
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Immunol. , 09 September 2022
Sec. Cancer Immunity and Immunotherapy
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.961105
 David G. Coffey1,2†
David G. Coffey1,2† Yuexin Xu1†
Yuexin Xu1† Andrea M. H. Towlerton1
Andrea M. H. Towlerton1 Marcin Kowanetz3
Marcin Kowanetz3 Priti Hegde4
Priti Hegde4 Martine Darwish5
Martine Darwish5 Mahesh Yadav5
Mahesh Yadav5 Craig Blanchette5
Craig Blanchette5 Shannon M. Ruppert5
Shannon M. Ruppert5 Sarah Bertino6
Sarah Bertino6 Qikai Xu6
Qikai Xu6 Andrew Ferretti6
Andrew Ferretti6 Adam Weinheimer6
Adam Weinheimer6 Matthew Hellmann7
Matthew Hellmann7 Angel Qin8
Angel Qin8 Dafydd Thomas9
Dafydd Thomas9 Edus H. Warren1
Edus H. Warren1 Nithya Ramnath8,10*
Nithya Ramnath8,10*Most patients with advanced non-small cell lung cancer (NSCLC) do not achieve a durable remission after treatment with immune checkpoint inhibitors. Here we report the clinical history of an exceptional responder to radiation and anti-program death-ligand 1 (PD-L1) monoclonal antibody, atezolizumab, for metastatic NSCLC who remains in a complete remission more than 8 years after treatment. Sequencing of the patient’s T cell repertoire from a metastatic lesion and the blood before and after anti-PD-L1 treatment revealed oligoclonal T cell expansion. Characterization of the dominant T cell clone, which comprised 10% of all clones and increased 10-fold in the blood post-treatment, revealed an activated CD8+ phenotype and reactivity against 4 HLA-A2 restricted neopeptides but not viral or wild-type human peptides, suggesting tumor reactivity. We hypothesize that the patient’s exceptional response to anti-PD-L1 therapy may have been achieved by increased tumor immunogenicity promoted by pre-treatment radiation therapy as well as long-term persistence of oligoclonal expanded circulating T cells.
Immunotherapy using immune checkpoint inhibitors has revolutionized treatment of various solid organ cancers, including non-small cell lung cancer (NSCLC). These therapies include monoclonal antibodies targeting immune checkpoint receptors anti-program death-1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) as well as tumor ligand anti-program death-ligand 1(PD-L1) (1). Durable clinical benefit with response lasting greater than 6 months is described in 20% of patients with stage IV NSCLC (2–5). Notably, 5-year survival of 15% has been reported with anti-PD1 based therapies for metastatic NSCLC (6, 7). Few studies have described long term persistence of expanded T-cell clonotypes in these long-term survivors. The current study provides a comprehensive evaluation of the T cell receptor β (TCRβ) repertoire and characterization of an expanded T cell clone in an exceptional responder to radiation and anti-PD-L1 antibody, atezolizumab. The patient is currently alive with no evidence of cancer 11 years following diagnosis of metastatic NSCLC.
A 63-year-old, asymptomatic woman with prior stage I, estrogen receptor positive, invasive ductal carcinoma of the left breast was found to have a right lower lobe lung nodule and enlarged right hilar lymph node upon imaging for breast cancer surveillance. She reported a prior history of smoking but had no family history of cancer. Full body imaging uncovered numerous bony lesions and a solitary right parietal lobe mass within the brain. Biopsy of the left iliac bone confirmed TTF-1 positive malignant cells consistent with adenocarcinoma of the lung. Targeted DNA sequencing of the tumor (FoundationOne) identified alterations in 23 of 236 assayed genes, including KRAS G12F and BRAC2 P1819S. No mutations were identified in EGFR. She was treated with stereotactic radiosurgery to the brain lesion followed by carboplatin, pemetrexed and bevacizumab chemotherapy. A year later, she underwent stereotactic body radiation therapy (SBRT) to a residual right lower lobe lung mass and achieved a near complete response.
