
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 26 August 2022
Sec. Cancer Immunity and Immunotherapy
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.922301
This article is part of the Research Topic Global Excellence in Translational Immunology: North America 2021 View all 7 articles
Messenger RNA (mRNA) vaccines constitute an emerging therapeutic method with the advantages of high safety and efficiency as well as easy synthesis; thus, they have been widely used in various human diseases, especially in malignant cancers. However, the mRNA vaccine technology has some limitations, such as instability and low transitive efficiency in vivo, which greatly restrict its application. The development of nanotechnology in the biomedical field offers new strategies and prospects for the early diagnosis and treatment of human cancers. Recent studies have demonstrated that Lipid nanoparticle (LNP)-based mRNA vaccines can address the poor preservation and targeted inaccuracy of mRNA vaccines. As an emerging cancer therapy, mRNA vaccines potentially have broad future applications. Unlike other treatments, cancer mRNA vaccines provide specific, safe, and tolerable treatments. Preclinical studies have used personalized vaccines to demonstrate the anti-tumor effect of mRNA vaccines in the treatment of various solid tumors, including colorectal and lung cancer, using these in a new era of therapeutic cancer vaccines. In this review, we have summarized the latest applications and progress of LNP-based mRNA vaccines in cancers, and discussed the prospects and limitations of these fields, thereby providing novel strategies for the targeted therapy of cancers.
Messenger RNA (mRNA) vaccines represent a newly studied method of immunotherapy that works by introducing exogenous mRNA encoding antigens into the body and translating and synthesizing antigens in the cells, which finally result in an immune response (1, 2). mRNA was first discovered in 1961 (3), and after a decade, researchers discovered how to translate proteins from isolated mRNA in living cells (4). In the 1990s, scientists began to use mRNA expression vectors to inject mRNA into mouse somatic cells to express luciferase, chloramphenicol acetyltransferase and β-galactosidase (2). Studies in 1992 showed that diabetes insipidus occurred in mutant Brattleboro rats owing to their inability to express and secrete vasopressin. However, when copies of purified or synthesized mRNA from the hypothalamus of normal rats were injected into the hypothalamus, it was found that large cell neurons selectively ingested, retrogradely transported, and expressed vasopressin, and their diabetes insipidus could be temporarily reversed within a few hours after injection for up to 5 days (5). In recent years, mRNA vaccines have attracted increasing attention for the clinical treatment of various diseases and have become one of the most effective drugs. However, mRNA vaccines have not been widely used thus far, mainly because of their instability (6, 7). Determining a suitable method to improve the stability of mRNA vaccines is one of the focus areas in research in recent years. The ideal way to deliver mRNA is to use a material that can protect it from degradation and can induce the mRNA to be effectively absorbed by cells after injection (8).
According to the materials science standards, the term “nanotechnology” is used to describe the manufacture of new materials with sizes of 1 to 100 nm, but with further study, the definition of these new materials has also expanded (9). Nanomedicine is the application of nanomaterials in clinics, including diagnosis, detection, control, and treatment of diseases (10). Unlike bulk materials, nanomaterials are a new type of material with unique physical and chemical properties, such as ultra-small size, high reactivity, and large surface-area-to-volume ratio, and can be used to overcome the limitations of traditional therapeutic reagents (11). Nanomaterials are also similar to biomolecules in scale and can be designed as drugs with various functions (12). Nanotechnology has great potential for application in medicine. Delicate designs and modifications enable nano-drugs to maintain better specificity and bioavailability, low cytotoxicity to normal tissue, larger drug loading, longer half-life, and unique drug release patterns than traditional drug formats (13). The frequently-used strategies of nano-carrier in tumor therapy are shown in Figure 1. Studies have shown that nanocarrier-based mRNA vaccines are widely used in the treatment of diseases. At present, lipid nanoparticles (LNP) are the main nanocarriers for cancer treatment. Phospholipids are one of the main components of cell membranes. Many kinds of lipids such as C12-200, cKK-E12, 5A2-SC8, 306Oi10 and BAMEA-O16B have been identified that can be formulated into LNP (14). The design of LNP as a vector for delivering mRNA can overcome the following disadvantages of mRNA therapy: first, as a negatively charged macromolecule, mRNA has difficulty in crossing the cell membrane (15); second, the average intracellular half-life of mRNA is only about 7 h (16); third, a large amount of mRNA is trapped in the endosome after entering, and cannot translocate into the cytoplasm to perform the translation function (17).
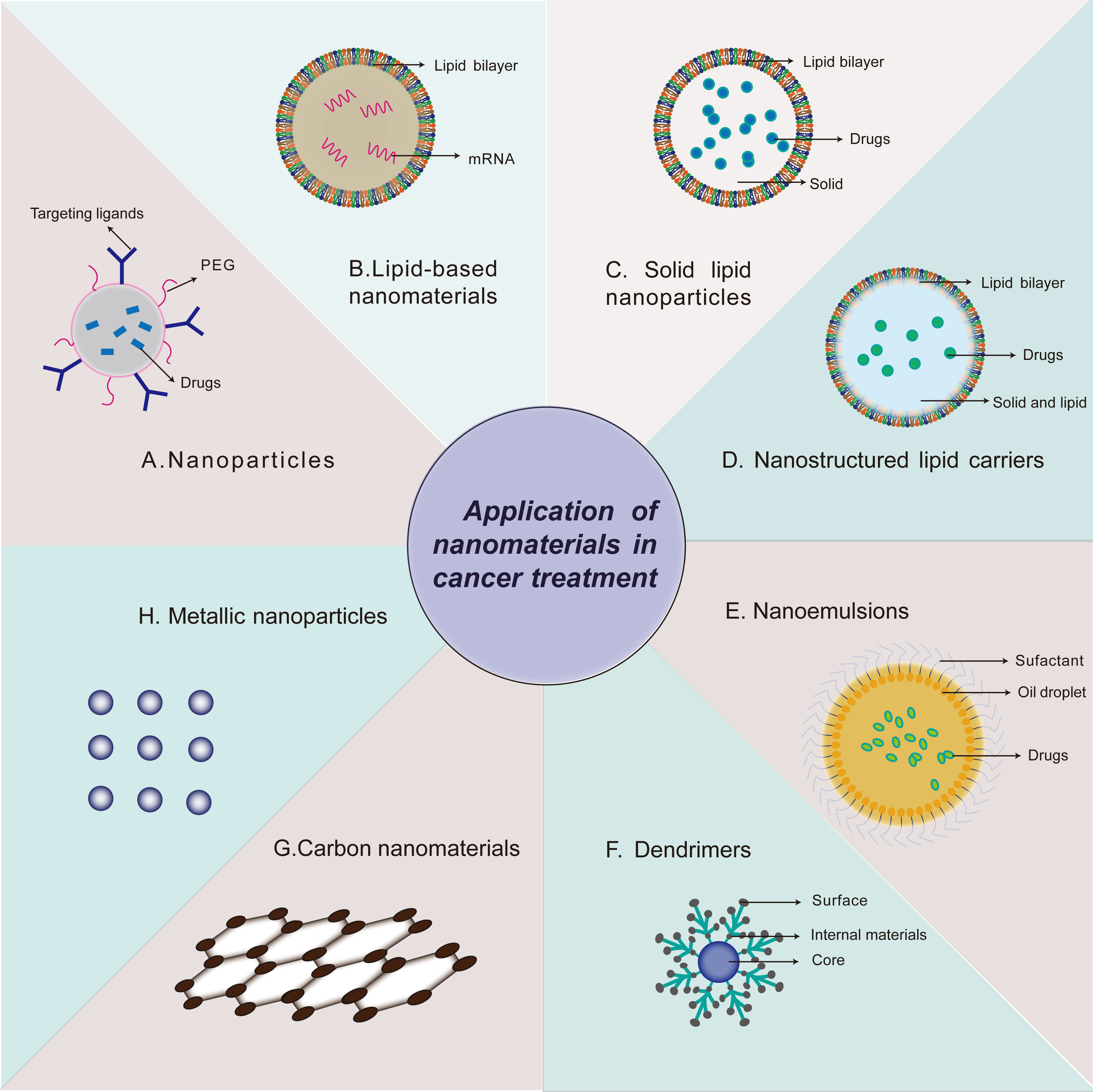
Figure 1 Strategies of nanomaterials applied in cancer treatment. Application of nanomaterials in cancer treatment, including Nanoparticles (A), Lipid-based nanomaterials (B), Solid lipid nanoparticles (C), Nanostructured lipid carriers (D), Nanoemulsions (E), Dendrimers (F), Carbon nanomaterials (G), Metallic nanoparticles (H).
Cancer is a severe disease that causes a global economic burden. Lung, colorectal, liver and breast cancers are the most common cancers (18). Various strategies can be used to deal with these serious diseases including surgery, radiotherapy, chemotherapy, immunotherapy, and microwave therapy, thereby greatly improving the therapeutic effect and quality of life of patients (19). However, for many patients, the above methods have obvious limitations owing to the serious side-effects and ineffectiveness of treatment. In recent years, nanotechnology has played an important role in vaccinology through the research on adjuvants and vaccine delivery systems (20–22). Moreover, some nanocarrier-based mRNA vaccines have been successfully applied to the treatment of coronavirus disease (COVID-19) (23–25), which has greatly enhanced the public’s confidence in the development of mRNA cancer vaccines. Further, some studies have reported the possibility of nanocarrier-based mRNA vaccines being used as cancer vaccines (26–32). Nanocarriers can address the instability of mRNA vaccines (33) and improve the targeting of drug therapy, which has good prospects through further research and the development of cancer nanocarrier-based mRNA vaccines.
