
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Immunol. , 01 July 2022
Sec. Viral Immunology
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.914498
This article is part of the Research Topic Human T cell Leukemia Virus-1 (HTLV-1) infection, associated pathology and response of the host View all 20 articles
Previous studies have demonstrated the development of pulmonary impairment in individuals infected with human T-lymphotropic virus type 1 (HTLV-1). Complications, such as alveolitis and bronchiectasis, were found in individuals who developed tropical spastic paraparesis/HTLV-1-associated myelopathy (TSP-HAM) due to chronic inflammation. These patients exhibited increased levels of lymphocytes (CD4+ and CD25+), cytokines (IL-2, IL-12, and IFN-γ), inflammatory chemokines (MIP-1α and IP-10), and cell adhesion molecules (ICAM-1) in the bronchoalveolar lavage fluid, with the result of chronic inflammation and lung injury. The main lesions observed at Chest high-resolution computed tomography were centrilobular nodules, parenchymal bands, lung cysts, bronchiectasis, ground-glass opacity, mosaic attenuation, and pleural thickening. It can lead to progressive changes in pulmonary function with the development of restrictive and obstructive diseases. Recent studies suggest a causal relationship between HTLV-1 and pulmonary diseases, with intensification of lesions and progressive decrease in pulmonary function. This summary updates a previous publication and addresses the general lack of knowledge regarding the relationship between TSP-HAM and pulmonary disease, providing direction for future work and the management of these individuals.
Human T-lymphotropic virus type 1 (HTLV-1) is a retrovirus with an incidence of approximately 20 million worldwide, with a higher prevalence in Africa, Japan, and America (1). In Latin America, Brazil has a high prevalence, mainly in the states of Maranhão, Bahia and Pará (2, 3). The virus is the etiological agent of tropical spastic paraparesis/HTLV-1-associated myelopathy (TSP-HAM) and adult T-cell lymphoma (ATL) (4).
There is a relationship between HTLV-1 and pulmonary diseases in individuals with TSP/HAM, these individuals exhibit pulmonary diseases with characteristics of lymphocytic inflammatory infiltrates (5–8). Individuals with ATL develop pneumopathies caused by opportunistic infections due to ATL cell proliferation, which leads to a low expression of naive T cells, increased expression of FoxP3+ and interleukin-10 (IL-10), and an increased number of Treg cells (CD4+ and CD25+), which suggests the development of immunodeficiency (9). Furthermore, HTLV-1 carriers, because of a mild immunodeficiency characterized by a low expression of IL-1b and IL-17 interleukins (10) have a higher risk of infection with Mycobacterium tuberculosis (11, 12), high mortality rates, and an increased likelihood of hospitalization for pulmonary tuberculosis (13) (Figure 1).
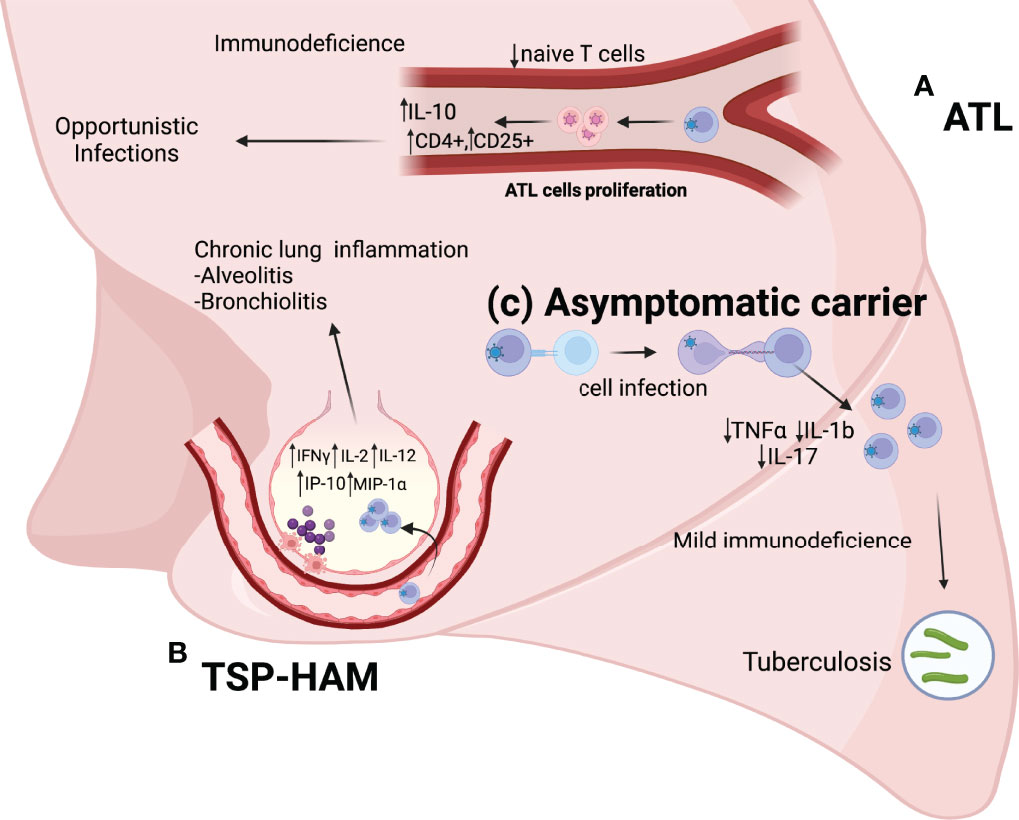
