
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol., 22 July 2022
Sec. Viral Immunology
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.957535
This article is part of the Research TopicHuman T cell Leukemia Virus-1 (HTLV-1) infection, associated pathology and response of the hostView all 20 articles
The Human T-cell Leukemia virus type 1 (HTLV-1) causes an array of pathologies, the most aggressive of which is adult T-cell leukemia (ATL), a fatal blood malignancy with dismal prognosis. The progression of these diseases is partly ascribed to the failure of the immune system in controlling the spread of virally infected cells. HTLV-1 infected subjects, whether asymptomatic carriers or symptomatic patients are prone to opportunistic infections. An increasing body of literature emphasizes the interplay between HTLV-1, its associated pathologies, and the pivotal role of the host innate and adoptive immune system, in shaping the progression of HTLV-1 associated diseases and their response to therapy. In this review, we will describe the modalities adopted by the malignant ATL cells to subvert the host innate immune response with emphasis on the role of the two viral oncoproteins Tax and HBZ in this process. We will also provide a comprehensive overview on the function of innate immunity in the therapeutic response to chemotherapy, anti-viral or targeted therapies in the pre-clinical and clinical settings.
The Human T-cell leukemia virus type 1 (HTLV-1) is the first oncogenic retrovirus associated with a human disease (1). HTLV-1 endemicity spans several continents, including Central and Latin America, the Caribbean islands, Southern Japan, Intertropical Africa, Romania, North-East Iran in the Middle East, Melanesia and Central Australia (2–6). Around 20 million people are infected worldwide, with only 5–10% who develop diseases, depending on their ethnic origin. HTLV-1-induced diseases range between inflammatory, neurodegenerative and malignant disorders. These include uveitis, dermatitis, arthritis, bronchiectasis (Reviewed in (3), and the HTLV-1 associated myelopathy/tropical spastic paraparesis (HAM/TSP), leading to a chronic neurological disease of the central nervous system (7, 8). Yet, the most aggressive form of HTLV-1-associated disorders is adult T cell leukemia (ATL) (9). ATL, discovered in Japan (9), is a hematological neoplasm with dismal prognosis. ATL develops after a very long latency period exceeding 50 years in some patients (reviewed in (10, 11). It is characterized by the clonal expansion of mature activated T cells (CD3+ CD4+ CD5+ CD7- CD8- CD25+) (12), and is subdivided into four clinical subtypes (acute, lymphoma, chronic, and smoldering) (13). “Indolent ATL” regroups the smoldering and chronic subtypes, while “aggressive ATL” describes the acute and lymphoma subtypes. Among all peripheral T cell lymphomas, ATL associates with the worst prognosis (14), with a 5-year OS predicted at 55, 31, 10 and 8% in the smoldering, chronic, lymphoma and acute subtypes respectively (15).
Undeniably, HTLV-1 also predisposes patients to profound immunosuppression and severe opportunistic microbial infections such as Pneumocystis jiroveci, Cryptosporidium parvum, fungal infections, activation of the Cytomegalovirus (16–18), Strongyloides stercoralis (19), Staphylococcus aureus (20), Mycobacterium tuberculosis (18), Sarcoptes scabiei (21). Moreover, higher bloodstream infections correlate with higher HTLV-1 proviral loads in patients (22).
It is intriguing how the same virus causes vastly distant diseases, and this process is highly modulated by the host/virus interplay. In that sense, host factors ostensibly play a key role in the different pathogenic outcomes of HTLV-1 infections. Not only HAM/TSP and ATL develop in different populations of HTLV-1 carriers but a flagrant immunological difference between the two categories of patients is well established. HTLV-1 carriers and HAM/TSP patients exhibit a Th-1 immune profile, while ATL patients display a Th-2/Treg response [reviewed in (23)]. It is also acknowledged that HTLV-1-specific cytotoxic T lymphocytes (CTLs) are highly activated in HAM/TSP patients, but are weaker in ATL patients, and these reduced CTLs predict a risk factor for the development of ATL (24–29). In addition, type-I interferon (IFN) plays a role in the differential suppression of HTLV-1 transcript levels between both types of patients (30), emphasizing a key role of the innate immune response, as another host determinant, in the modulation of HTLV-1 associated diseases (30). Moreover, the cytokine profile in the serum varies between HAM/TSP and ATL. Indeed, IL-10 levels are elevated in the serum of ATL patients (31), while IFN-γ, TNFα, CXCL9, and CXCL10 pro-inflammatory cytokines and chemokines are elevated in HAM/TSP patients (32).
At the viral level, the status of expression of viral proteins is critical in eliciting host immune responses, hence modulating HTLV-1 pathogenesis. Two main viral regulatory proteins, Tax and HBZ, play an essential role in this process. Recently, a dose-dependent increase in interferon (IFN)-γ and interleukin (IL)-8 was demonstrated in response to increasing doses of Tax+ HBZ+ small extracellular vesicles, and the expression of these two viral proteins in the small extracellular vesicles correlated with the proviral load and inflammatory markers in HTLV-1 carriers (33).
In this review, we will focus on ATL and on the interplay between these two viral proteins (Tax and HBZ) with the host innate immunity in modulating ATL leukemogenesis and its therapeutic responses.
The HTLV-1 provirus is flanked by the 5’ and 3’ “Long Terminal Repeat” sequences. HTLV-1 genome encodes for the characteristic structural retroviral genes (gag, pol, and env), in addition to numerous accessory and regulatory proteins. Indeed, the pX region of the provirus has six open reading frames, five on the plus-strand and one on the minus-strand. After alternative splicing, the encoded proteins include Tax, Rex, the HTLV-1 basic leucine zipper protein (HBZ), p8/p12 (where p8 is derived from proteolytic cleavage of p12), p13, p21 and p30 (reviewed in (34, 35). It is well documented that during the long latency period, Rex regulates the post-transcriptional viral gene expression and the stability of the viral transcripts, while p12, p13 and p30 contribute to viral persistence through degradation of Major Histocompatibility Class-1 (MHC-I), alteration of T-cell receptor signaling, and suppression of Tax expression (36). More recently, the effect of monocytes and NK cells, was investigated in primary HTLV-1 infection of macaques. Exposure of animals to an HTLV-1 p12 knock-out mutant demonstrated an impaired infectivity, which was fully restored only when NK cells were depleted. Moreover, the chief role of NK cells in primary infection and the role of p8/p12 in inducing viral persistence in monocytes and in offsetting the cytotoxic effect of NK and CD8+ T cells was demonstrated (37).
Among all described regulatory proteins, Tax and HBZ proteins were lengthily studied and are tightly allied to HTLV-1 pathogenesis (38–41). While Tax is encoded by a sense mRNA, and upregulates various host genes promoting cell activation and proliferation [reviewed in (42–45)], HBZ is encoded by the minus strand of the pX region, and plays several roles, mostly counteracting Tax-induced cellular phenomena (Table 1) (see sections below) (35, 47, 53, 64).
Tax is a 40 kDa protein exhibiting a key role in transformation and oligoclonal expansion of virally infected cells, hence ATL initiation and progression (12, 65). Tax protein is not detectable in most ATL cells (66–68), possibly due to multiple DNA methylations identified at its 5’LTR promoter or deletions of this 5’LTR [For a review (43)]. Some studies suggested that the undetectable Tax protein levels are also due to its strong immunogenic properties, ultimately leading to the rapid elimination of Tax expressing cells by the host immune system (69–71). Despite these undetectable levels, silencing of Tax in HTLV-1-infected and ATL derived cells results in cell death, pinpointing the dependence of these cells on Tax continuous expression (58, 72). Moreover, Tax sporadic bursts occur in a very small percentage (1-3%) of ATL-derived cells at a time, to maintain and ensure the survival of the whole malignant population (73).
Tax transactivates the plus-strand transcription by recruiting cAMP response element binding protein (CREB) and CBP/p300 and P/CAF transcriptional coactivators to Tax response elements (TREs) (74). Tax alters key cellular pathways controlling cell migration, virological synapses, and protein intracellular distribution (41–43, 74). In addition, Tax interferes with the cellular epigenetic machinery (75), down-regulates the expression of various microRNAs (76–79) and increases angiogenesis, invasion and extravasation of ATL cells, hence affecting the cellular microenvironment (80, 81).
Tax-mediated cellular consequences are partly due to its post-translational modifications (82–85), which allow its shuttling between different cellular compartments, enabling it to interfere with/activate a plethora of essential cellular regulators (41). Tax is primarily nuclear (Semmes and Jeang,1996; Bex et al.,1997), and colocalizes with various components of the NF-κB pathway (Bex et al.,1997), SUMO-1, 2, and 3 (Lamsoul et al., 2005; Nasr et al., 2006) and the SUMO-E2 ligase, Ubc-9 (Kfoury et al., 2011). Despite its abundant nuclear localization, Tax cytoplasmic expression was also described (Burton et al., 2000; Cheng et al., 2001). Indeed, Tax localizes with the microtubule organizing center, and with virological synapses (Igakura et al., 2003; Alefantis et al., 2005; Kfoury et al., 2008; Nejmeddine et al., 2009). More importantly, Tax cytoplasmic localization targets IκB-α/β for proteasomal-mediated degradation, to activate the NF-κB pathway (Nicot et al., 1998), paramount for the proliferation and survival of infected T cells (43, 84, 86–95). The activation of this pathway has pleotropic functions on top of which is the modulation of the host immune response (see section 2.1.3 below). Indeed, Tax-mediated-constitutive NF-κB activation occurs at the very early stages of HTLV-1 infection, and this pathway (canonical and non-canonical) remains constitutively activated in Tax expressing cells, ATL derived cell lines, and freshly isolated ATL cells. However, persistent Tax-induced NF-κB activation results in cellular senescence (53, 54, 96, 97), potentially offering a further explanation of the undetectable levels of Tax protein in vivo. Yet, recent studies suggested that the evasion from replicative senescence in HTLV-1 infected cells is achieved through reactivation of human telomerase (hTERT), and highlighted a role of Tax in the transcriptional activation of the hTERT promoter, but also in hTERT enzymatic activity, through Tax-mediated NF-κB activation (98, 99).
Tax also induces genomic instability through inhibition of cell cycle checkpoints (100–102), DNA repair mechanisms (103, 104), induction of chromosome instability (105) and aneuploidy (35, 106). Besides, Tax functionally inactivates p53 (107), and inhibits p53-induced apoptosis via cytoplasmic sequestration of CBP/p300 (108). Altogether, these Tax-mediated cellular phenomena result in increased proliferation and accumulation of somatic mutations due to profound genomic instability.
Finally, Tax oncogenic capacity is well recognized, as its sole expression transforms T cells in vitro, induces leukemia in transgenic mice (109–115) and transformation in Drosophila transgenic flies (116). Nevertheless, primary ATL cells display most properties of Tax expressing cells (117), and carry somatic mutations mimicking Tax cellular effects, in particular mutations targeting the T-cell receptor and the NF-κB pathways (106, 118).
The interplay between HTLV-1 and the innate immune system was well studied (For a review (119). In the cytoplasm of infected cells, HTLV-1 viral RNA carrying 5-triphosphate is detected by the pattern recognition receptor-1 (RIG-I), culminating in the transcription of the interferon response factor-3 (IRF3) (119). This triggers the activation of the interferon anti-viral response. HTLV-1 can also infect dendritic cells (DCs), which are the foremost producers of type I interferon (120, 121). Cell–cell HTLV-1 infection induces type-I IFN production in plasmacytoid DCs (122). Furthermore, in de novo infection with cell-free HTLV-1, pDCs or monocytes produce type I IFN through TLR7 or STING signaling pathways, seemingly recognizing HTLV-1 RNA or its reverse transcribed intermediate DNA (123, 124). To counteract this response, HTLV-1 induces the expression of the suppressor of cytokine signaling gene SOCS1. Indeed, Tax interacts with and stabilizes SOCS1, an inhibitor of interferon signaling to inhibit RIG-I-dependent antiviral signaling and hijacking anti-viral IFN signaling (125). Another effect of Tax counterpoising type I IFN responses was also described. Indeed, Tax suppresses the TBK1 kinase which phosphorylates IRF3 impairing the production of type I IFN (126).
