
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 21 September 2022
Sec. Vaccines and Molecular Therapeutics
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.941492
This article is part of the Research Topic Innate Immunity and Severe Asthma: From Microbiome to Target Therapy View all 8 articles
 Veronika Naumova1
Veronika Naumova1 Evgeny Beltyukov1
Evgeny Beltyukov1 Katarzyna Niespodziana2,3
Katarzyna Niespodziana2,3 Peter Errhalt3
Peter Errhalt3 Rudolf Valenta2,4,5,6*
Rudolf Valenta2,4,5,6* Alexander Karaulov5
Alexander Karaulov5 Darina Kiseleva1
Darina Kiseleva1Molecular therapies, including anti-IgE, biologicals and small molecules are increasingly used for treatment of asthma. The effectiveness of these therapies may be increased with biomarkers. Aim of this study was to assess the value of measuring cumulative IgE levels specific for respiratory allergens to increase the efficacy of anti-IgE therapy for severe bronchial asthma. One hundred and thirty seven patients with severe asthma were recruited from 2016 to 2022. Standard empirical allergy diagnosis (i.e., anamnesis, skin testing, allergen-specific IgE measurement), blood eosinophil counting, measurement of total IgE and of cumulative IgE-specific for respiratory allergens by Phadiatop™ were performed. Thirty four patients with severe allergic asthma, for whom all three diagnostic methods were performed, were then used to analyze the efficacy of anti-IgE treatment in patients stratified in two groups according to cumulative IgE levels specific for respiratory allergens determined by Phadiatop™. Group #1 patients (n = 8) had cumulative specific IgE values ≥ 0.35 and < 1.53 PAU/l while in group #2 patients (n = 26) they were ≥ 1.53 PAU/l. Treatment with Omalizumab was performed for at least 12 months. The level of asthma control (ACT questionnaire), the number of asthma exacerbations, the quality of life (AQLQ questionnaire), the need for systemic corticosteroids, and the respiratory function (FEV1) was determined by “before-after” analysis for each group, followed by a comparison of the dynamics between groups. In group 2 patients with an initial allergen-specific IgE level ≥ 1.53 kUA/L, the efficacy of Omalizumab treatment was better regarding asthma control, number of exacerbations, and quality of life than in group 1 patients. Our study provides evidence that measuring cumulative levels of IgE specific for respiratory allergens could be a useful screening method for detecting an allergic phenotype of severe asthma and may serve as biomarker to enhance the success of IgE-targeted therapy.
New opportunities have appeared in the treatment of severe bronchial asthma due to the development of targeted molecular therapies (1, 2). However, the effectiveness of molecular therapies depends on the correct diagnosis of the disease phenotype and the adequate drug selection (3–6). Accordingly, many researchers are looking for biomarkers for various asthma phenotypes (7–13). These biomarkers should be accurate, available in practice and cost-effective. To determine the allergic phenotype of asthma, it is important to prove clinically the presence of a clinically relevant allergen-specific IgE sensitization. This can be achieved by comparing allergic anamnesis and the results of standard diagnostic methods which are usually selected empirically (skin testing and/or measurement of specific IgE and/or allergen provocation testing) and/or anamnesis in combination with comprehensive assessment of multiple IgE sensitizations (7, 14–17). Skin tests are usually reliable, relatively inexpensive but do not necessarily prove the presence of allergen-specific IgE, while provocation tests are rarely used in patients with severe asthma because of the risk of severe systemic reactions. Furthermore, these methods are particularly performed with allergen extracts prepared from the natural allergen sources and thus represent mixtures of allergenic and non-allergenic substances. Although extract-based tests can provide information about the sensitizing allergen source, they do not inform to which specific allergenic component(s) present in the source the patient is sensitized to (18). Therefore, defined allergen components have been introduced in the diagnosis of allergy and different component-based tests have been developed to measure allergen-specific IgE levels in blood of a patient as biomarkers for a clinically relevant allergen exposure associated with symptoms of allergy (17, 19–21). Determination of allergen-specific IgE by in vitro methods is safe for patients but testing with single allergen molecules or micro-arrayed allergens might be expensive (17). One inexpensive possibility for simultaneous testing of IgE sensitizations to multiple respiratory allergen sources is the PhadiatopТМ test, which has been studied as a screening method for detecting allergen-specific IgE sensitization from the late 1980s (22–29) to the presence (30, 31). This test is based on simultaneous testing for serum specific IgE to a mixture of allergens causing common inhalant allergies. The high value of Phadiatop™ was evaluated in the general population to identify patients with allergic sensitizations (28, 29) among patients with symptoms of airway obstruction (26) and among children with suspected respiratory allergies, (32) among adults with asthma (30, 33) and children with asthma (34) among patients with allergic rhinitis (27, 31, 35), among patients with asthma and/or rhinitis (23, 24, 36–39). In these studies the PhadiatopТМ showed a sensitivity of 70% (29) to 100% (25, 33) and a specificity of 83% (33) to 100% (23, 26). The ineffectiveness of measuring total IgE for the precise diagnosis of allergic diseases has been shown in several studies (23, 26, 28, 31).
Today, patients with uncontrolled severe asthma are routinely considered as candidates for molecular therapies, which target specific inflammatory pathways involved in the pathogenesis of asthma (40). Omalizumab, a therapeutic anti-IgE antibody, is the first globally approved and most often used targeted molecular therapy of severe or moderate to severe allergic asthma. It is administered subcutaneously every two or four weeks at a dose determined according to the patient’s body weight and serum total IgE levels (30 – 1500 IU/mL), ranging from 75 to 600 mg. Clinical studies performed with Omalizumab in the last 10 years have confirmed its effectiveness and safety in the treatment of severe asthma by reducing symptoms, frequency of reliever use, and severe exacerbations but there are also non-responders to this treatment (41).
Accordingly, there is an unmet need for biomarkers which are useful for identifying patients suffering from allergic asthma and which at the same time can be used to predict the efficacy of anti-IgE treatment (42, 43). The measurement of total IgE levels has been suggested as one possible biomarker (42) but there seem to be no clear cut-off levels and it even was suggested that subjects with low total IgE levels benefit most from treatment (44). Another study performed a retrospective investigation of poly- versus monosensitized patients but the results were not clear (45). Yet another study demonstrated that the proportion of allergen-specific IgE antibodies in relation to total IgE may be useful to predict the efficacy of anti-IgE treatment indicating the importance of the measurement of allergen-specific IgE levels (46). However, two studies provided counter-intuitive information. One study found no relation between pretreatment specific IgE and response to treatment (47) and another study even showed that Omalizumab is also effective in non-atopic asthma (48).
We hypothesized that the measurement of the cumulative amount of IgE specific for respiratory allergens may be useful to identify patients suffering from severe allergic asthma and eventually may even serve as a biomarker to predict the efficacy of anti-IgE treatment.
One hundred and thirty seven adult patients, who were registered in the Sverdlovsk region, Russia from 2016 to April 2022, were enrolled in this study. Inclusion criteria included patients (18 years and older) with severe bronchial asthma, who were diagnosed according to the ATS/ERS criteria from 2014 (49) and GINA guidelines (50, 51). Asthma was considered as not controlled by GINA steps 4-5 (moderate or high ICS with a second baseline drug, continuous SGCS), or by the fact that this treatment was required to maintain control and to reduce the risk of exacerbations. Exclusion criteria included confirmed helminthic invasion, cytostatic therapy, pregnancy, severe somatic pathology (heart failure 3-4 functional classes, liver failure according to Child-Pugh class B and C, chronic kidney disease C3a and above) and the inability to meet the schedule of visits for injection, evaluation of efficacy and safety. All subjects provided their written informed consent. The study was approved by the ethics committee of the Ural State Medical University and conducted according to the Declaration of Helsinki.