Figure 1 (A) ATL: ATL cell proliferation leads to a reduction in the number of naive T cells, a increase in the number of T reg cells (CD4+ and CD25+), and increased expression of anti-inflammatory cytokines (IL-10). Pneumopathies in these individuals are mainly caused by opportunistic diseases. (B) TSP-HAM: The high expression of inflammatory cytokines (IFNγ, IL-2, and IL-12) and chemokines (IP-10 and MIP-1α) results in an exacerbated immune response and chronic lung inflammation (Alveolitis and bronchiolitis). (C) HTLV-1 asymptomatic carriers: The low expression of TNFα, IL-17, and IL-1b causes mild immunodeficiency in these individuals, with a higher risk of infection by Mycobacterium tuberculosis.
TSP-HAM individuals have a major risk to development of lung injuries, being the major radiological findings bronchiectasis, centrilobular nodules, and ground-glass opacities (14–16); lesions are attributable to chronic inflammation resulting from the effects of the virus in situ (17–20). Lung inflammation may be the causal agent of lung volume obstruction, flow limitation, and the development of restrictive and obstructive lung diseases in TSP-HAM patients (17, 19, 21).
Recent publications, including a systematic review and a cohort study developed by our research group, have suggested a causal relationship between HTLV-1 infection, the development of lung injury (20, 22), and the evolution of lung disease in HTLV-1 infected individuals (23). This scientific literature review aims to update our previous publication (19) with these recent findings on HTLV-1 pulmonary disease and the existing lack of knowledge regarding the effects of this infection on the respiratory system.
The chronic pulmonary inflammation in TSP-HAM individuals can be caused by an exacerbated immune response. The elevation of T lymphocytes in the bronchoalveolar lavage fluid (BALF) of HTLV-1 individuals pulmonary involvement is characterized by a cytokine storm, with high expression of soluble IL-2 receptors (IL-2R), as well as, interleukins (IL-2, IL-12), and interferon (IFN-γ) (8, 24, 25).
A selective T-cells infiltration occurs in the lungs, with an accumulation of HTLV-1-specific CD8+ T cells in BALF, and the occurrence of specific immune responses in lung tissues (7, 26). The high-expression of lymphocites, and its interaction with cytokines (IL-2, IL-12 and IFN-y) and chemokines (MIP-1a and IP-10) leads to chronic pulmonary inflammation and lung injury (25, 27). It is known that HTLV-1 infection induces an abnormal frequency and phenotype of FoxP3+CD4+T cells (28). The higher expression of Foxp3 mRNA in the BALF of patients with HTLV-1-related lung diseases suggests the involvement of regulatory T cells in the pathogenesis of lung injuries (8).
TSP-HAM individuals exhibit alveolitis, a high proviral load (29), and increased levels of cytokines and inflammatory chemokines in the BALF in comparison to asymptomatic carriers (8, 25, 27, 30). The lymphocytosis in the lungs results in a higher expression of proinflammatory cytokines (31–33).
