
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 30 June 2022
Sec. Alloimmunity and Transplantation
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.912749
This article is part of the Research Topic Alloimmunity and Immunotherapy in Renal Allograft Fibrosis View all 3 articles
 Jiali Wang1†
Jiali Wang1† Jinqi Liu2†
Jinqi Liu2† Wenrui Wu3†
Wenrui Wu3† Shicong Yang4
Shicong Yang4 Longshan Liu3,5,6
Longshan Liu3,5,6 Qian Fu3
Qian Fu3 Jun Li3
Jun Li3 Xutao Chen3
Xutao Chen3 Ronghai Deng3
Ronghai Deng3 Chenglin Wu3
Chenglin Wu3 Sizhe Long7
Sizhe Long7 Wujun Zhang7
Wujun Zhang7 Huanxi Zhang3*
Huanxi Zhang3* Haiping Mao1*
Haiping Mao1* Wenfang Chen4*
Wenfang Chen4*Background: We developed a pragmatic dichotomous grading criterion to stratify the acute tubular injury (ATI) of deceased-donor kidneys. We intended to verify the predictive value of this criterion for the prognosis of deceased-donor kidney transplantation.
Methods: The allografts with ATI were classified into severe and mild groups. Severe ATI was defined as the presence of extreme and diffuse flattening of the tubular epithelial cells, or denudement of the tubular basement membrane. The clinical delayed graft function (DGF) risk index was calculated based on a regression model for posttransplant DGF using 17 clinical parameters related to donor–recipient characteristics.
Results: A total of 140 recipients were enrolled: 18 severe and 122 mild ATI. Compared with the mild ATI group, the severe ATI group had more donors after cardiac death, higher median donor terminal serum creatinine level (dScr), and longer median cold ischemia time. Severe ATI had a higher DGF rate (55.6% vs 14.6%, p < 0.001), longer DGF recovery time (49.6 vs 26.3 days, p < 0.001), and a lower estimated glomerular filtration rate (eGFR) at 1 month (23.5 vs 54.0 ml/min/1.73 m2, p < 0.001), 3 months (40.4 vs 59.0, p = 0.001), and 6 months after transplant (46.8 vs 60.3, p = 0.033). However, there was no significant difference in eGFR at 1 year or beyond, graft, and patient survival. The predictive value of combined dScr with ATI severity for DGF rate and DGF recovery time was superior to that of dScr alone. The predictive value of the combined DGF risk index with ATI severity for DGF was also better than that of the DGF risk index alone; however, the association of the DGF risk index with DGF recovery time was not identified. Chronic lesions including glomerulosclerosis, interstitial fibrosis, arterial intimal fibrosis, and arteriolar hyalinosis were associated with declined posttransplant 1-year eGFR.
Conclusion: Based on our pragmatic dichotomous grading criterion for ATI in a preimplantation biopsy, donor kidneys with severe ATI increased DGF risk, prolonged DGF recovery, and decreased short-term graft function but demonstrated favorable long-term graft function. Our grading method can offer additive valuable information for assessing donor kidneys with acute kidney injury and may act as an effective supplementary index of the Banff criteria.
Kidney transplantation is the best treatment for patients with irreversible chronic kidney failure (1). An increasing number of transplantation candidates and a relative shortage of donor kidneys have resulted in the increasing use of marginal donor kidneys (2–3). However, marginal donor kidneys may be a risk factor for poor allograft outcomes (4–6). Thus, evaluating marginal donor kidneys and determining outcome predictors are important.
Kidneys with acute kidney injury (AKI) from deceased donors are considered marginal donor kidneys (7–10). Serum creatinine is a common clinical indicator of AKI, and the RIFLE (Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease), AKIN (Acute Kidney Injury Network), and KDIGO (Kidney Disease: Improving Global Outcomes) criteria all use it as a biomarker (11–13). Some studies have indicated that donor kidneys with AKI are associated with an increased incidence of delayed graft function (DGF), but there is no influence on long-term allograft function (14–18). AKI in these studies has typically been defined as an elevated donor terminal serum creatinine level (≥1.7 mg/dl) (14–18). However, using donor terminal creatinine only to assess AKI ignores other factors that cause damage to graft, including ischemia during the process of dying and the damage during organ procurement, transportation, and preservation (19–21). Moreover, terminal donor creatinine level does not distinguish between the influence of the AKI and that of chronic kidney disease. Neither can it exclude thrombosis and extensive infarction. An elevated creatinine level may be associated with reversible kidney injury and does not necessarily indicate there is a structural injury of the kidney (19–23). Donor serum creatinine is not a sensitive indicator and may not necessarily peak at the time of organ procurement, failing to reflect the severity of AKI. Therefore, to assess donor kidney AKI based solely on donor serum creatinine is insufficient. Apart from donor creatinine, some studies have also developed models for predicting short-term and long-term kidney transplantation outcomes based on multiple clinical parameters to assess donor kidney quality, but most of them have not been externally validated and thus cannot be generalized (24). Among them, the Irish 2010 model is a good externally validated model for predicting the incidence of DGF (25, 26), which requires clinical information from both donors and recipients. KDRI/KDPI (kidney donor risk index/ kidney donor profile index) is another reliable graft survival prediction model for donor kidney quality assessment using donor clinical characteristics (27). These multiparameter clinical models may offer more guidance for AKI donor kidney evaluation.
Another method to identify and quantify donor AKI is pretransplantation renal biopsy (28–34). All causes of acute injury to the kidney allograft can be reflected on histopathological examination of a pretransplantation biopsy, which mainly manifests as acute tubular injury (ATI) (19–21, 29, 33). Renal biopsy can also distinguish acute and chronic lesions of the donor kidneys and exclude diffuse thrombosis and extensive infarction. However, a pretransplantation biopsy is not routinely performed at many transplant centers. The results from the previous studies examining the relationship between pathological changes seen on pretransplantation biopsy and graft function after transplantation were contradictory. A multicenter study by Hall et al. (33) revealed no significant associations overall between preimplant biopsy-reported acute tubular necrosis (ATN) and the outcomes of DGF or graft failure. Matignon et al. (31) and Oberbauer et al. (28) both reported that ATI was not associated with DGF. On the other hand, Sulikowski et al. (5) found that biopsy-proven ATI was associated with DGF and primary non-graft function. A possible important reason for these differences in results is the different definitions and pathological descriptions of ATI or ATN. For example, Hall et al. (33) classified all degrees of ATI as ATN, which would dilute the impact of severe ATI. Currently, the international histopathological criteria for donor kidney evaluation are the Banff criteria (35). The grading of ATI/ATN is as follows: mild = epithelial flattening, tubule dilation, nuclear dropout, and loss of brush border; moderate = focal coagulative type necrosis; severe = infarction. Signs of necrosis are essential in moderate-to-severe degrees. It is understandable that the donor kidney Banff criteria are classified in this way because they are primarily used to determine whether a donor kidney should be discarded, and necrosis is one of the important determinants. However, very few of the donor kidneys we have received have met the criteria of moderate to severe. Instead, the vast majority fall in the mild category. The Banff criteria do not allow for more precise grading of ATI, and it is difficult to make prognostic judgments and risk grading of AKI donor kidneys. In addition, donor kidney evaluation often requires urgent and accurate judgment from the pathologist on a rush basis. Therefore, we need to develop refined and pragmatic ATI grading criteria that can be used to predict the short-term and long-term outcomes of kidney transplantation. A systematic review has summarized 16 pathological descriptions of acute renal tubular injury (36). After combining and screening the 16 pathological changes, we finally developed a simple and easy-to-use dichotomous (mild-to-severe) grading criterion for donor allograft ATI. We intend to clarify the discriminative and predictive ability of this grading criterion for the short-term and long-term prognoses by retrospective analysis.
