
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 01 July 2022
Sec. Cancer Immunity and Immunotherapy
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.937344
This article is part of the Research Topic The Role of Angiogenesis and Immune Response in Tumor Microenvironment of Solid Tumor View all 16 articles
Tumor immunotherapy has shown strong therapeutic potential for stimulating or reconstructing the immune system to control and kill tumor cells. It is a promising and effective anti-cancer treatment besides surgery, radiotherapy and chemotherapy. Presently, some immunotherapy methods have been approved for clinical application, and numerous others have demonstrated promising in vitro results and have entered clinical trial stages. Although immunotherapy has exhibited encouraging results in various cancer types, however, a large proportion of patients are limited from these benefits due to specific characteristics of the tumor microenvironment such as hypoxia, tumor vascular malformation and immune escape, and current limitations of immunotherapy such as off-target toxicity, insufficient drug penetration and accumulation and immune cell dysfunction. Ultrasound-target microbubble destruction (UTMD) treatment can help reduce immunotherapy-related adverse events. Using the ultrasonic cavitation effect of microstreaming, microjets and free radicals, UTMD can cause a series of changes in vascular endothelial cells, such as enhancing endothelial cells’ permeability, increasing intracellular calcium levels, regulating gene expression, and stimulating nitric oxide synthase activities. These effects have been shown to promote drug penetration, enhance blood perfusion, increase drug delivery and induce tumor cell death. UTMD, in combination with immunotherapy, has been used to treat melanoma, non-small cell lung cancer, bladder cancer, and ovarian cancer. In this review, we summarized the effects of UTMD on tumor angiogenesis and immune microenvironment, and discussed the application and progress of UTMD in tumor immunotherapy.
In the past decade, rapid advancements in tumor immunotherapy have established it as a crucial treatment for various kinds of cancers (1). Compared with surgery, radiotherapy and chemotherapy that directly act on the tumor itself, tumor immunotherapy stimulates the body’s immune system and indirectly attacks tumor cells by enhancing the immune defense mechanism against the tumor and reshaping the immune microenvironment (2, 3). On the one hand, it can enhance immune-mediated tumor cell death by promoting immune tumor cell recognition and eliminating target cells that carry tumor antigens, while on the other hand, it can eliminate or reduce immunosuppressive signals induced by tumor cells (4, 5).
At present, the common tumor immunotherapy includes tumor vaccines, tumor-agnostic therapies, gene therapies and adoptive cell immunotherapies. Nano-based drug delivery systems (6) and cell-inspired drug delivery platforms (7) are also being used in cancer immunotherapy. A variety of immunotherapy drugs have been approved for clinical use and are benefiting patients with lung cancer (8), bladder cancer (NEO-PV-01) (8), melanoma (NeoVax) (9), and ovarian cancer (OCDC) (10). However, some patients have poor responses to immunotherapy and may even develop hyper progressive disease after treatment. Positive responses to immunotherapy usually depend on the dynamic interactions between tumor cells and immunomodulators in the tumor microenvironment. Low immune responses are often associated with tumor angiogenesis and tumor-specific immunosuppressive microenvironments (11). In addition, complexities in the structures and functions of tumor angiogenesis make drug penetration very challenging, resulting in insufficient drug delivery (12).
Ultrasound-targeted microbubble destruction (UTMD) utilizes microstreaming, microjets and free radicals generated by ultrasonic cavitation to damage endothelial cells (ECs) (Figure 1). Similar to sonodynamic therapy, this regulates the tumor immunosuppressive microenvironment by causing microvascular rupture and tumor cell apoptosis, hindering tumor angiogenesis, and enhancing immunotherapy effects (14–16). Further, local ultrasound irradiation can trigger the targeted release of drugs and exogenous genes to achieve higher treatment efficiency (17). Therefore, UTMD has shown promising prospects in improving the therapeutic efficacies of immunotherapy.
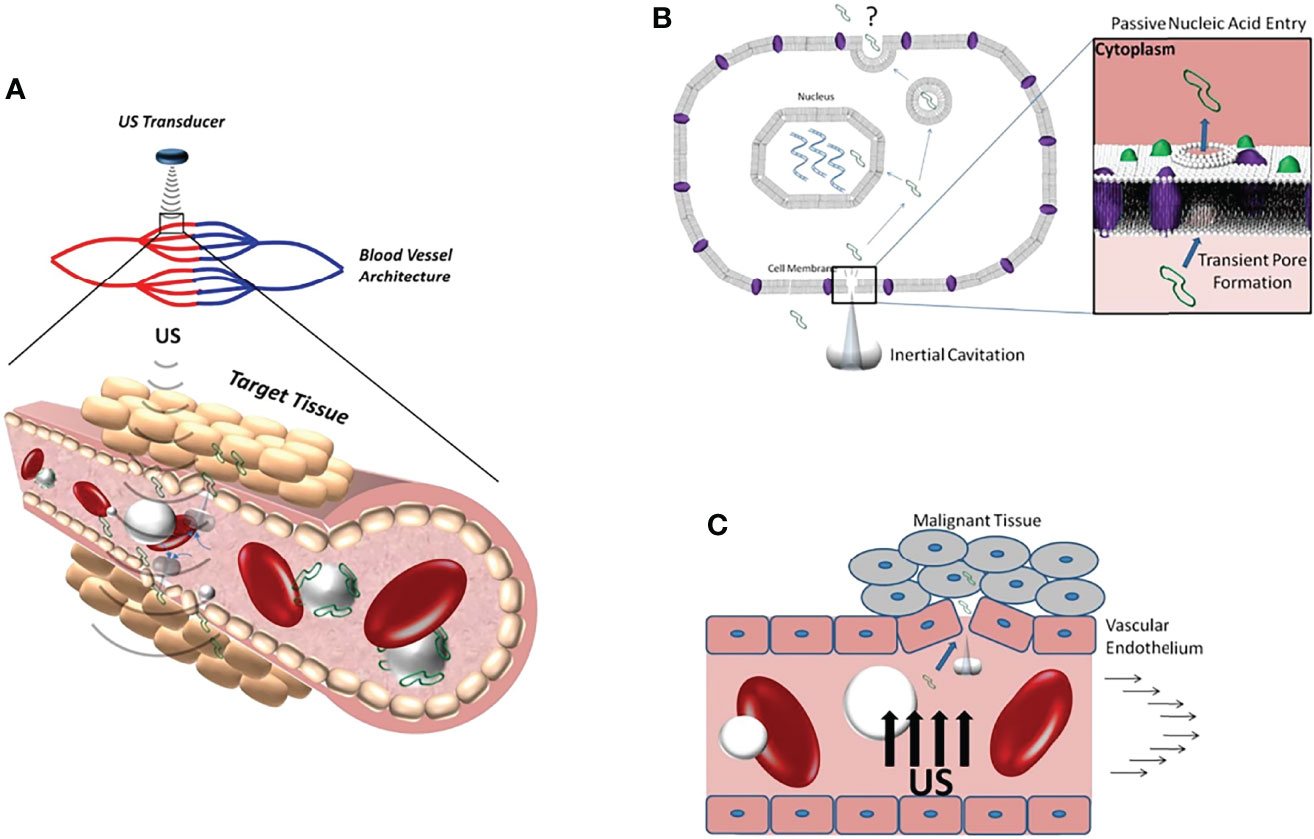
Figure 1 (A) Schematic diagram of ultrasonic cavitation promoting DNA (green) extravasation into tissue. (B) Microjets generated by inertial cavitation creates acoustic pores that allow DNA to enter the cytoplasm. (C) Inertial cavitation increases the permeability of blood vessels to DNA by damaging the integrity of the vascular endothelium. Reprinted with permission from ref (13). copyright © 2012 Sirsi and Borden.
In this article, we summarized the regulation of UTMD on tumor angiogenesis and the immune microenvironment. Then, we reviewed the practical application of UTMD in various tumor immunotherapies, including tumor vaccines antibody therapy, gene therapy and adoptive cell therapy, and their projected future developments (Table 1).
The tumor immune microenvironment has the following characteristics (29): (a) contains immune cells that lack antigenic stimulation, which could lead to ineffective inhibition of tumor growth and promote tumor immune escape; (b) decreased proliferative ability and insufficiency of immune cells to infiltrate tumoral tissues due to increased interstitial pressure caused by the tumor that acts as a physical barrier; (c) depletion or transient activation of antigen-specific T cells that cannot effectively inhibit tumor growth; (d) poor release of tumor antigens to peripheral lymph nodes, resulting in inadequate direct or indirect antigen presentation and insufficient T cell initiation, and; (e) failure to recognize and present tumor antigens due to the secretion of a variety of negative immune regulatory factors by tumor cells and immunosuppressive cells, leading to immune escape. All these immunosuppressive microenvironments characteristics contributed to the clinically observed drug resistance and off-target toxicity of immunotherapy.
Solid aggressive malignant tumors grow rapidly by inducing the release of various pro-angiogenic and anti-angiogenic factors that prompt the formations of tumor blood vessels to ensure their survival. Among them, the vascular endothelial growth factor (VEGF) has been found to have the most significant pro-angiogenic effect. It can promote vascular ECs division and support ECs migration for constructing more new vessels (30). In addition, VEGF can also induce the expression of adhesion molecules on ECs, mobilize bone marrow-derived cells, and directly or indirectly promote tumor angiogenesis.
Tumor-induced angiogenesis is characterized by structurally distorted tumor vessels and pericellular insufficiency (31). It can cause severe microenvironmental hypoxia, promote VEGF expression and induce vascular malformations (32), resulting in uneven distribution of blood flow and affecting drug penetration and delivery (12). Further, blood leakage from malformed vessels can increase interstitial pressure, which reduces the proliferation, infiltration and survival ability of immune cells. These hinder infiltration and lead to senescence and exhaustion and poor tumor-killing ability of immune cells (33).