Allergic phenotype was determined based on standard diagnostic methods for allergy including anamnesis, skin prick tests (52), total and specific IgE determination according to a traditional pathway (53), and Phadiatop™ ImmunoCAP measurements of IgE specific for a mix of inhalant allergen sources (hereinafter referred to as Phadiatop™). An allergic phenotype was defined by a combination of a positive allergic anamnesis and positive skin testresult or a positive allergic anamnesis and at least one positive specific IgE. An eosinophilic phenotype of bronchial asthma was defined in the case of negative allergic anamnesis, the absence of proven clinically relevant sensitization, and a concentration of eosinophils exceeding 150 cells/μL. The mixed phenotype of asthma was defined as a combination of a positive allergic anamnesis, late onset of asthma, eosinophilia of more than 300 cells/μL.
Anamnesis was assessed in a comprehensive manner according to the presence of a clinically relevant reaction of the patient (symptoms of asthma, rhinoconjunctivitis, urticaria and angioedema) upon contact with an allergen source, the effect of elimination of exposure and/or the presence of relatives suffering from allergic diseases. Skin tests were assessed retrospectively if patients had earlier positive skin test reactions specific for allergen sources (house dust, house dust mites, cat, dog, a mixture of trees, meadow grasses, weeds) with positive (histamine) and negative (saline) controls (52).
Determination of total IgE was performed using a chemiluminescent method (IMMULITE 2000, SIEMENS, Erlangen, Germany). Total IgE was considered as elevated at a level ≥ 100 IU/ml. Specific IgE was determined by the ImmunoCAP method (immunofluorescence on a three-dimensional porous solid phase, Phadia 250, Phadia) (Thermofisher, Uppsala, Sweden) to dog dandruff allergens (Dog dander e5), house dust mite allergens (Dermatophagoides pteronyssinus D1, Dermatophagoides farinae D2), epithelium of a cat (Cat Dander-Epithelium E1), house dust (Greer H1), mold (Penicillum notatum M1), birch pollen (Birch T3), a mixture of grass allergens (Orchard Grass, Meadow Fescue, Perennial Rye Grass, Timothy Grass, June Grass (Kentucky Blue) GP1), mugwort pollen (Artemisia vulgaris W6). A result was considered positive when specific IgE levels were ≥ 0.35 kUA/l. Phadiatop™ was performed using the ImmunoCAP technique (immunofluorescence on three-dimensional porous solid phase, Phadia 250, Phadia). Phadiatop™ level ≥ 0.35 PAU/l was reported as positive result, and < 0.35 PAU/l as negative result.
Sensitivity, specificity, accuracy, positive and negative predictive values of diagnostic methods were determined in four-field tables separately for each method and in combinations. For this analysis patients with severe allergic and non-allergic asthma were studied, in whom all 3 methods had been performed (i.e., taking allergic anamnesis, standard diagnostic methods such as skin testing and/or measuring allergen-specific IgE and/or Phadiatop™ testing). Patients with mixed bronchial asthma (n = 4) were not included in the analysis. Patients with the diagnosis of severe allergic asthma were selected for the treatment with Omalizumab (Novartis, Basel, Switzerland) for at least 12 months according to the instructions of the manufacturer, taking into account the patient’s body weight and the level of total IgE (the corresponding amount of mg of the drug 1 or 2 times a month). Each patient had evaluation visits before starting the treatment with Omalizumab (baseline), at 4 months of therapy, and at 12 months of therapy. At each of these visits, patients completed the ACT (Asthma Control Test), AQLQ (Asthma Quality of Life Questionnaire) questionnaires, spirometry (=respiratory function) was performed, and data on exacerbations were collected. Spirometry was done according to ATS/ERS recommendations 2005 and GINA guidelines (54). Exacerbation of asthma was defined as the worsening of symptoms requiring an increase in of therapy (increasing the dose of inhaled corticosteroids, prescribing systemic corticosteroids or increasing the dose of systemic corticosteroids) and/or hospitalization. Patients were asked about the number of exacerbations in the year before baseline and between visits after the initiation of Omalizumab therapy.
Statistical analysis was performed using StatTech v. 2.1.0 (Developer - StatTech LLC, Russia), NCSS Statistical Software 2021 (https://www.ncss.com/). Quantitative variables were assessed for normality using the Shapiro-Wilk test or the Kolmogorov-Smirnov test. Quantitative variables following a normal distribution were described using mean (M) and standard deviation (SD), 95% confidence interval (95% CI) for the mean were estimated. Quantitative variables following the non-normal distribution were described using median (Me) and lower and upper quartiles (Q1 – Q3). Student’s t-test or Mann-Whitney-U test were used for comparisons between two groups following the normal or non-normal distribution of the data, respectively. Comparisons of three or more groups were done using the Kruskal-Wallis test and Dunn’s criterion with Holm correction as a post-hoc method. P-value under the set up value of α = 0.05 (two-sided) was considered as statistically significant. ROC analysis was used to assess the diagnostic performance of quantitative variables in predicting a categorical outcome. The optimal cut-off value was estimated using the Youden’s index. Comparison of frequencies in the analysis of 2 by 2 contingency tables was performed using Pearson’s chi-square test (for expected values greater than 10), and Fisher’s exact test (for expected values less than 10). One-way repeated measures analysis of variance was used to compare three or more matched samples in regard to normally distributed quantitative variables. Statistical significance of dependent variable changes over time was assessed using the Pillai’s Trace and paired Student’s t-test with Holm correction as post hoc methods. When comparing three or more matched samples regarding to non-normally distributed quantitative variables Friedman test was used along with ConoverIman test with Holm correction as a post hoc method.
This study evaluated a total number of 137 subjects with severe bronchial asthma, who were included in the regional register of Sverdlovsk region from 2016 to April 2022. Table 1 shows a detailed characterization of these patients and the diagnostic methods performed in real clinical practice. According to the International Classification of Diseases (ICD), patients with severe asthma (SA) were divided into three groups: Allergic SA (J45.0; n = 57), non-allergic SA (J45.1; n = 61), and mixed SA (J45.8; n = 19). The median age of the participants was 52 years (range: 43 – 59) . One hundred and fourteen (i.e., 83.2%) were women, twenty three (i.e., 16.8%) were men and fifty seven (i.e., 41.6%) had an allergic phenotype of severe asthma. Phadiatop™ tests were performed in 107 (i.e., 78.1%) of the patients. The diagnostic methods performed in the patients are shown in Table 1. Phadiatop™ test results and total IgE levels for the severe asthma patients are shown in Table 2. Cumulative IgE levels specific for respiratory allergens as determined with Phadiatop™ testing in patients with allergic and mixed severe asthma were significantly higher than those in patients with non-allergic asthma (**p < 0.001). The same dynamics was detected for total IgE level (*p = 0.002) (Table 2). Data obtained by Phadiatop™ testing were then compared with the results of standard diagnostic methods for determining the allergic status of asthmatic patients. In groups of patients with a positive allergic anamnesis, positive skin tests or positive allergen-specific IgE obtained by standard empirical testing, specific IgE levels determined by the Phadiatop™ test were significantly higher than in the groups with negative results obtained by each of the three standard allergy test methods (Table 3). By contrast, total IgE levels in the groups with positive and negative allergic anamnesis, positive and negative skin test results did not differ significantly from each other. Statistically significant differences in regard to total IgE levels were only obtained for the groups with negative and positive allergen-specific IgE (*p = 0.047) (Table 3). To assess the diagnostic performance of Phadiatop™ test results for predicting a positive allergic anamnesis a ROC-curve analysis was performed (Figure 1A). The calculated area under the ROC curve comprised 0.750 ± 0.047 with 95% CI: 0.658 - 0.841 and the resulting model was statistically significant (**p < 0.001). A cut-off value of specific IgE levels (i.e., 1.530 PAU/l) measured by Phadiatop™ test was also determined and found to correspond to the highest Youden’s index (Figure 1B). If the cumulative IgE specific for respiratory allergens determined by Phadiatop™ was greater than or equal to 1.530 PAU/l, it predicted the presence of a positive allergic anamnesis. The sensitivity and specificity of the method was 62.5% and 86.1%, respectively (Figure 1A). In addition, if specific IgE levels determined by the Phadiatop™ test were ≥ 0.380 PAU/l positive skin test results were predicted with a sensitivity of 81.8% and with a specificity of 90.5%. If specific IgE levels measured by Phadiatop™ were ≥ 0.380 PAU/l the presence of specific IgE as determined by empirical individual testing of specific IgE was predicted with 90.0% sensitivity and 77.8% specificity. Furthermore, a comparison of different diagnostic methods was performed separately and in combinations depending on the phenotype of asthma (Table 4). Measurement of specific IgE by Phadiatop™ showed a sensitivity of 94.44%, which was the same as the sensitivity of allergic anamnesis and inferior to the standard empirical diagnostic methods, which showed 100% sensitivity (Table 4). However, the specificity of 83.78% of the Phadiatop™ test turned out to be higher than the specificity of allergic anamnesis and standard diagnostic methods, showing 64.86% and 62.16% of specificity, respectively (Table 4). The best results were obtained when Phadiatop™ testing was combined with allergic anamnesis (sensitivity 100%, specificity 87.5%, accuracy 94.64%, PPV 91.43%, NPV 100%) (Table 4).

