
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol., 17 August 2022
Sec. T Cell Biology
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.907172
Recently, accumulating evidence has elucidated the important role of T cells with stem-like characteristics in long-term maintenance of T cell responses and better patient outcomes after immunotherapy. The fate of TSL cells has been correlated with many physiological and pathological human processes. In this review, we described present advances demonstrating that stem-like T (TSL) cells are central players in human health and disease. We interpreted the evolutionary characteristics, mechanism and functions of TSL cells. Moreover, we discuss the import role of distinct niches and how they affect the stemness of TSL cells. Furthermore, we also outlined currently available strategies to generate TSL cells and associated affecting factors. Moreover, we summarized implication of TSL cells in therapies in two areas: stemness enhancement for vaccines, ICB, and adoptive T cell therapies, and stemness disruption for autoimmune disorders.
Physiologically, stem cells are responsible for tissue regeneration (1). They exist in multiple tissues, such as skin (2), cardiac muscle (3), lung (4), central nervous systems (5), intestine and blood (6, 7). In tumor, cancer stem cells (CSCs) sustained the heterogeneity of tumor cell populations (8, 9). Since hematopoietic stem cells (HSCs) are insufficient to support the high turnover of antigen-specific T cell for long-lasting immunological memory, which persisting for a lifetime without re-exposing to the antigen stimulation (10, 11). In 2001, Fearon’s team proposed stem cell-like properties of T cells to maintain T cell memory (12). Like HSCs, memory T cells also show some stem-like features like asymmetric T lymphocyte division (ASD) (13, 14). Possibly, the T cell population is originated from memory stem T cells.
Akin to stem cell in somatic tissues, these T cells generated and maintained T cell heterogeneity (14, 15), and shared a core transcriptional signature with HSCs (13). Recently, significant development can be seen in revealing the features and functions of stem-like T cells and how they can be harnessed to improve therapeutic outcomes (14, 16–31) (Figure 1).
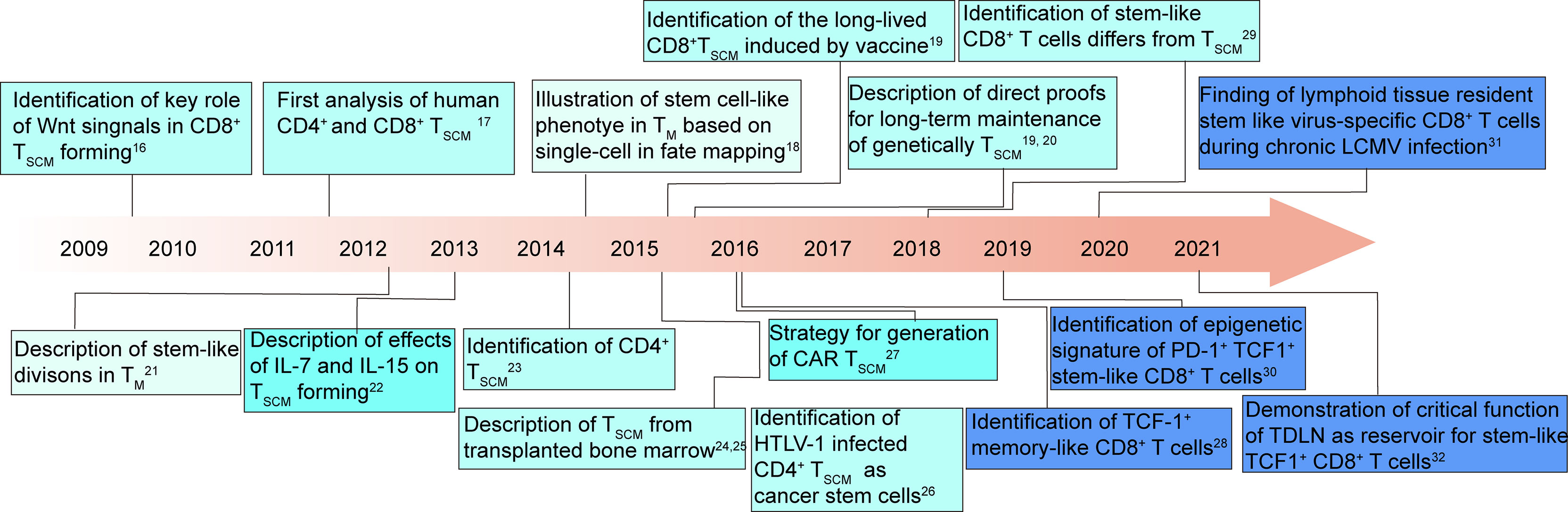
Figure 1 T cell stemness: milestones and key discoveries in the last 12 years (14, 16–31). TM, T memory cells; TSCM, T memory stem cells; HTLV-1, human T cell lymphotropic virus type 1; CAR, chimeric antigen receptor; LCMV, lymphocytic choriomeningitis virus; TDLN, tumor-draining lymph node.
The TCF1-positive stem-like T cells, differing from TSCM cells, have been found in chronic immune model. These stem-like T cells are memory-like progenitor exhausted T cells. They are essential to maintain the immune response of T cell and determine therapeutic effects of PD-1 antibody (31).
Traditionally, niche is defined as a specific microenvironment that contains intrinsic an extrinsic cues for stemness retaining and continuous division (32).
Niche provides a spatial associations, which surrounded by extracellular matrix, cytokines and chemokines, which are produced by stroma, are essential for cellular homeostasis, self-renewal or differentiation (33). Integrins are essential to regulate the interaction of stem cell with surrounding niches, and mediate stem cell location and motion. Depending on contacting via integrin, stem cells receive cues from niches to sustain their activities (34–39). Bone marrow provides physical and chemical confinement to keep anatomic structure of the stem cell niches (40–42). But there is no direct evidence to prove that it similarly serves as a TSCM cell niche.
Particularly, increasing evidence has shown that T cells need niches to maintain their long-lived memory and effectively respond to immunotherapy (40, 43). Further studies demonstrated that secondary lymphoid tissues (lymph nodes (LNs) and spleens etc.) provide homeostatic cues from fibroblastic reticular cell niches for TSCM cells (44). These organs are specialized to support immune cells in antigen presentation, initial activation and proliferation (45, 46). In triple negative breast cancer (TNBC) patients, stem-like T lymphocytes against tumor have been found to residue in tumor-draining lymph nodes (TDLNs) (47). TDLNs, tertiary lymphoid structures and other intra-tumoral niches have been demonstrated to house CD8 positive stem-like T cells (48–50).
In this review, we describe emerging findings demonstrating the characteristics of subset of stem-like T (TSL) cells, mainly including stem-like memory T (TSCM) cells and stem-like progenitor exhausted T cells. We highlight how they exist and functionalize for human health and disease. We further demonstrate the potential signaling pathways that maintain stemness of T cells. Moreover, we discuss the import role of distinct niches and how they affect the stemness of TSL cells. Furthermore, we also outlined currently available strategies to generate TSL cells and associated affecting factors. Finally, we envision how to confer stem cell-like properties to T cells that might be used for immunotherapies.
Box 1. Highlights
This box includes main points of view of this review.
● Stem-like T cells (TSC) are a subpopulation of mature T cells that display the stem-like properties, maintaining long-lasting immune effect even among exhausting clones.
● All unique function of stem-cell like T cells is not completed proven by experiments. Further experimental evidence is still needed to comprehensively dig out all their unique function before an exact definition can be made.
● Secondary lymphoid tissues provide survival niches for most stem-like T cells of the adaptive immune system; besides, intratumoral APC niches and tumor-associated tertiary lymphoid structures also house stem-like tumor-specific lymphocytes.
● Approaches to generate stem-like T cells mainly include; pharmacological, cytokine and costimulatory signal-based regulation (e.g.GSK3β inhibitor, MEK inhibitor AKT inhibitor, IL-21, 4-1BB agonist, metabolic taming and TCR controlling), and gene-based regulation (TSCM-associated transcription factors or miRNAs, NOTCH signaling).
● Currently, clinical clinical-grade memory stem cells are mainly derived from naïve T cells culturing in with cytokines like IL-7, IL-21, and or IL-15, however, many other experimental methods are still not accessible in clinic.
● A significant number of researches have demonstrated that approaches to control stem-like T cells are necessary to achieve better outcomes in immune related diseases, such as HIV infection, Type I diabetes and cancer.
● Evidence from both experiments and clinical trials has demonstrated stem-like T cells determine therapeutic effect of immunotherapies, especially like immune checkpoint blockade (ICB), vaccines and adoptive cell therapy (ACT).
Stem-like T cells (TSC) are a subpopulation of mature T cells that own self-renewal potential and can generate progenies by asymmetric division. These stem-like T cells exist in differentiating and even in exhausting process (Figure 2). The strength and duration of stimulatory signals decides the fate of naïve T cells: transform to Effector (TEFF) or exhausted (TEX) T cells.
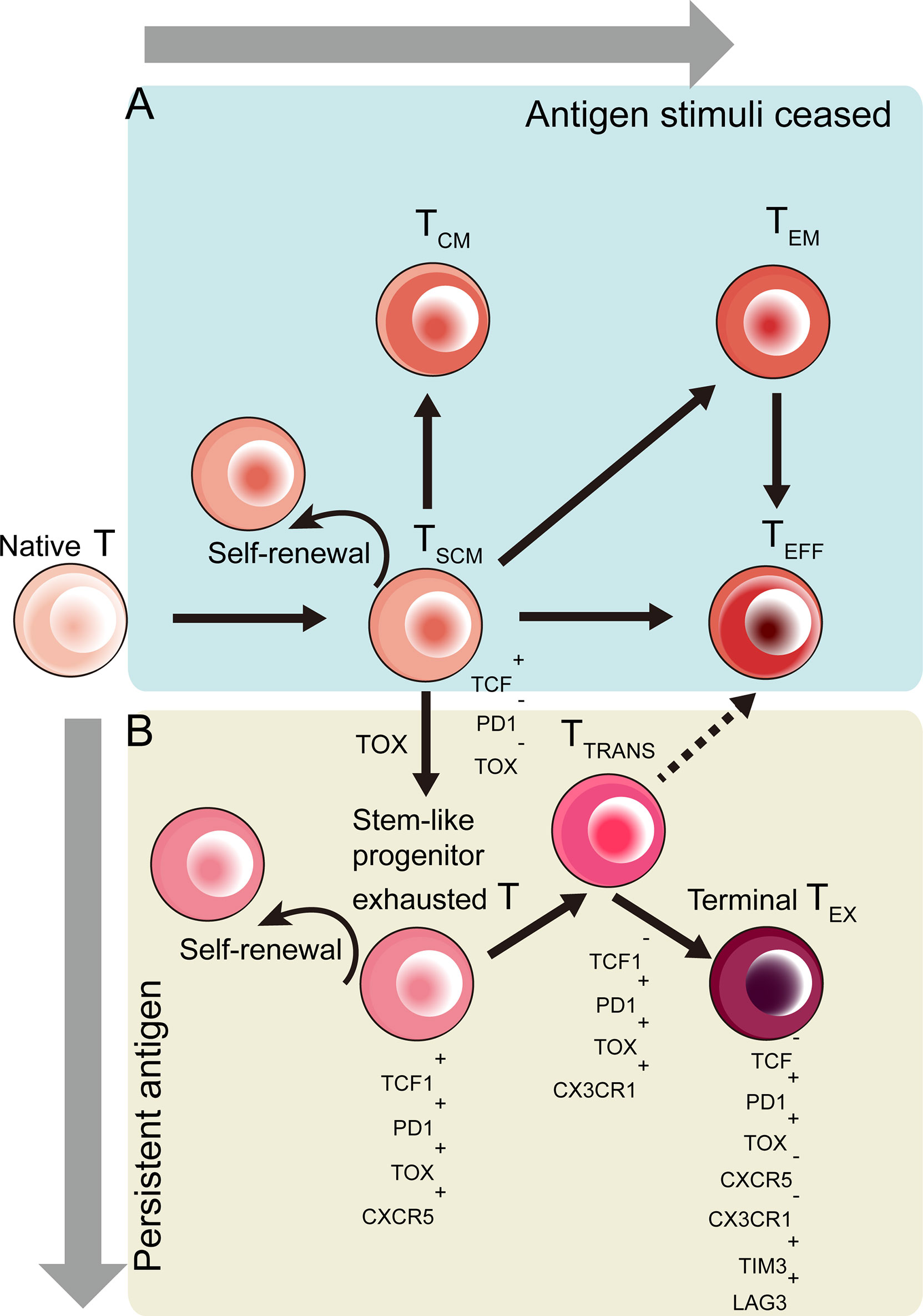
Figure 2 Developmental trajectory of CD8+ T cells toward effector (TEFF) and exhausted (TEX) CD8+ T cells in response to different antigen stimulation conditions. Stem-like T cells emerge during the T cell differentiation. (A) When antigen clear and stimuli cease, activated T cells differentiate into memory stem cell-like T cells (TSCM), central memory (TCM) T cells or effector memory (TEM) T cells in terms of signal strength (51). (B) Induced by TOX-based epigenetic regulation, TSCM cells become stem-like progenitor exhausted T cells co-expressed TCF1, PD1, TOX and CXCR5. These stem-like exhausted T cells then become TCF1-PD1+TOX+CX3CR1+ transitory exhausted T (TTRANS) cells, which give rise to terminally exhausted (TEX) T cells (52–56).
Responding to antigen stimuli, naïve T cells differentiated into memory T cells, like central memory T (TCM) cells and effector memory (TEM) T cells and terminally, into TEFF cells (51) (Figure 2A). The CD62L+ CCR7+ TCM cells were considered to display stem cell-like phenotypes and functions among all memory T cells (12). Later in mice model, a new group of memory T cells were discovered with more stem cell-like attributes in comparison with traditional TCM cells. Similar to naive T cells, these memory T cells have a similar CD44lowCD62Lhigh phenotype. Moreover, they are a new Sca-1+ Bcl-2+ IL-2Rβ+ CXCR3+ T cells subset (16, 57). These T cells were named memory stem T (TSCM) cells.
CD8+ TSCM cells are also found in humans, which highly express c-KIT indicating stemness, expel cellular toxins by ABCB1 (58). More recently, other studies have demonstrated highly expressed β-catenin as molecular clue to human CD8+ TSCM cells (16, 59). Therefore, TSCM cells can be regarded as stem-like memory T cells. Upon acute antigen stimulation, naïve T cells may differentiate into TSCM cells, TCM cells or TEM cells after antigen clear. In virus infection condition, TSCM cells, TCM cells and TEM cells can be found in latent or resolved infection (60).
However, primed T cells would differentiate into terminal T exhausted (TEX) cells upon chronical antigen stimulation (52). The exhausted PD-1 positive T cells are divided by differentiation hierarchy marked by their PD-1 expressing level, including less-differentiated PD-1intermediate T cell that are able to respond to PD-1 blockade, and terminally exhausted PD-1 high T cells, which have inefficient function (27, 53, 61). Jolanda Brummelman et al. conducted scRNA-seq and found that these stem-like T cells do not exhibit typical properties as seen in circulating TSCM cells (28).
These stem-like CD8+ T cells, which undergo TOX-driven epigenetic changes, are progenitor exhausted T cells co-expressing TCF1,PD1,TOX and CXCR5 (52–56) (Figure 2B).
Indeed, these stem-like CD8+ T cells are subset of exhausted T cells. But they differ from exhausted T cells by the expression of TCF-1 Under chronic antigen stimulation, Tex cell population can be generated independently of TCF-1-expressing Tpex cells, but they would be replaced by new Tex cells derived from TCF-1+exhausted T cells progenitor T cells, which continuously activated, proliferated and differentiated into Tex cells (60). Some researchers demonstrated the development relationship among different exhausted T cells (62, 63). There are four subsets of exhausted T cells distinguished by expression of Slamf6 and CD69, and the four subsets were defined as progenitor 1 (Slamf6+CD69+; Texprog1), progenitor 2 (Slamf6+CD69-; Texprog2), intermediate (Slamf6-CD69-;Texint), and terminal (Slamf6-CD69+; Texterm).Among them, the Texprog1 cells are stem-like progenitor exhausted T cells we mentioned above, which had potential to generate all other three subsets of exhausted T cells. Tex cells transited from Texprog1 to Texprog2 to Texint and to Texterm,accompanied by changes of TCF1,TOX, T-bet and Eomes expressions (from TCF1hiToxhi to TCF1intToxhi to TCF1negT-bethiToxint and finally to TCF1negT-betloToxhiEomeshi). Further, Yao et al. emphasized the role of transcriptional repressor BACH2 for stem-like T cells to maintain their stem-like feature (64). But the mechanism about how stem-like cells prevent terminally exhausted cell fate is not well understand till now. There is still much to do to address this issue.
According to Lugli’s depiction (65), TSCM cells are generated with stem-like proprerties like strong proliferation potential and multipotency under healthy homeostatic conditions. These cells can rapidly generate progeny that produce granzyme and other cytokines. Besides, they express naïve T markers-CD62L and CCR7, and self-renewal markers- TCF1 and LEF1.
Stem-like T cells exhibit long-term persistence and the ability to renew to generate more differentiated cells. To monitor the persistence of these cells, genetically engineered T cells have been infused into the host, in order to easily trace these infused cells as antigen-experienced cells over time. Based on this approach, TSCM cells have demonstrated decades of persistence in patients who suffer severe combined immunodeficiency (adenosine deaminase -deficient form) (20). Further studies demonstrated a strong correlation between TSCM cell numbers and enhance d immune reconstitution capacity and longevity in hematopoietic stem cell transplantation (HSCT) models and chimeric antigen receptor T (CAR-T) cell therapy (66, 67).
To track individual transgenic T-cell clones, TCR-α/TCR-β labeling and viral integration can be used to classify according to the patient’s infusion product and T-cell differentiation phenotype during long-term follow-up. Similarly, in patients infected with simian immunodeficiency virus (SIV), TSCM cells can be tracked for up to 70 days at high levels after infection (68). In another study, the number of TSCM cell types gradually increased in HIV-1 patients receiving antiretroviral therapy (ART). After treatment, terminally differentiated effector (TTE) cells shrank more than TEM cells and TSCM cells (19). After all, these studies prove that human TSCM cells have long-term self-renewal capacity and pluripotency.
Another feature of stem-like T cells is that they are also able to produce more differentiated progenies (69). Upon acute antigenic stimulation, TSCM cells directly transformed from naive T cells and further differentiated into TCM, TEM, and terminally differentiated effector T cells (23, 70–72). Pais Ferreira et al. found that stem cell-like TCF7high CD8+ T cells possess a central memory function and can quantitatively generate TCM cells (73). In fact, only naive T cells and TSCM cells were able to produce all memory T cell subsets (17, 19, 21, 51). Therefore, TSCM cells are a memory T cell subset sharing some phenotypes with naïve T cells.
Gattinoni’s group have been done a serial of studies to generate and identify TSCM cells (16, 17, 74, 75). They illustrated that TSCM cells are genetically similar to naïve T cells, showing only 75 differently expressed genes compared with naïve T cells. But they differed from naïve T cells by expression of CD95 and IL-2Rβ, and performing memory T cells functions, including low levels of T cell receptor rearrangement excision circles, replicative history, rapid acquisition of effector functions (IFN-γ, IL-2, and TNF-α production) upon antigen re-challenge. On the other hand, TSCM cells differ also differed from conventional memory T cells with enhanced self-renewal and multipotency.