Lymphocytosis and the presence of HTLV-1 provirus in the BALF (7), elevated levels of macrophage inflammatory protein (MIP-1α), interferon g-induced protein kDa (IP-10), and chemokines are linked with the activation and recruitment of inflammatory cells (30, 34). The pulmonary epithelium expresses intercellular adhesion molecule-1 (ICAM-1), a chemokine that facilitates the adhesion of neutrophils to cells of the respiratory epithelium (30, 34) and induces lung tissue injury and chronic inflammation in situ (16) (Figure 2).
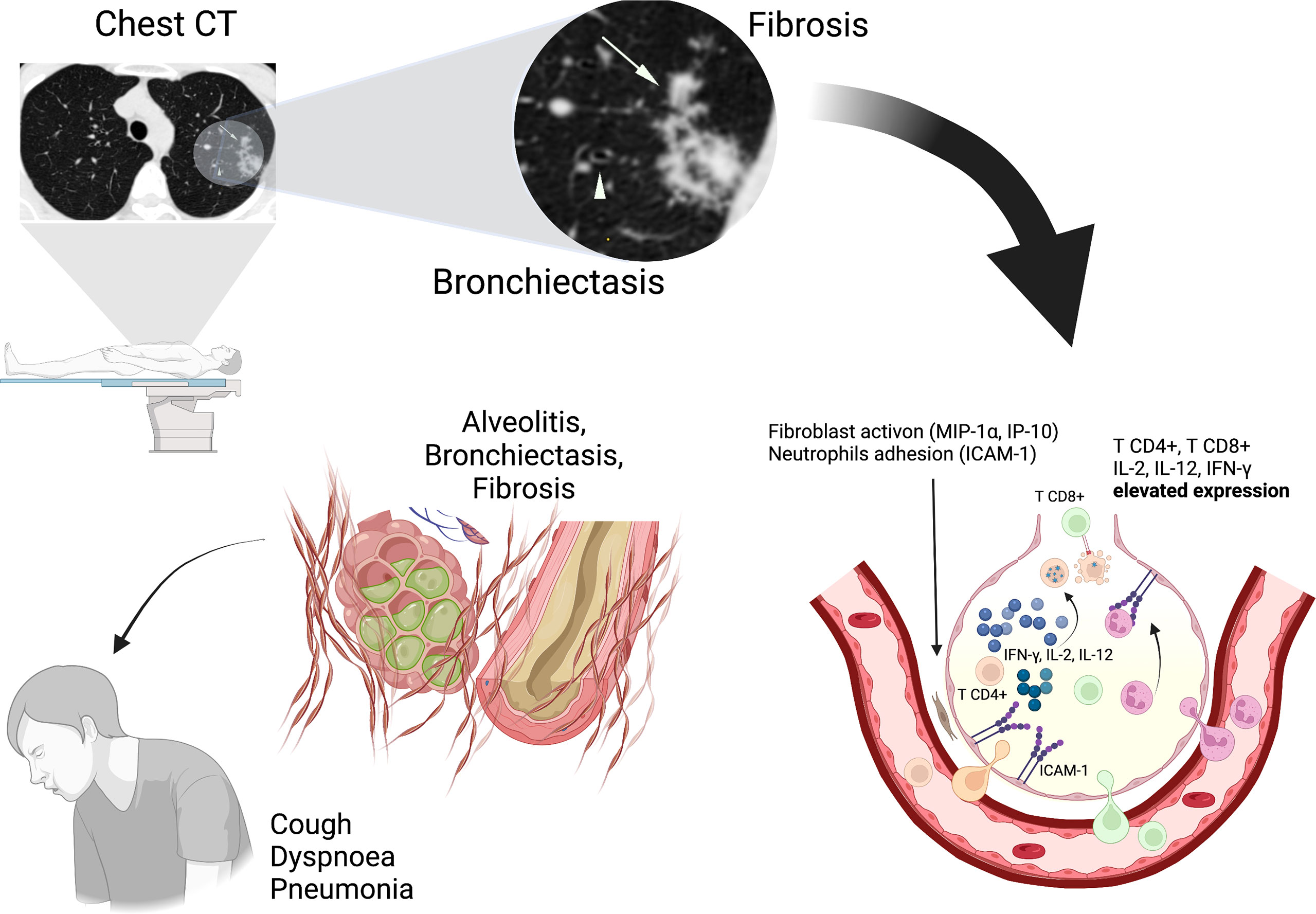
Figure 2 Pulmonary inflammation involves the interaction between cytokines (IL-2, IL-12, and IFN-γ) and chemokines (MIP-1α and IP-10) with HTLV-1 infected CD4+ T cells resulting in lung injuries (alveolitis, bronchiectasis). ICAM-1 facilitates the adhesion of neutrophils and potentiates the chronic inflammation. Chest CT imaging shows Bronchiectasis (Arrowhead) that indicates enlargement and deformation of airway and Centrilobular nodule (Arrows) that indicates Fibrosis, bronchiolitis and alveolitis at sites of injury. These alterations determine a chronic evolution with presence of symptoms (Cough, Dyspnoea, Pneumonia).
