- 1Graduate School, Beijing University of Chinese Medicine, Beijing, China
- 2Oncology Department of Integrated Traditional Chinese and Western Medicine, China-Japan Friendship Hospital, Beijing, China
Background: There is a crosstalk between gut microbiota (GM) and cancer immunotherapy (CI). The purpose of this study is to use bibliometric analysis to identify the highly cited papers relating to GM/CI and explore the research status and development trends of the GM/CI research.
Methods: A literature search regarding GM/CI publications from 2012 to 2021 was undertaken on July 4, 2022. The article titles, journals, authors, institutions, countries, total citations, keywords, and other information were extracted from the Science Citation Index Expanded (SCIE) of Web of Science Core Collection (WoSCC). The Bibliometrix of R package and VOSviewer were used for bibliometric analysis.
Results: A total of 665 papers were extracted. The number of papers has increased rapidly over the past decade, especially after 2018. The United States and China had the most publications and made great contributions to this field. Th5e Univ Texas MD Anderson Canc Ctr and Univ Paris Saclay were absolutely in the leading position in GM/CI. The most influential authors were Zitvogel L and Routy B. Frontiers in Immunology had the most publications and Science had the most total citations. Historical direct citation analysis explained the historical evolution in GM/CI. Highly cited papers and high-frequency keywords illustrated the current status and trends of GM/CI. Four clusters were identified and the important topics included the role of GM and antibiotics in CI, the methods of targeting GM to improve CI outcomes, the mechanism by which GM affects CI and the application of ICIs in melanoma. “Tumor microbiome”, “proton pump inhibitors” and “prognosis” may be the new focus of attention in the next few years.
Conclusion: This study filtered global publications on GM/CI correlation and analyzed their bibliometric characteristics, identified the most cited papers in GM/CI, and gained insight into the status, hotspots and trends of global GM/CI research, which may inform researchers and practitioners of future directions.
Introduction
In the last decade, cancer immunotherapy (CI) represented by immune checkpoint inhibitors (ICIs) has revolutionized clinical practice in oncology as an emerging therapeutic approach and ushered in a new era of cancer treatment (1–3). Immunotherapy has been shown to be effective in oncology treatment (4, 5), whether used alone or in combination with other anti-tumor methods (6, 7), significantly improving the health-related quality of life in cancer patients. However, its efficacy is still limited by the heterogeneity of the patients’ immune response and the heterogeneity among different tumors (8, 9), and some patients will experience primary or acquired drug resistance and related adverse events (10, 11) during treatment, which greatly hinder the widespread clinical application of anti-tumor immunotherapy.
Gut microbiome (GM) plays a key regulatory role in cancer development and treatment, as well as the immune response of the body, influencing anti-tumor immunosurveillance (12, 13). Preclinical and clinical studies in recent years also have found that GM can modulate anti-tumor immune response and affect the efficacy and toxicity of CI, especially ICIs (14, 15). In addition, GM can be involved in inducing inflammation or indirectly participating in cancer treatment through derived metabolites, ultimately affecting anti-tumor therapeutic effects, and can also be used as one of the biomarkers to predict tumor patients’ response to immunotherapy and potential prognosis (16). There is an intricate crosstalk among the GM, cancer immune response and immunotherapy (17).
Bibliometric analysis is a method of statistically evaluating the research status and trends, as well as the most influential studies in a specific field, and has been successfully applied to relevant research in medicine (18, 19). Citation analysis is one of the main methods of bibliometrics, which can evaluate the quality and recognition of articles, and better understand the discipline construction and development of a research field. At present, many immunotherapy-related areas have been well researched and explored through bibliometric analysis, such as the analyses of highly cited articles in programmed cell death protein 1 (PD-1) and programmed cell death ligand 1(PD-L1) immunotherapy (20), immunotherapy for hepatocellular carcinoma (21) and colorectal cancer (22), and emerging chimeric antigen receptor-based immunotherapy (23). However, currently, there is no published paper on the quantitative analysis of interactions between GM and CI. Therefore, this article aims to review the regulatory role of GM in CI especially ICIs immunotherapy in recent years, identify the related articles in GM/CI and analyze their characteristics, and look forward to providing references for researchers in the field of GM/CI.
Materials and methods
Data source and search strategy
Web of Science (http://www.webofknowledge.com) is an important database platform for obtaining global academic information. It includes a variety of influential international academic journals in the world, covering natural science, social science, art and humanities and other disciplines. In addition, Web of Science has a strict screening mechanism, which only includes important academic journals in various disciplines according to Bradford’s law in bibliometrics. Science Citation Index Expanded (SCIE) of Web of Science Core Collection (WoSCC) includes the most authoritative and influential mainstream academic journals in the field of natural science. Therefore, we chose SCIE of WoSCC as the search source.
All searches were performed on the same day (July 4, 2022) to avoid the bias caused by database updates. The data were retrieved from the SCIE of WoSCC database on July 4, 2022. Using the subject term “advanced search” method, the search terms were TS = “immunotherapy” and “gut microbiota” and “cancer” and their synonyms. Terms related to immunotherapy, gut microbiome and cancer entered into the WoS engine were extracted from the Medical Subject Headings (MeSH) in PubMed, and the wildcard “*” was used in place of any number of characters for the most comprehensive search of relevant literature. The detailed search strategy is in Supplementary Material S1. The selection criteria were as follows: (1) The period of the literature search was from January 1, 2012, to December 31, 2021, (2) Document types were limited to “article” and “review”, (3) The language type was set to only English. After screening, a total of 665 papers were finally obtained (Figure 1), of which 294 were “articles” and 371 were “reviews”. Two researchers (SY and SZ) independently performed the search and data extraction. We extracted all available information such as title, author, institution, country, publication year, and keywords from the raw data and exported records to the plain text file.
Data analysis and parameter query
Bibliometric analysis was performed using a specific program from Rstudio’s Bibliometrix R package (version 2022.03.10, RStudio team, Boston, MA, USA), VOSviewer (version 1.6.18, Leiden University Science and Technology Research Center, the Netherlands) and Microsoft Excel 2019 (Microsoft, Redmond, Washington, USA). VOSviewer is a software developed for building and visualizing bibliometric networks. The Bibliometrix (https://www.bibliometrix.org) is an open-source tool for quantitative research in scientometrics. Each software allows for the construction and visualization of bibliometric networks to facilitate understanding of the GM/CI research. Specifically, the distribution of each component analyzed in the bibliometric analysis was assessed by a software package applying machine learning. For this, we used the following variables: annual scientific production, average citations per year, most relevant journals, journals dynamics, most impact journals by H-index or total citations (TC), top journals’ production over time, most relevant authors, top authors’ production over time, author local impact, most relevant affiliations, relevant funding agencies, country scientific production, collaboration network of countries, corresponding author’s country, top countries’ production over time, historical direct citation network, most global cited papers, most relevant keywords and cluster analysis of keywords. The journals’ impact factor (IF) and partition refer to the “2020 Journal Citation Reports”.
Results
Distribution of annual documents in GM/CI research
Annual development trend of publications
Analyzing the distribution of published papers from time series can reflect the trend of research. Figure 2A shows the number of GM/CI research papers from 2012 to 2021. As we can see, the number of papers (Np) was relatively small in 2012-2014 (n = 5, 0.75%) and increased slowly in 2015-2017 (n = 58, 8.72%). The Np increased rapidly between 2018 and 2021 (n = 602, 90.53%). A polynomial regression model (f(x)=p0xn+p1xn−1+p2xn−2+p3xn−3+…+pn) was constructed by Microsoft Office Excel 2019 to predict the Np published in 2022. By fitting data, a time prediction curve model was constructed and the formula was y=5.1742x2-20842x+2E+07. We observed a statistically significant relationship between the year and the number of publications (R² = 0.9879), and the fitting degree was good. According to the fitting curve, we estimated that the number of publications will reach about 320 in 2022.

Figure 2 (A) Annual scientific production and the polynomial curve fitting of publications growth in GM/CI. (B) The number of average citations per year in GM/CI.
Annual development trend of citations
The average annual citations of articles showed dynamic changes, as evidenced by the average citation distribution of papers per year (Figure 2B). The years with the highest average annual citations were 2013, 2015 and 2018, about 45, 65 and 34 times (the three peaks in Figure 2B), respectively, indicating that the papers from these three years had important research significance. As shown in Table 1, the respective highly cited papers of Iida N (2013), Sivan A (2015), Vetizou M (2015), Routy B (2018), and Gopalakrishnan V (2018) may make important contributions to this. In 2015, the average annual citations were highest, which may be related to fewer publications and higher quality of papers. From 2018 to 2021, the average annual citations showed a downward trend, which was contrary to the annual Np, and may be related to the delayed citation of recently published papers.
Primary journals
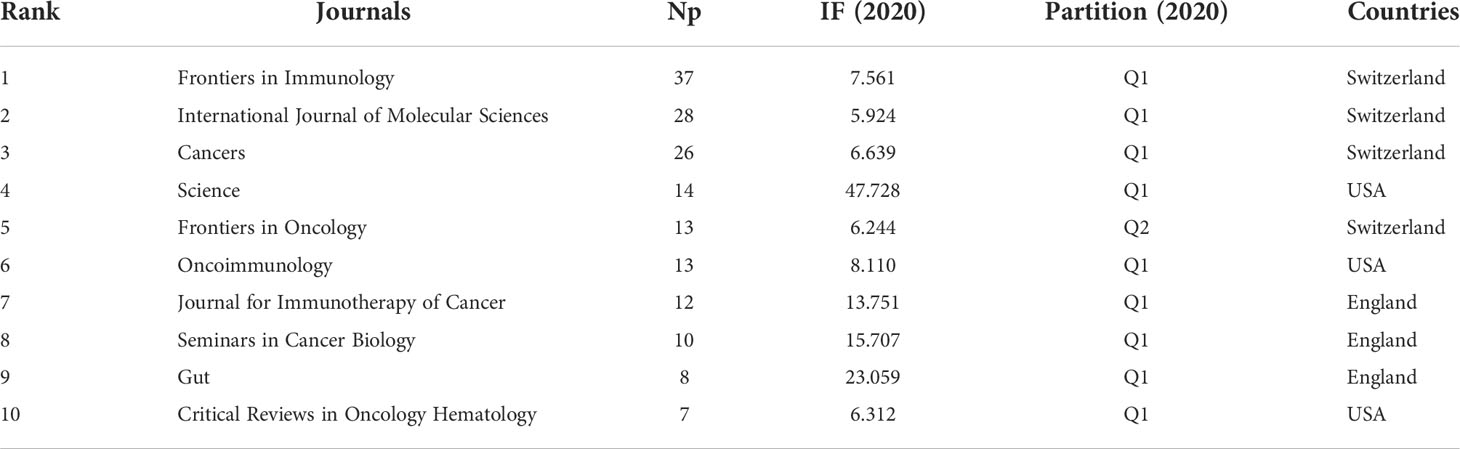
These papers were published in 295 journals. Table 2 shows the top 10 journals in the Np. It can be seen that Frontiers in Immunology had the most Np (n = 37, 5.56%), followed by International Journal of Molecular Sciences (n = 28, 4.21%), and Cancers (n = 26, 3.91%). Figure 3A depicts annual publications of the top 10 journals over time in GM/CI. Figure 3B summarizes the annual changes in the cumulative Np published in the top 10 journals. The cumulative Np published in these journals was 175, accounting for about 26.32% of all papers, indicating that these journals published most of the papers in GM/CI. The TC can show the importance of the journals and H-Index can evaluate the academic influence of journals. Table 3 shows the top 10 most cited journals, of which Science was the most cited, followed by Nature, Nature Reviews Cancer, Immunity and Annals of Oncology. Frontiers in Immunology has the highest H-index, followed by Science and International Journal of Molecular Sciences.
