- Department I for Internal Medicine, Center of Molecular Medicine Cologne (CMMC), University Hospital of Colgne, Cologne, Germany
CAR (Chimeric Antigen Receptor) T-cell therapy has revolutionized the field of oncology in recent years. This innovative shift in cancer treatment also provides the opportunity to improve therapies for many patients suffering from various autoimmune diseases. Recent studies have confirmed the therapeutic suppressive potential of regulatory T cells (Tregs) to modulate immune response in autoimmune diseases. However, the polyclonal character of regulatory T cells and their unknown TCR specificity impaired their therapeutic potency in clinical implementation. Genetical engineering of these immune modulating cells to express antigen-specific receptors and using them therapeutically is a logical step on the way to overcome present limitations of the Treg strategy for the treatment of autoimmune diseases. Encouraging preclinical studies successfully demonstrated immune modulating properties of CAR Tregs in various mouse models. Still, there are many concerns about targeted Treg therapies relating to CAR target selectivity, suppressive functions, phenotype stability and safety aspects. Here, we summarize recent developments in CAR design, Treg biology and future strategies and perspectives in CAR Treg immunotherapy aiming at clinical translation.
Introduction
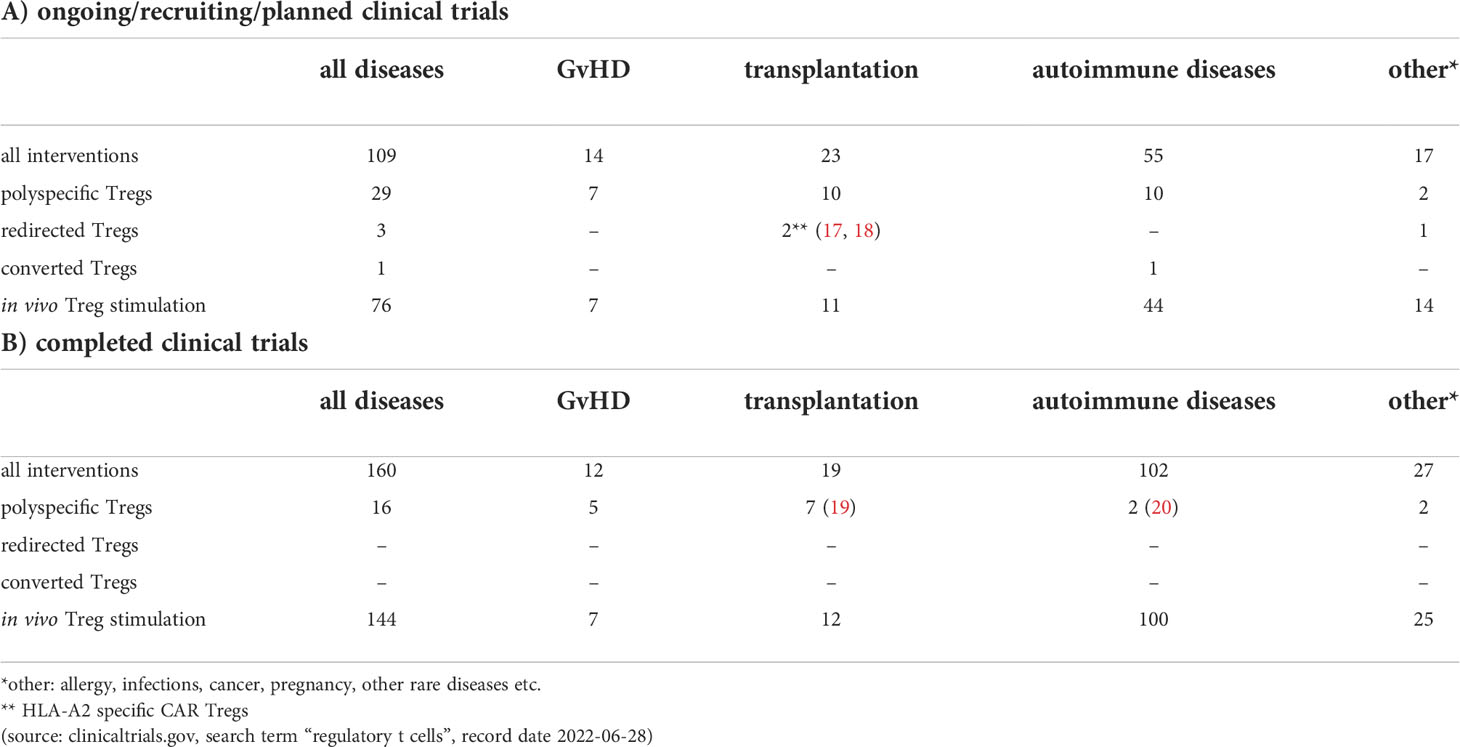
Regulatory T cells (Treg) are a critical CD4 T cell subset involved in the control of immune-tolerance. Tregs regulate immune-homeostasis and limit immune activation mediated by proinflammatory activities of CD4+ and CD8+ T cells, natural killer (NK) cells, and antigen-presenting cells (APC). Furthermore, Treg cells harbor powerful suppressive potential to promote tissue repair (1, 2) and modulate metabolic regulation (3). Thus, defects in Tregs that induce an imbalance of immune regulation often lead to autoimmune disorders (4). On the other hand, the involvement of immunosuppressive regulatory T cells plays a central role in tumor progression in both human and mice (5, 6). Accordingly, numerous groups have demonstrated an increased persistence of regulatory T cells in tumor tissue and surrounding tumor microenvironment (TME) (7). A continuous high expression of the transcription factor forkhead box protein P3 (FOXP3) in Treg cells is considered to be essential for their suppressive activity (8). Two main groups of FOXP3 Treg cells exist: natural Treg (nTreg) cells that develop in the thymus and represent a professional, stable T-cell lineage and peripherally induced Treg cells (pTreg) that differentiate from naïve CD4+ T cells in the periphery after antigen receptor stimulation in the presence of transforming growth factor beta (TGF-beta) (9). Lyon and colleagues demonstrated the importance of FOXP3 for Treg cell functions by their finding that mutation in the FoxP3 locus in mice leads to Treg dysfunction and severe autoimmunity (10). Bennett, as well as Wildin and colleagues, confirmed that IPEX syndrome is the human equivalent of the scurfy mouse phenotype by identifying mutations in the FOXP3 gene, the human homolog of the mouse gene FoxP3 (11, 12). Furthermore, Barnes and colleagues demonstrated that CTLA-4 crosslinking on the surface of regulatory T cells in the presence of TCR signal enhanced the generation of FOXP3+ T cells (13). Moreover, CTLA-4 engagement changed the effect of CD28 crosslinking from inhibiting to promoting FOXP3 expression (13). As demonstrated by Read and colleagues (14), inhibition of CTLA-4 receptor in mouse abrogates Treg cell-mediated protection and, similarly, blocking of CTLA-4 in cancer patients improves anti-tumor immune response but also boosts autoimmunity (15). Furthermore, human Treg cells affect surrounding immune cells by releasing immunosuppressive cytokines including TGF-beta, IL-10, and IL-35 (16). Because of their proven immunomodulatory properties, Treg cells became an attractive therapeutic tool for treating autoimmune diseases and modulating or preventing transplant rejection and graft vs. host disease (GvHD). In recent years, several phase I clinical trials aiming to investigate safety and feasibility of a Treg-based therapy were conducted, thereby revealing chances and challenges of this immunotherapeutic strategy (see Table 1). For example, autologous ex-vivo expanded polyclonal Tregs have been transferred in 2009 to respective recipients who suffered from either acute or chronic GvHD (21). Since then, the therapeutic potential of Treg cells was gradually widened to autoimmunological diseases such as Type 1 diabetes (22), cutaneous lupus (23), autoimmune hepatitis (24) or Crohn’s disease (25), and to prevent rejection in solid organ transplantation (26), but the treatment modalities changed only slightly. Novel technologies to alter the genome of the Treg cells might enhance functional activity, stability, persistence and antigen specificity, and could broaden the therapeutic capacity of this promising immunotherapeutic strategy. Numerous preclinical studies have revealed that antigen-specific or redirected Treg cells are superior as compared to classical polyclonal Treg cells in diverse mouse models (27–30). Redirected Treg cells predominantly localize at the site of target antigen expression, thereby reducing the risk of systemic immunosuppression. Thus, CAR or TCR redirected Treg cells seem to be more effective and safer than polyclonal Tregs cells. In addition, advances in the field of Treg biology open up new possibilities to generate Tregs from naïve T CD4+ T cells through targeted modifications, such as induction of FOXP3 expression (30, 31).