The development of lung injuries, mainly bronchiectasis and centrilobular nodules are related to alveolitis and bronchiolitis (27, 35). These lung injuries cause scarring in the lung tissue and fibrosis, which can induce traction bronchiectasis in a cycle of chronic lung injury (17). TSP-HAM individuals have a bronchiectasis relative risk of 8.4 (95% CI 2.7-26.1, p = 0.0002) in comparison to asymptomatic carriers and other HTLV-1 related diseases (16).
Other imaging findings reinforce the existence of a causal relationship between pulmonary diseases and HTLV-1; the centrilobular nodules indicate peripheral bronchiolitis and alveolitis at sites of injury, probably due to lymphocytosis (7). Ground-glass opacity is characteristic of pneumonia and has a higher prevalence among patients with HTLV-1 than in the general population (15).
Chest high-resolution computed tomography is the gold standard method to observe lung injuries. Previous studies have shown that the characteristic lesions observed in HTLV-1 infected individuals are bronchiectasis (8, 16, 17, 24, 25, 27), bronchiectasis is characterized by bronchial dilatation (36). Other lung injuries, such as centrilobular nodules, ground-glass opacity, pleural thickening, and parenchymal bands, were also found (14, 15, 17, 36) (Figure 2).
The studies about HTLV-1 related lung diseases shows that these abnormal CT findings are more common in TSP-HAM individuals than asymptomatic carriers (16, 17, 23), their higher frequency of lung injury can be explained by their major in situ inflammatory processes (8, 17, 24, 25, 27) and is associated with high HTLV-1 proviral load (37, 38). These individuals also exhibit three or more lesions types, and a combination between bronchiectasis and other lesions in HRCT, such as pleural thickening, parenchymal bands, interlobular septum thickening, centrilobular nodules, and parenchymal bands (17). A follow-up study shows the intensification of these lesions, and an increase in the frequency of four types: ground-glass opacity, bronchiectasis, centrilobular nodules, and pleural thickening between TSP-HAM individuals previous evaluated (23).
Individuals with TSP-HAM can develop changes in pulmonary function, due to pulmonary inflammation and lung lesions, which may progress to obstructive or restrictive lung disease (17, 21). An analysis of pulmonary function in these individuals showed a reduction in vital capacity (VC) and forced expiratory volume in one second (FEV1), these alterations are related to restrictive lung disease, and airway obstruction, respectively (17).
Other findings were a reduction in peak expiratory flow, which is very sensitive in most diseases that affect the lungs, alteration in the 50% Forced expiration flow (FEF50%), common alteration in the early stages of obstructive lung disease, and reduction in 25-75% Final Expiratory Flow (FEF 25-75), that is linked to histological changes in the peripheral airways and obstruction (17, 21, 23).
Finally, a reduction in maximum voluntary ventilation (MVV) was observed (17, 21, 23). Changes in MVV may be present both in diseases that affect the lungs and in adverse conditions that alter the mobility of the rib cage (39). HTLV-1 individuals tend to have decreased lung values and this may be related to the development of motor changes related to myelopathy associated with TSP-HAM (17).
