
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 29 August 2022
Sec. Nutritional Immunology
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.934617
This article is part of the Research Topic Effects of Alcohol Use on Immunity and Immune Responses View all 12 articles
Alcohol use is known to alter the function of both innate and adaptive immune cells, such as neutrophils, macrophages, B cells, and T cells. Immune dysfunction has been associated with alcohol-induced end-organ damage. The role of innate lymphocytes in alcohol-associated pathogenesis has become a focus of research, as liver-resident natural killer (NK) cells were found to play an important role in alcohol-associated liver damage pathogenesis. Innate lymphocytes play a critical role in immunity and homeostasis; they are necessary for an optimal host response against insults including infections and cancer. However, the role of innate lymphocytes, including NK cells, natural killer T (NKT) cells, mucosal associated invariant T (MAIT) cells, gamma delta T cells, and innate lymphoid cells (ILCs) type 1–3, remains ill-defined in the context of alcohol-induced end-organ damage. Innate-like B lymphocytes including marginal zone B cells and B-1 cells have also been identified; however, this review will address the effects of alcohol misuse on innate T lymphocytes, as well as the consequences of innate T-lymphocyte dysfunction on alcohol-induced tissue damage.
A complete immune response requires optimal and timely responses from both tissue-resident and circulating immune cell populations. Innate immune cells are often found in peripheral tissues and respond to various infectious challenges, cancers, and allergens through the expression of toll-like receptors (TLRs) and limit tissue injury via the production of a wide variety of TLR-dependent effectors. However, there is a growing appreciation and understanding of the scope and diversity of tissue-resident lymphocytes in peripheral organs, which suggests that myeloid cells may not be the primary, or only, immune response prior to the initiation of classical adaptive immunity (1). Innate lymphocytes are broken into two distinct groups: innate lymphoid cells (ILCs) and innate-like T lymphocytes. Innate lymphocytes have been shown to mediate normal host immune responses to various infectious challenges, cancers, and allergens, as well as provide immune regulatory and modulatory effects. Currently, ILCs are classified into five subsets within three major groups: natural killer (NK) cells, ILC1 (group 1), ILC2 (group 2), and ILC3 and LTi cells (group 3) (1). Innate-like T lymphocytes or unconventional T cells are typically classified as gamma delta (γδ) T cells, mucosal associated invariant T cells (MAIT), natural killer T cells (NKT), and invariant natural killer T cells (iNKT) and, similar to ILCs, mediate both immune responses and homeostasis (1). This review will address our current knowledge regarding the effects of alcohol misuse on these innate and innate-like lymphocytes (Figure 1), as well as the consequences of innate lymphocyte dysfunction on alcohol-induced end-organ damage.
ILCs utilize a variety of germline-encoded activating and inhibitory receptors, as opposed to conventional lymphocytes which express rearranged antigen receptors (2). ILCs are primarily located at epithelial barrier surfaces (i.e., intestine, lung, and skin) but can also be identified in lymphoid and other non-lymphoid tissues (3). ILCs are often classified based on their expression of transcription factors, cell surface markers, cytokines, and effector molecules. Following tissue injury due to infection or inflammation, as well as perturbation to the intestinal commensal microbiota, ILCs produce both proinflammatory and regulatory cytokines to combat the tissue insult (1, 3, 4). Nearly every organ has associated tissue-specific ILCs, which attests to their ability to support many critical functions necessary for immune homeostasis.
NK cells and type 1 ILCs (ILC1), which are defined based on the secretion of interferon (IFN)-γ, are the prototypical group 1 ILC. Group 1 ILCs are highly responsive to interleukin IL-15, IL-18, and IL-12 and are typically characterized by the expression of the surface receptors NKp46 and NK1.1 (mice) or CD56 (humans) (5). ILC1s are often considered to be more tissue specific/resident than NK cells and express higher levels of CD103, CD49a, and CD69, all of which are considered markers of tissue residency (1). Conversely, NK cells typically express surface markers that facilitate circulation, such as CCR7, S1PR, and CD62L (6). Likewise, NK cells are more cytolytic than ILC1s, as they have a higher expression of both perforin and granzymes (7). However, ILC1s also have the potential to be cytolytic via the production of the tumor necrosis factor-related apoptosis-inducing ligand. Innate lymphoid cell precursor (ILCP) differentiation into ILC1 requires the transcription factors T-bet and Hobit (8, 9); conversely, differentiation of precursor NK cells into mature NK cells is dependent on the transcription factors Eomes and T-bet (10). While these transcription factors are widely accepted for cellular development in rodents, the transcriptional profile for human ILC1s and NK-cell development is less defined. Eome expression is found in intestinal intraepithelial ILC1s (11) but, until recently, was not believed to contribute to liver-resident NK cells (12). Interestingly, however, it has been shown in humans that a liver-resident Eomeshi NK-cell population does exist (13). In fact, it appears that Eomes expression in humans is a factor for NK retention. Cuff et al. examined liver transplants from donors which were HLA mismatched (HLA-A2 or HLA-A3 mismatches). This allowed them to distinguish between donor liver–derived and recipient-derived leucocytes via antibody staining for the specific donor-recipient HLA mismatch. They found that Eomeslo NK cells circulate freely whereas Eomeshi NK cells were only observed in the liver and not found in blood samples. Cuff et al. went to further establish that liver NK-cell replenishment from the circulation can occur, possibly via Eomeslo NK cells being induced to upregulate Eomes expression. These data suggest that Eomeshi expression may be a characteristic of mature liver NK cells; however, the role of Eomes expression in NK development remains clouded. For example, some authors have described Eomes expression as part of NK-cell development but have also argued that the Eomeshi state is associated with immature NK cells. They argue instead that mature NK cells are more associated with an abundance of T-bet (14). Whatever the case may be, NK cells and ILC1s are best known for their critical role in the normal immune responses to viral infection through secretion of IFN-γ.
The secretion of the classical type-2 cytokines amphiregulin, IL-13, IL-9, and IL-5 in response to IL-33, IL-25, and TSLP secreted by parenchymal cells is one key defining feature of group 2 ILCs (3, 4, 15). ILC2s are also classically defined by the expression of CRTH2, KLRG1, ST2, and CD25 (16, 17). Interestingly, the expression of CD44 and CD161 on ILC2 seems to differ between mice and humans, as mouse ILC2s are CD44+ CD161-, while human ILC2s are CD44- CD161+ (18). Differentiation from ILCPs into mature ILC2s depends on the transcription factors GATA3 (also required for effector function), RORα, and TCF-1 (19–23). Recently, ILC2s have been sub-characterized via their ability to respond to IL-33 (natural ILC2s) and IL-25 (inflammatory ILC2s), or their ability to secrete IL-10 (ILC210) (24–26). The ILC2-mediated secretion of IL-13 and amphiregulin is critical for the repair of tissue damage following helminth or viral infections. IL-13 is also important for host-mediated removal of helminths.
Group 3 ILCs (innate counterparts of Th17 T cells) typically produce IL-22 and IL-17A following activation by IL-1β and IL-23. Furthermore, ILC3 can also secrete TNF-α and GM-CSF in response to stimulation (27, 28). ILC3 development from ILCPs is primarily driven by three key transcription factors: 1) aryl hydrocarbon receptor (AhR), 2) promyelocytic leukemia zinc finger (PLZF), and 3) retinoid-related orphan receptor γt (RORγt) (29–31). Similarly, lymphoid tissue inducer (LTi) cells, a unique subtype of ILC3s, also require RORγt for differentiation and similarly produce the cytokines IL-22 and IL-17; however, PLZF in not necessary for development (30). Similarly, ILC3s are classified by their expression of NKp46, CD127, c-Kit, and CCR6. However, in mice CCR6+NKp46− LTi cells as well as CCR6−NKp46− and CCR6−NKp46+ ILC3 cells have also been described. ILC3 through the production of IL-22 play an integral role in immune homeostasis, by stimulating antimicrobial peptide production by epithelial cells and goblet cell mucus secretion, both of which support barrier integrity. Additionally, the ILC3 secretion of IL-17 and GM-CSF promotes granulopoiesis, the production of neutrophil chemoattractant (32), as well as the generation and survival of myeloid cells, and tolerogenic T cells (27).