Table 2 Specific IgE measured by Phadiatop™ and total IgE levels in patients with different phenotypes of severe asthma.

Table 3 Phadiatop™ test results and standard diagnostic methods in different phenotypes of severe asthma.
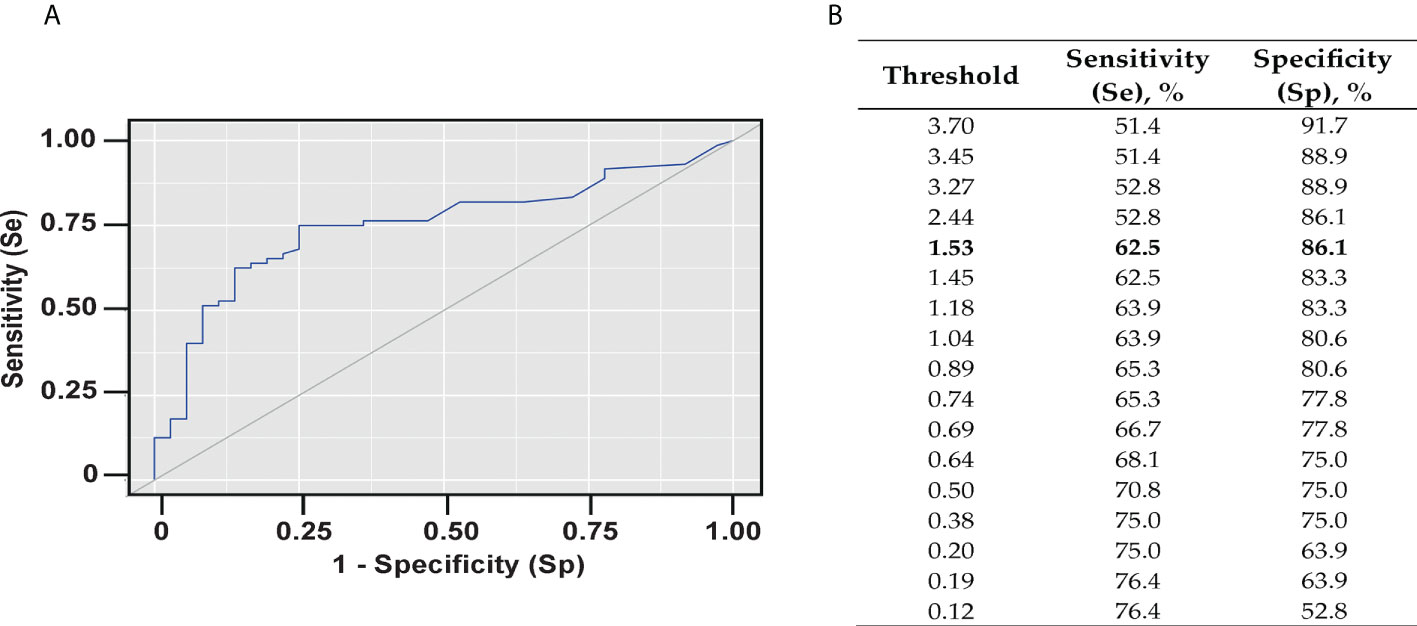
Figure 1 Diagnostic performance of Phadiatop™ test. (A) ROC-curve characterizing the probability of a positive allergic anamnesis depending on the results of the Phadiatop™ test. (B) Threshold values of the Phadiatop™ test. Sensitivity (Se) is defined as the proportion of positive tests among atopics. Specificity (Sp) is the percentage of negative tests among non-atopics.
Next, we investigated if the measurement of cumulative IgE values specific for respiratory allergens by Phadiatop™ can be useful to predict the efficacy of anti-IgE treatment in patients with severe asthma. The analysis included 34 patients with severe allergic asthma (J45.0) treated with Omalizumab for at least 12 months for whom all three diagnostic methods had been performed. The patients were divided into two groups. Group #1 (n = 8) included patients with cumulative IgE levels to respiratory allergens ≥ 0.35 and < 1.53 PAU/l while group #2 (n = 26) included patients with specific IgE levels ≥ 1.53 PAU/l. The efficacy of Omalizumab treatment was assessed by the level of asthma control (ACT questionnaire), the number of asthma exacerbations within one year before and one year after the initiation of therapy, the quality of life (AQLQ questionnaire), the need for systemic corticosteroids, and the respiratory function (FEV1). Patients were examined before the start of therapy, and after 4 and 12 months of therapy. “Before-after” analysis was performed for each group, followed by a comparison of the dynamics between groups.
The analysis of Omalizumab therapy efficacy in group #1 (Phadiatop™ test < 1.53 PAU/l) and group #2 (Phadiatop™ test ≥ 1.53 PAU/l) revealed a significant decrease in the proportion of uncontrolled bronchial asthma in both groups (*p = 0.018 and **p < 0.001, respectively) (Figure 2A). The reduction of uncontrolled asthma was more pronounced in group #2 where asthma was controlled in 26% of the patients (Figure 2A) However, the difference in asthma control levels between the groups observed after 12 months of therapy was not statistically significant (Figure 3A). Prior to the start of IgE-targeting therapy, 66.7% of patients in group #1 and 52.4% of patients in group #2, respectively, were taking systemic glucocorticosteroids (Figure 2B). After 4 months only 9% of patients in group #2 took systemic corticosteroids as compared to 50% in patients of group #1. After month 12 of Omalizumab therapy 83.3% of patients in group #1 and 85.7% of patients in group #2 did not require systemic glucocorticosteroids (p = 0.097 and **p < 0.001, respectively). There were no statistically significant differences in the dynamics between the groups (Figure 2B).
