- 1Tuberculosis Prevention and Control Key Laboratory, Senior Department of Tuberculosis, The 8th Medical Center of People's Liberation Army (PLA) General Hospital, Beijing, China
- 2Beijing Key Laboratory of New Techniques of Tuberculosis Diagnosis and Treatment, Senior Department of Tuberculosis, The 8th Medical Center of People’s Liberation Army (PLA) General Hospital, Beijing, China
- 3Huadong Research Institute for Medicine and Biotechniques, Nanjing, China
Introduction
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has inflicted an unprecedented and significant toll on global public health and the economy. During the fight against the pandemic, the century-old tuberculosis (TB) vaccine Bacille Calmette Guérin (BCG), which has been reported to protect against infections of various respiratory pathogens other than Mycobacterium tuberculosis by inducing non-specific immune responses, was found to play an essential role against COVID-19 in various ecological, analytical, and animal studies (1, 2). However, its effectiveness remains controversial due to the limited number of published clinical trials (2). The effect of BCG vaccines appears to be quite non-specific or speculative. Currently there is no clear or strong evidence to support the notion that BCG vaccination confer protection to COVID-19. In the absence of evidence, the World Health Organization (WHO) issued a Scientific Brief on April 12, 2020, against BCG vaccination to prevent COVID-19, and this recommendation has remained unchanged to date (3).
Non-Specific Immune Response Induced by the BCG
Our previous studies have reported that BCG vaccination could induce a non-specific immune response to fight against unrelated pathogens (2, 4–7). The non-specific immune response induced by BCG vaccination may be due to three potential mechanisms: trained immunity, heterologous immunity, and anti-inflammatory effect (2). Of the three possible mechanisms, trained immunity has received the most attention. Trained immunity is derived from immune memory in innate immune cells such as monocytes and macrophages. The primary immunization of BCG can activate innate immune cells to produce IL-1β, TNF-α, and IL-6, generating trained monocytes/macrophages with an “infection memory.” These trained monocytes/macrophages will respond rapidly by releasing higher levels of cytokines to combat the second unrelated pathogens’ invasion (8). The trained immunity induced by BCG has been demonstrated in both animal experiments and human clinical trials, and it may be beneficial for the prevention and treatment of SARS-CoV-2 infection (2, 4–7, 9–11).
Early Evidence on BCG Prevention of COVID-19
Early in the COVID-19 outbreak, the hypothesis that BCG could prevent COVID-19 infection was raised. Isabel N. Kantor was the first to publicly discuss whether BCG has preventive and protective effects against COVID-19 and how strong the association is between the “more BCG vaccination” and the “ less COVID-19 infection” (12). Then, the lead investigator of the BRACE trial (NCT04327206) and the BCG-CORONA trial (NCT04328441) as well as the Director General of WHO discussed this hypothesis, and they suggested that BCG might reduce viremia following SARS-COV-2 exposure, thereby reducing the severity of COVID-19 and recovering faster (13). Furthermore, Luke A. J. O’Neill and Mihai G. Netea claimed that the BCG vaccine might well be a bridge to a specific COVID-19 vaccine due to the trained immunity induced by BCG.
Although this hypothesis offered a glimmer of hope to the panicked and helpless people in the early days of the COVID-19 pandemic, it still needed a lot of evidence to prove it. In the early months of the COVID-19 pandemic, findings from the ecological and analytical studies indicated that countries with low BCG coverage had significantly higher rates of COVID-19 morbidity and mortality than countries with high BCG coverage (14–40). On the contrary, other ecological and analytical studies found that BCG vaccination could not provide adequate protection against COVID-19 infection (41–50). The results of these ecological and analytical studies were contradictory, and the heterogeneity of these findings may originate from some confounding factors, such as population density, ethnicity, age structure, income, healthcare access and quality index, COVID-19 transmission progression, COVID-19 testing rate, non-pharmaceutical interventions, and geographical distribution (2, 5, 51–53). Therefore, these findings should be investigated by randomized clinical trials.