As a simple, safe and non-invasive method, ultrasound has been widely used to diagnose and treat diseases. UTMD is a recently developed technology that can target the release of drugs and exogenous genes by augmenting ultrasonic cavitation effects, which has the advantages of being precise, highly efficient and safe, with good repeatability (34–36). The cavitation effect is a significant physical impact of ultrasound. When ultrasonic pressure reaches a certain threshold, the surrounding liquid is rapidly filled with small cavities of gas and steam, forming microbubbles (MBs), also known as cavitation nuclei. Under the activity of ultrasound, these MBs continue to vibrate, expand and contract, which, when finally burst and collapse (37, 38), release instantaneous energy and cause extreme physical phenomena such as luminescence, high temperature, high pressure, discharge, and microjet (39).
Cavitation effects can be divided into non-inertial cavitation (i.e., stable cavitation) and inertial cavitation (i.e., unstable cavitation) (40). When the ultrasonic amplitude is low, the bubbles can oscillate symmetrically around an equilibrium radius without bursting under the action of ultrasonic, producing microflows characterized by fluid flow (41). Microflows impose shear stress on cells while generating heat and lead to sound holes, which help open the tissue barrier formed by ECs. When the ultrasonic amplitude is large, the bubbles oscillate asymmetrically, their volume expands asymmetrically and collapses. The intense compression of gasses inside the bubbles and the huge fluctuation of local pressure generated by the surrounding fluid are called shock waves, which have substantial impacts on cells or tissues and can locally produce high temperature and high pressure. The energy generated by bubble collapse is then converted to kinetic energy, which allows the fluid to be ejected and leads to irreversible tissue or cell damage (42, 43). This significantly increases the permeability of the tumors’ cell membrane, causing damage and widening the gap of ECs, and DNA breakage, which eventually leads to microvascular rupture, hemorrhage and hemolysis (44). MBs can implode under certain acoustic pressure irradiation, which significantly increases the number of cavitation nuclei and enhances the cavitation effect. The fundamentals of cavitation effects are as follows (45): (a) exogenous MBs increase the number of cavitation nuclei, which then increase the intensity of the cavitation effects; (b) as the quantity of MBs increases, the energy required to produce cavitation decreases and the energy threshold for cavitation effect decreases. Moreover, immunotherapy drugs, exogenous genes, or acoustic sensitizers can be incorporated into MBs to target specific tissues. Ultrasound can irradiate the target tissue with a certain amount of radiation energy, destroying the MBs carrying the drugs and releasing the payload, thus achieving the targeted release of drugs or genes (46). Under the action of low-frequency ultrasound, the MB collapse process caused by the cavitation effect produces jet and releases energy, which can instantly break adjacent cell membranes, increasing their permeability and promoting the phagocytosis of the cell to drugs (47). Furthermore, UTMD can temporarily allow immunotherapy drugs to cross the blood-brain barrier (BBB) and blood-tumor barrier and reach the targeted tumor area (48). Therefore, UTMD-mediated antitumor drug release can reduce the off-target toxicity of tumor immunotherapy. As of now, many studies have utilized UTMD to enhance the efficiency of drug targeting and delivery in local tissues (49, 50).
Microstreaming and microjets from ultrasonic cavitation-related biological effects can cause ECs damage and microvascular rupture (51). Due to the rapid, loose, and irregular growth of tumor blood vessels and functional defects in their vascular architecture, UTMD can cause significant damage to tumor vascular ECs, manifesting as endothelial cell malformations or endothelial cell contractions (52). Under appropriate sound pressure, UTMD can damage tumor vascularization and exert its antitumor angiogenesis effects (53).
Liu et al. (54) used UTMD (acoustic pressure: 2.6 MPa and 4.8 MPa) to mechanically destroy tumor blood vessels in Walker 256 tumors. They found that contrast-enhanced ultrasound could disrupt tumor neovasculature and significantly decrease tumor perfusion compared with the control group. Histopathologically, the tumor microvascular were destroyed into diffuse hematomas. In a study by Jing et al. (55), the authors showed that the microcirculation of Walker 256 tumors treated with 4.8 MPa could be blocked for 24 h. In a previous study, the investigators used lipid shell MBs loaded with Endostar combined with UTMD to explore the anti-angiogenesis effect of UTMD in established nude mice breast cancer models. Compared with the Endostar group alone, they observed that after ultrasound targeted irradiation of drug-loaded MBs, the release of Endostar was significantly increased, and tumor VEGF expression was significantly down-regulated. Tumor growth inhibition rate was significantly increased, confirming that UTMD combined with drug-carrying MBs could improve the anti-angiogenesis effect of Endostar by downregulating VEGF expression, thus, achieving tumor growth inhibition. Meanwhile, UTMD can release targeted drugs that can accumulate in tumors. Yu et al. (56) treated rats inoculated with Walker 256 tumors using Endostar combined with UTMD and measured the microvascular density by contrast-enhanced ultrasound. They observed that UTMD could significantly lower tumor blood perfusion and had a significantly higher tumor growth inhibition rate than the control group, thus confirming that UTMD enhanced the anti-angiogenic effect of Endostar.
The potential of UTMD in anti-angiogenic therapy remains largely unknown. UTMD at higher energy intensity has been shown to promote apoptosis of ECs by regulating gene expression and contributing to microvascular destruction. Su et al. (57) demonstrated that UTMD (0.5 MHz, 210 mW/cm2) significantly promoted apoptosis and inhibited the angiogenesis of human umbilical vein ECs and human microvascular ECs through the phosphorylation of p38 mitogen-activated protein kinase and activated endoplasmic reticulum stress signal. These results demonstrate the potential value of UTMD in anti-angiogenic therapy.
Tumor vascular ECs are the first contact point of cavitation effects (58). UTMD was shown to enhance endothelial cell permeability in in vivo and in vitro settings, reversibly opening the BBB or blood-tumor barrier and facilitating extracellular drug transfer into the interstitial space. Hallow et al. (59) quantified the biological effects of UTMD on ECs using isolated live pig carotid arteries. Their results showed that relatively low ultrasound energy (700 kPa-1400 kPa) could target 9%-24% of the drug uptake of ECs. Lelu et al. (60) compared the effects of inertial and non-inertial cavitation on the monolayer resistance and permeability of pigs brain’s ECs in the presence of SonoVue. Their results demonstrated that non-inertial cavitation had better cell permeability than inertial cavitation, could reversibly open the BBB and promoted drug delivery. Wang et al. (61) showed that gambogic acid-loaded porous-lipid MBs in combination with UTMD could instantly increase BBB permeability and promote the release of gambogic acid into the stroma of human glioma (U251 cell line), and could also significantly inhibit the tumor’s growth in in vitro BBB model of mouse brain endothelial cell line. UTMD has also shown therapeutic potential in pancreatic cancer mouse models. In a study by Zhang et al. (62), the authors showed that UTMD enhanced the permeability of the hematoma barrier through cavitation effects. This promoted the delivery of drug-loaded MBs to the tumor matrix and inhibited the growth rate of pancreatic cancer by 89.8% during 21 days of treatment. Zhang et al. (63) utilized C6 glioma-bearing rats to study the mechanism of UTMD in improving BBB permeability. They observed that the enhanced BBB permeability could be associated with the downregulation of cellular junctional adhesion molecule-A and up-regulation of calcium-activated potassium channel expression, which affected the BBB tight connection.
It was shown that intermittent ultrasound irradiation, compared with continuous ultrasound irradiation, improved the permeability of BBB and promoted the extravasation of Evans Blue into the stromal tissues of C6 glioma membranes. Wang et al. (64) confirmed that microRNA-34a encapsulated with nanoparticles combined with UTMD exerted a significant inhibitory effect on castration-resistant prostate cancer by improving membrane permeability and capillary space and promoting the delivery of nanoparticles to prostate cancer xenograft.
UTMD promotes tumor cell death by regulating calcium levels and ceramide signaling pathways. Dying or stressed tumor cells release DAMPs, which act as adjuvants or immune recognition stimulants, which trigger immune responses (65, 66). Similarly, ultrasonic cavitation effects produce free radicals, which act as inducing factors that stimulate the release of DAMPs (67). DAMPs then activate inflammatory reaction pathways, lymphocytes, monocytes and macrophages release IL-1 and IL-18 inflammatory regulating cell factors, promoting tumor antigens presentation for induction of T cells adaptive responses, which improves tumor immune escape (68) (Figure 2).
