
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 27 July 2022
Sec. Cytokines and Soluble Mediators in Immunity
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.951107
Although numerous clinical trials have been implemented, an absolutely effective treatment against coronavirus disease 2019 (COVID-19) is still elusive. Interleukin-22 (IL-22) has attracted great interest over recent years, making it one of the best-studied cytokines of the interleukin-10 (IL-10) family. Unlike most interleukins, the major impact of IL-22 is exclusively on fibroblasts and epithelial cells due to the restricted expression of receptor. Numerous studies have suggested that IL-22 plays a crucial role in anti-viral infections through significantly ameliorating the immune cell-mediated inflammatory responses, and reducing tissue injury as well as further promoting epithelial repair and regeneration. Herein, we pay special attention to the role of IL-22 in the lungs. We summarize the latest progress in our understanding of IL-22 in lung health and disease and further discuss maneuvering this cytokine as potential immunotherapeutic strategy for the effective manage of COVID-19.
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection has affected more than 548 million coronavirus disease 2019 (COVID-19) patients and caused more than 6.3 million deaths globally (https://coronavirus.jhu.edu, Johns Hopkins Coronavirus Resource Center) up to July 2, 2022, with these cases continuously increasing and producing several variants of concern. COVID-19 patients develop a constellation of clinical features, ranging from mild respiratory symptoms to severe acute respiratory syndromes, and even death (1, 2). Multiple evidences have illustrated that cytokine release syndrome (CRS) is the pathological hallmark of critically ill COVID-19 patients, which is characterized by a rapid and sustained systemic increase of more than 20 inflammatory chemokines and cytokines. CRS induced secondary hemophagocytic lymphohistiocytosis and acute respiratory distress syndrome (ARDS) are regarded as main causes of organ injuries that drive the deterioration of COVID-19 (3–7). Therefore, besides direct supplemental oxygen and antiviral therapy for COVID-19 patients, immunotherapeutic strategies potentially alleviate COVID-19 progression and rescue severe or critical illness. Unfortunately, a recent double-blind, randomized Phase III trial (clinicaltrials.gov; NCT04320615) testing the treatment efficacy of an anti-interleukin (IL-6) receptor antibody (tocilizumab) in COVID-19 cases failed to show a significant reduced the mortality (8, 9). Thus, further studies aimed at exploration of novel immunomodulatory therapeutic strategies to treat COVID-19 and development corresponding regimens are urgently warranted.
Interleukin-22 (IL-22) has attracted great interest over recent years, making it one of the best-studied cytokines of the interleukin-10 (IL-10) family (10, 11). The function of IL-22 is mediated through directly interacting with its heterodimeric IL-22R1 and IL-10R2 receptor complex (12, 13). Unlike most interleukins, which directly regulate the function of hematopoietic cells, the major impact of IL-22 is exclusively on fibroblasts and epithelial cells due to the restricted expression of receptors in the kidney, lung, liver, pancreas, gastrointestinal tract, skin, and thymus (14). Therefore, IL-22 represents a main communication channel between specialized tissue cell types and the immune system. Signaling occurs via the activation of Jak1/Tyk2 and STAT3 pathway and then activation of anti-apoptotic, mitogenic and antioxidant molecules. Protein kinase B (AKT)/mechanistic target of rapamycin (mTOR), ERK1/2, P38, MAPK, STAT1, and STAT5 pathways are also activated by IL-22 (15–20). Of note, numerous of studies have illustrated that IL-22 plays a pivotal role in anti-viral infections through significantly ameliorating the immune cell-mediated inflammatory responses, as well as reducing lung injury and promoting further airway epithelial repair and regeneration (Figure 1) (21–23). Herein, we summarize recent progress in understanding the biology of IL-22 in lung health, suggesting more immunotherapeutic strategies to maneuver this cytokine for the effective manage of COVID-19 (24–28).
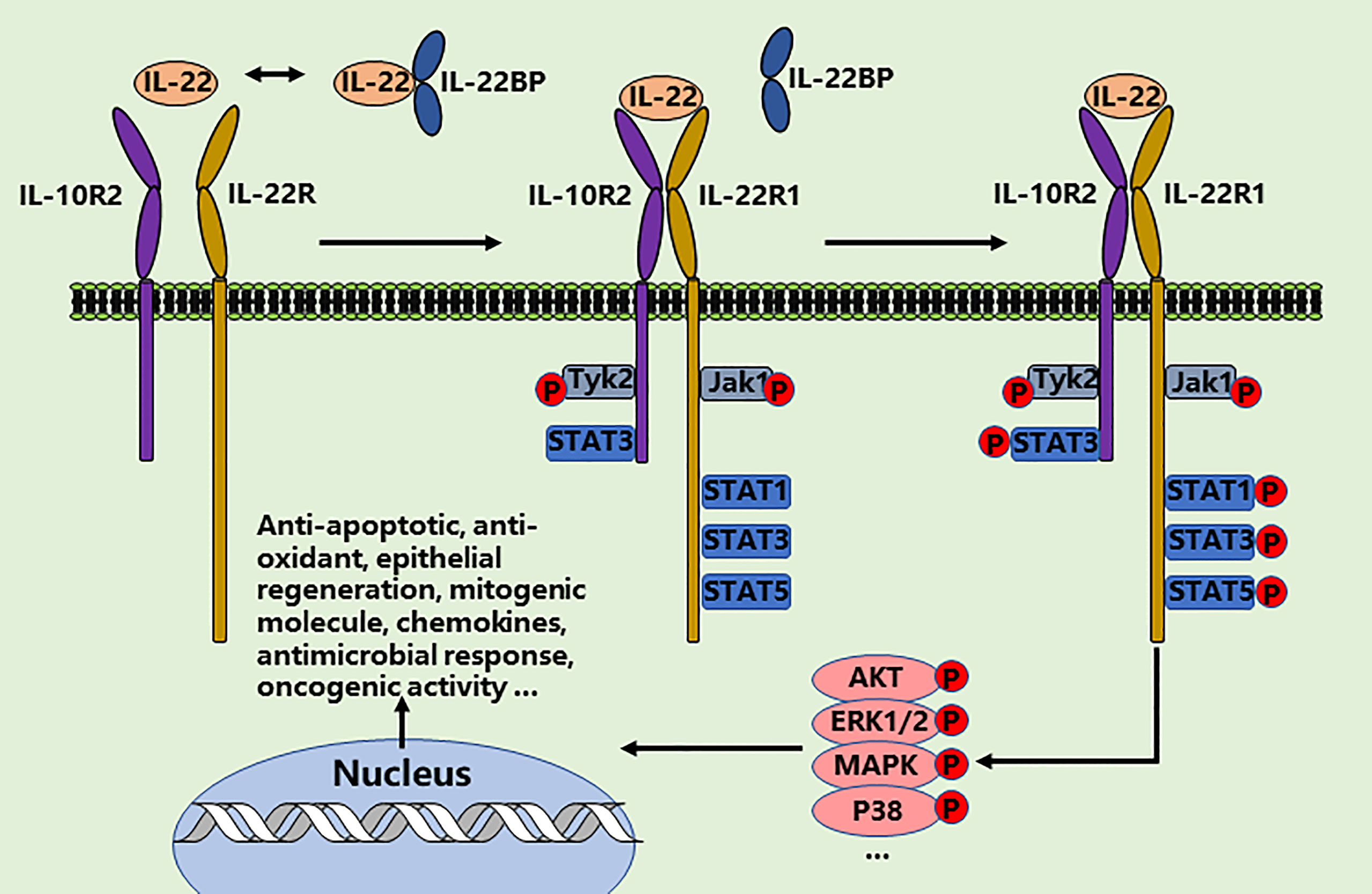
Figure 1 IL-22/IL-22-R1 signaling events. IL‐22 exerts the therapeutic effects in two steps through a receptor complex composed of IL‐10R2 and IL‐22R1. IL‐22-IL10R2/IL‐22R1 complex leads to the phosphorylation of JAK1 and TYK2 and then phosphorylates the tyrosine residues such as STATs, AKT, ERK1/2, MAPK, and P38 in the cytoplasmic domain of IL‐22R1. The cellular effects of IL-22 are involved in anti-apoptotic and anti-oxidant response, epithelial regeneration, mitogenic molecule expression, chemokines expression, antimicrobial response, and oncogenic activity.