Eight months later, she was found to have increasing lung nodules around the site of radiation. A positron emission tomography (PET) scan revealed increased uptake in the right lower lobe of the lung, right hilum, right axilla and numerous bony sites. Despite treatment with pemetrexed, she continued to progress and developed vaginal wall lesions and a new subcutaneous lesion within the right buttock. Biopsy of this lesion confirmed lung adenocarcinoma. She received palliative radiation to the right buttock and enrolled in a single-arm, phase II study evaluating the efficacy and safety of atezolizumab, an anti-PD-L1 inhibitor, in patients with locally advanced or metastatic NSCLC (NCT01846416) (8). Immunohistochemistry of her right buttock tumor biopsy confirmed 100% of her cancer cells expressed PD-L1 (C223 DAKO monoclonal antibody, Figure 1A). Repeat imaging prior to her enrollment in the clinical trial revealed further progression of her cancer with nodal and lymphangitic spread (Figure 1B).
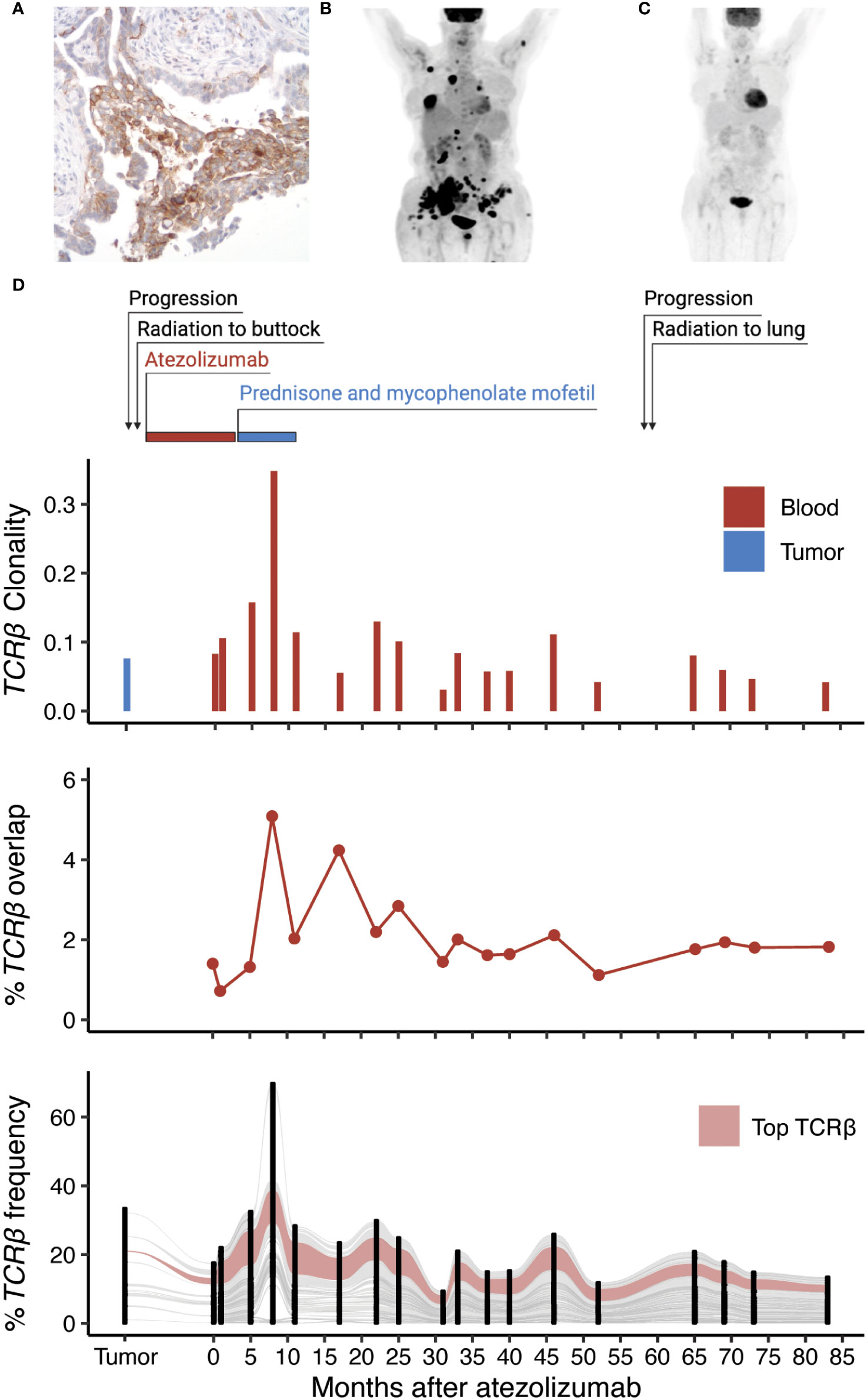
Figure 1 Identification of an expanded T cell clone following radiation and anti-PD-L1 therapy. (A) Expression of PD-L1 within the metastatic tumor biopsy by immunohistochemistry. (B) PET scan of the patient before and (C) one year of anti-PD-L1 therapy. (D) Treatment timeline of the patient in relation to TCRβ clonality (inverted Shannon entropy), percent overlap of all TCRβ sequences detected in the tumor and the blood, and an alluvial plot tracking the frequency of the top 100 TCRβ sequences shared by at least 2 samples across all collection time points.
After the third dose of atezolizumab, she developed symptoms of hypothyroidism that was treated thyroid replacement hormone. Subsequently, she noted hypopigmentation in the region of her right buttock, where she had previously been irradiated. She also developed skin tightening along her torso, forearms, antecubital fossa, and posterior legs. A skin biopsy of the left forearm showed slight collagen alteration and an eosinophilic infiltrate. She was found to have peripheral eosinophilia with absolute eosinophil count of 1.4 cells/µL (normal less than 0.5 cells/µL). She was diagnosed with eosinophilic fasciitis and recommended to discontinue atezolizumab since it was believed to be the cause of her skin reaction. She was treated with prednisone resulting in substantial improvement in the appearance and thickening of her skin and then transitioned