Latest Evidence on BCG Prevention of COVID-19
Recently, results of a double-blind, randomized, placebo-controlled clinical trial (NCT04379336), which aimed to evaluate the efficacy of BCG vaccination in delivering protection against COVID-19 in healthcare workers (HCWs) in South Africa, were published in the journal eClinicalMedicine and attracted wide attention (54). The study reported that BCG revaccination failed to protect HCWs from SARS-CoV-2 infection, severe COVID-19, hospitalization, and respiratory tract infections (RTIs), on the contrary, resulting in an unexpected trend toward more symptomatic and severe RTIs. Nevertheless, it is plausible that BCG offered protection from death. The results indicated that BCG had no statistically significant effect on COVID-19, which in our opinion, maybe still controversial.
A large number of COVID-19 immune-tolerant rather than sensitive participants recruited in the study may have influenced or masked the true protective effect of the BCG vaccine. However, the COVID-19-seropositive population, with a rate of 15.3% resistant to COVID-19-related severe disease, were not excluded. Considering that M. tuberculosis-infected mice were resistant to secondary SARS-CoV-2 infection (48), it’s not inconceivable that the latent tuberculosis infection in the HCWs occupied 48.5% of all the participants might play resistant roles and influence the significant difference between groups. However, they were not excluded, either. More importantly, all the participants had previously received at least one dose of the BCG vaccine due to the vaccination policy of South Africa before the trial, which also could decrease the variation between the BCG revaccination and placebo groups. Interestingly, the only clinical trial to obtain results similar to this one also recruited volunteers who had received the BCG vaccine before in Poland (55), indicating the limitation should not be ignored.
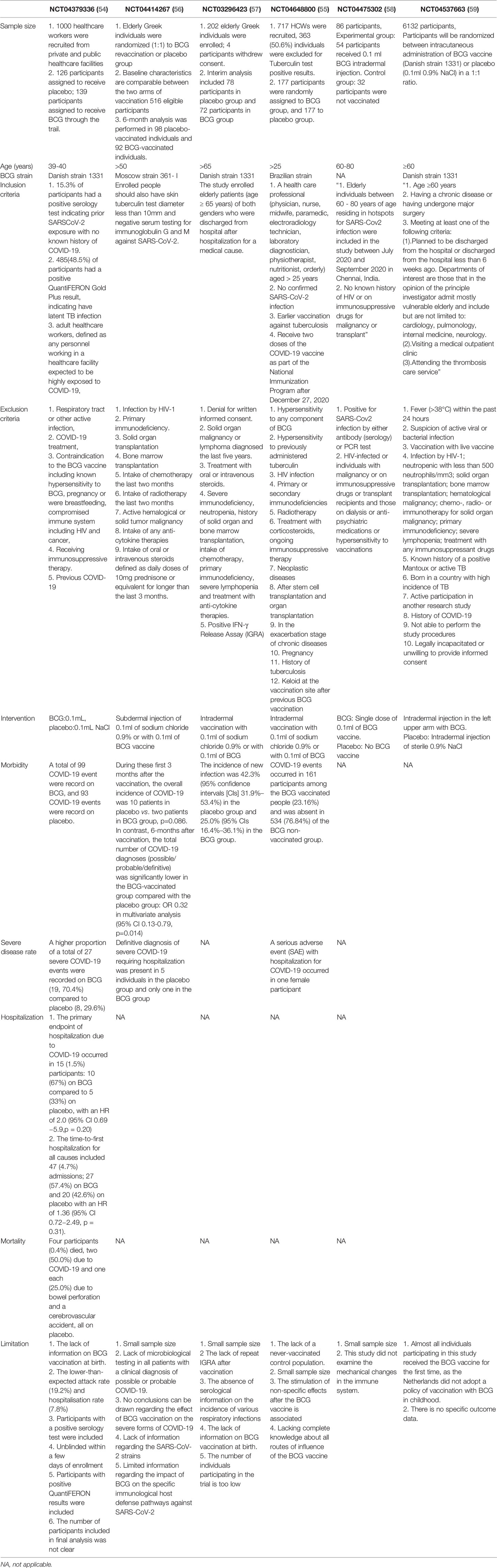
Considering that many clinical trials began in the early days of the pandemic like this study, there must have been various drawbacks in their design due to the lack of understanding of the disease and its interaction with the BCG vaccine at that time. For instance, the BCG vaccination-caused scars would expose the participants’ group and make the double-blind experimental design impossible. Furthermore, the young and middle-aged participants being relatively more resistant to COVID-19 than older people may mask the real efficacy of the BCG vaccine. Here we summarize the designs, results, and deficiencies of all published clinical studies (Table 1) (55–59) and hope to provide helpful information for more clinical studies involving the efficacy of BCG vaccination against COVID-19 or other virus infections in the future. After all, though more than 50 clinical trials have been registered, results of less than ten clinical trials have been published so far (2).