The mRNA contains the genetic information of organisms, which can serve as a direct template for protein biosynthesis. It is a bridge that connects genetic information in the DNA with protein translation and expression, thereby playing an important role in regulating the activities of life. The mRNA vaccine, a subtype of the nucleic acid vaccine, can be divided into two categories, non-replicating mRNA and self-amplified RNA (saRNA). The traditional non-replicative mRNA consists of a 5′ cap, a 5′ - untranslated region (UTR), an open reading frame, a 3′- UTR and a poly (A) tail encoding a vaccine antigen. Structural elements are very important for the transcriptional efficiency and stability of mRNA. These elements are also modifiable sites, which can prolong the half-life of mRNA in vivo and limit unnecessary immune responses (34). Compared to conventional mRNA, saRNA is another kind of mRNA molecule with a different structure. saRNA can effectively produce a large amount of protein of interest by exploiting the innate nature of alphaviruses (17). The basic components of saRNA are the 5′ cap, 5′-UTR, sequence coding for nonstructural proteins (NSP), subgenomic promoter sequence, open reading frame, 3′-UTR, and a 3′ poly(A) tail (35). After saRNA is transfected into the cell, the NSP sequence is translated into the NSP polyprotein, which functions as the precursor of the replicase complex. This complex transcribes the original positive-sense RNA strand into a negative-sense RNA strand, which is then used as the template for subsequent replication (36).
Currently, there are four types of cancer vaccines: viral vector vaccines, tumor cell- and immune cell-based vaccines, peptide-based vaccines, and nucleic acid-based vaccines (37). Nucleic acid-based vaccines are promising platforms for cancer vaccines for many reasons. First, nucleic acid vaccines can simultaneously transmit multiple antigens covering a series of tumor-associated antigens (TAAs) or somatic tumor mutations, while stimulating cell-mediated and humoral immune responses, which significantly increase the possibility of overcoming vaccine drug resistance. Second, nucleic acid vaccines are unlike polypeptide vaccines, which can encode full-length tumor antigens, allowing antigen-presenting cells (APCs) to simultaneously present or cross-present multiple epitopes of class I and II patient-specific human leukocyte antigen (HLA). As a result, nucleic acid vaccines are not limited by HLA type and are more likely to stimulate a wider range of T-cell responses (38, 39).
Some mRNA vaccines for non-cancerous diseases have been studied in clinical trials, especially in COVID-19 treatment (Table 1). Several preclinical and clinical studies have explored mRNA vaccines for anticancer use, either by adoptive transfer on dendritic cells (DCs) in vitro or by direct injection (47, 48). The basic principle of mRNA as a cancer vaccine is that it can deliver the target transcript encoding one or more tumor-specific antigens (TSAs) or TAAs to the cytoplasm of the host cell (especially in APCs), and then express it as the target antigen (49). Through a major histocompatibility complex, the expressed TSAs and TAAs can be presented on the cellular surface of APCs, thereby activating the anti-tumor immunity (50). When inoculated into host cells, mRNA can be translated into the corresponding antigens, imitating humoral and cellular immunity similar to a viral infection (51). mRNA vaccines enhance the antiviral and antitumor activity of the host by increasing the antigenic activity of T cells (52, 53). Most studies on T-cell immunity induced by antigen-encoded mRNA use autologous DCs, which are loaded with mRNA in vitro and reintroduced into patients. Although clinical trials have proved the safety and feasibility of this method, the in vitro operation of DCs requires complex personalized vaccination procedures, which seriously hinders its application in patients. Therefore, there is an urgent demand for new vaccination methods that can directly target DCs in vivo (1). We have described the process of using mRNA vaccines for drug treatment (Figure 2) (13).
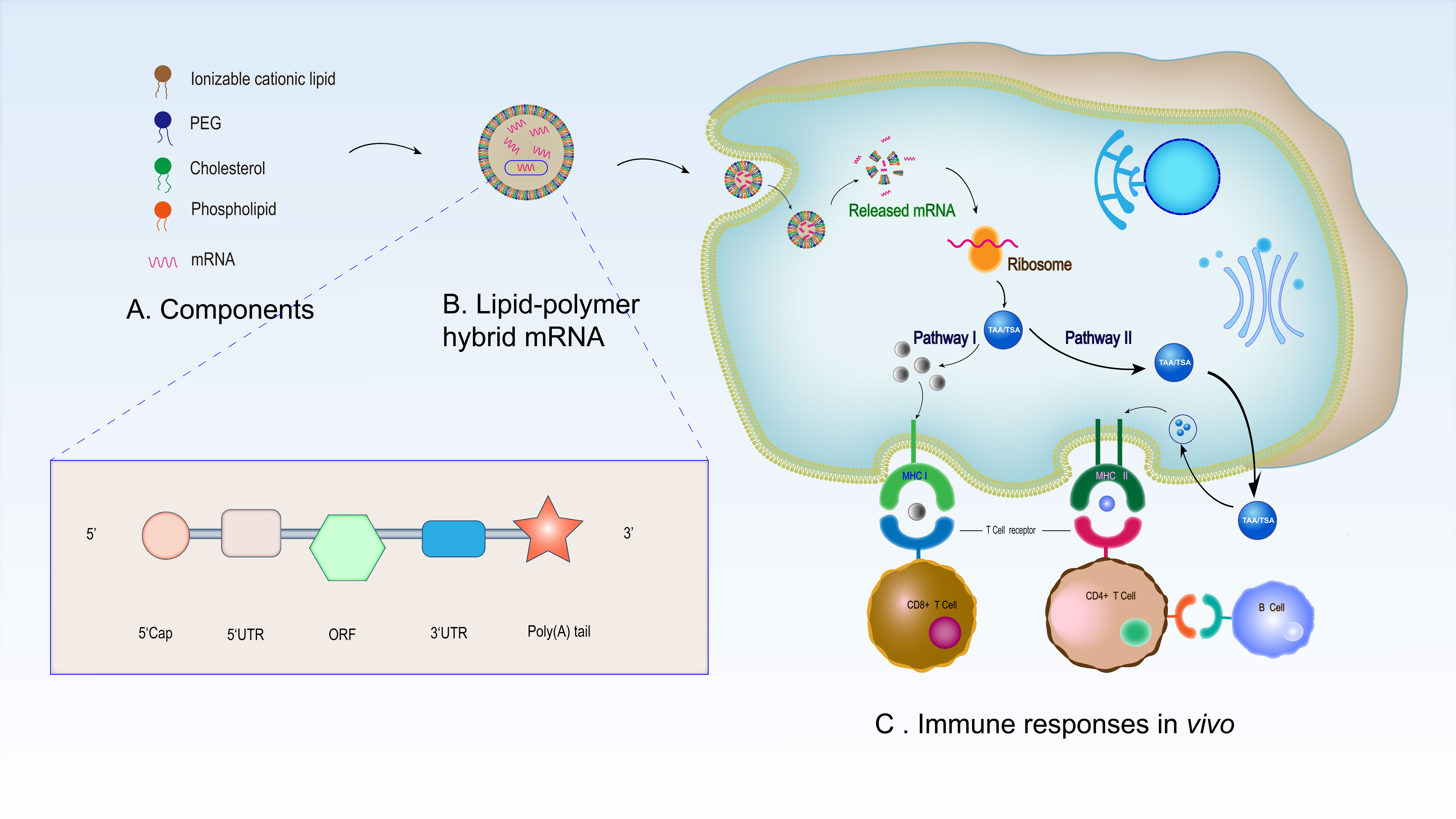
Figure 2 Synthesis and mechanisms of LNP-based mRNA vaccine in immunotherapy. (A) Synthetic raw materials of LNP -based mRNA vaccine. (B) Morphology of the assembled mRNA. The mRNA structure in the liposome is composed of a 5’cap structure, 5’- and 3’- UTR, a 3’ poly (A) tail and an open reading frame. (C) Immune responses of LNP-based mRNA vaccine in vivo. After entering the human body, the LNP-based mRNA vaccine enters antigen-presenting cells through endocytosis before releasing the mRNA in the cells. The released mRNA translates the target antigen in the ribosome. Subsequently, endogenous antigens are degraded into polypeptides and are presented by MHC I and activate CD8+ T cells (pathway I). In addition, secreted antigens can be taken up by cells, degraded inside endosomes, and presented on the cell surface to CD4+ T cells by MHC II. Finally, CD4+ T cells stimulate B cells to promote B cell maturation and then B cells produce specific antibodies to play an anti-tumor role (pathway II).
Many drugs use nanomaterials as carriers. Compared to other treatment with small molecules, DNA, oligonucleotides, viral systems and proteins (including antibodies), messenger RNA therapy has many advantages (54). In contrast to oligonucleotides and most small molecular drug targets, mRNA vaccines can simultaneously mediate stimulation and inhibition patterns, as well as express or replace defective proteins, thus expanding the range of their potential indications. Compared to DNA vaccines and lentiviral vector, mRNA vaccine only needs to enter the cytoplasmic ribosomal translation mechanism and not the nucleus; thus, there is no risk of genome integration (55–59). Furthermore, after mRNA vaccination, antigen expression is transient, thus avoiding T cell depletion caused by persistent antigen exposure (60). Compared to protein vaccines, mRNA vaccines have the advantages of simple synthesis, fast purification and low cost (61).
Because of the degradation of RNases in vivo and the innate immunogenicity of mRNA vaccines, mRNA is easily degraded before APCs are present. Under the influence of these factors, improving the stability of mRNA can ensure an expression effect. The common ways of improving the stability of mRNA translation are as follows (Table 2). For example, using synthetic cap analogues that by binding to the eukaryotic translation initiation factor 4e (eIF4E) stabilize mRNA and increase protein translation (73, 74); regulatory elements in the 5’- and 3’- UTR and poly (A) tail also can stabilize mRNA and increase protein translation (75, 76); nucleoside modification reduces the activation of innate immunity and enhances translation (77); and sequence and/or codon optimization increases translation (78). Although changing the codon composition or introducing modified nucleosides can positively regulate protein expression, these may affect the secondary structure of mRNA (79), kinetics and accuracy of protein folding and translation (80), and the expression of T cell epitopes in alternative reading frames (81). Collectively, these factors may affect the degree and specificity of the immune response.
