
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 02 September 2022
Sec. Cancer Immunity and Immunotherapy
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.961926
This article is part of the Research Topic Community Series in Combining Chemo/radio Therapy and Immunotherapy for Cancers—Perfect Mix of Old and New, volume II View all 31 articles
 Jian-Guo Zhou1,2,3,4†
Jian-Guo Zhou1,2,3,4† Ada Hang-Heng Wong5†
Ada Hang-Heng Wong5† Haitao Wang6†
Haitao Wang6† Su-Han Jin7
Su-Han Jin7 Fangya Tan8
Fangya Tan8 Yu-Zhong Chen9
Yu-Zhong Chen9 Si-Si He1
Si-Si He1 Gang Shen1
Gang Shen1 Benjamin Frey2,3,4
Benjamin Frey2,3,4 Rainer Fietkau3,4
Rainer Fietkau3,4 Markus Hecht3,4
Markus Hecht3,4 Shamus R. Carr6
Shamus R. Carr6 Ruihong Wang6
Ruihong Wang6 Bo Shen9
Bo Shen9 David S. Schrump6*‡
David S. Schrump6*‡ Hu Ma1*‡
Hu Ma1*‡ Udo S. Gaipl2,3,4*‡
Udo S. Gaipl2,3,4*‡Importance: Blood cell count test (BCT) is a robust method that provides direct quantification of various types of immune cells to reveal the immune landscape to predict atezolizumab treatment outcomes for clinicians to decide the next phase of treatment.
Objective: This study aims to define a new BCTscore model to predict atezolizumab treatment benefits in non-small lung cell cancer (NSCLC) patients.
Design, Setting, and Participants: This study analyzed four international, multicenter clinical trials (OAK, BIRCH, POPLAR, and FIR trials) to conduct post-hoc analyses of NSCLC patients undergoing atezolizumab (anti–PD-L1) single-agent treatment (n = 1,479) or docetaxel single-agent treatment (n = 707). BCT was conducted at three time points: pre-treatment (T1), the first day of treatment cycle 3 (T2), and first day of treatment cycle 5 (T3). Univariate and multivariate Cox regression analyses were conducted to identify early BCT biomarkers to predict atezolizumab treatment outcomes in NSCLC patients.
Main Outcomes and Measures: Overall survival (OS) was used as the primary end point, whereas progression-free survival (PFS) according to Response Evaluation Criteria in Solid Tumors (RECIST), clinical benefit (CB), and objective response rate (ORR) were used as secondary end points.
Results: The BCT biomarkers of neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) at time point T3 and neutrophil-to-monocyte ratio (NMR) at time point T2 with absolute cutoff values of NLR_T3 = 5, PLR_T3 = 180, and NMR_T2 = 6 were identified as strong predictive biomarkers for atezolizumab (Ate)–treated NSCLC patients in comparison with docetaxel (Dtx)–treated patients regarding OS (BCTscore low risk: HR Ate vs. Dtx = 1.54 (95% CI: 1.04–2.27), P = 0.031; high risk: HR Ate vs. Dtx = 0.84 (95% CI: 0.62–1.12), P = 0.235). The identified BCTscore model showed better OS AUC in the OAK (AUC12month = 0.696), BIRCH (AUC12month = 0.672) and POPLAR+FIR studies (AUC12month = 0.727) than that of each of the three single BCT biomarkers.
Conclusion and Relevance: The BCTscore model is a valid predictive and prognostic biomarker for early survival prediction in atezolizumab-treated NSCLC patients.
Non-small cell lung cancer (NSCLC) accounts for 84% of all lung cancer incidence, roughly accounting for 235,170 new cases in the United States in 2021 (1). Therapy for advanced NSCLC can include chemotherapy, radiotherapy, or tyrosine kinase inhibitors (TKIs) as first-line therapy for patients carrying genetic mutations in the genes of EGFR, ALK, ROS1, and NTRK (2). However, for patients without TKI-targeted mutations, safe and effective therapeutic options were limited. With the development of immune checkpoint inhibitors (ICIs), this has changed. ICIs were developed against programmed cell death ligand 1 (PD-L1) on cancer cells, such as atezolizumab, and the immune suppressive receptors programmed cell death 1 (PD-1), such as nivolumab and pembrolizumab, and cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), such as ipilimumab, being present on cytotoxic T cells (2).
These therapies have improved NSCLC treatment outcomes in patients with advanced disease (3–5). However, without the implementation of patient selection by predictive or prognostic biomarkers, no significant PFS or OS improvement by ICI therapy, as compared with chemotherapy, is observed (6). PD-L1 expression and tumor mutational burden (TMB) are often recommended for patient selection before treatment, but contrasting results are seen in clinical trials involving atezolizumab (3, 4, 7) and nivolumab (6). Other biomarkers such as chromosome instability, tumor microsatellite instability, and T-cell surface markers such as PD-11, CD38 and CD392, or tumor-infiltrating PD-1hi CD8+ T cells (8) might serve as prognostic and predictive biomarkers for ICI therapy (9, 10). However, genetic biomarkers require tumor biopsy samples, which are invasive and limit longitudinal analysis for continuous disease monitoring. Hence, liquid biopsy-based biomarkers are attracting more recent attention (11–15).
Blood cell count test (BCT) is a routine, regularly performed blood test conducted before and during treatment. The less invasiveness of BCT makes it potentially useful for longitudinal disease monitoring, especially at the end of each treatment cycle to decide if the treatment plan needs to be altered. BCT provides a direct overview of the immune landscape based on the counts of various types of immune cells. For instance, high pre-treatment neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) correlated with poor survival outcomes in NSCLC patients treated with ICIs, regardless of TMB (16–18). However, a limitation of most of the published studies is either small cohorts or analysis of multiple ICI therapies in diverse clinical settings, which may compromise the validity of findings. Consequently, this study focuses on survival data obtained from four international, multicenter clinical trials to conduct post-hoc analysis of NSCLC patients undergoing atezolizumab (Ate) single-agent treatment, whereas docetaxel (Dtx) single-agent treatment, the standard protocol for second-line NSCLC treatment at the time of clinical trial, served as control. BCT was conducted at three time points: baseline (T1), 6 weeks on-treatment (T2), and 12 weeks on-treatment (T3). The overarching goal was to identify a BCTscore as a biomarker that may predict overall survival in NSCLC patients treated with atezolizumab.
Pseudonymized individual participant data from the single-arm phase II studies FIR (NCT01846416) (19) and BIRCH (NCT02031458) (20) and the two-arm randomized controlled trials (RCTs) POPLAR phase II study (NCT01903993) (3) and OAK phase III study (NCT02008227) (4) were provided by Genentech Inc. and accessed through the secure Vivli online platform. Docetaxel was used in the POPLAR and OAK studies as chemotherapy control to the anti–PD-L1 immunotherapy atezolizumab. Raw data were extracted and compared with the available published data to ensure accuracy. Secondary analysis of the trial data was deemed to be of negligible risk and was approved by the Institutional Review Board of the Second Affiliated Hospital, Zunyi Medical University (No. YXLL(KY-R)-2021-010). Deidentified data were accessed according to Roche’s policy and process for Vivli. Data analyses were conducted from 2 March 2021 to 30 June 2021.
