
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Immunol., 25 August 2022
Sec. Nutritional Immunology
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.919402
 Farid Azizi Jalilian1,2
Farid Azizi Jalilian1,2 Gheisar Keshavarz3
Gheisar Keshavarz3 Salman Khazaei4
Salman Khazaei4 Manije Nezamdoost5
Manije Nezamdoost5 Seyed Hamid Hashemi6
Seyed Hamid Hashemi6 Mojgan Mamani6
Mojgan Mamani6 Nastaran Ansari2
Nastaran Ansari2 Razieh Amini1
Razieh Amini1 Aref Khalkhali7
Aref Khalkhali7 Arghavan Keshavarz8
Arghavan Keshavarz8 Erfan Ayubi9
Erfan Ayubi9 Maryam Fazeli2
Maryam Fazeli2 Rashid Heidari Moghadam4
Rashid Heidari Moghadam4 Saeid Alizadeh10
Saeid Alizadeh10 Behzad Pourhossein2
Behzad Pourhossein2 Ali Teimouri2
Ali Teimouri2 Fariba Keramat6*†
Fariba Keramat6*† Sajad Karampour11
Sajad Karampour11 Mohammadreza Khakzad12
Mohammadreza Khakzad12The present study aimed to evaluate the effects of Nutrition Bio-shield Superfood (NBS) powder on the immune system function and clinical manifestations in patients with COVID-19. We compare the effects of NBS powder on the immune system function and clinical manifestations among two different groups: 1) intervention group receiving standard treatment scheduled according to treatment guidelines plus NBS powder, and 2) control group receiving only the same standard treatment. The serum levels of IL-2, IL-6, IL-17, IFNγ, and TNFα were determined after four weeks of treatment by specific ELISA kits according to the manufacturer’s instructions. Finally, the level of immune system stimulation and inflammatory markers were compared at baseline and after intervention in both groups. Data were analyzed using SPSS (version 22). A p-value of ≤ 0.05 was set as significant. A total of 47 patients with COVID-19 (24 patients in the intervention group and 23 patients in the control group) were included in this study. Results showed that the differences in the mean decrease of IL-2, IL-6, and TNF-α in the intervention group in comparison to the control group were 0.93, 10.28, and 8.11 pg/ml, respectively (P<0.001). On the other hand, there was no difference in IL-17, IFNγ, monocytes, eosinophil, and other inflammatory indices between the intervention and control groups. Although NBS powder was able to significantly decrease the levels of some proinflammatory cytokines in patients with COVID-19, however, it is noteworthy that the course of the disease was to large part unaffected by NBS power and there was a reduction independent of treatment. The present study indicates that NBS powder could provide a beneficial anti-inflammatory effect in patients with COVID-19. Hence, NBS in treating patients with COVID-19 shows promise as an adjuvant to the current standard antiviral treatment of such patients.
Clinical Trial Registration: https://www.irct.ir, identifier IRCT20200426047206N1.
The ongoing pandemic of coronavirus disease 2019 (COVID-19) is considered a public health concern by World Health Organization (WHO) (1, 2). Globally, COVID-19 affects different people and is associated with significant mortality and morbidity. Till 7/July/2022 around 550,218,992 cases of COVID-19 and 6,343,783 deaths have been reported, worldwide. (https://www.worldometers.info/coronavirus/). Moreover, this new virus led to more than 7,244,694 confirmed cases and 141,420 deaths up to 8 July 2022 in Iran. (https://www.worldometers.info/coronavirus/country/iran/) (3). In general, host immune systems interact with microbial and non-microbial antigens and may stimulate cytokine production (4, 5). It was found that the immune response may play a vital role in the infectious disease caused by COVID-19. The secretion of inflammatory cytokines and other mediators of the immune system plays an important role against viral infections (6, 7). Pathogenic mechanisms of COVID-19 on the host immune systems are through decreasing the counts of lymphocytes, especially CD4+ T cell, CD8+ T cell, and B cell, and inducing excessive inflammatory reaction known as the cytokine storm phenomenon (8–10). An extremely robust cytokine storm due to an unsteady response can be extremely damaging to lung tissue and leads to lung capacity reduction, more severe disease, and even death (11). The increased level of some cytokines, including interleukin (IL)-1β, IL-2, IL-6, IL-7, IL-8, IL-10, granulocyte-colony stimulating factor (G-CSF), granulocyte macrophage-colony stimulating factor (GM-CSF), interferon-inducible protein-10 (IP10), monocyte