There were more TSCM cells retained their input phenotype than TCM cells did, indicating enhanced self-renewal capacity of TSCM cells. Researchers have demonstrated increased fraction of effector functional T cells from TN cells to TSCM cells to TCM cells to TEM cells (17). Besides, TSCM cells could generate all conventional memory T cells, including TCM cells and TEM cells. On the contrary, TCM cell could not generate TSCM cells. They further testified increased proliferation and survival of TSCM cells compared with naïve T, TCM and TEM cells in vivo. Verma et al. also confirmed that TSCM cells were distinct from TCM and T naive cells and possessed the characteristics of an intermediated state between naïve T and TCM cells (76). They demonstrated these TSCM cells had high FAO-mediated metabolic fitness, self-renewability, high proliferation, multipotent capacity and antigen recall responses. These TSCM cells are different from naïve T cells in capacity of antigen recall response. In other words, TSCM cells are cover function of both naïve T and TCM cells. Based on their findings that TSCM cells owned a higher degree of unmethylated CpG sites at Tcf7 and methylated CpG sites at Ifng and Prf1 sites, it can be reasonably inferred that more TSCM cells may be superior to TCM cells in stemness when compared with TCM cells.
Indeed, existing observations have provided some experimental evidence for specificity of stem-like T cells among all T cells. However, it may be not sufficient to completely isolate this subset of T cells out of memory T cells and defined as T stem cells. All unique function of stem-cell like T cells is not completed proven by experiments. Further experimental evidence is still needed to comprehensively dig out all their unique function before an exact definition can be made.
According to Lugli’s depiction (65), TSCM cells are generated with stem-like proprerties like strong proliferation potential and multipotency under healthy homeostatic conditions or acute antigen stimulation. While TSL cells herein specifically refers to exhausted precursor T cells with stem-like properties under the condition of chronic antigenic stimulation. Therefore, compared with TSCM cells, TSLs are a group of cells that are closer to exhausted T cells, and thus have certain characteristics of exhausted cells including increased expression of PD-1 and decreased secretion of TNF-α and IFN-γ (65).
TCF1 is the key factor to determine the recall expansion and self-renewal of stem-like PD-1+ T cells (77). These cells also express TIGIT, but not other negative regulators. Furthermore, sustained TCR stimulation is needed for the differentiation into terminal effector or exhausted T cells. Besides, conventional type 1 dendritic cells, CD28 co-stimulation signals, and the cytokine IL-12 in tumors are needed for TIL re-activation under treatment of immune checkpoint blockade, suggesting that these factors are key regulators for PD-1+ TCF1+ progenitor TILs. Besides, IL-27, c-MAF, PRDM1, NR4A1 and TOX support the immune effect and inhibit potential. However, how these factors affect less-differentiated PD-1+ TCF1+ T cells versus PD-1+ TCF1- T cells should be directly evaluated. During T cell priming, the expression level of TCF1 decides the fate of CD8+ T cells: enter into memory T cell pool or differentiated effector cell pool (16, 56, 73, 78–91).
CXCR5+exhausted CD8+ T cells play a key role in controlling replication of virus in lymphocytic choriomeningitis virus (LCMV)-infected mice (61). These CXCR5+ PD-1lo TIM-3lo KLRG1hi T cells are less exhausted than their CXCR5- counterparts. This result suggests that CXCR5+ KLRG1+CD8+ PD-1lo TIM-3lo T cells may have stem cell-like properties. Based on high-dimensional single-cell analysis, additional studies demonstrated that these T cells retained signal networks regulating stem cell-like properties and functions (28). Similarly, other chronic viral infection studies also discovered CXCR5+TCF1+PD1+ TIM3- T cells with stem-like features and CXCR5−TCF1- PD1+ TIM3+ T cells with terminally differentiated features (53, 54, 56). Therefore, CXCR5 may be another key marker for stem-like progenitor exhausted T cells.
Galletti’s group had illustrated difference between fwo subsets of stem-like CD8+ memory T cells progenitors (PD1-TIGIT- subsets versus PD1+TIGIT+ subsets). PD1-TIGIT- subsets were committed to functional progeny, while PD1+TIGIT+ subsets were committed to dysfunctional progeny (82). Acccording to their data about phenotype and transcription charaters, these two subsets correspond to TSCM cells and TSL progenitor exhausted cells as we described in this paper. In order to fast distinguish TSL progenitor exhausted cells and TSCM cells, we summarized serval key difference of them in Table 1 (60, 65). Besides, as TSCM cells are functionally similar to TCM cells, we also compared TSCM and TCM cells together in this Table 1. As shown in this table, TSL progenitor exhausted cells can be distinguished from TSCM cells mainly by the generation condition. TSCM cells were generated under physiology stimulation or acute antigen stimulation, while TSL progenitor exhausted cells. Besides,TSL cells cannot perform high proliferation unless treated by immune checkpoint blockade. Besides, the effector progency derived from TSL progenitor exhausted cells cannot produce Granzyme and express high level of PD1. Additionally, TSL exhausted progenitor cells maintained their survial independent of CD4+ T cell help, while TSCM cells needed the help of CD4+ T cells. On the other hand, although TSCM cells possessed most function that TCM cells also have, they can be differentiated from TCM cells by enhanced stem-like properoties, such as proliferation potential. In addition, TSCM cells are always CD45RA-positive, while TCM cells are CD45RA-positive.
When TSCM cells are abnormal and self-reactive, these T cells may cause pathogenic effects (83). For example, CD4+ TSCM cells may support viral replication and transcriptionally silenced forms of infection in HIV-1 infections (84). Furthermore, HIV-1 is able to use CD4+ TSCM cells as an extremely durable, self-renewing viral reservoir that persists despite antiretroviral therapy for up to approximately 277 months (85). Similar conclusion is reached in patients with HTLV-1 infection. HTLV-1 infected CD4+ TSCM cells can act as a cancer stem cells contributing to the spread and preservation of malignant cells infected by HTLV-1.
TSCM cells also exhibit negative effects in autoimmunity. Aplastic anemia is regulated by autoreactive CTLs that target hematopoietic progenitor cells. Compared with healthy people, patients with aplastic anemia have increased frequency and activation status of CD8+ TSCM cells. Furthermore, the growing number of CD8 positive TSCM cells after immunosuppressive therapy indicates bad prognosis (86). A recent genome-wide association study further pinpoints the effect of CD4+ TSCM cells in the autoimmune and other lymphoid diseases. It indicated that the number of CD4+ TSCM cells indicates those susceptible individuals to juvenile idiopathic arthritis or chronic lymphocytic leukemia (87). Till now, it can be hypothesized that self-renewal autoreactive TSCM cells may lead to long-lasting inflammatory responses, which may explain the long persistence of these diseases. But how TSCM cells affect autoimmune disease must be investigated in dedicated studies. In general, TSCM cells, especially CD4+ TSCM cells, play negative role in many virus-induced diseases and autoimmune diseases. However, how TSCM cells act in diseases like type 1 diabetes, thyroiditis and autoimmune hepatitis is currently poorly understood.
TSCM cells are vital in maintaining long-term protection in many acute and chronic infections (17, 19, 88, 89). However, how these TSCM cells generate during immune response remains unclear. It is difficult to dynamically study T cell activities in human by the limitation of knowing exact timing of infection. Active vaccination, especially smallpox and yellow fever vaccines, offer the possibility to induce an immune response in a supervised manner, are particularly suitable models for primary acute viral infection in humans (90). Based on this, further study has been made to entirely uncover how TSCM cell form and maintain for a long time by using the YF vaccine as a model system (19). In line with results on SIV infection in NHP (68), YF-specific TSCM cells existed early after receiving vaccine, and persisted at stable levels for decades (19). The frequency of TSCM cells are responsible for continuously replenishing exhausted effector T cells, so that to control persisting infections (19, 27, 55, 91).
Notably, some researches reveal that TSCM cells are unable to show positive effects in chronic viral and parasitic infections (88, 89, 92). However, TSCM cells are generally necessary for long-term immune effect acute and chronic microbial infections. The existence of SIV and HIV-1 infected TSCM cells is closely correlated to the development of symptomatic immune deficiency after virus infections (93, 94). In fact, SIV DNA copies were found in CD4-positive TSCM cells of rhesus monkeys, which normally exhibit AIDS-like clinical manifestations when untreated. But in SIV-infected CD4+ TSCM cells of sooty mangoes (a group of NHPs), SIV DNAs are not found. This group is more likely to be asymptomatic carriers even when large number of virus can be detected in circulation system (95, 96). Similar results are also found in viremic nonprogressors, who have less HIV-1 DNA carried CD4+ TSCM cells than ordinary patients. Despite their low frequency, TSCM cells are pivotal to produce the circulating CAR T cell pools substantially, and determine early anti-leukemic responses in patients (97).
Current vaccines are often not efficient enough to induce robust and long-term immunological effect, because they mainly focus on induce CD8+ TEM cells other than TSCM cells (98, 99). In fact, these T cell vaccines are inferior to vaccines that can induce protective antibodies (100, 101). In order to improve efficiency of T cell vaccines, methods should be developed considering induced stem or memory T cell pool at gene, transcription or metabolism levels (102).
Indeed, small number of TSCM cells can be induced after natural infections or administration of cancer vaccine, despite that effector cells are initially predominant cell subsets (103). On the other hand, TEM and tissue-resident memory cells should be reserved to guarantee immediate protection against re-infection (104–106). Altogether, ideal vaccines should be designed to reconstitute entire diversity of memory T cell subsets in vivo (107, 108).
In adult immune system, TCM cell pool has been considered as one cell subset with stem cell characteristics (16, 109). Bone marrow is crucial in sustaining the persistence of memory T cells (40, 41). However, it is unclear if the bone marrow plays role to support TSCM cell as their niche.
Memory T cells were previously thought to share some attributes with p HSCs (13). Further, the existence of two distinct BM niches, including quiescence niches and self- renewal niches, was found to be a main reservoir for memory T cells. Together with similar niches in other organs, BM niches maintain the pool of memory T cell (12, 110). Indeed, bone marrow provides pivotal cues, such as IL-7, to support long-lasting pathogenic CD4+ T cells (111). Most dividing T cells reside in BM other than in spleen or LNs, despite that only less than 2% memory CD8+T cells in the BM enter into proliferating cell phase (112, 113). In bone marrow, such dividing is antigen-independent and equality occurs among CD8+T cells that had experimentally primed long before or those not express antigen before (113, 114).
Notably, there are approximately 2% of the cells are cycling, and BM memory CD8+T cell in BM affect state of HSCs (115). Both HSCs memory CD8+T cells are regulated by two kinds of BM niches: quiescence and self- renewal niches. Under physiological conditions, quiescence niches not self- renewal niches are predominant niches for both HSCs and memory CD8+T cells. But in some diseases, like BM T cell cancer, self-renewal niches might grow (116). Memory phenotype cells may include both antigen-reactive T cells and bystander T cells (117). The frequency of these two groups of memory T cells changes according microenvironment. Based on this, someone observed different Ki67 staining results in animals-primates and mice, living in living in normal (118) or SPF environment (41).
In fact, the majority of memory T cells in bone morrow enter into the periapical tissue and are replenished by newly coming memory T cells. This conclusion was reached by many researches through methods including bromodeoxyuridine (BrdU) pulse-tracking (110), in situ labeling (119), and parabiosis experiment (120).
Taking together, the two niches in the BM not only maintain sustained recirculating memory T cells for re-circulation without antigen stimulation, but also quickly regulate division and migration of these cells when encounter disturbing (121). Upon perturbation, the number of BM niches grow to produce more relevant factors, which not only support T cell survival and growth but also attract more memory T cells into the self-renewal niches. Of note, the BM CD8+T cell proliferate in response to stimulators that boost innate immune effect. Someone found that polyI:C alone could evoke proliferation of memory CD8+T cell in vivo, particularly in the BM (122).
Connolly et al. found that T cells in tumor-draining lymph nodes (TDLNs) may be precursors of TCF1+ T cells and responsible for persistence of T cell responses in lung cancer. Therefore, TDLNs serve as reservoirs for TCF1+ tumor-specific CD8+ T cells throughout development (31). Similarly in triple negative breast cancer, Researchers demonstrated tumor-draining LNs (TDLN) worked as niches for CD8+T lymphocytes against lymph-draining antigen (48).
TSCM cell homeostasis may depend on cues from FRC-based lymphatic niches (44) (Figures 3A–C). Lymph nodes (LNs) depend on a number of stromal cells, such as fibroblastic reticular cells (FRCs), to build lymphatic macroniches and guide cell-cell interactions (141). These cells provide integrin, chemokine, and cytokine cues to keep multipotency of lymphocytes, while also give rise to differentiated cells upon pathogenic challenge. Therefore, lymphatic niches must maintain homeostasis so that to avoid inappropriate immune responses and autoimmunity.
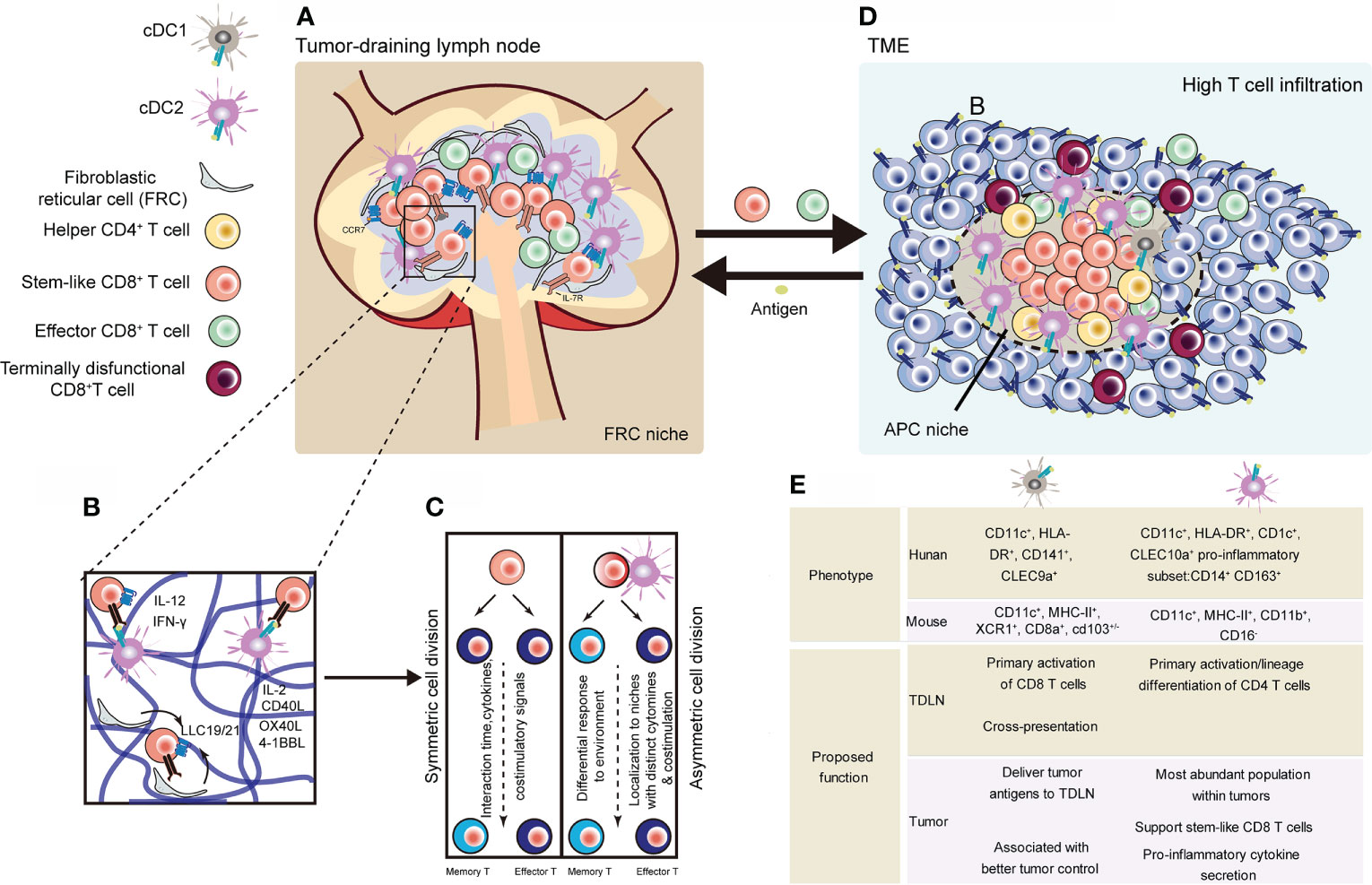
Figure 3 Representative niches supporting the maintenance of stem-like CD8+ T cells. (A) Stem-like CD8+ T cells in lymphoid niches differentiate into cytotoxic effector T cells, regulating tumor regression (44). (B) CCR7+ naïve CD8+ T cells localize and in the inner cortex (blue) and depend on homeostatic factors from niche supported by fibroblastic reticular cell. Activation process of CD8+ T cells can be divided into three phases, during which regulation signals are unleashed from surrounding, especially DCs (123–125). (C) After activation signals, T cells undergo symmetric or asymmetric cell division. Daughter cells transform into TEFF or memory T cells depending on their distance to DCs (32, 126). (D) Tumors with high dense of TILs show APC-niches, containing cDC1, cDC2 and helper CD4 T cells and stem-like CD8 T cells (50, 127–130). (E) Comparisons of human and mouse cDCs found in TDLNs and tumors (43, 49, 50, 127, 131–134). cDC, conventinal dendritic cell; TDLN, tumor-draining lymph node (129, 130, 135–140).
T cells mainly locate in cortex (including outer cortex and inner cortex) of LNs (141). The outer cortex is rich in CD4+ follicular T helper cells, while the inner cortex, also known as the T cell zone, is rich in both CD4+ and CD8+ T cells. Other important cells in T cell zone include dendritic cells (DCs), and fibroblastic reticular cells (FRCs) (142).
Medulla is innermost region, containing plasma cells and macrophages (143). Specific receptors and ligands in niches are important for niche development and maintenance. Once these receptors and ligands were knockout, it may change lymph node structure and alter cell localization and responses (144–146). Numerous key studies describe cell-cell interaction and their behavior in lymph nodes upon immune stimulation (147–150). In the paracortical macroniche, CCL21/19 secreted by FRC regulate the movement of DCs and T cells in FRC network, and FRC also produces survival and proliferation factors such as IL-7 and IL-15 (151–153). The process of T cell activation can be divided into three distinct phases according to T cells migration patterns along the FRC network (154).
The fate of CD8+ T cell may be conferred by T-APC interactions during early T cell activation (Figure 3B). APCs can generate inflammatory factors like IL-12, IFN-γ, and IFNαα, which control function and activities of T cells (155). In the third phase, CD8+ T cells transiently contact with APCs and received additional signals such as IL-2, CD40, CD27, 4-1BB, OX40, and TNFR2 (123–125). After activation signals, T cells undergo symmetric or asymmetric cell division (126) (Figure 3C). Moreover, the fate of T cells is tuned by CXCR3-mediated signals. Based on CXCR3-CXCL9 axis, T cells move out of center of lymph node and lose some stem-like memory features (131).