Conclusion
The hypothesis that BCG has a protective effect against COVID-19 has not been robustly verified. Therefore, the interpretation of any clinical trial results used to confirm this hypothesis should follow the principles of objectivity, science, and prudence. Considering the ongoing pandemic and the possibility of novel variants or other pathogens emerging, the potential effect of BCG on COVID-19 needs to be further confirmed in rigorous randomized clinical trials.
Author Contributions
Conceptualization: WG, YQ, and HA; Data curation: JW and PC; Formal analysis: YQ; Funding acquisition: WG; Methodology: HA, JW, and PC; Writing - original draft: WG and YQ; Writing - review and editing: HA, YQ, and WG. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the Beijing Municipal Science & Technology Commission (Grant No. 19L2065) and the Chinese PLA General Hospital (Grant No. QNC19047) to WG.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Parmar K, Siddiqui A, Nugent K. Bacillus Calmette-Guerin Vaccine and Nonspecific Immunity. Am J Med Sci (2021) 361:683–9. doi: 10.1016/j.amjms.2021.03.003
2. Gong W, Mao Y, Li Y, Qi Y. BCG Vaccination: A Potential Tool Against COVID-19 and COVID-19-Like Black Swan Incidents. Int Immunopharmacol (2022) 108:108870. doi: 10.1016/j.intimp.2022.108870
4. Gong W, Parkkila S, Wu X, Aspatwar A. SARS-CoV-2 Variants and COVID-19 Vaccines: Current Challenges and Future Strategies. Int Rev Immunol (2022) 41:1–22. doi: 10.1080/08830185.2022.2079642
5. Wang J, Zhang Q, Wang H, Gong W. The Potential Roles of BCG Vaccine in the Prevention or Treatment of COVID-19. Front Biosci (Landmark Ed) (2022) 27:157. doi: 10.31083/j.fbl2705157
6. Gong W, Aspatwar A, Wang S, Parkkila S, Wu X. COVID-19 Pandemic: SARS-CoV-2 Specific Vaccines and Challenges, Protection via BCG Trained Immunity, and Clinical Trials. Expert Rev Vaccines (2021) 20:857–80. doi: 10.1080/14760584.2021.1938550
7. Aspatwar A, Gong W, Wang S, Wu X, Parkkila S. Tuberculosis Vaccine BCG: The Magical Effect of the Old Vaccine in the Fight Against the COVID-19 Pandemic. Int Rev Immunol (2022) 41(2):283–96. doi: 10.1080/08830185.2021.1922685
8. Netea MG, Giamarellos-Bourboulis EJ, Domínguez-Andrés J, Curtis N, van Crevel R, van de Veerdonk FL, et al. Trained Immunity: A Tool for Reducing Susceptibility to and the Severity of SARS-CoV-2 Infection. Cell (2020) 181:969–77. doi: 10.1016/j.cell.2020.04.042
9. Brueggeman JM, Zhao J, Schank M, Yao ZQ, Moorman JP. Trained Immunity: An Overview and the Impact on COVID-19. Front Immunol (2022) 13:837524. doi: 10.3389/fimmu.2022.837524
