
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 29 November 2022
Sec. Autoimmune and Autoinflammatory Disorders
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.947341
 Jia Wang1,2
Jia Wang1,2 Sheng-Xiao Zhang1,2
Sheng-Xiao Zhang1,2 Jia-Song Chang2,3
Jia-Song Chang2,3 Ting Cheng1,2
Ting Cheng1,2 Xiao-Jing Jiang1,2
Xiao-Jing Jiang1,2 Qin-Yi Su1,2
Qin-Yi Su1,2 Jia-Qi Zhang1,2
Jia-Qi Zhang1,2 Jing Luo1,2
Jing Luo1,2 Xiao-Feng Li1,2*
Xiao-Feng Li1,2*Background: Regulatory T cells (Tregs) have been found to play crucial roles in immune tolerance. However, the status of Tregs in refractory rheumatoid arthritis (RA) is still unclear. Moreover, low-dose interleukin-2 (IL-2) has been reported to selectively promote the expansion of Tregs. This study investigated the status of CD4+ Tregs and low-dose IL-2 therapy in patients with refractory RA.
Methods: The absolute number of CD4+CD25+FOXP3+ Treg (CD4 Treg), CD4+IL17+ T (Th17), and other subsets in peripheral blood (PB) from 41 patients with refractory RA and 40 healthy donors was characterized by flow cytometry combined with an internal microsphere counting standard. Twenty-six patients with refractory RA were treated with daily subcutaneous injections of 0.5 million IU of human IL-2 for five consecutive days. Then, its effects on CD4 Treg and Th17 cells in PB were analyzed.
Results: A decrease in the absolute number of PB CD4 Tregs rather than the increase in the number of Th17 was found to contribute to an imbalance between Th17 and CD4 Tregs in these patients, suggesting an essential role of CD4 Tregs in sustained high disease activity. Low-dose IL-2 selectively increased the number of CD4 Tregs and rebalanced the ratio of Th17 and CD4 Tregs, leading to increased clinical symptom remission without the observed side effects.
Conclusions: An absolute decrease of PB CD4 Tregs in patients with refractory RA was associated with continuing disease activation but not the increase of Th17 cells. Low-dose IL-2, a potential therapeutic candidate, restored decreased CD4 Tregs and promoted the rapid remission of patients with refractory RA without overtreatment and the observed side effects.
Clinical trial registration: http://www.chictr.org.cn/showproj.aspx?proj=13909, identifier ChiCTR-INR-16009546.
Rheumatoid arthritis (RA) is a chronic inflammatory joint disease that potentially leads to cartilage, bone damage, and disability. Treatment algorithms involve measuring disease activity with composite indices, applying a treatment-to-target strategy, and using conventional, biological, and new non-biological disease-modifying antirheumatic drugs (DMARDs) (1). Though these strategies have good efficacy, up to 40% of patients did not benefit from these therapies due to lack of efficacy or development of resistance (2, 3) or treatment-related adverse events (4, 5). Adequate control of disease activity in RA is achieved in only approximately one-third of all RA patients with previous treatments (6, 7). Accordingly, new therapies are urgently required for refractory RA exposed to multiple DMARDs without necessarily benefitting from them (8).
Effector T cells, such as the Th17 subset of CD4+ T cells producing interleukin-17 (IL-17), have been reported to play important roles in inflammation (9). Th17 cells mediate the inflammation process by stimulating the production of cytokines and chemokines (10). IL-17 levels are extremely low or undetectable in normal human peripheral blood, while the levels are elevated in peripheral blood or synovial fluid in RA patients (11). The frequency of Th17 in peripheral blood was reported to increase in RA patients (12, 13), while the role of Th17 in refractory RA remains to be studied.
The pathogenesis of RA is further closely associated with the imbalance of pro-inflammatory Th17 cells and anti-inflammatory Treg cells (14). Treg cells are a distinct set of T cells responsible for suppressing autoreactive deleterious activities of effector T cells (15). Our previous study reported that reduced circulating Tregs (CD4+CD25+Foxp3+ Treg) might be involved in the pathogenesis and progression of RA (16). Although several studies (17) evaluated the proportion of Tregs in active RA and remission RA patients, the definition of Treg cells was different between these works. However, the status of Treg cells in refractory RA is still unclear.
IL-2, a cell growth factor, plays a critical role in regulating immune balance by providing a survival and proliferation signal for various types of T cells (18). However, high-dose IL-2 administration has been confirmed to enhance immune responses by activating effector T cells against cancer (19, 20), while low-dose IL-2 was evidenced to mainly stimulate Treg cell survival and expansion and thereby control autoimmunity and inflammation. Low-dose IL-2 at 0.5 million IU was widely used in the treatment of several autoimmune diseases, such as systemic lupus erythematosus (20, 21), psoriatic arthritis (22), and RA (16), but not refractory RA. However, due to the lack of evidence of decreased Treg cells in patients with refractory RA, the immune regulation therapy of IL-2 in these patients has not been examined.
The present study compared the status of PB lymphocyte and CD4+ T cell subsets of refractory RA with healthy donors. Furthermore, we explored whether low-dose IL-2 could effectively induce the remission of refractory RA by upregulating Treg cells.
A total of 41 patients with RA and 40 healthy individuals were enrolled in the study. The patients fulfilled the 2010 rheumatoid arthritis classification criteria (23). The main characteristics of RA patients and age- and sex-matched healthy donors are shown in Supplemental Table 1. These patients between the age of 18 and 65 had severely active rheumatoid arthritis [≥8 tender joints of 28 joints examined, ≥3 swollen joints of 28 joints examined, morning stiffness lasting longer than 60 min, a serum C-reactive protein (CRP) level that is at least 1.5 times of the upper limit or an erythrocyte sedimentation rate (ESR) that is at least 28 mm per hour, and DAS28 ≥5.1]. At study entry, patients must have been regularly taking one or more conventional DMARDs for at least the preceding 6 months but without disease activity remission. Patients must also have a negative pregnancy test and agree to use effective contraception during the study and for at least 6 months after stopping study treatment. They were able to comply with scheduled visits, treatment plans, laboratory tests, and other study procedures. The patients were excluded if they had a history of malignancy, were suffering from malignant disease within 5 years prior to study entry, or had a recent clinically significant infection. Patients in a pregnancy test who disagreed with using effective contraception during the study or at least 6 months after stopping study treatment were also excluded.
The patients were divided randomly into two groups: the non-IL-2 group (n = 15), who were still given conventional glucocorticoid and DMARD treatment, and the IL-2 group (n = 26), who were not only given the same conventional treatment but also injected subcutaneously IL-2 at 0.5 million IU per day for five consecutive days (24). Before and after the treatment, the patient’s peripheral lymphocyte subpopulation and CD4+ T subgroups were measured by flow cytometry. The absolute numbers of those cells were compared between the non-IL-2 and IL-2 groups before and after treatment.