Treg cells: Phenotype and function
Thymically derived FOXP3+ regulatory T cells (nTreg, formerly tTregs) constitute a unique T cell lineage that is essential for maintaining immune tolerance to self as well as innocuous environmental antigens and intra-tissue immune homeostasis. These cells develop from antigen-unexperienced naïve Tregs with expression of CD4+, CD25+, CD127dim/–, CD45RA+ (32) to natural Tregs (nTreg) which account for 1-4% of all white blood cells and express CD4+, CD25hi, CD127dim/– Helios+. However, FOXP3 can also be turned on in conventional T cells with effector functions (Teff) as consequence of antigen exposure in the periphery, under both non-inflammatory and inflammatory conditions. These so-called peripheral Treg cells (pTreg, formerly induced Tregs/iTregs) that involve both CD4+ and CD8+ pTreg cells are characterized by CD4+/CD8+, CD25hi, CD127dim/– expression and participate in the control of immunity at sites of inflammation. Although phenotypically and functionally similar, nTregs can be clearly discriminated from pTregs by their stable epigenetic modification of the Treg-specific demethylated region (TSDR) of the FOXP3 gene (33). Peripherally induced Tregs are also considered to be negative for Helios expression, in contrast to nTreg, but this is still controversially debated in the field (34–41). The both, nTreg and pTreg can control inflammation/immunity by multiple mechanisms, such as i) competition with Teff for IL-2, ii) through cAMP-mediated immunosuppression, iii) adenosine production via the ectoenzymes CD39 and CD79, iv) secretion of inhibitory cytokines (e.g. IL-10, TGF-beta, IL-33, IL-34), and v) cytolysis of Teff via granzyme/perforin-dependent mechanisms (42, 43).
Despite some other common specific cell surface markers like GITR and CTLA-4 (44), many additional molecules describe various subpopulations of Treg cells like CD39 (45, 46) and CD49d (47, 48). Although a stable and high FOXP3 expression seems to be crucial for Treg cell function, recent data point to a limited effect on functional characteristics of FOXP3-ablated Tregs (49). There is increasing evidence that intra-tissue antigen-driven activation and inflammation promotes FOXP3 instability even in Treg that expressed high amounts of FOXP3 before. Key factors of instability seem to be lack of IL-2, inflammatory cytokines (Stat3, NFkappaB pathways) and activation of certain costimulatory molecules, so called switch-points (50, 51). Diminished number and/or function of Treg, e.g. by malformation in IPEX syndrome, plus a misbalanced Teff/Treg ratio at the site of inflammation result in unwanted inflammation/immunity associated with autoimmunity, autoinflammation, and disturbed regeneration from trauma and ischemia/reperfusion (52). Consequently, agonistic targeting of Treg is a promising therapeutic option to combat undesired inflammation/immunity in a broad range of medical indications. Although in vivo Treg induction/expansion approaches such as blocking costimulatory signals during antigen exposure, tolerogenic dendritic cells, tolerogenic peptide vaccination, and low-dose IL-2 show some efficacy in preclinical models and first in human trials, their efficacy is limited and some adverse effects can be observed (53, 54). In many preclinical models, the adoptive transfer of Treg seems to be more effective but recent technological advances to isolate and expand human Treg under GMP compliant conditions now allow adoptive Treg therapy to be more and more introduced to the clinic, thereby opening up new opportunities.
Based on the analysis of very recent preclinical and clinical studies on 1st-generation Treg products, the main objectives to develop next-generation Treg approaches with enhanced efficacy are:
- To improve the antigen specificity of Treg products by generating i) Chimeric Antigen Receptor-expressing Treg (CAR-Treg) (27, 55–59) or ii) specific T cell receptor-expressing Treg (TCR-Treg) (60–65).
- To stabilize the in vivo suppressive function of Treg by i) epigenetic FOXP3 gene modification (66–69), ii) CRISPR/Cas9 mediated knocking-out of switch-point-receptors whose activation leads to loss of Treg function or even to the switch to effector cells (70, 71), or iii) mitochondrial modification (72–74) iv) exploring CD8+ Treg as an alternative and/or complementary to CD4+ Treg (75–81), v) inducing resistance to immunosuppressive drugs using CRISPR/Cas9 mediated knocking-out of their target molecules (82, 83), vi) insertion of an additional FOXP3 gene cassette, which also works as a safety mechanism to prevent Treg to Teff conversion (56, 84, 85), vii) development of Treg supporting IL-2 muteins or orthogonal IL-2 pairings (86–89).