As previously mentioned, Tax protein levels are undetectable in vivo (49, 66, 68, 127). Several mechanisms were proposed to explain this finding. Tax expression triggers a strong CTL response (128, 129) and HTLV-1 infected cells and ATL cells frequently reduce the expression of Tax, to evade this CTL-mediated lysis and maintain the in vivo viral persistence (64, 130–134). In addition to Tax specific CTLs, anti-Tax antibodies are reported in ATL patients, pointing to the expression of the protein in vivo, even if at undetectable levels (135). Moreover, donor derived anti-Tax CTL were described following allogeneic hematopoietic cell transplantation for ATL (136). Prominently, the efficacy of a Tax peptide-pulsed dendritic cell vaccine in treating Tax-positive ATL patients was highlighted, further capitalizing on in vivo expression of Tax (137).
More recently, the role of Tax in modulating three members of the Pim serine/threonine kinases to enhance survival and inhibit apoptosis, was elucidated. Indeed, Tax increased Pim-1 and Pim-3 expression and decreased Pim-2 expression, while the three members of Pim family bind Tax, to lessen its expression in response to increased CTL responses. This feedback regulatory loop between the viral and cellular proteins suggests a potential modulation by Pim kinases of the immune escape of HTLV-1-infected cells, through partial suppression of the host immunogenic responses favoring the persistence of the virally-infected cells (138).
Finally, targeted therapies against Tax led to selective growth arrest and apoptosis in vitro and in vivo. In that sense, treatment with arsenic trioxide (AS) and interferon-alpha (IFN), which induces Tax proteasomal degradation, resulted in selective cell death of ATL cells, eradicated murine ATL through abrogating the activity of ATL leukemia initiating cells (LIC), and ensured long-lasting responses in ATL patients (See section below) (31, 58, 109, 139–142).
Finally, Tax-mediated constitutive activation of the NF-κB pathway results in a significant expression in cytokines and their receptors (43, 90, 117, 143, 144), notably Interleukin IL-6/IL6R, IL-2/IL2R, IL-9, IL-15, IL-13, interferon-γ (IFN-γ), tumor necrosis factor-beta (TNF-β), and the chemokine (C-C motif) ligand 2 (CCL2), which contribute to inhibition of apoptosis and enhanced survival of HTLV-1 infected cells (145, 146).
HBZ, a bZIP nuclear factor, is encoded by the minus-strand of the HTLV-1 provirus (39, 64, 147). HBZ transcription occurs at the 3’LTR promoter, generating two transcripts, the spliced sHBZ and the unspliced usHBZ transcripts (64). The expression of sHBZ is four times higher than that of unHBZ in both HTLV-1 infected and ATL cells (148). Unlike Tax, HBZ is persistently expressed in vivo, but at a low level (50). This might be due to the absence of DNA methylation, the intact 3’ LTR promoter, and the lack of abortive mutations in hbz gene. In spite of the low expression levels and the low T cell immunogenicity (149), an effective CTL response to HBZ correlates with a low proviral load in vivo (56, 149, 150). Furthermore, the localization of HBZ differs according to different HTLV-1 associated diseases. While HBZ is exclusively localized in the cytoplasm of HTLV-1 asymptomatic carriers and HAM/TSP patients, it exhibits a nuclear localization in ATL cell lines. In ATL patients, HBZ localizes to the cytoplasm and the nucleus of cells irrespective of the clinical status, but with a pronounced preference for the cytoplasmic localization, suggesting a role of HBZ cytoplasmic/nuclear translocation in HTLV-1 oncogenesis (52).
HBZ belongs to the basic leucine zipper protein class. As such, it controls the DNA binding or transcriptional activities of CREB-2, JunB, and c-Jun (AP-1) (134). By binding CREB-2, HBZ bZIP interacts with CREB/CREB-2, preventing it from binding to Tax-responsive element (TRE) and CRE, hence inhibiting Tax-mediated HTLV-1 transcription from the 5’LTR (64, 151). HBZ also induces T-cell proliferation through interaction with the activator protein 1 (AP1) superfamily proteins, mostly JunD (152). HBZ/JunD heterodimer enhances the transcription of the human telomerase reverse transcriptase (hTERT), which may promote cell proliferation (152). HBZ also inhibits the canonical Wnt pathway, which is deleterious for ATL development, and upregulates the transcription of Wnt5a, promoting the proliferation of ATL cells (153). Importantly, HBZ knock-down (50) or knock-out (154) impede cell proliferation (155).
At the functional level, HBZ is almost as pleiotropic as Tax (156), and many HBZ functions oppose Tax-induced cellular effects (Table 1). Precisely, HBZ inhibits Tax-mediated transcriptional activation of CREB, AP-1, NF-κB, and Wnt (157, 158). In addition, HBZ inhibits the canonical NF-κB pathway (157), alleviating Tax-induced cellular senescence (97). In an in vivo Drosophila melanogaster fly model, HBZ expression failed to activate NF-κB or to induce transformation or senescence, yet HBZ successfully activated epigenetic core components leading to consequent epigenetic changes (53). Strikingly, HBZ expression in tax transgenic flies prohibited Tax-induced NF-κB activation, preventing both malignant proliferation and senescence (53).
HBZ induces the expression of CCR4 to promote cell migration and proliferation of HTLV-1-infected cells (159). As previously mentioned, Tax-expressing cells constitute a major target of CTL in vivo (160, 161), due to the elevated immunogenic properties of Tax. In contrast, HBZ is less immunogenic than Tax and anti-HBZ antibodies are rarely detected in infected patients (156). As such, through the continuous expression of HBZ, which offsets Tax expression (162), HTLV-1 infected cells lessen Tax expression to evade the host immune response (24, 57).
Despite its low immunogenicity, HBZ can induce inflammation. Indeed, the vast majority of hbz-transgenic mice develop a spontaneous systemic inflammatory disease (163). Interestingly, HBZ also stimulates the TGF-β/Smad pathway, upregulates Foxp3 expression, hence converting the T cell population into Tregs (164), to reduce the immune response (165). Likewise, HBZ promotes the secretion of IFN-ɣ in hbz transgenic mice, highlighting the role of HBZ in the induction of inflammation (166). Moreover, HBZ impairs cell-mediated immunity in hbz transgenic mice which fail to mount an optimal Th1 immune response upon challenge with Listeria monocytogenes or herpes simplex virus (150). In hbz transgenic flies, HBZ expression failed to activate NF-κB, a key pathway in the activation of the immune response (53). Indeed, HBZ attenuates the canonical NF-κB pathway, decreasing the expression of genes associated with innate immunity and inflammatory responses (157). Remarkably, HBZ totally abrogates Tax-activated canonical NF-κB, enabling cells to escape senescence and to proliferate incessantly (53, 167). HBZ also affects the transcription of several NF-κB target genes such as IL-8, IL-2RA, VEGF, CCND1, VCAM-1, and IRF4 (39, 168).
Interleukin-10 is an immunosuppressive cytokine exhibiting high levels in ATL patients and leading to an immunosuppressive profile (31, 169). IL-10 plays a role in the proliferative capacity of ATL cells through its downstream activation of STAT3 signaling (59). Recently, IL-10 was shown to be chiefly produced by the CD25+ cells (58), and a critical role of Tax in its production was depicted. Indeed, silencing Tax in HTLV-1 transformed or ATL derived cell lines abrogated IL-10 levels in these cells (58). Other cells and/or factors may also contribute to elevated IL-10 levels. Indeed, T helper cells, Tregs, monocytes, macrophages, and dendritic cells may produce IL-10. Moreover, the microbiome in HTLV-1 infected patients may contribute to these elevated IL-10 levels. In that sense, the predominant association of Strongyloides stercoralis with ATL may induce IL-10 and TGF-β (170). HBZ also modulates IL-10, through induction of expression and induced-promoter acetylation levels of TIGIT, Foxp3 and CCR4 (171). Moreover, the prolonged IFN activation by persistent viral infection can lead to an IL-10-predominant cytokine imbalance (172, 173).
ATL management remains intricate, after more than four decades of research. Attempts to tackle ATL by targeting leukemic cells with chemotherapy and monoclonal antibodies, without targeting HTLV-1, have failed [reviewed in (45)]. Despite slight improved outcomes with chemotherapy in newly diagnosed aggressive ATL, particularly the lymphoma subtype (174, 175), chemotherapy alone exhibits only a minimal effect on long-term survival, specifically in the acute subtype (11, 176). Allogeneic hematopoietic cell transplantation (HCT) is used in ATL (Iqbal et al., 2019), and improves the long-term survival in around one third of transplanted patients (177, 178). Yet, less that 10% of ATL patients can make it to transplant and hence the cure options using this approach do not exceed 5% of ATL patients (Hishizawa et al., 2010; Bazarbachi et al., 2014).
Since ATL is secondary to HTLV-1 infection, the combination of two antiviral agents, AZT and IFN was investigated in ATL. High response rates using this combination were achieved in newly diagnosed and relapsed ATL patients (10, 175, 176, 179–188). The smoldering and chronic subtypes benefited most from AZT/IFN which became the standard treatment of indolent ATL in most parts of the world (10, 175, 176, 180, 185, 189, 190). At the molecular level, AZT/IFN inhibits the reverse transcriptase activity and modifies the clonality pattern in responding ATL patients (191–193). Despite this clinical improvement, AZT/IFN was not curative and patients with acute and lymphoma ATL remained a population with unmet medical need.
Due to the importance of the host immune responses and the host microenvironment, in the progression of ATL, immunotherapy using monoclonal antibodies (mAb) and immune-modulatory drugs was investigated (10, 11, 194, 195). Tested mAbs mostly targeted CCR4, CD25, CD30, CD52 and the surface transferrin receptor (196–198). The humanized antibody mogamulizumab, targeting CCR4 expressed on ATL cells (197), was tested and phase I/II clinical trials proved its efficacy in patients with relapsed/refractory CCR4+ ATL (199). In newly diagnosed ATL patients, mogamulizumab combined with dose-intensified chemotherapy improved response rates in the peripheral blood, but failed to improve progression free survival or overall survival (200).
The efficacy of an anti-CD25 antibody, targeting CD25 highly expressed on ATL cells yielded some clinical response in indolent ATL (198). A24 mAb directed against the surface transferrin receptor induced apoptosis of ATL cell lines or primary ATL cells in vitro (201, 202) Alemtuzumab (Campath-1H), a chimeric humanized antibody that binds to the CD52 glycoprotein, led to promising, but short overall response rates in acute, chronic and lymphoma ATL (203). The anti-PD-1 antibody, nivolumab, was also investigated in several phase I/II clinical trials but unfortunately led to a rapid progression of ATL (204). Finally, the immunomodulatory drug, lenalidomide exhibited a significant anti-leukemic activity, in relapsed/recurrent ATL (205). Recently, low dose lenalidomide was proposed as a maintenance therapy of ATL, and resulted in continuous complete remission in a patient with acute ATL lasting more than 24 months (195). Finally, the anti-CD30 monoclonal antibody brentuximab vedotin (BV), used in several clinical trials including patients with relapsed/refractory CD30+ ATL patients, yielded promising results (196, 206).
