
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Immunol. , 19 July 2022
Sec. Alloimmunity and Transplantation
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.909008
This article is part of the Research Topic Novel treatments for Diffuse Large B-cell Lymphoma: the post-CART Era View all 6 articles
Diffuse large B-cell lymphoma is an aggressive and biologically heterogeneous disease. R-CHOP is the standard first line therapy and cures more than 60% of patients. Salvage high-dose chemotherapy with autologous stem cell transplant remains the standard second-line treatment for relapsed or refractory patients, and recently, three CD19 chimeric antigen receptor T cells (CART) cell products have been approved beyond 2 prior lines of systemic therapy. Nevertheless, some patients are not eligible for transplant or CARTs, or progress after these treatments. In this context, IgG-like bispecific antibodies (BsAbs) have been designed to treat B‐cell lymphomas. They combine two different monospecific antigen‐binding regions that target CD20 on B cells and engage T cells via CD3 in a 1:1 or 2:1 CD20:CD3 antigen binding fragment (Fab) format. The results of different phase 1 trials with BsAbs, including mosunetuzumab, glofitamab, epcoritamab and odeonextamab, have been recently published. They are infused intravenously or subcutaneously, and have a favorable toxicity profile, with reduced cytokine release syndrome and neurological toxicity. Moreover, these BsAbs have demonstrated very promising efficacy in B-cell lymphomas, including in aggressive lymphomas. New trials are currently ongoing to confirm BsAbs efficacy and tolerability, as well as to explore its efficacy in different lines of therapy or in combination with other drugs.
Diffuse large B-cell lymphoma (DLBCL), the most common subtype of non-Hodgkin lymphoma, is an aggressive and heterogeneous disease. Since the late 1990s, six to eight cycles of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) has been the standard of care (1). More than 60% of patients are cured with this regimen. There have been different trials trying to improve the results of R-CHOP without success, as those in which targeted therapies are added to the R-CHOP backbone: bortezomib (REMoDL-B trial) (2), ibrutinib (PHOENIX trial) (3), or lenalidomide (ROBUST trial) (4), or the trial in which rituximab is replaced by obinutuzumab (a glycoengineered, type II anti-CD20 monoclonal antibody, GOYA trial) (5). Nevertheless, in a recently published phase 3 trial, a modified regimen of R-CHOP (pola-R-CHP), in which vincristine was replaced by polatuzumab vedotin (anti-CD79b antibody-drug conjugate), was compared with the standard R-CHOP, in patients with previously untreated intermediate-risk or high-risk DLBCL, and progression-free survival (PFS) was significantly higher in the pola-R-CHP group than in the R-CHOP group (76.7% vs. 70.2% at 2 years, hazard ratio 0.73), with a similar safety profile in the two groups, although overall survival did not differ significantly (6).
Salvage high-dose chemotherapy with autologous stem cell transplant (ASCT) remains the standard second-line treatment for relapsed or refractory (R/R) patients. However, few patients are cured with this intensive approach, and applicability is limited by comorbidities and advanced age (7). Moreover, patients with refractory disease or relapse within 12 months of ASCT have poor outcomes even with this intense strategies, as it is shown in the SCHOLAR-1 multicenter retrospective study, in which the objective response rate (ORR) to the next line of therapy in such patients was 26% (CR, 7%), with a median overall survival (OS) rate of 6.3 months (8).
Recent novel immunotherapy approaches are changing the treatment landscape for these patients. CD19 chimeric antigen receptor T cells (CARTs), are autologous T cells that have been genetically reengineered using viral transduction to express an anti-CD19 single- chain variable fragment for antigen recognition. Three CD19 CART products have been approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) [axicabtagene ciloleucel (axi-cel), tisagenlecleucel (tisa-cel), and lisocabtagene maraleucel (liso-cel)], for the treatment of R/R aggressive B-cell lymphomas, including DLBCL, high-grade B-cell lymphoma, transformed follicular lymphoma, and primary mediastinal B-cell lymphoma, after ≥ 2 prior lines of systemic therapy, and they show high response rates with durable remissions (9–11). The most up-to-date data with axi-cel demonstrates an OS rate at 4 years of 44% (12).
Due to these impressive results beyond two lines of therapy, several trials tested CART therapy in second line in high risk DLBCL patients. Three randomized phase 3 clinical trials compared the second line treatment with high-dose chemotherapy followed by ASCT (standard arm), with CART therapy (experimental arm), in high-risk patients with DLBCL, refractory or in early relapse (during the first year after finishing the first line treatment) (13–15). An improvement in event-free survival compared with ASCT was demonstrated in 2 of them (13, 15). As a result of these trials, FDA has approved on April 1st, 2022, the use of axi-cel in second line for adult patients with DLBCL refractory or relapsed within 12 months after first-line chemoimmunotherapy. Therefore, CARTs have changed the treatment paradigm for R/R aggressive B-cell lymphomas, although significant toxicities are associated with this therapy, such as cytokine release syndrome (CRS) and immune effector cell-associated neurologic syndrome (ICANS).
