- 1Department of General Surgery, Tianjin Medical University General Hospital, Tianjin, China
- 2Tianjin General Surgery Institute, Tianjin Medical University General Hospital, Tianjin, China
Background: IL-37 is a recently identified cytokine with potent immunosuppressive functions. The research fronts of IL-37 are worth investigating, and there is no bibliometric analysis in this field. The purpose of this study is to construct the intellectual base and predict research hotspots of IL-37 research both quantitatively and qualitatively according to bibliometric analysis.
Methods: The articles were downloaded from the Web of Science Core Collection (WoSCC) database from the inception of the database to 1 April 2022. CiteSpace 5.8.R3 (64-bit, Drexel University, Philadelphia, PA, USA) and Online Analysis Platform of Literature Metrology (https://bibliometric.com/) were used to perform bibliometric and knowledge-map analyses.
Results: A total of 534 papers were included in 200 academic journals by 2,783 authors in 279 institutions from 50 countries/regions. The journal Cytokine published the most papers on IL-37, while Nature Immunology was the most co-cited journal. The publications belonged mainly to two categories of Immunology and Cell Biology. USA and China were the most productive countries. Meanwhile, the University of Colorado Denver in USA produced the highest number of publications followed by Radboud University Nijmegen in the Netherlands and Monash University in Australia. Charles A. Dinarello published the most papers, while Marcel F. Nold had the most co-citations. Top 10 co-citations on reviews, mechanisms, and diseases were regarded as the knowledge base. The keyword co-occurrence and co-citations of references revealed that the mechanisms and immune-related disorders were the main aspects of IL-37 research. Notably, the involvement of IL-37 in various disorders and the additional immunomodulatory mechanisms were two emerging hotspots in IL-37 research.
Conclusions: The research on IL-37 was thoroughly reviewed using bibliometrics and knowledge-map analyses. The present study is a benefit for academics to master the dynamic evolution of IL-37 and point out the direction for future research.
Introduction
IL-37, previously termed as IL-IF7, is a novel number of the IL-1 family discovered by computational prediction and renamed in 2010 (1). IL-37 is recently identified as a natural suppressor of innate immunity (2). The coding genes of IL-37, IL-1α, and IL-1β are also located on the long arm of chromosome 2 and are located closer following LPS stimulation (3). When pro-inflammatory signals induce the transcription of IL-1α and IL-1β genes, IL-37 transcription is also induced to limit excessive inflammation (3). The properties of this spatial structure are critical for the immunomodulatory effects of IL-37. Besides, it has been established that there is a “dual function” of IL-37 in that it is capable of acting intracellularly and extracellularly (4, 5). Above all, extracellular IL-37 bound to immobilized ligand-binding IL-18Rα and the decoy receptor IL-1R8 (5). Recombinant IL-37 resulted in a 16-fold increase in IL-1R8 mRNA levels in M1 macrophages. Beyond this, the expression level of TNF-α and IL-6 in mouse bone marrow-derived dendritic cells stimulated by LPS decreased by 50%–55% but not in an IL-1R8-deficient mice (5). The formation of the IL-37/IL-18Rα/IL-1R8 complex is a key mechanism by which IL-37 exerts its extracellular suppressive function (6). Notably, accumulating evidence indicated that endogenous IL-37 was effectively induced by inflammatory stimulation, which inhibited the production of IL-6, TNF-α, IL-1β, and protected mice against LPS-induced shock (7). Similar to other members of the IL-1 family, IL-37 has a caspase-1 cleavage domain at the aspartate site of the N-terminal sequence of exon 1 (8). The caspase-1 processes IL-37 precursors into mature IL-37 (9). The mature IL-37 then forms a compound with phosphorylated Smad3, which translocate into the nucleus exerting actual anti-inflammatory effects (10). It has been demonstrated that silencing Smad3 in IL-37-overexpressing RAW264.7 and THP-1 cell lines diminishes the anti-inflammatory role of IL-37, thereby inducing TNF-α and IL-6 expression (11). Similar findings have been validated in IL-37tg mice (11). Given broad immunomodulatory roles, IL-37 has been shown to be directly implicated in several inflammatory diseases, such as systemic lupus erythematosus and inflammatory bowel disease (12, 13). Due to its potent effect for inhibiting innate and adaptive immune responses, our recent publication revealed that IL-37 was also closely associated with cancer development such as oral squamous cell carcinoma, colorectal cancer, and hepatocellular carcinoma (14–16). With a constantly expanding number of articles, IL-37 has piqued the interest of academics. Many scholars have reviewed IL-37 research from various aspects. Su (17) summarized the expression and release of IL-37, IL-37-dependent intracellular and extracellular signaling, genetic variants, pathophysiological effects, IL-37-induced effects on cholesterol homeostasis, regulation of innate and acquired immunity, the role of IL-37 in mesenchymal stem cell, immune cells, autoimmune diseases, and cancer. Nevertheless, to the best of our knowledge, there are no reports with a comprehensive analysis of publication trends, authoritative author and institutions, and their cooperation, knowledge domain, and emerging trends in IL-37 research.
Bibliometrics refers to the quantitative analysis of scholar publications (18, 19). Bibliometrics could be used to qualitatively and quantitatively review relevant research from this period, comprehensively sort out the context of the IL-37 study, and encourage and offer new insights into IL-37 research (20). As far as we are aware, currently there is no bibliometric analysis of IL-37 research. This study aimed to use CiteSpace software and Online Analysis Platform of Literature Metrology to objectively describe the knowledge domain and emerging trends of IL-37 research. We quantified and identified the general information in IL-37 research such as annual publication trends, cooperation information, and the impact of author, institution, and country. Otherwise, we conducted the most co-cited references and keywords to assess the intellectual base of IL-37. Most of all, co-cited references, keyword bursts, and clustering were performed to detect the hotspots evolution and emerging topics.
Materials and methods
Data collection
Journal publications have strong timeliness and could accurately reflect the progression and changes of a research topic. The Web of Science Core Collection (WoSCC) bibliographic database is among the largest and most comprehensive electronic scientific literature databases worldwide (21). The retrieved documents in this database ensure the reliability and authority of the conclusions. The data were collected from the inception of the database to 1 April 2022 (22). The retrieval strategy used in this study was set to TS = (“IL-37” OR “IL-1F7” OR “interleukin 37” OR “interleukin 1 family, member 7 (zeta)” OR “IL1F7”). The article type was limited to Article or Review, and the language was confined to English. Then, the search results were recorded with the content of “Full Record and Cited References” in the format of “Plain Text.”
Data analysis and visualization
We exported the citation reports and search results from the WoSCC database including the number of annual published articles, the annual citation number, the number of publications and H-indexes in different counties and institutions, and the quantity of categories. The downloaded files were imported into Microsoft Excel 2021 (version 16.48), CiteSpace 5.8.R3 (64-bit, Drexel University, Philadelphia, PA, USA), and Online Analysis Platform of Literature Metrology (https://bibliometric.com/) for further analysis (23, 24). CiteSpace, developed by Prof. Chao-mei Chen, has been the most applied bibliometrics software particularly adept at detecting the intellectual structure, research frontiers and dynamics, publication trend, and cooperation (25). Therefore, CiteSpace was performed to analyze and visualize co-cited references, co-cited authors, co-cited journals, cited reference bursts, co-occurrence of keywords, citation bursts for keywords, dual maps of journals, keyword clusters, co-cited reference clusters, and timelines. The specific settings used in CiteSpace were established as follows: time slicing was from January 2001 to December 2022 and years per slice = 1. Text processing, node types, link strength and scope, and selection criteria followed the default. Minimum spanning trees and pruning sliced networks were selected during the analysis process of keyword co-occurrence and burst. Pathfinder and pruning sliced networks were selected in the analysis of the co-cited reference cluster and other indicators. Microsoft Excel 2021 was used to visualize the annual publication number and citation number. Online Analysis Platform of Literature Metrology provided the bibliometric analysis of scientific citation data in an intuitive and friendly manner. The publication numbers among authors, institutions, journals, and collaboration between countries were visualized and analyzed based on this platform.
Results
Obtaining relevant literature
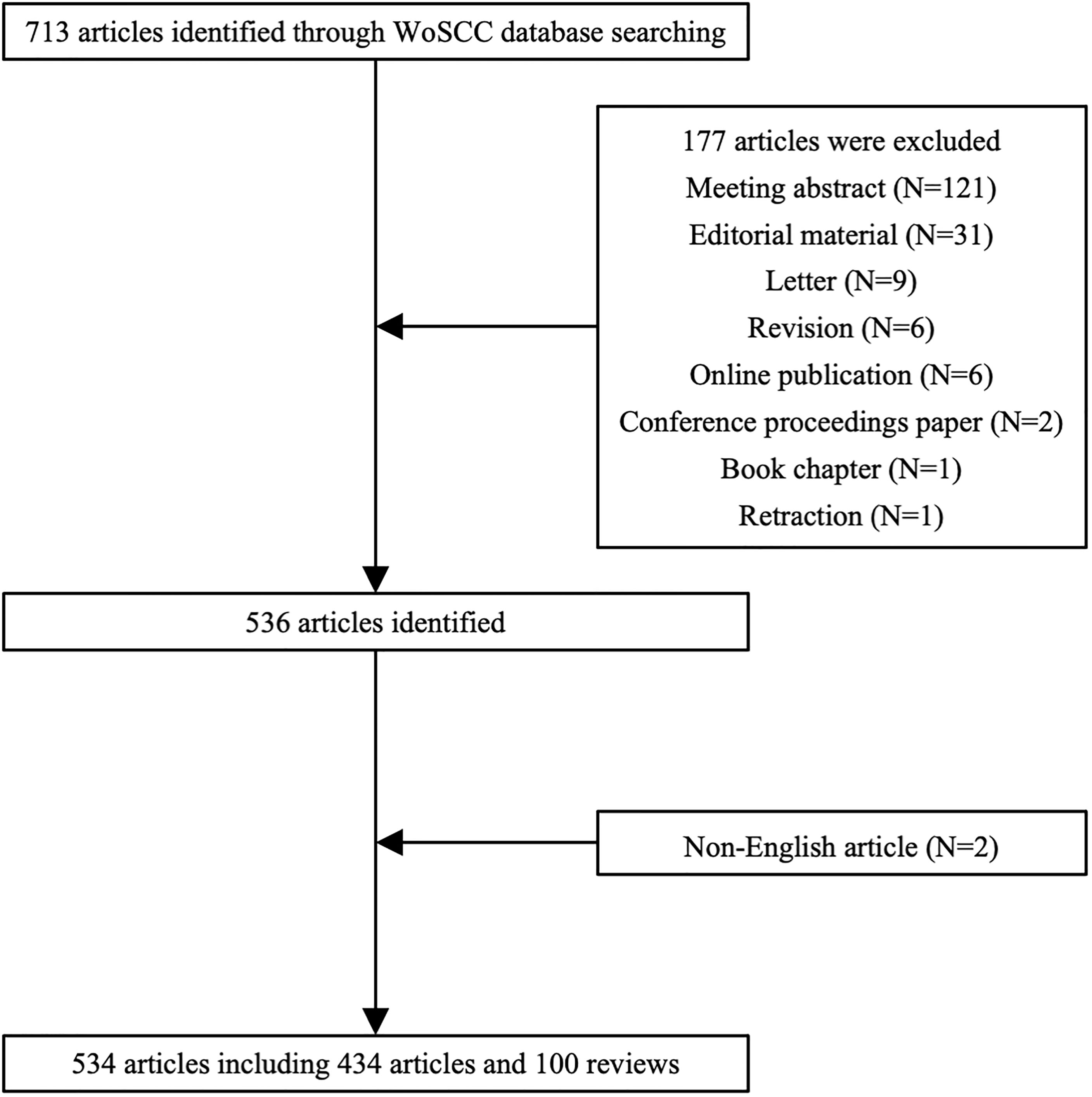
A total of 713 articles were identified through the WoSCC database initially. One hundred seventy-seven articles were ruled out based on the exclusion criteria including 121 meeting abstracts, 31 editorial materials, nine letters, six revisions, six online publications, two conference proceeding papers, one book chapter, one retraction, and two non-English articles. Five hundred thirty-four articles including 434 articles and 100 reviews were ultimately included in the present research (Figure 1).
