
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 22 July 2022
Sec. T Cell Biology
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.952338
This article is part of the Research TopicAdaptive Immunity in Local TissuesView all 12 articles
 Wenxing Su1,2,3†
Wenxing Su1,2,3† Ji Zhang1†
Ji Zhang1† Shun Yang1
Shun Yang1 Minhui Tang2
Minhui Tang2 Yu Shen4
Yu Shen4 Cuiping Liu4
Cuiping Liu4 Jiang Ji1*
Jiang Ji1* Marcus Maurer5,6*
Marcus Maurer5,6* Qingqing Jiao2*
Qingqing Jiao2*Background: Atopic dermatitis (AD), a common type 2 inflammatory disease, is driven by T helper (TH) 2/TH22polarization and cytokines.Galectin-9 (Gal-9), via its receptor T cell immunoglobulin- and mucin-domain-containing molecule-3 (TIM-3), can promote TH2/TH22 immunity. The relevance of this in AD is largely unclear.
Objectives: To characterize the role of TIM-3 and Gal-9 in the pathogenesis of AD and underlying mechanisms.
Methods: We assessed the expression of Gal-9 and TIM-3 in 30 AD patients, to compare them with those of 30 healthy controls (HC) and to explore possible links with disease features including AD activity (SCORAD), IgE levels, and circulating eosinophils and B cells. We also determined the effects of Gal-9 on T cells from the AD patients.
Results: Our AD patients had markedly higher levels of serum Gal-9 and circulating TIM-3-expressing TH1 and TH17 cells than HC. Gal-9 and TIM-3 were linked to high disease activity, IgE levels, and circulating eosinophils and/or B cells. The rates of circulating TIM-3-positive CD4+ cells were positively correlated with rates of TH2/TH22 cells and negatively correlated with rates of TH1/TH17 cells. Gal-9 inhibited the proliferation and induced the apoptosis of T cells in patients with AD, especially in those with severe AD.
Conclusion: Our findings suggest thatGal-9, via TIM-3, contributes to the pathogenesis of AD by augmenting TH2/TH22 polarization through the downregulation of TH1/TH17immunity. This makes Gal-9 and TIM-3 interesting to explore further, as possible drivers of disease and targets of novel AD treatment.
Atopic dermatitis (AD) is a common T-cell mediated skin inflammatory disease, with abnormal activation of several subpopulations of T helper (TH)cells (1–3). We and others have shown that AD, in many patients, is characterized by excess TH2/TH22 cell activity (4–8). The increased production of TH2 cytokines such as interleukin (IL)-4 and IL-13 initiates a complex immune cascade that includes the generation of allergen-specific IgE-producing B cells and eosinophil migration to AD skin lesions (9–14), two hallmark features of AD. Furthermore, TH2 and TH22 cytokines inhibit skin barrier protein-encoding genes such as filaggrin, loricrin, and involucrin (15) and the production of antimicrobial peptides, both of which are held to contribute to the increased susceptibility to skin infections in patients with AD (16). The key role of TH2/TH22 cytokines in the pathogenesis of AD is supported by the efficacy of treatment with the anti-IL-4 receptor antibody dupilumab and an anti-IL-22 antibody (ILV-094) (17–19). As of now, it is largely unclear what drives TH2/TH22 skewing in AD.
Galectin-9 (Gal-9) is a tandem-repeat type galectin with two carbohydrate-recognition domains, and it was first identified as an eosinophil chemoattractant and activation factor (20, 21). It is universally expressed in a wide range of immune and non-immune cells and is known to regulate different biological functions, such as cell adhesion, differentiation, aggregation, and cell death (22). Galectin-9 is a versatile immunomodulator that has recently been shown to be associated with the pathogenesis of AD. For example, the skin of AD patients exhibits increased levels of Gal-9, especially in the epidermis, and increased numbers of Gal-9 positive eosinophils and mast cells (23). Blood levels of Gal-9, in patients with AD, were reported to be significantly higher than in healthy controls (HC) and correlated with disease activity (24).
Gal-9 exerts its biological functions via multiple receptors, including CD44 and T-cell immunoglobulin and mucin containing-protein 3 (TIM-3). TIM-3 is expressed by several populations of immune cells including terminally differentiated TH1, TH17, and Tc1 lymphocytes as well as NK, monocytes, and myeloid cells, whereas TH2/TH22 cells do not express TIM-3 (25). Gal-9 signaling via TIM-3 is held to modulate immune responses and diseases. For example, we have previously shown upregulation of Gal-9 and TIM-3 in the serum and peripheral blood mononuclear cells of patients with systemic lupus erythematosus (SLE), and this was closely related to disease activity (26). Gal-9, via TIM-3, induces apoptosis in TH1 and TH17 cells (27, 28), is involved in tolerance induction and T cell exhaustion (25, 27, 29, 30), and downregulates TH1/TH17-biased immune responses resulting in TH2 polarization. Whether or not TIM-3 plays a role in AD is currently unknown.
To address this question, we investigated patients with AD and HC for their Gal-9 serum levels and rates of circulating TIM-3-positive cells, we characterized the clinical relevance of Gal-9 and TIM-3 in AD, and we explored potential mechanisms that underlie their role in the pathogenesis of AD.
Ethical approval from the Ethics Committee of The First Affiliated Hospital of Soochow University (Suzhou, China, No. 2014809026) was obtained prior to the study. All patients provided written informed consent in accordance with the Helsinki Declaration of the World Medical Association. AD was diagnosed in accordance with the criteria of Hanifin and Rajka and disease severity was evaluated using the SCORing Atopic Dermatitis index (SCORAD), with 0–24, 25-50, and >50 points reflecting mild, moderate, and severe AD, respectively (31). At the time of the study and for one month prior, none of the patients were treated with systemic steroids or other immunosuppressant treatments, or potent topical steroids, or topical corticosteroids as well as other medications (e.g. antibiotics, light therapy ect.). Patients with other allergic conditions, e.g., pollen allergy, food allergy, or allergic asthma, et al. were excluded. Age-matched healthy blood donors were recruited as controls, all of whom were without any allergic conditions (n =30, female: 19; mean age: 10.4 ± 4.7 years).Pediatric allergy specialists and trained field technicians performed the physical examinations, SCORAD score assessments, and collected blood samples.
Laboratory investigation including blood routine examination and IgE levels. Total and specific IgE levels measured at the central laboratory (Central Labor, The Second Affiliated Hospital of Soochow University) using Immuno CAP System (Phadia Laboratory Systems, Thermo Fisher Scientific Inc, Uppsala, Sweden).
PBMCs were immediately isolated and purified from drawn blood as previously published (32). Briefly, PBMCs were isolated from heparinized venous blood on Ficoll-Hypaque gradients (Pharmacia, Uppsala, Sweden) and re-suspended in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with gentamicin (40μg/mL) and 10% pooled type AB normal human serum (Sigma-Aldrich).