We retrospectively reviewed the medical records of adult (≥18 years old) kidney transplant recipients who received deceased-donor kidneys, including organ donation after brain death (DBD) and donation after circulatory death (DCD), in which a pretransplantation donor kidney biopsy was performed, at the First Affiliated Hospital of Sun-Yat Sen University from Jan 2012 to June 2017. Recipient and donor demographic, clinical, and pathological data were extracted from the medical records of the follow-up database. These recipients were followed up until 2021. This study was approved by the Institutional Review Board of the hospital, and because of the retrospective nature of the study, the requirement of informed patient consent was waived. No organs from executed prisoners were used.
Biopsies were performed immediately before implantation by using a 16-gauge biopsy needle. The biopsy was performed at the superior edge of the donor kidneys, and two cores of tissue were obtained. Frozen sections of one core were stained with H&E and examined to determine whether to use or discard the kidney. Multiple frozen sections were also saved for subsequent immunofluorescence stains for immunoglobulin and complements including IgG, IgM, IgA, C3, and C1q. The other core and the rest of the frozen tissue were fixed, paraffin-embedded, and stained with H&E, periodic acid–Schiff (PAS), periodic Schiff-methenamine silver (PASM), and Masson’s trichrome (MASSON). Stained biopsy specimens were examined retrospectively by 2 experienced pathologists for a comprehensive assessment of glomerular, tubular, interstitial, and vascular lesions in the donor kidneys.
Donor kidneys with tubular coagulative necrosis and parenchymal infarction were excluded from the study. The recipients enrolled were classified into 2 groups based on whether or not the pretransplantation renal biopsy showed severe ATI (Figure 1). Severe ATI was defined as the presence of extreme and diffuse (>50%) flattening of the tubular epithelial cells or denudement of tubular basement membrane (TBM) (Figures 2A, B). Mild ATI was defined as the absence of severe ATI and no significant effacement of tubular epithelial cells or only mild detachment of brush border. Demographic and clinical characteristics as well as the outcomes were compared between the 2 groups. The chronic kidney injury was assessed using the Remuzzi scoring system (37), including global glomerulosclerosis, interstitial fibrosis, tubular atrophy, arterial intimal fibrosis, and arteriolar hyalinosis.
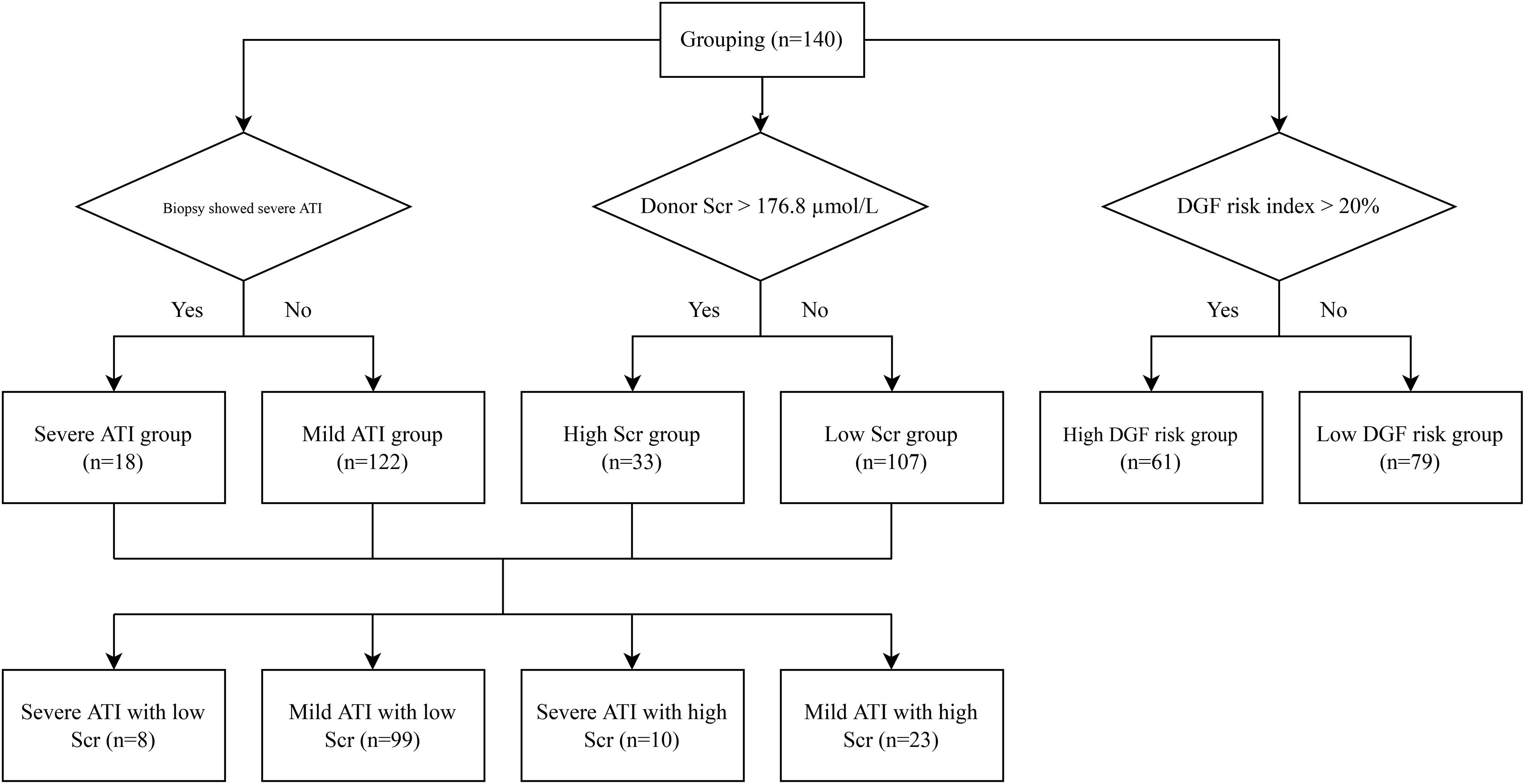
Figure 1 Grouping flowchart. ATI, acute tubular injury; Scr, serum creatinine; DGF, delayed graft function.