Alongside ILCs, innate-like T lymphocytes participate in host defense against tissue damage or pathogenic insult prior to the adaptive immune response. Unconventional T-cell subsets express restricted T-cell receptor (TCR) sequences. Consequently, unconventional T-cell stimulation occurs independent of the classical major histocompatibility complex (MHC) I and II-dependent presentation of microbial components and/or antigens (33). Like ILCs, the classification of unconventional T cells depends on cytokines, effector molecules, transcription factors, and surface markers (Table 1). Growing evidence supports an important role of unconventional T cells in the early immune response by providing an immediate cellular response and facilitating conventional T-cell responses (33).

Table 1 Innate-like immune cells: recognized surface markers, effectors, and transcription regulators.
Presentation of lipid antigens via CD1d is the major defining characteristic of NKT cells. NKT cells are classically subdivided into two distinct populations based on the expression of different TCR alpha chains (38–41). Type 1 NKT cells (iNKT cells) express an invariant TCRα chain and a limited TCRβ profile. Human iNKT cells typically express the TCRα chain Vα24-Jα18, while iNKT cells from mice expresses the TCRα chain Vα14-Jα18. NKT cells also possess cytotoxic capabilities due to the expression of perforin, CD95/CD95 L, and TNF (42). Conversely, type 2 NKT cells express an expanded TCRα and TCRβ profile (40, 43). Alpha-galactosylceramide (α-GalCer), a ceramide lipid attached to a polar galactose head, is a model CD1d antigen. iNKT cells react and expand rapidly in response to α-GalCer, which drives iNKT cells to a classical effector status characterized by the production of key immunoregulatory cytokines. Non-lipid antigen-specific responses in iNKT cells have also been reported; however, most iNKT cells drive innate and adaptive immune responses via tumor necrosis factor-α (TNF-α), IFN-γ, IL-17, and IL-4-mediated activation of antigen-presenting cells (APCs). Finally, iNKT cells can also be characterized based on specific cytokines and transcription factors unique to each subset. Specifically, iNKT cells are often subdivided into the following groups: 1) iNKT1 cells, which utilize T-bet and secrete IFN-γ, 2) iNKT2 cells, which are GATA-3 expressing and IL-4 secreting, and 3) iNKT17 cells, which are dependent of RORγt expression and produce IL-17 (44).
MAIT cells co-express a semi-invariant TCR alpha (α) and beta (β) chain and CD161. In humans, TCR Vα 7.2-Jα 33/12/20 and Vβ2/13 are the most common TCR αβ chains, while in mice TCR Vα 19-Jα 33 paired with Vβ6/20 classically defines MAIT cells (45, 46). Recognition of vitamin B (riboflavin and folic acid) metabolites via presentation through the highly conserved MHC class I-related molecule 1 (MR1) is widely viewed as one of the, if not the, main characteristic of MAIT cells. Upon stimulation, MAIT cells rapidly secrete IFN-γ, TNF-α, IL-2, and IL-17, as well as exhibit cytotoxic effects (45, 47–53). In addition to classical MAIT-cell activation via MR1 ligands, MAIT cells can be alternatively activated via IL-15, IL-18, and IL-12 without TCR engagement (54–56). RORγt and PLZF are the two key transcription factors for MAIT-cell development (57, 58). Interestingly, there appear to be tissue-specific populations of MAIT cells. For example, MAIT cells derived from the liver generally have higher levels of the tissue residency markers CD69 and CD103, as well as markers of cellular activation CD56, CD38, PD-1, and NKG2D, under normal physiological conditions (54, 55, 59). MAIT cells facilitate immune regulation both during normal physiological conditions and during pathogenic or antigenic insult.
γδ T cells represent a unique subset of unconventional T cells, as they exhibit characteristics of both innate and adaptive immune cells. For example, following insult γδ T cells respond rapidly and do not require clonal selection or TCR recognition-mediated differentiation (60). Additionally, γδ T cells can be further characterized into distinct populations based on their TCRδ chain expression. γδ T cells that express either the Vδ1 or Vδ2 TCR chain are the two most common populations. These γδ T-cell subsets seem to also display a tissue-specific tropic behavior. Vδ2+ γδ T cells are mainly located in the circulatory system, while Vδ1+ γδ T cells are primarily mucosal-associated (61). Vδ1+ γδ T cells are also long-lived cells that exhibit low levels of CD27 and high levels of granzyme B and CX3CR1 (62). In addition, Vδ1+ γδ T cells also retain their proliferative capacity and TCR sensitivity (62). Likewise, Vδ1+ γδ T cells secrete IFN-γ and TNF-α, as well as perforin and granzyme B following TCR or CD1d stimulation (60, 62, 63). Recently, Vδ3+ γδ T cells have been described and were found to be enriched with hepatic tissues. These cells are activated by CD1d stimulation, which drives the production of Th1, Th2, and Th17 cytokines. These cells were also demonstrated to exhibit cytotoxic activity (64). γδ T cells play an important role in host defense, especially within mucosal-associated tissues.
Alcohol use is known to alter the number and function of immune cells, such as macrophages, neutrophils, and T cells. This also appears to be true for innate lymphocytes (Table 2). Immune dysfunction has been associated with alcohol-induced end-organ damage (Figure 2). However, the role of innate lymphocytes remains ill-defined in the context of alcohol-induced end-organ damage. Below, we will highlight our current understanding of the effects of alcohol on innate lymphocyte populations.