to mycophenolate mofetil. Following her last dose of atezolizumab, PET imaging revealed a complete resolution of her cancer including the lymph nodes, primary tumor, and bone metastases (Figure 1C). Four years later, an enlarging right upper lobe lesion within the lung was identified and treated with empiric SBRT since the site was inaccessible for a biopsy. Over the subsequent three years the patient has remained in a complete remission with no detectable disease on annual radiographic imaging.
To characterize the T cell response of this exceptional responder, we performed sequencing of the TCRβ chain on genomic DNA extracted from the patient’s metastatic subcutaneous tumor biopsy in addition to 18 peripheral blood samples including one obtained before and 12 months after treatment with atezolizumab (Supplemental Methods). Additionally, TCRβ sequencing was performed on sorted CD3+CD4+ and CD3+CD8+ T cells from one sample obtained 19 months after immunotherapy. Pre-treatment metastatic skin lesions showed greater TCRβ diversity than the post-treatment blood samples (Figure 1D). Among the 2,163 productive TCRβ sequences detected in the pre-treatment metastatic skin lesions, 52.5% were also detected in at least one of the post-treatment blood samples. Most T cell clones present in the tumor increased in frequency from the pre-treatment to the post-treatment blood samples and were also detected in both the CD4+ and CD8+ sorted blood samples suggesting a polyclonal immune response. The most abundant peripheral blood complementarity-determining region 3 β (CDR3β) sequence detected (CASSLERGLAVSGANVLTF) increased 10-fold in frequency in the 12 months after anti-PD-L1 therapy and was present within the sorted CD8+ T cell population, but not the CD4+ T cell population. The frequency of this sequence among productive CDR3β sequences was 0.24% in the pre-treatment skin biopsy, 1.01% in the pre-treatment blood sample and increased to 10.5% 12 months after treatment (42 months post diagnosis). We continued to observe this sequence in every blood sample at relatively stable frequency, including the last sample 83 months after treatment (113 months post diagnosis). Sequencing of the TCRβ repertoire from the patient’s biopsy revealing eosinophilic fasciitis did not detect the dominant clone. Single-cell RNA sequencing (scRNAseq) of CD3+ sorted blood cells confirmed the dominant CDR3β sequence paired with the CDR3α sequence CIVGVHYGGSQGNLIF.