The study of mRNA vaccines based on nanomaterial delivery systems can overcome the limitations of in vivo delivery, such as insufficient protein expression in cells, insufficient antigen load, and maturation of APCs (82). Nanotechnology has made an important contribution to the development of effective vaccine adjuvants and delivery systems. LNP-based mRNA vaccines can protect the encapsulated mRNA from the host environment and release it continuously to induce a lasting immunostimulatory effect. Nanocarriers, including viral and non-viral carriers, have also been explored as potential tools in delivering vaccines because they are expected to elicit a wide range of immune responses in addition to cell-mediated immunity (83). Nanomaterial delivery systems mainly include nanoemulsion-based adjuvants, lipid nanocarriers, and adjuvants targeting pattern recognition receptors (83). The use of nanomaterials as drug carriers is effective in the treatment of many diseases such as pulmonary infectious diseases and psoriasis (84–86).
Currently, the main nanomaterials used to deliver mRNA vaccines are lipid nanoparticles (LNPs). LNPs constitute four components, including ionizable cationic lipids, lipid-linked polyethylene glycol, cholesterol, and naturally occurring phospholipids. The synthesis of LNPs is a crucial process, which directly affect the size and encapsulation efficiency of the LNPs. The crucial factor in the synthesis of fine and uniform LNPs is the rapid mixing of the excess water and ethanol-lipid phase. Using the staggered herringbone structure, microfluidic mixing is a selectable synthesis method, for the diverse-scale synthesis of LNPs (15). Once the mRNA vaccines enter the targeted cells, the process of their forming complexes with cationic lipids released is necessary for nucleic acid delivery. Through neutralizing the charge of their cationic lipid carriers, the cell’s anionic lipids can help LNPs release nucleic acids, thereby disrupting the electrostatic interactions between the lipid carriers and nucleic acids (87, 88).
The extracellular half-life of mRNA can be prolonged by encapsulating mRNA into liposome nanoparticles to prevent its degradation by ubiquitous RNA enzymes (89–92). Nanocarrier administration can also improve the half-life of biological agents by preventing the premature release and degradation of drugs and evading clearance from the kidneys and liver (93). Thus, a nanomaterial-based delivery system can maintain the extracellular stability of the mRNA vaccine.
LNP-based mRNA vaccines are more selective than other treatments. The use of nanomaterials for drug delivery can reduce adverse reactions by preventing the non-specific absorption of therapeutic agents in healthy tissues (94). Therefore, this is more suitable for patients who cannot tolerate traditional treatment because of their poor physical condition. Targeted therapy has also been proven to be an effective method for tumor therapy (95, 96). Because of their benign biocompatibility and low toxicity, endogenous carriers show great potential for the delivery of therapeutic nanoparticles (97, 98). An efficient way to deliver drugs to the lesion is to wrap anticancer agents in nanocarriers (99–101). The main advantage of the LNP-based strategy compared to naked mRNA is that it can deliver many drugs specifically by changing the pharmacokinetic properties of drugs, which increases drug intake in tumor tissues, improves the anti-tumor effect, and reduces non-specific toxicity (102, 103). Currently, many immunotherapies based on mRNA have been applied in clinical trials, and some confirmative results have shown the efficacy results of solid tumor treatments (54). Cancer therapies involve multi-disciplinary participation (104), and the vaccine is in the developing stage. LNP-based mRNA vaccines have obvious advantages, but they have not been widely used in clinical practice. LNP-based vaccines that are currently in the preclinical phase or have been used in clinical trials have been introduced below. Figure 3 shows the anti-cancer mechanism of LNP-based mRNA vaccines in lung cancer, colorectal cancer (CRC), and glioma.
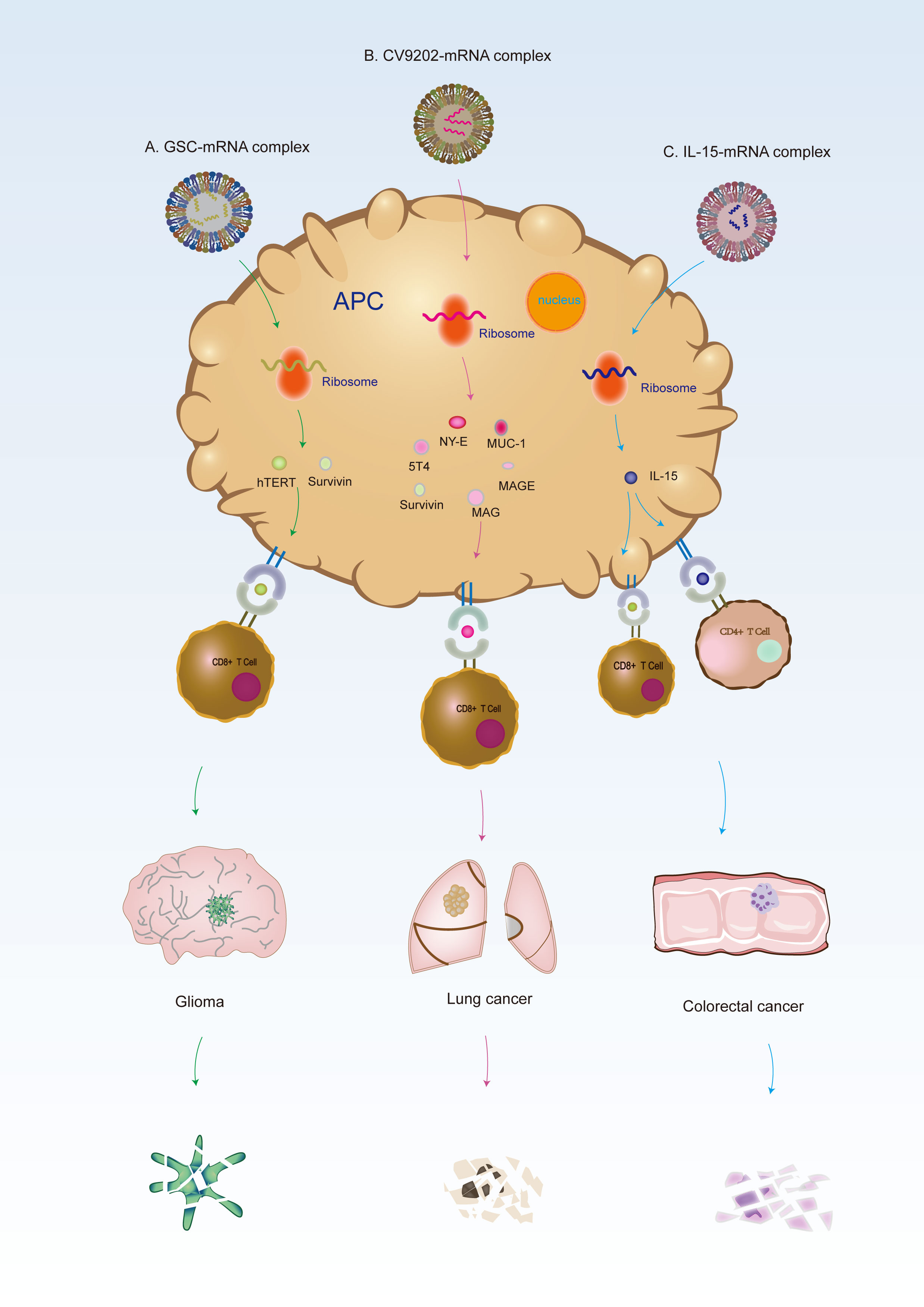
Figure 3 Mechanism of mRNA vaccines against lung cancer, CRC and glioma. (A) Glioma stem cells (GSC): Autologous dendritic cells transfected with autologous GSC-mRNA are utilized to induce an immune response against the GSC of patients. GSC-mRNA can translate hTERT and survivin to induce the immune response. (B) Lung cancer: CV9202 is a cancer immunotherapy with sequence-optimized mRNAs encoding different cancer antigens in free and complexed form with the CLPP; this facilitates antigen expression and activation of the immune system, essentially conferring self-adjuvant activity and subsequently inducing an adaptive cellular and humoral immune response. (C) CRC: After the CLPP/mRNA complex enters the body, CLPP can deliver the IL-15-mRNA to antigen-presenting cells (APCs), and then APCs will translate IL-15 protein and release it, causing cellular immune response and promoting the cell death of CRC.
Lung cancer is the most commonly diagnosed cancer, and the leading cause of cancer-related deaths (105). Non-small-cell lung cancer (NSCLC) is the most common type of lung cancer, with approximately 40% patients having stage IV metastatic disease when diagnosed (106). According to the type and stage of malignant tumors, the treatment of lung cancer usually requires a combination of chemotherapy, surgery, or radiotherapy. However, because of the inadequacy of early diagnosis, the cancer in most patients with lung cancers is detected at an advanced stage, with distant metastasis or local tumor invasion, and are not suitable for surgical treatment. With the progression of the disease, the survival time of patients in stage IV lung cancer gradually decreases to 4 months. Therefore, it is crucial to intervene at an early stage. Systemic cytotoxic therapy has become the main treatment for advanced NSCLC, but the benefits of chemotherapy have reached a plateau and new forms of treatment are needed (107). Systemic chemotherapy is the mainstream treatment for advanced lung cancer; it aims to prolong the survival time of patients and improve their quality of live (108). The standard first-line chemotherapy regimen for lung cancer includes platinum-based drugs (e.g. carboplatin and cisplatin) (109). However, platinum-based chemotherapy causes dose-limiting side effects, including intestinal damage, anemia, nephrotoxicity and cardiotoxicity, and peripheral neuropathy, with other symptoms such as nausea, restlessness, and fatigue (110). Moreover, the survival rate of traditional chemotherapy combinations has stabilized, and the median survival rate of patients with molecular changes targeted by new drugs is only approximately 2–3 years (111).
