcinoma) and hematologic malignancies (1–3).
Given that ICIs act via nonspecific activation of the immune system, treatment with ICIs can also induce immune-related toxicities that mimic autoimmune diseases. Theoretically, these immune-related adverse events can affect any organ system; however, the skin is one of the most common organs involved (4, 5). Clinical characteristics, skin manifestations, and severity of cutaneous immune-related adverse events (cirAEs) vary across ICI therapies and comorbid conditions (5, 6). Existing studies among the white population in several western countries have reported the incidence of cirAEs ranging from 13.1% to 52.3% and are more common in anti-CTLA-4 than in anti-PD-1/anti-PD-L1 (5, 7–12).
Despite the occurrence and patterns of cirAE-related ICI therapy that have been extensively reported, particularly in melanoma and lung cancer (5, 10–12), to our knowledge, limited evidence exists regarding the incidence and risk factors of cirAEs in different cancer types and non-white populations. To date, data on the epidemiology of cirAE-related ICI therapy among advanced cancer patients in Thailand are limited, with lung and liver cancers being the most common malignancies. To better understand and improve treatment care for patients receiving ICIs therapy, we aimed to investigate the incidence and risk factors of cirAEs among cancer patients in a large tertiary care referral center in northern Thailand.
Methods
Study design and patient population
This single-center, ambispective (retrospective and prospective) study was based on a cohort of cancer patients from the Maharaj Nakorn Chiang Mai Hospital, Chiang Mai University, a tertiary referral and university hospital in Northern Thailand. The study protocol was conducted according to the Declaration of Helsinki and reported following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement (13) along with the Reporting of studies Conducted using Observational Routinely collected health Data (RECORD) statement and extension to STROBE reporting guidelines (14). All consecutive cancer patients who received ICIs for treatment of any cancer type between January 1, 2017, and March 31, 2021, and screened for inclusion with follow-up through August 31, 2021, were included in the study. For prospective data collection, data were collected after the approval of the institutional review board of the Faculty of Medicine, Chiang Mai University (study code: MED-2562-06587). The requirement for informed consent for the retrospective phase was waived because of the nature of the study, and written informed consent was obtained for the prospective phase. No financial compensation was provided for participating in this study, and the patient information was de-identified.
Relevant information was gathered and merged using the local joint databases from the Maharaj Nakorn Chiang Mai Hospital based on (i) routine cancer care management profiles, including patient-level details, which comprised clinical characteristics, cancer types, cancer stage, Eastern Cooperative Oncology Group (ECOG) performance status, pre-ICI therapy (traditional chemotherapy, radiotherapy, targeted agent, concurrent chemoradiation therapy, traditional chemotherapy plus radiotherapy, or none), comorbidities (hypertension, diabetes, coronary artery [CAD], chronic kidney disease [CKD] stage 3-4, chronic pulmonary disease, cirrhosis, Charlon comorbidity index, and multimorbidity condition), history of drug allergy, ICI therapy (anti-PD-1/anti-PD-L1 and or anti-CTLA-4 compared), and patient monitoring and treatment follow-up; and (ii) electronic health records, claim databases that contain outpatient and inpatient data, pharmacy dispensing, and laboratory results. The final datasets were reviewed and cross-checked to ensure data quality and to limit the quantity of missing data.
Patients eligible for this study included new users aged 18 years or older with pathologically confirmed cancer diagnosed and treated with at least one dose of ICI therapy (monotherapy or based on two-drug combinations) regardless of cancer type. Exclusion criteria were (i) a history of a cutaneous event within the first three months before receiving ICI therapy; (ii) incomplete data on ICI treatment and monitoring during the follow-up period; (iii) a history of end-stage kidney disease, serum aminotransferase/serum alanine aminotransferase more than 3-times the reference upper limit of normal, or total bilirubin more than 2-times the upper limit of normal; and (iv) pregnancy or breastfeeding.
Outcome: cirAEs
We screened for possible cirAEs using a manual review of routine cancer care management profile information and medical records. All eligible cirAEs were based on clinical evaluation and judgment that established treatment-emergent reactions after ICI therapy based on cutaneous morphology, in which skin lesions exist for more than one day and involve a body surface area of at least 1% or more (10, 15). Details regarding the clinical consequences of cirAE-related ICI therapy were collected, including date of onset, the severity of symptoms using the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 (16), and clinical complications and associated hospitalization. Two or more study investigators, of which at least one is a board-certified dermatologist (MC), were critically reviewed, and all cirAE cases were ascertained in terms of causality related to ICI therapy.