T cells were stained with fluorescent-labeled monoclonal antibodies against CD-4-FITC (300538, Clone: RPA-T4, Biolegend), TIM-3-PE (345006, Clone: F38-2E2, Biolegend), IFN-γ-APC (502512, Clone: 4S.B3, Biolegend), IL-17A-APC (512334, Clone: BL168, Biolegend), IL-4-APC (500812, Clone: MP4-25D2, Biolegend), and IL-22-APC (366706, Clone: 2G12A41, Biolegend). Intracellular staining was performed as follows: Surface staining was performed for 20 minutes with CD-4-FITC and/or TIM-3-PE antibodies on ice. Cells were washed and resuspended in fixation/permeabilization solution (420801/421002, Biolegend) and stained with IFN-γ-APC, IL-17A-APC, IL-4-APC, and IL-22-APC. B cells were stained with CD19-PerCP (392510, Clone: 4G7, Biolegend). Armenian hamster IgG (400908, Clone: HTK88, Biolegend), mouse IgG1 (400108, Clone: RTK2071, Biolegend), mouse IgG2a (400246, Clone: MOPC-173, Biolegend), and mouse IgG 2b (400314, Clone: MPC-11, Biolegend) were used as isotype controls. Cells were analyzed with a Coulter Epics XL Flow cytometer (Beckman) and a Coulter FC 500 ANALYZER (Beckman Coulter); the relevant data were obtained and analyzed using FlowJo software, version 7.6.
For analysis of the Gal-9 level, serum was obtained by centrifuging peripheral blood samples (PBs) from patients with AD; the level of expression of Gal-9 in serum was detected using ELISA kits (AMS Biotechnology, UK), and the PBMC were separated by density gradient centrifugation. Cells from the interphase were collected and washed twice with Dulbecco’s PBS. For analysis of proliferation, apoptosis, cytokine production, freshly isolated PBMCs (1 × 105 cells/well) were cultured in RPMI 1640 medium (Gibco, USA) containing 10% human AB serum (Gibco) with Recombinant Gal-9 (0.5µg/mL, 1µg/mL, 2µg/mL, and 4µg/mL, ICA309Bo01, LMAI Bio) and LEAFTM Purified Anti-Human CD3 Antibody (100 ng/mL, BioLegend) in 96-well plates for 72 hours, respectively. For analysis of cell proliferation, cell viability was determined using a Cell Counting Kit-8 (CCK-8) assay kit (Beyotime Institute of Biotechnology, Beijing, China). Cells were stained with annexin V-FITC and PI to detect early apoptotic cells (annexin V positive, PI negative) and late apoptotic cells (annexin V positive, PI positive) by flow cytometry (BD PharMingen).
Statistical analysis and Figures were performed or made using GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA), respectively. The distribution of numerical variables were analyzed with the Kolmogorov-Smirnov test. Nonparametric tests were used for not normally distributed data. The relation between TIM-3 or Gal-9 expression level and clinical and laboratory characteristics was examined by Spearman’s or Pearson’s correlation coefficient rank test. Comparison analyses between the groups were carried out using the χ2 test, the Mann-Whitney U test, and the Friedman test. A P-value ≤ 0.05 was considered statistically significant.
A total of 30 AD patients (female: 20; mean age: 11.1 ± 6.0 years; aged 1-3 years, n=2; aged 3-5 years, n=5; aged 5-12 years, n = 10; aged 12-18 years, n=10; aged 18-20, n=3) were included after informed consent. As was previously reported, patients with AD had markedly higher serum levels of Gal-9, as compared to HC (3,030 ± 208 vs 1,330 ± 90 pg/ml, P<0.0001, Figure 1A). In addition, AD patients showed significantly higher rates of TIM-3-positive (TIM-3+) circulating CD4+ T cells (27.2 ± 2.9%vs 11± 1.1%, P<0.0001, Figure 1B and S1).In CD4+IFN-γ+ cells(TH1 cells), rates of TIM-3 expression were more than 3-fold higher in AD patients (17.7 ± 3.6% vs 4.9± 0.9%, P=0.003, Figure 1C, and S2), and rates in CD4+ IL-17A+ cells(TH17 cells) were twice as high, as compared to HC (12.5± 2.7%vs 6.1± 1.2%, P=0.033, Figure 1D and S3) (Table 1).
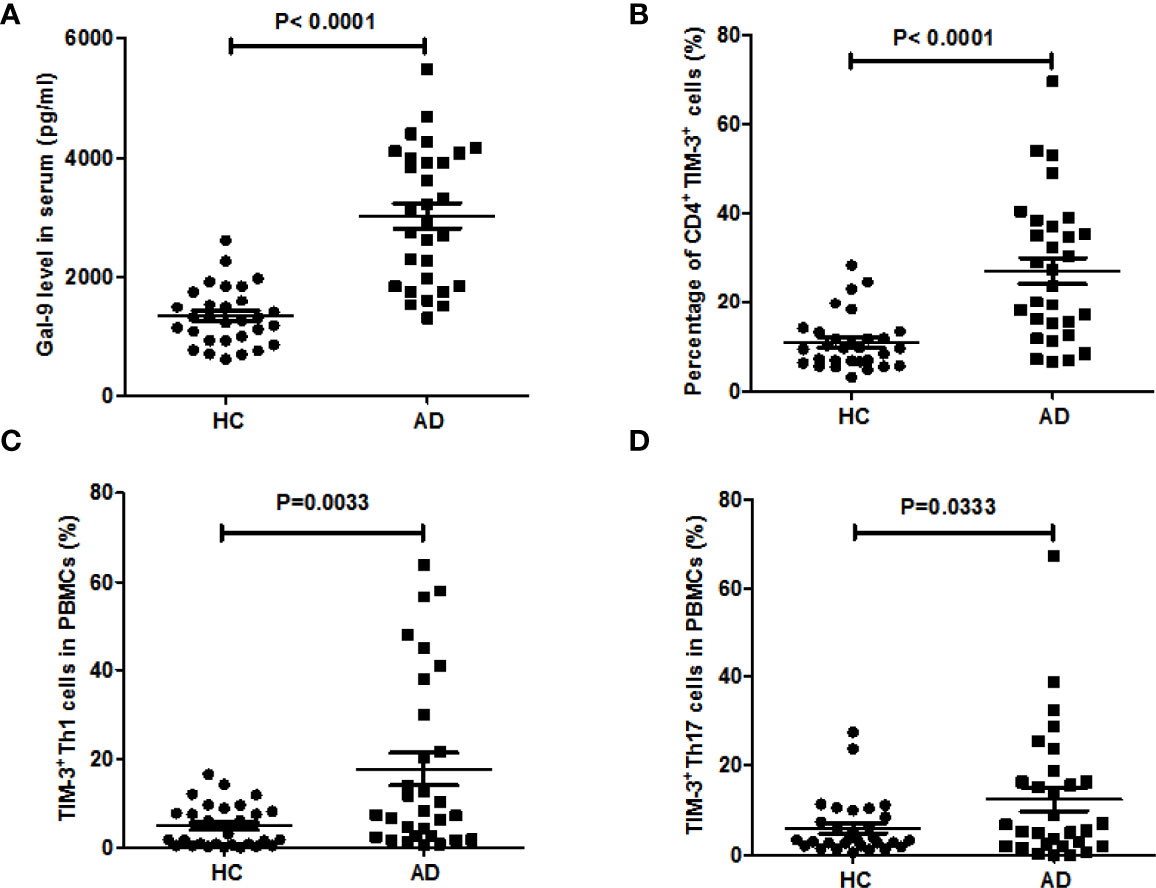
Figure 1 Blood levels of Gal-9 and TIM-3-positive T cells are markedly increased in patients with AD. (A) Serum Gal-9 levels of patients with atopic dermatitis involved in this study (AD, n = 30), as compared to healthy controls (HC, n = 30), (B) the percentage of CD4+TIM-3+ T cells in whole blood, the percentage of (C) TIM-3+TH1 cells and (D) TIM-3+TH17 cells in PBMCs of the above patients with AD compared to HC. These results are presented as means ± SEM.