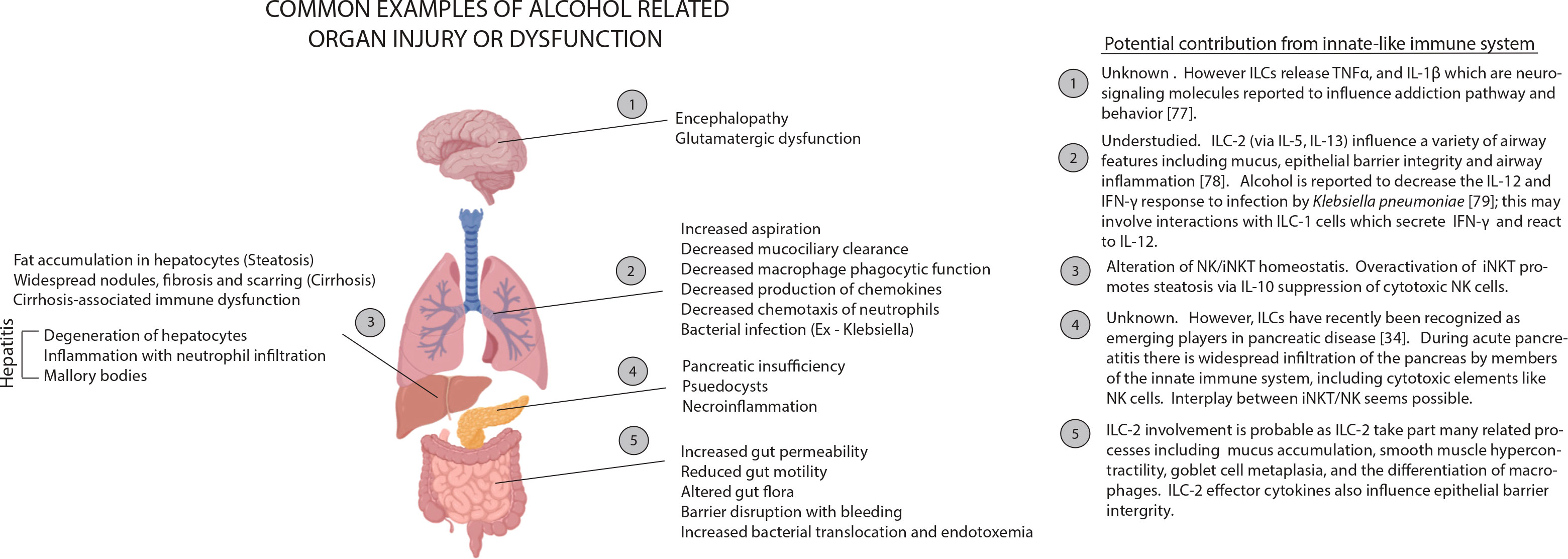
Figure 2 Examples of alcohol related organ injury with potential influences from the innate-like immune system. Many tissues rely on processes regulated by innate-like immune signaling to maintain homeostasis. Alcohol can perturb homeostasis by interfering with signal release (ex. decrease in IL-22 release by ILC-3 cells), by depleting or activating regulatory cells (ex. maturation of iNKT and inactivation of NK cells following alcohol exposure) or by interfering with effector cell function. Little has been rigorously established about how broad changes in the innate-like immune system result in tissue damage. However, we can make some informed inferences. Depletion of signals like IL-22 could facilitate injury in tissues like the lungs and small intestines (2, 5) because IL-22 is a fundamental mediator of inflammation, mucous production and tissue regeneration. During necrotic alcohol-associated tissue injuries (3, 4), there is often tissue infiltration by cytolytic elements including NK cells. In a healthy individual, the activity of these cytotoxic elements is kept in check by cytokine signaling by innate-like including iNKT cells. However, alcohol exposure can dysregulate this signaling and periods of hypo- and hyperactive cytolytic activity may result.
Chronic alcohol consumption has been demonstrated to decrease the abundance and function of NK cells in the periphery (65). Zhang et al. demonstrated that following chronic alcohol exposure, NK cells are arrested in their development at the CD27+CD11b+ stage (Figure 3). Further, cytotoxic NK cells (cNK) appear to accumulate in the bone marrow with a corresponding drop in the number of cNK cells in tissues (i.e., the spleen, lung, liver, and lymph nodes). Given that cNK cells produce IFN-γ and the cytotoxic effector molecules perforin and granzyme B, it is likely that the impairment of cNK maturation alters the release of IFN-γ and cytotoxic effector molecules, which has further downstream effects/impairments in other components of the innate immune system. Importantly, treatment with IL-15 and IL-15Rα restores the alcohol-mediated impairment of cNK development and maturation. This finding implies that the deleterious effects of alcohol might be traced to an impairment of upstream cells/pathways critical for the secretion of IL-15. For example, IL-15-producing CD11chi cells in the spleen are significantly decreased following chronic alcohol consumption. However, IL-15 can be produced from a variety of different cells including intestinal epithelial cells, thymic epithelial cells, keratinocytes, macrophages, and dendritic cells (80).
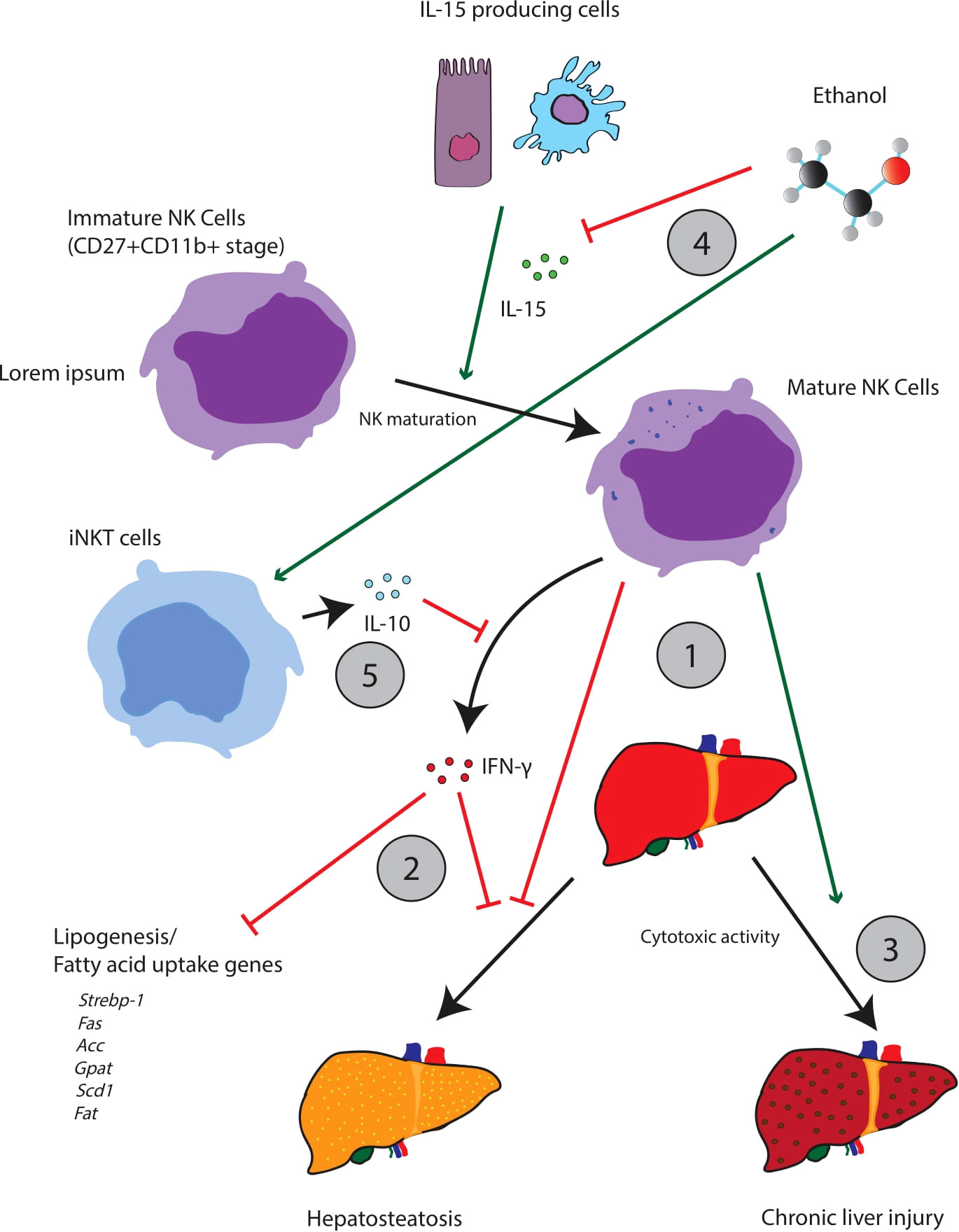
Figure 3 The interplay between iNKT cells and NK cells appears central to the pathogenesis of hepatic steatosis and other aspects of alcoholic liver disease. Mature NK cells appear to oppose hepatic steatosis, but also facilitate tissue injury through cytotoxic activity (1). Acutely, NK activity is thought to be beneficial; NK cells release IFN-γ which downregulates a variety of lipogenic and fatty uptake genes (2). NK cells can also promote beneficial remodeling and regeneration of the liver. Chronically however, over activation of NK cells may contribute to liver injury (3). Alcohol can perturb the iNKT/NK cell balance, favoring iNKT cell maturation while suppressing the maturation of NK cells (3). This change can be achieved through a variety of possible mechanisms. For example, ethanol impairs the release of IL-15 which promotes the maturation of NK cells (4). Ethanol can also promote the release of IL-10 from iNKT cells which will suppress NK activity (5).
