- 1Department of Rheumatology, the Second Hospital of Shanxi Medical University, Taiyuan, China
- 2Department of General Medicine, the Second Hospital of Shanxi Medical University, Taiyuan, China
- 3Department of Pathology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, United States
Objective: To search for the immunological risk factors of Psoriatic arthritis (PsA) combined with nonalcoholic fatty liver disease (NAFLD), development and assessment of predictive nomograms for NAFLD risk in patients with PsA, and to further explore the correlation between risk factors and dyslipidemia.
Methds: A total of 127 patients with PsA (46 with NAFLD and 81 without NAFLD) were included in this retrospective study. The clinical and serological parameters of the patients were collected. The percentage and the absolute number of lymphocytes and CD4+T cells were determined by Flow cytometry. Univariate and multivariate binary logistic regression analysis was used to screen independent risk factors of PsA complicated with NAFLD in the model population, and a nomogram prediction model was developed and assessed.
Results: (1) Univariate and multivariate logistic regression analysis of the modeling population showed that the percentage of peripheral blood T helper 1 cells (Th1%) (OR=1.12, P=0.001), body mass index (BMI) (OR=1.22, P=0.005) and triglycerides (TG) (OR=4.78, P=0.003) were independent risk factors for NAFLD in patients with PsA, which were incorporated and established a nomogram prediction model. The model has good discrimination and calibration, and also has certain clinical application value. (2) The number of peripheral blood NK cells in PsA patients was significantly positively correlated with serum triglyceride (TG) (r=0.489, P<0.001), cholesterol (CHOL) (r=0.314, P=0.003) and low-density lipoprotein (LDL) (r=0.362, P=0.001) levels.
Conclusions: Our study shows that the novel NAFLD nomogram could assess the risk of NAFLD in PsA patients with good efficiency. In addition, peripheral blood NK cell levels may be associated with dyslipidemia in patients with PsA.
Introduction
Psoriatic arthritis (PsA) is a chronic, immune-mediated inflammatory joint disease characterized by progressive inflammatory destruction of the axial and peripheral joints. In recent years, the inflammatory process of the pathophysiological changes of PsA has become the focus of attention. Chronic low-grade systemic inflammation associated with skin inflammation is thought to underlie many comorbidities of PsA, including cardiovascular disease, metabolic syndrome, and nonalcoholic fatty liver disease (NAFLD) (1).
NAFLD is a liver disease characterized by hepatic steatosis, with a global incidence of about 25%, and is currently one of the leading causes of liver cirrhosis and liver transplantation (2). A recent study found that even mild fatty liver disease increased a patient’s risk of all-cause death by 71%, and the risk was proportional to the severity of fatty liver disease (3). NAFLD is not only associated with increased liver-related mortality or morbidity but is a multisystem disease affecting multiple extrahepatic organs, including the heart and vascular system. Cardiovascular disease (CAD) is considered in some respects to be an inflammatory disease with an inflammatory pathway similar to NAFLD and is the leading cause of death in NAFLD (4, 5). Visceral adipose tissue promotes eotaxin release from atherosclerotic vascular smooth muscle cells by releasing IL-17, so IL-17 may be a key regulator of local inflammation in CAD lesions and a significant risk factor for CVD (6). According to histological form, NAFLD is divided into simple steatohepatitis and nonalcoholic steatohepatitis (NASH). NASH is a potentially progressive disease that causes cirrhosis in 12-25% of cases within 10 years, significantly increasing the risk of advanced liver disease (7). About 65% of patients with PsA have NAFLD (8). Compared with patients with PsA alone, patients with NAFLD tend to have more severe clinical symptoms, and NAFLD is significantly correlated with Psoriasis (PsO) lesions and disease severity index (PASI). In contrast, PsO, especially PsA, is an important predictor of NAFLD severity. When PsO is present, the likelihood of advanced NAFLD increases by approximately 60%, and progression to NASH is more likely. Furthermore, PsA is even a risk factor for advanced liver fibrosis (9). The pathogenesis of PsA combined with NAFLD is not fully understood, and previous studies have attributed it to a metabolic syndrome caused by factors such as dyslipidemia. Recent studies suggest that chronic low-level systemic inflammation is a common cause of both.
Similar to other autoimmune diseases, a variety of CD4+ T cells and their secreted cytokines are involved in the pathogenesis of PsA. Preliminary studies suggest that PsA is a Th1 cell-mediated disease as Th1 cells and their secretion of interferon-gamma (INF-γ) are significantly increased in skin lesions (10). Likewise, Th17 cells and their secreted cytokine interleukin 17A (IL-17A) are not only present in skin lesions (11), but also have an increased frequency in diseased joints (12). In addition to helper T cells, innate immune cells, especially NK cells, are also closely involved in the pathogenesis of PsA. INF-γ produced by NK cells in the skin is a potent factor for Th17 cell migration into skin lesions. IL-17 producing NK cells are also increased in synovial fluid from patients with undifferentiated spondyloarthropathy closely related to PsA (13).
An increasing number of studies have shown that NAFLD is not a simple liver disease and that the immune system is one of the main drivers of disease progression (14), each stage of NAFLD is accompanied by the accumulation of T cells and NK cells with different functions and phenotypes (15). For example, clinical and animal studies have shown that NAFLD patients have significantly increased liver and peripheral blood Th1 cells and their secreted cytokines IFN-γ, IL-12, and TNF-α (16). In addition, the number of Th17 cells in the liver and peripheral blood of NAFLD patients was also significantly increased (17), which not only mediates liver inflammation and liver injury but also has a significant effect on liver fibrosis (18). NK cells account for 30-55% of the total number of hepatic lymphocytes. Recent studies have found that the number of NK cells in NAFLD patients is reduced (19, 20), while the number of NK cells in the liver of NASH patients is significantly increased (21), which promotes the occurrence and development of liver fibrosis (22, 23), accelerates disease progression and leads to an increased risk of advanced liver disease.
Therefore, PsA and NAFLD share similar underlying inflammatory pathways, and inflammatory cells and pro-inflammatory cytokines associated with PsA may be involved in the occurrence and development of NAFLD through a common chronic inflammatory pathway. However, there is currently no research on the role of inflammatory cells in the pathogenesis of NAFLD and whether they are also involved in abnormal metabolic processes such as lipid disorders. In this study, by looking for the differences in peripheral blood lymphocyte subsets and CD4+T cells in PsA-NAFLD patients, we identified the immunological high-risk factors of PsA patients complicated with NAFLD, development, and assessment of predictive nomograms for NAFLD risk in patients with PsA, and to further explore the correlation between risk factors and dyslipidemia. Early identification and scientific management of risk factors can not only reduce the incidence of NAFLD and the risk of NAFLD-induced advanced liver disease but also effectively control the disease and improve the quality of life of patients.
Materials and methods
Clinical date
Our study collected 127 patients with PsA admitted to the Rheumatology Department of the Second Hospital of Shanxi Medical University from August 2016 to June 2021, including 53 males and 74 females, with an average age of 46.5 ± 13.26 years. All patients were diagnosed according to the CASPAR classification diagnostic criteria revised in 2006 (24). The diagnosis of NAFLD mainly relies on liver ultrasound. In addition, 56 healthy controls were recruited from the physical examination center of our hospital. Patients with other autoimmune diseases, serious infections especially viral hepatitis, tumors, and drug-induced hepatitis were excluded. Exclusion criteria also included alcohol consumption > 20 g/day, recreational drug use, and exposure to environments known to induce hepatic steatosis. Clinical and laboratory data, including blood cell analysis, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), liver enzymes, bilirubin, lipids, and immunoglobulins, were retrospectively collected from patients and healthy controls. All blood samples were collected on an empty stomach on the morning of admission. According to the presence or absence of NAFLD, the patients were divided into two groups, the PsA group (PsA) and PsA combined NAFLD group (PsA-NAFLD). This study was approved by the Ethics Committee of the Second Hospital of Shanxi Medical University (2019YX114). A written informed consent was obtained from every participant.
Flow cytometry for absolute counts of peripheral blood lymphocytes and CD4+T cell subsets
Whole blood samples from patients with PsA were collected in heparin anticoagulant tubes, and the peripheral blood mononuclear cell suspension was prepared by Ficoll-hypaque density gradient centrifugation. Different antibodies were added in turn for the combined staining of peripheral blood lymphocyte subsets and CD4+T cells. The staining protocol for peripheral blood lymphocyte subsets is as follows: anti-CD3-FITC/CD8-PE/CD45-PercP/CD4-APC antibody is used to label T lymphocytes, anti-CD3-FITC/CD16+56-PE/CD45-PercP/CD19-APC antibody is used to label B lymphocytes and NK cells. CD4+T cell subsets were stained and identified by the following protocols: anti-CD4-FITC/IFN-γ-APC (intracellular staining) for Th1 cells and anti-CD4-FITC/IL-4-PE (intracellular staining) for Th2 Cells, anti-CD4-FITC/IL-17-PE (intracellular staining) to detect Th17 cells, anti-CD4-FITC/CD25-APC/FOXP3-PE (intracellular staining) to identify and detect Treg cells. All immunofluorescent antibodies were purchased from BD Biosciences (BD Biosciences, Franklin Lakes, NJ, USA), blood samples were mixed, incubated, and washed according to the manufacturer’s recommendations, and stained samples were detected within 24 hours using FACSCalibur flow cytometer and BD Multitest software (BD Biosciences, Franklin Lakes, NJ, USA).