chemotactic protein 1 (MCP1), macrophage inflammation protein-1α, IFN-γ, and tumor necrosis factor-alpha (TNF-α) is known as the signs of cytokines storm caused by COVID-19 (12, 13). It was highlighted that COVID-19 could consequently lead to a weak production of type I interferons (IFN-Is) (14). Designing and conducting clinical trials to develop new laboratory technics and drugs, to detect and treat COVID-19 is an urgent need. Nutrition Bio-Shield (Agri Bio Nutrition®, Turkey) (NBS) powder is an herbal product mainly made from wheat germ (15). Wheat germ contains α-linolenic acid; glutathione; fibers; minerals; tocopherols; carotenoids; B group vitamins; phytosterols; policosanols; betaine; alkylresorcinol; and polyphenols such as flavonoids, lignans, and ferulic acid (16). This herb can be used with no side effects and it has an approved number as a dietary supplement (006633-14.11.2019). Moreover, it was issued by the Turkish ministry of agriculture and forestry. Herbal medicines are natural, so they appear to cause fewer side effects (15, 17). NBS has various health benefits and may be used as a good therapeutic target in patients with COVID-19. Administration of this viable herbal supplement could improve the functioning of the immune system (15). In general, many efforts are being made in all over the world to find appropriate herbal medicine for the treatment of COVID-19 (6). However, further clinical trials are needed to better understanding about the inflammatory responses associated with COVID-19 when applying newer treatment interventions. Therefore, the present randomized controlled trial study was designed with the aim of evaluating the effect of NBS powder on immune system markers in patients with COVID-19.
The current study was a double-blinded randomized controlled trial (RCT) study performed in Sina hospital, Hamadan, Iran from May to July 2020. In the first step, using simple randomization, all patients included were randomly assigned to one of two groups in an allocation ratio of 1:1, as follows: intervention group receiving standard treatment scheduled according to treatment guidelines plus NBS powder, and 2) control group receiving only the same standard treatment. Written informed consent was obtained from all patients. A randomized list was generated by an online randomization site. All included patients and researchers were blinded to the allocation assignments; however, the physician and clinicians team were aware of the group that was given the NBS powder.
A total of 47 patients with COVID-19 were included in the present study. All patients were divided into two groups, the intervention group (n=24) and the control group (n=23) for four weeks. The inclusion criteria were the confirmed COVID-19 patients through PCR over the age of 20 years and who are not allergic to the powder used. Moreover, exclusion criteria were disagreement of the patient or relatives to participate in the project.
Patients in the intervention group received NBS powder in addition to the standard antiviral treatment. The dosage of NBS was 500 mg capsules daily in four capsules (two grams) given in divided doses of one gram in the morning and one gram in the evening for 4 weeks. Patients in control groups received standard antiviral treatment only. Standard antiviral treatment includes a two-drug regimen: Hydroxychloroquine (Oxiklorin, Elyson Pharmaceutical Co. Ltd., Seoul, Korea) and Kaletra (Lopinavir + Ritonavir) (AbbVie Inc. North Chicago, Illinois, U.S.A).
For this purpose, at the end of the fourth week, blood samples were taken from each patient. We evaluated the serum levels of IL-2, IL-6, IL-17, IFNγ, and TNFα with ELISA kits (ZellBio Co., Germany) according to the manufacturer’s instructions. Results were analyzed by an ELISA microplate reader (Bio Tek E1800, USA), and the concentrations were calculated.
Data were analyzed using SPSS (version 22). A P-value of ≤ 0.05 was set as significant. Shapiro-Wilk test was used to check whether parameters were normally distributed. Parameters with normal and non-normal distribution were presented using mean (standard deviation; SD) and median (interquartile range; IQR) according to treatment groups, respectively. Paired t-test and Wilcoxon signed-rank test were used for comparison baseline vs. end line within each treatment group, as appropriate. Comparison between the treatment groups was carried out by independent t-test and Mann-Whitney test, as appropriate (6, 18, 19).