The key role of Tax and HBZ in ATL development and maintenance of the leukemic phenotype highlights the potential importance of ATL therapeutic approaches directly targeting these viral proteins or indirectly targeting their downstream cellular targets or inducing antiviral immunity. In that sense, the combination of arsenic trioxide (AS) and interferon-α (IFN) selectively induced cell cycle arrest and apoptosis of ATL cells in vitro (139). This was associated with a reversal of the constitutive activation of NF-κB and delayed shut down of cell cycle-regulated genes secondary to proteoasomal-mediated Tax degradation (207–209). In vivo, AS/IFN cured Tax-driven murine ATL through leukemia initiating cell (LIC) eradication (109). AS/IFN-induced abolition of ATL LIC activity required IL-10 expression shutoff. Indeed, loss of IL-10 secretion by ATL cells, triggered the production of inflammatory cytokines by the innate immune microenvironment, namely NK cells and macrophages, hence mediating the clearance of ATL cells. Strikingly, anti-IL-10 monoclonal antibodies significantly increased the efficiency of AS/IFN therapy (58), and treatment of murine ATL with the triple combination of AS/IFN/anti-IL-10 monoclonal antibody cured 80% of mice and significantly decreased LIC activity in serial transplantation assays (58). Overall, these results highlight the potential dual targeting of malignant ATL cells and their immune microenvironment and provide a strong rational to test the therapeutic effect of this triple combination in ATL patients.
The importance of such a dual targeting of viral oncoproteins and the immune microenvironment, was further strengthened by vaccination approaches against Tax, HBZ or both. A Tax peptide-pulsed dendritic cell (DC) vaccine, designed to augment Tax-specific CTL response, led to favorable clinical outcomes in a pilot clinical trial (137), and two patients survived for more than 4 years after vaccination (136). A recombinant vaccinia virus (rVV) that induced an HBZ-specific T-cell response, improved the survival of HBZ-induced lymphoma-challenged mice (210). And finally, THV02, comprising two lentiviral vectors encoding for a peptide deriving from the viral proteins Tax, HBZ, p12I and p30II, and to be used in a prime/boost regimen, induced a promising cellular response in animal models (Hermine et al. personal communication).
ATL is a virally-driven malignancy that associates with dismal prognosis. The clinical management of ATL remains difficult, partially due to the incomplete understanding of the intimate relationship between HTLV-1 and its induced forefront immune response. Indeed, the intricacy of disease mechanisms following HTLV-1 infection is a consequence of the interplay between the host immune responses in concert with HTLV-1 proteins including Tax and HBZ. Despite the intensive literature on the plethora of functions of these viral oncoproteins, Tax and HBZ fail to induce and sustain proliferation of malignant ATL cells without IL-10 (Figure 1). Both Tax and HBZ upregulate IL-10 production, inducing proliferation of HTLV-1-infected cells. This effect, along with the anti-inflammatory and immunosuppressive properties of IL-10 may play a key role in switching HTLV-1 induced inflammation towards ATL. The tremendously low but not silent levels of Tax protein expression in ATL patients and the efficacy of Tax-targeted therapeutic vaccine in ATL patients highlight the impact of Tax-specific CTLs on immune surveillance of HTLV-1 infected and ATL cells. Moreover, targeted therapies leading to Tax degradation proved selective and potent efficacy against ATL cells in vitro and in vivo. Murine preclinical models of ATL highpoint the importance of the dual targeting of the innate immune microenvironment and the viral oncoproteins. Adding pieces to the intriguing puzzle of host immunity/HTLV-1 infection is required, and future studies should include therapies that target the main driver of ATL, the HTLV-1 virus (Figure 1). These therapeutic options may target the viral proteins, their downstream cellular targets, along with the host immune microenvironment including HTLV-1 infected non-malignant cells.
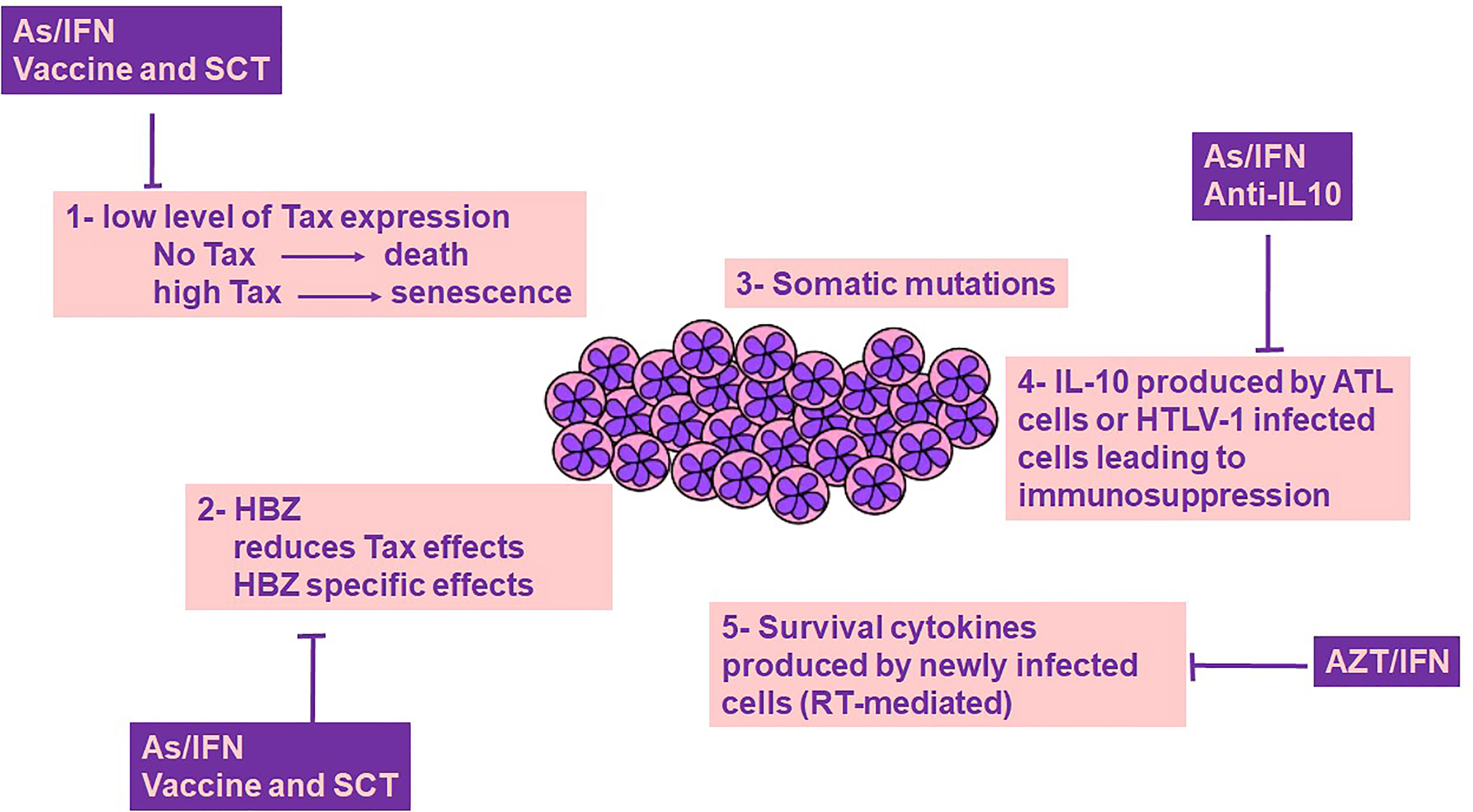
Figure 1 ATL cells survival: a cross-talk between genetics, viral proteins and immune-microenvironment. Survival of ATL cells requires Tax expression, yet Tax is highly immunogenic, and its expression at high levels drives senescence, a cellular fate counterbalanced by HBZ. Tax induced genetic instability results in the accumulation of somatic mutations. Both Tax and HBZ promote IL-10 expression, a key cytokine contributing to ATL cell survival and host immunosuppression. Newly infected T cells produce cytokines that contribute to the survival of ATL cells. The role of Tax/HBZ and IL-10 in ATL leukemogenesis highlights the importance of dual targeted therapies including anti-viral therapies and targeted therapies against viral oncoproteins and IL-10, as a promising curative avenue for ATL. AZT/IFN, Zidovudine and Interferon-alpha; As/IFN, Arsenic trioxide and Interferon-alpha; SCT, Stem Cell Transplantation; IL-10, Interleukin-10; RT, Reverse transcriptase.
HEH and AB equally contributed in writing this review. All authors read and approved the final manuscript.
The authors declare this review was written in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Poiesz BJ, Ruscetti FW, Reitz MS, Kalyanaraman VS, Gallo RC. Isolation of a new type c retrovirus (HTLV) in primary uncultured cells of a patient with sezary T-cell leukaemia. Nature (1981) 294:268–71. doi: 10.1038/294268a0
2. Afonso PV, Cassar O, Gessain A. Molecular epidemiology, genetic variability and evolution of HTLV-1 with special emphasis on African genotypes. Retrovirology (2019) 16:39. doi: 10.1186/s12977-019-0504-z
3. Gessain A. [Human retrovirus HTLV-1: descriptive and molecular epidemiology, origin, evolution, diagnosis and associated diseases]. Bull Soc Pathol Exot (2011) 104:167–80. doi: 10.1007/s13149-011-0174-4
4. Rafatpanah H, Hedayati-Moghaddam MR, Fathimoghadam F, Bidkhori HR, Shamsian SK, Ahmadi S, et al. High prevalence of HTLV-I infection in mashhad, northeast Iran: a population-based seroepidemiology survey. J Clin Virol (2011) 52:172–6. doi: 10.1016/j.jcv.2011.07.004
5. Ablain J, Nasr R, Bazarbachi A, de The H. The drug-induced degradation of oncoproteins: an unexpected achilles' heel of cancer cells? Cancer Discov (2011) 1:117–27. doi: 10.1158/2159-8290.CD-11-0087
6. Gessain A, Cassar O. Epidemiological aspects and world distribution of HTLV-1 infection. Front Microbiol (2012) 3:388. doi: 10.3389/fmicb.2012.00388
7. Gessain A, Francis H, Sonan T, Giordano C, Akani F, Piquemal M, et al. HTLV-I and tropical spastic paraparesis in Africa. Lancet (1986) 2:698. doi: 10.1016/S0140-6736(86)90218-7
8. Osame M, Igata A, Matsumoto M, Tara M. [HTLV-i-associated myelopathy]. Gan To Kagaku Ryoho (1987) 14:2411–6.
9. Takatsuki K, Uchiyama T, Sagawa K, Yodoi J. [Surface markers of malignant lymphoid cells in the classification of lymphoproliferative disorders, with special reference to adult T-cell leukemia (author's transl)]. Rinsho Ketsueki (1976) 17:416–21.