Furthermore, serum Gal-9 levels were strongly and positively correlated with rates of circulating TIM-3+CD4+ T cells (r=0.6364, P=0.0002, Figure 2A). AD patients with high serum levels of Gal-9 had markedly higher rates of TIM-3+CD4+ T cells as compared toAD patients with low serum levels ofGal-9 (32.5 ± 4%vs 19.3± 3.9%, P=0.029, Figure 2B).Vice versa, patients with high rates of TIM-3+CD4+ T cells had markedly higher serum levels of Gal-9 than patients with low rates (3,532 ± 253 vs 2,456 ± 273 pg/ml, P=0.0074, Figure 2C).
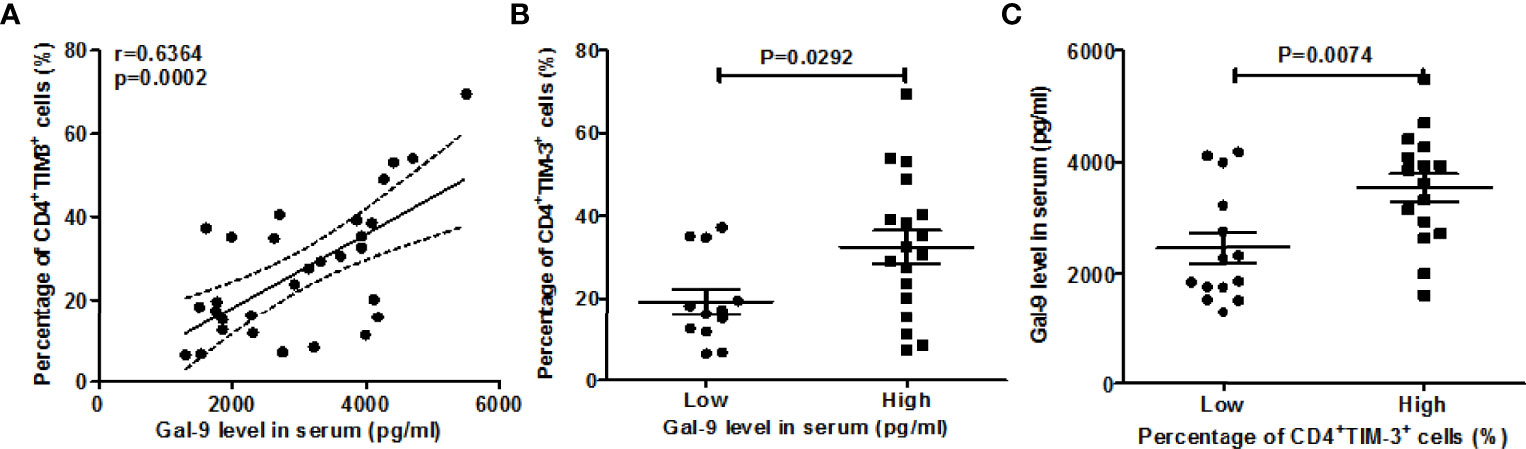
Figure 2 Correlation between Gal-9 levels and rates of TIM-3+CD4+ T cells. (A) Association between the percentage of TIM-3+CD4+ T cells and serum Gal-9 levels. For comparisons between groups, we divided the data based on Gal-9 level as low (<2659.13pg/ml), high (≥2659.13pg/m) and the frequency of TIM-3+ cells on CD4+ T cells as low (<21.9%), high (≥21.9%), respectively. The above cut-off values were 2 times the mean of HC test results. (B, C) The association of the serum Gal-9 level and the percentage of TIM-3+CD4+ T cells.
When we assessed these findings for their clinical relevance, increased circulating TIM-3+CD4+T cell populations in our AD patients were linked to higher disease activity, i.e. SCORAD values (r =0.6060, P=0.0004, Figure 3D), higher serum levels of total IgE (r =0.3633, P=0.048, Figure 3A), as well as higher number of circulating blood eosinophils (r =0.6126, P=0.0003, Figure 3B) and CD19+B cells (r =0.5120, P=0.0038, Figure 3C). Gal-9 serum levels showed similar links, albeit less pronounced (Figure 4), suggesting that TIM-3 and Gal-9 contribute to the course and pathogenesis of AD.
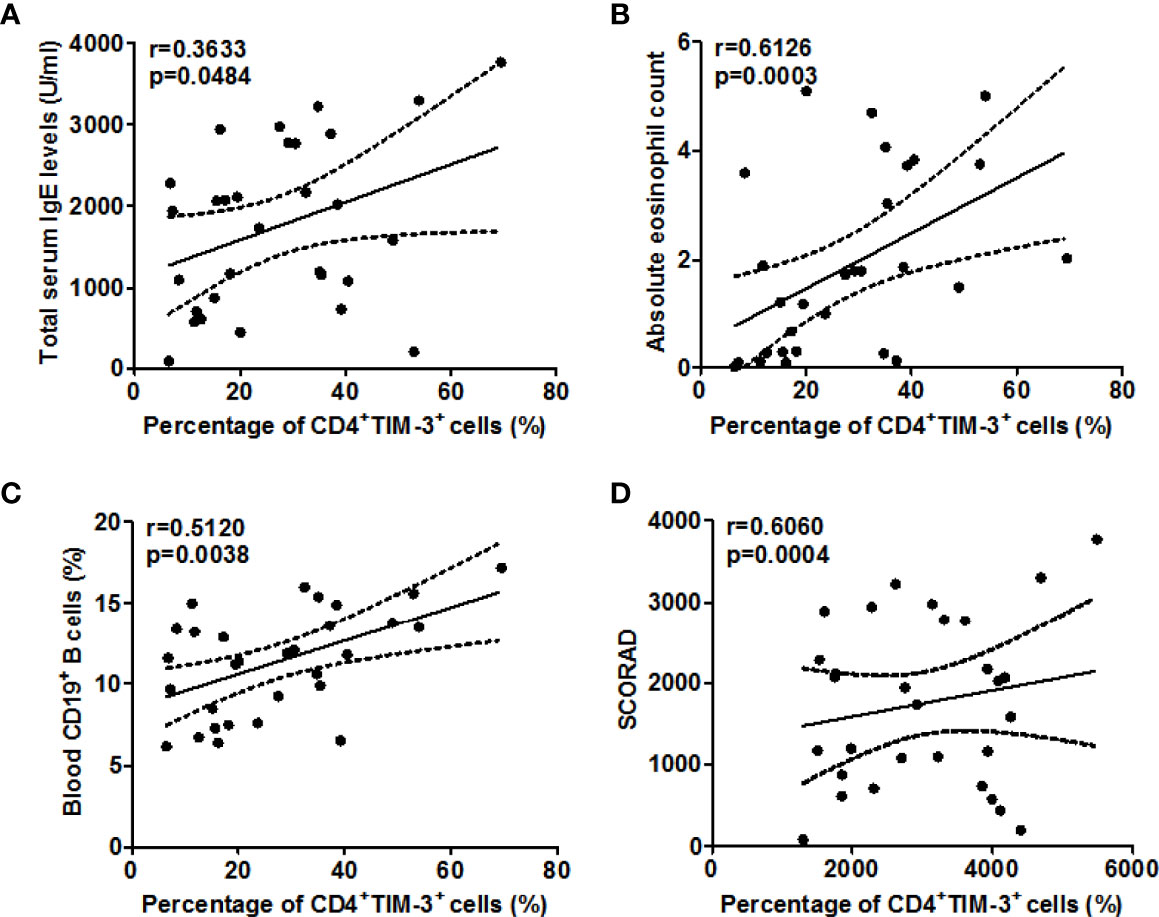
Figure 3 Association between the percentage of TIM-3+CD4+ T cells and total serum IgE (A), circulating eosinophils (B), blood CD19+ B cells (C), and disease activity as assessed by SCORAD (D) in patients with AD.