Annual publication growth trend
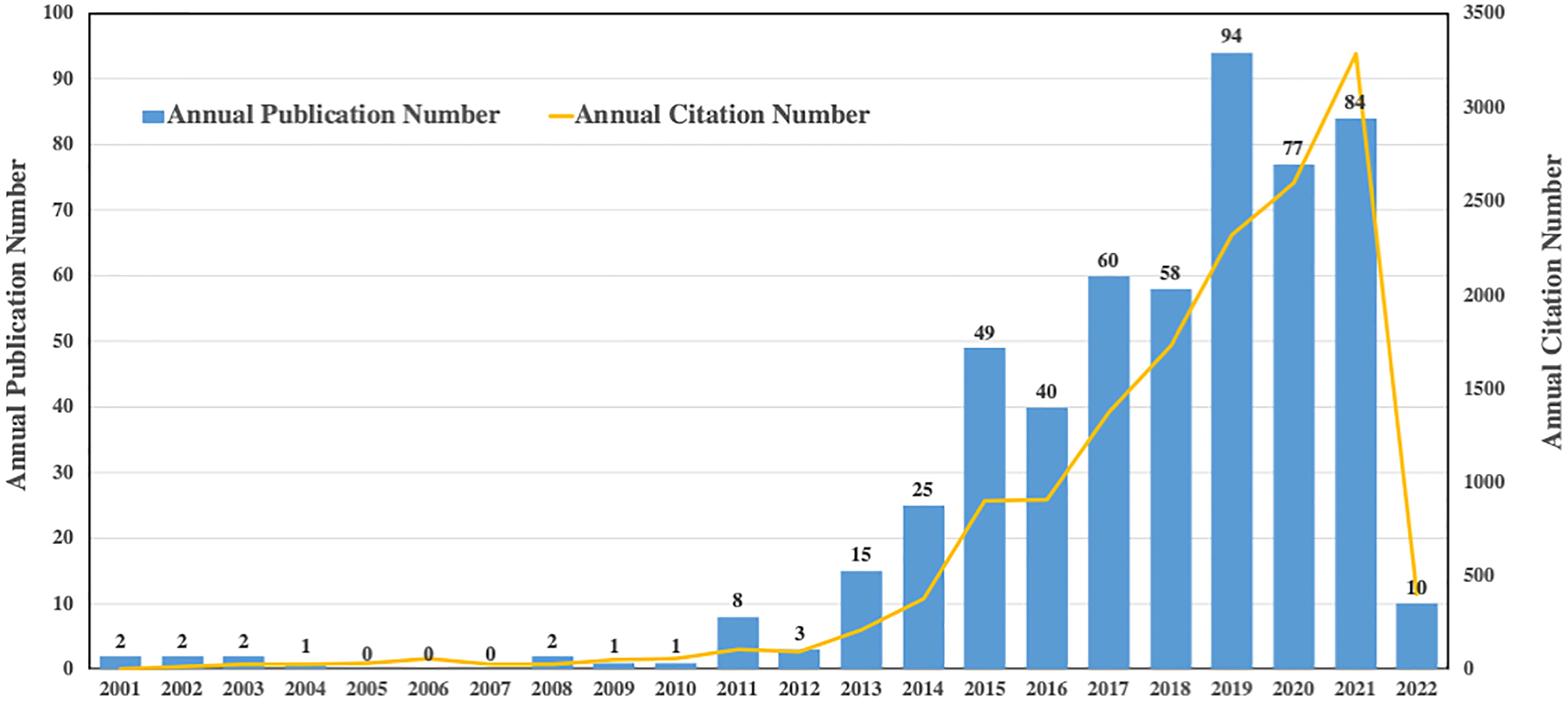
As we have known, IL-37 belongs to the IL-1 family and could also be referred to as IL-1F7 (26). The literature search yielded only 22 articles during the time period from 2001 to 2012 (Figure 2). Strikingly, the number of papers published on IL-37 topics overall continuously increased from 2013 to 2021 in a fluctuating manner, with a slight drop in 2016 and 2021. The highest annual number of published articles was more than 90. Phenomenally, the annual citation number has steadily increased from 2013 to 2021. Collectively, these results suggested that the research on IL-37 is appealing and that more prospective studies in the field of IL-37 are still needed in the future.
Spatial distribution map of countries/regions, institutions, and collaboration
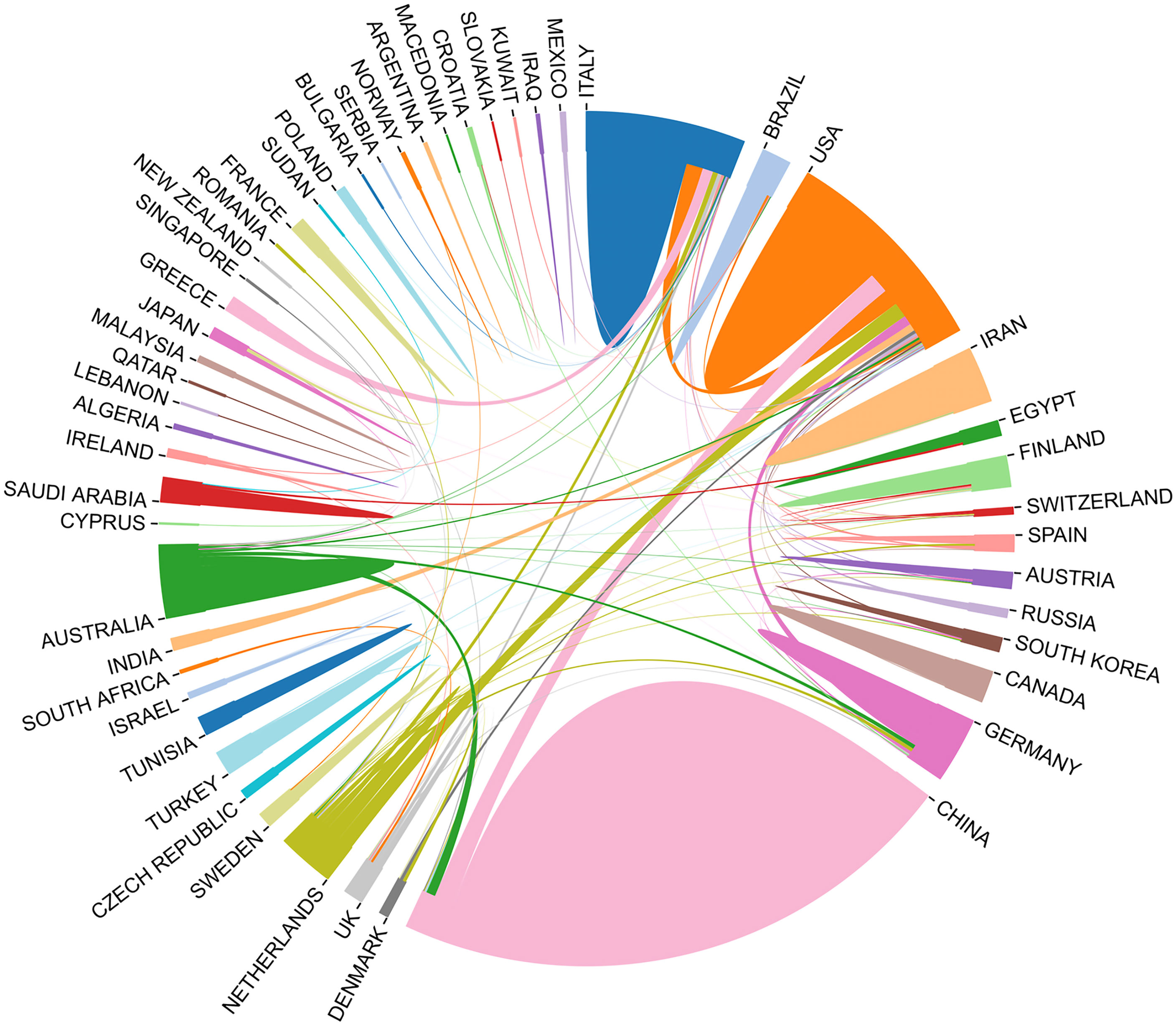
Fifty countries and 279 institutions have contributed research on the IL-37 field. As summarized in Table 1, the top 10 productive countries/regions and institutions were ranked. It is noteworthy that China, USA, Italy, Netherlands, and Germany are among the top 5 countries in the world in terms of IL-37 research, with China (257/48.1%) ranking first, followed by USA (23.2%) and Italy (10.5%). Although China has contributed nearly half the number of publications in the research of the IL-37 field, the total number of citations was 2,709, which is far less than that of USA (6455). The H-index was computed by taking the highest number of H such that H publications have at least H citations (27). Unsurprisingly, USA has the highest H index of 39, which is far higher than those of China (33) Italy (24), and Netherlands (23). The above data indicated that USA was the most influential country, far ahead of the rest in the research of the IL-37 field. The University of Colorado Denver (48, 9.0%) from USA produced the highest number of publications in IL-37 literature followed by Radboud University Nijmegen (47, 8.8%) in the Netherlands and Monash University (43, 8.1%). Besides, among the top 10 productive institutions, Fudan University, Shandong University, Huazhong University of Science and Technology, Guangdong Medical University, and Shenzhen University are all from China. It should also be noted that several affiliations such as the University of Colorado Denver (0.11), Radboud University Nijmegen (0.11), Huazhong University of Science and Technology (0.21), and Shenzhen University (0.21) showed high centrality, which means that these institutions occupy an important position in the research of the IL-37 field. As shown in Figure 3, collaboration network analysis revealed that the most frequent collaboration occurred between USA and China, followed by USA and the Netherlands.
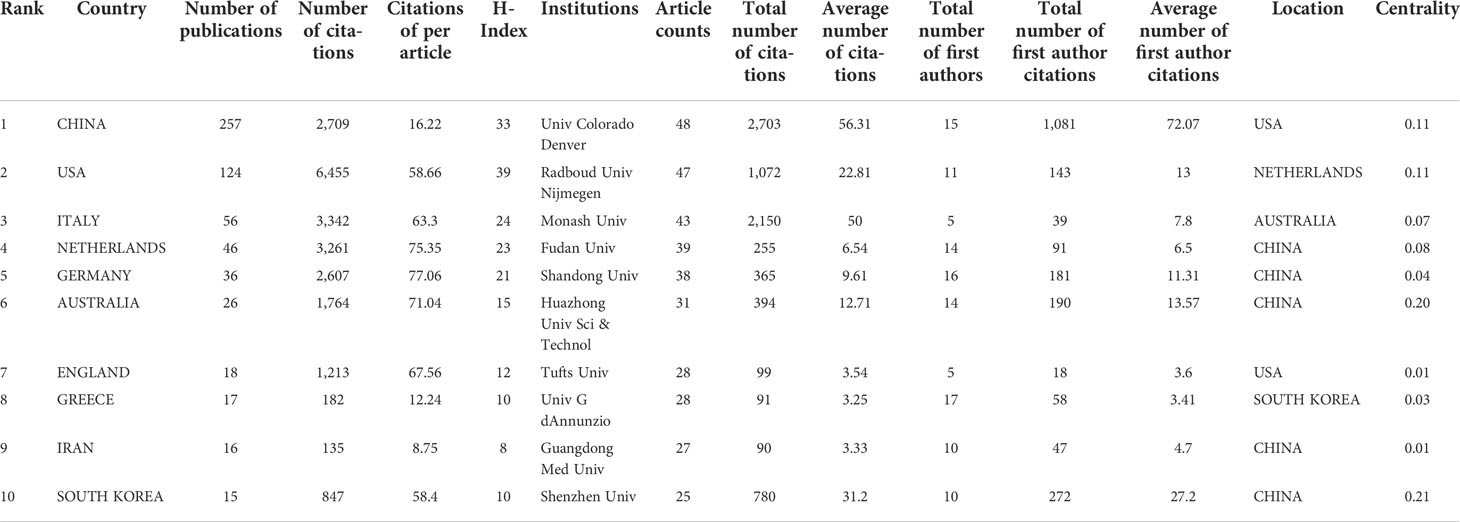
Table 1 The top 10 countries/regions and institutions contributing to publications of IL-37 research.
Visual analysis of journals and co-cited journals
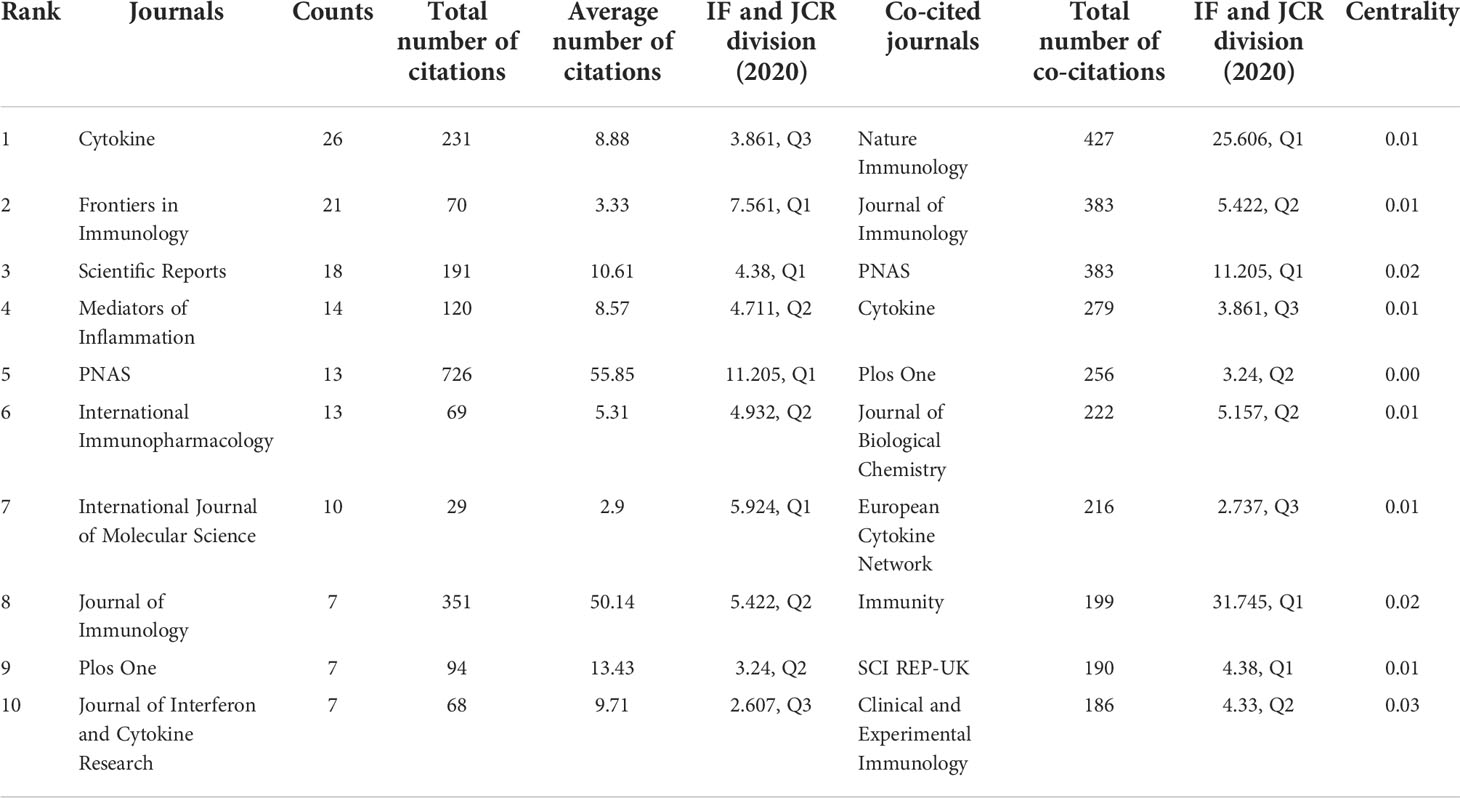
Online Analysis Platform of Literature Metrology was applied to identify the most productive and influential journals in the field of IL-37 research. The results show that 534 articles were published in 200 academic journals. As shown in Table 2, cytokine (3.861, Q3) published the most papers (26, 4.9%) with 231 total citations, followed by Frontiers in Immunology (7.561, Q1), Scientific Reports (4.38, Q1), and Mediators of Inflammation (4.711, Q2). Although only 13 articles were published in Proceedings of the National Academy of Sciences of the United States of America (PNAS, 11.205, Q1), it received 726 citations which are threefold more than the journal of Cytokine. Among the top 10 journals, four were at the Q1 JCR division and four had an impact factor (IF) of more than 5. Among 519 co-cited journals, seven journals had citations over 200. Nature Immunology (25.606, Q1) had the most co-citations, followed by Journal of Immunology (5.422, Q2), PNAS (11.205, Q1), and Cytokine (3.861, Q3). Among the top 10 co-cited journals, four were at the Q1 JCR division, and five had an IF of more than 5.