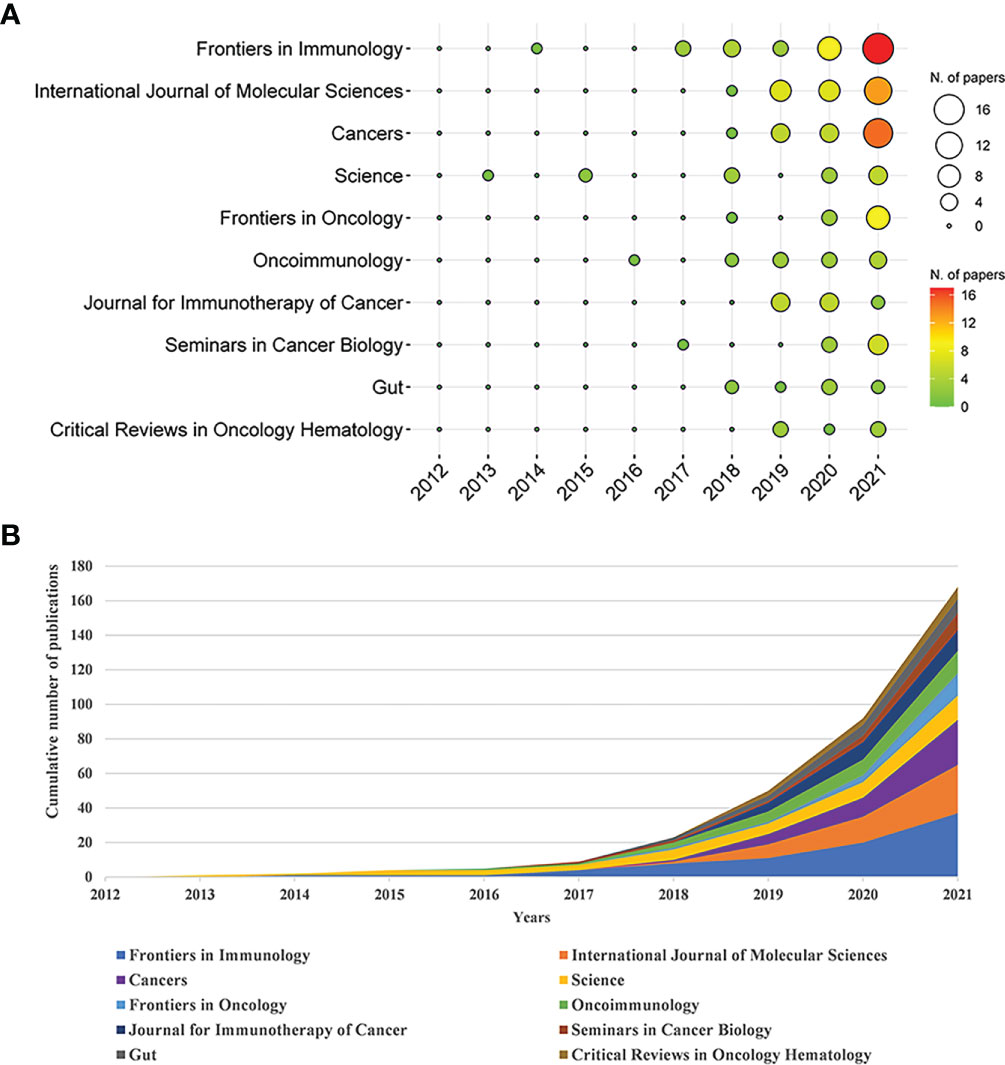
Figure 3 (A) The top 10 journals’ annual publications over time in GM/CI (the size of the circle represents the number of publications, and the larger the circle, the more the number of publications; the depth of the circle represents the average annual citation, and the darker the color, the more citations). (B) The cumulative number of publications of the top 10 journals in GM/CI.
Main authors
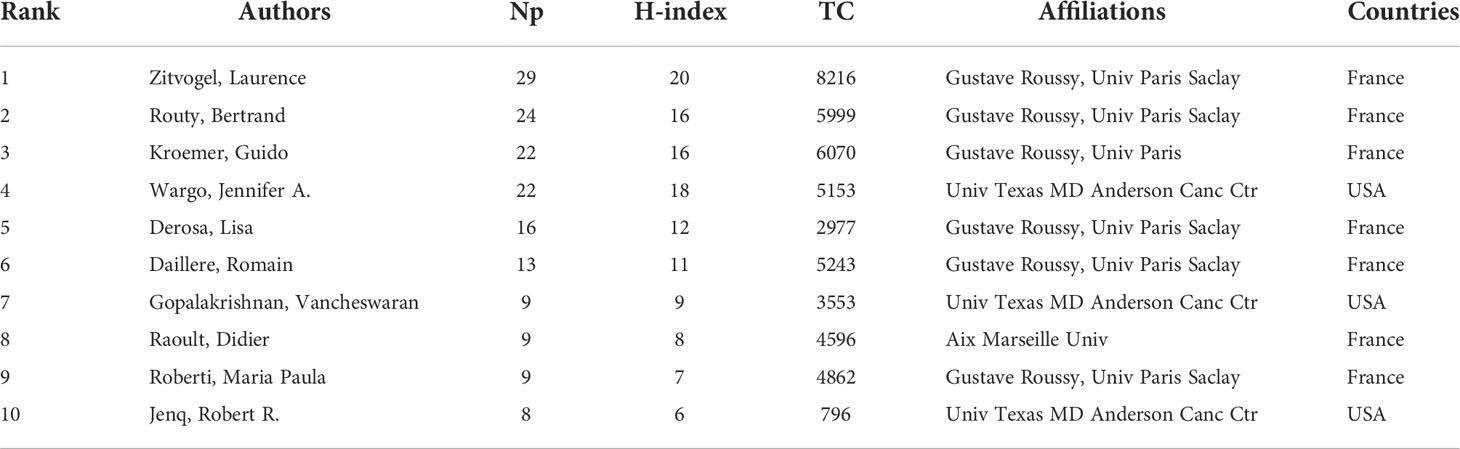
The dataset involved more than 4000 authors. To avoid the repetition caused by name abbreviation, we used the authors’ full names and Web of Science Researcher ID to extract and analyze the Np of authors. Table 4 lists the top 10 authors in the Np and their H-index, TC, affiliates and countries. As we can see, the top 10 authors were mainly from Univ Texas MD Anderson Canc Ctr in the United States and Gustave Roussy and Univ Paris Saclay in France. Zitvogel L (29/4.36%, 20), Routy B (24/3.61%, 16), Kroemer G (22/3.31%, 16), Wargo JA (22/3.31%, 18), and Derosa L (16/2.41%, 12) ranked the top five in the Np/percentage and H-index. Zitvogel L had the most Np (29), the highest H-index (20) and the most TC (8216), indicating that his papers were of high quality and had a great impact on GM/CI research. Figure 4A shows the change in the Np of the top 20 authors over time. Most authors started publishing related papers in 2015 and published the most papers in 2018 (darkest in the graph). Figure 4B shows the collaborations of the top 20 authors, we can see that Zitvogel L, Routy B, Kroemer G, Derosa L and Daillere R from Gustave Roussy in France had the closest cooperative relationship in GM/CI.
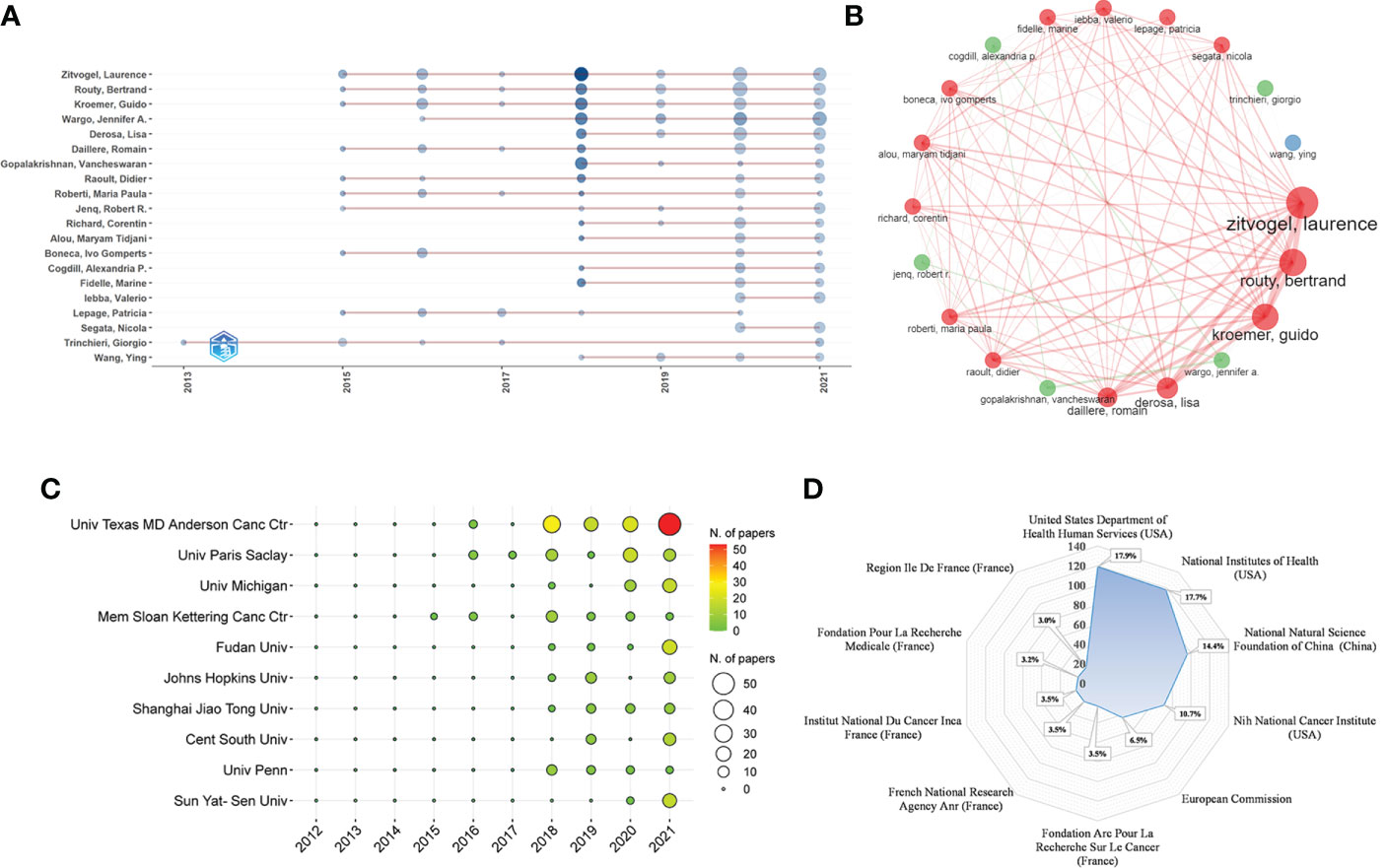
Figure 4 (A) The top 20 authors’ annual publication over time in GM/CI (the size of the circle represents the number of publications, and the larger the circle, the more the number of publications; the depth of the circle represents the average annual citation, and the darker the color, the more citations). (B) The top 20 authors’ co-authorship network in GM/CI (each node represents an author, the size of the node represents the number of published articles, the line represents the collaboration network between authors, and the thickness of the line represents the strength of collaboration). (C) The top 10 institutions’ annual publications over time in GM/CI. (D) The top 10 related funding agencies for the support of GM/CI research.
Major institutions and countries/regions
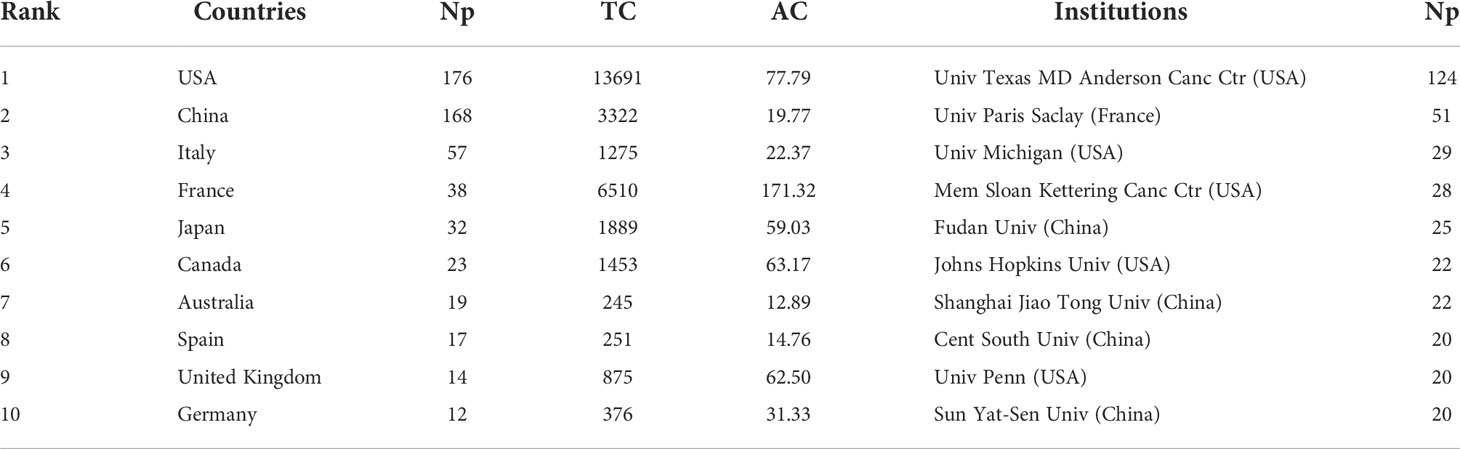
More than 1200 institutions were involved in this study, of which the top 10 most productive institutions were shown in Table 5. Among them, Univ Texas MD Anderson Canc Ctr, Univ Paris Saclay, Univ Michigan, Mem Sloan Kettering Canc Ctr and Fudan Univ were the top five. The distribution of the top 10 institutions was as follows: six institutions in the United States, three institutions in China and one institution in France. Figure 4C depicts the annual Np of the top 10 institutions over time. Among them, Mem Sloan Kettering Canc Ctr was the institution that started earlier (2015), while Univ Texas MD Anderson Canc Ctr published the most papers. Figure 4D depicts the main funding agencies, mainly from the US, China and France, indicating these countries have strong support for GM/CI research.