In this study, BD Trucount™ tubes with the lyophilized pellet of a known number of fluorescent beads were used for determining the absolute counts of T, B, NK, CD8+ T, and total CD4+ T cells in PB and calculating the percentage and the absolute number of CD4+ T subsets, especially that of Th17 and CD4 Tregs.
For the analysis of T, B, CD4+ T, and CD8+ T cells, 50 µl of fully blending whole anticoagulant blood was reversely added into two Trucount tubes carefully without touching the standard beads that are in the bottom of the tube. Both tubes were added with 20 μl of anti-CD3FITC/CD8PE/CD45PercP/CD4APC antibodies and anti-CD3FITC/CD16+56PE/CD45PercP/CD19APC antibodies, respectively, totally mixed by a vortex blender. Cells were stained with antibodies for CD3+ (T), CD3+/CD19+ (B), CD3+/CD4+ (CD4+ T), CD3+/CD8+ (CD8+ T), and CD3-CD16+CD56+ (NK). All antibodies were purchased from BD (USA) (Supplemental Table 2).
For the analysis of Th1, Th2, and Th17 cells, cells in 1 ml of heparin-anticoagulated venous blood were stimulated for 5 h with 10 μl of PMA, 10 μl of ionomycin (final concentration was 750 ng/ml), and 1 μl of GolgiStop, respectively, in a 37°C incubator. Stimulated cells (80 μl) were taken for surface staining, and the cells were fixed and permeabilized using fixation/permeabilization reagent (BD, USA) and then stained with anti-IFN-γ-APC (for Th1), anti-IL-4-PE (for Th2), and anti-IL-17A-PE (for Th17) monoclonal (Supplemental Table 2).
For the analysis of CD4 Tregs, cells in 80 μl of heparin-anticoagulated venous blood were aliquoted into tubes without PMA and ionomycin stimulation and surface-labeled with anti-CD4-FITC and anti-CD25-APC followed by fixation, permeabilization, and intracellular staining with anti-FoxP3-PE (all from BD, USA). The labeled cells were washed and analyzed with a FACSCalibur flow cytometer (Becton-Dickinson) using the CellQuest software (BD, USA). At least 5,000 to 10,000 cells were collected to calculate the percentage of these CD4+ T subsets, and the total number of CD4+ T cells was calculated using the internal microsphere counting standard.
In this study, we used an equation to calculate the absolute number of CD4+ T subsets: the absolute number of CD4+ T subsets = the percentage of each CD4+ T subset * the absolute number of total CD4+ T cells.
At the primary endpoint of IL-2 treatment, the number of tender swollen joints and disease activity were assessed using the 28-joint Disease Activity Score (based on the erythrocyte sedimentation rate, ESR). The primary comparison was between the group receiving conventional treatment and the group receiving IL-2 at a dose of 0.5 million IU for a 5-day course. Secondary measures included the level of high-sensitivity CRP and ESR.
Clinical laboratory tests, measurement of vital signs, and other safety assessments were performed at scheduled visits. The incidence and severity of all adverse events were recorded. The blood routine and the liver and renal function were detected before and after the treatment.
The demographic parameters of the healthy individuals and RA patients were compared using an unpaired t-test for parametric data (age, PB lymphocyte subpopulations, and CD4+ T subsets) and the χ2 test for proportions (sex). Blood routine, liver and renal function, and disease activity were measured in two different treatment groups before and after the treatment. The paired t-test was used to analyze the influence of IL-2 on peripheral lymphocytes and CD4+ T subsets in patients receiving IL-2 subcutaneous injection. Correlation analysis was performed using the Spearman correlation coefficient. All p-values reported herein are two-tailed. A p-value <0.05 was taken as statistical significance. The software used for the statistical analysis was SPSS 13.0 and GraphPad Prism 5.0.
CD4 Tregs and Th17 cells were analyzed by flow cytometry. Though Th17 cells are considered to be a leading actor in the autoimmunity scenario, our results showed that the number of peripheral Th17 had no significant difference between patients with refractory RA and healthy donors [9.0 (5.1–16.8) vs. 7.4 (4.3–10.8), p = 0.132]. Interestingly, the absolute number of CD4 Tregs was significantly lower in RA patients compared with that in healthy donors [19.5 (12.2–28.9) vs. 35.5 (24.6–46.7), p < 0.001]. Accordingly, the ratio between Th17 and CD4 Treg in RA patients was significantly elevated as compared with that in the control group [0.59 (0.24–1.07) vs. 0.20 (0.16–0.31), p < 0.001]. The absolute number of CD4 Tregs was much lower [19.5 (12.2–28.9)] in those RA patients, especially for those who received long-term immunosuppression by conventional glucocorticoid, NSAID, or DMARD treatment (data not shown). No significant difference was observed in terms of age (55.1 ± 13.83 vs. 50.9 ± 9.49, p = 0.111) and sex (p = 0.809) between RA patients and healthy donors. Except for Tregs and B cells, all the other populations also showed no significant changes (Supplemental Table 1).
As shown in Supplemental Table 3, the CD4 Treg values were found to be slightly negatively correlated with DAS28 (r = −0.625, p < 0.001), ESR (r = −0.408, p = 0.001), CRP (r = −0.344, p = 0.009), number of joint pain (r = −0.639, p < 0.001), and number of joint swollen (r = −0.538, p < 0.001). The ratio of Th17 cell to CD4 Treg cell was found to be correlated with DAS28 (r = 0.350, p = 0.004), number of joint pain (r = 0.393, p = 0.001), and number of joint swollen (r = 0.407, p = 0.001). These data point to a relevant contribution of the deficiency of CD4 Tregs and an imbalanced Th17/CD4 Treg homeostasis to disease-continuing activity.
Low-dose IL-2 can selectively expand CD4 Tregs in vivo (25). Therefore, we next evaluated whether the observed CD4 Treg defects in patients with refractory RA could be restored in vivo by subcutaneous injection of IL-2. Twenty-six of 41 patients received recombinant human IL-2 (aldesleukin) at a dose of 0.5 million IU for five consecutive days. Complete whole blood from patients was repetitively collected on days 1 and 5 of IL-2 stimulation. Before IL-2 treatment, there were no significant differences in the numbers of CD4+ T-cell subsets between the IL-2 group and the non-IL-2 group (Figures 1A, B and Supplemental Table 4). In contrast, the percentage of CD4 Treg cells was higher in the IL-2 group (Supplemental Figure 1). After IL-2 treatment (Figures 1C, D and Table 1), compared with before treatment as well as with non-IL-2 control, there was a significant increase in T cells [990 (799, 1,301) vs. 1,227 (954, 2,026) cells/µl, p = 0.015], B cells [144 (80, 213) vs. 218 (149, 445) cells/µl, p = 0.005], CD4+ T cells [564 (417, 832) vs. 823 (597, 1,334) cells/µl, p = 0.003], and total lymphocyte cells [1,282 (1,109, 1,761) vs. 1,798 (1,414, 2,600) cells/µl, p = 0.006)]. The absolute count and percentage of CD4 Tregs [23 (14, 30) vs. 65 (48, 102) cells/µl and 3.7% (2.7%, 5.1%) vs. 8.5% (6.2%, 10.7%), p < 0.001] were dramatically elevated by 3-fold (Table 1; Supplemental Figure 2). There was also an increase in the absolute number of Th17 cells [9 (4, 16) vs. 17 (9, 29) cells/µl, p = 0.008]. However, it is noteworthy that the CD4 Tregs were increased much more dramatically than the Th17 cells (Figures 1, 2), leading to a decrease in their ratio [0.40 (0.23, 0.76) vs. 0.23 (0.12, 0.44), p = 0.010].