Knowledge flow analysis is used to explore the evolutionary relationship of knowledge citations and co-citations between citing and cited journals (28). The dual-map overlay of journals can intuitively show the distribution of journals in various disciplines, the evolution of citation trajectories, and the drift of scientific research centers (28, 29). It is a new method to show the flow of knowledge at the journal level on the dual-map overlay of journals. The citing map is on the left and the cited map is on the right. All colored curves originating from the citing map and pointing to the ones cited are the citation connection lines, which completely show the ins and outs of the citation. In the citing map, the more papers the journal publishes, the longer the vertical axis of the ellipse, and the greater the number of authors, the longer the horizontal axis of the ellipse (28). Figure 4 in the present research indicates that the articles published in Molecular/Biology/Genetics journals are often cited by Medicine/Medical/Clinical journals and Molecular/Biology/Immunology journals.
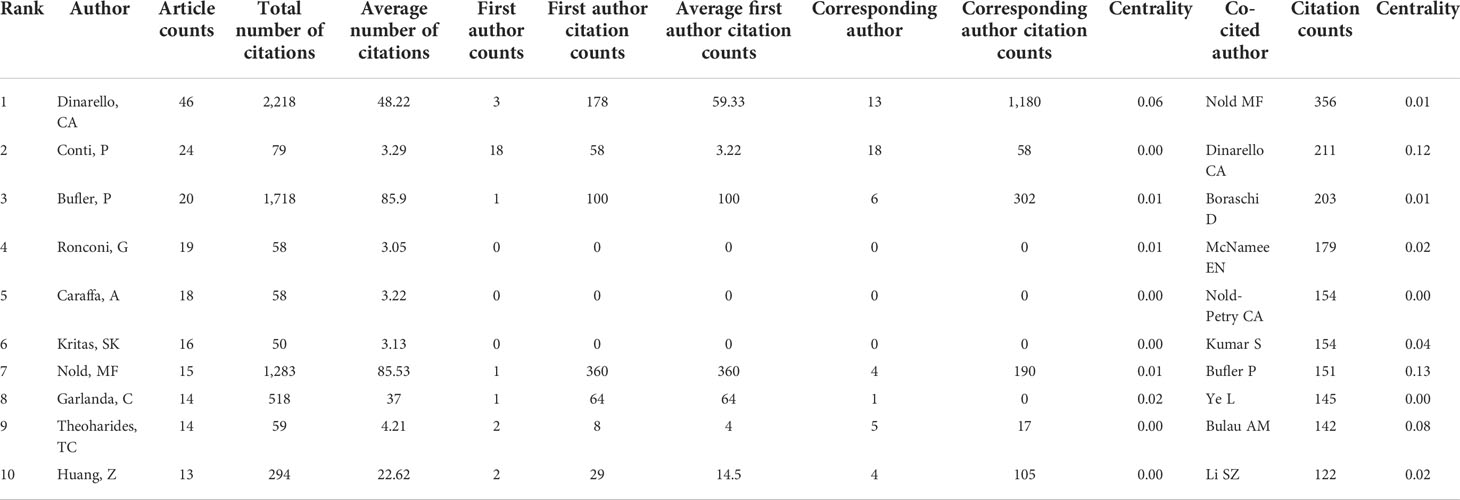
Analysis of authors and co-cited authors
Co-cited author analysis refers to when the literatures of two authors are simultaneously cited by a third author. The higher the number of co-citations, the closer their academic interests are, and the closer the research density is (30). Analysis of the most productive author and co-cited author in the IL-37 field research could intuitively reflect the research strength and hot topics of research. The 534 selected publications were produced by 2,783 authors, and the top 10 productive authors and co-cited authors are listed in Table 3. Charles A. Dinarello (Department of Medicine, Radboud University Medical Center and Department of Medicine, University of Colorado Denver) headed with 46 documents and 2,218 total citations, followed by Conti with 24 documents with a total of only 79 of citations. Bufler from the Department of Medicine, University of Colorado Denver ranked third in productive author with 20 documents and 1,718 total citations. We also note that although only 15 articles have been published by Marcel F. Nold (Department of Medicine, University of Colorado Denver), 1,283 citations were cited. As we have seen, Charles A. Dinarello, Bufler, and Marcel F. Nold, productive authors with the most total number of citations, are all from the University of Colorado Denver, implying that the University of Colorado Denver produced chief articles in this field. Meanwhile, we cannot ignore that five out of 10 authors have centrality less than 0.01, which means that these authors should strengthen cooperation with others. It is demonstrated in Table 3 that the most frequently co-cited author is Marcel F. Nold (356 citations), who is also the seventh most productive, right after whom Charles A. Dinarello appears with 211 citations, which is also the most productive author. The following authors are Boraschi (203 citations), McNamee (179 citations), and Claudia A. Nold-Petry (154 citations). Indeed, China has produced the largest number of literatures in this field. However, the most productive and co-cited authors are American scholars. It could be that numerous fundamental studies have been carried out by American scholars particularly at an early stage.
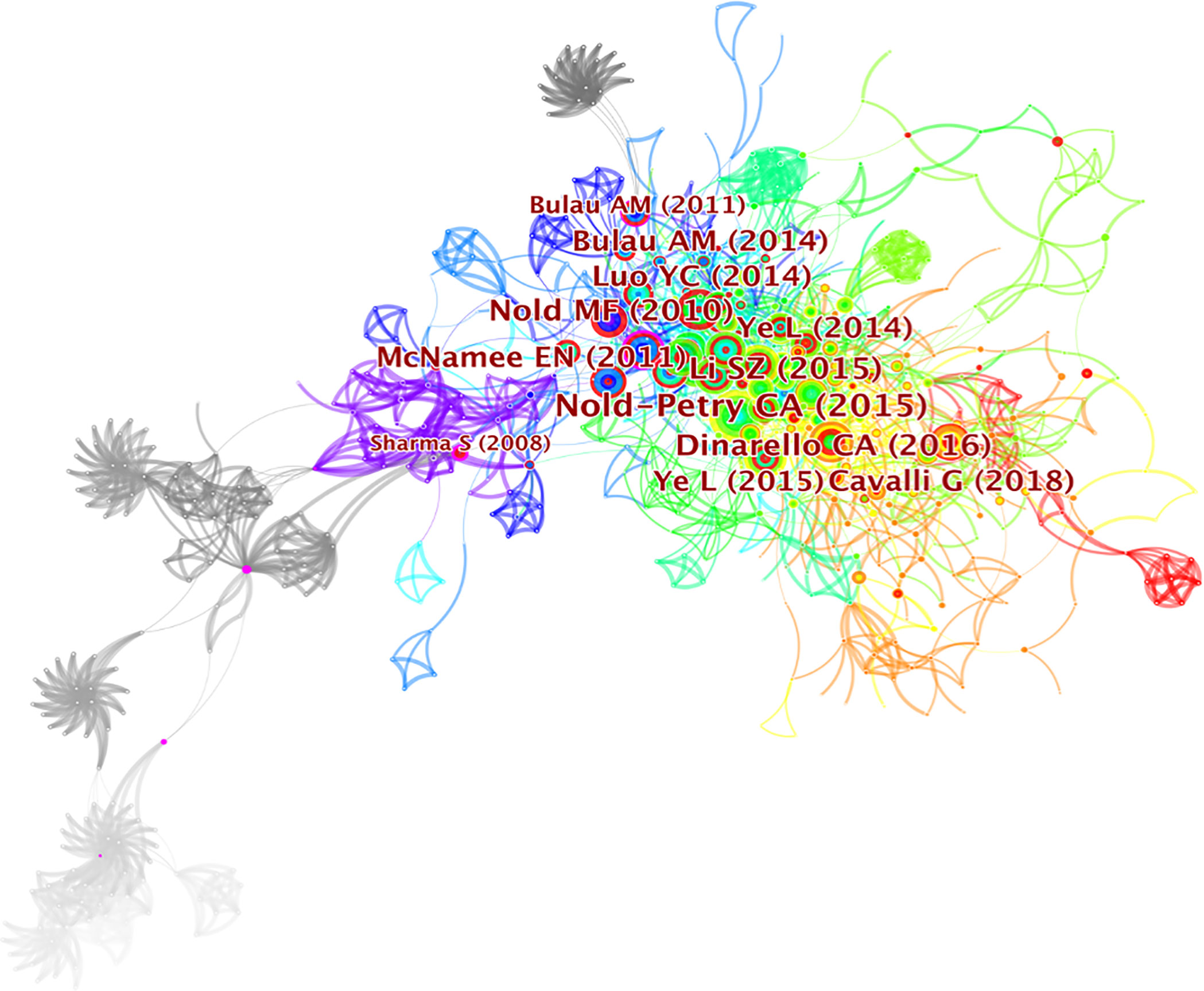
Co-cited reference analysis and clustering network
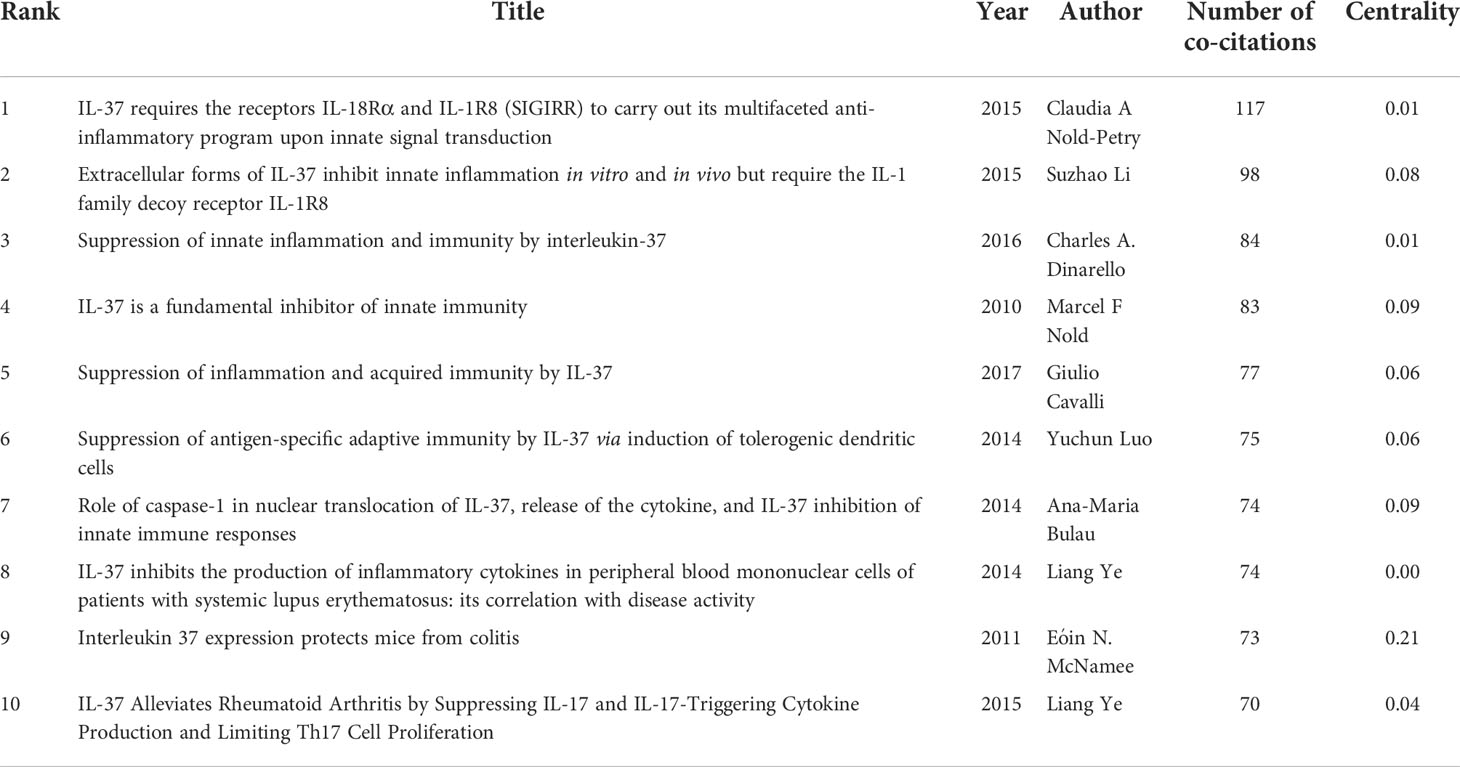
Literature co-citation analysis expresses the relationship between documents by analyzing the frequency at which they are cited (31). The knowledge base consists of a collection of co-cited articles, while research fronts consist of collections of citing articles (32). The clusters of co-cited references in CiteSpace were determined by extracting titles of citing articles using a log‐likelihood ratio algorithm (33). The clusters of co-cited references were deemed as research fronts (34). A total of 776 co-cited references were visualized by CiteSpace and are demonstrated in Figure 5. The first author and the top 12 most cited references are clearly seen on the graph. The size of circles is proportional to the co-citation frequency. The thickness of a ring of circles is directly proportional to the number of co-citations in the corresponding time zone. The thicker the ring, the more co-citations in the time zone. The color of the link between the two circles represents the year of the first co-citation for two references. Table 4 presents the details of top 10 co-cited references in the field of IL-37 research. The most co-cited reference performed by Claudia A. Nold-Petry et al. (35) in 2015 was an original article published in Nature Immunology, entitled “IL-37 requires the receptors IL-18Rα and IL-1R8 (SIGIRR) to carry out its multifaceted anti-inflammatory program upon innate signal transduction”, followed by an article entitled “Extracellular forms of IL-37 inhibit innate inflammation in vitro and in vivo but require the IL-1 family decoy receptor IL-1R8” (5). The two articles respectively discuss the extracellular mechanism of IL-37 and assessed its role in innate immunity. The results of two studies set the basis for further research on IL-37. The five frequently cited literatures are from USA, and the first five are from the University of Colorado Denver. Therefore, the University of Colorado Denver attached importance of IL-37 research not only to the quantity but also to the quality of the articles. In contrast, it is not ignored that the nine out of top 10 co-cited references with centrality less than 0.1, suggesting that the scholars on IL-37 research lack cooperation and academic exchanges. The co-cited reference entitled “Interleukin 37 expression protects mice from colitis” with high betweenness centrality (0.21) could be identified as a vital point that provides important bridging connections between two research interests.