Intratumoral tertiary lymphoid structures (TLSs) have been found in breast, colorectal, lung, hepatocellular and pancreatic cancers. TLSs might form through IL-22-, IL-23-, CXCL13-, CXCL19-, CXCL21- and LTα1β2-mediated signals (132). TLSs can be considered small ectopic lymphoid structures found within the tumor stroma. They also contain both B cell zone and T cell zone. TLSs may promote local antitumor immune responses, because TLSs can be linked to higher CD8+ T cell infiltration. Recently, several studies identified positive correlation of B cells found in TLSs with good outcomes of ICB treatment in patients (43, 133).
Contrary to tumors in absence of TLSs, metastatic melanoma tumors that own abundant TLS have larger number of less differentiated TCF1+ T cells (49). Dense APC niches were found in patients with kidney cancer. These niches keep phenotype of TCF-1 positive T cells in tumor (123). Jansen et al. also discovered dense antigen-presenting-cell niches within tumor to keep stem-like T cells, which support extensively infiltrating of T cells (50).
Although further researches are needed to uncover the role of these structures for effect on immunotherapeutic outcomes and tumor-specific CD8+ T cell function, it can be inferred that TLSs and APC niches are predictive markers of immune checkpoint blockade (ICB). Additionally, it will be important to develop immune-monitoring platforms to visualize the immune response organization inside the tumor before or during ICB therapy (134).
Both CD4+ T cells and DCs play important role in intratumoral niches to facilitate the response of CD8 T cells (50, 127–130) (Figure 3D). These intratumoral niches are immune cell rich structures, where stem-like CD8 T cells locate). DCs are mainly composed of Conventional dendritic (cDC) 1 cells (cDC1s) and cDC2s are two main types of DCs. cDC1s have been widely investigated in many kinds of cancer based on the fact that they can crossly present exogenous antigens and are prodominately APCs to prime CD8+ T cells (135, 136). Their function has been studied in many tumor models, as they are able to improve anti-tumor effect of CD8+T cells (129, 130). It has also been demonstrated that CCR7 promotes migration of cDC1s into TDLNs, where they present tumor antigen to CD8+ T cells (137). Resonating these results, other studies in human also observed that increased cDC1 associates with better overall survival in patients with cancers (129). However, the proportion of cDC1s is much lower than that of cDC2s within tumors (136, 138). And cDC2s preferentially perform as CD4+ T cells initial activator relying on MHC class II (MHC-II)-antigen complex (138). A recent study revealed that DC2s are a heterogenous population and can gain different properties (139). But their role in the tumor response remains unclear. A recent study focused on the interaction of cDC2s and CD8+ T cells within breast cancer. They found the presence of CD14-expressing cDC2 in tumors correlates with infiltration by tissue-resident CD8+ cells (140). Thus, cDC1s may differ from cDC2s in their roles for activating and differentiating antigen-specific CD8+ T cells in tumor. cDC1 population may mainly play its major role in TDLN, whereas the cDC2 population may maintain intratumoral CD8+ T cell responses. The different types of cDCs and their potential roles in the antitumor response are summed up in Figure 3E (129, 130, 135–140). Together, the presence of APC-rich niches and other intratumoral lymphoid structures (e.g. TLSs) may be an explanation of long-lasting T cell immune response in chronic antigen stimulation.
Asymmetric T lymphocyte division (ACD) ACD is speculated to be a mechanism for CD8+ memory T cell development (126). Stem-like memory cells are derived from one subset of daughter cells arising from ACD. Besides, TCM cells arising from ACD have ability to replenish the CD8+ effector T cells population in response to antigen exposure (16, 109). Driven by IL-2Rα and T‐BET, TCM cells differentiated into effector T cells (156, 157). T cells experience an effector fate resulting from the asymmetric segregation. Similar to other stem cells, TSCM cells rely on activity of telomerase to keep telomere length and replicative ability (158, 159). Thus, ACD can be considered as a conserved mechanism giving rise to a subset of stem-cell-like, less-differentiated memory precursor cells during antigen stimulation (32).
Upregulation of TCF-1 in follicular helper T (TFH) cell may induce expression of CXCR5 and PD-1. Meanwhile, TCF-1 enhances expression of BCL6, and further promotes early differentiation (160, 161). TCF-1 further represses expression of Blimp1 and IL2Ra, restricting differentiation toward TH1 pool (162, 163). Moreover, TCF-1 increases IL-6 responsiveness by its enrichment at the IL-6 receptor gene locus. LEF-1 is also a key regulator of T cells stemness, Both TCF-1 and LEF-1 is essential to control early development of TFH cells (160, 164). Additionally, TCF-1 keeps the expression of Achaete-scute homologue-2 (ASCL2), which downregulates expression of CCR7, leading T cell migration to the border of T and B cell zone (165). Besides, TCF-1 also regulates TFH cell commitment and growth by targeting costimulatory marker inducible T cell co-stimulator (ICOS) (160). Among the TH cells subsets, TFH cells are the main component to from memory pool to maintain stem-like properties against stress in chronic infection (166, 167). These cells help B cell helper re-initiate function (168). And TFH stemness was kept in the presence of TCF-1 (169), which drives development of CD8+ central memory precursors by increasing expression of Eomes.
TCF-1 plays similar role and relies on similar mechanism in CD8+ TSCM and TFH cell differentiation to regulate cell stemness and memory (170). For example, TCF-1 represses Tbx21 and Prdm1 but induces Bcl6 expression in memory precursor CD8+ T cells. Further, TCF-1 is also a key player in regulating sustaining immune memory and preserving T cell stemness against chronic or acute infections (27, 75, 171). Loss of TCF-1 hinders the generation of stem-like progenitor cells pool in CD8+ T cells, resulting in rapid decrease of CD8+ T cells and viral control impairment (53, 75). Together, TCF-1 plays a shared role between TFH and central memory TSCM cells for suppressing exhaustion and immunologic recall response.
A representative mechanism is shown in Figure 4, describing how T cells devote to self-renewal character and keep stemness on epigenetic level. In details, T cells also rely on WNT-β-catenin signaling to obtain stemness (Figure 4). Once WNT ligating to the Frizzled receptor and low-density lipoprotein receptor related proteins (LRP), Dishevelled protein (DVL) is activated to inhibit the formation of destruction complex (containing glycogen synthase kinase 3β (GSK3β) (172), which mediates degradation of β-catenin by proteasome. Then, β-catenin accumulates in cell nucleus, where it interacts with different kind of DNA-binding partners, such as members of the TCF and LEF family, leading chromatin remodeling and transcription modulating. TCF7 and LEF1, the WNT signaling transducers, which are found highly expressing in naïve T cells and CD8+ TSCM cells and lost in more differentiated T cells, may maintain the stemness of T cells (16, 109, 173, 174). WNT3A or GSK3β inhibitors have been shown to promote the generation of self-renewing TSCM and TCM cells, and inhibit the progressive differentiation of naïve T cells (175, 176). In line in with these findings, stabilizing expression of β-catenin also inhibited the acquisition of effector T cell functions (177). In several studies involved in infection, memory T cells were increased by overexpression of TCF1 and β-catenin stabilization (178). Contrarily, it could be found that TCM cells were depleted when TCF-1 was knocked out, which increased expressing levels of granzyme B and KLRG1 in T cells, but prevented the establishment of long-term T cell memory (80, 179). And further study revealed that TCF-1 regulate stemness depending on its binding capacity to β-catenin (80).
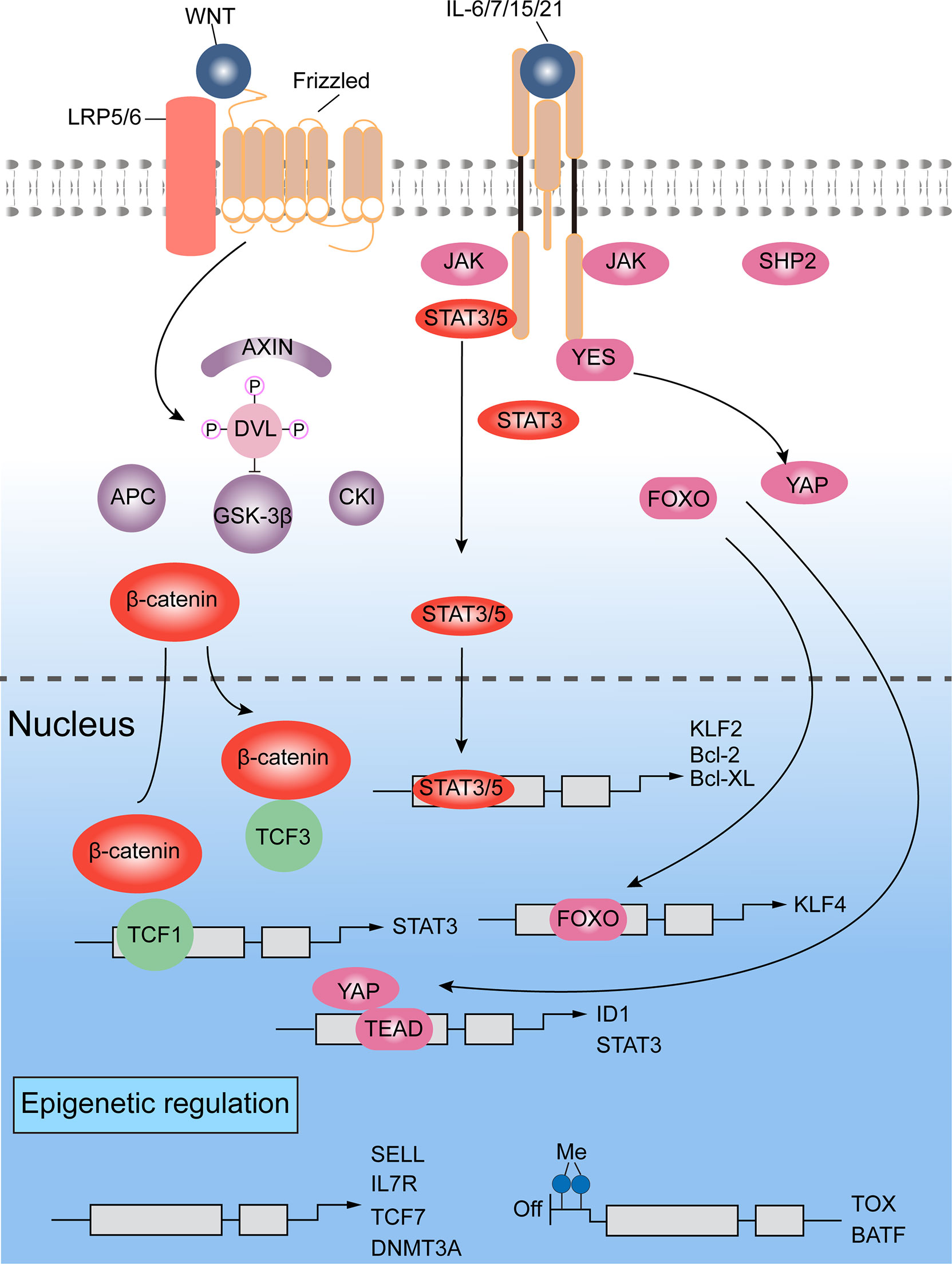
Figure 4 Signaling pathways and epigenetics regulating self-renewal of T lymphocytes (16, 80, 109, 172–181). WNT-β-catenin and transducer and activator of transcription 3 (STAT3) signals are mainly involved for the self-renewal regulation. Additionally, a crucial role is played by chromatin modifiers, which can add repressive histone marks to genes responsible for maintaining stemness, or catalyze DNA methylation on genes regulating differentiation.
Interestingly, the center role of β-catenin in Wnt/β-catenin pathway for memory regulation of CD8+ T cell was challenged by some researches. Someone observed arrest of effector differentiation by GSK3β inhibitors even without β-catenin (175). In a mouse model, T cell memory and cell function were not found to be impaired by T cell-specific deletion (182). However, WNT reporter activity was not hindered by β-catenin deleting, which suggested that there is compensation mechanism for lack of β-catenin in WNT signals (183). Of note, a 52 kDa fragment of β‐catenin, which mediates interaction with TCF protein, retains some functionality (184). In addition, γ-catenin, which is also regulated by the destruction complex, could substitute β-catenin to promote transcriptional activity of TCF and LEF in cells showing β-catenin deficiency (185).
Other signaling involved in self-renewal of T cells include STAT3, SMAD and Yes-associated protein (YAP). KLF4 and KLF5 are the downstream genes of LIF-STAT3 signaling (186). On the other hand, direct activating complex of LIF receptor and GP130 recruit SHP2 to exciting MAPK signals. But this pathway is mainly for transmitting pro-differentiation cues rather than self-renewal signals (187, 188). Indeed, mature T lymphocytes can achieve long-term memory by triggering STAT3 activity based on environmental signals, which are triggered by IL-6, IL-7, IL-15 and IL-21. For instance, IL-21 suppresses forming of CD8+ TEFF cells, maintaining TSCM pool and supporting long-term T cell survival (189). STAT3 might also limit cell differentiation by activating KLF, which keep quiescence state of cells and induce lymphoid-homing molecules expressing (190, 191).
YAP has been found to enhance expression of ID protein based on BMP-SMAD pathway, and induce binding of LIF to the transcription factor TEA domain, resulting in expressing of pluripotent genes. This process could promote ESC self-renewal (192–194). As AKT/Hippo signals regulate YAP negatively, activation of Hippo pathways by cytokine IL-2 resulted in YAP degrading and differentiation-associated molecules expressing, further preventing CD8+ T cells senescence in response to viral infection (195). In ESCs, YAP may also enhance stemness associated transcriptional regulators, including STAT3 and members of ID family, but further studies are needed to confirm this mechanism in T cells.
Once activated, the effector related genes of TN CD8+ cells are epigenetically regulated before they gain memory and effector features. As such, CD8+ TSCM cells show some effector features, while retain naive-associated transcriptional programs fundamental for self-renewal and for homing to lymphoid tissues (180). Therefore, epigenetic mechanisms are essential to regulate the differentiation of self-reactive CD8+ T cells. Epigenetic modifications not only regulate genes for silencing stemness, for example TCF7, SELL and CCR7, but also genes for memory and effector-associated factors (e.g. EOMES and TBX21) (180) (Figure 4). Furthermore, methylated modification happens on chromatin (like H3K9me3 and H3K27me3), resulting in suppression effect on gene expression (181).
By profiling genome-wide DNA methylation in normal versus autoimmune responses (196), Abdelsamed et al. built a whole epigenetic profile was made for entire human CD8+T cell population differentiation including TN, TSCM, TCM, TEM and TEFF cells. Based on this profile, the CD8+ T cell differentiation was further studied in type 1 diabetes exposed to chronic antigen stimulation. They discovered There were 3,000 most variable CpG loci found to distinguish different T cell subsets. In this way, TSCM cells are found to be equally distant from both TN and the TEFF cells.
In fact, autoreactive CD8+ T cells and TSCM cells show same methylated features on differentiation-associated genes like BATF, DNMT3A and TOX. TOX and BATF are responsible for CD8+ T cell exhaustion and effector function specification seperately (197, 198). These two factors are repressed by methylation in both TSCM and beta cell specific CD8+T cells, while they are free from methylation marks in more differentiated CD8+ T cells. On the other hand, DNA methyltransferase DNMT3A, which involves in self-renewal regulation of stem cells, exhibits opposite methylation patterns. Of note, researchers found a self-reactive CD8+ T cell subset, which is clearly different from TN and TEFF cells, showing a mixed epigenetic pattern. Indeed, both stemness-associated-genes (such as LEF1, TCF7 and DNMT3A), and differentiation-involved-genes (such as TBX21, INFG, PRF1 and GZMK) opened, leading mixture of stem-like effector features in the same cells. Above studies suggested that progressive differentiation of CD8+ T cell is accompanied by great changes in epigenetic modification, degree of gene loci openness, and stem- and effector-like programming.
Multiple signal pathways, deriving from factors like TCRs, cytokines, co-stimulatory receptors and growth factor receptors, regulate the fate of effector and memory T cells (199–201). These signal pathways can be regulated by many small molecular drugs-mTOR inhibitor rapamycin (201), AMPK agonist metformin (202), GSK3β inhibitor (16, 109) and AKT inhibitor (203), can be quickly applied in new clinical trials. Success clinical practice have been released in solid organ and HSC transplantation, type 2 diabetes treatment, and neurodegenerative diseases. Similar results can be achieve by using cytokines such as IL-7, IL-15, and IL-21 or by activating proper costimulatory receptors like 4-1BB, OX40 and CD27 (21, 204). Combined these studies, it shows that pharmacological, cytokine and costimulatory signal involved stemness can be used to generate antigen-specific stem-like T cells (Figure 5A).
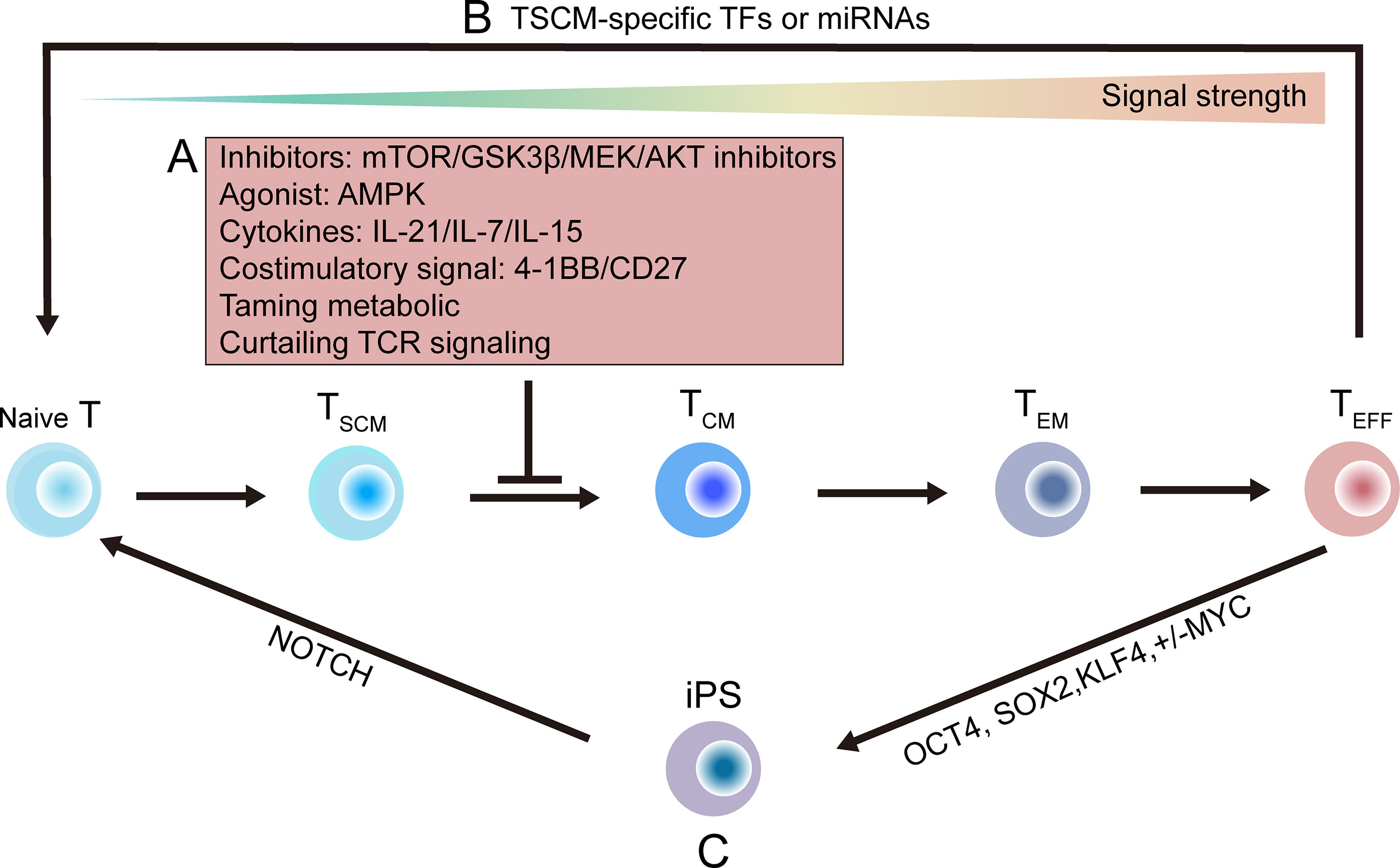
Figure 5 Strategies that might be utilized to generate stem-like T cells. (A) Progress of T cells differentiation toward effector T (TEFF) cell pool depends on the strength of stimulatory signals. Differentiating progress of primed TN cells can be delayed or suppressed by targeted inhibitors (such as mTOR, GSK3β, MEK, AKT inhibitor); or by using cytokines, such as interleukin‐21 (IL-21), interleukin-7 (IL-7), interleukin-15 (IL-15), or by using proper costimulatory signal; or by taming metabolic modulators; or by curtailing TCR signaling (16, 21, 109, 201–204). (B) Direct reprogramming methods using naïve- or stem-associated factors or miRNAs (205, 206). (C) A two-step induction method is shown. By enforcing expression of OCT4, SOX2, KLF4 and MYC, terminal TEFF cells can be programmed into iPS cells, which are then re-differentiated into TN cells by inducing NOTCH signals (207–214). GSK3β, glycogen synthase 3β; TN, naïve T; TEFF, TCM, effector T; iPS, induced pluripotent stem; OCT4, octamer-binding transcription factor 4; SOX2, sex determining region Y BOX 2; KLF4, Kruppel-like factor 4.