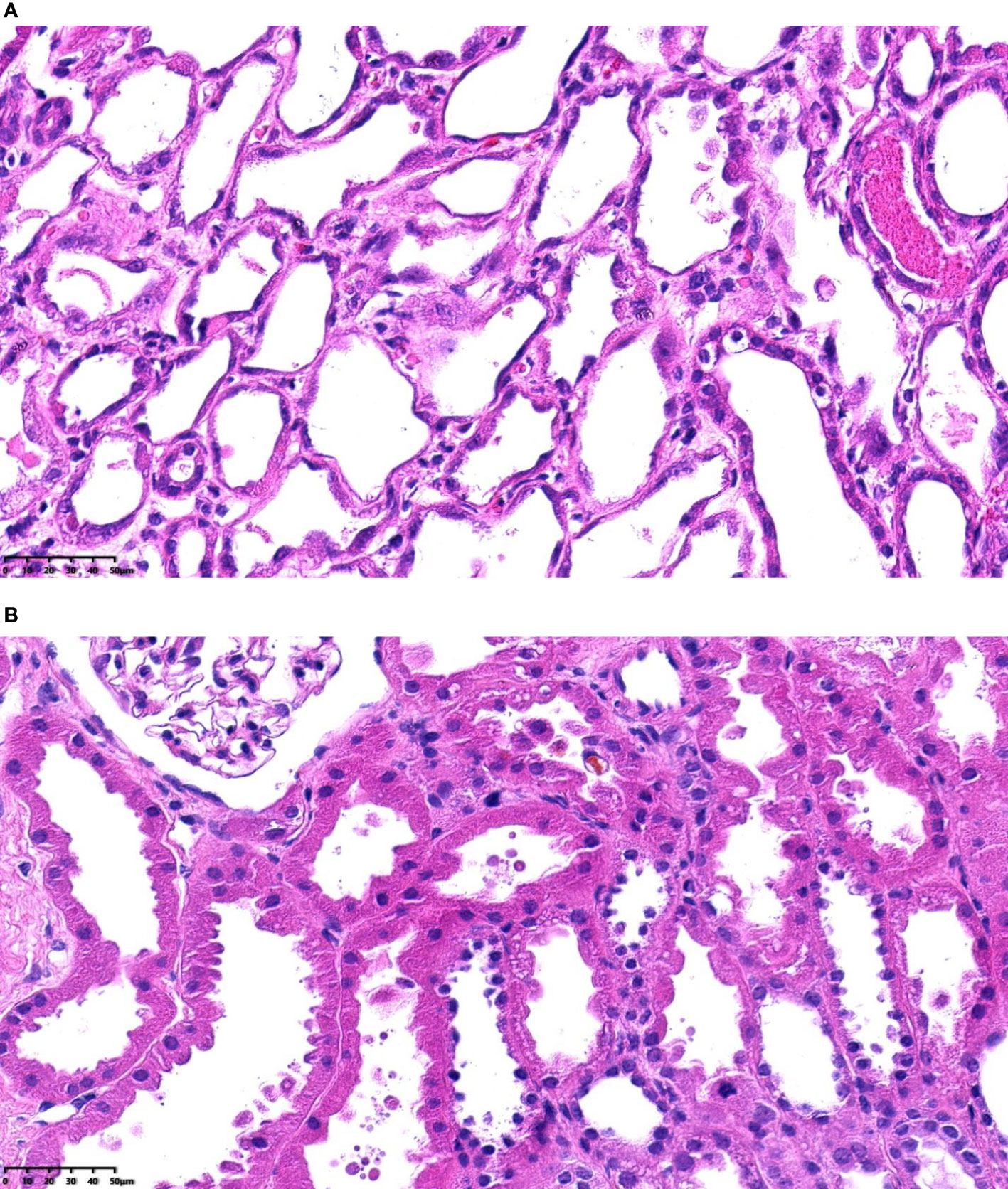
Figure 2 (A) Severe acute tubular injury, H&E-stained paraffin sections (magnification, ×400). (B) Mild acute tubular injury, H&E-stained paraffin sections (magnification, ×400).
The patients were divided into high and low donor serum creatinine groups based on donor terminal serum creatinine levels, with a cutoff value of 176.8 µmol/L (2 mg/dl). The high creatinine group was roughly equivalent to stage 2 and 3 AKI according to the KDIGO criteria (13). To assess whether pretransplant renal pathology has additive value in the assessment of the prognosis of AKI donor kidney transplantation compared with the use of serum creatinine alone, the patients were divided into four groups: 1) mild ATI with low terminal serum creatinine, 2) mild ATI with high terminal serum creatinine, 3) severe ATI with low terminal serum creatinine, and 4) severe ATI with high terminal serum creatinine.
Clinical prediction models are also a means to assess the prognosis of AKI donor kidneys, and we have previously validated models published internationally (26), with the Irish 2010 model performing best (25). In the present study, we calculated the predicted risk of DGF occurrence based on the Irish 2010 model by using 17 clinical parameters related to donor–recipient characteristics, including panel reactive antibody (PRA), duration of dialysis, recipient body mass index (BMI), HLA mismatch, cold ischemia time, warm ischemia time, donor terminal creatinine, donor age, donor weight, race, gender, previous transplant, recipient diabetes, recipient pretransplant transfusion, donation after cardiac death, donor history of hypertension, and donor cause of death. The patients were divided into high- and low-risk groups for DGF with 20% as the cutoff.
Measures of short-term posttransplantation allograft function were the occurrence of DGF, DGF recovery time, and estimated glomerular filtration rate (eGFR) at 3 and 6 months. Long-term graft function was evaluated by eGFR at 1–3 years after transplantation and death censored graft survival and patient survival. Glomerular filtration rate was estimated using the Modification of Diet in Renal Disease (MDRD) Study formula (38). If the patient died, the eGFR was counted as censored. If the patient lost his/her graft function, the eGFR was labeled as 0 in the calculation. DGF was defined as the need for dialysis within the first week after transplantation. DGF recovery time was defined as the time from posttransplantation day 1 to the day the recipient achieved a stable eGFR (eGFR did not change by more than 10% in the following week) (39), which was demonstrated to be associated with poor prognosis (rejection, infection, loss of graft function, and death) in our previous study.
We also collected posttransplant indication renal allograft biopsy results from the patients with severe ATI to observe the pathological change of renal tubular injury at different times after surgery to demonstrate AKI recovery.
Continuous data with normal distribution were presented as mean ± SD and compared using Student’s t-test. Continuous data without a normal distribution were expressed as median (interquartile range [IQR]) and compared using the Mann–Whitney U test. Categorical data were reported as counts and percentages and compared using a chi-square test or Fisher’s exact test. Comparison of continuous data among multiple groups was performed using one-way ANOVA or the Kruskal–Wallis test. Logistic regression and linear regression were used for univariate and multivariate analyses. The likelihood-ratio test was used for the comparison of model performance. All statistical tests were 2-sided, and values of p < 0.05 were considered to indicate statistical significance. Statistical analyses were performed with R software (version 3.6.1).
A total of 140 recipients were included in the analysis with a median follow-up time of 4.0 (IQR 3.1–5.0) years. Eighteen of the recipients received donor kidneys with severe ATI identified on pretransplantation biopsy, and 122 received kidneys with mild ATI (Table 1). Pretransplant PRA was absent in all recipients. Recipient characteristics were similar between the severe and mild ATI groups. With respect to donor characteristics, as compared with the mild ATI group, the severe ATI group had more DCD donors (77.8% vs 29.5%, p < 0.001), higher median donor terminal serum creatinine level (232 vs 94 µmol/L, p = 0.001), and longer median cold ischemia time (15 vs 10 h, p < 0.001).
Compared with the grafts with mild ATI, those with severe ATI had a significantly higher DGF rate, longer DGF recovery time, and a lower eGFR at 1, 3, and 6 months after transplant (Table 2). However, there was no significant difference in 1-year, 2-year, and 3-year eGFR between severe ATI and mild ATI groups. In contrast, higher donor serum creatinine was not associated with the DGF recovery time. The incidence of DGF was significantly higher in the group with high calculated risk group compared with low calculated risk, but the differences in DGF recovery time and in eGFR at different posttransplant time points were not statistically significant between the two groups (Table 3). No significant difference was identified in 3-year death-censored graft survival or patient survival between the two groups regardless of the method of grouping.