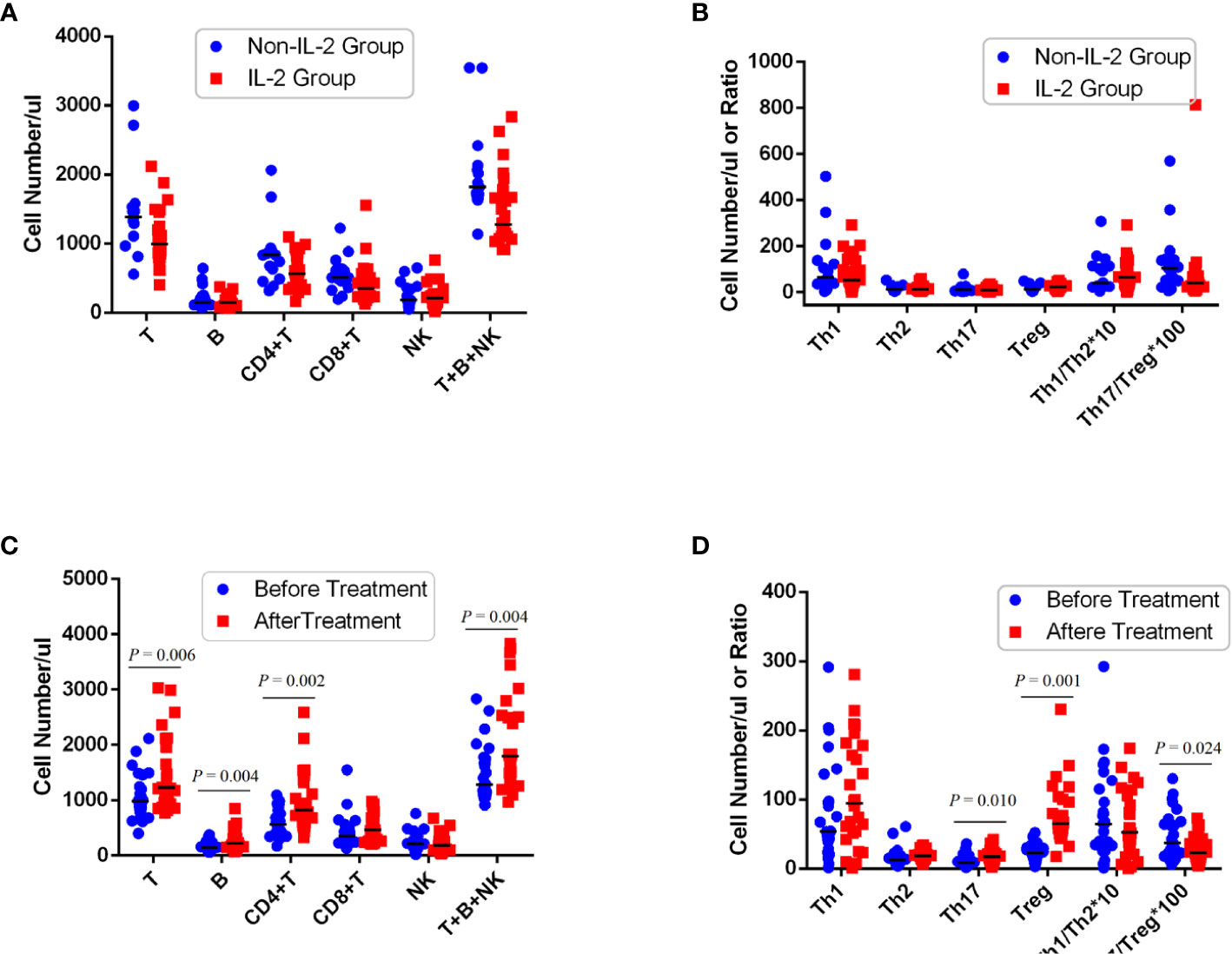
Figure 1 Peripheral CD4 Treg cells markedly decreased in refractory RA patients and restored after IL-2 treatment. Absolute number of lymphocyte subpopulations (A) or CD4+ T subsets (B) in PB of RA patients treated with or without IL-2 before this therapy. Status of peripheral lymphocyte subpopulations (C) or CD4+ T subsets (D) in patients with IL-2 before and after treatment. Absolute values of the cells (median, quartile range) are shown.