Metabolic tuning may be another way to confer stemness to T cells, based on the fact that stem-like T cells own distinct energetic and metabolic characteristics. For example, T cells with stemness show a decrease in mitochondrial membrane potential (ΔΨm) (215, 216). Besides, metabolism is closely related to T cell activity and fate (217).
TSCM cells usually show increased fatty acid oxidation, which further increasing mitochondrial biomass and spare respiratory capacity (218), while show decreased aerobic glycolysis, which devoting to end-effector differentiation. Therefore, strategies based on metabolic taming to directly confer these metabolic signatures to T cell would help harness TSCM cells. Besides, continuous TCR engagement should be control to help maintain long-lived memory CD8+ T cells (219). Future study areas of research getting deep insight into the global characterization of the TSCM cell metabolome would lay the better foundation to develop these strategies.
By inducing TSCM-specific transcription factors or microRNAs, tumor-specific TEFF cell can be reprogramming to display stem-like features (205, 206) (Figure 5B). This direct reprogramming method has also been applied to convert specific mature cells to generate other tissues, such as neurons, heart and liver, or stem-like blood progenitors (220–222).
Ideally, T cell can be de-differentiated into induced pluripotent stem (iPS) cells by inducing expression of stem-related transcription factors including OCT4, SOX2, KLF4 and MYC, then iPS cells can be induced into TN cells by NOTCH activation (207–210) (Figure 5C). Therefore, new group of less-differentiated T cells can be re-differentiated from ESC, HSC and iPS cells (211–214). However, the two-step method is now impractical, because its efficiency is limited by low success rate and long duration of reprogramming. Further studies are needed to solve these problems.
TSCM cells have no unique marker. And they shared given markers with TN and memory T cells. This indicates a limitation to isolate pure TSCM cells with high yield for following studies. To address this issue, Lugli et al. developed soring panels containing CD95, CCR7, CD45RA, CD27, CD62L, CD127, CD28 and CD11 (223).
Given the potential of harnessing TSCM cells, there is much work remained to be done about how cell-cell contact affect the formation of these cells.
Recently, many new technologies have been developed to reveal the relationship between transcriptional factors involved in cellular contact and T cell fate. The most powerful of these contains advances in imaging methods combine with new transcription analyze. One approach to aiming to harness interactions between T cells and DCs is achieved by targeting specific dendritic cell populations (224). This strategies target antigen to restrict presentation to a specific DCs type. The most promising strategy for this purpose is targeting DC-expressed molecules, such as CLEC-9A (expressed on cDC1s) and DEC-205 (expressed on DCs and langerin cells), by antigen to the Fc portion of an antibody heavy chain (224).
By targeting the two molecules with adjuvants, both CD4 positive and CD8 positive T cells show potent cellular responses with strong humoral responses. While without adjuvants, the responses are weak and regulatory T cells are activated (225). Surprisingly, CLEC-9A without adjuvants also show CD4+ T responses and humoral responses, which are stronger than DEC-205 targeting without adjuvants (226, 227). The mechanism is unclear about how these targeting strategies induce long-term memory. It is seems that different selection of adjuvants may show different results, which indicates that these strategies are flexible.
NICHE-seq is the most suitable technology to determine cell interactions (228). After dissociation of tissues, cells are separated to identify components of niche, and then investigated by single cell RNA-seq (scRNA-seq). Combined with two-photon laser scanning technology, this method has been used to CD4+T cell priming niche in response to viral infection. Further combined with a ligand-receptor bioinformatics analysis, this method widely revealed the cellular interaction within a specific zone (229). This method identifies conjugates of interacting cells before scRNA-seq analysis. Following conjugate RNA-seq, the interacting partners are bioinformatically separated. Applying these approaches to a specific lymphoid niche uncovers what the effects of cell interaction and inflammatory factors to T cell differentiation. Labeling immune cell partnerships by a proximity-dependent labeling system SorTagging intercellular contacts (LIPSTIC), which crosses interfaces of cells, has been developed to investigate CD40-CD40L interactions (230).
In order to study the interaction of ligand and receptors, Staphylococcus aureus transpeptidase sortase A (SrtA) or tag residue are usually used to modified them. When the interact, Based on substrate transfer onto tagged receptor catalyzed by Srt A, the interaction ligand and receptors can be tracked and detected by flow cytometry. Based on this method, someone developed a new method called FucolD, which relying on enzymatic fucosyl-biotinylation, offers alternative choice to label T cells in terms of cell interaction (231). Unlike LIPSTIC, this method is a proximity-based labeling system, allowing detection even without prior known receptor.
Despite that TSCM cells possess superior antitumor response and immune reconstitution capabilities, their rareness and lack of unique markers poses a major hurdle to their clinical application.
A panel was made to include optimal set of markers for human TSCM cells identification (223). Thereby, pure TSCM cells can be identified and separated with high efficiency and yield. It is possible to apply this direct isolation method in clinical application if this process can be completed under GMP grade A condition. However, it requires GMP-grade equipment that integrating with flow sorting function.
It is easier to generate TSCM cells from naive precursors. After selected by magnetic beads, naïve T cells can be induced with IL-15, IL-21 and IL-7, or in combination with small molecular drug (e.g. GS3K GSK-3β inhibitor). These conditions enabled the generation of large number of TSCM cells, which meet clinical needs. A representative scheme that illustrate how to produce and program clinical-grade TSCM cells based on present technologies, is shown in Figure 6 (26, 75, 223).
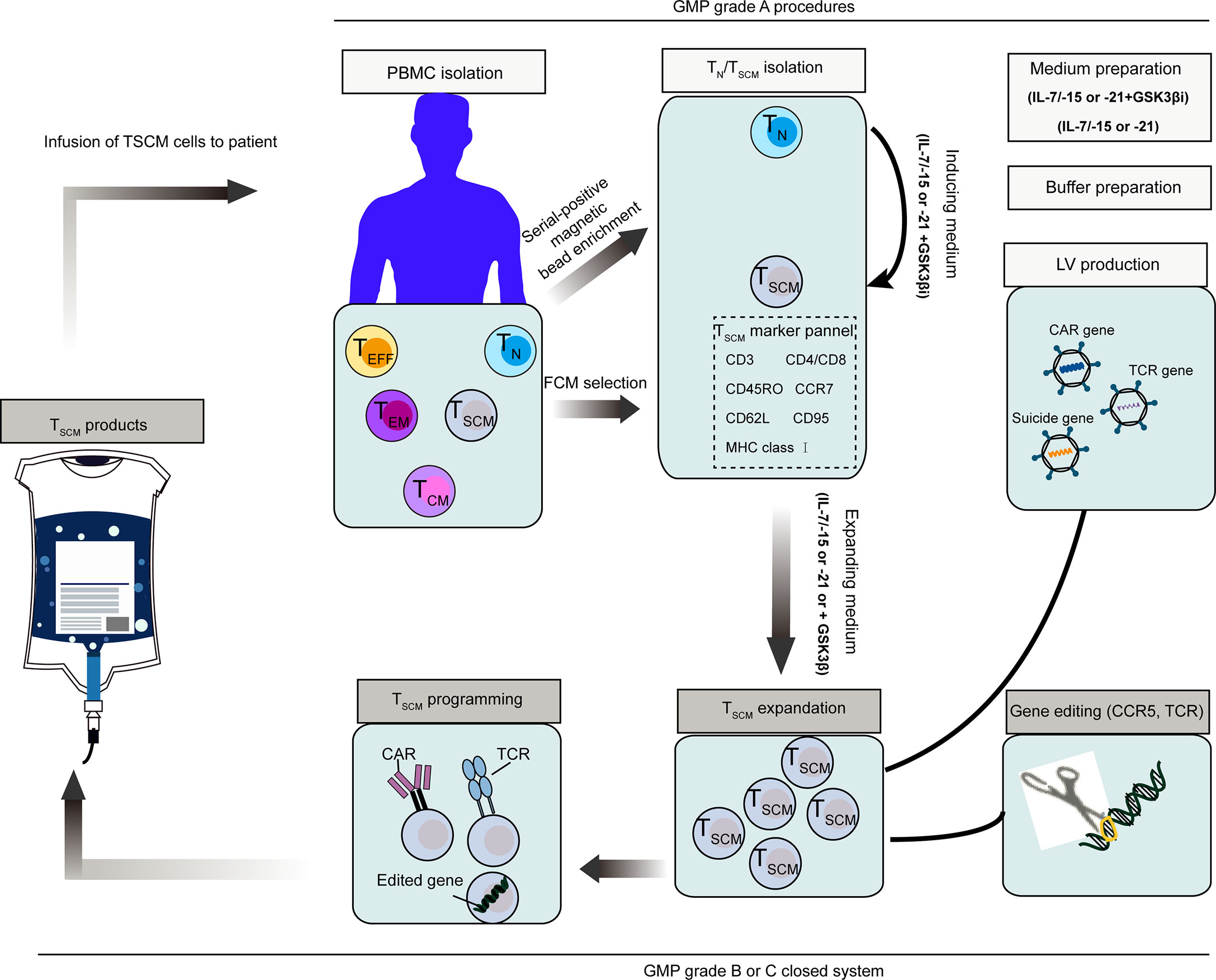
Figure 6 Schematic representation of clinical stem cell memory T (TSCM) cells product and programming process (26, 75, 243). Naïve T (TN) cells were then stimulated to transform to TSCM cells with IL-15, IL-21 and IL-7. To maximize the induction of TSCM, GSK-3β inhibitor can be used in addition to the above cytokines. TSCM cells can be also isolated directly from PBMCs by a set of markers. Representative programming methods include introducing exogenous genes like specific CAR, TCR and suicide genes, or directly editing endogenous genes. Requirements of GMP grade for main procedures are indicated in the figure. PBMC, peripheral blood mononuclear cells, CAR, chimeric antigen receptor, TCR, T cell receptor; GMP, good manufacturing practice.
CD4+ TSCM cells might be a new target in patients infected with HIV-1 and HTLV-1. In the context of HIV-1 infection, HIV-1-carried CD4+ TSCM cells serve as the source of the virus in HIV-1-infected patients. Although patients received antiretroviral therapy, these CD4+ TSCM cells continuously provide progenies infected with HIV-1. There are some essential factors determining self-renewing and expanding abilities of TSCM cells. Therefore, these factors might be new targets to reduce continuous existence of virus in CD4+ TSCM cells. For example, targeting WNT-β-catenin pathway, which regulates the homeostasis of TSCM cells (16), might reduce frequency of long-lasting HIV-1-loaed TSCM cells (75) (Table 2).
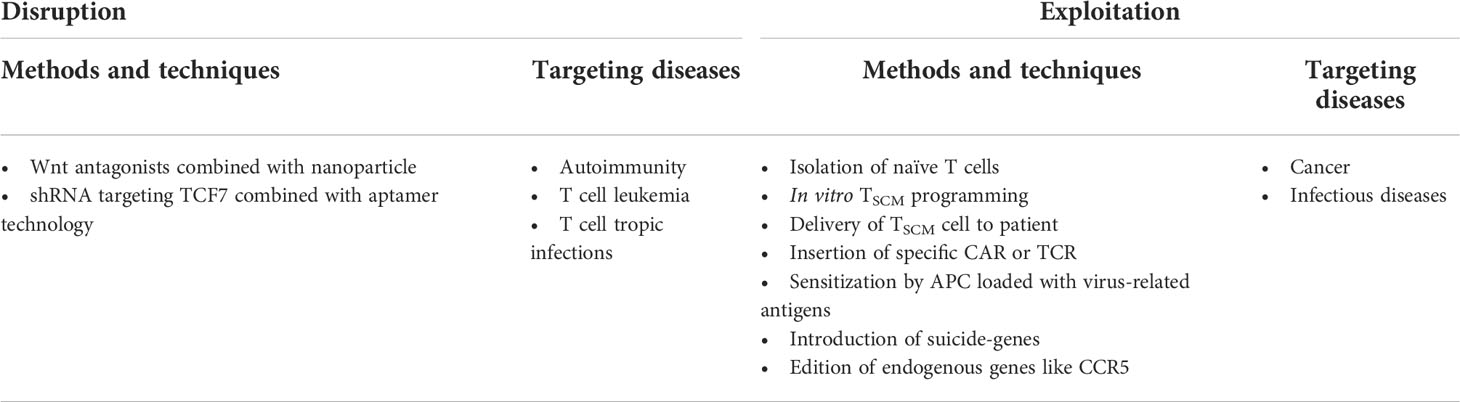
Table 2 Overview of researches relating to TSCM-cell-based applications for human diseases (21, 21, 26, 75, 232–237).
Current pharmacological inhibitors for Wnt-β-catenin targeting cancer stem cells can be also applied for eliminate HIV-1 infected CD4+ TSCM cells (238). By nanoparticles or aptamer-based targeting systems, it can be more precise to deliver Wnt inhibitors or shRNAs to viral host cells (232, 233) (Table 2). Based on similar strategies, TSCM cells can be disturbed in HTLV-1infection followed by T cell leukemia, or in autoimmune diseases.
In addition, ex vivo genetically modifying TSCM cells to make them more resistant to pathogen factors should be another way for the treatment purpose. For instance, knock-out of CCR5, which determines the ability of the virus to enter into host cell (239), therefore imitating the CCR5Δ32 mutation that make hosts resist to HIV-1 (234) (Table 2). Once these long-lasting CD4+ T cells become intrinsically HIV-1-resistant, they could be utilized to build long-term HIV-resistant immune system. Such population of cells might be helpful to remit HIV-1 infection in absence of drug treatment. Representative clinical trails relating to the adverse effects of TSCM cells are listed in Supplementary Table 1.
TSCM cell type is a promising target to promote immunotherapeutic strategies, because it shows several advantages: the robust proliferative potential, the extreme longevity, and the potential to produce various types of the T cells. Adoptive immunotherapy has really become a practical option for cancer treatment (240, 241). Despite some success in patients with advanced cancer, adoptive T cells are not robust enough to induce adequate response, which underscores the need for further improvements (240). Notably, experiments in mice have demonstrated the role of CD8+CD45RA+CCR7+ TSCM cells for supporting long-lasting proliferation of CD19-specific CAR T cells and T cells expressed suicide genes. Similarly, transferring naïve-like CD62L positive T cell population leading to increased number of long-lasting activated T cells, which lead to robust and continuous tumor suppression (16, 109, 242, 243).
TCM cells induce stronger antitumor immunity than highly differentiated TEM cells, and TSCM cells are more profound than TCM cells (244). These robust TSCM cells can be generated in vitro and used for clinical exploitation (21, 26) (Table 2). Although it is not easy to isolate naive T cells, this step is important to determine the therapeutic effect. Because the differentiation progress of naive T cell would be promoted by co-existed T cells at more differentiated status (245). Relying on the developments in clinical cell-sorting technologies, it is a real strategy to enrich specific cell subsets with high yield under GMP condition (246, 247). The combination strategy of IL-7 and IL-15 is ideal to produce TSCM cells without the need to redirect their specificity. Based on this strategy, naive cell precursors can be programed into tumor-specific, TCR-gene-edited, suicide-gene-modified or virus-specific TSCM cells (21, 235, 248) (Table 2).
Additionally, IL-21 also has been used to restrain T cell differentiation and keep their stemness (189), which is realized by activating STAT3 signaling and sustaining the expression of TCF7 and LEF1. A study reported that they induced CAR-modified TSCM cells by use of IL-21 together with inhibitor TWS119, which keep stability of β-catenin resulting in enhanced expression of TCF7 and LEF1 (26). These induced CAR-T cells perform metabolic features of TSCM cells (e.g. low glycolysis and high spare respiratory capacity) (249). CAR-modified TSCM cells can also be attractive for effective treatment of in the setting of chronic virus infection like HIV-1 infection (236, 237) (Table 2). Collective studies provide practical approaches for the use of TSCM cells in clinical practice of immunotherapy (250). Based on these approaches, it would be better to confer tumor reactivity to circulating T cells with less-differentiated features, other than directly select specific TILs at exhausted status (251, 252). Overall, the approach that relying on cytokines (such as IL-7, IL-15 and IL-21) is applicable to produce clinical-grade adoptive TSCM cells. And antigen-specific TSCM cells exhibit better clinical outcomes in immunotherapy (Supplementary Table 1).
Methods for sorting of specific stem-like T cells also ensure the yield and reproducibility of defined T cell products. Indeed, Unselected PBMC population from individuals with different age, pathogen and therapeutic treatment may show different outcomes owing to varied amplification rate (253–255). Moreover, the benefits of removing more differentiated T cells were offset by simultaneous depletion of TN and TSCM cells (256).
Several studies had successfully converted by infusing TSCM cells derived from circulating CD8+ T cells, and it showed robust cellular immune responses in the treatment of different cancers such as non-small cell lung cancer, acute myeloid leukemia and renal cell carcinoma (257–259). TSCM CAR-T cells, which were less likely to become exhausted, exhibited stronger antitumor response in mice model of Raji-ffluc lymphoma (260). Similar observation was achieved in yellow fever-vaccinated patients, when a subset of antigen-specific CD8+ T cells was found to display naïve-like phenotypes and play protection effect over 25 years (261). Since TSCM cells are responsible for long-lasting memory of immune effect, TSCM may be an ideal target for prediction and improvement of vaccine effectiveness. Combination use of HPV vaccine with CD40 and TLR3 agonist produces increased number of CD8+ TSCM cells, mediating better therapeutic and preventive effects (262).