Statistical analysis
Statistical analysis was performed using SPSS software (version 23.0; SPSS Inc., Chicago, IL, USA) and R software (4.1.2, USA). Count data were tested using the chi-square goodness-of-fit test. Measurement data were measured using the Kolmogorov-Smirnov test and Levene t-test; mean ± standard deviation was used to express normality and homogeneity of variance. The independent samples t-test was used for comparison between two groups, and the analysis of variance was used for comparison between groups. Non-normally distributed data are expressed as median (M) and interquartile range and tested using the Kruskal-Wallis H test. Correlation analysis was performed using Pearson’s or Spearman’s correlation. Univariate and multivariate binary logistic regression analysis was used to screen independent risk factors for PsA combined with NAFLD, and a nomogram prediction model was constructed using these risk factors. The discriminative power of the model was evaluated by the area under the receiver operating characteristic curve (AUROC) and the C-index; the calibration of the model was evaluated by the calibration curve and the Hosmer-Lemeshow test; the DCA curve was used to verify the clinical validity of the model.
Results
Comparison of demographic and clinical features and laboratory data between the PsA group and PsA-NFFLD group
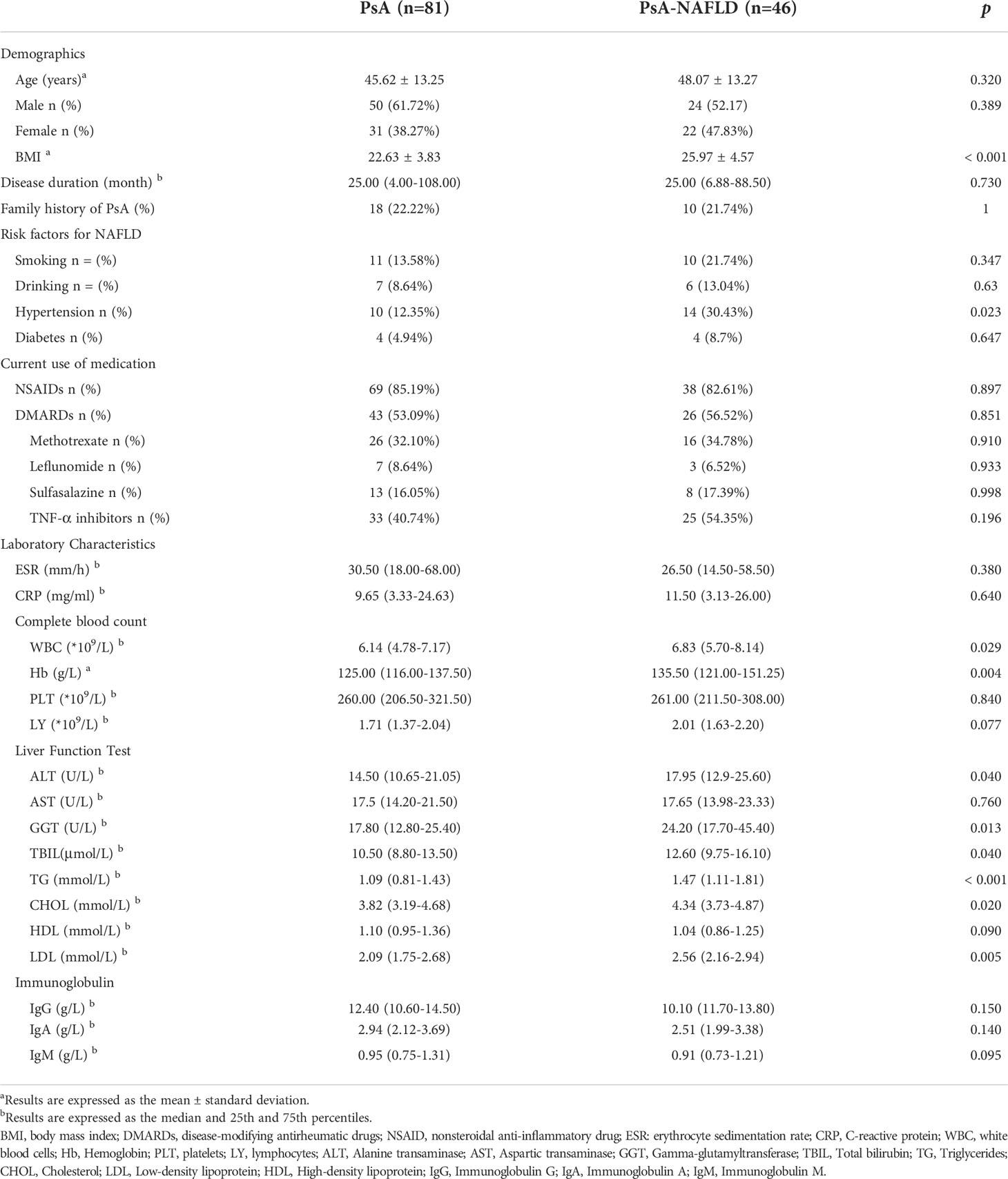
A total of 127 patients with PsA were included in this study, including 53 male patients and 74 female patients. The mean age of the patients was 46.5 ± 13.26 years. The main demographic characteristics, disease characteristics, and serological parameters of the two groups of patients are shown in Table 1. There were no significant differences between the PsA group and the PsA-NAFLD group in terms of sex, age, disease course, and family history. The BMI of PsA-NAFLD patients was significantly higher than that of PsA patients. Diabetes and hypertension are risk factors for NAFLD. The prevalence of hypertension in PsA-NAFLD was 30.43%, which was significantly higher than that in the PsA group (12.35%). In addition, there were no significant differences in medication use between the two groups. The use rate of tumor necrosis factor inhibitor (54.35%) was higher in the PsA-NAFLD group, but the difference was not statistically significant.
By comparing the differences in blood cell analysis between the two groups, it was found that the peripheral blood white blood cell count and hemoglobin content in the PsA group were significantly lower than those in the PsA-NAFLD group. In addition, there were significant differences in liver function-related indicators between the two groups. First, the partial liver enzymes (Alanine transaminase, Gamma-glutamyltransferase) and total bilirubin in the PsA-NAFLD group were significantly higher than those in the PsA group. Since the main diagnosis of NAFLD in this study is based on abdominal ultrasound, it is difficult to accurately determine the degree of NAFLD lesions, so we speculate that the reason for the elevated liver enzymes in PsA-NAFLD patients may be that some patients progress to early NASH. Second, the blood lipid levels in the PsA-NAFLD patients were higher, especially the levels of triglycerides, cholesterol, and low-density lipoprotein were significantly higher than those in the PsA group.
Peripheral Th1 cells and NK cells were elevated in PsA-NAFLD patients
We compared the numbers and percentages of peripheral blood lymphocyte subsets and CD4+ T cell subsets between the two groups of PsA patients and healthy controls. Compared with the healthy control group, the number of total T cells (P=0.001) and percentage (P<0.001), the number of total B cells (P=0.050), the number of CD4+T cells (P<0.001), and the percentage (P<0.001), Th1 cell number (P<0.001) Th17 cell number (P<0.001) and percentage (P=0.001), Th17/Treg (P<0.001) all increased in different degrees. The percentage of NK cells (P<0.001) and Treg cells (P<0.001) in the healthy control group were significantly higher than those in the two groups of PsA patients (Tables 2A, B, Figure 1A, B).