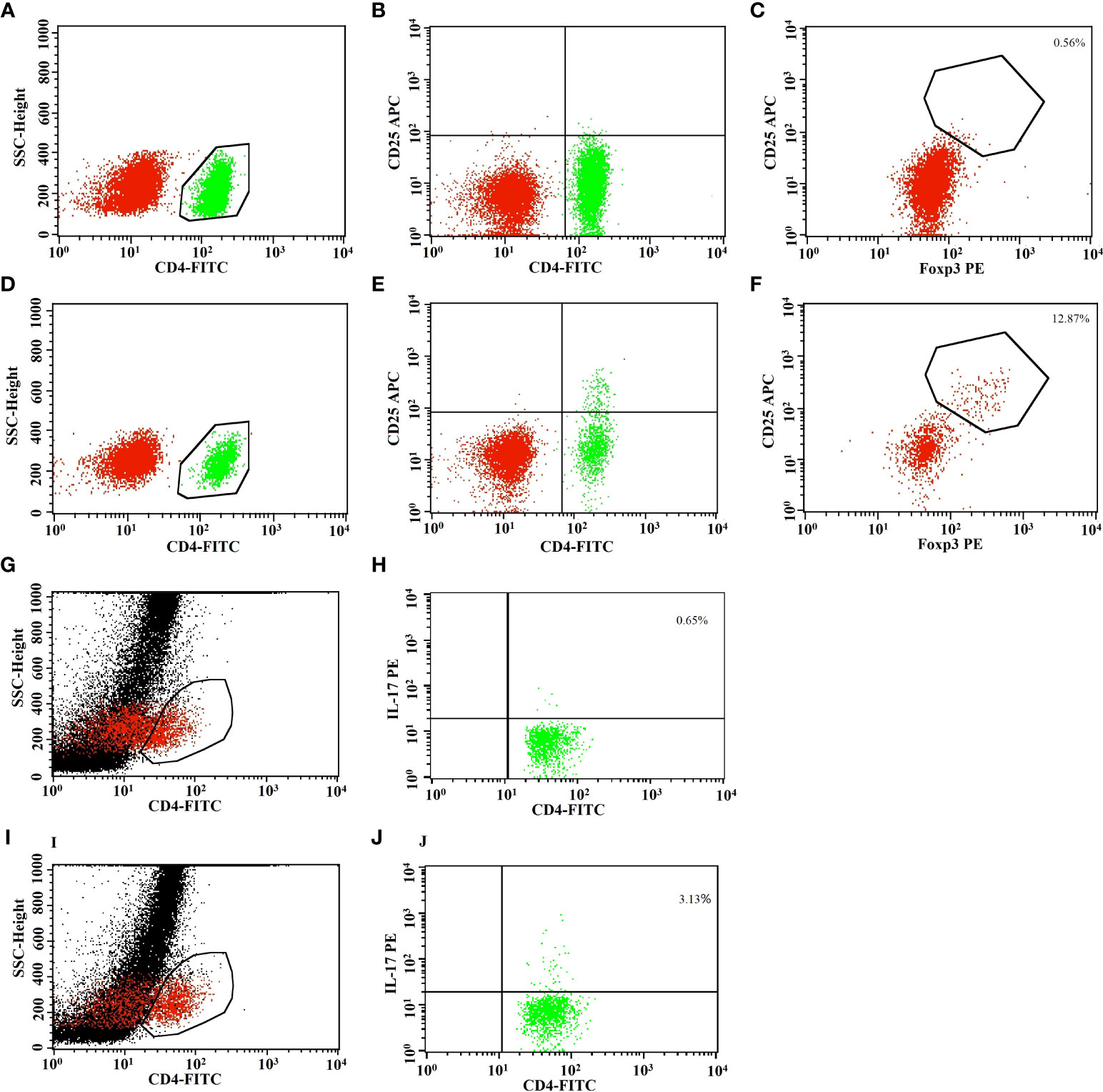
Figure 2 Representative flow cytometric analysis showed an obvious increase of CD4 Treg cells after low-dose IL-2 treatment. Representative flow cytometric analysis of peripheral CD4+ T, CD4+CD25+ T, CD4 Tregs (CD4+CD25+FOXP3+ T), and Th17 cells (CD4+IL17+ T) was shown in refractory RA after low-dose IL-2 treatment. The results revealed the increase of CD4+ T cells (A, D, G, I), CD4+CD25+ T cells (B, E), CD4 Tregs (C, F), or Th17 cells (H, J) in CD4+ T-cell population after IL-2 treatment (D–F, I, J) compared to before (A–C, G, H).
Before the treatment, there was no difference in disease activity measures between the IL-2 group and the non-IL-2 group (p > 0.05) (Table 2). But after IL-2 subcutaneous injection combined with the same conventional antirheumatic drugs, there was a significant decrease in DAS28 score (3.60 ± 0.96 vs. 2.85 ± 0.67, p = 0.005), 28 tender joint count (3.73 ± 2.79 vs. 0.94 ± 1.00, p = 0.001), and swollen joint count (1.40 ± 1.64 vs. 0.42 ± 0.70, p = 0.011) in the IL-2 group compared with the non-IL-2-treated individuals (Table 3). The above results suggest that although the numbers of T, B, CD4+ T, and Th17 cells were increased, as shown in Table 1, these changes contribute to tipping the immune status toward balance rather than inflammation.
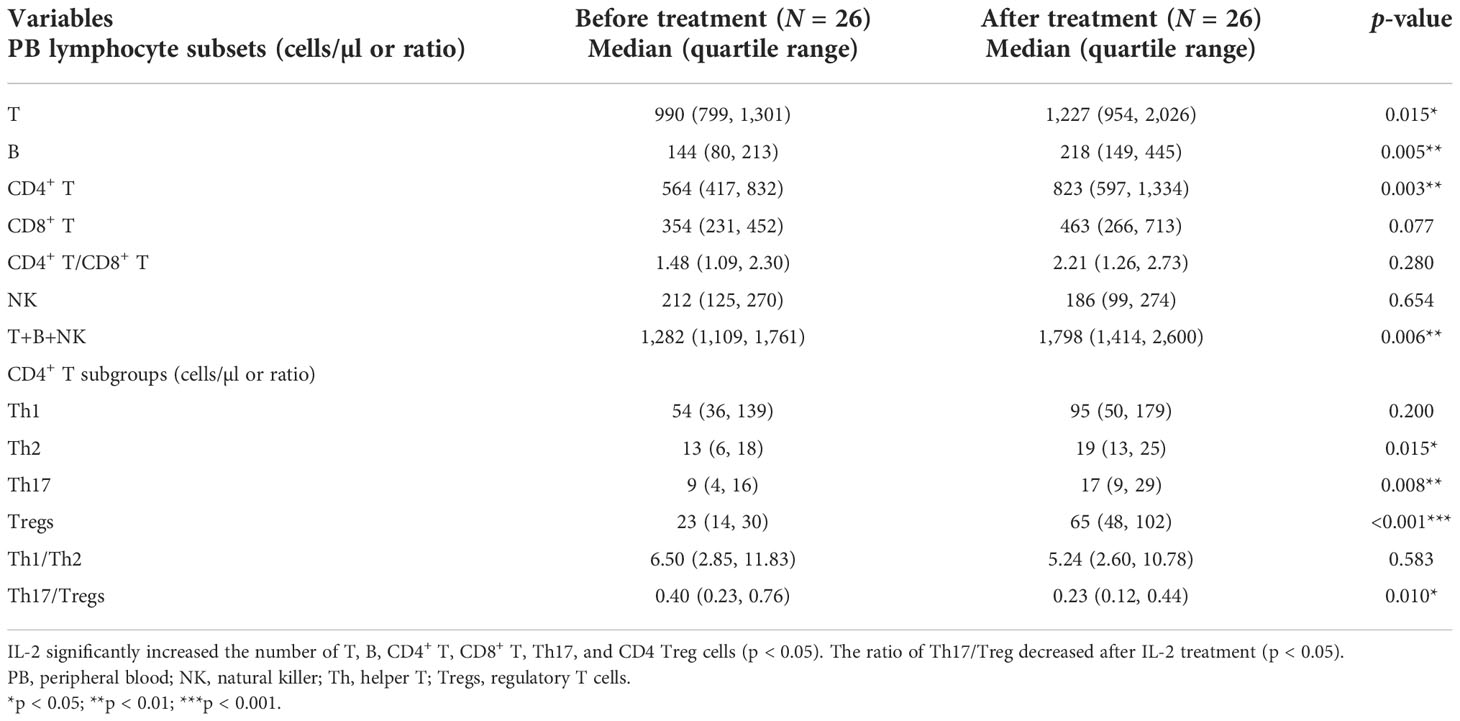
Table 1 Comparison of PB lymphocyte subsets and CD4+ T subsets in IL-2-treated patients before and after IL-2 treatment (N = 26).