Having identified the α and β CDR3 sequences of the dominant T cell clone that was present in the tumor and expanded in the blood after anti-PD-L1 therapy, we next characterized its phenotype and identified its antigenic specificity. Targeted single-cell RNA sequencing using a 23 gene panel, confirmed the T cell to be CD8+ and we identified increased differential expression of IFNG and reduced expression of IL13, PDCD1, and CTLA4 (Figure 2A) compared with other T cells derived from the tumor. Whole-exome sequencing of the tumor for neoantigen prediction identified 792 unique neopeptides encoded by 201 genes. We cloned the dominant αβ TCR (Figure 2B) and transduced it into donor CD8+ human T cells and screened it for reactivity against the top 165 HLA-A2 restricted neopeptides. Additionally, we constructed HLA-A2 specific tetramers using these 165 neopeptides by UV mediated peptide exchange (9) and screened for affinity to the dominant clone (Figure 2C). This combined analysis identified 6 candidate neopeptides which we validated using a chromium release assay (Figure 2D). This analysis revealed 4 statistically significant neopeptides with reactivity to the patient’s dominant T cell clone. These neopeptides are encoded by the genes NTM, UGT1A4, OPN1MW2, and NYX (Figure 2E). Finally, to exclude the possibility that the dominant T cell clone also reacts to a self-antigen, we performed a T-Scan analysis against the entire viral and human peptidome. No protein fragments were detected besides known fragments recognized by the included positive control TCR suggesting the dominant T cell clone does not recognize a self-antigen (Figure 2F) (10). Taken together, these results suggest that the expanded T cell clone was an activated, effector CD8+ T cell with reactivity against tumor-derived neopeptides.
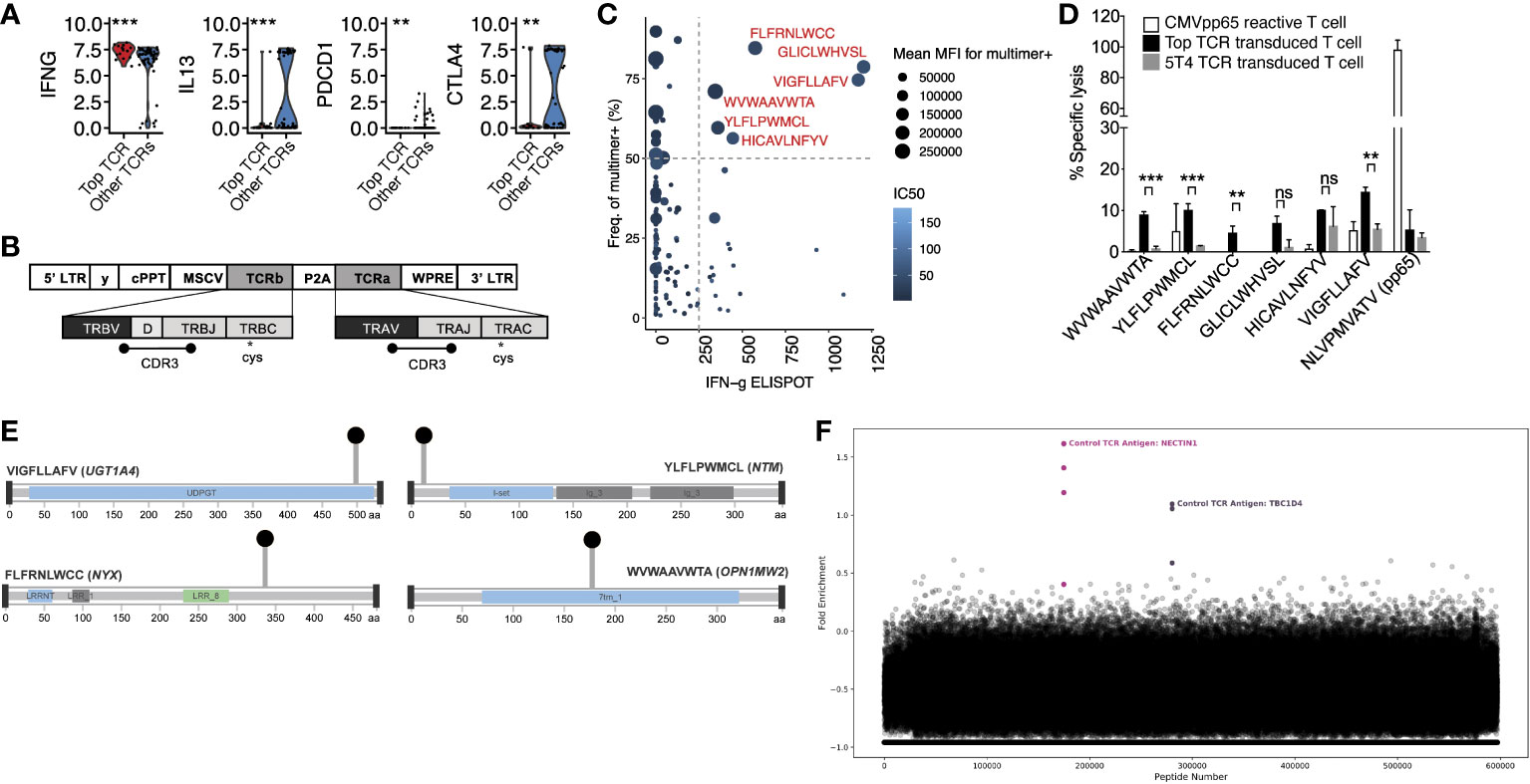