Despite the potential of ICB to induce long-term remission, most patients fail to achieve durable clinical responses, even in patients with metastatic solid tumors (263–265). Initially, the role of ICBs was thought to rejuvenate exhausted or dysfunctional tumor-infiltrating CD8+ T cells. However, to date it is unclear whether dysfunctional tumor-specific TILs can be reversed to activated state by ICB.
Conversely, it has been shown that exhausted CD8+ T cells (TEX) acquired a steady epigenetic feature different from that of TEFF cells and memory T cells that were minimally remodeled after PD-L1 blockade (266). Preliminary studies have revealed that increased expansion of lymphocytes in blood predicts more potent ICB responses (267), and T cell responses to ICB are derived from newly entered T cells from outside of tumor (268).
In addition, amplification of T cells in blood of patients with cancer can serve as a prediction marker for clinical response against PD-L1 antibody (269). Other studies have shown that tumor-reactive TILs are largely ineffective for ICB-mediated resuscitation prior to ICB. Whether there is a specific subpopulation of T cells determining the ICB responses is still unknown. Other studies identified a subset of TCF1+ CD8+TILs as target T cell group for ICB or vaccination treatment (77, 270). Sade Feldman et al. proved the correlation between TCF1+ TILs and enhanced response to ICB (271). But there is no evidence that whether these cells display stable stem-like attributes or not (272).
By scRNA and TCR sequencing, Li’s team studied tumor-reactive TILs in melanoma patients (273). They found that the intratumoral TCF1+ T cells include bystanders without tumor reactivity. Several studies have identified stem-like TCF1+ TILs in human tumors (28, 55, 77, 274). PDL1 blockade could induce CD8+ T cells antitumor effect mediated by TDLNs-derived stem-like PD1+ T cells, which interact with PDL1+ DCs. The existence of PD1-PDL1 interaction in TDLNs other than with tumors predict better outcomes in patients with melanoma (275). However, there may be some tumor-specific TILs that are capable to respond to ICB: those recently entered less-exhausted TCF1+T cells, or T cells that reside in intratumoral niches (50), tertiary lymphoid structures (TLSs) (132, 133), regions of antigen loss or specific metabolic niches (276). Tumeh et al. found that more PD1+CD8+ TILs localized at the margins of invasive tumors, where they interact with PD-L+ cells, are positively correlated with better ICB responses (277). The relationship between the existence of TCF1+ TSL cells in tumors and good prognosis may reflect the properties of these tumors-they have hot TME to promote infiltration of T cells without limitation of tumor antigen specificity. Such tumors were more likely to promote tumor-reactive T cell infiltration from the peripheral tissues, when treated with ICB. Collectively, these studies suggest that the stem-like TCF1+PD1+ T cells in tumor and peripheral compartment may determine ICB responses. They are the source of differentiated T cells in tumor (278). This enlightened researchers to find ways to active and expand stem-like/progenitor exhausted CD8+T cells in tumor in order to improve ICB efficacy. Some recent studies have proven to improve anti-PD-1 efficacy by combining with an alarmin HMGN1 peptide or anti-PD-L1 efficacy by combining with cyclophosphamide and vinorelbine (279, 280). These observations above solved an important question about the source of intratumoral stem-like T cellsm that is, they were mainly arised in LNs. Another import question is how they enter the tumor. A recent study pointed out that the existence of tumor-associated endothelial cells (TA-HECs) is essential to recruit stem-like T cells into tumor. And ICB would increase recruitement of stem-like T cells by increasing the maturation of TA-HECs (281). But further studies are still needed to illustrate this question.
On the other hand, ICB still have many shortcomings. Many clinical trials have found that ICB can lead to systemic immune disorders, and long-term treatment can also induce the occurrence of autoimmune diseases (282). In addition, the cessation of PD-1 inhibition may also lead to the enhancement of pathogenic immune responses, which is likely correlated with the loss of immune memory of CD8+ T cells (283). Memory CD8+ T cells persisting in the body for decades, are antigen-independent, pluripotent, and respond rapidly to secondary aggression. In some chronic viral infections and tumors, effector CD8 T cells alone are not sufficient to induce tumor elimination, and long-lived memory CD8 T cells are required to maintain sustained antitumor immunity. A few studies have found that PD-1 inhibitors reduce memory T cells (284). Therefore, how PD-1 affects memory CD8+ T cells deserves further study. Surojit Sarkar’s team found that PD-1 signaling can promote the development and homeostasis of long-lasting memory CD8+ T cells by balance metabolic ways relying on glycolysis and fatty acid oxidation. PD-1 deficiency results in memory T cells consumption (285). Although wide type T cells and PD-1 positive T cells had similar numbers of memory precursors during the early activation phase, PD-1-deficient T cells had similar numbers of memory precursors during the contraction and memory maintenance phases of CD8+ T cells. Both CD8+ TEFF cell numbers were markedly reduced, resulting in an approximately 100-fold reduction in final memory cell numbers in both lymphoid and non-lymphoid regions. The researchers re-stimulated these memory cells with antigen to follow longitudinally in vivo secondary responses. After antigen clearance, Loss of PD-1 impairs protection memory of effector T cells and results in the decreased number of antigen-specific cells. At the same time, the researchers also found that PD-1 signaling ensures the normal homeostatic renewal and maintenance of memory CD8 T cells. These results all imply that PD-1 is pivotal in the formation of long-time immune memory of CD8+ T cells.
Current immunotherapeutic approaches are often inefficient to control the progress of tumors, partly due to negative regulatory factors in TILs and tumor microenvironment. The important role of stem-like T cells has been underlined in recent studies in terms of immunotherapeutic effect of cancer. Therefore, it may take advantages of stem-like T cells to improve antitumor effect, based on complete understanding of how these specific T cells form, maintain and functionalize. We summarized our view about sem-like T cells in Box 1.
Additional work highlights the important relationship between several kinds of stem-like T cells and relevant niches, for example, studying interaction between CD8+ T cell and stroma, other immune-associated cells and cues provided by lymphatic niches. However, traditional methodology for studying T cell localization like immunohistochemistry limit further investigation due to the fact that the number of antibodies or fluorophores are limited for each imaging. Besides this destructive method disrupt the structure of tissue, losing spatial information of important epitopes. A new technique called CLARITY has been developed to implement antibody labeling and imaging within intact organ (286, 287). Based on multiple staining and visualizing of intact lymph node, it will be easier to elucidate stem-like T cell localization signals and its surrounding microenvironment.
Given the importance of niches to support stem-like T cells, we summarized current knowledge about several specific niches and their spatial location and environmental cues that maintain stemness and promote differentiation. While the secondary lymphoid niches that imprint TSCM cells fate have been illustrated, there is still much work left for further understanding of intratumoral stem-like T cells and niches. This is important and helpful to harness stem-like T cell for therapeutic interventions and develop methods for stem-like T cell generation; either to strength long-lasting T cell responses against chronic infection or cancer, especially for enhancing ICB treatment, or to amplify humoral responses.
LYi and LY conceived of the topics to include in the review. LYi was mainly responsible to write the review and design the figures. LY provided critical edits to the manuscript. All authors reviewed the manuscript and approved the final version.
Funding support was provided by the National Natural Science Foundation of China (No. 81903148, 82073366).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.907172/full#supplementary-material
1. Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. Cell (2000) 100(1):157–68. doi: 10.1016/S0092-8674(00)81692-X
2. Sun T-T, Green H. Differentiation of the epidermal keratinocyte in cell culture: formation of the cornified envelope. Cell (1976) 9(4):511–21. doi: 10.1016/0092-8674(76)90033-7
3. Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. cell (2003) 114(6):763–76. doi: 10.1016/S0092-8674(03)00687-1
4. Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, et al. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell (2009) 4(6):525–34. doi: 10.1016/j.stem.2009.04.002
5. Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science (1992) 255(5052):1707–10. doi: 10.1126/science.1553558
6. Barker N, Van Es JH, Kuipers J, Kujala P, Van Den Born M, Cozijnsen M, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature (2007) 449(7165):1003–7. doi: 10.1038/nature06196
7. Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science (1988) 241(4861):58–62. doi: 10.1126/science.2898810
8. Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature (2001) 414(6859):105–11. doi: 10.1038/35102167
9. Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: an evolving concept. Nat Rev Cancer (2012) 12(2):133–43. doi: 10.1038/nrc3184
10. Demkowicz WE, Littaua RA, Wang J, Ennis FA. Human cytotoxic T-cell memory: long-lived responses to vaccinia virus. J Virol (1996) 70(4):2627–31. doi: 10.1128/jvi.70.4.2627-2631.1996
11. Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, et al. Duration of antiviral immunity after smallpox vaccination. Nat Med (2003) 9(9):1131–7. doi: 10.1038/nm917
12. Fearon DT, Manders P, Wagner SD. Arrested differentiation, the self-renewing memory lymphocyte, and vaccination. Science (2001) 293(5528):248–50. doi: 10.1126/science.1062589
13. Luckey CJ, Bhattacharya D, Goldrath AW, Weissman IL, Benoist C, Mathis D. Memory T and memory b cells share a transcriptional program of self-renewal with long-term hematopoietic stem cells. Proc Natl Acad Sci U.S.A. (2006) 103(9):3304–9. doi: 10.1073/pnas.0511137103
14. Ciocca ML, Barnett BE, Burkhardt JK, Chang JT, Reiner SL. Cutting edge: Asymmetric memory T cell division in response to rechallenge. J Immunol (2012) 188(9):4145–8. doi: 10.4049/jimmunol.1200176
15. Gattinoni L, Klebanoff CA, Restifo NP. Paths to stemness: building the ultimate antitumour T cell. Nat Rev Cancer (2012) 12(10):671–84. doi: 10.1038/nrc3322
16. Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med (2009) 15(7):808–13. doi: 10.1038/nm.1982
17. Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, et al. A human memory T cell subset with stem cell–like properties. Nat Med (2011) 17(10):1290–7. doi: 10.1038/nm.2446
18. Graef P, Buchholz VR, Stemberger C, Flossdorf M, Henkel L, Schiemann M, et al. Serial transfer of single-cell-derived immunocompetence reveals stemness of CD8+ central memory T cells. Immunity (2014) 41(1):116–26. doi: 10.1016/j.immuni.2014.05.018
19. Fuertes Marraco SA, Soneson C, Cagnon L, Gannon PO, Allard M, Maillard SA, et al. Long-lasting stem cell–like memory CD8+ T cells with a naïve-like profile upon yellow fever vaccination. Sci Trans Med (2015) 7(282):282ra248–282ra248. doi: 10.1126/scitranslmed.aaa3700
20. Biasco L, Scala S, Basso Ricci L, Dionisio F, Baricordi C, Calabria A, et al. In vivo tracking of T cells in humans unveils decade-long survival and activity of genetically modified T memory stem cells. Sci Trans Med (2015) 7(273):273ra213–273ra213. doi: 10.1126/scitranslmed.3010314
21. Cieri N, Camisa B, Cocchiarella F, Forcato M, Oliveira G, Provasi E, et al. IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood (2013) 121(4):573–84. doi: 10.1182/blood-2012-05-431718
22. Buzon MJ, Sun H, Li C, Shaw A, Seiss K, Ouyang Z, et al. HIV-1 persistence in CD4+ T cells with stem cell–like properties. Nat Med (2014) 20(2):139–42. doi: 10.1038/nm.3445
23. Cieri N, Oliveira G, Greco R, Forcato M, Taccioli C, Cianciotti B, et al. Generation of human memory stem T cells after haploidentical T-replete hematopoietic stem cell transplantation. Blood J Am Soc Hematol (2015) 125(18):2865–74. doi: 10.1182/blood-2014-11-608539
24. Roberto A, Castagna L, Zanon V, Bramanti S, Crocchiolo R, McLaren JE, et al. Role of naive-derived T memory stem cells in T-cell reconstitution following allogeneic transplantation. Blood J Am Soc Hematol (2015) 125(18):2855–64. doi: 10.1182/blood-2014-11-608406
25. Nagai Y, Kawahara M, Hishizawa M, Shimazu Y, Sugino N, Fujii S, et al. T Memory stem cells are the hierarchical apex of adult T-cell leukemia. Blood J Am Soc Hematol (2015) 125(23):3527–35. doi: 10.1182/blood-2014-10-607465
26. Sabatino M, Hu J, Sommariva M, Gautam S, Fellowes V, Hocker JD, et al. Generation of clinical-grade CD19-specific CAR-modified CD8+ memory stem cells for the treatment of human b-cell malignancies. Blood (2016) 128(4):519–28. doi: 10.1182/blood-2015-11-683847
27. Utzschneider DT, Charmoy M, Chennupati V, Pousse L, Ferreira DP, Calderon-Copete S, et al. T Cell factor 1-expressing memory-like CD8(+) T cells sustain the immune response to chronic viral infections. Immunity (2016) 45(2):415–27. doi: 10.1016/j.immuni.2016.07.021
28. Brummelman J, Mazza EMC, Alvisi G, Colombo FS, Grilli A, Mikulak J, et al. High-dimensional single cell analysis identifies stem-like cytotoxic CD8(+) T cells infiltrating human tumors. J Exp Med (2018) 215(10):2520–35. doi: 10.1084/jem.20180684
29. Jadhav RR, Im SJ, Hu B, Hashimoto M, Goronzy J. Epigenetic signature of PD-1+ TCF1+ CD8 T cells that act as resource cells during chronic viral infection and respond to PD-1 blockade. Proc Natl Acad Sci (2019) 116(28):201903520. doi: 10.1073/pnas.1903520116
30. Im SJ, Konieczny BT, Hudson WH, Masopust D, Ahmed R. PD-1+ stemlike CD8 T cells are resident in lymphoid tissues during persistent LCMV infection. Proc Natl Acad Sci (2020) 117(8):4292–9. doi: 10.1073/pnas.1917298117
31. Connolly KA, Kuchroo M, Venkat A, Khatun A, Wang J, William I, et al. A reservoir of stem-like CD8(+) T cells in the tumor-draining lymph node preserves the ongoing antitumor immune response. Sci Immunol (2021) 6(64):eabg7836. doi: 10.1126/sciimmunol.abg7836
32. Capece T, Kim M. The role of lymphatic niches in T cell differentiation. Mol Cells (2016) 39(7):515–23. doi: 10.14348/molcells.2016.0089
33. Jones DL, Wagers AJ. No place like home: anatomy and function of the stem cell niche. Nat Rev Mol Cell Biol (2008) 9(1):11–21. doi: 10.1038/nrm2319
34. Goulas S, Conder R, Knoblich JA. The par complex and integrins direct asymmetric cell division in adult intestinal stem cells. Cell Stem Cell (2012) 11(4):529–40. doi: 10.1016/j.stem.2012.06.017
35. Gui L, Homer H. Spindle assembly checkpoint signalling is uncoupled from chromosomal position in mouse oocytes. Development (2012) 139(11):1941–6. doi: 10.1242/dev.078352
36. Petridou NI, Skourides PA. A ligand-independent integrin β1 mechanosensory complex guides spindle orientation. Nat Commun (2016) 7(1):1–15. doi: 10.1038/ncomms10899
37. Yang J, Plikus MV, Komarova NL. The role of symmetric stem cell divisions in tissue homeostasis. PloS Comput Biol (2015) 11(12):e1004629. doi: 10.1371/journal.pcbi.1004629
38. Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell (2004) 116(6):769–78. doi: 10.1016/S0092-8674(04)00255-7
39. Streuli CH. Integrins and cell-fate determination. J Cell Sci (2009) 122(2):171–7. doi: 10.1242/jcs.018945
40. Di Rosa F. Two niches in the bone marrow: A hypothesis on life-long T cell memory. Trends Immunol (2016) 37(8):503–12. doi: 10.1016/j.it.2016.05.004
41. Sercan Alp Ö, Durlanik S, Schulz D, McGrath M, Grün JR, Bardua M, et al. Memory CD8(+) T cells colocalize with IL-7(+) stromal cells in bone marrow and rest in terms of proliferation and transcription. Eur J Immunol (2015) 45(4):975–87. doi: 10.1002/eji.201445295
