- 1Department of Hepatopancreatobiliary Surgery, Mengchao Hepatobiliary Hospital of Fujian Medical University, Fuzhou, China
- 2College of Clinical Medicine for Oncology, Fujian Medical University, Fuzhou, China
- 3Department of Radiation Oncology, Fujian Cancer Hospital, Fujian Medical University Cancer Hospital, Fuzhou, China
- 4Department of Hepatopancreatobiliary Surgery, Fujian Cancer Hospital, Fujian Medical University Cancer Hospital, Fuzhou, China
- 5The United Innovation of Mengchao Hepatobiliary Technology Key Laboratory of Fujian Province, Mengchao Hepatobiliary Hospital of Fujian Medical University, Fuzhou, China
Background and Aims: Regardless of great progress in early detection of hepatocellular carcinoma (HCC), unresectable HCC (uHCC) still accounts for the majority of newly diagnosed HCC with poor prognosis. With the promising results of a double combination of transarterial chemo(embolization) and tyrosine kinase inhibitors (TKIs), and TKIs and immune checkpoint inhibitors (ICIs), a more aggressive strategy, a triple combination of transarterial chemo(embolization), TKIs, and ICIs has been tried in the recent years. Hence, we aimed to conduct a systematic review to verify the safety and efficacy of the triple therapy for uHCC.
Methods: PubMed, MedLine, Embase, the Cochrane Library, and Web of Knowledge were used to screen the eligible studies evaluating the clinical efficacy and safety of triple therapy for patients with uHCC up to April 25th 2022, as well as Chinese databases. The endpoints were the complete response (CR), objective response rate (ORR), disease control rate (DCR), conversion rate, progression-free survival (PFS) rate, overall survival (OS) rate, and the incidence of adverse events (AEs).
Results: A total of 15 studies were eligible with 741 patients receiving transarterial chemoembolization (TACE) or hepatic arterial infusion chemotherapy (HAIC) combined with TKIs and ICIs. The pooled rate and 95% confidence interval (CI) for CR, ORR, and DCR were 0.124 (0.069–0.190), 0.606 (0.528–0.682), and 0.885 (0.835–0.927). The pooled rates for PFS at 0.5 years and 1 year were 0.781 (0.688–0.862) and 0.387 (0.293–0.486), respectively. The pooled rates for OS at 1, 2, and 3 years were 0.690 (0.585–0.786), 0.212 (0.117–0.324), and 0.056 (0.028–0.091), respectively. In addition, the pooled rate and 95%CI for the conversion surgery was 0.359 (0.153–0.595). The subgroup analysis of control studies showed that triple therapy was superior to TACE+TKIs, TKIs+ICIs, and TKIs in CR, ORR, and DCR, conversion rate; PFS; and OS. No fatal AEs were reported, and the top three most common AEs were elevated ALT, elevated AST, and hypertension, as well as severe AEs (grading ≥3).
Conclusion: With the current data, we concluded that the triple therapy of TACE/HAIC, TKIs, and ICIs would provide a clinical benefit for uHCC both in short- and long-term outcomes without increasing severe AEs, but the conclusion needs further validation.
Systematic Review Registration: http://www.crd.york.ac.uk/PROSPERO/, Review registry: CRD42022321970.
Introduction
Primary liver cancer is the sixth most common cancer worldwide with approximately 906,000 newly diagnosed patients per year and more than 90% patients having hepatocellular carcinoma (HCC) (1). The prognosis of HCC patients remains far from satisfactory with the median overall survival (OS) of 25–30 months (2, 3). Radical surgery is still the most cost-effective curative treatment for HCC patients, but the majority have lost the chance of surgery, so called “unresectable” HCC (uHCC), mainly due to the absence of symptoms in the early stage of HCC (4–6).
There are no guidelines or consensus on the management of uHCC patients up to now (4–6) because this population is too heterogeneous. For uHCC patients at intermediate stage according to the Barcelona Clinic Liver Cancer stage (BCLC) (6), transarterial chemoembolization (TACE), as a classical modality of transarterial chemo(embolization), is strongly recommended with the overall objective response rate (ORR) beyond 50% (7, 8). In the recent years, another modality of transarterial chemo(embolization), hepatic arterial infusion chemotherapy (HAIC), has been identified as non-inferior to TACE in the management of HCC, and particularly, the advantage of HAIC over TACE has been verified among those with macrovascular invasion (9, 10). Nonetheless, the prognosis of patients receiving TACE/HAIC remains poor with the median progression-free survival (PFS) of 2.8–9.6 months and most of the patients will get resistant after repeated TACE (9, 11). For advanced uHCC patients including those with extrahepatic metastasis, systemic therapy is the preferred strategy (12, 13). With the advent of the novel tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors (ICIs), sorafenib is not the only option for advanced HCC (14, 15). In addition, the double-combination modality of systemic therapy, such as atezolizumab and bevacizumab (15), lenvatinib and pembrolizumab (16), durvalumab and tremelimumab (17), apatinib and camrelizumab (18), and novolumab and ipimumab (19), have exhibited promising results with manageable toxicity. However, the objective response rate (ORR) of systemic therapy is still poor, and the time to response might be too long.
The combination of locoregional and systemic treatments is another option for uHCC (3). In theory, locoregional treatment, such as TACE or HAIC, is efficient to achieve satisfactory local control (LC); however, it has not always translated into a long-term survival benefit. On the other hand, systemic therapy is the key to improve the long-term prognosis, but unsatisfactory LC will impair the long-term survival advantage. Preclinical studies have identified the synergistic effect of TACE/HAIC and systemic therapy (20, 21), which was also confirmed in practice of the combination of TACE and sorafenib (22), TACE and lenvatinib (23), HAIC and sorafenib (24), and HAIC plus lenvatinib and toripalimab (25), but it is not the end.
With the publication of the IMbrave 150 trial (15), HCC has entered the era of molecular and immune therapy. It is surprising that approximately 40% patients were found to receive previous TACE before randomization in the IMbrave 150 trial, which shed light on a more aggressive modality, the triple therapy of TACE/HAIC, TKIs, and ICIs. Furthermore, the current strategy is far from enough to satisfy the increasing demands of “conversion therapy” for uHCC. In the past 2 years, the triple therapy of TACE/HAIC, TKIs, and ICIs has been tried with encouraging results (20, 25, 26), but most of the studies were retrospective with a small sample size. Therefore, in this study, we comprehensively reviewed all the literature on the triple therapy of TACE/HAIC, TKIs, and ICIs and aimed to provide substantial clues for the subsequent studies.
Material and Method
This systematic review was conducted according to the preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline, which was also registered at http://www.crd.york.ac.uk/PROSPERO/ (Review registry 321970)
Literature Searching
Using PubMed, MedLine, Embase, the Cochrane Library, and Web of Science, all literature was searched on the triple combination of TACE/HAIC, TKIs, and ICIs for uHCC. The keywords included “primary liver cancer” or “liver tumor” or “hepatocellular carcinoma” or “HCC”, and “transcatheter arterial chemoembolization” or “transarterial chemoembolization” or “hepatic arterial infusion chemotherapy” or “chemotherapy” or “TACE” or “HAIC”, and “tyrosine kinase inhibitors” or “TKIS” and “immune check point inhibitors” or “ICIs” or “programmed cell death protein 1” or “programmed cell death ligand 1” or “PD-1” or “PD-L1” or “B7-H1.” The literature searching began in December 2021, and the last searching was April 25th 2022. Considering that TACE or HAIC is preferred in China, the Chinese database of China National Knowledge Infrastructure(CNKI) and Wanfang were also used to identify the eligible studies.
Selection Criteria
The inclusion criteria were as follows: i) patients diagnosed as HCC by image or biopsy, ii) unresectable after Multi-Disciplinary Team (MDT), and iii) received the triple combination modalities of TACE/HAIC, TKIs, and ICIs, regardless of sequence; iv) endpoints must consist at least one of the following items: complete response (CR), ORR, disease control rate (DCR), PFS, OS, conversion rate, and adverse events (AEs).
The exclusion criteria were as follows: i) combined with other treatment such as ablation or radiation, ii) duplicate report derived from the same cohort, iii) protocol, case reports or reviews, and iv) data unavailable.
Data Acquisition
According to the predefined forms, the information of the eligible studies include the surname of the first author, year of publication, design of the study, and study period. In addition, baseline characteristics in each study (sample size, age, sex, hepatitis B virus infection, Child–Pugh grade, AFP level, tumor number, tumor size, macrovascular invasion, extrahepatic metastasis, BCLC stage, mean PFS, mean OS and regimen of TACE/HAIC, TKIs, and ICIs) were extracted directly by two independent researchers (QK and FX). Endpoints including the CR, ORR, DCR, PFS, OS, conversion rate, and adverse events were obtained directly from the main text or supplementary files and were then cross-validated between the researchers. In case of any discrepancy, an MDT discussion including at least one senior doctor was introduced to reach the final decision. Of note, tumor responses were determined by the modified response evaluation criteria in solid tumors (mRECIST) or RECIST in this study, but we would choose the results evaluated by mRECIST if they were also evaluated by RECIST in each included study.
Quality Assessment
Considering that all the included studies were retrospective, the quality of each eligible study was assessed by the modified Newcastle–Ottawa Scale (NOS) (27). Briefly, the risk of bias was graphically presented as a proportion of all included studies. Evaluating elements included the following: i) whether the study reported a definite definition of the objective; ii) whether a clear triple combination of TACE/HAIC, TKIs, and ICIs was offered (including the technique, regimen, and course of TACE/HAIC and dosage and courses of TKIs and ICIs); iii) whether the criteria of response assessment was provided (i.e., RECIST or mRECIST); and iv) whether there was a clear definition of outcomes including THE CR, ORR, and DCR.
Statistical Analysis
A meta-analysis of the pooled rate was conducted using Rstudio and R (4.1.2); while a comparison analysis between two groups was conducted using RevMan Version 5.3. The pooled rate for the CR, ORR, and DCR and rates of PFS and OS at different time-points was used as an effect size with 95% confidence interval (CI). The χ2 test and I2 statistics were used to evaluate the heterogeneity among the included studies. If P>0.10 and I2<50%, there was no apparent heterogeneity, and the fixed-effect model was used to estimate the effect size; otherwise, the random-effect model was used (28, 29). Sensitivity analysis was carried out by removing each of the included studies one by one to determine the reliability of the results. Subgroup analyses were also conducted to decrease the heterogeneity among the included studies. Publication bias was determined using the funnel plot with Egger’s and Begg’s tests. In this study, a P-value <0.05 was considered significant.