COVID-19 is a complex disorder in which the respiratory performance is accompanied by systemic responses, illustrating viral infection generates widely dysfunctional immune reactions. Generally, the pathological feature of COVID-19 is bilateral diffuse alveolar damage with evidence of neutrophil extracellular traps, fibrin thrombi, and activated platelets in the vessels (25, 26). Infiltrating monocytes, macrophages, and neutrophils are clearly found in both lungs, accompanied by multinucleated syncytial cells infiltration in the alveoli that characterized by amphiphilic granular cytoplasm, large nuclei, and prominent nucleoli indicating viral cytopathic alters (27–31). Additionally, myocardial infarction, kidney damage, and persistent symptoms, such as depression, anxiety, palpitations, chest pain, sleep difficulty, dizziness, and weight loss, also represent the pathological features of critically COVID-19 cases (27–32). The immune profile in COVID-19 patients has been well reviewed, which shows dysfunction in both the innate and adaptive immune (33, 34). Specifically, CD16+ monocytes, γδ T cells, and NK cells are obviously activated, while the percentages of CD4+ T cells, CD8+ T cells, and natural killer (NK) cells are significantly decreased (34). Besides that, T cells show hyperinflammatory responses and enhanced migration abilities, along with evidently increased expression of inhibitory molecules (7, 33, 34). The clonality of B cells and the proportion of plasma B cell compartments are also elevated. The percentages of dendritic cells are obviously decreased; however, the IFN-response cell compartments are increased (34–38). For the mechanisms, multiple evidence has demonstrated that IL-1 axis and IL-6 axis are the most importantly relevant signal transductions in the SARS-CoV-2-induced hyperinflammatory responses (39).
Altogether, the pathological characteristics of COVID-19 are complex, comprising fibrotic processes, hyperinflammation, thromboembolic complications and endothelial cells and lymphocyte dysfunction in the lung. These processes between patients are also highly variable, may be due to the heterogeneity of host immune reaction, which urgently require stratified and novel immunotherapy strategies for COVID-19 management.
Despite numerous clinical trials have been implemented, an absolutely effective treatment against COVID-19 is still elusive. Immunomodulatory medications hold huge promise, but their administration should be cautiously considered so that the protective effects are appropriate for the dominant immunopathology and the disease stages to minimize the side-effects (Table 1).
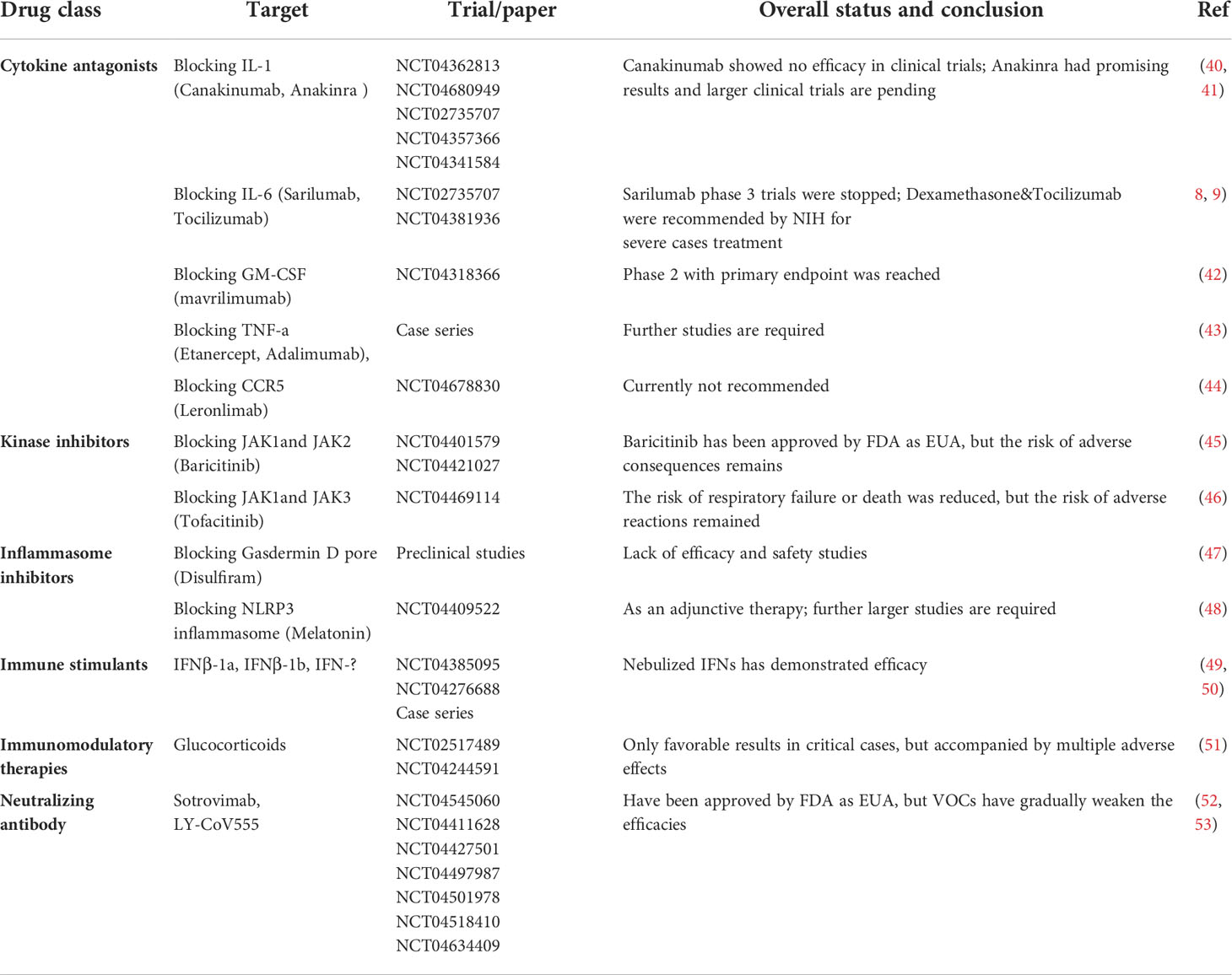
Table 1 Overview of the candidate immunotherapeutic strategies for the treatment of COVID-19/SARS-CoV-2 infection.
Of note, immunotherapeutic strategies blocking proinflammatory cytokines or their receptors are only meaningful in the hyperinflammatory stages; as in the early stages of mild lung injury or minimal inflammation, cytokines are largely desirable in fighting against virus infection. These include inhibitors of IL-1, IL-6, IL-18, TNF, CCR5 and GM-CSF or their receptors. However, some phase 2 or 3 results suggest no or little benefits in moderate and severe patients, and large-scale clinical data in COVID-19 are needed (8, 40–44, 54). Interferons (IFNs) have been used for many years to treat infectious disorders, cancers and multiple sclerosis, suggesting their potent antiviral effects for COVID-19. Although recent studies have found early nebulized IFN-α/β/𝜆 administration accelerated high-risk patients’ recovery and reduced mortality, the multinational clinical trials on hospitalized severe cases indicated subcutaneous IFNs injection with or without lopinavir treatment had minor effects on length of hospital stay and mortality (49, 50, 55, 56).
Small molecule inhibitors targeting cytokine-mediated signaling pathway, specifically of Janus kinases (JAKs), have also been well studied for treating COVID-19. For instance, Baricitinib and Tofacitinib were approved for emergency use authorization (EUA) because the clinical symptoms improved when co-treated with remdesivir during SARS-CoV-2 infection. But secondary infection and thromboembolism are the risk of these kinase inhibitors, which is highly dependent on administration time (45, 46). Glucocorticoids, such as dexamethasone, are broad-acting and powerful immunosuppressive and antiphlogistic therapies that can reduce mortality in critical COVID-19 cases (51). However, many clinical studies demonstrated that such efficacies are inevitably accompanied by multiple adverse effects, including viral reactivation, blocking of the host immune responses important for viral clearance, and others (51). In addition, it is indisputable that inflammasomes contribute to the pathogenesis of COVID-19, and trials on the GasderminD-pore inhibitor Disulfiram and the NLRP3 inhibitors Melatonin are ongoing (47, 48).
Monoclonal antibody (mAb) is one of most effective immunotherapeutic strategies for the treatment of serious viral infection; thus, lots of mAbs are identified purposing to inhibit SARS-CoV-2 infection through binding to the spike protein. So far, the FDA has approved EUA for LY-CoV555 and Sotrovimab in pediatric and high-risk patients to prophylaxis and treat COVID-19, as these antibodies can reduce mortality and hospitalization (52, 57). Nevertheless, viral mutations present in variants/variants of concern (VOCs) have begun to gradually weaken the efficacies of many mAbs, and it is likely some will become invalid without upgrading to compensate for SARS-CoV-2 evolution (53). Altogether, immune system dysfunction plays a key role in COVID-19 pathogenesis and immunotherapeutic strategies for SARS-CoV-2 infection are promising, future efforts to discover more effective agents are obviously warranted. Also note that COVID-19 remains a field of rapid progress, thus recommendations on drugs and biomarkers will continue to evolve.
