
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 01 July 2022
Sec. Autoimmune and Autoinflammatory Disorders
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.922111
This article is part of the Research Topic The Potential Drug Regulation in Arthritic Disorders View all 11 articles
Rheumatoid arthritis (RA) is a chronic autoimmune disease that endangers the health of approximately 1% of the global population. Current RA medications on the market mainly include non-steroidal anti-inflammatory drugs, biological agents, and disease-modifying drugs. These drugs aim to inhibit the overactivated immune response or inflammation of RA, but they cannot cure RA. A better understanding of the pathogenesis of RA will provide a new understanding to search for RA targets and for drug development. The infiltration of T cells and hyper-proliferation of fibroblast-like synoviocytes (FLS) in the synovium of patients with RA are significantly upregulated. Furthermore, the abnormal activation of these two types of cells has been confirmed to promote development of the course of A by many studies. This article systematically summarizes the interactions between T cells and FLS in RA synovial tissues, including one-way/mutual regulation and direct/indirect regulation between the two. It further aims to investigate the pathogenesis of RA from the perspective of mutual regulation between T cells and FLS and to provide new insights into RA research.
In the past few decades, extensive research has been conducted to illustrate the important role of T lymphocytes (T cell) in rheumatoid arthritis (RA) (1). In RA, T cell can interact with antigen-presenting cells, including dendritic cell, macrophage, B lymphocyte (B cell), and even non-professional antigen-presenting cell, such as fibroblast-like synoviocyte (FLS). During T cell activation, CD4+ T cells initially form contacts with human leukocyte antigen (HLA) or major histocompatibility class II (MHC-II) molecules and co-stimulatory molecules (e.g., CD28) of other cells, leading to the maturation of CD4+ cells (2). Subsequently, antigens presented by other cells promote the activation of CD8+ T cells, further exacerbating inflammation in RA (3). The interaction between T cells and other cellular components is a key factor in RA pathogenesis.
Apart from immune cells, non-immune cells of target organs also play a vital role in various autoimmune diseases, forming the foundation of the pathogeneses of these diseases (4). FLS are a special type of non-immune cells present in synovial tissue around joints. FLS play an important destructive role in the pathogenesis of RA; specifically, the numbers of FLS significantly increase and become an important part of the destructive pannus that characterizes the synovial membrane of patients with RA. In addition, FLS in RA exhibit an aggressive phenotype and mediate inflammation and joint destruction. Therefore, cellular crosstalk between FLS and other cellular components might also play an important role in RA, especially in the pathology of the joint synovium.
In this review, we summarize the pathophysiological features of T cells and FLS, which are two important cellular types in the joint synovium of patients with RA, at the functional and molecular level. Further, we outline the interactions between T cells and FLS in RA. Finally, we summarize the potential therapeutic options by explaining the roles of these cells in RA.
RA is an autoimmune disease associated with severe synovitis and the destruction of bone and cartilage. In the synovial tissues of patients with RA, T cells can interact with other immune cells, such as macrophages and B cells, and other non-immune cells, including FLS, leading to T cell recruitment, activation, and cytokine production (5). This section focuses on these functions of T cells mediated by FLS-secreted chemokines (Figure 1).

Figure 1 RA-FLS indirectly promotes survival and chemotaxis of T cells in joint synovium of patients with RA via by producing various chemokines, including CD13, SDF1, VCAM1, IL15, TNF, and type I IFN. SDF, Stromal cell-derived factor; VCAM, Vascular cell adhesion molecule; VLA,Integrins alpha; CXCR, CXC motif chemokine receptor; IL, interleukin; JAK, Janus kinase; STAT, signal transducer and activator of transcription; TNF, tumor necrosis factor; IFN, interferon.
CD13 released from FLS induces chemotaxis and T cell activation through a pertussis toxin-sensitive G protein-coupled receptor in RA (6). FLS-derived stromal cell-derived factor (SDF)-1 and vascular cell adhesion molecule (VCAM)-1 recruit T cells via their corresponding receptors, CXC motif chemokine receptor (CXCR)-4 and integrins alpha (VLA)-4, respectively, in RA (7). FLS can produce an abundance of proinflammatory cytokines in RA joints. For example, interleukin (IL)-15 is mainly responsible for local T cell activation and proliferation (8). The action of FLS-derived IL-7 is essential for lymphoid neogenesis in the RA synovium (9). The Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway in FLS is indirectly activated by the tumor necrosis factor (TNF) through the autocrine expression of type I interferon (IFN), resulting in IFN-α/β receptor engagement and the production of chemokines by T cells, which play a role in the effects of the JAK inhibitor CP-690550 (tofacitinib) in the treatment of RA. The reduction of chemokine synthesis mediated by FLS limits the recruitment of T cells and other infiltrating leucocytes (10).
In addition to recruiting and activating T cells, FLS can also promote the differentiation of proinflammatory subtypes and inhibit the differentiation of anti-inflammatory subtypes of T cells in the synovial joints of patients with RA (11) (Figure 2). FLS co-cultured with peripheral blood mononuclear cells (PBMCs) increase peripheral T follicular helper (Tfh) cell (CD4+CXCR5+ICOS+) count in patients with RA (12). Adiponectin-stimulated FLS can also promote Tfh generation, predominantly via IL-6 production in RA (13). P53 abrogates FLS-induced Th1 and Th17 cell differentiation in RA (14). Upregulated KAT7, an H4-specific histone acetylase in FLS, promotes Th17 cell differentiation in RA by inducing C–C motif chemokine ligand (CCL) 20 expression and the p44/42 mitogen-activated protein kinase pathway (15). Further, myeloid-related protein (MRP)8/MRP14 is an endogenous Toll-like receptor 4 (TLR4) ligand. MRP8 produced by FLS can promote Th17 differentiation by enhancing the expression of IL-6 in RA. MRP8 induces IL-6 secretion in FLS via TLR4/phosphoinositide 3-kinase (PI3K)/nuclear factor kappa B (NF-κB) and mitogen-activated protein kinase signaling pathways in RA (16). Moreover, IL-34/colony stimulating factor 1 receptor (CSF-1R) axis-induced FLS upregulate Th17 production through increased IL-6 in RA (17). In addition, cysteine-rich protein 61 (Cyr61) induces IL-6 production by FLS, promoting Th17 differentiation via the Avb5/Akt/NF-kB signaling pathway in RA (18). Co-cultured FLS enhance PBMC-secreted IL-17-A, IL-6, IFNγ, and IL-1β production in RA (19). FLS and macrophages are the main sources of IL-26 in RA joints. IL-26 induces production of the proinflammatory cytokines IL-1β, IL-6, and TNF-α in monocytes. IL-26-stimulated monocytes selectively promote the generation of RORγt+ Th17 cells through IL-1β secretion by monocytes. Therefore, IL-26 is constitutively produced by FLS, induces proinflammatory cytokines in myeloid cells, and promotes Th17 cell differentiation in RA (20). Synovial fluid and FLS from patients with RA suppress enhancer of zeste homolog 2 (EZH2) expression in CD4+ T cells. EZH2 deficiency attenuates regulatory T cells (Treg) differentiation in RA (21). Overall, IL-6 seems to be a key inflammatory factor released by FLS in RA. Thus, FLS indirectly affect the differentiation of T cells in the synovial joints of patients with RA through IL-6, promoting the differentiation of Th17 and Tfh cells.
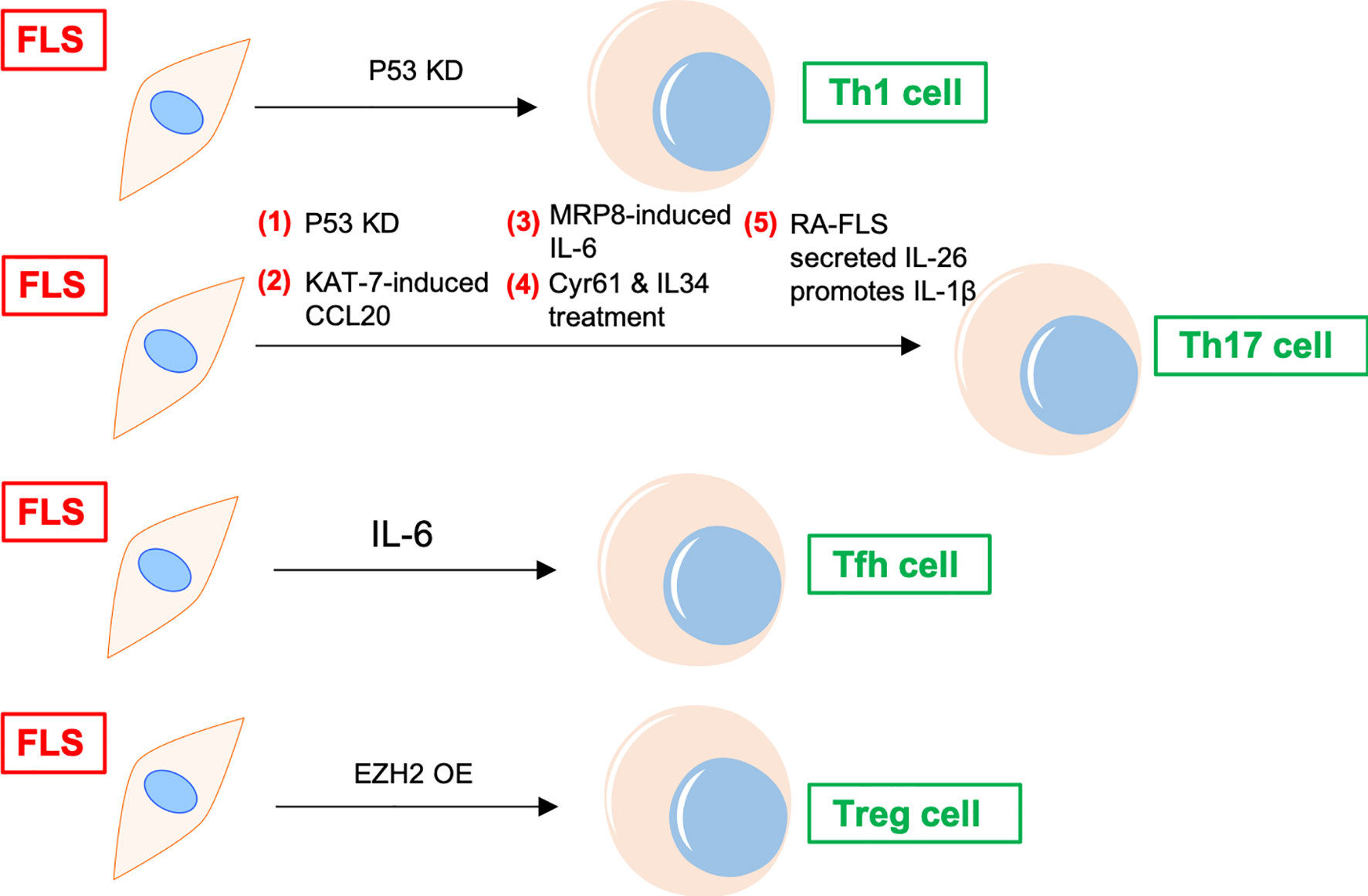
Figure 2 FLS regulates differentiation of CD4+ T cells in RA. P53 KD and EZH2 OE promote Th1 and Treg differentiation of T cells in RA synovium, respectively. FLS promotes Tfh differentiation of T cells via IL-6. FLS directly induces Th17 differentiation through cytokines, including CCL20, MRP8, IL-6, and IL-26, etc. KD, knockdown; OE, overexpression; Tfh, T follicular helper; CCL, C–C motif chemokine ligand; MRP, myeloid-related protein; TLR, Toll-like receptor; Cyr61, cysteine-rich protein 61; EZH, enhancer of zeste homolog; Treg, regulatory T cells.