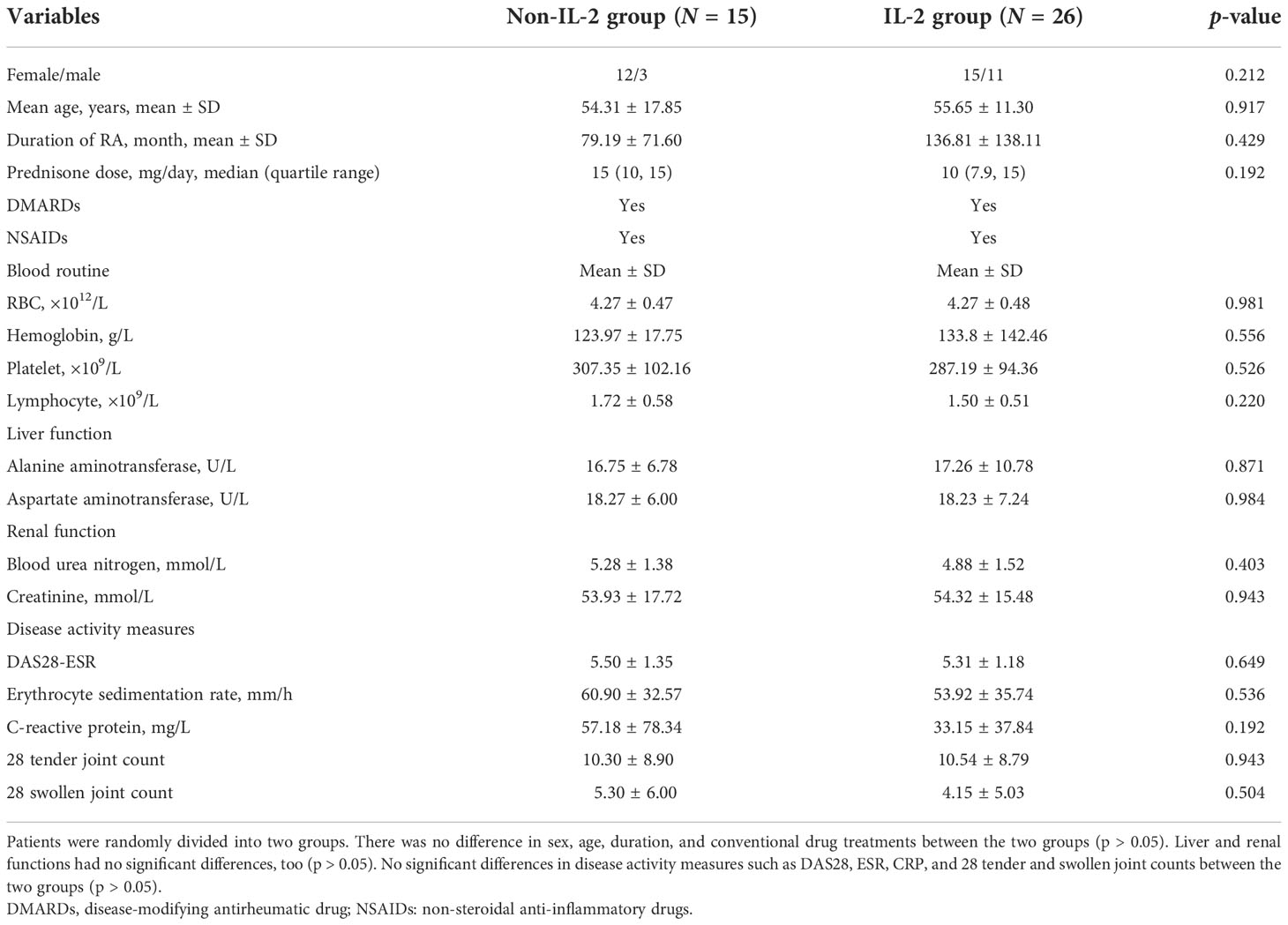
Table 2 Baseline characteristics and disease activity measures of patients before IL-2 treatment (N = 41).
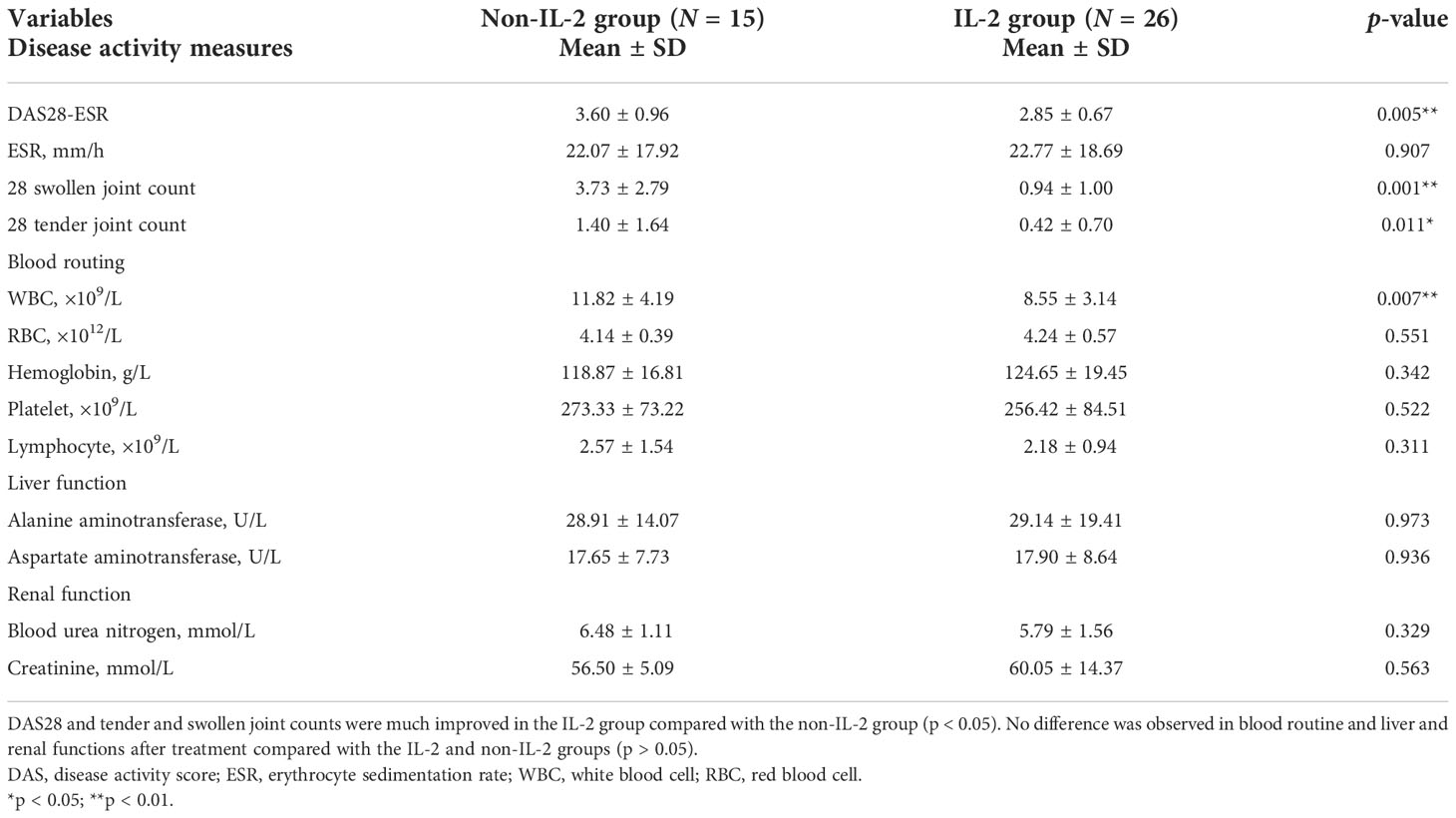
Table 3 Baseline characteristics and disease activity measures of patients after the treatment (N = 41).
