- 1Department of Breast Surgery, Fudan University Shanghai Cancer Center, Shanghai, China
- 2Shanghai Medical College, Fudan University, Shanghai, China
- 3Shanghai Key Laboratory of Breast Cancer, Shanghai, China
Background: The optimal (neo)adjuvant regimen for human epidermal growth factor receptor-2 (HER2)-positive breast cancer regarding survival outcomes remains unclear.
Methods: We searched Web of Science, PubMed, and the Cochrane Central Register of Controlled Trials systematically to find out randomized controlled studies, up to January 2022, that compared different anti-HER2 regimens in the (neo)adjuvant setting. The primary endpoint was disease-free survival (DFS). We used a Bayesian statistical model to combine direct and indirect comparisons and used odds ratios (ORs) to pool effect sizes and performed the surface under the cumulative ranking area (SUCRA) curves to estimate the ranking probabilities of various regimens. For survival outcomes, we performed two parallel analyses, one based on data from both neoadjuvant and adjuvant studies and the other specific to adjuvant studies. All statistics were two-sided.
Results: Fifteen studies were finally enrolled. Regarding DFS, the overall analysis indicated that the top two regimens for HER2-positive breast cancer were chemotherapy plus trastuzumab with lapatinib, and chemotherapy plus trastuzumab with pertuzumab (SUCAR of 81% and 79%, respectively), with the OR of 0.99 [95% confidence interval (CI), 0.59 to 1.54]; the parallel analysis specific to adjuvant trials indicated that the top two regimens were chemotherapy plus trastuzumab with sequential neratinib, and chemotherapy plus trastuzumab with pertuzumab (SUCRA of 80% and 76%, respectively), with the OR of 1.04 (95% CI, 0.63 to 1.73). The dual-target therapy that combines trastuzumab and pertuzumab showed the highest risk of inducing cardiac events, with an SUCRA of 92%.
Conclusions: Chemotherapy plus trastuzumab and pertuzumab might be the optimal regimen for HER2-positive breast cancer in improving the survival rate. However, the cardiotoxicity of this dual-target therapy should be taken care of.
Introduction
According to the 2020 global cancer statistics, female breast cancer has become the most commonly diagnosed cancer globally. It was estimated that there were 2.3 million new breast cancer cases in 2020 (1). Approximately 15% to 20% of breast cancer patients are human epidermal growth factor receptor 2 (HER2) positive (2), which are associated with high disease recurrence and poor prognosis (3). Since late 2006, trastuzumab, a HER2-targeted monoclonal antibody, has been the standard care for this breast cancer subtype, and this was based on the results from several landmark trials that demonstrated a significant association between chemotherapy plus trastuzumab and better overall survival (OS) and progression-free survival in HER2-positive breast cancer (4–6). However, it was reported that among patients with early-stage, HER2-positive breast cancer who received trastuzumab and adjuvant chemotherapy, there was still a recurrence rate of approximately 16%–22% (7, 8), and among patients with metastatic breast cancer, 22% to 25% of them displayed primary or secondary resistance to HER2-targeted therapies (9, 10). Therefore, the focus of research has now shifted to finding strategies that can overcome resistance to HER2-targeted therapies so as to further improve patient outcomes.
The currently available HER2-targeted agents that are approved by the US Food and Drug Administration for treating HER2-positive breast cancer mainly included the following categories: HER2-targeted monoclonal antibodies, such as trastuzumab and pertuzumab; tyrosine kinase inhibitors (TKIs), such as lapatinib, neratinib, and tucatinib; and antibody-drug conjugates, which include trastuzumab emtansine (T-DM1) and trastuzumab deruxtecan (11). These agents can be used either alone or in combination, which has created many treatment choices that prompted us to find out the optimal one for this disease. Two previously published network meta-analyses (NMA) on HER2-positive breast cancer tried to integrate efficacy and safety information of all neoadjuvant regimens tested in clinical trials by combining both direct and indirect evidence (12, 13). However, they mainly focused on the outcome of pathological complete response, while the more important survival information was not integrated yet. Therefore, focusing on the survival data from relevant clinical trials, we conducted the present NMA to provide an updated overview on the comparative efficacy of the currently available (neo)adjuvant regimens for HER2-positive breast cancer.
Methods
Search Strategy
The present study was reported according to the Preferred Reporting Items for Systematic Reviews incorporating Network Meta Analyses (PRISMA-NMA) statement (14). Web of Science, PubMed, and the Cochrane Central Register of Controlled Trials were searched systematically. Only English publications were selected, and the search algorithm was as follows: “(mammary OR breast) AND (tumor OR carcinoma OR cancer) AND (human epidermal growth factor receptor 2 OR HER2 OR ERBB2) AND (positive) AND (adjuvant OR neoadjuvant OR preoperative) AND (therapy OR regimen OR treatment).” References of relevant studies were also reviewed carefully to find other relevant trials. The last search was performed in January 2022.
Selection Criteria
The inclusion criteria were as follows: (a) randomized controlled trials that focused on (neo)adjuvant therapy for HER2-positive breast cancer; (b) trials included at least two treatment arms [one of the single-use or different combinations of monoclonal antibodies, TKIs, antibody–drug conjugates, and/or chemotherapy were considered as one arm]; and (c) reported the hazard ratio (HR) and its 95% confidence interval (CI) of survival outcomes. Studies that only recruited elderly patients (over 65 years old) were excluded. To reduce bias, studies that only reported the HR of partial participants (such as those who achieved pathological complete response) were also excluded. If multiple reports were available for the same trial, only the latest version that reported the corresponding survival data was enrolled.