Currently, only a few preclinical studies on LNP-based mRNA vaccines are available for lung cancer. In a clinical Phase Ib study, Papachristofilou et al. (112) demonstrated that BI1361849, an immunotherapeutic that included the five antigens encoded by CV9202, combined with local radiation treatment with or without pemetrexed was well tolerated with few side effects, and could induce a targeted immune response in patients with stage IV NSCLC. After vaccination, functional CD4+ and/or CD8+ T cells in 40% of patients increased at least twice as much as before treatment compared to the baseline. This treatment provided evidence to support that BI1361849 (CV9202) combined with immune checkpoint inhibitors benefitted the patients with NSCLC. Similarly, in a clincal phase I/IIa study, Sebastian et al. (113) concluded that the antigen-specific immunotherapy (CV9201) was well tolerated and had enhanced the immune response in patients with stage IIIB/IV NSCLC. Encouragingly, the efficacy and safety of mRNA vaccines (BI 1361849) combined with a checkpoint inhibitor, anti-CTLA-4(tremlimumab) and anti-PD-L1(duvalumab) in the treatment of NSCLC have been evaluated in an ongoing phase I/II study (NCT03164772) (114). These studies suggested the importance of mRNA-based immunotherapy combined with immune checkpoint inhibitors in the treatment of NSCLC.
In 2018, more than 1.8 million new CRC cases and 881,000 deaths were estimated to have occurred (105). Currently, most stage I or II CRC patients are treated surgically, and the standard surgical procedure for CRC is total mesenterectomy (115, 116). Surgery with chemotherapy is considered the standard treatment for patients with stage III CRC, whereas systemic chemotherapy or a combination of targeted biologics is often preferred for patients with metastatic CRC (117).
Regarding the close relationship between the incidence of CRC and heterogeneity (118), precision therapy has been used for treating CRC in recent years (19, 119–121). LNP -based mRNA vaccines display unique advantages for the therapy of CRC. Lei et al. (122) established a liposome/protamine system (CLPP) nano-delivery system to provide IL-15-encoded mRNA for colon cancer gene therapy. The results indicated that the IVTIL-15mRNA could be effectively and safely introduced into CT26 CRC cells through the prepared CLPP system. Local administration as well as systemic administration of the CLPP/mIL-15 complex showed significant anticancer effects in a C26 CRC cell model of subcutaneous metastasis, abdominal metastasis and lung metastasis through various mechanisms. Local or systemic administration of CLPP/mIL-15 complex had an obvious inhibitory effect on a variety of C26 CRC mouse models, with inhibition rates of 70%, 55%, and 69% in the CT26 abdominal metastasis model, subcutaneous metastasis model and in the lung metastasis model, respectively. They are all highly effective and safe. After vaccination, the activity of lymphocytes was significantly stimulated, and the magnitude of CD8+T cells increased significantly. These data proved that the CLPP/mIL-15 complex had high therapeutic potential in the immune gene therapy of CRC, indicating that CLPP is ideal for mRNA delivery, and the CLPP/mIL-15 complex is promising for cancer immune gene therapy.
Central nervous system tumors and peripheral nervous system tumors together constitute neurotumors. The formation of these tumors is mainly caused by improper nerve repair after a certain degree of nerve injury (123). Although only a small proportion of the neurotumor cases are the central nervous system tumors (105), their treatment is challenging. It is challenging to reach the tumor site surgically owing to the deep location of the central nervous system. Therefore, as the current treatment still has many limitations, exploring the regulatory mechanism and searching for biomarkers for glioma tumors is urgently need (124).
mRNA can be used to treat genetic diseases or repair tissues as they express certain functional proteins; thus, they can also be used for immunotherapy by expressing antigens, antibodies or receptors. Although these therapies are not sufficiently mature, they have broad research prospects. Vik-Mo et al. (125) successfully isolated brain tumor biopsy tissues and prepared a single-cell suspension. Autologous glioma stem cells (GSCs) were amplified into tumor spheres in vitro, and GSC-mRNA was amplified and transfected into monocyte-derived autologous DCs. Autologous DCs transfected with autologous GSC-mRNA were used to induce an immune response to the patient’s GSCs. Compared to somatic neural progenitor cells, GSCs had enhanced telomerase activity and highly expression of survivin, an inhibitor of apoptosis protein. Seven patients were treated with DC vaccine targeting GSCs in solid tumors, resulting in a 2.9 times increase in the progression-free survival of patients with glioma. In all seven patients, they found specific-induced lymphocyte proliferation upon stimulation with tumorsphere lysate, hTERT, or survivin peptides.
In 2010, Provenge (Sipuleucel-T), an immune cell-based vaccine approved by the United States Food and Drug Administration, served as the first therapeutic cancer vaccine for patients with hormone-resistant prostate cancer (126). Huang et al. (127) found that CD247, FCGR1A and TRRAP were potential antigens of mRNA vaccines against cholangiocarcinoma, especially for patients with the immune subtypes 2 (IS2) tumors, providing a theoretical foundation for the development of anti-cholangiocarcinoma mRNA vaccines and identifying suitable vaccination targets. Huang et al. (128) also reported that ADAM9, MET, TPX2, EFNB2, WNT7A, and TMOD3 were effective antigens of the anti-pancreatic cancer mRNA vaccine, and that patients with two immune subtypes IS4 and IS5 tumors were suitable for vaccination. The anti-cancer vaccines CV9103 and CV9104 can also be used for the treatment of prostate cancer (129).
Although the prospect of LNP-based mRNA vaccines as a cancer therapy is optimistic, currently, its application is mainly in the translational therapy stage. Translational medicine is usually defined as “the transfer of new understandings of disease mechanisms gained in the laboratory into the development of new methods for diagnosis, prevention, and therapy” (130). As shown in Figure 4, in the Pten-mutated melanoma and Pten-null prostate cancer mouse model, the combination of PTEN mRNA nanoparticles (mPTEN@NPs) can reverse the inhibition of antitumor immune responses by increasing the infiltration of CD8+T and CD3+T cells, and can combine with anti-programmed death-1 (anti-PD-1) antibody enhancing the therapeutic efficacy (131). Moreover, Zhang et al. (132) used paclitaxel aminolipid (PAL)-derived nanoparticles to integrate p53-mRNA and chemotherapy drug. In contrast to clinical drugs, PAL-p53-mRNA nanoparticles had higher paclitaxel loading. In addition, these nanoparticles showed the synergistic cytotoxicity of paclitaxel and P53-mRNA in cultured TNBC cells. They displayed the anti-tumor effect of PAL-p53-mRNA nanoparticles in vivo in a TNBC mouse model. In Table 3, we summarized the current application of LNP-based mRNA vaccines in conversion therapy, aiming to provide new methods and ideas for clinical treatment in the future. The applications of LNP-based mRNA vaccines in cancer transformation therapy are limited, while transformation therapy is an extremely important stage before the clinical application of drugs, which is the premise and basis of drugs entering clinical trials. Taken together, we hope that more drug therapeutic targets can be confirmed in transformation trials.
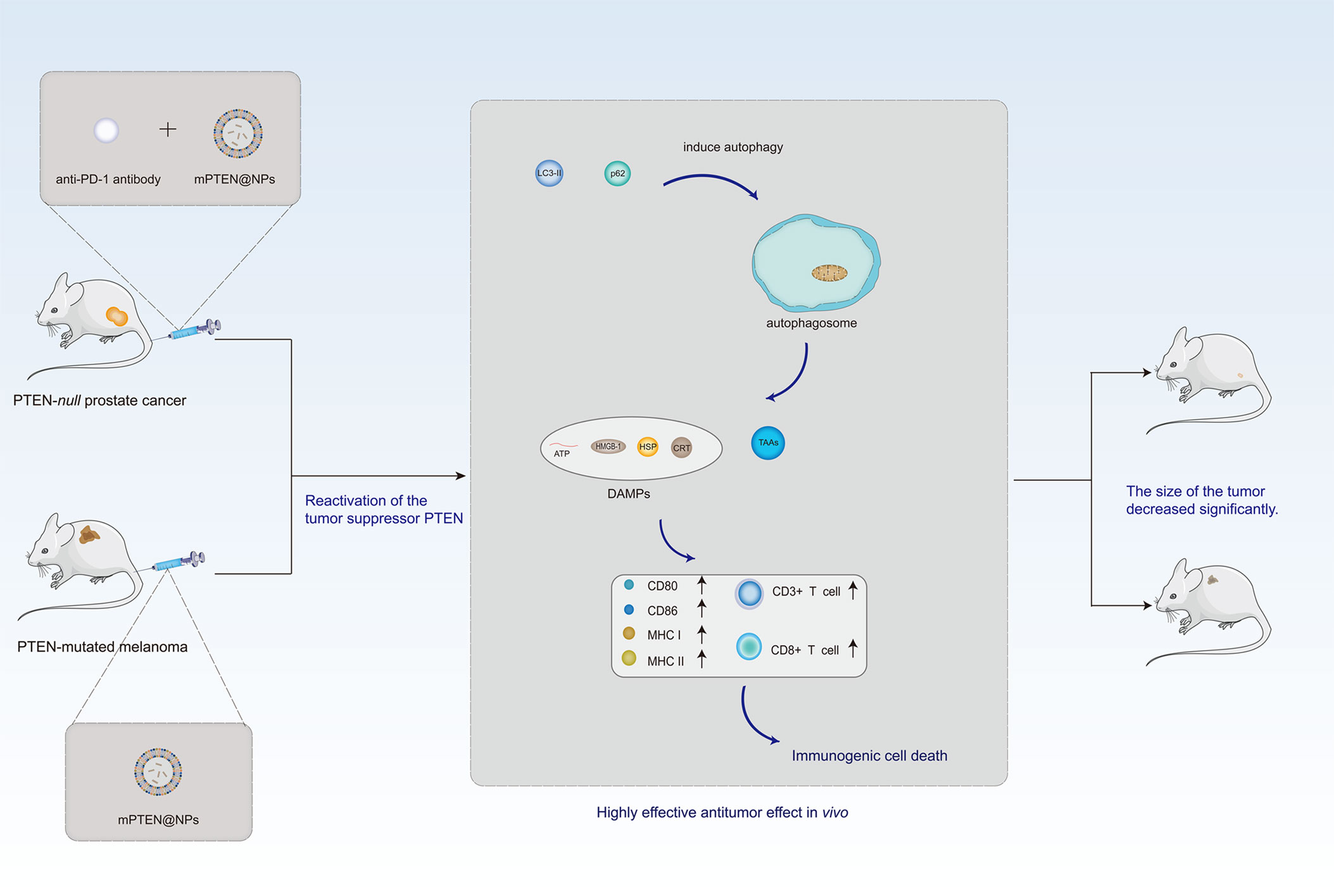
Figure 4 Antitumor function of PTEN can be reactivated by PTEN mRNA nanoparticles (mPTEN@NPs) in preclinical models. In the Pten-mutated melanoma and Pten-null prostate cancer mouse model, the combination of PTEN mRNA nanoparticles (mPTEN@NPs) can reverse the inhibition of antitumor immune responses by increasing the infiltration of CD8+T and CD3+T cells, as well as combine with anti-programmed death-1 antibody enhancing the therapeutic efficacy.