The safety of this treatment is shown in Tables 2, 3. There were no differences in blood routine, serum alanine transaminase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), and serum creatinine (Cr) between the two groups before the treatment (p > 0.05) (Table 2). After the treatment, these measurements were also comparable in these two groups (p > 0.05) (Table 3). Except for mild reactions at the site of injection in some subjects (2 of 26 patients), no other side effects were observed.
The status of T-cell subsets in peripheral blood in patients with RA was controversial, especially that of Th17 and Treg cells (26). A meta-analysis of all 36 selected studies (17) revealed no significant difference in the proportion of the so-called Tregs between RA patients and control subjects. The inconsistent definitions of Tregs have been used in these studies, leading to different results. As a major CD4+ T-cell subset of Treg cells that express CD4 and FOXP3 (a transcription factor that plays a vital role in the function of Tregs) (27), we studied CD4 Tregs (CD4+CD25+FOXP3+). Our study firstly showed that the reduction of the absolute number or proportion of CD4 Tregs in PB was associated with the disease activity of refractory RA but not that of Th17 cells.
It is reported that Th17 cells and IL-17 levels are low or undetectable in regular human PB, while the levels are elevated in PB or synovial fluid in RA patients (12). CD4+ T-cell subsets in the CD4+ T lymphocyte population of PB include Th1, Th2, Treg, and Th17 cells. Generally, the proportions of these subsets in PB CD4+ T cells are used to quantify their levels. However, we found that the number of CD4 Tregs was almost 5-folds higher than that of Th17 in PB of healthy controls. Therefore, the number of CD4 Tregs greatly affects the percentage of Th17 cells in the PB CD4+ T population. In previous studies, the increased frequencies in Th17 cells may not be considered a change in Th17. On the contrary, it may suggest a decrease in the CD4 Treg cells. Perhaps only absolute numbers can precisely reflect the actual status of cells but not their frequency. Some flow cytometers cannot directly provide the cell concentration or total counts of cells in a sample. Absolute cell counts can be obtained by adding an internal microsphere-counting standard to the flow cytometric sample (single platform testing). The single-platform method is preferred as it is technically less complicated as it avoids interlaboratory variation and underestimations, making it more accurate than multiple-platform testing. In this study, BD Trucount™ tubes with the lyophilized pellet of a known number of fluorescent beads were used for determining the absolute counts of T, B, NK, and total CD4+ T cells in PB and then calculating the absolute number of CD4+ T subsets, especially that of Th17 and CD4 Tregs.
Our results showed that Th17 cells did not increase in patients with refractory RA compared with healthy controls. Therefore, the change in the Th17 number should not be the direct cause of refractory RA. Indeed, for these patients who received the long-term immunosuppressor treatment, the normal levels of Th17 cells might represent the treatment results (28–31). On the other hand, immunosuppressants also decrease the number or function of Tregs non-selectively (32, 33). These changes may aggravate the disturbance of immune balance. Therefore, disease activity relapses after drug withdrawal due to insufficient regulatory T cells, which might be an essential cause of refractory RA (34).
Moreover, long-term immunosuppressive therapies also lead to drug resistance (35, 36), which may be another cause of refractory RA. Therapeutic unresponsiveness may be caused by various mechanisms, such as the rapid degradation of corticosteroids (CCS), the release of neutralizing antibodies against biological agents such as infliximab, or other factors (37, 38). Moreover, long-term immunosuppressants downregulated the level of effector T cells, leading to a higher risk of infection and malignancy (39, 40).
Therefore, we propose an immunoregulation therapy to induce and reconstitute immune tolerance by low-dose IL-2 subcutaneous injection instead of simple immunosuppression. CD4 Tregs express high levels of CD25 (alpha-chain of the IL-2 receptor), so IL-2 was able to increase the level of Tregs (41). Our results demonstrated that low-dose IL-2 effectively increased many kinds of lymphocyte cells. The number of Th17 cells increased slightly, while that of CD4 Tregs soared dramatically. The increased CD4 Tregs contributed to tipping the immune balance and inhibiting inflammation for RA patients. Recent studies have reported the capacity for plasticity of the Th17 and Treg phenotypes. There is growing evidence that IL-17(+)/FoxP3(+) Treg clones also exhibit plasticity to secrete pro-inflammatory cytokines, such as IL-17A, in inflammatory conditions and retain their suppressive function (42, 43), which probably supports the finding regarding the increasing number of Th17 in the study.
These increased lymphocytes in PB may also be beneficial by relieving patients’ clinical symptoms without the expected adverse effects. Though no significant difference in ESR and CRP was observed between these groups for this short-time observation, there were substantial decreases in DAS28 and tender and swollen joint counts in the IL-2 group, compared with the non-IL-2-treated individuals who received almost the same conventional antirheumatic drugs after the treatment. Therefore, we confirm that low-dose IL-2 injection can help the remission of RA disease activity rapidly. As for the safety of IL-2 injection, no severe adverse event was observed except for mild reactions at the injection site.
However, we only observed this study for 1 week, and a long-term study is needed. According to the levels of Th17 and CD4 Tregs, we are trying to maintain the balance of immune function by low-dose IL-2 once a week. We are also trying other methods to maintain a long-term immune balance and induce and reconstitute immune tolerance for patients with immunodeficiency. In addition, this study also has some limitations, such as being a single-center study, having a small sample size, and implementing short-term monitoring. A multicenter study, a maximum sample, and long-term clinical studies are still extremely necessary.