Outcomes
The primary endpoint of the present study was DFS, which was defined as the time from randomization to recurrence of invasive breast cancer at local, regional, or distant sites; contralateral invasive breast cancer; second non-breast malignancy; or death as a result of any cause, whichever occurred first. If not reported in a certain study, DFS would be substituted by event-free survival, progression-free survival, invasive disease-free survival, or distant DFS in order. Additionally, if any of the above candidate outcomes were reported as the primary outcome, it would be selected with priority. Secondary endpoints included OS, defined as the time from randomization to death as a result of any cause, and safety outcomes. Small differences in the definitions among enrolled studies were allowed.
Data Collection and Bias Assessment
Data from the enrolled studies were extracted by two authors independently. The following information was collected: first author’s name, year of publication, trial name, the phase of the study, treatment settings, sample size, treatment arms and their corresponding number of patients, hormone receptor status, treatment duration, follow-up time, primary outcome, and survival outcomes reported with HR with 95% CI. The numbers of grade 3 or 4 adverse events according to the National Cancer Institute Common Terminology Criteria, version 3.0, together with the number of patients treated were also collected. Other grading standards were substituted if not reported. Only the intention-to-treat data were collected. Also, for safety outcomes, only those that were reported by three or more studies and could form a closed loop would be analyzed and reported. The quality of enrolled studies was assessed by version 2 of the Cochrane risk-of-bias tool for randomized trials (RoB 2), which contains five domains for evaluating the risk of bias in randomized trials: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result (15). Disputes were resolved through discussion, or a third author would join to make a decision.
Statistical Analysis
Firstly, data (HR with 95% CI for survival outcomes and the total number of patients treated in each arm, together with the corresponding incidence of events for safety outcomes) comparing the same treatment arms in terms of the same outcomes were integrated using traditional meta-analyses. By recalling JAGS in R for Markov chain Monte Carlo (MCMC) sampling, the “gemtc” R package was then used to perform NMA, in a Bayesian random-effects model, and the odds ratios (ORs) for survival and safety outcomes were used to pool effect sizes. A total of 200,000 simulations were generated for each of the sets of different initial values, and the first 5,000 simulations, as an annealing process, were discarded. Brooks-Gelman-Rubin diagnostic and trace plots were then performed to check the convergence of the model (16).