A total of 2,316 patients were included from the four clinical trials, and after the exclusion of untreated patients and patients without pre-treatment BCT, 1,479 and 707 advanced NSCLC patients undergoing atezolizumab and docetaxel treatment, respectively, were included in this study (Figure 1). Of note, atezolizumab was administered either as first-line or second-line therapy after failure of prior chemotherapy in the four trials used in this study. Atezolizumab and docetaxel were both administered every 3 weeks in the two-arm RCTs POPLAR and OAK. BCT was obtained at three time points: pre-treatment baseline (T1), 6 weeks on-treatment (T2), and 12 weeks on-treatment (T3). Baseline was defined as within 28 days prior to the start of treatment. Time points T2 and T3 corresponded to the first day of treatment cycles 3 and 5, respectively.
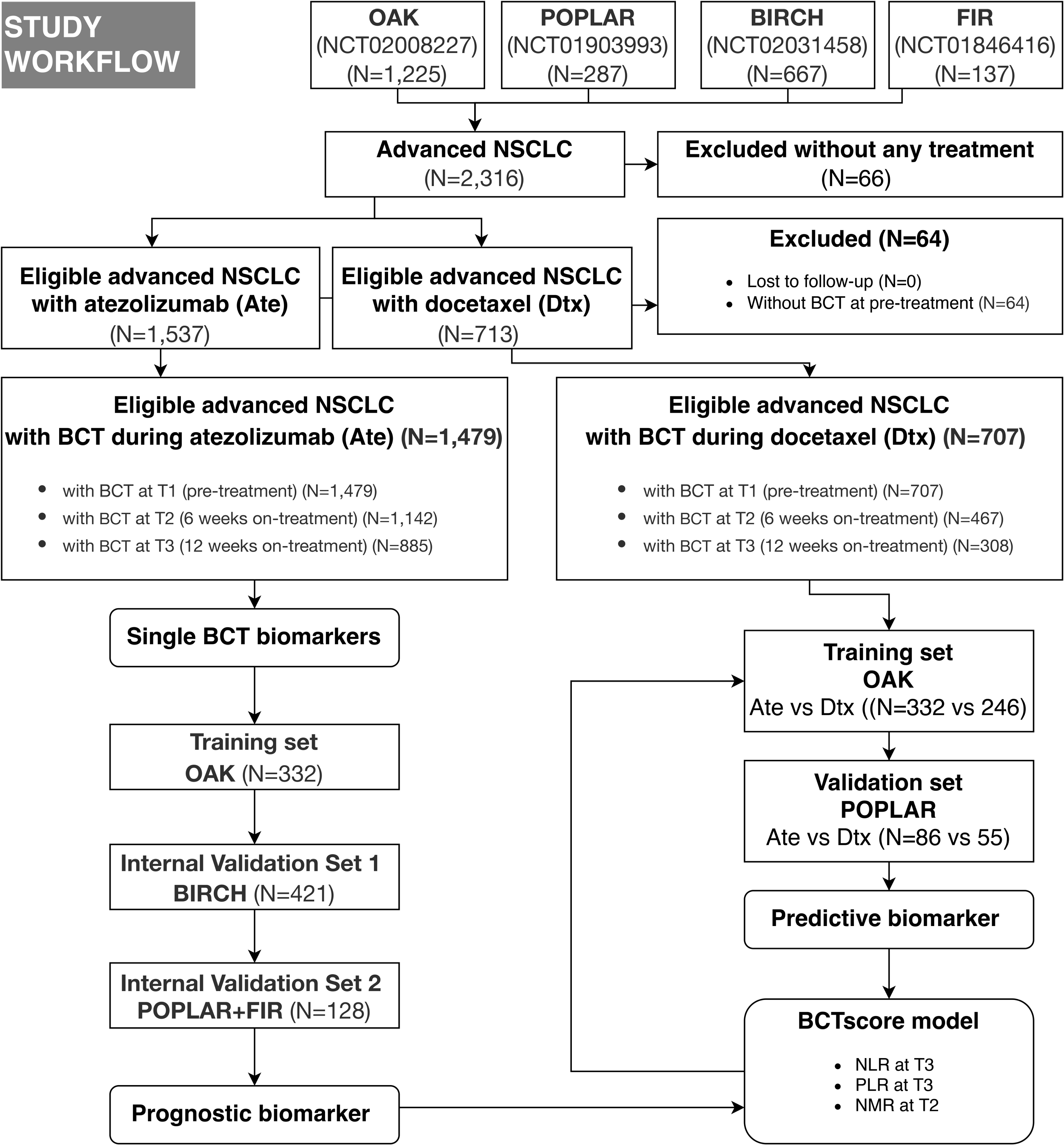
Figure 1 Flow chart demonstrating the patient cohorts of the indicated trials and the approach for the development of a blood cell count test (BCT)–based score (BCTscore). The internal cohorts are from four international, multicenter studies (OAK, POPLAR, BIRCH, and FIR). Ate, atezelizumab; Dtx, docetaxel.
The definitions of OS, PFS, clinical benefit (CB), and objective response rate (ORR) were detailed in each trial (3, 4, 19, 20). In this study, OS was used as the primary end point, whereas PFS, according to Response Evaluation Criteria in Solid Tumors (RECIST version 1.1), CB, and ORR was used as secondary end points.
All biomarkers acquired from BCT, such as absolute cell counts of red blood cells, white blood cells, platelets, as well as the ratios of immune cell subgroups were subjected to the biomarker screening. The BCT biomarkers of NLR, PLR, NMR, and lymphocyte-to-monocyte ratio (LMR) were calculated by dividing absolute cell counts of corresponding immune cells acquired from BCT. The identified biomarkers were named with the abbreviation of the immune cell ratios followed by the indication of the time points T1, T2, and T3, respectively.
Associations between BCT biomarkers and OS or PFS were calculated by the Cox proportional hazards regression model and reported as the mean of hazard ratio (HR) with two-sided 95% confidence interval (CI) and p-value as calculated by the Wald test. The Kaplan–Meier method was used to estimate median OS and PFS between risk groups with a stratified log-rank test at the two-sided significance level. Survival analysis was performed by the survival (V.3.2-11) and survminer (V.0.4.9) packages. To analyze the degree of discrimination of biomarkers, we performed time-dependent receiver-operating characteristic (ROC) analysis and calculated the area under curve (AUC) for the indicated survival outcomes by the timeROC (V.0.3) and pROC (V.1.17.0.1) packages. Comparisons of CB, ORR, or clinical factors between the specified groups were calculated by the generalized linear model (GLM) to report relative risk (RR) with 95% CI and p-value as calculated by the Pearson’s χ2-test or Fisher’s exact test. Comparisons of BCT biomarkers between the treatment groups or different time points of the same treatment group were performed using Wilcoxon signed-rank test. All statistical analyses were carried out in R V.3.6.1 (R Foundation for Statistical Computing). P ≤ 0.050 was considered to be statistically significant. All analyses were univariate except for the multivariate Cox analyses. In multivariate analysis, the BCT biomarker(s) and the clinical factors of sex (male/female), age, race (white/Asian/other), Eastern Cooperative Oncology Group performance status (ECOG PS), metastasis, and pre-treatment PD-L1 (high ≥ 1%/low < 1%; except for the BIRCH study, high ≥ 5%/low < 5%) were included; however, the additional biomarkers of body mass index (BMI) and smoker (never/previous/current) were insignificant as assessed by univariate Cox analysis and were, hence, removed from the analyses.