In conclusion, the disease activity of refractory RA is associated with the absolute number of CD4 Tregs but not that of Th17 cells and other populations. Low-dose IL-2 can effectively upregulate the level of CD4 Treg as well as that of Th17 to some degree and maintain the balance of Th17 and CD4 Tregs. Subcutaneous injection of IL-2 combined with traditional antirheumatic drugs may help in the rapid remission of RA patients’ symptoms without overtreatment and the expected side effects. However, the long-term benefits of this immune regulation therapy need to be clarified by conducting further studies.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
This study was approved by the Ethics Committee of the Second Hospital of Shanxi Medical University (2016 KY-007). The patients/participants provided their written informed consent to participate in this study.
Study design and manuscript writing: JW and S-XZ. Data extraction, quality assessment, analysis, and interpretation of data: JW, TC, J-SC, and X-JJ. All authors approved the final version to be published. X-FL had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis.
This work was supported by the National Natural Science Foundation of China (No. 82001740).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.947341/full#supplementary-material
1. Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet (2016) 388(10055):2023–38. doi: 10.1016/S0140-6736(16)30173-8
2. van der Heijden JW, Dijkmans BA, Scheper RJ, Jansen G. Drug insight: resistance to methotrexate and other disease-modifying antirheumatic drugs–from bench to bedside. Nat Clin Pract Rheumatol (2007) 3(1):26–34. doi: 10.1038/ncprheum0380
3. Muller IB, Lin M, Lems WF, Ter Wee MM, Wojtuszkiewicz A, Nurmohamed MT, et al. Association of altered folylpolyglutamate synthetase pre-mRNA splicing with methotrexate unresponsiveness in early rheumatoid arthritis. Rheumatology (2021) 60(3):1273–81. doi: 10.1093/rheumatology/keaa428
4. Chen AY, Wolchok JD, Bass AR. TNF in the era of immune checkpoint inhibitors: friend or foe? Nat Rev Rheumatol (2021) 17(4):213–23. doi: 10.1038/s41584-021-00584-4
5. Sherbini AA, Sharma SD, Gwinnutt JM, Hyrich KL, Verstappen SMM. Prevalence and predictors of adverse events with methotrexate mono- and combination-therapy for rheumatoid arthritis: A systematic review. Rheumatology (2021) 60(9):4001–17. doi: 10.1093/rheumatology/keab304
6. Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, van Zeben D, Kerstens PJ, Hazes JM, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): A randomized, controlled trial. Arthritis rheumatism (2008) 58(2 Suppl):S126–135. doi: 10.1002/art.23364
7. Nam JL, Villeneuve E, Hensor EM, Conaghan PG, Keen HI, Buch MH, et al. et al: Remission induction comparing infliximab and high-dose intravenous steroid, followed by treat-to-target: A double-blind, randomised, controlled trial in new-onset, treatment-naive, rheumatoid arthritis (the IDEA study). Ann rheumatic Dis (2014) 73(1):75–85. doi: 10.1136/annrheumdis-2013-203440
8. Buch MH, Eyre S, McGonagle D. Persistent inflammatory and non-inflammatory mechanisms in refractory rheumatoid arthritis. Nat Rev Rheumatol (2021) 17(1):17–33. doi: 10.1038/s41584-020-00541-7
9. Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. New Engl J Med (2009) 361(9):888–98. doi: 10.1056/NEJMra0707449
10. van Hamburg JP, Asmawidjaja PS, Davelaar N, Mus AM, Colin EM, Hazes JM, et al. Th17 cells, but not Th1 cells, from patients with early rheumatoid arthritis are potent inducers of matrix metalloproteinases and proinflammatory cytokines upon synovial fibroblast interaction, including autocrine interleukin-17A production. Arthritis Rheumatism (2011) 63(1):73–83. doi: 10.1002/art.30093
11. Cho ML, Yoon CH, Hwang SY, Park MK, Min SY, Lee SH, et al. Effector function of type II collagen-stimulated T cells from rheumatoid arthritis patients: cross-talk between T cells and synovial fibroblasts. Arthritis Rheumatism (2004) 50(3):776–84. doi: 10.1002/art.20106
12. Hull DN, Cooksley H, Chokshi S, Williams RO, Abraham S, Taylor PC. Increase in circulating Th17 cells during anti-TNF therapy is associated with ultrasonographic improvement of synovitis in rheumatoid arthritis. Arthritis Res Ther (2016) 18(1):303. doi: 10.1186/s13075-016-1197-5
13. Tu J, Han D, Fang Y, Jiang H, Tan X, Xu Z, et al. MicroRNA-10b promotes arthritis development by disrupting CD4(+) T cell subtypes. Mol Ther Nucleic Acids (2022) 27:733–50. doi: 10.1016/j.omtn.2021.12.022
14. Wang W, Shao S, Jiao Z, Guo M, Xu H, Wang S. The Th17/Treg imbalance and cytokine environment in peripheral blood of patients with rheumatoid arthritis. Rheumatol Int (2012) 32(4):887–93. doi: 10.1007/s00296-010-1710-0
15. Goswami TK, Singh M, Dhawan M, Mitra S, Emran TB, Rabaan AA, et al. Regulatory T cells (Tregs) and their therapeutic potential against autoimmune disorders - advances and challenges. Hum Vaccin Immunother (2022) 18(1):2035117. doi: 10.1080/21645515.2022.2035117
16. Zhang SX, Wang J, Wang CH, Jia RH, Yan M, Hu FY, et al. Low-dose IL-2 therapy limits the reduction in absolute numbers of circulating regulatory T cells in rheumatoid arthritis. Ther Adv Musculoskelet Dis (2021) 13:1759720X211011370. doi: 10.1177/1759720X211011370
17. Morita T, Shima Y, Wing JB, Sakaguchi S, Ogata A, Kumanogoh A. The proportion of regulatory T cells in patients with rheumatoid arthritis: A meta-analysis. PLos One (2016) 11(9):e0162306. doi: 10.1371/journal.pone.0162306
18. Wu R, Li N, Zhao X, Ding T, Xue H, Gao C, et al. Low-dose interleukin-2: Biology and therapeutic prospects in rheumatoid arthritis. Autoimmun Rev (2020) 19(10):102645. doi: 10.1016/j.autrev.2020.102645
19. Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol (2014) 192(12):5451–8. doi: 10.4049/jimmunol.1490019
20. Raker VK, Becker C, Landfester K, Steinbrink K. Targeted activation of T cells with IL-2-Coupled nanoparticles. Cells (2020) 9(9):2063. doi: 10.3390/cells9092063