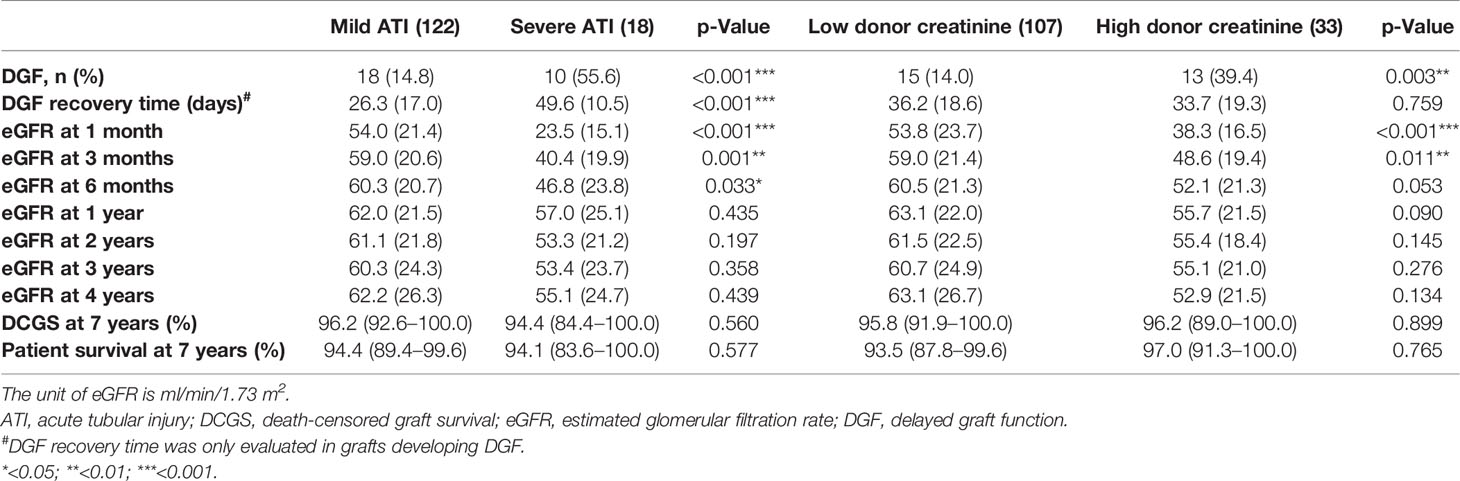
Table 2 Comparison of posttransplant allograft outcome based on the severity of ATI and the level of donor terminal serum creatinine.
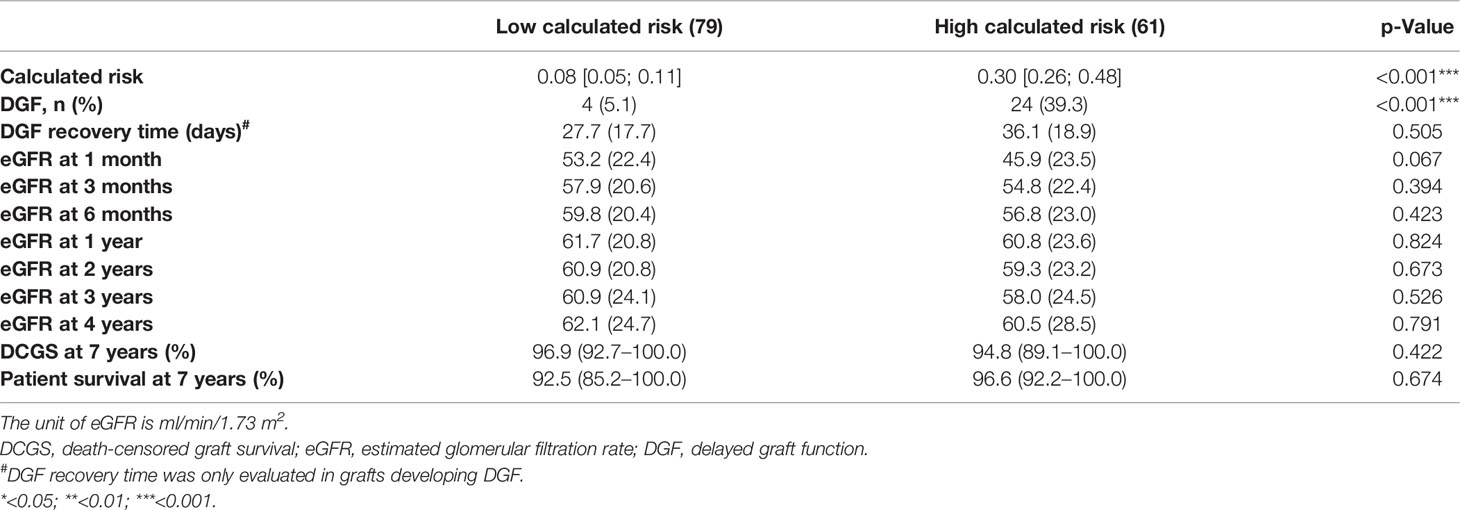
Table 3 Comparison of posttransplant allograft outcome based on the calculated DGF risk derived from Irish 2010 model.
Among the grafts with high donor terminal serum creatinine, those with severe ATI had higher DGF risk, longer DGF recovery time, and lower 1-month eGFR, as compared with those with mild ATI (DGF, 70.0% vs 26.1%, p = 0.026; DGF recovery time, 48.3 ± 11.2 vs 16.2 ± 8.6 days, p < 0.001; 1-month eGFR, 22.4 ± 11.5 vs 45.2 ± 13.4 ml/min/1.73 m2, p < 0.001) (Table 4). Among the grafts with low donor terminal serum creatinine, those with severe ATI had longer DGF recovery time and lower 1-month, 3-month, and 6-month eGFR, as compared with those with mild ATI (DGF recovery time, 52.0 ± 10.6 vs 31.4 ± 18.2 days, p = 0.049; 1-month eGFR, 24.8 ± 19.5 vs 56.1 ± 22.5 ml/min/1.73 m2, p = 0.002; 3-month eGFR, 36.5 ± 18.7 vs 60.9 ± 20.7 ml/min/1.73 m2, p = 0.007; 6-month eGFR, 40.9 ± 21.1 vs 62.1 ± 20.6 ml/min/1.73 m2, p = 0.025). The predictive ability of combined donor creatinine level with ATI severity for DGF was significantly better than that of donor creatinine level alone (likelihood-ratio test, p = 0.004) or that of ATI severity alone (p = 0.037) (Table 5). No interaction between ATI severity and donor creatinine level was identified. The predictive ability of combined calculated DGF risk with ATI severity for DGF was also better than that of calculated DGF risk alone (likelihood-ratio test, p = 0.012). ATI severity is considered the mediator variable between the risk factors for ATI (e.g., DCD and cold ischemia time) and allograft outcome; therefore, the risk factors for ATI were excluded from the multivariable analysis.