10. Lee MH, Kim BJ. COVID-19 Vaccine Development Based on Recombinant Viral and Bacterial Vector Systems: Combinatorial Effect of Adaptive and Trained Immunity. J Microbiol (Seoul Korea) (2022) 60:321–34. doi: 10.1007/s12275-022-1621-2
11. You M, Chen L, Zhang D, Zhao P, Chen Z, Qin EQ, et al. Single-Cell Epigenomic Landscape of Peripheral Immune Cells Reveals Establishment of Trained Immunity in Individuals Convalescing From COVID-19. Nat Cell Biol (2021) 23:620–30. doi: 10.1038/s41556-021-00690-1
13. Curtis N, Sparrow A, Ghebreyesus TA, Netea MG. Considering BCG Vaccination to Reduce the Impact of COVID-19. Lancet (2020) 395:1545–6. doi: 10.1016/S0140-6736(20)31025-4
14. Klinger D, Blass I, Rappoport N, Linial M. Significantly Improved COVID-19 Outcomes in Countries With Higher BCG Vaccination Coverage: A Multivariable Analysis. Vaccines (Basel) (2020) 8:378. doi: 10.3390/vaccines8030378
15. Miller A, Reandelar MJ, Fasciglione K, Roumenova V, Li Y, Otazu GH. Correlation Between Universal BCG Vaccination Policy and Reduced Morbidity and Mortality for COVID-19: An Epidemiological Study. medRxiv (2020). doi: 10.1101/2020.03.24.20042937
16. Gong W, Wu X. Is the Tuberculosis Vaccine BCG an Alternative Weapon for Developing Countries to Defeat COVID-19? Indian J tuberculosis (2021) 68:401–4. doi: 10.1016/j.ijtb.2020.10.012
17. Berg MK, Yu Q, Salvador CE, Melani I, Kitayama S. Mandated Bacillus Calmette-Guérin (BCG) Vaccination Predicts Flattened Curves for the Spread of COVID-19. Sci Advances (2020) 6:eabc1463. doi: 10.1126/sciadv.abc1463
18. Escobar LE, Molina-Cruz A, Barillas-Mury C. BCG Vaccine Protection From Severe Coronavirus Disease 2019 (COVID-19). Proc Natl Acad Sci USA (2020) 117:17720–6. doi: 10.1073/pnas.2008410117
19. Covián C, Retamal-Díaz A, Bueno SM, Kalergis AM. Could BCG Vaccination Induce Protective Trained Immunity for SARS-CoV-2? Front Immunol (2020) 11:970. doi: 10.3389/fimmu.2020.00970
20. Urashima M, Otani K, Hasegawa Y, Akutsu T. BCG Vaccination and Mortality of COVID-19 Across 173 Countries: An Ecological Study. Int J Environ Res Public Health (2020) 17(15):5589. doi: 10.3390/ijerph17155589
21. Joy M, Malavika B, Asirvatham ES, Sudarsanam TD, Jeyaseelan L. Is BCG Associated With Reduced Incidence of COVID-19? A Meta-Regression of Global Data From 160 Countries. Clin Epidemiol Global Health (2021) 9:202–3. doi: 10.1016/j.cegh.2020.08.015
22. Brooks NA, Puri A, Garg S, Nag S, Corbo J, Turabi AE, et al. The Association of Coronavirus Disease-19 Mortality and Prior Bacille Calmette-Guerin Vaccination: A Robust Ecological Analysis Using Unsupervised Machine Learning. Sci Rep (2021) 11:774. doi: 10.1038/s41598-020-80787-z