LNP-based mRNA vaccines are an excellent example of combining materials′ science with medicine, enhancing communication between different disciplines, and integrating the different innovative approaches. Antagonism between humans and cancer is perennial. As an emerging cancer therapy, mRNA vaccines have broad future applications because unlike other treatments, cancer mRNA vaccines provide specific, safe, and well-tolerated treatments. Preclinical studies have used personalized vaccines to evaluate the anti-tumor effect of mRNA vaccines in the therapy of several solid tumors, including lung cancer and CRC. To further improve the capacity of mRNA anti-cancer vaccines, many clinical trials are underway to evaluate the efficacy and safety of mRNA vaccines in combination with checkpoint inhibitor therapy or cytokine therapy. As a new method of cancer therapy, the significant advantages of LNP-based mRNA vaccines are reflected in the following aspects: (1) the significant advantage of mRNA vaccines compared to other treatment lies in the efficacy, safety, and productive efficiency of mRNA vaccines; (2) with the rapid development of nanotechnology, LNP-based mRNA vaccines are likely to greatly improve the stability of mRNA vaccines, prevent mRNA from decomposing prematurely in vivo, increase the half-life of drugs, and reduce the dose and frequency of drugs; (3) with an in-depth understanding of the mechanism of cancer, the targeting of LNP-based mRNA vaccines will be further improved. mRNA vaccines can treat diseases from the genetic level. Compared with other therapies, LNP-based mRNA vaccines are more scientific, have few side effects and will not cause other adverse effects in patients, which is a good embodiment of the benefits of precision medicine in cancer treatment.
However, the clinical application of LNP-based mRNA vaccines is limited by several factors. First is the high diversity of tumor antigens. Cancer can be caused by multiple genes, which is a challenge in the development of mRNA vaccines. Second limitation is determining the proper treatment targets. The correct treatment target requires accurate determination of the location and related information about the genes that cause the disease. Although the Human Genome Project has been completed, the locations of many disease-associated genes are still unknown, which renders mRNA vaccines lack effective therapeutic targets for diseases of unknown etiology, resulting in rare types of cancers that could potentially be treated with mRNA vaccines. Third limitation is the availability of suitable nanocarriers. Although liposomes are currently the most widely used nanocarriers of mRNA vaccines, they still have a certain toxicity and are not absolutely safe for the human body. It is difficult to search for a safer and more efficient nanocarrier because a suitable nanocarrier requires identifying materials that not only combine perfectly with the drugs, but also avoid rejection by human cells and need to be of low toxicity. This requires further development of materials′ science. Fourth limitation is the high capital needs. The research and development of mRNA vaccines are still in its infancy, with uncertain efficacy in animals, and requiring more clinical trials in humans to prove that they are efficacious; hence, this process requires considerable financing.
In conclusion, LNP-based mRNA vaccines have shown potential in the treatment and prevention of cancers because of their evident advantages, and are expected to be a promising approach in many cancer therapies. Moreover, it is reasonable to believe that LNP-based mRNA vaccines could play an important role in disease diagnosis and prognosis in the near future.
CO, JW and XH conceived manuscript. TH collected relevant references, drafted manuscript and finished the figures. All authors offered crucial content revision and language polishing. CO, JW and XH completed the final manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (No. 81602167 and 81903032), the China Postdoctoral Science Foundation (No. 2020M672520), the Outstanding Youth Foundation of Hunan Provincial Natural Science Foundation of China (No. 2022JJ20098), the Hunan Provincial Natural Science Foundation of China (No. 2021JJ31100 and 2021JJ41013), the Science and Technology Program Foundation of Changsha City (No. kq2004085) and the Youth Fund of Xiangya Hospital (No. 2018Q011).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
CRC, colorectal cancer; COVID-19, Corona Virus Disease 2019; UTR, untranslated region; TAAs: tumor-associated antigens; APCs: antigen-presenting cells; HLA, human leukocyte antigen; DCs, dendritic cells; TSAs, tumor-specific antigens; MHC, major histocompatibility complex; eIF4E, eukaryotic translation initiation factor 4E; m7G, 7-methylguanosine; LNPs, lipid nanoparticles; NSCLC, Non-small-cell lung cancer; CLPP, a liposome/protamine system; IL-15, Interleukin 15; GSCs, glioma stem cells; mPTEN@NPs, PTEN mRNA nanoparticles; PD-1, programmed death-1; PAL, paclitaxel aminolipid; TNBC, Triple-negative breast cancer.
1. Pollard C, De Koker S, Saelens X, Vanham G, Grooten J. Challenges and advances towards the rational design of mRNA vaccines. Trends Mol Med (2013) 19(12):705–13. doi: 10.1016/j.molmed.2013.09.002
2. Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, et al. Direct gene transfer into mouse muscle in vivo. Sci (New York NY) (1990) 247(4949 Pt 1):1465–8. doi: 10.1126/science.1690918
3. Brenner S, Jacob F, Meselson M. An unstable intermediate carrying information from genes to ribosomes for protein synthesis. Nature (1961) 190:576–81. doi: 10.1038/190576a0
4. Gurdon JB, Lane CD, Woodland HR, Marbaix G. Use of frog eggs and oocytes for the study of messenger RNA and its translation in living cells. Nature (1971) 233(5316):177–82. doi: 10.1038/233177a0
5. Jirikowski GF, Sanna PP, Maciejewski-Lenoir D, Bloom FE. Reversal of diabetes insipidus in brattleboro rats: Intrahypothalamic injection of vasopressin mRNA. Sci (New York NY) (1992) 255(5047):996–8. doi: 10.1126/science.1546298
6. Tavernier G, Andries O, Demeester J, Sanders NN, De Smedt SC, Rejman J. mRNA as gene therapeutic: How to control protein expression. J Controlled Release Off J Controlled Release Society (2011) 150(3):238–47. doi: 10.1016/j.jconrel.2010.10.020
7. Malone RW, Felgner PL, Verma IM. Cationic liposome-mediated RNA transfection. Proc Natl Acad Sci USA (1989) 86(16):6077–81. doi: 10.1073/pnas.86.16.6077
8. Pardi N, Hogan MJ, Weissman D. Recent advances in mRNA vaccine technology. Curr Opin Immunol (2020) 65:14–20. doi: 10.1016/j.coi.2020.01.008
9. Liu Y, Miyoshi H, Nakamura M. Nanomedicine for drug delivery and imaging: A promising avenue for cancer therapy and diagnosis using targeted functional nanoparticles. Int J Cancer (2007) 120(12):2527–37. doi: 10.1002/ijc.22709
10. Kim BY, Rutka JT, Chan WC. Nanomedicine. N Engl J Med (2010) 363(25):2434–43. doi: 10.1056/NEJMra0912273
11. Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles in medicine: Therapeutic applications and developments. Clin Pharmacol Ther (2008) 83(5):761–9. doi: 10.1038/sj.clpt.6100400
12. Cullis PR, Hope MJ. Lipid nanoparticle systems for enabling gene therapies. Mol Ther J Am Soc Gene Ther (2017) 25(7):1467–75. doi: 10.1016/j.ymthe.2017.03.013
13. Cheng Z, Li M, Dey R, Chen Y. Nanomaterials for cancer therapy: Current progress and perspectives. J Hematol Oncol (2021) 14(1):85. doi: 10.1186/s13045-021-01096-0
14. Wang C, Zhang Y, Dong Y. Lipid nanoparticle-mRNA formulations for therapeutic applications. Acc Chem Res (2021) 54(23):4283–93. doi: 10.1021/acs.accounts.1c00550
15. Reichmuth AM, Oberli MA, Jaklenec A, Langer R, Blankschtein D. mRNA vaccine delivery using lipid nanoparticles. Ther Deliv (2016) 7(5):319–34. doi: 10.4155/tde-2016-0006
16. Schlake T, Thess A, Fotin-Mleczek M, Kallen KJ. Developing mRNA-vaccine technologies. RNA Biol (2012) 9(11):1319–30. doi: 10.4161/rna.22269
17. Müntjes K, Devan SK, Reichert AS, Feldbrügge M. Linking transport and translation of mRNAs with endosomes and mitochondria. EMBO Rep (2021) 22(10):e52445. doi: 10.15252/embr.202152445
18. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA: A Cancer J Clin (2022) 72(1):7–33. doi: 10.3322/caac.21708
19. Wang Y, Nie H, He X, Liao Z, Zhou Y, Zhou J, et al. The emerging role of super enhancer-derived noncoding RNAs in human cancer. Theranostics (2020) 10(24):11049–62. doi: 10.7150/thno.49168