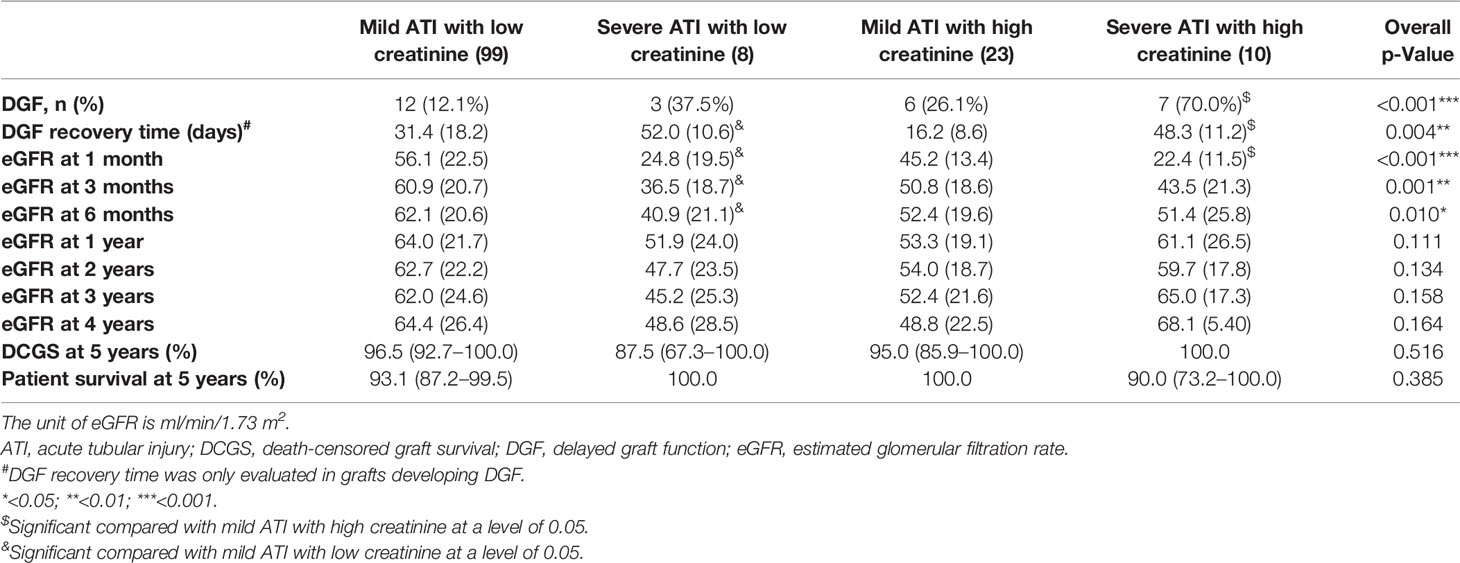
Table 4 Comparison of kidney allograft outcome among different combinations of donor terminal serum creatinine level and ATI severity.
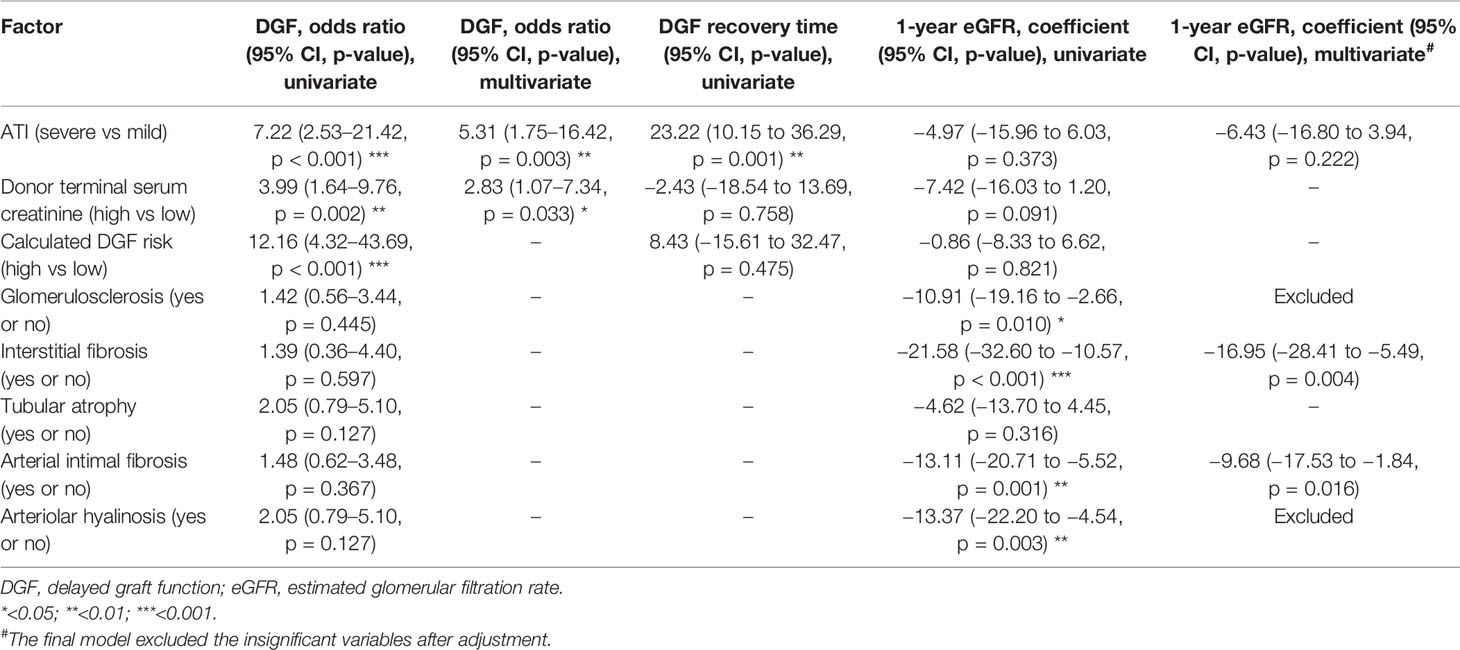
Table 5 Univariable and multivariable analyses of pathological factors affecting DGF and 1-year eGFR.
We also evaluated the effect of chronic pathological changes (including glomerulosclerosis, interstitial fibrosis, tubular atrophy, arterial intimal fibrosis, and arteriolar hyalinosis) in the pretransplant biopsy on the allograft outcome (Tables 5, 6). The existence of the chronic lesions except for tubular atrophy correlated with decreased posttransplant 1-year eGFR, while no effect was seen on the risk of DGF. After the confounding effect of the chronic pathological changes was adjusted, severe ATI was found to be not associated with 1-year eGFR.
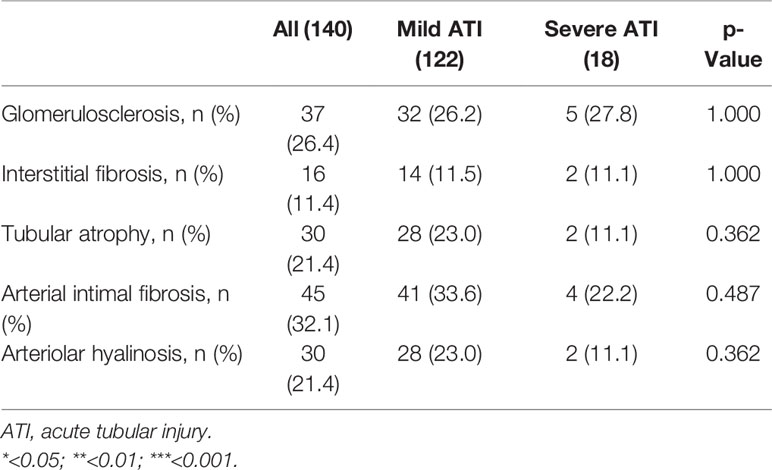
Table 6 The chronic pathological lesions in pretransplant biopsy of grafts with severe ATI and mild ATI.
Nine of the patients with severe ATI underwent 13 postoperative allograft biopsies, and the biopsy was performed from 2 weeks to 1 month posttransplant in 9 cases, 2 months in 2 cases, and 6 months in 2 cases. The pathological manifestations associated with renal tubular injury were described as tubular epithelium flattening, denudement of TBM, tubular cell calcification, granular cast, and regenerative changes. From 2 weeks to 1 month posttransplant, there was some degree of recovery from renal tubular injury, with severe lesions still present (denudement of TBM 22.2%) (Table 7), and most patients had begun to show regenerative changes (77.8%). At 2 months posttransplant, renal tubular injury was still present, but severe lesions had disappeared. At 6 months posttransplant, manifestations of renal tubular injury were hardly seen.