Other than the overall analysis that combined both the neoadjuvant and adjuvant data, a parallel analysis specific to adjuvant data was also performed to reduce bias. The overall analysis was based on more comprehensive and longer (including both the neoadjuvant and adjuvant periods) data compared with the parallel analysis. However, the primary endpoint of most neoadjuvant studies is pathological complete response, which might have caused some bias regarding survival data. In contrast, the parallel analysis specific to adjuvant studies, the primary endpoint of which is mostly survival outcomes, was based on more accurate survival data. Therefore, it is necessary to perform the two analyses simultaneously, and only therapies that pass the test of the two analyses would be considered to be truly effective.
Inconsistency tests were performed using the node-splitting method by separating the direct and indirect evidence of the same comparison (17). I2 tests were performed to assess the heterogeneity, with I2 > 50% indicating significant heterogeneity. Additionally, sensitivity analyses were also performed by omitting each article sequentially to test the robustness of the primary results. At last, the ranking probabilities of treatment arms in terms of different outcomes were estimated using the surface under the cumulative ranking area (SUCRA) (18). R software (version 4.1.1) and STATA (version 15.0, Stata MP) were used to perform the above statistical analyses and generate plots, with P value less than 0.05 considered statistically significant.
Results
Study Selection
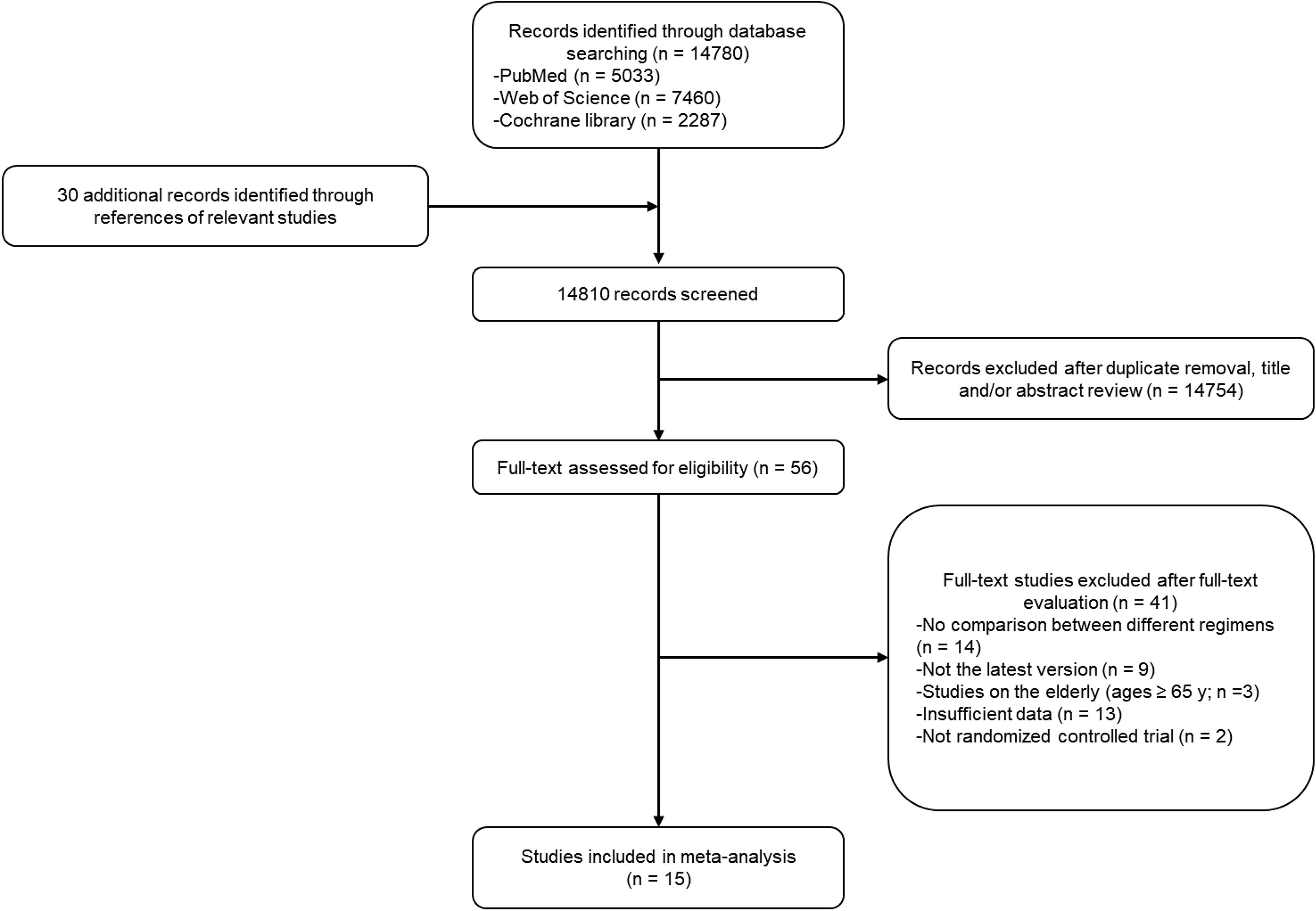
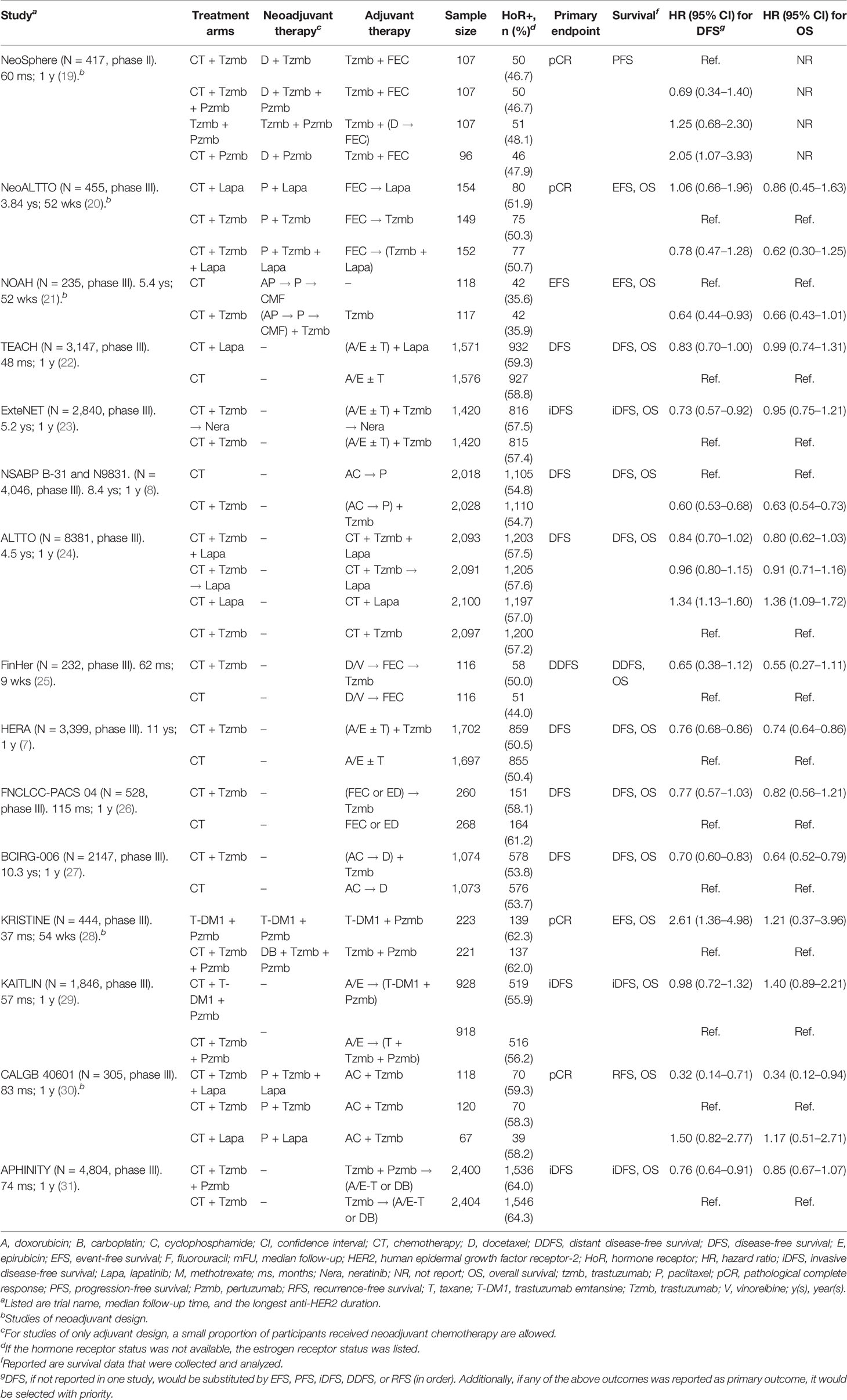
A total of 14,780 potentially relevant studies in the databases and 30 additional studies from references were filtered, from which only 15 studies were finally enrolled in the present analysis (7, 8, 19–31). The detailed selection process is shown in Figure 1. The enrolled studies were published from 2009 to 2021, and the sample size ranged from 232 to 8,381, with a total sample size of 33,226 in the overall analysis. Five of the enrolled studies were of neoadjuvant setting, while the remaining 10 studies were of adjuvant setting. The baseline characteristics of the enrolled studies are summarized in Table 1. The anti-HER2 duration of the majority of enrolled studies was 1 year, except that of the FinHER Trial (25) was 9 weeks. In general, the quality of enrolled studies was medium to high, as shown in Supplementary Material 1.