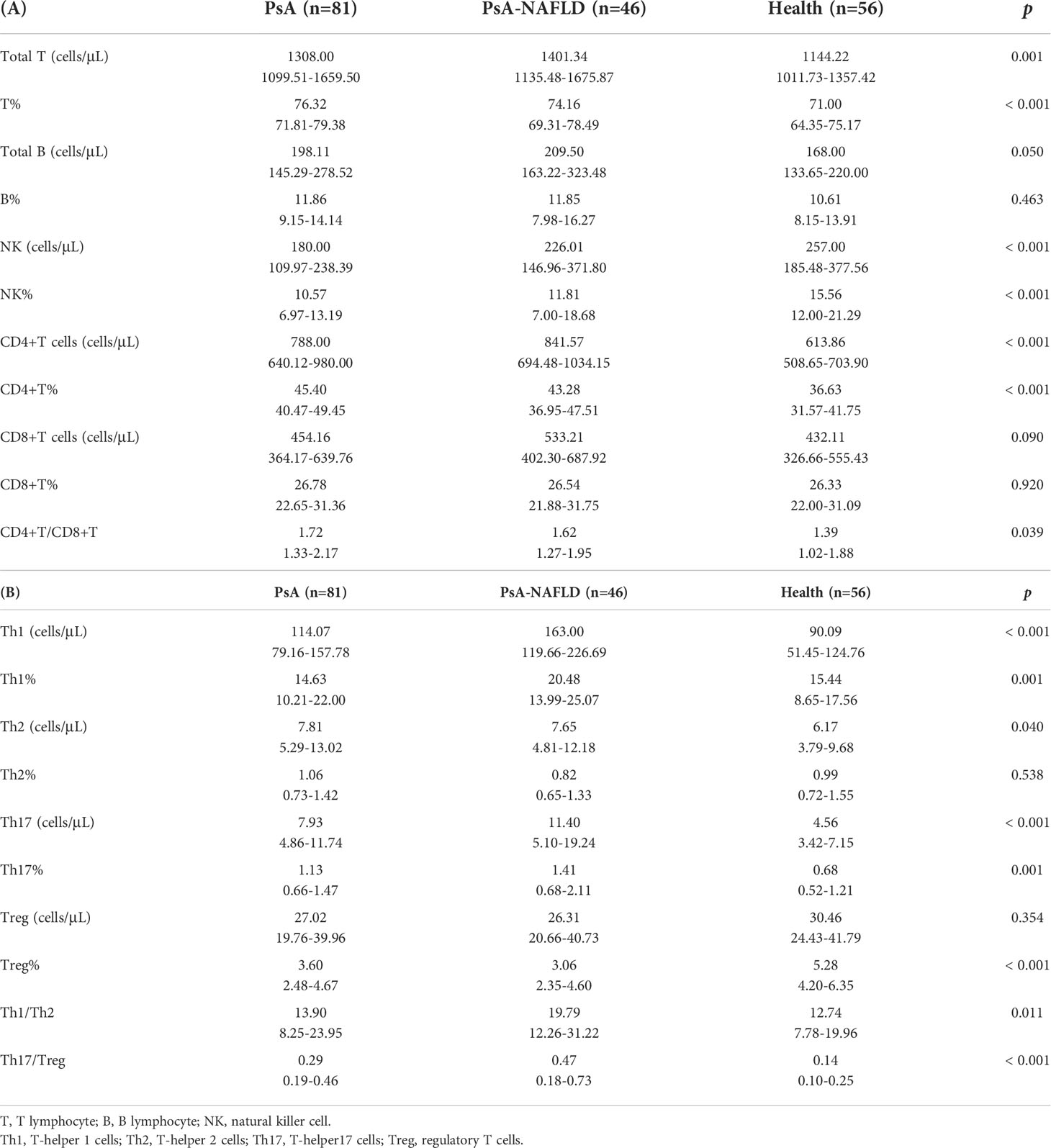
Table 2 Absolute counts and proportions of lymphocytes in the peripheral blood in the study participants.
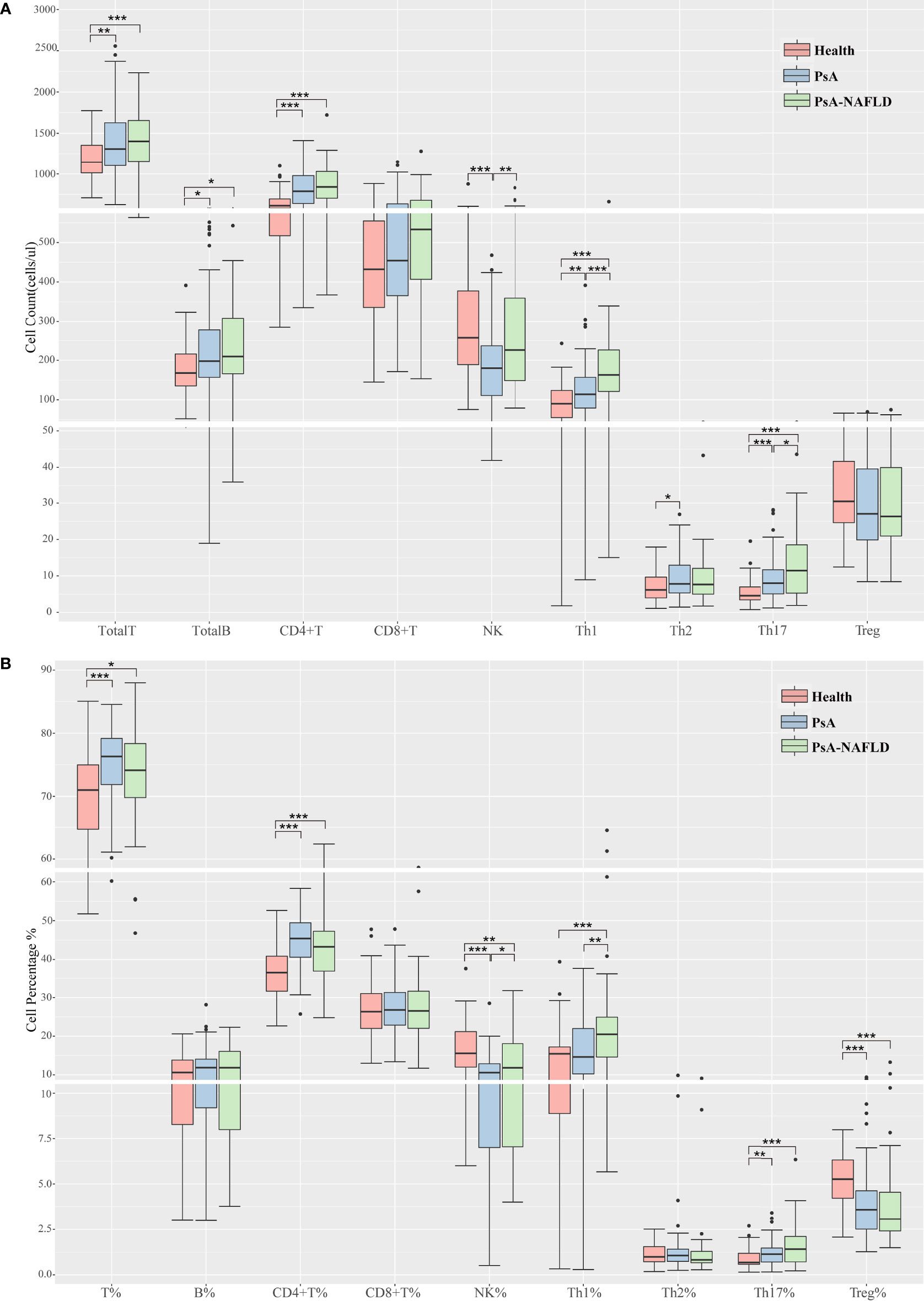
Figure 1 (A). Comparison of peripheral blood lymphocyte subsets and CD4+T cell counts among each study group. (B). Comparison of the proportion of peripheral blood lymphocyte subsets and CD4+ T cells among each study group. (*p < 0.05, **p < 0.01, ***p < 0.001).
In addition, we compared the number and percentage of peripheral blood lymphocyte subsets and CD4+T cell subsets in the two PsA groups and found that the number (P<0.001) and percentage (P=0.001) of peripheral blood Th1 cells, Th17 cells (P=0.037), Th1/Th2 ratio (P=0.021), NK cell numbers (P=0.007) and percentages (P=0.041) were significantly increased in the PsA-NAFLD group (Tables 2A, B, Figures 1A, B).
Development of the nomogram for prediction of nonalcoholic fatty liver disease in psoriatic arthritis
The 127 patients with PsA included in this study were divided into the modeling population (102 cases) and the validation population (25 cases) according to the ratio of 8:2 by the random number table method. The auxiliary examination results and other data were compared (P>0.05), the difference was not statistically significant, and the two groups were comparable. Univariate Logistic regression was used to screen the risk factors of NAFLD in PsA patients. The clinical indicators with the statistical difference (P<0.05) are shown in Figure 2. Risk factors with P<0.01 in the univariate logistic regression were included in the multivariate logistic stepwise regression analysis. The results showed that in addition to traditional risk factors such as BMI and TG, peripheral blood Th1% was also an independent risk factor for NAFLD in patients with PsA (Table 3). The above three independent risk factors were incorporated into the prediction model, and the individualized nomogram prediction model of PsA complicated with NAFLD was established (Figure 3A). Considering that peripheral blood Th1% is not a traditional risk factor for NAFLD, we removed Th1% from the above prediction model, re-established a new prediction model 2, and compared the test performance of the two models (Table 3).