Initially, the datasets of the four international, multicenter studies containing 1,479 atezolizumab-treated patients’ survival data (the baseline characteristics of these patients are summarized in Supplementary Table S1) were combined to identify 80 common BCT biomarkers that demonstrated correlations to PFS and OS in advanced NSCLC based on HR calculation. Next, we removed the 62 BCT biomarkers that we identified from analyses of the survival data of 707 advanced NSCLC patients who underwent docetaxel treatment. Moreover, all biomarkers containing absolute cell counts were eliminated to avoid sampling-based systemic errors. Hence, 11 BCT biomarkers unique to the atezolizumab-treated patient group remained. Consequently, based on existing knowledge of immune biomarkers, we selected the cell ratios of NLR, PLR, and LMR at 12 weeks on-treatment (NLR_T3, PLR_T3, and LMR_T3), and NMR at 6 and 12 weeks on-treatment (NMR_T2 and NMR_T3), for further analysis. Frequency distribution analysis of these four BCT biomarkers at pre-treatment (T1), followed by Wilcoxon signed-rank test of the absolute difference between the mean values of the two patient groups showed no significant difference between the atezolizumab and docetaxel treatment groups in OAK and POPLAR studies (Supplementary Figure S1). This suggested that baseline levels of these biomarkers were comparable between the two treatment groups. Hence, we deduced that changes in subsequent time points at T2 and T3 likely occurred after treatment started (Supplementary Figure S2).
Next, we performed univariate Cox analysis by decile patient fractions [Ref: Valero] at 10% intervals from 10% to 90% or by the quadrant percentiles of 25% and 75% for all five biomarkers in the combined datasets of atezolizumab-treated patients to calculate HRs for OS and PFS (Figure 2), respectively. NLR_T3 showed significant HR for all patient cutoffs examined in the atezolizumab-treated group and for both OS and PFS. PLR_T3 showed significant PFS HR for the > 10% patient fractions and significant OS HR for all defined patient fractions. LMR_T3 showed significant but inconsistent PFS HR between the 25% and 80% patient fractions and significant OS HR for the > 10% patient fraction. NMR_T2 showed significant PFS HR at the > 20% patient fractions and significant OS HR at the > 10% patient fractions. NMR_T3 showed significant PFS HR at the > 10% patient fractions and significant OS HR for all patient fractions.
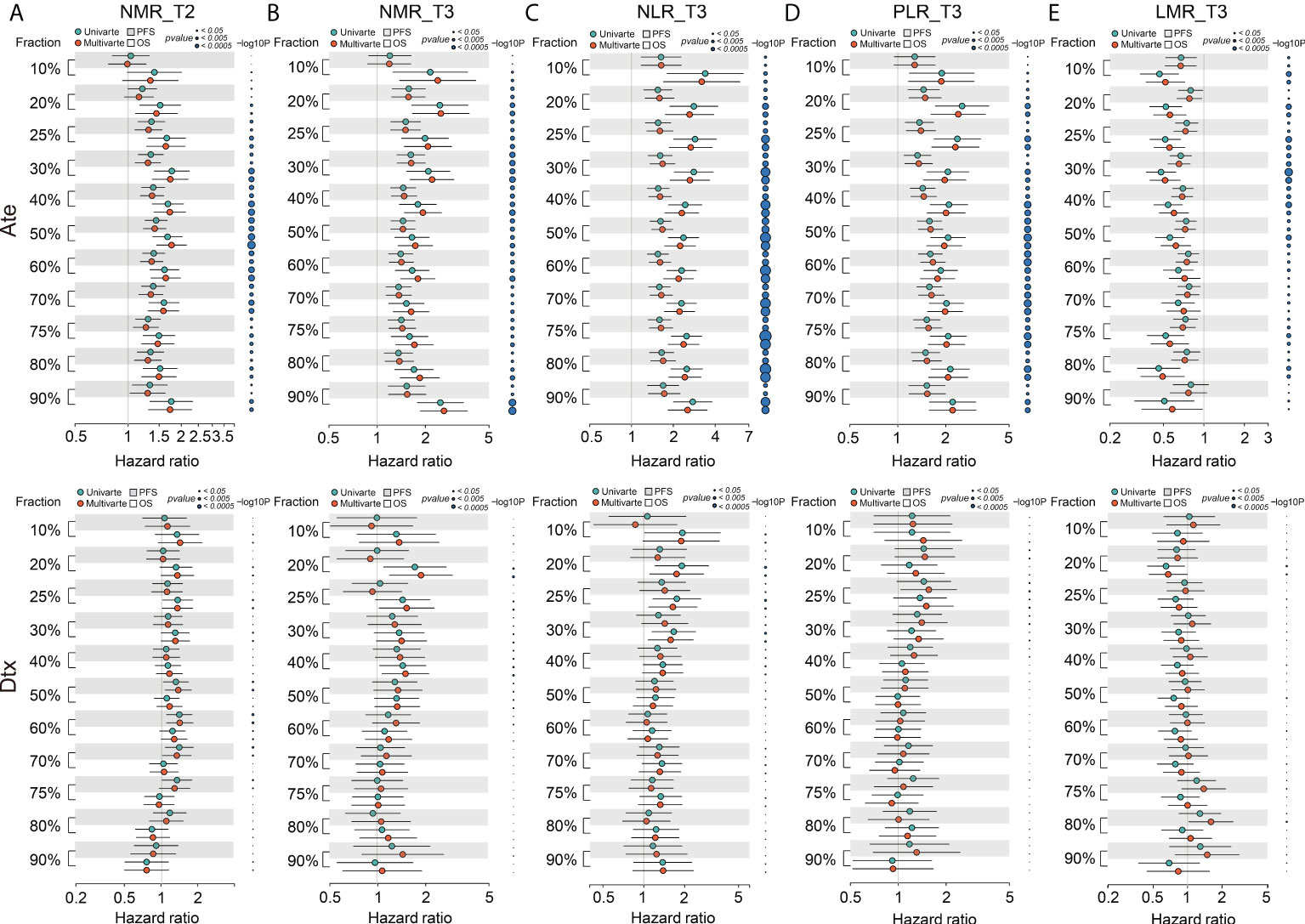
Figure 2 Forest plot of hazard ratio (HR) for overall survival (OS) and progression-free survival (PFS) of the BCT biomarkers (A) NMR_T2, (B) NMR_T3, (C) NLR_T3, (D) PLR_T3, and (E) LMR_T3 in decile patient fractions in the atezolizumab (Ate) or docetaxel (Dtx) treatment groups of the combined internal cohorts. Mean HRs for OS (white shade) or PFS (gray shade) under univariate (green) or multivariate (red) Cox analysis is indicated by the dots, the range of HR is indicated by the error bar of the forest plot; -log10 p-value of each calculated HR is indicated by the size of the blue dots adjacent to the forest plot.
On multivariate analysis, we initially screened for clinical factors that might confer to PFS and OS in atezolizumab-treated NSCLC patients (Supplementary Table S2). Similar to univariate analysis, we performed multivariate Cox analysis using the same patient fractions in the combined datasets for PFS and OS (Figure 2), respectively. NLR_T3, PLR_T3, NMR_T2, and NMR_T3 all depicted identical trends to univariate analysis. Alternatively, LMR_T3 showed significant PFS HR from 10% to 80% patient fractions and significant OS HR from 10% to 50% patient fractions. In contrast, all five BCT biomarkers showed non-significant HRs for both OS and PFS in the docetaxel-treated group (Figure 2). Collectively, these results suggested that LMR_T3 exhibited significant but inconsistent HRs as compared with the rest of the selected biomarkers. Furthermore, as deduced from its definition, LMR_T3 displayed HR < 1, whereas the other four biomarkers displayed HR > 1.