10. El Hajj H, Tsukasaki K, Cheminant M, Bazarbachi A, Watanabe T, Hermine O. Novel treatments of adult T cell leukemia lymphoma. Front Microbiol (2020) 11:1062. doi: 10.3389/fmicb.2020.01062
11. Tsukasaki K, Marcais A, Nasr R, Kato K, Fukuda T, Hermine O, et al. Diagnostic approaches and established treatments for adult T cell leukemia lymphoma. Front Microbiol (2020) 11:1207. doi: 10.3389/fmicb.2020.01207
12. Wattel E, Vartanian JP, Pannetier C, Wain-Hobson S. Clonal expansion of human T-cell leukemia virus type I-infected cells in asymptomatic and symptomatic carriers without malignancy. J Virol (1995) 69:2863–8. doi: 10.1128/jvi.69.5.2863-2868.1995
13. Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. a report from the lymphoma study group (1984-87). Br J Haematol (1991) 79:428–37. doi: 10.1111/j.1365-2141.1991.tb08051.x
14. Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol (2008) 26:4124–30. doi: 10.1200/JCO.2008.16.4558
15. Katsuya H, Ishitsuka K, Utsunomiya A, Hanada S, Eto T, Moriuchi Y, et al. Treatment and survival among 1594 patients with ATL. Blood (2015) 126:2570–7. doi: 10.1182/blood-2015-03-632489
16. Ueda N, Iwata K, Tokuoka H, Akagi T, Ito J, Mizushima M. Adult T-cell leukemia with generalized cytomegalic inclusion disease and pneumocystis carinii pneumonia. Acta Pathol Jpn (1979) 29:221–32. doi: 10.1111/j.1440-1827.1979.tb03176.x
17. Blayney DW, Jaffe ES, Blattner WA, Cossman J, Robert-Guroff M, Longo DL, et al. The human T-cell leukemia/lymphoma virus associated with American adult T-cell leukemia/lymphoma. Blood (1983) 62:401–5. doi: 10.1182/blood.V62.2.401.401
18. Verdonck K, Gonzalez E, Van Dooren S, Vandamme AM, Vanham G, Gotuzzo E. Human T-lymphotropic virus 1: recent knowledge about an ancient infection. Lancet Infect Dis (2007) 7:266–81. doi: 10.1016/S1473-3099(07)70081-6
19. Gotuzzo E, Terashima A, Alvarez H, Tello R, Infante R, Watts DM, et al. Strongyloides stercoralis hyperinfection associated with human T cell lymphotropic virus type-1 infection in Peru. Am J Trop Med Hyg (1999) 60:146–9. doi: 10.4269/ajtmh.1999.60.146
20. LaGrenade L, Hanchard B, Fletcher V, Cranston B, Blattner W. Infective dermatitis of Jamaican children: a marker for HTLV-I infection. Lancet (1990) 336:1345–7. doi: 10.1016/0140-6736(90)92896-P
21. Bittencourt AL, de Oliveira Mde F. Cutaneous manifestations associated with HTLV-1 infection. Int J Dermatol (2010) 49:1099–110. doi: 10.1111/j.1365-4632.2010.04568.x
22. Einsiedel L, Cassar O, Spelman T, Joseph S, Gessain A. Higher HTLV-1c proviral loads are associated with blood stream infections in an indigenous Australian population. J Clin Virol (2016) 78:93–8. doi: 10.1016/j.jcv.2016.03.006
23. Kannagi M, Hasegawa A, Nagano Y, Kimpara S, Suehiro Y. Impact of host immunity on HTLV-1 pathogenesis: potential of tax-targeted immunotherapy against ATL. Retrovirology (2019) 16:23. doi: 10.1186/s12977-019-0484-z
24. Jacobson S, Shida H, McFarlin DE, Fauci AS, Koenig S. Circulating CD8+ cytotoxic T lymphocytes specific for HTLV-I pX in patients with HTLV-I associated neurological disease. Nature (1990) 348:245–8. doi: 10.1038/348245a0
25. Kannagi M, Matsushita S, Harada S. Expression of the target antigen for cytotoxic T lymphocytes on adult T-cell-leukemia cells. Int J Cancer (1993) 54:582–8. doi: 10.1002/ijc.2910540411
26. Kannagi M, Matsushita S, Shida H, Harada S. Cytotoxic T cell response and expression of the target antigen in HTLV-I infection. Leukemia (1994) 8 Suppl 1:S54–9.
27. Parker CE, Daenke S, Nightingale S, Bangham CR. Activated, HTLV-1-specific cytotoxic T-lymphocytes are found in healthy seropositives as well as in patients with tropical spastic paraparesis. Virology (1992) 188:628–36. doi: 10.1016/0042-6822(92)90517-S
28. Bangham CR, Osame M. Cellular immune response to HTLV-1. Oncogene (2005) 24:6035–46. doi: 10.1038/sj.onc.1208970
29. Kannagi M. [Immunological aspects of human retrovirus infection]. Nihon Rinsho (2005) 63 Suppl 4:508–16.
30. Kinpara S, Hasegawa A, Utsunomiya A, Nishitsuji H, Furukawa H, Masuda T, et al. Stromal cell-mediated suppression of human T-cell leukemia virus type 1 expression in vitro and in vivo by type I interferon. J Virol (2009) 83:5101–8. doi: 10.1128/JVI.02564-08
31. Kchour G, Rezaee R, Farid R, Ghantous A, Rafatpanah H, Tarhini M, et al. The combination of arsenic, interferon-alpha, and zidovudine restores an "immunocompetent-like" cytokine expression profile in patients with adult T-cell leukemia lymphoma. Retrovirology (2013) 10:91. doi: 10.1186/1742-4690-10-91
32. Neco H, Teixeira V, da Trindade ACL, Magalhaes PMR, de Lorena VMB, Castellano LRC, et al. Mediators go together: High production of CXCL9, CXCL10, IFN-gamma, and TNF-alpha in HTLV-1-Associated Myelopathy/Tropical spastic paraparesis. AIDS Res Hum Retroviruses (2017) 33:1134–9. doi: 10.1089/aid.2016.0296
33. de La-Roque DGL, Santos EV, Rodrigues ES, da Costa PNM, Brauer VS, Almeida F, et al. The expression of tax and HBZ genes in serum-derived extracellular vesicles from HTLV-1 carriers correlates to proviral load and inflammatory markers. Front Microbiol (2022) 13:881634. doi: 10.3389/fmicb.2022.881634
34. Bai XT, Nicot C. Overview on HTLV-1 p12, p8, p30, p13: accomplices in persistent infection and viral pathogenesis. Front Microbiol (2012) 3:400. doi: 10.3389/fmicb.2012.00400
35. Matsuoka M, Jeang KT. Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat Rev Cancer (2007) 7:270–80. doi: 10.1038/nrc2111
36. Edwards D, Fenizia C, Gold H, de Castro-Amarante MF, Buchmann C, Pise-Masison CA, et al. Orf-I and orf-II-encoded proteins in HTLV-1 infection and persistence. Viruses (2011) 3:861–85. doi: 10.3390/v3060861
37. Moles R, Sarkis S, Galli V, Omsland M, Artesi M, Bissa M, et al. NK cells and monocytes modulate primary HTLV-1 infection. PloS Pathog (2022) 18:e1010416. doi: 10.1371/journal.ppat.1010416
38. Matsuoka M, Jeang KT. Human T-cell leukemia virus type 1 (HTLV-1) and leukemic transformation: viral infectivity, tax, HBZ and therapy. Oncogene (2011) 30:1379–89. doi: 10.1038/onc.2010.537
39. Giam CZ, Semmes OJ. HTLV-1 infection and adult T-cell Leukemia/Lymphoma-a tale of two proteins: Tax and HBZ. Viruses (2016) 8(6):161. doi: 10.3390/v8060161
40. Kfoury Y, Nasr R, Journo C, Mahieux R, Pique C, Bazarbachi A. The multifaceted oncoprotein tax: subcellular localization, posttranslational modifications, and NF-κB activation. Adv Cancer Res (2012) 113:85–120. doi: 10.1016/B978-0-12-394280-7.00003-8
41. Shirinian M, Kfoury Y, Dassouki Z, El-Hajj H, Bazarbachi A. Tax-1 and tax-2 similarities and differences: focus on post-translational modifications and NF-κB activation. Front Microbiol (2013) 4:231. doi: 10.3389/fmicb.2013.00231
42. Kfoury Y, Nasr R, Hermine O, de The H, Bazarbachi A. Proapoptotic regimes for HTLV-i-transformed cells: targeting tax and the NF-kappaB pathway. Cell Death Differ (2005) 12 Suppl 1:871–7. doi: 10.1038/sj.cdd.4401624
43. Mohanty S, Harhaj EW. Mechanisms of oncogenesis by HTLV-1 tax. Pathogens (2020) 9(7):543. doi: 10.3390/pathogens9070543
44. Shirinian M, Kfoury Y, Dassouki Z, El-Hajj H, Bazarbachi A. Tax-1 and tax-2 similarities and differences: focus on post-translational modifications and NF-kappaB activation. Front Microbiol (2013) 4:231. doi: 10.3389/fmicb.2013.00231
45. Hleihel R, Akkouche A, Skayneh H, Hermine O, Bazarbachi A, El Hajj H. Adult T-cell leukemia: a comprehensive overview on current and promising treatment modalities. Curr Oncol Rep (2021) 23:141. doi: 10.1007/s11912-021-01138-3
46. Yoshida M. Multiple viral strategies of HTLV-1 for dysregulation of cell growth control. Annu Rev Immunol (2001) 19:475–96. doi: 10.1146/annurev.immunol.19.1.475
47. Matsuoka M, Yasunaga J. Human T-cell leukemia virus type 1: replication, proliferation and propagation by tax and HTLV-1 bZIP factor. Curr Opin Virol (2013) 3:684–91. doi: 10.1016/j.coviro.2013.08.010
48. Kurihara K, Harashima N, Hanabuchi S, Masuda M, Utsunomiya A, Tanosaki R, et al. Potential immunogenicity of adult T cell leukemia cells in vivo. Int J Cancer (2005) 114:257–67. doi: 10.1002/ijc.20737
49. Kinoshita T, Shimoyama M, Tobinai K, Ito M, Ito S, Ikeda S, et al. Detection of mRNA for the tax1/rex1 gene of human T-cell leukemia virus type I in fresh peripheral blood mononuclear cells of adult T-cell leukemia patients and viral carriers by using the polymerase chain reaction. Proc Natl Acad Sci USA. (1989) 86:5620–4. doi: 10.1073/pnas.86.14.5620
50. Satou Y, Yasunaga J, Yoshida M, Matsuoka M. HTLV-I basic leucine zipper factor gene mRNA supports proliferation of adult T cell leukemia cells. Proc Natl Acad Sci USA (2006) 103:720–5. doi: 10.1073/pnas.0507631103
51. Raval GU, Bidoia C, Forlani G, Tosi G, Gessain A, Accolla RS. Localization, quantification and interaction with host factors of endogenous HTLV-1 HBZ protein in infected cells and ATL. Retrovirology (2015) 12:59. doi: 10.1186/s12977-015-0186-0
52. Forlani G, Shallak M, Tedeschi A, Cavallari I, Marcais A, Hermine O, et al. Dual cytoplasmic and nuclear localization of HTLV-1-encoded HBZ protein is a unique feature of adult T-cell leukemia. Haematologica (2021) 106:2076–85. doi: 10.3324/haematol.2020.272468
53. Akkouche A, Moodad S, Hleihel R, Skayneh H, Chambeyron S, El Hajj H, et al. In vivo antagonistic role of the human T-cell leukemia virus type 1 regulatory proteins tax and HBZ. PloS Pathog (2021) 17:e1009219. doi: 10.1371/journal.ppat.1009219
54. Kuo YL, Giam CZ. Activation of the anaphase promoting complex by HTLV-1 tax leads to senescence. EMBO J (2006) 25:1741–52. doi: 10.1038/sj.emboj.7601054
55. Ho YK, Zhi H, DeBiaso D, Philip S, Shih HM, Giam CZ. HTLV-1 tax-induced rapid senescence is driven by the transcriptional activity of NF-kappaB and depends on chronically activated IKKalpha and p65/RelA. J Virol (2012) 86:9474–83. doi: 10.1128/JVI.00158-12