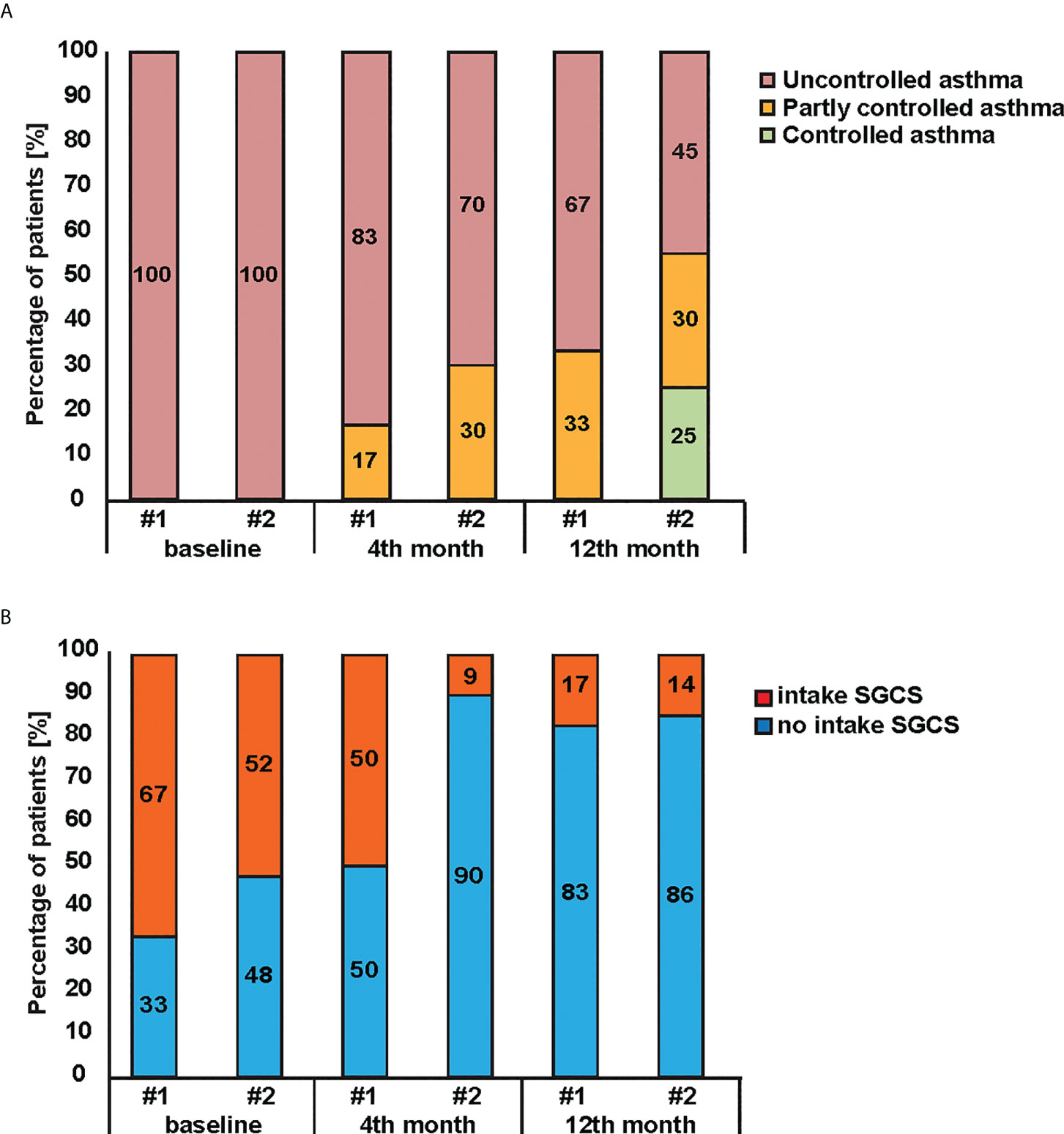
Figure 2 Effectiveness of Omalizumab therapy in patients initially diagnosed with Phadiatop™ test. Changes (y-axes) of (A) asthma control levels and (B) systemic glucocorticosteroids intake during Omalizumab therapy in patients with initial Phadiatop™ test value < 1.53 PAU/l (i.e. group #1) and > 1.53 PAU/l (i.e. group #2).
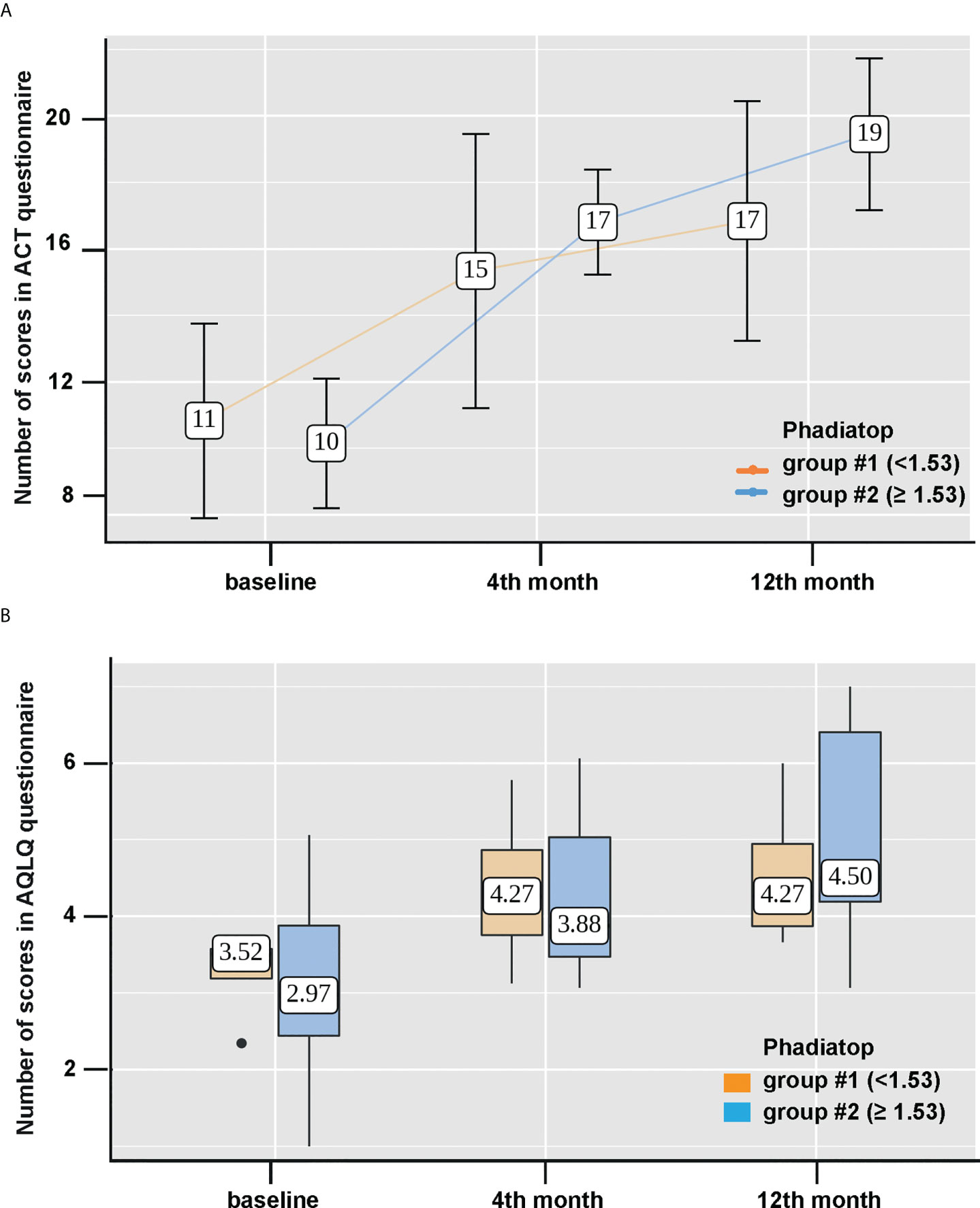
Figure 3 Improvement of asthma control level and the quality of life after Omalizumab treatment. (A) Changes of asthma control levels according to ACT questionnaire scores (points) and (B) dynamics of the quality of life according to AQLQ questionnaire scores (points) during Omalizumab therapy based on the initial value of the Phadiatop™ test (y-axes). Group 1, < 1.53 PAU/l (light brown, left) and group 2, > 1.53 PAU/l (blue, right) were compared. #stands for number in Figure 3.