42. Becker TC, Coley SM, Wherry EJ, Ahmed R. Bone marrow is a preferred site for homeostatic proliferation of memory CD8 T cells. J Immunol (2005) 174(3):1269–73. doi: 10.4049/jimmunol.174.3.1269
43. Petitprez F, de Reyniès A, Keung EZ, Chen TW, Sun CM, Calderaro J, et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature (2020) 577(7791):556–60. doi: 10.1038/s41586-019-1906-8
44. Takada K, Jameson SC. Naive T cell homeostasis: from awareness of space to a sense of place. Nat Rev Immunol (2009) 9(12):823–32. doi: 10.1038/nri2657
45. O'Melia MJ, Lund AW, Thomas SN. The biophysics of lymphatic transport: Engineering tools and immunological consequences. Iscience (2019) 22:28–43. doi: 10.1016/j.isci.2019.11.005
47. Wang T, Wang C, Wu J, He C, Zhang W, Liu J, et al. The different T-cell receptor repertoires in breast cancer tumors, draining lymph nodes, and adjacent tissues. Cancer Immunol Res (2017) 5(2):148–56. doi: 10.1158/2326-6066.CIR-16-0107
48. O'Melia MJ, Manspeaker MP, Thomas SN. Tumor-draining lymph nodes are survival niches that support T cell priming against lymphatic transported tumor antigen and effects of immune checkpoint blockade in TNBC. Cancer Immunol Immunother (2021) 70(8):2179–95. doi: 10.1007/s00262-020-02792-5
49. Cabrita R, Lauss M, Sanna A, Donia M, Skaarup Larsen M, Mitra S, et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature (2020) 577(7791):561–5. doi: 10.1038/s41586-019-1914-8
50. Jansen CS, Prokhnevska N, Master VA, Sanda MG, Carlisle JW, Bilen MA, et al. An intra-tumoral niche maintains and differentiates stem-like CD8 T cells. Nature (2019) 576(7787):465–70. doi: 10.1038/s41586-019-1836-5
51. Marraco SAF, Soneson C, Delorenzi M, Speiser DE. Genome-wide RNA profiling of long-lasting stem cell-like memory CD8 T cells induced by yellow fever vaccination in humans. Genomics Data (2015) 5:297–301. doi: 10.1016/j.gdata.2015.06.024
52. Buchholz VR, Busch DH. Back to the future: effector fate during T cell exhaustion. Immunity (2019) 51(6):970–2. doi: 10.1016/j.immuni.2019.11.007
53. Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature (2016) 537(7620):417–21. doi: 10.1038/nature19330
54. Leong YA, Chen Y, Ong HS, Wu D, Man K, Deleage C, et al. CXCR5+ follicular cytotoxic T cells control viral infection in b cell follicles. Nat Immunol (2016) 17(10):1187–96. doi: 10.1038/ni.3543
55. Wu T, Ji Y, Moseman EA, Xu HC, Manglani M, Kirby M, et al. The TCF1-Bcl6 axis counteracts type I interferon to repress exhaustion and maintain T cell stemness. Sci Immunol (2016) 1(6):eaai8593. doi: 10.1126/sciimmunol.aai8593
56. Wieland D, Kemming J, Schuch A, Emmerich F, Knolle P, Neumann-Haefelin C, et al. TCF1+ hepatitis c virus-specific CD8+ T cells are maintained after cessation of chronic antigen stimulation. Nat Commun (2017) 8(1):1–13. doi: 10.1038/ncomms15050
57. Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG. Host-reactive CD8+ memory stem cells in graft-versus-host disease. Nat Med (2005) 11(12):1299–305. doi: 10.1038/nm1326
58. Turtle CJ, Swanson HM, Fujii N, Estey EH, Riddell SR. A distinct subset of self-renewing human memory CD8+ T cells survives cytotoxic chemotherapy. Immunity (2009) 31(5):834–44. doi: 10.1016/j.immuni.2009.09.015
59. Schenkel JM, Zloza A, Li W, Narasipura SD, Al-Harthi L. β-catenin signaling mediates CD4 expression on mature CD8+ T cells. J Immunol (2010) 185(4):2013–9. doi: 10.4049/jimmunol.0902572
60. Zehn D, Thimme R, Lugli E, de Almeida GP, Oxenius A. ‘Stem-like’precursors are the fount to sustain persistent CD8+ T cell responses. Nat Immunol (2022) 23(6):1–12.doi: 10.1038/s41590-022-01219-w
61. He R, Hou S, Liu C, Zhang A, Bai Q, Han M, et al. Follicular CXCR5-expressing CD8+ T cells curtail chronic viral infection. Nature (2016) 537(7620):412–6. doi: 10.1038/nature19317
62. Beltra J-C, Manne S, Abdel-Hakeem MS, Kurachi M, Giles JR, Chen Z, et al. Developmental relationships of four exhausted CD8+ T cell subsets reveals underlying transcriptional and epigenetic landscape control mechanisms. Immunity (2020) 52(5):825–841.e828. doi: 10.1016/j.immuni.2020.04.014
63. Alanio C, Wherry EJ. Subsetting the subsets: Heterogeneity and developmental relationships of T cells in human tumors. Sci Immunol (2021) 6(61):eabj3067. doi: 10.1126/sciimmunol.abj3067
64. Yao C, Lou G, Sun H-W, Zhu Z, Sun Y, Chen Z, et al. BACH2 enforces the transcriptional and epigenetic programs of stem-like CD8+ T cells. Nat Immunol (2021) 22(3):370–80. doi: 10.1038/s41590-021-00868-7
65. Lugli E, Galletti G, Boi SK, Youngblood BA. Stem, effector, and hybrid states of memory CD8+ T cells. .Trends Immunol (2020) 41(1):17–28. doi: 10.1016/j.it.2019.11.004
66. Oliveira G, Ruggiero E, Stanghellini MTL, Cieri N, D’Agostino M, Fronza R, et al. Tracking genetically engineered lymphocytes long-term reveals the dynamics of T cell immunological memory. Sci Trans Med (2015) 7(317):317ra198–317ra198. doi: 10.1126/scitranslmed.aac8265
67. Xu Y, Zhang M, Ramos CA, Durett A, Liu E, Dakhova O, et al. Closely related T-memory stem cells correlate with in vivo expansion of CAR. CD19-T cells and are preserved by IL-7 and IL-15. Blood J Am Soc Hematol (2014) 123(24):3750–9. doi: .10.1182/blood-2014-01-552174
68. Lugli E, Dominguez MH, Gattinoni L, Chattopadhyay PK, Bolton DL, Song K, et al. Superior T memory stem cell persistence supports long-lived T cell memory. J Clin Invest (2013) 123(2):594–9. doi: 10.1172/JCI66327
69. Simons BD, Clevers H. Strategies for homeostatic stem cell self-renewal in adult tissues. Cell (2011) 145(6):851–62. doi: 10.1016/j.cell.2011.05.033
70. Ahmed R, Bevan MJ, Reiner SL, Fearon DT. The precursors of memory: models and controversies. Nat Rev Immunol (2009) 9(9):662–8. doi: 10.1038/nri2619
71. Buchholz VR, Flossdorf M, Hensel I, Kretschmer L, Weissbrich B, Gräf P, et al. Disparate individual fates compose robust CD8+ T cell immunity. Science (2013) 340(6132):630–5. doi: 10.1126/science.1235454
72. Lanzavecchia A, Sallusto F. Progressive differentiation and selection of the fittest in the immune response. Nat Rev Immunol (2002) 2(12):982–7. doi: 10.1038/nri959
73. Ferreira DP, Silva JG, Wyss T, Marraco SAF, Scarpellino L, Charmoy M, et al. Central memory CD8+ T cells derive from stem-like Tcf7hi effector cells in the absence of cytotoxic differentiation. Immunity (2020) 53(5):985–1000.e1011. doi: 10.1016/j.immuni.2020.09.005
74. Gattinoni L, Ji Y, Restifo NP. Wnt/β-catenin signaling in T-cell immunity and cancer ImmunotherapyWnt/β-catenin in T-cell biology and tumor immunotherapy. Clin Cancer Res (2010) 16(19):4695–701. doi: 10.1158/1078-0432.CCR-10-0356
75. Gattinoni L, Speiser DE, Lichterfeld M, Bonini C. T Memory stem cells in health and disease. Nat Med (2017) 23(1):18–27. doi: 10.1038/nm.4241
76. Verma V, Jafarzadeh N, Boi S, Kundu S, Jiang Z, Fan Y, et al. MEK inhibition reprograms CD8+ T lymphocytes into memory stem cells with potent antitumor effects. Nat Immunol (2021) 22(1):53–66. doi: 10.1038/s41590-020-00818-9
77. Siddiqui I, Schaeuble K, Chennupati V, Fuertes Marraco SA, Calderon-Copete S, Pais Ferreira D, et al. Intratumoral Tcf1(+)PD-1(+)CD8(+) T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity (2019) 50(1):195–211.e110. doi: 10.1016/j.immuni.2018.12.021
78. Verbeek S, Izon D, Hofhuis F, Robanus-Maandag E, Te Riele H, Watering M, et al. An HMG-box-containing T-cell factor required for thymocyte differentiation. Nature (1995) 374(6517):70–4. doi: 10.1038/374070a0
79. Escobar G, Mangani D, Anderson AC. T Cell factor 1: A master regulator of the T cell response in disease. Sci Immunol (2020) 5(53):eabb9726. doi: 10.1126/sciimmunol.abb9726
80. Jeannet G, Boudousquié C, Gardiol N, Kang J, Huelsken J, Held W. Essential role of the wnt pathway effector tcf-1 for the establishment of functional CD8 T cell memory. Proc Natl Acad Sci USA (2010) 107(21):9777–82. doi: 10.1073/pnas.0914127107
81. Lin W-HW, Nish SA, Yen B, Chen Y-H, Adams WC, Kratchmarov R, et al. CD8+ T lymphocyte self-renewal during effector cell determination. Cell Rep (2016) 17(7):1773–82. doi: 10.1016/j.celrep.2016.10.032
82. Galletti G, De Simone G, Mazza E, Puccio S, Mezzanotte C, Bi TM, et al. Two subsets of stem-like CD8+ memory T cell progenitors with distinct fate commitments in humans. Nat Immunol (2020) 21(12):1552–62. doi: 10.1038/s41590-020-0791-5
83. Gattinoni L. The dark side of T memory stem cells. Blood J Am Soc Hematol (2015) 125(23):3519–20. doi: 10.1182/blood-2015-04-640631
84. Tabler CO, Lucera MB, Haqqani AA, McDonald DJ, Migueles SA, Connors M, et al. CD4+ memory stem cells are infected by HIV-1 in a manner regulated in part by SAMHD1 expression. . J Virol (2014) 88(9):4976–86. doi: 10.1128/JVI.00324-14
85. Jaafoura S, De Goer De Herve M, Hernandez-Vargas EA, Hendel-Chavez H, Abdoh M, Mateo M, et al. Progressive contraction of the latent HIV reservoir around a core of less-differentiated CD4+ memory T cells. Nat Commun (2014) 5(1):1–8. doi: 10.1038/ncomms6407
86. Hosokawa K, Muranski P, Feng X, Townsley DM, Liu B, Knickelbein J, et al. Memory stem T cells in autoimmune disease: high frequency of circulating CD8+ memory stem cells in acquired aplastic anemia. J Immunol (2016) 196(4):1568–78. doi: 10.4049/jimmunol.1501739
87. Roederer M, Quaye L, Mangino M, Beddall MH, Mahnke Y, Chattopadhyay P, et al. The genetic architecture of the human immune system: a bioresource for autoimmunity and disease pathogenesis. Cell (2015) 161(2):387–403. doi: 10.1016/j.cell.2015.02.046
88. Mateus J, Lasso P, Pavia P, Rosas F, Roa N, Valencia-Hernández CA, et al. Low frequency of circulating CD8+ T stem cell memory cells in chronic chagasic patients with severe forms of the disease. PloS Negl Trop Dis (2015) 9(1):e3432. doi: 10.1371/journal.pntd.0003432
89. Vigano S, Negron J, Ouyang Z, Rosenberg ES, Walker BD, Lichterfeld M, et al. Prolonged antiretroviral therapy preserves HIV-1-specific CD8 T cells with stem cell-like properties. J Virol (2015) 89(15):7829–40. doi: 10.1128/JVI.00789-15
90. Ahmed R, Akondy RS. Insights into human CD8+ t‐cell memory using the yellow fever and smallpox vaccines. Immunol Cell Biol (2011) 89(3):340–5. doi: 10.1038/icb.2010.155
91. Speiser DE, Utzschneider DT, Oberle SG, Münz C, Romero P, Zehn D. T Cell differentiation in chronic infection and cancer: functional adaptation or exhaustion? Nat Rev Immunol (2014) 14(11):768–74. doi: 10.1038/nri3740
92. Ribeiro SP, Milush JM, Cunha-Neto E, Kallas EG, Kalil J, Somsouk M, et al. The CD8+ memory stem T cell (TSCM) subset is associated with improved prognosis in chronic HIV-1 infection. J Virol (2014) 88(23):13836–44. doi: 10.1128/JVI.01948-14
93. Cartwright EK, Mcgary CS, Cervasi B, Micci L, Lawson B, Elliott S, et al. Divergent CD4+ T memory stem cell dynamics in pathogenic and nonpathogenic simian immunodeficiency virus infections. J Immunol (2014) 192(10):4666–73. doi: 10.4049/jimmunol.1303193
94. Klatt NR, Bosinger SE, Peck M, Richert-Spuhler LE, Heigele A, Gile JP, et al. Limited HIV infection of central memory and stem cell memory CD4+ T cells is associated with lack of progression in viremic individuals. PloS Pathog (2014) 10(8): e1004345. doi: 10.1371/journal.ppat.1004345
95. Cartwright EK, Palesch D, Mavigner M, Paiardini M, Chahroudi A, Silvestri G. Initiation of antiretroviral therapy restores CD4+ TSCM homeostasis in SIV-infected macaques. J Virol (2016) 90(15):6699–708 .doi: 10.1128/JVI.00492-16
96. Calascibetta F, Micci L, Carnathan D, Lawson B, Vanderford TH, Bosinger SE, et al. Antiretroviral therapy in SIV-infected sooty mangabeys: implications for AIDS pathogenesis. J Virol (2016) 90(16):7541–51.doi: 10.1128/JVI.00598-16
97. Biasco L, Izotova N, Rivat C, Ghorashian S, Richardson R, Guvenel A, et al. Clonal expansion of T memory stem cells determines early anti-leukemic responses and long-term CAR T cell persistence in patients. Nat Cancer (2021) 2(6):629–42. doi: 10.1038/s43018-021-00207-7
98. Speiser DE, Liénard D, Rufer N, Rubio-Godoy V, Romero P. Rapid and strong human CD8+ T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J Clin Invest (2005) 115(3):739–46. doi: 10.1172/JCI23373
99. Melero I, Gaudernack G, Gerritsen W, Huber C, Mellstedt H. Therapeutic vaccines for cancer: An overview of clinical trials. Nat Rev Clin Oncol (2014) 11(9): 509–24 doi: 10.1038/nrclinonc.2014.111
100. Gregorio ED, Rappuoli R. Vaccines for the future: learning from human immunology. Microbial Biotechnol (2012) 5(2):149–55. doi: 10.1111/j.1751-7915.2011.00276.x
101. Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity (2010) 33(4):451–63. doi: 10.1016/j.immuni.2010.10.008
102. Chang JT, Wherry EJ, Goldrath AW. Molecular regulation of effector and memory T cell differentiation. Nat Immunol (2014) 15(12):1104–15. doi: 10.1038/ni.3031
103. Gannon P, Baumgaertner P, Huber A, Iancu EM, Cagnon L, Abed-Maillard S, et al. Rapid and continued T cell differentiation into long-term effector and memory stem cells in vaccinated melanoma patients. Clin Cancer Res Off J Am Assoc Cancer Res (2016) 23(13):3285–96. doi: 10.1158/1078-0432.CCR-16-1708.
104. Park CO, Kupper TS. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat Med (2015) 21(7):688–97. doi: 10.1038/nm.3883
105. Mackay LK, Wynne-Jones E, Freestone D, Pellicci DG, Mielke LA, Newman DM, et al. T-Box transcription factors combine with the cytokines TGF-β and IL-15 to control tissue-resident memory T cell fate. Immunity (2015) 43(6):1101–11. doi: 10.1016/j.immuni.2015.11.008
106. Zhang N, Bevan M. Transforming growth factor-β signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity (2013) 39(4):687–96. doi: 10.1016/j.immuni.2013.08.019
107. Jameson SC, Masopust D. Diversity in T cell memory: An embarrassment of riches. Immunity (2009) 31(6):859–71. doi: 10.1016/j.immuni.2009.11.007
108. Sathaliyawala T, Kubota M, Yudanin N, Turner D, Camp P, Thome JJ, et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity (2013) 38(1):187–97. doi: 10.1016/j.immuni.2012.09.020
109. Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, et al. A human memory T cell subset with stem cell-like properties. Nat Med (2011) 17(10):1290–7. doi: 10.1038/nm.2446
110. Di Rosa F, Gebhardt T. Bone marrow T cells and the integrated functions of recirculating and tissue-resident memory T cells. Front Immunol (2016) 7:51. doi: 10.3389/fimmu.2016.00051
111. Nemoto Y, Kanai T, Takahara M, Oshima S, Nakamura T, Okamoto R, et al. Bone marrow-mesenchymal stem cells are a major source of interleukin-7 and sustain colitis by forming the niche for colitogenic CD4 memory T cells. Gut (2013) 62(8):1142–52. doi: 10.1136/gutjnl-2012-302029
112. Parretta E, Cassese G, Barba P, Santoni A, Guardiola J, Di Rosa F. CD8 cell division maintaining cytotoxic memory occurs predominantly in the bone marrow. J Immunol (2005) 174(12):7654–64. doi: 10.4049/jimmunol.174.12.7654
113. Okhrimenko A, Grün JR, Westendorf K, Fang Z, Reinke S, von Roth P, et al. Human memory T cells from the bone marrow are resting and maintain long-lasting systemic memory. Proc Natl Acad Sci USA (2014) 111(25):9229–34. doi: 10.1073/pnas.1318731111
114. Parretta E, Cassese G, Santoni A, Guardiola J, Vecchio A, Di Rosa F. Kinetics of in vivo proliferation and death of memory and naive CD8 T cells: parameter estimation based on 5-bromo-2'-deoxyuridine incorporation in spleen, lymph nodes, and bone marrow. J Immunol (2008) 180(11):7230–9. doi: 10.4049/jimmunol.180.11.7230
115. Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell (2008) 135(6):1118–29. doi: 10.1016/j.cell.2008.10.048
116. Pitt LA, Tikhonova AN, Hu H, Trimarchi T, King B, Gong Y, et al. CXCL12-producing vascular endothelial niches control acute T cell leukemia maintenance. Cancer Cell (2015) 27(6):755–68. doi: 10.1016/j.ccell.2015.05.002
117. Murali-Krishna K, Ahmed R. Cutting edge: naive T cells masquerading as memory cells. J Immunol (2000) 165(4):1733–7. doi: 10.4049/jimmunol.165.4.1733
118. Paiardini M, Cervasi B, Engram JC, Gordon SN, Klatt NR, Muthukumar A, et al. Bone marrow-based homeostatic proliferation of mature T cells in nonhuman primates: implications for AIDS pathogenesis. Blood (2009) 113(3):612–21. doi: 10.1182/blood-2008-06-159442
119. Pabst R, Miyasaka M, Dudler L. Numbers and phenotype of lymphocytes emigrating from sheep bone marrow after in situ labelling with fluorescein isothiocyanate. Immunology (1986) 59(2):217–22.