Results
Searching Results
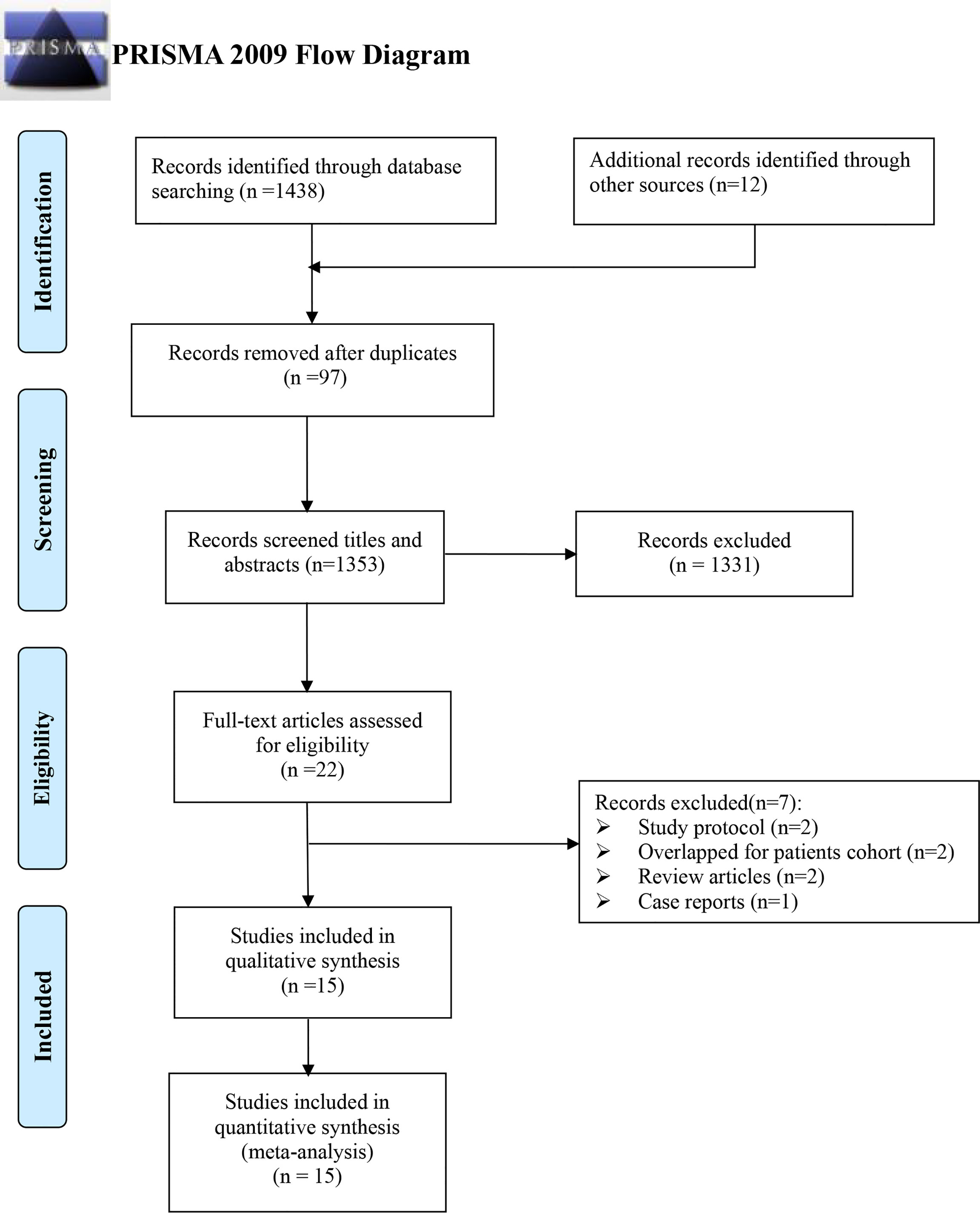
Initially, 1,450 records were identified using an electronic database, including 1,223 written in English and 227 in Chinese, respectively. A total of 97 were excluded by duplicating, 1,353 by screening titles and abstracts, and another 22 more by reading the full text. Finally, 15 records were assessed to be eligible for this meta-analysis (21, 25, 30–42) (Figure 1).
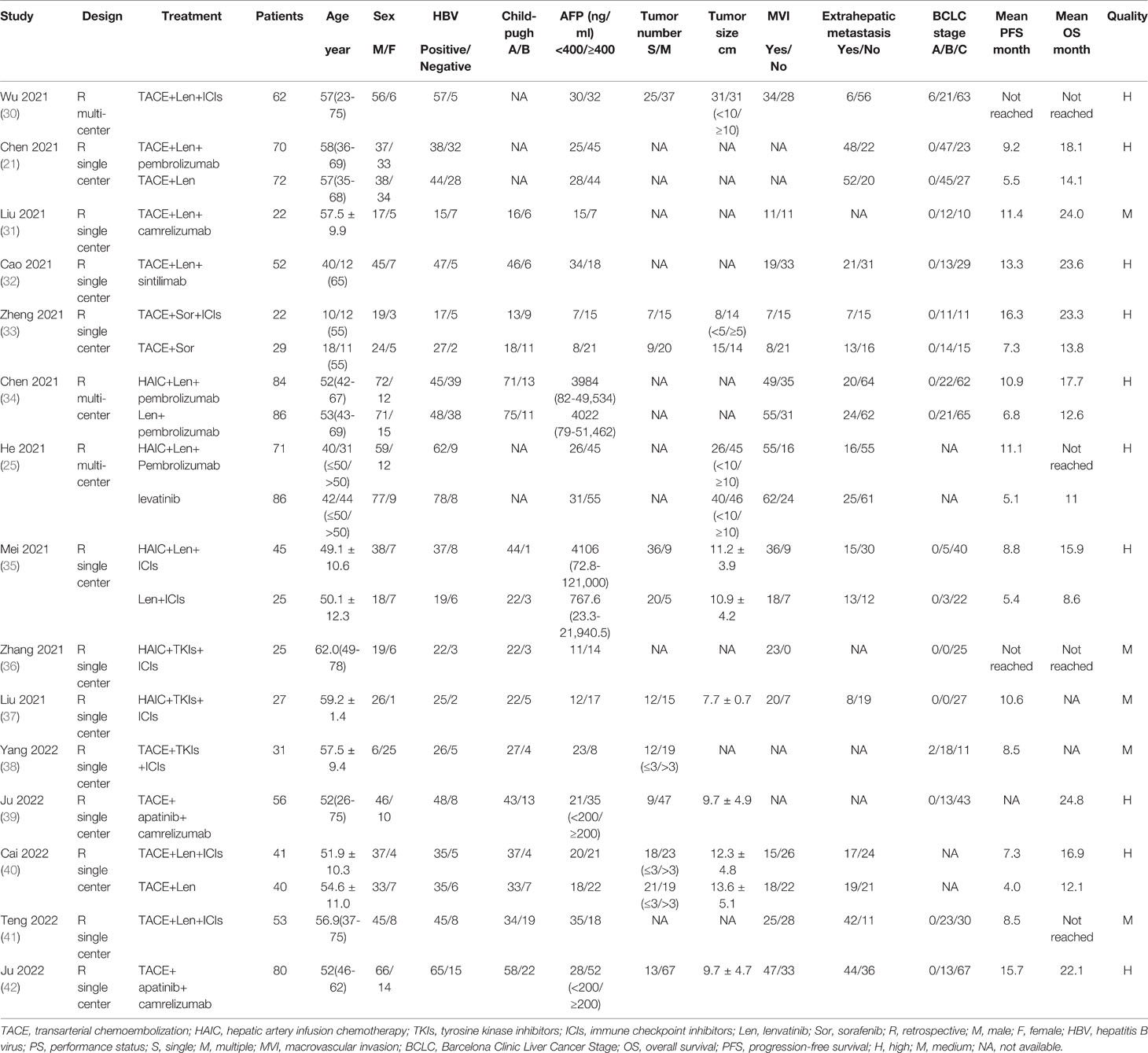
All of the included studies came from China, and all the studies were retrospective, three of which were multi-centered (25, 30, 34). The baseline characteristics in each study were depicted in Table 1, as well as the results of quality assessment. Of note, the baseline characteristics of the control arm in the comparing cohort studies were also depicted in Table 1.
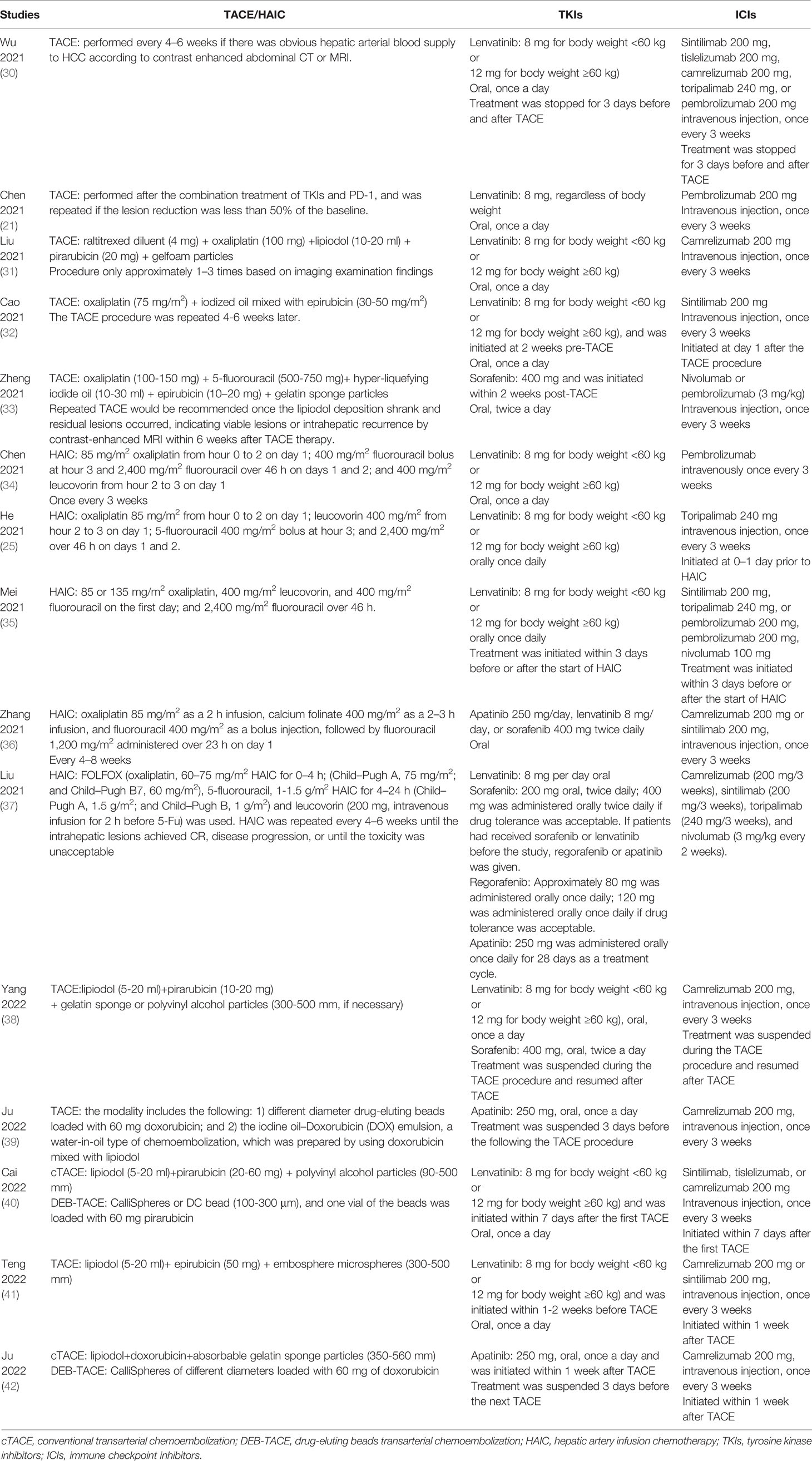
Considering that there was no consensus on the triple combination of TACE/HAIC, TKIs, and ICIs, the scheme in each study was a little different from each other. Table 2 exhibited the detailed information on the treatment scheme, including the technique of TACE/HAIC, drug regimens, and the sequence of local and systemic therapy.