Statistical analysis
The sample size of the study was estimated based on the occurrence of cirAEs among cancer patients who received ICI therapy and ranged from 13.1% to 52.3% (5, 7–12). To account for a type I error of 0.05 and compensate for 10% missing data, at least 99 cancer patients receiving ICI therapy were required for this study. Descriptive data are summarized as numbers with percentages for categorical variables and mean ± standard deviation (SD) or medians with interquartile range (IQR) as appropriate. Differences in cirAE-related ICI therapy status (with or without cirAEs) were assessed using Fisher’s exact test and independent t-test or Wilcoxon rank-sum test for categorical and continuous data, respectively. The incidence estimated with 95% confidence intervals (CIs) of the occurrence of cirAE-related ICI therapy, as well as cirAE-specific morphology, were calculated based on age (<65, 65-75, and >75 years), sex (male and female), cancer types (lung, liver, melanoma, and others), pre-ICI therapy (traditional chemotherapy, radiotherapy, targeted agents, concurrent chemoradiation therapy, and traditional chemotherapy plus radiotherapy), and ICI therapy (anti-PD-1, anti-PD-L1, and anti-CTLA-4 or anti-CTLA-4 plus anti-PD-1/PD-L1).
To identify and explore the predictors associated with the development of cirAE-related ICI therapy, candidate risk factors based on all available patient characteristics, cancer-related characteristics, comorbidities, history of drug allergy, laboratory profiles, and phase of data collection (retrospective vs. prospective) were employed using univariable modified Poisson regression models with robust standard errors to estimate crude relative risks (RR) and 95% CIs. Next, candidate predictors with a P-value <0.100 were analyzed in the multivariable modified Poisson regression models using the stepwise backward method. The final model was determined for multicollinearity by investigating the variance inflation factors of the risk factors in the multivariable model. Multiple imputation analysis was performed to account for missing values; however, variables with more than 20% missing data were excluded from the analysis. All analyses were performed using Stata version 16.0 (Stata Corporation, College Station, TX, USA). Two-tailed tests with a P-value <0.05 were considered statistically significant for all tests.
Results
Characteristics of cancer patients treated with ICI therapy
A total of 118 cancer patients were screened. Of these, four patients were excluded according to the study selection criteria, resulting in 112 patients (64 patients in the retrospective cohort and 48 patients in the prospective cohort) being included in this study (Supplementary Figure S1). Overall, most patients were male (59.8%), with a mean age of 65.9 ± 10.3 years. The included patients were mainly diagnosed with lung cancer (56.3%), followed by liver cancer (19.6%). According to cancer stage, 72.1% of the patients had stage IV cancer, while only 15.2% had an ECOG performance of grade 0. Regarding cancer management, traditional chemotherapy (50.5%) and anti-PD-1 therapy (nivolumab and pembrolizumab, 56.2%) were the most commonly prescribed pre-ICI and ICI therapies, respectively. Table 1 describes the details of patient characteristics according to cirAE-related ICI therapy status.
Incidence of cirAEs among ICI recipients
The overall unadjusted incidence for cirAE-related ICI therapy was 32.1% (95% CI, 24.1-41.4; Table 2). According to ICI therapy, the unadjusted incidences of cirAE-related ICI therapy were 33.3% (95% CI, 22.7-45.9) for anti-PD-1, 29.5% (95% CI, 17.9-44.7) for anti-PD-L1, and 40.0% (95% CI, 9.8-80.3) for anti-CTLA-4 or anti-CTLA-4 plus anti-PD-1/PD-L1. Remarkably, a high incidence of cirAE-related ICI therapy was observed among individuals aged >75 years (50.0%; 95% CI, 27.1-72.9). However, there was no substantial difference in the sex and type of cancer-specific incidence of cirAE-related ICI therapy.