56. Hilburn S, Rowan A, Demontis MA, MacNamara A, Asquith B, Bangham CR, et al. In vivo expression of human T-lymphotropic virus type 1 basic leucine-zipper protein generates specific CD8+ and CD4+ T-lymphocyte responses that correlate with clinical outcome. J Infect Dis (2011) 203:529–36. doi: 10.1093/infdis/jiq078
57. Kannagi M, Harada S, Maruyama I, Inoko H, Igarashi H, Kuwashima G, et al. Predominant recognition of human T cell leukemia virus type I (HTLV-I) pX gene products by human CD8+ cytotoxic T cells directed against HTLV-i-infected cells. Int Immunol (1991) 3:761–7. doi: 10.1093/intimm/3.8.761
58. El Hajj H, Hleihel R, El Sabban M, Bruneau J, Zaatari G, Cheminant M, et al. Loss of interleukin-10 activates innate immunity to eradicate adult T-cell leukemia-initiating cells. Haematologica (2021) 106:1443–56. doi: 10.3324/haematol.2020.264523
59. Sawada L, Nagano Y, Hasegawa A, Kanai H, Nogami K, Ito S, et al. IL-10-mediated signals act as a switch for lymphoproliferation in human T-cell leukemia virus type-1 infection by activating the STAT3 and IRF4 pathways. PloS Pathog (2017) 13:e1006597. doi: 10.1371/journal.ppat.1006597
60. Mori N, Gill PS, Mougdil T, Murakami S, Eto S, Prager D. Interleukin-10 gene expression in adult T-cell leukemia. Blood (1996) 88:1035–45. doi: 10.1182/blood.V88.3.1035.bloodjournal8831035
61. Kim SJ, Kehrl JH, Burton J, Tendler CL, Jeang KT, Danielpour D, et al. Transactivation of the transforming growth factor beta 1 (TGF-beta 1) gene by human T lymphotropic virus type 1 tax: a potential mechanism for the increased production of TGF-beta 1 in adult T cell leukemia. J Exp Med (1990) 172:121–9. doi: 10.1084/jem.172.1.121
62. Mori N, Morishita M, Tsukazaki T, Giam CZ, Kumatori A, Tanaka Y, et al. Human T-cell leukemia virus type I oncoprotein tax represses smad-dependent transforming growth factor beta signaling through interaction with CREB-binding protein/p300. Blood (2001) 97:2137–44. doi: 10.1182/blood.V97.7.2137
63. Zhao T, Satou Y, Sugata K, Miyazato P, Green PL, Imamura T, et al. HTLV-1 bZIP factor enhances TGF-beta signaling through p300 coactivator. Blood (2011) 118:1865–76. doi: 10.1182/blood-2010-12-326199
64. Gaudray G, Gachon F, Basbous J, Biard-Piechaczyk M, Devaux C, Mesnard JM. The complementary strand of the human T-cell leukemia virus type 1 RNA genome encodes a bZIP transcription factor that down-regulates viral transcription. J Virol (2002) 76:12813–22. doi: 10.1128/JVI.76.24.12813-12822.2002
65. Mortreux F, Leclercq I, Gabet AS, Leroy A, Westhof E, Gessain A, et al. Somatic mutation in human T-cell leukemia virus type 1 provirus and flanking cellular sequences during clonal expansion in vivo. J Natl Cancer Inst (2001) 93:367–77. doi: 10.1093/jnci/93.5.367
66. Franchini G, Wong-Staal F, Gallo RC. Human T-cell leukemia virus (HTLV-I) transcripts in fresh and cultured cells of patients with adult T-cell leukemia. Proc Natl Acad Sci USA (1984) 81:6207–11. doi: 10.1073/pnas.81.19.6207/
67. Masaki A, Ishida T, Suzuki S, Ito A, Narita T, Kinoshita S, et al. Human T-cell lymphotropic/leukemia virus type 1 (HTLV-1) tax-specific T-cell exhaustion in HTLV-1-infected individuals. Cancer Sci (2018) 109:2383–90. doi: 10.1111/cas.13654
68. Gessain A, Louie A, Gout O, Gallo RC, Franchini G. Human T-cell leukemia-lymphoma virus type I (HTLV-I) expression in fresh peripheral blood mononuclear cells from patients with tropical spastic paraparesis/HTLV-i-associated myelopathy. J Virol (1991) 65:1628–33. doi: 10.1128/jvi.65.3.1628-1633.1991
69. Yamano Y, Nagai M, Brennan M, Mora CA, Soldan SS, Tomaru U, et al. Correlation of human T-cell lymphotropic virus type 1 (HTLV-1) mRNA with proviral DNA load, virus-specific CD8(+) T cells, and disease severity in HTLV-1-associated myelopathy (HAM/TSP). Blood (2002) 99:88–94. doi: 10.1182/blood.V99.1.88
70. Goon PK, Biancardi A, Fast N, Igakura T, Hanon E, Mosley AJ, et al. Human T cell lymphotropic virus (HTLV) type-1-specific CD8+ T cells: frequency and immunodominance hierarchy. J Infect Dis (2004) 189:2294–8. doi: 10.1086/420832
71. Goon PK, Igakura T, Hanon E, Mosley AJ, Barfield A, Barnard AL, et al. Human T cell lymphotropic virus type I (HTLV-i)-specific CD4+ T cells: immunodominance hierarchy and preferential infection with HTLV-I. J Immunol (2004) 172:1735–43. doi: 10.4049/jimmunol.172.3.1735
72. Dassouki Z, Sahin U, El Hajj H, Jollivet F, Kfoury Y, Lallemand-Breitenbach V, et al. ATL response to arsenic/interferon therapy is triggered by SUMO/PML/RNF4-dependent tax degradation. Blood (2015) 125:474–82. doi: 10.1182/blood-2014-04-572750
73. Mahgoub M, Yasunaga JI, Iwami S, Nakaoka S, Koizumi Y, Shimura K, et al. Sporadic on/off switching of HTLV-1 tax expression is crucial to maintain the whole population of virus-induced leukemic cells. Proc Natl Acad Sci USA (2018) 115:E1269–78. doi: 10.1073/pnas.1715724115
74. Currer R, Van Duyne R, Jaworski E, Guendel I, Sampey G, Das R, et al. HTLV tax: a fascinating multifunctional co-regulator of viral and cellular pathways. Front Microbiol (2012) 3:406. doi: 10.3389/fmicb.2012.00406
75. Fujikawa D, Nakagawa S, Hori M, Kurokawa N, Soejima A, Nakano K, et al. Polycomb-dependent epigenetic landscape in adult T-cell leukemia. Blood (2016) 127:1790–802. doi: 10.1182/blood-2015-08-662593
76. Yamagishi M, Watanabe T. miRNA in HTLV-1 related disease. Uirusu (2012) 62:9–18. doi: 10.2222/jsv.62.9
77. Bellon M, Lepelletier Y, Hermine O, Nicot C. Deregulation of microRNA involved in hematopoiesis and the immune response in HTLV-I adult T-cell leukemia. Blood (2009) 113:4914–7. doi: 10.1182/blood-2008-11-189845
78. Fochi S, Ciminale V, Trabetti E, Bertazzoni U, D'Agostino DM, Zipeto D, et al. NF-κB and MicroRNA deregulation mediated by HTLV-1 tax and HBZ. Pathogens (2019) 8(4):290. doi: 10.3390/pathogens8040290
79. Yeung ML, Yasunaga J, Bennasser Y, Dusetti N, Harris D, Ahmad N, et al. Roles for microRNAs, miR-93 and miR-130b, and tumor protein 53-induced nuclear protein 1 tumor suppressor in cell growth dysregulation by human T-cell lymphotrophic virus 1. Cancer Res (2008) 68:8976–85. doi: 10.1158/0008-5472.CAN-08-0769
80. El-Sabban ME, Merhi RA, Haidar HA, Arnulf B, Khoury H, Basbous J, et al. Human T-cell lymphotropic virus type 1-transformed cells induce angiogenesis and establish functional gap junctions with endothelial cells. Blood (2002) 99:3383–9. doi: 10.1182/blood.V99.9.3383
81. Bazarbachi A, Abou Merhi R, Gessain A, Talhouk R, El-Khoury H, Nasr R, et al. Human T-cell lymphotropic virus type I-infected cells extravasate through the endothelial barrier by a local angiogenesis-like mechanism. Cancer Res (2004) 64:2039–46. doi: 10.1158/0008-5472.CAN-03-2390
82. Hleihel R, Khoshnood B, Dacklin I, Omran H, Mouawad C, Dassouki Z, et al. The HTLV-1 oncoprotein tax is modified by the ubiquitin related modifier 1 (Urm1). Retrovirology (2018) 15:33. doi: 10.1186/s12977-018-0415-4
83. Kfoury Y, Setterblad N, El-Sabban M, Zamborlini A, Dassouki Z, El Hajj H, et al. Tax ubiquitylation and SUMOylation control the dynamic shuttling of tax and NEMO between Ubc9 nuclear bodies and the centrosome. Retrovirology (2011) 8:A146. doi: 10.1182/blood-2010-05-285742
84. Nasr R, Chiari E, El-Sabban M, Mahieux R, Kfoury Y, Abdulhay M, et al. Tax ubiquitylation and sumoylation control critical cytoplasmic and nuclear steps of NF-kappaB activation. Blood (2006) 107:4021–9. doi: 10.1182/blood-2005-09-3572
85. Lamsoul I, Lodewick J, Lebrun S, Brasseur R, Burny A, Gaynor RB, et al. Exclusive ubiquitination and sumoylation on overlapping lysine residues mediate NF-kappaB activation by the human T-cell leukemia virus tax oncoprotein. Mol Cell Biol (2005) 25:10391–406. doi: 10.1128/MCB.25.23.10391-10406.2005
86. Duyao MP, Kessler DJ, Spicer DB, Bartholomew C, Cleveland JL, Siekevitz M, et al. Transactivation of the c-myc promoter by human T cell leukemia virus type 1 tax is mediated by NF kappa b. J Biol Chem (1992) 267:16288–91. doi: 10.1016/S0021-9258(18)41998-9
87. Li M, Siekevitz M. A cis element required for induction of the interleukin 2 enhancer by human T-cell leukemia virus type I binds a novel tax-inducible nuclear protein. Mol Cell Biol (1993) 13:6490–500. doi: 10.1128/mcb.13.10.6490-6500.1993
88. Bohnlein E, Siekevitz M, Ballard DW, Lowenthal JW, Rimsky L, Bogerd H, et al. Stimulation of the human immunodeficiency virus type 1 enhancer by the human T-cell leukemia virus type I tax gene product involves the action of inducible cellular proteins. J Virol (1989) 63:1578–86. doi: 10.1128/jvi.63.4.1578-1586.1989
89. Beraud C, Sun SC, Ganchi P, Ballard DW, Greene WC. Human T-cell leukemia virus type I tax associates with and is negatively regulated by the NF-kappa B2 p100 gene product: implications for viral latency. Mol Cell Biol (1994) 14:1374–82. doi: 10.1128/mcb.14.2.1374-1382.1994
90. Sun SC, Elwood J, Beraud C, Greene WC. Human T-cell leukemia virus type I tax activation of NF-kappa B/Rel involves phosphorylation and degradation of I kappa b alpha and RelA (p65)-mediated induction of the c-rel gene. Mol Cell Biol (1994) 14:7377–84. doi: 10.1128/mcb.14.11.7377-7384.1994
91. Mori N, Shirakawa F, Saito K, Murakami S, Oda S, Eto S. Transactivation of the interleukin-1 alpha gene promoter by human T-cell leukemia virus type I tax in T cells. Blood (1994) 84:1688–9. doi: 10.1182/blood.V84.5.1688.1688
92. Harhaj NS, Sun SC, Harhaj EW. Activation of NF-kappa b by the human T cell leukemia virus type I tax oncoprotein is associated with ubiquitin-dependent relocalization of I kappa b kinase. J Biol Chem (2007) 282:4185–92. doi: 10.1074/jbc.M611031200
93. Hirai H, Fujisawa J, Suzuki T, Ueda K, Muramatsu M, Tsuboi A, et al. Transcriptional activator tax of HTLV-1 binds to the NF-kappa b precursor p105. Oncogene (1992) 7:1737–42.
