
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 25 July 2022
Sec. Autoimmune and Autoinflammatory Disorders
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.909783
This article is part of the Research Topic Osteoarticular-immunological Interplay in Response to Disease and Therapy View all 11 articles
 Kevin Sheng-Kai Ma1,2,3
Kevin Sheng-Kai Ma1,2,3 Jung-Nien Lai4,5
Jung-Nien Lai4,5 Eshwar Thota3
Eshwar Thota3 Hei-Tung Yip6,7
Hei-Tung Yip6,7 Ning-Chien Chin8,9*
Ning-Chien Chin8,9* James Cheng-Chung Wei10,11,12*
James Cheng-Chung Wei10,11,12* Thomas E. Van Dyke13,14*
Thomas E. Van Dyke13,14*Objective: To identify the relationship between osteoarthritis and periodontitis.
Methods: 144,788 periodontitis patients and 144,788 propensity score-matched controls without history of periodontitis were enrolled in this cohort study. A Cox proportional hazard model was used to estimate the risk of osteoarthritis. Survival analysis was utilized to assess the time-dependent effect of periodontitis on osteoarthritis. Age and gender were stratified to identify subgroups at risk. A symmetrical case-control analysis was designed to determine the relationship between present periodontitis and history of osteoarthritis.
Results: Patients with periodontitis had higher risk of osteoarthritis (hazard ratio, HR =1.15, 95% CI =1.12–1.17, p < 0.001) and severe osteoarthritis that led to total knee replacement or total hip replacement (TKR/THR) (HR =1.12, 95% CI =1.03–1.21, p < 0.01) than controls, which was time-dependent (log-rank test p < 0.01). The effect of periodontitis on osteoarthritis was significant in both genders and age subgroups over 30 years-old (all p < 0.001). Among them, females (HR=1.27, 95% CI = 1.13–1.42, p < 0.001) and patients aged over 51 (HR= 1.21, 95% CI =1.10-1.33, p < 0.001) with periodontitis were predisposed to severe osteoarthritis. In addition, periodontitis patients were more likely to have a history of osteoarthritis (odds ratio = 1.11, 95% CI = 1.06 - 1.17, p < 0.001).
Conclusions: These findings suggest an association between periodontitis and a higher risk of osteoarthritis, including severe osteoarthritis that led to TKR/THR. Likewise, periodontitis is more likely to develop following osteoarthritis. A bidirectional relationship between osteoarthritis and periodontitis was observed.
1. Periodontitis was independently associated with a significantly higher risk of osteoarthritis than did controls.
2. Females and those aged over 50 with periodontitis were predisposed to severe osteoarthritis that needed surgery.
3. An independent bidirectional relationship between osteoarthritis and periodontitis was observed.
Osteoarthritis is characterized by progressive deterioration of articular cartilage with remodelling and proliferation of the bone beneath it (1–3), which serves as one of the leading causes of disability (1). Following the deterioration of articular cartilage, worsening osteoarthritis is one major indication for total joint replacement, with other indications including rheumatoid arthritis (RA) (2, 3). Among well-known etiological factors of osteoarthritis, primary osteoarthritis is caused by the malfunctioning synthetic function of hyaline cartilage, for which obesity and overloading contribute to its progression (1). Osteoarthritis can as well be secondary to causes such as instability of joint due to ligamentous laxity, neuropathies following diabetes mellitus (DM) (4, 5), inflammatory conditions following RA (6) or gout (3, 7), and aseptic necrosis (8). Mechanical insults in the form of trauma also fall into the secondary form of post-traumatic osteoarthritis, in which mechanical overload is then associated with a failure of the axis in the sense of valgosity and varosity of the knee joint (1). Apart from these recognized etiologies, it has been recently proposed that complement-mediated inflammatory cascades play a central role in osteoarthritis progression (9). That is, in addition to the conventional degenerative model (1), our knowledge of osteoarthritis pathogenesis has been expanded with an inflammation-dependent theory (9).
Periodontitis is an oral disease characterized by progressive inflammatory destruction of the periodontium and alveolar bone caused by biofilm-forming micro-organisms (10, 11). Such destructive inflammation is driven by complement-dependent mechanisms following oral microbial dysbiosis (12), and may translocate out of the oral cavity (10, 13–15). Clinical signs of periodontitis include gingival inflammation, alveolar bone loss, tooth mobility and eventually tooth loss (16). Periodontitis has been suggested to be a risk factor for systemic inflammatory conditions such as RA, even after considering known comorbidities (17); the underlying mechanism of which involve anti–citrullinated protein antibody (ACPA) formation by the periodontitis-associated pathogen Porphyromonas gingivalis (11). Likewise, periodontitis has been demonstrated to predispose to type 2 diabetes (14, 18–20), through mechanisms including metastatic inflammation in response to periodontal bacteria invasion (10, 18). These findings suggest a role for periodontal micro-organisms in systemic inflammation (17, 18) that may serve as the etiology of osteoarthritis acting through both complement cascades and periodontitis-associated systemic inflammation.
Failure of knee joint prosthesis may result from bacterial infection (2, 3), including oral bacteria that exist in the joint synovial samples from both natural and artificial joint tissues (13, 15). That is, there is an association between periodontitis and worse prognosis of total knee replacement (TKR) surgery (13, 15, 21, 22). Moreover, mechanical debridement to reduce oral bacteria has been proposed to reduce TKR failure (21, 22). This is consistent with our knowledge that periodontitis is associated with high risk of peri-prosthetic infection of alveolar bone implants, also known as peri-implantitis; as well as our knowledge of the proven efficacy of mechanical debridement for peri-implantitis management (23). Although the effect of periodontitis on risk of RA (24) and worse prognosis of TKR (13, 15, 21, 22) has been suggested, so far no studies have reported whether periodontitis as an chronic low-grade inflammatory event may be an independent risk factor of osteoarthritis in the general population. Given both the fact that bacterial invasion to the synovium could trigger complement cascades, and that periodontitis may exacerbate systemic inflammatory diseases that may drive osteoarthritis (11, 17, 25, 26), we conducted this population-based cohort study to identify whether periodontitis is a risk factor for osteoarthritis.
This retrospective cohort study used data from the Longitudinal Health Insurance Database (LHID), a registry that includes all claimed diagnoses and treatments from outpatient visits, as well as emergency and hospitalization medical records. From LHID, one million randomly sampled eligible patients with periodontitis diagnosed between 1997-2013 were enrolled.