CAR-History
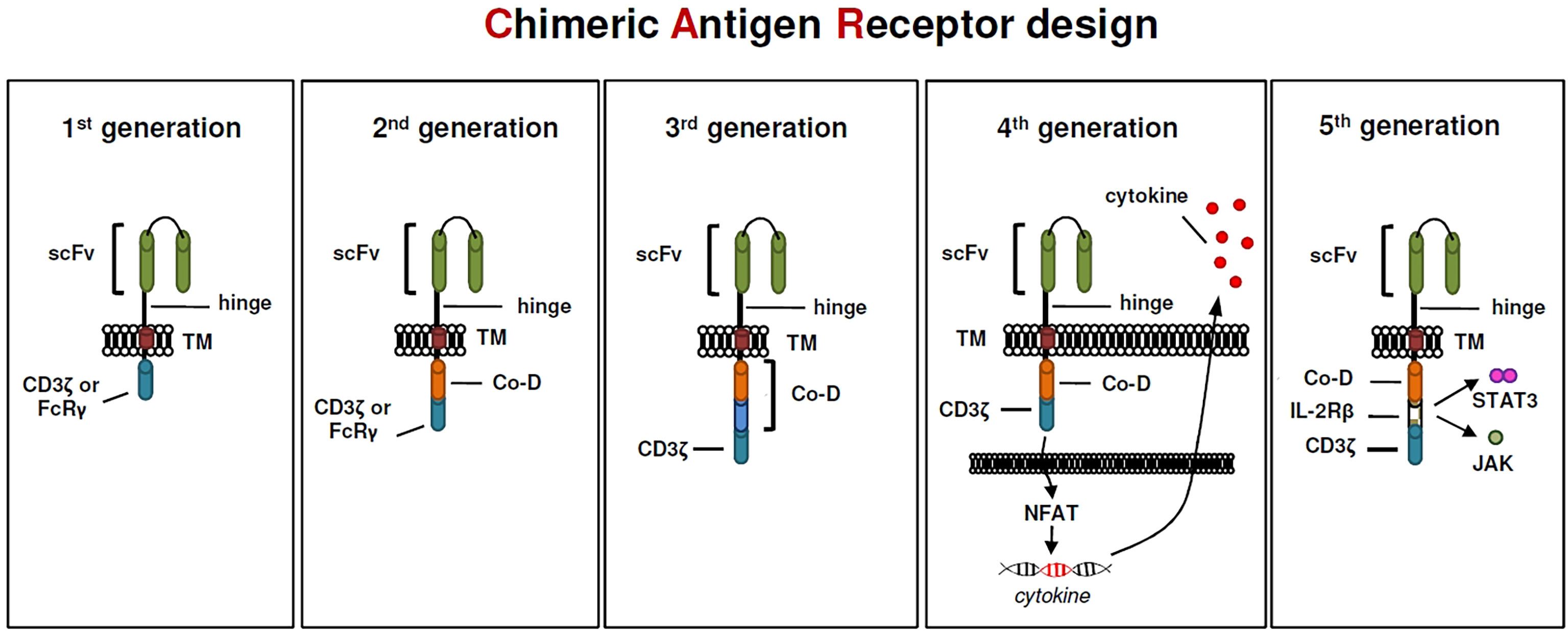
Although, to many of us, it seems that CAR T cells have only recently entered the world stage, their origin dates back forty years. In 1982, almost unnoticed by the research community, Zelig Eshhar demonstrated for the first time a CAR prototype, so called “T-body”, that conceptually has a lot in common with today’s CAR constructs (90). The first CAR constructs designed by Eshhar and colleagues contained a TNP-specific scFv binding domain linked to either the CD3zeta or the FcRgamma signaling domain for T cell activation (90). These first-generation CARs (Figure 1) did not provide an additional costimulatory domain that is essential for full T-cell activation. CAR constructs designed this way were still functional because T cells could be able to replace the missing coactivating signal (also known as signal 2) via the endogenous CD28-B7.1/B7.2 interaction between T cell and target cell (91). Subsequently, the logical conclusion by the early CAR pioneers was the integration of a coactivating domain into the signaling moiety of CAR constructs (Figure 1), which became a common feature of second generation CARs (92, 93). This resulted in two basic CAR designs that are still relevant for cancer treatment, one using CD28 and a second using 4-1BB as a coactivating domain. In preclinical studies effector T cells expressing CARs harboring a CD28 costimulatory domain have repeatedly shown higher proliferation rates and released higher quantities of the cytokines IL-2, IFN-gamma, and TNF-alpha, than T cells expressing 4-1BB-costimulated CARs (94–96). Interestingly, preclinical data showed, that the CD28zeta format has also a positive effect on regulatory T cells in the tumor microenvironment because of its high capacity to induce IL-2 secretion (97). Hence, this format might be the favored one in the field to create highly active CAR Tregs. Subsequently, the question arose whether the integration of an additional costimulatory domain could have a positive impact on CAR T-cell efficacy, especially since the problem of tumor microenvironment-mediated T-cell exhaustion in cancer treatment became more and more evident. However, third-generation CARs containing an scFv, a CD3zeta domain and two costimulatory domains in tandem (Figure 1) have been tested in clinical trials involving small cohorts of patients but, thus far, they have not been associated with enhanced anti-tumor activity to that of second-generation CARs (98). Despite a promising development of CAR T cell-based immunotherapy, B cell malignancies such as ALL or DLBCL, most attempts to induce long-lasting anti-tumor effects with second-generation CAR constructs in solid tumors were less successful (99, 100). In order to enhance the efficacy of redirected T cells in solid tumors CAR T cells were genetically modified to release a transgenic cytokine upon CAR signaling in the targeted tumor tissue (101). The TRUCK strategy (“T cells Redirected for Antigen-Unrestricted Cytokine-initiated Killing”), also called “4th Generation” (Figure 1) unites the direct anti-tumor attack of the CAR T cell with the tumor microenvironment-modulating capabilities of a proinflammatory cytokine. Binding to the CAR cognate antigen on the tumor cell induces NFAT phosphorylation, migration to the nucleus and induction of the NFAT-responsive/IL-2 minimal promoter that drives transgene expression (101). TRUCKs could also potentially be of interest in autoimmune diseases, since the proinflammatory payload could be replaced by an anti-inflammatory cytokine such as IL-10 or TGF-beta. Interestingly, contrary to CD4 expressing helper T cells, activated NFAT1 in Treg cells forms a ternary complex with FOXP3 at the IL2 promoter that replaces AP-1 (Jun/Fos) in the NFAT : AP-1 complex present in effector T cells (102, 103). FOXP3 thus transforms a transcriptionally activating NFAT : AP-1 complex in effector T cells into a repressive NFAT : FOXP3 complex in regulatory T cells (103). The fifth generation of CARs (Figure 1), is also based on the second generation of CARs, with the addition of intracellular domains of cytokine receptors. In this context, Kagoya and colleagues presented a CD19-specific CAR construct containing a truncated cytoplasmic domain from the interleukin IL-2R beta-chain (IL-2Rbeta) and a STAT3-binding tyrosine-X-X-glutamine (YXXQ) motif that was incorporated into CD28-CD3zeta activation moiety (104).
Antigen-specific regulatory T cells
As demonstrated by various groups, antigen-specific Treg cells showed higher potency in immunosuppression than polyclonal T cells in diverse preclinical mouse models (105–107). However, the low Treg cell frequency often limits the expansion of endogenous antigen-specific Treg cells and hence prevent their therapeutic use. For this reason, in recent years technical solutions have been sought to generate antigen-specific Treg cells in sufficient quantities and qualities. Promising current methods include redirecting regulatory T cells using synthetic receptors on the one hand and converting antigen-specific effector T cells into regulatory T cells using FOXP3 overexpression on the other hand. Although both CAR and T cell receptor (TCR) based constructs are available for Treg redirection, CAR constructs are mainly used. Unlike MHC-restricted TCR targets, potential antigens recognized by CARs also include non-protein targets such as carbohydrates and glycolipid molecules (108, 109). However, we must also note that CAR constructs only recognize and target cell surface antigens, while TCR constructs can target both MHC-restricted cell surface and intracellular antigens. Moreover, CAR redirected T cells reveal promising results in the treatment of hematological malignancies (110) but demonstrate little effects on solid tumors (111), while T cells engineered to express TCR constructs display encouraging curative outcomes in the therapy of solid tumors (112). Some preclinical studies suggest that the antigen density recognized by the CAR must be high on the target cell to initiate T cell activation (113, 114). Additionally, cross-presentation of the antigen in nearby/draining lymph nodes might be an important factor for TCR redirected cells to build up a reservoir and maintain activation (115). Therefore, the use of TCR redirected T cells could be appropriate for antigens expressed in low densities whereas T cells modified to display CAR constructs better target overexpressed antigens. A safety problem of the TCR Treg cell strategy is also caused by potential TCR mispairing with the endogenous TCR resulting in T cells with unpredicted specificity. To avoid this, replacement of the endogenous TCR by using gene editing technology as recently reported by Stadtmauer might be necessary (116). Finally, although no cytotoxicity was demonstrated in the initial preclinical CAR-Treg cell studies, first Macdonald and then Boroughs and colleagues reported that CAR-stimulated Tregs might also exhibit cytotoxic activity (57, 117). Thus, the cytotoxic activity of CAR Treg cells needs to be studied in more detail to avoid unexpected adverse effects in the future. The very first preclinical study with antigen-specific CAR redirected Tregs was already performed in 2008 by the Eshhar’s group. As demonstrated by the authors, CAR redirected Treg cells accumulated at colonic inflammatory lesions and suppressed effector T cells in a specific, non-MHC-restricted manner, resulting in significant amelioration of colitis (55). Interestingly, the authors were already using a second-generation CAR containing a CD28-gamma signaling domain. The CAR design has changed only slightly until today, whereby the gamma-signaling unit was replaced by the CD3zeta signaling domain. In this context, Dawson and colleagues reported a comprehensive comparison of coreceptor signaling domain CAR variants