In the lung, stimulation of CD11b+-DCs and alveolar macrophages via their innate pattern recognition receptors leads to the secretion of related proinflammatory cytokines, expression of RORγt, differentiation of ILC3, γδT, Th17 and NKT cells, and production of IL-22 (57). Conversely, CD103+-DCs and plasmacytoid can be stimulated through viral antigens, resulting in the secretion of IL-27, IFN-γ and type I IFNs that block IL-22 expression in lymphocytes (58).
Multiple studies have shown that IL-22 is involved in lung repair, recovery and regeneration during lung infection or injury. From these studies, it is concluded that IL-22 can be protective or proinflammatory, where IL-22/IL-22R axis is important for host protective immunity to both viral infections and bacterial infections. The tissue protective nature of IL-22 via enhancing wound healing and the epithelial barrier through promoting antimicrobial peptides expression, enhancing anti-apoptosis and antiviral effects and alleviating airway inflammation, as well as inhibiting proinflammatory cytokines produced by airway epithelial cells (Figure 2). In contrast, the proinflammatory of IL-22 is evidenced by its ability to promote the expression of chemokines, and inflammatory cytokines such as IL-1β, IL-17, and TNF-α. Whether IL-22 has a protective or proinflammatory effect seems to depend on the related cytokines co-produced by the relevant cells during different stages of the diseases (10, 14).
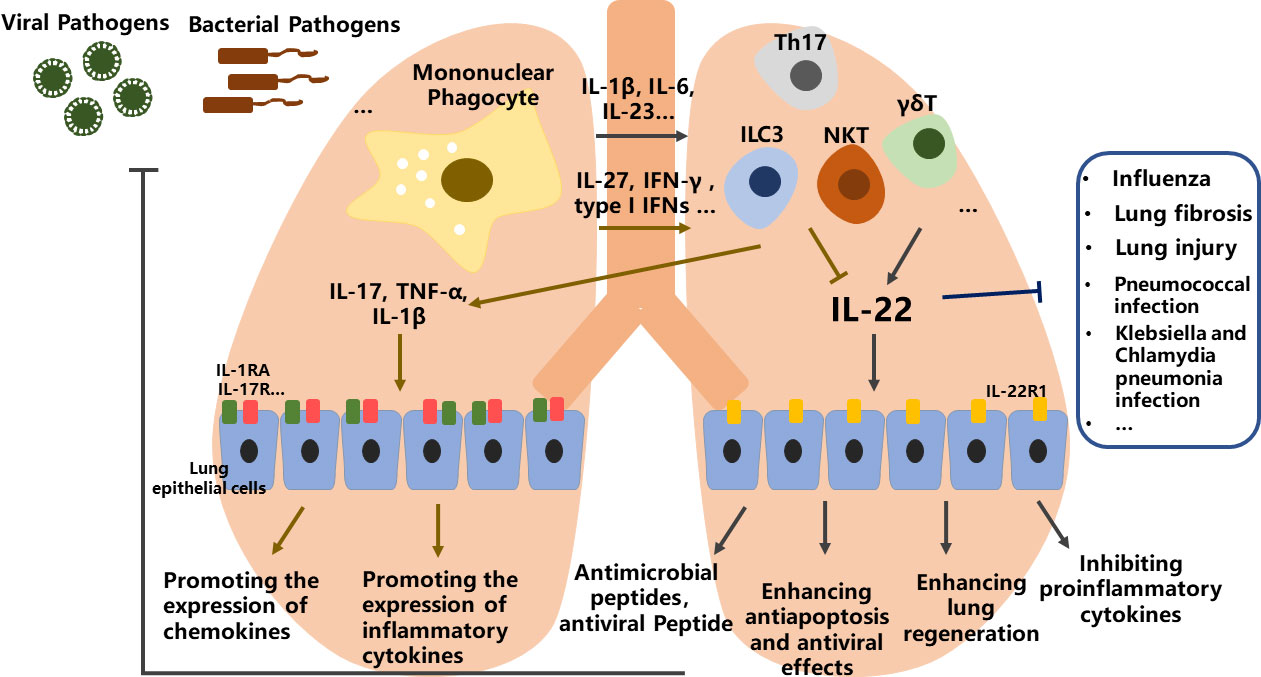
Figure 2 The role of IL-22 in lung health and diseases. In the lung, stimulation of mononuclear phagocyte via viral or bacterial pathogens leads to the secretion of related proinflammatory cytokines, then differentiation of ILC3, γδT, Th17 and NKT lymphocytes, and promoting or blocking of IL-22 expression. IL-22 can be protective or proinflammatory through multiple effects, where IL-22/IL-22R axis is important for host protective immunity to both viral infections and bacterial infections.
Transcription factors play crucial roles in controlling the production of IL-22 in lungs (15, 59–61). Interestingly, studies indicate that these transcription factors, such as aryl hydrocarbon receptor (AhR), c-Maf, Batf, and Notch, can sometimes control IL-22 even in the same cells. These transcription factors form complex regulatory networks and regulate the production of IL-22 in context-dependent manners (62–64). As a cytosolic transcription factor, AhR is a harbor that converges several different environmental and cellular signaling to regulate the development of many Th cell lines, including Tr1, Th17 and Treg cells via recognizing multiple natural molecules and small xenobiotics (62–65). AhR directly binds to cMaf and synergistically enhances IL-22 production in ILC3s, Th17 cells, γδ T cells and Th22 cells. Nevertheless, under some certain conditions, AhR may not produce IL-22, and AhR-/- mice can develop severe skin inflammation associated with higher IL-22 and IL-17 expression. The Notch signaling also promotes IL-22 expression via AhR induction in Th17 cells (65). Additionally, prostaglandin E2 (PGE2) enhances IL-22 production from both T cells and ILC3 via binding EP4 and EP2 receptors and blocking IRF-4, as well as activating AhR and cAMP signaling (66, 67) (Figure 3). In the lungs, certain viral or bacterial microorganisms can convert tryptophan (Trp) into derivatives that promote IL-22 secretion. Of importance, IL-22 production from γδ T cells is manipulated through AhR- and Try-dependent mechanism via CD69 (68). And CD69 regulates LAT-1 expression, which determines the intracellular quantity of AhR and the uptake of Trp (Figure 3). Moreover, both CARD-/- and IDO-/- mice can alter downstream Trp metabolites, for instance 3-IAID, with one depriving IL-22-promoting derivatives and the other favoring them, respectively (69). Taken together, these findings illustrate that controlling AhR signaling and Trp metabolites may be a powerful tool for regulating IL-22 functions.
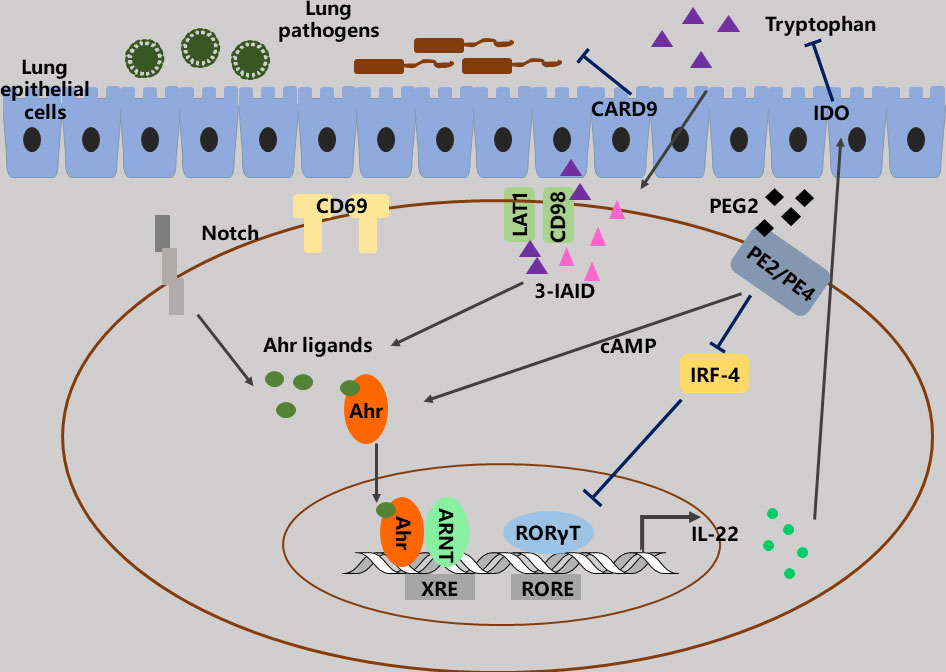
Figure 3 The functions and regulation of IL-22. AhR plays importance roles in the functions and regulation of IL-22 in lungs. A lot of signaling, such as PGE2, LAT1, CD69, CD98 and Notch can converge to AhR signaling to regulate IL-22 production via modulating of AhR ligand.