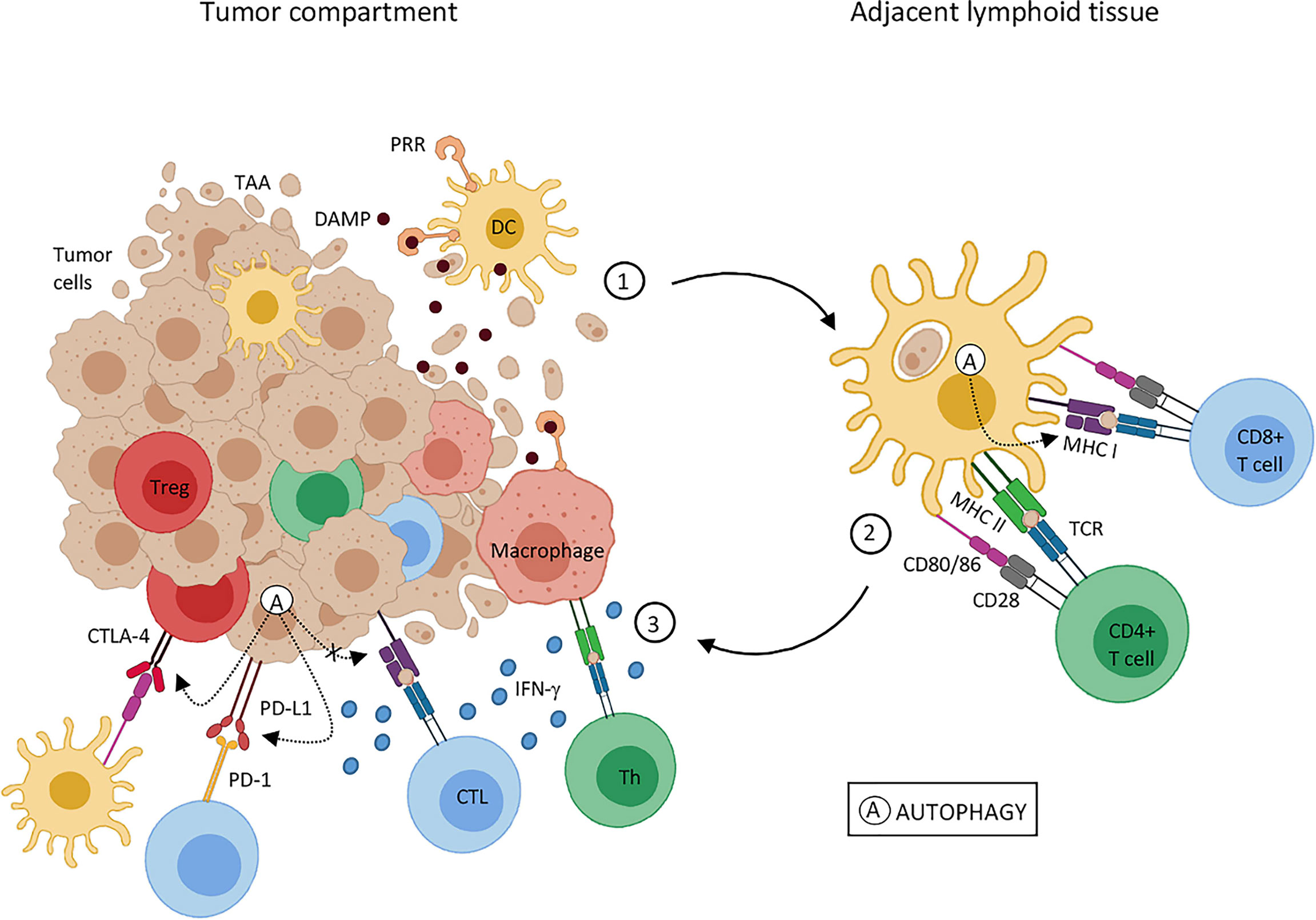
Figure 2 Effects of tumor cell death on tumor-associated antigen presentation. Tumor-associated antigens (TAA); dendritic cells (DC); major histocompatibility complex (MHC); damage-associated molecular pattern (DAMP); cytotoxic T lymphocytes (CTL); T regulatory cells (Treg); pattern recognition receptor (PRR); T-cell receptor (TCR); helper T cell (Th); tumor necrosis factor (TNF); programmed cell death protein 1 (PD-1); programmed cell death-ligand 1 (PD-L1). Reprinted with permission from ref (69). copyright © 2020 de Souza, Gonçalves, Lepique and de Araujo-Souza.
Ca2+ plays a key role in cell integrity, membrane encapsulation, and intercellular signaling. Ultrasonic cavitation can increase intracellular Ca2+ levels by inducing adjacent intracellular Ca2+ increase via intercellular signaling to neighboring cells (70). Beekers et al. (71) showed that an MB oscillation amplitude between 0.75 μm and 1 μm could maintain stable cell viability, but increasing the amplitude oscillation to greater than 1 μm would cause dramatic fluctuation in Ca2+ concentration. They also showed that contact between adjacent cells was opened when irreversible Ca2+ fluctuations were caused by ultrasound-induced MB oscillation, suggesting that the opening of intercellular contact is a biological response caused by elevated Ca2+ levels; a mechanism that also facilitates drug passage through the BBB (72). Thus, increasing oscillation amplitude increases the degree of pore damage and decreases the ability of cell membranes to reseal. This leads to activation of voltage-sensitive Ca2+ channel, through which extracellular Ca2+ flows into the cell, causing drastic Ca2+ fluctuations and Ca2+ overload.
Some endonucleases responsible for DNA fragments are Ca2+-dependent, and once Ca2+ concentration increases, the enzyme is activated and degrades DNA to induce apoptosis (73). Similarly, Shi et al. (74) found that Ca2+-dependent endonucleases and protease activation could lead to the apoptosis of hepatocellular carcinoma cells, SMMC-7221, by opening their mitochondrial pores and increasing membrane permeability. The ceramide signaling pathway instigated by ECs injury has a significant role in controlling cancer cell demise (14). Al-mahrouki et al. (75) confirmed that UTMD-induced ceramide accumulation was caused by the downregulation of UDP glycosyltransferase-8. The anti-apoptotic function of UDP glycosyltransferase-8 was achieved by disrupting the ceramide signaling pathway and converting ceramide to galactose ceramide.
In a study by Hu et al. (18), the authors compared the antitumor effect of ultrasound-stimulated nanobubbles alone and in combination with an anti-programmed cell death protein 1 (aPD-1) in RM1 (prostate cancer), MC38 (colon cancer), and B16 (melanoma) xenograft mouse models. They found that ultrasound-stimulated nanobubbles combined with aPD-1 induced tumor cell necrosis, significantly increased the release of DAMP and tumor antigen presentation, and promoted the invasion and antitumor activity of CD8+ T cells (Figure 3). Thus, with this strategy, immunogenicity can be improved by remodeling the tumor immune microenvironment and sensitizing poorly immunogenic solid tumors to aPD-1 treatment.
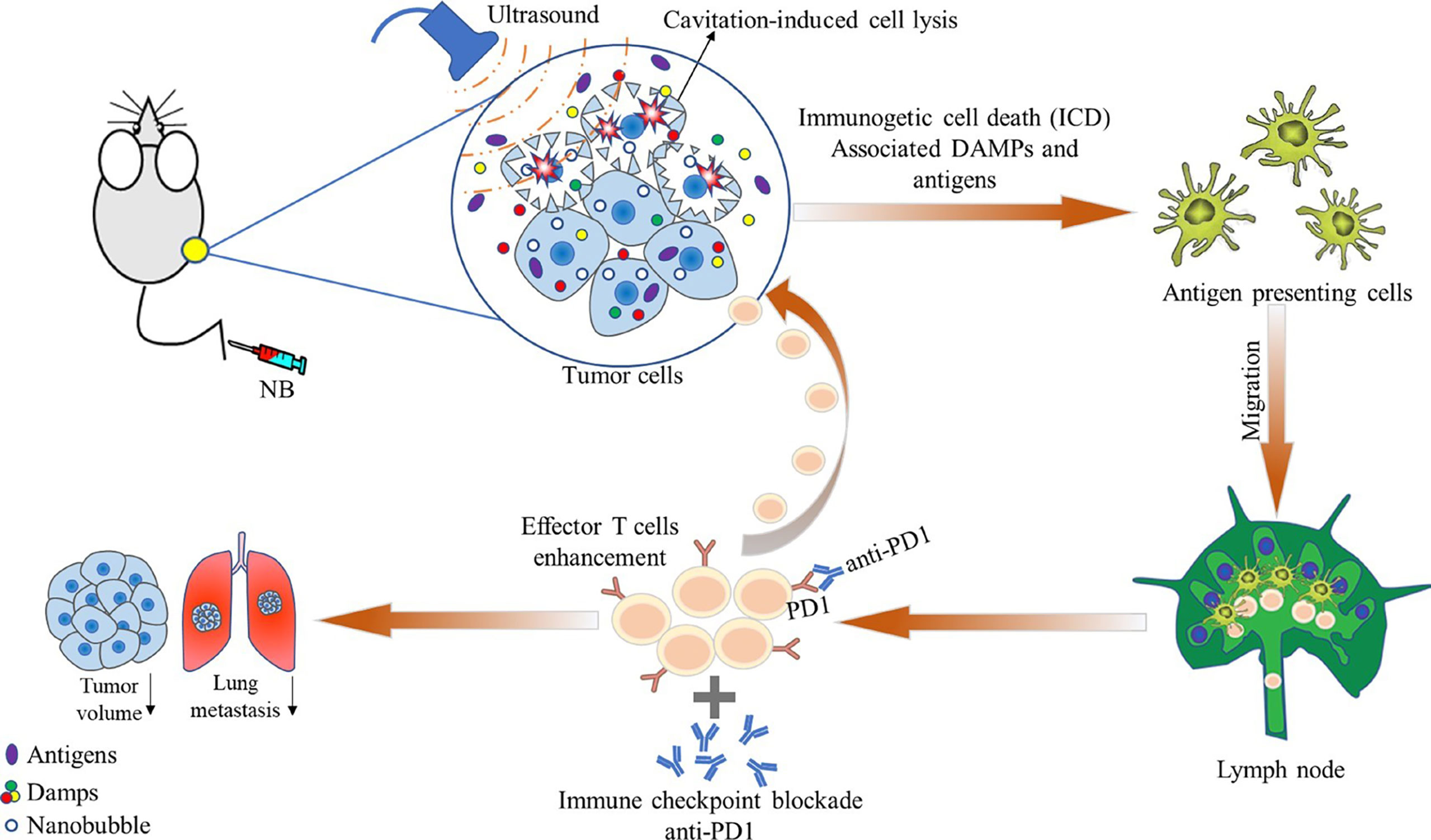
Figure 3 Schematic illustration showing the effects of ultrasound-stimulated nanobubbles (USNBs) on the mouse tumor model. USNBs can induce tumor cell necrosis, which can release immunogenic substances, further activate innate and adaptive immune cells, and finally activate CD8+ T cells. This leads to systemic anti-tumor immunity, enhancing the efficacy of anti-PD1 therapy and promoting immune memory. Reprinted (adapted) with permission from ref (18). copyright © 2022 Hu, He, Wang, Zhao, Fang, Dong, Chen, Zhang, Zhang, Wang, Tan, Wang, Zi, Liu, Liang, Guo, Ou.