To select non-periodontitis controls without bias, propensity score matching was applied. The propensity score match using strata was performed by adjusting for age, gender, socioeconomic variables, including income, occupation, and healthcare accessibility in the residential area, underlying comorbidities, including systemic lupus erythematosus (SLE), RA, obesity, DM, hypertension, hyperlipidaemia, chronic liver disease, osteonecrosis, Paget’s disease, and hypothyroidism, and the year of periodontitis onset or matched-year for controls, to control for confounding factors between the periodontitis and non-periodontitis groups. The propensity score is a probability estimated through logistic regression (27–30), with which the cases and controls were matched on a 1:1 basis. To ensure the selection of the matched periodontitis and non-periodontitis cases was not biased, standard mean difference (SMD) was derived for the comparison between the cases and the controls. When the SMDs were equal to or less than 0.05, the characteristics of both groups were considered similar (25).
To assure the validity and consistency of the diagnosis used in the cohort, both osteoarthritis and periodontitis were confirmed in at least two outpatient visits or at least one inpatient discharge note within one year; all medical records, along with the diagnoses, were peer-reviewed for quality control by rheumatologists and dentists, respectively. Moreover, the validity of diagnoses has been confirmed in previous studies (31–33).The database was de-identified, and the current study was approved by the Institutional Review Board of Chung Shan Medical University Hospital (approval number CS15134).
To determine whether periodontitis was associated with higher risks of osteoarthritis, medical records of patients diagnosed with periodontitis during 1997 to 2013 were retrieved from LHID. The occurrence of osteoarthritis after periodontitis onset was compared with that of propensity score-matched controls without periodontitis. The diagnosis of periodontitis was made following periodontal examinations that evaluated: (1) probing depth ≥ 5 mm in at least 4 teeth with each ≥ 1 site, (2) clinical attachment level (CAL) loss ≥5 mm at the same site, and (3) observed bleeding upon stimulus. The periodontal evaluations and diagnoses of periodontal diseases were made by dentists from medical centres, community hospitals and private dental clinics; the diagnosis of chronic periodontitis was confirmed in at least two outpatient visits within two years and peer-reviewed by other dentists to ensure its validity and consistency. The enrolled participants were followed up until the occurrence of osteoarthritis, December, 2013, or withdrawal, whichever occurred first.
To ensure each observed osteoarthritis case developed after periodontitis, we excluded the following patients: (1) patients with missing data throughout the period, including demographic information, comorbidity covariates, and death or loss to follow-up, (2) edentulous patients, (3) patients with tobacco addiction or alcoholism, (4) patients who throughout the study period had been diagnosed with osteoarthritis before periodontitis onset, (5) those whose diagnoses were made before 2000 or after 2012.
The diagnosis of osteoarthritis, which was the primary outcome of the periodontitis cohort, was made following the American College of Rheumatology (ACR) diagnostic criteria for osteoarthritis of the hand (26), hip (27), and knee (28, 29). A sensitivity analysis restricting the outcome of interest to only severe osteoarthritis that resulted in TKR or total hip replacement (THR) was conducted, for which osteoarthritis had to be the main diagnosis in the discharge notes of TKR/THR. Accordingly, the subsequent risks of receiving TKR/THR for osteoarthritis following periodontitis throughout the study period, as parameterized by adjusted hazard ratios (aHRs), was derived.
To identify the subgroups at risk of developing osteoarthritis and severe osteoarthritis following periodontitis, stratification of the analyses based on age and sex were carried out.
To identify whether the relationship between osteoarthritis and periodontitis was bidirectional or unidirectional, a symmetrical case-control analysis was designed parallel to the periodontitis cohort. Cases eligible for osteoarthritis case-control analysis were patients with periodontitis, for which their past history of osteoarthritis was retrieved from LHID. Propensity score-matched controls in the case-control analysis were selected based on demographic characteristics and comorbidities at baseline of periodontitis onset. Adjusted variables in propensity score matching were identical to those matched in the above-mentioned cohort design, and in the above-mentioned sensitivity analysis. The primary outcome, set as the history of osteoarthritis before periodontitis onset, was compared with that of propensity score-matched non-periodontitis controls, for which the odds ratios (ORs) were derived. Patients who had been diagnosed with periodontitis prior to osteoarthritis onset were excluded.
Baseline demographics and comorbidities at periodontitis onset were compared through chi-square tests for categorical variables and t-tests for continuous variables. Kaplan-Meier survival analysis (34) was used to compute the cumulative incidence of osteoarthritis, and a log-rank test was used to test the significance of differences between cases of periodontitis and propensity score-matched controls. Cox proportional hazard regression with time-dependent periodontitis and comorbidity information using the counting process was used to produce the HRs of osteoarthritis between periodontitis cases and propensity score-matched non-periodontitis controls. All analyses were conducted using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
For the periodontitis cohort, a total of 148,224 eligible patients who were newly diagnosed with periodontitis were identified from LHID (Figure 1). After propensity score matching, 144,788 subjects from periodontitis and the non-periodontitis group were selected (Figure 1) with a matched mean age of 39 ± 15 years for the final cohort (Table 1). There was no statistically significant difference for demographic variables and incidence of comorbidities between the periodontitis group and the non-periodontitis group (all SMDs>0.05) (Table 1).