Different subtypes of CD4+ T cells can be detected in the synovial joints of patients with RA (22). Th17 promotes the development of RA and is an important aspect of the proinflammatory function of FLS. Transcripts of IL-17R, as well as those of IL-17RB, C, and D, have been previously detected in the FLS of patients with RA (23) (Figure 3A).
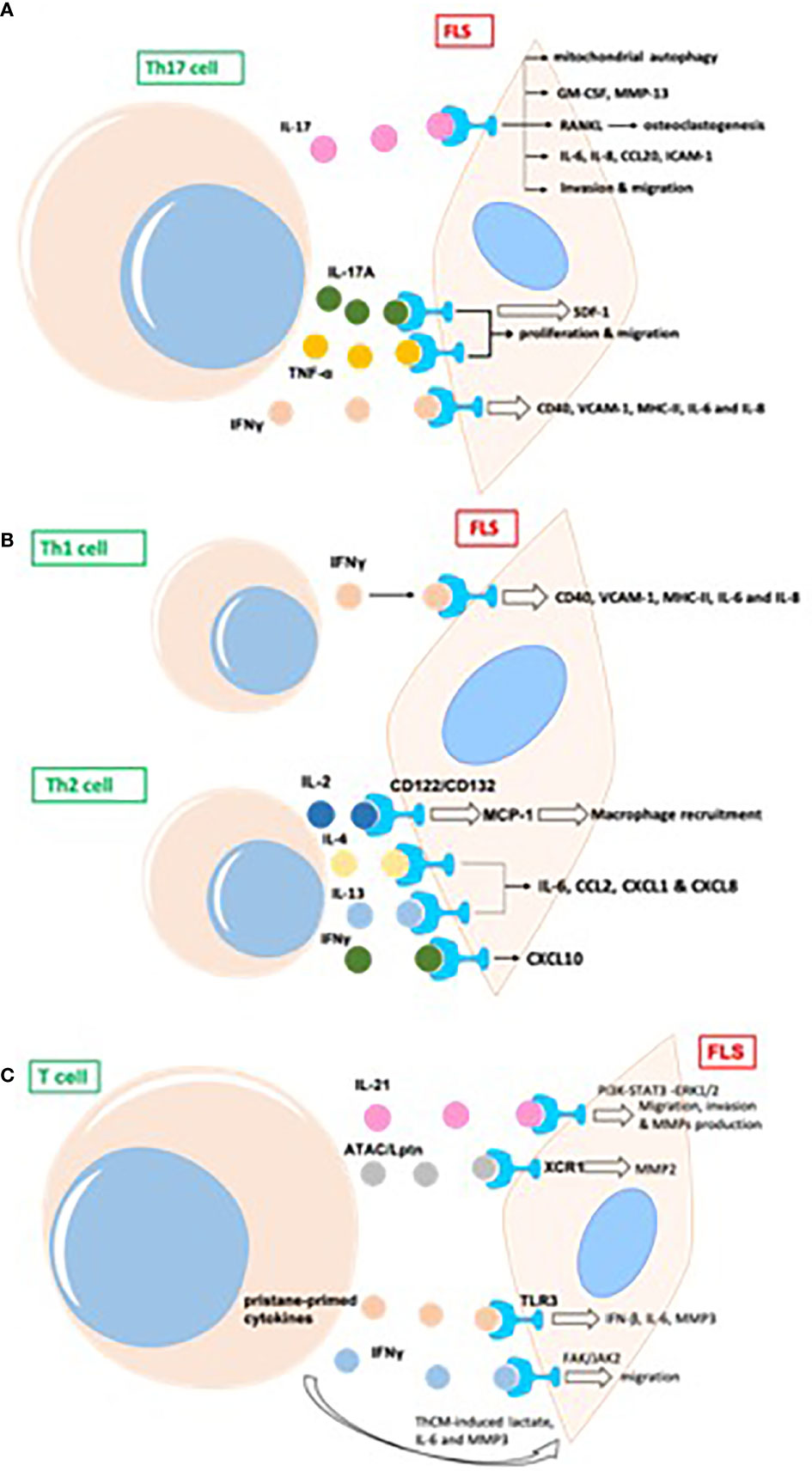
Figure 3 T cells promote inflammatory phenotype of FLS in RA, mainly including proliferation, migration, invasion, and production of proinflammatory cytokines and destructive MMPs in joint synovium. (A) The indirect effects of Th17 cells on FLS in RA; (B) The indirect effects of Th1/Th2 cells on FLS in RA; (C) The indirect effects of other T cells on FLS in RA. GM-CSF, granulocyte-macrophage colony-stimulating factor; MMP, matrix metalloproteinase; RANKL, receptor activator of NF-κB ligand; TRAF, TNF receptor-associated factor; AP, activator protein; ICAM, intercellular adhesion molecule; MIF, migration inhibitory factor; ATAC, activation-induced, T cell-derived, and chemokine-related cytokine; Lptn, lymphotactin; FAK,focal adhesion kinase.
Th17 cells induce secretion of the cytokine granulocyte-macrophage colony-stimulating factor (GM-CSF) in synovial stroma and innate lymphoid cells to initiate and augment autoimmune arthritis (24). Th17 cells and IL-17 increase autophagy of FLS by causing mitochondrial dysfunction in RA (25). The blockade of IL-17 alleviates inflammation in rat arthritis and matrix metalloproteinase (MMP)-13 expression from FLS (26). In addition, IL-17-induced receptor activator of NF-κB ligand (RANKL) expression is decreased by the inhibition of Act1, TNF receptor-associated factor 6 (TRAF6), and activator protein (AP)-1. In the absence of RANKL, IL-17-prestimulated FLS induce osteoclastogenesis from monocytes, which is repressed by the inhibition of TNF-α (27). FLS express two types of phospholipase D, namely PLD1 and PLD2. PLD regulates the Th17-promoted production of proinflammatory cytokines by FLS (28). The dihydroartemisinin derivative DC32 inhibits the Th17-induced invasion and migration of FLS by decreasing the secretion of MMPs (MMP-2, MMP-3) in vitro (29).
Th17-cell-secreted IL-17A and TNF-α have synergistic effects on promoting the production of inflammatory cytokines in FLS from patients with RA, the human leukemia cell line THP-1, and the rheumatoid synovial fibroblast cell line MH7A. IL-17A and TNF-α also promote the proliferation and migration of MH7A cells. However, a novel dual targeting fusion protein (targeting TNF-α and IL-17A) was found to be more efficient in inhibiting these synergistic effects when compared to the effects of etanercept (30). Stromal cell-derived factor 1 (SDF-1) is overproduced in RA FLS, and IL-17 upregulates the expression of SDF-1 in RA FLS via pathways mediated by PI3K, NF-κB, and AP-1 (31).
Apart from Th17, Th1 and CXCR3+ Th2 phenotypes are the main subtypes of T helper cells in the synovium of patients with RA; IL-4 and IL-13 induce FLS to produce a series of inflammatory cytokines, such as IL-6, CCL2, CXCL1, and CXCL8, whereas IFNγ promotes the expression of CXCL10 (32). Both Th1 and Th17 cells produce IL-17 and IFNγ. The expression of CD40, intercellular adhesion molecule 1 (ICAM-1), and MHC-II in FLS is upregulated upon co-culture with Th1 cells, whereas Th17 cells induce only ICAM-1 in FLS. Both T cell subsets promote the production of IL-6 and IL-8 by FLS from patients with RA (33). T cell-derived IL-2 might activate FLS (via IL-2 receptor (CD122) and (CD132) chains) to produce MCP-1, thus recruiting macrophages into the rheumatoid synovium and promoting inflammation (34). Both Th1 and Th2 cells express macrophage migration inhibitory factor (MIF). MMPs are induced by FLS after co-culture with Th1 and Th2 cells, and activated T helper cells are more effective than resting cells. The neutralization of MIF by an anti-MIF antibody leads to the downregulation of MMP in both Th1- and Th2-stimulated FLS (35) (Figure 3B).
IL-21 is produced primarily by CD4+ T cells and natural killer T cells. IL-21 induces the migration, invasion, and production of MMPs (MMP-2, MMP-3, MMP-9, MMP-13) in FLS from patients with RA (36). IL-21 promotes activation of the PI3K, STAT3, and ERK1/2 pathways in FLS, and the inhibition of these pathways attenuates IL-21-mediated migration and the production of MMPs (36). The percentage of T cells from the synovial fluid in patients with RA is upregulated relative to that in patients with psoriatic arthritis (37). The proportion of IL-21+CD4+ T cells from peripheral blood in patients with RA is positively associated with IgM-rheumatoid factor, serum anticyclic citrullinated peptide antibodies, and disease activity score 28 (DAS28). IL-21 expression in synovial fluid is correlated with MMPs; IL-21 significantly induces the production of MMPs in synovial biopsies from patients with RA (37). CD4+IL-21+ T cells sorted from synovial fluid promote the secretion of MMPs by FLS to a greater extent than medium or CD4+IL-21− T cells in an in vitro co-culture system. The blockage of IL-21 and TNF leads to the downregulation of MMPs from FLS (37).