in human Treg cells and revealed that inclusion of the CD28 costimulatory domain was essential for potent function (28). Moreover, CARs encoding domains from TNFR family members, such as 4-1BB, were unable to confer a protective effect in comparison to irrelevant Ag-specific control Treg cells (28). As shown by Lamarthée and colleagues, ligand-independent CAR tonic signaling significantly affects the biology of CAR-Tregs and thereby compromises their suppressive function (118). The authors demonstrated in their study that the negative effects of 4-1BB tonic signaling in Treg cells could be mitigated by transient mTOR inhibition (118). Currently, the range of applications using redirected Treg cells has been expanded to other diseases such as Graft versus hosed disease (GVHD), type 1 diabetes, multiple sclerosis, vitiligo, asthma or haemophilia (27, 56, 119–122) (see also Table 1). Based on preclinical but also clinical results, we can already notice today that CAR Treg cells are slowly emerging as a promising strategy for the treatment of autoimmune diseases and as adjunctive therapy in transplantation. Many clinical studies were already performed using polyclonal Treg cells (19, 20, 22, 26, 123, 124) with important lessons learned from, e.g. production procedures, Treg stability, cell doses, circulation in patients, tolerability and combination with immunosuppressive drugs. Some clinical efficacy could be seen by successful weaning of immunosuppressives or, for diabetes, prolonged c-peptide production. However, the suppressive activity at the site of inflammation could not be detected and there was no improvement in metabolic function or rejection rate, respectively. Preclinical data indicate that engineered Treg cells (CAR or TCR) might be more efficacious to treat autoimmune diseases and transplantation rejection, but the clinical use of redirected (CAR) Treg cells is only just beginning (Table 1). The purpose of the first in human study with redirected CAR Treg cells is to evaluate the safety and tolerability of HLA-A2-specific CAR Treg cells (TX200-TR101) and its effects on the donated kidney in living donor kidney transplant recipients (17). There is another HLA-A2-specific CAR Treg study (LIBERATE, Phase I/II) starting in 2022, which, most interestingly, will also address clinical outcome and immunosuppression in liver transplantation (QEL-001) (18). In this context, HLA-A2-specific CAR redirected (CD4 or CD8) Treg cells have been already used in different pre-clinical studies of skin transplantation demonstrating superior suppression of human skin graft rejection and reduced GvHD in humanized mouse models (58, 125, 126).
Discussion
Although the immunomodulatory properties of Treg cells in the field of autoimmune diseases and transplantation medicine has been repeatedly demonstrated in many preclinical studies and clinical trials, some limitations have prevented the widespread use of this form of immunotherapy. This includes, on the one hand, the selection of the suitable Treg cell population to enhance efficacy. On the other hand, the targeted immunosuppressive effect on the local immune response is not supposed to cause global immunosuppression. Recently, technical progress such as redirection of T cells by CAR constructs, conversion of T cell into Treg cell using FOXP3 transfer or targeted gene editing using CRISPR/Cas9 allow to design Treg cells with a defined specificity and functionality in a rapid and efficient manner. New applications of Treg cells outside autoimmune diseases and transplantations demonstrate multiple uses of Treg-mediated immune modulation. As demonstrated by numerous groups, in many injured tissues, so-called ‘repair’ Treg cells are recruited to the damaged site to facilitate inflammation resolution and to regulate immunity after injury (127–129). Furthermore, Baek and colleagues demonstrated that Treg cell administration has a neuroprotective effect on pathology and cognitive function in a mouse model of Alzheimer’s disease. In detail, Treg cells had an impact on cognitive function, decreasing amyloid-beta deposition and inflammatory cytokine levels (130). In the future, the use of redirected CAR-Treg cells can potentially increase the efficacy and prevent global immunosuppression of Treg-based immunotherapies and thus make an important contribution to clinical implementation (17, 18), giving new hope for a cure to millions of suffering patients.
Author contributions
MC and TR participated in manuscript writing, editing and cooperation of its submission. All authors contributed to the article and approved the submitted version.
Funding
The authors received funding from Deutsche Krebshilfe (DKH) grant 3641 0237 21 and Deutsche Forschungsgemeinschaft (DFG) grant CH 2463/1-1. This work was further supported by the SFB1530.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y, et al. A special population of regulatory T cells potentiates muscle repair. Cell (2013) 155(6):1282–95. doi: 10.1016/j.cell.2013.10.054
2. Arpaia N, Green JA, Moltedo B, Arvey A, Hemmers S, Yuan S, et al. A distinct function of regulatory t cells in tissue protection. Cell (2015) 162(5):1078–89. doi: 10.1016/j.cell.2015.08.021
3. Kempkes RWM, Joosten I, Koenen H, He X. Metabolic pathways involved in regulatory t cell functionality. Front Immunol (2019) 10:2839. doi: 10.3389/fimmu.2019.02839
4. Dejaco C, Duftner C, Grubeck-Loebenstein B, Schirmer M. Imbalance of regulatory T cells in human autoimmune diseases. Immunology (2006) 117(3):289–300. doi: 10.1111/j.1365-2567.2005.02317.x
5. Kos K, Aslam MA, Wellenstein MD, Pieters W, van Weverwijk A, Duits DEM, et al. Tumor-educated tregs drive organ-specific metastasis in breast cancer by impairing NK cells in the lymph node niche. Cell Rep (2022) 38(9):110447. doi: 10.1016/j.celrep.2022.110447
6. Kidani Y, Nogami W, Yasumizu Y, Kawashima A, Tanaka A, Sonoda Y, et al. CCR8-targeted specific depletion of clonally expanded treg cells in tumor tissues evokes potent tumor immunity with long-lasting memory. Proc Natl Acad Sci USA (2022) 119(7):e2114282119. doi: 10.1073/pnas.2114282119
7. Li C, Jiang P, Wei S, Xu X, Wang J. Regulatory T cells in tumor microenvironment: new mechanisms, potential therapeutic strategies and future prospects. Mol Cancer (2020) 19(1):116. doi: 10.1158/1557-3125.HIPPO19-B11
8. Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol (2010) 11(1):7–13. doi: 10.1038/ni.1818
9. Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity (2009) 30(5):626–35. doi: 10.1016/j.immuni.2009.05.002
10. Lyon MF, Peters J, Glenister PH, Ball S, Wright E. The scurfy mouse mutant has previously unrecognized hematological abnormalities and resembles wiskott-Aldrich syndrome. Proc Natl Acad Sci USA (1990) 87(7):2433–7. doi: 10.1073/pnas.87.7.2433
11. Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet (2001) 27(1):20–1. doi: 10.1038/83713
12. Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, et al. X-Linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet (2001) 27(1):18–20. doi: 10.1038/83707
13. Barnes MJ, Griseri T, Johnson AM, Young W, Powrie F, Izcue A. CTLA-4 promotes Foxp3 induction and regulatory T cell accumulation in the intestinal lamina propria. Mucosal Immunol (2013) 6(2):324–34. doi: 10.1038/mi.2012.75
14. Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med (2000) 192(2):295–302. doi: 10.1084/jem.192.2.295
15. Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA (2003) 100(14):8372–7. doi: 10.1073/pnas.1533209100
16. Gravano DM, Vignali DA. The battle against immunopathology: infectious tolerance mediated by regulatory T cells. Cell Mol Life Sci (2012) 69(12):1997–2008. doi: 10.1007/s00018-011-0907-z
17. Safety & tolerability study of chimeric antigen receptor T-reg cell therapy in living donor renal transplant recipients. Available at: https://ClinicalTrials.gov/show/NCT04817774.