DFS Network
In the overall analysis, a total of 15 studies (7, 8, 19–31) with 11 treatment arms were involved in the NMA of DFS, as shown in Figure 2A (top panel). According to the SUCRA estimates, the top two regimens for HER2-positive breast cancer were trastuzumab plus lapatinib with chemotherapy and trastuzumab plus pertuzumab with chemotherapy [SUCRA of 81% and 79%, respectively; Figure 2B (top panel)], with the cross-comparison OR of 0.99 [95% CI: 0.59 to 1.54; Figure 2C (top panel)]. Of note, there was significant heterogeneity in the traditional pair-wise comparison of trastuzumab plus lapatinib with chemotherapy versus lapatinib plus chemotherapy (I2 = 85.5%), as shown in Supplementary Material 2. No significant inconsistency was observed between direct and indirect evidence, as shown in Supplementary Material 3. A total of 10 studies (7, 8, 22–27, 29, 31) with eight treatment arms were involved in the parallel analysis specific to adjuvant studies, as illustrated in Figure 2A (bottom panel). According to the SUCRA estimates, the top two therapies were trastuzumab with sequential neratinib based on chemotherapy and trastuzumab plus pertuzumab with chemotherapy [SUCRA of 80% and 76%, respectively; Figure 2B (bottom panel)], with the OR of the latter regimen compared with the former of 1.04 [95% CI: 0.63 to 1.73; Figure 2C (bottom panel)].
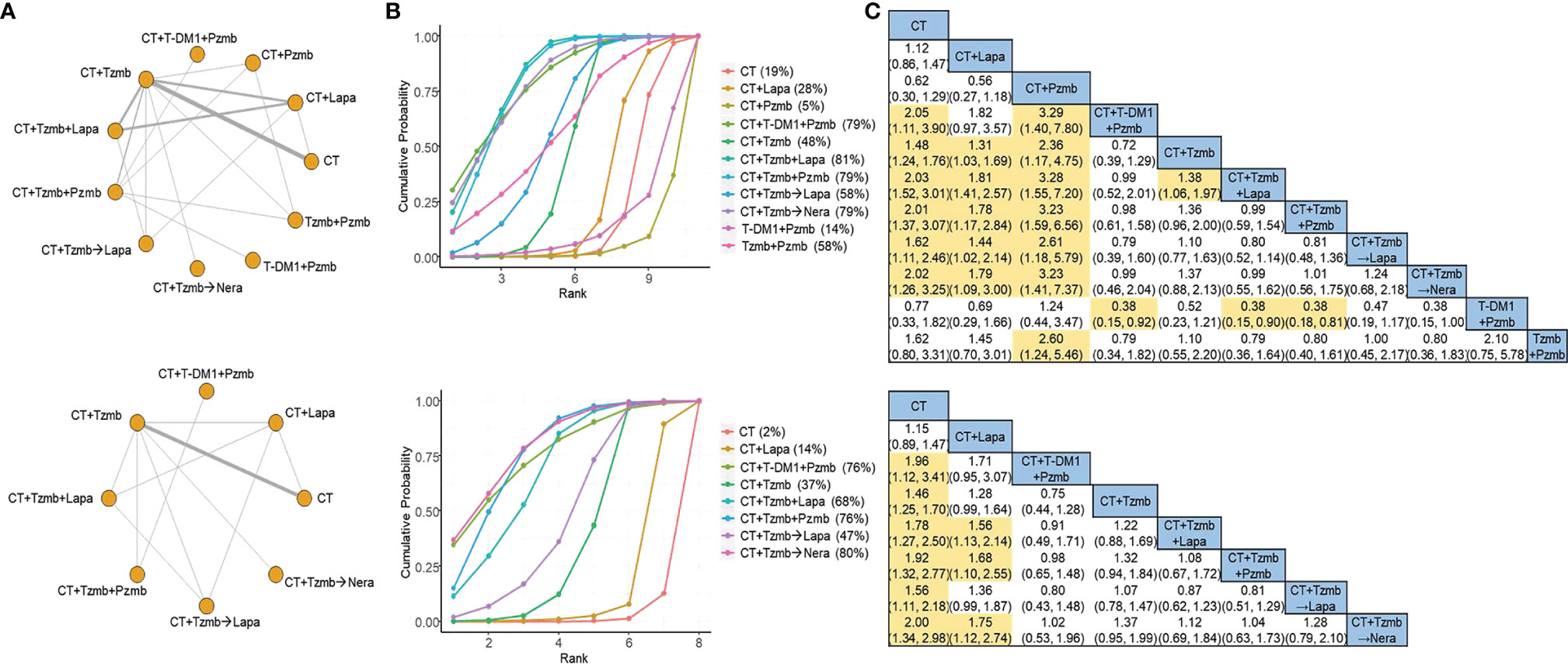
Figure 2 Network meta-analysis for disease-free survival. (A) Network plots for disease-free survival in the overall analysis (top panel) and in the parallel analysis (bottom panel). (B) Ranking probabilities of surface under the cumulative ranking curve for disease-free survival in the overall analysis (top panel) and in the parallel analysis (bottom panel). (C) Cross-comparison odds ratios and their corresponding 95% confidence intervals for disease-free survival in the overall analysis (top panel) and in the parallel analysis (bottom panel). CT, chemotherapy; Lapa, lapatinib; Nera, neratinib; Pzmb, pertuzumab; T-DM1, trastuzumab emtansine; Tzmb, trastuzumab.