The cluster analysis of literature co-citation could objectively show the knowledge structure of the research field. On this basis, we further constructed a network map to visualize the top 10 clusters of co-cited references (Figure 6A). Pathfinder and pruning sliced networks were selected to remain the most salient network structure when the co-cited references were clustered. Modularity was 0.799, and the weighted mean silhouette was 0.9156. Therefore, the results of the cluster in the present research are compelling. Clearly, all the clusters shown in Table 5 are highly homogeneous with silhouette values above 0.7, suggesting a reliable clustering result. Cluster #0, namely, cardiovascular disease, was the largest cluster consisting of 123 references, followed by disease activity (cluster #1), differential expression (cluster #2), new insight (cluster #3), complex (cluster #4) and related cytokine (cluster #5), mast cell (cluster #6), disease pathogenesis (cluster #7), epidermal barrier (cluster #8), and IL-1f5 (cluster #9). To reveal the research trends and hotspots over time, we conducted a timeline view of co-cited references (Figure 6B). It is obvious that clusters#1 have a high concentration of nodes with citation bursts from 2010. Cluster #0 has a sustained period of about 10 years from 2010 to 2020, whereas cluster #2 is short-lived with an associated period of 7 years from 2008 to 2015. The results exhibited that cluster #1 has the largest nodes scattered along the timeline and contains 8 of the 10 most frequently cited references (2, 4, 5, 12, 35–38). Besides, it is obvious that the research on IL-1F5 (SIGIRR, cluster #9) with the size of 21 publications started earlier and relevant studies has a certain interruption period. Furthermore, cluster #4 and cluster#9 are separate from other clusters with infrequent citation links connecting to other clusters.
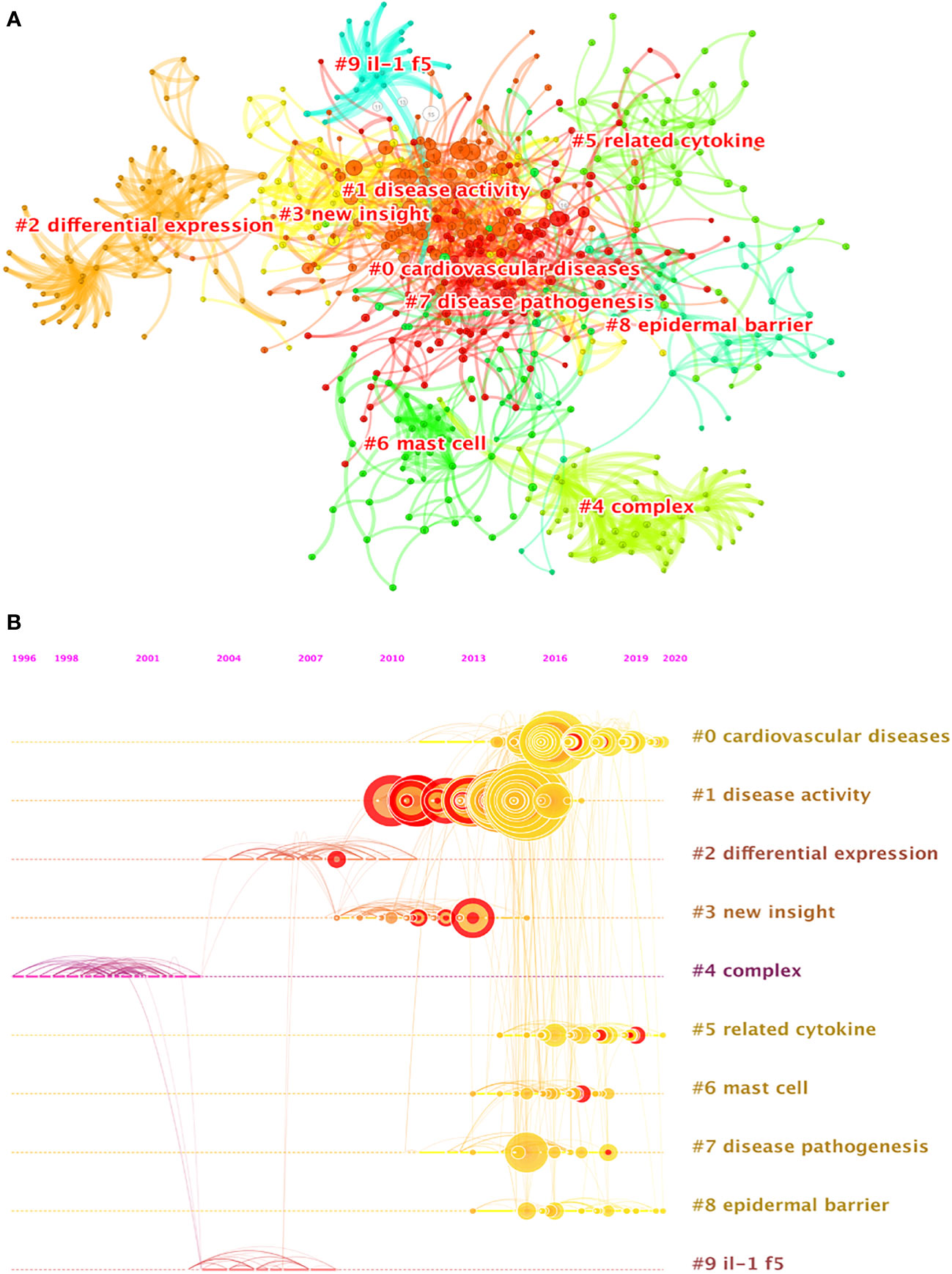
Figure 6 (A) The clustered network map of co-cited references on IL-37 research. (B) The timeline view of co-citation clusters.
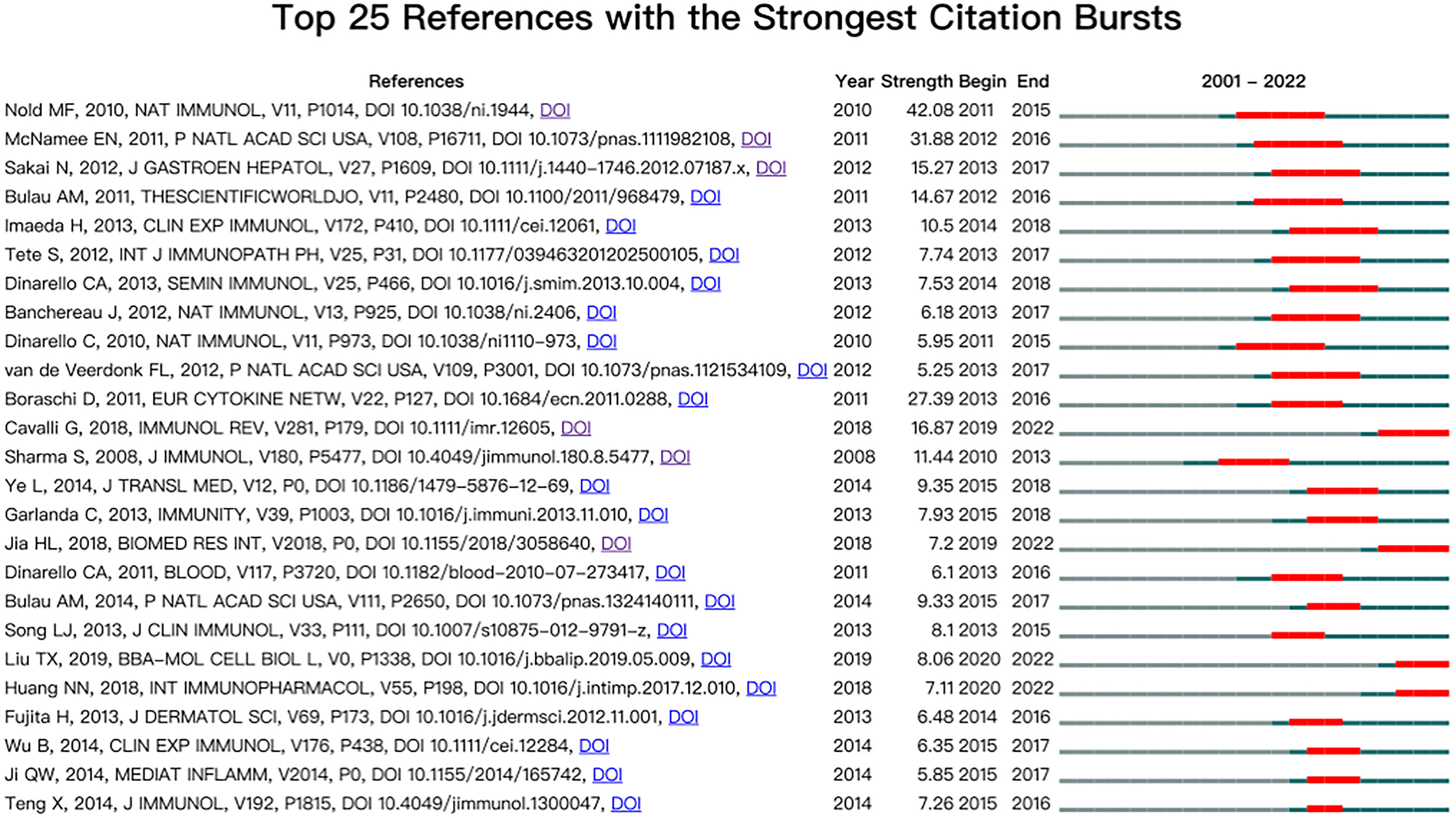
Burst detection of co-cited references
Co-cited reference bursts provide helpful insight into the research footprint of the research focus (39, 40). In CiteSpace, the number of states was stetted to 2, γ [0,1] = 1.0, and minimum duration = 1. The top 25 co-cited references with strong bursts were recorded (Figure 7). The first co-citation burst began in 2001, entitled “The IL-1 Family Member 7b Translocates to the Nucleus and Down-Regulates Proinflammatory Cytokines” (9). The paper with the strongest burst (strength = 42.08) was entitled “IL-37 is a fundamental inhibitor of innate immunity”, published in Nature Immunology by Marcel F. Nold in 2010, with bursts from 2011 to 2015 (2). The result showed that 34.55% (19/25) of the references showed citation burstiness in 2014, followed by 2013 (2/25, 27.8%) and 2015 (5/25, 24.0%). Remarkably, four references were in burstiness until 2022 which indicated that research related to IL-37 may continue to be explored in the future.