These papers were from 43 countries (Figure 5A). Table 5 shows that the papers were mainly published in the United States (176) and China (168), accounting for about 52% of the total output. Analysis of national cooperation in the top 20 countries was carried out through the collaboration network (Figure 5B). We can see that the United States was at the center of international cooperation and had the closest cooperation with China. A total of 42 papers in the United States came from international cooperation, followed by China with 19 papers (Figure 5C). More than half of the papers of France and Canada come from international cooperation in the top 10 countries. From the time distribution of papers (Figure 5D), before 2019, the United States ranked first overall in the annual Np. Since 2019, China’s annual publications had exceeded the United States.
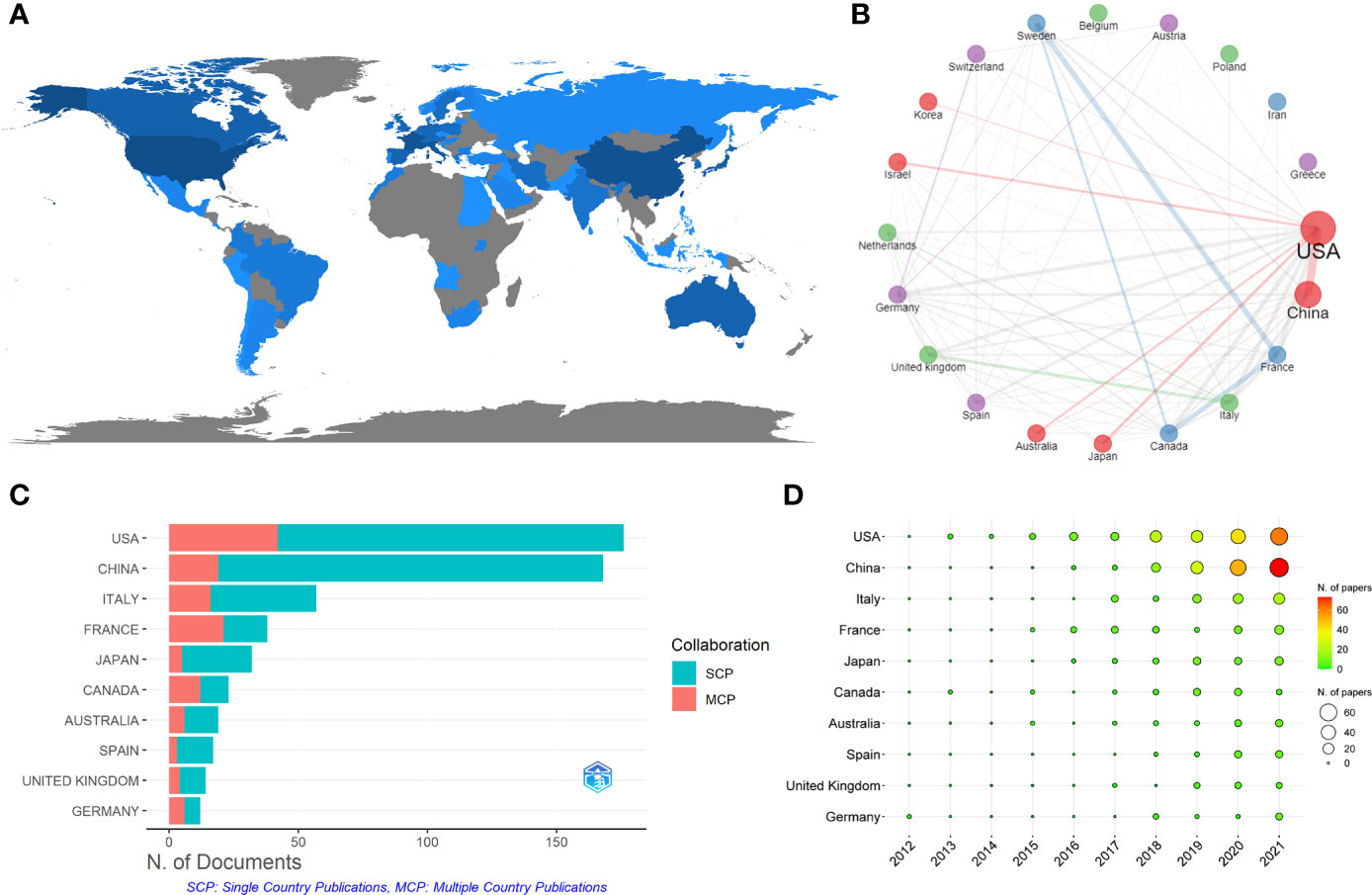
Figure 5 (A) Distribution of publications from different countries/regions in GM/CI. (B) International collaboration network of the top 20 countries in GM/CI. (C) The top 10 countries’ papers partnerships in GM/CI. (D) The top 10 countries’ annual publications over time in GM/CI.
Analysis of highly cited papers in GM/CI research
Historical cited papers of GM/CI research
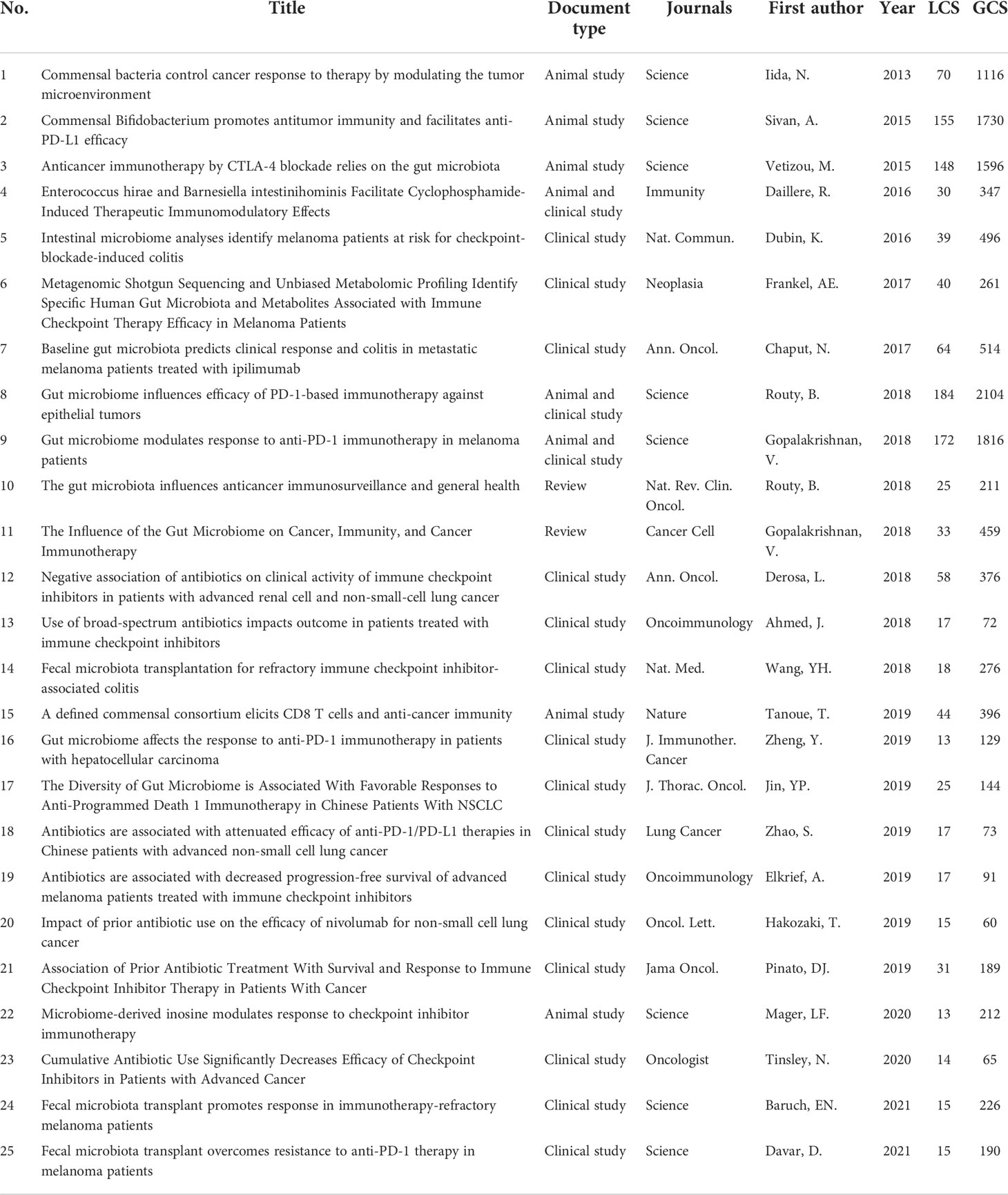
Using the historical cited paper visualization analysis in the Bibliometrix of R package, we found some classic papers in GM/CI (Figure 6). To examine the quality of research contents of the classic papers, two metrics, LCS and GCS, were used. LCS is for local citation score, and it corresponds to the number of citations of an article in the downloaded dataset. GCS is for global citation score, which represents the times an article has been cited by all documents in the WoS database (Table 6).
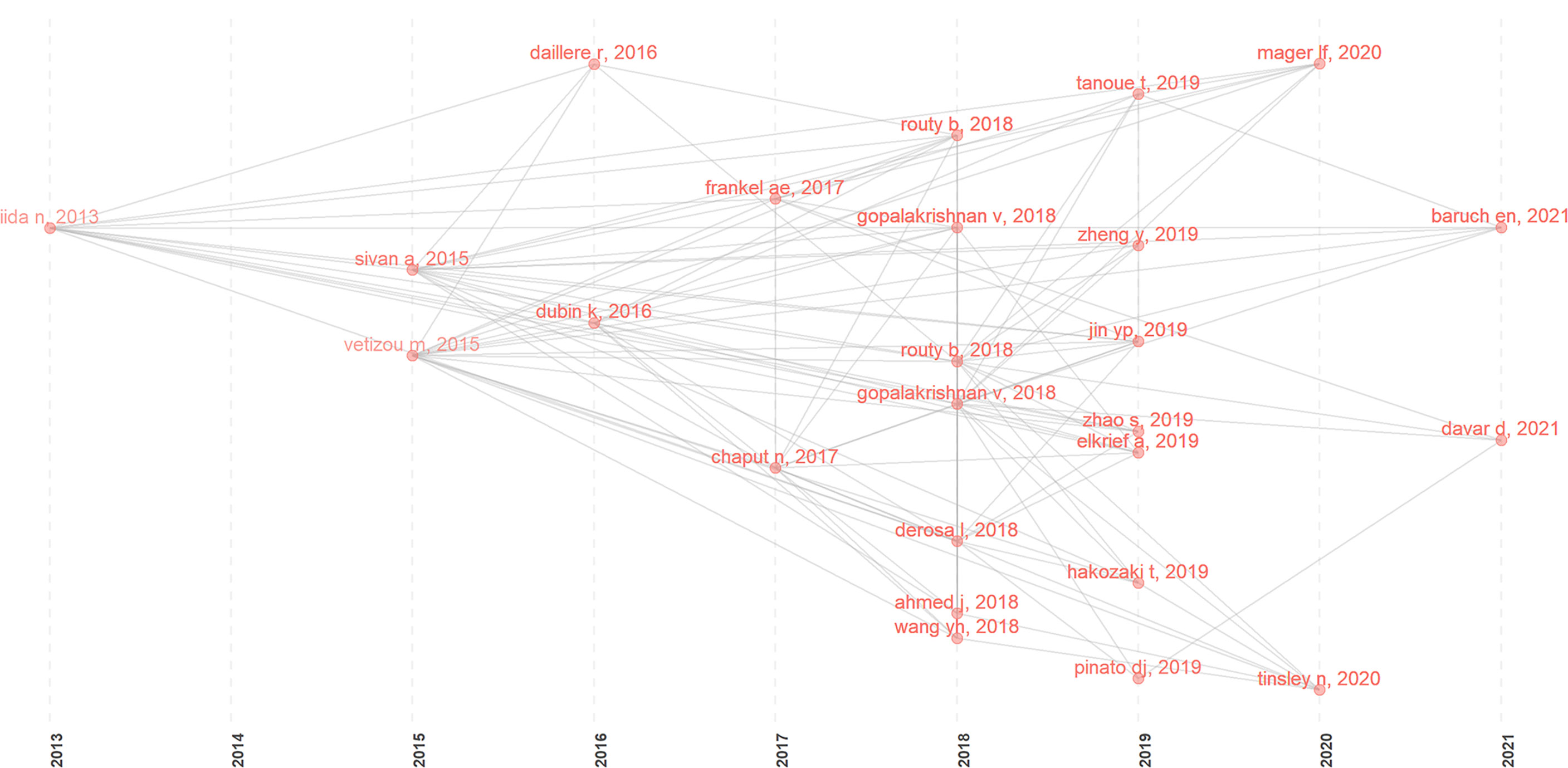
Figure 6 Historical direct citation network in GM/CI research (the gray lines between the dots indicate the citation relationship, and each dot represents an article, distinguished by author name and published year).