Therapeutic cancer vaccines are an active immunotherapy approach to induce durable antitumor immunity. These include tumor cell vaccines, dendritic cell (DC) vaccines, viral vector vaccines, and molecular vaccines composed of peptides, DNA or RNA (76, 77). DC vaccines are most commonly used in tumor immunotherapy due to their high antigen presentation effect. High-efficiency antigen-presenting cells DC load tumor-associated antigens into the body and activate T cells. Some of them are activated and proliferate into cytotoxic T lymphocytes, causing a strong immune response, while some become long-term memory T cells, producing immune memory. Since the approval of PROVENGE (Sipuleucel-T), the first DC vaccine, to treat advanced resistant prostate cancer by the Food and Drug Administration (FDA) in 2010 (78), several cancer vaccines have been developed against melanoma (NeoVax) and non-small cell lung cancer. Some of them have shown promising benefits and are being further tested in clinical trial settings. However, due to the low efficiency of traditional antigen infusion methods, inducing an effective immune effect with tumor vaccines has been very challenging (79). Therefore, the key of current research is to deliver an adequate concentration of antigens to DC for effective activation of antitumor immunity and preventing the degradation of antigen.
Suzuki et al. (23) delivered antigen into DC using an ultrasonic approach combined with foam liposomes, which was found to act similarly to ultrasonic MBs. The antigen passed through the transient pore produced by cavitation effects without entering the cytoplasm of DC through the endocytosis pathway. This delivery method directly enabled the model antigen (ovalbumin, OVA) to enter the major histocompatibility complex (MHC) class I presentation pathway and activated exogenous antigen-specific cytotoxic T lymphocytes. Further, Oda et al. (22) demonstrated that UTMD combined with immunotherapy could deliver tumor extracted antigen to DC and reduce the incidence of pulmonary metastasis of melanoma by four times. These indicate that bubble liposomes combined with ultrasound could be an effective method to transport antigen to DC. Additionally, studies have shown that immersion of nano-cavitated nuclei with model antigen (OVA) in hydrogel and exposure to ultrasound could significantly increase the transdermal delivery dose and enhance vaccine model antigen penetration, which was associated with highly-specific effects on anti-OVA IgG antibody levels in mice. These results indicate that ultrasound combined with nano cavitation nucleus has potential prospects in adjuvant percutaneous needle-free tumor vaccine vaccination (80). Meng et al. (81) designed an injectable self-healing hydrogel system loaded with nano-vaccines which could be converted into a sol state after ultrasonic treatment, allowing the release of the vaccine and then self-healing into a gel. Thus, multiple ultrasound treatments can repeatedly release nano-vaccines and produce effective antitumor immune responses, allowing one-time ultrasonic mediated inoculation and multiple effective treatments.
Monoclonal antibodies are among the most successful and important strategies for treating patients with hematological malignancies and solid tumors. Due to rapid developments in the field of immunology and protein engineering, monoclonal antibodies are currently the fastest-growing type of immunotherapy (82, 83). Monoclonal antibodies exert their tumor-killing effects via complement-mediated cytotoxicity and antibody-dependent cytotoxicity, while immune checkpoint inhibitors exert their antitumor effects by blocking immunosuppressive signals. In addition, antibody-coupled drugs can specifically bind to tumor surface antigens, releasing drugs that kill tumor cells and activate the immune system. UTMD can assist in the targeting and releasing of antibodies to target tissues, increase treatment efficiency and reduce systemic toxicity.
To investigate the therapeutic effect of UTMD-mediated chemotherapy drugs combined with monoclonal antibodies in multiple myeloma tumor stem cell transplantation mouse models, Shi et al. (84) developed lipoid MBs loaded with epirubicin and combined them with anti-ABCG2 monoclonal antibody. They found that, compared with no ultrasound irradiation, the combined approach could effectively inhibit the growth of multiple myeloma, prolong the survival time of mice, and alleviate the symptoms of multiple myeloma. In addition, the approach was more targeted than epirubicin therapy alone and was associated with reduced cardiac toxicity in mice models. Sun et al. (20) constructed an ultrasonic MB loaded with trastuzumab coupled with acoustic sensitizer nanoparticles. They found that the delivery and treatment efficiency with nanoparticles was improved with UTMD and successfully inhibited the proliferation of tumor cells, achieving a targeted combination of sonodynamic therapy and antibody therapy with nanoparticles for treating HER2-positive gastric cancer (Figure 4).
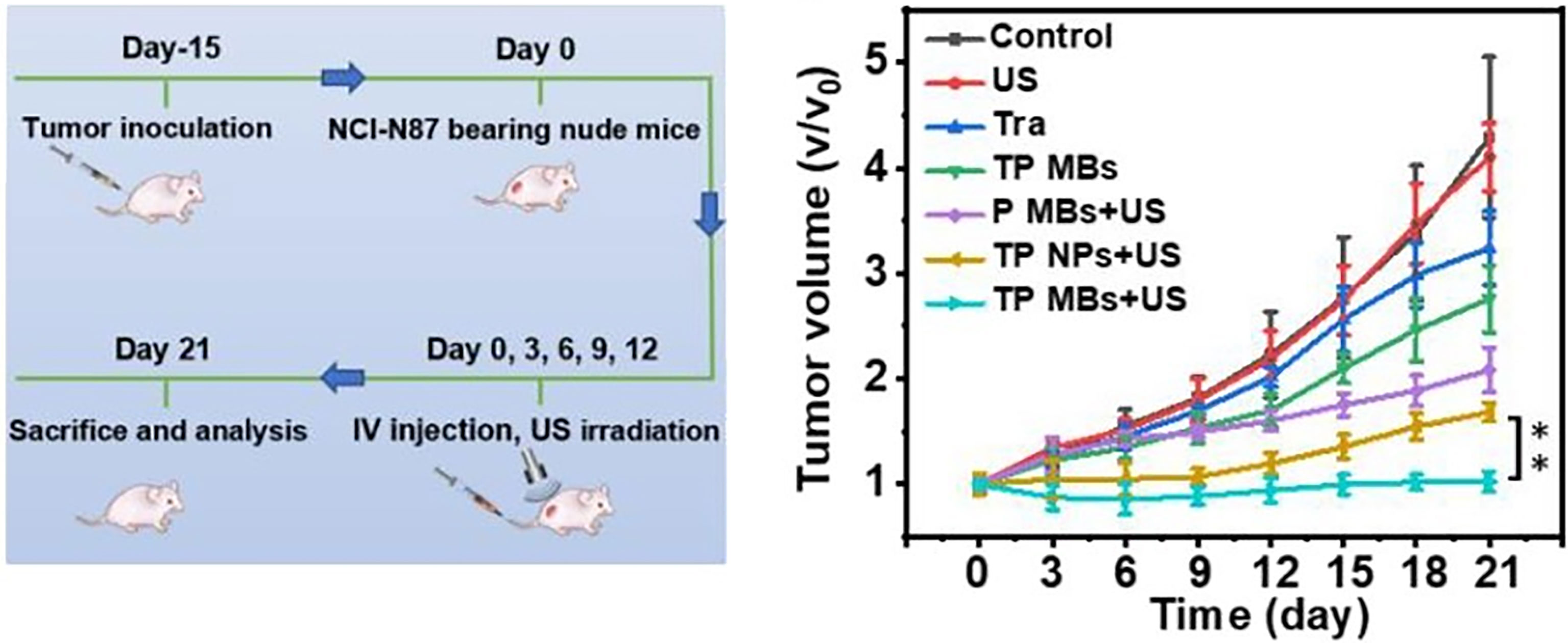
Figure 4 Ultrasound microbubbles mediated sonosensitizer and trastuzumab (TP MBs) treatment significantly inhibited the proliferation of tumor cells. The TP MBs + US group showed the best therapeutic effect with almost no tumor volume change for 21 days. Reprinted (adapted) with permission from ref (20). copyright © 2022 American Chemical Society. **p<0.01.
UTMD can increase antigen release, heat shock protein expression, calreticulin levels and pro-phagocytic signals, affecting the tumor microenvironment and comprehensively stimulating tumor immunity (85, 86). In a study by Ye et al. (21), the authors evaluated the ability of UTMD to enhance the targeted accumulation of aPD-L1 in the brain stem. They used UTMD to deliver the study drug to a brain of a mouse glioma model via the intranasal route. Anti-programmed cell death-ligand1 antibody (aPD-L1) was alternately dropped through the nostril, followed by MBs injected through the tail vein and an immediate head ultrasonic irradiation. Their results showed that, compared with intranasal administration alone, UTMD enhanced the targeted accumulation of aPD-L1 in the brain stem after intranasal administration and improved the penetration depth and transmission efficiency of aPD-L1 in the brain parenchyma. The accumulation rates in mice with and without tumor were similar, suggesting that the effect of UTMD-mediated intranasal brain drug delivery was not affected by the tumor microenvironment.
Gene therapy methods include viral vector transfection and non-viral transfection. Retrovirus and adenovirus transfection methods have systemic toxicity, insertion mutation and other problems (87, 88). In contrast, non-viral chemical and physical transfection methods seem safer, have lesser toxicity and are more specific than viral vector transfection methods (89). Thus, UTMD-mediated gene transfer is one of the most promising non-viral physical delivery methods.