Nevertheless, despite the high efficacy of CART therapy, many patients do not respond or relapse, representing a new unmet clinical need. Results from retrospective studies show a median survival for these patients of around 6 months (16–18). There are no standard treatment options. Check points inhibitors have been used in early relapses, resulting In ORR of 40%, as well as lenalidomide based regimens, chemotherapy and radiotherapy (17). Loncastuximab tesirine, a humanized anti-CD19 antibody drug conjugated, has been used in the LOTIS 2 trial, in 13 patients after CART failure, with an ORR of 46% (19). For those patients who achieve a response, a consolidation with allogeneic transplantation should be considered (17).
Other new targeted approaches for R/R DLBCL have been recently approved, as the combination of tafasitamab (anti-CD19 monoclonal antibody) and lenalidomide (20), the combination of polatuzumab vedotin with bendamustine and rituximab (21), and selinexor, an oral inhibitor of exportin 1 (approved by the FDA but not by the EMA) (22). Each of these options should be considered for patients who are poor candidates for ASCT or for CARTs.
The observations that CART cells are capable of achieving very durable remissions supported the T cell‐mediated approach to therapy. With this in mind, a new type of monoclonal antibodies called bispecific antibodies (BsAbs) has been designed. BsAbs combine two different monospecific antigen‐binding regions from different antibodies to achieve a single antibody‐derived molecule with bispecific antigen binding. Currently, there are under development BsAbs that target CD20 on B cells and engage CD3 on T cells in a 1:1 or 2:1 CD20:CD3 antigen binding fragment (Fab) format, to treat B‐cell lymphomas (Table 1). There are different types of BsAbs, those that are IgG-like are large, and contain the fragment crystallizable (Fc) region linking the two antibody binding domains, which is an advantage as they have pharmacokinetic profiles that allow intermittent dosing. Thus, BsAbs have emerged as a novel class of off-the-shelf immunotherapies with clear efficacy in R/R aggressive B-cell lymphomas, including for those patients relapsing after CART therapy. This review describes updated data on the toxicity and efficacy of the IgG-like BsAbs that are currently being studied in lymphomas, especially in DLBCL.
Clinical data available of the four BsAbs that are in clinical development for DLBCL will be discussed, as well as the clinical trials that are ongoing.
Mosunetuzumab is a fully humanized IgG1 BsAb against CD20 and CD3 and has been developed in both an intravenous (IV) and subcutaneous (SC) formulation.
The results of the first-in-human trial have been recently published (23). This trial evaluated the safety and efficacy of mosunetuzumab in patients with R/R B-NHL and established the recommended phase II dose. Single-agent mosunetuzumab was administered IV in 3-week cycles, at full dose in cycle 1 day 1 (group A) or with ascending (step-up) doses during cycle 1 on days 1, 8, and 15 (group B), to reduce CRS, for eight or 17 cycles on the basis of tumor response. Two hundred thirty patients were enrolled, 129 with aggressive B-NHL, with a median of 3 previous lines of therapy, 82% of whom were refractory to last therapy. Fifteen patients were treated after failure to CART therapy. Common adverse events of the whole group were neutropenia (28.4%), CRS (27.4%), hypophosphatemia (23.4%), fatigue (22.8%), and diarrhea (21.8%). CRS was mostly low-grade (grade ≥ 3: 1.0%) and mainly confined to cycle 1. In group B, most neurologic adverse events were grade 1-2: headache (17.8%), insomnia (11.2%), and dizziness (10.2%). Grade 3 occurred in 4.1% of patients; however, only two (1.0%) were considered treatment-related; there were no grade 4 or 5 neurologic events. For patients with aggressive B-NHL, ORR was 34.9%, complete remission (CR) was 19.4%, and median duration of response (DoR) was 22.8 months. In patients who were refractory to prior CART therapy (15 with aggressive NHL and 4 with indolent NHL), ORR was 36.8%, and CR rate was 26.3%. The authors conclude that mosunetuzumab has a manageable safety profile and induces durable complete responses, and select the dose of 1/2/60/60/30 mg for the expansion stage of the study. Preliminary results of the SC formulation have been presented, with similar response rates (24).
These promising results have led to different new studies that are showed in Table 2. One trial has been design to evaluate mosunetuzumab after failure to CART therapy, others in R/R patients in combination with other drugs as lenalidomide, atezolizumab, polatuzumab, or GemOx (gemcitabine and oxaliplatin); and some trials are designed in the upfront setting combined with CHOP. Some preliminary results from some of these ongoing trials, that confirm the efficacy of mosunetuzumab in different settings with a good safety profile, have been already reported (25–28).