Keyword co-occurrence and burst
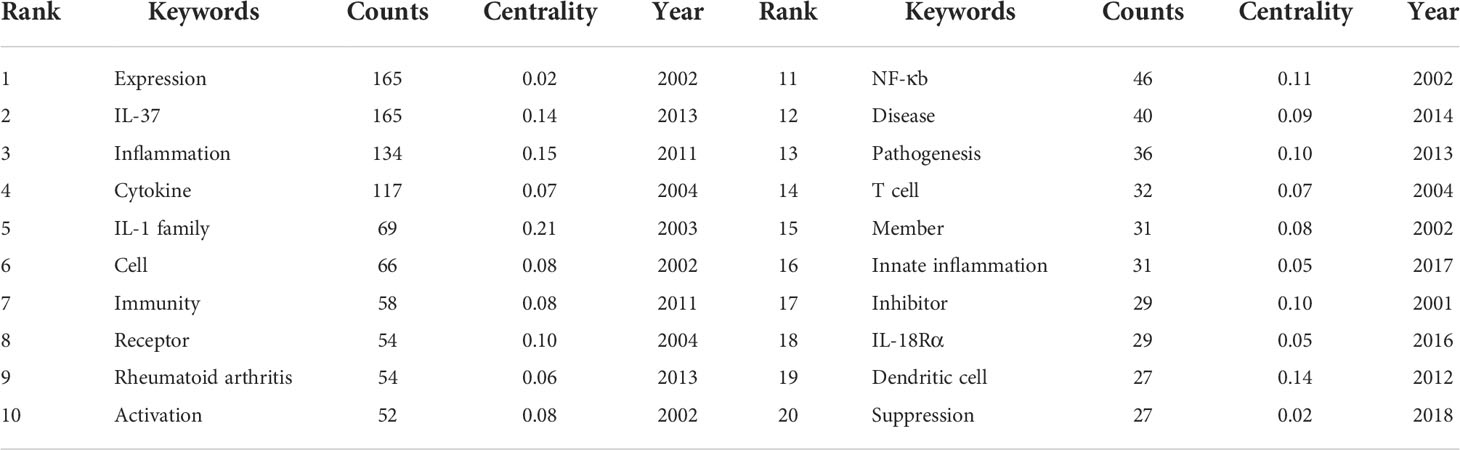
In bibliometrics, keyword co-occurrence and burst (Figures 8A, B) can reflect the emerging concepts that increased abruptly over time. A total of 423 keywords were extracted, of which 57 keywords appeared more than 10 times. As demonstrated in Figure 8A and Table 6, “IL-37” and “expression” were the most important terms with 165 co-occurrences, followed by “inflammation”, “cytokine”, “IL-1 family”, “cell”, and “immunity”. In addition, the centrality of “IL-37”, “inflammation”, “IL-1 family”, “receptor”, “NF-κB”, “pathogenesis”, “pathogenesis”, and “inhibitor” was greater than or equal to 0.1. Also, the burst for keywords revealed that “Suppression” is still the research front of IL-37. Considering the immunomodulatory role of IL-37, the function of IL-37 in inhibiting the potentiating immune responses in different disorders should be verified more in the future.
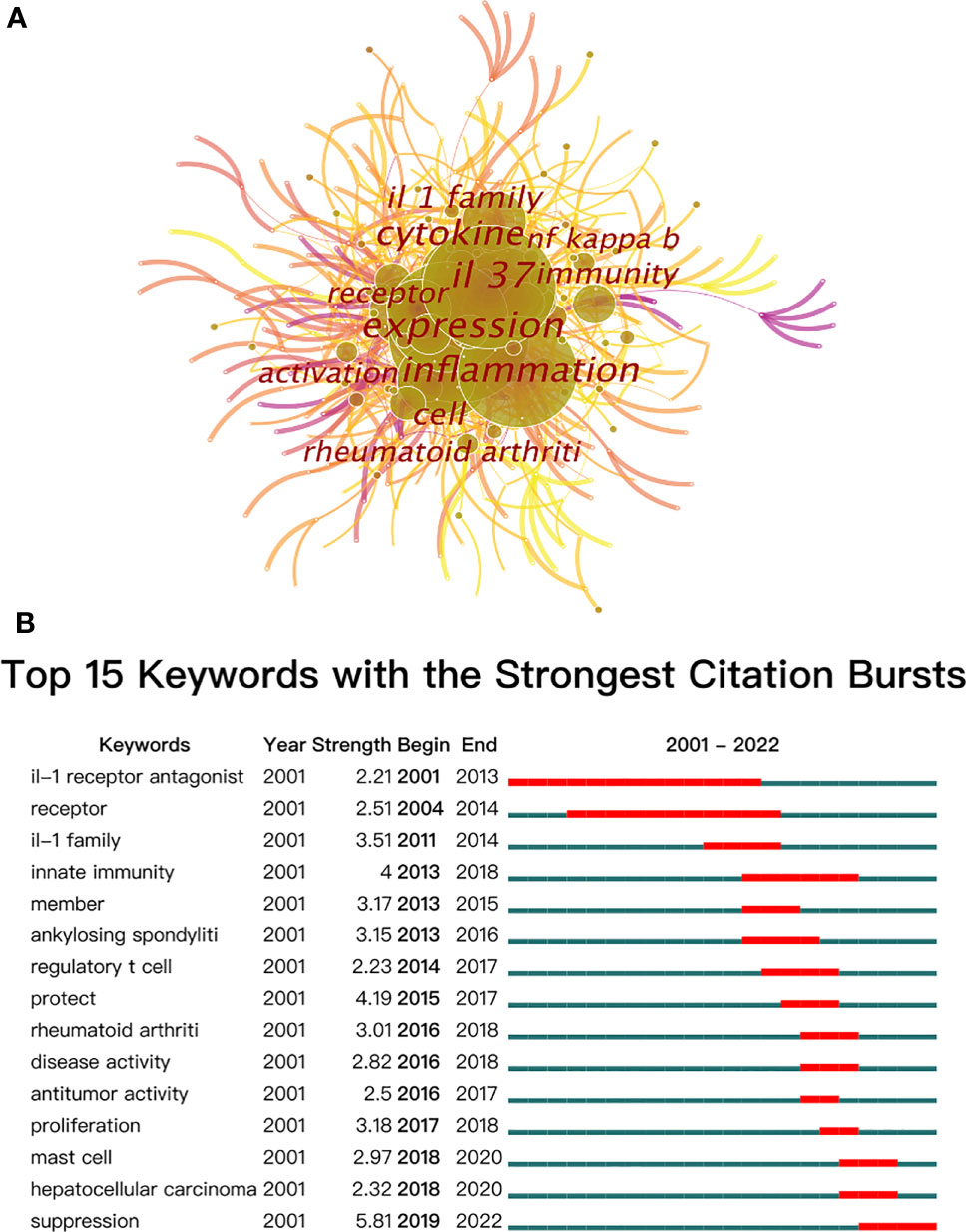
Figure 8 (A) The co-occurrence map of keywords on IL-37 research. (B) Top 15 keywords with the strongest citation bursts.
Subject categories
According to the results of WOS, the categories of Immunity and Cell Biology account for almost 50% of IL-37 research followed by Biochemistry Molecular Biology (16.854%), Medicine Research Experimental (9.738%), and Multidisciplinary Sciences (8.24%). The immunomodulatory effect of IL-37 and its role in different cells remain a major focus of IL-37 research (Table 7).
Discussion
To our knowledge, this would be the first bibliometric analysis of the intellectual base and research fronts of IL-37. As of 1 April 2022, 534 records were identified in 200 journals by 2,783 authors in 279 institutions from 50 countries/regions. The annual number of publications is an important indicator to measure the strength and development trend of IL-37 over a specific period (41, 42). An increasing trend of IL-37 research officially started in 2001. IL-37, previously termed IL-1F7, was discovered by computational cloning in 2000 (43). Before 2013, the number of studies investigating IL-37 function was relatively few. After that, the publications continued to rise in IL-37 research, which implies that IL-37 has also gained much attention. Increased levels of circulating IL-37 have been reported in the patients with rheumatoid arthritis (37, 44–48), multiple sclerosis (49–51), and systemic lupus erythematosus (12, 52–54). Patients with rheumatoid arthritis demonstrated an increased level of synovial IL-1R8, which is essential for the anti-inflammatory effects of IL-37. Not only that, it has been proven that IL-37 induces apoptosis in rheumatoid arthritis fibroblast-like synoviocytes (17, 55). Multiple sclerosis is a central nervous system-demyelinating illness that primarily affects young people (56). By acting through IL-18Rα and IL-1R8, neurological deficits and myelin loss in the experimental autoimmune encephalomyelitis model, an extensively used model for multiple sclerosis, were protected by the treatment of rhIL-37 (57). The MSCs overexpressing IL-37 decreased the levels of IL-1, TNF-α, IL-17, IL-6, anti-dsDNA, and anti-ANA in systemic lupus erythematosus mice (58). On the contrary, the abundance of circulating IL-37 decreased in patients with inflammatory bowel disease compared to healthy subjects (13). Likely, IL-37b gene transfer enhances the therapeutic efficacy of MSCs in DSS-induced colitis mice (59). In addition to the disorders described above, an increasing number of studies were performed to develop a better understanding regarding the role of IL-37 in other diseases including necrotizing enterocolitis (60), temporomandibular joint inflammation (61, 62), Behcet’s disease (63, 64), periodontitis (65), idiopathic pulmonary fibrosis (66), type 2 diabetes mellitus (67, 68), calcific aortic valve disease (69, 70), asthma (71–73), liver inflammation, and fibrosis (74). With increased in-depth IL-37 research, the role of IL-37 in different cells and relevant cell signaling pathways captured attention. Macrophage-orchestrated chronic inflammation plays a vital role in accelerating the development of calcific aortic valve disease. It has been proven that rhIL-37 shifts macrophage polarization from the pro-inflammatory M1 phenotype to the anti-inflammatory M2 phenotype by inhibiting the Notch1 and NF-kB pathways to suppress the progression of calcific aortic valve disease (75). In vitro, DCs from IL-37tg mice with downregulation of MHC II and CD40 molecular have diminished the ability to stimulate naïve T cells and activate antigen-specific T cells (36). Anti-inflammatory actions of IL-37 depend on inhibition of various pro-inflammatory actions and activation of anti-inflammatory signaling mediators. It has been proven that IL-37 binding to IL-1R8 inhibits the pro-inflammatory properties of the signaling molecules, including FAK, STAT1, p53, p38, paxillin, Pyk2, Syk, NLRP3, and SHP-2. Furthermore, PTEN is upregulated by IL-37 and thus may inhibit PI3K-AKT-mTOR, MAPK, and FADE pathways (2, 35, 76).
As shown in Table 2, the journals of Cytokine and Nature Immunology published the most papers about IL-37 research and received the largest number of co-citations, respectively. Most of the studies related to IL-37 research belong to the subject of biology and immunology, which is consistent with the dual-map analysis (Figure 4). Given the broad regulatory roles of IL-37 in innate and adaptive immunity, some journals concentrating on immunology research are enthusiastic about IL-37 research. It is worth noting that although Cytokine published the most IL-37 research, it took the fourth place in the journals ranking with the total number of co-citations.
These three journals, namely, Nature Immunology, Journal of Immunology, and PNAS, dominate the number of citations in the field with approximately 50% of all the co-citations. This is because fundamental IL-37 research has been published in these journals and gained tremendous attention. The spatial distribution map of institutions revealed that the University of Colorado Denver located in the USA is the most productive institution with 48 publications, 2,703 total citations, and high centrality, which plays a key bridge role in institution cooperation networks. Of note, the five most productive institutions Fudan University, Shandong University, Huazhong University of Science and Technology, Guangdong Medical University, and Shenzhen University are from China, of which Shenzhen University and Huazhong University of Science and Technology had high centrality. Although the five institutions are from China, interagency cooperation among them seems to be lacking. Moreover, we found active collaboration among the University of Colorado Denver and Radboud University Nijmegen, implying their significant contributions to the IL-37 field. A comprehensive look at the above results in Table 1 demonstrates that USA and China are productive and influential countries in IL-37 research. Furthermore, USA was the earliest country to conduct the IL-37 study, followed by Germany, Italy, South Korea, and Australia, the top 10 productive countries. Although China started later than in the above four countries, it has emerged as one of the most productive contributors. This also provides a potential interpretation for the greatest number of publications, almost twice that of USA.
Detecting the top co-occurrences and co-cited papers in the specific field could benefit the research guideline and further directions (77). Three writers were co-cited more than 200 times among the total co-cited authors, with Marcel F. Nold receiving the most co-citations (356 citations), followed by Charles A. Dinarello (211 citations) and Boraschi (203 citations). In addition, Marcel F. Nold, Bufler, and Charles A. Dinarello were not only the top 10 productive authors but also the top 10 co-cited authors, indicating that their work contributed immensely to understanding the role of IL-37. In particular, Marcel F. Nold ranks seventh in the co-occurrence of authors but with the most co-citations. A vital paper entitled “IL-37 is a fundamental inhibitor of innate immunity” published by Marcel F. Nold et al. (2) in 2010 was constructive and profoundly significant to the IL-37 research. It has to be mentioned that although Chinese academics contribute a large quantity of publications of IL-37, the productive authors are especially scarce. The more meticulous research on IL-37 would be the need for Chinese scholars.
Co-cited references appear together in the bibliography of a third citing paper, and the two papers constitute a co-citation relationship (29, 78). The intellectual base and research fronts are the integration of co-cited references cited by the research collectives (79, 80). The co-citation relationship of documents changed over time, predicting the development and evolution of a discipline through document co-citation networks (81). The numbers of top 10 co-cited references in IL-37 literatures are presented in Table 5. In 2015, the most co-cited paper entitled “IL-37 requires the receptors IL-18Rα and IL-1R8 (SIGIRR) to carry out its multifaceted anti-inflammatory program upon innate signal transduction” published by Claudia A. Nold-Petry (35) in Nature Immunology demonstrated that the tripartite ligand–receptor complex IL-37–IL-1R8–IL-18Rα scanned by three-dimensional wide-field fluorescence imaging is a prominent form of endogenous IL-37 in the innate immune response. The author clarified that mice with a transgenic expression of IL-37 (IL-37tg) were protected against endotoxemia, whereas IL-1R8-deficient IL-37tg mice were not. Lipopolysaccharide rapidly stimulated the tripartite complex assembled on the surface of peripheral blood mononuclear cells. Therefore, this research not only demonstrated that the extracellular form of IL-37 exerts an immunomodulatory effect but also suggested that it could exert an immunosuppressive role in an inflammatory environment. That same year, Suzhao Li (5) from the University of Colorado Denver further emphasized that IL-1R8 is required for the immunosuppressive effect of extracellular IL-37. Mechanistically, it has been proven that extracellular IL-37 binds to IL-18Rα and recruits IL-1R8 to activate downstream signaling pathways, while the intracellular matured IL-37 binds to Smad3 in the cytoplasm and traffics to the nucleus to regulate gene transcription (14). Although the IL-37/IL-18Rα/IL-1R8 trimeric complex is the extracellular form of immunomodulatory effect of IL-37, it is controversial whether there is another unique receptor for IL-37.