Mice depleted of NK cells by anti-AsGM1 antibody treatment displayed increased hepatic triglyceride levels and decreased serum alanine aminotransferase (ALT) levels following chronic ethanol exposure in mice, suggesting that NK cells mediate, in part, liver steatosis and injury. These data are also consistent with research that suggests that NK activation is beneficial in the short run, by increasing host defense against fibrosis and hepatic steatosis through selective cytotoxic activity. However, it is clear that chronic NK-cell activation contributes to liver damage (81). Cui et al. argued that the hepato-specific effects of NK cells were partially mediated by IFN-γ. IFN-γ downregulated the expression of several genes related to lipogenesis and fatty uptake including Srebp-1, Fas, Acc, Gpat, Scd1, and Fat (82). In addition, IFN-γ genetic knockout mice exhibited significantly more severe steatosis than WT mice. Finally, in recent work from our group we found that mice fed a binge-on-chronic ethanol diet exhibited reduced recruitment of NK cells and T cells to the lungs in response to bacterial pneumonia compared to control mice (83). Importantly, indole or probiotics supplementation restored pulmonary immune cell recruitment (NK cells and T cells) to the lungs of alcohol-fed mice and was dependent on AhR signaling, suggesting that alcohol-mediated intestinal dysbiosis and loss of specific microbial metabolites impairs recruitment of NK cells and T cells to the lungs to combat pathogenic insult (83). While NK-cell numbers and function are detrimentally affected by alcohol, it does not appear to affect the frequency of group I ILC.
In summary, alcohol arrests the development of NK cells in CD27+CD11b+ which could contribute to systemic dysregulation via interference with NK-driven IFN-γ signaling. Such dysregulation can contribute to the development of alcoholic liver disease, and studies on the depletion of cNK cells (via the anti-AsGM1 antibody) show increased steatohepatitis. Interestingly, NK-cell maturation can be rescued by the administration of IL-15 which suggests that IL-15 signaling is disrupted following alcohol administration. However, we have not rigorously identified which specific IL-15 producers are involved.
To our knowledge, there are no studies that have evaluated the effects of alcohol on ILC2 cells in any tissue. However, it is likely that ILC2s are affected by alcohol and contribute to alcohol-induced end-organ damage. ILC2 are critically important for type 2 inflammation and the regulation of normal host physiological responses, such as eosinophil and mast-cell recruitment, mucus accumulation, smooth-muscle hypercontractility, goblet-cell metaplasia, and the differentiation of macrophages toward an M2 phenotype. Alcohol is known to impair goblet-cell metaplasia and mucus accumulation (84–86), smooth-muscle hypercontractility (87), eosinophil and mast-cell recruitment (88–90), and alternative macrophage activation (91, 92). It follows that ILC2 dysregulation may contribute to alcohol-induced impairment of these processes. However, the role of ILC2 cells in these processes in the context of alcohol use is unknown and primed for future research.
While ILC3s have become a hot topic in immune research, little is known about the role of ILC3 in alcohol-induced end-organ damage. However, this is an ever-growing interest in the field. To date, only one study has examined the effects of alcohol on ILC3. Specifically, ethanol feeding was found to impair IL-22 production by ILC3s in the gastrointestinal tract (69). Loss of ILC3-mediated IL-22 production was driven by alcohol-associated dysbiosis and reduced levels of indole-3-acetic acid (I3AA). Noteworthily, supplementation of alcohol-fed mice with I3AA protected mice from steatohepatitis via increased expression of IL-22 and REG3G, as well as decreased bacterial translocation to the liver (69). Given that the importance of ILC3 in immune homeostasis is continually expanding, it is likely that ILC3 dysregulation may be a contributing factor during alcohol-induced end-organ damage. However, the role of ILC3 cells in the context of alcohol use is still understudied and primed for future research.
The effects of alcohol on traditional NK (discussed above), NKT, and iNKT cells are the most well studied of effects on innate lymphocyte populations. However, given the ever-growing role and understanding of innate lymphocytes, even our knowledge of the effects of alcohol on these cell types is most likely in its infancy. Further, there are differences in the effects of acute and chronic alcohol consumption and there are likely subtle differences in the effects of alcohol across specific iNKT subsets. Broadly speaking, alcohol appears to increase immature iNKT-cell proliferation and maturation in the thymus with a corresponding increase in IFN-γ-producing iNKT-1 cells (65). In vivo, this facilitates a Th1-dominant immune response. This activation is interesting as it contrasts strongly to the inhibitory effects that alcohol exhibits on NK cells (discussed above).
Some have hypothesized that NK and iNKT cells may be interlinked through a system of contra-regulation (71). A significant fraction of iNKT cells produce interleukin-10 (IL-10). IL-10 is an interleukin known to antagonize the action of NK cells (72). For example, in contrast to NK-cell activity, iNKT cells promote hepatic steatosis by inhibiting the accumulation of NK cells and the release of IFN-γ (71). In addition, Jα18-/- mice (a knockout model deficient in iNKT cells) demonstrated significantly higher levels of total NK-cell count and IFN-γ release following alcohol exposure, while WT mice exhibited a loss of total NK cells and IFN-γ. Likewise, iNKT-deficient Jα18-/- mice appeared relatively protected from hepatic steatosis, but if these mice were also depleted of their NK cells by using the anti-AsGM1 antibody, alcoholic liver injury steatosis was significantly aggravated. Further, hepatic IL-10 was significantly upregulated, but no changes in TGF-β or IL-4 were noted. As noted above, iNKT cells are known for generating IL-10, which can inhibit NK activation and recruitment. In support of this cross talk, steatosis and liver damage were also alleviated in IL-10 KO mice, presumably via the suppression of NK cells.
In summary, alcohol enhances the development of iNKT cells, which promotes a Th1-dominant immune response. The extent to which the altered abundance of iNKT alters host health is unclear. However, dysregulation of iNKT may account for reports of alcohol-related signaling dysfunction involving IL-10 and other iNKT-derived cytokines. There is also evidence that increased iNKT activity promotes alcohol steatosis.
Alcohol consumption influences MAIT-cell numbers and function through a variety of mechanisms. For example, chronic alcohol use is associated with impaired intestinal transport of riboflavin, as well as other B vitamins (93), which likely contributes to MAIT-cell depletion following chronic alcohol consumption. Work done by Zhang et al. demonstrates that there is a decrease in the abundance of MAIT cells in subjects with chronic alcoholic liver disease (78). Changes in MAIT-cell numbers also appear to depend on chronic ethanol consumption, as changes in MAIT-cell numbers were not observed following short-term binge drinking or short-term abstinence. Furthermore, the levels of peripheral MAIT cells were decreased and exhibited reduced antibacterial activity in subjects with alcoholic cirrhosis or severe alcoholic hepatitis (79). The hepatic expressions of the key transcription factors RORγt, PLZF, and Eomes were all reduced in subjects with severe alcoholic hepatitis (79).