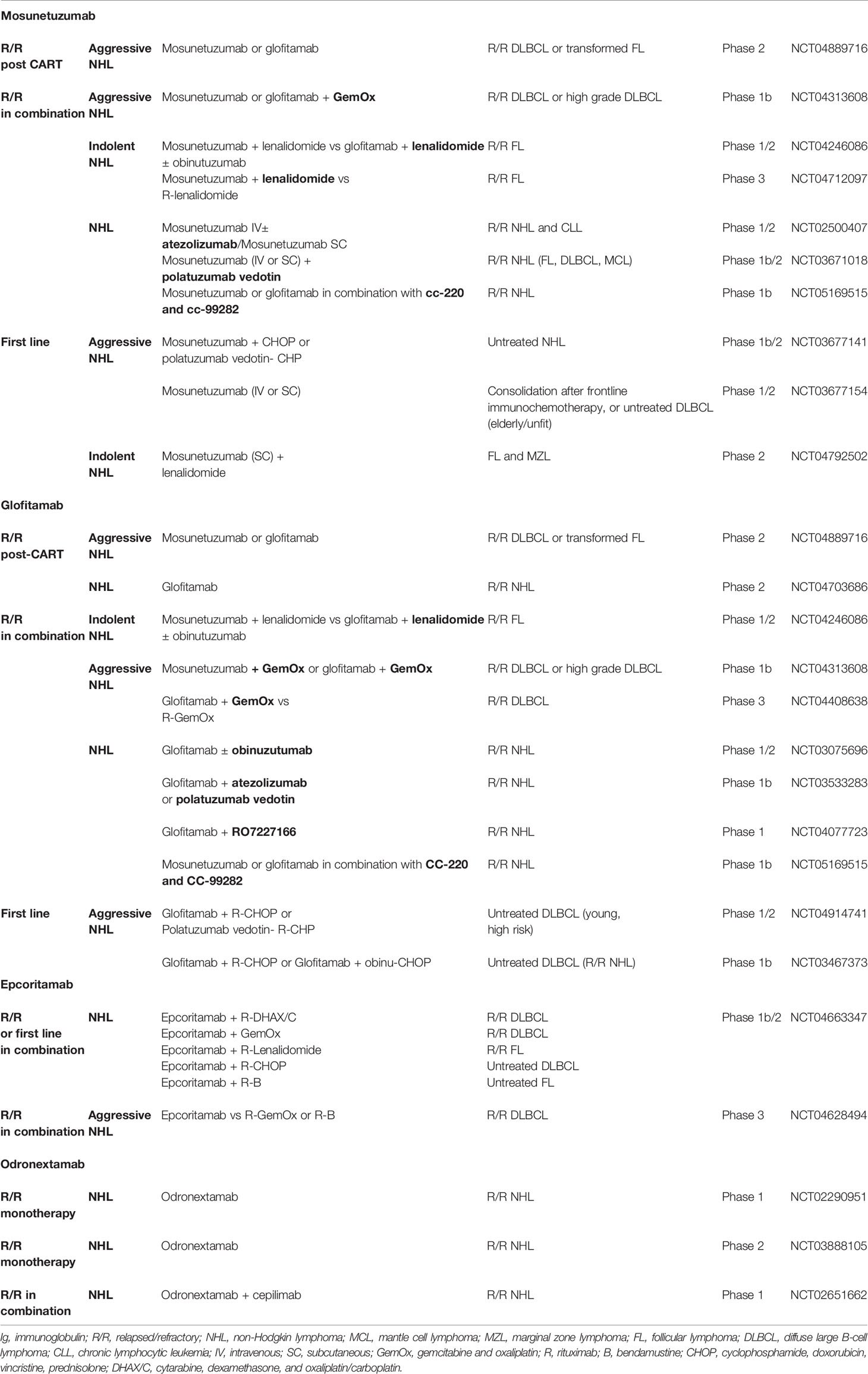
Table 2 Ongoing trials with IgG-like bispecific antibodies in lymphoma, with the clinical trial identifier.
Glofitamab is a full-length BsAb with a 2:1 configuration with bivalency for CD20 on B cells and monovalency for CD3 on T cells. Results of the first-in-human phase I study have been published (29). R/R B-cell NHL patients were treated with single-agent in 14- or 21-day cycles (with obinutuzumab pretreatment 7 days before the first dose of glofitamab to reduce toxicity). One hundred seventy-one patients were treated, 74% with aggressive NHL. This trial included heavily pretreated patients, most refractory to prior therapy (90.6%) and with a median of 3 prior therapies. CRS occurred in 50.3% patients (grade 3 or 4: 3.5%); 1.2% experienced grade 3 ICAN. The ORR was 65.7% (CR, 57%) in those dosed at the recommended phase II dose. Of 63 patients with CR, 53 (84.1%) have ongoing CR with a maximum of 27.4 months observation. In patients with aggressive NHL treated with ≥ 10 mg, ORR was 61% with CR of 49%. Therefore, glofitamab demonstrated frequent and durable CRs with a manageable tolerability profile for patients with refractory B-NHL.