During the early stage of influenza infection, studies has previously illustrated that IL22 is overexpressed by NK and NKT cells in the lung, resulting in the airway and parenchymal epithelium regeneration. Within two days after viral infection, IL-22 gene transcription in Tγδ, Tαβ and innate lymphoid cells is also increased in lung (Table 2). However, IL22BP gene transcription is decreased. During the sublethal stage of viral infection, IL-22 can inhibit lung inflammation, reduce secondary infection and preserve the integrity of lung epithelium (70). Whereas IL-22-/- mice infected with viral shows enhanced collagen accumulation and a defect in epithelial regeneration (70). Moreover, IL-22 can also prevent pulmonary fibrosis in a Bacillus subtilis induced model. Administration of IL-22 alleviates lung fibrosis, while inhibition of IL-22 leads to increased collagen accumulation in the lungs (71). In pneumococcal pneumonia, IL-22 gene is found to increase in the lung together with other cytokines, for example TNFα, IL-6, and IL-1. The absence of IL-22 makes the host vulnerable to pneumococcal infection, which indicates that the presence of IL22 is very important to control pneumococcal infection (72). IL-22 also plays a key role in host resistance to many other lung pathogens. During Klebsiella pneumonia infection, NK cells activation results in IL-22 production to protect lung tissue, and during infection with Streptococcus pneumoniae, TLR5 and DC cells activation leads to a repaid accumulation of ILC3s in the lungs to express IL-22 and defense against bacterial infection (73–75). During Chlamydia muridarum infection, IL-22 levels are rapidly increased in the lungs. Neutralization of IL-22 with anti-IL-22 mAbs can lead to impaired Th17 responses and deterioration of disease. Intranasal injection of IL-22 can significantly enhance Th17 response and have a protective effect following Chlamydia pneumoniae infection (76). After Aspergillus fumigatus pulmonary infection, the fungal burden is increased in IL-22-/- mice, suggesting the critical role of IL-22 in the elimination of this pathogen (77). Additionally, IL-22 can also play a protective role in the barotrauma model, thereby reducing pulmonary edema and disintegration (78).
In the animal model of bleomycin induced lung injury, it is demonstrated that IL-22 and IL-17 are predominantly produced by Th17 cells. Inhibiting IL-22 during bleomycin injection can improve airway inflammation, indicating IL-22 has a proinflammatory effect; nevertheless, airway inflammation is also significantly reduced in IL-17-/- mice with still high levels of IL-22 produced by T cells, suggesting IL-22 has a protective function for pulmonary fibrosis in deficiency of IL-17 (79, 80). Therefore, the proinflammatory effect of IL-22 may depend on the synergistic effect with IL-17 (Table 2).
In peripheral blood of asthmatic patients and preclinical asthma models, serum IL-22 levels are increased. The recent studies have indicated IL-22 can induce the occurrence of asthma in preclinical models, but it can ameliorate inflammation during asthma exacerbation (81, 82). In ovalbumin induced asthma animal model, IL-22 neutralization with mAbs during sensitization stage of disease, which is similar to the pulmonary fibrosis research just mentioned above, evidently alleviated lung pathology and airway inflammation. Subcutaneous administration of IL-22 during sensitization leads to worse lung pathology and inflammation (83–85). Conversely, IL-22 neutralization after sensitization can result in increased lung inflammation, whereas IL-22 injection decreases chemokine expression, goblet cell hyperplasia, production of IL-25, eosinophil infiltration and constriction of the airway (82–85). The mechanism behind this dual effect in allergic airway inflammation is unclear, but it may involve inhibiting the production of IL-25 and CCL17 through airway epithelial repair, which may involve IL-10.
In conclusion, IL-22 is involved in a variety of lung diseases, making it a promising target for clinical development.
The emergence of SARS-CoV-2, which leads to COVID-19, is one of the most serious public health and epidemics crises in this century. As an emerging virus, there are multiple problems to be clarified in distinguishing the effective immune response of mild and severe diseases.
Although IL-22 does not appear to reduce virus titer during infections, studies have illustrated that IL-22 can reduce the pneumonia severity via the immune regulation and tissue protective or regenerative functions (86, 87). As COVID-19 is a respiratory disorder with similar pathological characteristics and symptoms to other serious pulmonary virus infections, it is reasonable to speculate that IL-22 may also serve to limit the severity of this disease. More importantly, recent studies have shown that IL-22 has potent immune boosting, antiviral, and antibacterial properties to respiratory syncytial virus, which could also extend to manage SARS-CoV-2 infection (88). Accordingly, compared with healthy control individuals, the IL-22+-Tc22 and IL-22+-Th22 numbers in adult-COVID-19 patients increased significantly, whether it is asymptomatic pneumonia, mild pneumonia, or severe pneumonia (Figure 4). These findings further suggest that in the 0-12-year-old age group with asymptomatic disease course and uncomplicated adult cases, IL-22 expressed Tc22 cells are higher, which indicates that IL-22 has a protective effect (89). In contrast, the association between the increase of Tc17 cells and the severity of COVID-19 may reveal the destructive effect of co-expression of IL-17 and IL-22.

Figure 4 The therapeutic potential of IL-22 for COVID-19. SARS-CoV-2 virus first occupied the airway epithelial barrier; mononuclear inflammatory infiltration increased; and then platelets activation, cytokine storm, fibrin thrombi and diffuse alveolar injury followed. IL-22 can reduce the pneumonia severity through immunomodulation, anti-inflammation and tissue regenerative and repair functions, but the detailed mechanism underlying the therapeutic consequences for COVID-19 remains to be fully clarified.
As the IL-22/IL-22R1 axis is involved in inflammation during virus infection, the expression patterns of IL-22/IL-22R1 on blood hematopoietic cells in SARS-CoV-2 infection have been well studied (90–93). The numbers of IL-22R1+ myeloid DC1, myeloid DC2, and plasmacytoid DC and the proportions of IL-22R1+ intermediate, non-classical, and classical monocytes higher in COVID-19 patients than controls at the presented day. Moreover, high proportions of mDC2 and IL-22R1+ non-classical monocytes show high HLA-DR expression and are therefore activated. Multivariate analysis is performed on all IL-22R1+ myeloid cells to distinguish the disease severity. However, correlation analysis between the concentration of plasma chemokines and IL-22R1+ cell subsets indicates that some subsets have protective effects, while others have pro-inflammatory effects (93, 94). Without stimulation, CD4+-T and NK lymphocytes produced IL-22. CD4+-T cells expressed IL-22R1, and its expression density defines two different functional subsets. The number of IL-22R1+ intermediate monocytes is negatively correlated with IFN-α, CRP and IL-6 in non-serious SARS-CoV-2 infection, whereas pDC and IL-22R1+ classical monocytes are positively correlated with pro-inflammatory chemokines IP-10 and MCP-1 in serious SARS-CoV-2 infections. Besides, researchers further demonstrate that IL-22 can reduce the expression of viral entry receptors, such as ACE2, TMPRSS2, and increase the expression of anti-viral proteins through IL-22/IL-22R1 pathway (Figure 4) (95). Thus, these findings suggest that the IL-22/IL-22R1 signaling is involved in the pathological process of COVID-19, which can be protective during SARS-CoV-2 infections (93–95).
Recently, studies have demonstrated the roles of ILCs (innate lymphoid cells) in COVID-19 patients. Blood from severely infected COVID-19 patients were found that had fewer ILC precursor cells and ILCs than those mild cases. Of importance, in these severely infected COVID-19 patients, the expression of CD69 in ILCs was higher, which as a marker for tissue homing and activation. ILCs-CD69+ increased and blood ILCs decreased in severe infections, indicating that severely infected COVID-19 patients have more lung homing and activation (96). Further findings corroborate these results by confirming that higher ILCs abundance in blood was associated with shorter hospitalization. Additionally, the number of ILCs in the blood of hospitalized patients with SARS-CoV-2 infection decreased by 1.8 times (97). Collectively, these studies demonstrate that there are correlations between severe COVID-19 and decreased ILCs in the blood. As IL-22 plays critical functions in epithelial barrier integrity and ILCs are identified as the major source of IL-22 in response to lung pathogens stimulation (98), further studies are urgently needed to assess their exact roles in SARS-CoV-2 infection since current results regarding IL-22 and ILCs in COVID-19 individuals are obtained via analysis of blood periphery (Figure 4).