Figure 2 Phenotypic characterization and antigen specificity of the dominant T cell clone. (A) Differential gene expression from targeted scRNAseq comparing the dominant (“Top TCR”) to non-dominant clones. Significance was measured by T test. (B) Construction of TCRαβ clone. (C) Interferon-gamma elispot in relation to mean fluorescence intensity (MFI) of neopeptide generated tetramers. Dashed lines define threshold for reactive peptides (show in red) (D) Chromium release assay of candidate neopeptides. Mean with standard deviations are plotted along with the level of significance from T tests. (E) Lollipop plots showing the location of non-synonymous mutations within the protein amino acid sequence of candidate neoantigens. (F) T-Scan results showing the fold enrichment of each protein fragment in the T-Scan peptidome library. Each point represents an individual protein fragment. Enriched protein fragments with a common epitope are highlighted and labeled. ns: P > 0.05, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
We present a case of a patient with widely metastatic EGFR wild-type lung adenocarcinoma who achieved a durable complete response following radiation and anti-PD-L1 therapy. The mutation of a DNA repair gene within her tumor cells may have increased the mutational burden within her tumor, resulting in a higher neoantigen load. Additionally, we hypothesize that the radiation therapy administered prior to atezolizumab may have acted synergistically with the immunotherapy to augment her response. Prior studies have shown that radiation therapy can enhance tumor immunogenicity, overcome the immunosuppressive effects of the tumor microenvironment, and increase recruitment of antigen-presenting and immune effector cells to the tumor microenvironment (11). Additionally, multiple clinical studies have demonstrated improved treatment outcomes among patients with lung cancer receiving radiation combined with immune checkpoint inhibitors compared to immune checkpoint inhibitors alone (12–14). Serial TCRβ sequencing of the blood revealed polyclonal expansion of CD8+ and CD4+ T cell clones within the pre-treatment metastatic tumor biopsy including a single CD8+ T cell clone that expanded more than 10-fold. Single-cell and antigen characterization of the dominant T cell clone demonstrated the T cell was likely tumor specific based on its activated, IFNG expressing CD8+ phenotype and neopeptide reactivity.