Then, we assessed the scores on the ACT questionnaire in both groups and the dynamics of the results was statistically significant from 11 (Q1-Q3: 8-14) to 17 (Q1-Q3: 13-20) points in group #1 and from 10 (Q1-Q3: 8 -12) to 19 (Q1-Q3: 17-22) points in group #2 (p = 0.015 and **p < 0.001, respectively) (Figure 3A). However, the changes were more pronounced in group #2, starting from month 4 of the therapy (Figure 3A). A significant improvement in the quality of life according to AQLQ questionnaire for a year of Omalizumab therapy was also observed in both groups (p = 0.039 and **p < 0.001). However, between the groups the differences by 12 months of therapy were not statistically significant (Figure 3B).
In addition, we also observed a decrease in the number of exacerbations from 1.68 initially to 0.35 at month 12 of therapy (**p < 0.001) in group #2 while in group #1 there was no significant reduction in the number of asthma exacerbations per year per patient of Omalizumab therapy (p = 0.277) (Figure 4).

Figure 4 Changes of the number of asthma exacerbations during Omalizumab therapy depending on the initial value of the Phadiatop™ test (Group 1, < 1.53 PAU/l light brown and group 2, > 1.53 PAU/l blue).
Finally, we also assessed respiratory function by FEV1. Group #1 showed an increase from 46% at baseline (95% CI 44.9 – 52.2) to 59.1% (95% CI 56.0 – 62.8) in month 12 (p = 0.311). Group #2 also had an increase from 69% (95% CI 48.0 – 77.1) at baseline to 78% (95% CI 56.0 – 94.0) after 12 months (p = 0.0233). Regarding FEV1 there were no statistically significant differences between the groups (data not shown).
Molecular testing using defined recombinant allergen molecules has revolutionized the understanding and diagnosis of allergy (17, 53). Serological tests containing a broad panel of allergen molecules have been developed to discriminate subjects with and without IgE sensitizations and to support accurate prescription of targeted immunotherapies (55, 56). It has been suggested that the assessment of allergic sensitization and antibodies specific for respiratory viruses, in particular to rhinovirus can be useful to identify forms of asthma triggered by allergen exposure and/or infection by respiratory viruses for personalized asthma treatment according to the responsible trigger factors (21, 55). Determination of virus-triggered forms of asthma can be achieved with chips containing micro-arrayed viral proteins and/or peptides which allow measuring increases of virus-specific antibody levels after the asthma attack or by measuring cumulative virus-specific antibody levels (57, 58). For the assessment of allergic sensitization tests are needed which allow to assess IgE sensitization to a broad panel of allergen molecules or alternatively by using a simple screening test combining allergens from a large panel of allergen sources such as the Phadiatop™ test (29, 30, 59). Previous studies have investigated the sensitivity and specificity of the Phadiatop™ test among the general population, among patients with allergic rhinitis and/or bronchial asthma to detect allergic IgE sensitization (23, 26, 27, 30–39). However, we did not find studies which have evaluated the usefulness of the measurement of cumulative IgE specific for respiratory allergens (e.g., by Phadiatop™) for a group of patients with severe asthma aiming at the determination of asthma phenotypes and for the assessment if cumulative IgE levels specific for respiratory allergens could be a biomarker predicting the success of anti-IgE therapy. By contrast, two studies would have rather suggested the opposite. One study reported that the assessment of pretreatment specific IgE levels is not helpful in predicting the outcome of anti-IgE treatment (47) and a second even suggested that Omalizumab treatment is also effective in non-atopic asthma (48). Moreover, there is growing interest for specific IgE in response prediction also in other settings, such as in atopic dermatitis patients treated with Dupilumab, where Malassezia specific-IgE seems to be associated with the development of Dupilumab-induced facial redness dermatitis (60). However, complex dynamics, possibly involving eosinophils (61), are thought to play a role in the latter.
Other studies suggested that determination of total IgE levels maybe useful to predict efficacy of anti-IgE treatement (42, 44) and a post-hoc analysis of poly- versus monosensitized subjects did not provide unequivocal results regarding sensitization status (45).
We hypothesized that the measurement of cumulative IgE levels specific for respiratory allergens by Phadiatop™ testing may be useful for asthma phenotyping and in particular to identify patients with allergen-triggered asthma to choose IgE-targeted strategies for the 5th stage of therapy for severe asthma. The use of the Phadiatop™ test for this purpose seemed justified to us by the fact that it detects increased levels of specific IgE to respiratory allergens to suggest respiratory allergen exposure as possible asthma trigger factor.
It is common practice among clinicians to use total IgE levels as a biomarker that can be used to determine the allergic phenotype of asthma. However, it often turns out, and the results of our study support this assumption, that total IgE measurements are not sufficient and additional testing for allergen-specific IgE is required, which ultimately is more costly than using the Phadiatop™ as first line screening test. Skin tests with allergens are in principle cheaper than in vitro determination of specific IgE, but only a limited number of allergen sources can be tested and the procedure is time consuming and may require multiple visits of the patient. Furthermore, in vivo tests can be performed in controlled asthma patients and a FEV1 level > 70%, but may be risky in patients suffering from severe asthma. Obtaining a profound and complete anamnesis regarding allergy status is not always possible in routine clinical practice and needs to be linked with a deep diagnostic work-up regarding allergy by targeted allergy diagnosis selecting the serological tests for measuring allergen-specific IgE and provocation with the correct allergen extracts according to empiric knowledge. Since Phadiatop™ is a simple and inexpensive biomarker for IgE sensitization to respiratory allergens the selection of skin tests with allergens and/or laboratory tests determining the level of specific IgE to preselected allergens, according to the allergic anamnesis data may be reduced or even avoided. Similar results may be achieved with allergen chips containing a large panel of micro-arrayed allergens but the costs for chip testing are currently considerably higher (17).
Our study indicates that Phadiatop™ testing may indeed be useful to identify patients who are suitable for IgE-targeted molecular therapy and demonstrates that patients with allergen-specific levels over a certain threshold benefit more than those below this threshold. The study also showed that the level of total IgE did not depend on the positive/negative allergic anamnesis (p = 0.218) and the result of skin tests (p = 0.1), which has been also reported by other researchers (23, 26, 27, 31). Statistically significant differences in the level of total IgE were obtained depending on the result of specific IgE (p = 0.047), but with wide confidence intervals, which does not allow relying on the accuracy of the method. Cumulative IgE levels specific for respiratory allergens as measured by Phadiatop™ were statistically significantly higher in groups of patients with positive allergic anamnesis, positive skin tests and positive sIgE results than in groups with no allergic anamnesis, negative skin tests and negative sIgE results (**p <0.001, ). The increase in the total IgE level was associated with different phenotypes of the asthma, whereas cumulative IgE levels specific for respiratory allergens were significantly higher in patients with an allergic component in the pathogenesis of asthma (J45.0 and J45.8).
It is generally considered that a Phadiatop™ test result ≥ 0.35 PAU/l is positive. However, in practice, it is often observed that the patient does not have a clinically relevant reaction with a weakly positive result in the Phadiatop™ test. For our set of patients a cut-off point of 1.53 PAU/l was obtained (Se 62.5%, Spec 86.1%). Due to the low sensitivity and high specificity, if the result is less than 1.53 PAU/l in the patient it can be predicted that the patient will not have clinically relevant sensitization. The cut-off level found by us is higher than that of Zeng et al., 2018 (0.53, Se 89.4, Spec 97.5%) (30) which is possibly due to differences in the study groups and the allergen molecules responsible for sensitization in the two study populations. In fact, house dust mite allergy is very common in the Chinese population (62) whereas it is rare in allergic subjects in Russia due to climate differences (63). Accordingly, it is likely that cut-off levels for specific IgE levels determined by PhadiatopTM test as biomarker may vary in subjects with different IgE sensitization profiles.