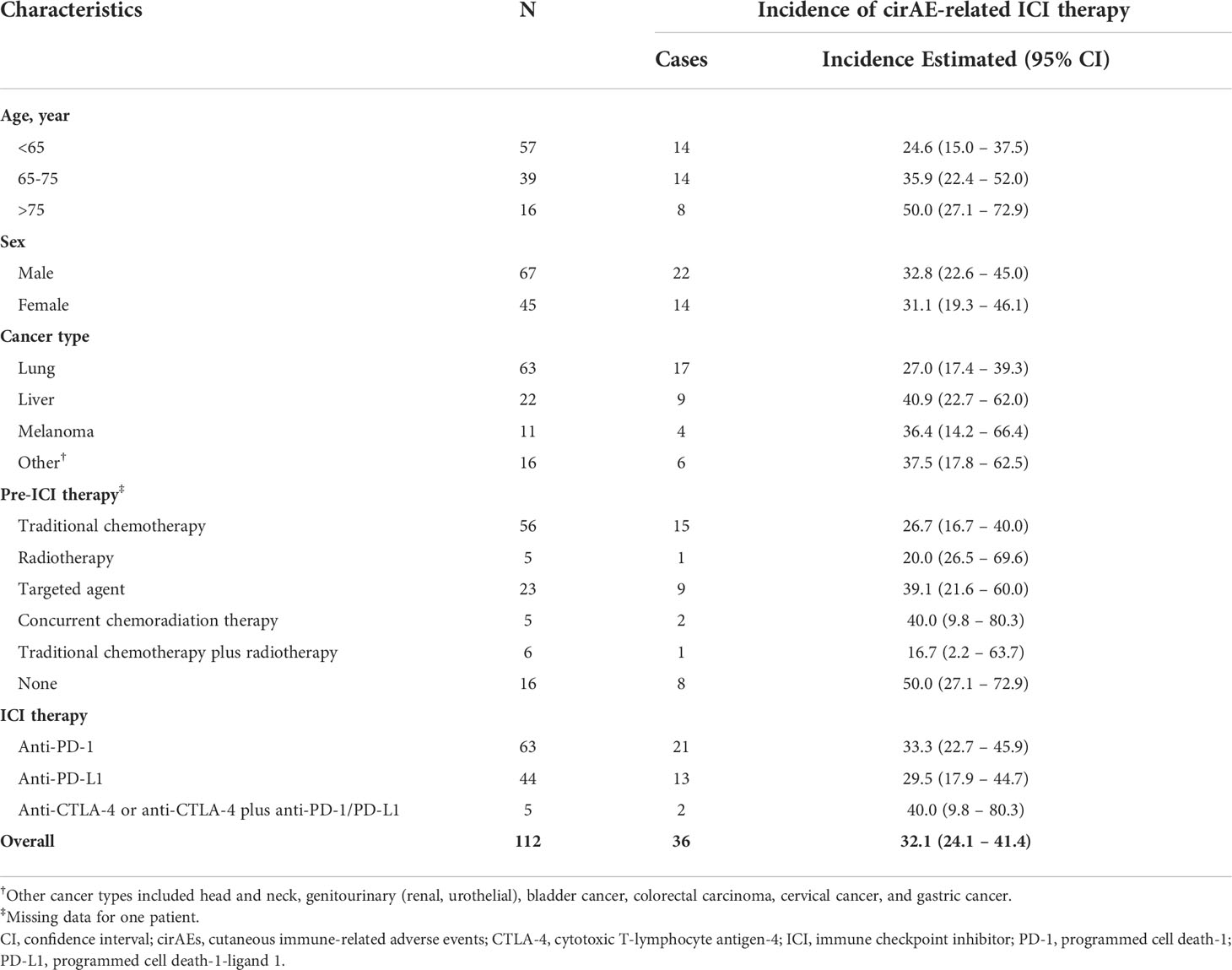
Table 2 Age-, sex-, and cancer-specific conditions incidence of cirAE in patients treated with ICIs.
No statistically significant differences were observed with respect to individual ICI therapies (Figure 1). Based on morphology-specific cirAEs (Figure 1), isolated pruritus was the most cirAE-related to ICI therapy (23.2%; 95% CI, 16.2-32.0), followed by eczematous/dermatitis (6.2%; 95%, 3.0-12.6), and skin xerosis (4.5%; 95% CI, 1.8-10.4). Moreover, rash and other non-specific eruption (3.6%; 95% CI, 1.3-9.2), lichenoid (2.7%; 95%, 0.1-8.1), and vitiligo (0.9%; 95% CI, 0.1-6.2) were also diagnosed as cirAEs among ICI recipients.