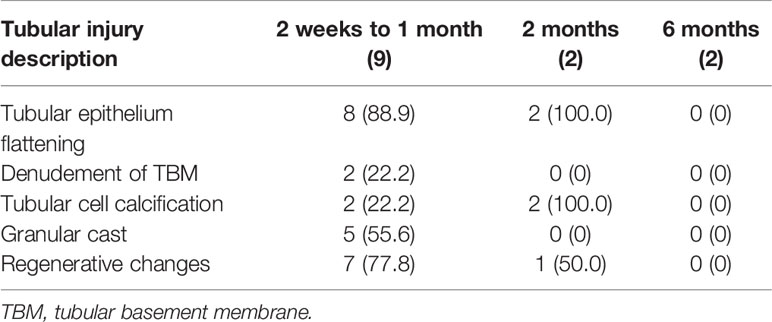
Table 7 Histopathological change of renal tubule in severe tubular injury after kidney transplantation.
In this study, we developed a pragmatic pathological grading criterion for ATI. Severe ATI compromised short-term posttransplantation graft function; severe ATI was associated with an increased DGF rate, prolonged DGF recovery time, and lower eGFR before 6 months posttransplant. However, the kidney allograft function at 1 year and beyond posttransplant and the graft survival was not related to the severity of ATI. It was noteworthy that the performance of the prediction model for DGF including both donor creatinine and ATI severity was the best among all prediction models.
Long-term graft kidney function was closely related to the preexisting chronic lesions of the donor kidneys (e.g., glomerulosclerosis, interstitial fibrosis, arterial intimal fibrosis, and arteriolar hyalinosis). By analyzing the biopsy results at different times posttransplant, we found that at 2–4 weeks posttransplant, the donor kidney transplanted in the recipient still showed significant tubular injury but started to recover as regenerative change occurred. By 6 months posttransplant, the manifestations of renal tubular injury were almost absent. This reflected that severe ATI can be successfully recovered, and thus renal function can gradually return to normal. These results suggested that donor kidneys with ATI and no chronic lesions for renal transplantation have a good long-term prognosis, which is similar to the findings of previous studies (18). However, patients with severe ATI require special attention for perioperative and postoperative follow-up management. Patients with severe ATI are at high risk of DGF and, more importantly, have a significantly longer recovery time from DGF (taking an average of 50 days), which not only prolongs the hospital stay but also increases the risk of multiple complications such as postoperative infection and rejection (39), increasing the financial and psychological burden on the patient, which can affect the long-term prognosis if not managed promptly.
Our results indicated that ATI was associated with DCD status and cold ischemia time. This finding may be explained by the mechanisms by which ATI develops. ATI in a donor kidney is primarily the result of hypoxic–ischemic damage and nephrotoxic insults (20, 21, 23). The major pathological changes due to hypoxic–ischemic damage are reflected in the tubules through a sequence of events: vacuolation, swelling of the epithelium, loss of the brush border and flattening of the epithelium, and eventually cell necrosis and exposure of the basal membrane. As such, DCD status and cold ischemia time, which are related to hypoxic–ischemic damage, were identified as risk factors for developing severe ATI. We did not find a relation between severe ATI and warm ischemia time. This might be explained by the relatively short mean warm ischemia time in our study sample. Our findings are consistent with those of Hall et al. (40).
Prior studies that have examined whether or not ATI was related to DGF have provided conflicting results. We identified several studies that focused on the association of DGF and ATI or ATN (5, 28, 29, 31, 33, 35). Of the 6 studies, 3 found an association between ATN/ATI and DGF, and 3 did not find an association. An important explanation is that the definition of ATI/ATN varied among the studies (36). For example, a multicenter study by Hall et al. (33) with the largest sample size (N = 651) reveals no significant associations overall between preimplant biopsy-reported ATN and the outcomes of DGF. In the study, Hall et al. (33) classified all degrees of ATI as ATN, which would dilute the impact of severe ATI. In addition, 541 of the 651 grafts were considered to have normal tubules on pretransplantation biopsies. However, in our sample, few grafts were reported to have entirely normal tubules—most of the grafts had at least vacuolization. Several other studies did not give a specific definition of ATI/ATN, and it was not possible to evaluate the impact of their definitions.
Assessment of the donor kidneys with donor terminal serum creatinine or model prediction scores could only distinguish whether DGF would occur, but was not well enough to distinguish whether DGF recovery was fast or slow. The relationship between ATI severity and DGF recovery time has been poorly studied in the past. We have previously found a correlation between DGF recovery time and poor prognoses such as rejection, infection, or kidney allograft failure (33). In the study, we found that severe ATI prolongs DGF recovery time. The tubules regenerate through cellular proliferation, migration, and subsequent hypertrophy of a new population of proximal tubule cells (41). This process takes time; thus, severe ATI influences short-term allograft function, but in the long-term, the proximal tubules regenerate and graft function normalizes.
Our results showed that pretransplantation biopsy could provide additional valuable information to the quality assessment of the donor kidneys. First, severe ATI combined with high donor terminal serum creatinine was associated with a higher risk of DGF occurrence and a longer recovery time from DGF compared with high donor terminal serum creatinine alone. The model predictive ability of combining either donor serum creatinine level or calculated DGF risk with ATI severity for DGF rate was also better than that of donor serum creatinine level or calculated DGF risk alone. ATI severity can well predict DGF recovery time, while donor serum creatinine level or calculated DGF risk is not that effective. Therefore, the combination of clinical and pathological information allows for a comprehensive assessment of AKI in the donor kidneys. Second, a part of donor kidneys with low terminal serum creatinine will have severe tubular damage, possibly due to other factors such as ischemia during the process of dying, interventions during donor procurement, and damage during organ transportation and preservation. Acute injury to donor kidneys may not be identified in these donors if a pretransplant biopsy is not performed. Third, the chronic lesions in the donor kidneys could be evaluated in the biopsy, which were associated with the long-term allograft function in our study and prior studies (24). Therefore, it is necessary to perform a pretransplant renal allograft biopsy to clarify the severity of the ATI and the chronic lesions, especially for donors with high serum creatinine, DCD donors, or donors with long cold ischemia time.
We chose extremely and diffusely flattened renal tubular epithelial cells or denudement of TBM when developing the criteria for ATI severity for the following reasons. First, these manifestations can be easily identified in the frozen section. Second, a quick diagnosis is required in pretransplant donor kidney biopsy, so the simpler and more practical the criteria, the better. Third, a previous study on the relationship between different ATI pathological descriptions and the severity of clinical AKI found that only simplification (defined as flattened tubular cell cytoplasm with complete loss of brush border), mitosis, and cell sloughing (defined as a free-floating cell in the tubular lumen without attachment to adjacent cells or basement membrane) were able to independently predict severe AKI (42). This was consistent with our criteria. Mitosis was less frequent in the early stage of acute kidney injury and thus was not included in our criteria.
In our cohort, severe ATI appeared slightly worse in long-term eGFR compared to mild ATI, but not statistically significant. Kidney allograft fibrosis is an important cause of late graft loss, and previous studies suggest that ATI may be associated with renal fibrosis by combined mechanisms (43). Whether severe ATI can lead to deterioration of long-term renal allograft function by promoting renal fibrosis remains unanswered. Few clinical studies were found to explore the relationship between ATI and renal fibrosis in the background of kidney transplantation.