94. Kfoury Y, Nasr R, Favre-Bonvin A, El-Sabban M, Renault N, Giron ML, et al. Ubiquitylated tax targets and binds the IKK signalosome at the centrosome. Oncogene (2008) 27:1665–76. doi: 10.1038/sj.onc.1210804
95. Kfoury Y, Setterblad N, El-Sabban M, Zamborlini A, Dassouki Z, El Hajj H, et al. Tax ubiquitylation and SUMOylation control the dynamic shuttling of tax and NEMO between Ubc9 nuclear bodies and the centrosome. Blood (2011) 117:190–9. doi: 10.1182/blood-2010-05-285742
96. Yang L, Kotomura N, Ho YK, Zhi H, Bixler S, Schell MJ, et al. Complex cell cycle abnormalities caused by human T-lymphotropic virus type 1 tax. J Virol (2011) 85:3001–9. doi: 10.1128/JVI.00086-10
97. Zhi H, Yang L, Kuo YL, Ho YK, Shih HM, Giam CZ. NF-kappaB hyper-activation by HTLV-1 tax induces cellular senescence, but can be alleviated by the viral anti-sense protein HBZ. PloS Pathog (2011) 7:e1002025. doi: 10.1371/journal.ppat.1002025
98. Bellon M, Nicot C. Regulation of telomerase and telomeres: human tumor viruses take control. J Natl Cancer Inst (2008) 100:98–108. doi: 10.1093/jnci/djm269
99. Bellon M, Yuan Y, Nicot C. Transcription independent stimulation of telomerase enzymatic activity by HTLV-I tax through stimulation of IKK. J Cancer Sci (2021) 8(1):10. doi: 10.13188/2377-9292.1000024
100. Nicot C. HTLV-I tax-mediated inactivation of cell cycle checkpoints and DNA repair pathways contribute to cellular transformation: "A random mutagenesis model". J Cancer Sci (2015) 2(2):6. doi: 10.13188/2377-9292.1000009
101. Haller K, Wu Y, Derow E, Schmitt I, Jeang KT, Grassmann R. Physical interaction of human T-cell leukemia virus type 1 tax with cyclin-dependent kinase 4 stimulates the phosphorylation of retinoblastoma protein. Mol Cell Biol (2002) 22:3327–38. doi: 10.1128/MCB.22.10.3327-3338.2002
102. Lemasson I, Thébault S, Sardet C, Devaux C, Mesnard JM. Activation of E2F-mediated transcription by human T-cell leukemia virus type I tax protein in a p16(INK4A)-negative T-cell line. J Biol Chem (1998) 273:23598–604. doi: 10.1074/jbc.273.36.23598
103. Kibler KV, Jeang KT. Taxing the cellular capacity for repair: human T-cell leukemia virus type 1, DNA damage, and adult T-cell leukemia. J Natl Cancer Inst (1999) 91:903–4. doi: 10.1093/jnci/91.11.903
104. Philpott SM, Buehring GC. Defective DNA repair in cells with human T-cell leukemia/bovine leukemia viruses: role of tax gene. J Natl Cancer Inst (1999) 91:933–42. doi: 10.1093/jnci/91.11.933
105. Kao SY, Lemoine FJ, Mariott SJ. HTLV-1 tax protein sensitizes cells to apoptotic cell death induced by DNA damaging agents. Oncogene (2000) 19:2240–8. doi: 10.1038/sj.onc.1203559
106. Kataoka K, Nagata Y, Kitanaka A, Shiraishi Y, Shimamura T, Yasunaga J, et al. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat Genet (2015) 47:1304–15. doi: 10.1038/ng.3415
107. Pise-Masison CA, Mahieux R, Jiang H, Ashcroft M, Radonovich M, Duvall J, et al. Inactivation of p53 by human T-cell lymphotropic virus type 1 tax requires activation of the NF-kappaB pathway and is dependent on p53 phosphorylation. Mol Cell Biol (2000) 20:3377–86. doi: 10.1128/MCB.20.10.3377-3386.2000
108. Suzuki T, Uchida-Toita M, Yoshida M. Tax protein of HTLV-1 inhibits CBP/p300-mediated transcription by interfering with recruitment of CBP/p300 onto DNA element of e-box or p53 binding site. Oncogene (1999) 18:4137–43. doi: 10.1038/sj.onc.1202766
109. El Hajj H, El-Sabban M, Hasegawa H, Zaatari G, Ablain J, Saab ST, et al. Therapy-induced selective loss of leukemia-initiating activity in murine adult T cell leukemia. J Exp Med (2010) 207:2785–92. doi: 10.1084/jem.20101095
110. Hasegawa H, Sawa H, Lewis MJ, Orba Y, Sheehy N, Yamamoto Y, et al. Thymus-derived leukemia-lymphoma in mice transgenic for the tax gene of human T-lymphotropic virus type I. Nat Med (2006) 12:466–72. doi: 10.1038/nm1389
111. Portis T, Grossman WJ, Harding JC, Hess JL, Ratner L. Analysis of p53 inactivation in a human T-cell leukemia virus type 1 tax transgenic mouse model. J Virol (2001) 75:2185–93. doi: 10.1128/JVI.75.5.2185-2193.2001
112. Portis T, Harding JC, Ratner L. The contribution of NF-kappa b activity to spontaneous proliferation and resistance to apoptosis in human T-cell leukemia virus type 1 tax-induced tumors. Blood (2001) 98:1200–8. doi: 10.1182/blood.V98.4.1200
113. Banerjee P, Rochford R, Antel J, Canute G, Wrzesinski S, Sieburg M, et al. Proinflammatory cytokine gene induction by human T-cell leukemia virus type 1 (HTLV-1) and HTLV-2 tax in primary human glial cells. J Virol (2007) 81:1690–700. doi: 10.1128/JVI.01513-06
114. Ohsugi T, Kumasaka T, Okada S, Urano T. The tax protein of HTLV-1 promotes oncogenesis in not only immature T cells but also mature T cells. Nat Med (2007) 13:527–8. doi: 10.1038/nm0507-527
115. Moodad S, Akkouche A, Hleihel R, Darwiche N, El-Sabban M, Bazarbachi A, et al. Mouse models that enhanced our understanding of adult T cell leukemia. Front Microbiol (2018) 9:558. doi: 10.3389/fmicb.2018.00558
116. Shirinian M, Kambris Z, Hamadeh L, Grabbe C, Journo C, Mahieux R, et al. A transgenic drosophila melanogaster model to study human T-lymphotropic virus oncoprotein tax-1-Driven transformation in vivo. J Virol (2015) 89:8092–5. doi: 10.1128/JVI.00918-15
117. Mori N, Fujii M, Ikeda S, Yamada Y, Tomonaga M, Ballard DW, et al. Constitutive activation of NF-kappaB in primary adult T-cell leukemia cells. Blood (1999) 93:2360–8.
118. Yamagishi M, Kubokawa M, Kuze Y, Suzuki A, Yokomizo A, Kobayashi S, et al. Chronological genome and single-cell transcriptome integration characterizes the evolutionary process of adult T cell leukemia-lymphoma. Nat Commun (2021) 12:4821. doi: 10.1038/s41467-021-25101-9
119. Journo C, Mahieux R. HTLV-1 and innate immunity. Viruses (2011) 3:1374–94. doi: 10.3390/v3081374
120. Tanaka Y, Tanaka R, Terada E, Koyanagi Y, Miyano-Kurosaki N, Yamamoto N, et al. Induction of antibody responses that neutralize human T-cell leukemia virus type I infection in vitro and in vivo by peptide immunization. J Virol (1994) 68:6323–31. doi: 10.1128/jvi.68.10.6323-6331.1994
121. Macatonia SE, Cruickshank JK, Rudge P, Knight SC. Dendritic cells from patients with tropical spastic paraparesis are infected with HTLV-1 and stimulate autologous lymphocyte proliferation. AIDS Res Hum Retroviruses (1992) 8:1699–706. doi: 10.1089/aid.1992.8.1699
122. Assil S, Futsch N, Decembre E, Alais S, Gessain A, Cosset FL, et al. Sensing of cell-associated HTLV by plasmacytoid dendritic cells is regulated by dense beta-galactoside glycosylation. PloS Pathog (2019) 15:e1007589. doi: 10.1371/journal.ppat.1007589
123. Colisson R, Barblu L, Gras C, Raynaud F, Hadj-Slimane R, Pique C, et al. Free HTLV-1 induces TLR7-dependent innate immune response and TRAIL relocalization in killer plasmacytoid dendritic cells. Blood (2010) 115:2177–85. doi: 10.1182/blood-2009-06-224741
124. Sze A, Belgnaoui SM, Olagnier D, Lin R, Hiscott J, van Grevenynghe J. Host restriction factor SAMHD1 limits human T cell leukemia virus type 1 infection of monocytes via STING-mediated apoptosis. Cell Host Microbe (2013) 14:422–34. doi: 10.1016/j.chom.2013.09.009
125. Charoenthongtrakul S, Zhou Q, Shembade N, Harhaj NS, Harhaj EW. Human T cell leukemia virus type 1 tax inhibits innate antiviral signaling via NF-kappaB-dependent induction of SOCS1. J Virol (2011) 85:6955–62. doi: 10.1128/JVI.00007-11
126. Yuen CK, Chan CP, Fung SY, Wang PH, Wong WM, Tang HV, et al. Suppression of type I interferon production by human T-cell leukemia virus type 1 oncoprotein tax through inhibition of IRF3 phosphorylation. J Virol (2016) 90:3902–12. doi: 10.1128/JVI.00129-16
127. Asquith B, Hanon E, Taylor GP, Bangham CR. Is human T-cell lymphotropic virus type I really silent? Philos Trans R Soc Lond B Biol Sci (2000) 355:1013–9. doi: 10.1098/rstb.2000.0638
128. Masaki A, Ishida T, Suzuki S, Ito A, Mori F, Sato F, et al. Autologous tax-specific CTL therapy in a primary adult T cell leukemia/lymphoma cell-bearing NOD/Shi-scid, IL-2Rgammanull mouse model. J Immunol (2013) 191:135–44. doi: 10.4049/jimmunol.1202692
129. Kawamura K, Tanaka Y, Nakasone H, Ishihara Y, Kako S, Kobayashi S, et al. Development of a unique T cell receptor gene-transferred tax-redirected T cell immunotherapy for adult T cell leukemia. Biol Blood Marrow Transplant (2020) 26:1377–85. doi: 10.1016/j.bbmt.2020.04.006
130. Tamiya S, Matsuoka M, Etoh K, Watanabe T, Kamihira S, Yamaguchi K, et al. Two types of defective human T-lymphotropic virus type I provirus in adult T-cell leukemia. Blood (1996) 88:3065–73. doi: 10.1182/blood.V88.8.3065.bloodjournal8883065
131. Takeda S, Maeda M, Morikawa S, Taniguchi Y, Yasunaga J, Nosaka K, et al. Genetic and epigenetic inactivation of tax gene in adult T-cell leukemia cells. Int J Cancer (2004) 109:559–67. doi: 10.1002/ijc.20007
132. Koiwa T, Hamano-Usami A, Ishida T, Okayama A, Yamaguchi K, Kamihira S, et al. 5'-long terminal repeat-selective CpG methylation of latent human T-cell leukemia virus type 1 provirus in vitro and in vivo. J Virol (2002) 76:9389–97. doi: 10.1128/JVI.76.18.9389-9397.2002
133. Furukawa Y, Kubota R, Tara M, Izumo S, Osame M. Existence of escape mutant in HTLV-I tax during the development of adult T-cell leukemia. Blood (2001) 97:987–93. doi: 10.1182/blood.V97.4.987
134. Basbous J, Arpin C, Gaudray G, Piechaczyk M, Devaux C, Mesnard JM. The HBZ factor of human T-cell leukemia virus type I dimerizes with transcription factors JunB and c-jun and modulates their transcriptional activity. J Biol Chem (2003) 278:43620–7. doi: 10.1074/jbc.M307275200