In phorbol myristate acetate/ionomycin-stimulated PBMCs, activation-induced, T cell-derived, and chemokine-related cytokine (ATAC)/lymphotactin (Lptn) is detected in CD8+ T cells and is upregulated in CD4+CD28− T cells from patients with RA as compared with their levels in healthy controls (38). FLS express the ATAC/Lptn receptor XCR1 in the RA synovium. ATAC/Lptn leads to the marked downregulation of MMP2 production in FLS (38). TLR3 is induced in the synovium of rats with pristane-induced arthritis (39). In addition, activation of the TLR3 signaling pathway promotes the development of this arthritis model. Interestingly, pristane-primed T cell-derived cytokines further promote FLS activation (39).
IFNγ produced by T cell stimulation promotes the phosphorylation of focal adhesion kinase (FAK)-Y925, which is important for cell migration (40). SiRNA-mediated knockdown of JAK2, but not JAK1, substantially suppresses FAK activation via IFNγ. IFNγ-induced FAK activation and invasion of FLS are also blocked by baricitinib (JAK inhibitor) (40). Soluble mediators released by Th cells drive synovial fluid towards a glycolytic and proinflammatory phenotype. Targeting JAKs or glycolytic enzymes modulates synovial fluid glucose metabolism and decreases the secretion of IL-6 and MMP3 (41). Therefore, targeting glycolytic pathways represents a potential therapeutic strategy to treat inflammation in synovial fluid (41) (Figure 3C).
In addition to indirect regulation through cytokines and chemokines, there is a direct interaction between T cells and FLS in the synovium of patients with RA. Different antigen-presenting cells, including B cells, macrophages, and dendritic cells, interact directly with T cells. FLS, as non-immune cells, also have antigen-presenting capabilities. This section summarizes the direct communication between T cells and FLS in the synovium of patients with RA (Figure 4).
Despite not being professional antigen-presenting cells, FLS can also present peptides, such as human cartilage gp-39 and human type II collagen (CII), derived from autoantigens discovered in the joint tissues of patients with RA, to activated T cells in vitro in an IFN-dependent and MHC-restricted manner (42). Cell–cell contact between T cells and FLS induce the lymphocytic expression of aminopeptidase N/CD13 and results in lymphocytic activation (43). Both FLS (production of SDF-1) and CD8/CD4+ T cells (expression of CXCR4) play a positive role in the recruitment of T cells in the joint synovium (44). CD4+ T cells abnormally express CX3CR1 in the synovium of patients with RA. Fractalkine (FKN) induces the adhesion of CD4+ T cells and survival signals and co-stimulates the secretion of inflammatory cytokines and granules. CD4+ T cells accept primary stimulatory and co-stimulatory signals from non-professional antigen-presenting cells, such as FLS, in the RA synovial microenvironment (45).
A previous study showed the effects of FLS on the recruitment, activation, and expansion of T cells in RA in a CD47-TSP1 (thrombospondin-1)-dependent manner (46). TSP1-mediated co-stimulation is achieved through its independent interaction with CD36 on antigen-presenting cells and with CD47 on T cells. A CD47–TSP1–CD36 trimolecular complex is a new co-stimulatory pathway that represses the activation of T cells. Because the lesions in rheumatoid synovitis are sites of antigenic recognition, the identification of TSP1 on antigen-presenting cells such as FLS suggests the central role of TSP1 in the expansion of T cells in RA (47). Direct contact between T cells and FLS induces the expression of HLA-DR on FLS and CD69 on T cells in an allogeneic and autologous manner. The addition of DAP3.B7 cells to co-cultures of T cells and FLS alleviates the repressed allogeneic activation of T cells (48). The allogeneic response by T cells to FLS in the presence of DAP3.B7 cells can be blocked by inhibiting CD80 with CTLA-4 Ig (48). Strong expression of B7-H3 was detected on FLS in synovial tissue of a patient with RA (49). Cells expressing B7-H3 are distinct from but very close to cells expressing CD45, CD3, and CD20. In addition, FLS and T cell co-cultures show localization of B7-H3 in the contact section between them but this is distinct from CD11a/CD18 (LFA-1)+ T cells and ICAM-1+ FLS. Blocking B7-H3 on FLS affects the interactions between FLS and T cells. Resting T cells have upregulated IL-2, TNF-α, and IFN-γ, whereas cytokine-activated T cells exhibit downregulated cytokine production. However, cytokine production by T cells activated via TCR is not affected by B7-H3 (49).
Direct contact between activated CD4+ T cells and an FLS-facilitated hGITR–GITRL interaction lead to the upregulation of MMP-13 (50). CII-reactive T cells induce the secretion of chemokines (IL-8, MCP-1, and MIP-1α) through interactions with FLS in RA joints, which is mediated by CD40L–CD40 communication (51). FKN–CX3CR1 receptor–ligand interactions affect FLS growth and T cell functions. FLS promote autocrine growth by releasing FKN and triggering the activity of CX3CR1. This growth-promotion loop is amplified by CX3CR1+ T cell-produced TNF-α upon stimulation by FKN+ FLS (52).
Mutual activation of T cells and FLS results in increased proliferation and expression of ICAM-1 and VCAM-1 by both CD4+ T cells and FLS (53). The interaction between CD4+ T cells and FLS results in the upregulation of TNF-α, IFN-γ, and IL-17A from CD4+ T cells and the secretion of other cytokines, including IL-6, IL-8, and vascular endothelial growth factor (VEGF). Moreover, CD4+ T cells cultured in conditional medium promote invasiveness and glycolysis in FLS while repressing oxidative phosphorylation, with the effects paralleled by induced glucose transporters GLUT1 and GLUT3, key glycolytic enzymes GSK3A, HK2, LDHA, and PFKFB3, VEGF, and MMPs, which is alleviated by the glycolytic inhibitor 2-DG and adenosine monophosphate analogue 5-aminoimidazole-4-carboxamide ribonucleotide (53).
Co-culture with T cells induces the phosphorylation of protein kinase Akt (Ser473) and downstream mediators, including GSK-3α/β, FoxO1/3a, and mouse double minute (MDM)-2, in FLS from patients with RA (54). Co-cultured T cells also promote the proliferation of FLS and the production of IL-6, which is repressed by blocking antibodies to CD11a and ICAM-2. T cell-mediated phospho-Akt upregulation is unique to FLS because no such effect is observed in B cells and dendritic cells. Selective involvement of the LFA-1–ICAM-2 pathway has been confirmed based on increased ezrin phosphorylation at Tyr353 downstream of ICAM-2, which supports cell survival through Akt activation (54).
The rapid and robust adhesion of cytokine-activated T cells (Tck) and super antigen-activated T cells to FLS leads to flattening and a crawling movement in T cells on the cellular surface of FLS (55). Tck activates FLS to secret IL-6 and IL-8 in a cell contact-dependent manner, which is further activated by IL-17. Antibody blocking of membrane TNF-α on the Tck surface inhibits cytokine production by FLS, demonstrating a novel mechanism of TNF-α during T cells–FLS interactions in the RA synovium (55) (Figure 5).
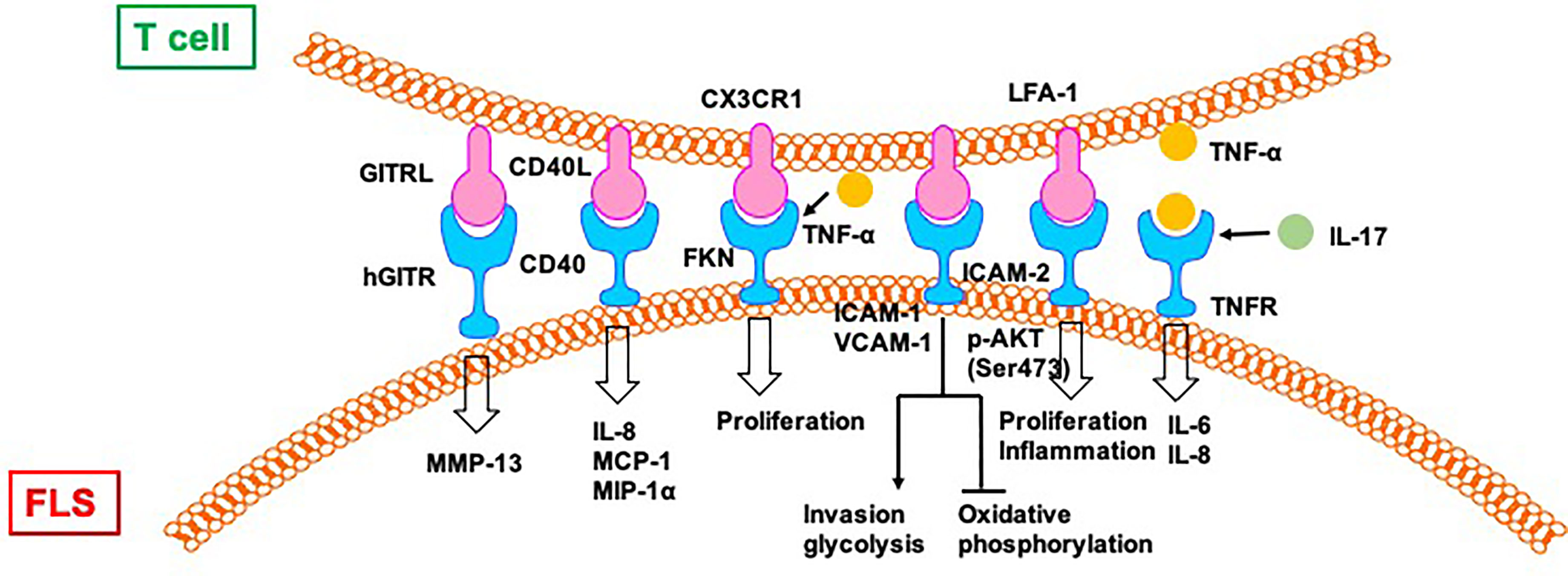
Figure 5 T cells directly regulate FLS in RA. CII:type II collagen; FKN, Fractalkine; TSP, thrombospondin.