23. Li WX. Worldwide Inverse Correlation Between Bacille Calmette-Guérin (BCG) Immunization and COVID-19 Mortality. Infection (2021) 49:463–73. doi: 10.1007/s15010-020-01566-6
24. Jirjees FJ, Dallal Bashi YH, Al-Obaidi HJ. COVID-19 Death and BCG Vaccination Programs Worldwide. Tuberc Respir Dis (Seoul) (2021) 84:13–21. doi: 10.4046/trd.2020.0063
25. Islam MZ, Zahan MK, Al-Bari MAA. Convergence Between Global BCG Vaccination and COVID-19 Pandemic. J Med virol (2021) 93:1496–505. doi: 10.1002/jmv.26450
26. Senoo Y, Suzuki Y, Tsuda K, Tanimoto T, Takahashi K. Association Between COVID-19 Morbidity and Mortality Rates and BCG Vaccination Policies in OECD Countries. J Infect Prev (2021) 22:91–3. doi: 10.1177/1757177420976812
27. Kinoshita M, Tanaka M. Impact of Routine Infant BCG Vaccination on COVID-19. J Infect (2020) 81:625–33. doi: 10.1016/j.jinf.2020.08.013
28. Singh S, Maurya RP, Singh RK. "Trained Immunity" From Mycobacterium Spp. Exposure or BCG Vaccination and COVID-19 Outcomes. PloS pathogens (2020) 16:e1008969. doi: 10.1371/journal.ppat.1008969
29. Raham TF. Influence of Malaria Endemicity and Tuberculosis Prevalence on COVID-19 Mortality. Public Health (2021) 194:33–5. doi: 10.1016/j.puhe.2021.02.018
30. Inoue K, Kashima S. Association of the Past Epidemic of Mycobacterium Tuberculosis With Mortality and Incidence of COVID-19. PloS One (2021) 16:e0253169. doi: 10.1371/journal.pone.0253169
31. Chaudhari VL, Godbole CJ, Gandhe PP, Gogtay NJ, Thatte UM. Association of Bacillus Calmette Guerin Vaccine Strains With COVID-19 Morbidity and Mortality - Evaluation of Global Data. Indian J Community Med (2021) 46:727–30. doi: 10.4103/ijcm.IJCM_103_21
32. Kumar A, Misra S, Verma V, Vishwakarma RK, Kamal VK, Nath M, et al. Global Impact of Environmental Temperature and BCG Vaccination Coverage on the Transmissibility and Fatality Rate of COVID-19. PloS One (2020) 15:e0240710. doi: 10.1371/journal.pone.0240710
33. Abdulah DM, Hassan AB. Exploration of Association Between Respiratory Vaccinations With Infection and Mortality Rates of COVID-19. Disaster Med Public Health Prep (2021), 1–16. doi: 10.1017/dmp.2021.47
34. Pathak S, Jolly MK, Nandi D. Countries With High Deaths Due to Flu and Tuberculosis Demonstrate Lower COVID-19 Mortality: Roles of Vaccinations. Hum Vaccin Immunother (2021) 17:2851–62. doi: 10.1080/21645515.2021.1908058
35. Hidvégi M, Nichelatti M. Bacillus Calmette-Guerin Vaccination Policy and Consumption of Ammonium Chloride-Enriched Confectioneries May Be Factors Reducing COVID-19 Death Rates in Europe. Israel Med Assoc J IMAJ (2020) 8:435–8.