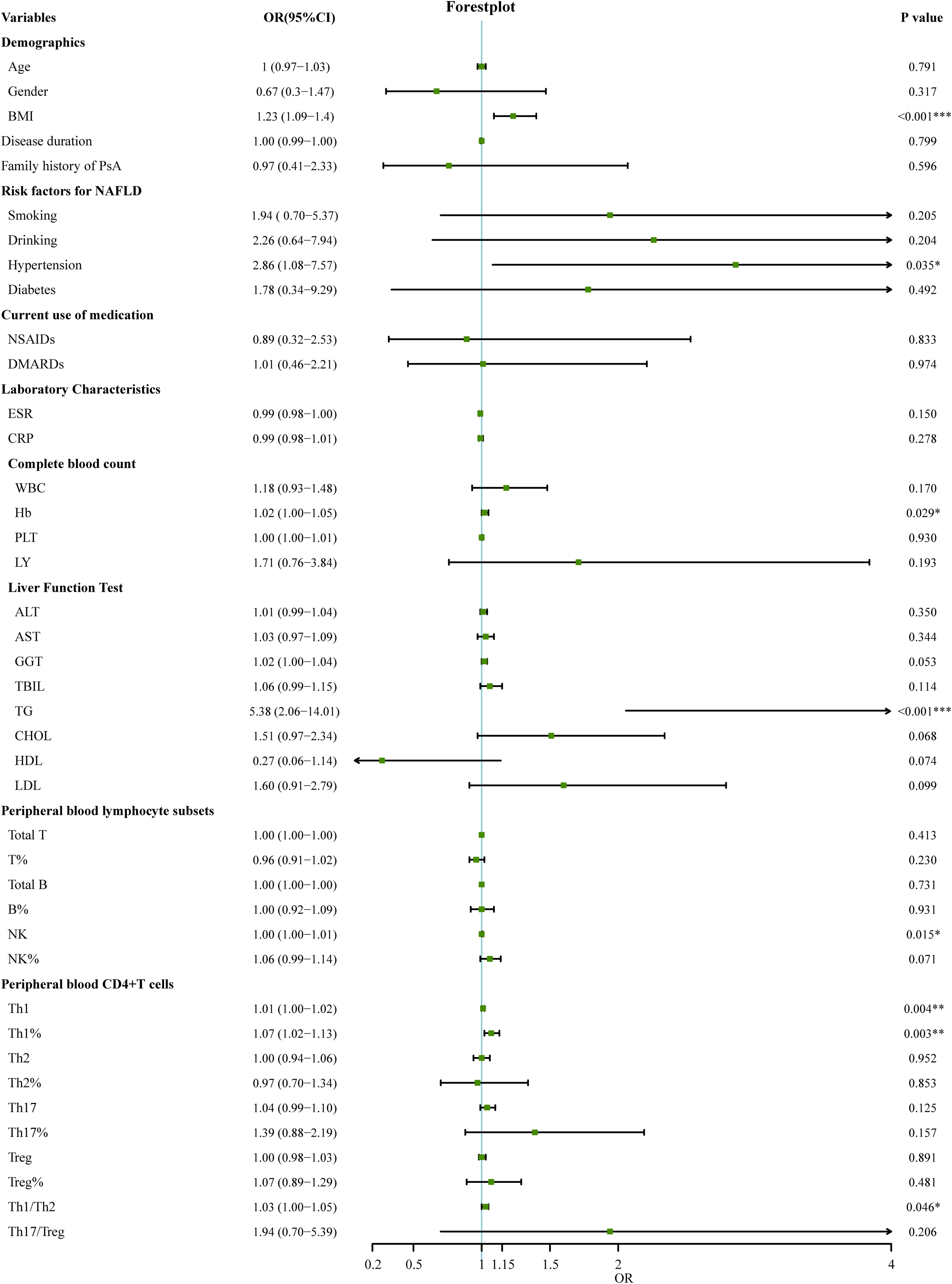
Figure 2 Univariate logistic regression analyses for factors associated with the presence of NAFLD in PsA patients. (*p < 0.05, **p < 0.01, ***p < 0.001). OR, odds ratio; 95%CI:95% confidence interval; BMI, Body Mass Index; DMARDs, disease-modifying antirheumatic drugs; NSAID, nonsteroidal anti-inflammatory drug; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; WBC, white blood cells; Hb, Hemoglobin; PLT, platelets; LY, lymphocytes; ALT, Alanine transaminase; AST, Aspartic transaminase; GGT, Gamma-glutamyltransferase; TBIL, Total bilirubin; TG, Triglycerides; CHOL, Cholesterol; LDL, Low-density lipoprotein; HDL, High-density lipoprotein; T, T lymphocyte; B, B lymphocyte; NK, natural killer cell; Th1, T-helper 1 cells; Th2, T-helper 2 cells; Th17, T-helper17 cells; Treg, regulatory T cells.
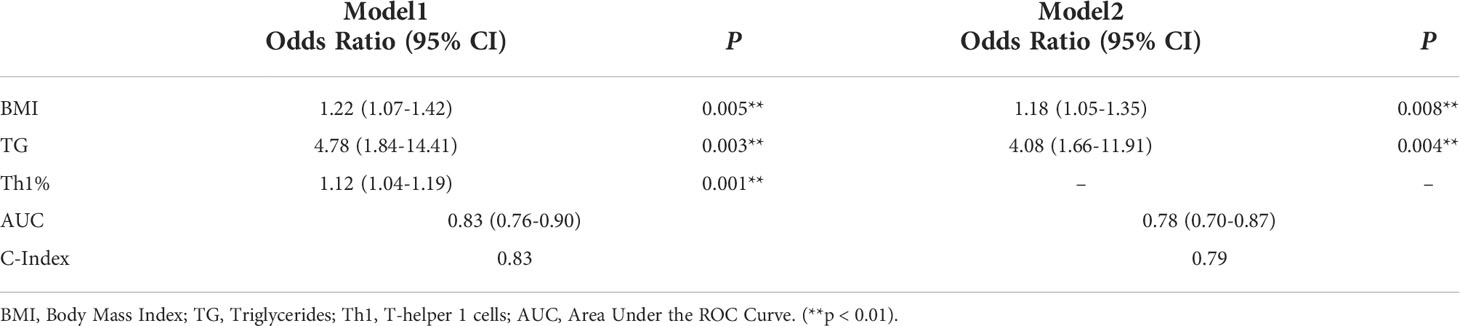
Table 3 Univariate logistic regression analyses for factors associated with the presence of NAFLD in PsA patients.
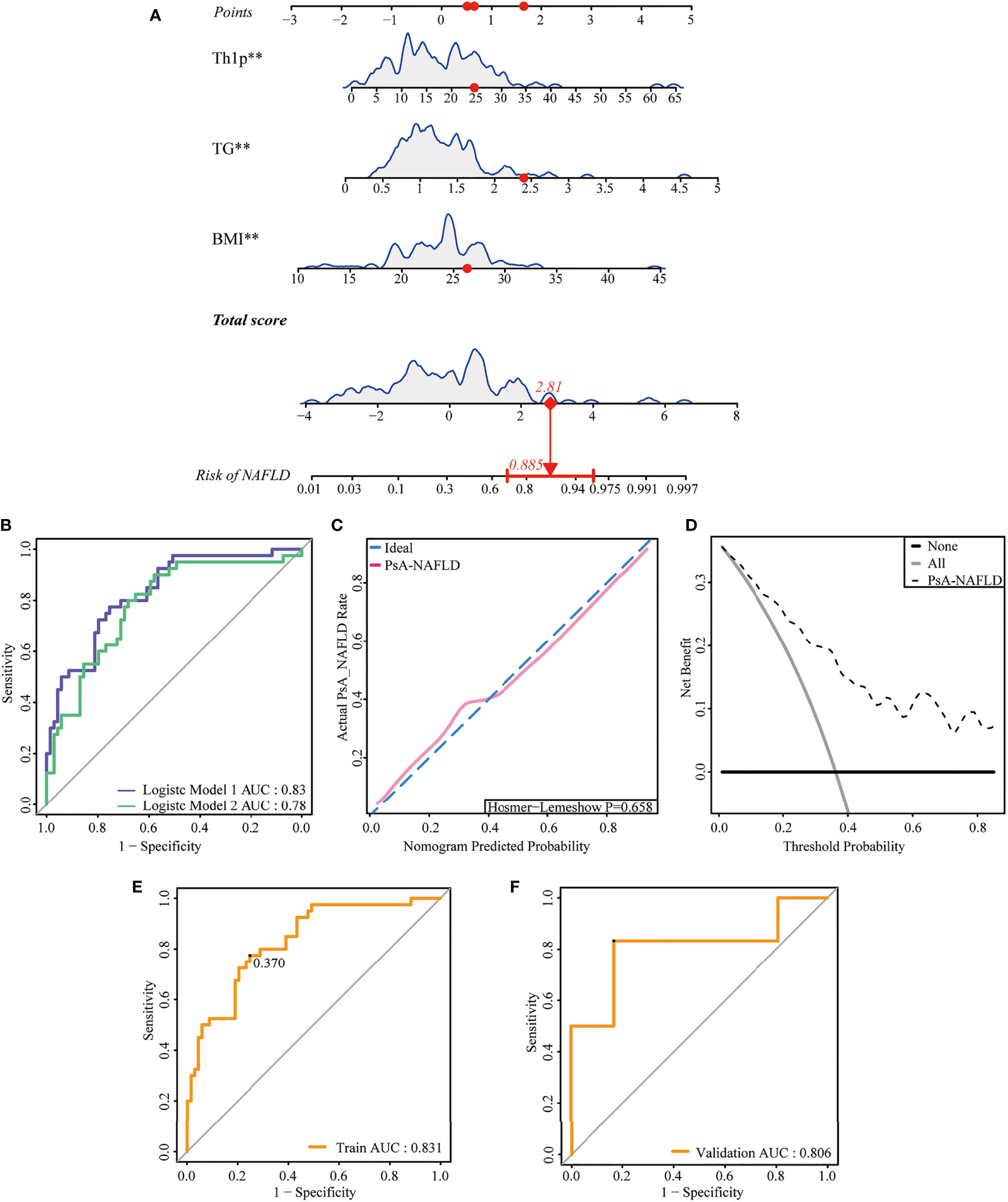
Figure 3 Development and assessment of a nomogram for prediction of NAFLD in PsA. (A). Example of prediction nomogram for risk of NAFLD in PsA patients. The nomogram incorporates the risk factors of Th1%, TG, and BMI. (B). The receiver operating characteristic (ROC) curve for the discrimination of the nomogram to predict the risk of NAFLD in PsA patients. Model 1: The nomogram incorporating the risk factors of Th1%, TG, and BMI; Model 2: The nomogram incorporating the risk factors of TG and BMI. (C). Calibration curve for predicting the risk of NAFLD in PsA patients. The red line along the dashed line indicates that the predicted prevalence is close to the actual prevalence. (D). Decision curve analysis for predicting the risk of NAFLD in PsA patients. (E) The receiver operating characteristic (ROC) curve for the discrimination of the nomogram to predict the risk of NAFLD in modeling population. (F) The receiver operating characteristic (ROC) curve for the discrimination of the nomogram to predict the risk of NAFLD in validation population **p < 0.01.