Apart from these significant findings, the mechanisms of IL-37 mediating resistance to innate response have been studied intensively in another publication entitled “IL-37 is a fundamental inhibitor of innate immunity” with 83 co-citations ranking the fourth in the 10 most productive co-cited references published by Marcel F. Nold (2) in 2010. The expression of pro-inflammatory cytokines IL-1α and IL-6 decreased by 88% and 86%, respectively, in RAW cells, and this immunomodulatory role of IL-37 depends on Smad3. Ana-Maria Bulau (4) has proven the role of caspase-1 in the nuclear translocation and function of immunosuppression of IL-37, as well as the process of secretion of mature IL-37. These four studies elaborate on the mechanisms of the intracellular and extracellular immunosuppressive roles of IL-37 in innate response, while having served as a foundation for this new cytokine of IL-37 research in future studies. Since DCs bridge innate and adaptive immunity, DCs from IL-37tg mice exhibited a characterization of immunological tolerance with a lower ability to stimulate syngeneic and allogeneic naive T cells as well as stimulate the increase of Treg (36). In addition to mechanism research, three productive co-cited papers determined the effect of IL-37 in systemic lupus erythematosus, colitis, and rheumatoid arthritis (12, 37, 38). Besides, two reviews of the top 10 co-cited references focused on a summary of the role of IL-37 in innate and acquired immunity (76, 82).
The occurrence of keywords and burst of the network reflect the hotspots of the specific field (83). The high-frequency keywords include “expression”, “IL-37”, “inflammation”, “cytokine”, “IL-1 family”, “cell”, “immunity”, “receptor”, “rheumatoid arthritis”, and “activation”. IL-37, the search term with the frequency of 165, should be excluded from further analysis. Combined with the strongest citation bursts of keywords, the majority of relevant publications during the budding stage from 2000 to 2004 concentrated on the basic structure and mechanism of IL-37. The article entitled “Identification and Initial Characterization of Four Novel Members of the Interleukin-1 Family” published by Sanjay Kumar (43) in 2000 identified the four novel members of the IL-1 family which were designated as IL-1 homologue 1–4 (IL-1H1-4) for the first time and involved in the pathogenesis of immune processes. On the basis of this study, Sanjay Kumar (8) published an article that revealed that caspase-1 cleaves IL–1H4 (renamed IL-1F7b) to attain a mature status. Furthermore, both precursors and mature IL-1F7b could bind to the IL-18Rα receptor of the cell surface. After that, the academics were almost silent on IL-37 research until its reemergence in 2010. Insufficient attention was paid to IL-37 until some scholars at the University of Colorado Denver have conducted in-depth research on the mechanism of IL-37. As showcased in Figure 1, the study of IL-37 has been in the rapid development phase since 2010. The keywords with high co-occurrence, “Inflammation”, “Dendritic cell”, “Rheumatoid arthritis”, “Pathogenesis”, and “Disease”, proved that the role of IL-37 in different cellular immune responses and immunological diseases has grown into a main focus of research in the past few years. Emerging evidence revealed that IL-37 suppresses the maturation of DCs and the production of IL-6, IL-1α, and TNF-α in innate immune cells (17). The co-occurrence of keywords predicts that the mechanistic role of IL-37 in a variety of diseases remains the most attractive research subject. The research fronts of IL-37 are still to be confirmed by citation bursts of the keywords. The keyword “IL-1 receptor antagonist (with a burst strength of 2.21)” occurred in 2001 and lasted 13 years, indicating that the research on the inhibitory effect of IL-37 for the IL-1 receptor family persisted over long periods. Likewise, the burst of “receptor (with a burst strength of 2.51)” lasted 11 years. IL-37 weakly binds to IL-18Rα and requires the IL-1 family decoy receptor IL-1R8 constituting the tripartite ligand–receptor complex to exert function (84). Therefore, the studies concerning the IL-18Rα and IL-1R8 lasted for a long time. In addition, the keyword “suppression” with the largest burst strength of 5.81 started to burst from 2019 to the present, which implies that the detection of immune effects of IL-37 in a wide variety of immune disorders still remains a research hotspot in future studies.
The analysis of clusters of co-citations contributed to our understanding of the knowledge structure and dynamic evolution of IL-37 research. Cluster#4 (complex) and cluster#9 (IL-1f5) initiated early, indicating that they are fundamental and matured theoretical knowledge of IL-37 research. The nodes scattered along the timeline of cluster#4 and cluster#9 contain few citations. The main topic involved in the clusters is to identify the role of IL-37 and its receptor from 1996 to 2008. In this period, the article with the most citations published by Philip Bufler (85) discovered that recombinant human IL-37 sharing two conserved amino (Glu-35 and Lys-124) acids with IL-18 was shown to bind to IL-18Rα but failed to recruit the IL-18Rβ chain to form a functionally active complex. Another study in this cluster with the highest betweenness centrality (0.23) analyzed the antitumor activity of human IL-37 by adenovirus-mediated gene transfer (adIL-37) and found that the direct single intra-tumoral injection of adIL-37 resulted in a significant growth suppression of mouse fibrosarcoma at the first attempt (86). Notably, the article with the strongest burst of 3.34 originally identified the initial characterization of four novel members of the IL-1 family, namely, IL-1H1, IL-1H2, IL-1H3, and IL-1H4, which was renamed as IL-37 in 2010 (43). Above all, the integrated cluster with 69 references provides a new vision of IL-37. Cluster #1 (disease activity) has the largest nodes and contains eight of the 10 most frequently cited references from 2010 to 2017. The evolution of this specialty can be divided into two parts. On the one side, the specific mechanism of IL-37 in innate response was further confirmed by transgenic mouse and visualized experiment techniques. On the other hand, a growing body of research has been performed to identify the correlation of IL-37 in several disorders such as mycobacterial infection, colitis, SLE, RA, insulin resistance, and psoriasis (12, 37, 38, 87–89) Similarly, cluster #7 (disease pathogenesis) and cluster #1 (disease activity) still focus on the underlying mechanisms. Cluster #0 (cardiovascular disease) is the largest cluster with 124 references from 2010 to the present. The underlying mechanisms of IL-37 regulating immune responses have been mainly discussed in cluster#1. Therefore, core contents in this cluster still refer to immune-related diseases, especially cardiovascular diseases. The reference with the strongest burst (8.06) demonstrated that IL-37 protects against the development of atherosclerosis in ApoE-/- mice by eliminating the maturation of DCs (90). Similarly, DCs from human atherosclerosis and acute coronary syndrome patients pretreated with rhIL-37 induce tolerance by converting immature DCs into tolerogenic DCs (91). IL-37 has recently been considered to enhance Treg abundance and the production of anti-inflammatory cytokines in patients with atherosclerosis and acute coronary syndrome (1, 92). The levels of IL-10 and TGF-β secreted by T cells isolated from patients were decreased in the treatment of rhIL-37 (91). Although the role of IL-37 in the animal model and clinical patients with atherosclerosis (93–96), acute coronary syndrome (1, 97), coronary artery calcification (98), coronary heart disease (99), chronic heart failure (100), atrial fibrillation (101), abdominal aortic aneurysm (102), hypertension (103), and other cardiovascular system diseases (91) has been identified, the timeline view of co-citation clusters revealed that the relationships between IL-37 and the cardiovascular system diseases remain the future hotspots. Cluster #0 seems to be the transfer of the second part of cluster #1 which has been undertaken in terms of research content. The content from clusters #0 and #1 proved that the specific role of IL-37 in several diseases remains an important direction for future research, consistent with the findings from keyword occurrence and burst. The majority of studies in cluster#0 were all conducted prior to 2010. Therefore, the contents of cluster #2 (differential expression) probed the mechanism of IL-37. Although clusters #2 and #1 partially overlap in content, the reference in cluster#2 has fewer citations. Cluster #5 (related cytokine) is a comprehensive literature collection of the IL-1 family of cytokines. As we know, IL-37 is a newly discovered member of the IL-1 family (104). IL-1α, IL-1β, IL-1Ra, IL-18, IL-33, IL-36α, IL-36β, IL-36γ, L-36Ra, IL-37, and IL-38 have always been summarized in the same article (105, 106). Cluster #6 (mast cell) contains fewer nodes on the timeline, suggesting that the research theme was a previous research hotspot. The research in cluster #8 (epidermal barrier) started in 2013 and continued to the present, implying that the role of IL-37 on the epidermal barrier is a research front and predominant aspect in the following study. An infant with non-functional IL-37 had suffered from inflammatory bowel disease, proving the importance of IL-37 in the function of mucosal immunity and epidermal barrier (107). However, related studies are very scarce and should be further explored. Taken together, we found that the role of IL-37 has received much attention in the past 20 years since its finding. Currently, the broad immune regulatory function of IL-37 is still the focus and continues to be a hotspot in future research.
There are still some limitations in the study. Although some articles referring to IL-37 research included in the WoSCC database may lead to few comprehensive research results, there is no denying that the retrieved literature obtained from the WoSCC database accompanied the features with the reliability of the publications and citations. Furthermore, specific data formats downloaded from the WoSCC database are more convenient and widely accepted for processing in the CiteSpace software. The overwhelming majority of bibliometrics articles have utilized the data from WoSCC. Notably, terms extracted from title, abstract, and keywords exhibit a high discrepancy in the process of clustering analysis due to the certain limitations inherent in CiteSpace software. Besides, some terms with the same meaning should be incorporated using CiteSpace in the co-occurrence of keywords.
Conclusion
In summary, IL-37 research is undergoing rapid development worldwide, of which USA stands out as the most important contributor to this field. By detecting the most recent burst keywords and co-cited reference clusters up until 2022, emerging trends in IL-37 research were discerned. It is believed that thorough analyses of the immune function of IL-37 in different disorders will be an emerging focus. This bibliometric analysis provides the visualization to the field of IL-37, contributing to tracking the intellectual structure of specific knowledge domains and research fronts of IL-37.
Author contributions
Y-FQ: conception and design, data analysis and interpretation, manuscript writing. S-HR and BS: collection and assembly of data, data analysis, and interpretation. HQ, HW, G-ML, Y-LZ, C-LS, CL, and J-YZ: collection of data and data analysis. HW: conception and design, financial support, administrative support, manuscript writing, and final approval of the manuscript. All the authors have read and approved the final content of this manuscript.