In addition to the one-way direct regulation, there is a direct and mutual crosstalk between T cells and FLS in the synovium of patients with RA. This two-way communication further leads to the development of RA (56) (Figure 6). The T cells from patients with RA with a stronger response to CII show higher expression of inflammatory mediators, including IL-15, TNF-α, IFN, and IL-17. When co-incubated with RA FLS, T cells can stimulate the secretion of TNF-α, IL-15, and IL-18 from FLS during CII stimulation. In contrast, T cells also produce higher amounts of IL-17 and IFN-γ during co-culture with RA FLS. The crosstalk between T cells and FLS requires direct cell–cell contact and occurs in a CD40L-CD40 dependent manner (57).
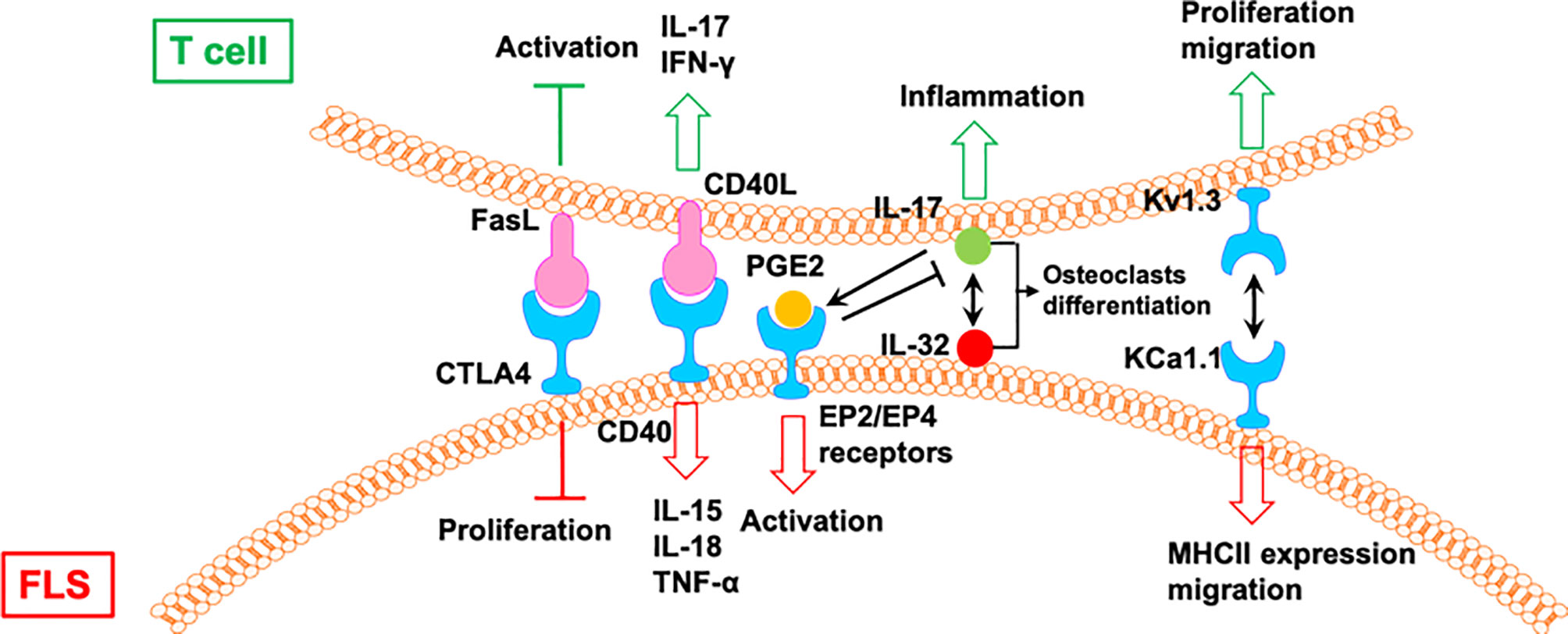
Figure 6 The mutual regulation of T cells and FLS in RA. CIA, collagen-induced arthritis; Tem: effector memory T; AIA, adjuvant-induced arthritis.
IL-17 induces the expression of IL-32 in FLS from patients with RA, which activates the secretion of IL-17 from CD4+ T cells (58). IL-17 and IL-32 are co-localized near tartrate resistant acid phosphatase-positive areas in joints from patients with RA. IL-32 and IL-17 promote osteoclast differentiation in a synergistic manner, and both promote osteoclast resorption via RANKL (58).
The interactions between FLS from rats with collagen-induced arthritis (CIA) and rat CCR7− effector memory T (Tem) cells is regulated by KCa1.1 and Kv1.3 (59). Blocking KCa1.1 on FLS reduces the promoting effects of FLS on the proliferation and migration of Tem cells, and blocking Kv1.3 on Tem cells reduces the effects of Tem cells on the expression of KCa1.1 and MHCII and the invasion of FLS. Furthermore, combination therapies comprising selective KCa1.1 and Kv1.3 inhibitors are more efficacious than monotherapies in alleviating disease features of rat arthritis models (59).
Macrophage-produced PGE2 is a response to IL-17 of T cells, which negatively regulates the expression of TNF-α and IL-17, as well as the TNF-α/IL-1-mediated activation of FLS via EP2 and EP4 receptors, resulting in the modulation of proinflammatory cascades in RA (60). A CTLA4-FasL fusion protein suppresses FLS proliferation and the development of adjuvant-induced arthritis (AIA) in rats. However, CTLA4-FasL also acts as an effective inhibitor for T cells; it not only inhibits the activation of T cells but also promotes activated T cell death (61).
The numbers of FLS and T cells in the synovial tissue of patients with RA are closely associated with joint damage (62). RA naiüve T cells share hypermethylation sites with FLS. FLS-representative DNA methylation signatures derived from blood might serve as biomarkers of RA risk or disease status (63). In the following section, we summarize some recent studies reporting that some treatments for RA (Table 1) or the regulation of a specific gene/protein (Table 2) can affect the function of both T cells and FLS in RA.
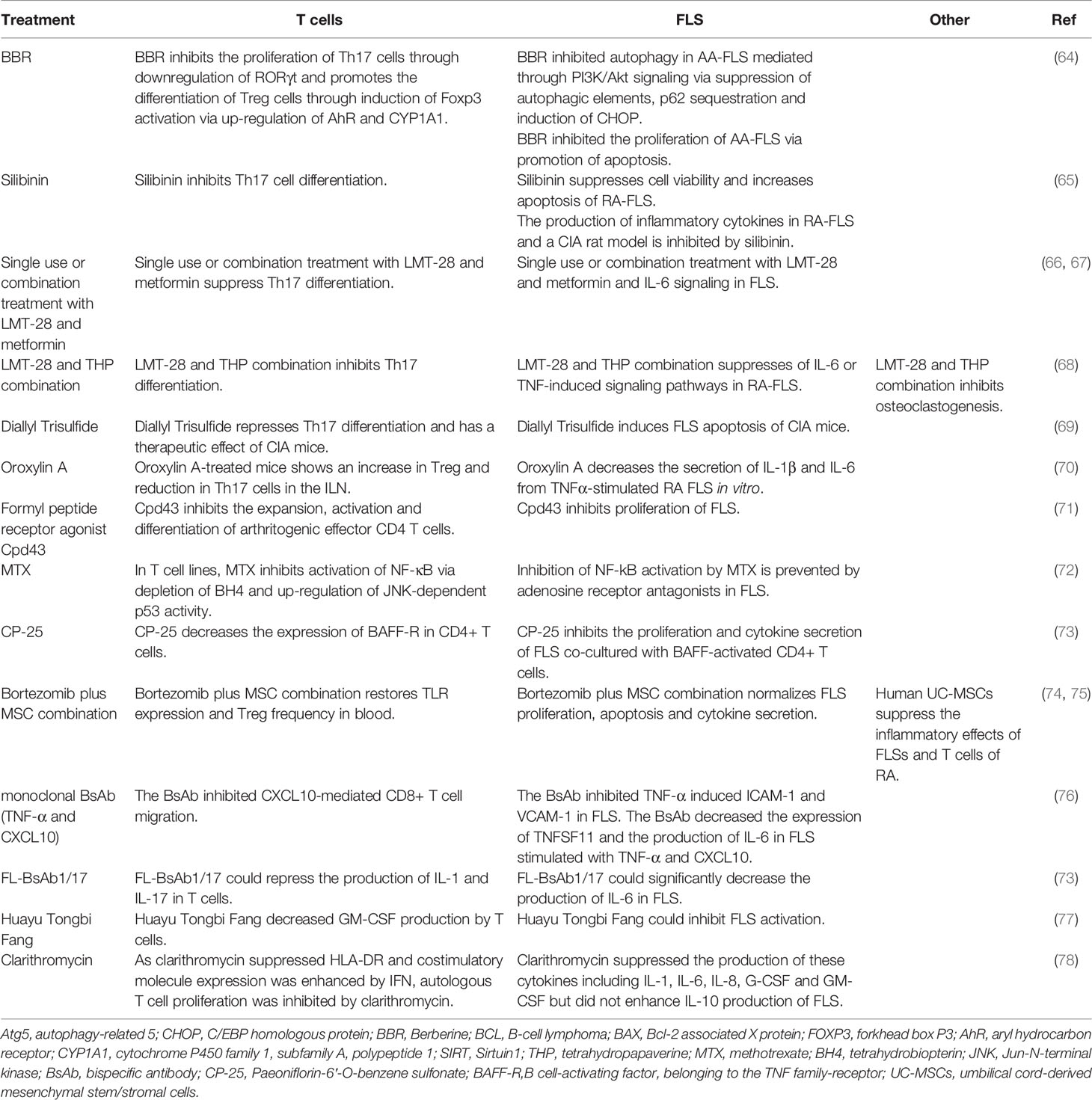
Table 1 The medicinal treatment regimens that can affect the functions of both T cells and FLS in RA.