Endpoints
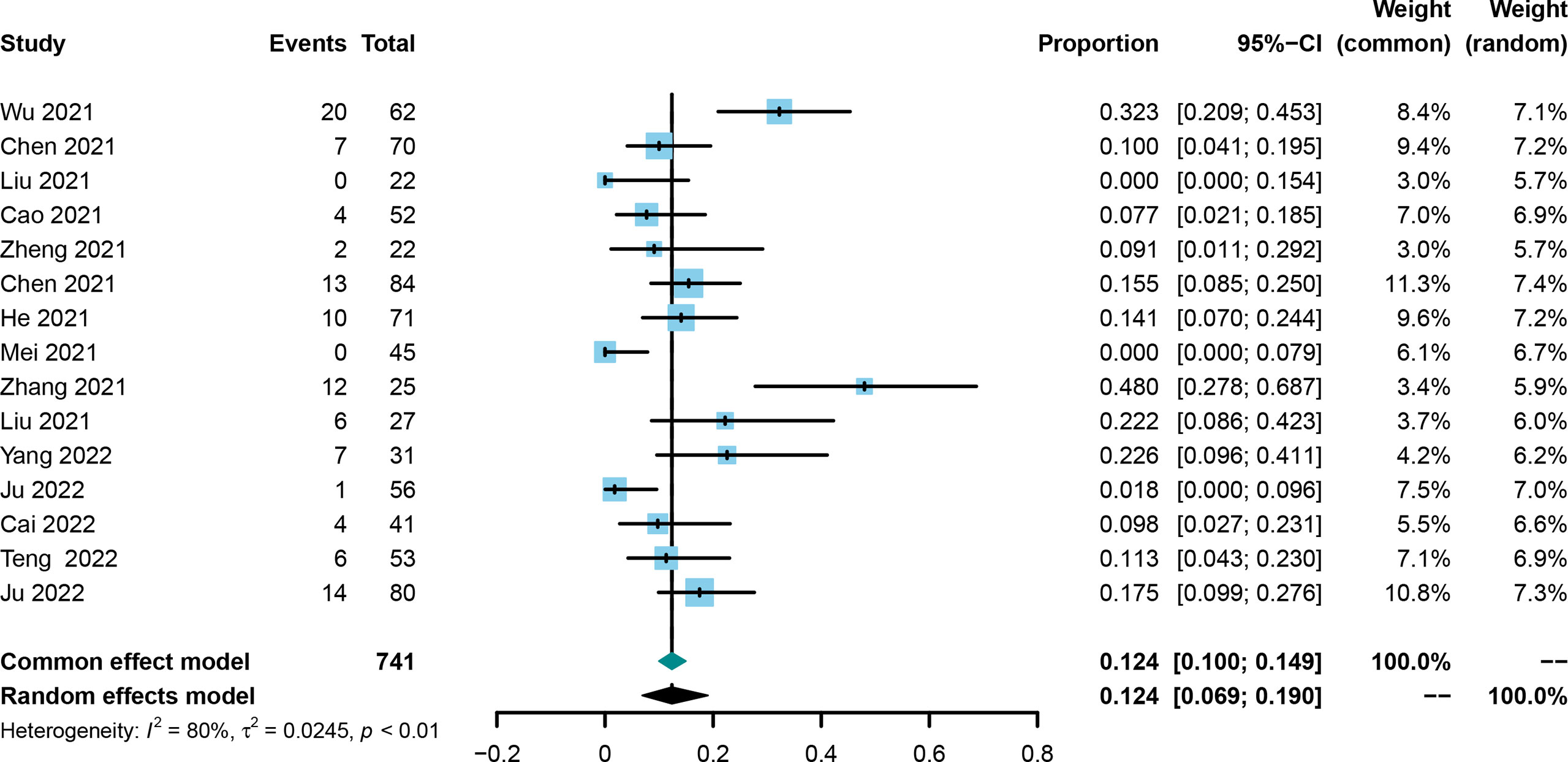
The CR was evaluated in all included trials (21, 25, 30–42), and the corresponding rates ranged from 0% to 48.0%. Using the random effect model, the pooled rate and 95%CI for CR was 0.124 (0.069-0.190, Figure 2). Asymmetry was not observed by the funnel plot (Supplementary Figure 1) with the Egger’s test of 0.9846 and Begg’s test of 0.7662. Sensitivity analysis showed that the results did not change greatly after removing any included single study (Supplementary Figure 2).
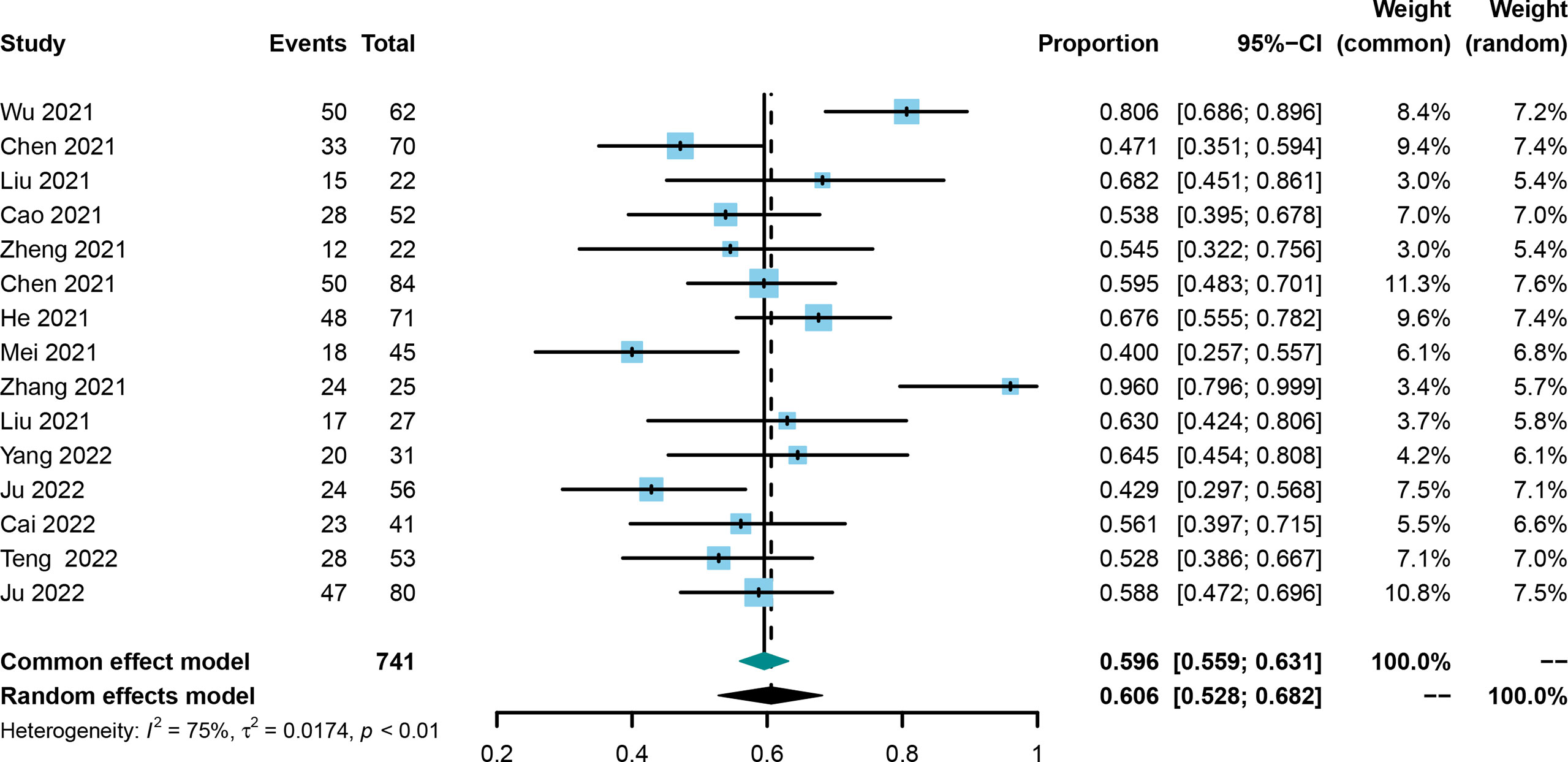
ORR was evaluated in all included trials (21, 25, 30–42), and the pooled ORR and 95% CI was 0.606 (0.528-0.682, Figure 3) using the random effect model. No publication bias was identified using the funnel plot (Supplementary Figure 3) and Egger’s and Begg’s tests (0.4223 and 0.4879, respectively), and the result was not influenced by any one of the included studies (Supplementary Figure 4).