20. Chauhan G, Madou MJ, Kalra S, Chopra V, Ghosh D, Martinez-Chapa SO. Nanotechnology for COVID-19: Therapeutics and vaccine research. ACS Nano (2020) 14(7):7760–82. doi: 10.1021/acsnano.0c04006
21. Han Y, Wang D, Peng L, Huang T, He X, Wang J, et al. Single-cell sequencing: A promising approach for uncovering the mechanisms of tumor metastasis. J Hematol Oncol (2022) 15(1):59. doi: 10.1186/s13045-022-01280-w
22. Peng L, Wang D, Han Y, Huang T, He X, Wang J, et al. Emerging role of cancer-associated fibroblasts-derived exosomes in tumorigenesis. Front Immunol (2022) 12:795372. doi: 10.3389/fimmu.2021.795372
23. Sahin U, Muik A, Derhovanessian E, Vogler I, Kranz LM, Vormehr M, et al. COVID-19 vaccine BNT162b1 elicits human antibody and T(H)1 T cell responses. Nature (2020) 586(7830):594–9. doi: 10.1038/s41586-020-2814-7
24. Jackson LA, Roberts PC, Graham BS. A SARS-CoV-2 mRNA vaccine - preliminary report. Reply. N Engl J Med (2020) 383(12):1191–2. doi: 10.1056/NEJMoa2022483
25. Corbett KS, Edwards DK, Leist SR, Abiona OM, Boyoglu-Barnum S, Gillespie RA, et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature (2020) 586(7830):567–71. doi: 10.1038/s41586-020-2622-0
26. Billingsley MM, Singh N, Ravikumar P, Zhang R, June CH, Mitchell MJ. Ionizable lipid nanoparticle-mediated mRNA delivery for human CAR T cell engineering. Nano Lett (2020) 20(3):1578–89. doi: 10.1021/acs.nanolett.9b04246
27. Zhu G, Zhang F, Ni Q, Niu G, Chen X. Efficient nanovaccine delivery in cancer immunotherapy. ACS Nano (2017) 11(3):2387–92. doi: 10.1021/acsnano.7b00978
28. Verbeke R, Lentacker I, Breckpot K, Janssens J, Van Calenbergh S, De Smedt SC, et al. Broadening the message: A nanovaccine Co-loaded with messenger RNA and α-GalCer induces antitumor immunity through conventional and natural killer T cells. ACS Nano (2019) 13(2):1655–69. doi: 10.1021/acsnano.8b07660
29. Son S, Nam J, Zenkov I, Ochyl LJ, Xu Y, Scheetz L, et al. Sugar-nanocapsules imprinted with microbial molecular patterns for mRNA vaccination. Nano Lett (2020) 20(3):1499–509. doi: 10.1021/acs.nanolett.9b03483
30. Li N, Yang H, Pan W, Diao W, Tang B. A tumour mRNA-triggered nanocarrier for multimodal cancer cell imaging and therapy. Chem Commun (2014) 50(56):7473–6. doi: 10.1039/C4CC01009D
31. De Beuckelaer A, Pollard C, Van Lint S, Roose K, Van Hoecke L, Naessens T, et al. Type I interferons interfere with the capacity of mRNA lipoplex vaccines to elicit cytolytic T cell responses. Mol Ther J Am Soc Gene Ther (2016) 24(11):2012–20. doi: 10.1038/mt.2016.161
32. Cafri G, Gartner JJ, Zaks T, Hopson K, Levin N, Paria BC, et al. mRNA vaccine–induced neoantigen-specific T cell immunity in patients with gastrointestinal cancer. J Clin Invest (2020) 130(11):5976–88. doi: 10.1172/JCI134915
33. Xiong Q, Lee GY, Ding J, Li W, Shi J. Biomedical applications of mRNA nanomedicine. Nano Res (2018) 11(10):5281–309. doi: 10.1007/s12274-018-2146-1
34. Sullenger BA, Nair S. From the RNA world to the clinic. Sci (New York NY) (2016) 352(6292):1417–20. doi: 10.1126/science.aad8709
35. McCullough KC, Bassi I, Milona P, Suter R, Thomann-Harwood L, Englezou P, et al. Self-replicating replicon-RNA delivery to dendritic cells by chitosan-nanoparticles for translation In vitro and in vivo. Mol Ther Nucleic Acids (2014) 3(7):e173. doi: 10.1038/mtna.2014.24
36. Geall AJ, Verma A, Otten GR, Shaw CA, Hekele A, Banerjee K, et al. Nonviral delivery of self-amplifying RNA vaccines. Proc Natl Acad Sci U S A (2012) 109(36):14604–9. doi: 10.1073/pnas.1209367109
37. Faghfuri E, Pourfarzi F, Faghfouri AH, Abdoli Shadbad M, Hajiasgharzadeh K, Baradaran B. Recent developments of RNA-based vaccines in cancer immunotherapy. Expert Opin Biol Ther (2021) 21(2):201–18. doi: 10.1080/14712598.2020.1815704
38. Van Nuffel AM, Wilgenhof S, Thielemans K, Bonehill A. Overcoming HLA restriction in clinical trials: Immune monitoring of mRNA-loaded DC therapy. Oncoimmunology (2012) 1(8):1392–4. doi: 10.4161/onci.20926
39. Miao L, Zhang Y, Huang L. mRNA vaccine for cancer immunotherapy. Mol Cancer (2021) 20(1):41. doi: 10.1186/s12943-021-01335-5
40. Li J, Hui A, Zhang X, Yang Y, Tang R, Ye H, et al. Safety and immunogenicity of the SARS-CoV-2 BNT162b1 mRNA vaccine in younger and older Chinese adults: A randomized, placebo-controlled, double-blind phase 1 study. Nat Med (2021) 27(6):1062–70. doi: 10.1038/s41591-021-01330-9
41. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med (2020) 383(27):2603–15. doi: 10.1056/NEJMoa2034577
42. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med (2021) 384(5):403–16. doi: 10.1056/NEJMoa2035389
43. de Jong W, Aerts J, Allard S, Brander C, Buyze J, Florence E, et al. iHIVARNA phase IIa, a randomized, placebo-controlled, double-blinded trial to evaluate the safety and immunogenicity of iHIVARNA-01 in chronically HIV-infected patients under stable combined antiretroviral therapy. Trials (2019) 20(1):361. doi: 10.1186/s13063-019-3409-1
44. Alberer M, Gnad-Vogt U, Hong HS, Mehr KT, Backert L, Finak G, et al. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: An open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet (London England) (2017) 390(10101):1511–20. doi: 10.1016/S0140-6736(17)31665-3
45. Aldrich C, Leroux-Roels I, Huang KB, Bica MA, Loeliger E, Schoenborn-Kellenberger O, et al. Proof-of-concept of a low-dose unmodified mRNA-based rabies vaccine formulated with lipid nanoparticles in human volunteers: A phase 1 trial. Vaccine (2021) 39(8):1310–8. doi: 10.1016/j.vaccine.2020.12.070
46. Aliprantis AO, Shaw CA, Griffin P, Farinola N, Railkar RA, Cao X, et al. A phase 1, randomized, placebo-controlled study to evaluate the safety and immunogenicity of an mRNA-based RSV prefusion f protein vaccine in healthy younger and older adults. Hum Vaccin Immunother (2021) 17(5):1248–61. doi: 10.1080/21645515.2020.1829899
47. Islam MA, Rice J, Reesor E, Zope H, Tao W, Lim M, et al. Adjuvant-pulsed mRNA vaccine nanoparticle for immunoprophylactic and therapeutic tumor suppression in mice. Biomaterials (2021) 266:120431. doi: 10.1016/j.biomaterials.2020.120431
48. Rittig SM, Haentschel M, Weimer KJ, Heine A, Müller MR, Brugger W, et al. Long-term survival correlates with immunological responses in renal cell carcinoma patients treated with mRNA-based immunotherapy. Oncoimmunology (2016) 5(5):e1108511. doi: 10.1080/2162402X.2015.1108511
49. Cobb M. Who discovered messenger RNA? Curr Biol CB (2015) 25(13):R526–32. doi: 10.1016/j.cub.2015.05.032
50. Van der Jeught K, De Koker S, Bialkowski L, Heirman C, Tjok Joe P, Perche F, et al. Dendritic cell targeting mRNA lipopolyplexes combine strong antitumor T-cell immunity with improved inflammatory safety. ACS Nano (2018) 12(10):9815–29. doi: 10.1021/acsnano.8b00966
51. Yokokawa H, Higashino A, Suzuki S, Moriyama M, Nakamura N, Suzuki T, et al. Induction of humoural and cellular immunity by immunisation with HCV particle vaccine in a non-human primate model. Gut (2018) 67(2):372–9. doi: 10.1136/gutjnl-2016-312208
52. Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer (2012) 12(4):265–77. doi: 10.1038/nrc3258
53. Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature (2011) 480(7378):480–9. doi: 10.1038/nature10673
54. Buschmann MD, Carrasco MJ, Alishetty S, Paige M, Alameh MG, Weissman D. Nanomaterial delivery systems for mRNA vaccines. Vaccines (2021) 9(1):65. doi: 10.3390/vaccines9010065
55. Pascolo S. Vaccination with messenger RNA (mRNA). Handb Exp Pharmacol (2008) 183):221–35. doi: 10.1007/978-3-540-72167-3_11
56. Monopoli MP, Aberg C, Salvati A, Dawson KA. Biomolecular coronas provide the biological identity of nanosized materials. Nat Nanotechnol (2012) 7(12):779–86. doi: 10.1038/nnano.2012.207
57. Lee H, Lytton-Jean AK, Chen Y, Love KT, Park AI, Karagiannis ED, et al. Molecularly self-assembled nucleic acid nanoparticles for targeted in vivo siRNA delivery. Nat Nanotechnol (2012) 7(6):389–93. doi: 10.1038/nnano.2012.73
58. Mockey M, Gonçalves C, Dupuy FP, Lemoine FM, Pichon C, Midoux P. mRNA transfection of dendritic cells: Synergistic effect of ARCA mRNA capping with Poly(A) chains in cis and in trans for a high protein expression level. Biochem Biophys Res Commun (2006) 340(4):1062–8. doi: 10.1016/j.bbrc.2005.12.105
59. Van Tendeloo VF, Ponsaerts P, Berneman ZN. mRNA-based gene transfer as a tool for gene and cell therapy. Curr Opin Mol Ther (2007) 9(5):423–31.