The cavitation effect of ultrasound produces instantaneous pores on the cell membrane. Different ultrasonic peak negative pressures can create instantaneous pores of different sizes and mediate the entry of plasmids through these pores into cells (90–92). After ultrasonic irradiation, the membrane regains its integrity, seals all the pores, and traps the material delivered inside the cell (93). Plasmid DNA uptake induced by UTMD is a rapid, multi-mechanistic process that is not limited to the site of MB attachment (94). The continuity and fluidity of membrane lipid bilayer and the interaction between membrane and cytoskeleton may also be associated with plasmid DNA uptake (46). Due to low toxicity, low immunogenicity and high targeting efficiency, UTMD-mediated gene transfer has shown great application prospects in clinical gene therapy, and has been successfully applied in various tissues and organs, including muscles (95), kidneys (96), liver (97), parotid gland (98) and retina (99), and.
Zhang et al. (24) introduced plasmids into tumor cells using UTMD of different MB sizes. They observed that the transfection rate of the larger MBs (4.23 ± 2.27 μm) was significantly higher than those of smaller MBs (1.27 ± 0.89 μm), and 29.7% of the tumor cells were transfected into the DNA plasmid. Further, after 48 h, the gene expression in tumors using UTMD with larger MBs was more than tripled that of smaller MBs, and had greater infiltration of CD8 T cells and F4/80 macrophages. Dong et al. (25) investigated the effectiveness of in vivo UTMD (ultrasonic peak negative pressure: 5.5 MPa) delivery of pre-miRNAs plasmids, and observed that UTMD could effectively inhibit subcutaneous tumor growth in a mouse liver cancer model. They also found that the plasmid delivery efficiency and cell viability were positively correlated with peak negative ultrasound pressure. In a study by Ilovitsh et al. (26), the authors found that combining UTMD with intraperitoneal administration of checkpoint inhibition and IFN-β plasmid transfection could significantly reduce tumor volume and enhance T cell infiltration by recruiting effective local and distant tumor site immune cells.
Adoptive cell therapy is a passive immunotherapy method in which a large number of amplified and activated immune cells after in vitro genetic engineering or screening activation are transfused back into the patient to enhance immune responses in the tumor microenvironment and directly or indirectly achieve tumor-killing effects (100). Unlike T cells and B cells, natural killer (NK) cells can express high levels of effector molecules with cytotoxicity, including perforin and granase B, making NK cells the most widely used in adoptive immunotherapy (100, 101). However, the antitumor functions of NK cells in solid tumors are still unclear. The main reason is that the injection of NK cells cannot fully home at the tumor site, leading to a low number of NK cells targeting tumor cells and inadequate effective immune responses. Thus, improving the homing of NK cells at tumor sites could improve therapeutic outcomes (102).
Studies have shown that the stable cavitation effects produced by low-intensity focused ultrasound combined with MB therapy could promote the homing of various cells, including CD8+ cytotoxic T lymphocytes, dendritic cells, NK cells, neutrophils and macrophages, and could induce effective immune responses by disrupting the tight junctions of endothelial cells, increasing vesicular transport and changing ECs membrane proteins (103–105). Thus, UTMD has the potential to provide effective targeted delivery of adoptive cells to tumor lesions.
Alkins et al. (27) demonstrated that MRI-guided low-intensity focused ultrasound combined with MBs (peak sound pressure: 0.33 MPa) could target the implantation of NK-92 cells specifically expressing HER2 into the brain of nude mice before the BBB is destroyed, thereby increasing the high number of effector cells at the metastatic brain tumor site. Intravenous injection of HER2-specific NK-92-scFv (FRP5) zeta cell line, in early tumor developmental stages before BBB disruption, using MRI-guided focused-ultrasound combined with MB local irradiation to the tumor inhibited tumor growth in metastatic breast cancer model by amplifying HER2. This led to a significant reduction in the mean tumor volume, measured on the 28th day, and prolonged the survival of the mice. In a study by Yang et al. (102), the authors investigated the tumor-shrinking efficacy of UTMD combined with NK-92MI versus NK-92MI alone. They observed that although the addition of UTMD demonstrated an accumulation of adoptive NK-92MI cells from blood vessels to the tumor site, the difference in tumor volume reduction between the two groups was not statistically significant. The reason for such observation could be related to an insufficient number of NK cells entering the tumor, leading to low tumor-killing efficacy. Therefore, to improve the transfer efficiency of NK-92MI cells in the treatment of solid tumors, it is still necessary to further optimize the MB dose, ultrasound irradiation time and treatment frequency in future studies.
Sta Maria et al. (28) treated xenograft tumors of human colorectal adenocarcinoma mice with low dose focused ultrasound with MBs (peak sound pressure: 0.50 MPa) and injected MBs plus NK cells into the mouse tail vein. They observed that within 24 h of treatment, the aggregation of NK cells in the 0.5 MPa low-dose ultrasound group was significantly greater than in the non-low-dose ultrasound group, while no NK cell aggregation was observed in the 0.25 MPa low-dose ultrasound group. These observations suggest that sound pressure could be an important factor affecting the local homing of NK cells to tumor sites and the systemic effects of NK cells.
Immunotherapy has enormous potential in cancer treatment and has offered patients with advanced malignant tumors new and promising treatment options. The recent combined application of UTMD with tumor immunotherapy has shown great potential in amplifying immunotherapy outcomes as nanometer MB can extend the time for payload drug activities or gene foam half-life, thereby increasing the bioavailability, specificity, and specificity durability of immunotherapy to the tumor site, whilst decreasing systemic toxicity. However, the optimal dose of MB, time for ultrasonic irradiation, and treatment frequency are still undetermined and should be further explored. Different cancer types and individual genetic background need to be taken into account in UTMD combined immunotherapy (106). At present, UTMD-mediated tumor immunotherapy is mainly in an investigational stage in in vitro experiments. Many potential mechanisms of the biological effects of vascular ECs induced by UTMD have not yet been deeply explored, and further clarifications on their underlying mechanisms are still needed to better assist tumor immunotherapy.
In the future, the cross fusion between tumor immunotherapy and other therapeutic methods could further improve the outcomes of tumor immunotherapy. The prospects of combining new technologies and methods with immunotherapy to safely and effectively destroy tumor cells and ultimately achieve the goal of a non-toxic and lasting cure remain promising.
YH, HW, JH, and WL contributed to conception and design of the study. JS wrote the first draft of the manuscript. XW, YH, JH, and WL wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Pang B, Neefjes J. Coupled for Cross-Presentation in Tumor Immunotherapy. Sci Trans Med (2010) 2(44):44ps0. doi: 10.1126/scitranslmed.3001245
2. Yang Y. Cancer Immunotherapy: Harnessing the Immune System to Battle Cancer. J Clin Invest (2015) 125(9):3335–7. doi: 10.1172/JCI83871
3. Liu Q, Sun Z, Chen L. Memory T Cells: Strategies for Optimizing Tumor Immunotherapy. Protein Cell (2020) 11(8):549–64. doi: 10.1007/s13238-020-00707-9
4. Frankel T, Lanfranca M, Zou W. The Role of Tumor Microenvironment in Cancer Immunotherapy. Adv Exp Med Biol (2017) 1036:51–64. doi: 10.1007/978-3-319-67577-0_4