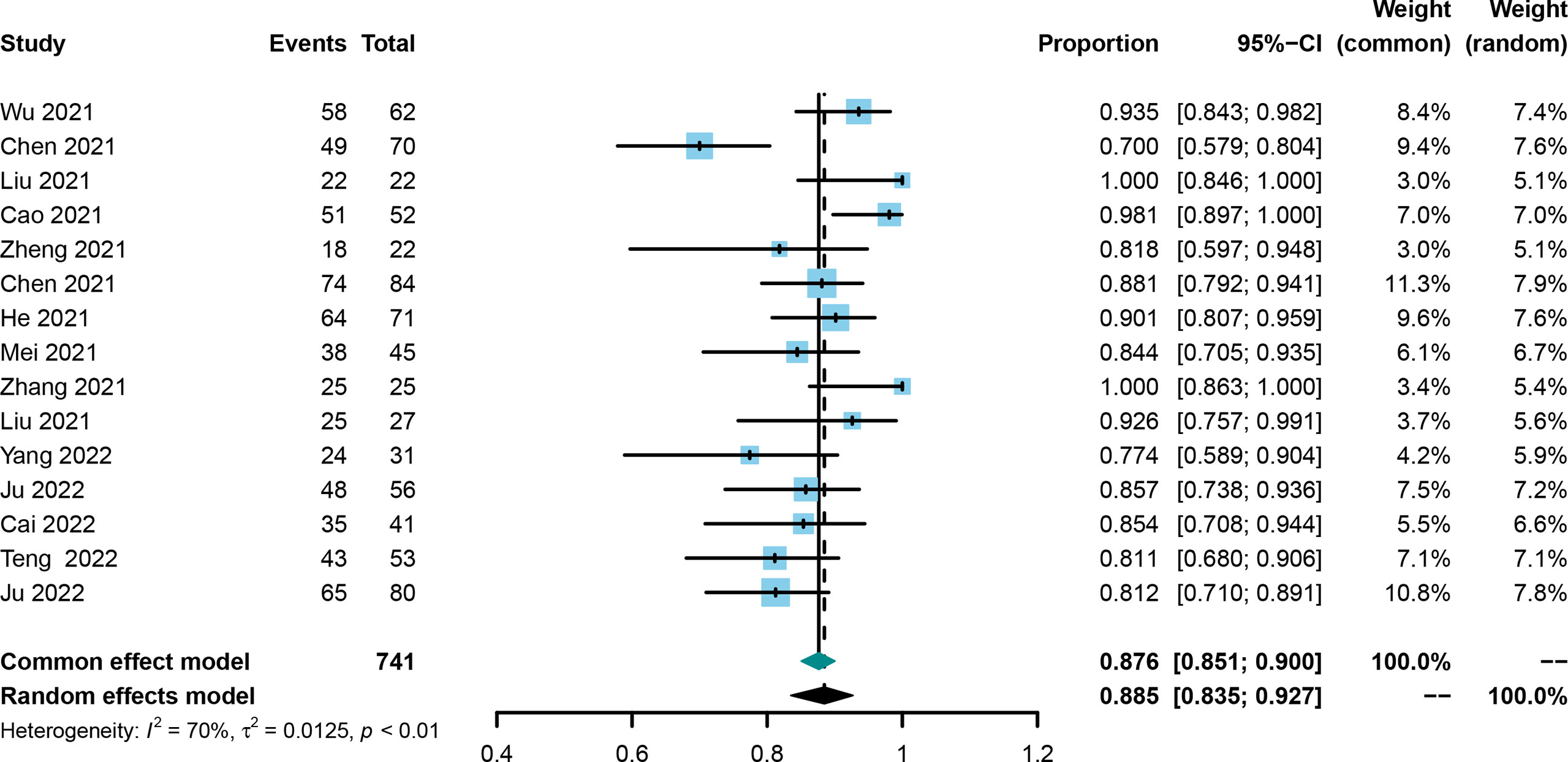
The DCR was also evaluated in all included trials (21, 25, 30–42), and the corresponding rates ranged from 70.0% to 100%. Using the random effect model, the pooled DCR was 0.885 with the 95%CI of 0.835–0.927 (Figure 4). Publication bias was not observed among the included studies (Supplementary Figure 5), and the stability of the result was confirmed by sensitivity analysis (Supplementary Figure 6).
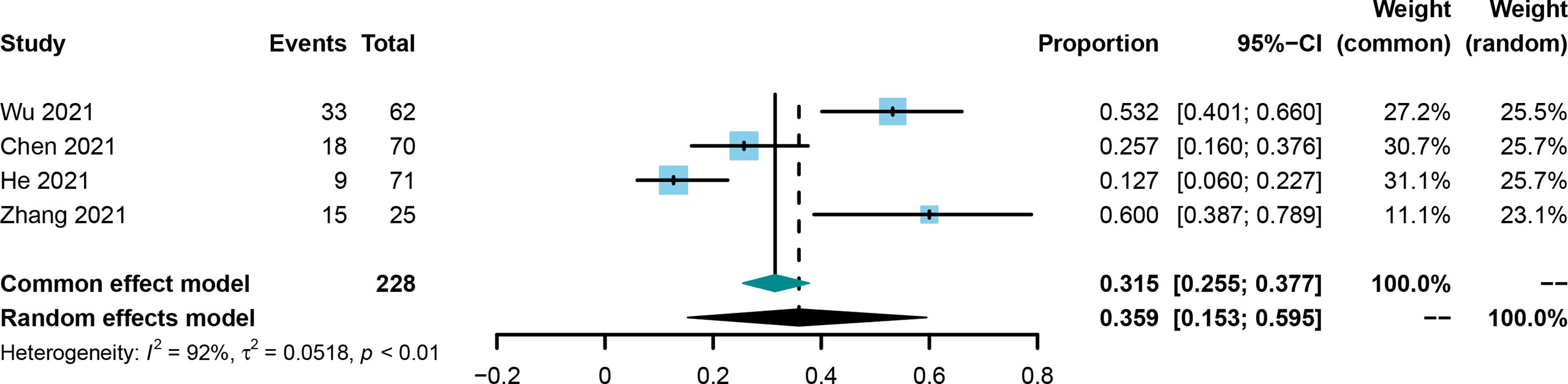
The conversion rate was evaluated in four included studies (21, 25, 30, 36). The conversion rate in the included studies was from 12.7% to 60.0%, and the pooled rate and 95%CI was 0.359 (0.153-0.595, Figure 5) using the random effect model.
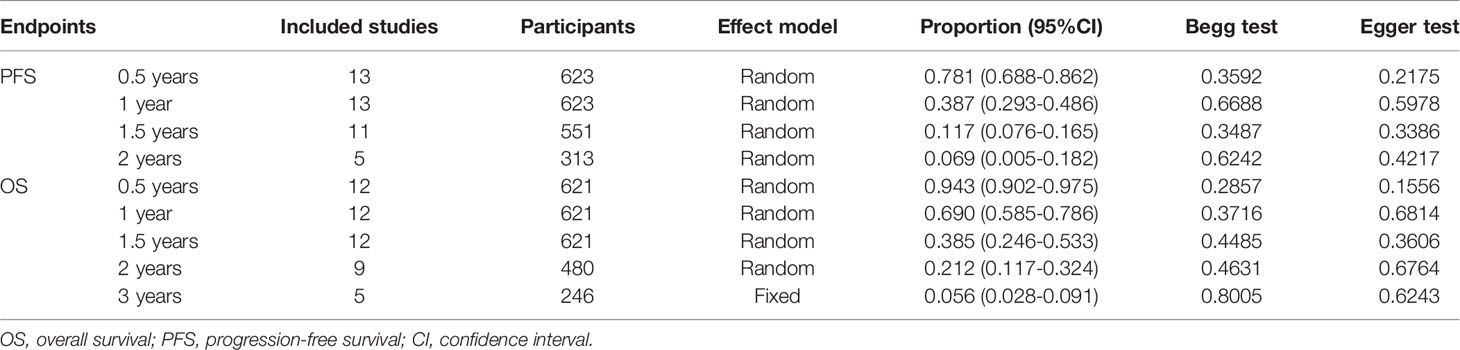
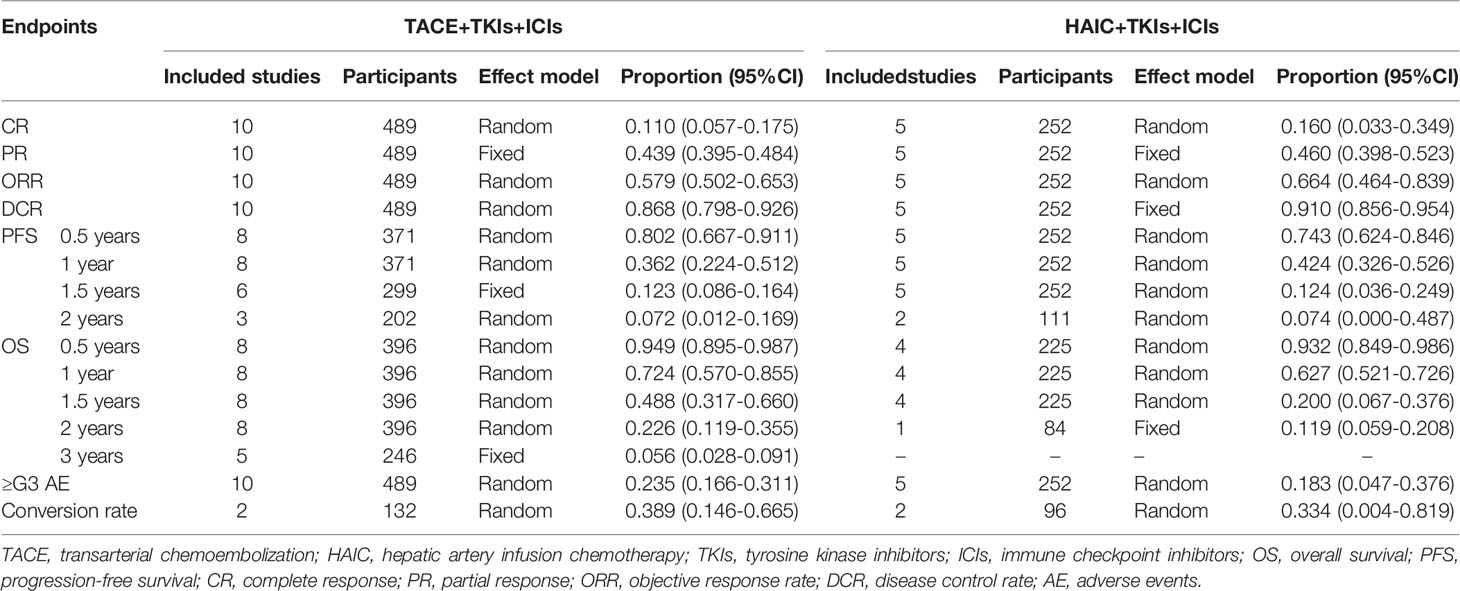
PFS at 0.5, 1, 1.5, and 2 years were evaluated in 13 (21, 25, 31–38, 40–42), 13 (21, 25, 31–38, 40–42), 11 (21, 25, 31–37, 41, 42), and 5 studies (21, 32, 34, 37, 42), and the pooled rates and 95%CI were 0.781 (0.688-0.862), 0.387 (0.293-0.486), 0.117 (0.076-0.165), and 0.069 (0.005-0.182), respectively (Table 3). OS at 0.5, 1, 1.5, 2, and 3 years were evaluated in 12 (21, 25, 31–36, 39–42), 12 (21, 31–34, 39–42), 12 (21, 31–34, 39–42), 9 (21, 31–34, 39–42), and 5 studies (21, 31–33, 42), and the pooled rates and 95%CI were 0.943 (0.902-0.975), 0.690 (0.585-0.786), 0.385 (0.246-0.533), 0.212 (0.117-0.324), and 0.056 (0.028-0.091), respectively (Table 3).