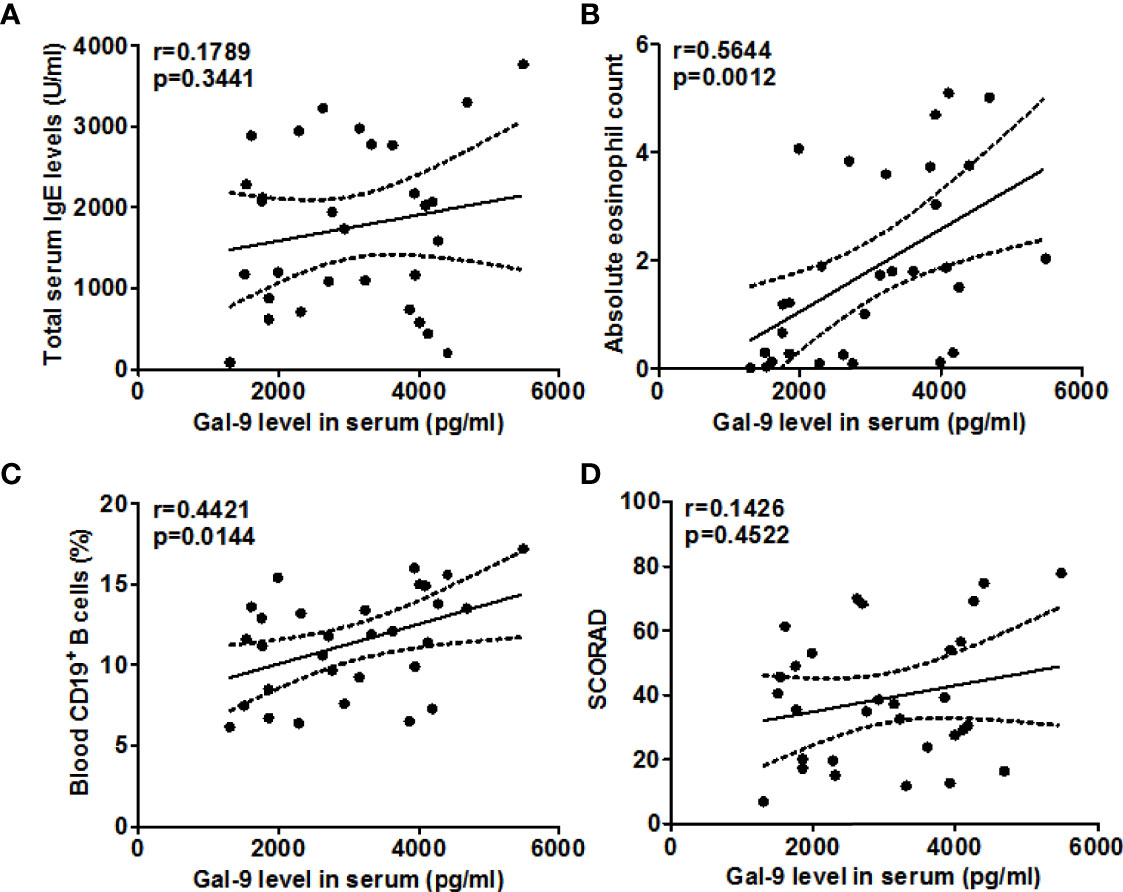
Figure 4 Association between the serum Gal-9 levels and total IgE (A), eosinophils (B), CD19+ B cells (C), and SCORAD (D) in patients with AD.
Next, we explored the role of TIM-3 and possible underlying mechanisms in AD. High rates of TIM-3+CD4+T cells in the blood of AD patients were strongly linked with high rates of TH22 cells (r=0.7633, P<0.0001, Figure 5A), and, in addition, with those of TH2 cells (r =0.5481, P <0.01, Figure 5B). In contrast, the rates of TIM-3+CD4+ T cells in the blood of our AD patients were negatively correlated, albeit weakly, with those of TH17 cells (r =-0.4372, P <0.05, Figure 5C), and, additionally, with those of TH1 cells (r =-0.4652, P <0.01, Figure 5D). Serum levels of Gal-9, in our AD patients, were also positively and negatively correlated with circulating TH22 cells (r=0.5904, P <0.001, Figure 6A) and TH17 cells (r =-0.4647, P <0.01, Figure 6C), respectively, suggesting that Gal-9, via TIM-3, downregulates TH1/TH17-immunity and drives TH2/TH22 polarization. However, there were no significant correlations between Gal-9 serum levels and circulating TH2 cells (Figure 6B) and TH1 cells (Figure 6D).
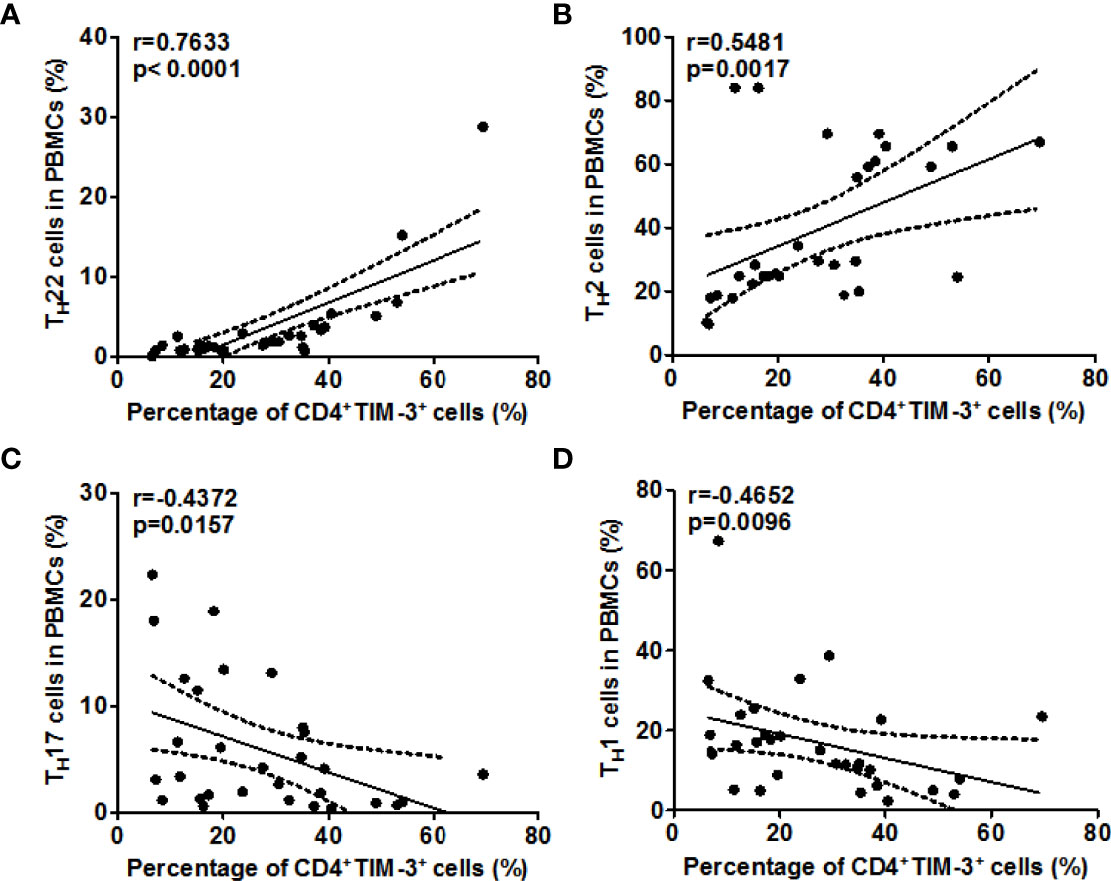
Figure 5 The correlation between TIM-3 levels and TH2/TH22 as well as TH1/TH17 cell ratios in AD. Association between the percentage of TIM-3+CD4+ T cells and the frequency of TH1 cells (A), TH2 cells (B), TH17 cells (C), and TH1 cells (D) in patients with AD.