36. Ebina-Shibuya R, Horita N, Namkoong H, Kaneko T. Current National Policies for Infant Universal Bacille Calmette-Guérin Vaccination Were Associated With Lower Mortality From Coronavirus Disease 2019. Clin Exp Vaccine Res (2020) 9:179–82. doi: 10.7774/cevr.2020.9.2.179
37. Szigeti R, Kellermayer D, Trakimas G, Kellermayer R. BCG Epidemiology Supports its Protection Against COVID-19? A Word of Caution. PloS One (2020) 15:e0240203. doi: 10.1371/journal.pone.0240203
38. Lerm M. On the Relationship Between BCG Coverage and National COVID-19 Outcome: Could 'Heterologous' Herd Immunity Explain Why Some Countries Are Better Off? J Intern Med (2020) 288:682–8. doi: 10.1111/joim.13198
39. Hauer J, Fischer U, Auer F, Borkhardt A. Regional BCG Vaccination Policy in Former East- and West Germany May Impact on Both Severity of SARS-CoV-2 and Incidence of Childhood Leukemia. Leukemia (2020) 34:2217–9. doi: 10.1038/s41375-020-0871-4
40. Weng CH, Saal A, Butt WW, Bica N, Fisher JQ, Tao J, et al. Bacillus Calmette-Guérin Vaccination and Clinical Characteristics and Outcomes of COVID-19 in Rhode Island, United States: A Cohort Study. Epidemiol infect (2020) 148:e140. doi: 10.1017/S0950268820001569
41. Hamiel U, Kozer E, Youngster I. SARS-CoV-2 Rates in BCG-Vaccinated and Unvaccinated Young Adults. Jama (2020) 323:2340–1. doi: 10.1001/jama.2020.8189
42. Sayed A, Challa KT, Arja S. Epidemiological Differences of COVID-19 Over the World. Cureus (2020) 12:e10316. doi: 10.7759/cureus.10316
43. Hensel J, McAndrews KM, McGrail DJ, Dowlatshahi DP, LeBleu VS, Kalluri R. Protection Against SARS-CoV-2 by BCG Vaccination Is Not Supported by Epidemiological Analyses. Sci Rep (2020) 10:18377. doi: 10.1038/s41598-020-75491-x
44. Wassenaar TM, Buzard GS, Newman DJ. BCG Vaccination Early in Life Does Not Improve COVID-19 Outcome of Elderly Populations, Based on Nationally Reported Data. Lett Appl Microbiol (2020) 71:498–505. doi: 10.1111/lam.13365
45. Chimoyi L, Velen K, Churchyard GJ, Wallis R, Lewis JJ, Charalambous S. An Ecological Study to Evaluate the Association of Bacillus Calmette-Guerin (BCG) Vaccination on Cases of SARS-CoV2 Infection and Mortality From COVID-19. PloS One (2020) 15:e0243707. doi: 10.1371/journal.pone.0243707
46. de Chaisemartin C, de Chaisemartin L. Bacille Calmette-Guérin Vaccination in Infancy Does Not Protect Against Coronavirus Disease 2019 (COVID-19): Evidence From a Natural Experiment in Sweden. Clin Infect Dis (2021) 72:e501–e5. doi: 10.1093/cid/ciaa1223
47. Patella V, Sanduzzi A, Bruzzese D, Florio G, Brancaccio R, Fabbrocini G, et al. A Survey Among Italian Physicians During COVID-19 Outbreak. Could Bacillus Calmette-Guérin Vaccine Be Effective Against SARS-Cov2? Front Pharmacol (2021) 12:646570. doi: 10.3389/fphar.2021.646570
48. Chauhan A, Singh M, Agarwal A, Jaiswal N, M Lakshmi PV, Singh M. Exploring the Role of Bacillus Calmette-Guerin Vaccination in Protection Against COVID-19. Int J mycobacteriol (2021) 10:433–6. doi: 10.4103/ijmy.ijmy_179_21
49. Cerqueira S, Deps PD, Cunha DV, Bezerra NVF, Barroso DH, Pinheiro ABS, et al. The Influence of Leprosy-Related Clinical and Epidemiological Variables in the Occurrence and Severity of COVID-19: A Prospective Real-World Cohort Study. PloS Negl Trop Dis (2021) 15:e0009635. doi: 10.1371/journal.pntd.0009635
50. Bates MN, Herron TJ, Lwi SJ, Baldo JV. BCG Vaccination at Birth and COVID-19: A Case-Control Study Among U.S. Military Veterans. Hum Vaccin Immunother (2021) 18(1):1981084. doi: 10.1080/21645515.2021.1981084
51. Ogimi C, Qu P, Boeckh M, Bender Ignacio RA, Zangeneh SZ. Association Between Live Childhood Vaccines and COVID-19 Outcomes: A National-Level Analysis. Epidemiol Infect (2021) 149:e75. doi: 10.1017/S0950268821000571
52. Lindestam Arlehamn CS, Sette A, Peters B. Lack of Evidence for BCG Vaccine Protection From Severe COVID-19. Proc Natl Acad Sci USA (2020) 117:25203–4. doi: 10.1073/pnas.2016733117
53. Ritchie H, Mathieu E, Rodés-Guirao L, Appel C, Giattino C, Ortiz-Ospina E, et al. Responses to the Coronavirus Pandemic. In: School OM, editor. Oxford: Oxford Martin School (2022).