Subgroup Analysis Stratified by Chemo(Embolization) Technique
TACE was adopted in 10 studies (21, 30–33, 38–42), and HAIC were in 5 studies (25, 34–37), respectively. Table 4 exhibited the outcomes using TACE+TKIs+ICIs and HAIC+TKIs+ICIs, respectively. Briefly, the rates of CR, ORR, and DCR were slightly higher in the HAIC+TKIs+ICIs group than those in the TACE+TKIs+ICIs group, but on the contrary, a mild increase was observed in the rates of conversion, PFS at 0.5 years and 1 year, and OS at 1 and 2 years. Of note, the pooled rates of AEs grading exceeding three for TACE+TKIs+ICIs and HAIC+TKIs+ICIs were 0.235 (0.166-0.311) and 0.183 (0.047-0.376), respectively (Table 4).
Subgroup Analysis of Control Studies
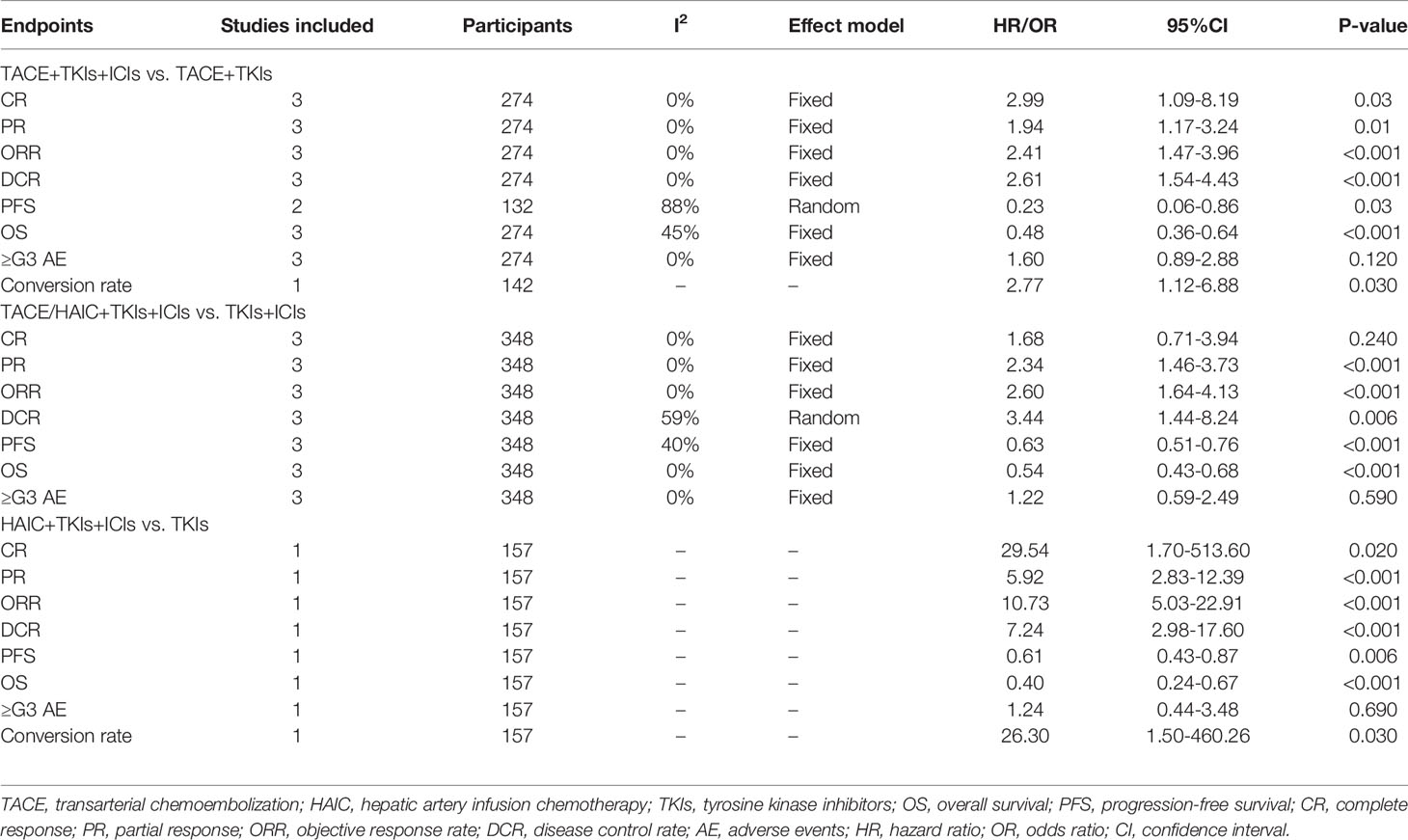
There were 7 studies incorporating the control group (21, 25, 33–35, 39, 40), and the control group was TACE+TKIs in three studies (21, 33, 40), TKIs+ICIs in three (34, 35, 39), and TKIs in one (25), respectively. Results showed that TACE/HAIC+TKIs+ICIs was superior to TKIs alone in all fields (Table 5), and similar advantage were also observed compared to TACE+TKIs and TKIs+ICIs (all P<0.05, Table 5). Of note, there was no significant difference between TACE/HAIC+TKIs+ICIs and TKIs+ICIs in terms of the CR rate (P>0.05, Table 5) using a fixed effect model, and increasing severe AEs were not observed in the triple combination regimens.
Adverse Events
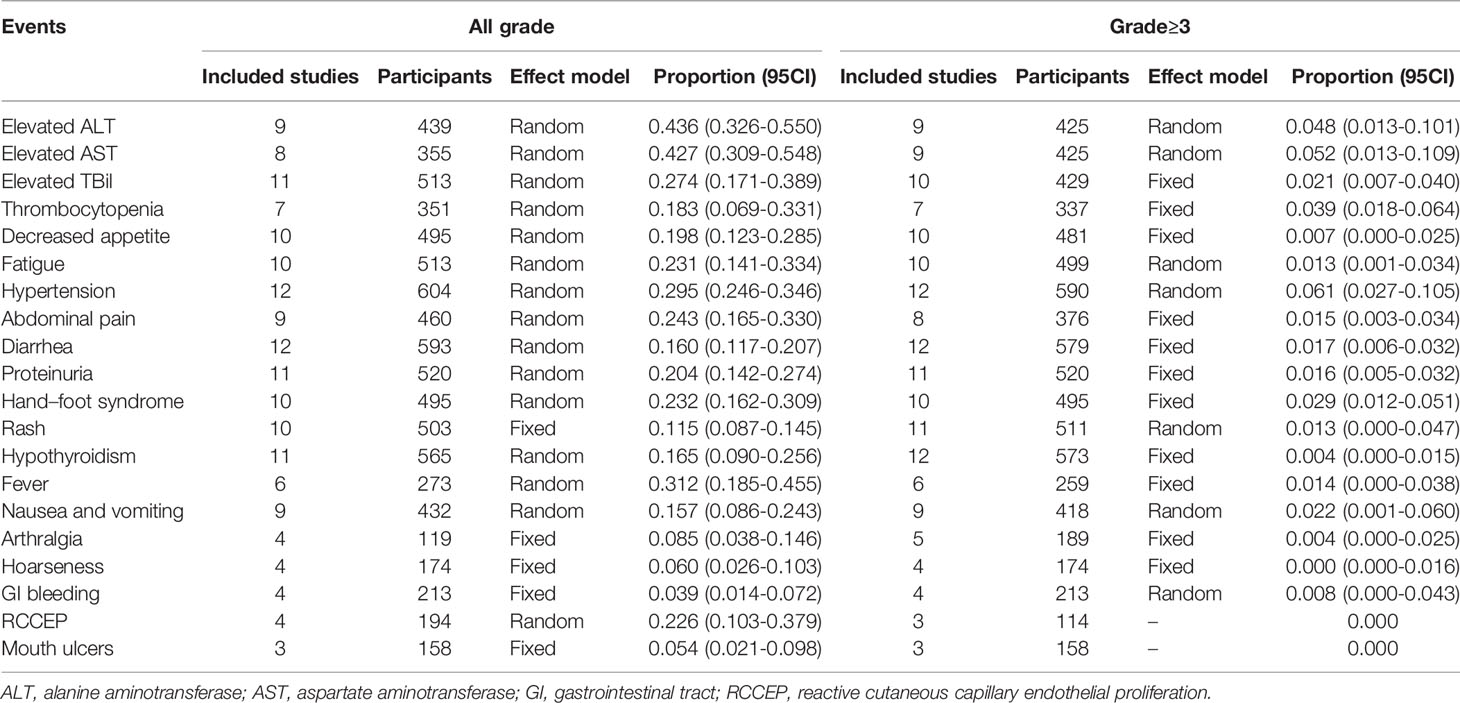
The pooled rates for the treatment related AEs were depicted in Table 6. No fatal AEs were reported in all the included studies. The top three most common AEs were elevated ALT (rate=0.436, 95%CI=0.326-0.550), elevated AST (rate=0.427, 95%CI=0.309-0.548), and hypertension (rate=0.295, 95%CI=0.246-0.346), respectively. The severe AEs were rarely reported, and the top three most common severe adverse events were hypertension (rate=0.061, 95%CI=0.027-0.105), elevated AST (rate=0.052, 95%CI=0.013-0.109), and elevated ALT (rate=0.048, 95%CI=0.013-0.101), respectively.