Glofitamab is also being tested in a number of combination trials for R/R and untreated B-cell NHL, such as in combination with chemotherapy as R-GemOx for R/R DLBCL or R-CHOP or R-CHP-polatuzumab vedotin in untreated DLBCL, in combination with monoclonal antibodies as atezolizumab or polatuzumab vedotin for R/R NHL, in combination with other BsAbs as the CD19 × CD3 RO7227166 for R/R NHL (Table 2).
Epcoritamab is a full-length IgG1 BsAb targeting CD3 and CD20 that is administered SC, leading to a gradual increase in drug levels and a lower peak in plasma cytokine levels. Results of the phase 1 trial have been also recently published (30). The primary objectives were to determine the maximum tolerated dose (MTD) and the recommended phase 2 dose. Sixty eight patients received escalating full doses (0·0128–60 mg) of epcoritamab, administered in 28-day cycles. No dose-limiting toxic effects were observed, and the MTD was not reached; the full dose of 48 mg was identified as the recommended phase 2 dose. Common adverse events were pyrexia 69%, CRS 59%, (all grade 1–2), and injection site reactions 47%. No discontinuations occurred due to treatment related adverse events. ORR was 68%, with 45% CR at full doses of 12–60 mg. Forty-eight patients had R/R DLBCL, with a median of 3 previous lines of therapy, 89% refractory to the previous line of therapy and 5 (11%) had relapsed to CART therapy. At 48 mg, ORR was 88%, with 38% CR. In conclusion, epcoritamab showed potent, single-agent, antitumor activity and an overall manageable safety profile, along with an easy way of administration.
Epcoritamab is currently being studied in two trials. The first one is a phase 1/2 study, in which epcoritamab is used in combination with other therapies in the relapsed or refractory setting as well as in previously untreated patients. Results of the first 9 patients treated with epcoritamab-R-CHOP have been presented. Preliminary data suggest that the combination has a manageable safety profile and all evaluable patients achieved early responses (31). It is also being evaluated in a phase 3 trial in patients with relapsed or refractory DLBCL (epcoritamab versus investigator choice of standard-of-care chemotherapy: R-GemOx or R-Bendamustine) (Table 2).
Odronextamab is a fully human IgG4-based bispecific antibody targeting CD3 and CD20. Phase I data have been presented for R/R NHL (32). Odronextamab was administered using a step-up dose schedule consisting of an initial dose at week 1, an intermediate dose at week 2, and thereafter, a fixed weekly dose until week 12 followed by maintenance every other week dosing. One hundred twenty-seven patients with R/R B-NHL were treated at doses ranging from 0.03–320 mg (71 patients with DLBCL). Most patients were refractory to last therapy (80.3%) and had received a median of 3 (range: 1−11) prior lines of therapy; 29 (22.8%) patients had received prior CART therapy. No dose limiting toxicities were reported during dose escalation and MTD was not reached. The most frequent treatment-related adverse events were pyrexia (76.4%), CRS (62.2%), and chills (48.0%). Grade 3 CRS occurred in 8 (6.3%) patients and a grade 4 CRS occurred in 1 patient. Most of the CRS events occurred during the first 2 weeks of step-up dosing. Grade 3 neurologic events were noted in 5 (4.0%) patients; there were no grade 4 or higher neurologic adverse events. None of these events required treatment discontinuation. R/R DLBCL patients who had not received prior CART therapy, treated at doses ≥80 mg (n=10), ORR and CR rate were 60%; median observed DoR was 10.3 months (range 2.9–18.6+). In DLBCL patients who were refractory to prior CAR T therapy, treated at doses ≥80 mg (n=21), ORR was 33.3%, and CR rate was 23.8%; median observed DoR was 2.8 months (range 0 -18.9).
These results leaded to the pivotal phase 2 study currently enrolling for different disease groups, and a trial with the combination of odronextamab with the anti-PD-1 antibody cepilimab (Table 2).
Fortunately, new therapeutic strategies are available today to treat high risk R/R DLBCL patients. Three CD19 CART products, axi-cel, tisa-cel, and liso-cel, have been approved by FDA and EMA, to treat R/R DLBCL patients beyond 2 lines, and axi-cel has been recently approved by FDA as second line for adult patients with DLBCL refractory or relapsed within 12 months after first-line chemoimmunotherapy.
On the other hand, BsAbs have shown very promising preliminary results in the first published trials. Advantages and disadvantages of each of these strategies will be discussed, and a treatment algorithm is proposed for the treatment of R/R DLBCL (Figure 1).