Several classic articles appeared from 2013 to 2021 (Figure 6 clearly shows the citation relationship), most of which were published in 2018 and 2019. As we can see, the earliest node was a paper in 2013, which found that disruption of the commensal microbiota could impair subcutaneous tumor response to CpG-oligonucleotide immunotherapy and platinum chemotherapy (24). In 2015, two studies (25, 26) in Science showed that the GM can influence the efficacy of ICIs, and manipulating the GM may modulate ICIs. In 2016, a prospective study demonstrated that increased representation of bacteria belonging to the Bacteroidetes phylum is correlated with resistance to the development of ICI-induced colitis (27). Another study showed that Enterococcus hirae and Barnesiella intestinihominis can promote anti-tumor immune response (28). In 2017, an article showed that baseline GM enriched with Faecalibacterium and other Firmicutes were associated with a beneficial response to ipilimumab and a higher incidence of ipilimumab-induced colitis (29). A clinical study identified specific human GM and metabolites associated with the efficacy of ICIs in melanoma patients (30).
In 2018, two studies (31, 32) published in Science revealed that primary resistance to ICIs can be attributed to GM, significant differences were observed in the diversity and composition of the GM between responders and non-responders, antibiotics and fecal microbiota transplantation (FMT) can influence the anti-tumor effect of ICIs. Two clinical studies (33, 34) showed that antibiotic use is associated with the reduced clinical benefit of ICIs in cancer patients. Simultaneously, a clinical study (35) showed that FMT can treat refractory ICIs-associated colitis. Two reviews (12, 36) further summarized the impact of the GM on CI. In 2019, Tanoue T et al. (37) identified 11 healthy human-associated bacterial strains that work together to induce interferon-γ-producing CD8 T cells, which in turn, together with ICIs, effectively inhibit tumor growth. Two clinical studies (38, 39) revealed a strong correlation between GM diversity and response to anti-PD-1 immunotherapy in hepatocellular carcinoma and non-small cell lung cancer (NSCLC) patients. Four clinical studies (40–43) found that antibiotic therapy is associated with reduced response to ICIs in routine practice and affects the prognosis of patients treated with ICIs. In 2020, an article (44) showed that microbiome-derived inosine can modulate response to ICIs immunotherapy. In 2021, two clinical studies (45, 46) showed that FMT can promote melanoma patients’ response to CI and overcome resistance to anti-PD-1 therapy.
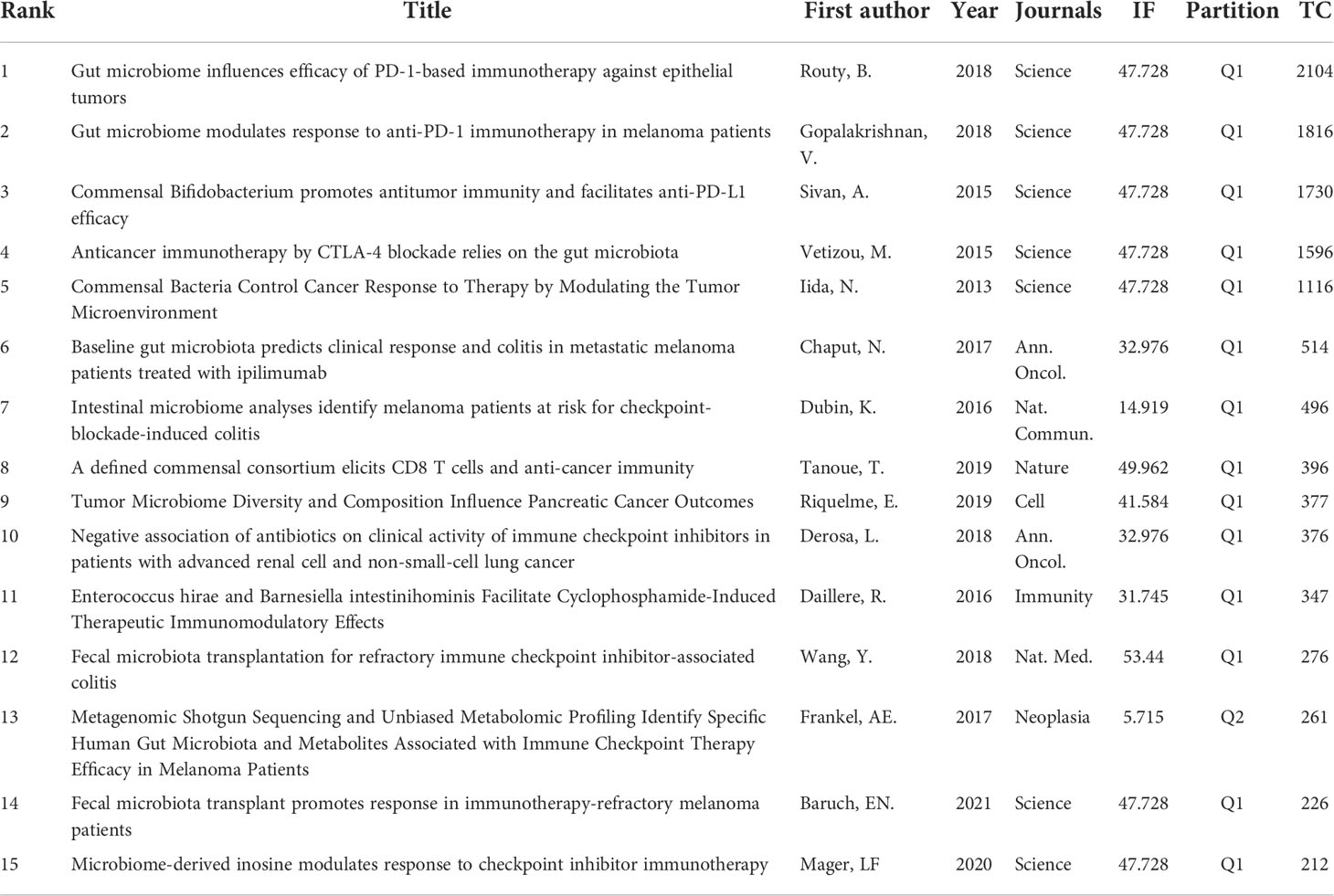
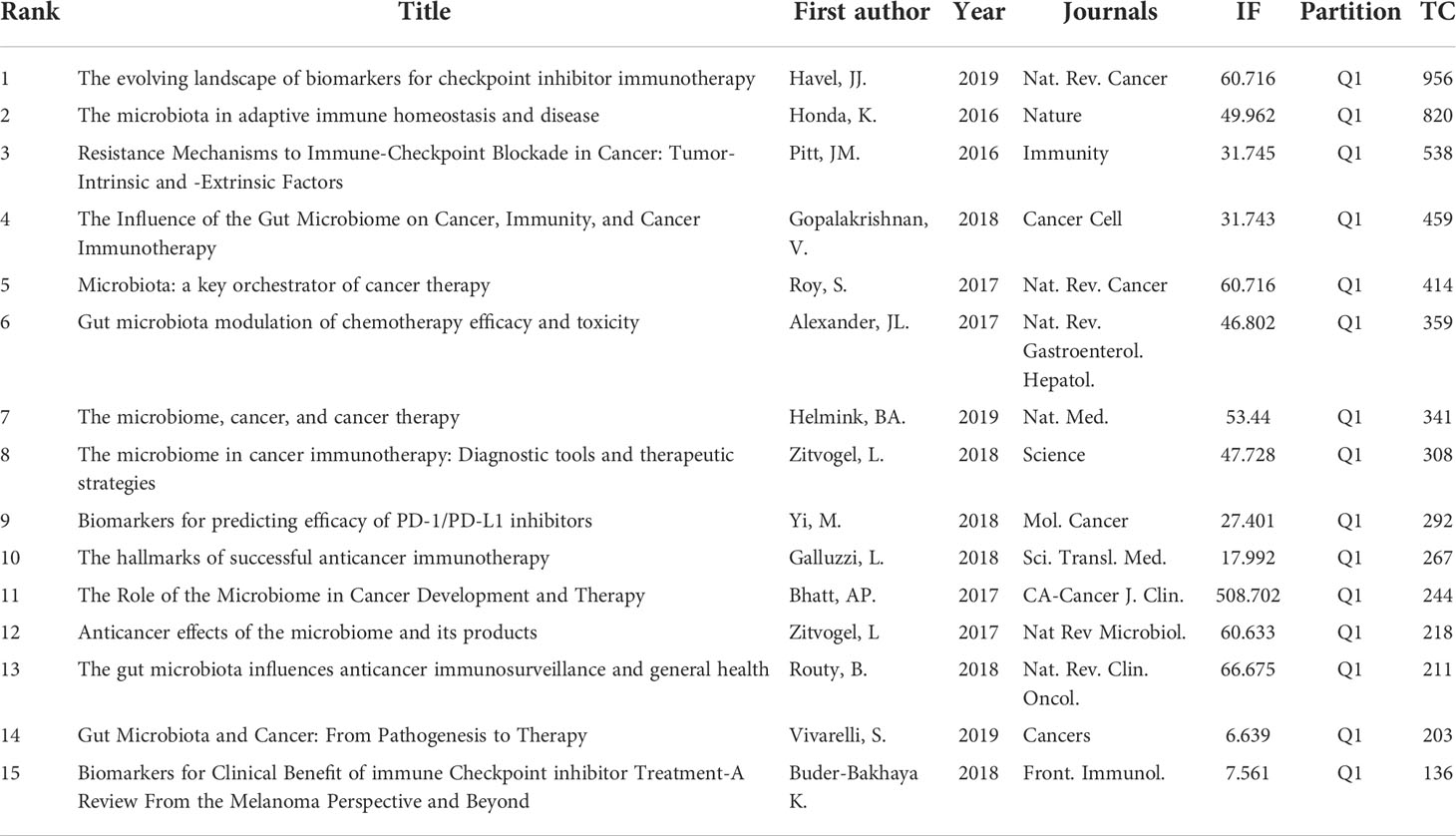
Top 15 most cited papers in GM/CI research
Highly cited articles are generally articles with extremely high academic value and great professional influence in a field, which are also one of the most valuable indicators in bibliometric methods. Table 7 and Table 8 list the 15 most cited papers in original research and reviews worldwide.
The top 15 most cited original articles were mainly published between 2013 and 2021, nearly half of which were from the Science, with an average IF of 39.828. Some original research has been outlined above, including the efficacy of ICIs targeting cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) (26), PD-1 (31, 32) and PD-L1 (25) were closely related to GM; the GM can predict clinical response and adverse effects in cancer patients treated with ICIs (27, 29, 30); antibiotics can alter the GM and affect the clinical efficacy of ICIs (33); specific gut bacteria (28, 37), microbial metabolites such as inosine (44) and probiotics such as Bifidobacterium (25) can enhance host resistance and the efficacy of ICIs; FMT can play an active role in altering the GM to modulate CI, which can not only promote response in patients with immunotherapy-refractory melanoma (45), but also successfully treat refractory ICI-related colitis (35). Aside from that, the GM has a regulatory effect on the tumor microenvironment. Related studies showed that the GM can mediate its effects by modulating myeloid-derived cell function in the tumor microenvironment (24), and the interactions of the pancreatic adenocarcinoma microbiota and the GM can influence the host immune response and tumor growth (47).
A review is an informative document condensed on the basis of a large number of collected papers, which can provide researchers with comprehensive information, guide scientific research, help readers understand the new progress, existing problems and future directions of the discipline, and provide timely guidance for further research on the basis of grasping the dynamics of the discipline. The top 15 most cited review articles were mainly published between 2016 and 2019, nearly half of their articles were from Nature and its sub-journals, with an average IF of 71.897. Among them, four review articles (48–51) mentioned GM can be used as a biomarker for predicting the efficacy of ICIs immunotherapy. Several review articles (12, 13, 24, 36, 52–56) outlined the effect of the GM on oncology, immunity, and the efficacy and adverse effects of CI. There is a crosstalk between GM and immune cells. A paper (57) outlined the interactions of T and B cells of the immune system and the GM. A review (58) mentioned interventions to modify the microbiome to aid in cancer treatment, including FMT, antibiotic therapy, prebiotics, dietary interventions, or drugs that alter the composition of the GM.
Given that high-cited papers were mostly published in the high IF-journals and were mainly original research, we aggregated GM/CI-related original research articles published in high IF-journals (may get more citations in the future) and ranked them by TC (Supplementary Material S2). A total of 20 articles were extracted, of which Science had the most publications (n=12), accounting for 60%, Nature and Cell each published three papers, each accounting for 15%, and Nature Medicine published two papers, accounting for 10%. Compared with previous studies (24–26, 31, 32), which mainly focused on the relationship between GM and CI outcomes, the mechanism research of the GM/CI correlation from the perspective of metabolism (44, 59) and immunity (60–62) and the intervention research that alter the GM to improve the efficacy of CI, such as FMT (45, 46), dietary fiber and probiotics (63), Enterococcus peptidoglycan (64) and Enterococcus bacteriophage (65), have gradually become the focus of GM/CI research in high-IF journals in the last few years.
Analysis of keywords
Analysis of high-frequency keywords in GM/CI
To determine the hotspots in GM/CI, it is necessary to examine an important index in the literature—keywords. The frequency of keywords and the cluster analysis of high-frequency keywords based on the co-occurrence of keywords can indicate the current research hotspots and themes in a certain field. In this study, a total of 2447 keywords (1109 author’s keywords and 1138 keywords plus) were extracted from the imported papers. The author’s keywords and keywords plus with the top 50 frequencies were represented in wordcloud via the R tool’s Bibliometrix packages (Figure 7A, B). Among author’s keywords, the most used keywords (exclude search terms) are “efficacy”, “antibiotics”, “probiotics”, “biomarkers”, “fecal microbiota transplantation”, “diet”, etc. Among keywords plus, the most used keywords (exclude search terms) are “Fusobacterium-nucleatum”, “inflammation”, “regulatory t-cells”, “short chain fatty-acids”, “resistance”, “dendritic cells”, “survival”, “metabolites”, etc.