Discussion
In the era of systemic therapy for intermediate–advanced HCC, is there still a niche for locoregional treatment including TACE and HAIC? In this systematic review, 741 patients in the 15 studies received the triple combination of TACE/HAIC, TKIs, and ICIs. Results showed that the triple combination provided a substantial CR rate of 0.124 (0.069-0.190), ORR of 0.606 (0.528-0.682), DCR of 0.885 (0.835-0.927), and prolonged PFS and OS without increased severe AEs. Further meta-analysis of control studies exhibited the superiority of the triple combination modality to TACE+TKIs, TKIs+ICIs, and TKIs alone.
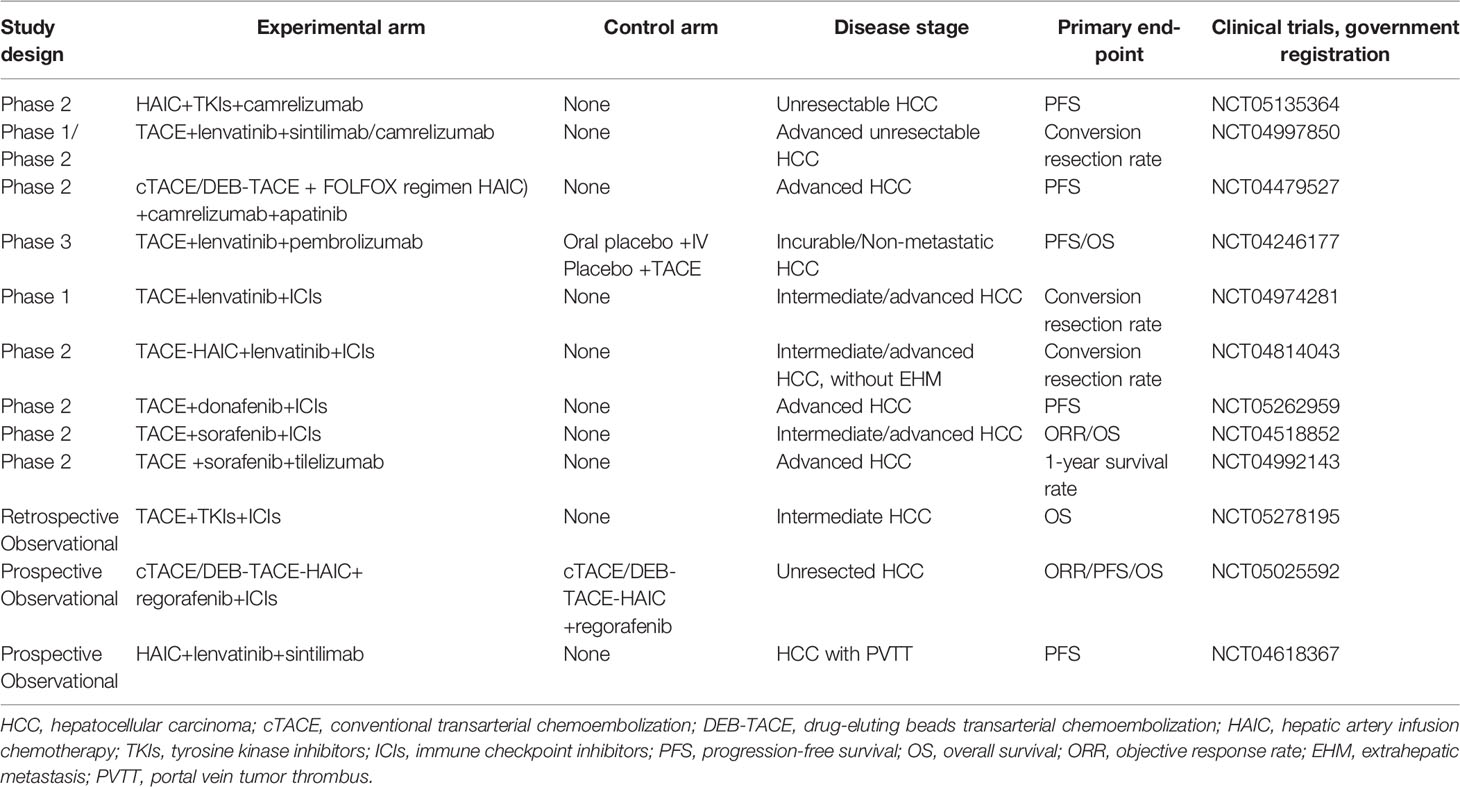
Triple combination modality was firstly reported by Liu et al. (31) in 2021. A total of 22 patients with advanced HCC received TACE plus lenvatinib and camrelizumab, and at the first month, the ORR reached as high as 68.2%. The median PFS and OS were 11.4 and 24 months, respectively, without severe AEs during the treatment. From then on, more studies have been published with promising results. In this systematic review, 15 studies were identified with 741 patients receiving TACE/HAIC+TKIs+ICIs, and initial analysis showed encouraging results. However, the CR rate ranged from 0.069 to 0.190, the ORR ranged from 0.528 to 0.682, and the DCR ranged from 0.835 to 0.927, as well as the mean PFS (4.0-16.3 months) and mean OS (8.6-24.8 months). The divergences between the included studies might be attributed to the following reasons: 1) the sample size of all eligible studies was small, which meant that II error is hard to avoid; 2) there is substantial heterogeneity among uHCC patients, not to mention primary or recurrent HCC; 3) the regimen of triple therapy was very different from each other, including transarterial therapy modality (conventional TACE, DEB-TACE, or HAIC), TKI agents (sorafenib, Lenvatinib, or apatinib), and ICI agents (pembrolizumab, camrelizumab, tislelizumab, sintilimab, toripalimab, or nivolumab); 4) triple therapy as first-line treatment or not, which greatly influenced on tumor responses, median PFS and OS; 5) different treatment goals, for example, successful conversion and subsequent curative resection would have better prognosis than those with palliative treatment. Hence, the conclusion needs further validation, and Table 7 exhibited ongoing prospective trials evaluating the clinical efficacy of the triple combination modality.
Conversion therapy is well concerned nowadays in the field of uHCC (43, 44). Evidence suggests that R0 resection is a crucial independent protective factor of long-term survival (45). Shindoh et al. (46) found that advanced HCC patients after conversion therapy receiving R0 resection could have comparable prognosis with initially resectable HCC patients. Previous studies found that the successful conversion rate was 42.4% by lenvatinib and ICIs (47), 14.8% by TACE+sorafenib (48), and 12.8%-14.3% by HAIC+sorafenib (24, 49), respectively. Using the triple combination modality of HAIC+TKIs+ICIs, the conversion rate was reported to be as high as 60% by Zhang et al. (36), and in this systematic review, the pooled rate for the conversion surgery was 35.9%. The underlying mechanism of the synergistic effect of the triple combination might be as follows: 1) TACE or HAIC could improve the tumor immune microenvironment, induce continuous exposure to tumor antigens caused by continuous drug penetration, and thus enhance the efficacy of systemic therapies and 2) the anti-tumor angiogenesis effect of TKIs and ICIs will help eliminate tumor angiogenesis and tumor recurrence followed by TACE/HAIC, but both of them lack of validation in practice. Yang et al. (20) firstly identified that the triple therapy could not only activate cell immunity but also stimulate humoral immunity, and circulating Ig G, Ig λ, and Ig κ could serve as potential biomarkers of triple therapy. In the future, more attention should be paid on the triple combination modality for uHCC, especially for those with a strong willingness to receive radical resection.
It has been yet to be known which is the optimal modality of transarterial chemo(embolization) because there are rare reports comparing TACE, DEB-ATCE, and HAIC in the triple therapy for unresectable HCC. TACE has always been the cornerstone for intermediate-stage HCC (6), which was repeatedly confirmed by a recent systematic review with a median OS of 19.4 months (50). However, repeated TACE may lead to liver function impairment and even TACE resistance, and TACE alone is unsatisfactory for patients in advanced stage, especially portal vein invasion or extrahepatic spread (9, 10). Drug-eluting beads TACE (DEB-TACE) was found to yield better tumor responses and a similar safety profile compared to conventional TACE (5). Ren et al. (51) firstly compared the efficacy of DEB-TACE combined with ICI versus conventional TACE combined with ICI for unresectable HCC. Results showed that DEB-TACE was a safe and well-tolerated treatment and produced better PFS and tumor response in patients with unresectable HCC than conventional TACE. On the other hand, HAIC has been identified to be non-inferior to TACE in local control and even had a weak advantage over TACE in long-term prognosis (9–11). In this systematic review, a slight advantage of HAIC+TKIs+ICIs over TACE+TKIs+ICIs was observed in CR, ORR, and DCR, but it did not translate into survival benefit in PFS and OS. In addition, an apparent inferiority of HAIC+TKIs+ICIs to TACE+TKIs+ICIs was also found in the conversion rate (33.4% vs. 38.9%). Hence, it remains controversial in the choice of conventional TACE or DEB-TACE or HAIC among the triple combination, and “head-to-head” prospective trials might be the answer in the future.
As a saying goes, one size does not fit for all, and not all unresectable HCCs will benefit from the triple therapy. Ju et al. (39) found that TACE+apatinib+camrelizumab provided a clinical benefit for the subgroups of age <65 years old, men, PS score of 1, Child–Pugh classification of B, liver cirrhosis, hepatitis B infection, and AFP >200 mg/ml (all P<0.05), and similar findings were observed in the study of Zheng et al. (33). Mei et al. (35) found that HAIC+lenvatinib+ICIs exhibited a clinical benefit in patients with large, multiple HCCs (all P<0.05), but it failed in those with main portal vein tumor thrombus or extrahepatic metastasis (P>0.05), which was confirmed in a study of HAIC+lenvatinib+toripalimab. Further, Chen et al. (21) revealed that increased survival benefits with TACE+TKIs+ICIs was associated with the PD-L1 CPS score. Hence, identifying the potential beneficiary of the triple combination modality is an urgent agenda.