After that, we applied the univariate and multivariate Cox analysis with decile patient fractions to the cohort of atezolizumab-treated NSCLC patients in the four individual trials, respectively (Supplementary Figure S3). In concordance to the joint analyses, all of the five biomarkers showed no significant HRs for both PFS and OS in the docetaxel treatment group. In contrast, positive results, consistent to the combined cohort, were obtained for all biomarkers in the BIRCH and OAK cohorts for both PFS and OS. This was also true of the POPLAR cohort, except for LMR_T3. However, in the FIR cohort none of the biomarkers demonstrated significant HRs for either PFS or OS, but this is most likely because of the small sample size (21). Consequently, absolute integer cutoff values were set for the combined cohort using the patient fractions of 25–50% for all five biomarkers to establish a BCTscore model. The application of these variables to univariate and multivariate Cox analysis of each trial’s cohort succeeded in narrowing the range of each biomarker’s integer cutoff values to uncover the significant range (Supplementary Figure S4). NLR_T3, PLR_T3, and NMR_T2 confirmed consistently significant PFS and OS HRs in the cohorts of BIRCH, OAK, and POPLAR. In contrast, all cutoff values of LMR_T3 did not. Because LMR_T3 showed consistently poor prognostic power in all of the above outlined analyses, it was removed from the biomarker selection for the BCTscore.
Hence, all combinations of the three selected BCT biomarkers, namely, NLR_T3, PLR_T3, and NMR_T2, formed the 16 BCTscore candidates subjected to further optimization for clinical application (Supplementary Table S3).
To establish the BCTscore model, the OAK study was used as our training cohort. Next, the BIRCH study was used as internal validation cohort 1, and the POPLAR combined with the FIR study as internal validation cohort 2. Univariate and multivariate Cox analysis demonstrated that all of the 16 BCTscore candidates demonstrated significant HRs in both OS and PFS, as well as RR for CB and ORR (Supplementary Figure S5). To further narrow down the BCTscore candidates, we performed ROC analysis for OS, PFS, CB, and ORR. The BCTscore candidate 2 (BCTscore #2) was the only candidate that had good AUC for OS, PFS, CB, and ORR in all of the three internal cohorts (Supplementary Table S4). Hence, BCTscore candidate 2, composing of the BCT biomarkers of NLR and PLR at 12 weeks on-treatment (T3) and NMR at 6 weeks on-treatment (T2) with absolute cutoff values of NLR_T3 = 5, PLR_T3 = 180, and NMR_T2 = 6, respectively, was selected as the BCTscore model for NSCLC.
This BCTscore model displayed significant OS and PFS HRs in both univariate and multivariate Cox analysis in all of the three cohorts (Supplementary Figure S5A). The OAK cohort’s RR for CB (univariate = 0.60 [95% CI: 0.39–0.93], P = 0.024; multivariate = 0.56 [95% CI: 0.35–0.88], P = 0.014) (Supplementary Figure S5B) and ORR (univariate = 0.53 [95% CI: 0.31–0.91], P = 0.22; multivariate = 0.58 [95% CI: 0.37–0.88], P = 0.013) with BCTscore stratification (Supplementary Figure S5B) were good. The rate of CB (high risk = 38%, low risk = 51%) and ORR (high risk = 17%, low risk = 28%) of the low-risk atezolizumab-treated patients in the OAK cohort after BCTscore stratification (Supplementary Table S5) were also higher than the 48% CB and 14% ORR reported in the original study (4). Furthermore, survival analysis also showed that our newly identified BCTscore model presented significant difference in both OS and PFS between high- and low-risk patients in the atezolizumab-treated group (Figure 3). ROC analysis resulted in a BCTscore model that consistently exhibited better OS AUC in the OAK (AUC12month = 0.696), BIRCH (AUC12month = 0.672), and POPLAR+FIR studies (AUC12month = 0.727) than that of each of the three single BCT biomarkers in these studies (Figure 4). However, the AUCs of the BCTscore model were lower than those of NLR_T3 for PFS (Supplementary Figure S6), CB (Supplementary Figure S7), and ORR (Supplementary Figure S7) in the OAK cohort, whereas the BCTscore model depicted better AUCs than the standalone BCT biomarkers for these survival indicators in the BIRCH and POPLAR+FIR cohorts.
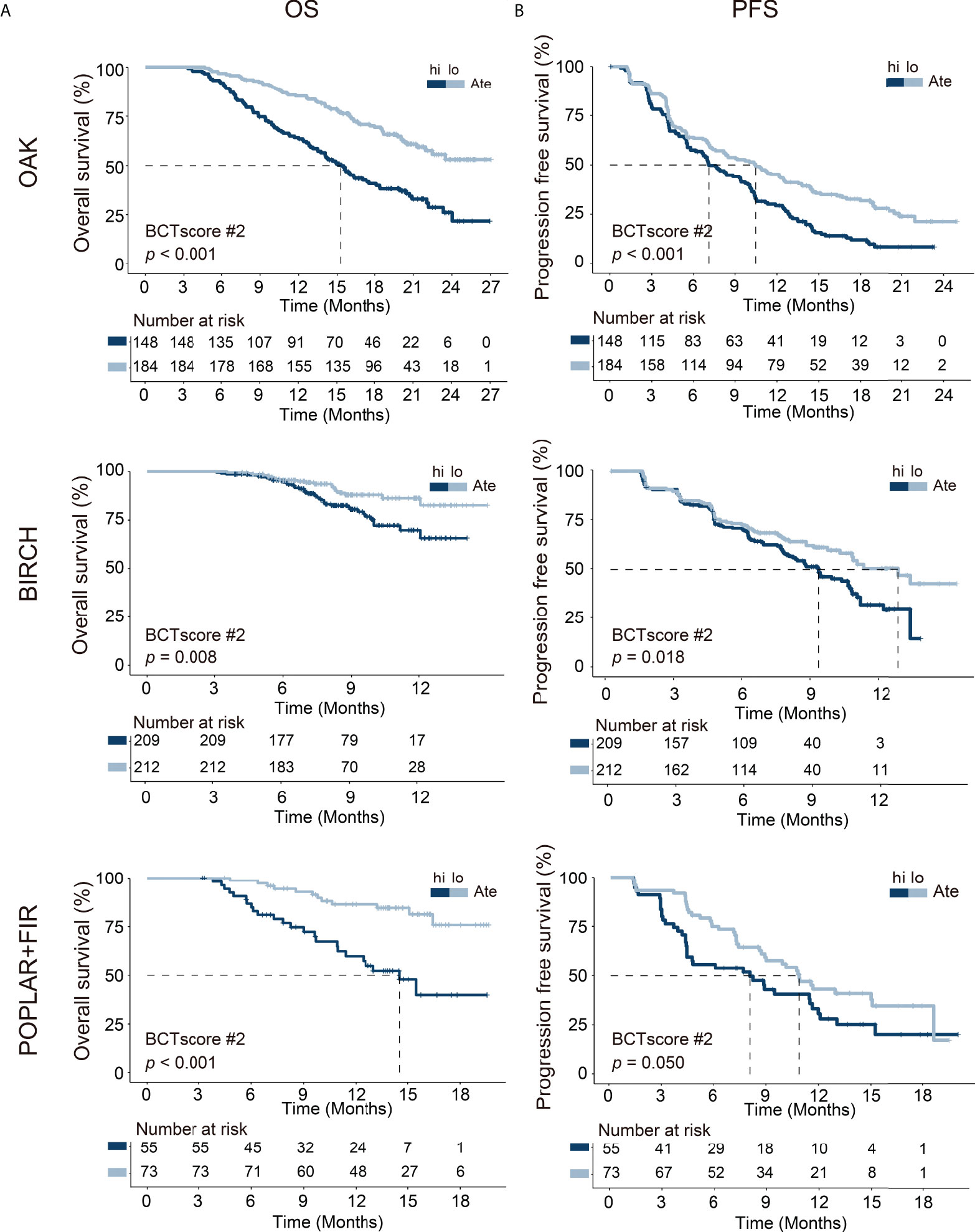
Figure 3 Kaplan–Meier analysis of (A) overall survival (OS) and (B) progression-free survival (PFS) between high-risk (hi) and low-risk (lo) patients, as defined with the identified BCTscore candidate 2 (BCTscore #2), treated with atezolizumab (Ate) of the training cohort (OAK) and the internal validation cohorts (BIRCH and POPLAR + FIR). The percentage of survival of high-risk (dark blue) and low-risk (light blue) patients is plotted against the time in months.