The downward trend in VC, FVC, and FEV1, with the maintenance of a normal ratio of forced expiratory volume in one second to forced vital capacity (FEV1/FVC) values, may indicate the development of restrictive lung disease; however, this restriction must be confirmed by measuring lung values and documenting total lung capacity below normal limits (40). The MVV measure is related to the level of physical activity in daily life and is applied to individuals with chronic obstructive pulmonary disease (41). Abnormal CT findings, with airway and lung scarring lesions observed in HTLV-1 individuals, associated with the low mobility that affects patients with TSP-HAM may play a key role in pulmonary function changes (17).
A follow-up study showed a decrease in lung function related to lung injuries observed by chest CT; the patient group with lung injury showed a tendency of decline in VC, FVC, FEV1, FEF25-75%, and MVV values (23). As shown in previous studies, lung injury and altered lung function are more common in TSP-HAM individuals (17, 21), with a major degree of lung involvement among those who developed TSP-HAM. It is possible that bronchiectasis and pleural thickening play key roles in the development of obstructive and restrictive lung disease, respectively (17).
The studies with Chest CT imaging shows that lung lesions are more common in TSP-HAM patients than asymptomatic individuals, suggesting that lesions at the pulmonary level follow the systemic inflammatory process. HTLV-1 infection is a systemic inflammatory disease characterized by chronic evolution. Observational studies conducted on these individuals do not allow for the determination of the pathophysiological mechanisms and their links to specific clinical presentations of patients infected with HTLV-1.
The development of lung lesions in HTLV-1 infected individuals has been described in several studies, but some points, such as the actual mechanism of action of the virus in the pulmonary system, the role of epigenetic factors and inflammatory imbalance in lung injury, and the death rate among those infected, remain unclear. These studies have a limited scope and describe only isolated clinical cases. They do not answer the question about the evolution and physiopathology of HTLV-1-related pulmonary disease.
There are a few prospective studies, such as follow-up and case-control studies, but they suggest a progressive characteristic of HTLV-1 pulmonary disease, and more studies are necessary to better understand the mechanisms of pulmonary involvement. Screening of these patients is very important to show the evolution of chronic inflammation at the pulmonary level, parenchymal lesions, and the development of new lung lesions in individuals with TSP-HAM. Periodic pulmonary evaluation is needed to improve the clinical management of these individuals. This review intends to update a review previously published by our research group, contributing to providing directions for future investigations.
AD, LF, and JQ contributed to conception and design of the study. AD, and LF wrote the sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to thank the support of Federal University of Pará for this publication through the “Programa de Apoio a Publicação Qualificada”.
1. Gessain A, Cassar O. Epidemiological Aspects and World Distribution of HTLV-1 Infection. Front Microbiol (2012) 3:1. doi: 10.3389/fmicb.2012.00001