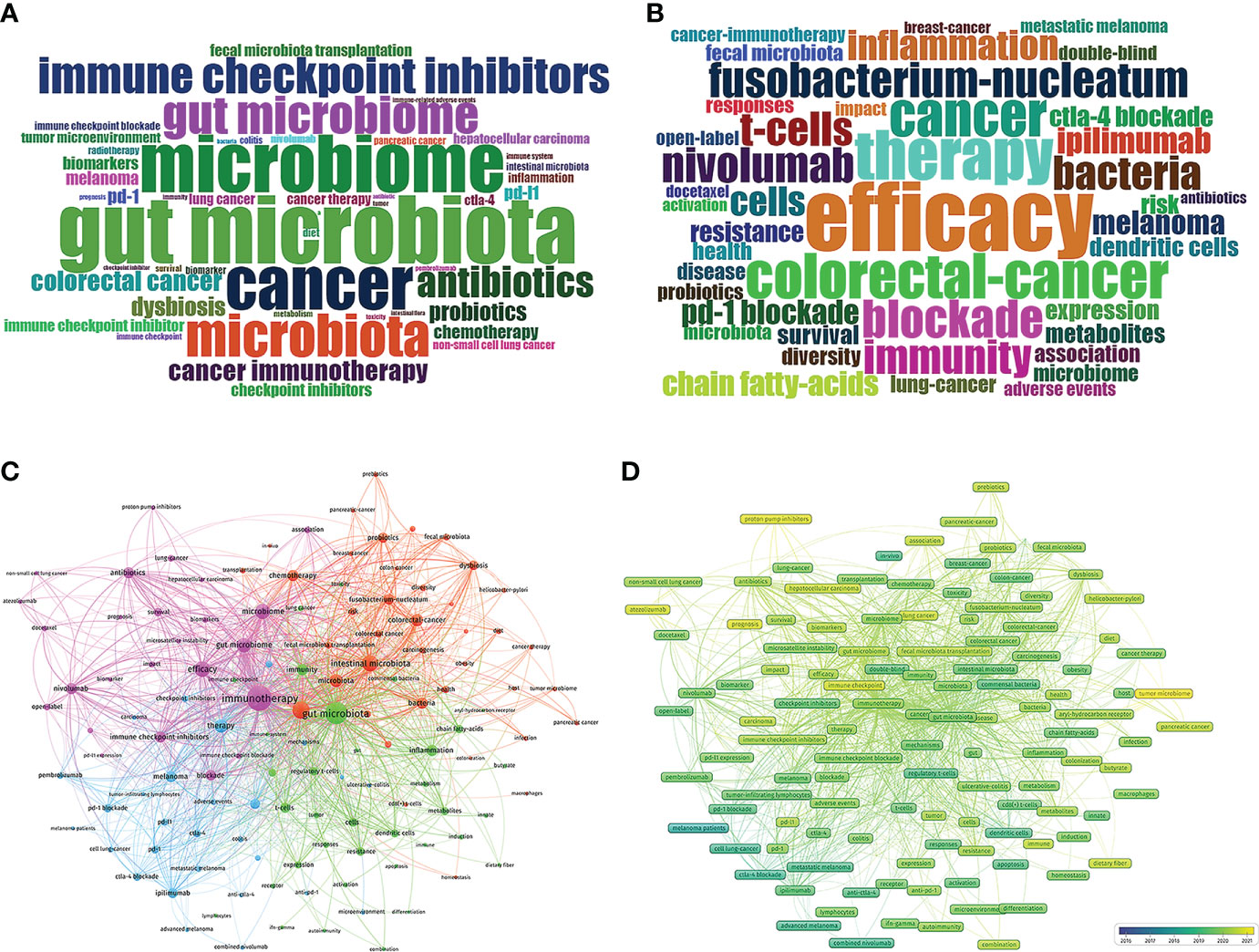
Figure 7 (A) Distribution of top 50 Author Keywords in GM/CI. (B) Distribution of top 50 Keywords Plus in GM/CI. (C) Cluster analysis of high-frequency keywords (frequency ≥10) based on all keywords of publications in GM/CI (different colors represent different clusters, the size of the circle represents the frequency the keywords appear, and the thickness of the line represents the total link strength between keywords). (D) Trends in keywords (frequency ≥ 10) over time based on all keywords of publications in GM/CI (the blue boxes represent the earliest keywords and the yellow boxes represent the latest keywords).
Based on the number of publications with co-occurrence keywords and the association strength of keywords, a clustering analysis was performed to assess the relationship between the identified items via VOSviewer. This study treated each clustered keywords as a category and classifies all individuals based on the same color. As shown in Figure 7C, we divided common keywords (frequency ≥ 10, 127 items) into 4 clusters based on the total link strength between keywords and represented them with different colors.
Cluster 1 (purple topic, 25 items) is mainly related to the association between GM and efficacy of CI (such as GM as a biomarker to predict the efficacy and prognosis of CI) and the effect of some drugs such as antibiotics on efficacy of CI. Cluster 2 (red topic, 39 items) is primarily related to the links between GM and cancer (such as Fusobacterium-nucleatum and colorectal-cancer), as well as the important role of diet, FMT, probiotics and prebiotics in cancer and CI. Cluster 3 (green topic, 35 items) is mainly related to the mechanism by which the GM affects cancer and immunotherapy, including immunity (such as regulatory T cells and dendritic cells), inflammation, and metabolism (such as short chain fatty acids and butyrate). Cluster 4 (blue topic, 28 items) is related to specific applications of ICIs in melanoma and adverse events such as colitis. The previous research on the links between GM and CI mainly focused on melanoma may be related to ICIs were the first approved for melanoma treatment.
Analysis of the development trends of high-frequency keywords in GM/CI
The development and change of keywords over time can reflect the evolution of frontier knowledge to a certain extent and lead the future research direction in a field. Through the Overlay Visualization of VOSviewer, similar to concurrency graphs, we predicted the trends for the next few years in GM/CI. VOSviewer uses different colors for each keyword in the image based on the average year they appear and frequency (year. frequency) in all included publications, as shown in Figure 7D, where the blue boxes represent the earliest keywords and the yellow boxes represent keywords appear in recent years and may be future research directions. From 2016 to 2021, there were relatively unbalanced development dynamics in four clusters, more yellow nodes were in the cluster 1 and 2 and the main keywords for yellow nodes included “tumor microbiome (2020.93)”, “proton pump inhibitors (2020.69)”, “prognosis (2020.64)”, “atezolizumab (2020.55)”, “immune checkpoint” (2020.50), “immune checkpoint inhibitor” (2020.48), “hepatocellular carcinoma” (2020.44), “lung cancer” (2020.44), “pancreatic cancer (2020.33)”, etc.
Discussion
At present, immunotherapy plays an increasingly important role in tumor treatment, among which ICIs have received the most attention, but there are still some problems that could lead to treatment delay and even early termination, such as low response rate to ICIs and immune-related adverse events in some cancer patient. Over the past decade, with the deepening understanding of GM and the increasing evidence which suggest GM is one of the modulators that can alter the efficacy and toxicity of CI, the studies on improving the efficacy of CI by modulating GM have gradually increased. Based on the above reasons, this paper conducts a bibliometric analysis on the role of GM in CI for the first time and allows researchers to have a general understanding of the current status of the links between GM and CI.
Analysis of characteristics of literature
The number and trend of publications in a certain field can reflect the development stage it has gone through. From the annual Np, 2012-2014 and before 2012 (only 5 articles in 2004-2011) belonged to the initial stage of GM/CI research, 2015-2017 was in a steady development stage, and 2018-2021 was in a stage of rapid development. In 2010, a human gut microbial gene catalog established by metagenomic sequencing opened up a new direction for the GM research. In 2011, the first ICI Ipilimumab was approved by the US FDA for the second-line treatment of advanced melanoma, marking a new era of CI. Since then, GM and CI began to collide. In 2015, two blockbuster studies (25, 26) found that GM can influence the efficacy of ICIs. Correspondingly, the correlation research between GM and CI began to increase. Since 2018, the GM/CI research has received increasing attention from scholars, which may be due to the rapid development of ICIs and GM research and widespread clinical application of ICIs.
Our analysis showed that Frontiers in Immunology, International Journal of Molecular Sciences and Cancers published the most papers. Moreover, Frontiers in Immunology had the highest H-index. Frontiers in Immunology is the official journal of the International Union of Immunological Societies (IUIS), supports full open access, and is a leading journal in the field of immunology. The higher the academic quality of an academic journal, the easier it will attract the attention of the academic community and be read by more experts and scholars, and the greater its influence may be. The most cited articles were published in Science, followed by Nature, Nature Reviews Cancer, Immunity, and Annals of Oncology. These journals are internationally renowned and have greater international influence. Among them, Science has the highest TC, and the top 5 highly cited papers were all from the journal, indicating this influential and prestigious journal is more likely to publish high-quality research in the future. Nature and its related sub-journals published most of the highly cited reviews, indicating that these journals are more likely to publish high-quality review articles in the future.
Countries, institutions and authors may not affect the quality of papers, but bibliometrics can show their contributions to a particular field. These papers mainly came from the United States and China, followed by Italy, France and Japan. The United States had the most Np and was at the core of global cooperation. China had seen a surge in the Np and had outnumbered the United States in terms of annual Np. This can be related to the high attention and financial support of the government and the community of these two countries on the GM program and CI research. For example, the United States launched the Human Microbiome Project and the National Microbiome Initiative in 2007 and 2016, respectively, and China launched the Chinese Academy of Sciences Microbiome Program in 2017. Furthermore, a 2020 study (66) showed that the United States and China dominated cancer cell therapy in the world and China had surpassed the United States in the number of clinical trials. Notably, despite the Np in China was considerable, the average citations were far lower than that of other countries and the highly cited papers were lacking, suggesting that China still needs to further improve the quality of articles.
The top 10 institutions were from the United States, China and France, half of which were in the United States, demonstrating its good scientific research capabilities in GM/CI. Univ Texas MD Anderson Canc Ctr, one of the three earliest comprehensive cancer treatment centers designated by the National Cancer Program in the United States and one of the most authoritative cancer hospitals in the world, had published the most articles. Most of the top 10 authors were from Gustave Roussy and Univ Paris Saclay in France. The former is the first cancer center in Europe, and the latter is a comprehensive and world-class research university jointly built by a number of French educational institutions and national institutes. The most published author was Laurence Zitvogel, an immunologist from France, who has contributed to the field of CI and is a leading pioneer in the study of the relationship between GM and CI, with the highest H-index and TC. In terms of the impact of papers, Routy B is the first author of the most cited article and Zitvogel L is its corresponding author. In addition, Gopalakrishnan V, Sivan A, Vetizou M and Iida N also participated in the writing of this highest cited paper, and they respectively contributed a high cited paper as the first author. They can be considered as outstanding contributioners.
Historical evolution in the GM/CI research
Through highly cited papers and historical citation network analysis, we have a general understanding of the development process of GM/CI research. From 2012 to 2014, animal studies (24, 67) showed that GM can influence local and systemic inflammation and stimulate anti-tumor immune responses of CpG-oligonucleotide immunotherapy and chemotherapy. In 2015, two blockbuster preclinical animal studies (25, 26) were the first to link GM to ICIs response. One of them found that the composition of GM can affect the treatment response of PD-L1 and oral administration of Bifidobacterium can improve anti-tumor effects of PD-L1 by enhancing the function of dendritic cells (25). The other one (26) showed that the anti-tumor effect of CTLA-4 was inhibited in germ-free or antibiotic-treated melanoma mice, and the supplementation with Bacteroides fragilis could overcome this defect, which was related to the activation of Th1 cells in tumor draining lymph node and the induction of maturation of DCs in tumors. Since then, more and more research efforts have been devoted to GM/CI studies. In 2018, correlation studies of GM and CI began to shift from preclinical mouse models to cancer patients. Two representative studies (31, 32) showed that composition and diversity of GM can predict response to ICIs immunotherapy, which were of milestone significance in the field of GM/CI. One of the studies with the highest TC clearly demonstrated the significant impact of GM on CI, revealed that primary resistance to ICIs can be attributed to aberrant GM composition, antibiotics inhibited the clinical benefit of ICIs and FMT can improved the anti-tumor response of ICIs, and suggested that regulation of specific gut bacteria can avoid the primary resistance of ICIs (31). The other study (32) found that in melanoma patients receiving PD-1 immunotherapy, significant differences were observed in the diversity and composition of the GM between responders and non-responders. Systemic anti-tumor immunity was enhanced in responder patients and germ-free mice with favorable GM that received FMT from responders. From 2019 to 2020, many clinical studies began to appear, some retrospective clinical studies (40–43) have shown that antibiotics are associated with decreased survival and reduced response to ICIs in cancer patients. Some prospective clinical studies (38, 39) confirmed a significant association between GM and ICIs outcomes in advanced solid tumors. Simultaneously, mechanistic studies are also further in-depth to microbial metabolites. In 2021, clinical research went further into the therapeutic area. Two clinical trials (45, 46) showed that FMT from ICIs responders combined with anti-PD-1 therapy can overcome the resistance to PD-1 in melanoma patients.