Comparison of the efficacy parameters of the Phadiatop™ test when used as isolated biomarker with the collection of allergic anamnesis, skin tests and the determination of specific IgE does not allow us to conclude that the Phadiatop™ test has a clear advantage for asthma phenotyping. However, the characteristics of the combination of the Phadiatop™ test with allergic anamnesis were superior to both allergic anamnesis and standard methods separate and in combination. Thus, Phadiatop™, in combination with the collection of an allergic anamnesis, can be used instead of cumber-some standard skin testing and hypothesis-driven determination of specific IgE.
Indeed, response prediction to Omalizumab is challenging. This is true also in the setting of chronic spontaneous urticaria, where low total IgE is a strong predictor of poor response (64, 65), but specific IgE levels against a number of autoallergens (e.g., Fc epsilon R1, IL-24 and others) also may have some role in response prediction - although the evidence at the moment is conflicting (66). To evaluate the effectiveness of the proposed method for selecting patients for molecular therapy targeting IgE, we conducted a comparative analysis of treatment results with an anti-IgE drug in two groups of patients, with an initial value of the Phadiatop™ test < 1.53 PAU/l and ≥ 1.53 PAU/l. The dynamics of Omalizumab therapy results in the group with initial Phadiatop™ ≥ 1.53 PAU/l was superior to the results of therapy in the group with initial Phadiatop™ ≥ 0.35 and < 1.53 in terms of the level of asthma control (the proportion of uncontrolled asthma and an increase in AСT scores), a decrease in the number of exacerbations of asthma, improvement of the quality of life (according to AQLQ questionnaire).
It has been suggested that typing of asthma according to underlying trigger factors may be useful for guiding newly emerging forms of molecular and personalized treatment of patients suffering from asthma. The limitations of our study are associated with the retrospective analysis and the absence of a control group comprisig patients with non-allergic severe asthma treated with Omalizumab. Furthermore, we were able to analyze only a relatively small number of patients with severe asthma for whom all diagnostic methods were performed and who were only treated with Omalizumab. However, it must be acknowledged that severe asthma patients account only for approximately 5% to 10% of patients with asthma, which makes it very difficult to find them in real clinical practice (67). Nevertheless, our study demonstrated clearly that patients with IgE levels above 1.53 PAU/l achieved better asthma control with molecular therapy targeting IgE than patients with allergen-specific IgE levels below 1.53 PAU/l. Our study thus demonstrates that it may be possible to use Phadiatop™ as biomarker to determine cut-off levels of allergen-specific IgE above which clinical effects of IgE-targeted molecular therapy are better than in severe asthma patients with allergen-specific IgE levels below the cut-off. Therefore, the measurement of cumulative IgE levels with a screening test comprising respiratory allergens (i.e., Phadiatop™) seems superior to the measurement of total IgE levels and classical hypothesis-driven methods for allergy diagnosis for identifying patients with severe allergic asthma and can enhance the clinical efficacy of IgE-targeted asthma therapy. Our results were obtained in a single region pilot study with a relatively simple and crude test for measuring allergen-specific IgE. Accordingly, further studies involving larger numbers of patients with severe asthma and more sophisticated tests (e.g., chips containing micro-arrayed allergen molecules) for measuring allergen-specific IgE will be needed to further investigate the usefulness of measuring allergen-specific IgE as biomarker for prescription and prediction of efficacy of IgE-targeted therapies in asthma.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committee of the Ural State Medical University. The patients/participants provided their written informed consent to participate in this study.
Conceptualization, VN, EB, DK, AK and RV. Methodology, VN, DK. Formal analysis, VN, EB, DK, and RV. Investigation, VN, EB, DK, and RV. Resources, VN, EB, DK, and RV. Data curation, VN, EB, DK, and RV. Writing — original draft preparation, VN, EB, DK. Writing — review and editing, KN, PE, and RV. Visualization, VN, EB, DK, KN. All authors have read and agreed to the published version of the manuscript.
RV is a recipient of a Megagrant of the Government of the Russian Federation, grant number 14.W03.31.0024. This work was partially supported by the “Danube Allergy Research Cluster” Program of the Lower Austria (330950005).
RV has received research grants from HVD Life-Sciences, Vienna, Austria, and WORG Pharmaceuticals, Hangzhou, China and from Viravaxx AG, Vienna, Austria. Furthermore, he serves as consultant for WORG and Viravaxx AG.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. GINA. Global initiative for asthma – GINA 2021. Bethesda: Global Strategy for Asthma Management and Prevention (2021). Available at: www.ginasthma.org.
2. Ridolo E, Pucciarini F, Nizi MC, Makri E, Kihlgren P, Panella L, Incorvaia C. Mabs for treating asthma: omalizumab, mepolizumab, reslizumab, benralizumab, dupilumab. Hum Vaccin Immunother (2020) 16:2349–23561753440. doi: 10.1080/21645515.2020
3. Fajt ML, Wenzel SE. Asthma phenotypes and the use of biologic medications in asthma and allergic disease: The next steps toward personalized care. J Allergy Clin Immunol (2015) 135:299–311. doi: 10.1016/j.jaci.2014.12.1871
4. Hamilton D, Lehman H. Asthma phenotypes as a guide for current and future biologic therapies. Clin Rev Allergy Immunol (2020) 59:160–74. doi: 10.1007/s12016-019-08760-x
5. Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med (2012) 18:716–25. doi: 10.1038/nm.2678
6. Schoettler N, Strek ME. Recent advances in severe asthma: From phenotypes to personalized medicine. Chest (2020) 157:516–28. doi: 10.1016/j.chest.2019.10.009
7. Diamant Z, Vijverberg S, Alving K, Bakirtas A, Bjermer L, Custovic A, et al. Toward clinically applicable biomarkers for asthma: An EAACI position paper. Allergy (2019) 74:1835–51. doi: 10.1111/all.13806