IL-21 induces the expression of Beclin-1, autophagy-related 5 (Atg5), and LC3-phosphatidylethanolamine conjugate 3-II (LC3-II) through the inhibition of C/EBP homologous protein (CHOP) in FLS from rats with adjuvant-induced arthritis. Berberine (BBR), an alkaloid derivative predominantly present in Oregon grapes and shoots of barberry, represses FLS autophagy via PI3K/Akt signaling by inhibiting autophagic elements, p62 sequestration, and the induction of CHOP. In addition, IL-21 induces the hyper-proliferation of FLS by upregulating the B-cell lymphoma-2 (Bcl-2)/Bcl-2 associated X protein (BAX) ratio, which can be reversed by BBR. IL-21 also promotes CD4+ CD196+ Th17 cell expansion via the PI3K/Akt pathway, and BBR can repress the expansion of Th17 cells by repressing the specific transcriptional factor RORγt in Th17 cells in a PI3K/AKT-dependent manner. Furthermore, BBR promotes the expansion of CD4+CD25+ Treg cells, which exerts an effect opposite to that of Th17 cells, through induction of a specific Treg transcriptional factor, forkhead box P3 (Foxp3), via aryl hydrocarbon receptor (AhR) and the upregulation of cytochrome P450 family 1, subfamily A, polypeptide 1 (CYP1A1) (64).
Silibinin, a natural polyphenolic flavonoid, represses cell proliferation and induces the apoptosis of FLS from patients with RA in an NF-κB pathway-dependent manner. Silibinin also represses Sirtuin1 (SIRT1), and SIRT1 knockdown enhances silibinin-induced apoptosis in FLS. Silibinin also inhibits arthritis development in a CIA rat model and the secretion of inflammatory cytokines in FLS from patients with RA. In addition, it inhibits the differentiation of Th17 cells in vitro (65).
Single-use or combination treatment with LMT-28 (a derivative of oxazolidinone) and metformin significantly ameliorates arthritic signs in rats with CIA by suppressing Th17 differentiation and IL-6 signaling in FLS (66, 67). A combination of LMT-28 and tetrahydropapaverine (THP, benzylisoquinoline alkaloid) could attenuate RA through the suppression of Th17 differentiation in T cells and proinflammatory cytokine-induced inflammation in FLS (68). Diallyl trisulfide induces FLS apoptosis, represses Th17 differentiation, and has a therapeutic effect on mice with CIA by blocking NF-κB and Wnt pathways (69). Oroxylin A-treated CIA mice demonstrate an upregulation of Treg cells and downregulation of Th17 cells in the inguinal lymph nodes. Oroxylin A also represses the production of IL-1β and IL-6 from TNFα-stimulated FLS in vitro (70). The formyl peptide receptor agonist Cpd43 inhibits the expansion of arthritogenic effector CD4 T cells and FLS and reduces joint damage in CIA and AIA mice (71).
Previous results also showed that methotrexate (MTX) represses the NF-κB pathway in T cells and FLS. In T cell lines, MTX blocks the NF-κB pathway by repressing tetrahydrobiopterin (BH4) and inducing p53 in a Jun-N-terminal kinase (JNK)-dependent manner (72). Levels of phosphorylated RelA are decreased in low-dose MTX-treated patients with RA. However, the MTX-mediated inhibition of the NF-κB pathway is completely prevented by adenosine receptor antagonists in FLS from patients with RA but not via BH4 and JNK (72). Clarithromycin represses the secretion of cytokines such as IL-1, IL-6, IL-8, G-CSF, and GM-CSF but does not enhance the production of IL-10 by FLS. As clarithromycin suppresses HLA-DR and co-stimulatory molecule expression is enhanced by IFN, the proliferation of autologous T cells is markedly inhibited by clarithromycin. Clarithromycin exerts a considerable immunosuppressive effect on FLS by inhibiting co-stimulatory molecule expression, cytokine production, and antigen-specific T cell proliferation induced by FLS (78).
The effects of a monoclonal bispecific antibody (BsAb) targeting TNF-α and CXCL10 was also evaluated in RA (76). BsAb repressed the CXCL10-mediated migration of CD8+ T cells. Further, the effect of binding of the BsAb to TNF-α was comparable to that of adalimumab; BsAb also repressed TNF-α-mediated cell death and the expression of VCAM-1 and ICAM-1 in FLS. BsAb was also found to inhibit TNFSF11 and IL-6 in TNF-α- and CXCL10-stimulated FLS (76). Another recombinant IgG-like bispecific antibody (FL-BsAb1/17) targeting IL-1β and IL-17A also showed considerable effects for RA treatment, which could repress the secretion of IL-6 in FLS from patients with RA (73). Paeoniflorin-6′-O-benzene sulfonate (CP-25) decreases the expression of B cell-activating factor, belonging to the TNF family-receptor (BAFF-R), in CD4+ T cells and represses cell proliferation and cytokine production in FLS co-cultured with BAFF-activated CD4+ T cells (85). A Chinese herbal formula, Huayu Tongbi Fang, also represses FL-mediated inflammation in rats by suppressing T cells and FLS-producing GM-CSF (77). Human umbilical cord-derived mesenchymal stem/stromal cells (UC-MSCs) inhibit the inflammatory features of FLS and T cells from patients with RA and alleviate the progression of CIA, implying that UC-MSCs can be used as a potential therapeutic strategy for RA (74). The combination of bortezomib and MSCs rescues TLR expression and the ratio of Treg cells in peripheral blood and normalizes FLS proliferation, apoptosis, and cytokine secretion (75).
CCL3 enhances the expression of proinflammatory cytokines (including IL-6, IL-1β, TNF-α, and RANKL) in RA-FLS by activating the PI3K/AKT signaling pathway. Moreover, CCL3 can upregulate CD4+ T cells to mediate the inflammatory response in RA (86). Cobrotoxin (CTX) suppresses the abnormal increase in CD4+/CD8+ T cells and inhibits T cell proliferation. CTX also inhibits the proliferation of cultured FLS by inhibiting the NF-κB signaling pathway (79).
rhIL23R-CHR can be used to inhibit the IL-23-related pathway to explore the role of IL-23 in the dysfunction of Th17/Th9/Treg cells in rats with CIA. CIA rats demonstrate downregulation of the production of IL-9 and IL-17 and upregulation of FoxP3 upon rhIL23R-CHR treatment, implying that IL-23 could alleviate the dysfunctions of Th17/Th9/Treg cells. Furthermore, IL-23 also promotes the proinflammatory features of FLS in vitro, showing synergetic outcomes with TNF-α (82).
RANKL is expressed by both FLS and sub-lining T lymphocytes (87). IL-21 promotes RANKL in CD4+ T cells from CIA and in CD4+ T cells and FLS from patients with RA. IL-21 also induces osteoclastogenesis by inducing RANKL expression in CD4+ T cells and FLS in vitro (80). Another study detected RANKL+ cells in FLS and infiltrating mononuclear cells of synovial tissue of patients with RA (81). Double immunostaining detected RANKL+ cells in CD3+ and CD4+ T cells. RANKL is elevated and osteoprotegerin is lowered in the synovial fluid of patients with RA. The ratio of the concentration of RANKL to that of osteoprotegerin is also upregulated in the synovial fluid of patients with RA compared to that in the synovial fluid of patients administered oroxylin A or with gout. In addition, RANKL+ T cells promote osteoclastogenesis from peripheral monocytes. The promoting function of RANKL osteoclastogenesis was confirmed by osteoprotegerin-mediated inhibition in a dose-dependent manner (81).
Dendrobium huoshanense stem polysaccharide (cDHPS) alleviates the imbalance in Th17/Treg cells; represses the production of FLS activation-associated proinflammatory cytokines, damage to articular cartilage, the formation of osteoclasts, and angiogenesis; reduces HIF-1α; and induces anti-inflammatory cytokines in joint synovium and serum of CIA mice (83). Delphinidin represses the histone acetyltransferase activities of p300/CBP and p65 acetylation in MH7A cells, which are a human RA FLS cell line (84). Delphinidin-mediated hypoacetylation is characterized by the cytosolic accumulation of NF-κB activator p65 and nuclear localization of the NF-κB inhibitor IKBα. Delphinidin suppresses the TNF-α-induced upregulation of the NF-κB pathway in MH7A cells. It also represses LPS-induced proinflammatory cytokine production in Jurkat T lymphocytes, implying that a histone acetyltransferase inhibitor can efficiently suppress cytokine-mediated immune responses (84).
T cells and FLS play an important role in the pathogenesis of RA. T cells show a systematic disorder in patients with RA, and FLS promote inflammation and damage the joints locally in the joint synovium of patients with RA. However, since T cells can be recruited to the joint synovium through blood and lymphatic circulation, there is a possibility of interactions between the two cellular components in the joint synovium. Recent publications have confirmed many means of communication between T cells and FLS in the joint synovium in RA, including direct or indirect interactions and one-way or two-way interactions, further amplifying the severity of synovitis. Therefore, blocking this key interaction has the potential to relieve the symptoms of RA or even completely treat RA.
Many agents can directly affect both FLS and T cells in RA. The dual effect of those potential drugs on FLS and T cells presents a promising solution for the treatment of RA and thus, should be further studied in the future. For example, blocking the proinflammatory cytokine (CCL3, IL-21, and IL-23) pathways will block the activation of T cells and FLS-mediated proinflammatory effects because their receptors are commonly expressed on T cells and FLS (88). In addition, for some pathways that can act mutually between T cells and FLS, such as PGE2/EP receptors and Kv1.3/KCa1.1, inhibitors that stop these bidirectional effects should be designed and tested to prevent the cascading proinflammatory effects and relieve the symptoms of RA (89).
But there are still some unsolved issues with the current research, which leads to obstacles to potential application in the future. For example, FLS is not professional APC, and it is not clear whether the molecular mechanism of the signals that activate T cells is exactly the same as that of APCs, and the interaction between FLS and different subtypes of CD4+ T cells is also not entirely clear. Secondly, in the joint synovial tissue of RA patients, in addition to FLS and T cells, there are many other important cell types, including B cells, macrophages, etc., and the interaction network between these cells also needs to be further clarified. Finally, the interaction between T cells and FLS in most of the literature mentioned in this review was confirmed by in vitro experiments, and whether the same regulatory patterns still exist in the in vivo environment require better in vivo models to confirm. All of these issues need further in-depth study before clinic application.