In addition, as can be seen from the International Clinical Trials Registry Platform (ICTRP, https://trialsearch.who.int/), the GM/CI-related clinical observational trials (ChiCTR2100047045, ChiCTR2100047044, NCT04682327, NCT05008861, NCT05065515, JPRN-UMIN000041822) and interventional trials especially FMT-related studies (ChiCTR2100043472, NCT04163289, NCT04758507, NCT04924374, NCT05273255, NCT05279677, NCT05286294) have also gradually increased in recent years. All in all, the GM/CI research has gone through the process from basic research to clinical application. Notably, since 2020, the COVID-19 pandemic has challenged the status of immunotherapy (68) and had an important impact on GM/CI research. A study (69) conducted in the early stages of the pandemic showed that scientists were spending significantly less time on research than they did before the pandemic and scientific output for many researchers has declined in 2020 compared to 2019. Moreover, affected by the COVID-19 pandemic, some researchers have also begun to link GM/CI research with the COVID-19 (70–72), for example, immunocompromised cancer patients, including those with COVID-19, may restore their anti-tumor immune responses when receiving ICIs therapy (71), COVID-19 may affect the GM, and then could affect the efficacy of immunotherapy (70).
Research hotspots and trends of GM/CI
Hotspots are determined by high-frequency keywords, cluster analysis of keywords and highly cited papers. By analyzing these elements, this study found that the current hotspots of GM/CI research are mainly concentrated in three aspects: (1) GM can be used as a biomarker to predict the efficacy and toxicity of CI. (2) GM can affect the efficacy of CI and some potential mechanisms may explain this phenomenon. (3) Modulation of GM can improve or reduce the efficacy of CI.
Firstly, currently available immunotherapy drugs are expensive and only some cancer patients respond to CI, requiring the identification of reliable predictive biomarkers to improve the accuracy of treatment selection. Some articles (48–51) and a meta-analysis (73) have mentioned that GM can be used as a biomarker to distinguish whether patients will respond to ICIs or not and predict the efficacy and adverse reaction of CI. In general, the higher the diversity of GM, the higher the response to CI (32). A 2017 study (29) showed that baseline GM may predict clinical response and colitis in patients with metastatic melanoma treated with ipilimumab. A review (74) summarizing the relationship between the GM and immunotherapy of different cancers indicated that the GM can be used as a predictive biomarker for clinical response in CI. Some bacterial species such as Akkermansia muciniphila, Bacteroides fragilis, Bifidobacterium spp., Faecalibacterium spp., Ruminococcaceae spp., Eubacterium limosum and Enterococcus hirae have been associated with favorable anti-tumor immune response (12, 39, 75). Moreover, a prospective study (27) demonstrated that increased abundance of the Bacteroidetes phylum is correlated with resistance to the development of ICIs-induced colitis. Another study (76) showed that the toxicity of ICIs was associated with a significant increase in the abundance of Bacteroides intestinalis.
Secondly, the efficacy is the most critical factor to evaluate the anti-tumor treatment, and it is also the most concerned part of doctors and patients. Therefore, most studies on the correlation between the GM and CI mainly focus on the effect of GM on CI outcomes. It has been confirmed that the GM has a significant relationship with CI outcomes in melanoma (77), NSCLC (78), hepatocellular carcinoma (39) and renal cell carcinoma (79). Many studies (25, 26, 31) have shown that the GM can affect the host’s response to PD-1, PD-L1 and CTLA-4, and the supplementation of specific bacterial species can restore or enhance the response to ICIs (75). Furthermore, the GM is also associated with response and toxicity of chimeric antigen receptor (CAR) T-cells therapy (80). As far as the current studies are concerned, the GM has an important impact on CI mainly by affecting immunity and metabolism. On the one hand, the effect of GM on CI acts through the local and systemic immune systems and the production of anti-inflammatory cytokines (13, 75). T and B cells of the immune system interact with the GM to influence CI (57). The GM plays a role in inducing the formation or reprogramming of immune cells such as regulatory T cells (81), interferon-γ-producing CD8 T cells (37) and interferon-dependent monocyte (61), which are associated with anti-tumor response. On the other hand, the GM can impact local and systemic anti-tumor immune responses by means of metabolites, such as inosine (44) and short-chain fatty acids (82), which may be the link between GM and CI efficacy. Moreover, the GM can modulate the outcomes of ICIs through antigen-specific mechanisms and antigen-independent mechanisms (83). The immuno-oncology-microbiome axis, also known as cancer-microbiome-immune axis, has been proposed to explain this phenomenon (84, 85).
Thirdly, the GM is a potential target for enhanced efficacy and reduced toxicity of CI. Modifying GM can not only improve clinical efficacy of CI, but also reduce the occurrence of adverse events. Microbiota-centric interventions could be the next breakthrough in CI. At present, the related research on intervening the GM to adjust CI mainly includes the following aspects: (1) Fecal microbiota transplantation: FMT is a common method for clinically altering GM, which can improve the efficacy of ICIs and reduce their side effects (86). In 2018, a clinical study (35) in Natural Medicine demonstrated that FMT can effectively treat ICIs-associated colitis. In 2021, two clinical trials in Science found that FMT can promote the response in immunotherapy-refractory melanoma patients (45) and FMT from ICIs-responders combined with anti-PD-1 therapy can overcome resistance to PD-1 blockade in melanoma patients (46). (2) Provision of specific microbiota: Microbiota with immune-enhancing effects or modified beneficial microbiota can be used as adjuvants for immunotherapy. For example, a consortium of 11 bacterial strains isolated from healthy human feces can enhance the efficacy of ICIs (37). Enterococcus peptidoglycan (64) and Enterococcus bacteriophage (65) can also improve curative effect of CI. (3) Probiotics and Prebiotics: Probiotic use is associated with favorable clinical outcomes in patients receiving anti-PD-1 therapy (87). Oral administration of Bifidobacterium combined with anti-PD-1 therapy can improve the effect of PD-L1 by enhancing the function of dendritic cells (25) and Bifidobacterium can reduce the toxicity of CTLA-4 by relying on regulatory T cells (88). Administration of adjunctive probiotic Lactobacillus rhamnosus Probio-M9 can enhance the efficacy of anti-PD-1 by restoring antibiotic-disrupted GM (89) and Lactobacillus rhamnosus GG can enhance anti-tumor immune responses in a CD8 T cell-dependent manner (90). (4) Diet and Dietary fiber: The interactions between dietary patterns and GM can affect the inflammatory response and the efficacy of CI. For instance, Western diet has negative effects on both the GM and immune system, while the Mediterranean diet is the exact opposite (91); Microbiota modulation with a high-fiber diet can trigger the intratumoral monocyte reprogramming and improve the efficacy of ICIs (61). Furthermore, dietary fiber can also influence GM and anti-tumor immune response (63). Combining dietary and nutritional approaches that alter the GM with immunotherapy could provide a new avenue for cancer therapy (68). Several studies (NCT04645680, NCT04866810, NCT05356182) are investigating the relationship between dietary interventions and ICIs. (5) Antibiotics: Unlike the above, antibiotics mainly play a negative role in CI. Antibiotics can disrupt the homeostasis of GM, thereby affecting the efficacy of CI (31, 33). At present, several clinical studies (40–43) have shown that antibiotic use is associated with reduced response to ICIs and affects the prognosis of patients treated with ICIs. Two meta-analyses also showed that antibiotics use is associated with worse clinical outcomes in cancer patients treated with ICIs (92, 93).
Keywords analysis showed that some emerging research fields (the yellow nodes in Figure 7D) are becoming the focus of scholars and may be future research directions. (1) Tumor microbiome (TM): Although the correlation of the GM and CI outcomes has been well studied, data on the role of the local TM, an important part of the tumor microenvironment, are insufficient. Some studies have explored the correlation between the TM and CI and found the TM can also promote oncogenesis (94) and affect the treatment effect (95). The interaction of the TM and the GM influenced the host immune response and tumor growth (47). A newly published article showed that the GM can affect the TM through translocation or other manners (15). (2) Proton pump inhibitors (PPIs): Antacids such as PPIs are often used in cancer patients, and these drugs have the potential to alter GM and the efficacy of anti-tumor therapy (96, 97). Two meta-analyses demonstrated that concomitant antacid could alter the activity of ICIs, PPIs were inversely associated with progression free survival and overall survival (98, 99). (3) Prognosis: By measuring the abundance and diversity of GM, we can quantify the relative proportions of recognized “beneficial” or “harmful” bacteria, which can predict treatment outcomes and prognosis, and help guide treatment decisions and regimens. In the future, the composition of the GM may be combined with other known outcome-related factors to predict the prognosis of CI. (4) Immune checkpoint inhibitors: As shown in the above keywords analysis, ICIs are not only a current research hotspot, but also a research focus in the next few years. ICIs are booming in the field of tumor treatment with good efficacy and safety. In the future, with the wide application of ICIs among different tumor types, there will be increasing papers related to GM and ICIs. (5) Hepatocellular carcinoma, lung cancer and pancreatic cancer: Previous research have focused on melanoma, and as indications for ICIs expand, more cancer types are being studied. For example, some studies show that the GM is associated with clinical response to CI in pancreatic cancer and hepatocellular carcinoma (38, 94, 100), and its composition may predict response to ICIs in patients with these cancers and influence the efficacy of CI (38, 100).
Limitations of the article
Our research still has some limitations. First, only the publications included in the SCIE of WoSCC were searched and English articles were included, although it represents a certain level of research, it cannot cover all studies in different languages around the world. This is mainly because English is the most widely used language in the world and the WoS is one of the most authoritative citation index databases for global academic information. Moreover, it is difficult to mix documents of different languages and databases for bibliometric analysis simultaneously in the same software. Meanwhile, we also searched other document types of papers (such as editorial material, meeting abstract) and other languages and found that they had few citations and low impact. In view of this, this study makes this choice. Second, bibliometric analysis tools cannot analyze the complete text of publications at present, and some information may be ignored, which can also be used as a shortcoming. Therefore, we analyzed research content of highly cited papers and provide an overview of the mechanisms by which GM and CI interact and how to modulate the GM to enhance the efficacy of CI to make up for the shortcomings and flaws of this paper. Third, this study is only an analysis of papers at the current stage. Even though we have found the top cited articles, newly published articles may also have higher influence but may be cited less currently. With the rapid development of research on the correlation between GM and CI, more papers will be available for analysis.
Conclusion
This study shows that the research attention of GM/CI has gradually increased in recent 10 years; specific GM can be used as a biomarker to distinguish patients who respond to ICIs, determine the effectiveness of ICIs, and predict the efficacy and toxicity of CI. Modifying GM can not only improve cancer patients’ clinical efficacy but also reduce the occurrence of adverse events, thereby improving the patients’ survival and prognosis. Microbiota-centric interventions can be the next breakthrough in CI. Concretely speaking, we can change the GM of patients who do not respond to CI or drug-resistant patients by diet adjustment, probiotics use, FMT and so on, so as to restore the response of patients to CI and improve their survival. Research trend terms include TM, PPIs, prognosis and ICIs. In addition to finding gut-specific microbial signatures associated with CI, identifying tumor-specific microbial characteristics is also necessary and promising. Understanding the mechanism of interactions between GM and TM, and then modulating TM by regulating GM or directly regulating TM to enhance the efficacy of CI is an attractive research direction. Moreover, relevant clinical research on the role of FMT in CI is still limited to small sample case reports. With the continuous development of FMT-related research, it may have broad application prospects in CI. In the future, more in-depth research is needed to identify specific bacteria that affect CI and transform them for clinical application, so as to predict and improve the efficacy of CI and reduce the incidence of adverse reactions.