Development of autoimmune vitiligo after immunotherapy in our patient may have been an early indicator of effective treatment response. While vitiligo is not a commonly reported adverse effect of checkpoint inhibitor therapy in lung cancer (15, 16), it is a relatively common event in melanoma (17) and associated with improved treatment outcome (18, 19). Resident memory T cells (TRM) specific to melanoma antigens have been reported to be maintained in vitiligo-affected skin of melanoma patients (20). One study demonstrated long-term persistence of TRM cell clones in the skin and effector memory T (TEM) cells in the blood among vitiligo-affected patients with melanoma and exceptional responses to immunotherapy (21). TRM are also associated with long-lived protection against infection (22) as wells inflammatory conditions of the skin (23). These observations may suggest that the expanded T cell clones we detected in the patient’s metastatic skin biopsy may have had a memory phenotype that contributed to their long term persistence. Although we did not detect reactivity of the patients dominate T cell clone to viral peptides screened by the T-Scan assay, it is possible some of the tumor infiltrating T cell clones may have cross reactivity to microbial antigens which would explain their long-term persistence. Others have reported that virus-specific memory T cells can extend their surveillance to neoantigens expressed by the tumor cells (24–26).
The observation that the patient’s dominant T cell clone reacted to multiple neopeptide-MHC complexes is not surprising. In fact, it is believed that a single TCR can recognize >106 different MHC-associated epitopes (27). Its ability to cross-react with multiple tumor antigens may have increased its probability of reacting to the tumor, especially if the mutations encoding the neopeptides were not present in all tumor subclones. Perhaps it is for this reason that the T cell exhibited the highest degree of expansion, enabling it to become the dominant clone.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
This study was reviewed and approved by University of Michigan Institutional Review Board. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Study conception and design: DC, YX, EW, NR. Data collection: DC, YX, AT, MK, MD, MY, CB, SR, SB, QX, AF, AW, MH, DT. Analysis and interpretation of results: DC, YX, AT, MK, MD, MY, CB, SR, SB, QX, AF, AW, MH, EW, NR. Draft manuscript preparation: DC, YX, AT, CB, AF, AQ, EW, NR. All authors contributed to the article and approved the submitted version.
This research was supported by funding from the Cancer Therapeutics Endowment. This research was also supported by the Flow Cytometry and Genomics Shared Resources of the Fred Hutch/University of Washington Cancer Consortium (P30 CA015704). Bioinformatic analysis was supported by the Scientific Computing Infrastructure at Fred Hutch funded by ORIP grant S10OD028685.