60. Hovav AH, Panas MW, Rahman S, Sircar P, Gillard G, Cayabyab MJ, et al. Duration of antigen expression in vivo following DNA immunization modifies the magnitude, contraction, and secondary responses of CD8+ T lymphocytes. J Immunol (Baltimore Md 1950) (2007) 179(10):6725–33. doi: 10.4049/jimmunol.179.10.6725
61. Zhang R, Tang L, Tian Y, Ji X, Hu Q, Zhou B, et al. DP7-c-modified liposomes enhance immune responses and the antitumor effect of a neoantigen-based mRNA vaccine. J Controlled Release Off J Controlled Release Society (2020) 328:210–21. doi: 10.1016/j.jconrel.2020.08.023
62. Wojtczak BA, Sikorski PJ, Fac-Dabrowska K, Nowicka A, Warminski M, Kubacka D, et al. 5′-phosphorothiolate dinucleotide cap analogues: Reagents for messenger RNA modification and potent small-molecular inhibitors of decapping enzymes. J Am Chem Society (2018) 140(18):5987–99. doi: 10.1021/jacs.8b02597
63. Wei C, Moss B. 5'-terminal capping of RNA by guanylyltransferase from HeLa cell nuclei. Proc Natl Acad Sci USA (1977) 74(9):3758–61. doi: 10.1073/pnas.74.9.3758
64. Wojtczak BA, Sikorski PJ, Fac-Dabrowska K, Nowicka A, Warminski M, Kubacka D, et al. 5'-phosphorothiolate dinucleotide cap analogues: Reagents for messenger RNA modification and potent small-molecular inhibitors of decapping enzymes. J Am Chem Soc (2018) 140(18):5987–99. doi: 10.1021/jacs.8b02597
65. Mignone F. UTRdb and UTRsite: A collection of sequences and regulatory motifs of the untranslated regions of eukaryotic mRNAs. Nucleic Acids Res (2004) 33(Database issue):D141–D6. doi: 10.1093/nar/gki021
66. Trepotec Z, Aneja MK, Geiger J, Hasenpusch G, Plank C, Rudolph C. Maximizing the translational yield of mRNA therapeutics by minimizing 5'-UTRs. Tissue Eng Part A (2019) 25(1-2):69–79. doi: 10.1089/ten.tea.2017.0485
67. Zeng C, Hou X, Yan J, Zhang C, Li W, Zhao W, et al. Leveraging mRNA sequences and nanoparticles to deliver SARS-CoV-2 antigens in vivo. Advanced Materials (Deerfield Beach Fla) (2020) 32(40):e2004452. doi: 10.1002/adma.202004452
68. Grosset C, Chen C-YA, Xu N, Sonenberg N, Jacquemin-Sablon H, Shyu A-B. A mechanism for translationally coupled mRNA turnover. Cell (2000) 103(1):29–40. doi: 10.1016/S0092-8674(00)00102-1
69. Goldstrohm AC, Wickens M. Multifunctional deadenylase complexes diversify mRNA control. Nat Rev Mol Cell Biol (2008) 9(4):337–44. doi: 10.1038/nrm2370
70. Presnyak V, Alhusaini N, Chen Y-H, Martin S, Morris N, Kline N, et al. Codon optimality is a major determinant of mRNA stability. Cell (2015) 160(6):1111–24. doi: 10.1016/j.cell.2015.02.029
71. Roundtree IA, Evans ME, Pan T, He C. Dynamic RNA modifications in gene expression regulation. Cell (2017) 169(7):1187–200. doi: 10.1016/j.cell.2017.05.045
72. Karikó K, Muramatsu H, Welsh FA, Ludwig J, Kato H, Akira S, et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol Ther J Am Soc Gene Ther (2008) 16(11):1833–40. doi: 10.1038/mt.2008.200
73. Martin SA, Paoletti E, Moss B. Purification of mRNA guanylyltransferase and mRNA (guanine-7-) methyltransferase from vaccinia virions. J Biol Chem (1975) 250(24):9322–9. doi: 10.1016/S0021-9258(19)40646-7
74. Rydzik AM, Lukaszewicz M, Zuberek J, Kowalska J, Darzynkiewicz ZM, Darzynkiewicz E, et al. Synthetic dinucleotide mRNA cap analogs with tetraphosphate 5',5' bridge containing methylenebis(phosphonate) modification. Organic Biomol Chem (2009) 7(22):4763–76. doi: 10.1039/b911347a
75. Ross J, Sullivan TD. Half-lives of beta and gamma globin messenger RNAs and of protein synthetic capacity in cultured human reticulocytes. Blood (1985) 66(5):1149–54. doi: 10.1182/blood.V66.5.1149.1149
76. Gallie DR. The cap and poly(A) tail function synergistically to regulate mRNA translational efficiency. Genes Dev (1991) 5(11):2108–16. doi: 10.1101/gad.5.11.2108
77. Kauffman KJ, Webber MJ, Anderson DG. Materials for non-viral intracellular delivery of messenger RNA therapeutics. J Controlled Release Off J Controlled Release Society (2016) 240:227–34. doi: 10.1016/j.jconrel.2015.12.032
78. Gustafsson C, Govindarajan S, Minshull J. Codon bias and heterologous protein expression. Trends Biotechnol (2004) 22(7):346–53. doi: 10.1016/j.tibtech.2004.04.006
79. Kudla G, Murray AW, Tollervey D, Plotkin JB. Coding-sequence determinants of gene expression in escherichia coli. Sci (New York NY) (2009) 324(5924):255–8. doi: 10.1126/science.1170160
80. Buhr F, Jha S, Thommen M, Mittelstaet J, Kutz F, Schwalbe H, et al. Synonymous codons direct cotranslational folding toward different protein conformations. Mol Cell (2016) 61(3):341–51. doi: 10.1016/j.molcel.2016.01.008
81. Mauro VP, Chappell SA. A critical analysis of codon optimization in human therapeutics. Trends Mol Med (2014) 20(11):604–13. doi: 10.1016/j.molmed.2014.09.003
82. Batty CJ, Heise MT, Bachelder EM, Ainslie KM. Vaccine formulations in clinical development for the prevention of severe acute respiratory syndrome coronavirus 2 infection. Advanced Drug Deliv Rev (2021) 169:168–89. doi: 10.1016/j.addr.2020.12.006
83. Petkar KC, Patil SM, Chavhan SS, Kaneko K, Sawant KK, Kunda NK, et al. An overview of nanocarrier-based adjuvants for vaccine delivery. Pharmaceutics (2021) 13(4):455. doi: 10.3390/pharmaceutics13040455
84. Liu J, Meng J, Cao L, Li Y, Deng P, Pan P, et al. Synthesis and investigations of ciprofloxacin loaded engineered selenium lipid nanocarriers for effective drug delivery system for preventing lung infections of interstitial lung disease. J Photochem Photobiol B Biol (2019) 197:111510. doi: 10.1016/j.jphotobiol.2019.05.007
85. Feng H, Wu R, Zhang S, Kong Y, Liu Z, Wu H, et al. Topical administration of nanocarrier miRNA-210 antisense ameliorates imiquimod-induced psoriasis-like dermatitis in mice. J Dermatol (2020) 47(2):147–54. doi: 10.1111/1346-8138.15149
86. Yang W, Zhang F, Deng H, Lin L, Wang S, Kang F, et al. Smart nanovesicle-mediated immunogenic cell death through tumor microenvironment modulation for effective photodynamic immunotherapy. ACS Nano (2020) 14(1):620–31. doi: 10.1021/acsnano.9b07212
87. Tarahovsky YS, Arsenault AL, MacDonald RC, McIntosh TJ, Epand RM. Electrostatic control of phospholipid polymorphism. Biophys J (2000) 79(6):3193–200. doi: 10.1016/S0006-3495(00)76552-0
88. Tarahovsky YS, Koynova R, MacDonald RC. DNA Release from lipoplexes by anionic lipids: Correlation with lipid mesomorphism, interfacial curvature, and membrane fusion. Biophys J (2004) 87(2):1054–64. doi: 10.1529/biophysj.104.042895
89. Martinon F, Krishnan S, Lenzen G, Magné R, Gomard E, Guillet JG, et al. Induction of virus-specific cytotoxic T lymphocytes in vivo by liposome-entrapped mRNA. Eur J Immunol (1993) 23(7):1719–22. doi: 10.1002/eji.1830230749
90. Hoerr I, Obst R, Rammensee HG, Jung G. In vivo application of RNA leads to induction of specific cytotoxic T lymphocytes and antibodies. Eur J Immunol (2000) 30(1):1–7. doi: 10.1002/1521-4141(200001)30:1<1::AID-IMMU1>3.0.CO;2-#
91. Scheel B, Aulwurm S, Probst J, Stitz L, Hoerr I, Rammensee HG, et al. Therapeutic anti-tumor immunity triggered by injections of immunostimulating single-stranded RNA. Eur J Immunol (2006) 36(10):2807–16. doi: 10.1002/eji.200635910
92. Lu D, Benjamin R, Kim M, Conry RM, Curiel DT. Optimization of methods to achieve mRNA-mediated transfection of tumor cells in vitro and in vivo employing cationic liposome vectors. Cancer Gene Ther (1994) 1(4):245–52.