Among the participants, 24 patients received NBS powder in addition to the standard antiviral treatment (intervention group) randomly, and 23 patients received standard treatment as the control group (Figure 1). Mean ± SD age of all patients in intervention and control groups were 51.54 ± 16.66 years (range; 22 to 87 years) and 46.26 ± 18.69 (range; 22 to 82 years), respectively. Table 1 illustrates the characteristics of the patients. There were no significant differences in all characteristics of patients between the two treatment groups. Age-sex distribution of patients was similar between the two groups. The frequency of smoking among patients in the control group was higher than those in another group (17.39% vs. 12.50%). Approximately 70% of the patients had underlying diseases in the two groups.
Results of comparing the level of parameters in the baseline and after the intervention within the groups were presented in Table 2 and Figure 2. A significant difference was found between the baseline and after the intervention in all of the studied parameters (P<0.05) except for the hemoglobin test (Hb), mean corpuscular volume (MCV), and mean corpuscular hemoglobin (MCH). Table 3 illustrated the comparing of difference in the level of parameters between the two groups at the end of follow-up. Statistical analysis showed a greater mean reduction in the level of cytokines including IL-2, IL-6, and TNF-α in the intervention group than in the control group (P<0.001), e.g. Δ mean difference for IL-2, IL-6, IL-17, and TNF-α were 0.93, 10.28, and 8.11, respectively. Although NBS powder was able to significantly decrease the levels of some proinflammatory cytokines in patients with COVID-19, however, it is noteworthy that the course of the disease was to large part unaffected by NBS power compared to the standard treatment. In