18. Safety and clinical activity of QEL-001 in A2-mismatch liver transplant patients. Available at: https://ClinicalTrials.gov/show/NCT05234190.
19. The ONE study nTreg trial (ONEnTreg13). Available at: https://ClinicalTrials.gov/show/NCT02371434.
20. T1DM immunotherapy using CD4+CD127lo/-CD25+ polyclonal tregs. Available at: https://ClinicalTrials.gov/show/NCT01210664.
21. Trzonkowski P, Bieniaszewska M, Juscinska J, Dobyszuk A, Krzystyniak A, Marek N, et al. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127- T regulatory cells. Clin Immunol (2009) 133(1):22–6. doi: 10.1016/j.clim.2009.06.001
22. Marek-Trzonkowska N, Myśliwiec M, Dobyszuk A, Grabowska M, Derkowska I, Juścińska J, et al. Therapy of type 1 diabetes with CD4(+)CD25(high)CD127-regulatory T cells prolongs survival of pancreatic islets - results of one year follow-up. Clin Immunol (2014) 153(1):23–30. doi: 10.1016/j.clim.2014.03.016
23. Dall’Era M, Pauli ML, Remedios K, Taravati K, Sandova PM, Putnam AL, et al. Adoptive treg cell therapy in a patient with systemic lupus erythematosus. Arthritis Rheumatol (2019) 71(3):431–40. doi: 10.1002/art.40737
24. Safety and efficacy study of regulatory T cells in treating autoimmune hepatitis. Available at: https://ClinicalTrials.gov/show/NCT02704338.
25. Goldberg R, Scotta C, Cooper D, Nissim-Eliraz E, Nir E, Tasker S, et al. Correction of defective t-regulatory cells from patients with crohn’s disease by ex vivo ligation of retinoic acid receptor-alpha. Gastroenterology (2019) 156(6):1775–87. doi: 10.1053/j.gastro.2019.01.025
26. Safety and efficacy study of regulatory T cell therapy in liver transplant patients. Available at: https://ClinicalTrials.gov/show/NCT02166177.
27. Skuljec J, Chmielewski M, Happle C, Habener A, Busse M, Abken H, et al. Chimeric antigen receptor-redirected regulatory t cells suppress experimental allergic airway inflammation, a model of asthma. Front Immunol (2017) 8:1125. doi: 10.3389/fimmu.2017.01125
28. Dawson NAJ, Rosado-Sanchez I, Novakovsky GE, Fung VCW, Huang Q, McIver E, et al. Functional effects of chimeric antigen receptor co-receptor signaling domains in human regulatory T cells. Sci Transl Med (2020) 12(557):eaaz3866. doi: 10.1126/scitranslmed.aaz3866
29. Yeh WI, Seay HR, Newby B, Posgai AL, Moniz FB, Michels A, et al. Avidity and bystander suppressive capacity of human regulatory T cells expressing De novo autoreactive T-cell receptors in type 1 diabetes. Front Immunol (2017) 8:1313. doi: 10.3389/fimmu.2017.01313
30. Fu RY, Chen AC, Lyle MJ, Chen CY, Liu CL, Miao CH. CD4(+) T cells engineered with FVIII-CAR and murine Foxp3 suppress anti-factor VIII immune responses in hemophilia a mice. Cell Immunol (2020) 358:104216. doi: 10.1016/j.cellimm.2020.104216
31. Xu Y, Liu E, Xie X, Wang J, Zheng H, Ju Y, et al. Induction of Foxp3 and activation of tregs by HSP gp96 for treatment of autoimmune diseases. iScience (2021) 24(12):103445. doi: 10.1016/j.isci.2021.103445
32. Seddiki N, Santner-Nanan B, Tangye SG, Alexander SI, Solomon M, Lee S, et al. Persistence of naive CD45RA+ regulatory T cells in adult life. Blood (2006) 107(7):2830–8. doi: 10.1182/blood-2005-06-2403
33. Baron U, Floess S, Wieczorek G, Baumann K, Grutzkau A, Dong J, et al. DNA Demethylation in the human FOXP3 locus discriminates regulatory T cells from activated FOXP3(+) conventional T cells. Eur J Immunol (2007) 37(9):2378–89. doi: 10.1002/eji.200737594
34. Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, et al. Expression of Helios, an ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol (2010) 184(7):3433–41. doi: 10.4049/jimmunol.0904028
35. Thornton AM, Lu J, Korty PE, Kim YC, Martens C, Sun PD, et al. Helios(+) and helios(-) treg subpopulations are phenotypically and functionally distinct and express dissimilar TCR repertoires. Eur J Immunol (2019) 49(3):398–412. doi: 10.1002/eji.201847935
36. Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW, Santacruz N, et al. Peripheral education of the immune system by colonic commensal microbiota. Nature (2011) 478(7368):250–4. doi: 10.1038/nature10434
37. Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, et al. Induction of colonic regulatory T cells by indigenous clostridium species. Science (2011) 331(6015):337–41. doi: 10.1126/science.1198469
38. Verhagen J, Wraith DC. Comment on “Expression of Helios, an ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol (2010) 185(12):7129. doi: 10.4049/jimmunol.1090105
39. Gottschalk RA, Corse E, Allison JP. Expression of Helios in peripherally induced Foxp3+ regulatory T cells. J Immunol (2012) 188(3):976–80. doi: 10.4049/jimmunol.1102964
40. Elkord E. Comment on “Expression of Helios in peripherally induced Foxp3+ regulatory T cells. J Immunol (2012) 189(2):500. doi: 10.4049/jimmunol.1290033
41. Szurek E, Cebula A, Wojciech L, Pietrzak M, Rempala G, Kisielow P, et al. Differences in expression level of helios and neuropilin-1 do not distinguish thymus-derived from extrathymically-induced cd4+foxp3+ regulatory t cells. PLoS One (2015) 10(10):e0141161. doi: 10.1371/journal.pone.0141161
42. Zhao H, Liao X, Kang Y. Tregs: Where we are and what comes next? Front Immunol (2017) 8:1578. doi: 10.3389/fimmu.2017.01578
43. Biswas M, Kumar SRP, Terhorst C, Herzog RW. Gene therapy with regulatory t cells: a beneficial alliance. Front Immunol (2018) 9:554. doi: 10.3389/fimmu.2018.00554
44. Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol (2012) 30:531–64. doi: 10.1146/annurev.immunol.25.022106.141623
45. Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, et al. Expression of ectonucleotidase CD39 by Foxp3+ treg cells: hydrolysis of extracellular ATP and immune suppression. Blood (2007) 110(4):1225–32. doi: 10.1182/blood-2006-12-064527
46. Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med (2007) 204(6):1257–65. doi: 10.1084/jem.20062512
47. Kleinewietfeld M, Starke M, Di Mitri D, Borsellino G, Battistini L, Rotzschke O, et al. CD49d provides access to “untouched” human Foxp3+ treg free of contaminating effector cells. Blood (2009) 113(4):827–36. doi: 10.1182/blood-2008-04-150524
48. Kraczyk B, Remus R, Hardt C. CD49d treg cells with high suppressive capacity are remarkably less efficient on activated CD45RA- than on naive CD45RA+ teff cells. Cell Physiol Biochem (2014) 34(2):346–55. doi: 10.1159/000363004
49. Lam AJ, Lin DTS, Gillies JK, Uday P, Pesenacker AM, Kobor MS, et al. Optimized CRISPR-mediated gene knockin reveals FOXP3-independent maintenance of human treg identity. Cell Rep (2021) 36(5):109494. doi: 10.1016/j.celrep.2021.109494