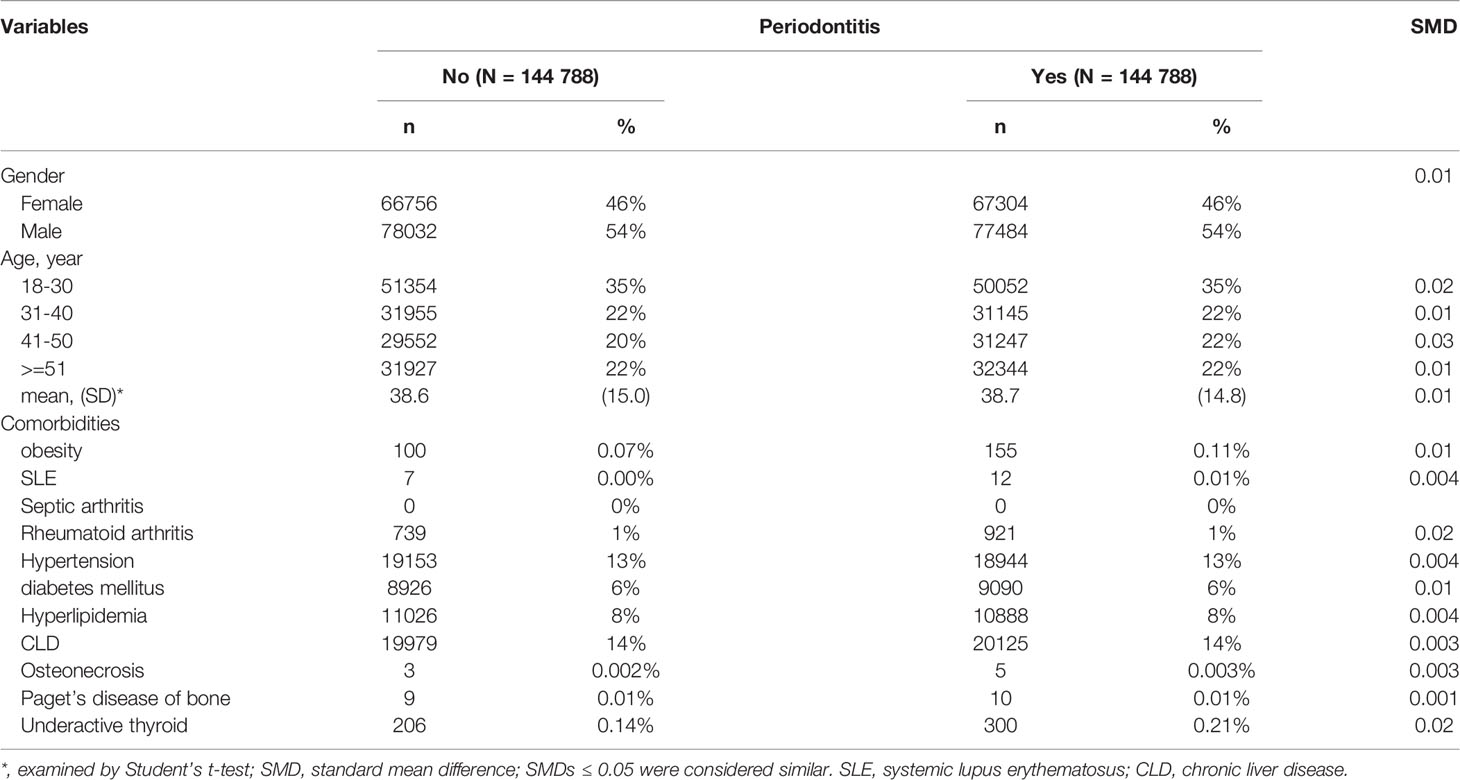
Table 1 Baseline characteristics for the periodontitis and the non-periodontitis cohorts after propensity score matching.
Among the 144,788 periodontitis cases enrolled in this study, 21,021 cases developed osteoarthritis after the onset of periodontitis, which was identified over 1,218,644 observed person-years. The incidence rate (IR) of osteoarthritis in patients who had periodontitis was significantly higher than the non-periodontitis cohort (1.72 v.s. 1.48 per 1,000 person-years) (p <0.001). Patients who had periodontitis had a significantly higher risk of osteoarthritis when compared to the non-periodontitis controls (adjusted HR=1.15, 95%CI=1.12-1.17) (Table 2). To sum up, the cumulative incidence of osteoarthritis in the periodontitis group was significantly higher than that of the non-periodontitis group, which was statistically significant (log-rank test p < 0.01) (Figure 2).
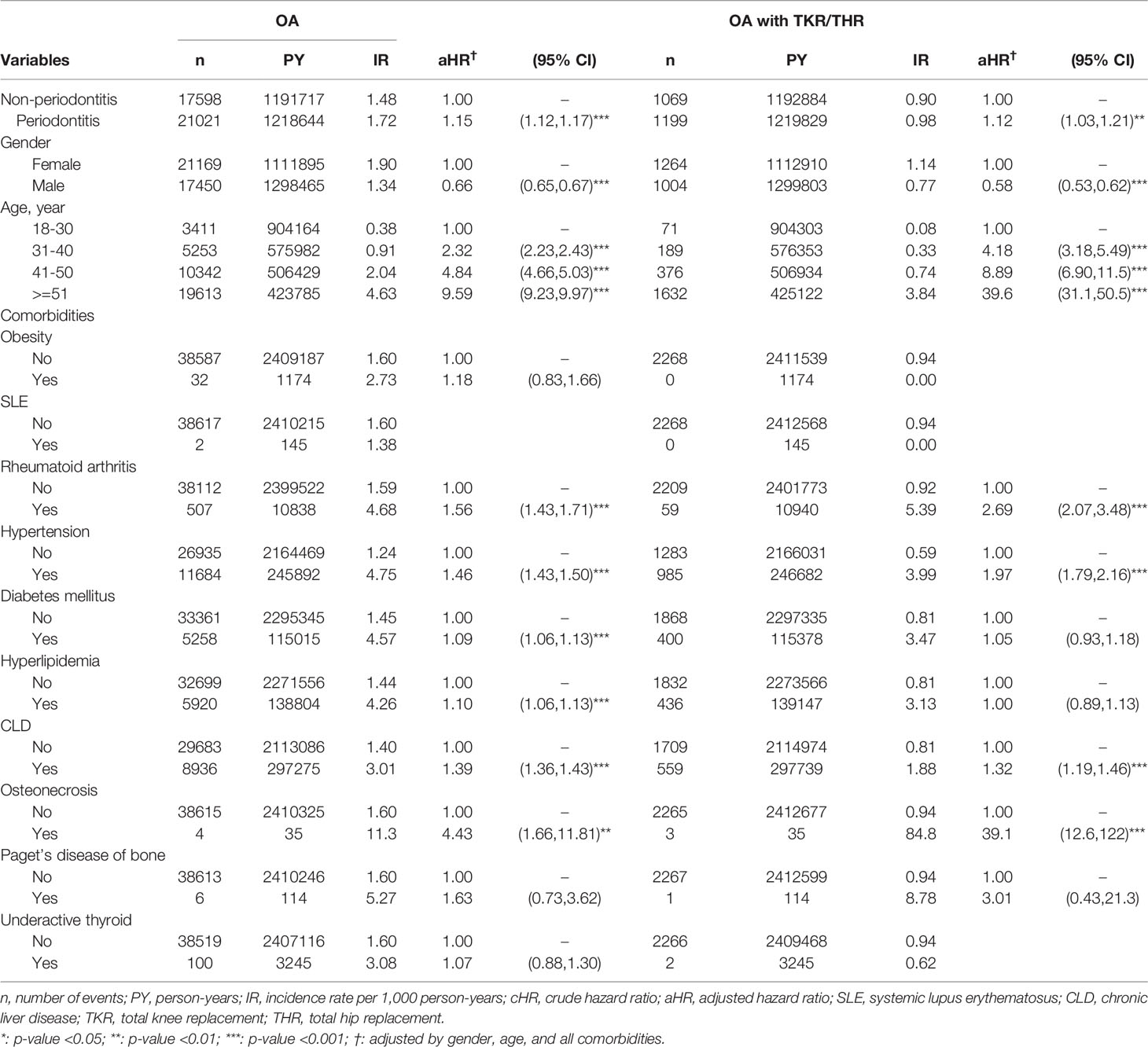
Table 2 Multiple Cox proportional model for risks of osteoarthritis (OA) in the periodontitis cohort.