Although alcohol can directly affect immune cells, it is worth noting that alcohol-related effects on intestinal bacterial antigens and/or metabolites, independent of ethanol, can deplete MAIT cells (79), which suggests that impairment in hepatic and circulating MAIT cells in patients with severe alcoholic hepatis is more likely due to chronic exposure to bacteria than to alcohol. Recent studies from our group have found that the number of MAIT cells in the mucosal tissues was significantly decreased in mice following binge-on-chronic alcohol feeding (47). However, CD69 expression was increased following alcohol feeding. Interestingly, the expression levels of Th1-specific cytokines and transcription factors were tissue specific. Th1-specific responses were decreased in the intestinal tract but enhanced in the lung and liver (47). Like previous studies which found a critical association of the gut microbiota with MAIT cells, we found that transplantation of the fecal microbiota from alcohol-fed mice into alcohol-naïve mice resulted in a MAIT-cell profile similar to those seen in our alcohol-feeding model (47). Importantly, the differences observed between MAIT cells from alcohol- and control-fed mice were mitigated by antibiotic treatment. Further, in subjects with alcohol-associated liver disease, as well as in rodent ethanol-feeding models, there is increased intestinal permeability with systemic distribution of bacterial products, such as LPS and bacterially derived riboflavin (94, 95). Riboflavin is known to activate MAIT cells, at least in the short term; however, long-term exposure may lead to MAIT-cell exhaustion, which has been reported in chronic conditions like HIV (96, 97).
In summary, alcohol decreases the function and abundance of MAIT cells. However, these deficiencies are not caused solely by the direct effects of alcohol and its metabolites on the eukaryotic cells of the host. Rather, it appears that alcohol-related changes to the microbiota can produce MAIT-cell dysfunction independent and in addition to changes caused directly by alcohol.
Like ILC3 cells, there is a paucity of data regarding the effects of alcohol on γδ T cells. Currently, the effects of alcohol on dermal immunological responses, particularly γδ T cells, are the most well characterized. In a murine model, chronic EtOH feeding leads to a loss of specific subsets of dermal T cells, including Foxp3+ regulatory T cells and both CD3hiVγ3+ and CD3intVγ3-γδ T cells (73). EtOH was also correlated with an impaired functional capacity of dermal γδ T cells (the prototypical dermal cells that produce IL-17). Precisely, IL-17 production following anti-CD3 stimulation was significantly reduced in dermal γδ T cells (73). Further, lymph node-associated γδ T cells isolated from EtOH-fed mice also exhibited diminished IL-17 production following stimulation (73). In similar studies, hepatic IL-17A production was found to be cell type specific depending on alcohol exposure. In alcohol-naïve mice, IL-17 is produced primarily by hepatic γδ T cells. However, following acute-on-chronic EtOH consumption, the secretion of IL-17A was shifted to a more CD4+ T-cell mediated response (74). Noteworthily, these results were not seen in TLR3 KO or Kupffer cell-depleted mice, which suggest that TLR3 activation in Kupffer cells leads to an elevated IL-1β expression, thus driving IL-17A secretion by γδ T cells early during alcohol-associated liver disease and increased CD4+ T-cell secretion of IL-17A during the end stage of alcohol-associated liver disease (74).
Alcohol is known to impair immune function and perturb immune homeostasis. It follows that organ systems which rely on immune signaling for proper functioning are also impaired. Some systems, like the nervous system may be altered in subtle ways that alter behavior (75). In contrast, systems which directly encounter pathogens from the environment can become more susceptible to infection and injury (76, 77). However, our understanding of the mechanisms by which this occurs remains in its infancy. Multiple researchers have reported that alcohol use can deplete critical cell subpopulations, by impairing cell maturation and chemotaxis. The depletion of these cells can propagate multiple deleterious effects. For example, a depletion of NK cells (cytotoxic, IFN-γ secreting) would be expected to impair immune responses reliant on cytotoxicity; however, NK depletion would also be expected to impact tissue IFN-γ levels and thereby attenuate responses by the adaptive and innate arms of the immune system. Examples of cell depletion and signaling disruption have been reported for many types of innate immune cells. However, it is also worth recognizing that alcohol and alcohol-related metabolites can interact with a variety of lymphocytes in nuanced ways through mechanisms other than cellular depletion. Some cell types, such as MAIT cells, may be mediated through indirect pathways that involve the microbiota. This review was primarily concerned with innate-like T lymphocytes, and therefore, we emphasized examples like the observation that alcohol increases iNKT IL-10 secretion. Consider however that IL-10 signaling is also heavily utilized by innate-like B cells, a group important for IgM and as the first line of defense against infection (98). It would be worth exploring the effects of alcohol on these cells, both in their secretion of IL-10 and in their ability to repel infection. At the time of this review, we found little research discussing the effects of alcohol on innate-like B cells. Overall, research on the effect of alcohol on all innate-like lymphocytes remains underdeveloped.
DV, KR-C, and DS reviewed the literature and wrote the manuscript. All authors contributed to the article and approved the submitted version.
The work was supported by the National Institute on Alcohol Abuse and Alcoholism Grants: #K99-AA026336 and #R00-AA026336. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Vivier E, Artis M, Colonna A, Diefenbach JP, Di Santo G, Eberl S, et al. Innate lymphoid cells: 10 years on. Cell (2018) 174(5):1054–66. doi: 10.1016/j.cell.2018.07.017
2. Spits D, Artis M, Colonna A, Diefenbach JP, Di Santo G, Eberl S, et al. Innate lymphoid cells–a proposal for uniform nomenclature. Nat Rev Immunol (2013) 13(2):145–9. doi: 10.1038/nri3365
3. Panda SK, Colonna M. Innate lymphoid cells in mucosal immunity. Front Immunol (2019) 10:861. doi: 10.3389/fimmu.2019.00861
4. Castellanos JG, Longman RS. Innate lymphoid cells link gut microbes with mucosal T cell immunity. Gut Microbes (2020) 11(2):231–6. doi: 10.1080/19490976.2019.1638725
5. Fuchs A, Vermi JS, Lee S, Lonardi S, Gilfillan RD, Newberry M, et al. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-gamma-producing cells. Immunity (2013) 38(4):769–81. doi: 10.1016/j.immuni.2013.02.010
6. Cortez VS, Robinette ML, Colonna M. Innate lymphoid cells: new insights into function and development. Curr Opin Immunol (2015) 32:71–7. doi: 10.1016/j.coi.2015.01.004
7. Bernink JH, Peters CP, Munneke te Velde MAA, Meijer SL, te Velde AA, Weijer K, et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat Immunol (2013) 14(3):221–9. doi: 10.1038/ni.2534
8. Klose CSN, Flach M, Mohle L., Rogell L, Hoyler T, Ebert K, et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell (2014) 157(2):340–56. doi: 10.1016/j.cell.2014.03.030
9. Mackay LK, Minnich M., Kragten NA, Liao Y, Nota B, Seillet C, et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science (2016) 352(6284):459–63. doi: 10.1126/science.aad2035
10. Gordon SM, Chaix J, Rupp LJ, Wu J, Madera S, Sun JC, et al. The transcription factors T-bet and eomes control key checkpoints of natural killer cell maturation. Immunity (2012) 36(1):55–67. doi: 10.1016/j.immuni.2011.11.016
11. Bernink JH, Krabbendam L, Germar K., de Jong E, Gronke K, Kofoed-Nielsen M, et al. Interleukin-12 and -23 control plasticity of CD127(+) group 1 and group 3 innate lymphoid cells in the intestinal lamina propria. Immunity (2015) 43(1):146–60. doi: 10.1016/j.immuni.2015.06.019
12. Marquardt N, Beziat S, Nystrom J, Hengst MA, Ivarsson E, Kekalainen H, et al. Cutting edge: identification and characterization of human intrahepatic CD49a+ NK cells. J Immunol (2015) 194(6):2467–71. doi: 10.4049/jimmunol.1402756
13. Cuff AO, Robertson FP, Stegmann KA, Pallett LJ, Maini MK, Davidson BR, et al. Eomeshi NK cells in human liver are long-lived and do not recirculate but can be replenished from the circulation. J Immunol (2016) 197(11):4283–91. doi: 10.4049/jimmunol.1601424
14. Collins A, Rothman N, Liu K, Reiner SL. Eomesodermin and T-bet mark developmentally distinct human natural killer cells. JCI Insight (2017) 2(5):e90063. doi: 10.1172/jci.insight.90063
15. Zwirner NW, Fuertes MB, Domaica CI. Innate lymphoid cells. new players in tissue homeostasis and inflammatory responses. Medicina (B Aires) (2019) 79(Spec 6/1):564–9.