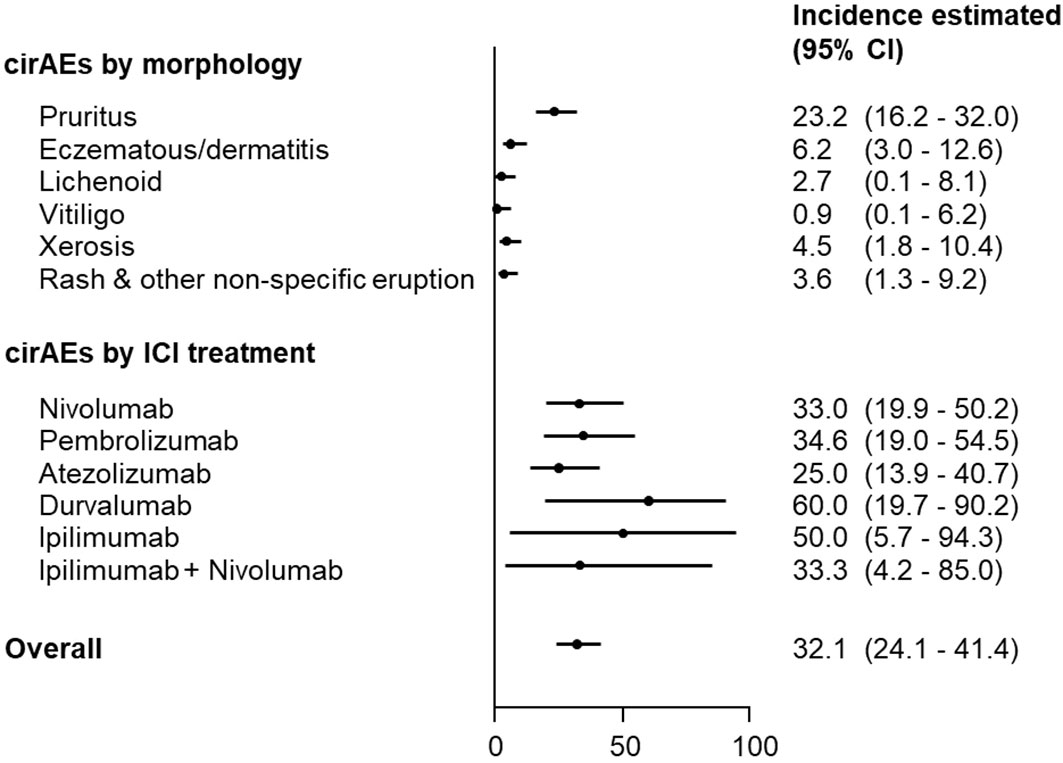
Figure 1 Incidence of cirAE-related ICI therapy according to morphology and individual ICI therapy. CI, confidence interval; cirAEs, cutaneous immune-related adverse events; ICI, immune checkpoint inhibitor.
Among individuals with cirAE-related ICI therapy, initial adverse cutaneous reactions emerged with a median time of 67 days (IQR, 21-146 days) after ICI initiation. Specifically, the median time of cirAEs was 42 days (IQR, 15-84 days) for anti-PD-1; 150.5 days (91-371.5 days) for anti-PD-L1; and 87 days (IQR, 45-782 days) for anti-CTL4 or anti-CTLA-4 plus anti-PD-1/PD-L1 (P=0.941). Nevertheless, no patients who required hospitalization or emergency department visits due to cirAE-related symptoms were identified. Most of the cirAE-related ICI therapy was grade 1, based on CTCAE version 5.0.
Risk factors of cirAEs among ICI recipients
Based on patient demographics, the univariable modified Poisson regression identified seven candidate predictors with P<0.100. These included age, pre-ICI therapy, diabetes, CAD, CKD stage 3-4, neutrophil-to-lymphocyte ratio, and data collection (Supplementary Table S1). Subsequently, two independent significant risk factors of cirAE-related ICI therapy were identified based on the multivariable modified Poisson regression (Table 3): (i) older age over 75 years (adjusted RR, 2.13; 95% CI, 1.09-4.15; P=0.027) and (ii) history of CKD stage 3-4 (adjusted RR, 3.52; 95% CI, 2.33-5.31; P<0.001).