JT and WH drafted the manuscript. JT, TL, WZ, and CZ revised the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (Grant No. 81871788 and 31900616), the Project for Science and Technology leader of Anhui Province (Grant No. 2018H177), the Scientific Research Fund of Anhui Education (Grant No. 2017jyxm1097), the Anhui Provincial Postdoctoral Science Foundation (Grant No. 2019B302), Youth Program of the Provincial Natural Science Foundation of Anhui (2008085MH247), The project of improvement of scientific ability of Anhui Medical University(2020xkjT009) and the Sanming Project of Medicine in Shenzhen (SZSM201812041).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Tu J, Huang W, Zhang W, Mei J, Zhu C. A Tale of Two Immune Cells in Rheumatoid Arthritis: The Crosstalk Between Macrophages and T Cells in the Synovium. Front Immunol (2021) 12:655477. doi: 10.3389/fimmu.2021.655477
2. Mørch AM, Bálint Š, Santos AM, Davis SJ, Dustin ML. Coreceptors and TCR Signaling – the Strong and the Weak of it. Front Cell Dev Biol (2020) 8:597627. doi: 10.3389/fcell.2020.597627
3. Malmström V, Trollmo C, Klareskog L. Modulating Co-Stimulation: A Rational Strategy in the Treatment of Rheumatoid Arthritis? Arthritis Res Ther (2005) 7 (Suppl 2):S15–20. doi: 10.1186/ar1505
4. Tu J, Hong W, Zhang P, Wang X, Körner H, Wei W. Ontology and Function of Fibroblast-Like and Macrophage-Like Synoviocytes: How do They Talk to Each Other and Can They Be Targeted for Rheumatoid Arthritis Therapy? Front Immunol (2018) 9:1467. doi: 10.3389/fimmu.2018.01467
5. Schonfeldova B, Zec K, Udalova IA. Synovial Single-Cell Heterogeneity, Zonation and Interactions: A Patchwork of Effectors in Arthritis. Rheumatol (United Kingdom) (2022) 61:913–25. doi: 10.1093/rheumatology/keab721
6. Morgan R, Endres J, Behbahani-Nejad N, Phillips K, Ruth JH, Friday SC, et al. Expression and Function of Aminopeptidase N/CD13 Produced by Fibroblast-Like Synoviocytes in Rheumatoid Arthritis: Role of CD13 in Chemotaxis of Cytokine-Activated T Cells Independent of Enzymatic Activity. Arthritis Rheumatol (2015) 67:74–85. doi: 10.1002/art.38878
7. Bryant J, Ahern DJ, Brennan FM. CXCR4 and Vascular Cell Adhesion Molecule 1 are Key Chemokine/Adhesion Receptors in the Migration of Cytokine-Activated T Cells. Arthritis Rheum (2012) 64:2137–46. doi: 10.1002/art.34394
8. Harada S, Yamamura M, Okamoto H, Morita Y, Kawashima M, Aita T, et al. Production of Interleukin-7 and Interleukin-15 by Fibroblast-Like Synoviocytes From Patients With Rheumatoid Arthritis. Arthritis Rheum (1999) 42:1508–16. doi: 10.1002/1529-0131(199907)42:7<1508::AID-ANR26>3.0.CO;2-L
9. Timmer TCG, Baltus B, Vondenhoff M, Huizinga TWJ, Tak PP, Verweij CL, et al. Inflammation and Ectopic Lymphoid Structures in Rheumatoid Arthritis Synovial Tissues Dissected by Genomics Technology: Identification of the Interleukin-7 Signaling Pathway in Tissues With Lymphoid Neogenesis. Arthritis Rheum (2007) 56:2492–502. doi: 10.1002/art.22748
10. Rosengren S, Corr M, Firestein GS, Boyle DL. The JAK Inhibitor CP-690,550 (Tofacitinib) Inhibits TNF-Induced Chemokine Expression in Fibroblast-Like Synoviocytes: Autocrine Role of Type I Interferon. Ann Rheum Dis (2012) 71:440–7. doi: 10.1136/ard.2011.150284
11. Bustamante MF, Garcia-Carbonell R, Whisenant KD, Guma M. Fibroblast-Like Synoviocyte Metabolism in the Pathogenesis of Rheumatoid Arthritis. Arthritis Res Ther (2017) 19:1–12. doi: 10.1186/s13075-017-1303-3
12. Tang Y, Wang B, Sun X, Li H, Ouyang X, Wei J, et al. Rheumatoid Arthritis Fibroblast-Like Synoviocytes Co-Cultured With PBMC Increased Peripheral CD4+CXCR5+ICOS+ T Cell Numbers. Clin Exp Immunol (2017) 190:384–93. doi: 10.1111/cei.13025
13. Liu R, Zhao P, Zhang Q, Che N, Xu L, Qian J, et al. Adiponectin Promotes Fibroblast-Like Synoviocytes Producing IL-6 to Enhance T Follicular Helper Cells Response in Rheumatoid Arthritis. Clin Exp Rheumatol (2020) 38:11–8.
14. Tang BX, You X, Zhao LD, Li Y, Zhang X, Tang FL, et al. P53 in Fibroblast-Like Synoviocytes can Regulate T Helper Cell Functions in Patients With Active Rheumatoid Arthritis. Chin Med J (Engl) (2011) 124:364–8. doi: 10.3760/cma.j.issn.0366-6999.2011.03.008
15. Gao S, Qi X, Li J, Sang L. Upregulated KAT7 in Synovial Fibroblasts Promotes Th17 Cell Differentiation and Infiltration in Rheumatoid Arthritis. Biochem Biophys Res Commun (2017) 489:235–41. doi: 10.1016/j.bbrc.2017.05.143
16. Lee DG, Woo JW, Kwok SK, La CM, Park SH. MRP8 Promotes Th17 Differentiation via Upregulation of IL-6 Production by Fibroblast-Like Synoviocytes in Rheumatoid Arthritis. Exp Mol Med (2013) 45:1–9. doi: 10.1038/emm.2013.39
17. Wang B, Ma Z, Wang M, Sun X, Tang Y, Li M, et al. IL-34 Upregulated Th17 Production Through Increased IL-6 Expression by Rheumatoid Fibroblast-Like Synoviocytes. Mediators Inflammation (2017) 2017:1567120. doi: 10.1155/2017/1567120
18. Lin J, Zhou Z, Huo R, Xiao L, Ouyang G, Wang L, et al. Cyr61 Induces IL-6 Production by Fibroblast-Like Synoviocytes Promoting Th17 Differentiation in Rheumatoid Arthritis. J Immunol (2012) 188:5776–84. doi: 10.4049/jimmunol.1103201
19. Eljaafari A, Tartelin M-L, Aissaoui H, Chevrel G, Osta B, Lavocat F, et al. Bone Marrow- and Synovium-Derived Mesenchymal Cells Promote Th-17 Cells Through Caspase-1 Activation: Contribution to Rheumatoid Arthritis Chronicity. Arthritis Rheum (2012) 64(7):2147–57. doi: 10.1002/art
20. Corvaisier M, Delneste Y, Jeanvoine H, Preisser L, Blanchard S, Garo E, et al. IL-26 Is Overexpressed in Rheumatoid Arthritis and Induces Proinflammatory Cytokine Production and Th17 Cell Generation. PLoS Biol (2012) 10(9):e1001395.21. doi: 10.1371/journal.pbio.1001395
21. Xiao X, Li Y, Jiang X, Ji X, Lu X, Yang B, et al. EZH2 Deficiency Attenuates Treg Diff Erentiation in Rheumatoid Arthritis. J Autoimmun (2019) 108:102404. doi: 10.1016/j.jaut.2020.102404
22. Wu X, Liu Y, Jin S, Wang M, Jiao Y, Yang B, et al. Single-Cell Sequencing of Immune Cells From Anticitrullinated Peptide Antibody Positive and Negative Rheumatoid Arthritis. Nat Commun (2021) 12(1):4977. doi: 10.1038/s41467-021-25246-7
23. Hwang SY, Kim HY. Expression of IL-17 Homologs and Their Receptors in the Synovial Cells of Rheumatoid Arthritis Patients. Mol Cells (2005) 19:180–4.
24. Hirota K, Hashimoto M, Ito Y, Matsuura M, Ito H, Tanaka M, et al. Autoimmune Th17 Cells Induced Synovial Stromal and Innate Lymphoid Cell Secretion of the Cytokine GM-CSF to Initiate and Augment Autoimmune Arthritis. Immunity (2018) 48:1220–32.e5. doi: 10.1016/j.immuni.2018.04.009
25. Kim EK, Kwon JE, Lee SY, Lee EJ, Kim DS, Moon SJ, et al. IL-17-Mediated Mitochondrial Dysfunction Impairs Apoptosis in Rheumatoid Arthritis Synovial Fibroblasts Through Activation of Autophagy. Cell Death Dis (2017) 8(1):e2565. doi: 10.1038/cddis.2016.490
26. Shui XL, Lin W, Mao CW, Feng YZ, Kong JZ, Chen SM. Blockade of IL-17 Alleviated Inflammation in Rat Arthritis and MMP-13 Expression. Eur Rev Med Pharmacol Sci (2017) 21:2329–37.