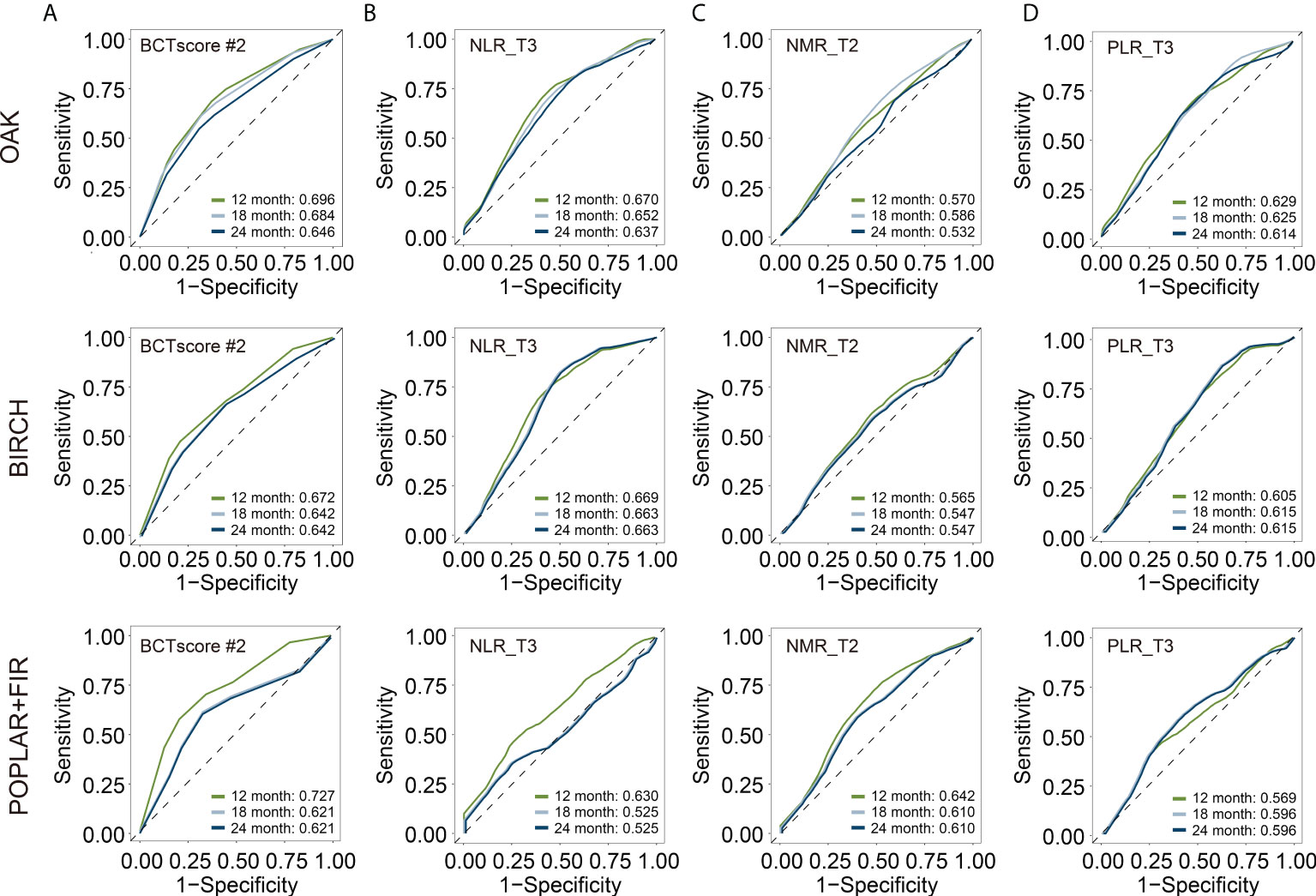
Figure 4 Time-dependent receiver-operating characteristic (ROC) analysis for overall survival (OS) to obtain the area under curve (AUC) of (A) BCTscore candidate 2 (BCTscore #2) and the BCT biomarkers (B) NLR_T3, (C) NMR_T2, and (D) PLR_T3 of the atezolizumab-treated patients of the training cohort (OAK) and the internal validation cohorts (BIRCH and POPLAR + FIR). Sensitivity is plotted against specificity.
Last, in order to test whether the newly identified BCTscore model could serve as a predictive biomarker, we performed survival analysis on the OAK and POPLAR RCTs. The Ate versus Dtx HRs of each BCT biomarker’s absolute cutoff value or decile fractionated BCTscore candidates above and below the cutoff were also calculated to determine whether each BCTscore candidate was a predictive biomarker (22). Results showed that NLR_T3 presented significant PFS prognosis in the OAK study and may be prognostic of OS in the POPLAR study, whereas PLR_T3 and NMR_T2 achieved no significant results in both RCTs (Supplementary Table S6). On the other hand, all the 16 BCTscore candidates had some predictive power in > 75% fractions in the OAK and the POPLAR RCTs (Supplementary Table S7).
In this regard, our newly developed BCTscore model is a strong predictive model specific to atezolizumab-treated NSCLC patients in comparison to docetaxel-treated patients for OS in the cohorts of OAK (BCTscore low risk: HR Ate vs. Dtx = 1.54 [95% CI: 1.04–2.27], P = 0.031; high risk: HR Ate vs. Dtx = 0.84 [95% CI: 0.62–1.12], P = 0.235) (Figure 5) and POPLAR (BCTscore low risk: HR Ate vs. Dtx = 2.93 [95% CI: 1.21–7.10], P = 0.013; high risk: HR Ate vs. Dtx = 0.56 (95% CI: 0.29-1.07), P = 0.074) (Supplementary Figure S8).