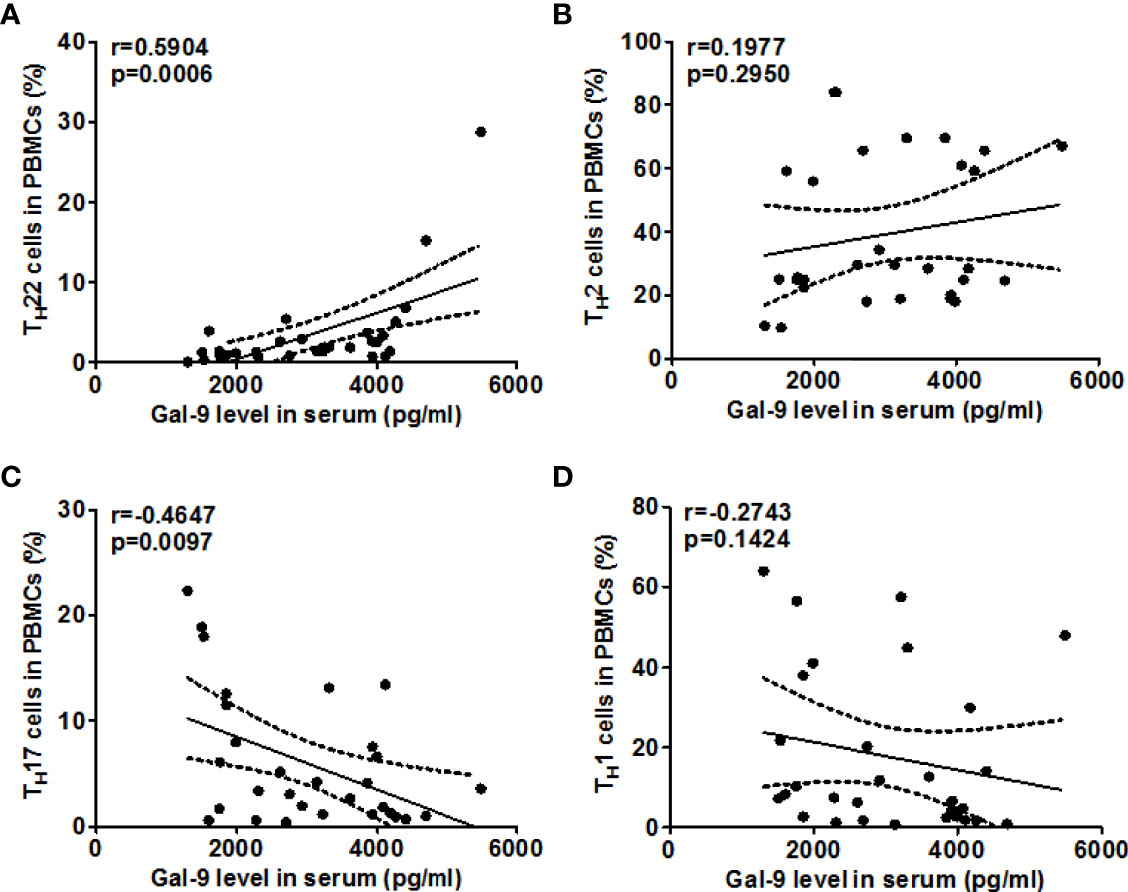
Figure 6 The correlation between Gal-9 levels and TH2/TH22 as well as TH1/TH17 cell ratios in AD. Association of Gal-9 levels and the percentage of TIM-3+CD4+ T cells and frequency of TH1 cells (A), TH2 cells (B), TH17 cells (C), and TH22 cells (D) in patients with AD.
Finally, we characterized the effects of TIM-3 activation of T cells by Gal-9 in AD and their clinical relevance. To this end, we stimulated circulating T cells in PBMC samples of patients with mild, moderate, or severe AD with Gal-9 and anti-CD3 and then assessed their proliferation and apoptosis. Gal-9 dose-dependently inhibited proliferation (Figure 7A) and induced apoptosis (Figure 7B) in PBMCs of AD patients. The inhibition of proliferation and induction of apoptosis by Gal-9 were highest in PBMCs of patients with severe AD and lowest in patients with mild AD (Figures 7A, B), linking Gal-9 effects on T cells to AD disease severity.
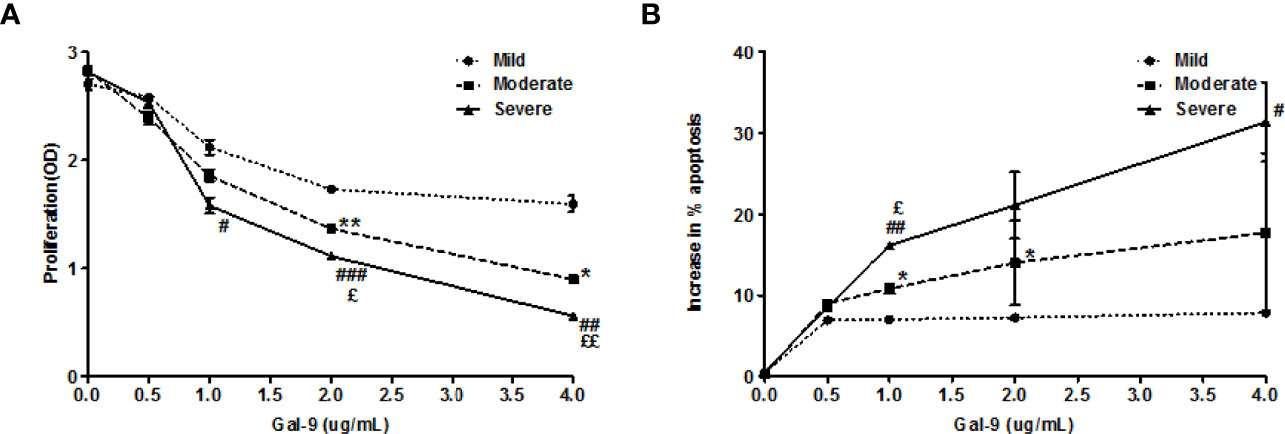
Figure 7 Gal-9 inhibits the proliferation and induces apoptosis in PBMCs from AD patients. (A) CCK-8 assay and (B) Apoptosis assay of PBMCs isolated from AD patients (Mild AD, n=11; Moderate AD, n=9; Severe AD, n=10) under graded doses of Gal-9 (0, 1.5, 5, 15, and 50 nM) and anti-CD3(100ng/ml) for 72 h, respectively. These results are presented as means ± SEM. *P <0.05, **P <0.01, ***P <0.001 Mild AD vs. Moderate AD; #P < 0.05, ##P < 0.01, ###P < 0.001 Mild AD vs. Severe AD. ζP < 0.05, ζζP < 0.01, ζζζP < 0.001 Moderate AD vs. Severe AD.