50. Bailey-Bucktrout SL, Martinez-Llordella M, Zhou X, Anthony B, Rosenthal W, Luche H, et al. Self-antigen-driven activation induces instability of regulatory T cells during an inflammatory autoimmune response. Immunity (2013) 39(5):949–62. doi: 10.1016/j.immuni.2013.10.016
51. Polesso F, Sarker M, Anderson A, Parker DC, Murray SE. Constitutive expression of NF-kappaB inducing kinase in regulatory T cells impairs suppressive function and promotes instability and pro-inflammatory cytokine production. Sci Rep (2017) 7(1):14779. doi: 10.1038/s41598-017-14965-x
52. Passerini L, Bacchetta R. Forkhead-Box-P3 gene transfer in human cd4(+) t conventional cells for the generation of stable and efficient regulatory t cells, suitable for immune modulatory therapy. Front Immunol (2017) 8:1282. doi: 10.3389/fimmu.2017.01282
53. Landwehr-Kenzel S, Zobel A, Schmitt-Knosalla I, Forke A, Hoffmann H, Schmueck-Henneresse M, et al. Cyclosporine a but not corticosteroids support efficacy of ex vivo expanded, adoptively transferred human tregs in gvhd. Front Immunol (2021) 12:716629. doi: 10.3389/fimmu.2021.716629
54. Landwehr-Kenzel S, Mueller-Jensen L, Kuehl JS, Abou-El-Enein M, Hoffmann H, Muench S, et al. Adoptive transfer of ex vivo expanded regulatory T-cells improves immune cell engraftment and therapy-refractory chronic GvHD. Mol Ther (2022) 30(6):2298–314. doi: 10.1016/j.ymthe.2022.02.025
55. Elinav E, Waks T, Eshhar Z. Redirection of regulatory T cells with predetermined specificity for the treatment of experimental colitis in mice. Gastroenterology (2008) 134(7):2014–24. doi: 10.1053/j.gastro.2008.02.060
56. Fransson M, Piras E, Burman J, Nilsson B, Essand M, Lu B, et al. CAR/FoxP3-engineered T regulatory cells target the CNS and suppress EAE upon intranasal delivery. J Neuroinflamm (2012) 9:112. doi: 10.1186/1742-2094-9-112
57. MacDonald KG, Hoeppli RE, Huang Q, Gillies J, Luciani DS, Orban PC, et al. Alloantigen-specific regulatory T cells generated with a chimeric antigen receptor. J Clin Invest (2016) 126(4):1413–24. doi: 10.1172/JCI82771
58. Noyan F, Zimmermann K, Hardtke-Wolenski M, Knoefel A, Schulde E, Geffers R, et al. Prevention of allograft rejection by use of regulatory t cells with an mhc-specific chimeric antigen receptor. Am J Transplant (2017) 17(4):917–30. doi: 10.1111/ajt.14175
59. Muller YD, Ferreira LMR, Ronin E, Ho P, Nguyen V, Faleo G, et al. Precision engineering of an anti-hla-a2 chimeric antigen receptor in regulatory t cells for transplant immune tolerance. Front Immunol (2021) 12:686439. doi: 10.3389/fimmu.2021.686439
60. Attridge K, Walker LS. Homeostasis and function of regulatory T cells (Tregs) in vivo: lessons from TCR-transgenic tregs. Immunol Rev (2014) 259(1):23–39. doi: 10.1111/imr.12165
61. Smith BM, Lyle MJ, Chen AC, Miao CH. Antigen-specific in vitro expansion of factor VIII-specific regulatory T cells induces tolerance in hemophilia a mice. J Thromb Haemost (2020) 18(2):328–40. doi: 10.1111/jth.14659
62. Brusko TM, Koya RC, Zhu S, Lee MR, Putnam AL, McClymont SA, et al. Human antigen-specific regulatory T cells generated by T cell receptor gene transfer. PloS One (2010) 5(7):e11726. doi: 10.1371/journal.pone.0011726
63. Putnam AL, Safinia N, Medvec A, Laszkowska M, Wray M, Mintz MA, et al. Clinical grade manufacturing of human alloantigen-reactive regulatory T cells for use in transplantation. Am J Transplant (2013) 13(11):3010–20. doi: 10.1111/ajt.12433
64. Landwehr-Kenzel S, Issa F, Luu SH, Schmuck M, Lei H, Zobel A, et al. Novel GMP-compatible protocol employing an allogeneic b cell bank for clonal expansion of allospecific natural regulatory T cells. Am J Transplant (2014) 14(3):594–606. doi: 10.1111/ajt.12629
65. Wagner DL, Amini L, Wendering DJ, Burkhardt LM, Akyuz L, Reinke P, et al. High prevalence of streptococcus pyogenes Cas9-reactive T cells within the adult human population. Nat Med (2019) 25(2):242–8. doi: 10.1038/s41591-018-0204-6
66. Floess S, Freyer J, Siewert C, Baron U, Olek S, Polansky J, et al. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol (2007) 5(2):e38. doi: 10.1371/journal.pbio.0050038
67. Polansky JK, Kretschmer K, Freyer J, Floess S, Garbe A, Baron U, et al. DNA Methylation controls Foxp3 gene expression. Eur J Immunol (2008) 38(6):1654–63. doi: 10.1002/eji.200838105
68. Polansky JK, Schreiber L, Thelemann C, Ludwig L, Kruger M, Baumgrass R, et al. Methylation matters: binding of ets-1 to the demethylated Foxp3 gene contributes to the stabilization of Foxp3 expression in regulatory T cells. J Mol Med (Berl) (2010) 88(10):1029–40. doi: 10.1007/s00109-010-0642-1
69. Huehn J, Polan-sky JK, Hamann A. Epigenetic control of FOXP3 expression: the key to a stable regulatory T-cell lineage? Nat Rev Immunol (2009) 9(2):83–9. doi: 10.1038/nri2474
70. Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, et al. mTOR regulates memory CD8 T-cell differentiation. Nature (2009) 460(7251):108–12. doi: 10.1038/nature08155
71. Akhmetzyanova I, Zelinskyy G, Littwitz-Salomon E, Malyshkina A, Dietze KK, Streeck H, et al. CD137 agonist therapy can reprogram regulatory T cells into cytotoxic CD4+ T cells with antitumor activity. J Immunol (2016) 196(1):484–92. doi: 10.4049/jimmunol.1403039
72. Buck MD, O’Sullivan D, Klein Geltink RI, Curtis JD, Chang CH, Sanin DE, et al. Mitochondrial dynamics controls t cell fate through metabolic programming. Cell (2016) 166(1):63–76. doi: 10.1016/j.cell.2016.05.035
73. Gerriets VA, Kishton RJ, Johnson MO, Cohen S, Siska PJ, Nichols AG, et al. Foxp3 and toll-like receptor signaling balance t(reg) cell anabolic metabolism for suppression. Nat Immunol (2016) 17(12):1459–66. doi: 10.1038/ni.3577
74. Kishore M, Cheung KCP, Fu H, Bonacina F, Wang G, Coe D, et al. Regulatory T cell migration is dependent on glucokinase-mediated glycolysis. Immunity (2018) 48(4):831–2. doi: 10.1016/j.immuni.2018.03.034
75. Bezie S, Picarda E, Ossart J, Tesson L, Usal C, Renaudin K, et al. IL-34 is a treg-specific cytokine and mediates transplant tolerance. J Clin Invest (2015) 125(10):3952–64. doi: 10.1172/JCI81227
76. Bézie S, Anegon I, Guillonneau C. Advances on cd8+ treg cells and their potential in transplantation. Transplantation (2018) 102(9):1467–78. doi: 10.1097/TP.0000000000002258
77. Guillonneau C, Hill M, Hubert FX, Chiffoleau E, Herve C, Li XL, et al. CD40Ig treatment results in allograft acceptance mediated by CD8CD45RC T cells, IFN-gamma, and indoleamine 2,3-dioxygenase. J Clin Invest (2007) 117(4):1096–106. doi: 10.1172/JCI28801
78. Li XL, Menoret S, Bezie S, Caron L, Chabannes D, Hill M, et al. Mechanism and localization of CD8 regulatory T cells in a heart transplant model of tolerance. J Immunol (2010) 185(2):823–33. doi: 10.4049/jimmunol.1000120