Findings of the subgroup analysis suggested that the association between periodontitis and the risk of developing osteoarthritis was significant for both genders. Among them, the risk of osteoarthritis for 9,626 male periodontitis patients (aHR=1.18, 95% CI=1.15-1.22, p < 0.001) was higher than that for 11,395 female periodontitis patients (aHR=1.11, 95% CI = 1.09-1.15, p < 0.001) (Table 3).
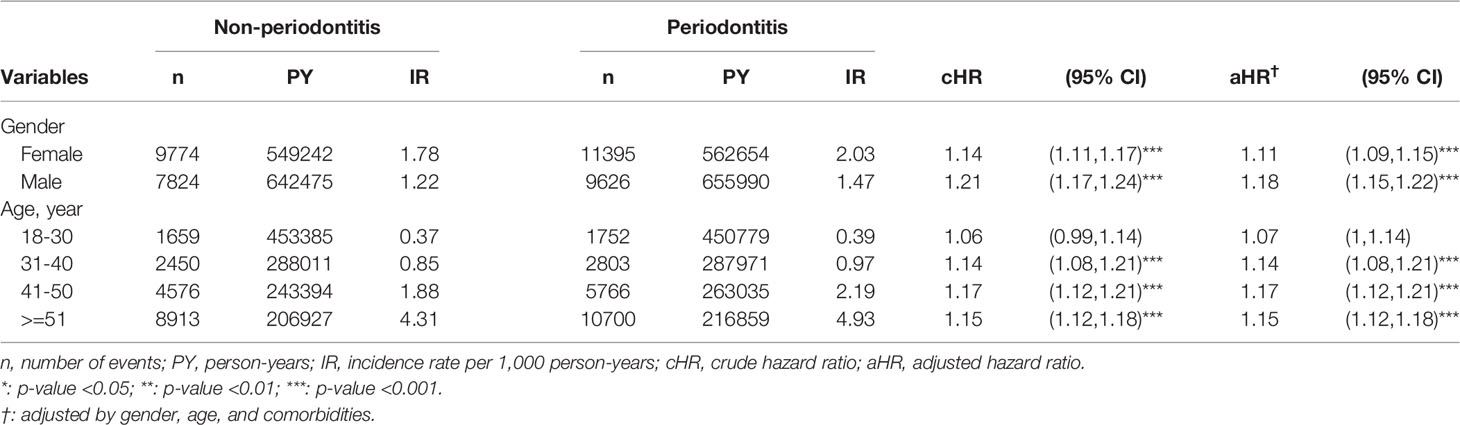
Table 3 Subgroup analyses on risks of osteoarthritis following periodontitis, based on sex and age stratification.
Likewise, subgroup analysis based on age stratification showed that the risk of osteoarthritis in the periodontitis group was particularly high among patients aged over 30. Specifically, periodontitis patients aged between 31-40 (n=2,803, aHR=1.14, 95% CI = 1.08-1.21, p < 0.001), aged between 41-50 (n=5,766, aHR=1.17, 95% CI=1.12-1.21, p < 0.001), and aged over 51 (n=10,700, aHR = 1.15, 95% CI = 1.12-1.18, p < 0.001) had significantly higher risk of developing osteoarthritis. In comparison, the association between periodontitis and risk of osteoarthritis in those aged 18-30 (n = 1,752, aHR = 1.07, 95% CI = 1-1.14) was not significant (Table 3).
Among the 21,021 cases that developed osteoarthritis following periodontitis onset, 1,199 patients received TKR/THR. The risk of osteoarthritis-associated TKR/THR in periodontitis patients was significantly higher than the non-periodontitis controls (aHR = 1.12, 95% CI = 1.03-1.21, p < 0.01) (Table 2). Likewise, the incidence rate (IR) of severe osteoarthritis that needed TKR/THR in patients who had periodontitis was significantly higher than the non-periodontitis controls (0.98 v.s. 0.90 per 1,000 person years) (p <0.01) (Table 2).
The subgroup analysis suggested that the association between periodontitis and the risk of severe osteoarthritis that needed TKR/THR was particularly significant for females (aHR = 1.27; 95% CI = 1.13 – 1.42) (Table 4). Likewise, subgroup analysis based on age stratification showed that the risk of osteoarthritis in the periodontitis group was significantly higher among patients aged over 51 (n = 878, aHR = 1.21, 95% CI = 1.10-1.33, p < 0.001). In comparison, the association between periodontitis and risks of osteoarthritis for those aged below 50 and males was not significant (Table 4).
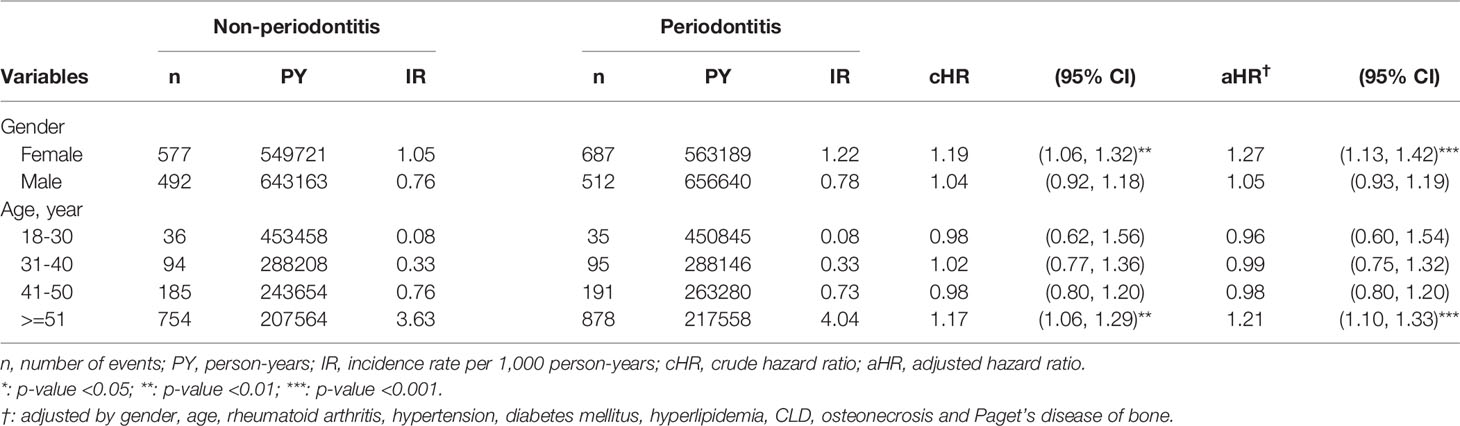
Table 4 Subgroup analyses on risks of severe osteoarthritis that led to total knee or total hip replacement following periodontitis, based on sex and age stratification.