MK was employed by ArriVent. PH was employed by Foundation Medicine. MD, MY, CB, and SB were employed by Genetech. QX, AF, AW were employed by Tscan.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.961105/full#supplementary-material
1. Qin A, Coffey DG, Warren EH, Ramnath N. Mechanisms of immune evasion and current status of checkpoint inhibitors in non-small cell lung cancer. Cancer Med (2016) 5:2567–78. doi: 10.1002/cam4.819
2. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non–Small-Cell lung cancer. New Engl J Med (2015) 373:123–35. doi: 10.1056/nejmoa1504627
3. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non–Small-Cell lung cancer. New Engl J Med (2015) 372:2018–28. doi: 10.1056/nejmoa1501824
4. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet (2017) 389:255–65. doi: 10.1016/s0140-6736(16)32517-x
5. Hellmann MD, Paz-Ares L, Caro RB, Zurawski B, Kim S-W, Costa EC, et al. Nivolumab plus ipilimumab in advanced non–Small-Cell lung cancer. New Engl J Med (2019) 381:2020–31. doi: 10.1056/nejmoa1910231
6. Gettinger S, Horn L, Jackman D, Spigel D, Antonia S, Hellmann M, et al. Five-year follow-up of nivolumab in previously treated advanced non-Small-Cell lung cancer: Results from the CA209-003 study. J Clin Oncol Off J Am Soc Clin Oncol (2018) 36:1675–84. doi: 10.1200/jco.2017.77.0412
7. Garon EB, Hellmann MD, Rizvi NA, Carcereny E, Leighl NB, Ahn M-J, et al. Five-year overall survival for patients with advanced Non−Small-cell lung cancer treated with pembrolizumab: Results from the phase I KEYNOTE-001 study. J Clin Oncol (2019) 37:2518–27. doi: 10.1200/jco.19.00934
8. Spigel DR, Chaft JE, Gettinger S, Chao BH, Dirix L, Schmid P, et al. FIR: Efficacy, safety, and biomarker analysis of a phase II open-label study of atezolizumab in PD-L1–selected patients with NSCLC. J Thorac Oncol (2018) 13:1733–42. doi: 10.1016/j.jtho.2018.05.004
9. Darwish M, Wichner S, Li J, Chang JC, Tam C, Franke Y, et al. High-throughput identification of conditional MHCI ligands and scaled-up production of conditional MHCI complexes. Protein Sci (2021) 30:1169–83. doi: 10.1002/pro.4082
10. Kula T, Dezfulian MH, Wang CI, Abdelfattah NS, Hartman ZC, Wucherpfennig KW, et al. T-Scan: A genome-wide method for the systematic discovery of T cell epitopes. Cell (2019) 178:1016–1028.e13. doi: 10.1016/j.cell.2019.07.009
11. Ko EC, Formenti SC. Radiotherapy and checkpoint inhibitors: a winning new combination? Ther Adv Med Oncol (2018) 10:1758835918768240. doi: 10.1177/1758835918768240
12. Fiorica F, Belluomini L, Stefanelli A, Santini A, Urbini B, Giorgi C, et al. Immune checkpoint inhibitor nivolumab and radiotherapy in pretreated lung cancer patients. Am J Clin Oncol (2018) 41:1101–5. doi: 10.1097/coc.0000000000000428
13. Zhou L, Yu M, Chen L, Zhang Y, Jiang Z, Liu Y, et al. Marvelous objective response of low dose radiotherapy plus ICIs for extended stage small cell lung cancer. J Clin Oncol (2020) 38:e21097. doi: 10.1200/jco.2020.38.15_suppl.e21097
14. Yamaguchi O, Kaira K, Hashimoto K, Mouri A, Miura Y, Shiono A, et al. Radiotherapy is an independent prognostic marker of favorable prognosis in non-small cell lung cancer patients after treatment with the immune checkpoint inhibitor, nivolumab. Thorac Cancer (2019) 10:992–1000. doi: 10.1111/1759-7714.13044
15. Uenami T, Hosono Y, Ishijima M, Kanazu M, Akazawa Y, Yano Y, et al. Vitiligo in a patient with lung adenocarcinoma treated with nivolumab: A case report. Lung Cancer (2017) 109:42–4. doi: 10.1016/j.lungcan.2017.04.019