5. Wu H, Li H, Liu Y, Liang J, Liu Q, Xu Z, et al. Blockading a New NSCLC Immunosuppressive Target by Pluripotent Autologous Tumor Vaccines Magnifies Sequential Immunotherapy. Bioactive materials (2022) 13:223–38. doi: 10.1016/j.bioactmat.2021.10.048
6. Riley R, June C, Langer R, Mitchell M. Delivery Technologies for Cancer Immunotherapy. Nat Rev Drug discovery (2019) 18(3):175–96. doi: 10.1038/s41573-018-0006-z
7. Ding Y, Wang Y, Hu Q. Recent Advances in Overcoming Barriers to Cell-Based Delivery Systems for Cancer Immunotherapy. Exploration (2022) 00:20210106. doi: 10.1002/EXP.20210106
8. Ott P, Hu-Lieskovan S, Chmielowski B, Govindan R, Naing A, Bhardwaj N, et al. A Phase Ib Trial of Personalized Neoantigen Therapy Plus Anti-PD-1 in Patients With Advanced Melanoma, Non-Small Cell Lung Cancer, or Bladder Cancer. Cell (2020) 183(2):347–62.e24. doi: 10.1016/j.cell.2020.08.053
9. Hu Z, Ott P, Wu C. Towards Personalized, Tumour-Specific, Therapeutic Vaccines for Cancer. Nat Rev Immunol (2018) 18(3):168–82. doi: 10.1038/nri.2017.131
10. Chiang C, Kandalaft L, Tanyi J, Hagemann A, Motz G, Svoronos N, et al. A Dendritic Cell Vaccine Pulsed With Autologous Hypochlorous Acid-Oxidized Ovarian Cancer Lysate Primes Effective Broad Antitumor Immunity: From Bench to Bedside. Clin Cancer Res an Off J Am Assoc Cancer Res (2013) 19(17):4801–15. doi: 10.1158/1078-0432.CCR-13-1185
11. Li J, Wang Q, Nie Y, Xiao Y, Lin T, Han R, et al. A Multi-Element Expression Score Is A Prognostic Factor In Glioblastoma Multiforme. Cancer Manage Res (2019) 11:8977–89. doi: 10.2147/CMAR.S228174
12. Li N, Tang J, Yang J, Zhu B, Wang X, Luo Y, et al. Tumor Perfusion Enhancement by Ultrasound Stimulated Microbubbles Potentiates PD-L1 Blockade of MC38 Colon Cancer in Mice. Cancer letters (2021) 498:121–9. doi: 10.1016/j.canlet.2020.10.046
13. Sirsi S, Borden M. Advances in Ultrasound Mediated Gene Therapy Using Microbubble Contrast Agents. Theranostics (2012) 2(12):1208–22. doi: 10.7150/thno.4306
14. Karthikesh M, Yang X. The Effect of Ultrasound Cavitation on Endothelial Cells. Exp Biol Med (Maywood NJ). (2021) 246(7):758–70. doi: 10.1177/1535370220982301
15. Yin Y, Jiang X, Sun L, Li H, Su C, Zhang Y, et al. Continuous Inertial Cavitation Evokes Massive ROS for Reinforcing Sonodynamic Therapy and Immunogenic Cell Death Against Breast Carcinoma. Nano Today (2021) 36:101009. doi: 10.1016/j.nantod.2020.101009
16. Sun L, Cao Y, Lu Z, Ding P, Wang Z, Ma F, et al. A Hypoxia-Irrelevant Fe-Doped Multivalent Manganese Oxide Sonosensitizer via a Vacancy Engineering Strategy for Enhanced Sonodynamic Therapy. Nano Today (2022) 43:101434. doi: 10.1016/j.nantod.2022.101434
17. Liu Z, Zhang J, Tian Y, Zhang L, Han X, Wang Q, et al. Targeted Delivery of Reduced Graphene Oxide Nanosheets Using Multifunctional Ultrasound Nanobubbles for Visualization and Enhanced Photothermal Therapy. Int J nanomedicine (2018) 13:7859–72. doi: 10.2147/IJN.S181268
18. Hu J, He J, Wang Y, Zhao Y, Fang K, Dong Y, et al. Ultrasound Combined With Nanobubbles Promotes Systemic Anticancer Immunity and Augments Anti-PD1 Efficacy. J immunother Cancer (2022) 10(3):e003408. doi: 10.1136/jitc-2021-003408
19. Park E, Zhang Y, Vykhodtseva N, McDannold N. Ultrasound-Mediated Blood-Brain/Blood-Tumor Barrier Disruption Improves Outcomes With Trastuzumab in a Breast Cancer Brain Metastasis Model. J Controlled release (2012) 163(3):277–84. doi: 10.1016/j.jconrel.2012.09.007
20. Sun L, Zhang J, Xu M, Zhang L, Tang Q, Chen J, et al. Ultrasound Microbubbles Mediated Sonosensitizer and Antibody Co-Delivery for Highly Efficient Synergistic Therapy on HER2-Positive Gastric Cancer. ACS Appl materials interfaces (2022) 14(1):452–63. doi: 10.1021/acsami.1c21924
21. Ye D, Yuan J, Yue Y, Rubin J, Chen H. Focused Ultrasound-Enhanced Delivery of Intranasally Administered Anti-Programmed Cell Death-Ligand 1 Antibody to an Intracranial Murine Glioma Model. Pharmaceutics (2021) 13(2):190. doi: 10.3390/pharmaceutics13020190
22. Oda Y, Suzuki R, Otake S, Nishiie N, Hirata K, Koshima R, et al. Prophylactic Immunization With Bubble Liposomes and Ultrasound-Treated Dendritic Cells Provided a Four-Fold Decrease in the Frequency of Melanoma Lung Metastasis. J Controlled release (2012) 160(2):362–6. doi: 10.1016/j.jconrel.2011.12.003
23. Suzuki R, Oda Y, Utoguchi N, Namai E, Taira Y, Okada N, et al. A Novel Strategy Utilizing Ultrasound for Antigen Delivery in Dendritic Cell-Based Cancer Immunotherapy. J Controlled release (2009) 133(3):198–205. doi: 10.1016/j.jconrel.2008.10.015
24. Zhang N, Foiret J, Kheirolomoom A, Liu P, Feng Y, Tumbale S, et al. Optimization of Microbubble-Based DNA Vaccination With Low-Frequency Ultrasound for Enhanced Cancer Immunotherapy. Advanced Ther (2021) 4(9):2100033. doi: 10.1002/adtp.202100033
25. Dong W, Wu P, Zhou D, Huang J, Qin M, Yang X, et al. Ultrasound-Mediated Gene Therapy of Hepatocellular Carcinoma Using Pre-microRNA Plasmid-Loaded Nanodroplets. Ultrasound Med Biol (2020) 46(1):90–107. doi: 10.1016/j.ultrasmedbio.2019.09.016
26. Ilovitsh T, Feng Y, Foiret J, Kheirolomoom A, Zhang H, Ingham E, et al. Low-Frequency Ultrasound-Mediated Cytokine Transfection Enhances T Cell Recruitment at Local and Distant Tumor Sites. Proc Natl Acad Sci United States America (2020) 117(23):12674–85. doi: 10.1073/pnas.1914906117
27. Alkins R, Burgess A, Kerbel R, Wels W, Hynynen K. Early Treatment of HER2-Amplified Brain Tumors With Targeted NK-92 Cells and Focused Ultrasound Improves Survival. Neuro-oncology (2016) 18(7):974–81. doi: 10.1093/neuonc/nov318
28. Sta Maria N, Barnes S, Weist M, Colcher D, Raubitschek A, Jacobs R. Low Dose Focused Ultrasound Induces Enhanced Tumor Accumulation of Natural Killer Cells. PLoS One (2015) 10(11):e0142767. doi: 10.1371/journal.pone.0142767
29. Tang H, Qiao J, Fu Y. Immunotherapy and Tumor Microenvironment. Cancer letters (2016) 370(1):85–90. doi: 10.1016/j.canlet.2015.10.009
30. Ferrara N, Gerber H, LeCouter J. The Biology of VEGF and its Receptors. Nat Med (2003) 9(6):669–76. doi: 10.1038/nm0603-669
31. Schaaf M, Garg A, Agostinis P. Defining the Role of the Tumor Vasculature in Antitumor Immunity and Immunotherapy. Cell Death disease (2018) 9(2):115. doi: 10.1038/s41419-017-0061-0
32. Ma J, Chen C, Blute T, Waxman D. Antiangiogenesis Enhances Intratumoral Drug Retention. Cancer Res (2011) 71(7):2675–85. doi: 10.1158/0008-5472.CAN-10-3242
33. Pei J, Zhang C, Yusupu M, Zhang C, Dai D. Screening and Validation of the Hypoxia-Related Signature of Evaluating Tumor Immune Microenvironment and Predicting Prognosis in Gastric Cancer. Front Immunol (2021) 12:705511. doi: 10.3389/fimmu.2021.705511
34. Wang T, Choe J, Pu K, Devulapally R, Bachawal S, Machtaler S, et al. Ultrasound-Guided Delivery of microRNA Loaded Nanoparticles Into Cancer. J Controlled release (2015) 203:99–108. doi: 10.1016/j.jconrel.2015.02.018
35. Qiu Y, Luo Y, Zhang Y, Cui W, Zhang D, Wu J, et al. The Correlation Between Acoustic Cavitation and Sonoporation Involved in Ultrasound-Mediated DNA Transfection With Polyethylenimine (PEI) In Vitro. J Controlled release (2010) 145(1):40–8. doi: 10.1016/j.jconrel.2010.04.010
36. Bazan-Peregrino M, Rifai B, Carlisle R, Choi J, Arvanitis C, Seymour L, et al. Cavitation-Enhanced Delivery of a Replicating Oncolytic Adenovirus to Tumors Using Focused Ultrasound. J Controlled release (2013) 169:40–7. doi: 10.1016/j.jconrel.2013.03.017
37. Wu P, Wang X, Lin W, Bai L. Acoustic Characterization of Cavitation Intensity: A Review. Ultrasonics sonochem (2022) 82:105878. doi: 10.1016/j.ultsonch.2021.105878
38. Ashokkumar M. The Characterization of Acoustic Cavitation Bubbles - an Overview. Ultrasonics sonochem (2011) 18(4):864–72. doi: 10.1016/j.ultsonch.2010.11.016
39. Ji Q, Yu X, Yagoub A, Chen L, Fakayode O, Zhou C. Synergism of Sweeping Frequency Ultrasound and Deep Eutectic Solvents Pretreatment for Fractionation of Sugarcane Bagasse and Enhancing Enzymatic Hydrolysis. Ultrasonics sonochem (2021) 73:105470. doi: 10.1016/j.ultsonch.2021.105470