OS network
For OS outcome, a total of 14 studies (7, 8, 20–31) were involved in the overall analysis and 10 studies (7, 8, 22–27, 29, 31) in the parallel analysis, as shown in Figure 3A (top panel) and Figure 3A (bottom panel), respectively. Both of the two analyses indicated that the top two therapies were trastuzumab plus lapatinib with chemotherapy and trastuzumab plus pertuzumab with chemotherapy [SUCRA of 88% and 74%, respectively, in the overall analysis (Figure 3B, top panel) and 84% and 79%, respectively, in the parallel analysis (Figure 3B, bottom panel)]. The cross-comparison OR was 0.88 (95% CI: 0.57 to 1.33) in the overall analysis (Figure 3C, top panel) and 0.96 (95% CI: 0.59 to 1.56) in the parallel analysis (Figure 3C, bottom panel), respectively. No significant heterogeneity was observed in the traditional pair-wise comparisons of OS, and the direct and indirect evidence was generally consistent, as shown in Supplementary Materials 2, 3, respectively.
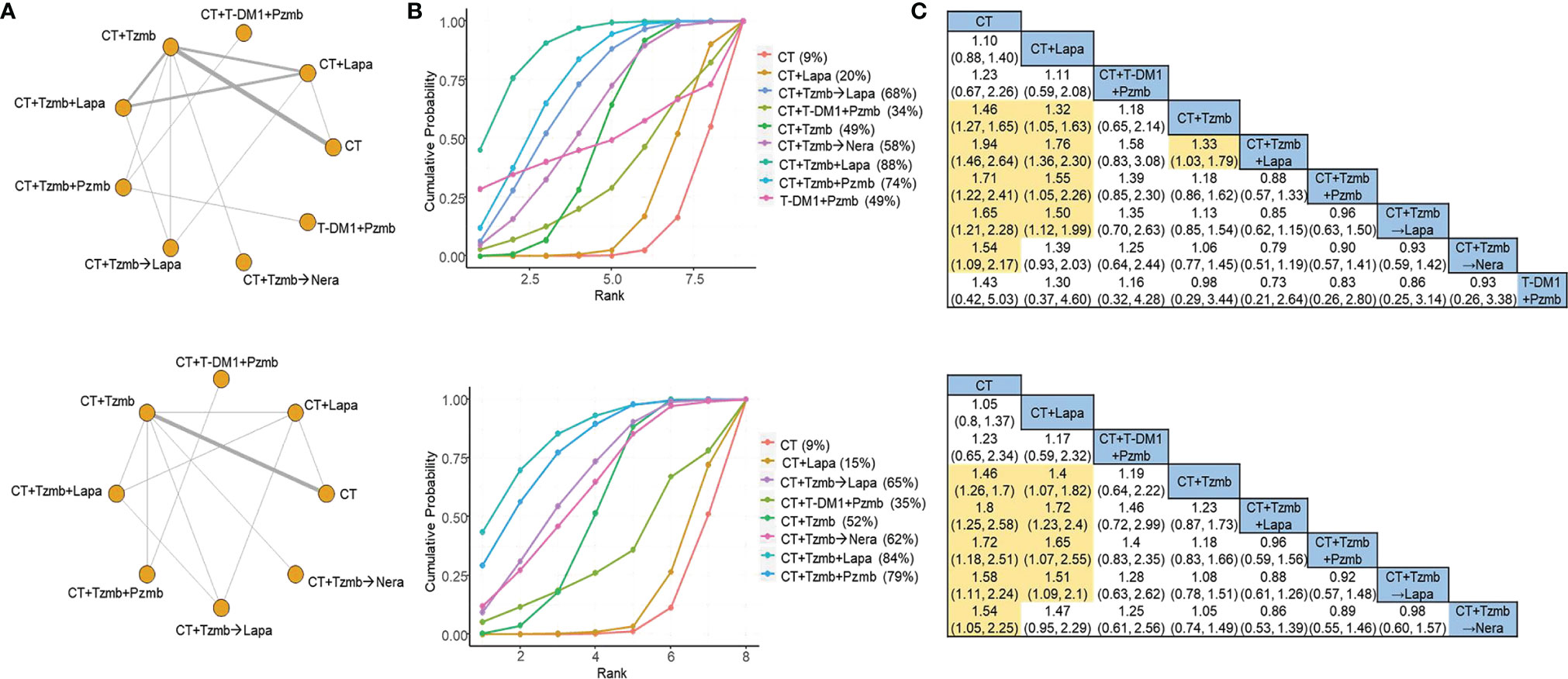
Figure 3 Network meta-analysis for overall survival. (A) Network plots for overall survival in the overall analysis (top panel) and in the parallel analysis (bottom panel). (B) Ranking probabilities of surface under the cumulative ranking curve for overall survival in the overall analysis (top panel) and in the parallel analysis (bottom panel). (C) Cross-comparison odds ratios and their corresponding 95% confidence intervals for overall survival in the overall analysis (top panel) and in the parallel analysis (bottom panel). CT, chemotherapy; Lapa, lapatinib; Nera, neratinib; Pzmb, pertuzumab; T-DM1, trastuzumab emtansine; Tzmb, trastuzumab.