16. Kosche C, Mohindra N, Choi JN. Vitiligo in a patient undergoing nivolumab treatment for non–small cell lung cancer. Jaad Case Rep (2018) 4:1042–4. doi: 10.1016/j.jdcr.2018.08.009
17. Boniface K, Dutriaux C, Prey S, Taieb A, Seneschal J. Vitiligo-like lesions in patients receiving anti–programmed cell death-1 therapies are distinct from spontaneously occurring active vitiligo. J Am Acad Dermatol (2018) 78:e17–8. doi: 10.1016/j.jaad.2017.08.028
18. Nakamura Y, Tanaka R, Asami Y, Teramoto Y, Imamura T, Sato S, et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: A multi-institutional retrospective study. J Dermatol (2017) 44:117–22. doi: 10.1111/1346-8138.13520
19. Teulings H-E, Limpens J, Jansen SN, Zwinderman AH, Reitsma JB, Spuls PI, et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: A systematic review and meta-analysis. J Clin Oncol (2015) 33:773–81. doi: 10.1200/jco.2014.57.4756
20. Malik BT, Byrne KT, Vella JL, Zhang P, Shabaneh TB, Steinberg SM, et al. Resident memory T cells in the skin mediate durable immunity to melanoma. Sci Immunol (2017) 2:1–11. doi: 10.1126/sciimmunol.aam6346
21. Han J, Zhao Y, Shirai K, Molodtsov A, Kolling FW, Fisher JL, et al. Resident and circulating memory T cells persist for years in melanoma patients with durable responses to immunotherapy. Nat Cancer (2021) 2:300–11. doi: 10.1038/s43018-021-00180-1
22. Zhu J, Peng T, Johnston C, Phasouk K, Kask AS, Klock A, et al. Immune surveillance by CD8αα+ skin-resident T cells in human herpes virus infection. Nature (2013) 497:494–7. doi: 10.1038/nature12110
23. Campbell JJ, Clark RA, Watanabe R, Kupper TS. Sézary syndrome and mycosis fungoides arise from distinct T-cell subsets: a biologic rationale for their distinct clinical behaviors. Blood (2010) 116:767–71. doi: 10.1182/blood-2009-11-251926
24. Simoni Y, Becht E, Fehlings M, Loh CY, Koo S-L, Teng KWW, et al. Bystander CD8+ T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature (2018) 557:575–9. doi: 10.1038/s41586-018-0130-2
25. Rosato PC, Wijeyesinghe S, Stolley JM, Nelson CE, Davis RL, Manlove LS, et al. Virus-specific memory T cells populate tumors and can be repurposed for tumor immunotherapy. Nat Commun (2019) 10:567. doi: 10.1038/s41467-019-08534-1
26. Chiou S-H, Tseng D, Reuben A, Mallajosyula V, Molina IS, Conley S, et al. Global analysis of shared T cell specificities in human non-small cell lung cancer enables HLA inference and antigen discovery. Immunity (2021) 54:586–602.e8. doi: 10.1016/j.immuni.2021.02.014
Keywords: case report, immunotherapy, programmed death-ligand 1, t lymphocytes, lung neoplasms
Citation: Coffey DG, Xu Y, Towlerton AMH, Kowanetz M, Hegde P, Darwish M, Yadav M, Blanchette C, Ruppert SM, Bertino S, Xu Q, Ferretti A, Weinheimer A, Hellmann M, Qin A, Thomas D, Warren EH and Ramnath N (2022) Case report: A persistently expanded T cell response in an exceptional responder to radiation and atezolizumab for metastatic non-small cell lung cancer. Front. Immunol. 13:961105. doi: 10.3389/fimmu.2022.961105
Received: 03 June 2022; Accepted: 22 August 2022;
Published: 09 September 2022.
Edited by:
Mary A Markiewicz, University of Kansas Medical Center, United StatesReviewed by:
Sally Lau, New York University, United StatesCopyright © 2022 Coffey, Xu, Towlerton, Kowanetz, Hegde, Darwish, Yadav, Blanchette, Ruppert, Bertino, Xu, Ferretti, Weinheimer, Hellmann, Qin, Thomas, Warren and Ramnath. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nithya Ramnath, bml0aHlhckBtZWQudW1pY2guZWR1
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.