40. Zheng Y, Ye J, Li Z, Chen H, Gao Y. Recent Progress in Sono-Photodynamic Cancer Therapy: From Developed New Sensitizers to Nanotechnology-Based Efficacy-Enhancing Strategies. Acta Pharm Sin B (2021) 11(8):2197–219. doi: 10.1016/j.apsb.2020.12.016
41. Manzano M, Vallet-Regí M. Ultrasound Responsive Mesoporous Silica Nanoparticles for Biomedical Applications. Chem Commun (Cambridge England) (2019) 55(19):2731–40. doi: 10.1039/C8CC09389J
42. Husseini G, Diaz de la Rosa M, Richardson E, Christensen D, Pitt W. The Role of Cavitation in Acoustically Activated Drug Delivery. J Controlled release (2005) 107(2):253–61. doi: 10.1016/j.jconrel.2005.06.015
43. Mitragotri S. Healing Sound: The Use of Ultrasound in Drug Delivery and Other Therapeutic Applications. Nat Rev Drug discovery (2005) 4(3):255–60. doi: 10.1038/nrd1662
44. Miller D, Averkiou M, Brayman A, Everbach E, Holland C, Wible J, et al. Bioeffects Considerations for Diagnostic Ultrasound Contrast Agents. J ultrasound Med Off J Am Inst Ultrasound Med (2008) 27(4):611–32. doi: 10.7863/jum.2008.27.4.611
45. Thomas E, Menon J, Owen J, Skaripa-Koukelli I, Wallington S, Gray M, et al. Ultrasound-Mediated Cavitation Enhances the Delivery of an EGFR-Targeting Liposomal Formulation Designed for Chemo-Radionuclide Therapy. Theranostics (2019) 9(19):5595–609. doi: 10.7150/thno.34669
46. Rong N, Zhou H, Liu R, Wang Y, Fan Z. Ultrasound and Microbubble Mediated Plasmid DNA Uptake: A Fast, Global and Multi-Mechanisms Involved Process. J Controlled release (2018) 273:40–50. doi: 10.1016/j.jconrel.2018.01.014
47. Yang Q, Tang P, He G, Ge S, Liu L, Zhou X. Hemocoagulase Combined With Microbubble-Enhanced Ultrasound Cavitation for Augmented Ablation of Microvasculature in Rabbit VX2 Liver Tumors. Ultrasound Med Biol (2017) 43(8):1658–70. doi: 10.1016/j.ultrasmedbio.2017.03.013
48. Zhao Y, Lin Q, Wong H, Shen X, Yang W, Xu H, et al. Glioma-Targeted Therapy Using Cilengitide Nanoparticles Combined With UTMD Enhanced Delivery. J Controlled release (2016) 224:112–25. doi: 10.1016/j.jconrel.2016.01.015
49. Abrahao A, Meng Y, Llinas M, Huang Y, Hamani C, Mainprize T, et al. First-In-Human Trial of Blood-Brain Barrier Opening in Amyotrophic Lateral Sclerosis Using MR-Guided Focused Ultrasound. Nat Commun (2019) 10(1):4373. doi: 10.1038/s41467-019-12426-9
50. Downs M, Buch A, Sierra C, Karakatsani M, Teichert T, Chen S, et al. Long-Term Safety of Repeated Blood-Brain Barrier Opening via Focused Ultrasound With Microbubbles in Non-Human Primates Performing a Cognitive Task. PLoS One (2015) 10(5):e0125911. doi: 10.1371/journal.pone.0125911
51. Samuel S, Cooper M, Bull J, Fowlkes J, Miller D. An Ex Vivo Study of the Correlation Between Acoustic Emission and Microvascular Damage. Ultrasound Med Biol (2009) 35(9):1574–86. doi: 10.1016/j.ultrasmedbio.2009.04.013
52. Hwang J, Tu J, Brayman A, Matula T, Crum L. Correlation Between Inertial Cavitation Dose and Endothelial Cell Damage In Vivo. Ultrasound Med Biol (2006) 32(10):1611–9. doi: 10.1016/j.ultrasmedbio.2006.07.016
53. Wang J, Zhao Z, Shen S, Zhang C, Guo S, Lu Y, et al. Selective Depletion of Tumor Neovasculature by Microbubble Destruction With Appropriate Ultrasound Pressure. Int J cancer (2015) 137(10):2478–91. doi: 10.1002/ijc.29597
54. Liu Z, Gao S, Zhao Y, Li P, Liu J, Li P, et al. Disruption of Tumor Neovasculature by Microbubble Enhanced Ultrasound: A Potential New Physical Therapy of Anti-Angiogenesis. Ultrasound Med Biol (2012) 38(2):253–61. doi: 10.1016/j.ultrasmedbio.2011.11.007
55. Jing Y, Xiu-Juan Z, Hong-Jiao C, Zhi-Kui C, Qing-Fu Q, En-Sheng X, et al. Ultrasound-Targeted Microbubble Destruction Improved the Antiangiogenic Effect of Endostar in Triple-Negative Breast Carcinoma Xenografts. J Cancer Res Clin Oncol (2019) 145(5):1191–200. doi: 10.1007/s00432-019-02866-7
56. Yu Y, Qiao W, Feng S, Yi C, Liu Z. Inhibition of Walker-256 Tumor Growth by Combining Microbubble-Enhanced Ultrasound and Endostar. J ultrasound Med (2022). doi: 10.1002/jum.15949
57. Su Z, Xu T, Wang Y, Guo X, Tu J, Zhang D, et al. Low−intensity Pulsed Ultrasound Promotes Apoptosis and Inhibits Angiogenesis via P38 Signaling−Mediated Endoplasmic Reticulum Stress in Human Endothelial Cells. Mol Med Rep (2019) 19(6):4645–54. doi: 10.3892/mmr.2019.10136
58. Hwang J, Brayman A, Reidy M, Matula T, Kimmey M, Crum L. Vascular Effects Induced by Combined 1-MHz Ultrasound and Microbubble Contrast Agent Treatments In Vivo. Ultrasound Med Biol (2005) 31(4):553–64. doi: 10.1016/j.ultrasmedbio.2004.12.014
59. Hallow D, Mahajan A, Prausnitz M. Ultrasonically Targeted Delivery Into Endothelial and Smooth Muscle Cells in Ex Vivo Arteries. J Controlled release (2007) 118(3):285–93. doi: 10.1016/j.jconrel.2006.12.029
60. Lelu S, Afadzi M, Berg S, Aslund A, Torp S, Sattler W, et al. Primary Porcine Brain Endothelial Cells as In Vitro Model to Study Effects of Ultrasound and Microbubbles on Blood-Brain Barrier Function. IEEE Trans ultrasonics ferroelectrics frequency control (2017) 64(1):281–90. doi: 10.1109/TUFFC.2016.2597004
61. Wang F, Dong L, Wei X, Wang Y, Chang L, Wu H, et al. Effect of Gambogic Acid-Loaded Porous-Lipid/PLGA Microbubbles in Combination With Ultrasound-Triggered Microbubble Destruction on Human Glioma. Front bioeng Biotechnol (2021) 9:711787. doi: 10.3389/fbioe.2021.711787
62. Zhang L, Sun L, Tang Q, Sun S, Zeng L, Ma J, et al. Viacascade Drug Delivery Through Tumor Barriers of Pancreatic Cancer Ultrasound in Combination With Functional Microbubbles. ACS biomaterials Sci eng (2022) 8(4):1583–95. doi: 10.1021/acsbiomaterials.2c00069
63. Zhang J, Liu H, Du X, Guo Y, Chen X, Wang S, et al. Increasing of Blood-Brain Tumor Barrier Permeability Through Transcellular and Paracellular Pathways by Microbubble-Enhanced Diagnostic Ultrasound in a C6 Glioma Model. Front Neurosci (2017) 11:86. doi: 10.3389/fnins.2017.00086
64. Wang Z, Chen C, Tao Y, Zou P, Gao F, Jia C, et al. Ultrasound-Induced Microbubble Cavitation Combined With miR-34a-Loaded Nanoparticles for the Treatment of Castration-Resistant Prostate Cancer. J Biomed nanotechnol (2021) 17(1):78–89. doi: 10.1166/jbn.2021.3020
65. Zhu L, Ren S, Daniels M, Qiu W, Song L, You T, et al. Exogenous HMGB1 Promotes the Proliferation and Metastasis of Pancreatic Cancer Cells. Front Med (2021) 8:756988. doi: 10.3389/fmed.2021.756988
66. Wang L, Li G, Cao L, Dong Y, Wang Y, Wang S, et al. An Ultrasound-Driven Immune-Boosting Molecular Machine for Systemic Tumor Suppression. Sci Adv (2021) 7(43):eabj4796. doi: 10.1126/sciadv.abj4796
67. Han H, Desert R, Das S, Song Z, Athavale D, Ge X, et al. Danger Signals in Liver Injury and Restoration of Homeostasis. J hepatol (2020) 73(4):933–51. doi: 10.1016/j.jhep.2020.04.033
68. Cai R, Wang Q, Zhu G, Zhu L, Tao Z. Increased Expression of Caspase 1 During Active Phase of Connective Tissue Disease. PeerJ (2019) 7:e7321. doi: 10.7717/peerj.7321
69. de Souza A, Gonçalves L, Lepique A, de Araujo-Souza P. The Role of Autophagy in Tumor Immunology-Complex Mechanisms That May Be Explored Therapeutically. Front Oncol (2020) 10:603661. doi: 10.3389/fonc.2020.603661
70. Leybaert L, Sanderson M. Intercellular Ca(2+) Waves: Mechanisms and Function. Physiol Rev (2012) 92(3):1359–92. doi: 10.1152/physrev.00029.2011
71. Beekers I, Mastik F, Beurskens R, Tang P, Vegter M, van der Steen A, et al. High-Resolution Imaging of Intracellular Calcium Fluctuations Caused by Oscillating Microbubbles. Ultrasound Med Biol (2020) 46(8):2017–29. doi: 10.1016/j.ultrasmedbio.2020.03.029
72. Konofagou E. Optimization of the Ultrasound-Induced Blood-Brain Barrier Opening. Theranostics (2012) 2(12):1223–37. doi: 10.7150/thno.5576
73. Honda H, Kondo T, Zhao Q, Feril L, Kitagawa H. Role of Intracellular Calcium Ions and Reactive Oxygen Species in Apoptosis Induced by Ultrasound. Ultrasound Med Biol (2004) 30(5):683–92. doi: 10.1016/j.ultrasmedbio.2004.02.008
74. Shi M, Liu B, Liu G, Wang P, Yang M, Li Y, et al. Low Intensity-Pulsed Ultrasound Induced Apoptosis of Human Hepatocellular Carcinoma Cells In Vitro. Ultrasonics (2016) 64:43–53. doi: 10.1016/j.ultras.2015.07.011