Safety Network
The safety data of cardiac events, neutropenia, febrile neutropenia, diarrhea, vomiting, rash or erythema, hepatobiliary disorders, arthralgia, and fatigue were collected and analyzed in the study. The network and SUCRA plots for the above safety outcomes are shown in Supplementary Materials 4, 5, respectively. The combination of trastuzumab and pertuzumab with or without chemotherapy showed the highest risk of inducing cardiac events, with a SUCRA of 92%. Trastuzumab with sequential neratinib based on chemotherapy had the highest risk of causing diarrhea, vomiting, rash or erythema, and fatigue, with the SUCRA of 94%, 92%, 98%, and 94%, respectively, while chemotherapy plus lapatinib had the highest risk of inducing neutropenia and hepatobiliary, with the SUCRA of 90% and 82%, respectively. Pertuzumab plus chemotherapy was most likely to cause febrile neutropenia, while chemotherapy alone was most likely to cause arthralgia, with the SUCRA of 79% and 74%, respectively.
Sensitivity Analysis
Sensitivity analyses were performed by omitting a single study sequentially, and the results were generally consistent with the primary results (data not shown). Of note, the heterogeneity observed in the comparison of DFS of trastuzumab plus lapatinib with chemotherapy versus lapatinib plus chemotherapy became non-significant when the CALGB 40601 Trial (30) was omitted.
Discussion
With the increase of anti-HER2 therapies and related head-to-head studies, it is vital to perform a comprehensive analysis to integrate relevant data. The previous NMA on HER2-positive breast cancer focused on either only neoadjuvant regimens using pathologic complete response as primary outcome (13, 32) or only adjuvant regimens using OS as primary outcome (33). In our study, however, more recent and complete studies were enrolled to compare regimens in both the neoadjuvant and adjuvant settings, and DFS was set as the primary efficacy indicator, which enhances the reliability of our results.
In the overall analysis that combined both neoadjuvant and adjuvant studies, our ranking results indicated that in terms of inducing DFS, the top two therapies were, in order, the combination of trastuzumab and TKI, and the dual-target anti-HER2 therapy that combines trastuzumab and pertuzumab, both on the basis of chemotherapy. For the neoadjuvant studies, although they had the advantages of longer treatment duration and more comprehensive data (including both the neoadjuvant and adjuvant periods), its primary endpoint was often pathological complete response rather than survival outcomes, which might have caused some bias regarding survival outcomes. Therefore, a parallel analysis that was specific to adjuvant studies was further performed. As a result, the top two regimens for HER2-positive breast cancer in terms of inducing DFS were trastuzumab with sequential lapatinib and the combination of trastuzumab and pertuzumab, both on the basis of chemotherapy. The combination of trastuzumab and TKI showed good efficacy only in the overall survival, and trastuzumab with sequential lapatinib with chemotherapy showed good efficacy only in the parallel analysis. Both of the above two therapies failed to pass the test of both analyses simultaneously, while only trastuzumab plus pertuzumab with chemotherapy succeeded. Therefore, we tended to infer that chemotherapy with dual-target anti-HER2 therapy that combines trastuzumab and pertuzumab was the optimal therapy for HER2-positive early breast cancer. This finding was consistent with the results of a previous NMA, in which therapies containing trastuzumab and pertuzumab were found to be most likely to be the best therapy in terms of achieving pathological complete response (13).
The sensitivity analyses indicated that the observed heterogeneity was mainly induced by the CALGB 40601 Trial. A potential reason might be the long follow-up time of CALGB 40601 Trial (nearly 7 years) (30) than that of the other two trials (less than 5 years) (20, 24). Nevertheless, there are still some limitations of our study. First, although the present NMA mainly focused on survival data, the primary outcome of some enrolled studies was not survival outcome, which, to a certain extent, reduced the statistical efficiency. Second, the definitions of outcomes, the dose and duration of therapies, the baseline chemotherapy regimens, and the follow-up time in the enrolled studies were not completely consistent, which might be a partial source of heterogeneity. Third, due to the limited data, we could not make further subgroup analysis regarding important clinical factors, such as hormone receptor status. Fourth, a total of 11 regimens were compared, but only 15 studies were enrolled; therefore, the majority of pair-wise comparisons was composed of only one or two studies. Fifth, we did not register the NMA prospectively. All the above limitations should be considered with caution when extrapolating our findings.