Patients with periodontitis were more likely to have a history of osteoarthritis (adjusted OR, aOR = 1.11, 95% CI = 1.06 - 1.17, p < 0.001), compared to non-periodontitis controls. This was identified in the symmetrical case-control analysis consisting of 51,551 periodontitis patients (Supplementary Table S1), in which 4,783 cases (9.3%) had osteoarthritis before periodontitis onset. The OR of osteoarthritis history was significantly higher among periodontitis patients than that of non-periodontitis controls (9.3% v.s. 8.0%) (Table 5).
Among all enrolled patients, 330 patients (1.0%) below the age of 30 had osteoarthritis before periodontitis onset. Compared with those aged below 30, patients with periodontitis aged between 31-40 (n=396, aOR=2.11, 95% CI = 1.82-2.45), patients aged between 41-50 (n=979, aOR=3.59, 95% CI = 3.16-4.08), and aged over 51 (n=7,202, aOR=12.2, 95% CI = 10.9-13.7) were associated with higher incidence of osteoarthritis history (Table 5). Moreover, females were associated with higher incidence of osteoarthritis history (n=5,425, 11%), compared to males (n=3,482, aOR= 0.56, 95% CI =0.53-0.59) (Table 5). Both the age and sex distribution of osteoarthritis were in line with previous studies (1, 4).
The findings of this cohort study suggest that periodontitis and osteoarthritis are linked in a bidirectional pattern. After propensity score matching for potential confounding factors including common risk factors for osteoarthritis, the Cox proportional hazard model revealed that the risk of osteoarthritis was significantly higher for patients who had periodontitis than that for the non-periodontitis controls. These findings were time-dependent, as suggested by the log-rank test of the Kaplan–Meier curve, and persisted in the sensitivity analysis, in which the criteria of osteoarthritis was confined to surgically treated osteoarthritis with TKR/THR. Patients of both genders and all age groups aged over 30 had higher risks for osteoarthritis following periodontitis; among them, women and those aged over 51 had higher risks for severe osteoarthritis that led to TKR/THR. In line with the epidemiology of osteoarthritis (1–4), the incidence rates of osteoarthritis were higher among females and the elders in our study. To the contrary, the association between periodontitis and the risk for osteoarthritis in males was higher following periodontitis. This is interesting as periodontitis is also more frequent and severe in males as opposed to females (35, 36). In the symmetrical case-control analysis, where the incidences of osteoarthritis history among periodontitis versus non-periodontitis participants were compared, periodontitis patients were more likely to have a history of osteoarthritis. Overall, increased risks of osteoarthritis and periodontitis were observed for patients with either disease.
Based on its etiologies, osteoarthritis could be classified into primary and secondary osteoarthritis. Primary osteoarthritis, caused by a disorder in the synthetic function of hyaline cartilage, comprises most of the cases. Although conventionally considered as a non-inflammatory arthritis (1), osteoarthritis has lately been suggested to be driven by complement-mediated inflammatory processes (9, 37) and has been managed as an inflammatory disease with medications including nonsteroidal antiinflammatory drugs (NSAIDs) (38). Hence, a paradigm shift of osteoarthritis pathogenesis has occurred towards an inflammatory-associated progression, which expands our understanding of the wear-and-tear theory (1). At the same time, infections originating in the oral cavity, with pathogens being isolated from the synovial fluid of patients operated for peri-prosthetic joint infection (PJI), have been suggested to contribute to a worse prognosis for TKR/THR (13, 22). These concepts (13, 15) have been applied to promote prophylactic management of periodontal diseases prior to joint replacement surgeries (21, 22). Given the fact that periodontopathic bacteria have been identified in joint synovium (10, 13–15), and that inflammatory complement cascades may be initiated by bacterial infection (39–41), it is possible that periodontitis could lead to or exacerbate the progression of osteoarthritis. In this first hypothesis, instead of obvious infection and inflammation that develops into septic arthritis (42), periodontal pathogens may cause or aggravate the inflammatory process in the joints, finally developing into osteoarthritis, or even severe osteoarthritis that requires surgical treatment.
Apart from being involved in idiopathic or primary osteoarthritis, periodontitis and periodontopathic bacterial invasion (43) have been shown to increase the risk of RA (7, 11, 17) and DM (5, 10, 14, 18, 20, 44, 45). These clinically evident correlations provide a plausible causal interpretation that may connect periodontitis to secondary osteoarthritis. Periodontal pathogens may trigger osteoarthritis indirectly through mechanisms including autoantibodies such as ACPA (11, 46), or humoral immunity (47–50) that involves ANCA and B cell activation upon bacterial infection (25).
Osteoarthritis could develop following periodontal pathogen invasion, which may further indicate an infection-associated model for osteoarthritis progression. The underlying mechanism of this relationship could be similar to odontogenic infections that lead to metastatic infection, metastatic inflammation, or endotoxin-driven metastatic injury (10, 14, 51). In this sense, our findings could be interpreted that periodontitis is an etiologic factor for osteoarthritis, which may trigger joint degeneration once metastatic injury is initiated. To further explore this potential relationship, intervention studies are required to determine whether mechanical debridement and surgical management for periodontal diseases leads to the primary prevention of osteoarthritis, the clinical improvement of osteoarthritis, the prevention of PJI in patients who had received TKR/THR.
Periodontitis has been suggested to be a comorbidity with knee osteoarthritis in a cross-sectional study (52). Although the underlying mechanisms are yet to be studied, potential common denominators might include underlying inflammatory traits that made those individuals susceptible to both osteoarthritis and periodontitis, with the two diseases being regarded as inflammatory diseases. Our findings may be interpreted as periodontitis serving as an early sign, or a predictor of osteoarthritis, which may be clinically identified prior to osteoarthritis onset.
Findings in the present study supported that osteoarthritis also increased the risk for future periodontitis. Whether this is a direct common susceptibility of related to impaired motor function is unknown. Osteoarthritis of the hands, knees, and hip has been suggested to result in impaired functional ability (40) that may result in poor oral hygiene due to a lack of daily activity and patient education (53), with excess biofilm formation on teeth and the periodontium, followed by increased risks of dental caries (54, 55) and periodontal diseases. This may explain our findings in the case-control analysis of the correlation between present periodontitis and a history of osteoarthritis. Prospective studies including more parameters for osteoarthritis measurement, such as the Kellgren-Lawrence (K&L) grading scale (56), is warranted, to precisely depict the observed association between osteoarthritis and periodontitis.