Discussion
This study investigated the occurrence of cirAEs in adult cancer patients treated with ICI therapy at a large tertiary care referral center in northern Thailand. We found that cirAEs are common immune-related toxicities among ICI recipients (estimated incidence occurred in approximately one-third) and mainly include isolated pruritus, eczematous/dermatitis, and skin xerosis. However, there was no significant difference in the incidence of cirAEs according to sex, type of cancer, or ICI therapy. We also found that individuals aged >75 years and with a history of CKD stage 3-4 are at a higher risk of cirAE-related ICI therapy. Based on a clinical perspective, our findings can help inform decision-making management and provide insights for the routine surveillance of cancer patients receiving ICI therapy.
To our knowledge, several mechanisms responsible for cirAE-related ICI therapy, particularly immunopathogenesis via the generation of autoreactive T and B cells, have been proposed (3, 6). The estimated incidence of cirAE-related ICI treatment in this study was comparable to the previous literature in melanoma and non-melanoma patients, as well as in Asian and non-Asian patients (5, 7–12, 17). The overall incidence of cirAE-related ICI therapy among cancer patients was 13.1% to 52.3% (this study estimated incidence at 32.1% [95% CI, 24.1-41.4]) (5, 7–12). However, these disparities across different settings could be attributed to variations in the terminology of cirAEs and different types of ICI therapies, such as the use of anti-CTLA-4 or combination therapy. With a wide and diverse range, the reporting of cirAE-related ICI therapy was based on generic terms and skin manifestations (i.e., skin rash or dermatitis) rather than on clinical pathogenesis, immunohistopathological, and phenotypical characteristics. However, for clinical practicability, histopathological examination is considered only in cases with atypical or persistent forms and severe clinical presentation. Taken together, our findings are in line with existing studies that an extensive range of cirAEs could be observed among patients who received ICI therapy, suggesting that our estimated incidence has general relevance (5, 7–12, 17).
Although several cancer-specific risk factors (Supplementary Table S1) were investigated, we did not find an association between individual ICI therapies or cancer types and the risk of cirAEs. With respect to a population-based study in the United States, Wongvibulsin et al. (5) suggested that individuals with melanoma (odds ratio [OR], 2.47; 95% CI, 2.11-2.89; P<0.001) or renal cell carcinoma (OR, 1.65; 95% CI, 1.36-2.00; P<0.001) and use of combination ICI therapy (OR, 1.53; 95% CI, 1.25-1.88; P<0.001) had a significantly higher risk of cirAEs compared to other cancer types (lung cancer) or other ICI therapy (pembrolizumab), respectively. Apart from non-white populations, the main reason for the lack of this relationship could be attributable to the study power of analysis, different cancer types, and practice patterns of ICI utilization in our practice. Based on general patient characteristics (Supplementary Table S1), we did not observe any association of comorbid conditions such as diabetes, coronary artery disease, and chronic pulmonary disease with subsequent cirAE-related ICI therapy. These findings are supported by several studies that revealed no substantially pre-existing comorbidities and the risk of both cirAEs and non-cirAEs (5, 18, 19). Unfortunately, there is currently no identified specific biomarker for cirAEs; however, if novel biomarkers that predict cirAEs-related ICI therapy are available and established, they could be meaningful in clinical practice.
Unlike a previous study, based on common risk factors, we found that older age (>75 years) and pre-existing CKD stage 3-4 were identified as prognostic risk factors for developing of cirAEs following ICI therapy, which has not been fully elucidated previously. Besides a high rate of multimorbidity, elderly cancer patients also have remodeling of the immune system (20–22). Concerning multiple factors, we postulated that elderly patients could be at risk of cirAEs, in part reflecting the functional changes in immunosenescence and inflammaging. Notably, pre-existing CKD stage 3-4 was also recognized as a predictor of developing cirAE-related ICI therapy in our study (approximately 3-fold compared to those with an estimated glomerular filtration rate >60 mL/min/1.73m2). In recent years, Kartolo et al. (23) found that CKD stage 3 or greater was associated with an increased risk of any immune-related adverse events (OR, 10.66; 95% CI, 2.41-47.12; P=0.025) among 78 patients receiving ICI therapy. Collectively, our findings may expand and support a previous study demonstrating that poor kidney function may increase the risk of cirAEs via the major immune systems, both innate and adaptive responses (24).