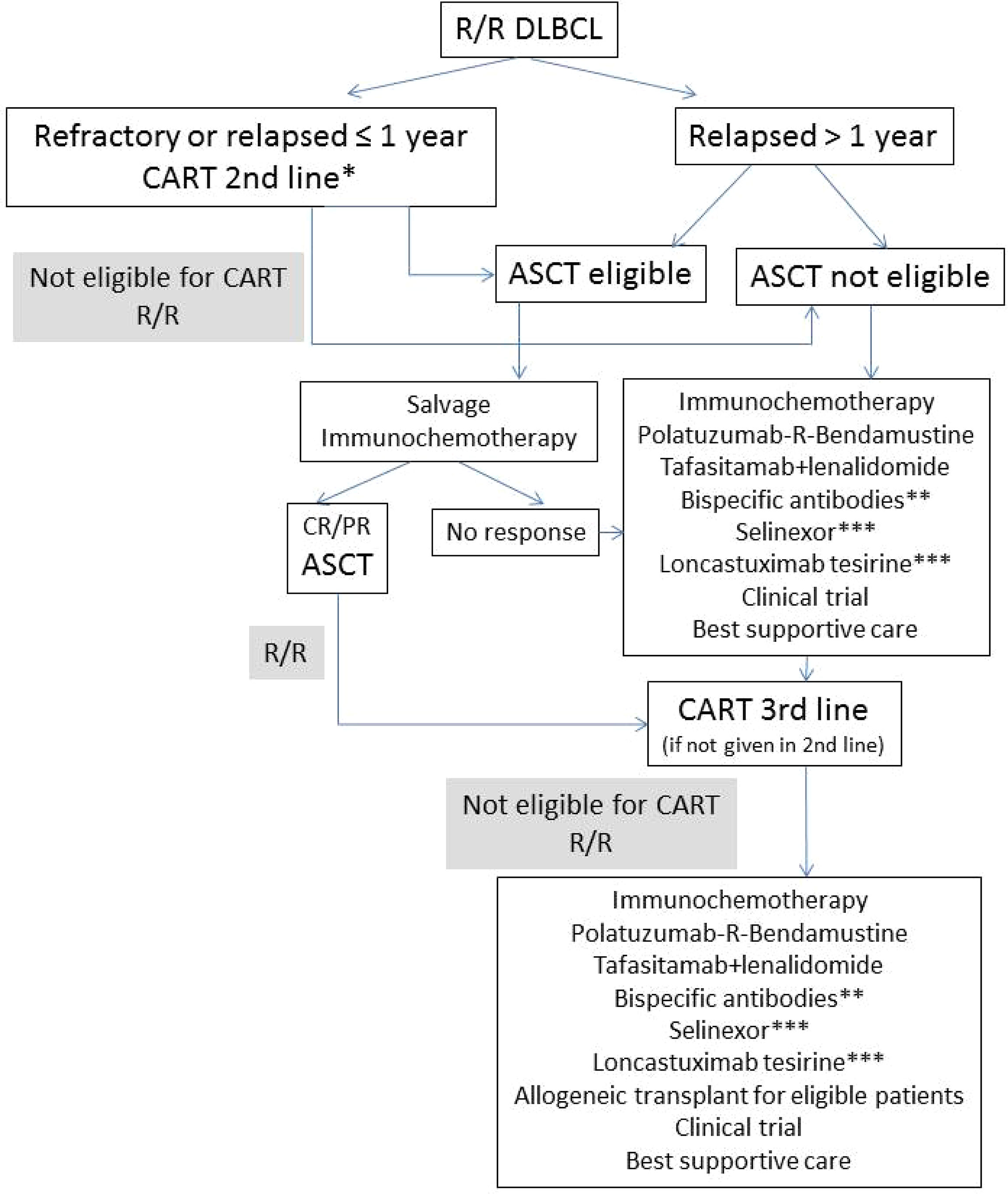
Figure 1 Proposed algorithm for the treatment of R/R DLBCL. R/R, relapsed or refractory; ASCT, autologous stem-cell transplant; CR, complete remission, PR, partial remission; *axi-cel approved by FDA; **currently available only in clinical trials; ***approved by FDA after ≥2 lines of previous therapy.
CARTs have to be engineered for each individual patient, with a potential for logistical delays from the time of patient identification to CART infusion, as well as a risk of manufacturing failure (9–11). Moreover, CARTs may not be feasible for patients with rapidly progressive disease. These patients need bridging therapy during the manufacturing period, but it is not known what treatments are the best to be used. On the other hand, BsAbs, as an off-the-shelf option, allow immediate treatment. These agents are administered in an ease way, every 1 to 4 weeks either IV or even SC, and most can be administered in an outpatient basis. However, CART therapy consists in only one IV infusion, while treatment duration with BsAbs is prolonged.