120. Klonowski KD, Williams KJ, Marzo AL, Blair DA, Lingenheld EG, Lefrançois L. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity (2004) 20(5):551–62. doi: 10.1016/s1074-7613(04)00103-7
121. Di Rosa F. -lymphocyte interaction with stromal, bone and hematopoietic cells in the bone marrow. Immunol Cell Biol (2009) 87(1):20–9. doi: 10.1038/icb.2008.84
122. Cassese G, Parretta E, Pisapia L, Santoni A, Guardiola J, Di Rosa F. Bone marrow CD8 cells down-modulate membrane IL-7Ralpha expression and exhibit increased STAT-5 and p38 MAPK phosphorylation in the organ environment. Blood (2007) 110(6):1960–9. doi: 10.1182/blood-2006-09-045807
123. Hendriks J, Xiao Y, Borst J. CD27 promotes survival of activated T cells and complements CD28 in generation and establishment of the effector T cell pool. J Exp Med (2003) 198(9):1369–80. doi: 10.1084/jem.20030916
124. Redmond WL, Ruby CE, Weinberg AD. The role of OX40-mediated co-stimulation in T-cell activation and survival. Crit Rev Immunol (2009) 29(3):187–201. doi: 10.1615/critrevimmunol.v29.i3.10
125. Twu YC, Gold MR, Teh HS. TNFR1 delivers pro-survival signals that are required for limiting TNFR2-dependent activation-induced cell death (AICD) in CD8+ T cells. Eur J Immunol (2011) 41(2):335–44. doi: 10.1002/eji.201040639
126. Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. science (2007) 315(5819):1687–91. doi: 10.1126/science.1139393
127. Borst J, Ahrends T, Bąbała N, Melief CJM, Kastenmüller W. CD4(+) T cell help in cancer immunology and immunotherapy. Nat Rev Immunol (2018) 18(10):635–47. doi: 10.1038/s41577-018-0044-0
128. Muranski P, Restifo NP. Adoptive immunotherapy of cancer using CD4(+) T cells. Curr Opin Immunol (2009) 21(2):200–8. doi: 10.1016/j.coi.2009.02.004
129. Broz ML, Binnewies M, Boldajipour B, Nelson AE, Pollack JL, Erle DJ, et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell (2014) 26(6):938. doi: 10.1016/j.ccell.2014.11.010
130. Spranger S, Dai D, Horton B, Gajewski TF. Tumor-residing Batf3 dendritic cells are required for effector T cell trafficking and adoptive T cell therapy. Cancer Cell (2017) 31(5):711–723.e714. doi: 10.1016/j.ccell.2017.04.003
131. Duckworth BC, Lafouresse F, Wimmer VC, Broomfield BJ, Dalit L, Alexandre YO, et al. Effector and stem-like memory cell fates are imprinted in distinct lymph node niches directed by CXCR3 ligands. Nat Immunol (2021) 22(4):434–48. doi: 10.1038/s41590-021-00878-5
132. Sautès-Fridman C, Petitprez F, Calderaro J, Fridman WH. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer (2019) 19(6):307–25. doi: 10.1038/s41568-019-0144-6
133. Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature (2020) 577(7791):549–55. doi: 10.1038/s41586-019-1922-8
134. Voabil P, de Bruijn M, Roelofsen LM, Hendriks SH, Brokamp S, van den Braber M, et al. An ex vivo tumor fragment platform to dissect response to PD-1 blockade in cancer. Nat Med (2021) 27(7):1250–61. doi: 10.1038/s41591-021-01398-3
135. Theisen DJ, Davidson JT, Briseño CG, Gargaro M, Lauron EJ, Wang Q, et al. WDFY4 is required for cross-presentation in response to viral and tumor antigens. Science (2018) 362(6415):694–9. doi: 10.1126/science.aat5030
136. Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, et al. Differential antigen processing by dendritic cell subsets in vivo. Science (2007) 315(5808):107–11. doi: 10.1126/science.1136080
137. Roberts EW, Broz ML, Binnewies M, Headley MB, Nelson AE, Wolf DM, et al. Critical role for CD103(+)/CD141(+) dendritic cells bearing CCR7 for tumor antigen trafficking and priming of T cell immunity in melanoma. Cancer Cell (2016) 30(2):324–36. doi: 10.1016/j.ccell.2016.06.003
138. Laoui D, Keirsse J, Morias Y, Van Overmeire E, Geeraerts X, Elkrim Y, et al. The tumour microenvironment harbours ontogenically distinct dendritic cell populations with opposing effects on tumour immunity. Nat Commun (2016) 7:13720. doi: 10.1038/ncomms13720
139. Dutertre CA, Becht E, Irac SE, Khalilnezhad A, Narang V, Khalilnezhad S, et al. Single-cell analysis of human mononuclear phagocytes reveals subset-defining markers and identifies circulating inflammatory dendritic cells. Immunity (2019) 51(3):573–589.e578. doi: 10.1016/j.immuni.2019.08.008
140. Bourdely P, Anselmi G, Vaivode K, Ramos RN, Missolo-Koussou Y, Hidalgo S, et al. Transcriptional and functional analysis of CD1c(+) human dendritic cells identifies a CD163(+) subset priming CD8(+)CD103(+) T cells. Immunity (2020) 53(2):335–352.e338. doi: 10.1016/j.immuni.2020.06.002
141. von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol (2003) 3(11):867–78. doi: 10.1038/nri1222
142. Mondino A, Khoruts A, Jenkins MK. The anatomy of T-cell activation and tolerance. Proc Natl Acad Sci USA (1996) 93(6):2245–52. doi: 10.1073/pnas.93.6.2245
143. M'Rini C, Cheng G, Schweitzer C, Cavanagh LL, Palframan RT, Mempel TR, et al. A novel endothelial l-selectin ligand activity in lymph node medulla that is regulated by alpha(1,3)-fucosyltransferase-IV. J Exp Med (2003) 198(9):1301–12. doi: 10.1084/jem.20030182
144. Okada T, Ngo VN, Ekland EH, Förster R, Lipp M, Littman DR, et al. Chemokine requirements for b cell entry to lymph nodes and peyer's patches. J Exp Med (2002) 196(1):65–75. doi: 10.1084/jem.20020201
145. Junt T, Fink K, Förster R, Senn B, Lipp M, Muramatsu M, et al. CXCR5-dependent seeding of follicular niches by b and Th cells augments antiviral b cell responses. J Immunol (2005) 175(11):7109–16. doi: 10.4049/jimmunol.175.11.7109
146. Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during mycobacterium tuberculosis challenge. Nat Immunol (2007) 8(4):369–77. doi: 10.1038/ni1449
147. Bajénoff M, Egen JG, Koo LY, Laugier JP, Brau F, Glaichenhaus N, et al. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity (2006) 25(6):989–1001. doi: 10.1016/j.immuni.2006.10.011
148. Beuneu H, Garcia Z, Bousso P. Cutting edge: cognate CD4 help promotes recruitment of antigen-specific CD8 T cells around dendritic cells. J Immunol (2006) 177(3):1406–10. doi: 10.4049/jimmunol.177.3.1406
149. Garcia Z, Pradelli E, Celli S, Beuneu H, Simon A, Bousso P. Competition for antigen determines the stability of T cell-dendritic cell interactions during clonal expansion. Proc Natl Acad Sci USA (2007) 104(11):4553–8. doi: 10.1073/pnas.0610019104
150. Henrickson SE, Mempel TR, Mazo IB, Liu B, Artyomov MN, Zheng H, et al. T Cell sensing of antigen dose governs interactive behavior with dendritic cells and sets a threshold for T cell activation. Nat Immunol (2008) 9(3):282–91. doi: 10.1038/ni1559
151. Luther SA, Tang HL, Hyman PL, Farr AG, Cyster JG. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc Natl Acad Sci USA (2000) 97(23):12694–9. doi: 10.1073/pnas.97.23.12694
152. Link A, Vogt TK, Favre S, Britschgi MR, Acha-Orbea H, Hinz B, et al. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol (2007) 8(11):1255–65. doi: 10.1038/ni1513
153. Seth S, Oberdörfer L, Hyde R, Hoff K, Thies V, Worbs T, et al. CCR7 essentially contributes to the homing of plasmacytoid dendritic cells to lymph nodes under steady-state as well as inflammatory conditions. J Immunol (2011) 186(6):3364–72. doi: 10.4049/jimmunol.1002598
154. Mempel TR, Henrickson SE, Von Andrian UH. -cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature (2004) 427(6970):154–9. doi: 10.1038/nature02238
155. Joshi NS, Kaech SM. Effector CD8 T cell development: a balancing act between memory cell potential and terminal differentiation. J Immunol (2008) 180(3):1309–15. doi: 10.4049/jimmunol.180.3.1309
156. Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, Ahmed R. Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity (2010) 32(1):91–103. doi: 10.1016/j.immuni.2009.11.010
157. Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity (2007) 27(2):281–95. doi: 10.1016/j.immuni.2007.07.010
158. Morrison SJ, Prowse KR, Ho P, Weissman IL. Telomerase activity in hematopoietic cells is associated with self-renewal potential. Immunity (1996) 5(3):207–16. doi: 10.1016/s1074-7613(00)80316-7
159. Hodes RJ, Hathcock KS, Weng NP. Telomeres in T and b cells. Nat Rev Immunol (2002) 2(9):699–706. doi: 10.1038/nri890
160. Xu L, Cao Y, Xie Z, Huang Q, Bai Q, Yang X, et al. The transcription factor TCF-1 initiates the differentiation of T(FH) cells during acute viral infection. Nat Immunol (2015) 16(9):991–9. doi: 10.1038/ni.3229
161. Choi YS, Gullicksrud JA, Xing S, Zeng Z, Shan Q, Li F, et al. LEF-1 and TCF-1 orchestrate T(FH) differentiation by regulating differentiation circuits upstream of the transcriptional repressor Bcl6. Nat Immunol (2015) 16(9):980–90. doi: 10.1038/ni.3226
162. Ballesteros-Tato A, León B, Graf BA, Moquin A, Adams PS, Lund FE, et al. Interleukin-2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity (2012) 36(5):847–56. doi: 10.1016/j.immuni.2012.02.012
163. Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, et al. Bcl6 and blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science (2009) 325(5943):1006–10. doi: 10.1126/science.1175870
164. Choi YS, Eto D, Yang JA, Lao C, Crotty S. Cutting edge: STAT1 is required for IL-6-mediated Bcl6 induction for early follicular helper cell differentiation. J Immunol (2013) 190(7):3049–53. doi: 10.4049/jimmunol.1203032
165. Liu X, Chen X, Zhong B, Wang A, Wang X, Chu F, et al. Transcription factor achaete-scute homologue 2 initiates follicular T-helper-cell development. Nature (2014) 507(7493):513–8. doi: 10.1038/nature12910
166. Choi YS, Yang JA, Yusuf I, Johnston RJ, Greenbaum J, Peters B, et al. Bcl6 expressing follicular helper CD4 T cells are fate committed early and have the capacity to form memory. J Immunol (2013) 190(8):4014–26. doi: 10.4049/jimmunol.1202963
167. Künzli M, Schreiner D, Pereboom TC, Swarnalekha N, Litzler LC, Lötscher J, et al. Long-lived T follicular helper cells retain plasticity and help sustain humoral immunity. Sci Immunol (2020) 5(45):eaay5552–eaay5552. doi: 10.1126/sciimmunol.aay5552
168. Hale JS, Youngblood B, Latner DR, Mohammed AU, Ye L, Akondy RS, et al. Distinct memory CD4+ T cells with commitment to T follicular helper- and T helper 1-cell lineages are generated after acute viral infection. Immunity (2013) 38(4):805–17. doi: 10.1016/j.immuni.2013.02.020
169. Gullicksrud JA, Li F, Xing S, Zeng Z, Peng W, Badovinac VP, et al. Differential requirements for Tcf1 long isoforms in CD8(+) and CD4(+) T cell responses to acute viral infection. J Immunol (2017) 199(3):911–9. doi: 10.4049/jimmunol.1700595
170. Fahey LM, Wilson EB, Elsaesser H, Fistonich CD, McGavern DB, Brooks DG. Viral persistence redirects CD4 T cell differentiation toward T follicular helper cells. J Exp Med (2011) 208(5):987–99. doi: 10.1084/jem.20101773
171. Gautam S, Fioravanti J, Zhu W, Le Gall JB, Brohawn P, Lacey NE, et al. The transcription factor c-myb regulates CD8(+) T cell stemness and antitumor immunity. Nat Immunol (2019) 20(3):337–49. doi: 10.1038/s41590-018-0311-z
172. Clevers H. Wnt/beta-catenin signaling in development and disease. Cell (2006) 127(3):469–80. doi: 10.1016/j.cell.2006.10.018
173. Wirth TC, Xue HH, Rai D, Sabel JT, Bair T, Harty JT, et al. Repetitive antigen stimulation induces stepwise transcriptome diversification but preserves a core signature of memory CD8(+) T cell differentiation. Immunity (2010) 33(1):128–40. doi: 10.1016/j.immuni.2010.06.014
174. Willinger T, Freeman T, Herbert M, Hasegawa H, McMichael AJ, Callan MF. Human naive CD8 T cells down-regulate expression of the WNT pathway transcription factors lymphoid enhancer binding factor 1 and transcription factor 7 (T cell factor-1) following antigen encounter in vitro and in vivo. J Immunol (2006) 176(3):1439–46. doi: 10.4049/jimmunol.176.3.1439
175. Driessens G, Zheng Y, Gajewski TF. Beta-catenin does not regulate memory T cell phenotype. Nat Med (2010) 16(5):513-4–514-5. doi: 10.1038/nm0510-513
176. Muralidharan S, Hanley PJ, Liu E, Chakraborty R, Bollard C, Shpall E, et al. Activation of wnt signaling arrests effector differentiation in human peripheral and cord blood-derived T lymphocytes. J Immunol (2011) 187(10):5221–32. doi: 10.4049/jimmunol.1101585
177. Driessens G, Zheng Y, Locke F, Cannon JL, Gounari F, Gajewski TF. Beta-catenin inhibits T cell activation by selective interference with linker for activation of T cells-phospholipase c-γ1 phosphorylation. J Immunol (2011) 186(2):784–90. doi: 10.4049/jimmunol.1001562
178. Zhao DM, Yu S, Zhou X, Haring JS, Held W, Badovinac VP, et al. Constitutive activation of wnt signaling favors generation of memory CD8 T cells. J Immunol (2010) 184(3):1191–9. doi: 10.4049/jimmunol.0901199
179. Zhou X, Yu S, Zhao DM, Harty JT, Badovinac VP, Xue HH. Differentiation and persistence of memory CD8(+) T cells depend on T cell factor 1. Immunity (2010) 33(2):229–40. doi: 10.1016/j.immuni.2010.08.002
180. Henning AN, Roychoudhuri R, Restifo NP. Epigenetic control of CD8(+) T cell differentiation. Nat Rev Immunol (2018) 18(5):340–56. doi: 10.1038/nri.2017.146
181. Pace L, Amigorena S. Epigenetics of T cell fate decision. Curr Opin Immunol (2020) 63:43–50. doi: 10.1016/j.coi.2020.01.002
182. Prlic M, Bevan MJ. Cutting edge: β-catenin is dispensable for T cell effector differentiation, memory formation, and recall responses. J Immunol (2011) 187(4):1542–6. doi: 10.4049/jimmunol.1100907
183. Jeannet G, Scheller M, Scarpellino L, Duboux S, Gardiol N, Back J, et al. Long-term, multilineage hematopoiesis occurs in the combined absence of beta-catenin and gamma-catenin. Blood (2008) 111(1):142–9. doi: 10.1182/blood-2007-07-102558
184. Huelsken J, Held W. Canonical wnt signalling plays essential roles. Eur J Immunol (2009) 39(12):3582–3. doi: 10.1002/eji.200838982. author reply 3583-3584.
185. Maeda O, Usami N, Kondo M, Takahashi M, Goto H, Shimokata K, et al. Plakoglobin (gamma-catenin) has TCF/LEF family-dependent transcriptional activity in beta-catenin-deficient cell line. Oncogene (2004) 23(4):964–72. doi: 10.1038/sj.onc.1207254
186. Hall J, Guo G, Wray J, Eyres I, Nichols J, Grotewold L, et al. Oct4 and LIF/Stat3 additively induce krüppel factors to sustain embryonic stem cell self-renewal. Cell Stem Cell (2009) 5(6):597–609. doi: 10.1016/j.stem.2009.11.003
187. Burdon T, Stracey C, Chambers I, Nichols J, Smith A. Suppression of SHP-2 and ERK signalling promotes self-renewal of mouse embryonic stem cells. Dev Biol (1999) 210(1):30–43. doi: 10.1006/dbio.1999.9265
188. Qu CK, Feng GS. Shp-2 has a positive regulatory role in ES cell differentiation and proliferation. Oncogene (1998) 17(4):433–9. doi: 10.1038/sj.onc.1201920
189. Hinrichs CS, Spolski R, Paulos CM, Gattinoni L, Kerstann KW, Palmer DC, et al. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood (2008) 111(11):5326–33. doi: 10.1182/blood-2007-09-113050
190. Yamada T, Park CS, Mamonkin M, Lacorazza HD. Transcription factor ELF4 controls the proliferation and homing of CD8+ T cells via the krüppel-like factors KLF4 and KLF2. Nat Immunol (2009) 10(6):618–26. doi: 10.1038/ni.1730
191. Weinreich MA, Takada K, Skon C, Reiner SL, Jameson SC, Hogquist KA. KLF2 transcription-factor deficiency in T cells results in unrestrained cytokine production and upregulation of bystander chemokine receptors. Immunity (2009) 31(1):122–30. doi: 10.1016/j.immuni.2009.05.011
192. Alarcón C, Zaromytidou AI, Xi Q, Gao S, Yu J, Fujisawa S, et al. Nuclear CDKs drive smad transcriptional activation and turnover in BMP and TGF-beta pathways. Cell (2009) 139(4):757–69. doi: 10.1016/j.cell.2009.09.035
193. Lian I, Kim J, Okazawa H, Zhao J, Zhao B, Yu J, et al. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev (2010) 24(11):1106–18. doi: 10.1101/gad.1903310
194. Tamm C, Böwer N, Annerén C. Regulation of mouse embryonic stem cell self-renewal by a yes-YAP-TEAD2 signaling pathway downstream of LIF. J Cell Sci (2011) 124(Pt 7):1136–44. doi: 10.1242/jcs.075796
195. Thaventhiran JE, Hoffmann A, Magiera L, de la Roche M, Lingel H, Brunner-Weinzierl M, et al. Activation of the hippo pathway by CTLA-4 regulates the expression of blimp-1 in the CD8+ T cell. Proc Natl Acad Sci USA (2012) 109(33):E2223–2229. doi: 10.1073/pnas.1209115109
196. Abdelsamed HA, Zebley CC, Nguyen H, Rutishauser RL, Fan Y, Ghoneim HE, et al. Beta cell-specific CD8(+) T cells maintain stem cell memory-associated epigenetic programs during type 1 diabetes. Nat Immunol (2020) 21(5):578–87. doi: 10.1038/s41590-020-0633-5
197. Scott AC, Dündar F, Zumbo P, Chandran SS, Klebanoff CA, Shakiba M, et al. TOX is a critical regulator of tumour-specific T cell differentiation. Nature (2019) 571(7764):270–4. doi: 10.1038/s41586-019-1324-y
198. Kurachi M, Barnitz RA, Yosef N, Odorizzi PM, DiIorio MA, Lemieux ME, et al. The transcription factor BATF operates as an essential differentiation checkpoint in early effector CD8+ T cells. Nat Immunol (2014) 15(4):373–83. doi: 10.1038/ni.2834
199. Gattinoni L, Ji Y, Restifo NP. Wnt/beta-catenin signaling in T-cell immunity and cancer immunotherapy. Clin Cancer Res (2010) 16(19):4695–701. doi: 10.1158/1078-0432.ccr-10-0356
200. Pearce EL. Metabolism in T cell activation and differentiation. Curr Opin Immunol (2010) 22(3):314–20. doi: 10.1016/j.coi.2010.01.018
201. Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nat Rev Immunol (2012) 12(5):325–38. doi: 10.1038/nri3198
202. Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature (2009) 460(7251):103–7. doi: 10.1038/nature08097