Validation of the nomogram
First, we compared the discriminability between the two models. The AUC of Model 1 was 0.83 (95%CI: 0.76~0.90), and the C-index was 0.83. Model 2 had an AUC of 0.79 (95% CI: 0.70–0.87) and a C-index of 0.79, which was worse than the aforementioned models (Table 3, Figure 3B). This suggests that peripheral blood Th1% in PsA patients plays an important role in predictive models of NAFLD onset.
The discriminability of the model was verified by plotting the ROC curves of the two groups of people. The AUC of the modeling population was 0.83 (95% CI: 0.76-0.90), the C-index was 0.83 (Figure 3E), and the AUC of the validation population was 0.81 (95% CI: 0.57-0.87), and the C-index was 0.81 (Figure 3F), the C-index of the prediction model in both populations is > 0.75, and the model has good discriminative power.
Next, we plotted the calibration curve of the diagnostic model. The prediction curve of the model is close to the actual observation curve, indicating that the calibration ability of the model is good. The Hosmer-Lemeshow test was further performed on the diagnostic model, and its P-value was 0.658 (P>0.05). The difference was not statistically significant (Figure 3C). Furthermore, by plotting the clinical decision curve (DCA), it was shown that the Cutoff value (37.0%) obtained by the ROC analysis (Figure 3E) was within the threshold probability range of the DCA curve. Further analysis showed that when 37.0% was set as the threshold probability of diagnosing PsA with NAFLD, 21 out of 100 PsA patients at risk for NAFLD diagnosed using this model could benefit from it without harming others (Figure 3D). Therefore, the nomogram of the prediction model has a better prediction effect on the risk of NAFLD in PsA patients and has a certain clinical application value.
Peripheral blood NK cell levels are associated with dyslipidemia in patients With PsA
Dyslipidemia is a traditional risk factor for NAFLD. Our study also showed that the levels of some blood lipids (TG, CHOL, LDL) were significantly higher in the PsA-NAFLD group than in the PsA group. Further analysis of the correlation between common lipids in serum of PsA patients and peripheral blood lymphocyte subsets revealed that the number and percentage of peripheral blood NK cells were positively correlated with CHOL, TG, and LDL levels. Among them, the correlation between the number of NK cells and the level of TG is more obvious (Figures 4A, B). This suggests that NK cells may be involved in the occurrence of dyslipidemia in PsA patients.
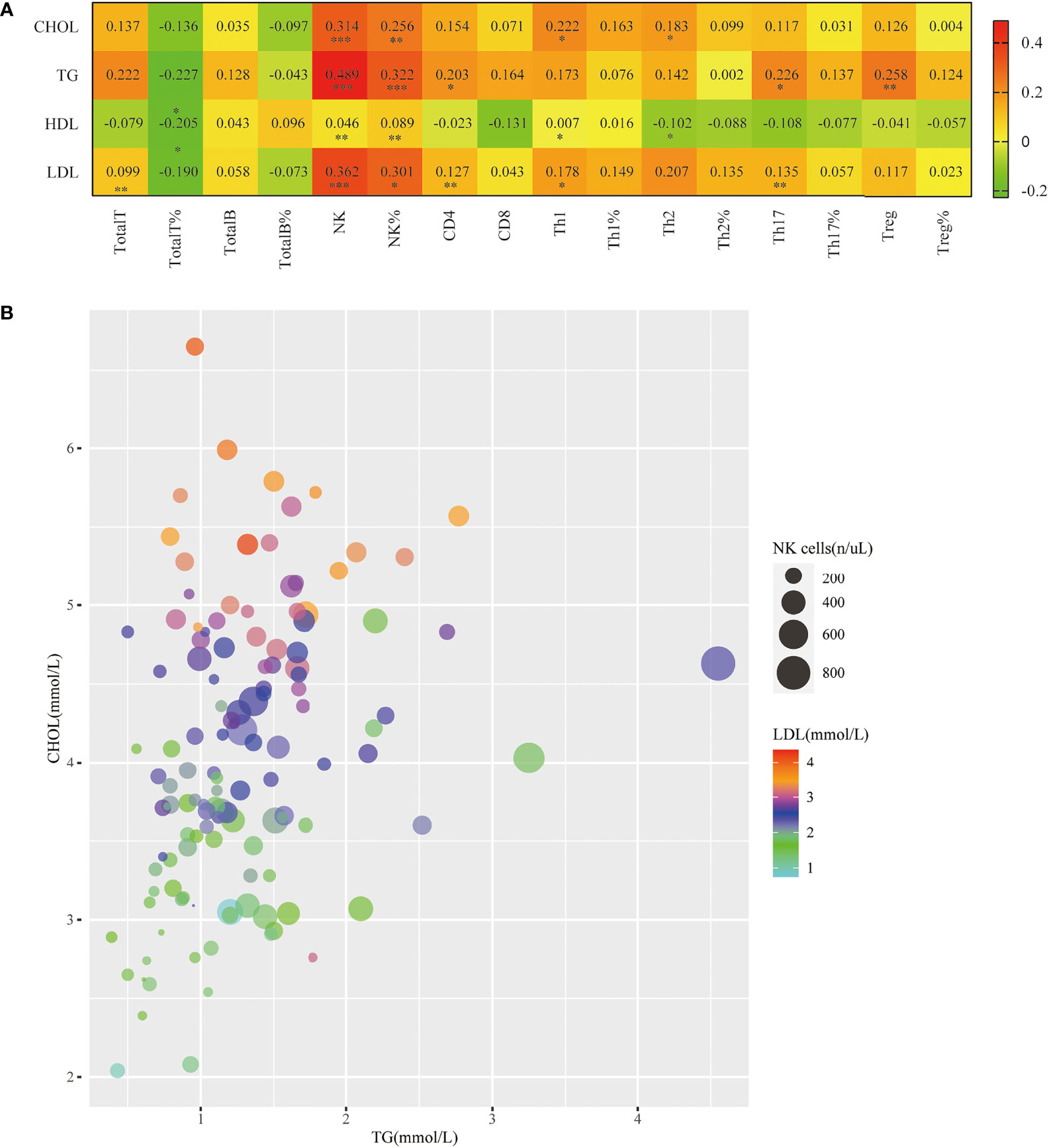
Figure 4 Correlation of peripheral blood NK cell levels with hyperlipidemia in patients with PsA. (A). Heatmap of correlation of the serum lipid levels (TG, CHOL, LDL, HDL) with Total T, Total T%, Total B, Total B%, NK, NK%, CD4+T, CD8+T, Th1, Th1%, Th2, Th2%, Th17, Th17%, Treg, Treg%. (*p < 0.05, **p < 0.01, ***p < 0.001; by Spearman correlation test). (B). Correlation bubble plot of TG, CHOL, LDL, and peripheral blood NK cell count.
In addition, we also found that the numbers of Th1 cells, Th2 cells, Th17 cells, and Treg cells in the peripheral blood of PsA patients were all correlated with certain blood lipids, but the correlation was weak (Figure 4A).
Discussion
Previous research on PsA has focused on the skin and joints. In the past ten years, with the in-depth study of the comorbidities of PsA patients, the pathophysiological relationship between PsA and cardiovascular disease, metabolic syndrome, insulin resistance, and mental illness has gradually been recognized. The occurrence of PsA-related comorbidities and their impact on mortality have received increasing attention from scholars (9, 25). Many recent studies have shifted the focus of PsA complications to the liver, especially NAFLD (26, 27). Because NAFLD is not only highly prevalent in patients with PsA, but more importantly, when PsA and NAFLD co-occur, the severity of both diseases may increase significantly. Therefore, early identification and intervention of NAFLD in PsA patients are particularly important.