This study tiesGal-9 and its receptor, TIM-3, to the pathogenesis of AD. Both are upregulated in patients in AD and linked to disease features and activity. Our findings support the notion that Gal-9, via TIM-3, augments TH2/TH22 polarization and down regulates TH1/TH17immunityvia effects on CD4+ TH1 and TH17 cells.
That Gal-9 levels are elevated in AD is not a new finding (23, 33). In contrast, what our study shows for the first time, is that levels of CD4+ T cells that express the Gal-9 receptor TIM-3 are also markedly increased in patients with AD. TIM-3 is specifically expressed in TH1 and TH17 cells, but not in TH2 (33), and our AD patients showed triple and double the rate of TIM-3-expressing TH1 and TH17 cells, respectively, as compared to HC. These findings go against those reported by Kanai and coworkers, who reported numbers of TIM-3-expressing CD4+T cells to be similar in 9 AD patients as compared to HC (34). Possible explanations for this discrepancy include differences in patient populations, i.e. young Han Chinese patients in our study vs middle-aged Japanese patients, and the small number of patients studied. In addition to age, factors such as gender, genetics, and environmental factors also will influence the immunological profile of patients with AD (35).
Why are Gal-9 levels and rates of TIM-3+CD4+ T cells both upregulated in AD? Our study does and cannot answer this question and was not meant to. Further studies are needed to identify the underlying mechanisms. At least four scenarios could be relevant. First, elevated Gal-9 could increase the rate of TIM-3+CD4+ T cells. Second, TIM-3+CD4+ T cells could drive Gal-9 levels. Third, Gal-9 and TIM-3 expression may be upregulated by independent mechanisms. Fourth, increased Gal-9 and TIM-3 expression may be driven by the same signals. The first scenario is unlikely since Gal-9 inhibits the proliferation and induces apoptosis of TIM-3+ cells, as previously reported (36)and demonstrated by our findings in AD. That TIM-3+cells produce or induce the production of Gal-9, i.e. scenario two, is also unlikely. CD4+T cells have been reported to produce Gal-9 (37, 38), but other cells such as keratinocytes and mast cells are probably much more relevant sources of Gal-9 in AD (24). As for the third and fourth options, the fact that Gal-9 levels and rates of TIM-3+CD4+T cells are strongly correlated suggests that the mechanisms that drive the elevation of both are shared, at least in part, rather than independent. Since both are not only correlated with each other, but also linked to disease activity and, to a lesser extent, AD features such as IgE and blood eosinophils and B cells, it appears likely that what drives the increase in Gal-9 levels and rates of TIM-3+CD4+T cells in AD is AD itself. Thus, Gal-9 and TIM-3 may act as amplifiers of AD pathogenesis. This notion is supported by the observation that effective treatment of AD can result in the decline of Gal-9 levels.
Our results clearly show that, regardless of the cause, high rates of circulating TIM-3+T cells are linked to high AD disease activity, IgE levels, numbers of circulating eosinophils and B cells, as well as high rates of TH2/TH22 cells and low rates of TH1/TH17 cells. This was also so for Gal-9, albeit less pronounced. What explains this, at least in part, is that Gal-9, in our AD patients, inhibits T cell proliferation and induces T cell apoptosis and that both effects are linked to AD severity. The vicious feedback loop suggested by our results looks like this: High levels of Gal-9 and high levels of TIM-3 expressing TH1/TH17 cells make for strong inhibition of TH1/TH17 immunity and for TH2/TH22 polarization, which in turn comes with high levels of disease activity and inflammatory signals that may drive further Gal-9 and TIM-3 expressions.
As two target glycoproteins of Gal-9 have been identified, TIM-3 and CD44. Whether Gal-9 downregulates TH1/TH17 immunity via TIM-3 in AD? First, we observed that both Gal-9 level and the rate of TIM-3+CD4+ T cells are elevated in AD patients. Second, Gal-9 levels and rates of TIM-3+CD4+T cells are strongly correlated in our patients with AD. Third, in our AD patients, Gal-9 significantly inhibited T cell proliferation and induced T cell apoptosis. These results indicate that Gal-9 might via TIM-3 contributes to the inhibition of TH1/TH17 activation in AD. However, further experimental evidence is still needed, such as TIM-3 block experiment. And additional experiments with galectin inhibitors also need to be performed to clarify the specific mechanism of Gal-9-mediated suppression in AD.
Our study has several strengths and a few limitations. As for the former, for example, we assessed Gal-9 and TIM-3 in a sizeable and well-characterized patient population, together with clinical and other molecular markers. A major limitation of our study is its monocentric approach, which calls for confirmation of our results in a broader and more heterogeneous group of patients. A minor limitation of our study is that we only investigates the expression of Gal-9 and TIM-3 in blood samples. This is mainly due to blood samples are relatively easy to obtain, and blood source indicators have the potential to be developed into biomarkers in the later stage for AD. As skin biopsy is not a routine test for patients with AD. Besides, two studies have been reported that, increased Gal-9 expression in the skin lesions of AD patients (23, 24). Whereas, comparative studies on Gal-9 and TIM-3 expression in peripheral blood and lesions of AD are still needed.
Taken together, as summarized in Figure 8, upregulation of TIM-3/Gal-9 interaction, in AD, comes with downregulation of TH1/TH17 responses and more pronounced TH2/TH22 immunity. Our data suggest that the TIM-3/Gal-9 pathway may play an important role in the pathogenesis of AD, given that levels of TIM-3/Gal-9 are closely associated with disease activity, total serum IgE levels as well as blood eosinophil and B cell count. Further research is needed to clarify the molecular mechanisms that drive increased TIM-3/Gal-9 expression of TH1/TH17 cells in AD. In addition, future studies should aim to characterize TIM-3/Gal-9 expression on Tc1, NK, and myeloid cells as well as their levels in skin lesions of patients in AD.

Figure 8 Proposed model of the role of Gal-9 and TIM-3 in the pathogenesis of AD. Gal-9, via TIM-3 expressed by TH1/TH17 cells, downregulates their numbers, by inhibiting proliferation and the induction of apoptosis (1). The reduction of TH1/TH17 immunity leads to TH2/TH22 polarization (2). Increased TH2/TH22 immunity and cytokines drive type 2 inflammation and disease activity (3) with higher numbers of eosinophils(4) and B cell class switching to IgE and elevated IgE levels (5). This, in turn, may drive further upregulation of Gal-9 and TIM-3 expression (6). MBP (Major basic protein), ECP (Eosinophil cationic protein), EPO (Eosinophilperoxidase), SCF (Stem cell factor), VEGF-A (Vascular endothelial growth factor-A), NGF (Nerve growth factor).