16. Lim AI, Menegatti S, Bustamante J, Le Bourhis L, Allez M, Rogge L, et al. IL-12 drives functional plasticity of human group 2 innate lymphoid cells. J Exp Med (2016) 213(4):569–83. doi: 10.1084/jem.20151750
17. Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet Fokkens BWJ, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol (2011) 12(11):1055–62. doi: 10.1038/ni.2104
18. Hurrell BP, Shafiei Jahani P, Akbari O. Social networking of group two innate lymphoid cells in allergy and asthma. Front Immunol (2018) 9:2694. doi: 10.3389/fimmu.2018.02694
19. Halim TY, MacLaren A, Romanish MT, Gold MJ, McNagny KM, Takei F. Retinoic-acid-receptor-related orphan nuclear receptor alpha is required for natural helper cell development and allergic inflammation. Immunity (2012) 37(3):463–74. doi: 10.1016/j.immuni.2012.06.012
20. Yang Q, Monticelli LA, Saenz SA, Chi AW, Sonnenberg GF, Tang J, et al. T Cell factor 1 is required for group 2 innate lymphoid cell generation. Immunity (2013) 38(4):694–704. doi: 10.1016/j.immuni.2012.12.003
21. Hoyler T, Klose CS, Souabni A, Turqueti-Neves A, Pfeifer D, Rawlins EL, et al. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity (2012) 37(4):634–48. doi: 10.1016/j.immuni.2012.06.020
22. Yagi R, Zhong C, Northrup DL, Yu F, Bouladoux N, Spencer S, et al. The transcription factor GATA3 is critical for the development of all IL-7Ralpha-expressing innate lymphoid cells. Immunity (2014) 40(3):378–88. doi: 10.1016/j.immuni.2014.01.012
23. Mjosberg J, et al. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity (2012) 37(4):649–59. doi: 10.1016/j.immuni.2012.08.015
24. Seehus CR, et al. Alternative activation generates IL-10 producing type 2 innate lymphoid cells. Nat Commun (2017) 8(1):1900. doi: 10.1038/s41467-017-02023-z
25. Huang Y, et al. IL-25-responsive, lineage-negative KLRG1(hi) cells are multipotential 'inflammatory' type 2 innate lymphoid cells. Nat Immunol (2015) 16(2):161–9. doi: 10.1038/ni.3078
26. Huang Y, Paul WE. Inflammatory group 2 innate lymphoid cells. Int Immunol (2016) 28(1):23–8. doi: 10.1093/intimm/dxv044
27. Mortha A, Chudnovskiy A, Hashimoto D, Bogunovic M, Spencer SP, Belkaid Y, et al. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science (2014) 343(6178):1249288. doi: 10.1126/science.1249288
28. Cella M, Chudnovskiy A, Hashimoto D, Bogunovic M, Spencer SP, Belkaid Y, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature (2009) 457(7230):722–5. doi: 10.1038/nature07537
29. Lee JS, Cella M, McDonald KG, Garlanda C, Kennedy GD, Nukaya M, et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of notch. Nat Immunol (2011) 13(2):144–51. doi: 10.1038/ni.2187
30. van de Pavert SA. Lymphoid tissue inducer (LTi) cell ontogeny and functioning in embryo and adult. BioMed J (2021) 44(2):123–32. doi: 10.1016/j.bj.2020.12.003
31. Sanos SL, Bui VL, Mortha A, Oberle K, Heners C, Johner C, et al. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol (2009) 10(1):83–91. doi: 10.1038/ni.1684
32. Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity (2005) 22(3):285–94. doi: 10.1016/j.immuni.2005.01.011
33. Godfrey DI, Le Nours J, Andrews DM, Uldrich AP, Rossjohn J. Unconventional T cell targets for cancer immunotherapy. Immunity (2018) 48(3):453–73. doi: 10.1016/j.immuni.2018.03.009
34. Shi S, Ye L, Jin K, Xiao Z, and Yu X, Wu W. Innate lymphoid cells: Emerging players in pancreatic disease. Int J Mol Sci (2022) 23(7):3748. doi: 10.3390/ijms23073748
35. Lamichhane R, Schneider M, Harpe SM, Harrop TWR, Hannaway RF, Dearden PK, et al. TCR- or cytokine-activated CD8+ mucosal-associated invariant T cells are rapid polyfunctional effectors that can coordinate immune responses. Cell Rep (2019) 28(12):3061–76.e5. doi: 10.1016/j.celrep.2019.08.054
36. Jiao Y, Huntington ND, and Belz GT, Seillet C. Type 1 innate lymphoid cell biology: Lessons learnt from natural killer cells. Front Immunol (2016) 7:426–6. doi: 10.3389/fimmu.2016.00426
37. Sawa S, Lochner M, Satoh-Takayama N, Dulauroy S, Bérard M, Kleinschek M, et al. RORγt+ innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota. Nat Immunol (2011) 12(4):320–6. doi: 10.1038/ni.2002
38. Nishioka Y, Masuda S, Tomaru U, Ishizu A. CD1d-restricted type II NKT cells reactive with endogenous hydrophobic peptides. Front Immunol (2018) 9:548. doi: 10.3389/fimmu.2018.00548
39. Ambrosino E, Terabe M, Halder RC, Peng J, Takaku S, Miyake S, et al. Cross-regulation between type I and type II NKT cells in regulating tumor immunity: a new immunoregulatory axis. J Immunol (2007) 179(8):5126–36. doi: 10.4049/jimmunol.179.8.5126
40. Weng X, He Y, Visvabharathy L, Liao CM, Tan X, Balakumar A, et al. Crosstalk between type II NKT cells and T cells leads to spontaneous chronic inflammatory liver disease. J Hepatol (2017) 67(4):791–800. doi: 10.1016/j.jhep.2017.05.024
41. Miko E, Barakonyi A, Meggyes M, Szereday L. The role of type I and type II NKT cells in materno-fetal immunity. Biomedicines (2021) 9(12):1901.