2. Catalan-Soares B, Carneiro-Proietti ABF, Proietti FA, Interdisciplinary HTLV Research Group. Heterogeneous Geographic Distribution of Human T-Cell Lymphotropic Viruses I and II (HTLV I/II): Serological Screening Prevalence Rates in Blood Donors From Large Urban Areas in Brazil. Cad Saúde Pública (2005) 21:926–31. doi: 10.1590/s0102-311x2005000300027
3. Silva IC, Pinheiro BT, Nobre AFS, Coelho JS, Pereira CCC, Ferreira LSC, et al. Moderate Endemicity of the Human T-Lymphotropic Virus Infection in the Metropolitan Region of Belém, Pará, Brazil. Rev Bras Epidemiol (2018) 21:e180018. doi: 10.1590/1980-549720180018
4. Richardson JH, Edwards J, Cruickshank JK, Rudge P, Dalgleish AG. In Vivo Cellular Tropism of Human T-Cell Leukemia Virus Type 1. J Virol (1990) 64:5682–7. doi: 10.1128/jvi.64.11.5682-5687.1990
5. Sugimoto M, Nakashima H, Watanabe S, Uyama E, Tanaka F, Ando M, et al. T-Lymphocyte Alveolitis in HTLV-1 Associated Myelopathy. Lancet (1987) 2:1220. doi: 10.1016/S0140-6736(87)91362-6
6. Teruya H, Tomita M, Senba M, Ishikawa C, Tamayose M, Miyazato A, et al. Human T-Cell Leukemia Virus Type I Infects Human Lung Epithelial Cells and Induces Gene Expression of Cytokines, Chemokines and Cell Adhesion Molecules. Retrovirology (2008) 5:1–10. doi: 10.1186/1742-4690-5-86
7. Kawabata T, Higashimoto I, Takashima H, Izumo S, Kubota R. Human T-Lymphotropic Virus Type I (HTLV-I) Specific CD8+ Cells Accumulate in the Lungs of Patients Infected With HTLV-I With Pulmonary Involvement. J Med Virol (2012) 84:1120–7. doi: 10.1002/jmv.23307
8. Nakayama Y, Yamazato Y, Tamayose M, Atsumi E, Yara S, Higa F, et al. Increased Expression of HBZ and Foxp3 mRNA in Bronchoalveolar Lavage Cells Taken From Human T-Lymphotropic Virus Type 1-Associated Lung Disorder Patients. Intern Med (2013) 52:2599–609. doi: 10.2169/internalmedicine.52.0845
9. Taylor GP, Matsuoka M. Natural History of Adult T Cell Leukemia/Lymphoma and Approaches to Therapy. Oncogene (2005) 24:6047–57. doi: 10.1038/sj.onc.1208979
10. Carvalho NB, Bastos ML, Souza AS, Netto EM, Arruda S, Santos SB, et al. Impaired TNF, IL-1β, and IL-17 Production and Increased Susceptiblity to Mycobacterium Tuberculosis Infection in HTLV-1 Infected Individuals. Tuberculosis (2018) 108:35–40. doi: 10.1016/j.tube.2017.10.004
11. Marinho J, Galvão-Castro B, Rodrigues LC, Barreto ML. Increased Risk of Tuberculosis With Human T-Lymphotropic Virus-1 Infection: A Case Control Study. J Acquir Immune Defic Syndr (2005) 40:625–8. doi: 10.1097/01.qai.0000174252.73516.7a
12. Grassi MFR, dos Santos NP, Lirio M, Kritski AL, Almeida MCC, Santana LP, et al. Tuberculosis Incidence in a Cohort of Individuals Infected With Human T-Lymphotropic Vírus Type 1 (HTLV-1) in Salvador, Brazil. BMC Infect Dis (2016) 16:491. doi: 10.1186/s12879-016-1428-z
13. Bastos ML, Santos SB, Souza A, Finkmoore B, Bispo O, Barreto T, et al. Influence of HTLV-1 on the Clinical, Microbiologic and Immunologic Presentation of Tuberculosis. BMC Infect Dis (2012) 12:199. doi: 10.1186/1471-2334-12-199
14. Okada F, Ando Y, Yoshitake S, Yotsumoto S, Matsumoto S, Wakisaka M, et al. Pulmonary CT Findings in 320 Carriers of Human T-Lymphotropic Virus Type 1. Radiology (2006) 240:559–64. doi: 10.1148/radiol.2402050886
15. Yamashiro T, Kamiya H, Myiara T, Gibo S, Ogawa K, Akamine T, et al. CT Scans of the Chest in Carriers of Human T-Cell Lymphotropic Virus Type 1: Presence of Interstitial Pneumonia. Academ Radiol (2012) 19:952–7. doi: 10.1016/j.acra.2012.03.020