This analysis is limited by its retrospective nature and may be subject to selection bias, as the choice of donor kidney biopsy before transplantation was subjective and at the surgeon’s discretion.
In summary, based on our pragmatic dichotomous (mild-to-severe) grading criterion for ATI in a preimplantation biopsy, kidney allograft with severe ATI increased the risk of DGF, prolonged the DGF recovery time, and decreased the short-term graft function but demonstrated favorable long-term graft function. ATI severity in combination with donor terminal serum creatinine level performs better in predicting DGF occurrence compared with either ATI severity or donor terminal serum creatinine level alone. Pretransplant biopsy offered additive and valuable information in the assessment of AKI in the donor kidneys.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Institutional Review Board of the First Affiliated Hospital of Sun-Yat Sen University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
JW, JQL, WW, and HZ designed the study, analyzed the data, and co-wrote the paper. SY and WC assessed the biopsies. LL, QF, JL, RD, CW, and WZ: performed data acquisition and interpretation. HZ, WW, XC, and SL performed the statistical analyses. HZ, HM, and WC supervised the research and critically reviewed it. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
This study was supported by the Science and Technology Planning Project of Guangdong Province, China (2015B020226002, 2017A020215012), National Natural Science Foundation of China (81870511, 82072824, 81772701), Key Scientific and Technological Program of Guangzhou City (201803040011), Guangdong Basic and Applied Basic Research Foundation (2020A1515010884), Guangdong Natural Science Foundation (2018A030313016), Clinical Research 5010 Programme of Sun Yat-Sen University, Guangdong Provincial Key Laboratory on Organ Donation and Transplant Immunology (2017B030314018, 2020B1212060026), and Guangdong Provincial International Cooperation Base of Science and Technology (Organ Transplantation, 2020A0505020003).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Abecassis M, Bartlett ST, Collins AJ, Davis CL, Delmonico FL, Friedewald JJ, et al. Kidney Transplantation as Primary Therapy for End-Stage Renal Disease: A National Kidney Foundation/Kidney Disease Outcomes Quality Initiative (NKF/KDOQITM) Conference. Clin J Am Soc Nephrol (2008) 3(2):471–80. doi: 10.2215/CJN.05021107
2. Gridelli B, Remuzzi G. Strategies for Making More Organs Available for Transplantation. N Engl J Med (2000) 343(6):404–10. doi: 10.1056/NEJM200008103430606
3. Heilman RL, Mathur A, Smith ML, Kaplan B, Reddy KS. Increasing the Use of Kidneys From Unconventional and High-Risk Deceased Donors. Am J Transplant (2016) 16(11):3086–92. doi: 10.1111/ajt.13867
4. Port FK, Bragg-Gresham JL, Metzger RA, Dykstra DM, Gillespie BW, Young EW, et al. Donor Characteristics Associated With Reduced Graft Survival: An Approach to Expanding the Pool of Kidney Donors. Transplantation (2002) 74(9):1281–6. doi: 10.1097/00007890-200211150-00014
5. Sulikowski T, Tejchman K, Zietek Z, Urasinska E, Domanski L, Sienko J, et al. Histopathologic Evaluation of Pretransplantation Biopsy as a Factor Influencing Graft Function After Kidney Transplantation in 3-Year Observation. Transplant Proc (2010) 42(9):3375–81. doi: 10.1016/j.transproceed.2010.08.060
6. Nagaraja P, Roberts GW, Stephens M, Horvath S, Kaposztas Z, Chavez R, et al. Impact of Expanded Criteria Variables on Outcomes of Kidney Transplantation From Donors After Cardiac Death. Transplantation (2015) 99(1):226–31. doi: 10.1097/TP.0000000000000304
7. Ali T, Khan I, Simpson W, Prescott G, Townend J, Smith W, et al. Incidence and Outcomes in Acute Kidney Injury: A Comprehensive Population-Based Study. J Am Soc Nephrol (2007) 18(4):1292–8. doi: 10.1681/ASN.2006070756
8. Boffa C, van de Leemkolk F, Curnow E, Homan VDHJ, Gilbert J, Sharples E, et al. Transplantation of Kidneys From Donors With Acute Kidney Injury: Friend or Foe? Am J Transplant (2017) 17(2):411–9. doi: 10.1111/ajt.13966
9. Tomita Y, Iwadoh K, Ogawa Y, Miki K, Kai K, Sannomiya A, et al. Single Graft Utilization From Donors With Severe Acute Kidney Injury After Circulatory Death. Transplant Direct (2018) 4(4):e355. doi: 10.1097/TXD.0000000000000768
10. Heilman RL, Smith ML, Smith BH, Kumar A, Srinivasan A, Huskey JL, et al. Long-Term Outcomes Following Kidney Transplantation From Donors With Acute Kidney Injury. Transplantation (2019) 103(9):e263–72. doi: 10.1097/TP.0000000000002792
11. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute Renal Failure - Definition, Outcome Measures, Animal Models, Fluid Therapy and Information Technology Needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care (2004) 8(4):R204–12. doi: 10.1186/cc2872
12. Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG. Acute Kidney Injury Network: Report of an Initiative to Improve Outcomes in Acute Kidney Injury. Crit Care (2007) 11(2):R31. doi: 10.1186/cc5713
13. Khwaja A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron Clin Pract (2012) 120(4):c179–84. doi: 10.1159/000339789
14. Al KA, Shaheen FA, Attar BA, Elamin KM, Al GF, Jondeby M. Successful Use of Kidneys From Deceased Donors With Acute Renal Failure. Prog Transplant (2007) 17(4):258–63. doi: 10.1177/152692480701700402
15. Kayler LK, Garzon P, Magliocca J, Fujita S, Kim RD, Hemming AW, et al. Outcomes and Utilization of Kidneys From Deceased Donors With Acute Kidney Injury. Am J Transplant (2009) 9(2):367–73. doi: 10.1111/j.1600-6143.2008.02505.x
16. Farney AC, Rogers J, Orlando G, Al-Geizawi S, Buckley M, Farooq U, et al. Evolving Experience Using Kidneys From Deceased Donors With Terminal Acute Kidney Injury. J Am Coll Surg (2013) 216(4):645–55. doi: 10.1016/j.jamcollsurg.2012.12.020
17. Lee MH, Jeong EG, Chang JY, Kim Y, Kim JI, Moon IS, et al. Clinical Outcome of Kidney Transplantation From Deceased Donors With Acute Kidney Injury by Acute Kidney Injury Network Criteria. J Crit Care (2014) 29(3):432–7. doi: 10.1016/j.jcrc.2013.12.016
18. Zheng YT, Chen CB, Yuan XP, Wang CX. Impact of Acute Kidney Injury in Donors on Renal Graft Survival: A Systematic Review and Meta-Analysis. Ren Fail (2018) 40(1):649–56. doi: 10.1080/0886022X.2018.1535982