addition, the level of SGPT, SGOT, NEU, ALP, and CPK tends to decrease more rapidly in the patients in the intervention group than in those in the control group (P<0.05). The statistical analysis showed that the level of white blood cells (WBC) and lymphocytes (LYM) in the intervention group increased significantly more than in the control group e.g. Δ mean difference for WBC and LYM was 1.05 and 5.12, respectively.
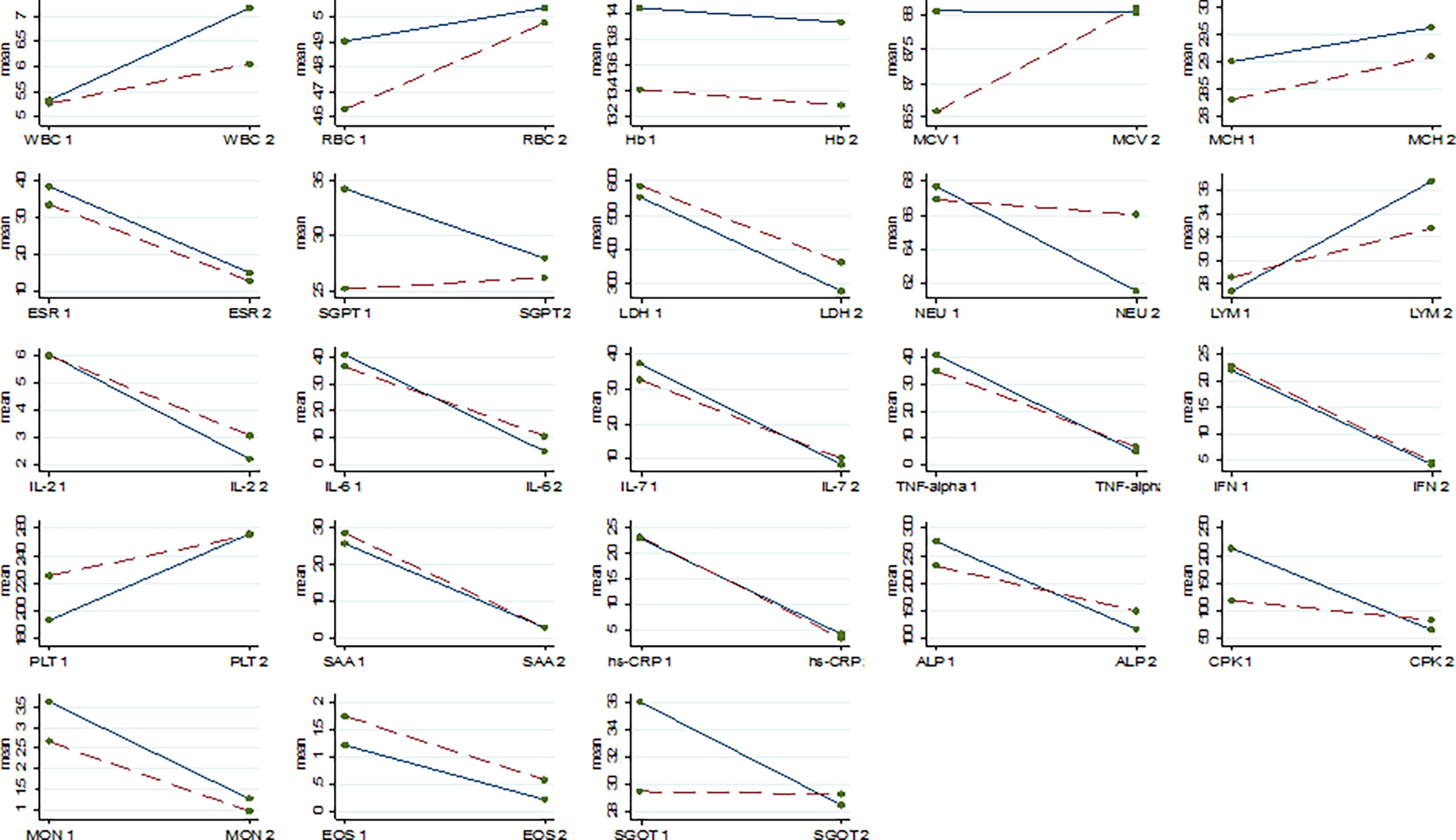
Figure 2 The change of mean of laboratory parameters from baseline (1) to end line (2); White Blood Cell (WBC), Red Blood Cell (RBC), Hemoglobin (HB), Mean Corpuscular Volume (MCV), Mean Corpuscular Hemoglobin (MCH), Erythrocyte Sedimentation Rate (ESR), Serum Glutamate-Pyruvate Transaminase (SGPT), Lactate Dehydrogenase (LDH), Neutrophil (NEU), Lymphocyte (LYM), Interleukin-2 (IL-2), Interleukin 6 (IL-6), Interleukin 7 (IL-7), Tumor Necrosis Factor (TNF)-alpha, Interferon (IFN), Platelet (PLT), Serum Amyloid A (SAA), High-Sensitivity C-Reactive Protein (hs-CRP), Serum Glutamic-Oxaloacetic Transaminase (SGOT), Alkaline Phosphatase (ALP), Creatine Phosphokinase (CPK), Monocytes (MON), Eosinophil (EOS); the change for Standard + NBS treatment group is solid line, the change for Standard treatment only group is Dash line.
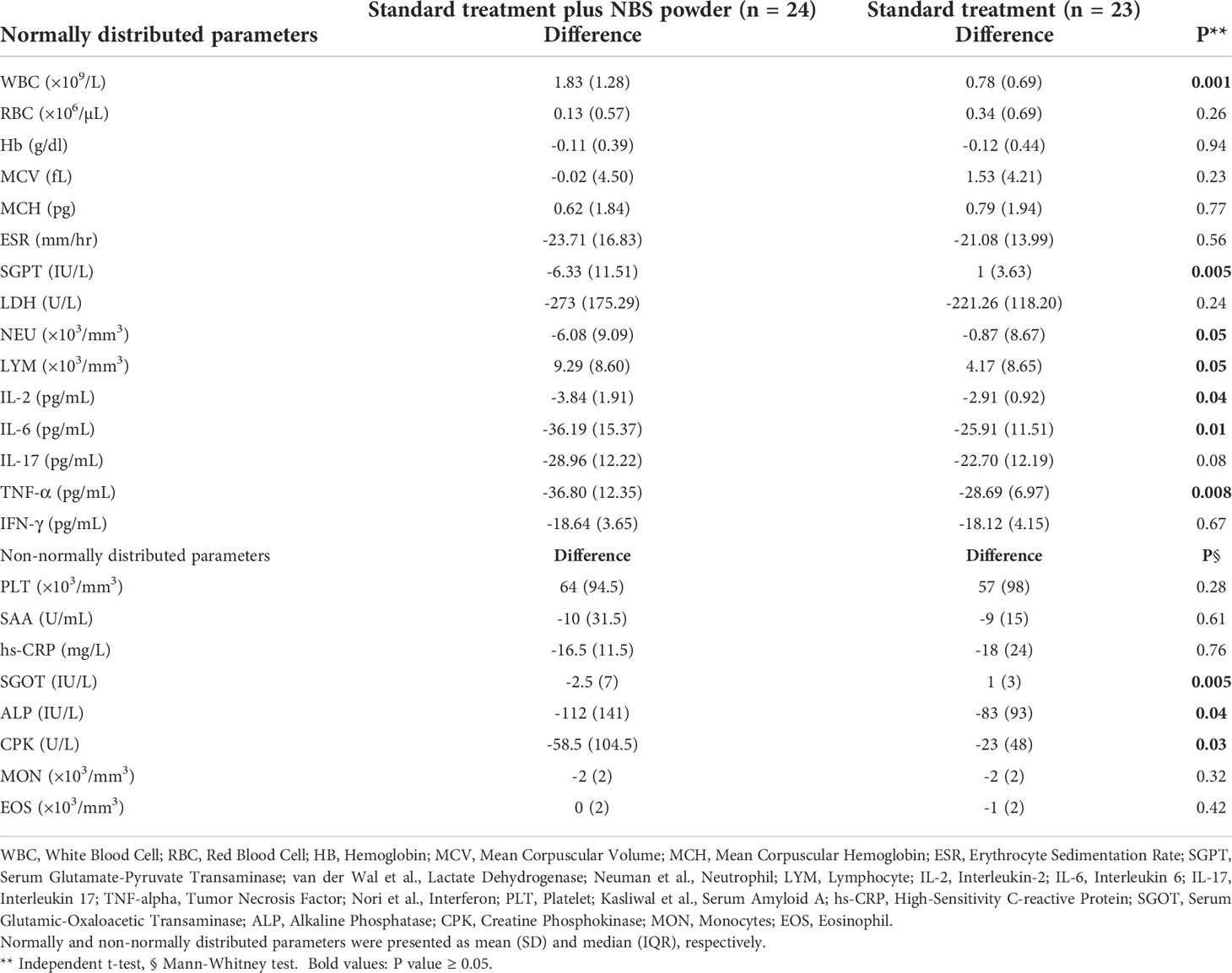
Table 3 Comparing of difference in level of parameters between the intervention and control groups at the end of follow up.
Multiple organ dysfunction syndrome, acute cardiac complications, acute respiratory distress syndrome, septic shock, and death are the severe complications of COVID-19 infection (20, 21). It is revealed that these manifestations and complications are related to viral replication that induces an abnormally strong release of cytokines and an excessive inflammatory reaction known as the cytokine storm (22). Several previously published studies revealed that optimal dietary patterns and adequate nutrient status are significant to modulate inflammatory reaction and oxidative stress processes. Moreover, in most cases, an optimal immune response is related to optimal dietary and nutritional constituents and is fundamental to preventing infection (23–25). In general, different plant-based foods can exert anti-inflammatory and antioxidant properties (26, 27). In the present RCT study, we aimed to evaluate the effects of NBS powder on the immune system function and clinical manifestations in patients with COVID-19. As far as we are concerned, the current study is the first research to have evaluated the effect of NBS powder, on immune system function in patients with COVID-19. In the present study we found a difference in the reduction of levels of IL-2, IL-6, and TNF-α. Results of our study showed that inflammatory responses in the patients who received NBS powder for four weeks with standard treatment (intervention group) were induced statistically fewer than in the patients who received only the standard treatment. Our finding revealed that compared to the control group, the means of IL-2, IL-6, and TNF-α concentrations were reduced by 0.93, 10.28, and 8.11 per unit during 4 weeks follow-up when NBS powder was added to the standard treatment in the intervention group.
Likewise, a similar pattern was found for both IL-17 and IFN-γ; however, the result was not statistically significant. Results of the current study revealed that NBS powder significantly decreased the levels of some proinflammatory cytokines in patients with COVID-19, however, it is noteworthy that the course of the disease was to large part unaffected by NBS power compared to the standard treatment. Therefore, there was a reduction independent of treatment.