Although IL-22 possesses definite immunomodulatory properties and tissue-protective effects, mainly via inhibiting apoptosis and promoting proliferation of epithelial cells, these same effects have also been involved in pathological states such as psoriasis, rheumatoid arthritis, and malignant tumors (99, 100). Additionally, functions of IL-22 have been implicated in host defense within barrier-tissues such as the skin, oral mucosa, and intestine (100–102). The functional outcomes of IL-22 in immunomodulation may be either protective or pathologic, suggesting that it is an extremely controversial interleukin (96–98). Systemic administration of IL-22 can upregulate the expression of proinflammatory cytokines including G-CSF and IL-6, and chemokines like chemokine (C-X-C motif) ligands CXCL1, CXCL5, CXCL9, which is sufficient to cause an inflammatory response (99). Studies indicate that IL-22 serum levels in psoriasis and rheumatoid arthritis patients are much higher than that the health individuals, and these also correlate with the disease severity (103, 104). Of note, IL-22-/- mice significantly alleviate collagen induced arthritis (105). In rheumatoid arthritis tissues, synovial fibroblasts are considered to be the key cellular target of IL-22, which may drive the proliferation of this cell type through STAT3 (106). Besides, synovial-fibroblasts activation via IL-22 can upregulate the expression of NF-KB ligand and CCL2, which promote inflammation and joint destruction (106, 107). Inhibition of IL-22 biological activity can also reduce the severity of psoriasis, which is also consistent with the psoriasis like symptoms in IL-22 transgenic mice (108–110). Keratinocytes are targets of IL-22 in psoriasis, and IL-22 regulates the expression of key inflammatory parameters by keratinocytes, including matrix metalloproteinases-1, IL-20 and CXCL5 (108–111). It is worth mentioning that studies have also suggested that IL-22 may also promote lung pathology during chronic exposure to Aspergillus fumigatus (112). As overactivation of STAT3 in a variety of human tumors, IL-22 is considered to be associated with many tumorigeneses (113, 114). Similarly, elevated levels of IL-22 can also be detected in human cancers, including hepatocellular carcinoma and gastric cancer as well as non-small cell lung cancer (15). The related consequences of high levels of IL-22 have been fully illustrated in the diethylnitrosamin induced hepatocellular carcinoma model, where IL-22 transgenic mice show increased and IL–22-/- mice decreased tumor formation (115). These complicated biological functions result in the difficulties of IL-22 clinical application.
In summary, it can be concluded that IL-22 is a crucial modulator of epithelial homeostasis and a regulator of host defense in the lung. It provides communication channels that allow hematopoietic cells, especially lymphocytes, to trigger pleiotropic responses in the epithelium to maintain barrier homeostasis against pulmonary pathogens while protecting the lungs from invasion or damage. IL-22 participates in various lung diseases through epithelial protection or regeneration, making it an extremely attractive cytokine for the treatment of COVID-19. Of note, the FDA has approved several research groups to study the efficacy of IL-22 during SARS-CoV-2 infection (116–118). These clinical trials suggest that IL-22 treatment can shorten the duration of Intensive Care Unit (ICU) stay. Therefore, it is necessary to further comprehensively understand the specific mechanisms and functions of IL-22 in regulating the lung microenvironment, which could enable to identify novel immunotherapeutic strategy for COVID-19. However, IL-22 has been demonstrated to promote tumor growth and accelerate inflammation in a few animal models. The therapeutic consequences of IL-22 in either direction for COVID-19 treatment should be evaluated, which will provide useful insights into its role in lung health.
WC and SF wrote the manuscript. WC, YL and DJ designed the structures and supervised the work. The final version of this paper has been approved by all authors.
Our work is partially supported by Shanghai Municipal Science and Technology Major Project [No.2018SHZDZX01] and National Natural Science Foundation of China.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in wuhan. China Lancet (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
2. Vabret N, Britton GJ, Gruber C, Hegde S, Kim J, Kuksin M, et al. Immunology of COVID-19: current state of the science. Immunity (2020) 52:910–41. doi: 10.1016/j.immuni.2020.05.002
3. Wong LR, Perlman S. Immune dysregulation and immunopathology induced by SARS-CoV-2 and related coronaviruses - are we our own worst enemy? Nat Rev Immunol Nov (2021) 22:47–56. doi: 10.1038/s41577-021-00656-2
4. Li J, Lai S, Gao GF, Shi. The emergence W. Genomic diversity and global spread of SARS-CoV-2. Nature (2021) 600:408–18. doi: 10.1038/s41586-021-04188-6
5. Mathew D, Giles JR, Baxter AE, Oldridge DA, Greenplate AR, Wu JE, et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science (2020) 369:eabc8511. doi: 10.1126/science.abc8511
6. Kuri-Cervantes L, Pampena MB, Meng W, Rosenfeld AM, Ittner CAG, Weisman AR, et al. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci Immunol (2020) 5:eabd7114. doi: 10.1126/sciimmunol.abd7114
7. Song J, Zhang C, Fan X, Meng F, Xu Z, Xia P, et al. Immunological and inflammatory profiles in mild and severe cases of COVID-19. Nat Commun (2020) 11:3410. doi: 10.1038/s41467-020-17240-2
8. Salama C, Han J, Yau L, Reiss WG, Kramer B, Neidhartet JD, et al. Tocilizumab in patients hospitalized with covid-19 pneumonia. N Engl J Med (2021) 384:20–30. doi: 10.1056/NEJMoa2030340
9. Brown MJ, Alazawi W, Kanoni S. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med (2021) 384:1491–502. doi: 10.1056/NEJMc2108482
10. Sabat R, Ouyang W, Wolk K. Therapeutic opportunities of the IL-22-IL-22R1 system. Nat Rev Drug Discovery (2014) 13:21–38. doi: 10.1038/nrd4176
11. Dumoutier L, Louahed J, Renauld JC. Cloning and characterization of IL‐10‐related T cell‐derived inducible factor (IL‐TIF), a novel cytokine structurally related to IL‐10 and inducible by IL‐9. J Immunol (2000) 164:1814–9. doi: 10.4049/jimmunol.164.4.1814
12. Xie MH, Aggarwal S, Ho WH, Foster J, Zhang Z, Stinson J, et al. Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptorrelated proteins CRF2-4 and IL-22R. J Biol Chem (2000) 275:31335–9. doi: 10.1074/jbc.M005304200
13. Kotenko SV, Izotova LS, Mirochnitchenko OV, Esterova E, Dickensheets H, Donnelly RP, et al. Identification of the functional interleukin-22 (IL-22) receptor complex: the IL-10R2 chain (IL-10Rβ) is a common chain of both the IL-10 and IL-22 (IL-10-related T cell-derived inducible factor, IL-TIF) receptor complexes. J Biol Chem (2001) 276:2725–32. doi: 10.1074/jbc.M007837200
14. Rutz S, Wang X, Ouyang W. The IL-20 subfamily of cytokines–from host defence to tissue homeostasis. Nat Rev Immunol (2014) 14:783–95. doi: 10.1038/nri3766
15. Ouyang W, O'Garra A. IL-10 family cytokines IL-10 and IL-22: from basic science to clinical translation. Immunity (2019) 50:871–91. doi: 10.1016/j.immuni.2019.03.020
16. Wang S, Yao Y, Yao M, Fu P, Wang W. Interleukin-22 promotes triple negative breast cancer cells migration and paclitaxel resistance through JAK-STAT3/MAPKs/AKT signaling pathways. Biochem Biophys Res Commun (2018) 503:1605–9. doi: 10.1016/j.bbrc.2018.07.088