Collectively, the results suggested that chemotherapy plus dual-target anti-HER2 therapy that combines trastuzumab and pertuzumab was the optimal regimen for HER2-positive breast cancer. The cardiotoxicity of this dual-target therapy should be taken care of, and the cardiac function of patients receiving this therapy is recommended to be regularly reviewed. Studies of a larger scale involving more participants still need to validate our results further.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
Conception and design: K-DY; administrative support: K-DY and Z-MS; provision of study materials or patients: K-DY; collection and assembly of data: Y-WC and K-DY; data analysis and interpretation: Y-WC; manuscript writing: Y-WC and K-DY; final approval of manuscript: all authors. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.919369/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch Pathol Lab Med (2018) 142(11):1364–82. doi: 10.5858/arpa.2018-0902-SA
3. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human Breast Cancer: Correlation of Relapse and Survival With Amplification of the HER-2/Neu Oncogene. Science (New York NY) (1987) 235(4785):177–82. doi: 10.1126/science.3798106
4. Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr., Davidson NE, et al. Trastuzumab Plus Adjuvant Chemotherapy for Operable HER2-Positive Breast Cancer. N Engl J Med (2005) 353(16):1673–84. doi: 10.1056/NEJMoa052122
5. Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab After Adjuvant Chemotherapy in HER2-Positive Breast Cancer. N Engl J Med (2005) 353(16):1659–72. doi: 10.1056/NEJMoa052306
6. Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of Chemotherapy Plus a Monoclonal Antibody Against HER2 for Metastatic Breast Cancer That Overexpresses HER2. N Engl J Med (2001) 344(11):783–92. doi: 10.1056/NEJM200103153441101
7. Cameron D, Piccart-Gebhart MJ, Gelber RD, Procter M, Goldhirsch A, de Azambuja E, et al. 11 Years' Follow-Up of Trastuzumab After Adjuvant Chemotherapy in HER2-Positive Early Breast Cancer: Final Analysis of the HERceptin Adjuvant (HERA) Trial. Lancet (London England) (2017) 389(10075):1195–205. doi: 10.1016/S0140-6736(16)32616-2
8. Perez EA, Romond EH, Suman VJ, Jeong JH, Sledge G, Geyer CE Jr., et al. Trastuzumab Plus Adjuvant Chemotherapy for Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: Planned Joint Analysis of Overall Survival From NSABP B-31 and NCCTG N9831. J Clin Oncol (2014) 32(33):3744–52. doi: 10.1200/JCO.2014.55.5730
9. Lux MP, Nabieva N, Hartkopf AD, Huober J, Volz B, Taran FA, et al. Therapy Landscape in Patients With Metastatic HER2-Positive Breast Cancer: Data From the PRAEGNANT Real-World Breast Cancer Registry. Cancers (2018) 11(1):10. doi: 10.3390/cancers11010010
10. Vernieri C, Milano M, Brambilla M, Mennitto A, Maggi C, Cona MS, et al. Resistance Mechanisms to Anti-HER2 Therapies in HER2-Positive Breast Cancer: Current Knowledge, New Research Directions and Therapeutic Perspectives. Crit Rev Oncol Hematol (2019) 139:53–66. doi: 10.1016/j.critrevonc.2019.05.001
11. Choong GM, Cullen GD, O'Sullivan CC. Evolving Standards of Care and New Challenges in the Management of HER2-Positive Breast Cancer. CA Cancer J Clin (2020) 70(5):355–74. doi: 10.3322/caac.21634
12. Nagayama A, Hayashida T, Jinno H, Takahashi M, Seki T, Matsumoto A, et al. Comparative Effectiveness of Neoadjuvant Therapy for HER2-Positive Breast Cancer: A Network Meta-Analysis. J Natl Cancer Inst (2014) 106(9):dju203. doi: 10.1093/jnci/dju203
13. Zhang J, Yu Y, Lin Y, Kang S, Lv X, Liu Y, et al. Efficacy and Safety of Neoadjuvant Therapy for HER2-Positive Early Breast Cancer: A Network Meta-Analysis. Ther Adv Med Oncol (2021) 13:17588359211006948. doi: 10.1177/17588359211006948
14. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-Analyses of Health Care Interventions: Checklist and Explanations. Ann Internal Med (2015) 162(11):777–84. doi: 10.7326/M14-2385
15. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ (2019) 366:l4898. doi: 10.1136/bmj.l4898
16. Gelman BA. General Methods for Monitoring Convergence of Iterative Simulations. J Comput Graph Stat (1998) 7(4):434–55. doi: 10.1080/10618600.1998.10474787
17. Spiegelhalter DJ, Best NG, Carlin BP, van der Linde A. Bayesian Measures of Model Complexity and Fit. J R Stat Soc Ser B (2002) 64(4):583–639. doi: 10.1111/1467-9868.00353
18. Salanti G, Ades AE, Ioannidis JP. Graphical Methods and Numerical Summaries for Presenting Results From Multiple-Treatment Meta-Analysis: An Overview and Tutorial. J Clin Epidemiol (2011) 64(2):163–71. doi: 10.1016/j.jclinepi.2010.03.016
19. Gianni L, Pienkowski T, Im YH, Tseng LM, Liu MC, Lluch A, et al. 5-Year Analysis of Neoadjuvant Pertuzumab and Trastuzumab in Patients With Locally Advanced, Inflammatory, or Early-Stage HER2-Positive Breast Cancer (NeoSphere): A Multicentre, Open-Label, Phase 2 Randomised Trial. Lancet Oncol (2016) 17(6):791–800. doi: 10.1016/S1470-2045(16)00163-7