19. Bonventre JV, Yang L. Cellular Pathophysiology of Ischemic Acute Kidney Injury. J Clin Invest (2011) 121(11):4210–21. doi: 10.1172/JCI45161
20. Gonsalez SR, Cortes AL, Silva R, Lowe J, Prieto MC, Silva LL. Acute Kidney Injury Overview: From Basic Findings to New Prevention and Therapy Strategies. Pharmacol Ther (2019) 200:1–12. doi: 10.1016/j.pharmthera.2019.04.001
21. Salvadori M, Rosso G, Bertoni E. Update on Ischemia-Reperfusion Injury in Kidney Transplantation: Pathogenesis and Treatment. World J Transplant (2015) 5(2):52–67. doi: 10.5500/wjt.v5.i2.52
22. Basile DP, Donohoe D, Roethe K, Osborn JL. Renal Ischemic Injury Results in Permanent Damage to Peritubular Capillaries and Influences Long-Term Function. Am J Physiol Renal Physiol (2001) 281(5):F887–99. doi: 10.1152/ajprenal.2001.281.5.F887
23. Situmorang GR, Sheerin NS. Ischaemia Reperfusion Injury: Mechanisms of Progression to Chronic Graft Dysfunction. Pediatr Nephrol (2019) 34(6):951–63. doi: 10.1007/s00467-018-3940-4
24. Salvadori M, Tsalouchos A. Histological and Clinical Evaluation of Marginal Donor Kidneys Before Transplantation: Which Is Best? World J Transplant (2019) 9(4):62–80. doi: 10.5500/wjt.v9.i4.62
25. Irish WD, Ilsley JN, Schnitzler MA, Feng S, Brennan DC. A Risk Prediction Model for Delayed Graft Function in the Current Era of Deceased Donor Renal Transplantation. Am J Transplant (2010) 10(10):2279–86. doi: 10.1111/j.1600-6143.2010.03179.x
26. Zhang H, Zheng L, Qin S, Liu L, Yuan X, Fu Q, et al. Evaluation of Predictive Models for Delayed Graft Function of Deceased Kidney Transplantation. Oncotarget (2018) 9(2):1735–44. doi: 10.18632/oncotarget.22711
27. Rao PS, Schaubel DE, Guidinger MK, Andreoni KA, Wolfe RA, Merion RM, et al. A Comprehensive Risk Quantification Score for Deceased Donor Kidneys: The Kidney Donor Risk Index. Transplantation (2009) 88(2):231–6. doi: 10.1097/TP.0b013e3181ac620b
28. Oberbauer R, Rohrmoser M, Regele H, Muhlbacher F, Mayer G. Apoptosis of Tubular Epithelial Cells in Donor Kidney Biopsies Predicts Early Renal Allograft Function. J Am Soc Nephrol (1999) 10(9):2006–13. doi: 10.1681/ASN.V1092006
29. Oda A, Morozumi K, Uchida K. Histological Factors of 1-H Biopsy Influencing the Delayed Renal Function and Outcome in Cadaveric Renal Allografts. Clin Transplant (1999) 13 Suppl 1:6–12.
30. Pokorna E, Vitko S, Chadimova M, Schuck O, Ekberg H. Proportion of Glomerulosclerosis in Procurement Wedge Renal Biopsy Cannot Alone Discriminate for Acceptance of Marginal Donors. Transplantation (2000) 69(1):36–43. doi: 10.1097/00007890-200001150-00008
31. Matignon M, Desvaux D, Noel LH, Roudot-Thoraval F, Thervet E, Audard V, et al. Arteriolar Hyalinization Predicts Delayed Graft Function in Deceased Donor Renal Transplantation. Transplantation (2008) 86(7):1002–5. doi: 10.1097/TP.0b013e31818776b2
32. Goumenos DS, Kalliakmani P, Tsamandas AC, Maroulis I, Savidaki E, Fokaefs E, et al. The Prognostic Value of Frozen Section Preimplantation Graft Biopsy in the Outcome of Renal Transplantation. Ren Fail (2010) 32(4):434–9. doi: 10.3109/08860221003658241
33. Hall IE, Reese PP, Weng FL, Schroppel B, Doshi MD, Hasz RD, et al. Preimplant Histologic Acute Tubular Necrosis and Allograft Outcomes. Clin J Am Soc Nephrol (2014) 9(3):573–82. doi: 10.2215/CJN.08270813
34. Naesens M. Zero-Time Renal Transplant Biopsies: A Comprehensive Review. Transplantation (2016) 100(7):1425–39. doi: 10.1097/TP.0000000000001018
35. Liapis H, Gaut JP, Klein C, Bagnasco S, Kraus E, Farris AR, et al. Banff Histopathological Consensus Criteria for Preimplantation Kidney Biopsies. Am J Transplant (2017) 17(1):140–50. doi: 10.1111/ajt.13929
36. Wen Y, Yang C, Menez SP, Rosenberg AZ, Parikh CR. A Systematic Review of Clinical Characteristics and Histologic Descriptions of Acute Tubular Injury. Kidney Int Rep (2020) 5(11):1993–2001. doi: 10.1016/j.ekir.2020.08.026
37. Remuzzi G, Grinyo J, Ruggenenti P, Beatini M, Cole EH, Milford EL, et al. Early Experience With Dual Kidney Transplantation in Adults Using Expanded Donor Criteria. Double Kidney Transplant Group (DKG). J Am Soc Nephrol (1999) 10(12):2591–8. doi: 10.1681/ASN.V10122591
38. Stevens LA, Manzi J, Levey AS, Chen J, Deysher AE, Greene T, et al. Impact of Creatinine Calibration on Performance of GFR Estimating Equations in a Pooled Individual Patient Database. Am J Kidney Dis (2007) 50(1):21–35. doi: 10.1053/j.ajkd.2007.04.004
39. Zhang H, Fu Q, Liu J, Li J, Deng R, Wu C, et al. Risk Factors and Outcomes of Prolonged Recovery From Delayed Graft Function After Deceased Kidney Transplantation. Ren Fail (2020) 42(1):792–8. doi: 10.1080/0886022X.2020.1803084
40. Hall IE, Schroppel B, Doshi MD, Ficek J, Weng FL, Hasz RD, et al. Associations of Deceased Donor Kidney Injury With Kidney Discard and Function After Transplantation. Am J Transplant (2015) 15(6):1623–31. doi: 10.1111/ajt.13144
41. Lombardi D, Becherucci F, Romagnani P. How Much can the Tubule Regenerate and Who Does It? An Open Question. Nephrol Dial Transplant (2016) 31(8):1243–50. doi: 10.1093/ndt/gfv262
42. Kudose S, Hoshi M, Jain S, Gaut JP. Renal Histopathologic Findings Associated With Severity of Clinical Acute Kidney Injury. Am J Surg Pathol (2018) 42(5):625–35. doi: 10.1097/PAS.0000000000001028
Keywords: kidney transplantation, pretransplant biopsy, acute tubular injury, delayed graft function, deceased donor
Citation: Wang J, Liu J, Wu W, Yang S, Liu L, Fu Q, Li J, Chen X, Deng R, Wu C, Long S, Zhang W, Zhang H, Mao H and Chen W (2022) Combining Clinical Parameters and Acute Tubular Injury Grading Is Superior in Predicting the Prognosis of Deceased-Donor Kidney Transplantation: A 7-Year Observational Study. Front. Immunol. 13:912749. doi: 10.3389/fimmu.2022.912749
Received: 04 April 2022; Accepted: 16 May 2022;
Published: 30 June 2022.
Edited by:
Helong Dai, Central South University, ChinaReviewed by:
Su-xia Wang, Peking University, ChinaCopyright © 2022 Wang, Liu, Wu, Yang, Liu, Fu, Li, Chen, Deng, Wu, Long, Zhang, Zhang, Mao and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenfang Chen, Y2h3ZmFuZ0BtYWlsLnN5c3UuZWR1LmNu; Haiping Mao, bWFvaHBAbWFpbC5zeXN1LmVkdS5jbg==; Huanxi Zhang, emhhbmdoeDM3QG1haWwuc3lzdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.