This retrospective study analyzed the characteristics of peripheral blood lymphocyte subsets and CD4+T cells in patients with PsA and PsA-NAFLD and used univariate and multivariate Logistic regression to find high-risk immunological factors for NAFLD. The results of the analysis showed that Th1% was an independent risk factor for NAFLD. Combined with other risk factors BMI and TG, a nomogram prediction model of NAFLD risk in PsA patients was established and validated, hoping to predict and identify NAFLD early. Furthermore, the relationship between lymphocyte subsets in peripheral blood and serum lipid profile was further discussed to seek the correlation between immune cells and lipid metabolism abnormalities.
PsA is a chronic inflammatory arthritis associated with PsO. Many studies have shown that both PsA and PsO are chronic inflammations mediated by abnormal T cells, and various pro-inflammatory cytokines play key roles in their development (28). Interactions between T-cell subsets, stromal cells, and cytokines in the local microenvironment determine disease characteristics, including colitis, synovitis, bone and cartilage loss, and new bone formation in the axial and peripheral musculoskeletal systems (29). PsA was originally thought to be a Th1 cell-mediated disease. Since the level of Th1 cells and their secreted INF-γ in skin lesions was significantly increased, in vitro experiments showed that IFN-γ could enhance the proliferation of keratinocytes (30). And in successfully treated PsO patients, the levels of Th1 cells in skin lesions and peripheral blood were significantly reduced (31). Recent studies suggest that Th17 cells and their secreted IL-23/IL-17 play a central role in the pathogenesis of PsA, similar to other autoimmune diseases (32). The levels of Th17 cells and IL-17 in the skin lesions of PsA patients were much higher than those in healthy skin, especially IL-17A played a key role in maintaining PsO plaque inflammation, and IL-17A mRNA levels correlated with disease activity. Th17 cells and IL-17 levels are also elevated in the synovial fluid of patients with PsA (33). Randomized clinical trials show that IL-17A and IL-17F antibodies have good efficacy and are potential therapeutic targets (34). Notably, these two types of CD4+T cells promote and activate each other during the pathogenesis of PsA. IFN-γ secreted by Th1 cells can induce Th17 cells through IL-1 and IL-23, and the overactivity of Th17 cells can lead to the exacerbation of Th1 immune responses and the development of chronic inflammatory states (33). Thus, Th1 cells and Th17 cells play a central role in systemic chronic inflammation in PsA. Our study also showed that the levels of Th1 cells and Th17 in the peripheral blood of PsA patients were significantly higher than those of healthy people, suggesting that their abnormal levels may be involved in the pathogenesis of PsA.
Treg cells, a subset of CD4+T lymphocytes, can suppress effector T cells and inflammation, and play an important role in maintaining autoimmune tolerance. Existing studies on the level of Treg cells in the lesional skin and peripheral blood of PsO are still controversial, but most studies have shown that the number and function of Treg cells in peripheral blood are defective (35), and the ratio of Th17/Treg was significantly positively correlated with PASI score (36). Our study also demonstrated decreased Treg% and Th17/Treg cell imbalance in peripheral blood of PsA patients.
In addition to CD4+T cells, NK cells also play a role in the skin lesions of PsA. Studies have shown that cellular infiltrates in acute psoriatic plaques include 5-8% NK cells and are primarily confined to the middle and papillary dermis. NK cells also function by secreting cytokines such as INF-γ, TNF, and IL-22. Once NK cells enter the diseased skin, they will produce INF-γ, which is an effective factor for Th17 cells to migrate to the skin lesions (37). It is unclear whether peripheral blood NK cells are involved in the pathogenesis of PsO, but multiple studies have shown that peripheral blood NK cells are reduced in PsO patients. Our findings also showed that both the number and percentage of NK in the peripheral blood of patients with PsA decreased.
Notably, chronic systemic inflammation caused by abnormal levels of CD4+T cells and NK cells in PsA patients promotes the occurrence and development of hepatic steatosis, especially NAFLD, and significantly increases the risk of advanced liver disease. Because the two diseases share a common inflammatory pathway, which plays a bidirectional promoting role in the course of both diseases. Therefore, identifying key links in the inflammatory pathway and intervening early can not only prevent the occurrence of NAFLD but also help control the disease of PsA. Our study showed that the level of CD4+T cells in peripheral blood of PsA-NAFLD patients was significantly different from that of PsA patients, especially the number and percentage of Th1 cells, and the number of Th17 cells and the ratio of Th1/Th2 were significantly increased. In addition, the number and percentage of NK cells also showed a significant upward trend.
CD4+T cells are involved in the pathological process of NAFLD and promote the occurrence of liver fibrosis and even liver cancer. A recent study explored the role of CD4+T cells in a high-fat diet-induced (HFD) mouse model of humanized NAFLD and found that it has an important role in promoting liver fibrosis. In HFD mice, marked infiltration of CD4+T cells was found and the levels of proinflammatory cytokines IFN-γ, IL-6, IL-17A, and IL-18 were elevated. However, depletion of CD4+T cells in HFD mice not only prevented liver steatosis but also significantly reduced immune infiltration and liver fibrosis (38). Th1 cells, as an important part of CD4+T cells, play an important role in the pathogenesis of NAFLD. Levels of hepatic Th1 cells and related cytokines IFN-γ, IL-12, and TNF-α are elevated in steatotic mice on a choline-deficient diet, a process associated with increased STAT4 and T-bet expression (39). In addition, elevated Th1 cells may be involved in the development of liver fibrosis in the advanced stages of the disease. Human studies also found elevated levels of Th1 cells in peripheral blood and liver in NAFLD patients, with no significant difference between NAFLD and NASH (16).
It has been confirmed that Th1 cells are involved in the pathological process of obesity-related adipose tissue inflammation. Th1 cells are abundant in subcutaneous and visceral adipose tissue in an HFD-fed obese mouse model (40, 41), and blocking Th1 function can reduce adipose tissue inflammation and alleviate symptoms of impaired glucose tolerance (42). Furthermore, in morbidly obese adult patients, genes involved in T cell activation and Th1 cell phenotype switching were already upregulated in the liver before the characteristic leukocyte infiltration occurred (43), this series of evidence suggests that Th1 cells may be involved in the early stages of NAFLD. Through multivariate logistic regression analysis, our study found that peripheral blood Th1% in PsA patients was an independent risk factor for NAFLD. Combined with other risk factors BMI and TG, a nomogram prediction model of NAFLD risk in PsA patients was constructed. Considering that Th1% is not a traditional risk factor for NAFLD, a new predictive model was reconstructed after removing Th1% from the model. By plotting the ROC curve, it is found that the discriminative power of the new model is worse than the original model, so Th1% plays an important role in the prediction model. When we used the cutoff value of the ROC curve of the prediction model (37.0%) as the threshold of the DCA curve, the clinical net benefit rate of patients was higher than the two extreme methods of no intervention and all intervention. This suggests that patients will benefit from immediate intervention when the model predicts risk of NADLD higher than 37.0%, and if the risk is lower than 37.0%, the intervention is temporarily withheld. Therefore, this model is beneficial to the formulation of clinical decision-making programs for PsA patients.
The role of Th17 cells in NAFLD has been extensively studied, and relevant animal and human studies have shown that Th17 cells are elevated in peripheral blood and liver tissue (14), especially in NASH patients (44). IL-17 secreted by Th17 cells is an important factor in promoting hepatic steatosis. Numerous animal and in vitro studies have found that hepatic steatosis increases when IL-17 is present but decreases after blocking IL-17 function (39, 45). In addition, Th17 cells also have obvious effects on liver fibrosis, and the possible mechanism is that IL-17 directly acts on hepatic stellate cells in a JNK- and STAT3-dependent manner to induce collagen production (46).
To date, the level and function of NK cells in NAFLD remain controversial. Recent studies have shown that NAFLD patients have a lower frequency of CD56dim NK cells and lower expression of the activating receptor NKG2D compared to healthy individuals (22). Furthermore, NK cell function is impaired, resulting in reduced granzyme/perforin and INF-γ production, ultimately reducing cytotoxicity and tumor lethality (19). However, in NASH patients, the level of NK cells in the liver parenchyma is increased, which directly activates hepatic stellate cells through the activation of receptors NKG2D, NKp46, and p38/PI3K/AKT pathways, and promotes the development of liver fibrosis (23, 47, 48). Our study also showed that the percentage of peripheral blood NK in PsA-NAFLD patients was lower than that in healthy people, but it is worth noting that the number and percentage of peripheral blood NK increased in the PsA-NAFLD group compared with the PsA group. We speculate that it may be associated with higher levels of systemic inflammation in patients with PsA-NAFLD. Considering the role of NK cells in NASH and liver fibrosis, the occurrence of NASH and advanced liver disease in PsA-NAFLD patients should be monitored in clinical practice.