135. Akimoto M, Kozako T, Sawada T, Matsushita K, Ozaki A, Hamada H, et al. Anti-HTLV-1 tax antibody and tax-specific cytotoxic T lymphocyte are associated with a reduction in HTLV-1 proviral load in asymptomatic carriers. J Med Virol (2007) 79:977–86. doi: 10.1002/jmv.20807
136. Kannagi M, Hasegawa A, Nagano Y, Iino T, Okamura J, Suehiro Y. Maintenance of long remission in adult T-cell leukemia by tax-targeted vaccine: A hope for disease-preventive therapy. Cancer Sci (2019) 110:849–57. doi: 10.1111/cas.13948
137. Suehiro Y, Hasegawa A, Iino T, Sasada A, Watanabe N, Matsuoka M, et al. Clinical outcomes of a novel therapeutic vaccine with tax peptide-pulsed dendritic cells for adult T cell leukaemia/lymphoma in a pilot study. Br J Haematol (2015) 169:356–67. doi: 10.1111/bjh.13302
138. Bellon M, Nicot C. Feedback loop regulation between pim kinases and tax keeps human T-cell leukemia virus type 1 viral replication in check. J Virol (2022) 96:e0196021. doi: 10.1128/jvi.01960-21
139. Bazarbachi A, El-Sabban ME, Nasr R, Quignon F, Awaraji C, Kersual J, et al. Arsenic trioxide and interferon-alpha synergize to induce cell cycle arrest and apoptosis in human T-cell lymphotropic virus type I-transformed cells. Blood (1999) 93:278–83. doi: 10.1182/blood.V93.1.278
140. Kchour G, Tarhini M, Kooshyar MM, El Hajj H, Wattel E, Mahmoudi M, et al. Phase 2 study of the efficacy and safety of the combination of arsenic trioxide, interferon alpha, and zidovudine in newly diagnosed chronic adult T-cell leukemia/lymphoma (ATL). Blood (2009) 113:6528–32. doi: 10.1182/blood-2009-03-211821
141. Mahieux R, Pise-Masison C, Gessain A, Brady JN, Olivier R, Perret E, et al. Arsenic trioxide induces apoptosis in human T-cell leukemia virus type 1- and type 2-infected cells by a caspase-3-dependent mechanism involving bcl-2 cleavage. Blood (2001) 98:3762–9. doi: 10.1182/blood.V98.13.3762
142. Marcais A, Cook L, Witkover A, Asnafi V, Avettand-Fenoel V, Delarue R, et al. Arsenic trioxide (As2O3) as a maintenance therapy for adult T cell leukemia/lymphoma. Retrovirology (2020) 17:5. doi: 10.1186/s12977-020-0513-y
143. Qu Z, Xiao G. Human T-cell lymphotropic virus: a model of NF-κB-associated tumorigenesis. Viruses (2011) 3:714–49. doi: 10.3390/v3060714
144. Siekevitz M, Feinberg MB, Holbrook N, Wong-Staal F, Greene WC. Activation of interleukin 2 and interleukin 2 receptor (Tac) promoter expression by the trans-activator (tat) gene product of human T-cell leukemia virus, type I. Proc Natl Acad Sci USA (1987) 84:5389–93. doi: 10.1073/pnas.84.15.5389
145. Horiuchi S, Yamamoto N, Dewan MZ, Takahashi Y, Yamashita A, Yoshida T, et al. Human T-cell leukemia virus type-I tax induces expression of interleukin-6 receptor (IL-6R): Shedding of soluble IL-6R and activation of STAT3 signaling. Int J Cancer (2006) 119:823–30. doi: 10.1002/ijc.21918
146. Leung K, Nabel GJ. HTLV-1 transactivator induces interleukin-2 receptor expression through an NF-kappa b-like factor. Nature (1988) 333:776–8. doi: 10.1038/333776a0
147. Larocca D, Chao LA, Seto MH, Brunck TK. Human T-cell leukemia virus minus strand transcription in infected T-cells. Biochem Biophys Res Commun (1989) 163:1006–13. doi: 10.1016/0006-291X(89)92322-X
148. Usui T, Yanagihara K, Tsukasaki K, Murata K, Hasegawa H, Yamada Y, et al. Characteristic expression of HTLV-1 basic zipper factor (HBZ) transcripts in HTLV-1 provirus-positive cells. Retrovirology (2008) 5:34. doi: 10.1186/1742-4690-5-34
149. Macnamara A, Rowan A, Hilburn S, Kadolsky U, Fujiwara H, Suemori K, et al. HLA class I binding of HBZ determines outcome in HTLV-1 infection. PloS Pathog (2010) 6:e1001117. doi: 10.1371/journal.ppat.1001117
150. Sugata K, Satou Y, Yasunaga J, Hara H, Ohshima K, Utsunomiya A, et al. HTLV-1 bZIP factor impairs cell-mediated immunity by suppressing production of Th1 cytokines. Blood (2012) 119:434–44. doi: 10.1182/blood-2011-05-357459
151. Lemasson I, Lewis MR, Polakowski N, Hivin P, Cavanagh MH, Thébault S, et al. Human T-cell leukemia virus type 1 (HTLV-1) bZIP protein interacts with the cellular transcription factor CREB to inhibit HTLV-1 transcription. J Virol (2007) 81:1543–53. doi: 10.1128/JVI.00480-06
152. Thébault S, Basbous J, Hivin P, Devaux C, Mesnard JM. HBZ interacts with JunD and stimulates its transcriptional activity. FEBS Lett (2004) 562:165–70. doi: 10.1016/S0014-5793(04)00225-X
153. Ma G, Yasunaga J, Fan J, Yanagawa S, Matsuoka M. HTLV-1 bZIP factor dysregulates the wnt pathways to support proliferation and migration of adult T-cell leukemia cells. Oncogene (2013) 32:4222–30. doi: 10.1038/onc.2012.450
154. Nakagawa M, Shaffer AL 3rd, Ceribelli M, Zhang M, Wright G, Huang DW, et al. Targeting the HTLV-I-Regulated BATF3/IRF4 transcriptional network in adult T cell Leukemia/Lymphoma. Cancer Cell (2018) 34:286–97.e10. doi: 10.1016/j.ccell.2018.06.014
155. Arnold J, Zimmerman B, Li M, Lairmore MD, Green PL. Human T-cell leukemia virus type-1 antisense-encoded gene, hbz, promotes T-lymphocyte proliferation. Blood (2008) 112:3788–97. doi: 10.1182/blood-2008-04-154286
156. Ma G, Yasunaga J, Matsuoka M. Multifaceted functions and roles of HBZ in HTLV-1 pathogenesis. Retrovirology (2016) 13:16. doi: 10.1186/s12977-016-0249-x
157. Zhao T, Yasunaga J, Satou Y, Nakao M, Takahashi M, Fujii M, et al. Human T-cell leukemia virus type 1 bZIP factor selectively suppresses the classical pathway of NF-kappaB. Blood (2009) 113:2755–64. doi: 10.1182/blood-2008-06-161729
158. Bangham CRM. Human T cell leukemia virus type 1: Persistence and pathogenesis. Annu Rev Immunol (2018) 36:43–71. doi: 10.1146/annurev-immunol-042617-053222
159. Sugata K, Yasunaga J, Kinosada H, Mitobe Y, Furuta R, Mahgoub M, et al. HTLV-1 viral factor HBZ induces CCR4 to promote T-cell migration and proliferation. Cancer Res (2016) 76:5068–79. doi: 10.1158/0008-5472.CAN-16-0361
160. Asquith B, Bangham CR. How does HTLV-I persist despite a strong cell-mediated immune response? Trends Immunol (2008) 29:4–11. doi: 10.1016/j.it.2007.09.006
161. Hanon E, Hall S, Taylor GP, Saito M, Davis R, Tanaka Y, et al. Abundant tax protein expression in CD4+ T cells infected with human T-cell lymphotropic virus type I (HTLV-I) is prevented by cytotoxic T lymphocytes. Blood (2000) 95:1386–92. doi: 10.1182/blood.V95.4.1386.004k22_1386_1392
162. Baratella M, Forlani G, Accolla RS. HTLV-1 HBZ viral protein: A key player in HTLV-1 mediated diseases. Front Microbiol (2017) 8:2615. doi: 10.3389/fmicb.2017.02615
163. Satou Y, Yasunaga J, Zhao T, Yoshida M, Miyazato P, Takai K, et al. HTLV-1 bZIP factor induces T-cell lymphoma and systemic inflammation in vivo. PloS Pathog (2011) 7:e1001274. doi: 10.1371/journal.ppat.1001274
164. Zhao T, Satou Y, Sugata K, Miyazato P, Green PL, Imamura T, et al. HTLV-1 bZIP factor enhances TGF-β signaling through p300 coactivator. Blood (2011) 118:1865–76. doi: 10.1182/blood-2010-12-326199
165. Yamamoto-Taguchi N, Satou Y, Miyazato P, Ohshima K, Nakagawa M, Katagiri K, et al. HTLV-1 bZIP factor induces inflammation through labile Foxp3 expression. PloS Pathog (2013) 9:e1003630. doi: 10.1371/journal.ppat.1003630
166. Mitagami Y, Yasunaga J, Kinosada H, Ohshima K, Matsuoka M. Interferon-γ promotes inflammation and development of T-cell lymphoma in HTLV-1 bZIP factor transgenic mice. PloS Pathog (2015) 11:e1005120. doi: 10.1371/journal.ppat.1005120
167. Zhi H, Yang L, Kuo YL, Ho YK, Shih HM, Giam CZ. NF-κB hyper-activation by HTLV-1 tax induces cellular senescence, but can be alleviated by the viral anti-sense protein HBZ. PloS Pathog (2011) 7:e1002025. doi: 10.1371/journal.ppat.1002025
168. Fochi S, Mutascio S, Bertazzoni U, Zipeto D, Romanelli MG. HTLV deregulation of the NF-κB pathway: An update on tax and antisense proteins role. Front Microbiol (2018) 9:285. doi: 10.3389/fmicb.2018.00285
169. Kagdi H, Demontis MA, Ramos JC, Taylor GP. Switching and loss of cellular cytokine producing capacity characterize in vivo viral infection and malignant transformation in human T- lymphotropic virus type 1 infection. PloS Pathog (2018) 14:e1006861. doi: 10.1371/journal.ppat.1006861
170. Anuradha R, Munisankar S, Bhootra Y, Jagannathan J, Dolla C, Kumaran P, et al. Systemic cytokine profiles in strongyloides stercoralis infection and alterations following treatment. Infect Immun (2016) 84:425–31. doi: 10.1128/IAI.01354-15
171. Yasuma K, Yasunaga J, Takemoto K, Sugata K, Mitobe Y, Takenouchi N, et al. HTLV-1 bZIP factor impairs anti-viral immunity by inducing Co-inhibitory molecule, T cell immunoglobulin and ITIM domain (TIGIT). PloS Pathog (2016) 12:e1005372. doi: 10.1371/journal.ppat.1005372
172. Teijaro JR, Ng C, Lee AM, Sullivan BM, Sheehan KC, Welch M, et al. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science (2013) 340:207–11. doi: 10.1126/science.1235214
173. Wilson EB, Yamada DH, Elsaesser H, Herskovitz J, Deng J, Cheng G, et al. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science (2013) 340:202–7. doi: 10.1126/science.1235208
174. Tsukasaki K, Utsunomiya A, Fukuda H, Shibata T, Fukushima T, Takatsuka Y, et al. VCAP-AMP-VECP compared with biweekly CHOP for adult T-cell leukemia-lymphoma: Japan clinical oncology group study JCOG9801. J Clin Oncol (2007) 25:5458–64. doi: 10.1200/JCO.2007.11.9958
175. Malpica L, Pimentel A, Reis IM, Gotuzzo E, Lekakis L, Komanduri K, et al. Epidemiology, clinical features, and outcome of HTLV-1-related ATLL in an area of prevalence in the United States. Blood Adv (2018) 2:607–20. doi: 10.1182/bloodadvances.2017011106