17. Calautti E, Avalle L, Poli V. Psoriasis: A STAT3-centric view. Int J Mol Sci (2018) 19:171. doi: 10.3390/ijms19010171
18. Bai L, Fang H, Xia S, Zhang R, Li L, Ochando J, et al. STAT1 activation represses IL-22 gene expression and psoriasis pathogenesis. Biochem Biophys Res Commun (2018) 501:563–9. doi: 10.1016/j.bbrc.2018.05.042
19. Chen W, Zhang X, Fan J, Zai W, Luan J, Li Y, et al. Tethering interleukin-22 to apolipoprotein a-I ameliorates mice from acetaminophen-induced liver injury. Theranostics (2017) 7:4135–48. doi: 10.7150/thno.20955
20. Chen W, Luan J, Wei G, Zhang X, Fan J, Zai W, et al. In vivo hepatocellular expression of interleukin-22 using penetratin-based hybrid nanoparticles as potential anti-hepatitis therapeutics. Biomaterials (2018) 187:66–80. doi: 10.1016/j.biomaterials.2018.09.046
21. Zhang Z, Zou J, Shi Z, Zhang B, Etienne-Mesmin L, Wang Y, et al. IL-22-induced cell extrusion and IL-18-induced cell death prevent and cure rotavirus infection. Sci Immunol (2020) 5:eabd2876. doi: 10.1126/sciimmunol.abd2876
22. Neil JA, Matsuzawa-Ishimoto Y, Kernbauer-Hölzl E, Schuster SL, Sota S, Venzon M, et al. IFN-I and IL-22 mediate protective effects of intestinal viral infection. Nat Microbiol (2019) 4:1737–49. doi: 10.1038/s41564-019-0470-1
23. Shabgah AG, Navashenaq JG, Shabgah OG, Mohammadi H, Sahebkar A. Interleukin-22 in human inflammatory diseases and viral infections. Autoimmun Rev (2017) 16:1209–18. doi: 10.1016/j.autrev.2017.10.004
24. Shi Z, Zou J, Zhang X, Zhao X, Noriega J, Zhang B, et al. Segmented filamentous bacteria prevent and cure rotavirus infection. Cell (2019) 179:644–58. doi: 10.1016/j.cell.2019.09.028
25. Fox SE, Akmatbekov A, Harbert JL, Li G, Brown JQ, Heide RSV. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from new Orleans. Lancet Respir Med (2020) 8:681–6. doi: 10.1016/S2213-2600(20)30243-5
26. Keam S, Megawati D, Patel SK, Tiwari R, Dhama K, Harapan H. Immunopathology and immunotherapeutic strategies in severe acute respiratory syndrome coronavirus 2 infection. Rev Med Virol (2020) 30:e2123. doi: 10.1002/rmv.2123
27. Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med (2020) 8:420–2. doi: 10.1016/S2213-2600(20)30076-X
28. Cevik M, Kuppalli K, Kindrachuk J, Peiris M. Virology, transmission, and pathogenesis of SARS-CoV-2. BMJ (2020) 371:m3862. doi: 10.1136/bmj.m3862
29. Behnood SA, Shafran R, Bennett SD, Zhang AXD, Mahoney LL, Stephenson TJ, et al. Persistent symptoms following SARS-CoV-2 infection amongst children and young people: A meta-analysis of controlled and uncontrolled studies. J Infect (2022) 84:158–70. doi: 10.1016/j.jinf.2021.11.011
30. Fahriani M, Ilmawan M, Fajar JK, Maliga HA, Frediansyah A, Masyeni S, et al. Persistence of long COVID symptoms in COVID-19 survivors worldwide and its potential pathogenesis - a systematic review and meta-analysis. Narra J (2021) 1:e36. doi: 10.52225/narraj.v1i2.36
31. Fajar JK, Ilmawan M, Mamada S, Mutiawati E, Husnah M, Yusuf H, et al. Global prevalence of persistent neuromuscular symptoms and the possible pathomechanisms in COVID-19 recovered individuals: A systematic review and meta-analysis. Narra J (2021) 1:e48. doi: 10.52225/narra.v1i3.48
32. Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med (2020) 383:590–2. doi: 10.1056/NEJMc2011400
33. Zhang Z, Shen Q. H, Chang, vaccines for COVID-19: A systematic review of immunogenicity, current development, and future prospects. Front Immunol (2022) 13:843928. doi: 10.3389/fimmu.2022.843928
34. Antia R, Halloran ME. Transition to endemicity: Understanding COVID-19. Immunity (2021) 54:2172–6. doi: 10.1016/j.immuni.2021.09.019
35. Ogega CO, Skinner NE, Blair PW, Park H, Littlefield K, Ganesan A, et al. Durable SARS-CoV-2 b cell immunity after mild or severe disease. J Clin Invest (2021) 131:e145516. doi: 10.1172/JCI145516
36. Siracusano G, Pastori C, Lopalco L. Humoral immune responses in COVID-19 patients: a window on the state of the art. Front Immunol (2020) 11:1049. doi: 10.3389/fimmu.2020.01049
37. Boyton RJ, Altmann DM. The immunology of asymptomatic SARS-CoV-2 infection: what are the key questions? Nat Rev Immunol (2021) 21:762–8. doi: 10.1038/s41577-021-00631-x
38. Shi L, Wang L, Xu R, Zhang C, Xie Y, Liu K, et al. Mesenchymal stem cell therapy for severe COVID-19. Signal Transduct Target Ther (2021) 6:339. doi: 10.1038/s41392-021-00754-6
39. Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe (2020) 27:992–1000. doi: 10.1016/j.chom.2020.04.009
40. Caricchio R, Abbate A, Gordeev I, Meng J, Hsue PY, Neogi T, et al. Effect of canakinumab vs placebo on survival without invasive mechanical ventilation in patients hospitalized with severe COVID-19. JAMA (2021) 326:230–9. doi: 10.1001/jama.2021.9508
41. Zhou T, Damsky W, Weizman OE, McGeary MK, Hartmann KP, Rosen CE, et al. IL-18BP is a secreted immune checkpoint and barrier to IL-18 immunotherapy. Nature (2020) 583:609–14. doi: 10.1038/s41586-020-2422-6
42. Luca GD, Cavalli G, Campochiaro C, Della-Torre E, Angelillo P, Tomelleri A, et al. GM-CSF blockade with mavrilimumab in severe COVID-19 pneumonia and systemic hyperinflammation: A single-centre, prospective cohort study. Lancet Rheumatol (2020) 2:e465–73. doi: 10.1016/S2665-9913(20)30170-3
43. Duret PM, Sebbag E, Mallick A, Gravier S, Spielmann L, Messer L. Recovery from COVID-19 in a patient with spondyloarthritis treated with TNF-alpha inhibitor etanercept. Ann Rheum Dis (2020) 79:1251–2. doi: 10.1136/annrheumdis-2020-217362
44. Patterson BK, Seethamraju H, Dhody K, Corley MJ, Kazempour K, Lalezari JP, et al. Disruption of the CCL5/RANTES-CCR5 pathway restores immune homeostasis and reduces plasma viral load in critical COVID-19. medRxiv (2020) 2020:20084673. doi: 10.1101/2020.05.02.20084673
45. Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, et al. ACTT-2 study group members, baricitinib plus remdesivir for hospitalized adults with covid-19. N Engl J Med (2020) 384:795–807. doi: 10.1056/NEJMoa2031994
46. Veerdonk FL, Giamarellos-Bourboulis E, Pickkers P, Derde L, Leavis H, Crevel R, et al. A guide to immunotherapy for COVID-19. Nat Med 28(2022) 28:39–50. doi: 10.1038/s41591-021-01643-9
47. Adrover JM, Carrau L, Daßler-Plenker J, Bram Y, Chandar V, Houghton S, et al. Disulfiram inhibits neutrophil extracellular trap formation and protects rodents from acute lung injury and SARS-CoV-2 infection. JCI Insight (2022) 7:e157342. doi: 10.1172/jci.insight.157342
48. Farnoosh G, Akbariqomi M, Badri T, Bagheri M, Izadi M, Saeedi-Boroujeni A, et al. Efficacy of a low dose of melatonin as an adjunctive therapy in hospitalized patients with COVID-19: A randomized, double-blind clinical trial. Arch Med Res (2022) 53:79–85. doi: 10.1016/j.arcmed.2021.06.006
49. Monk PD, Marsden RJ, Tear VJ, Brookes J, Batten TN, Mankowski M, et al. Inhaled interferon beta COVID-19 study group, safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: A randomised, double-blind, placebo-controlled, phase 2 trial, lancet respir. Med (2020) 9:196–206. doi: 10.1016/S2213-2600(20)30511-7
50. Wang N, Zhan Y, Zhu L, Hou Z, Liu F, Song P, et al. Retrospective multicenter cohort study shows early interferon therapy is associated with favorable clinical responses in COVID-19 patients. Cell Host Microbe (2020) 28:455–64. doi: 10.1016/j.chom.2020.07.005
51. The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: A meta-analysis. JAMA (2020) 324:1330–41. doi: 10.1001/jama.2020.17023
52. Hatzl S, Krause R, Schilcher G. Early treatment with sotrovimab for covid-19. N Engl J Med (2022) 386:1480. doi: 10.1056/NEJMc2201606