8. Kuruvilla ME, Lee FEH, Lee GB. Understanding asthma phenotypes, endotypes, and mechanisms of disease. Clin Rev Allergy Immunol (2019) 56:219–33. doi: 10.1007/s12016-018-8712-1
9. Agache I, Akdis CA. Endotypes of allergic diseases and asthma: An important step in building blocks for the future of precision medicine. Allergol Int (2016) 65:243–52. doi: 10.1016/j.alit.2016.04.011
10. Santos A, Pité H, Chaves-Loureiro C, Rocha SM, Taborda-Barata L, et al. Metabolic phenotypes in asthmatic adults: Relationship with inflammatory and clinical phenotypes and prognostic implications. Metabolites (2021) 11:534. doi: 10.3390/metabo11080534
11. Ricciardolo FLM, Sorbello V, Ciprandi G. FeNO as biomarker for asthma phenotyping and management. Allergy Asthma Proc (2015) 36:e1–8. doi: 10.2500/aap.2015.36.3805
12. Kunc P, Fabry J, Lucanska M, Pecova R. Biomarkers of bronchial asthma. Physiol Res (2020) 69:S29–34. doi: 10.33549/physiolres.934398
13. Karaulov AV, Garib V, Garib F, Valenta R. Protein biomarkers in asthma. Int Arch Allergy Immunol (2018) 175:189–208. doi: 10.1159/000486856
14. Kowalski ML, Agache I, Bavbek S, Agache I, Bavbek S, Bakirtas A, Blanca M, Bochenek G, et al. Diagnosis and management of NSAID-exacerbated respiratory disease (N-ERD)-a EAACI position paper. Allergy (2019) 74:28–39. doi: 10.1111/all.13599
15. Augé J, Vent J, Agache I, Airaksinen L, Campo Mozo P, et al. EAACI position paper on the standardization of nasal allergen challenges. Allergy (2018) 73:1597–608. doi: 10.1111/all.13416
16. Del Giacco SR, Bakirtas A, Bel E, Custovic A, Diamant Z, Hamelmann E, et al. Allergy in severe asthma. Allergy (2017) 72:207–20. doi: 10.1111/all.13072
17. Huang HJ, Campana R, Akinfenwa O, Curin M, Sarzsinszky E, Karsonova A, et al. Microarray-based allergy diagnosis: Quo vadis? Front Immunol (2021) 11:594978. doi: 10.3389/fimmu.2020.594978
18. Önell A, Whiteman A, Nordlund B, Mazzoleni .Baldracchini F, Hedlin G, G, et al. Allergy testing in children with persistent asthma: comparison of four diagnostic methods. Allergy (2017) 72:590–7. doi: 10.1111/all.13047
19. Valenta R, Lidholm J, Niederberger V, Hayek B, Kraft D, Grönlund H. The recombinant allergen-based concept of component-resolved diagnostics and immunotherapy (CRD and CRIT). Clin Exp Allergy (1999) 29:896–904. doi: 10.1046/j.1365-2222.1999.00653.x
20. Fedenko E, Elisyutina O, Shtyrbul O, Pampura A, Valenta R, Lupinek C, Khaitov M. Microarray-based IgE serology improves management of severe atopic dermatitis in two children. Pediatr Allergy Immunol (2016) 27:645–9. doi: 10.1111/pai.12572
21. Niespodziana K, Stenberg-Hammar K, Papadopoulos NG, Focke-Tejkl M, Errhalt P, Konradsen JR, et al. Microarray technology may reveal the contribution of allergen exposure and rhinovirus infections as possible triggers for acute wheezing attacks in preschool children. Viruses (2021) 13:915. doi: 10.3390/v13050915
22. Merrrett J, Merret TG. Phadiatop–a novel IgE antibody screening test. Clin Allergy (1987) 17:409–16. doi: 10.1111/j.1365-2222.1987.tb02034.x
23. Gustafsson D, Danielsson D. In vitro diagnosis of atopic allergy in children. Allergy (1988) 43:105–8. doi: 10.1111/j.1398-9995.1988.tb00402.x
24. Eriksson NE. Allergy screening with phadiatop ® and CAP phadiatop ® in combination with a questionnaire in adults with asthma and rhinitis. Allergy (1990) 45:285–92. doi: 10.1111/j.1398-9995.1990.tb00497.x
25. Matricardi PM, Nisini R, Pizzolo JG, D'Amelio R. The use of phadiatop® in mass-screening programmes of inhalant allergies: advantages and limitations. Clin Exp Allergy (1990) 20:151–5. doi: 10.1111/j.1365-2222.1990.tb02660.x
26. Wever AMJ, Wever-Hess J, van Schayck CP, van Weel C. Evaluation of the phadiatop ® test in an epidemiological study. Allergy (1990) 45:92–7. doi: 10.1111/j.1398-9995.1990.tb00464.x
27. Crobach MJJS, Kaptein AA, Kramps JA, Hermans J, Ridderikhoff J, Mulder JD. The phadiatop ® test compared with RAST, with the CAP system; proposal for a third phadiatop outcome : “inconclusive”. Allergy (1994) 49:170–6. doi: 10.1111/j.1398-9995.1994.tb00821.x
28. Tschopp JM, Schindler .Sistek D, Leuenberger C, Perruchoud P, Wüthrich AP, Brutsche B, M, et al. Current allergic asthma and rhinitis : diagnostic efficiency of three commonly used atopic markers (IgE, skin prick tests, and phadiatop ®) results from 8329 randomized adults from the SAPALDIA study. Allergy (1998) 53:608–13. doi: 10.1111/j.1398-9995.1998.tb03937.x
29. Vidal C, Gude F, Boquete O, Fernández-Merino MC, Meijide LM, Rey J, et al. Evaluation of the phadiatop™ test in the diagnosis of allergic sensitization in a general adult population. J Investig Allergol Clin Immunol (2005) 15:124–30.
30. Zeng G, Hu H, Zheng P, Wu G, Wei N, Liang X, et al. The practical benefit of phadiatop test as the first-line in vitro allergen-specific immunoglobulin e (sIgE) screening of aeroallergens among Chinese asthmatics : a validation study. Ann Transl Med (2018) 6:151. doi: 10.21037/atm.2018.04.06
31. Chang YC, Lee TJ, Huang CC, Chang PH, Chen YW, Fu CH. The role of phadiatop tests and total immunoglobulin e levels in screening aeroallergens: A hospital-based cohort study. J Asthma Allergy (2021) 14:135–40. doi: 10.2147/JAA.S292710
32. Salkie ML. The phadiatop test allows adequate screening for atopy with a marked reduction in cost. J Clin Lab Anal (1991) 5:226–7. doi: 10.1002/jcla.1860050313
33. Dekker FW, Mulder Dzn JD, Kramps JA, Kaptein AA, Vandenbroucke JP, Dijkman JH. The phadiatop in vitro test for allergy in general practice: Is it useful? Fam Pract (1990) 7:144–8. doi: 10.1093/fampra/7.2.144
34. Zimmerman B, Forsyth S. Diagnosis of allergy in different age groups of children: use of mixed allergen RAST discs, phadiatop and paediatric mix. Clin Allergy (1988) 18:581–7. doi: 10.1111/j.1365-2222.1988.tb02909.x
35. Bujía J, Rasp G. Determination of the diagnostic efficacy of the multi-RAST test (Phadiotop) in allergic rhinitis. Acta Otor-rinolaringol Esp (1995) 46:19–21.
36. Cantani A, Ferrara M, Barbieri C, Monteleone A, Businco L. Evaluation of new test (Phadiatop(™)) for the screening of respiratory allergic disorders in children. Ann Allergy (1990) 64:158–61.