Safety is a bottleneck of the triple combination modality. The most common AEs are impaired liver function, fever, and abdominal pain related to TACE/HAIC (9–11); hypertension; diarrhea; and hand–foot syndrome to TKIs (52, 53) and rash, fatigue, and pruritus to ICIs (14, 19), respectively. A combination of TACE/HAIC and TKIs often increases the AEs of hypertension, hand–foot syndrome, and diarrhea, and a combination of TKIs and ICIs increases the risk of fatigue, rash, and hypothyroidism (20, 25, 31). As for the triple combination modality, safety can never be overemphasized. In this systematic review, the most common AEs were still elevated ALT and/or AST and hypertension, as well as the most severe AEs, but no mortality caused by the triple combination modality was reported. These results indicated that liver function might be selection criteria for the triple modality, and patients with impaired liver function will be contradicted to this modality.
Generally, the triple combination of TACE/HAIC, TKIs, and ICIs for uHCC needs a long way to go. Apart from the triple modality itself including the scheme and sequence, more factors should be of concern: 1) how many additional survival benefit, 2) how much cost-effectiveness, and 3) how about AEs with an intensified regimen. In addition, the accessibility of the medical care is another decision-making factor. In China, TKIs like sorafenib and lenvatinib and ICIs like camrelizumab and sintilimab have been enrolled into a healthcare insurance, which are much cheaper than nivolumab, pembrolizumab, and atezolizumab. Furthermore, TACE is much more preferred than HAIC, owing to its high compliance. Hence, more factors should be taken into consideration in the future trials.
There were several limitations in this systematic review. First, all of the studies were retrospective, in which recalling bias was hard to avoid. Second, considering that all the published studies came from China, the conclusion would not be applicable for the western patients due to the apparent heterogeneity in etiology between the East and the West. Third, data on the TACE/HAIC, TKIs, and ICIs were not available in several included studies; hence, the corresponding subgroup analysis could not be conducted. Fourth, considering that some studies came from the same center, the patient’s cohort might be the presence of overlap. Last but not the least, the sequential order of the triple modality was not unified among the included studies, and in the future, an extensive consensus should be reached on this issue.
Conclusion
With the current data, we concluded that the triple combination of TACE/HAIC, TKIs, and ICIs would provide a clinical benefit for uHCC both in short- and long-term outcomes without increasing severe AEs. However, more is unknown on the optimal regimen, potential beneficiary, and latent AEs. Future RCTs with a larger sample size and cross-regional centers will aid in better clarifying the role of the triple modality for uHCC.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author Contributions
QK, FX, and HF: acquisition of data, analysis and interpretation of data. QK and LW: conception and design of the study. QK and LW: drafting the article. YZ and JL: critical revision, final approval. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Fujian Provincial Clinical Research Center for Hepatobiliary and Pancreatic Tumors, Fujian, P.R.C. (2020Y2013), the Key Clinical Specialty Discipline Construction Program of Fuzhou, Fujian, P.R.C. (201912002), and the Startup Fund for scientific research, Fujian Medical University, Fujian, P.R.C. (2020QH1242).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.913464/full#supplementary-material
Supplementary Figure 1 | Funnel plot of complete response
Supplementary Figure 2 | Sensitivity analysis of complete response
Supplementary Figure 3 | Funnel plot of objective response rate
Supplementary Figure 4 | Sensitivity analysis of objective response rate
Supplementary Figure 5 | Funnel plot of disease control rate
Supplementary Figure 6 | Sensitivity analysis of disease control rate
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular Carcinoma. Nat Rev Dis Primers (2021) 7(1):6. doi: 10.1038/s41572-020-00240-3
3. Llovet JM, De Baere T, Kulik L, Haber PK, Greten TF, Meyer T, et al. Locoregional Therapies in the Era of Molecular and Immune Treatments for Hepatocellular Carcinoma. Nat Rev Gastroenterol Hepatol (2021) 18(5):293–313. doi: 10.1038/s41575-020-00395-0
4. Zhou J, Sun H, Wang Z, Cong W, Wang J, Zeng M, et al. Guidelines for the Diagnosis and Treatment of Hepatocellular Carcinoma (2019 Edition). Liver Cancer (2020) 9(6):682–720. doi: 10.1159/000509424
5. Benson AB, D'Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, et al. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw (2021) 19(5):541–65. doi: 10.6004/jnccn.2021.0022
6. Reig M, Forner A, Rimola J, Ferrer-Fabrega J, Burrel M, Garcia-Criado A, et al. BCLC Strategy for Prognosis Prediction and Treatment Recommendation: The 2022 Update. J Hepatol (2022) 76(3):681–93. doi: 10.1016/j.jhep.2021.11.018
7. Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, et al. Randomized Controlled Trial of Transarterial Lipiodol Chemoembolization for Unresectable Hepatocellular Carcinoma. Hepatology (2002) 35(5):1164–71. doi: 10.1053/jhep.2002.33156
8. Llovet JM, Real MI, Montana X, Planas R, Coll S, Aponte J, et al. Arterial Embolisation or Chemoembolisation Versus Symptomatic Treatment in Patients With Unresectable Hepatocellular Carcinoma: A Randomised Controlled Trial. Lancet (2002) 359(9319):1734–9. doi: 10.1016/S0140-6736(02)08649-X
9. Li QJ, He MK, Chen HW, Fang WQ, Zhou YM, Xu L, et al. Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin Versus Transarterial Chemoembolization for Large Hepatocellular Carcinoma: A Randomized Phase III Trial. J Clin Oncol (2022) 40(2):150–60. doi: 10.1200/JCO.21.00608
10. Li S, Mei J, Wang Q, Shi F, Liu H, Zhao M, et al. Transarterial Infusion Chemotherapy With FOLFOX for Advanced Hepatocellular Carcinoma: A Multi-Center Propensity Score Matched Analysis of Real-World Practice. Hepatobiliary Surg Nutr (2021) 10(5):631–45. doi: 10.21037/hbsn.2020.03.14
11. Li S, Lyu N, Han X, Li J, Lai J, He M, et al. Hepatic Artery Infusion Chemotherapy Using Fluorouracil, Leucovorin, and Oxaliplatin Versus Transarterial Chemoembolization as Initial Treatment for Locally Advanced Hepatocellular Carcinoma: A Propensity Score-Matching Analysis. J Vasc Interv Radiol (2021) 32(9):1267–76. doi: 10.1016/j.jvir.2021.06.008
12. Su GL, Altayar O, O'Shea R, Shah R, Estfan B, Wenzell C, et al. AGA Clinical Practice Guideline on Systemic Therapy for Hepatocellular Carcinoma. Gastroenterology (2022) 162(3):920–34. doi: 10.1053/j.gastro.2021.12.276
13. Bruix J, Chan SL, Galle PR, Rimassa L, Sangro B. Systemic Treatment of Hepatocellular Carcinoma: An EASL Position Paper. J Hepatol (2021) 75(4):960–74. doi: 10.1016/j.jhep.2021.07.004
14. Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, et al. Nivolumab Versus Sorafenib in Advanced Hepatocellular Carcinoma (CheckMate 459): A Randomised, Multicentre, Open-Label, Phase 3 Trial. Lancet Oncol (2022) 23(1):77–90. doi: 10.1016/S1470-2045(21)00604-5
15. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab Plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med (2020) 382(20):1894–905. doi: 10.1056/NEJMoa1915745
16. Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J Clin Oncol (2020) 38(26):2960–70. doi: 10.1200/JCO.20.00808
17. Kelley RK, Sangro B, Harris W, Ikeda M, Okusaka T, Kang YK, et al. Safety, Efficacy, and Pharmacodynamics of Tremelimumab Plus Durvalumab for Patients With Unresectable Hepatocellular Carcinoma: Randomized Expansion of a Phase I/II Study. J Clin Oncol (2021) 39(27):2991–3001. doi: 10.1200/JCO.20.03555
18. Xu J, Shen J, Gu S, Zhang Y, Wu L, Wu J, et al. Camrelizumab in Combination With Apatinib in Patients With Advanced Hepatocellular Carcinoma (RESCUE): A Nonrandomized, Open-Label, Phase II Trial. Clin Cancer Res (2021) 27(4):1003–11. doi: 10.1158/1078-0432.CCR-20-2571
19. Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol (2020) 6(11):e204564. doi: 10.1001/jamaoncol.2020.4564
20. Yang F, Xu GL, Huang JT, Yin Y, Xiang W, Zhong BY, et al. Transarterial Chemoembolization Combined With Immune Checkpoint Inhibitors and Tyrosine Kinase Inhibitors for Unresectable Hepatocellular Carcinoma: Efficacy and Systemic Immune Response. Front Immunol (2022) 13:847601. doi: 10.3389/fimmu.2022.847601
21. Chen S, Wu Z, Shi F, Mai Q, Wang L, Wang F, et al. Lenvatinib Plus TACE With or Without Pembrolizumab for the Treatment of Initially Unresectable Hepatocellular Carcinoma Harbouring PD-L1 Expression: A Retrospective Study. J Cancer Res Clin Oncol (2021). doi: 10.1007/s00432-021-03767-4
22. Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, et al. Randomised, Multicentre Prospective Trial of Transarterial Chemoembolisation (TACE) Plus Sorafenib as Compared With TACE Alone in Patients With Hepatocellular Carcinoma: TACTICS Trial. Gut (2020) 69(8):1492–501. doi: 10.1136/gutjnl-2019-318934