The observed change in other inflammatory and immunity indices supports the clinical benefits of adding NBS powder to the standard treatment. The evidence also indicated that the severity of COVID-19 increases with the levels of inflammatory mediators including cytokines and chemokines such as IL-2, IL-7, IL-10, and TNF-α in the blood due to COVID-19 infection (28, 29). Significantly, by comparing survivors and non-survivors of COVID-19, it was observed that IL-6 plays a more important role in mortality than the other increased inflammatory factors (30). No statistical significance of Δ mean difference for IL-17 between the two groups may be due to the small sample size of the study that reduced the power of the study, thus there is a possibility of type II error in the results of this study. In the previously published RCT study (31), the researchers found that patients with COVID-19 receiving lopinavir-ritonavir along with interferon beta-1b and ribavirin had a significantly lower level of IL-6 at days 2, 6, and 8 in comparison with patients who only received lopinavir and ritonavir. Moreover, their results for TNF-α and IL-10 concentrations were not significant. To date, several studies have been performed on the effects of wheat germ (as the main component of NBS) and its derivatives on immune responses. Hussein et al. (2014) reported that pretreatment with wheat germ oil in a rat model of endotoxemia led to the suppression of serum levels of TNF-α and IL-6 (32). This finding is consistent with the results obtained in our study. In another study (2019) by Ojo et al., it was shown that wheat germ could reduce serum concentrations of IL-1b, IL-6, INF-γ, and TNF-α in C57BL6 mice. This study suggested that the addition of this nutrient to the diet may be vital in preventing diet-induced comorbidities (33). The present study was a double-blinded, randomized, controlled clinical trial, which could be considered a prerequisite to detect a treatment effect of an intervention. In addition, the distribution of the patients’ characteristics at baseline was balanced across the two groups, which decreased the chance of confounding in the results of the study. It was observed that the prevention of inflammatory responses to cytokines or their dependent receptors by antibodies or neutralizing compounds can decrease immune-mediated damage (34). The obtained data from the treatment with NBS in the patients of the test group showed a decrease in concentration of inflammatory cytokine. We showed that NBS significantly reduced the levels of IL-2, IL-6, and TNF-α; therefore, it can be considered as a potential treatment option that may reduce side effects of the COVID-19 disease.
The results of the present RCT show that NBS powder combined with the standard treatment might prevent inflammatory responses against COVID-19 infection. In this study, the standard treatment, which could preclude detecting the action mechanism of NBS, was administered to both groups. Moreover, our findings are preliminary. Therefore, larger RCTs with repeated measurements and large and different intervention groups are needed to determine the beneficial effects of NBS on the immune system markers.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The trial was approved by the Ethics committee of Hamadan University of Medical Sciences (IR.UMSHA.REC.1399.046; approval date: 2020-05-19) and conducted according to the Helsinki Declaration. The patients/participants provided their written informed consent to participate in this study.
FK, FAJ, GK, SKh, and MN: Conceptualization; Data curation; Formal analysis; and Writing – original draft. SH, MM, NA, RA, AKh, and AKe: Conceptualization; Methodology; Project administration; and Writing – original draft. EA, MF, RH, SA, and BP: Data curation; Formal analysis; Writing – original draft; and Writing – review and editing. AT, FK, SKa, and MK: Language editing. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Azimi T, Hamidi-Farahani R, Asgari A, Rajabi J, Ahmadi M, Darvishi M, et al. Molecular detection and clinical characteristics of bacterial and viral main etiological agents causing respiratory tract infections in Tehran, Iran. Gene Rep (2021) 24:101267. doi: 10.1016/j.genrep.2021.101267