20. de Azambuja E, Holmes AP, Piccart-Gebhart M, Holmes E, Di Cosimo S, Swaby RF, et al. Lapatinib With Trastuzumab for HER2-Positive Early Breast Cancer (NeoALTTO): Survival Outcomes of a Randomised, Open-Label, Multicentre, Phase 3 Trial and Their Association With Pathological Complete Response. Lancet Oncol (2014) 15(10):1137–46. doi: 10.1016/S1470-2045(14)70320-1
21. Gianni L, Eiermann W, Semiglazov V, Lluch A, Tjulandin S, Zambetti M, et al. Neoadjuvant and Adjuvant Trastuzumab in Patients With HER2-Positive Locally Advanced Breast Cancer (NOAH): Follow-Up of a Randomised Controlled Superiority Trial With a Parallel HER2-Negative Cohort. Lancet Oncol (2014) 15(6):640–7. doi: 10.1016/S1470-2045(14)70080-4
22. Goss PE, Smith IE, O'Shaughnessy J, Ejlertsen B, Kaufmann M, Boyle F, et al. Adjuvant Lapatinib for Women With Early-Stage HER2-Positive Breast Cancer: A Randomised, Controlled, Phase 3 Trial. Lancet Oncol (2013) 14(1):88–96. doi: 10.1016/S1470-2045(12)70508-9
23. Martin M, Holmes FA, Ejlertsen B, Delaloge S, Moy B, Iwata H, et al. Neratinib After Trastuzumab-Based Adjuvant Therapy in HER2-Positive Breast Cancer (ExteNET): 5-Year Analysis of a Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol (2017) 18(12):1688–700. doi: 10.1016/S1470-2045(17)30717-9
24. Piccart-Gebhart M, Holmes E, Baselga J, de Azambuja E, Dueck AC, Viale G, et al. Adjuvant Lapatinib and Trastuzumab for Early Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: Results From the Randomized Phase III Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization Trial. J Clin Oncol (2016) 34(10):1034–42. doi: 10.1200/JCO.2015.62.1797
25. Joensuu H, Bono P, Kataja V, Alanko T, Kokko R, Asola R, et al. Fluorouracil, Epirubicin, and Cyclophosphamide With Either Docetaxel or Vinorelbine, With or Without Trastuzumab, as Adjuvant Treatments of Breast Cancer: Final Results of the FinHer Trial. J Clin Oncol (2009) 27(34):5685–92. doi: 10.1200/JCO.2008.21.4577
26. D'Hondt V, Canon JL, Roca L, Levy C, Pierga JY, Le Du F, et al. UCBG 2-04: Long-Term Results of the PACS 04 Trial Evaluating Adjuvant Epirubicin Plus Docetaxel in Node-Positive Breast Cancer and Trastuzumab in the Human Epidermal Growth Factor Receptor 2-Positive Subgroup. Eur J Cancer (Oxford Engl 1990) (2019) 122:91–100. doi: 10.1016/j.ejca.2019.09.014
27. Slamon DJ, Eiermann W, Robert NJ, Giermek J, Martin M, Jasiowka M, et al. Ten Year Follow-Up of BCIRG-006 Comparing Doxorubicin Plus Cyclophosphamide Followed by Docetaxel (AC -> T) With Doxorubicin Plus Cyclophosphamide Followed by Docetaxel and Trastuzumab (AC -> TH) With Docetaxel, Carboplatin and Trastuzumab (TCH) in HER2+early Breast Cancer. Cancer Res (2016) 76:2. doi: 10.1158/1538-7445.SABCS15-S5-04
28. Hurvitz SA, Martin M, Jung KH, Huang CS, Harbeck N, Valero V, et al. Neoadjuvant Trastuzumab Emtansine and Pertuzumab in Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: Three-Year Outcomes From the Phase III KRISTINE Study. J Clin Oncol (2019) 37(25):2206–16. doi: 10.1200/JCO.19.00882
29. Krop IE, Im SA, Barrios C, Bonnefoi H, Gralow J, Toi M, et al. Trastuzumab Emtansine Plus Pertuzumab Versus Taxane Plus Trastuzumab Plus Pertuzumab After Anthracycline for High-Risk Human Epidermal Growth Factor Receptor 2-Positive Early Breast Cancer: The Phase III KAITLIN Study. J Clin Oncol (2021) 40(5):438–48\. doi: 10.1200/JCO.21.00896
30. Fernandez-Martinez A, Krop IE, Hillman DW, Polley MY, Parker JS, Huebner L, et al. Survival, Pathologic Response, and Genomics in CALGB 40601 (Alliance), a Neoadjuvant Phase III Trial of Paclitaxel-Trastuzumab With or Without Lapatinib in HER2-Positive Breast Cancer. J Clin Oncol (2020) 38(35):4184–93. doi: 10.1200/JCO.20.01276
31. Piccart M, Procter M, Fumagalli D, de Azambuja E, Clark E, Ewer MS, et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer in the APHINITY Trial: 6 Years' Follow-Up. J Clin Oncol (2021) 39(13):1448–57. doi: 10.1200/JCO.20.01204
32. Nakashoji A, Hayashida T, Yokoe T, Maeda H, Toyota T, Kikuchi M, et al. The Updated Network Meta-Analysis of Neoadjuvant Therapy for HER2-Positive Breast Cancer. Cancer Treat Rev (2018) 62:9–17. doi: 10.1016/j.ctrv.2017.10.009
Keywords: breast cancer, (neo)adjuvant, HER2-positive, disease-free survival, network meta-analysis
Citation: Cai Y-W, Shao Z-M and Yu K-D (2022) Determining the Optimal (Neo)Adjuvant Regimen for Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer Regarding Survival Outcome: A Network Meta-Analysis. Front. Immunol. 13:919369. doi: 10.3389/fimmu.2022.919369
Received: 13 April 2022; Accepted: 26 May 2022;
Published: 30 June 2022.
Edited by:
Pravin Kaumaya, The Ohio State University, United StatesReviewed by:
José Bines, National Cancer Institute (INCA), BrazilChao Ni, Zhejiang University, China
Wenbin Zhou, Nanjing Medical University, China
Yingying Xu, The First Affiliated Hospital of China Medical University, China
Kun Wang, Guangdong Provincial People’s Hospital, China
Copyright © 2022 Cai, Shao and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ke-Da Yu, eXVrZWRhQGZ1ZGFuLmVkdS5jbg==; orcid.org/0000-0002-2883-1282
 Yu-Wen Cai
Yu-Wen Cai Zhi-Ming Shao1,3
Zhi-Ming Shao1,3 Ke-Da Yu
Ke-Da Yu