Some limitations of this study include the lack of periodontal charting data, including probing depth measurments, clinical attachment level measurments, or relevant local inflammatory biomarker measurments. These parameters may be of interest as they represent the degree of periodontal destruction underlying periodontitis (57), through which their simultaneous correlation with K&L scores would be reflective of the above-propsed reciprocal or bidirectional model. Additionally, even though we have observed the temporal association between periodontitis and risk of osteoarthritis through time-dependent survival analysis, we cannot infer causation as bacterial cultures based on synovial samples and inflammatory biomarkers in pateints with osteoarthritis were not available in our database. Accordingly, we advocate studies with both K&L scores and synovial samples from osteoarthritis-involved joints to validate our findings. Despite these potential limitations, in relation to the previous studies, which were mainly cross-sectional, our major strengths include a fairly large sample size in a real-world setting (58, 59). Also, propensity score matching was adopted in this longitudinal study to minimize the effect of potential confounding factors (60) on associations.
To our knowledge, this is the first study suggesting a long-term association between periodontitis and osteoarthritis. After adjusting for known comorbidities and covariates, the association between periodontitis and risk of osteoarthritis was significant, for both sexes, and all periodontitis patients aged over 30 years. Females with periodontitis and those aged over 50 with periodontitis were predisposed to severe osteoarthritis that led to TKR/THR. We advocate for more research on infection-associated osteoarthritis pathogenesis and clinical studies.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by The institutional review board, chung shan medical university hospital. The ethics committee waived the requirement of written informed consent for participation.
KM , JW, and TD conceived and designed the study. KM and H-TY contributed to data analysis and interpretation. KM, J-NL, ET, and N-CC wrote the manuscript. All authors approved the final version of the manuscript to be published.
This work was supported by a research grant from International Team for Implantology (fund no. 1577_2021 to KSM).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.909783/full#supplementary-material
1. Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet (2019) 393:1745–59. doi: 10.1016/S0140-6736(19)30417-9
2. Lenguerrand E, Whitehouse MR, Beswick AD, Kunutsor SK, Foguet P, Porter M, et al. Risk Factors Associated With Revision for Prosthetic Joint Infection Following Knee Replacement: An Observational Cohort Study From England and Wales. Lancet Infect Dis (2019) 19:589–600. doi: 10.1016/S1473-3099(18)30755-2
3. Sodhi N, Mont MA. Survival of Total Hip Replacements. Lancet (2019) 393:613. doi: 10.1016/S0140-6736(18)31859-2
4. Swain S, Sarmanova A, Mallen C, Kuo CF, Coupland C, Doherty M, et al. Trends in Incidence and Prevalence of Osteoarthritis in the United Kingdom: Findings From the Clinical Practice Research Datalink (CPRD). Osteoarthr Cartilage (2020) 28:792–801. doi: 10.1016/j.joca.2020.03.004
5. Berenbaum F. Diabetes-Induced Osteoarthritis: From a New Paradigm to a New Phenotype. Postgrad Med J (2012) 88:240–2. doi: 10.1136/pgmj.2010.146399rep
6. Ma KS, Wang LT, Tsai SY. Correspondence to: 'Combination of Human Umbilical Cord Mesenchymal (Stromal) Stem Cell Transplantation With IFN-Gamma Treatment Synergistically Improves the Clinical Outcomes of Patients With Rheumatoid Arthritis'. Ann Rheum Dis (2020). doi: 10.1136/annrheumdis-2020-218704
7. Figueiredo CP, Simon D, Englbrecht M, Haschka J, Kleyer A, Bayat S, et al. Q.uantification and Impact of Secondary Osteoarthritis in Patients With Anti-Citrullinated Protein Antibody-Positive Rheumatoid Arthritis. Arthritis Rheumatol (2016) 68:2114–21. doi: 10.1002/art.39698
8. Bullough PG, DiCarlo EF. Subchondral Avascular Necrosis: A Common Cause of Arthritis. Ann Rheum Dis (1990) 49:412–20. doi: 10.1136/ard.49.6.412
9. Wang Q, Rozelle AL, Lepus CM, Scanzello CR, Song JJ, Larsen DM, et al. Identification of a Central Role for Complement in Osteoarthritis. Nat Med (2011) 17:1674–9. doi: 10.1038/nm.2543
10. Van Dyke TE, Starr JR. Unraveling the Link Between Periodontitis and Cardiovascular Disease. J Am Heart Assoc (2013) 2:e000657. doi: 10.1161/JAHA.113.000657
11. Ma KS, Chiang CH, Chen YW, Wang LT. Correspondence to 'Bacterial Citrullinated Epitopes Generated by Porphyromonas Gingivalis Infection-A Missing Link for ACPA Production'. Ann Rheum Dis (2021), 219255. doi: 10.1136/annrheumdis-2020-219255
12. Hajishengallis G, Kajikawa T, Hajishengallis E, Maekawa T, Reis ES, Mastellos DC, et al. Complement-Dependent Mechanisms and Interventions in Periodontal Disease. Front Immunol (2019) 10:406. doi: 10.3389/fimmu.2019.00406
13. Temoin S, Chakaki A, Askari A, El-Halaby A, Fitzgerald S, Marcus RE, et al. Identification of Oral Bacterial DNA in Synovial Fluid of Patients With Arthritis With Native and Failed Prosthetic Joints. J Clin Rheumatol (2012) 18:117–21. doi: 10.1097/RHU.0b013e3182500c95
14. Van Dyke TE, van Winkelhoff AJ. Infection and Inflammatory Mechanisms. J Clin Periodontol (2013) 40 Suppl 14:S1–7. doi; 10.1111/jcpe.12088
15. Adamkiewicz K, Platek AE, Legosz P, Czerniuk MR, Maldyk P, Szymanski FM. Evaluation of the Prevalence of Periodontal Disease as a non-Classical Risk Factor in the Group of Patients Undergoing Hip and/or Knee Arthroplasty. Kardiol Pol (2018) 76:633–36. doi: 10.5603/KP.a2017.0263