2. Ciotti M, Ciccozzi M, Terrinoni A, Jiang W-C, Wang C-B, Bernardini S. The COVID-19 pandemic. Crit Rev Clin Lab Sci (2020) 57(6):365–88. doi: 10.1080/10408363.2020.1783198
3. Weidmann MD, Ofori K, Rai AJ. Laboratory biomarkers in the management of patients with covid-19. Am J Clin Pathol (2021) 155(3):333–42. doi: 10.1093/ajcp/aqaa205
4. Behrens EM, Koretzky GA. Cytokine storm syndrome: Looking toward the precision medicine era. Arthritis Rheumatol (2017) 69(6):1135–43. doi: 10.1002/art.40071
5. Tang Y, Liu J, Zhang D, Xu Z, Ji J, Wen C. Cytokine storm in COVID-19: The current evidence and treatment strategies. Front Immunol (2020) 11:1708. doi: 10.3389/fimmu.2020.01708
6. Beigmohammadi MT, Bitarafan S, Hoseindokht A, Abdollahi A, Amoozadeh L, Soltani D. The effect of supplementation with vitamins a, b, c, d, and e on disease severity and inflammatory responses in patients with COVID-19: a randomized clinical trial. Trials (2021) 22(1):1–9. doi: 10.1186/s13063-021-05795-4
7. Mortaz E, Bezemer G, Alipoor SD, Varahram M, Mumby S, Folkerts G, et al. Nutritional impact and its potential consequences on COVID-19 severity. Front Nutr (2021) 8:698617. doi: 10.3389/fnut.2021.698617
8. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in wuhan, China: a descriptive study. Lancet (2020) 395(10223):507–13. doi: 10.1016/S0140-6736(20)30211-7
9. Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in wuhan, China. Clin Infect Dis (2020) 71(15):762–8. doi: 10.1093/cid/ciaa248
10. Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet (2020) 395(10236):1569–78. doi: 10.1016/S0140-6736(20)31022-9
11. Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R. The COVID-19 cytokine storm; what we know so far. Front Immunol (2020) 16(11):1446. doi: 10.3389/fimmu.2020.01446
12. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in wuhan, China. Lancet (2020) 395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5
13. Liu J, Li S, Liu J, Liang B, Wang X, Wang H, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine (2020) 55:102763. doi: 10.1016/j.ebiom.2020.102763
14. Sa Ribero M, Jouvenet N, Dreux M, Nisole S. Interplay between SARS-CoV-2 and the type I interferon response. PloS Pathog (2020) 16(7):e1008737. doi: 10.1371/journal.ppat.1008737
15. Bayat A, Khalkhali A, Mahjoub AR. Nutrition bio-shield superfood: Healthy and live herbal supplement for immune system enhancement. Int J Nutr Food Eng (2014) 15(1):6–9.
16. Fardet A. New hypotheses for the health-protective mechanisms of whole-grain cereals: what is beyond fibre? Nutr Res Rev (2010) 23(1):65–134. doi: 10.1017/S0954422410000041
17. Tourkochristou E, Triantos C, Mouzaki A. The influence of nutritional factors on immunological outcomes. Front Immunol (2021) 12:665968. doi: 10.3389/fimmu.2021.665968
18. Gadotti AC, de Castro Deus M, Telles JP, Wind R, Goes M, Ossoski RGC, et al. IFN-γ is an independent risk factor associated with mortality in patients with moderate and severe COVID-19 infection. Virus Res (2020) 289:198171. doi: 10.1016/j.virusres.2020.198171
19. Leulseged TW, Hassen IS, Ayele BT, Tsegay YG, Abebe DS, Edo MG, et al. Laboratory biomarkers of COVID-19 disease severity and outcome: Findings from a developing country. PloS One (2021) 16(3):e0246087. doi: 10.1371/journal.pone.0246087
20. Yoo J, Kim JH, Jeon J, Kim J, Song T-J. Risk of COVID-19 infection and of severe complications among people with epilepsy: a nationwide cohort study. Neurology (2022) 98(19):e1886–e92. doi: 10.1212/WNL.0000000000200195