53. Hoffmann M, Arora P, Gross R, Seidel A, Hörnich BF, Hahn AS, et al. SARS-CoV-2 variants B.1.351 and P.1 escape from neutralizing antibodies. Cell (2021) 184:2384–93. doi: 10.1016/j.cell.2021.03.036
54. Kooistra EJ, Waalders NJB, Grondman I, Janssen NAF, de Nooijer AH, Netea MG, et al. RCI-COVID-19 study group, anakinra treatment in critically ill COVID-19 patients: A prospective cohort study. Crit Care (2020) 24:688. doi: 10.1186/s13054-020-03364-w
55. WHO Solidarity Trial Consortium, Pan H, Peto R, Henao-Restrepo A, Preziosi M, Sathiyamoorthy V, et al. Repurposed antiviral drugs for Covid-19–interim WHO solidarity trial results. N Engl J Med (2020) 384:497–511. doi: 10.1056/NEJMoa2023184
56. Andreakos E, Tsiodras S. COVID-19: Lambda interferon against viral load and hyperinflammation. EMBO Mol Med (2020) 12:e12465. doi: 10.15252/emmm.202012465
57. Jones BE, Brown-Augsburger PL, Corbett KS, Westendorf K, Davies J, Cujec TP, et al. The neutralizing antibody, LY-CoV555, protects against SARS-CoV-2 infection in nonhuman primates. Sci Transl Med (2021) 13:eabf1906. doi: 10.1126/scitranslmed.abf1906
58. Dudakov JA, Hanash AM, van den Brink MR. Interleukin 22: immunobiology and pathology. Annu Rev Immunol (2015) 33:747–85. doi: 10.1146/annurev-immunol-032414-112123
59. Apetoh L, Quintana FJ, Pot C, Joller N, Xiao S, Kumar D, et al. The aryl hydrocarbon receptor interacts with c-maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol (2010) 11:854–61. doi: 10.1038/ni.1912
60. Gandhi R, Kumar D, Burns EJ, Nadeau M, Dake B, Laroni A, et al. Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3(+) regulatory T cells. Nat Immunol (2010) 11:846–53. doi: 10.1038/ni.1915
61. Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, et al. Control of t(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature (2008) 453:65–71. doi: 10.1038/nature06880
62. Qiu J, Guo X, Chen ZM, He J, Sonnenberg GF, Artis D, et al. Group 3 innate lymphoid cells inhibit T-cell-mediated intestinal inflammation through aryl hydrocarbon receptor signaling and regulation of microflora. Immunity (2013) 39:386–99. doi: 10.1016/j.immuni.2013.08.002
63. Stockinger B, Meglio PD, Gialitakis M, Duarte JH. The aryl hydrocarbon receptor: multitasking in the immune system. Annu Rev Immunol (2014) 32:403–32. doi: 10.1146/annurev-immunol-032713-120245
64. Lee JS, Cella M, McDonald KG, Garlanda C, Kennedy GD, Nukaya M, et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of notch. Nat Immunol (2011) 13:144–51. doi: 10.1038/ni.2187
65. Alam MS, Maekawa Y, Kitamura A, Tanigaki K, Yoshimoto T, Kishihara K, et al. Notch signaling drives IL-22 secretion in CD4+ T cells by stimulating the aryl hydrocarbon receptor. Proc Natl Acad Sci USA (2010) 107:5943–8. doi: 10.1073/pnas.0911755107
66. Robb CT, McSorley HJ, Lee J, Aoki T, Yu C, Crittenden S, et al. Prostaglandin E2 stimulates adaptive IL-22 production and promotes allergic contact dermatitis. J Allergy Clin Immunol (2018) 141:152–62. doi: 10.1016/j.jaci.2017.04.045
67. Duffin R, O’Connor RA, Crittenden S, Forster T, Yu C, Zheng X, et al. Prostaglandin E2 constrains systemic inflammation through an innate lymphoid cell-IL-22 axis. Science (2016) 351:1333–8. doi: 10.1126/science.aad9903
68. Araújo EF, Preite NW, Veldhoen M, Loures FV, Calich VLG. Pulmonary paracoccidioidomycosis in AhR deficient hosts is severe and associated with defective treg and Th22 responses. Sci Rep (2020) 10:11312. doi: 10.1038/s41598-020-68322-6
69. Michaudel C, Bataille F, Maillet I, Fauconnier L, Colas C, Sokol H, et al. Ozone-induced aryl hydrocarbon receptor activation controls lung inflammation via interleukin-22 modulation. Front Immunol (2020) 11:144. doi: 10.3389/fimmu.2020.00144
70. Ivanov S, Renneson J, Fontaine J, Barthelemy A, Paget C, Fernandez EM, et al. Interleukin-22 reduces lung inflammation during influenza a virus infection and protects against secondary bacterial infection. J Virol (2013) 87:6911–24. doi: 10.1128/JVI.02943-12
71. Simonian PL, Wehrmann F, Roark CL, Born WK, O'Brien RL, Fontenot AP. γδ T cells protect against lung fibrosis via IL-22. J Exp Med (2010) 207:2239–53. doi: 10.1084/jem.20100061
72. Trevejo-Nunez G, Elsegeiny W, Conboy P, Chen K, Kolls JK. Critical role of IL-22/IL22-RA1 signaling in pneumococcal pneumonia. J Immunol (2016) 197:1877–83. doi: 10.4049/jimmunol.1600528
73. Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, et al. IL-22 mediates mucosal host defense against gram-negative bacterial pneumonia. Nat Med (2008) 14:275–81. doi: 10.1038/nm1710
74. Maele LV, Carnoy C, Cayet D, Ivanov S, Porte R, Deruy E, et al. Activation of type 3 innate lymphoid cells and interleukin 22 secretion in the lungs during streptococcus pneumoniae infection. J Infect Dis (2014) 210:493–503. doi: 10.1093/infdis/jiu106
75. Xu X, Weiss ID, Zhang HH, Singh SP, Wynn TA, Wilson MS, et al. Conventional NK cells can produce IL-22 and promote host defense in klebsiella pneumoniae pneumonia. J Immunol (2014) 192:1778–86. doi: 10.4049/jimmunol.1300039
76. Peng Y, Gao X, Yang J, Shekhar S, Wang S S, Zhao W, et al. IL-22 promotes Th1/Th17 immunity in chlamydial lung infection. Mol Med (2014) 20:109–19. doi: 10.2119/molmed.2013.00115
77. Gessner MA, Werner JL, Lilly LM, Nelson MP, Metz AE, Dunaway CW, et al. Dectin-1-dependent interleukin-22 contributes to early innate lung defense against aspergillus fumigatus. Infect Immun (2012) 80:410–17. doi: 10.1128/IAI.05939-11
78. Hoegl S, Bachmann M, Scheiermann P, Goren I, Hofstetter C, Pfeilschifter J, et al. Protective properties of inhaled IL-22 in a model of ventilator-induced lung injury. Am J Respir Cell Mol Biol (2011) 44:369–76. doi: 10.1165/rcmb.2009-0440OC
79. Sonnenberg GF, Nair MG, Kirn TJ, Zaph C, Fouser LA, Artis D. Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. J Exp Med (2010) 207:1293–305. doi: 10.1084/jem.20092054
80. Gu P, Wang D, Zhang J, Wang X, Chen Z, Gu L, et al. Protective function of interleukin-22 in pulmonary fibrosis. Clin Transl Med (2021) 11:e509. doi: 10.1002/ctm2.509
81. Farfariello V, Amantini C, Nabissi M, Morelli MB, Aperio C, Caprodossi S, et al. IL-22 mRNA in peripheral blood mononuclear cells from allergic rhinitic and asthmatic pediatric patients. Pediatr Allergy Immunol (2011) 22:419–23. doi: 10.1111/j.1399-3038.2010.01116.x
82. Besnard AG, Sabat R, Dumoutier L, Renauld J, Willart M, Lambrecht B, et al. Dual role of IL-22 in allergic airway inflammation and its cross-talk with IL-17A, am. J Respir Crit Care Med (2011) 183:1153–63. doi: 10.1164/rccm.201008-1383OC
83. Nakagome K, Imamura M, Kawahata K, Harada H, Okunishi K, Matsumoto T, et al. High expression of IL-22 suppresses antigen-induced immune responses and eosinophilic airway inflammation via an IL-10-associated mechanism. J Immunol (2011) 187:5077–89. doi: 10.4049/jimmunol.1001560
84. Takahashi K, Hirose K, Kawashima S, Niwa Y, Wakashin H, Iwata A, et al. IL-22 attenuates IL-25 production by lung epithelial cells and inhibits antigeninduced eosinophilic airway inflammation. J Allergy Clin Immunol (2011) 128:1067–76. doi: 10.1016/j.jaci.2011.06.018
85. Badi YE, Pavel AB, Pavlidis S, Riley JH, Bates S, Kermani NZ, et al. Mapping atopic dermatitis and anti-IL-22 response signatures to type 2-low severe neutrophilic asthma. J Allergy Clin Immunol (2022) 149:89–101. doi: 10.1016/j.jaci.2021.04.010