Study strengths and limitations
Our study was based on patient-level data through the retrieval and linking of routinely collected data, providing detailed information on oncology and dermatology practices. Moreover, this study delivers previously under-recognized data on the prevalence and risk factors of cirAEs among Thai cancer patients treated with ICI therapy for cancer types other than melanoma and lung cancer.
Nevertheless, our findings should be exercised in the context of study limitations. First, this study was conducted within a single center; therapeutic strategies and treatment protocols, reimbursement schemes, and access to therapeutic options may differ from other settings, which may limit the generalizability of our findings. Second, given the ambispective design, some information regarding cirAEs was retrospectively collected in some patients; as such, information bias remains. Moreover, an inherent heterogeneous terminology of cirAEs may be present, particularly on the basis of retrospective chart reviews. Generally, reporting cirAEs among patients treated with ICI therapy does not involve dermatologists. However, to address the causality related to ICI therapy, all incidences of cirAEs were based on the ascertainment of a board-certified dermatologist. Third, based on a relatively small number of patients and residual risk factors, associations for the identified risk factors should be interpreted as exploratory. Therefore, validation of our risk findings, including comprehensive sets of candidate risk factors, is necessary. Moreover, subgroup analyses regarding the individual use of ICI therapy and cancer types cannot be performed because of the limited sample size. Fourth, evaluating the use of ICI therapy and severe or potentially life-threatening cirAEs (i.e., severe cutaneous adverse reactions) was not possible because the rates of these events were rare in our setting. Finally, although this study represents real-world evidence in clinical practice, the chronicity of observations and dynamic risk prediction for cirAEs cannot be established over time. Under these circumstances, these findings should not supersede the clinical context and judgment.
Implications for practice and future research
Despite the study limitations, our findings expand the incidence of cirAEs among broad cancer types other than the white population and suggest the need for routine proactive surveillance of the risk factors. According to the European Academy of Dermatology and Venereology Task Force for Dermatology for Cancer Patients (25), early identification and promptly appropriate treatment strategies are essential to minimize treatment discontinuation and improve patient health outcomes and oncologic treatment. Ultimately, collaborative management-based approaches across oncology and dermatology care practice should be targeted based on comprehensive system assessment concepts to identify cancer patients at high risk of developing cirAEs during ICI therapy.
To date, the development of ICI therapy has emerged as a treatment option in the management of people living with malignancy, resulting in an increasing number of patients receiving ICI therapy; thus, the occurrence of cirAEs is expected to increase. Interestingly, contemporary evidence also suggests that cirAEs may improve the survival of patients treated with ICI therapy (17, 26, 27). Pragmatic trials, along with large proactive pharmacoepidemiology surveillance studies are warranted to fully understand the risk of cirAEs among patients treated with ICI therapy. We underscore the need for a harmonized definition for cirAEs after ICI therapy, which will help inform international pharmacovigilance comparisons and guidelines for managing cirAEs. Moreover, further studies should address the impact of cirAEs on patients’ quality of life in both clinical trials and daily practice to account for the cirAE burden among patients treated with ICI therapy.
In conclusion, we found a relatively common incidence of cirAEs among Thai cancer patients who received ICI therapy. Early identification, particularly in elderly patients and those with CKD, may help optimize therapeutic decision-making, personalized follow-up strategies, collaborative management, and improve health-related quality of life in patients treated with ICI therapy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of the Faculty of Medicine, Chiang Mai University (study code: MED-2562-06587). The patients/participants provided their written informed consent to participate in this study.
Author contributions
Conceptual design: AL, MC. Data collection: AL, SK, MC, TK, CC, TS, BC, NT, SC, BC. Manuscript writing: AL, SN, SK, MC. Manuscript revision: AL, SK, SN, MC, TK, CC, TS, BC, NT, SC. Study supervision: SN, MC. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by a Faculty of Medicine Research Fund grant, Chiang Mai University (no.87/2563). This work was also partially supported by a grant from the Pharmacoepidemiology and Statistics Research Center (PESRC) through the Chiang Mai University (ORA2564/635).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.965550/full#supplementary-material
Abbreviations
CI, confidence interval; cirAEs, cutaneous immune-related adverse events; ICI, immune checkpoint inhibitor.