21. He J, Zhang X, Wei Y, Sun X, Chen Y, Deng J, et al. Low-dose interleukin-2 treatment selectively modulates CD4(+) T cell subsets in patients with systemic lupus erythematosus. Nat Med (2016) 22(9):991–3. doi: 10.1038/nm.4148
22. Wang J, Zhang SX, Hao YF, Qiu MT, Luo J, Li YY, et al. The numbers of peripheral regulatory T cells are reduced in patients with psoriatic arthritis and are restored by low-dose interleukin-2. Ther Adv Chronic Dis (2020) 11:2040622320916014. doi: 10.1177/2040622320916014
23. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010 rheumatoid arthritis classification criteria: an American college of Rheumatology/European league against rheumatism collaborative initiative. Ann rheumatic Dis (2010) 69(9):1580–8. doi: 10.1136/ard.2010.138461
24. Miao M, Hao Z, Guo Y, Zhang X, Zhang S, Luo J, et al. Short-term and low-dose IL-2 therapy restores the Th17/Treg balance in the peripheral blood of patients with primary sjogren's syndrome. Ann Rheum Dis (2018) 77(12):1838–40. doi: 10.1136/annrheumdis-2018-213036
25. Arenas-Ramirez N, Woytschak J, Boyman O. Interleukin-2: Biology, design and application. Trends Immunol (2015) 36(12):763–77. doi: 10.1016/j.it.2015.10.003
26. Alunno A, Manetti M, Caterbi S, Ibba-Manneschi L, Bistoni O, Bartoloni E, et al. Altered immunoregulation in rheumatoid arthritis: the role of regulatory T cells and proinflammatory Th17 cells and therapeutic implications. Mediators Inflammation (2015) 2015:751793. doi: 10.1155/2015/751793
27. Fontenot JD, Gavin MA, Rudensky AY. Pillars article: Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol (2003) 4:330–6. doi: 10.1038/ni904
28. Schewitz-Bowers LP, Lait PJ, Copland DA, Chen P, Wu W, Dhanda AD, et al. Glucocorticoid-resistant Th17 cells are selectively attenuated by cyclosporine a. Proc Natl Acad Sci United States America (2015) 112(13):4080–5. doi: 10.1073/pnas.1418316112
29. Kotake S, Nanke Y, Yago T, Kawamoto M, Kobashigawa T, Yamanaka H. Elevated ratio of Th17 cell-derived Th1 cells (CD161(+)Th1 cells) to CD161(+)Th17 cells in peripheral blood of early-onset rheumatoid arthritis patients. BioMed Res Int (2016) 2016:4186027. doi: 10.1155/2016/4186027
30. Zhu M, Xu Q, Li XL, He Q, Wang WF. Modulating effects of leflunomide on the balance of Th17/Treg cells in collageninduced arthritis DBA/1 mice. Central-European J Immunol (2014) 39(2):152–8. doi: 10.5114/ceji.2014.43714
31. Silva JC, Mariz HA, Rocha LF Jr., Oliveira PS, Dantas AT, Duarte AL, et al. Hydroxychloroquine decreases Th17-related cytokines in systemic lupus erythematosus and rheumatoid arthritis patients. Clinics (2013) 68(6):766–71. doi: 10.6061/clinics/2013(06)07
32. Oh JS, Kim YG, Lee SG, So MW, Choi SW, Lee CK, et al. The effect of various disease-modifying anti-rheumatic drugs on the suppressive function of CD4(+)CD25(+) regulatory T cells. Rheumatol Int (2013) 33(2):381–8. doi: 10.1007/s00296-012-2365-9
33. Li X, Xu H, Huang J, Luo D, Lv S, Lu X, et al. Dysfunctions, molecular mechanisms, and therapeutic strategies of regulatory T cells in rheumatoid arthritis. Front Pharmacol (2021) 12:716081. doi: 10.3389/fphar.2021.716081
34. Kanjana K, Chevaisrakul P, Matangkasombut P, Paisooksantivatana K, Lumjiaktase P. Regulatory T cell suppressive activity predicts disease relapse during disease-modifying anti-rheumatic drug dose reduction in rheumatoid arthritis: A prospective cohort study. Front Med (Lausanne) (2020) 7:25. doi: 10.3389/fmed.2020.00025
35. Ramesh R, Kozhaya L, McKevitt K, Djuretic IM, Carlson TJ, Quintero MA, et al. Pro-inflammatory human Th17 cells selectively express p-glycoprotein and are refractory to glucocorticoids. J Exp Med (2014) 211(1):89–104. doi: 10.1084/jem.20130301
36. Gottesman MM, Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem (1993) 62:385–427. doi: 10.1146/annurev.bi.62.070193.002125
37. Radstake TR, Svenson M, Eijsbouts AM, van den Hoogen FH, Enevold C, van Riel PL, et al. Formation of antibodies against infliximab and adalimumab strongly correlates with functional drug levels and clinical responses in rheumatoid arthritis. Ann Rheumatic Dis (2009) 68(11):1739–45. doi: 10.1136/ard.2008.092833
38. Wang Z, Huang J, Xie D, He D, Lu A, Liang C. Toward overcoming treatment failure in rheumatoid arthritis. Front Immunol (2021) 12:755844. doi: 10.3389/fimmu.2021.755844
39. Simon TA, Thompson A, Gandhi KK, Hochberg MC, Suissa S. Erratum to: Incidence of malignancy in adult patients with rheumatoid arthritis: a meta-analysis. Arthritis Res Ther (2016) 18(1):100. doi: 10.1186/s13075-016-0990-5
40. Salliot C, van der Heijde D. Long-term safety of methotrexate monotherapy in patients with rheumatoid arthritis: a systematic literature research. Ann Rheumatic Dis (2009) 68(7):1100–4. doi: 10.1136/ard.2008.093690
41. Kolios AGA, Tsokos GC, Klatzmann D. Interleukin-2 and regulatory T cells in rheumatic diseases. Nat Rev Rheumatol (2021) 17(12):749–66. doi: 10.1038/s41584-021-00707-x
42. Beriou G, Costantino CM, Ashley CW, Yang L, Kuchroo VK, Baecher-Allan C, et al. IL-17-producing human peripheral regulatory T cells retain suppressive function. Blood (2009) 113(18):4240–9. doi: 10.1182/blood-2008-10-183251
Keywords: rheumatoid arthritis, refractory, interleukin-2, Th17, CD4 Tregs, immunoregulation
Citation: Wang J, Zhang S-X, Chang J-S, Cheng T, Jiang X-J, Su Q-Y, Zhang J-Q, Luo J and Li X-F (2022) Low-dose IL-2 improved clinical symptoms by restoring reduced regulatory T cells in patients with refractory rheumatoid arthritis: A randomized controlled trial. Front. Immunol. 13:947341. doi: 10.3389/fimmu.2022.947341
Received: 18 May 2022; Accepted: 07 November 2022;
Published: 29 November 2022.
Edited by:
James A. Lederer, Brigham and Women’s Hospital and Harvard Medical School, United StatesReviewed by:
Agnieszka Bojarska-Junak, Medical University of Lublin, PolandCopyright © 2022 Wang, Zhang, Chang, Cheng, Jiang, Su, Zhang, Luo and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao-Feng Li, bHhmXzk4NTlAc3htdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.