● Atopic dermatitis (AD) is driven by TH2/TH22 polarization and cytokines.
● Galectin-9 (Gal-9) can promote TH2/TH22 immunity, via its receptor T cell immunoglobulin- and mucin-domain-containing molecule-3 (TIM-3).
● Gal-9 and TIM-3 are markedly upregulated in AD and linked to disease features.
● Gal-9 and TIM-3 levels are positively correlated with rates of TH2/TH22 cells and negatively correlated with rates of TH1/TH17 cells.
● TIM-3/Gal-9 inhibits the proliferation and induces apoptosis in AD T cells, and both effects are linked to disease severity.
The original contributions presented in the study are included in the article/Supplementary material. Further inquiries can be directed to the corresponding author/s.
This study was reviewed and approved by The Ethics Committee of The First Affiliated Hospital of Soochow University (Suzhou, China, No. 2014809026). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
WS, JZ, SY, YS, and CL performed experiments and analyzed the data. MT and JJ recruited patients. QJ, JJ, and MM analyzed data and wrote the manuscript. MM contributed to the critical revision of the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by grants from the National Natural Science Foundation of China (No. 82073434), the Medical Scientific Research Project of Jiangsu Health Commission 2020 (Z2020017), Suzhou Minsheng Technology-Medical and Health Application Foundation (SYS2020135), The Opening Project of State Key Laboratory of Radiation Medicine and Protection (GZK1201912), and The Assistance Project of Suzhou Key Medical Discipline (SZFCXK202118).
Author WS was employed by China National Nuclear Corporation 416 Hospital.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.952338/full#supplementary-material
Supplementary Figure 1 | Representative flow cytometry analysis of TIM-3 expression on CD4+T cells in whole blood of patients with AD and HC. Cells were initially gated on CD4+ T cells. Subsequently, the frequency of TIM-3+ cells CD4+ T cells was analyzed by flow cytometry in the whole blood of AD patients and HC, respectively.
Supplementary Figure 2 | Representative flow cytometry analysis of TIM-3 expression on CD4+IFN-γ+ T cells in PBMCs from AD patients and HC. Cells were initially gated on CD4+ T cells. Then IFN-γ+cells were selected out of the gated CD4+ T cells. Subsequently, the frequency of TIM-3+ cells on CD4+IFN-γ+ T cells was analyzed by flow cytometry in PBMCs from AD patients and HC, respectively.
Supplementary Figure 3 | Representative flow cytometry analysis of TIM-3 expression on CD4+IL-17A+ T cells in PBMCs from AD patients and HC. The cells were initially gated on CD4+ T cells. Then IL-17A+ cells were selected out of the gated CD4+ T cells. Subsequently, the frequency of TIM-3+ cells on CD4+IL-17A+ T cells was analyzed by flow cytometry in PBMCs from AD patients and HC, respectively.
Supplementary Figure 4 | Representative flow cytometry analysis of TH22 and TH22 in PBMCs from AD patients. (A) TH22 cells: Cells were initially gated on CD4+ T cells. Then IL-22+cells were selected out of the gated CD4+ T cells. (B) TH2: Cells were initially gated on CD4+ T cells. Then IL-4+cells were selected out of the gated CD4+ T cells.
AD, Atopic dermatitis; TH2, T helper TH 2; TIM-3, T cell immunoglobulin- and mucin-domain-containing molecules-3; Gal-9, Galectin-9; HC, Healthy controls; TIM, T cell immunoglobulin mucin; PBMCs, Peripheral Blood Mononuclear Cells; AV/PI, Annexin V/propidiumiodide.
1. Ogg G. Role of T cells in the pathogenesis of atopic dermatitis. Clin Exp Allergy (2009) 39:310–6. doi: 10.1111/j.1365-2222.2008.03146.x
2. Honda T, Kabashima K. Reconciling innate and acquired immunity in atopic dermatitis. J Allergy Clin Immunol (2020) 145:1136–7. doi: 10.1016/j.jaci.2020.02.008
3. Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet (2020) 396:345–60. doi: 10.1016/S0140-6736(20)31286-1
4. Howell MD, Parker ML, Mustelin T, Ranade K. Past, present, and future for biologic intervention in atopic dermatitis. Allergy (2015) 70:887–96. doi: 10.1111/all.12632
5. Jin M, Yoon J. From bench to clinic: the potential of therapeutic targeting of the IL-22 signaling pathway in atopic dermatitis. Immune Netw (2018) 18:e42. doi: 10.4110/in.2018.18.e42
6. Sanyal RD, Pavel AB, Glickman J, Chan TC, Zheng X, Zhang N, et al. Atopic dermatitis in African American patients is TH2/TH22-skewed with TH1/TH17 attenuation. Ann Allergy Asthma Immunol (2019) 122:99–110.e116. doi: 10.1016/j.anai.2018.08.024
7. Jiao Q, Qian Q, Liu C, Luo Y, Fang F, Wang M, et al. T Helper 22 cells from han Chinese patients with atopic dermatitis exhibit high expression of inducible T-cell costimulator. Br J Dermatol (2020) 182:648–57. doi: 10.1111/bjd.18040
8. Wang S, Zhu R, Gu C, Zou Y, Yin H, Xu J, et al. Distinct clinical features and serum cytokine pattern of elderly atopic dermatitis in China. J Eur Acad Dermatol Venereol (2020) 34:2346–52. doi: 10.1111/jdv.16346
9. Punnonen J, Cocks BG, De Vries JE. IL-4 induces germ-line IgE heavy chain gene transcription in human fetal pre-b cells. evidence for differential expression of functional IL-4 and IL-13 receptors during b cell ontogeny. J Immunol (1995) 155:4248–54.