Side effects associated with CARTs include CRS and ICANS, prolonged cytopenia, and impairment of humoral immunity with increased risk of infection (9–11). CRS is the most common and has been described in 42% to 93% of patients, with grade ≥3 events occurring in 2% to 22% of patients. BsAbs can also produce CRS and neurologic toxicities, seemingly at lower frequencies and severity, although toxicity data are not yet mature. Rates of CRS for BsAbs range from 27% (mosunetuzumab) to 62% (odronexamab), with grade ≥3 events in 0% (epcoritamab) to 7% (odronexamab) of patients (23, 29–31). Most of the CRS events occurred during the first 2 cycles. To mitigate CRS, different strategies are being employed, as the step-up dosing (23), the use SC formulation (24, 31), or the use a cytoreductive anti-CD20 monoclonal antibody before the BsAb infusion (29). Rates of ICANS of grade ≥3 range from 1% (glofitamab) to 4% (mosunetuzumab and odronexamab) with BsAbs. Additional toxicities described for BsAbs with an incidence of ≥10% include pyrexia, reaction at the injection site, and cytopenia. Like CARTs, BsAbs have been used safely in older patients and in patients with comorbidities (25).
Response rates for CARTs in patients with DLBCL range from 52% to 82%, with CR rates of 40% to 54%, and these responses are durable (9–12). For axi-cel, the CART product with a longer follow-up, with a median follow-up greater than 4 years, the OS at 4 years is 44% (12). For liso-cel, with a median follow-up of 40 months, the PFS at 3 years is 31%. For BsAbs, response rates in aggressive lymphomas range from 35% to 88%, with CRs of 19% to 60%, for those patients not exposed to prior CART. Nevertheless, follow-up with BsAbs is still very short, and most of the data regarding efficacy come from phase 1 trials. Therefore, efficacy data, although promising, are still immature, and the results of the new trials that are ongoing are needed to confirm them. Although CARTs offer durable responses, some patients will still relapse. BsAbs have been used in patients relapsed after CARTs, and durable responses have been observed (23, 31).
BsAbs have emerged as a novel class of off-the-shelf immunotherapies with a manageable safety profile, and clear efficacy in R/R aggressive B-cell lymphomas, including in those patients relapsing after CART therapy. There are still many open questions, and new trials are currently ongoing to confirm BsAbs efficacy and tolerability, as well as to explore its efficacy in different lines of therapy or in combination with other drugs.
EG performed the review, critically analyzed the data and wrote the manuscript.
We thank CERCA Programme/Generalitat de Catalunya for institutional support.
EG declares having received lecture fees and advisory board fees from Janssen, Abbvie, Gilead, Kiowa, EUSAPharma, Incyte, Lilly, Beigene, Novartis, Abbvie, Takeda, and Roche.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP Chemotherapy Plus Rituximab Compared With CHOP Alone in Elderly Patients With Diffuse Large-B-Cell Lymphoma. N Engl J Med (2002) 346:235–42. doi: 10.1056/nejmoa011795
2. Davies A, Cummin TE, Barrans S, Maishman T, Mamot C, Novak U, et al. Gene Expression Profiling of Bortezomib Added to Standard Chemoimmunotherapy for Diffuse Large B-Cell Lymphoma (REMoDL-B): An Open-Label, Randomised, Phase 3 Trial. Lancet Oncol (2019) 20(5):649–62. doi: 10.1016/S1470-2045(18)30935-5
3. Younes A, Sehn LH, Johnson P, Zinzani PL, Hong, Zhu J, et al. Randomized Phase III Trial of Ibrutinib and Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Non-Germinal Center B-Cell Diffuse Large B-Cell Lymphoma. J Clin Oncol (2019) 37(15):1285–95. doi: 10.1200/JCO.18.02403
4. Nowakowski GS, Chiappella A, Gascoyne RD, Scott DW, Zhang Q, Jurczak W, et al. ROBUST: A Phase III Study of Lenalidomide Plus R-CHOP Versus Placebo Plus R-CHOP in Previously Untreated Patients With ABC-Type Diffuse Large B-Cell Lymphoma. J Clin Oncol (2021) 39(12):1317–28. doi: 10.1200/JCO.20.01366
5. Vitolo U, Trneny M, Belada D, Burke JM, Carella AM, Chua N, et al. Obinutuzumab or Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Previously Untreated Diffuse Large B-Cell Lymphoma. J Clin Oncol (2017) 35(31):3529–37. doi: 10.1200/JCO.2017.73.3402
6. Tilly H, Morschhauser F, Sehn LH, Friedberg JW, Trněný M, Sharman JP, et al. Polatuzumab Vedotin in Previously Untreated Diffuse Large B-Cell Lymphoma. N Engl J Med (2022) 386(4):351–63. doi: 10.1056/nejmoa2115304
7. Gisselbrecht C, Glass B, Mounier N, Singh Gill D, Linch DC, Trneny M, et al. Salvage Regimens With Autologous Transplantation for Relapsed Large B-Cell Lymphoma in the Rituximab Era. J Clin Oncol (2010) 28(27):4184–90. doi: 10.1200/jco.2010.28.1618
8. Crump M, Neelapu SS, Farooq U, Van Den Neste E, Kuruvilla J, Westin J, et al. Outcomes in Refractory Diffuse Large B-Cell Lymphoma: Results From the International SCHOLAR-1 Study. Blood (2017) 130(16):1800–8. doi: 10.1182/blood-2017-11-817775
9. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel CAR Tcell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med (2017) 377(26):2531–44. doi: 10.1056/nejmoa1707447
10. Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med (2019) 380(1):45–5. doi: 10.1056/nejmoa1804980
11. Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagenemaraleucel for Patients With Relapsed or Refractory Large B-Cell Lymphomas (TRANSCEND NHL 001): A Multicentreseamless Design Study. Lancet (London England) (2020) 396(10254):839–5. doi: 10.1016/s0140-6736(20)31366-0
12. Jacobson CA. Highlights in CAR T-Cell Therapy From the 62nd American Society of Hematology Annual Meeting and Exposition: Commentary. Clin Adv Hematol Oncol (2021) 19 Suppl;10(3):19–23.