21. Ata F, Iqbal P, Choudry H, Muthanna B, Younas HW, Tabar OSA, et al. Long-term moderate to severe complications of COVID-19 infection since 2019 to date: A protocol for systematic review and/or meta-analysis. Med: Case Rep Study Protoc (2021) 2(11):e0159. doi: 10.1097/MD9.0000000000000159
22. Xie P, Ma W, Tang H, Liu D. Severe COVID-19: a review of recent progress with a look toward the future. Front Public Health (2020) 8:189. doi: 10.3389/fpubh.2020.00189
23. Jahns L, Conrad Z, Johnson LK, Whigham LD, Wu D, Claycombe-Larson KJ. A diet high in carotenoid-rich vegetables and fruits favorably impacts inflammation status by increasing plasma concentrations of IFN-α2 and decreasing MIP-1β and TNF-α in healthy individuals during a controlled feeding trial. Nutr Res (2018) 52:98–104. doi: 10.1016/j.nutres.2018.02.005
24. Chacko SA, Song Y, Nathan L, Tinker L, De Boer IH, Tylavsky F, et al. Relations of dietary magnesium intake to biomarkers of inflammation and endothelial dysfunction in an ethnically diverse cohort of postmenopausal women. Diabetes Care (2010) 33(2):304–10. doi: 10.2337/dc09-1402
25. George SM, Neuhouser ML, Mayne ST, Irwin ML, Albanes D, Gail MH, et al. Postdiagnosis diet quality is inversely related to a biomarker of inflammation among breast cancer survivors. Cancer Epidemiol Prev Biomarkers (2010) 19(9):2220–8. doi: 10.1158/1055-9965.EPI-10-0464
26. Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr (2014) 17(8):1689–96. doi: 10.1017/S1368980013002115
27. Rubin LP, Ross AC, Stephensen CB, Bohn T, Tanumihardjo SA. Metabolic effects of inflammation on vitamin a and carotenoids in humans and animal models. Adv Nutr (2017) 8(2):197–212. doi: 10.3945/an.116.014167
28. Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci (2020) 63(3):364–74. doi: 10.1007/s11427-020-1643-8
29. Kim JY, Ko J-H, Kim Y, Kim Y-J, Kim J-M, Chung Y-S, et al. Viral load kinetics of SARS-CoV-2 infection in first two patients in Korea. J Korean Med Sci (2019) 35(7):1–7. doi: 10.3346/jkms.2020.35.e86
30. Liu B, Li M, Zhou Z, Guan X, Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J Autoimmun (2020) 111:102452. doi: 10.1016/j.jaut.2020.102452
31. Hung IF, Lung KC, Tso EY, Liu R, Chung TW, Chu MY, et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet (2020) 395(10238):1695–704. doi: 10.1016/S0140-6736(20)31042-4
32. Hussein SA, Abdel-Aal S, Elghwab A. Biochemical role of wheat germ oil on biomarkers of oxidative stress and inflammatory response in a rat model of endotoxemia. Benha Vet Med J (2014) 27(2):157–67.
33. Ojo BA, O'Hara C, Wu L, El-Rassi GD, Ritchey JW, Chowanadisai W, et al. Wheat germ supplementation increases lactobacillaceae and promotes an anti-inflammatory gut milieu in C57BL/6 mice fed a high-fat, high-sucrose diet. J Nutr (2019) 149(7):1107–15. doi: 10.1093/jn/nxz061
Keywords: nutrition bio-safety powder, immunity system function, COVID-19, ELISA - enzyme-linked immunosorbent assay, Iran
Citation: Azizi Jalilian F, Keshavarz G, Khazaei S, Nezamdoost M, Hashemi SH, Mamani M, Ansari N, Amini R, Khalkhali A, Keshavarz A, Ayubi E, Fazeli M, Heidari Moghadam R, Alizadeh S, Pourhossein B, Teimouri A, Keramat F, Karampour S and Khakzad M (2022) The effects of nutrition bio-shield superfood powder on immune system function: A clinical trial study among patients with COVID-19. Front. Immunol. 13:919402. doi: 10.3389/fimmu.2022.919402
Received: 14 April 2022; Accepted: 26 July 2022;
Published: 25 August 2022.
Edited by:
Jonas Bystrom, Queen Mary University of London, United KingdomReviewed by:
Kawa Amin, Uppsala University, SwedenCopyright © 2022 Azizi Jalilian, Keshavarz, Khazaei, Nezamdoost, Hashemi, Mamani, Ansari, Amini, Khalkhali, Keshavarz, Ayubi, Fazeli, Heidari Moghadam, Alizadeh, Pourhossein, Teimouri, Keramat, Karampour and Khakzad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fariba Keramat, ZmFyaWJha2VyYW1hdEB5YWhvby5jb20=, ZmFyaWJhLmtlcm1hdDIwMjFAZ21haWwuY29t
†ORCID: Fariba Keramat, orcid.org/0000-0003-2174-2495
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.