27. Kim KW, Kim HR, Kim BM, La CM, Lee SH. Th17 Cytokines Regulate Osteoclastogenesis in Rheumatoid Arthritis. Am J Pathol (2015) 185:3011–24. doi: 10.1016/j.ajpath.2015.07.017
28. Friday SC, Fox DA. Phospholipase D Enzymes Facilitate IL-17- and Tnfα-Induced Expression of Proinflammatory Genes in Rheumatoid Arthritis Synovial Fibroblasts (RASF). Immunol Lett (2016) 174:9–18. doi: 10.1016/j.imlet.2016.04.001
29. Fan M, Li Y, Yao C, Liu X, Liu X, Liu J. Dihydroartemisinin Derivative DC32 Attenuates Collagen-Induced Arthritis in Mice by Restoring the Treg/Th17 Balance and Inhibiting Synovitis Through Down-Regulation of IL-6. Int Immunopharmacol (2018) 65:233–43. doi: 10.1016/j.intimp.2018.10.015
30. Liu Z, Song L, Wang Y, Xu P, Guo X, Yang J, et al. A Novel Fusion Protein Attenuates Collagen–Induced Arthritis by Targeting Interleukin 17A and Tumor Necrosis Factor α. Int J Pharm (2018) 547:72–82. doi: 10.1016/j.ijpharm.2018.05.058
31. Kim KW, La CM, HR K, JH Ju, MK P, HJ Oh, et al. Up-Regulation of Stromal Cell-Derived Factor 1 (CXCL12) Production in Rheumatoid Synovial Fibroblasts Through Interactions With T Lymphocytes: Role of Interleukin-17 and CD40L-CD40 Interaction. Arthritis Rheum (2007) 56:1076–86. doi: 10.1002/art.22439
32. Aldridge J, Ekwall AKH, Mark L, Bergström B, Andersson K, Gjertsson I, et al. T Helper Cells in Synovial Fluid of Patients With Rheumatoid Arthritis Primarily Have a Th1 and a CXCR3+Th2 Phenotype. Arthritis Res Ther (2020) 22:1–11. doi: 10.1186/s13075-020-02349-y
33. Kato H, Endres J, Fox DA. The Roles of IFN-γ Versus IL-17 in Pathogenic Effects of Human Th17 Cells on Synovial Fibroblasts. Mod Rheumatol (2013) 23:1140–50. doi: 10.1007/s10165-012-0811-x
34. Corrigall VM, Arastu M, Khan S, Shah C, Fife M, Tak PP, et al. Functional IL-2 Receptor β (CD122) and γ (CD132) Chains Are Expressed by Fibroblast-Like Synoviocytes: Activation by IL-2 Stimulates Monocyte Chemoattractant Protein-1 Production. J Immunol (2001) 166:4141–7. doi: 10.4049/jimmunol.1201455
35. Schurigt U, Pfirschke C, Irmler IM, Hückel M, Gajda M, Janik T, et al. Interactions of T Helper Cells With Fibroblast-Like Synoviocytes: Up-Regulation of Matrix Metalloproteinases by Macrophage Migration Inhibitory Factor From Both Th1 and Th2 Cells. Arthritis Rheum (2008) 58:3030–40. doi: 10.1002/art.23904
36. Saadatmand S, Vos JR, Hooning MJ, Oosterwijk JC, Koppert LB, De BGH, et al. Interleukin-21 Induces Migration and Invasion of Fibroblast-Like Synoviocytes From Patients With Rheumatoid Arthritis. Clin Exp Immunol (2016) 184:2–31. doi: 10.1111/cei.12751
37. Lebre MC, Vieira PL, Tang MW, Aarrass S, Helder B, Newsom-Davis T, et al. Synovial IL-21/TNF-Producing CD4+ T Cells Induce Joint Destruction in Rheumatoid Arthritis by Inducing Matrix Metalloproteinase Production by Fibroblast-Like Synoviocytes. J Leukoc Biol (2017) 101:775–83. doi: 10.1189/jlb.5a0516-217rr
38. Blaschke S, Middel P, Dorner BG, Blaschke V, Hummel KM, Kroczek RA, et al. Expression of Activation-Induced, T Cell-Derived, and Chemokine-Related Cytokine/Lymphotactin and Its Functional Role in Rheumatoid Arthritis. Arthritis Rheum (2003) 48:1858–72. doi: 10.1002/art.11171
39. Zhu W, Meng L, Jiang C, He X, Hou W, Xu P, et al. Arthritis is Associated With T-Cell-Induced Upregulation of Toll-Like Receptor 3 on Synovial Fibroblasts. Arthritis Res Ther (2011) 13(3):R103. doi: 10.1186/ar3384
40. Karonitsch T, Beckmann D, Dalwigk K, Niederreiter B, Studenic P, Byrne RA, et al. Targeted Inhibition of Janus Kinases Abates Interfon Gamma-Induced Invasive Behaviour of Fibroblast-Like Synoviocytes. Rheumatol (United Kingdom) (2018) 57:572–7. doi: 10.1093/rheumatology/kex426
41. Kvacskay P, Yao N, Schnotz JH, Scarpone R, Carvalho R de A, Klika KD, et al. Increase of Aerobic Glycolysis Mediated by Activated T Helper Cells Drives Synovial Fibroblasts Towards an Inflammatory Phenotype: New Targets for Therapy? Arthritis Res Ther (2021) 23:1–15. doi: 10.1186/s13075-021-02437-7
42. Tran CN, Davis MJ, Tesmer LA, Endres JL, Motyl CD, Smuda C, et al. Presentation of Arthritogenic Peptide to Antigen-Specific T Cells by Fibroblast-Like Synoviocytes. Arthritis Rheum (2007) 56:1497–506. doi: 10.1002/art.22573
43. Riemann D, Röntsch J, Hause B, Langner J, Kehlen A. Cell-Cell Contact Between Lymphocytes and Fibroblast-Like Synoviocytes Induces Lymphocytic Expression of Aminopeptidase N/CD13 and Results in Lymphocytic Activation. Adv Exp Med Biol (2000) 477:57–66. doi: 10.1007/0-306-46826-3_6
44. Bradfield PF, Amft N, Vernon-Wilson E, Exley AE, Parsonage G, Rainger GE, et al. Rheumatoid Fibroblast-Like Synoviocytes Overexpress the Chemokine Stromal Cell-Derived Factor 1 (CXCL12), Which Supports Distinct Patterns and Rates of CD4+ and CD8+ T Cell Migration Within Synovial Tissue. Arthritis Rheum (2003) 48:2472–82. doi: 10.1002/art.11219
45. Sawai H, Park YW, Roberson J, Imai T, Goronzy JJ, Weyand CM. T Cell Costimulation by Fractalkine-Expressing Synoviocytes in Rheumatoid Arthritis. Arthritis Rheum (2005) 52:1392–401. doi: 10.1002/art.21140
46. Vallejo AN, Yang H, Klimiuk PA, Weyand CM, Goronzy JJ. Synoviocyte-Mediated Expansion of Inflammatory T Cells in Rheumatoid Synovitis Is Dependent on CD47-Thrombospondin 1 Interaction. J Immunol (2003) 171:1732–40. doi: 10.4049/jimmunol.171.4.1732
47. Vallejo AN, Mügge LO, Klimiuk PA, Weyand CM, Goronzy JJ. Central Role of Thrombospondin-1 in the Activation and Clonal Expansion of Inflammatory T Cells. J Immunol (2000) 164:2947–54. doi: 10.4049/jimmunol.164.6.2947
48. Corrigall VM, Solau-Gervais E, Panayi GS. Lack of CD80 Expression by Fibroblast-Like Synoviocytes Leading to Anergy in T Lymphocytes. Arthritis Rheum (2000) 43:1606–15. doi: 10.1002/1529-0131(200007)43:7<1606::AID-ANR26>3.0.CO;2-O
49. Tran CN, Thacker SG, Louie DM, Oliver J, White PT, Endres JL, et al. Interactions of T Cells With Fibroblast-Like Synoviocytes: Role of the B7 Family Costimulatory Ligand B7-H3. J Immunol (2008) 180:2989–98. doi: 10.4049/jimmunol.180.5.2989
50. Kim SJ, Shin HH, Park SY, Lee DS, Lee EA, Cho SD, et al. Induction of MMP-13 Expression by Soluble Human Glucocorticoid-Induced Tumor Necrosis Factor Receptor in Fibroblast-Like Synovial Cells. Osteoarthr Cartil (2006) 14:146–53. doi: 10.1016/j.joca.2005.08.012
51. Min DJ, Cho ML, SH L, SY M, WU K, JK M, et al. Augmented Production of Chemokines by the Interaction of Type II Collagen-Reactive T Cells With Rheumatoid Synovial Fibroblasts. Arthritis Rheum (2004) 50:1146–55. doi: 10.1002/art.20133
52. Sawai H, Park YW, He X, Goronzy JJ, Weyand CM. Fractalkine Mediates T Cell-Dependent Proliferation of Synovial Fibroblasts in Rheumatoid Arthritis. Arthritis Rheum (2007) 56:3215–25. doi: 10.1002/art.22919
53. Petrasca A, Phelan JJ, Ansboro S, Veale DJ, Fearon U, Fletcher JM. Targeting Bioenergetics Prevents CD4 T Cell-Mediated Activation of Synovial Fibroblasts in Rheumatoid Arthritis. Rheumatol (United Kingdom) (2020) 59:2816–28. doi: 10.1093/rheumatology/kez682
54. Singh K, Colmegna I, He X, Weyand CM, Goronzy JJ. Synoviocyte Stimulation by the LFA-1–Intercellular Adhesion Molecule-2–Ezrin–Akt Pathway in Rheumatoid Arthritis. J Immunol (2008) 180:1971–8. doi: 10.4049/jimmunol.180.3.1971
55. Tran CN, Lundy SK, White PT, Endres JL, Motyl CD, Gupta R, et al. Molecular Interactions Between T Cells and Fibroblast-Like Synoviocytes: Role of Membrane Tumor Necrosis Factor-α on Cytokine-Activated T Cells. Am J Pathol (2007) 171:1588–98. doi: 10.2353/ajpath.2007.070004
56. Bombara MP, Webb DL, Conrad P, Marlor CW, Sarr T, Ranges GE, et al. Cell Contact Between T Cells and Synovial Fibroblasts Causes Induction of Adhesion Molecules and Cytokines. J Leukoc Biol (1993) 54:399–406. doi: 10.1002/jlb.54.5.399
57. Cho ML, Yoon CH, Hwang SY, Park MK, Min SY, Lee SH, et al. Effector Function of Type II Collagen-Stimulated T Cells From Rheumatoid Arthritis Patients: Cross-Talk Between T Cells and Synovial Fibroblasts. Arthritis Rheum (2004) 50:776–84. doi: 10.1002/art.20106
58. Moon YM, Yoon BY, Her YM, Oh HJ, Lee JS, Kim KW, et al. IL-32 and IL-17 Interact and Have the Potential to Aggravate Osteoclastogenesis in Rheumatoid Arthritis. Arthritis Res Ther (2012) 14(6):R246. doi: 10.1186/ar4089