23. Ding X, Sun W, Li W, Shen Y, Guo X, Teng Y, et al. Transarterial Chemoembolization Plus Lenvatinib Versus Transarterial Chemoembolization Plus Sorafenib as First-Line Treatment for Hepatocellular Carcinoma With Portal Vein Tumor Thrombus: A Prospective Randomized Study. Cancer-Am Cancer Soc (2021) 127(20):3782–93. doi: 10.1002/cncr.33677
24. He M, Li Q, Zou R, Shen J, Fang W, Tan G, et al. Sorafenib Plus Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin vs Sorafenib Alone for Hepatocellular Carcinoma With Portal Vein Invasion: A Randomized Clinical Trial. JAMA Oncol (2019) 5(7):953–60. doi: 10.1001/jamaoncol.2019.0250
25. He MK, Liang RB, Zhao Y, Xu YJ, Chen HW, Zhou YM, et al. Lenvatinib, Toripalimab, Plus Hepatic Arterial Infusion Chemotherapy Versus Lenvatinib Alone for Advanced Hepatocellular Carcinoma. Ther Adv Med Oncol (2021) 13:17552544. doi: 10.1177/17588359211002720
26. Xu ZN, Huang JJ, Zhou J, Huang WS, Guo YJ, Cai MY, et al. Efficacy and Safety of Anti-PD-1 Monoclonal Antibody in Advanced Hepatocellular Carcinoma After TACE Combined With TKI Therapy. Zhonghua Nei Ke Za Zhi (2021) 60(7):630–6. doi: 10.3760/cma.j.cn112138-20200928-00841
27. Stang A. Critical Evaluation of the Newcastle-Ottawa Scale for the Assessment of the Quality of Nonrandomized Studies in Meta-Analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
28. Deeks JJ, Altman DG. Analysing Data and Undertaking Meta-Analyses. In: Cochrane Handbook for Systematic Reviews of Interventions Cochrane Book, vol. 9. Hoboken, New Jersey: John Wiley & Sons Inc. (2011). doi: 10.1002/9780470712184.ch9
29. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring Inconsistency in Meta-Analyses. BMJ (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
30. Wu JY, Yin ZY, Bai YN, Chen YF, Zhou SQ, Wang SJ, et al. Lenvatinib Combined With Anti-PD-1 Antibodies Plus Transcatheter Arterial Chemoembolization for Unresectable Hepatocellular Carcinoma: A Multicenter Retrospective Study. J Hepatocell Carcinoma (2021) 8:1233–40. doi: 10.2147/JHC.S332420
31. Liu J, Li Z, Zhang W, Lu H, Sun Z, Wang G, et al. Comprehensive Treatment of Trans-Arterial Chemoembolization Plus Lenvatinib Followed by Camrelizumab for Advanced Hepatocellular Carcinoma Patients. Front Pharmacol (2021) 12:709060. doi: 10.3389/fphar.2021.709060
32. Cao F, Yang Y, Si T, Luo J, Zeng H, Zhang Z, et al. The Efficacy of TACE Combined With Lenvatinib Plus Sintilimab in Unresectable Hepatocellular Carcinoma: A Multicenter Retrospective Study. Front Oncol (2021) 11:783480. doi: 10.3389/fonc.2021.783480
33. Zheng L, Fang S, Wu F, Chen W, Chen M, Weng Q, et al. Efficacy and Safety of TACE Combined With Sorafenib Plus Immune Checkpoint Inhibitors for the Treatment of Intermediate and Advanced TACE-Refractory Hepatocellular Carcinoma: A Retrospective Study. Front Mol Biosci (2021) 7:609322. doi: 10.3389/fmolb.2020.609322
34. Chen S, Xu B, Wu Z, Wang P, Yu W, Liu Z, et al. Pembrolizumab Plus Lenvatinib With or Without Hepatic Arterial Infusion Chemotherapy in Selected Populations of Patients With Treatment-Naive Unresectable Hepatocellular Carcinoma Exhibiting PD-L1 Staining: A Multicenter Retrospective Study. BMC Cancer (2021) 21(1):1126. doi: 10.1186/s12885-021-08858-6
35. Mei J, Tang YH, Wei W, Shi M, Zheng L, Li SH, et al. Hepatic Arterial Infusion Chemotherapy Combined With PD-1 Inhibitors Plus Lenvatinib Versus PD-1 Inhibitors Plus Lenvatinib for Advanced Hepatocellular Carcinoma. Front Oncol (2021) 11:618206. doi: 10.3389/fonc.2021.618206
36. Zhang J, Zhang X, Mu H, Yu G, Xing W, Wang L, et al. Surgical Conversion for Initially Unresectable Locally Advanced Hepatocellular Carcinoma Using a Triple Combination of Angiogenesis Inhibitors, Anti-PD-1 Antibodies, and Hepatic Arterial Infusion Chemotherapy: A Retrospective Study. Front Oncol (2021) 11:729764. doi: 10.3389/fonc.2021.729764
37. Liu BJ, Gao S, Zhu X, Guo JH, Kou FX, Liu SX, et al. Real-World Study of Hepatic Artery Infusion Chemotherapy Combined With Anti-PD-1 Immunotherapy and Tyrosine Kinase Inhibitors for Advanced Hepatocellular Carcinoma. Immunotherapy-Uk (2021) 13(17):1395–405. doi: 10.2217/imt-2021-0192
38. Yang F, Yang J, Xiang W, Zhong BY, Li WC, Shen J, et al. Safety and Efficacy of Transarterial Chemoembolization Combined With Immune Checkpoint Inhibitors and Tyrosine Kinase Inhibitors for Hepatocellular Carcinoma. Front Oncol (2022) 11:657512. doi: 10.3389/fonc.2021.657512
39. Ju S, Zhou C, Yang C, Wang C, Liu J, Wang Y, et al. Apatinib Plus Camrelizumab With/Without Chemoembolization for Hepatocellular Carcinoma: A Real-World Experience of a Single Center. Front Oncol (2022) 11:835889. doi: 10.3389/fonc.2021.835889
40. Cai M, Huang W, Huang J, Shi W, Guo Y, Liang L, et al. Transarterial Chemoembolization Combined With Lenvatinib Plus PD-1 Inhibitor for Advanced Hepatocellular Carcinoma: A Retrospective Cohort Study. Front Immunol (2022) 13:848387. doi: 10.3389/fimmu.2022.848387
41. Teng Y, Ding X, Li W, Sun W, Chen J. A Retrospective Study on Therapeutic Efficacy of Transarterial Chemoembolization Combined With Immune Checkpoint Inhibitors Plus Lenvatinib in Patients With Unresectable Hepatocellular Carcinoma. Technol Cancer Res Treat (2022) 21:2081150234. doi: 10.1177/15330338221075174
42. Ju S, Zhou C, Hu J, Wang Y, Wang C, Liu J, et al. Late Combination of Transarterial Chemoembolization With Apatinib and Camrelizumab for Unresectable Hepatocellular Carcinoma is Superior to Early Combination. BMC Cancer (2022) 22(1):335. doi: 10.1186/s12885-022-09451-1
43. Arita J, Ichida A, Nagata R, Mihara Y, Kawaguchi Y, Ishizawa T, et al. Conversion Surgery After Preoperative Therapy for Advanced Hepatocellular Carcinoma in the Era of Molecular Targeted Therapy and Immune Checkpoint Inhibitors. J Hepatobiliary Pancreat Sci (2022). doi: 10.1002/jhbp.1135
44. Zhao HT, Cai JQ. Chinese Expert Consensus on Neoadjuvant and Conversion Therapies for Hepatocellular Carcinoma. World J Gastroenterol (2021) 27(47):8069–80. doi: 10.3748/wjg.v27.i47.8069
45. Xie QS, Chen ZX, Zhao YJ, Gu H, Geng XP, Liu FB. Systematic Review of Outcomes and Meta-Analysis of Risk Factors for Prognosis After Liver Resection for Hepatocellular Carcinoma Without Cirrhosis. Asian J Surg (2021) 44(1):36–45. doi: 10.1016/j.asjsur.2020.08.019
46. Shindoh J, Kawamura Y, Kobayashi Y, Kobayashi M, Akuta N, Okubo S, et al. Prognostic Impact of Surgical Intervention After Lenvatinib Treatment for Advanced Hepatocellular Carcinoma. Ann Surg Oncol (2021) 28(12):7663–72. doi: 10.1245/s10434-021-09974-0
47. Zhang WW, Hu BY, Han J, Wang HG, Wang ZB, Ye HY, et al. A Real-World Study of PD-1 Inhibitors Combined With TKIs for HCC With Major Vascular Invasion as the Conversion Therapy: A Prospective, Non-Randomized, Open-Label Cohort Study. Ann Oncol (2020) 31:S1307. doi: 10.1016/j.annonc.2020.10.195
48. Li CJ, Zhou J. Initial Report of a Two-Stage Resection for Hepatocellular Carcinoma Following Downstaging With Transarterial Chemoembolization and Sorafenib. Fubu Waike (2017) 30: 295-8+301. doi: 10.3969/j.issn.1003-5591.2017.04.015
49. He MK, Zou RH, Li QJ, Zhou ZG, Shen JX, Zhang YF, et al. Phase II Study of Sorafenib Combined With Concurrent Hepatic Arterial Infusion of Oxaliplatin, 5-Fluorouracil and Leucovorin for Unresectable Hepatocellular Carcinoma With Major Portal Vein Thrombosis. Cardiovasc Intervent Radiol (2018) 41(5):734–43. doi: 10.1007/s00270-017-1874-z
50. Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol Transarterial Chemoembolization for Hepatocellular Carcinoma: A Systematic Review of Efficacy and Safety Data. Hepatology (2016) 64(1):106–16. doi: 10.1002/hep.28453
51. Ren Y, Guo Y, Chen L, Sun T, Zhang W, Sun B, et al. Efficacy of Drug-Eluting Beads Transarterial Chemoembolization Plus Camrelizumab Compared With Conventional Transarterial Chemoembolization Plus Camrelizumab for Unresectable Hepatocellular Carcinoma. Cancer Control (2022) 29:1389472442. doi: 10.1177/10732748221076806
52. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib Versus Sorafenib in First-Line Treatment of Patients With Unresectable Hepatocellular Carcinoma: A Randomised Phase 3 non-Inferiority Trial. Lancet (2018) 391(10126):1163–73. doi: 10.1016/S0140-6736(18)30207-1
Keywords: hepatocellular carcinoma, transarterial chemotherapy, hepatic arterial infusion chemotherapy, tyrosine kinase inhibitors, immune checkpoint inhibitors, systematic review
Citation: Ke Q, Xin F, Fang H, Zeng Y, Wang L and Liu J (2022) The Significance of Transarterial Chemo(Embolization) Combined With Tyrosine Kinase Inhibitors and Immune Checkpoint Inhibitors for Unresectable Hepatocellular Carcinoma in the Era of Systemic Therapy: A Systematic Review. Front. Immunol. 13:913464. doi: 10.3389/fimmu.2022.913464
Received: 05 April 2022; Accepted: 26 April 2022;
Published: 23 May 2022.
Edited by:
Peter Hamar, Semmelweis University, HungaryReviewed by:
Yanqiao Ren, Huazhong University of Science and Technology, ChinaQin Shi, Fudan University, China
Copyright © 2022 Ke, Xin, Fang, Zeng, Wang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongyi Zeng, bGFtcDE5NzMxMUAxMjYuY29t; Lei Wang, d2FuZ2xlaXkwMDFAMTI2LmNvbQ==; Jingfeng Liu, ZHJqaW5nZmVuZ0AxMjYuY29t
†These authors have contributed equally to this work
 Qiao Ke
Qiao Ke Fuli Xin1,2†
Fuli Xin1,2† Yongyi Zeng
Yongyi Zeng Lei Wang
Lei Wang Jingfeng Liu
Jingfeng Liu