13. Locke FL, Miklos DB, Jacobson CA, Perales MA, Kersten MJ, Oluwole OO, et al. Axicabtagene Ciloleucel as Second-Line Therapy for Large B-Cell Lymphoma. N Engl J Med (2022) 386(7):640–54. doi: 10.1056/NEJMoa2116133
14. Bishop MR, Dickinson M, Purtill D, Barba P, Santoro A, Hamad N, et al. Second-Line Tisagenlecleucel or Standard Care in Aggressive B-Cell Lymphoma. N Engl J Med (2022) 386(7):629–39. doi: 10.1056/nejmoa2116596
15. Kamdar M, Solomon SR, Arnason JE, Johnston PB, Glass B, Bachanova V, et al. Lisocabtagene Maraleucel (Liso-Cel), a CD19-Directed Chimeric Antigen Receptor (CAR) T Cell Therapy, Versus Standard of Care (SOC) With Salvage Chemotherapy (CT) Followed By Autologous Stem Cell Transplantation (ASCT) As Second-Line (2l) Treatment in Patients (Pts) With Relapsed or Refractory (R/R) Large B-Cell Lymphoma (LBCL): Results From the Randomized Phase 3 Transform Study. Blood (2021) 138(Supplement 1):91. doi: 10.1182/blood-2021-147913
16. Nastoupil LJ, Jain MD, Feng L, Spiegel JY, Ghobadi A, Lin Y, et al. Standardof-Care Axicabtagene Ciloleucel for Relapsed or Refractory Large B-Cell Lymphoma: Results From the US Lymphoma CAR T Consortium. J Clin Oncol (2020) 38(27):3119–28. doi: 10.1200/JCO.19.02104
17. Spiegel JY, Dahiya S, Jain MD, Tamaresis J, Nastoupil LJ, Jacobs MT, et al. Outcomes of Patients With Large B-Cell Lymphoma Progressing After Axicabtagene Ciloleucel Therapy. Blood (2021) 137(13):1832–5. doi: 10.1182/blood.2020006245
18. Chow VA, Gopal AK, Maloney DG, Turtle CJ, Smith SD, Ujjaniet CS, et al. Outcomes of Patients With Large B-Cell Lymphomas and Progressive Disease Following CD19-Specifc CAR T-Cell Therapy. Am J Hematol (2019) 94(8):E209–13. doi: 10.1002/ajh.25505
19. Hamadani M, Radford J, Carlo-Stella C, Caimi PF, Reid E, O’Connor OA, et al. Final Results of a Phase 1 Study of Loncastuximab Tesirine in Relapsed/Refractory B-Cell Non-Hodgkin Lymphoma. Blood (2021) 137(19):2634–45. doi: 10.1182/blood.2020007512
20. Salles G, Duell J, González Barca E, Tournilhac O, Jurczak W, Liberati AM, et al. Tafasitamab Plus Lenalidomide in Relapsed or Refractory Diffuse Large B-Cell Lymphoma (L-MIND): A Multicentre, Prospective, Single-Arm, Phase 2 Study. Lancet Oncol (2020) 21(7):978–88. doi: 10.1016/s1470-2045(20)30225-4
21. Sehn LH, Herrera AF, Flowers CR, Kamdar MK, McMillan A, Hertzberg M, et al. Polatuzumab Vedotin in Relapsed or Refractory Diffuse Large B-Cell Lymphoma. J Clin Oncol (2020) 38(2):155–65. doi: 10.1200/jco.19.00172
22. Kalakonda N, Maerevoet M, Cavallo F, Follows G, Goy A, Vermaat JSP, et al. Selinexor in Patients With Relapsed or Refractory Diffuse Large B-Cell Lymphoma (SADAL): A Singlearm, Multinational, Multicentre, Open-Label, Phase 2 Trial. Lancet Haematol (2020) 7(7):e511–22. doi: 10.1016/S2352-3026(20)30120-4
23. Budde LE, Assouline S, Sehn LH, Schuster SJ, Yoon S, Yoon DH, et al. Single-Agent Mosunetuzumab Shows Durable Complete Responses in Patients With Relapsed or Refractory B-Cell Lymphomas: Phase I Dose-Escalation Study. J Clin Oncol (2021) 40:481–91. doi: 10.1200/jco.21.00931
24. Matasar MJ, Cheah CY, Yoon DH, Assouline SE, Bartlett NL, Ku M, et al. Subcutaneous Mosunetuzumab in Relapsed or Refractory B-Cell Lymphoma: Promising Safety and Encouraging Efficacy in Dose Escalation Cohorts. Blood (2020) 136(Supplement 1):45–6. doi: 10.1182/blood-2020-135818