86. Pociask DA, Scheller EV, Mandalapu S, McHugh KJ, Enelow RI, Fattman CL, et al. Il-22 is essential for lung epithelial repair following influenza infection. Am J Pathol (2013) 182:1286–96. doi: 10.1016/j.ajpath.2012.12.007
87. Kumar P, Thakar MS, Ouyang W, Malarkannan S. IL-22 from conventional nk cells is epithelial regenerative and inflammation protective during influenza infection. Mucosal Immunol (2013) 6:69–82. doi: 10.1038/mi.2012.49
88. Das S, Croix CS, Good M, Chen J, Zhao J, Hu S, et al. Interleukin-22 inhibits respiratory syncytial virus production by blocking virus-mediated subversion of cellular autophagy. iScience (2020) 23:101256. doi: 10.1016/j.isci.2020.101256
89. Cagan E, Tezcan G, Simsek A, Kizmaz MA, Dombaz F, Asan A, et al. The age-dependent role of th22, tc22, and tc17 cells in the severity of pneumonia in covid-19 immunopathogenesis. Viral Immunol (2022) (35):318–27. doi: 10.1089/vim.2021.0132
90. Hebert KD, Mclaughlin N, Zhang Z, Cipriani A, Alcorn JF, Pociask DA. Il-22ra1 is induced during influenza infection by direct and indirect tlr3 induction of STAT1. Respir Res (2019) 20:184. doi: 10.1186/s12931-019-1153-4
91. Brias SG, Stack G, Stacey MA, Redwood AJ, Humphreys IR. The role of il-22 in viral infections: paradigms and paradoxes. Front Immunol (2016) 7:211. doi: 10.3389/fimmu.2016.00211
92. Zenewicz LA. Il-22: there is a gap in our knowledge. ImmunoHorizons (2018) 2:198–207. doi: 10.4049/immunohorizons.1800006
93. Albayrak N, Cano CO, Karimi S, Dogahe D, Praet AV, Godefroid A, et al. Distinct expression patterns of interleukin-22 receptor 1 on blood hematopoietic cells in SARS-CoV-2 infection. Front Immunol (2022) 13:769839. doi: 10.3389/fimmu.2022.769839
94. Savan R, McFarland AP, Reynolds DA, Feigenbaum L, Ramakrishnan K, Karwan M, et al. A novel role for il-22r1 as a driver of inflammation. Blood (2011) 117:575–84. doi: 10.1182/blood-2010-05-285908
95. Klooster JPT, Bol-Schoenmakers M, van Summeren K, van Vliet ALW, de Haan CAM, van Kuppeveld FJM, et al. Enterocytes, fibroblasts and myeloid cells synergize in anti-bacterial and anti-viral pathways with IL22 as the central cytokine. Commun Biol (2021) (4):631. doi: 10.1038/s42003-021-02176-0
96. Garcıa M, Kokkinou E, Garća AC, Parrot T, Palma Medina LM, Maleki KT, et al. Innate lymphoid cell composition associates with covid-19 disease severity. Clin Transl Immunol (2020) 9:e1224. doi: 10.1002/cti2.1224
97. Hoffmann JP, Kolls JK, McCombs JE. Regulation and function of ILC3s in pulmonary infections. Front Immunol (2021) 12:672523. doi: 10.3389/fimmu.2021.672523
98. Alcorn JF. Il-22 plays a critical role in maintaining epithelial integrity during pulmonary infection. Front Immunol (2020) 11:1160. doi: 10.3389/fimmu.2020.01160
99. Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by il-22. Nat Imm (2011) 12:383–90. doi: 10.1038/ni.2025
100. Sonnenberg GF, Fouser LA, Artis D. Functional biology of the IL-22-IL-22R pathway in regulating immunity and inflammation at barrier surfaces. Adv Immunol (2010) 107:1–29. doi: 10.1016/B978-0-12-381300-8.00001-0
101. Fabre T, Molina MF, Soucy G, Goulet JP, Willems B, Villeneuve JP, et al. Type 3 cytokines IL-17A and IL-22 drive TGF-beta-dependent liver fibrosis. Sci Immunol (2018) 3:eaar7754. doi: 10.1126/sciimmunol.aar7754
102. Powell N, Pantazi E, Pavlidis P, Tsakmaki A, Li K, Yang F, et al. Interleukin-22 orchestrates a pathological endoplasmic reticulum stress response transcriptional programme in colonic epithelial cells. Gut (2020) 69:578–90. doi: 10.1136/gutjnl-2019-318483
103. Nakajima H, Nakajima K, Tarutani M, Morishige R, Sano S. Kinetics of circulating Th17 cytokines and adipokines in psoriasis patients. Arch Dermatol Res (2011) 303:451–5. doi: 10.1007/s00403-011-1159-3
104. Leipe J, Schramm MA, Grunke M, Baeuerle M, Dechant C, Nigg AP, et al. Interleukin 22 serum levels are associated with radiographic progression in rheumatoid arthritis. Ann Rheum Dis (2011) 70:1453–7. doi: 10.1136/ard.2011.152074
105. Geboes L, Dumoutier L, Kelchtermans H, Schurgers E, Mitera T, Renauld JC, et al. Proinflammatory role of the Th17 cytokine interleukin-22 in collagen-induced arthritis in C57BL/6 mice. Arthritis Rheumatol (2009) 60:390–5. doi: 10.1002/art.24220
106. Ikeuchi H, Kuroiwa T, Hiramatsu N, Kaneko Y, Hiromura K, Ueki K, et al. Expression of interleukin-22 in rheumatoid arthritis:potential role as a proinflammatory cytokine. Arthritis Rheumatol (2005) 52:1037–46. doi: 10.1002/art.20965
107. Kim KW, Kim HR, Park JY, Park JS, Oh HJ, Woo YJ, et al. Interleukin-22 promotes osteoclastogenesis in rheumatoid arthritis through induction of RANKL in human synovial fibroblasts. Arthritis Rheumatol (2012) 64:1015–23. doi: 10.1002/art.33446
108. Van Belle AB, de Heusch M, Lemaire MM, Hendrickx E, Warnier G, Dunussi Joannopoulos K, et al. IL-22 is required for imiquimod-induced psoriasiform skin inflammation in mice. J Immunol (2012) 188:462–9. doi: 10.4049/jimmunol.1102224
109. Park O, Wang H, Weng H, Feigenbaum L, Li H, Yin S, et al. In vivo consequences of liver-specific interleukin-22 expression in mice: implications for human liver disease progression. Hepatology (2011) 54:252–61. doi: 10.1002/hep.24339
110. Li C, Xu M, Coyne J J, Wang W, Davila ML, Wang Y, et al. Psoriasis-associated impairment of CCL27/CCR10-derived regulation leads to IL-17A/IL-22-producing skin T-cell overactivation. J Allergy Clin Immunol (2021) 147:759–63. doi: 10.1016/j.jaci.2020.05.044
111. Bellone M, Brevi A, Huber S. Microbiota-propelled t helper 17 cells in inflammatory diseases and cancer. Microbiol Mol Biol Rev (2020) (84):e00064-19. doi: 10.1128/MMBR.00064-19
112. Lilly LM, Gessner MA, Dunaway CW, Metz AE, Schwiebert L, Weaver CT, et al. The β-glucan receptor dectin-1 promotes lung immunopathology during fungal allergy via IL-22. J Immunol (2012) 189:3653–60. doi: 10.4049/jimmunol.1201797
113. Johnston PA, Grandis JR. STAT3 signaling: anticancer strategies and challenges. Mol Interv (2011) 11:18–26. doi: 10.1124/mi.11.1.4
114. Thilakasiri PS, Dmello RS, Nero TL, Parker MW, Ernst M, Chand AL. Repurposing of drugs as STAT3 inhibitors for cancer therapy. Semin Cancer Biol (2021) 68:31–46. doi: 10.1016/j.semcancer.2019.09.022
115. Jiang R, Tan Z, Deng L, Chen Y, Xia Y, Gao Y, et al. Interleukin-22 promotes human hepatocellular carcinoma by activation of STAT3. Hepatology (2011) 54:900–9. doi: 10.1002/hep.24486
116. ClinicalTrials.gov. A study to evaluate the safety and efficacy of mstt1041a (astegolimab) or uttr1147a in patients with severe covid-19 pneumonia (covastil) (2020). Available at: https://clinicaltrials.gov/ct2/show/NCT04386616.
117. Clinicaltrials.gov. Study of f-652 (il-22: igg2 fusion protein) in patients with moderate to severe COVID-19 (2020). Available at: https://clinicaltrials.gov/ct2/show/NCT04498377.
Keywords: interleukin-22, immunotherapeutic strategy, lung, COVID-19, SARS-CoV-2 infection
Citation: Fang S, Ju D, Lin Y and Chen W (2022) The role of interleukin-22 in lung health and its therapeutic potential for COVID-19. Front. Immunol. 13:951107. doi: 10.3389/fimmu.2022.951107
Received: 23 May 2022; Accepted: 04 July 2022;
Published: 27 July 2022.
Edited by:
Hui-Rong Jiang, University of Strathclyde, United KingdomReviewed by:
Hussein Kadhem Al-Hakeim, University of Kufa, IraqCopyright © 2022 Fang, Ju, Lin and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Chen, d2VpY2hlbmZkQGZ1ZGFuLmVkdS5jbg==; Yong Lin, bGlueW9uZzcwMDdAMTYzLmNvbQ==; Dianwen Ju, ZGlhbndlbmp1QGZ1ZGFuLmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.