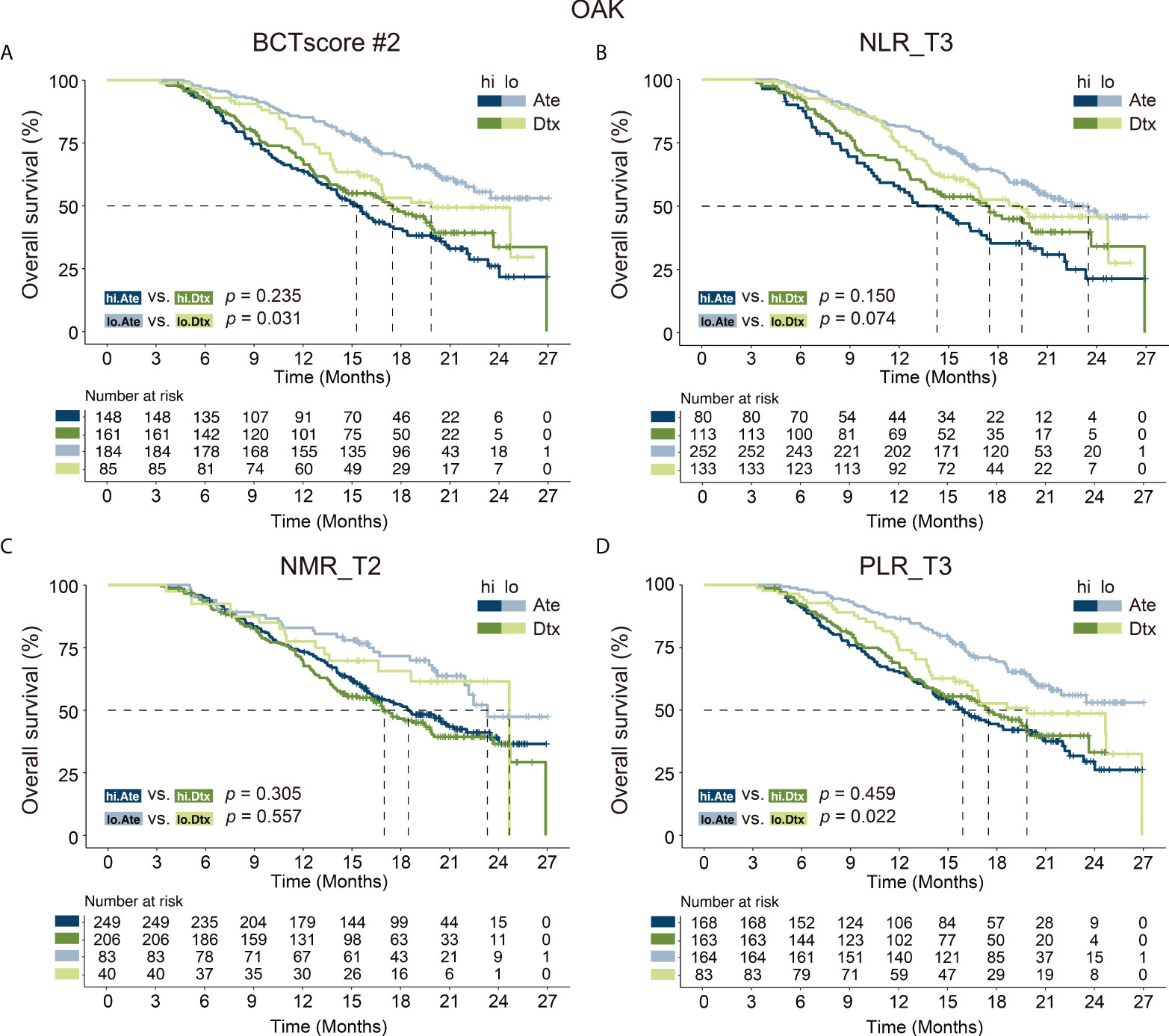
Figure 5 Kaplan–Meier analysis of overall survival (OS) for (A) BCTscore candidate 2 (BCTscore #2) and the BCT biomarkers (B) NLR_T3, (C) NMR_T2, and (D) PLR_T3 comparing atezolizumab (Ate)–treated patients (dark blue) against docetaxel (Dtx)–treated patients (dark green) in the high-risk (hi) group, and comparing between Ate-treated patients (light blue) against Dtx-treated patients (light green) in the low-risk (lo) group of the training cohort (OAK).
In contrast, no significant difference was observed in PFS between the atezolizumab and docetaxel treatment groups in both the OAK (BCTscore low risk: HR Ate vs. Dtx = 1.22 [95% CI: 0.85–1.75], P = 0.267; high risk: HR Ate vs. Dtx = 0.79 [95% CI: 0.60–1.04], P = 0.092) (Supplementary Figure S9) and POPLAR studies (BCTscore low risk: HR Ate vs. Dtx = 1.06 [95% CI: 0.50–2.24], P = 0.877; high risk: HR Ate vs. Dtx = 0.87 (95% CI: 0.47–1.59), P = 0.652) (Supplementary Figure S10), in consistence to the findings of the two studies (3, 4). Similarly, analysis of the relative response rate suggested that our BCTscore model did not distinguish between the atezolizumab- and docetaxel-treated patients in both CB (OAK high risk: Ate versus Dtx = 1.25, low risk: Ate versus Dtx = 0.85; POPLAR high risk: Ate versus Dtx = 0.67, low risk: Ate versus Dtx = 0.95) and ORR (OAK high risk: Ate versus Dtx = 0.96, low risk: Ate versus Dtx = 0.95; POPLAR high risk: Ate versus Dtx = 0.79, low risk: Ate versus Dtx = 0.78) (Supplementary Table S8), reinforcing the fact that our newly defined BCTscore model is a predictive and prognostic biomarker particularly for OS.
Taken together, these results indicate that our BCTscore model can predict the overall survival of NSCLC patients treated with anti–PD-L1 atezolizumab therapy at 12 weeks on-treatment to decide whether treatment can be terminated or alternative treatment plans should be devised.
In spite of the success of ICI therapy in NSCLC treatment, robust prediction of treatment response remains one of the biggest challenges (23). BCT, which is a routine clinical procedure, provides an unbiased overview of the immune landscape for patient stratification and longitudinal ICI efficacy assessment without the need for specialized analysis. This study showed that the BCTscore model serves as both a strong prognostic and predictive biomarkers of ICI efficacy, especially the prediction of overall survival beyond the date of the BCT test. The strengths of this study are manyfold. First, the OS AUCs of our newly identified BCTscore model surpassed that of PD-L1 (24) and TMB (25, 26), both of which can only be applied by more invasive tissue biopsy procedures. Hence, our BCTscore model complements PD-L1 and TMB at later stages to predict survival beyond the initial treatment cycle. Next, an important feature of our analyses is easily obtainable longitudinal data. We presume that the immune landscape alters during treatment, so each time point signifies a discrete event (27). Simultaneously, we removed any BCT biomarker at any time point that is correlated to patient survival after docetaxel treatment during initial biomarker screening, thus selecting atezolizumab-specific BCT biomarkers. Our assumption is that BCT biomarkers typical to cancer prognosis regardless of biological mechanism will show significant HR in the docetaxel patient group and, hence, should be removed from subsequent analyses. Hence, prognostic biomarkers were ruled out using our approach.
Furthermore, delays in immune response have been frequently observed during ICI therapy (28, 29). That is why later time points are hypothesized to have better indicative power as compared with earlier time points. Nevertheless, the biological nature of the BCT biomarker still holds the key to a successful predictive biomarker. For example, both NMR_T2 and NMR_T3 depicted significant HRs in our preliminary analysis. However, NMR_T2 showed better results in the survival analysis of the OAK and POPLAR studies than NMR_T3, whereas NLR_T3 and PLR_T3 displayed good results in the same analysis. This result demonstrated that after the number of neutrophils increased at 6 weeks on-treatment (T2), subsequent increase had little impact on disease prognosis and prediction; instead, the reduction of lymphocytes and increase in platelet numbers at 12 weeks on-treatment (T3) come into play. No prior studies have examined this, to our understanding, in the context of ICI, and the biological mechanism on the temporal changes of the immune landscape during ICI treatment remains elusive.