16. Honarbakhsh S, Taylor GP. High Prevalence of Bronchiectasis is Linked to HTLV 1-Associated Inflammatory Disease. BMC Infect Dis (2015) 15:1–7. doi: 10.1186/s12879-015-1002-0
17. Falcão LFM, Falcão ASC, Sousa RCM, Vieira WB, Oliveira RTM, Normando VMF, et al. CT Chest and Pulmonar Functional Changes in Patients With HTLV Associated Myelopathy in the Eastern Brazilian Amazon. PloS One (2017) 12:e0186055. doi: 10.1371/journal.pone.0186055
18. Einsiedel L, Pham H, Wilson K, Walley R, Turpin J, Bangham C, et al. Human T-Lymphotropic Virus Type 1c Subtype Proviral Loads, Chronic Lung Disease and Survival in a Prospective Cohort of Indigenous Aus- Tralians. PloS Negl Trop Dis (2018) 12:e0006281. doi: 10.1371/journal.pntd.0006281
19. Dias ARN, Falcão LFM, Falcão ASC, Normando VMF, Quaresma JAS. Human T Lymphotropic Virus and Pulmonary Diseases. Front Microbiol (2018) 9:1879. doi: 10.3389/fmicb.2018.01879
20. Normando VMF, Dias ARN, Silva ALSE, Pinto DS, Santos MCS, Rodrigues CL, et al. HTLV-I Induces Lesions in Pulmonar System: A Systematic Review. Life Sci (2020) 256:117979. doi: 10.1016/j.lfs.2020.117979
21. Normando VMF, Falcão LFM, Vieira WB, Oliveira R, Santos M, Fuzii H, et al. Changes in Lung Function in Patients With Human T-Cell Lymphotropic Virus (HTLV) Associated Myelopathy Residents in the Eastern Brazilian Amazon. ERS J (2016) 48:PA4442. doi: 10.1183/13993003.congress-2016.PA4442
22. Einsiedel L, Chiong F, Jersmann H, Taylor GP. Human T-Cell Leukaemia Virus Type 1 Associated Pulmonary Disease: Clinical and Pathological Features of an Under-Recognised Complication of HTLV-1 Infection. Retrovirology (2021) 18:1. doi: 10.1186/s12977-020-00543-z
23. Dias ARN, Vieira WB, Normando VMF, Franco KMVS, Falcão ASC, de Sousa RCM, et al. Computed Tomography With 6-Year Follow- Up Demonstrates the Evolution of HTLV-1 Related Lung Injuries: A Cohort Study. PloS One (2021) 16(12):e0261864. doi: 10.1371/journal.pone.0261864
24. Sugimoto M, Nakashima H, Matsumoto M, Uyama E, Ando M, Araki S. Pulmonary Involvement in Patients With HTLV-1 Associated Myelopathy: Increased Soluble IL-2 Receptors in Bronchoalveolar Lavage Fluid. Am Rev Respir Dis (1989) 139:1329–35. doi: 10.1164/ajrccm/139.6.1329
25. Yamazato Y, Miyazato A, Kawakami K, Yara S, Kaneshima H, Saito A. High Expression of P40tax and Pro-Inflammatory Cytokines and Chemokines in the Lungs of Human T-Lymphotropic Virus Type 1- Related Bronchopulmonary Disorders. Chest (2003) 124:2283–92. doi: 10.1378/chest.124.6.2283
26. Mori S, Mizoguchi A, Kawabata W, Fukunaga H, Usuku K, Maruyama I, et al. Bronchoalveolar Lymphocytosis Correlates With Human T Lymphotropic Viírus Type I (HTLV-1) Proviral DNA Load in HTLV-1 Carriers. Thorax (2005) 60:138–43. doi: 10.1136/thx.2004.021667
27. Nakayama Y, Ishikawa C, Tamaki K, Senba M, Fujita J, Mori N. Interleukin-1 Alpha Produced by Human T-Cell Leukaemia Vírus Type 1- Infected T Cells Induces Intercellular Adhesion Molecule-1 Expression on Lung Epithelial Cells. J Med Microbiol (2011) 60:1750–61. doi: 10.1099/jmm.0.033456-0
28. Satou Y, Utsunomiya A, Tanabe J, Nakagawa M, Nosaka K, Matsuoka M. HTLV-1 Modulates the Frequency and Phenotype of Foxp3+CD4+T Cells in Virus-Infected Individuals. Retrovirology (2012) 9:46. doi: 10.1186/1742-4690-9-46