In summary, with the help of scientometrics and visual analysis methods, this study preliminarily shows the global research status and trends of interactions between GM and CI, provides researchers in related fields with a clearer understanding of the development process of GM/CI through historical direct citation analysis, and provides references for in-depth research in the GM/CI field by summarizing and forecasting current research hotspots and future development directions.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
SY: writing-original draft preparation, manuscript, investigation, and figure preparation. SZ: manuscript, investigation, and figure preparation. YY: investigation and figure preparation. LJ: investigation, methodology, supervision. YL: conceptualization, methodology, supervision. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China Youth Science Foundation Project (No. 81904003) Personnel Training Program of China-Japan Friendship Hospital Elite Project (No. ZRJY2021-TD05) and Beijing Natural Science Foundation Project (No. 7202184).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.952546/full#supplementary-material
References
1. Christofi T, Baritaki S, Falzone L, Libra M, Zaravinos A. Current perspectives in cancer immunotherapy. Cancers (Basel) (2019) 11(10):1472. doi: 10.3390/cancers11101472
2. Lee JB, Kim HR, Ha SJ. Immune checkpoint inhibitors in 10 years: Contribution of basic research and clinical application in cancer immunotherapy. Immune Netw (2022) 22(1):e2. doi: 10.4110/in.2022.22.e2
3. Falzone L, Salomone S, Libra M. Evolution of cancer pharmacological treatments at the turn of the third millennium. Front Pharmacol (2018) 9:1300. doi: 10.3389/fphar.2018.01300
4. Dubois M, Liscia N, Brunetti O, Ziranu P, Lai E, Argentiero A, et al. The role of immune checkpoint inhibitors in the treatment sequence of advanced gastric or gastro-esophageal junction cancer: A systematic review and meta-analysis of randomized trials. Crit Rev Oncol Hematol (2022) 173:103674. doi: 10.1016/j.critrevonc.2022.103674
5. Juarez-Garcia A, Sharma R, Hunger M, Kayaniyil S, Penrod JR, Chouaïd C. Real-world effectiveness of immunotherapies in pre-treated, advanced non-small cell lung cancer patients: A systematic literature review. Lung Cancer (2022) 166:205–20. doi: 10.1016/j.lungcan.2022.03.008
6. Chai Y, Wu X, Bai H, Duan J. Combined immunotherapy with chemotherapy versus bevacizumab with chemotherapy in first-line treatment of driver-Gene-Negative non-squamous non-small cell lung cancer: An updated systematic review and network meta-analysis. J Clin Med (2022) 11(6):1655. doi: 10.3390/jcm11061655
7. Yu H, Chen P, Xia L, Fu S, Chen C, Zhang X, et al. PD-1/PD-L1 inhibitor plus chemotherapy versus bevacizumab plus chemotherapy in first-line treatment for non-squamous non-small-cell lung cancer. J Immunother Cancer (2021) 9(11):e003431. doi: 10.1136/jitc-2021-003431
8. Li Y, Ma Y, Wu Z, Zeng F, Song B, Zhang Y, et al. Tumor mutational burden predicting the efficacy of immune checkpoint inhibitors in colorectal cancer: A systematic review and meta-analysis. Front Immunol (2021) 12:751407. doi: 10.3389/fimmu.2021.751407
9. Zhang QJ, Luan JC, Song LB, Cong R, Ji CJ, Zhou X, et al. Age-related differences in molecular profiles for immune checkpoint blockade therapy. Front Immunol (2021) 12:657575. doi: 10.3389/fimmu.2021.657575
10. Wu Z, Chen Q, Qu L, Li M, Wang L, Mir MC, et al. Adverse events of immune checkpoint inhibitors therapy for urologic cancer patients in clinical trials: A collaborative systematic review and meta-analysis. Eur Urol (2022) 81(4):414–25. doi: 10.1016/j.eururo.2022.01.028
11. Nadelmann ER, Yeh JE, Chen ST. Management of cutaneous immune-related adverse events in patients with cancer treated with immune checkpoint inhibitors: A systematic review. JAMA Oncol (2022) 8(1):130–8. doi: 10.1001/jamaoncol.2021.4318
12. Routy B, Gopalakrishnan V, Daillère R, Zitvogel L, Wargo JA, Kroemer G. The gut microbiota influences anticancer immunosurveillance and general health. Nat Rev Clin Oncol (2018) 15(6):382–96. doi: 10.1038/s41571-018-0006-2
13. Bhatt AP, Redinbo MR, Bultman SJ. The role of the microbiome in cancer development and therapy. CA Cancer J Clin (2017) 67(4):326–44. doi: 10.3322/caac.21398
14. Lu Y, Yuan X, Wang M, He Z, Li H, Wang J, et al. Gut microbiota influence immunotherapy responses: Mechanisms and therapeutic strategies. J Hematol Oncol (2022) 15(1):47. doi: 10.1186/s13045-022-01273-9
15. Park EM, Chelvanambi M, Bhutiani N, Kroemer G, Zitvogel L, Wargo JA. Targeting the gut and tumor microbiota in cancer. Nat Med (2022) 28(4):690–703. doi: 10.1038/s41591-022-01779-2
16. Kim J, Lee HK. Potential role of the gut microbiome in colorectal cancer progression. Front Immunol (2021) 12:807648. doi: 10.3389/fimmu.2021.807648
17. Zhou CB, Zhou YL, Fang JY. Gut microbiota in cancer immune response and immunotherapy. Trends Cancer (2021) 7(7):647–60. doi: 10.1016/j.trecan.2021.01.010
18. Li K, Feng C, Chen H, Feng Y, Li J. Trends in worldwide research in inflammatory bowel disease over the period 2012-2021: A bibliometric study. Front Med (Lausanne) (2022) 9:880553. doi: 10.3389/fmed.2022.880553
19. Zhao J, Lu Y, Qian Y, Luo Y, Yang W. Emerging trends and research foci in artificial intelligence for retinal diseases: Bibliometric and visualization study. J Med Internet Res (2022) 24(6):e37532. doi: 10.2196/37532
20. Liu Y, Xu Y, Cheng X, Lin Y, Jiang S, Yu H, et al. Research trends and most influential clinical studies on anti-PD1/PDL1 immunotherapy for cancers: A bibliometric analysis. Front Immunol (2022) 13:862084. doi: 10.3389/fimmu.2022.862084
21. Shen J, Shen H, Ke L, Chen J, Dang X, Liu B, et al. Knowledge mapping of immunotherapy for hepatocellular carcinoma: A bibliometric study. Front Immunol (2022) 13:815575. doi: 10.3389/fimmu.2022.815575
22. Ma L, Ma J, Teng M, Li Y. Visual analysis of colorectal cancer immunotherapy: A bibliometric analysis from 2012 to 2021. Front Immunol (2022) 13:843106. doi: 10.3389/fimmu.2022.843106
23. Ou Z, Qiu L, Rong H, Li B, Ren S, Kuang S, et al. Bibliometric analysis of chimeric antigen receptor-based immunotherapy in cancers from 2001 to 2021. Front Immunol (2022) 13:822004. doi: 10.3389/fimmu.2022.822004
24. Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science (2013) 342(6161):967–70. doi: 10.1126/science.1240527
25. Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, et al. Commensal bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science (2015) 350(6264):1084–9. doi: 10.1126/science.aac4255
26. Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science (2015) 350(6264):1079–84. doi: 10.1126/science.aad1329
27. Dubin K, Callahan MK, Ren B, Khanin R, Viale A, Ling L, et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun (2016) 7:10391. doi: 10.1038/ncomms10391
28. Daillère R, Vétizou M, Waldschmitt N, Yamazaki T, Isnard C, Poirier-Colame V, et al. Enterococcus hirae and barnesiella intestinihominis facilitate cyclophosphamide-induced therapeutic immunomodulatory effects. Immunity (2016) 45(4):931–43. doi: 10.1016/j.immuni.2016.09.009
29. Chaput N, Lepage P, Coutzac C, Soularue E, Le Roux K, Monot C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol (2017) 28(6):1368–79. doi: 10.1093/annonc/mdx108
30. Frankel AE, Coughlin LA, Kim J, Froehlich TW, Xie Y, Frenkel EP, et al. Metagenomic shotgun sequencing and unbiased metabolomic profiling identify specific human gut microbiota and metabolites associated with immune checkpoint therapy efficacy in melanoma patients. Neoplasia (2017) 19(10):848–55. doi: 10.1016/j.neo.2017.08.004
31. Routy B, Le Chatelier E, Derosa L, Duong C, Alou MT, Daillère R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science (2018) 359(6371):91–7. doi: 10.1126/science.aan3706
32. Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science (2018) 359(6371):97–103. doi: 10.1126/science.aan4236
33. Derosa L, Hellmann MD, Spaziano M, Halpenny D, Fidelle M, Rizvi H, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann Oncol (2018) 29(6):1437–44. doi: 10.1093/annonc/mdy103
34. Ahmed J, Kumar A, Parikh K, Anwar A, Knoll BM, Puccio C, et al. Use of broad-spectrum antibiotics impacts outcome in patients treated with immune checkpoint inhibitors. Oncoimmunology (2018) 7(11):e1507670. doi: 10.1080/2162402X.2018.1507670
35. Wang Y, Wiesnoski DH, Helmink BA, Gopalakrishnan V, Choi K, DuPont HL, et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat Med (2018) 24(12):1804–8. doi: 10.1038/s41591-018-0238-9
36. Gopalakrishnan V, Helmink BA, Spencer CN, Reuben A, Wargo JA. The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell (2018) 33(4):570–80. doi: 10.1016/j.ccell.2018.03.015
37. Tanoue T, Morita S, Plichta DR, Skelly AN, Suda W, Sugiura Y, et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature (2019) 565(7741):600–5. doi: 10.1038/s41586-019-0878-z
38. Jin Y, Dong H, Xia L, Yang Y, Zhu Y, Shen Y, et al. The diversity of gut microbiome is associated with favorable responses to anti-programmed death 1 immunotherapy in Chinese patients with NSCLC. J Thorac Oncol (2019) 14(8):1378–89. doi: 10.1016/j.jtho.2019.04.007
39. Zheng Y, Wang T, Tu X, Huang Y, Zhang H, Tan D, et al. Gut microbiome affects the response to anti-PD-1 immunotherapy in patients with hepatocellular carcinoma. J Immunother Cancer (2019) 7(1):193. doi: 10.1186/s40425-019-0650-9
40. Pinato DJ, Howlett S, Ottaviani D, Urus H, Patel A, Mineo T, et al. Association of prior antibiotic treatment with survival and response to immune checkpoint inhibitor therapy in patients with cancer. JAMA Oncol (2019) 5(12):1774–8. doi: 10.1001/jamaoncol.2019.2785
41. Zhao S, Gao G, Li W, Li X, Zhao C, Jiang T, et al. Antibiotics are associated with attenuated efficacy of anti-PD-1/PD-L1 therapies in Chinese patients with advanced non-small cell lung cancer. Lung Cancer (2019) 130:10–7. doi: 10.1016/j.lungcan.2019.01.017
42. Elkrief A, El Raichani L, Richard C, Messaoudene M, Belkaid W, Malo J, et al. Antibiotics are associated with decreased progression-free survival of advanced melanoma patients treated with immune checkpoint inhibitors. Oncoimmunology (2019) 8(4):e1568812. doi: 10.1080/2162402X.2019.1568812
43. Hakozaki T, Okuma Y, Omori M, Hosomi Y. Impact of prior antibiotic use on the efficacy of nivolumab for non-small cell lung cancer. Oncol Lett (2019) 17(3):2946–52. doi: 10.3892/ol.2019.9899
44. Mager LF, Burkhard R, Pett N, Cooke N, Brown K, Ramay H, et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science (2020) 369(6510):1481–9. doi: 10.1126/science.abc3421
45. Baruch EN, Youngster I, Ben-Betzalel G, Ortenberg R, Lahat A, Katz L, et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science (2021) 371(6529):602–9. doi: 10.1126/science.abb5920
46. Davar D, Dzutsev AK, McCulloch JA, Rodrigues RR, Chauvin JM, Morrison RM, et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science (2021) 371(6529):595–602. doi: 10.1126/science.abf3363
47. Riquelme E, Zhang Y, Zhang L, Montiel M, Zoltan M, Dong W, et al. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell (2019) 178(4):795–806.e12. doi: 10.1016/j.cell.2019.07.008
48. Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer (2019) 19(3):133–50. doi: 10.1038/s41568-019-0116-x
49. Yi M, Jiao D, Xu H, Liu Q, Zhao W, Han X, et al. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol Cancer (2018) 17(1):129. doi: 10.1186/s12943-018-0864-3
50. Galluzzi L, Chan TA, Kroemer G, Wolchok JD, López-Soto A. The hallmarks of successful anticancer immunotherapy. Sci Transl Med (2018) 10(459):eaat7807. doi: 10.1126/scitranslmed.aat7807
51. Buder-Bakhaya K, Hassel JC. Biomarkers for clinical benefit of immune checkpoint inhibitor treatment-a review from the melanoma perspective and beyond. Front Immunol (2018) 9:1474. doi: 10.3389/fimmu.2018.01474
52. Roy S, Trinchieri G. Microbiota: a key orchestrator of cancer therapy. Nat Rev Cancer (2017) 17(5):271–85. doi: 10.1038/nrc.2017.13