79. Picarda E, Bezie S, Venturi V, Echasserieau K, Merieau E, Delhumeau A, et al. MHC-derived allopeptide activates TCR-biased CD8+ tregs and suppresses organ rejection. J Clin Invest (2014) 124(6):2497–512. doi: 10.1172/JCI71533
80. Picarda E, Bezie S, Boucault L, Autrusseau E, Kilens S, Meistermann D, et al. Transient antibody targeting of CD45RC induces transplant tolerance and potent antigen-specific regulatory T cells. JCI Insight (2017) 2(3):e90088. doi: 10.1172/jci.insight.90088
81. Picarda E, Bezie S, Usero L, Ossart J, Besnard M, Halim H, et al. Cross-reactive donor-specific cd8(+) tregs efficiently prevent transplant rejection. Cell Rep (2019) 29(13):4245–55.e6. doi: 10.1016/j.celrep.2019.11.106
82. Gundry MC, Brunetti L, Lin A, Mayle AE, Kitano A, Wagner D, et al. Highly efficient genome editing of murine and human hematopoietic progenitor cells by crispr/cas9. Cell Rep (2016) 17(5):1453–61. doi: 10.1016/j.celrep.2016.09.092
83. Amini L, Wagner DL, Rossler U, Zarrinrad G, Wagner LF, Vollmer T, et al. CRISPR-Cas9-edited tacrolimus-resistant antiviral t cells for advanced adoptive immunotherapy in transplant recipients. Mol Ther (2021) 29(1):32–46. doi: 10.1016/j.ymthe.2020.09.011
84. Allan SE, Alstad AN, Merindol N, Crellin NK, Amendola M, Bacchetta R, et al. Generation of potent and stable human CD4+ T regulatory cells by activation-independent expression of FOXP3. Mol Ther (2008) 16(1):194–202. doi: 10.1038/sj.mt.6300341
85. Tenspolde M, Zimmermann K, Weber LC, Hapke M, Lieber M, Dywicki J, et al. Regulatory T cells engineered with a novel insulin-specific chimeric antigen receptor as a candidate immunotherapy for type 1 diabetes. J Autoimmun (2019) 103:102289. doi: 10.1016/j.jaut.2019.05.017
86. Peterson LB, Bell CJM, Howlett SK, Pekalski ML, Brady K, Hinton H, et al. A long-lived IL-2 mutein that selectively activates and expands regulatory T cells as a therapy for autoimmune disease. J Autoimmun (2018) 95:1–14. doi: 10.1016/j.jaut.2018.10.017
87. Khoryati L, Pham MN, Sherve M, Kumari S, Cook K, Pearson J, et al. An IL-2 mutein engineered to promote expansion of regulatory T cells arrests ongoing autoimmunity in mice. Sci Immunol (2020) 5(50):eaba5264. doi: 10.1126/sciimmunol.aba5264
88. Hirai T, Ramos TL, Lin PY, Simonetta F, Su LL, Picton LK, et al. Selective expansion of regulatory T cells using an orthogonal IL-2/IL-2 receptor system facilitates transplantation tolerance. J Clin Invest (2021) 131(8):e139991. doi: 10.1172/JCI139991
89. Sockolosky JT, Trotta E, Parisi G, Picton L, Su LL, Le AC, et al. Selective targeting of engineered T cells using orthogonal IL-2 cytokine-receptor complexes. Science (2018) 359(6379):1037–42. doi: 10.1126/science.aar3246
90. Eshhar Z, Waks T, Oren T, Berke G, Kaufmann Y. Cytotoxic T cell hybridomas: generation and characterization. Curr Top Microbiol Immunol (1982) 100:11–8. doi: 10.1007/978-3-642-68586-6_2
91. Harding FA, Allison JP. CD28-B7 interactions allow the induction of CD8+ cytotoxic T lymphocytes in the absence of exogenous help. J Exp Med (1993) 177(6):1791–6. doi: 10.1084/jem.177.6.1791
92. Finney HM, Lawson AD, Bebbington CR, Weir AN. Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J Immunol (1998) 161(6):2791–7.
93. Tammana S, Huang X, Wong M, Milone MC, Ma L, Levine BL, et al. 4-1BB and CD28 signaling plays a synergistic role in redirecting umbilical cord blood T cells against b-cell malignancies. Hum Gene Ther (2010) 21(1):75–86. doi: 10.1089/hum.2009.122
94. Guedan S, Posey AD Jr., Shaw C, Wing A, Da T, Patel PR, et al. Enhancing CAR T cell persistence through ICOS and 4-1BB costimulation. JCI Insight (2018) 3(1):e96976. doi: 10.1172/jci.insight.96976
95. Milone MC, Fish JD, Carpenito C, Carroll RG, Binder GK, Teachey D, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther (2009) 17(8):1453–64. doi: 10.1038/mt.2009.83
96. Salter AI, Ivey RG, Kennedy JJ, Voillet V, Rajan A, Alderman EJ, et al. Phosphoproteomic analysis of chimeric antigen receptor signaling reveals kinetic and quantitative differences that affect cell function. Sci Signal (2018) 11(544):eaat6753. doi: 10.1126/scisignal.aat6753
97. Kofler DM, Chmielewski M, Rappl G, Hombach A, Riet T, Schmidt A, et al. CD28 costimulation impairs the efficacy of a redirected t-cell antitumor attack in the presence of regulatory t cells which can be overcome by preventing lck activation. Mol Ther (2011) 19(4):760–7. doi: 10.1038/mt.2011.9
98. Enblad G, Karlsson H, Gammelgård G, Wenthe J, Lövgren T, Amini RM, et al. A phase I/IIa trial using CD19-targeted third-generation CAR T cells for lymphoma and leukemia. Clin Cancer Res (2018) 24(24):6185–94. doi: 10.1158/1078-0432.CCR-18-0426
99. Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan DA, Feldman SA, et al. T Cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther (2011) 19(3):620–6. doi: 10.1038/mt.2010.272
100. Ahmed N, Brawley VS, Hegde M, Robertson C, Ghazi A, Gerken C, et al. Human epidermal growth factor receptor 2 (her2) -specific chimeric antigen receptor-modified t cells for the immunotherapy of her2-positive sarcoma. J Clin Oncol (2015) 33(15):1688–96. doi: 10.1200/JCO.2014.58.0225
101. Chmielewski M, Kopecky C, Hombach AA, Abken H. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res (2011) 71(17):5697–706. doi: 10.1158/0008-5472.CAN-11-0103
102. Bandukwala HS, Wu Y, Feuerer M, Chen Y, Barboza B, Ghosh S, et al. Structure of a domain-swapped FOXP3 dimer on DNA and its function in regulatory T cells. Immunity (2011) 34(4):479–91. doi: 10.1016/j.immuni.2011.02.017
103. Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell (2006) 126(2):375–87. doi: 10.1016/j.cell.2006.05.042
104. Kagoya Y, Tanaka S, Guo T, Anczurowski M, Wang CH, Saso K, et al. A novel chimeric antigen receptor containing a JAK-STAT signaling domain mediates superior antitumor effects. Nat Med (2018) 24(3):352–9. doi: 10.1038/nm.4478
105. Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med (2004) 199(11):1455–65. doi: 10.1084/jem.20040139
106. Stephens LA, Malpass KH, Anderton SM. Curing CNS autoimmune disease with myelin-reactive Foxp3+ treg. Eur J Immunol (2009) 39(4):1108–17. doi: 10.1002/eji.200839073
107. Sagoo P, Ali N, Garg G, Nestle FO, Lechler RI, Lombardi G. Human regulatory T cells with alloantigen specificity are more potent inhibitors of alloimmune skin graft damage than polyclonal regulatory T cells. Sci Transl Med (2011) 3(83):83ra42. doi: 10.1126/scitranslmed.3002076
108. Mezzanzanica D, Canevari S, Mazzoni A, Figini M, Colnaghi MI, Waks T, et al. Transfer of chimeric receptor gene made of variable regions of tumor-specific antibody confers anticarbohydrate specificity on T cells. Cancer Gene Ther (1998) 5(6):401–7.