Additionally, we picked immune cell ratios over absolute cell numbers to avoid systemic errors during blood sampling and sample analysis performed in different medical centers. The cell ratios used for our analysis were selected based on cell lineage and published data. For example, NMR and NLR were picked to distinguish changes in the neutrophil population against the immune cell lineages of monocytes and lymphocytes. It is known that lymphocytes are directly involved in tumor killing, whereas monocytes have more diverse biological roles. Indeed, the fact that NLR outperforms all other cell ratios as a single BCT biomarker reinforces previous observations that increasing neutrophil numbers and decreasing lymphocyte numbers result in poor cancer prognosis (30, 31). Alternatively, the poor correlation between LMR and survival supports the hypothesis that neutrophils, but not the entire monocyte population, contribute to cancer prognosis. Next, the improvement in prognostic ability by combining multiple immune cell ratios suggests that many factors play a role in ICI treatment; for instance, neutrophils were shown to promote tumor metastases (32, 33); platelets present antigens to trigger immune evasion (34). We deduce that future immunological studies will deepen our knowledge of the correlation between the immune landscape and ICI treatment success to unravel more effective and accurate biomarkers (35).
Finally, analysis of the four international, multicenter clinical trials consisting of 1,479 NSCLC patients treated with atezolizumab provides strong statistical evidence to support our findings. We observed statistically significant associations for OS, PFS, ORR, and CB with powerful diagnostic abilities, suggesting that the newly defined BCTscore has prognostic and predictive value in the context of anti–PD-L1 atezolizumab therapy. Nevertheless, because the mechanistic role of atezolizumab is restricted to tumor recognition by T cells, the application of our model to other ICI therapies, such as anti–PD-1 and anti–CTLA-4 therapies, remains to be tested.
A limitation of our study is the lack of TMB measurements in our dataset that would enable direct comparison between the predictive power of our newly defined BCTscore model and TMB. We were restricted in the validation of our results because of the shortage of patients treated by atezolizumab alone. Future studies will focus on the optimization of the absolute cutoff values of each BCT biomarker for different ICI therapies as well as combination therapies.
In summary, we demonstrated, for the first time, via a post-hoc analysis of four clinical trials, the predictive value of longitudinal blood cell count ratio for NSCLC patients treated with atezolizumab. Together, this study proved that the BCTscore combination of NLR at 12 weeks, PLR at 12 weeks, and NMR at 6 weeks provides prognostic and predictive information without the need to re-biopsy patients undergoing anti–PD-L1 atezolizumab monotherapy. Future studies utilizing our BCTscore model may demonstrate its broader versatility as a prognostic and predictive biomarker in all lung cancer patients undergoing atezolizumab treatment.
Data are available upon reasonable request from lead investigator: J-GZ,amlhbmd1by56aG91QHlhaG9vLmNvbQ==.
The studies involving human participants were reviewed and approved by Zunyi Medical University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Conceptualization, J-GZ, AHW, HM, UG, S-HJ, and DS. Methodology, J-GZ, AHW, and FT. Validation, Y-ZC, BS, RF, MH, HM, UG, and J-GZ. Data curation, J-GZ, AHW, GS, S-SH, HM, SC, and UG. Writing—original draft preparation, AHW, HW, J-GZ, and HW. Writing—review and editing, UG, HM, BF, SC, DS, and MH. Visualization, J-GZ and HW. Supervision, HM, UG, J-GZ, and DS. Project administration, J-GZ, HM, UG, and RW. Funding acquisition, J-GZ, S-HJ, and AHW. All authors contributed to the article and approved the submitted version.
This research was funded by the National Natural Science Foundation of China (Grant No. 82060475), the National Natural Science Foundation of Guizhou Province (Grant Nos. [2020]1Z062, ZK2021-YB435, and ZK2022-YB632), Lian Yun Gang Shi Hui Lan Public Foundation (Grant No. HL-HS2020-92). AW Medical Company Limited received startup funding from the University Development Fund of University of Macau.
We would like to thank all of the patients, investigators, and staff involved in the FIR, BIRCH, POPLAR, and OAK studies who released and shared their data. This publication is based on research using data from data contributors, Roche, that has been made available through Vivli, Inc. (Data Request ID: 5935; Lead Investigator: J-GZ). Vivli has not contributed to or approved, and is not in any way responsible for, the contents of this publication. The present work was performed by J-GZ in partial fulfillment of the requirements for containing the degree “Dr. rer. biol. hum.”
MH and UG reports collaborations with Merck Serono (advisory role, speakers’ bureau, honoraria, travel expenses, and research funding), MSD (advisory role, speakers’ bureau, honoraria, travel expenses, and research funding), AstraZeneca (research funding), Novartis (research funding), BMS (advisory role, honoraria, speakers’ bureau), Teva (travel expenses). AHW is a founder and shareholder of AW Medical Co. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.961926/full#supplementary-material
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA: A Cancer J Clin (2021) 71:7–33. doi: 10.3322/caac.21654
2. Duma N, Santana-Davila R, Molina JR. Non-small cell lung cancer: Epidemiology, screening, diagnosis, and treatment. Mayo Clinic Proc (2019) 94:1623–40. doi: 10.1016/j.mayocp.2019.01.013
3. Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet (2016) 387:1837–46. doi: 10.1016/S0140-6736(16)00587-0
4. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet (2017) 389:255–65. doi: 10.1016/S0140-6736(16)32517-X
5. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1-Positive non-Small-Cell lung cancer. New Engl J Med (2016) 375:1823–33. doi: 10.1056/NEJMoa1606774
6. Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, et al. First-line nivolumab in stage IV or recurrent non-Small-Cell lung cancer. N Engl J Med (2017) 376:2415–26. doi: 10.1056/NEJMoa1613493
7. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-Small-Cell lung cancer. N Engl J Med (2015) 373:123–35. doi: 10.1056/NEJMoa1504627
8. Hecht M, Gostian AO, Eckstein M, Rutzner S, von der Grün J, Illmer T, et al. Safety and efficacy of single cycle induction treatment with cisplatin/docetaxel/ durvalumab/tremelimumab in locally advanced HNSCC: first results of CheckRad-CD8. J Immunother Cancer (2020) 8(2):e001378. doi: 10.1136/jitc-2020-001378
9. Jamal-Hanjani M, Wilson GA, McGranahan N, Birkbak NJ, Watkins TBK, Veeriah S, et al. Tracking the evolution of non-Small-Cell lung cancer. N Engl J Med (2017) 376:2109–21. doi: 10.1056/NEJMoa1616288