25. Olszewski AJ, Avigdor A, Babu S, Levi I, Abadi U, Holmes H, et al. Single-Agent Mosunetuzumab Is a Promising Safe and Efficacious Chemotherapy-Free Regimen for Elderly/Unfit Patients With Previously Untreated Diffuse Large B-Cell Lymphoma. Blood (2020) 136(Supplement 1):43–5. doi: 10.1182/blood-2020-136255
26. Phillips TJ, Olszewski AJ, Munoz J, Kim TM, Yoon DH, Greil R, et al. Mosunetuzumab, a Novel CD20/CD3 Bispecific Antibody, in Combination With CHOP Confers High Response Rates in Patients With Diffuse Large B Cell Lymphoma. Blood (2020) 136(Supplement 1):37–8. doi: 10.1182/blood-2020-136295
27. Budde LE, Olszewski AJ, Assouline S, Kamdar M, Diefenbach CS, Ghosh N, et al. Mosunetuzumab Plus Polatuzumab Vedotin Has Promising Efficacy and a Favorable Safety Profile in Patients With Relapsed/Refractory Aggressive B-Cell Non-Hodgkin Lymphoma: Updated Results From a Phase Ib/II Study. Blood (2021) 138(Supplement 1):533.
28. Bartlett NL, Giri P, Budde E, Schuster SJ, Assouline S, Matasar M, et al. Subcutaneous (SC) Administration of Mosunetuzumab With Cycle 1 Step-Up Dosing Is Tolerable and Active in Patients With Relapsed/Refractory B-Cell Non-Hodgkin Lymphomas (R/R B-NHL): Initial Results From a Phase I/II Study. Blood (2021) 138(Supplement 1):3573. doi: 10.1182/blood-2021-147937
29. Hutchings M, Morschhauser F, Iacoboni G, Carlo-Stella C, Offner FC, Sureda A, et al. Glofitamab, a Novel, Bivalent CD20-Targeting T-Cell–Engaging Bispecific Antibody, Induces Durable Complete Remissions in Relapsed or Refractory B-Cell Lymphoma: A Phase I Trial. J Clin Oncol (2021) 39:1959–70. doi: 10.1200/jco.20.03175
30. Hutchings M, Mous R, Clausen MR, Johnson P, Linton KM, Chamuleau MED, et al. Dose Escalation of Subcutaneous Epcoritamab in Patients With Relapsed or Refractory B-Cell Non-Hodgkin Lymphoma: An Open-Label, Phase 1/2 Study. Lancet (2021) 398:1157–69. doi: 10.1016/s0140-6736(21)00889-8
31. Belada D, Christensen JH, Drott K, Snauwaert S, Brody J, Narkhede M, et al. Subcutaneous Epcoritamab in Combination With R-CHOP in Patients With Previously Untreated High-Risk Diffuse Large B-Cell Lymphoma: Preliminary Results From a Phase 1/2 Trial. Blood (2021) 138(Supplement 1):1413. doi: 10.1182/blood-2021-146569
32. Bannerji R, Allan JN, Arnason JE, Brown JR, Advani R, Ansell SM, et al. Odronextamab (REGN1979), a Human CD20 X CD3 Bispecific Antibody, Induces Durable, Complete Responses in Patients With Highly Refractory B-Cell Non-Hodgkin Lymphoma, Including Patients Refractory to CAR T Therapy. Blood (2020) 136(Supplement 1):42–3. doi: 10.1182/blood-2020-136659
Keywords: diffuse large B-cell lymphoma, relapsed/refractory, bispecific antibodies, non-Hodgkin lymphoma, post CART therapy
Citation: González Barca E (2022) Role of Bispecific Antibodies in Relapsed/Refractory Diffuse Large B-Cell Lymphoma in the CART Era. Front. Immunol. 13:909008. doi: 10.3389/fimmu.2022.909008
Received: 31 March 2022; Accepted: 23 May 2022;
Published: 19 July 2022.
Edited by:
Sarkozy Clementine, Gustave Roussy Cancer Campus, FranceReviewed by:
Narendranath Epperla, The Ohio State University, United StatesCopyright © 2022 González Barca. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eva González Barca, ZS5nb256YWxlekBpY29uY29sb2dpYS5uZXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.