16. Wang CY, Lee BS, Jhang YT, Ma KS, Huang CP, Fu KL, et al. Er:YAG Laser Irradiation Enhances Bacterial and Lipopolysaccharide Clearance and Human Gingival Fibroblast Adhesion on Titanium Discs. Sci Rep (2021) 11:23954. doi: 10.1038/s41598-021-03434-1
17. Chen HH, Huang N, Chen YM, Chen TJ, Chou P, Lee YL, et al. Association Between a History of Periodontitis and the Risk of Rheumatoid Arthritis: A Nationwide, Population-Based, Case-Control Study. Ann Rheum Dis (2013) 72:1206–11. doi: 10.1136/annrheumdis-2012-201593
18. Preshaw PM, Alba AL, Herrera D, Jepsen S, Konstantinidis A, Makrilakis K, et al. Periodontitis and Diabetes: A Two-Way Relationship. Diabetologia (2012) 55:21–31. doi: 10.1007/s00125-011-2342-y
19. Chapple IL, Genco R, working group 2 of the joint EFPAAPw. Diabetes and Periodontal Diseases: Consensus Report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Periodontol (2013) 84:S106–12. doi: 10.1902/jop.2013.1340011
20. Engebretson SP, Hyman LG, Michalowicz BS, Schoenfeld ER, Gelato MC, Hou W, et al. The Effect of Nonsurgical Periodontal Therapy on Hemoglobin A1c Levels in Persons With Type 2 Diabetes and Chronic Periodontitis: A Randomized Clinical Trial. JAMA (2013) 310:2523–32. doi: 10.1001/jama.2013.282431
21. Tai TW, Lin TC, Ho CJ, Kao Yang YH, Yang CY. Frequent Dental Scaling Is Associated With a Reduced Risk of Periprosthetic Infection Following Total Knee Arthroplasty: A Nationwide Population-Based Nested Case-Control Study. PloS One (2016) 11:e0158096. doi: 10.1371/journal.pone.0158096
22. Frey C, Navarro SM, Blackwell T, Lidner C, Del Schutte H Jr. Impact of Dental Clearance on Total Joint Arthroplasty: A Systematic Review. World J Orthop (2019) 10:416–23. doi: 10.5312/wjo.v10.i12.416
23. Ferreira SD, Martins CC, Amaral SA, Vieira TR, Albuquerque BN, Cota LOM, et al. Periodontitis as a Risk Factor for Peri-Implantitis: Systematic Review and Meta-Analysis of Observational Studies. J Dent (2018) 79:1–10. doi: 10.1016/j.jdent.2018.09.010
24. Luan YZ, Chen BS, Ma KS. Sequencing of the 16S Ribosomal DNA Gene and Virulence of the Oral Microbiome in Patients With Rheumatoid Arthritis. Arthritis Rheumatol (2022). doi: 10.1002/art.42106
25. Novo E, Garcia-MacGregor E, Viera N, Chaparro N, Crozzoli Y. Periodontitis and Anti-Neutrophil Cytoplasmic Antibodies in Systemic Lupus Erythematosus and Rheumatoid Arthritis: A Comparative Study. J Periodontol (1999) 70:185–8. doi: 10.1902/jop.1999.70.2.185
26. Wu YD, Lin CH, Chao WC, Liao TL, Chen DY, Chen HH. Association Between a History of Periodontitis and the Risk of Systemic Lupus Erythematosus in Taiwan: A Nationwide, Population-Based, Case-Control Study. PloS One (2017) 12:e0187075. doi: 10.1371/journal.pone.0187075
27. Ma KS, Thota E, Huang JY, Huang YF, Wei JC. Onset of Oral Lichen Planus Following Dental Treatments: A Nested Case-Control Study. Oral Dis (2021). doi: 10.1111/odi.14115
28. Ma KS, Illescas Ralda MM, Veeravalli JJ, Wang LT, Thota E, Huang JY, et al. Patients With Juvenile Idiopathic Arthritis are at Increased Risk for Obstructive Sleep Apnoea: A Population-Based Cohort Study. Eur J Orthod (2021) 44(2):226–31. doi: 10.1093/ejo/cjab050
29. Wu MC, Ma KS, Wang YH, Wei JC. Impact of Tonsillectomy on Irritable Bowel Syndrome: A Nationwide Population-Based Cohort Study. PloS One (2020) 15:e0238242. doi: 10.1371/journal.pone.0238242
30. Wu MC, Ma KS, Chen HH, Huang JY, Wei JC. Relationship Between Helicobacter Pylori Infection and Psoriasis: A Nationwide Population-Based Longitudinal Cohort Study. Med (Baltimore) (2020) 99:e20632. doi: 10.1097/MD.0000000000020632
31. Ma KS, Lai JN, Veeravalli JJ, Chiu LT, Van Dyke TE, Wei JC. Fibromyalgia and Periodontitis: Bidirectional Associations in Population-Based 15-Year Retrospective Cohorts. J Periodontol (2021) 93(6):877–87. doi: 10.1002/JPER.21-0256
32. Ma KS, Wu MC, Thota E, Wang YH, Alqaderi HE, Wei JC. Tonsillectomy as a Risk Factor of Periodontitis: A Population-Based Cohort Study. J Periodontol (2021) 93(5):721–31. doi: 10.1002/JPER.21-0215
33. Ma KS, Hasturk H, Carreras I, Dedeoglu A, Veeravalli JJ, Huang JY, et al. Dementia and the Risk of Periodontitis: A Population-Based Cohort Study. J Dent Res (2021) 101(3):270–7. doi: 10.1177/00220345211037220
34. Ma KS. Screening programs incorporating big data analytics. In: Keikhosrokiani P, editor. Big Data Analytics for Healthcare: Datasets, Techniques, Life Cycles, Management, and Applications. Academic Press: Elsevier (2022). p. (pp. 313–327). doi: 10.1016/B978-0-323-91907-4.00023-6
35. Shiau HJ, Reynolds MA. Sex Differences in Destructive Periodontal Disease: A Systematic Review. J Periodontol (2010) 81:1379–89. doi: 10.1902/jop.2010.100044
36. Eke PI, Dye BA, Wei L, Slade GD, Thornton-Evans GO, Borgnakke WS, et al. Update on Prevalence of Periodontitis in Adults in the United States: NHANES 2009 to 2012. J Periodontol (2015) 86:611–22. doi: 10.1902/jop.2015.140520
37. Wang LT, Ma KS. Correspondence to 'Normal Human Enthesis Harbours Conventional CD4+ and CD8+ T Cells With Regulatory Features and Inducible IL-17A and TNF Expression'. Ann Rheum Dis (2020). doi: 10.1136/annrheumdis-2020-217309
38. Haile Z, Khatua S. Beyond Osteoarthritis: Recognizing and Treating Infectious and Other Inflammatory Arthropathies in Your Practice. Prim Care (2010) 37:713–27. doi: 10.1016/j.pop.2010.07.004
39. Walport MJ. Complement. First of Two Parts. N Engl J Med (2001) 344:1058–66. doi: 10.1056/NEJM200104053441406