93. Luo C, Sun J, Sun B, He Z. Prodrug-based nanoparticulate drug delivery strategies for cancer therapy. Trends Pharmacol Sci (2014) 35(11):556–66. doi: 10.1016/j.tips.2014.09.008
94. Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, et al. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci USA (1998) 95(8):4607–12. doi: 10.1073/pnas.95.8.4607
95. Nurunnabi M, Khatun Z, Badruddoza AZM, McCarthy JR, Lee Y-K, Huh KM. Biomaterials and bioengineering approaches for mitochondria and nuclear targeting drug delivery. ACS Biomater Sci Engineering (2019) 5(4):1645–60. doi: 10.1021/acsbiomaterials.8b01615
96. Tran VA, Vo VG, Shim K, Lee SW, An SSA. Multimodal mesoporous silica nanocarriers for dual stimuli-responsive drug release and excellent photothermal ablation of cancer cells. Int J Nanomed (2020) 15:7667–85. doi: 10.2147/IJN.S254344
97. Parayath NN, Amiji MM. Therapeutic targeting strategies using endogenous cells and proteins. J Controlled Release Off J Controlled Release Society (2017) 258:81–94. doi: 10.1016/j.jconrel.2017.05.004
98. Zhang Y, Guo Z, Cao Z, Zhou W, Zhang Y, Chen Q, et al. Endogenous albumin-mediated delivery of redox-responsive paclitaxel-loaded micelles for targeted cancer therapy. Biomaterials (2018) 183:243–57. doi: 10.1016/j.biomaterials.2018.06.002
99. Daraee H, Eatemadi A, Abbasi E, Fekri Aval S, Kouhi M, Akbarzadeh A. Application of gold nanoparticles in biomedical and drug delivery. Artif Cells Nanomed Biotechnol (2016) 44(1):410–22. doi: 10.3109/21691401.2014.955107
100. De Jong WH, Borm PJ. Drug delivery and nanoparticles: Applications and hazards. Int J Nanomed (2008) 3(2):133–49. doi: 10.2147/IJN.S596
101. Deirram N, Zhang C, Kermaniyan SS, Johnston APR, Such GK. pH-responsive polymer nanoparticles for drug delivery. Macromol Rapid Commun (2019) 40(10):e1800917. doi: 10.1002/marc.201800917
102. Allen TM. Drug delivery systems: Entering the mainstream. Sci (New York NY) (2004) 303(5665):1818–22. doi: 10.1126/science.1095833
103. Hussain S, Plückthun A, Allen TM, Zangemeister-Wittke U. Antitumor activity of an epithelial cell adhesion molecule targeted nanovesicular drug delivery system. Mol Cancer Ther (2007) 6(11):3019–27. doi: 10.1158/1535-7163.MCT-07-0615
104. Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet (2014) 383(9927):1490–502. doi: 10.1016/S0140-6736(13)61649-9
105. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
106. Socinski MA, Crowell R, Hensing TE, Langer CJ, Lilenbaum R, Sandler AB, et al. Treatment of non-small cell lung cancer, stage IV: ACCP evidence-based clinical practice guidelines (2nd edition). Chest (2007) 132(3 Suppl):277s–89s. doi: 10.1378/chest.07-1381
107. Steven A, Fisher SA, Robinson BW. Immunotherapy for lung cancer. Respirol (Carlton Vic) (2016) 21(5):821–33. doi: 10.1111/resp.12789
108. Yano T, Okamoto T, Fukuyama S, Maehara Y. Therapeutic strategy for postoperative recurrence in patients with non-small cell lung cancer. World J Clin Oncol (2014) 5(5):1048–54. doi: 10.5306/wjco.v5.i5.1048
109. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clinic Proc (2008) 83(5):584–94. doi: 10.1016/S0025-6196(11)60735-0
110. Yao X, Panichpisal K, Kurtzman N, Nugent K. Cisplatin nephrotoxicity: A review. Am J Med Sci (2007) 334(2):115–24. doi: 10.1097/MAJ.0b013e31812dfe1e
111. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med (2010) 362(25):2380–8. doi: 10.1056/NEJMoa0909530
112. Papachristofilou A, Hipp MM, Klinkhardt U, Früh M, Sebastian M, Weiss C, et al. Phase ib evaluation of a self-adjuvanted protamine formulated mRNA-based active cancer immunotherapy, BI1361849 (CV9202), combined with local radiation treatment in patients with stage IV non-small cell lung cancer. J Immunother Cancer (2019) 7(1):38. doi: 10.1186/s40425-019-0520-5
113. Sebastian M, Schröder A, Scheel B, Hong HS, Muth A, von Boehmer L, et al. A phase I/IIa study of the mRNA-based cancer immunotherapy CV9201 in patients with stage IIIB/IV non-small cell lung cancer. Cancer immunol Immunother CII (2019) 68(5):799–812. doi: 10.1007/s00262-019-02315-x
114. Khan P, Siddiqui JA, Lakshmanan I, Ganti AK, Salgia R, Jain M, et al. RNA-Based therapies: A cog in the wheel of lung cancer defense. Mol Cancer (2021) 20(1):54. doi: 10.1186/s12943-021-01338-2
115. Wang Y, He X, Nie H, Zhou J, Cao P, Ou C. Application of artificial intelligence to the diagnosis and therapy of colorectal cancer. Am J Cancer Res (2020) 10(11):3575–98.
116. Liao Z, Nie H, Wang Y, Luo J, Zhou J, Ou C. The emerging landscape of long non-coding RNAs in colorectal cancer metastasis. Front Oncol (2021) 11:641343. doi: 10.3389/fonc.2021.641343
117. Papamichael D, Audisio RA, Glimelius B, de Gramont A, Glynne-Jones R, Haller D, et al. Treatment of colorectal cancer in older patients: International society of geriatric oncology (SIOG) consensus recommendations 2013. Ann Oncol Off J Eur Soc Med Oncol (2015) 26(3):463–76. doi: 10.1093/annonc/mdu253
118. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell (2011) 144(5):646–74. doi: 10.1016/j.cell.2011.02.013
119. Caspi M, Wittenstein A, Kazelnik M, Shor-Nareznoy Y, Rosin-Arbesfeld R. Therapeutic targeting of the oncogenic wnt signaling pathway for treating colorectal cancer and other colonic disorders. Advanced Drug Deliv Rev (2021) 169:118–36. doi: 10.1016/j.addr.2020.12.010
120. Xia Y, He J, Zhang H, Wang H, Tetz G, Maguire CA, et al. AAV-mediated gene transfer of DNase I in the liver of mice with colorectal cancer reduces liver metastasis and restores local innate and adaptive immune response. Mol Oncol (2020) 14(11):2920–35. doi: 10.1002/1878-0261.12787
121. Ou C, Sun Z, He X, Li X, Fan S, Zheng X, et al. Targeting YAP1/LINC00152/FSCN1 signaling axis prevents the progression of colorectal cancer. Advanced Sci (2020) 7(3):1901380. doi: 10.1002/advs.201901380
122. Lei S, Zhang X, Men K, Gao Y, Yang X, Wu S, et al. Efficient colorectal cancer gene therapy with IL-15 mRNA nanoformulation. Mol Pharm (2020) 17(9):3378–91. doi: 10.1021/acs.molpharmaceut.0c00451
123. Neumeister MW, Winters JN. Neuroma. Clinics Plast Surg (2020) 47(2):279–83. doi: 10.1016/j.cps.2019.12.008
124. Zheng Y, Luo Y, Chen X, Li H, Huang B, Zhou B, et al. The role of mRNA in the development, diagnosis, treatment and prognosis of neural tumors. Mol Cancer (2021) 20(1):49. doi: 10.1186/s12943-021-01341-7
125. Vik-Mo EO, Nyakas M, Mikkelsen BV, Moe MC, Due-Tønnesen P, Suso EM, et al. Therapeutic vaccination against autologous cancer stem cells with mRNA-transfected dendritic cells in patients with glioblastoma. Cancer Immunol Immunother CII (2013) 62(9):1499–509. doi: 10.1007/s00262-013-1453-3
126. Cheever MA, Higano CS. PROVENGE (Sipuleucel-T) in prostate cancer: The first FDA-approved therapeutic cancer vaccine. Clin Cancer Res an Off J Am Assoc Cancer Res (2011) 17(11):3520–6. doi: 10.1158/1078-0432.CCR-10-3126
127. Huang X, Tang T, Zhang G, Liang T. Identification of tumor antigens and immune subtypes of cholangiocarcinoma for mRNA vaccine development. Mol Cancer (2021) 20(1):50. doi: 10.1186/s12943-021-01342-6
128. Huang X, Zhang G, Tang T, Liang T. Identification of tumor antigens and immune subtypes of pancreatic adenocarcinoma for mRNA vaccine development. Mol Cancer (2021) 20(1):44. doi: 10.1186/s12943-021-01310-0
129. Rausch S, Schwentner C, Stenzl A, Bedke J. mRNA vaccine CV9103 and CV9104 for the treatment of prostate cancer. Hum Vaccines Immunother (2014) 10(11):3146–52. doi: 10.4161/hv.29553
130. Sung NS, Crowley WF Jr., Genel M, Salber P, Sandy L, Sherwood LM, et al. Central challenges facing the national clinical research enterprise. Jama (2003) 289(10):1278–87. doi: 10.1001/jama.289.10.1278
131. Lin Y-X, Wang Y, Ding J, Jiang A, Wang J, Yu M, et al. Reactivation of the tumor suppressor PTEN by mRNA nanoparticles enhances antitumor immunity in preclinical models. Sci Trans Med (2021) 13(599):eaba9772. doi: 10.1126/scitranslmed.aba9772
132. Zhang C, Zhang X, Zhao W, Zeng C, Li W, Li B, et al. Chemotherapy drugs derived nanoparticles encapsulating mRNA encoding tumor suppressor proteins to treat triple-negative breast cancer. Nano Res (2019) 12(4):855–61. doi: 10.1007/s12274-019-2308-9
133. Islam MA, Xu Y, Tao W, Ubellacker JM, Lim M, Aum D, et al. Restoration of tumour-growth suppression in vivo via systemic nanoparticle-mediated delivery of PTEN mRNA. Nat Biomed Engineering (2018) 2(11):850–64. doi: 10.1038/s41551-018-0284-0
134. Liu L, Wang Y, Miao L, Liu Q, Musetti S, Li J, et al. Combination immunotherapy of MUC1 mRNA nano-vaccine and CTLA-4 blockade effectively inhibits growth of triple negative breast cancer. Mol Ther (2018) 26(1):45–55. doi: 10.1016/j.ymthe.2017.10.020
Keywords: mRNA vaccine, nanocarrier, therapeutic target, delivery system, cancer therapy
Citation: Huang T, Peng L, Han Y, Wang D, He X, Wang J and Ou C (2022) Lipid nanoparticle-based mRNA vaccines in cancers: Current advances and future prospects. Front. Immunol. 13:922301. doi: 10.3389/fimmu.2022.922301
Received: 17 April 2022; Accepted: 08 August 2022;
Published: 26 August 2022.
Edited by:
Sudha Kumari, Indian Institute of Science (IISc), IndiaReviewed by:
Srujan Marepally, Center for Stem Cell Research (CSCR), IndiaCopyright © 2022 Huang, Peng, Han, Wang, He, Wang and Ou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyun He, aGV4aWFveXVuQGNzdS5lZHUuY24=; Junpu Wang, d2FuZy1qcDIwMTNAY3N1LmVkdS5jbg==; Chunlin Ou, b3VjaHVubGluQGNzdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.