References
1. Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol (2015) 33(17):1974–82. doi: 10.1200/jco.2014.59.4358
2. Twomey JD, Zhang B. Cancer immunotherapy update: Fda-approved checkpoint inhibitors and companion diagnostics. AAPS J (2021) 23(2):39. doi: 10.1208/s12248-021-00574-0
3. Goleva E, Lyubchenko T, Kraehenbuehl L, Lacouture ME, Leung DYM, Kern JA. Our current understanding of checkpoint inhibitor therapy in cancer immunotherapy. Ann Allergy Asthma Immunol (2021) 126(6):630–8. doi: 10.1016/j.anai.2021.03.003
4. Muntyanu A, Netchiporouk E, Gerstein W, Gniadecki R, Litvinov IV. Cutaneous immune-related adverse events (irAEs) to immune checkpoint inhibitors: A dermatology perspective on management. J Cutan Med Surg (2021) 25(1):59–76. doi: 10.1177/1203475420943260
5. Wongvibulsin S, Pahalyants V, Kalinich M, Murphy W, Yu KH, Wang F, et al. Epidemiology and risk factors for the development of cutaneous toxicities in patients treated with immune-checkpoint inhibitors: A united states population-level analysis. J Am Acad Dermatol (2022) 86(3):563–72. doi: 10.1016/j.jaad.2021.03.094
6. Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat Rev Clin Oncol (2019) 16(9):563–80. doi: 10.1038/s41571-019-0218-0
7. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med (2015) 373(1):23–34. doi: 10.1056/NEJMoa1504030
8. Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med (2015) 372(26):2521–32. doi: 10.1056/NEJMoa1503093
9. Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Cowey CL, et al. Adjuvant nivolumab versus ipilimumab in resected stage iii or iv melanoma. N Engl J Med (2017) 377(19):1824–35. doi: 10.1056/NEJMoa1709030
10. Thompson LL, Krasnow NA, Chang MS, Yoon J, Li EB, Polyakov NJ, et al. Patterns of cutaneous and noncutaneous immune-related adverse events among patients with advanced cancer. JAMA Dermatol (2021) 157(5):577–82. doi: 10.1001/jamadermatol.2021.0326
11. Le TK, Kaul S, Cappelli LC, Naidoo J, Semenov YR, Kwatra SG. Cutaneous adverse events of immune checkpoint inhibitor therapy: Incidence and types of reactive dermatoses. J Dermatolog Treat (2021) 33:1–5. doi: 10.1080/09546634.2021.1898529
12. Patel AB, Farooq S, Welborn M, Amaria RN, Chon SY, Diab A, et al. Cutaneous adverse events in 155 patients with metastatic melanoma consecutively treated with anti-Ctla4 and anti-Pd1 combination immunotherapy: Incidence, management, and clinical benefit. Cancer (2022) 128(5):975–83. doi: 10.1002/cncr.34004
13. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. Strengthening the reporting of observational studies in epidemiology (Strobe) statement: Guidelines for reporting observational studies. BMJ (2007) 335(7624):806–8. doi: 10.1136/bmj.39335.541782.AD
14. Benchimol EI, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, et al. The reporting of studies conducted using observational routinely-collected health data (Record) statement. PloS Med (2015) 12(10):e1001885. doi: 10.1371/journal.pmed.1001885
15. Thompson LL, Li EB, Krasnow NA, Chang MS, Said JT, Molina GE, et al. Effect of dermatological consultation on survival in patients with checkpoint inhibitor-associated cutaneous toxicity. Br J Dermatol (2021) 185(3):627–35. doi: 10.1111/bjd.20074
16. U.S. Department of Health and Human Services. Common terminology criteria for adverse events (CTCAE) version 5.0 (2017). Available at: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf.