Dyslipidemia is a common risk factor for both diseases, in addition to the common chronic low-grade systemic inflammation. PsA patients are often accompanied by a variety of lipid metabolism abnormalities, such as increased TG and decreased High-density lipoprotein cholesterol (HDL-C), which are more prevalent during disease activity (49), suggesting a potential link between inflammation and lipid profiles. Abnormal lipid metabolism is also closely associated with NAFLD, manifested by increased levels of TG and low-density lipoprotein cholesterol (LDL-C) and decreased high-density lipoprotein cholesterol (HDL-C) levels (50), therefore PsA and NAFLD have similar lipid profiles. However, the lipid profile of PsA-NAFLD patients has not been reported. In our study, serum TG, CHOL, and LDL levels were significantly higher in the PsA-NAFLD group than in the PsA group. It is worth noting that the number and percentage of NK cells in peripheral blood were positively correlated with these three lipids, especially the correlation between the number of NK cells and TG was more significant. At present, the research on the relationship between NK cells and lipid metabolism such as TG is still relatively limited. Recent studies have found that in the NASH mouse model, liver NK cells are significantly increased, and after NK cell depletion, liver TG content is reduced, and the symptoms of hepatitis in mice are significantly relieved, suggesting that NK cells are involved in the process of liver lipid accumulation (51). Therefore, given the correlation of NK cell levels with differential lipids, we speculate that NK cells may be involved in the occurrence of dyslipidemia in PsA patients, thereby increasing the susceptibility to NAFLD. The exact relationship between peripheral blood NK cells and dyslipidemia in PsA patients still requires epidemiological investigations with large samples.
Of course, our study has certain limitations. First, the modeling data came from the same medical center and lacked validation from external data. If patient data from multiple centers can be incorporated at a later stage, with simultaneous external validation, the model can be further adjusted and strengthened. Second, the independent risk factors in the nomogram prediction model are all continuous measurement variables, which are not clinically concise. In the later stage, the method of optimal scale regression can be considered to group the continuous measurement data variables and convert them into categorical variables, which is convenient for the clinical application of the nomogram prediction model. Finally, because this study was a retrospective clinical analysis, an accurate assessment of PsA disease activity and NAFLD severity was lacking.
In conclusion, NAFLD is a common complication of PsA. If not detected and intervened early, it may lead to the progression of PsA and even increase the risk of advanced liver disease. Our study found that Th1% in peripheral blood of PsA patients was an independent risk factor for NAFLD. Combined with the traditional risk factors of hyperlipidemia and obesity, an individualized nomogram prediction model was constructed to predict the risk of NAFLD in PsA at an early stage. The prediction model has good discrimination, calibration, and clinical validity, and the visualized prediction model is convenient for clinical operation. In addition, the level of NK cells in peripheral blood is related to dyslipidemia in patients with PsA, and may also be involved in the pathogenesis of NAFLD. Therefore, Th1 cells and NK cells may be potential biomarkers to detect NAFLD in PsA patients. In the future, we will analyze the levels of inflammatory cytokines downstream of Th1 and NK cells to explore their molecular biological mechanisms.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was approved by the Ethics Committee of the Second Hospital of Shanxi Medical University (2019YX114). A written informed consent was obtained from every participant.
Author contributions
Individual authors’ contributions are as follows: BL performed the data analyses and wrote the manuscript. HY and JL participated in the collection of samples and clinical data. RS participated in the performance of the research and statistical analysis. CG and XL participated in the study design and revising of the manuscript. CW provided intellectual input and supervision throughout the study and made a substantial contribution to manuscript drafting. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the National Natural Science Foundation of China (No. 81971543), National Natural Science Foundation of China (No. 81471618), and Key Research and Development (R&D) Projects of Shanxi Province (201803D31119).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Prussick RB, Miele L. Nonalcoholic fatty liver disease in patients with psoriasis: a consequence of systemic inflammatory burden? Br J Dermatol (2018) 179(1):16–29. doi: 10.1111/bjd.16239
2. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol (2018) 15(1):11–20. doi: 10.1038/nrgastro.2017.109
3. Tilg H, Targher G. NAFLD-related mortality: simple hepatic steatosis is not as 'benign' as thought. Gut (2021) 70(7):1212–3. doi: 10.1136/gutjnl-2020-323188
4. Eder L, Dey A, Joshi AA, Boehncke WH, Mehta NN, Szentpetery A. Cardiovascular diseases in psoriasis and psoriatic arthritis. J Rheumatol Suppl (2019) 95:20–7. doi: 10.3899/jrheum.190114
5. Mantovani A, Gisondi P, Lonardo A, Targher G. Relationship between non-alcoholic fatty liver disease and psoriasis: A novel hepato-dermal axis? Int J Mol Sci (2016) 17(2):217. doi: 10.3390/ijms17020217
6. Tarantino G, Costantini S, Finelli C, Capone F, Guerriero E, La Sala N, et al. Is serum interleukin-17 associated with early atherosclerosis in obese patients? J Transl Med (2014) 12:214. doi: 10.1186/s12967-014-0214-1
7. Balak DMW, Piaserico S, Kasujee I. Non-alcoholic fatty liver disease (NAFLD) in patients with psoriasis: A review of the hepatic effects of systemic therapies. Psoriasis (Auckl) (2021) 11:151–68. doi: 10.2147/PTT.S342911
8. Scriffignano S, Perrotta FM, De Socio A, Lubrano E. Role of comorbidities in spondyloarthritis including psoriatic arthritis. Clin Rheumatol (2019) 38(1):3–10. doi: 10.1007/s10067-018-4332-7
9. Heitmann J, Frings VG, Geier A, Goebeler M, Kerstan A. Non-alcoholic fatty liver disease and psoriasis - is there a shared proinflammatory network? J Dtsch Dermatol Ges (2021) 19(4):517–28. doi: 10.1111/ddg.14425
10. Diani M, Altomare G, Reali E. T helper cell subsets in clinical manifestations of psoriasis. J Immunol Res (2016) 2016:7692024. doi: 10.1155/2016/7692024
11. Benham H, Norris P, Goodall J, Wechalekar MD, FitzGerald O, Szentpetery A, et al. Th17 and Th22 cells in psoriatic arthritis and psoriasis. Arthritis Res Ther (2013) 15(5):R136. doi: 10.1186/ar4317
12. Jansen DT, Hameetman M, van Bergen J, Huizinga TW, van der Heijde D, Toes RE, et al. IL-17-producing CD4+ T cells are increased in early, active axial spondyloarthritis including patients without imaging abnormalities. Rheumatology (2015) 54(4):728–35. doi: 10.1093/rheumatology/keu382
13. Chowdhury AC, Chaurasia S, Mishra SK, Aggarwal A, Misra R. IL-17 and IFN-γ producing NK and γδ-T cells are preferentially expanded in synovial fluid of patients with reactive arthritis and undifferentiated spondyloarthritis. Clin Immunol (2017) 183:207–12. doi: 10.1016/j.clim.2017.03.016
14. Van Herck MA, Weyler J, Kwanten WJ, Dirinck EL, De Winter BY, Francque SM, et al. The differential roles of t cells in non-alcoholic fatty liver disease and obesity. Front Immunol (2019) 10:82. doi: 10.3389/fimmu.2019.00082
15. Gebru YA, Gupta H, Kim HS, Eom JA, Kwon GH, Park E, et al. T Cell subsets and natural killer cells in the pathogenesis of nonalcoholic fatty liver disease. Int J Mol Sci (2021) 22(22):12190. doi: 10.3390/ijms222212190
16. Rau M, Schilling AK, Meertens J, Hering I, Weiss J, Jurowich C, et al. Progression from nonalcoholic fatty liver to nonalcoholic steatohepatitis is marked by a higher frequency of th17 cells in the liver and an increased th17/resting regulatory t cell ratio in peripheral blood and in the liver. J Immunol (2016) 196(1):97–105. doi: 10.4049/jimmunol