53. Alexander JL, Wilson ID, Teare J, Marchesi JR, Nicholson JK, Kinross JM. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat Rev Gastroenterol Hepatol (2017) 14(6):356–65. doi: 10.1038/nrgastro.2017.20
54. Helmink BA, Khan M, Hermann A, Gopalakrishnan V, Wargo JA. The microbiome, cancer, and cancer therapy. Nat Med (2019) 25(3):377–88. doi: 10.1038/s41591-019-0377-7
55. Zitvogel L, Ma Y, Raoult D, Kroemer G, Gajewski TF. The microbiome in cancer immunotherapy: Diagnostic tools and therapeutic strategies. Science (2018) 359(6382):1366–70. doi: 10.1126/science.aar6918
56. Vivarelli S, Salemi R, Candido S, Falzone L, Santagati M, Stefani S, et al. Gut microbiota and cancer: From pathogenesis to therapy. Cancers (Basel) (2019) 11(1):38. doi: 10.3390/cancers11010038
57. Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature (2016) 535(7610):75–84. doi: 10.1038/nature18848
58. Zitvogel L, Daillère R, Roberti MP, Routy B, Kroemer G. Anticancer effects of the microbiome and its products. Nat Rev Microbiol (2017) 15(8):465–78. doi: 10.1038/nrmicro.2017.44
59. Han S, Van Treuren W, Fischer CR, Merrill BD, DeFelice BC, Sanchez JM, et al. A metabolomics pipeline for the mechanistic interrogation of the gut microbiome. Nature (2021) 595(7867):415–20. doi: 10.1038/s41586-021-03707-9
60. Schluter J, Peled JU, Taylor BP, Markey KA, Smith M, Taur Y, et al. The gut microbiota is associated with immune cell dynamics in humans. Nature (2020) 588(7837):303–7. doi: 10.1038/s41586-020-2971-8
61. Lam KC, Araya RE, Huang A, Chen Q, Di Modica M, Rodrigues RR, et al. Microbiota triggers STING-type I IFN-dependent monocyte reprogramming of the tumor microenvironment. Cell (2021) 184(21):5338–56.e21. doi: 10.1016/j.cell.2021.09.019
62. Goc J, Lv M, Bessman NJ, Flamar AL, Sahota S, Suzuki H, et al. Dysregulation of ILC3s unleashes progression and immunotherapy resistance in colon cancer. Cell (2021) 184(19):5015–30.e16. doi: 10.1016/j.cell.2021.07.029
63. Spencer CN, McQuade JL, Gopalakrishnan V, McCulloch JA, Vetizou M, Cogdill AP, et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science (2021) 374(6575):1632–40. doi: 10.1126/science.aaz7015
64. Griffin ME, Espinosa J, Becker JL, Luo JD, Carroll TS, Jha JK, et al. Enterococcus peptidoglycan remodeling promotes checkpoint inhibitor cancer immunotherapy. Science (2021) 373(6558):1040–6. doi: 10.1126/science.abc9113
65. Fluckiger A, Daillère R, Sassi M, Sixt BS, Liu P, Loos F, et al. Cross-reactivity between tumor MHC class I-restricted antigens and an enterococcal bacteriophage. Science (2020) 369(6506):936–42. doi: 10.1126/science.aax0701
66. Yu JX, Upadhaya S, Tatake R, Barkalow F, Hubbard-Lucey VM. Cancer cell therapies: the clinical trial landscape. Nat Rev Drug Discov (2020) 19(9):583–4. doi: 10.1038/d41573-020-00099-9
67. Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillère R, Hannani D, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science (2013) 342(6161):971–6. doi: 10.1126/science.1240537
68. Mokhtari RB, Sambi M, Qorri B, Baluch N, Ashayeri N, Kumar S, et al. The next-generation of combination cancer immunotherapy: Epigenetic immunomodulators transmogrify immune training to enhance immunotherapy. Cancers (Basel) (2021) 13(14):3596. doi: 10.3390/cancers13143596
69. Gao J, Yin Y, Myers KR, Lakhani KR, Wang D. Potentially long-lasting effects of the pandemic on scientists. Nat Commun (2021) 12(1):6188. doi: 10.1038/s41467-021-26428-z
70. Thompson NA, Stewart GD, Welsh SJ, Doherty GJ, Robinson MJ, Neville BA, et al. The MITRE trial protocol: A study to evaluate the microbiome as a biomarker of efficacy and toxicity in cancer patients receiving immune checkpoint inhibitor therapy. BMC Cancer (2022) 22(1):99. doi: 10.1186/s12885-021-09156-x
71. Vivarelli S, Falzone L, Torino F, Scandurra G, Russo G, Bordonaro R, et al. Immune-checkpoint inhibitors from cancer to COVID−19: A promising avenue for the treatment of patients with COVID−19 (Review). Int J Oncol (2021) 58(2):145–57. doi: 10.3892/ijo.2020.5159
72. Belluomini L, Caldart A, Avancini A, Dodi A, Trestini I, Kadrija D, et al. Infections and immunotherapy in lung cancer: A bad relationship. Int J Mol Sci (2020) 22(1):42. doi: 10.3390/ijms22010042
73. Limeta A, Ji B, Levin M, Gatto F, Nielsen J. Meta-analysis of the gut microbiota in predicting response to cancer immunotherapy in metastatic melanoma. JCI Insight (2020) 5(23):e140940. doi: 10.1172/jci.insight.140940
74. Oh B, Boyle F, Pavlakis N, Clarke S, Eade T, Hruby G, et al. The gut microbiome and cancer immunotherapy: Can we use the gut microbiome as a predictive biomarker for clinical response in cancer immunotherapy. Cancers (Basel) (2021) 13(19):4824. doi: 10.3390/cancers13194824
75. Wu J, Wang S, Zheng B, Qiu X, Wang H, Chen L. Modulation of gut microbiota to enhance effect of checkpoint inhibitor immunotherapy. Front Immunol (2021) 12:669150. doi: 10.3389/fimmu.2021.669150
76. Andrews MC, Duong C, Gopalakrishnan V, Iebba V, Chen WS, Derosa L, et al. Gut microbiota signatures are associated with toxicity to combined CTLA-4 and PD-1 blockade. Nat Med (2021) 27(8):1432–41. doi: 10.1038/s41591-021-01406-6
77. Peters BA, Wilson M, Moran U, Pavlick A, Izsak A, Wechter T, et al. Relating the gut metagenome and metatranscriptome to immunotherapy responses in melanoma patients. Genome Med (2019) 11(1):61. doi: 10.1186/s13073-019-0672-4
78. Hakozaki T, Richard C, Elkrief A, Hosomi Y, Benlaïfaoui M, Mimpen I, et al. The gut microbiome associates with immune checkpoint inhibition outcomes in patients with advanced non-small cell lung cancer. Cancer Immunol Res (2020) 8(10):1243–50. doi: 10.1158/2326-6066.CIR-20-0196
79. Derosa L, Routy B, Fidelle M, Iebba V, Alla L, Pasolli E, et al. Gut bacteria composition drives primary resistance to cancer immunotherapy in renal cell carcinoma patients. Eur Urol (2020) 78(2):195–206. doi: 10.1016/j.eururo.2020.04.044
80. Innao V, Allegra AG, Musolino C, Allegra A. New frontiers about the role of human microbiota in immunotherapy: The immune checkpoint inhibitors and CAR T-cell therapy era. Int J Mol Sci (2020) 21(23):8902. doi: 10.3390/ijms21238902
81. Liu Q, Sun Z, Chen L. Memory T cells: strategies for optimizing tumor immunotherapy. Protein Cell (2020) 11(8):549–64. doi: 10.1007/s13238-020-00707-9
82. Nomura M, Nagatomo R, Doi K, Shimizu J, Baba K, Saito T, et al. Association of short-chain fatty acids in the gut microbiome with clinical response to treatment with nivolumab or pembrolizumab in patients with solid cancer tumors. JAMA Netw Open (2020) 3(4):e202895. doi: 10.1001/jamanetworkopen.2020.2895
83. Hayase E, Jenq RR. Role of the intestinal microbiome and microbial-derived metabolites in immune checkpoint blockade immunotherapy of cancer. Genome Med (2021) 13(1):107. doi: 10.1186/s13073-021-00923-w
84. Sepich-Poore GD, Zitvogel L, Straussman R, Hasty J, Wargo JA, Knight R. The microbiome and human cancer. Science (2021) 371(6536):eabc4552. doi: 10.1126/science.abc4552
85. Jain T, Sharma P, Are AC, Vickers SM, Dudeja V. New insights into the cancer-Microbiome-Immune axis: Decrypting a decade of discoveries. Front Immunol (2021) 12:622064. doi: 10.3389/fimmu.2021.622064
86. Kang YB, Cai Y. Faecal microbiota transplantation enhances efficacy of immune checkpoint inhibitors therapy against cancer. World J Gastroenterol (2021) 27(32):5362–75. doi: 10.3748/wjg.v27.i32.5362
87. Takada K, Shimokawa M, Takamori S, Shimamatsu S, Hirai F, Tagawa T, et al. Clinical impact of probiotics on the efficacy of anti-PD-1 monotherapy in patients with nonsmall cell lung cancer: A multicenter retrospective survival analysis study with inverse probability of treatment weighting. Int J Cancer (2021) 149(2):473–82. doi: 10.1002/ijc.33557
88. Wang F, Yin Q, Chen L, Davis MM. Bifidobacterium can mitigate intestinal immunopathology in the context of CTLA-4 blockade. Proc Natl Acad Sci USA (2018) 115(1):157–61. doi: 10.1073/pnas.1712901115
89. Gao G, Ma T, Zhang T, Jin H, Li Y, Kwok LY, et al. Adjunctive probiotic lactobacillus rhamnosus probio-M9 administration enhances the effect of anti-PD-1 antitumor therapy via restoring antibiotic-disrupted gut microbiota. Front Immunol (2021) 12:772532. doi: 10.3389/fimmu.2021.772532
90. Owens JA, Saeedi BJ, Naudin CR, Hunter-Chang S, Barbian ME, Eboka RU, et al. Lactobacillus rhamnosus GG orchestrates an antitumor immune response. Cell Mol Gastroenterol Hepatol (2021) 12(4):1311–27. doi: 10.1016/j.jcmgh.2021.06.001
91. García-Montero C, Fraile-Martínez O, Gómez-Lahoz AM, Pekarek L, Castellanos AJ, Noguerales-Fraguas F, et al. Nutritional components in Western diet versus Mediterranean diet at the gut microbiota-immune system interplay. Implications Health Disease Nutrients (2021) 13(2):699. doi: 10.3390/nu13020699
92. Chen H, Han KD, He ZJ, Huang YS. How to choose a survival period? the impact of antibiotic use on OS or PFS in NSCLC patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Technol Cancer Res Treat (2021) 20:15330338211033498. doi: 10.1177/15330338211033498
93. Tsikala-Vafea M, Belani N, Vieira K, Khan H, Farmakiotis D. Use of antibiotics is associated with worse clinical outcomes in patients with cancer treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Int J Infect Dis (2021) 106:142–54. doi: 10.1016/j.ijid.2021.03.063
94. Pushalkar S, Hundeyin M, Daley D, Zambirinis CP, Kurz E, Mishra A, et al. The pancreatic cancer microbiome promotes oncogenesis by induction of innate and adaptive immune suppression. Cancer Discov (2018) 8(4):403–16. doi: 10.1158/2159-8290.CD-17-1134
95. Boesch M, Baty F, Albrich WC, Flatz L, Rodriguez R, Rothschild SI, et al. Local tumor microbial signatures and response to checkpoint blockade in non-small cell lung cancer. Oncoimmunology (2021) 10(1):1988403. doi: 10.1080/2162402X.2021.1988403
96. Hopkins AM, Kichenadasse G, McKinnon RA, Abuhelwa AY, Logan JM, Badaoui S, et al. Efficacy of first-line atezolizumab combination therapy in patients with non-small cell lung cancer receiving proton pump inhibitors: post hoc analysis of IMpower150. Br J Cancer (2022) 126(1):42–7. doi: 10.1038/s41416-021-01606-4
97. Qin BD, Jiao XD, Zhou XC, Shi B, Wang J, Liu K, et al. Effects of concomitant proton pump inhibitor use on immune checkpoint inhibitor efficacy among patients with advanced cancer. Oncoimmunology (2021) 10(1):1929727. doi: 10.1080/2162402X.2021.1929727
98. Rizzo A, Cusmai A, Giovannelli F, Acquafredda S, Rinaldi L, Misino A, et al. Impact of proton pump inhibitors and histamine-2-Receptor antagonists on non-small cell lung cancer immunotherapy: A systematic review and meta-analysis. Cancers (Basel) (2022) 14(6):1404. doi: 10.3390/cancers14061404
99. Wei N, Zheng B, Que W, Zhang J, Liu M. The association between proton pump inhibitor use and systemic anti-tumour therapy on survival outcomes in patients with advanced non-small cell lung cancer: A systematic review and meta-analysis. Br J Clin Pharmacol (2022) 88(7):3052–63. doi: 10.1111/bcp.15276
Keywords: immunotherapy, gut microbiota, cancer, research trends, highly cited papers, bibliometrics
Citation: Yang S, Zhao S, Ye Y, Jia L and Lou Y (2022) Global research trends on the links between gut microbiota and cancer immunotherapy: A bibliometric analysis (2012-2021). Front. Immunol. 13:952546. doi: 10.3389/fimmu.2022.952546
Received: 25 May 2022; Accepted: 05 August 2022;
Published: 24 August 2022.
Edited by:
Giandomenico Roviello, University of Firenze, ItalyReviewed by:
Peter Kokol, University of Maribor, SloveniaLuca Falzone, G. Pascale National Cancer Institute Foundation (IRCCS), Italy
Hilal Bashir, Institute of Microbial Technology (CSIR), India
Copyright © 2022 Yang, Zhao, Ye, Jia and Lou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liqun Jia, TGlxdW4tamlhQGhvdG1haWwuY29t; Yanni Lou, bG91eWFubmlAaG90bWFpbC5jb20=
 Shanshan Yang
Shanshan Yang Suya Zhao
Suya Zhao Yixiang Ye
Yixiang Ye Liqun Jia2*
Liqun Jia2*