54. Upton CM, van Wijk RC, Mockeliunas L, Simonsson USH, McHarry K, van den Hoogen G, et al. Safety and Efficacy of BCG Re-Vaccination in Relation to COVID-19 Morbidity in Healthcare Workers: A Double-Blind, Randomised, Controlled, Phase 3 Trial. EClinicalMedicine (2022) 48:101414. doi: 10.1016/j.eclinm.2022.101414
55. Czajka H, Zapolnik P, Krzych Ł, Kmiecik W, Stopyra L, Nowakowska A, et al. A Multi-Center, Randomised, Double-Blind, Placebo-Controlled Phase III Clinical Trial Evaluating the Impact of BCG Re-Vaccination on the Incidence and Severity of SARS-CoV-2 Infections Among Symptomatic Healthcare Professionals During the COVID-19 Pandemic in Poland-First Results. Vaccines (Basel) (2022) 10(2):314. doi: 10.3390/vaccines10020314
56. Tsilika M, Taks E, Dolianitis K, Kotsaki A, Leventogiannis K, Damoulari C, et al. ACTIVATE-2: A Double-Blind Randomized Trial of BCG Vaccination Against COVID-19 in Individuals at Risk. medRxiv (2021). doi: 10.1101/2021.05.20.21257520
57. Giamarellos-Bourboulis EJ, Tsilika M, Moorlag S, Antonakos N, Kotsaki A, Domínguez-Andrés J, et al. Activate: Randomized Clinical Trial of BCG Vaccination Against Infection in the Elderly. Cell (2020) 183:315–23.e9. doi: 10.1016/j.cell.2020.08.051
58. Kumar NP, Padmapriyadarsini C, Rajamanickam A, Bhavani PK, Nancy A, Jeyadeepa B, et al. BCG Vaccination Induces Enhanced Frequencies of Dendritic Cells and Altered Plasma Levels of Type I and Type III Interferons in Elderly Individuals. Int J Infect Dis IJID (2021) 110:98–104. doi: 10.1016/j.ijid.2021.07.041
59. Dos Anjos LRB, da Costa AC, Cardoso A, Guimarães RA, Rodrigues RL, Ribeiro KM, et al. Efficacy and Safety of BCG Revaccination With M. Bovis BCG Moscow to Prevent COVID-19 Infection in Health Care Workers: A Randomized Phase II Clinical Trial. Front Immunol (2022) 13:841868. doi: 10.3389/fimmu.2022.841868
Keywords: BCG (Bacille Calmette-Guérin), COVID-19, Mycobacterium tuberculosis, clinical trial, hospitalization, protective effect
Citation: Gong W, An H, Wang J, Cheng P and Qi Y (2022) The Natural Effect of BCG Vaccination on COVID-19: The Debate Continues. Front. Immunol. 13:953228. doi: 10.3389/fimmu.2022.953228
Received: 26 May 2022; Accepted: 20 June 2022;
Published: 08 July 2022.
Edited by:
Julia Y. Wang, Curandis Inc., United StatesReviewed by:
Irina V. Kiseleva, Institute of Experimental Medicine (RAS), RussiaCopyright © 2022 Gong, An, Wang, Cheng and Qi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenping Gong, Z3dwODkxMDE1QHdodS5lZHUuY24=; Yong Qi, cXNsYXJrQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work
 Wenping Gong
Wenping Gong Huiru An
Huiru An Jie Wang
Jie Wang Peng Cheng
Peng Cheng Yong Qi
Yong Qi