42. Berzins SP, Smyth MJ, Baxter AG. Presumed guilty: natural killer T cell defects and human disease. Nat Rev Immunol (2011) 11(2):131–42. doi: 10.3390/biomedicines9121901
43. Terabe M, Berzofsky JA. The immunoregulatory role of type I and type II NKT cells in cancer and other diseases. Cancer Immunol Immunother (2014) 63(3):199–213. doi: 10.1007/s00262-013-1509-4
44. Gapin L. Development of invariant natural killer T cells. Curr Opin Immunol (2016) 39:68–74. doi: 10.1016/j.coi.2016.01.001
45. Rahimpour A, Koay H.F, Enders A, Clanchy R, Eckle SB, Meehan B, et al. Identification of phenotypically and functionally heterogeneous mouse mucosal-associated invariant T cells using MR1 tetramers. J Exp Med (2015) 212(7):1095–108. doi: 10.1084/jem.20142110
46. Martin E, Treiner E, Duban L, Guerri L, Laude H, Toly C, et al. Stepwise development of MAIT cells in mouse and human. PloS Biol (2009) 7(3):e54. doi: 10.1371/journal.pbio.1000054
47. Gu M, Samuelson DR, Taylor CM, Molina PE, Luo M, Siggins RW, et al. Alcohol-associated intestinal dysbiosis alters mucosal-associated invariant T-cell phenotype and function. Alcohol Clin Exp Res (2021) 45(5):934–47. doi: 10.1111/acer.14589
48. Kulicke C, Karamooz E, Lewinsohn D, Harriff M. Covering all the bases: Complementary MR1 antigen presentation pathways sample diverse antigens and intracellular compartments. Front Immunol (2020) 11:2034. doi: 10.3389/fimmu.2020.02034
49. Huang S, et al. Evidence for MR1 antigen presentation to mucosal-associated invariant T cells. J Biol Chem (2005) 280(22):21183–93. doi: 10.1074/jbc.M501087200
50. McWilliam HE, Eckle SB, Theodossis A, Liu L, Chen Z, Wubben JM, et al. The intracellular pathway for the presentation of vitamin b-related antigens by the antigen-presenting molecule MR1. Nat Immunol (2016) 17(5):531–7. doi: 10.1038/ni.3416
51. McWilliam HE, Villadangos JA. MR1 antigen presentation to MAIT cells: new ligands, diverse pathways? Curr Opin Immunol (2018) 52:108–13. doi: 10.1016/j.coi.2018.04.022
52. Huang S, Martin E, Kim S, Yu L, Soudais C, Fremont DH, et al. MR1 antigen presentation to mucosal-associated invariant T cells was highly conserved in evolution. Proc Natl Acad Sci U.S.A. (2009) 106(20):8290–5. doi: 10.1073/pnas.0903196106
53. Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, et al. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature (2003) 422(6928):164–9. doi: 10.1038/nature01433
54. Rha MS, Han JW, Kim JH, Koh JY, Park HJ, et al. Human liver CD8(+) MAIT cells exert TCR/MR1-independent innate-like cytotoxicity in response to IL-15. J Hepatol (2020) 73(3):640–50. doi: 10.1016/j.jhep.2020.03.033
55. Jo J, Tan AT, Ussher JE, Sandalova E, Tang XZ, Tan-Garcia A, et al. Toll-like receptor 8 agonist and bacteria trigger potent activation of innate immune cells in human liver. PloS Pathog (2014) 10(6):e1004210. doi: 10.1371/journal.ppat.1004210
56. Liu J, Brutkiewicz RR. The toll-like receptor 9 signalling pathway regulates MR1-mediated bacterial antigen presentation in b cells. Immunology (2017) 152(2):232–42. doi: 10.1111/imm.12759
57. Savage AK, Constantinides MG, Han J, Picard D, Martin E, Li B, et al. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity (2008) 29(3):391–403. doi: 10.1016/j.immuni.2008.07.011
58. Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell (2006) 126(6):1121–33. doi: 10.1016/j.cell.2006.07.035
59. Tang XZ, Jo J, Tan AT, Sandalova E, Chia A, Tan KC, et al. IL-7 licenses activation of human liver intrasinusoidal mucosal-associated invariant T cells. J Immunol (2013) 190(7):3142–52. doi: 10.4049/jimmunol.1203218
60. Bonneville M, O'Brien RL, Born WK. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol (2010) 10(7):467–78. doi: 10.1038/nri2781
61. Davey MS, Willcox CR, Hunter S, Kasatskaya SA, Remmerswaal EBM, Salim M, et al. The human Vdelta2(+) T-cell compartment comprises distinct innate-like Vgamma9(+) and adaptive Vgamma9(-) subsets. Nat Commun (2018) 9(1):1760. doi: 10.1038/s41467-018-04076-0
62. Davey MS, Willcox CR, Joyce SP, Ladell K, Kasatskaya SA, McLaren JE, et al. Clonal selection in the human Vdelta1 T cell repertoire indicates gammadelta TCR-dependent adaptive immune surveillance. Nat Commun (2017) 8:14760. doi: 10.1038/ncomms14760
63. Hunter S, Willcox CR, Davey MS, Kasatskaya SA, Jeffery HC, Chudakov DM, et al. Human liver infiltrating gammadelta T cells are composed of clonally expanded circulating and tissue-resident populations. J Hepatol (2018) 69(3):654–65. doi: 10.1016/j.jhep.2018.05.007
64. Mangan BA, Dunne MR, O'Reilly VP, Dunne PJ, Exley MA, O'Shea D, et al. Cutting edge: CD1d restriction and Th1/Th2/Th17 cytokine secretion by human Vdelta3 T cells. J Immunol (2013) 191(1):30–4. doi: 10.4049/jimmunol.1300121
65. Zhang H, Zhang F, Zhu Z, Luong D, Meadows GG. Chronic alcohol consumption enhances iNKT cell maturation and activation. Toxicol Appl Pharmacol (2015) 282(2):139–50. doi: 10.1016/j.taap.2014.11.013
66. Zhang F, Little A, Zhang H. Chronic alcohol consumption inhibits peripheral NK cell development and maturation by decreasing the availability of IL-15. J Leukoc Biol (2017) 101(4):1015–27. doi: 10.1189/jlb.1A0716-298RR
67. Spitzer JH, Meadows GG. Modulation of perforin, granzyme a, and granzyme b in murine natural killer (NK), IL2 stimulated NK, and lymphokine-activated killer cells by alcohol consumption. Cell Immunol (1999) 194(2):205–12. doi: 10.1006/cimm.1999.1511
68. Ito I, Asai A, McCalla CT, Kobayashi M, Suzuki F. A role of lamina propria ILC1 in the impaired host antibacterial resistance of chronic alcohol consuming mice. J Immunol (2016) 196(1 Supplement):131.5–5.