40. Kemper C, Mitchell LM, Zhang L, Hourcade DE. The Complement Protein Properdin Binds Apoptotic T Cells and Promotes Complement Activation and Phagocytosis. Proc Natl Acad Sci U.S.A. (2008) 105:9023–8. doi: 10.1073/pnas.0801015105
41. Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: A Key System for Immune Surveillance and Homeostasis. Nat Immunol (2010) 11:785–97. doi: 10.1038/ni.1923
42. Mathews CJ, Weston VC, Jones A, Field M, Coakley G. Bacterial Septic Arthritis in Adults. Lancet (2010) 375:846–55. doi: 10.1016/S0140-6736(09)61595-6
43. Ma KS, Chiang C, Lopez AAV, Wang L, Tsai S. Identifying Mechanisms Underlying the Association Between Cardiovascular Diseases and Periodontitis Using in Silico Analysis of Canonical Pathways. Am Heart J (2020) 229:172–3. doi: 10.1016/j.ahj.2020.10.038
44. Thota E, Veeravalli JJ, Manchala SK, Lakkepuram BP, Kodapaneni J, Chen YW, et al. Age-Dependent Oral Manifestations of Neurofibromatosis Type 1: A Case-Control Study. Orphanet J Rare Dis (2022) 17:93. doi: 10.1186/s13023-022-02223-x
45. Ma KSK, Liou YJ, Huang PH, Lin PS, Chen YW, Chang RF. (2021). Identifying Medically-Compromised Patients With Periodontitis-Associated Cardiovascular Diseases Using Convolutional Neural Network-Facilitated Multilabel Classification of Panoramic Radiographs. in: International Conference on Applied Artificial Intelligence (ICAPAI), (2021). pp. 1–4. doi: 10.1109/ICAPAI49758.2021.9462069
46. Wu KJ, Tu CC, Hu JX, Chu PH, Ma KS, Chiu HY, et al. Severity of Periodontitis and Salivary Interleukin-1beta Are Associated With Psoriasis Involvement. J Formos Med Assoc (2022) 29:S0929-6646(22)00037-7. doi: 10.1016/j.jfma.2022.01.017
47. Ma KS, Lee CC, Liu KJ, Wei JC, Lee YT, Wang LT. Safety and Seroconversion of Immunotherapies against SARS-CoV-2 Infection: A Systematic Review and Meta-Analysis of Clinical Trials. Pathogens (2021) 10(12):1537. doi: 10.3390/pathogens10121537
48. Ma KS, Saeed HN, Chodosh J, Wang CW, Chung YC, Wei LC, et al. Ocular manifestations of anti-neoplastic immune checkpoint inhibitor-associated Stevens-Johnson syndrome/toxic epidermal necrolysis in cancer patients. Ocul Surf. (2021) 22:47–50. doi: 10.1016/j.jtos.2021.06.010
49. Huang JW, Kuo CL, Wang LT, Ma KS, Huang WY, Liu FC, et al. Case Report: In Situ Vaccination by Autologous CD16+ Dendritic Cells and Anti-PD-L 1 Antibody Synergized With Radiotherapy To Boost T Cells-Mediated Antitumor Efficacy In A Psoriatic Patient With Cutaneous Squamous Cell Carcinoma. Front Immunol (2021) 12:752563. doi: 10.3389/fimmu.2021.752563
50. Ma KS. (2021). Deep neural networks for prediction and detection of ocular sequelae among survivors of Stevens-Johnson syndrome/toxic epidermal necrolysis, in: 2021 IEEE 17th International Conference on Intelligent Computer Communication and Processing (ICCP), . pp. 463–7. doi: 10.1109/ICCP53602.2021.9733636
51. Li X, Kolltveit KM, Tronstad L, Olsen I. Systemic Diseases Caused by Oral Infection. Clin Microbiol Rev (2000) 13:547–58. doi: 10.1128/CMR.13.4.547
52. Kim JW, Chung MK, Lee J, Kwok SK, Kim WU, Park SH, et al. Association of Periodontitis With Radiographic Knee Osteoarthritis. J Periodontol (2020) 91:369–76. doi: 10.1002/JPER.19-0068
53. Ma KS, Chang HC, Krupat E. Teaching Evidence-Based Medicine With Electronic Databases for Preclinical Education. Adv Physiol Educ (2021) 45:849–55. doi: 10.1152/advan.00057.2021
54. Ma KS, Wang LT, Blatz MB. Efficacy of Adhesive Strategies for Restorative Dentistry: A Systematic Review and Network Meta-analysis of Double-blind Randomized Controlled Trials Over 12 Months of Follow-up. J Prosthodont Res (2022). doi: 10.2186/jpr.JPR_D_21_00279
55. Ma KSK. Dose-dependent relationship between history of dental caries and increased risk of newly-onset systemic lupus erythematosus: a nationwide population-based cohort study. Ann Rheumatic Dis (2021) 80:648. doi: 10.1136/annrheumdis-2021-eular.3945
56. El-Sherif HE, Kamal R, Moawyah O. Hand Osteoarthritis and Bone Mineral Density in Postmenopausal Women; Clinical Relevance to Hand Function, Pain and Disability. Osteoarthr Cartilage (2008) 16:12–7. doi: 10.1016/j.joca.2007.05.011
57. Caton JG, Armitage G, Berglundh T, Chapple ILC, Jepsen S, Kornman KS, et al. A New Classification Scheme for Periodontal and Peri-Implant Diseases and Conditions - Introduction and Key Changes From the 1999 Classification. J Clin Periodontol (2018) 45 (Suppl 20):S1–8. doi: 10.1111/jcpe.12935
58. Ma KS, Tsai SY. Big Data-Driven Personal Protective Equipment Stockpiling Framework Under Universal Healthcare for Disease Control and Prevention in the COVID-19 Era. Int J Surg (2020) 79:290–1. doi: 10.1016/j.ijsu.2020.05.091
59. Ma KS. Integrating Travel History via Big Data Analytics Under Universal Healthcare Framework for Disease Control and Prevention in the COVID-19 Pandemic. J Clin Epidemiol (2021) 130:147–8. doi: 10.1016/j.jclinepi.2020.08.016
Keywords: cohort study, periodontitis, osteoarthritis, total knee replacement, total hip replacement, inflammation
Citation: Ma KS-K, Lai J-N, Thota E, Yip H-T, Chin N-C, Wei JC-C and Van Dyke TE (2022) Bidirectional Relationship Between Osteoarthritis and Periodontitis: A Population-Based Cohort Study Over a 15-year Follow-Up. Front. Immunol. 13:909783. doi: 10.3389/fimmu.2022.909783
Received: 31 March 2022; Accepted: 10 June 2022;
Published: 25 July 2022.
Edited by:
Michal Tomcik, Institute of Rheumatology, Prague, CzechiaCopyright © 2022 Ma, Lai, Thota, Yip, Chin, Wei and Van Dyke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning-Chien Chin, b3J0aG9idXR0ZXJmbHlAZ21haWwuY29t; James Cheng-Chung Wei, d2VpMzIyOEBnbWFpbC5jb20=; Thomas E. Van Dyke, dHZhbmR5a2VAZm9yc3l0aC5vcmc=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.