17. Ma C, Kesarwala AH, Eggert T, Medina-Echeverz J, Kleiner DE, Jin P, et al. NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature (2016) 531(7593):253–7. doi: 10.1038/nature16969
18. Pellicoro A, Ramachandran P, Iredale JP, Fallowfield JA. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol (2014) 14(3):181–94. doi: 10.1038/nri3623
19. Michelet X, Dyck L, Hogan A, Loftus RM, Duquette D, Wei K, et al. Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat Immunol (2018) 19(12):1330–40. doi: 10.1038/s41590-018-0251-7
20. Cuff AO, Sillito F, Dertschnig S, Hall A, Luong TV, Chakraverty R, et al. The obese liver environment mediates conversion of nk cells to a less cytotoxic ilc1-like phenotype. Front Immunol (2019) 10:2180. doi: 10.3389/fimmu.2019.02180
21. Viel S, Besson L, Charrier E, Marçais A, Disse E, Bienvenu J, et al. Alteration of natural killer cell phenotype and function in obese individuals. Clin Immunol (2017) 177:12–7. doi: 10.1016/j.clim.2016.01.007
22. Martínez-Chantar ML, Delgado TC, Beraza N. Revisiting the role of natural killer cells in non-alcoholic fatty liver disease. Front Immunol (2021) 12:640869. doi: 10.3389/fimmu.2021.640869
23. Li T, Yang Y, Song H, Li H, Cui A, Liu Y, et al. Activated NK cells kill hepatic stellate cells via p38/PI3K signaling in a TRAIL-involved degranulation manner. J Leukoc Biol (2019) 105(4):695–704. doi: 10.1002/JLB.2A0118-031RR
24. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American college of Rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis (2010) 69(9):1580–8. doi: 10.1136/ard.2010.138461
25. Boehncke WH. Systemic inflammation and cardiovascular comorbidity in psoriasis patients: Causes and consequences. Front Immunol (2018) 9:579. doi: 10.3389/fimmu.2018.00579
26. Lønnberg AS, Skov L. Co-Morbidity in psoriasis: mechanisms and implications for treatment. Expert Rev Clin Immunol (2017) 13(1):27–34. doi: 10.1080/1744666X.2016.1213631
27. Elmets CA, Leonardi CL, Davis DMR, Gelfand JM, Lichten J, Mehta NN, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol (2019) 80(4):1073–113. doi: 10.1016/j.jaad.2018.11.058
28. FitzGerald O, Ogdie A, Chandran V, Coates LC, Kavanaugh A, Tillett W, et al. Psoriatic arthritis. Nat Rev Dis Primers (2021) 7(1):59. doi: 10.1038/s41572-021-00293-y
29. Jadon DR, Stober C, Pennington SR, FitzGerald O. Applying precision medicine to unmet clinical needs in psoriatic disease. Nat Rev Rheumatol (2020) 16(11):609–27. doi: 10.1038/s41584-020-00507-9
30. Hu P, Wang M, Gao H, Zheng A, Li J, Mu D, et al. The role of helper t cells in psoriasis. Front Immunol (2021) 12:788940. doi: 10.3389/fimmu.2021.788940
31. Lew W, Bowcock AM, Krueger JG. Psoriasis vulgaris: cutaneous lymphoid tissue supports T-cell activation and "Type 1" inflammatory gene expression. Trends Immunol (2004) 25(6):295–305. doi: 10.1016/j.it.2004.03.006
32. O'Brien-Gore C, Gray EH, Durham LE, Taams LS, Kirkham BW. Drivers of inflammation in psoriatic arthritis: the old and the new. Curr Rheumatol Rep (2021) 23(6):40. doi: 10.1007/s11926-021-01005-x
33. Karczewski J, Dobrowolska A, Rychlewska-Hańczewska A, Adamski Z. New insights into the role of T cells in pathogenesis of psoriasis and psoriatic arthritis. Autoimmunity (2016) 49(7):435–50. doi: 10.3109/08916934.2016.1166214
34. Leonardi C, Maari C, Philipp S, Goldblum O, Zhang L, Burkhardt N, et al. Maintenance of skin clearance with ixekizumab treatment of psoriasis: Three-year results from the UNCOVER-3 study. J Am Acad Dermatol (2018) 79(5):824–30.e2. doi: 10.1016/j.jaad.2018.05.032
35. Li B, Lei J, Yang L, Gao C, Dang E, Cao T, et al. Dysregulation of akt-FOXO1 pathway leads to dysfunction of regulatory t cells in patients with psoriasis. J Invest Dermatol (2018) 139(10):2098–107. doi: 10.1016/j.jid.2018.12.035
36. Wang XY, Chen XY, Li J, Zhang HY, Liu J, Sun LD. MiR-200a expression in CD4+ T cells correlates with the expression of Th17/Treg cells and relevant cytokines in psoriasis vulgaris: A case control study. BioMed Pharmacother (2017) 93:1158–64. doi: 10.1016/j.biopha.2017.06.055
37. Tobin AM, Lynch L, Kirby B, O'Farrelly C. Natural killer cells in psoriasis. J Innate Immun (2011) 3(4):403–10. doi: 10.1159/000328011
38. Her Z, Tan JHL, Lim YS, Tan SY, Chan XY, Tan WWS, et al. CD4+ T cells mediate the development of liver fibrosis in high fat diet-induced nafld in humanized mice. Front Immunol (2020) 11:580968. doi: 10.3389/fimmu.2020.580968
39. Rolla S, Alchera E, Imarisio C, Bardina V, Valente G, Cappello P, et al. The balance between IL-17 and IL-22 produced by liver-infiltrating T-helper cells critically controls NASH development in mice. Clin Sci (Lond) (2016) 130(3):193–203. doi: 10.1042/CS20150405
40. Khan IM, Dai Perrard XY, Perrard JL, Mansoori A, Wayne Smith C, Wu H, et al. Attenuated adipose tissue and skeletal muscle inflammation in obese mice with combined CD4+ and CD8+ T cell deficiency. Atherosclerosis (2014) 233(2):419–28. doi: 10.1016/j.atherosclerosis.2014.01.011
41. Hong CP, Park A, Yang BG, Yun CH, Kwak MJ, Lee GW, et al. Gut-specific delivery of t-helper 17 cells reduces obesity and insulin resistance in mice. Gastroenterology (2014) 152(8):1998–2010. doi: 10.1053/j.gastro.2017.02.016
42. Stolarczyk E, Vong CT, Perucha E, Jackson I, Cawthorne MA, Wargent ET, et al. Improved insulin sensitivity despite increased visceral adiposity in mice deficient for the immune cell transcription factor T-bet. Cell Metab (2013) 17(4):520–33. doi: 10.1016/j.cmet.2013.02.019
43. Bertola A, Bonnafous S, Anty R, Patouraux S, Saint-Paul MC, Iannelli A, et al. Hepatic expression patterns of inflammatory and immune response genes associated with obesity and NASH in morbidly obese patients. PloS One (2010) 5(10):e13577. doi: 10.1371/journal.pone.0013577
44. Vonghia L, Ruyssers N, Schrijvers D, Pelckmans P, Michielsen P, De Clerck L, et al. CD4+ROR γ t++ and tregs in a mouse model of diet-induced nonalcoholic steatohepatitis. Mediators Inflammation (2015) 2015:239623. doi: 10.1155/2015/239623
45. Gomes AL, Teijeiro A, Burén S, Tummala KS, Yilmaz M, Waisman A, et al. Metabolic inflammation-associated il-17a causes non-alcoholic steatohepatitis and hepatocellular carcinoma. Cancer Cell (2016) 30(1):161–75. doi: 10.1016/j.ccell.2016.05.020
46. Muscate F, Woestemeier A, Gagliani N. Functional heterogeneity of CD4+ T cells in liver inflammation. Semin Immunopathol (2021) 43(4):549–61. doi: 10.1007/s00281-021-00881-w
47. Radaeva S, Sun R, Jaruga B, Nguyen VT, Tian Z, Gao B. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology (2006) 130(2):435–52. doi: 10.1053/j.gastro.2005.10.055
48. Gur C, Doron S, Kfir-Erenfeld S, Horwitz E, Abu-Tair L, Safadi R, et al. NKp46-mediated killing of human and mouse hepatic stellate cells attenuates liver fibrosis. Gut (2011) 61(6):885–93. doi: 10.1136/gutjnl-2011-301400
49. Perez-Chada LM, Merola JF. Comorbidities associated with psoriatic arthritis: Review and update. Clin Immunol (2020) 214:108397. doi: 10.1016/j.clim.2020.108397
50. Katsiki N, Mikhailidis DP, Mantzoros CS. Non-alcoholic fatty liver disease and dyslipidemia: An update. Metabolism (2016) 65(8):1109–23. doi: 10.1016/j.metabol.2016.05.003
Keywords: psoriatic arthritis, nonalcoholic fatty liver disease, nomogram, dyslipidemia, T helper-1 cells, natural killer cells
Citation: Li B, Su R, Yan H, Liu J, Gao C, Li X and Wang C (2022) Immunological risk factors for nonalcoholic fatty liver disease in patients with psoriatic arthritis: New predictive nomograms and natural killer cells. Front. Immunol. 13:907729. doi: 10.3389/fimmu.2022.907729
Received: 30 March 2022; Accepted: 27 June 2022;
Published: 22 July 2022.
Edited by:
Raphaela Goldbach-Mansky, National Institutes of Health (NIH), United StatesReviewed by:
Giovanni Tarantino, University of Naples Federico II, ItalyMarija Takic, University of Belgrade, Serbia
Ali Akbar Malekirad, Payame Noor University, Iran
Copyright © 2022 Li, Su, Yan, Liu, Gao, Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Caihong Wang, c253Y2hAc2luYS5jb20=
 Baochen Li
Baochen Li Rui Su
Rui Su Huanhuan Yan
Huanhuan Yan Juanjuan Liu
Juanjuan Liu Chong Gao
Chong Gao Xiaofeng Li
Xiaofeng Li Caihong Wang
Caihong Wang