69. Hendrikx T, Duan Y, Wang Y, Oh JH, Alexander LM, Huang W, et al. Bacteria engineered to produce IL-22 in intestine induce expression of REG3G to reduce ethanol-induced liver disease in mice. Gut (2019) 68(8):1504–15. doi: 10.1136/gutjnl-2018-317232
70. Lane P, Gaspal F, McConnell F, Withers D, Anderson G. Lymphoid tissue inducer cells: Pivotal cells in the evolution of CD4 immunity and tolerance? Front Immunol (2012) 3. doi: 10.3389/fimmu.2012.00024
71. Cui K, Yan G, Zheng X, Bai L, Wei H, Sun R, et al. Suppression of natural killer cell activity by regulatory NKT10 cells aggravates alcoholic hepatosteatosis. Front Immunol (2017) 8. doi: 10.3389/fimmu.2017.01414
72. Scott MJ, Hoth JJ, Turina M, Woods DR, Cheadle WG. Interleukin-10 suppresses natural killer cell but not natural killer T cell activation during bacterial infection. Cytokine (2006) 33(2):79–86. doi: 10.1016/j.cyto.2005.12.002
73. Parlet CP, Waldschmidt TJ, Schlueter AJ. Chronic ethanol feeding induces subset loss and hyporesponsiveness in skin T cells. Alcohol Clin Exp Res (2014) 38(5):1356–64. doi: 10.1111/acer.12358
74. Lee JH, Shim YR, Seo W, Kim MH, Choi WM, Kim HH, et al. Mitochondrial double-stranded RNA in exosome promotes interleukin-17 production through toll-like receptor 3 in alcohol-associated liver injury. Hepatology (2020) 72(2):609–25. doi: 10.1002/hep.31041
75. Zhang Y, et al. Persistent deficiency of mucosa-associated invariant T (MAIT) cells during alcohol-related liver disease. Cell bioscience (2021) 11(1):148–8. doi: 10.1186/s13578-021-00664-8
76. Riva A, Patel V, Kurioka A, Jeffery HC, Wright G, Tarff S, et al. Mucosa-associated invariant T cells link intestinal immunity with antibacterial immune defects in alcoholic liver disease. Gut (2018) 67(5):918–30. doi: 10.1136/gutjnl-2017-314458
77. Coleman LG Jr., Crews FT. Innate immune signaling and alcohol use disorders. Handb Exp Pharmacol (2018) 248:369–96. doi: 10.1007/164_2018_92
78. Bartemes KR, Kita H. Roles of innate lymphoid cells (ILCs) in allergic diseases: The 10-year anniversary for ILC2s. J Allergy Clin Immunol (2021) 147(5):1531–47. doi: 10.1016/j.jaci.2021.03.015
79. Zisman DA, Strieter RM, Kunkel SL, Tsai WC, Wilkowski JM, Bucknell KA, et al. Ethanol feeding impairs innate immunity and alters the expression of Th1- and Th2-phenotype cytokines in murine klebsiella pneumonia. Alcohol Clin Exp Res (1998) 22(3):621–7. doi: 10.1111/j.1530-0277.1998.tb04303.x
80. Santana Carrero RM, Beceren-Braun F, Rivas SC, Hegde SM, Gangadharan A, Plote D, et al. IL-15 is a component of the inflammatory milieu in the tumor microenvironment promoting antitumor responses. Proc Natl Acad Sci (2019) 116(2):599–608. doi: 10.1073/pnas.1814642116
81. Tosello-Trampont A, Surette FA, Ewald SE, Hahn YS. Immunoregulatory role of NK cells in tissue inflammation and regeneration. Front Immunol (2017) 8. doi: 10.3389/fimmu.2017.00301
82. Cui K, Yan G, Xu C, Chen Y, Wang J, Zhou R, et al. Invariant NKT cells promote alcohol-induced steatohepatitis through interleukin-1beta in mice. J Hepatol (2015) 62(6):1311–8. doi: 10.1016/j.jhep.2014.12.027
83. Samuelson DR, Gu M, Shellito JE, Molina PE, Taylor CM, Luo M, et al. Pulmonary immune cell trafficking promotes host defense against alcohol-associated klebsiella pneumonia. Commun Biol (2021) 4(1):997. doi: 10.1038/s42003-021-02524-0
84. Hartmann P, Chen P, Wang HJ, Wang L, McCole DF, Brandl K, et al. Deficiency of intestinal mucin-2 ameliorates experimental alcoholic liver disease in mice. Hepatology (2013) 58(1):108–19. doi: 10.1002/hep.26321
85. Brozinsky S, Fani K, Grosberg SJ, Wapnick S. Alcohol ingestion-induced changes in the human rectal mucosa: light and electron microscopic studies. Dis Colon Rectum (1978) 21(5):329–35. doi: 10.1007/BF02586661
86. Grewal RK, Mahmood A. The effects of ethanol administration on brush border membrane glycolipids in rat intestine. Alcohol (2010) 44(6):515–22. doi: 10.1016/j.alcohol.2010.07.008
87. Alleyne J, Dopico AM. Alcohol use disorders and their harmful effects on the contractility of skeletal, cardiac and smooth muscles. Adv Drug Alcohol Res (2021) 1:10011. doi: 10.3389/adar.2021.10011
88. Draberova L, Paulenda T, Halova I, Potuckova L, Bugajev V, Bambouskova M, et al. Ethanol inhibits high-affinity immunoglobulin e receptor (FcepsilonRI) signaling in mast cells by suppressing the function of FcepsilonRI-cholesterol signalosome. PloS One (2015) 10(12):e0144596. doi: 10.1371/journal.pone.0144596
89. Nishida K, Yamasaki S, Ito Y, Kabu K, Hattori K, Tezuka T, et al. Fc{epsilon}RI-mediated mast cell degranulation requires calcium-independent microtubule-dependent translocation of granules to the plasma membrane. J Cell Biol (2005) 170(1):115–26. doi: 10.1083/jcb.200501111
90. Toivari M, Maki T, Suutarla S, Eklund KK. Ethanol inhibits IgE-induced degranulation and cytokine production in cultured mouse and human mast cells. Life Sci (2000) 67(23):2795–806. doi: 10.1016/S0024-3205(00)00863-8
91. Brown SD, Brown LA. Ethanol (EtOH)-induced TGF-beta1 and reactive oxygen species production are necessary for EtOH-induced alveolar macrophage dysfunction and induction of alternative activation. Alcohol Clin Exp Res (2012) 36(11):1952–62. doi: 10.1111/j.1530-0277.2012.01825.x
92. Pan XY, Wang L, You HM, Cheng M, Yang Y, Huang C. Alternative activation of macrophages by prostacyclin synthase ameliorates alcohol induced liver injury. Lab Invest (2021) 101(9):1210–24. doi: 10.1038/s41374-021-00531-7
93. Subramanian VS, Subramanya SB, Ghosal A, Said HM. Chronic alcohol feeding inhibits physiological and molecular parameters of intestinal and renal riboflavin transport. American journal of physiology. Cell Physiol (2013) 305(5):C539–46. doi: 10.1152/ajpcell.00089.2013
94. Parlesak A, Schäfer C, Schütz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol (2000) 32(5):742–7. doi: 10.1016/S0168-8278(00)80242-1
95. Bode C, Kugler V, Bode JC. Endotoxemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. J Hepatol (1987) 4(1):8–14. doi: 10.1016/S0168-8278(87)80003-X
96. Leeansyah E, Ganesh A, Quigley MF, Sönnerborg A, Andersson J, Hunt PW, et al. Activation, exhaustion, and persistent decline of the antimicrobial MR1-restricted MAIT-cell population in chronic HIV-1 infection. Blood (2013) 121(7):1124–35. doi: 10.1182/blood-2012-07-445429
97. Rodin W, Sundström P, Ahlmanner F, Szeponik L, Zajt KK, Wettergren Y, et al. Exhaustion in tumor-infiltrating mucosal-associated invariant T (MAIT) cells from colon cancer patients. Cancer Immunol Immunother (2021) 70(12):3461–75. doi: 10.1007/s00262-021-02939-y
Keywords: pneumonia, bacteria, alcohol, innate immunity, innate lymphocytes
Citation: Ruiz-Cortes K, Villageliu DN and Samuelson DR (2022) Innate lymphocytes: Role in alcohol-induced immune dysfunction. Front. Immunol. 13:934617. doi: 10.3389/fimmu.2022.934617
Received: 02 May 2022; Accepted: 25 July 2022;
Published: 29 August 2022.
Edited by:
Jochen Mattner, University of Erlangen Nuremberg, GermanyReviewed by:
Luc Van Kaer, Vanderbilt University Medical Center, United StatesCopyright © 2022 Ruiz-Cortes, Villageliu and Samuelson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Derrick R. Samuelson, ZGVycmljay5zYW11ZWxzb25AdW5tYy5lZHU=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.