10. Poulsen LK, Hummelshoj L. Triggers of IgE class switching and allergy development. Ann Med (2007) 39:440–56. doi: 10.1080/07853890701449354
11. Bagnasco D, Ferrando M, Caminati M, Bragantini A, Puggioni F, Varricchi G, et al. Targeting interleukin-5 or interleukin-5Ralpha: Safety considerations. Drug Saf (2017) 40:559–70. doi: 10.1007/s40264-017-0522-5
12. Junttila IS. Tuning the cytokine responses: An update on interleukin (IL)-4 and IL-13 receptor complexes. Front Immunol (2018) 9:888. doi: 10.3389/fimmu.2018.00888
13. Akdis CA, Arkwright PD, Bruggen MC, Busse W, Gadina M, Guttman-Yassky E, et al. Type 2 immunity in the skin and lungs. Allergy (2020) 75:1582–605. doi: 10.1111/all.14318
14. Shen ZJ, Hu J, O'neal MA, Malter JS. Pin1 regulates IL-5 induced eosinophil polarization and migration. Cells (2021) 10. doi: 10.3390/cells10020211
15. Furue M. Regulation of filaggrin, loricrin, and involucrin by IL-4, IL-13, IL-17A, IL-22, AHR, and NRF2: Pathogenic implications in atopic dermatitis. Int J Mol Sci (2020) 21. doi: 10.3390/ijms21155382
16. Ong PY, Leung DY. Bacterial and viral infections in atopic dermatitis: a comprehensive review. Clin Rev Allergy Immunol (2016) 51:329–37. doi: 10.1007/s12016-016-8548-5
17. Brunner PM, Pavel AB, Khattri S, Leonard A, Malik K, Rose S, et al. Baseline IL-22 expression in patients with atopic dermatitis stratifies tissue responses to fezakinumab. J Allergy Clin Immunol (2019) 143:142–54. doi: 10.1016/j.jaci.2018.07.028
18. Ariens LFM, van der Schaft J, Bakker DS, Balak D, Romeijn MLE, Kouwenhoven T, et al. Dupilumab is very effective in a large cohort of difficult-to-treat adult atopic dermatitis patients: First clinical and biomarker results from the BioDay registry. Allergy (2020) 75:116–26. doi: 10.1111/all.14080
19. Igelman S, Kurta AO, Sheikh U, Mcwilliams A, Armbrecht E, Jackson Cullison SR, et al. Off-label use of dupilumab for pediatric patients with atopic dermatitis: A multicenter retrospective review. J Am Acad Dermatol (2020) 82:407–11. doi: 10.1016/j.jaad.2019.10.010
20. Asakura H, Kashio Y, Nakamura K, Seki M, Dai S, Shirato Y, et al. Selective eosinophil adhesion to fibroblast via IFN-gamma-induced galectin-9. J Immunol (2002) 169:5912–8. doi: 10.4049/jimmunol.169.10.5912
21. Igawa K, Satoh T, Hirashima M, Yokozeki H. Regulatory mechanisms of galectin-9 and eotaxin-3 synthesis in epidermal keratinocytes: possible involvement of galectin-9 in dermal eosinophilia of Th1-polarized skin inflammation. Allergy (2006) 61:1385–91. doi: 10.1111/j.1398-9995.2006.01130.x
22. Hirashima M, Kashio Y, Nishi N, Yamauchi A, Imaizumi TA, Kageshita T, et al. Galectin-9 in physiological and pathological conditions. Glycoconj J (2002) 19:593–600. doi: 10.1023/B:GLYC.0000014090.63206.2f
23. Farag AGA, Al-Sharaky DR, Allam SS, Khaled HN. Role of galectin-9 in atopic dermatitis - is it mediated through e selectin? a clinical and immunohistochemical study. Clin Cosmet Investig Dermatol (2020) 13:11–9. doi: 10.2147/CCID.S229393
24. Nakajima R, Miyagaki T, Oka T, Nakao M, Kawaguchi M, Suga H, et al. Elevated serum galectin-9 levels in patients with atopic dermatitis. J Dermatol (2015) 42:723–6. doi: 10.1111/1346-8138.12884
25. Tang R, Rangachari M, Kuchroo VK. Tim-3: A co-receptor with diverse roles in T cell exhaustion and tolerance. Semin Immunol (2019) 42:101302. doi: 10.1016/j.smim.2019.101302
26. Jiao Q, Qian Q, Zhao Z, Fang F, Hu X, An J, et al. Expression of human T cell immunoglobulin domain and mucin-3 (TIM-3) and TIM-3 ligands in peripheral blood from patients with systemic lupus erythematosus. Arch Dermatol Res (2016) 308:553–61. doi: 10.1007/s00403-016-1665-4
27. Han G, Chen G, Shen B, Li Y. Tim-3: an activation marker and activation limiter of innate immune cells. Front Immunol (2013) 4:449. doi: 10.3389/fimmu.2013.00449
28. Zhang Q, Luan H, Wang L, He F, Zhou H, Xu X, et al. Galectin-9 ameliorates anti-GBM glomerulonephritis by inhibiting Th1 and Th17 immune responses in mice. Am J Physiol Renal Physiol (2014) 306:F822–832. doi: 10.1152/ajprenal.00294.2013
29. Page NS, Jones G, Stewart GJ. Genetic association studies between the T cell immunoglobulin mucin (TIM) gene locus and childhood atopic dermatitis. Int Arch Allergy Immunol (2006) 141:331–6. doi: 10.1159/000095459
30. Rodriguez-Manzanet R, Dekruyff R, Kuchroo VK, Umetsu DT. The costimulatory role of TIM molecules. Immunol Rev (2009) 229:259–70. doi: 10.1111/j.1600-065X.2009.00772.x
31. Severity scoring of atopic dermatitis: the SCORAD index. consensus report of the European task force on atopic dermatitis. Dermatology (1993) 186:23–31. doi: 10.1159/000247298
32. Jiao Q, Wang H, Hu Z, Zhuang Y, Yang W, Li M, et al. Lidocaine inhibits staphylococcal enterotoxin-stimulated activation of peripheral blood mononuclear cells from patients with atopic dermatitis. Arch Dermatol Res (2013) 305:629–36. doi: 10.1007/s00403-013-1339-4
33. Nakae S, Iikura M, Suto H, Akiba H, Umetsu DT, Dekruyff RH, et al. TIM-1 and TIM-3 enhancement of Th2 cytokine production by mast cells. Blood (2007) 110:2565–8. doi: 10.1182/blood-2006-11-058800
34. Kanai Y, Satoh T, Igawa K, Yokozeki H. Impaired expression of Tim-3 on Th17 and Th1 cells in psoriasis. Acta Derm Venereol (2012) 92:367–71. doi: 10.2340/00015555-1285
35. Suaini NHA, Tan CPT, Loo EXL, Tham EH. Global differences in atopic dermatitis. Pediatr Allergy Immunol (2021) 32:23–33. doi: 10.1111/pai.13335
36. Lee J, Park EJ, Noh JW, Hwang JW, Bae EK, Ahn JK, et al. Underexpression of TIM-3 and blunted galectin-9-induced apoptosis of CD4+ T cells in rheumatoid arthritis. Inflammation (2012) 35:633–7. doi: 10.1007/s10753-011-9355-z
37. Oomizu S, Arikawa T, Niki T, Kadowaki T, Ueno M, Nishi N, et al. Cell surface galectin-9 expressing Th cells regulate Th17 and Foxp3+ treg development by galectin-9 secretion. PloS One (2012) 7:e48574. doi: 10.1371/journal.pone.0048574
Keywords: T cell immunoglobulin- and mucin-domain-containing molecules-3 (TIM-3), Galectin-9 (Gal-9), TH1 cells, TH2 cells, TH17 cells, TH22 cells, atopic dermatitis
Citation: Su W, Zhang J, Yang S, Tang M, Shen Y, Liu C, Ji J, Maurer M and Jiao Q (2022) Galectin-9 contributes to the pathogenesis of atopic dermatitis via T cell immunoglobulin mucin-3. Front. Immunol. 13:952338. doi: 10.3389/fimmu.2022.952338
Received: 25 May 2022; Accepted: 27 June 2022;
Published: 22 July 2022.
Edited by:
Yuekang Xu, Anhui Normal University, ChinaReviewed by:
Xiaomin Zhang, University of Arkansas for Medical Sciences, United StatesCopyright © 2022 Su, Zhang, Yang, Tang, Shen, Liu, Ji, Maurer and Jiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiang Ji, amlqaWFuZ0BzdWRhLmVkdS5jbg==; Marcus Maurer, bWFyY3VzLm1hdXJlckBjaGFyaXRlLmRl; Qingqing Jiao, cWluZ3FpbmdqaWFvQHN1ZGEuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.