Funding
This work was supported by grants to HW from the National Natural Science Foundation of China (No. 82071802), Science and Technology Project of Tianjin Health Commission (No. TJWJ2021MS004), Tianjin Key Medical Discipline (Specialty) Construction Project, and Natural Science Foundation of Tianjin (No. 19JCYBJC27100).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mao X, Zhu R, Zhang F, Zhong Y, Yu K, Wei Y, et al. IL-37 plays a beneficial role in patients with acute coronary syndrome. Mediators Inflammation (2019) 2019:9515346. doi: 10.1155/2019/9515346
2. Nold MF, Nold-Petry CA, Zepp JA, Palmer BE, Bufler P, Dinarello CA. IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol (2010) 11(11):1014–22. doi: 10.1038/ni.1944
3. Sharaf N, Nicklin MJ, di Giovine FS. Long-range DNA interactions at the IL-1/IL-36/IL-37 gene cluster (2q13) are induced by activation of monocytes. Cytokine (2014) 68(1):16–22. doi: 10.1016/j.cyto.2014.03.002
4. Bulau AM, Nold MF, Li S, Nold-Petry CA, Fink M, Mansell A, et al. Role of caspase-1 in nuclear translocation of IL-37, release of the cytokine, and IL-37 inhibition of innate immune responses. Proc Natl Acad Sci USA (2014) 111(7):2650–5. doi: 10.1073/pnas.1324140111
5. Li S, Neff CP, Barber K, Hong J, Luo Y, Azam T, et al. Extracellular forms of IL-37 inhibit innate inflammation in vitro and in vivo but require the IL-1 family decoy receptor IL-1R8. Proc Natl Acad Sci USA (2015) 112(8):2497–502. doi: 10.1073/pnas.1424626112
6. Jia C, Zhuge Y, Zhang S, Ni C, Wang L, Wu R, et al. IL-37b alleviates endothelial cell apoptosis and inflammation in Kawasaki disease through IL-1R8 pathway. Cell Death Dis (2021) 12(6):575. doi: 10.1038/s41419-021-03852-z
7. Boraschi D, Lucchesi D, Hainzl S, Leitner M, Maier E, Mangelberger D, et al. IL-37: a new anti-inflammatory cytokine of the IL-1 family. Eur Cytokine Netw (2011) 22(3):127–47. doi: 10.1684/ecn.2011.0288
8. Kumar S, Hanning CR, Brigham-Burke MR, Rieman DJ, Lehr R, Khandekar S, et al. Interleukin-1F7B (IL-1H4/IL-1F7) is processed by caspase-1 and mature IL-1F7B binds to the IL-18 receptor but does not induce IFN-gamma production. Cytokine (2002) 18(2):61–71. doi: 10.1006/cyto.2002.0873
9. Sharma S, Kulk N, Nold MF, Gräf R, Kim SH, Reinhardt D, et al. The IL-1 family member 7b translocates to the nucleus and down-regulates proinflammatory cytokines. J Immunol (2008) 180(8):5477–82. doi: 10.4049/jimmunol.180.8.5477
10. Grimsby S, Jaensson H, Dubrovska A, Lomnytska M, Hellman U, Souchelnytskyi S. Proteomics-based identification of proteins interacting with Smad3: SREBP-2 forms a complex with Smad3 and inhibits its transcriptional activity. FEBS Lett (2004) 577(1-2):93–100. doi: 10.1016/j.febslet.2004.09.069
11. Quirk S, Agrawal DK. Immunobiology of IL-37: Mechanism of action and clinical perspectives. Expert Rev Clin Immunol (2014) 10(12):1703–9. doi: 10.1586/1744666x.2014.971014
12. Ye L, Ji L, Wen Z, Zhou Y, Hu D, Li Y, et al. IL-37 inhibits the production of inflammatory cytokines in peripheral blood mononuclear cells of patients with systemic lupus erythematosus: its correlation with disease activity. J Transl Med (2014) 12:69. doi: 10.1186/1479-5876-12-69
13. Li Y, Wang Y, Liu Y, Wang Y, Zuo X, Li Y, et al. The possible role of the novel cytokines il-35 and il-37 in inflammatory bowel disease. Mediators Inflammation (2014) 2014:136329. doi: 10.1155/2014/136329
14. Zhu Y, Qin H, Ye K, Sun C, Qin Y, Li G, et al. Dual role of IL-37 in the progression of tumors. Cytokine (2022) 150:155760. doi: 10.1016/j.cyto.2021.155760
15. Zhao JJ, Pan QZ, Pan K, Weng DS, Wang QJ, Li JJ, et al. Interleukin-37 mediates the antitumor activity in hepatocellular carcinoma: Role for CD57+ NK cells. Sci Rep (2014) 4:5177. doi: 10.1038/srep05177
16. Guo H, Li P, Su L, Wu K, Huang K, Lai R, et al. Low expression of IL-37 protein is correlated with high Oct4 protein expression in hepatocellular carcinoma. Gene (2020) 737:144445. doi: 10.1016/j.gene.2020.144445
17. Su Z, Tao X. Current understanding of IL-37 in human health and disease. Front Immunol (2021) 12:696605. doi: 10.3389/fimmu.2021.696605
18. Moed HF. New developments in the use of citation analysis in research evaluation. Arch Immunol Ther Exp (Warsz) (2009) 57(1):13–8. doi: 10.1007/s00005-009-0001-5
19. Gazzaz ZJ, Butt NS, Zubairi NA, Malik AA. Scientometric evaluation of research productivity on diabetes from the kingdom of Saudi Arabia over the last two decades (2000-2019). J Diabetes Res (2020) 2020:1514282. doi: 10.1155/2020/1514282
20. Yang Y, Ma Y, Chen L, Liu Y, Zhang Y. The 100 top-cited systematic Reviews/Meta-analyses on diabetic research. J Diabetes Res (2020) 2020:5767582. doi: 10.1155/2020/5767582
21. Ma C, Su H, Li H. Global research trends on prostate diseases and erectile dysfunction: A bibliometric and visualized study. Front Oncol (2020) 10:627891. doi: 10.3389/fonc.2020.627891
22. Rosewell A, Ropa B, Randall H, Dagina R, Hurim S, Bieb S, et al. Mobile phone-based syndromic surveillance system, Papua New Guinea. Emerg Infect Dis (2013) 19(11):1811–8. doi: 10.3201/eid1911.121843
23. Liang YD, Li Y, Zhao J, Wang XY, Zhu HZ, Chen XH. Study of acupuncture for low back pain in recent 20 years: A bibliometric analysis via CiteSpace. J Pain Res (2017) 10:951–64. doi: 10.2147/jpr.S132808
24. Qin Y, Chen S, Zhang Y, Liu W, Lin Y, Chi X, et al. A bibliometric analysis of endoscopic sedation research: 2001-2020. Front Med (Lausanne) (2021) 8:775495. doi: 10.3389/fmed.2021.775495
25. Chen C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci USA (2004) 101 Suppl 1(Suppl 1):5303–10. doi: 10.1073/pnas.0307513100
26. Mu X, Zhao Q, Chen W, Zhao Y, Yan Q, Peng R, et al. IL-37 confers anti-tumor activity by regulation of m6A methylation. Front Oncol (2020) 10:526866. doi: 10.3389/fonc.2020.526866
27. Bertran K, Cortey M, Díaz I. The use of h-index to assess research priorities in poultry diseases. Poult Sci (2020) 99(12):6503–12. doi: 10.1016/j.psj.2020.09.017
28. Chen CM, Leydesdorff L. Patterns of connections and movements in dual-map overlays: A new method of publication portfolio analysis. J Assoc Inf Sci Technol (2014) 65(2):334–51. doi: 10.1002/asi.22968
29. Zhang J, Song L, Xu L, Fan Y, Wang T, Tian W, et al. Knowledge domain and emerging trends in ferroptosis research: A bibliometric and knowledge-map analysis. Front Oncol (2021) 11:686726. doi: 10.3389/fonc.2021.686726
30. Cheng P, Tang H, Dong Y, Liu K, Jiang P, Liu Y. Knowledge mapping of research on land use change and food security: A visual analysis using CiteSpace and VOSviewer. Int J Environ Res Public Health (2021) 18(24):13065. doi: 10.3390/ijerph182413065
31. Yang X, Wang X, Li X, Gu D, Liang C, Li K, et al. Exploring emerging IoT technologies in smart health research: A knowledge graph analysis. BMC Med Inform Decis Mak (2020) 20(1):260. doi: 10.1186/s12911-020-01278-9
32. Chen S, Bie R, Lai Y, Shi H, Ung COL, Hu H. Trends and development in enteral nutrition application for ventilator associated pneumonia: A scientometric research study (1996-2018). Front Pharmacol (2019) 10:246. doi: 10.3389/fphar.2019.00246
33. Zhu X, Hu J, Deng S, Tan Y, Qiu C, Zhang M, et al. Comprehensive bibliometric analysis of the kynurenine pathway in mood disorders: Focus on gut microbiota research. Front Pharmacol (2021) 12:687757. doi: 10.3389/fphar.2021.687757
34. Yang DW, Wang XP, Wang ZC, Yang ZH, Bian XF. A scientometric analysis on hepatocellular carcinoma magnetic resonance imaging research from 2008 to 2017. Quant Imaging Med Surg (2019) 9(3):465–76. doi: 10.21037/qims.2019.02.10
35. Nold-Petry CA, Lo CY, Rudloff I, Elgass KD, Li S, Gantier MP, et al. IL-37 requires the receptors IL-18Rα and IL-1R8 (SIGIRR) to carry out its multifaceted anti-inflammatory program upon innate signal transduction. Nat Immunol (2015) 16(4):354–65. doi: 10.1038/ni.3103
36. Luo Y, Cai X, Liu S, Wang S, Nold-Petry CA, Nold MF, et al. Suppression of antigen-specific adaptive immunity by IL-37 via induction of tolerogenic dendritic cells. Proc Natl Acad Sci USA (2014) 111(42):15178–83. doi: 10.1073/pnas.1416714111
37. Ye L, Jiang B, Deng J, Du J, Xiong W, Guan Y, et al. IL-37 alleviates rheumatoid arthritis by suppressing IL-17 and IL-17-Triggering cytokine production and limiting Th17 cell proliferation. J Immunol (2015) 194(11):5110–9. doi: 10.4049/jimmunol.1401810
38. McNamee EN, Masterson JC, Jedlicka P, McManus M, Grenz A, Collins CB, et al. Interleukin 37 expression protects mice from colitis. Proc Natl Acad Sci USA (2011) 108(40):16711–6. doi: 10.1073/pnas.1111982108
39. Chen C, Hu Z, Liu S, Tseng H. Emerging trends in regenerative medicine: A scientometric analysis in CiteSpace. Expert Opin Biol Ther (2012) 12(5):593–608. doi: 10.1517/14712598.2012.674507
40. Zhang XL, Zheng Y, Xia ML, Wu YN, Liu XJ, Xie SK, et al. Knowledge domain and emerging trends in vinegar research: A bibliometric review of the literature from WoSCC. Foods (2020) 9(2):166. doi: 10.3390/foods9020166
41. Qin Y, Zhang Q, Liu Y. Analysis of knowledge bases and research focuses of cerebral ischemia-reperfusion from the perspective of mapping knowledge domain. Brain Res Bull (2020) 156:15–24. doi: 10.1016/j.brainresbull.2019.12.004
42. Gao Y, Shi S, Ma W, Chen J, Cai Y, Ge L, et al. Bibliometric analysis of global research on PD-1 and PD-L1 in the field of cancer. Int Immunopharmacol (2019) 72:374–84. doi: 10.1016/j.intimp.2019.03.045
43. Kumar S, McDonnell PC, Lehr R, Tierney L, Tzimas MN, Griswold DE, et al. Identification and initial characterization of four novel members of the interleukin-1 family. J Biol Chem (2000) 275(14):10308–14. doi: 10.1074/jbc.275.14.10308
44. Yang L, Zhang J, Tao J, Lu T. Elevated serum levels of interleukin-37 are associated with inflammatory cytokines and disease activity in rheumatoid arthritis. Apmis (2015) 123(12):1025–31. doi: 10.1111/apm.12467
45. Song L, Wang Y, Sui Y, Sun J, Li D, Li G, et al. High interleukin-37 (IL-37) expression and increased mucin-domain containing-3 (TIM-3) on peripheral T cells in patients with rheumatoid arthritis. Med Sci Monit (2018) 24:5660–7. doi: 10.12659/msm.909254
46. Xia L, Shen H, Lu J. Elevated serum and synovial fluid levels of interleukin-37 in patients with rheumatoid arthritis: Attenuated the production of inflammatory cytokines. Cytokine (2015) 76(2):553–7. doi: 10.1016/j.cyto.2015.06.005
47. Ragab D, Mobasher S, Shabaan E. Elevated levels of IL-37 correlate with T cell activation status in rheumatoid arthritis patients. Cytokine (2019) 113:305–10. doi: 10.1016/j.cyto.2018.07.027
48. Xia T, Zheng XF, Qian BH, Fang H, Wang JJ, Zhang LL, et al. Plasma interleukin-37 is elevated in patients with rheumatoid arthritis: Its correlation with disease activity and Th1/Th2/Th17-related cytokines. Dis Markers (2015) 2015:795043. doi: 10.1155/2015/795043
49. Kouchaki E, Tamtaji OR, Dadgostar E, Karami M, Nikoueinejad H, Akbari H. Correlation of serum levels of IL-33, IL-37, soluble form of vascular endothelial growth factor receptor 2 (VEGFR2), and circulatory frequency of VEGFR2-expressing cells with multiple sclerosis severity. Iran J Allergy Asthma Immunol (2017) 16(4):329–37.