75. Al-Mahrouki A, Giles A, Hashim A, Kim H, El-Falou A, Rowe-Magnus D, et al. Microbubble-Based Enhancement of Radiation Effect: Role of Cell Membrane Ceramide Metabolism. PLoS One (2017) 12(7):e0181951. doi: 10.1371/journal.pone.0181951
76. Song Q, Zhang C, Wu X. Therapeutic Cancer Vaccines: From Initial Findings to Prospects. Immunol letters (2018) 196:11–21. doi: 10.1016/j.imlet.2018.01.011
77. McNamara M, Nair S, Holl E. RNA-Based Vaccines in Cancer Immunotherapy. J Immunol Res (2015) 2015:794528. doi: 10.1155/2015/794528
78. Cheever M, Higano C. PROVENGE (Sipuleucel-T) in Prostate Cancer: The First FDA-Approved Therapeutic Cancer Vaccine. Clin Cancer Res (2011) 17(11):3520–6. doi: 10.1158/1078-0432.CCR-10-3126
79. Sakurai F, Inoue R, Nishino Y, Okuda A, Matsumoto O, Taga T, et al. Effect of DNA/liposome Mixing Ratio on the Physicochemical Characteristics, Cellular Uptake and Intracellular Trafficking of Plasmid DNA/cationic Liposome Complexes and Subsequent Gene Expression. J Controlled release (2000) 66:255–69. doi: 10.1016/S0168-3659(99)00280-1
80. Bhatnagar S, Kwan J, Shah A, Coussios C, Carlisle R. Exploitation of Sub-Micron Cavitation Nuclei to Enhance Ultrasound-Mediated Transdermal Transport and Penetration of Vaccines. J Controlled release (2016) 238:22–30. doi: 10.1016/j.jconrel.2016.07.016
81. Meng Z, Zhang Y, She J, Zhou X, Xu J, Han X, et al. Ultrasound-Mediated Remotely Controlled Nanovaccine Delivery for Tumor Vaccination and Individualized Cancer Immunotherapy. Nano letters (2021) 21(3):1228–37. doi: 10.1021/acs.nanolett.0c03646
82. Briani C, Visentin A. Therapeutic Monoclonal Antibody Therapies in Chronic Autoimmune Demyelinating Neuropathies. Neurotherapeutics (2022). doi: 10.1007/s13311-022-01222-x
83. Isazadeh A, Hajazimian S, Garshasbi H, Shadman B, Baghbanzadeh A, Chavoshi R, et al. Resistance Mechanisms to Immune Checkpoints Blockade by Monoclonal Antibody Drugs in Cancer Immunotherapy: Focus on Myeloma. J Cell Physiol (2021) 236(2):791–805. doi: 10.1002/jcp.29905
84. Shi F, Li M, Wu S, Yang F, Di W, Pan M, et al. Enhancing the Anti-Multiple Myeloma Efficiency in a Cancer Stem Cell Xenograft Model by Conjugating the ABCG2 Antibody With Microbubbles for a Targeted Delivery of Ultrasound Mediated Epirubicin. Biochem Pharmacol (2017) 132:18–28. doi: 10.1016/j.bcp.2017.02.014
85. de Smet M, Hijnen N, Langereis S, Elevelt A, Heijman E, Dubois L, et al. Magnetic Resonance Guided High-Intensity Focused Ultrasound Mediated Hyperthermia Improves the Intratumoral Distribution of Temperature-Sensitive Liposomal Doxorubicin. Invest radiol (2013) 48(6):395–405. doi: 10.1097/RLI.0b013e3182806940
86. Formenti S, Demaria S. Combining Radiotherapy and Cancer Immunotherapy: A Paradigm Shift. J Natl Cancer Institute (2013) 105(4):256–65. doi: 10.1093/jnci/djs629
87. Hudry E, Vandenberghe L. Therapeutic AAV Gene Transfer to the Nervous System: A Clinical Reality. Neuron (2019) 101(5):839–62. doi: 10.1016/j.neuron.2019.02.017
88. Manno C, Pierce G, Arruda V, Glader B, Ragni M, Rasko J, et al. Successful Transduction of Liver in Hemophilia by AAV-Factor IX and Limitations Imposed by the Host Immune Response. Nat Med (2006) 12(3):342–7. doi: 10.1038/nm1358
89. Delalande A, Kotopoulis S, Postema M, Midoux P, Pichon C. Sonoporation: Mechanistic Insights and Ongoing Challenges for Gene Transfer. Gene (2013) 525(2):191–9. doi: 10.1016/j.gene.2013.03.095
90. Shapiro G, Wong A, Bez M, Yang F, Tam S, Even L, et al. Multiparameter Evaluation of In Vivo Gene Delivery Using Ultrasound-Guided, Microbubble-Enhanced Sonoporation. J Controlled release (2016) 223:157–64. doi: 10.1016/j.jconrel.2015.12.001
91. Lentacker I, De Cock I, Deckers R, De Smedt S, Moonen C. Understanding Ultrasound Induced Sonoporation: Definitions and Underlying Mechanisms. Advanced Drug delivery Rev (2014) 72:49–64. doi: 10.1016/j.addr.2013.11.008
92. van Wamel A, Kooiman K, Harteveld M, Emmer M, ten Cate F, Versluis M, et al. Vibrating Microbubbles Poking Individual Cells: Drug Transfer Into Cells via Sonoporation. J Controlled release (2006) 112(2):149–55. doi: 10.1016/j.jconrel.2006.02.007
93. Zhao Y, Luo Y, Lu C, Xu J, Tang J, Zhang M, et al. Phospholipids-Based Microbubbles Sonoporation Pore Size and Reseal of Cell Membrane Cultured In Vitro. J Drug Targeting (2008) 16(1):18–25. doi: 10.1080/10611860701637792
94. Sun M, Northup N, Marga F, Huber T, Byfield F, Levitan I, et al. The Effect of Cellular Cholesterol on Membrane-Cytoskeleton Adhesion. J Cell science (2007) 120:2223–31. doi: 10.1242/jcs.001370
95. Li Y, Wang Y, Wang J, Chong K, Xu J, Liu Z, et al. Expression of Neprilysin in Skeletal Muscle by Ultrasound-Mediated Gene Transfer (Sonoporation) Reduces Amyloid Burden for AD. Mol Ther Methods Clin Dev (2020) 17:300–8. doi: 10.1016/j.omtm.2019.12.012
96. Huang S, Ren Y, Wang X, Lazar L, Ma S, Weng G, et al. Application of Ultrasound-Targeted Microbubble Destruction-Mediated Exogenous Gene Transfer in Treating Various Renal Diseases. Hum Gene Ther (2019) 30(2):127–38. doi: 10.1089/hum.2018.070
97. Xie A, Wu M, Cigarroa G, Belcik J, Ammi A, Moccetti F, et al. Influence of DNA-Microbubble Coupling on Contrast Ultrasound-Mediated Gene Transfection in Muscle and Liver. J Am Soc Echocardiogr (2016) 29(8):812–8. doi: 10.1016/j.echo.2016.04.011
98. Wang Z, Zourelias L, Wu C, Edwards P, Trombetta M, Passineau M. Ultrasound-Assisted Nonviral Gene Transfer of AQP1 to the Irradiated Minipig Parotid Gland Restores Fluid Secretion. Gene Ther (2015) 22(9):739–49. doi: 10.1038/gt.2015.36
99. Li H, Qian J, Yao C, Wan C, Li F. Combined Ultrasound-Targeted Microbubble Destruction and Polyethylenimine-Mediated Plasmid DNA Delivery to the Rat Retina: Enhanced Efficiency and Accelerated Expression. J Gene Med (2016) 18:47–56. doi: 10.1002/jgm.2875
100. Saetersmoen M, Hammer Q, Valamehr B, Kaufman D, Malmberg K. Off-The-Shelf Cell Therapy With Induced Pluripotent Stem Cell-Derived Natural Killer Cells. Semin immunopathol (2019) 41(1):59–68. doi: 10.1007/s00281-018-0721-x
101. Li X, Liu M, Zhao J, Ren T, Yan X, Zhang L, et al. Research Progress About Glioma Stem Cells in the Immune Microenvironment of Glioma. Front Pharmacol (2021) 12:750857. doi: 10.3389/fphar.2021.750857
102. Yang C, Du M, Yan F, Chen Z. Focused Ultrasound Improves NK-92mi Cells Infiltration Into Tumors. Front Pharmacol (2019) 10:326. doi: 10.3389/fphar.2019.00326
103. Chen P, Liu H, Hua M, Yang H, Huang C, Chu P, et al. Novel Magnetic/Ultrasound Focusing System Enhances Nanoparticle Drug Delivery for Glioma Treatment. Neuro-oncology (2010) 12(10):1050–60. doi: 10.1093/neuonc/noq054
104. Kinoshita M, McDannold N, Jolesz F, Hynynen K. Noninvasive Localized Delivery of Herceptin to the Mouse Brain by MRI-Guided Focused Ultrasound-Induced Blood-Brain Barrier Disruption. Proc Natl Acad Sci United States America (2006) 103(31):11719–23. doi: 10.1073/pnas.0604318103
105. Watson K, Lai C, Qin S, Kruse D, Lin Y, Seo J, et al. Ultrasound Increases Nanoparticle Delivery by Reducing Intratumoral Pressure and Increasing Transport in Epithelial and Epithelial-Mesenchymal Transition Tumors. Cancer Res (2012) 72(6):1485–93. doi: 10.1158/0008-5472.CAN-11-3232
Keywords: ultrasound-targeted microbubble destruction, tumor microenvironment, tumor angiogenesis, ultrasonic cavitation, tumor immunotherapy, endothelial cells
Citation: Han Y, Sun J, Wei H, Hao J, Liu W and Wang X (2022) Ultrasound-Targeted Microbubble Destruction: Modulation in the Tumor Microenvironment and Application in Tumor Immunotherapy. Front. Immunol. 13:937344. doi: 10.3389/fimmu.2022.937344
Received: 06 May 2022; Accepted: 27 May 2022;
Published: 01 July 2022.
Edited by:
Xi Cheng, Shanghai Jiao Tong University, ChinaReviewed by:
Wang Zheng, Suzhou Institute of Nano-tech and Nano-bionics (CAS), ChinaCopyright © 2022 Han, Sun, Wei, Hao, Liu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaolei Wang, d3hsZ2hiQDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.