17. Cho YT, Lin YT, Yang CW, Chu CY. Cutaneous immune-related adverse events among Taiwanese cancer patients receiving immune checkpoint inhibitors link to a survival benefit. Sci Rep (2022) 12(1):7021. doi: 10.1038/s41598-022-11128-5
18. Suazo-Zepeda E, Bokern M, Vinke PC, Hiltermann TJN, de Bock GH, Sidorenkov G. Risk factors for adverse events induced by immune checkpoint inhibitors in patients with non-Small-Cell lung cancer: A systematic review and meta-analysis. Cancer Immunol Immunother (2021) 70(11):3069–80. doi: 10.1007/s00262-021-02996-3
19. Okada N, Matsuoka R, Sakurada T, Goda M, Chuma M, Yagi K, et al. Risk factors of immune checkpoint inhibitor-related interstitial lung disease in patients with lung cancer: A single-institution retrospective study. Sci Rep (2020) 10(1):13773. doi: 10.1038/s41598-020-70743-2
20. Pawelec G. Immunosenescence and cancer. Biogerontology (2017) 18(4):717–21. doi: 10.1007/s10522-017-9682-z
21. Hong H, Wang Q, Li J, Liu H, Meng X, Zhang H. Aging, cancer and immunity. J Cancer (2019) 10(13):3021–7. doi: 10.7150/jca.30723
22. Aiello A, Farzaneh F, Candore G, Caruso C, Davinelli S, Gambino CM, et al. Immunosenescence and its hallmarks: How to oppose aging strategically? a review of potential options for therapeutic intervention. Front Immunol (2019) 10:2247. doi: 10.3389/fimmu.2019.02247
23. Kartolo A, Sattar J, Sahai V, Baetz T, Lakoff JM. Predictors of immunotherapy-induced immune-related adverse events. Curr Oncol (2018) 25(5):e403–e10. doi: 10.3747/co.25.4047
24. Kato S, Chmielewski M, Honda H, Pecoits-Filho R, Matsuo S, Yuzawa Y, et al. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol (2008) 3(5):1526–33. doi: 10.2215/cjn.00950208
25. Apalla Z, Nikolaou V, Fattore D, Fabbrocini G, Freites-Martinez A, Sollena P, et al. European Recommendations for management of immune checkpoint inhibitors-derived dermatologic adverse events. the eadv task force 'Dermatology for cancer patients' position statement. J Eur Acad Dermatol Venereol (2022) 36(3):332–50. doi: 10.1111/jdv.17855
26. Tang K, Seo J, Tiu BC, Le TK, Pahalyants V, Raval NS, et al. Association of cutaneous immune-related adverse events with increased survival in patients treated with anti-programmed cell death 1 and anti-programmed cell death ligand 1 therapy. JAMA Dermatol (2022) 158(2):189–93. doi: 10.1001/jamadermatol.2021.5476
Keywords: anti-programmed cell death-1, anti-programmed cell death ligand-1, cutaneous immune-related adverse event, cytotoxic T-lymphocyte antigen-4 inhibitors, immune checkpoint inhibitors
Citation: Luangnara A, Kiratikanon S, Ketpueak T, Suksombooncharoen T, Charoentum C, Chewaskulyong B, Tovanabutra N, Chiewchanvit S, Nochaiwong S and Chuamanochan M (2022) Incidence and factors associated with cutaneous immune-related adverse events to immune check point inhibitors: An ambispective cohort study. Front. Immunol. 13:965550. doi: 10.3389/fimmu.2022.965550
Received: 09 June 2022; Accepted: 05 October 2022;
Published: 20 October 2022.
Edited by:
Laura Ridolfi, Scientific Institute of Romagna for the Study and Treatment of Tumors (IRCCS), ItalyReviewed by:
Wayne Robert Thomas, University of Western Australia, AustraliaXin Li, Shanghai University of Traditional Chinese Medicine, China
Copyright © 2022 Luangnara, Kiratikanon, Ketpueak, Suksombooncharoen, Charoentum, Chewaskulyong, Tovanabutra, Chiewchanvit, Nochaiwong and Chuamanochan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mati Chuamanochan, bWF0aS5jQGNtdS5hYy50aA==; Surapon Nochaiwong, c3VyYXBvbi5ub2NoYWl3b25nQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Athitaya Luangnara
Athitaya Luangnara Salin Kiratikanon
Salin Kiratikanon Thanika Ketpueak
Thanika Ketpueak Thatthan Suksombooncharoen
Thatthan Suksombooncharoen Chaiyut Charoentum
Chaiyut Charoentum Busyamas Chewaskulyong3
Busyamas Chewaskulyong3 Napatra Tovanabutra
Napatra Tovanabutra Siri Chiewchanvit
Siri Chiewchanvit Surapon Nochaiwong
Surapon Nochaiwong Mati Chuamanochan
Mati Chuamanochan