50. Farrokhi M, Rezaei A, Amani-Beni A, Etemadifar M, Kouchaki E, Zahedi A. Increased serum level of IL-37 in patients with multiple sclerosis and neuromyelitis optica. Acta Neurol Belg (2015) 115(4):609–14. doi: 10.1007/s13760-015-0491-3
51. Cavalli E, Mazzon E, Basile MS, Mammana S, Pennisi M, Fagone P, et al. In silico and in vivo analysis of IL37 in multiple sclerosis reveals its probable homeostatic role on the clinical activity, disability, and treatment with fingolimod. Molecules (2019) 25(1):20. doi: 10.3390/molecules25010020
52. Tawfik MG, Nasef SI, Omar HH, Ghaly MS. Serum interleukin-37: a new player in lupus nephritis? Int J Rheum Dis (2017) 20(8):996–1001. doi: 10.1111/1756-185x.13122
53. Song L, Qiu F, Fan Y, Ding F, Liu H, Shu Q, et al. Glucocorticoid regulates interleukin-37 in systemic lupus erythematosus. J Clin Immunol (2013) 33(1):111–7. doi: 10.1007/s10875-012-9791-z
54. Godsell J, Rudloff I, Kandane-Rathnayake R, Hoi A, Nold MF, Morand EF, et al. Clinical associations of IL-10 and IL-37 in systemic lupus erythematosus. Sci Rep (2016) 6:34604. doi: 10.1038/srep34604
55. Cavalli G, Koenders M, Kalabokis V, Kim J, Tan AC, Garlanda C, et al. Treating experimental arthritis with the innate immune inhibitor interleukin-37 reduces joint and systemic inflammation. Rheumatol (Oxford) (2016) 55(12):2220–9. doi: 10.1093/rheumatology/kew325
56. Genovese AV, Hagemeier J, Bergsland N, Jakimovski D, Dwyer MG, Ramasamy DP, et al. Atrophied brain T2 lesion volume at MRI is associated with disability progression and conversion to secondary progressive multiple sclerosis. Radiology (2019) 293(2):424–33. doi: 10.1148/radiol.2019190306
57. Sánchez-Fernández A, Zandee S, Amo-Aparicio J, Charabati M, Prat A, Garlanda C, et al. IL-37 exerts therapeutic effects in experimental autoimmune encephalomyelitis through the receptor complex IL-1R5/IL-1R8. Theranostics (2021) 11(1):1–13. doi: 10.7150/thno.47435
58. Xu J, Chen J, Li W, Lian W, Huang J, Lai B, et al. Additive therapeutic effects of mesenchymal stem cells and IL-37 for systemic lupus erythematosus. J Am Soc Nephrol (2020) 31(1):54–65. doi: 10.1681/asn.2019050545
59. Wang WQ, Dong K, Zhou L, Jiao GH, Zhu CZ, Li WW, et al. IL-37b gene transfer enhances the therapeutic efficacy of mesenchumal stromal cells in DSS-induced colitis mice. Acta Pharmacol Sin (2015) 36(11):1377–87. doi: 10.1038/aps.2015.51
60. Cho SX, Rudloff I, Lao JC, Pang MA, Goldberg R, Bui CB, et al. Characterization of the pathoimmunology of necrotizing enterocolitis reveals novel therapeutic opportunities. Nat Commun (2020) 11(1):5794. doi: 10.1038/s41467-020-19400-w
61. Luo P, Feng C, Jiang C, Ren X, Gou L, Ji P, et al. IL-37b alleviates inflammation in the temporomandibular joint cartilage via IL-1R8 pathway. Cell Prolif (2019) 52(6):e12692. doi: 10.1111/cpr.12692
62. Luo P, Peng S, Yan Y, Ji P, Xu J. IL-37 inhibits M1-like macrophage activation to ameliorate temporomandibular joint inflammation through the NLRP3 pathway. Rheumatol (Oxford) (2020) 59(10):3070–80. doi: 10.1093/rheumatology/keaa192
63. Özgüçlü S, Duman T, Ateş F, Küçükşahin O, Çolak S, Ölmez Ü. Serum interleukin-37 level and interleukin-37 gene polymorphism in patients with behçet disease. Clin Rheumatol (2019) 38(2):495–502. doi: 10.1007/s10067-018-4288-7
64. Kacem O, Kaabachi W, Dhifallah IB, Hamzaoui A, Hamzaoui K. Elevated expression of TSLP and IL-33 in behçet's disease skin lesions: IL-37 alleviate inflammatory effect of TSLP. Clin Immunol (2018) 192:14–9. doi: 10.1016/j.clim.2018.03.016
65. Jing L, Kim S, Sun L, Wang L, Mildner E, Divaris K, et al. IL-37- and IL-35/IL-37-Producing plasma cells in chronic periodontitis. J Dent Res (2019) 98(7):813–21. doi: 10.1177/0022034519847443
66. Kim MS, Baek AR, Lee JH, Jang AS, Kim DJ, Chin SS, et al. IL-37 attenuates lung fibrosis by inducing autophagy and regulating TGF-β1 production in mice. J Immunol (2019) 203(8):2265–75. doi: 10.4049/jimmunol.1801515
67. Li T, Li H, Li W, Chen S, Feng T, Jiao W, et al. Interleukin-37 sensitize the elderly type 2 diabetic patients to insulin therapy through suppressing the gut microbiota dysbiosis. Mol Immunol (2019) 112:322–9. doi: 10.1016/j.molimm.2019.06.008
68. Wang P, Wang H, Li C, Zhang X, Xiu X, Teng P, et al. Dysregulation of microRNA-657 influences inflammatory response via targeting interleukin-37 in gestational diabetes mellitus. J Cell Physiol (2019) 234(5):7141–8. doi: 10.1002/jcp.27468
69. Kapelouzou A, Kontogiannis C, Tsilimigras DI, Georgiopoulos G, Kaklamanis L, Tsourelis L, et al. Differential expression patterns of toll like receptors and interleukin-37 between calcific aortic and mitral valve cusps in humans. Cytokine (2019) 116:150–60. doi: 10.1016/j.cyto.2019.01.009
70. Zeng Q, Song R, Fullerton DA, Ao L, Zhai Y, Li S, et al. Interleukin-37 suppresses the osteogenic responses of human aortic valve interstitial cells in vitro and alleviates valve lesions in mice. Proc Natl Acad Sci USA (2017) 114(7):1631–6. doi: 10.1073/pnas.1619667114
71. Huang N, Liu K, Liu J, Gao X, Zeng Z, Zhang Y, et al. Interleukin-37 alleviates airway inflammation and remodeling in asthma via inhibiting the activation of NF-κB and STAT3 signalings. Int Immunopharmacol (2018) 55:198–204. doi: 10.1016/j.intimp.2017.12.010
72. Meng P, Chen ZG, Zhang TT, Liang ZZ, Zou XL, Yang HL, et al. IL-37 alleviates house dust mite-induced chronic allergic asthma by targeting TSLP through the NF-κB and ERK1/2 signaling pathways. Immunol Cell Biol (2019) 97(4):403–15. doi: 10.1111/imcb.12223
73. Lv J, Xiong Y, Li W, Cui X, Cheng X, Leng Q, et al. IL-37 inhibits IL-4/IL-13-induced CCL11 production and lung eosinophilia in murine allergic asthma. Allergy (2018) 73(8):1642–52. doi: 10.1111/all.13395
74. Mountford S, Effenberger M, Noll-Puchta H, Griessmair L, Ringleb A, Haas S, et al. Modulation of liver inflammation and fibrosis by interleukin-37. Front Immunol (2021) 12:603649. doi: 10.3389/fimmu.2021.603649
75. Zhou P, Li Q, Su S, Dong W, Zong S, Ma Q, et al. Interleukin 37 suppresses M1 macrophage polarization through inhibition of the Notch1 and nuclear factor kappa b pathways. Front Cell Dev Biol (2020) 8:56. doi: 10.3389/fcell.2020.00056
76. Cavalli G, Dinarello CA. Suppression of inflammation and acquired immunity by IL-37. Immunol Rev (2018) 281(1):179–90. doi: 10.1111/imr.12605
77. Kodonas K, Fardi A, Gogos C, Economides N. Scientometric analysis of vital pulp therapy studies. Int Endod J (2021) 54(2):220–30. doi: 10.1111/iej.13422
78. Lu C, Liu M, Shang W, Yuan Y, Li M, Deng X, et al. Knowledge mapping of angelica sinensis (Oliv.) diels (Danggui) research: A scientometric study. Front Pharmacol (2020) 11:294. doi: 10.3389/fphar.2020.00294
79. Chen YL, Zhao C, Zhang L, Li B, Wu CH, Mu W, et al. Toward evidence-based Chinese medicine: Status quo, opportunities and challenges. Chin J Integr Med (2018) 24(3):163–70. doi: 10.1007/s11655-017-2795-2
80. Xiao F, Li C, Sun J, Zhang L. Knowledge domain and emerging trends in organic photovoltaic technology: A scientometric review based on CiteSpace analysis. Front Chem (2017) 5:67. doi: 10.3389/fchem.2017.00067
81. Wang M, Liu P, Gu Z, Cheng H, Li X. A scientometric review of resource recycling industry. Int J Environ Res Public Health (2019) 16(23):4654. doi: 10.3390/ijerph16234654
82. Dinarello CA, Nold-Petry C, Nold M, Fujita M, Li S, Kim S, et al. Suppression of innate inflammation and immunity by interleukin-37. Eur J Immunol (2016) 46(5):1067–81. doi: 10.1002/eji.201545828
83. Liu G, Jiang R, Jin Y. Sciatic nerve injury repair: A visualized analysis of research fronts and development trends. Neural Regener Res (2014) 9(18):1716–22. doi: 10.4103/1673-5374.141810
84. Tomasoni R, Morini R, Lopez-Atalaya JP, Corradini I, Canzi A, Rasile M, et al. Lack of IL-1R8 in neurons causes hyperactivation of IL-1 receptor pathway and induces MECP2-dependent synaptic defects. Elife (2017) 6:e21735. doi: 10.7554/eLife.21735
85. Bufler P, Azam T, Gamboni-Robertson F, Reznikov LL, Kumar S, Dinarello CA, et al. A complex of the IL-1 homologue IL-1F7b and IL-18-binding protein reduces IL-18 activity. Proc Natl Acad Sci USA (2002) 99(21):13723–8. doi: 10.1073/pnas.212519099
86. Gao W, Kumar S, Lotze MT, Hanning C, Robbins PD, Gambotto A. Innate immunity mediated by the cytokine IL-1 homologue 4 (IL-1H4/IL-1F7) induces IL-12-dependent adaptive and profound antitumor immunity. J Immunol (2003) 170(1):107–13. doi: 10.4049/jimmunol.170.1.107
87. Liu H, Zheng R, Wang P, Yang H, He X, Ji Q, et al. IL-37 confers protection against mycobacterial infection involving suppressing inflammation and modulating T cell activation. PLoS One (2017) 12(1):e0169922. doi: 10.1371/journal.pone.0169922
88. Ballak DB, van Diepen JA, Moschen AR, Jansen HJ, Hijmans A, Groenhof GJ, et al. IL-37 protects against obesity-induced inflammation and insulin resistance. Nat Commun (2014) 5:4711. doi: 10.1038/ncomms5711
89. Teng X, Hu Z, Wei X, Wang Z, Guan T, Liu N, et al. Li: IL-37 ameliorates the inflammatory process in psoriasis by suppressing proinflammatory cytokine production. J Immunol (2014) 192(4):1815–23. doi: 10.4049/jimmunol.1300047
90. Liu T, Liu J, Lin Y, Que B, Chang C, Zhang J, et al. IL-37 inhibits the maturation of dendritic cells through the IL-1R8-TLR4-NF-κB pathway. Biochim Biophys Acta Mol Cell Biol Lipids (2019) 1864(10):1338–49. doi: 10.1016/j.bbalip.2019.05.009
91. McCurdy S, Yap J, Irei J, Lozano J, Boisvert WA. IL-37-a putative therapeutic agent in cardiovascular diseases. Qjm (2021) 24:hcab011. doi: 10.1093/qjmed/hcab011
92. Lotfy H, Moaaz M, Moaaz M. The novel role of IL-37 to enhance the anti-inflammatory response of regulatory T cells in patients with peripheral atherosclerosis. Vascular (2020) 28(5):629–42. doi: 10.1177/1708538120921735
93. Xie Y, Li Y, Cai X, Wang X, Li J. Interleukin-37 suppresses ICAM-1 expression in parallel with NF-κB down-regulation following TLR2 activation of human coronary artery endothelial cells. Int Immunopharmacol (2016) 38:26–30. doi: 10.1016/j.intimp.2016.05.003
94. Chai M, Ji Q, Zhang H, Zhou Y, Yang Q, Zhou Y, et al. The protective effect of interleukin-37 on vascular calcification and atherosclerosis in apolipoprotein e-deficient mice with diabetes. J Interferon Cytokine Res (2015) 35(7):530–9. doi: 10.1089/jir.2014.0212
95. McCurdy S, Baumer Y, Toulmin E, Lee BH, Boisvert WA. Macrophage-specific expression of IL-37 in hyperlipidemic mice attenuates atherosclerosis. J Immunol (2017) 199(10):3604–13. doi: 10.4049/jimmunol.1601907
96. Ji Q, Meng K, Yu K, Huang S, Huang Y, Min X, et al. Exogenous interleukin 37 ameliorates atherosclerosis via inducing the treg response in ApoE-deficient mice. Sci Rep (2017) 7(1):3310. doi: 10.1038/s41598-017-02987-4
97. Liu K, Tang Q, Zhu X, Yang X. IL-37 increased in patients with acute coronary syndrome and associated with a worse clinical outcome after ST-segment elevation acute myocardial infarction. Clin Chim Acta (2017) 468:140–4. doi: 10.1016/j.cca.2017.02.017
98. Chai M, Zhang HT, Zhou YJ, Ji QW, Yang Q, Liu YY, et al. Elevated IL-37 levels in the plasma of patients with severe coronary artery calcification. J Geriatr Cardiol (2017) 14(5):285–91. doi: 10.11909/j.issn.1671-5411.2017.05.013
99. Li H, Shen C, Chen B, Du J, Peng B, Wang W, et al. Interleukin−37 is increased in peripheral blood mononuclear cells of coronary heart disease patients and inhibits the inflammatory reaction. Mol Med Rep (2020) 21(1):151–60. doi: 10.3892/mmr.2019.10805
100. Shou X, Lin J, Xie C, Wang Y, Sun C. Plasma IL-37 elevated in patients with chronic heart failure and predicted major adverse cardiac events: A 1-year follow-up study. Dis Markers (2017) 2017:9134079. doi: 10.1155/2017/9134079
101. Racca V, Torri A, Grati P, Panzarino C, Marventano I, Saresella M, et al. Inflammatory cytokines during cardiac rehabilitation after heart surgery and their association to postoperative atrial fibrillation. Sci Rep (2020) 10(1):8618. doi: 10.1038/s41598-020-65581-1
102. Ding Y, Wang Y, Cai Y, Pan C, Yang C, Wang M, et al. IL-37 expression in patients with abdominal aortic aneurysm and its role in the necroptosis of vascular smooth muscle cells. Oxid Med Cell Longev (2022) 2022:1806513. doi: 10.1155/2022/1806513
103. Ye J, Wang Y, Wang Z, Lin Y, Liu L, Zhou Q, et al. Circulating IL-37 levels are elevated in patients with hypertension. Exp Ther Med (2021) 21(6):558. doi: 10.3892/etm.2021.9990
104. Dinarello CA. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev (2018) 281(1):8–27. doi: 10.1111/imr.12621
105. Kaplanski G. Interleukin-18: Biological properties and role in disease pathogenesis. Immunol Rev (2018) 281(1):138–53. doi: 10.1111/imr.12616
106. Mantovani A, Dinarello CA, Molgora M, Garlanda C. Interleukin-1 and related cytokines in the regulation of inflammation and immunity. Immunity (2019) 50(4):778–95. doi: 10.1016/j.immuni.2019.03.012
Keywords: IL-37, bibliometric analysis, visualization, CiteSpace, immunity
Citation: Qin Y-f, Ren S-h, Shao B, Qin H, Wang H-d, Li G-m, Zhu Y-l, Sun C-l, Li C, Zhang J-y and Wang H (2022) The intellectual base and research fronts of IL-37: A bibliometric review of the literature from WoSCC. Front. Immunol. 13:931783. doi: 10.3389/fimmu.2022.931783
Received: 29 April 2022; Accepted: 28 June 2022;
Published: 22 July 2022.
Edited by:
Parameswaran Ramakrishnan, Case Western Reserve University, United StatesReviewed by:
Zhengping Huang, Guangdong Second Provincial General Hospital, ChinaKunwu Yu, Huazhong University of Science and Technology, China
Copyright © 2022 Qin, Ren, Shao, Qin, Wang, Li, Zhu, Sun, Li, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Wang, aHdhbmdjYTI3MkBob3RtYWlsLmNvbQ==; aHdhbmcxQHRtdS5lZHUuY24=
†These authors share first authorship
 Ya-fei Qin
Ya-fei Qin Shao-hua Ren
Shao-hua Ren Bo Shao
Bo Shao Hong Qin1,2
Hong Qin1,2 Guang-ming Li
Guang-ming Li Chuan Li
Chuan Li Hao Wang
Hao Wang