37. Köhl C, Debelić M. In vitro screening for inhalant allergy with multi SX 1 RAST® (Phadiatop®). Allergy (1991) 46:245–50. doi: 10.1111/j.1398-9995.1991.tb00581.x
38. Paganelli R, Ansotegui IJ, Sastre J, Lange CE, Roovers MH, de Groot H, et al. Specific IgE antibodies in the diagnosis of atopic disease. clinical evaluation of a new in vitro test system, UniCAP(™), in six European allergy clinics. Allergy (1998) 53:763–8. doi: 10.1111/j.1398-9995.1998.tb03972.x
39. Williams PB, Siegel C, Portnoy J. Efficacy of a single diagnostic test for sensitization to common inhalant allergens. Ann Allergy Asthma Immunol (2001) 86:196–202. doi: 10.1016/S1081-1206(10)62691-9
40. McGregor MC, Krings JG, Nair P, Castro M. Role of biologics in asthma. Am J Respir Crit Care Med (2019) 199:433–45. doi: 10.1164/rccm.201810-1944CI
41. Okayama Y, Matsumoto H, Odajima H, Takahagi S, Hide M, Okubo K. Roles of omalizumab in various allergic diseases. Allergol Int (2020) 69:167–77. doi: 10.1016/j.alit.2020.01.004
42. Bousquet J, Rabe K, Humbert M, Chung KF, Berger W, Fox H, et al. Predicting and evaluating response to omalizumab in patients with severe allergic asthma. Respir Med (2007) 101:1483–92. doi: 10.1016/j.rmed.2007.01.011
43. Probst M, Gogolka A, Krüll M, Noga O. In search of clinically relevant parameters to monitor successful omalizumab therapy in allergic asthma. Allergol Select (2019) 2:49–55. doi: 10.5414/ALX01377E
44. Ankerst J, Nopp A, Johansson SG, Adédoyin J, Oman H. Xolair is effective in allergics with a low serum IgE level. Int Arch Allergy Immunol (2010) 152:71–4. doi: 10.1159/000260086
45. Vaník P, Novosad J, Kirchnerová O, Krčmová I, Teřl M, Czech Anti-IgE Registry collaborators. Effect of individual allergen sensitization on omalizumab treatment outcomes in patients with severe allergic asthma determined using data from the Czech anti-IgE registry. Allergy Asthma Clin Immunol (2020) 16:81. doi: 10.1186/s13223-020-00479-1
46. Johansson SGO, Nopp A, Öman H, Ankerst J, Cardell LO, Grönneberg R, et al. The size of the disease relevant IgE antibody fraction in relation to ‘total-IgE’ predicts the efficacy of anti-IgE (Xolair) treatment. Allergy (2009) 64:1472–7. doi: 10.1111/j.1398-9995.2009.02051.x
47. Wahn U, Martin C, Freeman P, Blogg M, Jimenez P. Relationship between pretreatment specific IgE and the response to omalizumab therapy. Allergy (2009) 64:1780–7. doi: 10.1111/j.1398-9995.2009.02119.x
48. Pillai P, Chan YC, Wu SY, Ohm-Laursen L, Thomas C, Durham SR, et al. Omalizumab reduces bronchial mucosal IgE and improves lung function in non-atopic asthma. Eur Respir J (2016) 48:1593–601. doi: 10.1183/13993003.01501-2015
49. Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J (2014) 43:343–73. doi: 10.1183/09031936.00202013
50. GINA. Difficult-to-treat asthma in adolescent and adult patients: Diagnosis and management - a GINA pocket guide for health professionals. global strategy for asthma management and prevention. (2018). https://ginasthma.org/difficult-to-treat-and-severe-asthma-guide/
51. GINA. Difficult-to-treat asthma in adolescent and adult patients: Diagnosis and management - a GINA pocket guide for health professionals. global strategy for asthma management and prevention, Vol. 2. (2019). https://ginasthma.org/wp-content/uploads/2019/04/GINA-Severe-asthma-Pocket-Guide-v2.0-wms-1.pdf
52. Bousquet J, Heinzerling L, Bachert C, Papadopoulos NG, Bousquet PJ, Burney PG, et al. Practical guide to skin prick tests in allergy to aeroallergens. global allergy and asthma European network; allergic rhinitis and its impact on asthma. Allergy (2012) 67:18–24. doi: 10.1111/j.1398-9995.2011.02728.x
53. Matricardi PM, Kleine-Tebbe J, Hoffmann HJ, Valenta R, Hilger C, Hofmaier S, et al. EAACI molecular allergology user’s guide. Pediatr Allergy Immunol (2016) 23:1–250. doi: 10.1111/pai.12563
54. Miller MR, Hankinson J, Barjaktarevic IZ, Cooper BG, Hall GL, et al. Standardization of spirometry 2019 update. an official American thoracic society and European respiratory society technical statement. Am J Respir Crit Care Med (2019) 200:e70–88. doi: 10.1164/rccm.201908-1590ST
55. Niespodziana K, Borochova K, Pazderova P, Schlederer T, Astafyeva N, Baranovskaya T, et al. Toward personalization of asthma treatment according to trigger factors. J Allergy Clin Immunol (2020) 145:1529–34. doi: 10.1016/j.jaci.2020.02.001
56. Incorvaia C, Ridolo E, Bagnasco D, Scurati S, Canonica GW. Personalized medicine and allergen immunotherapy: the beginning of a new era? Clin Mol Allergy (2021) 19:10. doi: 10.1186/s12948-021-00150-z
57. Niespodziana K, Stenberg-Hammar K, Megremis S, Cabauatan CR, Napora-Wijata K, Vacal PC, et al. PreDicta chip-based high resolution diagnosis of rhinovirus-induced wheeze. Nat Commun (2018) 9:2382. doi: 10.1038/s41467-018-04591-0
58. Megremis S, Niespodziana K, Cabauatan C, Xepapadaki P, Kowalski ML, Jartti T, et al. Rhinovirus species-specific antibodies differentially reflect clinical outcomes in health and asthma. Am J Respir Crit Care Med (2018) 198:1490–9. doi: 10.1164/rccm.201803-0575OC
59. Lupinek C, Hochwallner H, Hochwallner H, Johansson C, Mie A, Rigler E, Scheynius A, et al. Maternal allergen-specific IgG might protect the child against allergic sensitization. J Allergy Clin Immunol (2019) 144:536–48. doi: 10.1016/j.jaci.2018.11.051
60. Kozera E, Stewart T, Gill K, de la Vega MA, Frew JW. Dupilumab-associated head and neck dermatitis is associated with elevated pretreatment serum malassezia-specific IgE: a multicentre, prospective cohort study. Br J Dermatol (2022) 186:1050–2. doi: 10.1111/bjd.21019
61. Ferrucci S, Angileri L, Tavecchio S, Fumagalli S, Iurlo A, Cattaneo D, Marzano AV, Maronese CA, et al. Elevation of peripheral blood eosinophils during dupilumab treatment for atopic dermatitis is associated with baseline comorbidities and development of facial redness dermatitis and ocular surface disease. J Dermatolog Treat (2022), 31:1–6. doi: 10.1080/09546634.2022.2049588
62. D’souza N, Weber M, Sarzsinszky E, Vrtala S, Curin M, Schaar M, et al. The molecular allergen recognition profile in China as basis for allergen-specific immunotherapy. Front Immunol (2021) 12:719573. doi: 10.3389/fimmu.2021.719573
63. Elisyutina O, Lupinek C, Fedenko E, Litovkina A, Smolnikov E, Ilina N, et al. IgE-reactivity profiles to allergen molecules in Russian children with and without symptoms of allergy revealed by micro-array analysis. Pediatr Allergy Immunol (2021) 32:251–63. doi: 10.1111/pai.13354
64. Fok JS, Kolkhir P, Church MK, Maurer M. Predictors of treatment response in chronic spontaneous urticaria. Allergy (2021) 76:2965–81. doi: 10.1111/all.14757
65. Marzano AV, Genovese G, Casazza G, Genovese G, Casazza G, Fierro MT, et al. Predictors of response to omalizumab and relapse in chronic spontaneous urticaria: a study of 470 patients. J Eur Acad Dermatol Venereol (2019) 33:918–24. doi: 10.1111/jdv.15350
66. Asero R, Marzano AV, Ferrucci S, Lorini M, Carbonelli V, Cugno M. Co-Occurrence of IgE and IgG autoantibodies in patients with chronic spontaneous urticaria. Clin Exp Immunol (2020) 200:242–9. doi: 10.1111/cei.13428
Keywords: asthma biomarker, Omalizumab, biologicals, molecular therapy, targeted therapy
Citation: Naumova V, Beltyukov E, Niespodziana K, Errhalt P, Valenta R, Karaulov A and Kiseleva D (2022) Cumulative IgE-levels specific for respiratory allergens as biomarker to predict efficacy of anti-IgE-based treatment of severe asthma. Front. Immunol. 13:941492. doi: 10.3389/fimmu.2022.941492
Received: 11 May 2022; Accepted: 29 August 2022;
Published: 21 September 2022.
Edited by:
Arzu Didem Yalcin, Academia Sinica, TaiwanReviewed by:
Onur Turan, Izmir Katip Celebi University, TurkeyCopyright © 2022 Naumova, Beltyukov, Niespodziana, Errhalt, Valenta, Karaulov and Kiseleva. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rudolf Valenta, cnVkb2xmLnZhbGVudGFAbWVkdW5pd2llbi5hYy5hdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.