176. Bazarbachi A, Suarez F, Fields P, Hermine O. How I treat adult T-cell leukemia/lymphoma. Blood (2011) 118:1736–45. doi: 10.1182/blood-2011-03-345702
177. Bazarbachi A, Cwynarski K, Boumendil A, Finel H, Fields P, Raj K, et al. Outcome of patients with HTLV-1-associated adult T-cell leukemia/lymphoma after SCT: a retrospective study by the EBMT LWP. Bone Marrow Transplant (2014) 49:1266–8. doi: 10.1038/bmt.2014.143
178. Hishizawa M, Kanda J, Utsunomiya A, Taniguchi S, Eto T, Moriuchi Y, et al. Transplantation of allogeneic hematopoietic stem cells for adult T-cell leukemia: a nationwide retrospective study. Blood (2010) 116:1369–76. doi: 10.1182/blood-2009-10-247510
179. Bazarbachi A, Hermine O. Treatment with a combination of zidovudine and alpha-interferon in naive and pretreated adult T-cell leukemia/lymphoma patients. J Acquir Immune Defic Syndr Hum Retrovirol (1996) 13 (Suppl 1):S186–90. doi: 10.1097/00042560-199600001-00028
180. Bazarbachi A, Plumelle Y, Carlos Ramos J, Tortevoye P, Otrock Z, Taylor G, et al. Meta-analysis on the use of zidovudine and interferon-alfa in adult T-cell leukemia/lymphoma showing improved survival in the leukemic subtypes. J Clin Oncol (2010) 28:4177–83. doi: 10.1200/JCO.2010.28.0669
181. Gill PS, Harrington W Jr., Kaplan MH, Ribeiro RC, Bennett JM, Liebman HA, et al. Treatment of adult T-cell leukemia-lymphoma with a combination of interferon alfa and zidovudine. N Engl J Med (1995) 332:1744–8. doi: 10.1056/NEJM199506293322603
182. Hermine O, Allard I, Levy V, Arnulf B, Gessain A, Bazarbachi A, et al. A prospective phase II clinical trial with the use of zidovudine and interferon-alpha in the acute and lymphoma forms of adult T-cell leukemia/lymphoma. Hematol J (2002) 3:276–82. doi: 10.1038/sj.thj.6200195
183. Hermine O, Bouscary D, Gessain A, Turlure P, Leblond V, Franck N, et al. Brief report: treatment of adult T-cell leukemia-lymphoma with zidovudine and interferon alfa. N Engl J Med (1995) 332:1749–51. doi: 10.1056/NEJM199506293322604
184. Kchour G, Makhoul NJ, Mahmoudi M, Kooshyar MM, Shirdel A, Rastin M, et al. Zidovudine and interferon-alpha treatment induces a high response rate and reduces HTLV-1 proviral load and VEGF plasma levels in patients with adult T-cell leukemia from north East Iran. Leuk Lymphoma (2007) 48:330–6. doi: 10.1080/10428190601071717
185. Kinpara S, Kijiyama M, Takamori A, Hasegawa A, Sasada A, Masuda T, et al. Interferon-alpha (IFN-alpha) suppresses HTLV-1 gene expression and cell cycling, while IFN-alpha combined with zidovudine induces p53 signaling and apoptosis in HTLV-1-infected cells. Retrovirology (2013) 10:52. doi: 10.1186/1742-4690-10-52
186. Matutes E, Taylor GP, Cavenagh J, Pagliuca A, Bareford D, Domingo A, et al. Interferon alpha and zidovudine therapy in adult T-cell leukaemia lymphoma: response and outcome in 15 patients. Br J Haematol (2001) 113:779–84. doi: 10.1046/j.1365-2141.2001.02794.x
187. Ratner L, Harrington W, Feng X, Grant C, Jacobson S, Noy A, et al. Human T cell leukemia virus reactivation with progression of adult T-cell leukemia-lymphoma. PloS One (2009) 4:e4420. doi: 10.1371/journal.pone.0004420
188. White JD, Wharfe G, Stewart DM, Maher VE, Eicher D, Herring B, et al. The combination of zidovudine and interferon alpha-2B in the treatment of adult T-cell leukemia/lymphoma. Leuk Lymphoma (2001) 40:287–94. doi: 10.3109/10428190109057927
189. Cook LB, Fuji S, Hermine O, Bazarbachi A, Ramos JC, Ratner L, et al. Revised adult T-cell leukemia-lymphoma international consensus meeting report. J Clin Oncol (2019) 37:677–87. doi: 10.1200/JCO.18.00501
190. Tsukasaki K, Hermine O, Bazarbachi A, Ratner L, Ramos JC, Harrington W Jr., et al. Definition, prognostic factors, treatment, and response criteria of adult T-cell leukemia-lymphoma: a proposal from an international consensus meeting. J Clin Oncol (2009) 27:453–9. doi: 10.1200/JCO.2008.18.2428
191. Macchi B, Balestrieri E, Frezza C, Grelli S, Valletta E, Marcais A, et al. Quantification of HTLV-1 reverse transcriptase activity in ATL patients treated with zidovudine and interferon-alpha. Blood Adv (2017) 1:748–52. doi: 10.1182/bloodadvances.2016001370
192. Bazarbachi A, Nasr R, El-Sabban ME, Mahé A, Mahieux R, Gessain A, et al. Evidence against a direct cytotoxic effect of alpha interferon and zidovudine in HTLV-I associated adult T cell leukemia/lymphoma. Leukemia (2000) 14:716–21. doi: 10.1038/sj.leu.2401742
193. Nasr R, El Hajj H, Kfoury Y, de Thé H, Hermine O, Bazarbachi A. Controversies in targeted therapy of adult T cell leukemia/lymphoma: ON target or OFF target effects? Viruses (2011) 3:750–69. doi: 10.3390/v3060750
194. Ogura M, Imaizumi Y, Uike N, Asou N, Utsunomiya A, Uchida T, et al. Lenalidomide in relapsed adult T-cell leukaemia-lymphoma or peripheral T-cell lymphoma (ATLL-001): a phase 1, multicentre, dose-escalation study. Lancet Haematol (2016) 3:e107–18. doi: 10.1016/S2352-3026(15)00284-7
195. Oka S, Ono K, Nohgawa M. Effective maintenance treatment with lenalidomide for a patient with aggressive adult T cell leukemia after chemotherapy. Leuk Res Rep (2019) 11:21–3. doi: 10.1016/j.lrr.2019.04.001
196. Horwitz S, O'Connor OA, Pro B, Illidge T, Fanale M, Advani R, et al. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet (2019) 393:229–40. doi: 10.1016/S0140-6736(18)32984-2
197. Tobinai K, Takahashi T, Akinaga S. Targeting chemokine receptor CCR4 in adult T-cell leukemia-lymphoma and other T-cell lymphomas. Curr Hematol Malig Rep (2012) 7:235–40. doi: 10.1007/s11899-012-0124-3
198. Waldmann TA, White JD, Goldman CK, Top L, Grant A, Bamford R, et al. The interleukin-2 receptor: a target for monoclonal antibody treatment of human T-cell lymphotrophic virus I-induced adult T-cell leukemia. Blood (1993) 82:1701–12. doi: 10.1182/blood.V82.6.1701.1701
199. Ishida T, Joh T, Uike N, Yamamoto K, Utsunomiya A, Yoshida S, et al. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: a multicenter phase II study. J Clin Oncol (2012) 30:837–42. doi: 10.1200/JCO.2011.37.3472
200. Ishida T, Jo T, Takemoto S, Suzushima H, Uozumi K, Yamamoto K, et al. Dose-intensified chemotherapy alone or in combination with mogamulizumab in newly diagnosed aggressive adult T-cell leukaemia-lymphoma: a randomized phase II study. Br J Haematol (2015) 169:672–82. doi: 10.1111/bjh.13338
201. Moura IC, Lepelletier Y, Arnulf B, England P, Baude C, Beaumont C, et al. A neutralizing monoclonal antibody (mAb A24) directed against the transferrin receptor induces apoptosis of tumor T lymphocytes from ATL patients. Blood (2004) 103:1838–45. doi: 10.1182/blood-2003-07-2440
202. Callens C, Moura IC, Lepelletier Y, Coulon S, Renand A, Dussiot M, et al. Recent advances in adult T-cell leukemia therapy: focus on a new anti-transferrin receptor monoclonal antibody. Leukemia (2008) 22:42–8. doi: 10.1038/sj.leu.2404958
203. Sharma K, Janik JE, O'Mahony D, Stewart D, Pittaluga S, Stetler-Stevenson M, et al. Phase II study of alemtuzumab (CAMPATH-1) in patients with HTLV-1-Associated adult T-cell leukemia/lymphoma. Clin Cancer Res (2017) 23:35–42. doi: 10.1158/1078-0432.CCR-16-1022
204. Ratner L, the A. PD-1 inhibitor therapy in adult T-cell leukemia-lymphoma. N Engl J Med (2018) 379:696–7. doi: 10.1056/NEJMc1807852
205. Ishida T, Fujiwara H, Nosaka K, Taira N, Abe Y, Imaizumi Y, et al. Multicenter phase II study of lenalidomide in relapsed or recurrent adult T-cell Leukemia/Lymphoma: ATLL-002. J Clin Oncol (2016) 34:4086–93. doi: 10.1200/JCO.2016.67.7732
206. Horwitz S, O'Connor OA, Pro B, Trumper L, Iyer S, Advani R, et al. The ECHELON-2 trial: 5-year results of a randomized, phase III study of brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma. Ann Oncol (2022) 33:288–98. doi: 10.1016/j.annonc.2021.12.002
207. El-Sabban ME, Nasr R, Dbaibo G, Hermine O, Abboushi N, Quignon F, et al. Arsenic-interferon-alpha-triggered apoptosis in HTLV-I transformed cells is associated with tax down-regulation and reversal of NF-kappa b activation. Blood (2000) 96:2849–55.
208. Nasr R, Rosenwald A, El-Sabban ME, Arnulf B, Zalloua P, Lepelletier Y, et al. Arsenic/interferon specifically reverses 2 distinct gene networks critical for the survival of HTLV-1-infected leukemic cells. Blood (2003) 101:4576–82. doi: 10.1182/blood-2002-09-2986
209. Dbaibo GS, Kfoury Y, Darwiche N, Panjarian S, Kozhaya L, Nasr R, et al. Arsenic trioxide induces accumulation of cytotoxic levels of ceramide in acute promyelocytic leukemia and adult T-cell leukemia/lymphoma cells through de novo ceramide synthesis and inhibition of glucosylceramide synthase activity. Haematologica (2007) 92:753–62. doi: 10.3324/haematol.10968
Keywords: HTLV-1/HTLV-1, ATL/ATLL, Tax, HBZ, innate immunity, interleukin-10 (IL-10), targeted therapy/therapies
Citation: El Hajj H and Bazarbachi A (2022) Interplay between innate immunity and the viral oncoproteins Tax and HBZ in the pathogenesis and therapeutic response of HTLV-1 associated adult T cell leukemia. Front. Immunol. 13:957535. doi: 10.3389/fimmu.2022.957535
Received: 31 May 2022; Accepted: 27 June 2022;
Published: 22 July 2022.
Edited by:
Roberto S. Accolla, University of Insubria, ItalyReviewed by:
Juan Carlos Ramos, University of Miami, United StatesCopyright © 2022 El Hajj and Bazarbachi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ali Bazarbachi, YmF6YXJiYWNAYXViLmVkdS5sYg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.