10. Peters S, Reck M, Smit EF, Mok T, Hellmann MD. How to make the best use of immunotherapy as first-line treatment of advanced/metastatic non-small-cell lung cancer. Ann Oncol Off J Eur Soc Med Oncol (2019) 30:884–96. doi: 10.1093/annonc/mdz109
11. Bonaventura A, Grossi F, Carbone F, Vecchié A, Minetti S, Bardi N, et al. Serum PCSK9 levels at the second nivolumab cycle predict overall survival in elderly patients with NSCLC: a pilot study. Cancer Immunology Immunotherapy CII (2019) 68:1351–8. doi: 10.1007/s00262-019-02367-z
12. Anagnostou V, Forde PM, White JR, Niknafs N, Hruban C, Naidoo J, et al. Dynamics of tumor and immune responses during immune checkpoint blockade in non-small cell lung cancer. Cancer Res (2019) 79:1214–25. doi: 10.1158/0008-5472.CAN-18-1127
13. Thommen DS, Koelzer VH, Herzig P, Roller A, Trefny M, Dimeloe S, et al. A transcriptionally and functionally distinct PD-1(+) CD8(+) T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat Med (2018) 24:994–1004. doi: 10.1038/s41591-018-0057-z
14. Yu H, Guan Z, Cuk K, Zhang Y, Brenner H. Circulating MicroRNA biomarkers for lung cancer detection in East Asian populations. Cancers (2019) 11(3):415. doi: 10.3390/cancers11030415
15. Zhou JG, Donaubauer AJ, Frey B, Becker I, Rutzner S, Eckstein M, et al. Prospective development and validation of a liquid immune profile-based signature (LIPS) to predict response of patients with recurrent/metastatic cancer to immune checkpoint inhibitors. J Immunother Cancer (2021) 9(2):e001845. doi: 10.1136/jitc-2020-001845
16. Valero C, Lee M, Hoen D, Weiss K, Kelly DW, Adusumilli PS, et al. Pretreatment neutrophil-to-lymphocyte ratio and mutational burden as biomarkers of tumor response to immune checkpoint inhibitors. Nat Commun (2021) 12:729. doi: 10.1038/s41467-021-20935-9
17. Diem S, Schmid S, Krapf M, Flatz L, Born D, Jochum W, et al. Neutrophil-to-Lymphocyte ratio (NLR) and platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer (Amsterdam Netherlands) (2017) 111:176–81. doi: 10.1016/j.lungcan.2017.07.024
18. Russo A, Russano M, Franchina T, Migliorino MR, Aprile G, Mansueto G, et al. Neutrophil-to-Lymphocyte ratio (NLR), platelet-to-Lymphocyte ratio (PLR), and outcomes with nivolumab in pretreated non-small cell lung cancer (NSCLC): A Large retrospective multicenter study. Adv Ther (2020) 37:1145–55. doi: 10.1007/s12325-020-01229-w
19. Spigel DR, Chaft JE, Gettinger S, Chao BH, Dirix L, Schmid P, et al. FIR: Efficacy, safety, and biomarker analysis of a phase II open-label study of atezolizumab in PD-L1–selected patients with NSCLC. J Thorac Oncol (2018) 13:1733–42. doi: 10.1016/j.jtho.2018.05.004
20. Peters S, Gettinger S, Johnson ML, Jänne PA, Garassino MC, Christoph D, et al. Phase II trial of atezolizumab as first-line or subsequent therapy for patients with programmed death-ligand 1–selected advanced non–Small-Cell lung cancer (BIRCH). J Clin Oncol (2017) 35:2781–9. doi: 10.1200/JCO.2016.71.9476
21. Button KS, Ioannidis JPA, Mokrysz C, Nosek BA, Flint J, Robinson ESJ, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci (2013) 14:365–76. doi: 10.1038/nrn3475
22. Polley MY, Freidlin B, Korn EL, Conley BA, Abrams JS, McShane LM. Statistical and practical considerations for clinical evaluation of predictive biomarkers. J Natl Cancer Institute (2013) 105:1677–83. doi: 10.1093/jnci/djt282
23. Hegde PS, Chen DS. Top 10 challenges in cancer immunotherapy. Immunity (2020) 52:17–35. doi: 10.1016/j.immuni.2019.12.011
24. Ugurel S, Schadendorf D, Horny K, Sucker A, Schramm S, Utikal J, et al. Elevated baseline serum PD-1 or PD-L1 predicts poor outcome of PD-1 inhibition therapy in metastatic melanoma. Ann Oncol (2020) 31:144–52. doi: 10.1016/j.annonc.2019.09.005
25. Alborelli I, Leonards K, Rothschild SI, Leuenberger LP, Savic Prince S, Mertz KD, et al. Tumor mutational burden assessed by targeted NGS predicts clinical benefit from immune checkpoint inhibitors in non-small cell lung cancer. J Pathol (2020) 250:19–29. doi: 10.1002/path.5344
26. Yu Y, Zeng D, Ou Q, Liu S, Li A, Chen Y, et al. Association of survival and immune-related biomarkers with immunotherapy in patients with non–small cell lung cancer: A meta-analysis and individual patient–level analysis. JAMA Network Open (2019) 2:e196879–e196879. doi: 10.1001/jamanetworkopen.2019.6879
27. Hiam-Galvez KJ, Allen BM, Spitzer MH. Systemic immunity in cancer. Nat Rev Cancer (2021) 21:345–59. doi: 10.1038/s41568-021-00347-z
28. Owen CN, Bai X, Quah T, Lo SN, Allayous C, Callaghan S, et al. Delayed immune-related adverse events with anti-PD-1-based immunotherapy in melanoma. Ann Oncol (2021) 32:917–25. doi: 10.1016/j.annonc.2021.03.204
29. Couey MA, Bell RB, Patel AA, Romba MC, Crittenden MR, Curti BD, et al. Delayed immune-related events (DIRE) after discontinuation of immunotherapy: diagnostic hazard of autoimmunity at a distance. J ImmunoTherapy Cancer (2019) 7:165. doi: 10.1186/s40425-019-0645-6
30. Grecian R, Whyte MKB, Walmsley SR. The role of neutrophils in cancer. Br Med Bull (2018) 128:5–14. doi: 10.1093/bmb/ldy029
31. Shaul ME, Fridlender ZG. Tumour-associated neutrophils in patients with cancer. Nat Rev Clin Oncol (2019) 16:601–20. doi: 10.1038/s41571-019-0222-4
32. Park J, Wysocki RW, Amoozgar Z, Maiorino L, Fein MR, Jorns J, et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci Trans Med (2016) 8:361ra138. doi: 10.1126/scitranslmed.aag1711
33. Wu M, Ma M, Tan Z, Zheng H, Liu X. Neutrophil: A new player in metastatic cancers. Front Immunol (2020) 11:2198. doi: 10.3389/fimmu.2020.565165
34. Menter DG, Tucker SC, Kopetz S, Sood AK, Crissman JD, Honn KV. Platelets and cancer: a casual or causal relationship: revisited. Cancer Metastasis Rev (2014) 33:231–69. doi: 10.1007/s10555-014-9498-0
Keywords: NSCLC, atezolizumab, biomarker, blood cell count, clinical trial
Citation: Zhou J-G, Wong AH-H, Wang H, Jin S-H, Tan F, Chen Y-Z, He S-S, Shen G, Frey B, Fietkau R, Hecht M, Carr SR, Wang R, Shen B, Schrump DS, Ma H and Gaipl US (2022) Definition of a new blood cell count score for early survival prediction for non-small cell lung cancer patients treated with atezolizumab: Integrated analysis of four multicenter clinical trials. Front. Immunol. 13:961926. doi: 10.3389/fimmu.2022.961926
Received: 05 June 2022; Accepted: 15 August 2022;
Published: 02 September 2022.
Edited by:
Jian Zhang, Southern Medical University, ChinaReviewed by:
Quan Cheng, Central South University, ChinaCopyright © 2022 Zhou, Wong, Wang, Jin, Tan, Chen, He, Shen, Frey, Fietkau, Hecht, Carr, Wang, Shen, Schrump, Ma and Gaipl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Udo S. Gaipl, dWRvLmdhaXBsQHVrLWVybGFuZ2VuLmRl; Hu Ma, bWFodWFiQDE2My5jb20=; David S. Schrump, RGF2aWRfU2NocnVtcEBuaWguZ292
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.