29. Desgranges C, Bechet JM, Couderc LJ, Caubarrere I, Vernant JC. Detection of HTLV-1 DNA by Polymerase Chain Reaction in Alveolar Lymphocytes of Patients With Tropical Spastic Paraparesis. J Infect Dis (1989) 160:162–3. doi: 10.1093/infdis/160.1.162
30. Matsuyama W, Kawabata M, Mizoguchi A, Iwami F, Wakimoto J, Osame M. Influence of Human T Lymphotrophic Virus Type I on Cryptogenic Fibrosing Alveolitis - HTLV-I Associated Fibrosing Alveolitis: Proposal of a New Clinical Entity. Clin Exp Immunol (2003) 133:397–403. doi: 10.1046/j.1365-2249.2003.02240.x
31. Yamamoto M, Matsuyama W, Oonakahara K, Watanabe M, Higashimoto I, Kawabata M, et al. Influence of Human T Lymphotropic Virus Type I on Diffuse Pan-Bronchiolitis, Clin. Exp Immunol (2004) 136:513–20. doi: 10.1111/j.1365-2249.2004.02485.x
32. Seki M, Kadota JI, Higashiyama Y, Lida K, Iwashita T, Sasaki E, et al. Elevated Levels of Beta-Chemokines in Bronchoalveolar Lavage Fluid (BALF) of Individuals Infected With Human T Lymphotropic Virus Type-1 (HTLV-1), Clin. Exp Immunol (1999) 118:417–22. doi: 10.1046/j.1365-2249.1999.01093.x
33. Tendler CL, Greensberg SJ, Burton JD, Danielpour D, Kim SJ, Blattner WA, et al. Cytokine Induction in HTLV-1 Associated Myelopathy and Adult T Cell Leukemia: Alternate Molecular Mechanisms Underlying Retroviral Pathogenesis. J Cell Biochem (1991) 46:302–11. doi: 10.1002/jcb.240460405
34. Miyazato A, Kawakami K, Iwakura Y, Saito A. Chemokine Synthesis and Cellular Inflammatory Changes in Lungs of Mice Bearing P40tax of Human T-Lymphotropic Virus Type 1. Clin Exp Immunol (2000) 120:113–24. doi: 10.1046/j.1365-2249.2000.01197.x
35. Mortreux F, Gabet AS, Wattel E. Molecular and Cellular Aspects of HTLV-1 Associated Leukemogenesis In Vivo. Leukemia (2003) 17:26–38. doi: 10.1038/sj.leu.2402777
36. Hansell DM, Bankier AA, Macmahon H, Mcloud TC, Mueller NL, Remy J, et al. Fleischner Society: Glossary of Terms for Thoracic Imaging. Radiology (2008) 246:697–722. doi: 10.1148/radiol
37. Einsiedel L, Pham H, Au V, Hatami S, Wilson K, Spelman T, et al. Predictors of non-Cystic Fibrosis Bronchiectasis in Indigenous Adult Residents of Central Australia: Results of a Case-Control Study. ERJ Open Res (2019) 5:00001–2019. doi: 10.1183/23120541.00001-2019
38. Einsiedel L, Pham H, Talukder MRR, Liddle J, Taylor K, Wilson K, et al. Pulmonary Disease Is Associated With Human T-Cell Leukemia Virus Type 1c Infection: A Cross-Sectional Survey in Remote Aboriginal Communities. Clin Infect Dis (2021) 73:e1498–506. doi: 10.1093/cid/ciaa1401
39. Miller MR, Hankinson JATS, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of Spirometry. Eur Respir J (2005) 26:319–38. doi: 10.1183/09031936.05.00034805
40. Haynes JM. Basic Spirometry Testing and Interpretation for the Primary Care Provider. Can J Respir Ther (2018) 54:4. doi: 10.29390/cjrt-2018-017
Keywords: HTLV-1, HAM/TSP, chest CT, pulmonary disease, pulmonary function
Citation: Dias ÁRN, Falcão LFM and Quaresma JAS (2022) An Overview of Human T-Lymphotropic Virus Type 1 Lung Injury. Front. Immunol. 13:914498. doi: 10.3389/fimmu.2022.914498
Received: 06 April 2022; Accepted: 07 June 2022;
Published: 01 July 2022.
Edited by:
Steven Jacobson, National Institute of Neurological Disorders and Stroke (NIH), United StatesReviewed by:
Yoshihisa Yamano, St. Marianna University School of Medicine, JapanCopyright © 2022 Dias, Falcão and Quaresma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juarez Antônio Simões Quaresma, anVhcmV6LnF1YXJlc21hQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.