59. Tanner MR, Pennington MW, Chauhan SS, Laragione T, Gulko PS, Beeton C. KCa1.1 and Kv1.3 Channels Regulate the Interactions Between Fibroblast-Like Synoviocytes and T Lymphocytes During Rheumatoid Arthritis. Arthritis Res Ther (2019) 21:1–21. doi: 10.1186/s13075-018-1783-9
60. Akaogi J, Nozaki T, Satoh M, Yamada H. Role of PGE2 and EP Receptors in the Pathogenesis of Rheumatoid Arthritis and as a Novel Therapeutic Strategy. Endocrine Metab Immune Disord - Drug Targets (2012) 6:383–94. doi: 10.2174/187153006779025711
61. Zhang W, Wang B, Wang F, Zhang J, Yu J. CTLA4-FasL Fusion Product Suppresses Proliferation of Fibroblast-Like Synoviocytes and Progression of Adjuvant-Induced Arthritis in Rats. Mol Immunol (2012) 50:150–9. doi: 10.1016/j.molimm.2012.01.007
62. Kraan MC, Haringman JJ, Weedon H, Barg EC, Smith MD, Ahern MJ, et al. T Cells, Fibroblast-Like Synoviocytes, and Granzyme B+ Cytotoxic Cells are Associated With Joint Damage in Patients With Recent Onset Rheumatoid Arthritis. Ann Rheum Dis (2004) 63:483–8. doi: 10.1136/ard.2003.009225
63. Brooke A, Bs R, Mph CH, Ma MC, Shao X, Ba HLQ, et al. Rheumatoid Arthritis Naïve T Cells Share Hypermethylation Sites With Synoviocytes. Arthritis Rheumatol (2017) 69:550–9. doi: 10.1002/art
64. Dinesh P, Rasool MK. Berberine Mitigates IL-21/IL-21R Mediated Autophagic Influx in Fibroblast-Like Synoviocytes and Regulates Th17/Treg Imbalance in Rheumatoid Arthritis. Apoptosis (2019) 24:644–61. doi: 10.1007/s10495-019-01548-6
65. Tong WW, Zhang C, Hong T, Liu DH, Wang C, Li J, et al. Silibinin Alleviates Inflammation and Induces Apoptosis in Human Rheumatoid Arthritis Fibroblast-Like Synoviocytes and has a Therapeutic Effect on Arthritis in Rats. Sci Rep (2018) 8:1–12. doi: 10.1038/s41598-018-21674-6
66. Park YH, Jang YJ, Choi Y, Lee K, Kim HJ, Cho O, et al. Combination of LMT-28 and Metformin Improves Beneficial Anti-Inflammatory Effect in Collagen-Induced Arthritis. Pharmacology (2021) 106:53–9. doi: 10.1159/000507451
67. Park YH, Kim HJ, Heo TH. A Directly GP130-Targeting Small Molecule Ameliorates Collagen-Induced Arthritis (CIA) by Inhibiting IL-6/GP130 Signalling and Th17 Differentiation. Clin Exp Pharmacol Physiol (2020) 47:628–39. doi: 10.1111/1440-1681.13215
68. Park Y, Jung H, Lee K, Choi Y, Heo T. Combination of Gp130-Targeting and TNF-Targeting Small Molecules in Alleviating Arthritis Through the Down-Regulation of Th17 Differentiation and Osteoclastogenesis. Biochem Biophys Res Commun (2019) 522(4):1030–36. doi: 10.1016/j.bbrc.2019.11.183
69. Liang JJ, Li HR, Chen Y, Zhang C, Chen DG, Liang ZC, et al. Diallyl Trisulfide can Induce Fibroblast-Like Synovial Apoptosis and has a Therapeutic Effect on Collagen-Induced Arthritis in Mice via Blocking NF-κb and Wnt Pathways. Int Immunopharmacol (2019) 71:132–8. doi: 10.1016/j.intimp.2019.03.024
70. Wang YL, Gao JM, Xing LZ. Therapeutic Potential of Oroxylin A in Rheumatoid Arthritis. Int Immunopharmacol (2016) 40:294–9. doi: 10.1016/j.intimp.2016.09.006
71. Odobasic D, Jia Y, Kao W, Fan H, Wei X, Gu R, et al. Formyl Peptide Receptor Activation Inhibits the Expansion of Effector T Cells and Synovial Fibroblasts and Attenuates Joint Injury in Models of Rheumatoid Arthritis. Int Immunopharmacol (2018) 61:140–9. doi: 10.1016/j.intimp.2018.05.028
72. Spurlock CF, Gass HM, Bryant CJ, Wells BC, Olsen NJ, Aune TM. Methotrexate-Mediated Inhibition of Nuclear Factor κb Activation by Distinct Pathways in T Cells and Fibroblast-Like Synoviocytes. Rheumatol (United Kingdom) (2014) 54:178–87. doi: 10.1093/rheumatology/keu279
73. Wang Y, Wu Q, Liu Z, Guo X, Zhou L, Wang Y, et al. A Recombinant IgG-Like Bispecific Antibody Acting as Interleukin-1β and Interleukin-17A Inhibitor Exhibits a Promising Efficacy for Rheumatoid Arthritis. BioMed Pharmacother (2017) 89:426–37. doi: 10.1016/j.biopha.2017.02.045
74. Liu Y, Mu R, Wang S, Long L, Liu X, Li R, et al. Therapeutic Potential of Human Umbilical Cord Mesenchymal Stem Cells in the Treatment of Rheumatoid Arthritis. Arthritis Res Ther (2010) 12:1–13. doi: 10.1186/ar3187
75. Papadopoulou A, Yiangou M, Athanasiou E, Zogas N, Kaloyannidis P, Batsis I, et al. Mesenchymal Stem Cells are Conditionally Therapeutic in Preclinical Models of Rheumatoid Arthritis. Ann Rheum Dis (2012) 71:1733–40. doi: 10.1136/annrheumdis-2011-200985
76. Kang SE, Park JK, Yoo HJ, Kang HS, Park YW, Park BC, et al. Efficacy of Novel Bispecific Antibody Targeting TNF-α/CXCL10 in the Treatment of Experimental Arthritis. Transl Res (2021) 232:75–87. doi: 10.1016/j.trsl.2021.01.004
77. Chen H, Wang C, Li J, Huandike M, Liu J, Huang Q, et al. Chinese Herbal Formula, Huayu Tongbi Fang, Attenuates Inflammatory Proliferation of Rat Synoviocytes Induced by IL-1 β by Regulating Proliferation and Differentiation of T Lymphocytes. Evidence-Based Complement Altern Med (2020) 2020:1706837. doi: 10.1155/2020/1706837
78. Matsuoka N, Eguchi K, Kawakami A, Tsuboi M, Kawabe Y, Aoyagi T, et al. Inhibitory Effect of Clarithromycin on Costimulatory Molecule Expression and Cytokine Production by Synovial Fibroblast-Like Cells. Clin Exp Immunol (1996) 104:501–8. doi: 10.1046/j.1365-2249.1996.46752.x
79. Zhu Q, Huang J, Wang SZ, Qin ZH, Lin F. Cobrotoxin Extracted From Naja Atra Venom Relieves Arthritis Symptoms Through Anti-Inflammation and Immunosuppression Effects in Rat Arthritis Model. J Ethnopharmacol (2016) 194:1087–95. doi: 10.1016/j.jep.2016.11.009
80. Kwok SK, Cho ML, Park MK, Oh HJ, Park JS, Her YM, et al. Interleukin-21 Promotes Osteoclastogenesis in Humans With Rheumatoid Arthritis and in Mice With Collagen-Induced Arthritis. Arthritis Rheum (2012) 64:740–51. doi: 10.1002/art.33390
81. Kotake S, Udagawa N, Hakoda M, Mogi M, Yano K, Tsuda E, et al. Activated Human T Cells Directly Induce Osteoclastogenesis From Human Monocytes: Possible Role of T Cells in Bone Destruction in Rheumatoid Arthritis Patients. Arthritis Rheum (2001) 44:1003–12. doi: 10.1002/1529-0131(200105)44:5<1003::aid-anr179>3.0.co;2-%23
82. Guo W, Yu D, Wang X, Luo C, Chen Y, Lei W, et al. Anti-Inflammatory Effects of Interleukin-23 Receptor Cytokinebinding Homology Region Rebalance T Cell Distribution in Rodent Collagen-Induced Arthritis. Oncotarget (2016) 7:31800–13. doi: 10.18632/oncotarget.9309
83. Shang ZZ, Qin DY, Li QM, Zha XQ, Pan LH, Peng DY, et al. Dendrobium Huoshanense Stem Polysaccharide Ameliorates Rheumatoid Arthritis in Mice via Inhibition of Inflammatory Signaling Pathways. Carbohydr Polym (2021) 58(3):718–29. doi: 10.1016/j.carbpol.2021.117657
84. Seong AR, Yoo JY, Choi KC, Lee MH, Lee YH, Lee J, et al. Delphinidin, a Specific Inhibitor of Histone Acetyltransferase, Suppresses Inflammatory Signaling via Prevention of NF-κb Acetylation in Fibroblast-Like Synoviocyte MH7A Cells. Biochem Biophys Res Commun (2011) 410:581–6. doi: 10.1016/j.bbrc.2011.06.029
85. Jia X, Wei F, Sun X, Chang Y, Xu S, Yang X, et al. CP-25 Attenuates the in Flammatory Response of Fibroblast-Like Synoviocytes Co-Cultured With BAFF-Activated CD4+ T Cells. J Ethnopharmacol J (2016) 189:194–201. doi: 10.1016/j.jep.2016.05.034
86. Zhang G, Liu HB, Zhou L, Cui XQ, Fan XH. CCL3 Participates in the Development of Rheumatoid Arthritis by Activating AKT. Eur Rev Med Pharmacol Sci (2018) 22:6625–32. doi: 10.26355/eurrev_201810_16137
87. Vandooren B, Cantaert T, Noordenbos T, Tak PP, Baeten D. The Abundant Synovial Expression of the RANK/RANKL/osteoprotegerin System in Peripheral Spondylarthritis is Partially Disconnected From Inflammation. Arthritis Rheum (2008) 58:718–29. doi: 10.1002/art.23290
88. Fitch E, Harper E, Skorcheva I, Kurtz SE, Blauvelt A. Pathophysiology of Psoriasis: Recent Advances on IL-23 and Th17 Cytokines. Curr Rheumatol R (2007) 9:461–7. doi: 10.1007/s11926-007-0075-1
Keywords: RA, T cells, FLS, cellular interaction, therapy
Citation: Tu J, Huang W, Zhang W, Mei J and Zhu C (2022) Two Main Cellular Components in Rheumatoid Arthritis: Communication Between T Cells and Fibroblast-Like Synoviocytes in the Joint Synovium. Front. Immunol. 13:922111. doi: 10.3389/fimmu.2022.922111
Received: 17 April 2022; Accepted: 25 May 2022;
Published: 01 July 2022.
Edited by:
Heinrich Korner, University of Tasmania, AustraliaReviewed by:
Yujie Deng, Guangzhou Regenerative Medicine and Health Guangdong Laboratory, ChinaCopyright © 2022 Tu, Huang, Zhang, Mei and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chen Zhu, emh1Y2hlbmFAdXN0Yy5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.