109. Pfeifer R, Lock D, Aloia A, Bosio A, Kaiser A, Hardt O, et al. Sialyl glycolipid stage-specific embryonic antigen 4 (SSEA4) - a novel target for CAR T cell therapy of solid cancers. Mol Ther (2016) 24:S259. doi: 10.1016/S1525-0016(16)33461-X
110. Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med (2011) 365(8):725–33. doi: 10.1056/NEJMoa1103849
111. Katz SC, Burga RA, McCormack E, Wang LJ, Mooring W, Point GR, et al. Phase i hepatic immunotherapy for metastases study of intra-arterial chimeric antigen receptor-modified t-cell therapy for cea+ liver metastases. Clin Cancer Res (2015) 21(14):3149–59. doi: 10.1158/1078-0432.CCR-14-1421
112. D’Angelo SP, Melchiori L, Merchant MS, Bernstein D, Glod J, Kaplan R, et al. Antitumor activity associated with prolonged persistence of adoptively transferred ny-eso-1 (c259)t cells in synovial sarcoma. Cancer Discovery (2018) 8(8):944–57. doi: 10.1158/2159-8290.CD-17-1417
113. Walker AJ, Majzner RG, Zhang L, Wanhainen K, Long AH, Nguyen SM, et al. Tumor antigen and receptor densities regulate efficacy of a chimeric antigen receptor targeting anaplastic lymphoma kinase. Mol Ther (2017) 25(9):2189–201. doi: 10.1016/j.ymthe.2017.06.008
114. Fry TJ, Shah NN, Orentas RJ, Stetler-Stevenson M, Yuan CM, Ramakrishna S, et al. CD22-targeted CAR T cells induce remission in b-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med (2018) 24(1):20–8. doi: 10.1038/nm.4441
115. Alvarez D, Vollmann EH, von Andrian UH. Mechanisms and consequences of dendritic cell migration. Immunity (2008) 29(3):325–42. doi: 10.1016/j.immuni.2008.08.006
116. Stadtmauer EA, Fraietta JA, Davis MM, Cohen AD, Weber KL, Lancaster E, et al. CRISPR-engineered T cells in patients with refractory cancer. Science (2020) 367(6481):eaba7365. doi: 10.1126/science.aba7365
117. Boroughs AC, Larson RC, Choi BD, Bouffard AA, Riley LS, Schiferle E, et al. Chimeric antigen receptor costimulation domains modulate human regulatory T cell function. JCI Insight (2019) 5:e126194. doi: 10.1172/jci.insight.126194
118. Lamarthee B, Marchal A, Charbonnier S, Blein T, Leon J, Martin E, et al. Transient mTOR inhibition rescues 4-1BB CAR-tregs from tonic signal-induced dysfunction. Nat Commun (2021) 12(1):6446. doi: 10.1038/s41467-021-26844-1
119. Imura Y, Ando M, Kondo T, Ito M, Yoshimura A. CD19-targeted CAR regulatory T cells suppress b cell pathology without GvHD. JCI Insight (2020) 5(14):e136185. doi: 10.1172/jci.insight.136185
120. Radichev IA, Yoon J, Scott DW, Griffin K, Savinov AY. Towards antigen-specific tregs for type 1 diabetes: Construction and functional assessment of pancreatic endocrine marker, HPi2-based chimeric antigen receptor. Cell Immunol (2020) 358:104224. doi: 10.1016/j.cellimm.2020.104224
121. Mukhatayev Z, Dellacecca ER, Cosgrove C, Shivde R, Jaishankar D, Pontarolo-Maag K, et al. Antigen specificity enhances disease control by tregs in vitiligo. Front Immunol (2020) 11:581433. doi: 10.3389/fimmu.2020.581433
122. Rana J, Perry DJ, Kumar SRP, Munoz-Melero M, Saboungi R, Brusko TM, et al. CAR- and TRuC-redirected regulatory T cells differ in capacity to control adaptive immunity to FVIII. Mol Ther (2021) 29(9):2660–76. doi: 10.1016/j.ymthe.2021.04.034
123. Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK, et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med (2015) 7(315):315ra189. doi: 10.1126/scitranslmed.aad4134
124. Sawitzki B, Harden PN, Reinke P, Moreau A, Hutchinson JA, Game DS, et al. Regulatory cell therapy in kidney transplantation (The ONE study): a harmonised design and analysis of seven non-randomised, single-arm, phase 1/2A trials. Lancet (2020) 395(10237):1627–39. doi: 10.1016/S0140-6736(20)30167-7
125. Boardman DA, Philippeos C, Fruhwirth GO, Ibrahim MA, Hannen RF, Cooper D, et al. Expression of a chimeric antigen receptor specific for donor hla class i enhances the potency of human regulatory t cells in preventing human skin transplant rejection. Am J Transplant (2017) 17(4):931–43. doi: 10.1111/ajt.14185
126. Bézie S, Charreau B, Vimond N, Lasselin J, Gérard N, Nerrière-Daguin V, et al. Human CD8+ tregs expressing a MHC-specific CAR display enhanced suppression of human skin rejection and GVHD in NSG mice. Blood Adv (2019) 3(22):3522–38. doi: 10.1182/bloodadvances.2019000411
127. Murphy TJ, Ni Choileain N, Zang Y, Mannick JA, Lederer JA. CD4+CD25+ regulatory T cells control innate immune reactivity after injury. J Immunol (2005) 174(5):2957–63. doi: 10.4049/jimmunol.174.5.2957
128. Zhang C, Li L, Feng K, Fan D, Xue W, Lu J. ‘Repair’ treg cells in tissue injury. Cell Physiol Biochem (2017) 43(6):2155–69. doi: 10.1159/000484295
129. Popovic B, Golemac M, Podlech J, Zeleznjak J, Bilic-Zulle L, Lukic ML, et al. IL-33/ST2 pathway drives regulatory T cell dependent suppression of liver damage upon cytomegalovirus infection. PLoS Pathog (2017) 13(4):e1006345. doi: 10.1371/journal.ppat.1006345
Keywords: regulatory (Treg) cell, chimeric antigen receptor (CAR), autoimmune diseases, adoptive therapy, immunosuppressive therapy
Citation: Riet T and Chmielewski M (2022) Regulatory CAR-T cells in autoimmune diseases: Progress and current challenges. Front. Immunol. 13:934343. doi: 10.3389/fimmu.2022.934343
Received: 02 May 2022; Accepted: 19 July 2022;
Published: 10 August 2022.
Edited by:
Anne L. Astier, INSERM UMR1291 – , Toulouse III, FranceReviewed by:
Joshua Daniel Ooi, Monash University, AustraliaCopyright © 2022 Riet and Chmielewski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Markus Chmielewski, bWFya3VzLmNobWllbGV3c2tpQHVrLWtvZWxuLmRl
 Tobias Riet
Tobias Riet Markus Chmielewski
Markus Chmielewski