203. Macintyre AN, Finlay D, Preston G, Sinclair LV, Waugh CM, Tamas P, et al. Protein kinase b controls transcriptional programs that direct cytotoxic T cell fate but is dispensable for T cell metabolism. Immunity (2011) 34(2):224–36. doi: 10.1016/j.immuni.2011.01.012
204. Van Braeckel-Budimir N, Dolina JS, Wei J, Wang X, Chen SH, Santiago P, et al. Combinatorial immunotherapy induces tumor-infiltrating CD8(+) T cells with distinct functional, migratory, and stem-like properties. J Immunother Cancer (2021) 9(12): e003614–e003614. doi: 10.1136/jitc-2021-003614
205. Marro S, Pang ZP, Yang N, Tsai MC, Qu K, Chang HY, et al. Direct lineage conversion of terminally differentiated hepatocytes to functional neurons. Cell Stem Cell (2011) 9(4):374–82. doi: 10.1016/j.stem.2011.09.002
206. Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Südhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature (2010) 463(7284):1035–41. doi: 10.1038/nature08797
207. Eminli S, Foudi A, Stadtfeld M, Maherali N, Ahfeldt T, Mostoslavsky G, et al. Differentiation stage determines potential of hematopoietic cells for reprogramming into induced pluripotent stem cells. Nat Genet (2009) 41(9):968–76. doi: 10.1038/ng.428
208. Loh YH, Hartung O, Li H, Guo C, Sahalie JM, Manos PD, et al. Reprogramming of T cells from human peripheral blood. Cell Stem Cell (2010) 7(1):15–9. doi: 10.1016/j.stem.2010.06.004
209. Staerk J, Dawlaty MM, Gao Q, Maetzel D, Hanna J, Sommer CA, et al. Reprogramming of human peripheral blood cells to induced pluripotent stem cells. Cell Stem Cell (2010) 7(1):20–4. doi: 10.1016/j.stem.2010.06.002
210. Seki T, Yuasa S, Oda M, Egashira T, Yae K, Kusumoto D, et al. Generation of induced pluripotent stem cells from human terminally differentiated circulating T cells. Cell Stem Cell (2010) 7(1):11–4. doi: 10.1016/j.stem.2010.06.003
211. Schmitt TM, de Pooter RF, Gronski MA, Cho SK, Ohashi PS, Zúñiga-Pflücker JC. Induction of T cell development and establishment of T cell competence from embryonic stem cells differentiated in vitro. Nat Immunol (2004) 5(4):410–7. doi: 10.1038/ni1055
212. Schmitt TM, Zúñiga-Pflücker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity (2002) 17(6):749–56. doi: 10.1016/s1074-7613(02)00474-0
213. Zhao Y, Parkhurst MR, Zheng Z, Cohen CJ, Riley JP, Gattinoni L, et al. Extrathymic generation of tumor-specific T cells from genetically engineered human hematopoietic stem cells via notch signaling. Cancer Res (2007) 67(6):2425–9. doi: 10.1158/0008-5472.can-06-3977
214. Lei F, Haque R, Weiler L, Vrana KE, Song J. T Lineage differentiation from induced pluripotent stem cells. Cell Immunol (2009) 260(1):1–5. doi: 10.1016/j.cellimm.2009.09.005
215. Sukumar M, Liu J, Mehta GU, Patel SJ, Roychoudhuri R, Crompton JG, et al. Mitochondrial membrane potential identifies cells with enhanced stemness for cellular therapy. Cell Metab (2016) 23(1):63–76. doi: 10.1016/j.cmet.2015.11.002
216. Scholz G, Jandus C, Zhang L, Grandclément C, Lopez-Mejia IC, Soneson C, et al. Modulation of mTOR signalling triggers the formation of stem cell-like memory T cells. EBioMedicine (2016) 4:50–61. doi: 10.1016/j.ebiom.2016.01.019
217. Pearce EL, Poffenberger MC, Chang CH, Jones RG. Fueling immunity: insights into metabolism and lymphocyte function. Science (2013) 342(6155):1242454. doi: 10.1126/science.1242454
218. van der Windt GJ, O'Sullivan D, Everts B, Huang SC, Buck MD, Curtis JD, et al. CD8 memory T cells have a bioenergetic advantage that underlies their rapid recall ability. Proc Natl Acad Sci USA (2013) 110(35):14336–41. doi: 10.1073/pnas.1221740110
219. Yang Y, Kohler ME, Chien CD, Sauter CT, Jacoby E, Yan C, et al. TCR engagement negatively affects CD8 but not CD4 CAR T cell expansion and leukemic clearance. Sci Transl Med (2017) 9(417)eaag1209–eaag1209. doi: 10.1126/scitranslmed.aag1209
220. Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell (2010) 142(3):375–86. doi: 10.1016/j.cell.2010.07.002
221. Szabo E, Rampalli S, Risueño RM, Schnerch A, Mitchell R, Fiebig-Comyn A, et al. Direct conversion of human fibroblasts to multilineage blood progenitors. Nature (2010) 468(7323):521–6. doi: 10.1038/nature09591
222. Sekiya S, Suzuki A. Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature (2011) 475(7356):390–3. doi: 10.1038/nature10263
223. Lugli E, Gattinoni L, Roberto A, Mavilio D, Price DA, Restifo NP, et al. Identification, isolation and in vitro expansion of human and nonhuman primate T stem cell memory cells. Nat Protoc (2013) 8(1):33–42. doi: 10.1038/nprot.2012.143
224. Macri C, Dumont C, Johnston AP, Mintern JD. Targeting dendritic cells: a promising strategy to improve vaccine effectiveness. Clin Transl Immunol (2016) 5(3):e66. doi: 10.1038/cti.2016.6
225. Mahnke K, Qian Y, Knop J, Enk AH. Induction of CD4+/CD25+ regulatory T cells by targeting of antigens to immature dendritic cells. Blood (2003) 101(12):4862–9. doi: 10.1182/blood-2002-10-3229
226. Kato Y, Zaid A, Davey GM, Mueller SN, Nutt SL, Zotos D, et al. Targeting antigen to Clec9A primes follicular Th cell memory responses capable of robust recall. J Immunol (2015) 195(3):1006–14. doi: 10.4049/jimmunol.1500767
227. Lahoud MH, Ahmet F, Kitsoulis S, Wan SS, Vremec D, Lee CN, et al. Targeting antigen to mouse dendritic cells via Clec9A induces potent CD4 T cell responses biased toward a follicular helper phenotype. J Immunol (2011) 187(2):842–50. doi: 10.4049/jimmunol.1101176
228. Medaglia C, Giladi A, Stoler-Barak L, De Giovanni M, Salame TM, Biram A, et al. Spatial reconstruction of immune niches by combining photoactivatable reporters and scRNA-seq. Science (2017) 358(6370):1622–6. doi: 10.1126/science.aao4277
229. Cohen M, Giladi A, Gorki AD, Solodkin DG, Zada M, Hladik A, et al. Lung single-cell signaling interaction map reveals basophil role in macrophage imprinting. Cell (2018) 175(4):1031–44:e1018. doi: 10.1016/j.cell.2018.09.009
230. Pasqual G, Chudnovskiy A, Tas JMJ, Agudelo M, Schweitzer LD, Cui A, et al. Monitoring T cell-dendritic cell interactions in vivo by intercellular enzymatic labelling. Nature (2018) 553(7689):496–500. doi: 10.1038/nature25442
231. Liu Z, Li JP, Chen M, Wu M, Shi Y, Li W, et al. Detecting tumor antigen-specific T cells via interaction-dependent fucosyl-biotinylation. Cell (2020) 183(4):1117–33.e1119. doi: 10.1016/j.cell.2020.09.048
232. Ramishetti S, Kedmi R, Goldsmith M, Leonard F, Sprague AG, Godin B, et al. Systemic gene silencing in primary T lymphocytes using targeted lipid nanoparticles. ACS Nano (2015) 9(7):6706–16. doi: 10.1021/acsnano.5b02796
233. Zhou J, Swiderski P, Li H, Zhang J, Neff CP, Akkina R, et al. Selection, characterization and application of new RNA HIV gp 120 aptamers for facile delivery of dicer substrate siRNAs into HIV infected cells. Nucleic Acids Res (2009) 37(9):3094–109. doi: 10.1093/nar/gkp185
234. Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, Nagashima KA, et al. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature (1996) 381(6584):667–73. doi: 10.1038/381667a0
235. Stemberger C, Graef P, Odendahl M, Albrecht J, Dössinger G, Anderl F, et al. Lowest numbers of primary CD8(+) T cells can reconstitute protective immunity upon adoptive immunotherapy. Blood (2014) 124(4):628–37. doi: 10.1182/blood-2013-12-547349
236. Liu L, Patel B, Ghanem MH, Bundoc V, Zheng Z, Morgan RA, et al. Novel CD4-based bispecific chimeric antigen receptor designed for enhanced anti-HIV potency and absence of HIV entry receptor activity. J Virol (2015) 89(13):6685–94. doi: 10.1128/jvi.00474-15
237. Ali A, Kitchen SG, Chen ISY, Ng HL, Zack JA, Yang OO. HIV-1-Specific chimeric antigen receptors based on broadly neutralizing antibodies. J Virol (2016) 90(15):6999–7006. doi: 10.1128/jvi.00805-16
238. Kahn M. Can we safely target the WNT pathway? Nat Rev Drug Discovery (2014) 13(7):513–32. doi: 10.1038/nrd4233
239. Tebas P, Stein D, Tang WW, Frank I, Wang SQ, Lee G, et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med (2014) 370(10):901–10. doi: 10.1056/NEJMoa1300662
240. Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science (2015) 348(6230):62–8. doi: 10.1126/science.aaa4967
241. June CH, Riddell SR, Schumacher TN. Adoptive cellular therapy: a race to the finish line. Sci Transl Med (2015) 7(280):280ps287. doi: 10.1126/scitranslmed.aaa3643
242. Wang X, Wong CW, Urak R, Taus E, Aguilar B, Chang WC, et al. Comparison of naïve and central memory derived CD8(+) effector cell engraftment fitness and function following adoptive transfer. Oncoimmunology (2016) 5(1):e1072671. doi: 10.1080/2162402x.2015.1072671
243. Sommermeyer D, Hudecek M, Kosasih PL, Gogishvili T, Maloney DG, Turtle CJ, et al. Chimeric antigen receptor-modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo. Leukemia (2016) 30(2):492–500. doi: 10.1038/leu.2015.247
244. Klebanoff CA, Gattinoni L, Palmer DC, Muranski P, Ji Y, Hinrichs CS, et al. Determinants of successful CD8+ T-cell adoptive immunotherapy for large established tumors in mice. Clin Cancer Res (2011) 17(16):5343–52. doi: 10.1158/1078-0432.ccr-11-0503
245. Klebanoff CA, Scott CD, Leonardi AJ, Yamamoto TN, Cruz AC, Ouyang C, et al. Memory T cell-driven differentiation of naive cells impairs adoptive immunotherapy. J Clin Invest (2016) 126(1):318–34. doi: 10.1172/jci81217
246. Klebanoff CA, Gattinoni L, Restifo NP. Sorting through subsets: which T-cell populations mediate highly effective adoptive immunotherapy? J Immunother (2012) 35(9):651–60. doi: 10.1097/CJI.0b013e31827806e6
247. Busch DH, Fräßle SP, Sommermeyer D, Buchholz VR, Riddell SR. Role of memory T cell subsets for adoptive immunotherapy. Semin Immunol (2016) 28(1):28–34. doi: 10.1016/j.smim.2016.02.001
248. Provasi E, Genovese P, Lombardo A, Magnani Z, Liu PQ, Reik A, et al. Editing T cell specificity towards leukemia by zinc finger nucleases and lentiviral gene transfer. Nat Med (2012) 18(5):807–15. doi: 10.1038/nm.2700
249. Sukumar M, Liu J, Ji Y, Subramanian M, Crompton JG, Yu Z, et al. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J Clin Invest (2013) 123(10):4479–88. doi: 10.1172/jci69589
250. Gattinoni L, Restifo NP. Moving T memory stem cells to the clinic. Blood (2013) 121(4):567–8. doi: 10.1182/blood-2012-11-468660
251. Baitsch L, Baumgaertner P, Devêvre E, Raghav SK, Legat A, Barba L, et al. Exhaustion of tumor-specific CD8+ T cells in metastases from melanoma patients. J Clin Invest (2011) 121(6):2350–60. doi: 10.1172/jci46102
252. Gros A, Robbins PF, Yao X, Li YF, Turcotte S, Tran E, et al. PD-1 identifies the patient-specific CD8+ tumor-reactive repertoire infiltrating human tumors. J Clin Invest (2014) 124(5):2246–59. doi: 10.1172/jci73639
253. Lugli E, Pinti M, Nasi M, Troiano L, Ferraresi R, Mussi C, et al. Subject classification obtained by cluster analysis and principal component analysis applied to flow cytometric data. Cytometry A (2007) 71(5):334–44. doi: 10.1002/cyto.a.20387
254. Mackall CL, Fleisher TA, Brown MR, Andrich MP, Chen CC, Feuerstein IM, et al. Distinctions between CD8+ and CD4+ T-cell regenerative pathways result in prolonged T-cell subset imbalance after intensive chemotherapy. Blood (1997) 89(10):3700–7. doi: 10.1182/blood.V89.10.3700
255. Singh N, Perazzelli J, Grupp SA, Barrett DM. Early memory phenotypes drive T cell proliferation in patients with pediatric malignancies. Sci Transl Med (2016) 8(320):320ra323. doi: 10.1126/scitranslmed.aad5222
256. Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult b cell ALL patients. J Clin Invest (2016) 126(6):2123–38. doi: 10.1172/jci85309
257. Chen Y, Yu F, Jiang Y, Chen J, Wu K, Chen X, et al. Adoptive transfer of interleukin-21-stimulated human CD8+ T memory stem cells efficiently inhibits tumor growth. J Immunother (2018) 41(6):274–83. doi: 10.1097/cji.0000000000000229
258. Teo YWB, Linn YC, Goh YT, Li S, Ho LP. Tumor infiltrating lymphocytes from acute myeloid leukemia marrow can be reverted to CD45RA+ central memory state by reactivation in SIP (Simulated infective protocol). Immunobiology (2019) 224(4):526–31. doi: 10.1016/j.imbio.2019.05.001
259. Yan C, Chang J, Song X, Yan F, Yu W, An Y, et al. Memory stem T cells generated by wnt signaling from blood of human renal clear cell carcinoma patients. Cancer Biol Med (2019) 16(1):109–24. doi: 10.20892/j.issn.2095-3941.2018.0118
260. Alizadeh D, Wong RA, Yang X, Wang D, Pecoraro JR, Kuo CF, et al. IL15 enhances CAR-T cell antitumor activity by reducing mTORC1 activity and preserving their stem cell memory phenotype. Cancer Immunol Res (2019) 7(5):759–72. doi: 10.1158/2326-6066.cir-18-0466
261. Minervina AA, Pogorelyy MV, Komech EA, Karnaukhov VK, Bacher P, Rosati E, et al. Primary and secondary anti-viral response captured by the dynamics and phenotype of individual T cell clones. Elife (2020) 9:e53704–e53704. doi: 10.7554/eLife.53704
262. Zhang Y, Wang N, Ding M, Yang Y, Wang Z, Huang L, et al. CD40 accelerates the antigen-specific stem-like memory CD8(+) T cells formation and human papilloma virus (HPV)-positive tumor eradication. Front Immunol (2020) 11:1012. doi: 10.3389/fimmu.2020.01012
263. Haslam A, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open (2019) 2(5):e192535. doi: 10.1001/jamanetworkopen.2019.2535
264. Kalbasi A, Ribas A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat Rev Immunol (2020) 20(1):25–39. doi: 10.1038/s41577-019-0218-4
265. Fares CM, Van Allen EM, Drake CG, Allison JP, Hu-Lieskovan S. Mechanisms of resistance to immune checkpoint blockade: Why does checkpoint inhibitor immunotherapy not work for all patients? Am Soc Clin Oncol Educ Book (2019) 39:147–64. doi: 10.1200/edbk_240837
266. Pauken KE, Sammons MA, Odorizzi PM, Manne S, Godec J, Khan O, et al. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science (2016) 354(6316):1160–5. doi: 10.1126/science.aaf2807
267. Huang AC, Postow MA, Orlowski RJ, Mick R, Bengsch B, Manne S, et al. T-Cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature (2017) 545(7652):60–5. doi: 10.1038/nature22079
268. Yost KE, Satpathy AT, Wells DK, Qi Y, Wang C, Kageyama R, et al. Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat Med (2019) 25(8):1251–9. doi: 10.1038/s41591-019-0522-3
269. Wu TD, Madireddi S, de Almeida PE, Banchereau R, Chen YJ, Chitre AS, et al. Peripheral T cell expansion predicts tumour infiltration and clinical response. Nature (2020) 579(7798):274–8. doi: 10.1038/s41586-020-2056-8
270. Miller BC, Sen DR, Al Abosy R, Bi K, Virkud YV, LaFleur MW, et al. Subsets of exhausted CD8(+) T cells differentially mediate tumor control and respond to checkpoint blockade. Nat Immunol (2019) 20(3):326–36. doi: 10.1038/s41590-019-0312-6
271. Sade-Feldman M, Yizhak K, Bjorgaard SL, Ray JP, de Boer CG, Jenkins RW, et al. Defining T cell states associated with response to checkpoint immunotherapy in melanoma. Cell (2018) 175(4):998–1013.e1020. doi: 10.1016/j.cell.2018.10.038
272. Philip M, Fairchild L, Sun L, Horste EL, Camara S, Shakiba M, et al. Chromatin states define tumour-specific T cell dysfunction and reprogramming. Nature (2017) 545(7655):452–6. doi: 10.1038/nature22367
273. Li H, van der Leun AM, Yofe I, Lubling Y, Gelbard-Solodkin D, van Akkooi ACJ, et al. Dysfunctional CD8 T cells form a proliferative, dynamically regulated compartment within human melanoma. Cell (2019) 176(4):775–89.e718. doi: 10.1016/j.cell.2018.11.043
274. Kurtulus S, Madi A, Escobar G, Klapholz M, Nyman J, Christian E, et al. Checkpoint blockade immunotherapy induces dynamic changes in PD-1(-)CD8(+) tumor-infiltrating T cells. Immunity (2019) 50(1):181–94.e186. doi: 10.1016/j.immuni.2018.11.014
275. Dammeijer F, van Gulijk M, Mulder EE, Lukkes M, Klaase L, van den Bosch T, et al. The PD-1/PD-L1-Checkpoint restrains T cell immunity in tumor-draining lymph nodes. Cancer Cell (2020) 38(5):685–700.e688. doi: 10.1016/j.ccell.2020.09.001
276. Vodnala SK, Eil R, Kishton RJ, Sukumar M, Yamamoto TN, Ha NH, et al. T Cell stemness and dysfunction in tumors are triggered by a common mechanism. Science (2019) 363(6434):eaau0135–eaau0135. doi: 10.1126/science.aau0135
277. Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature (2014) 515(7528):568–71. doi: 10.1038/nature13954
278. Harjes U. The source within− intratumoural stem-like T cells give rise to differentiated T cells. Nat Rev Cancer (2020) 20(3):140. doi: 10.1038/s41568-020-0239-0
279. Falvo P, Orecchioni S, Hillje R, Raveane A, Mancuso P, Camisaschi C, et al. Cyclophosphamide and vinorelbine activate stem-like CD8+ T cells and improve anti-PD-1 efficacy in triple-negative breast cancer. Cancer Res (2021) 81(3):685–97. doi: 10.1158/0008-5472.CAN-20-1818
280. Chen C-Y, Ueha S, Ishiwata Y, Shichino S, Yokochi S, Yang D, et al. Combining an alarmin HMGN1 peptide with PD-L1 blockade results in robust antitumor effects with a concomitant increase of stem-like/progenitor exhausted CD8+ T cells. Cancer Immunol Res (2021) 9(10):1214–28. doi: 10.1158/2326-6066.CIR-21-0265
281. Held W, Luther SA, Petrova TV. Stem-cell-like T cells have a specific entry gate to the tumor. Cancer Cell (2022) 40(3):243–5. doi: 10.1016/j.ccell.2022.02.004
282. Pauken KE, Dougan M, Rose NR, Lichtman AH, Sharpe AH. Adverse events following cancer immunotherapy: Obstacles and opportunities. Trends Immunol (2019) 40(6):511–23. doi: 10.1016/j.it.2019.04.002
283. Barber DL, Sakai S, Kudchadkar RR, Fling SP, Day TA, Vergara JA, et al. Tuberculosis following PD-1 blockade for cancer immunotherapy. Sci Transl Med (2019) 11(475): eaat2702–eaat2702. doi: 10.1126/scitranslmed.aat2702
284. Ahn E, Araki K, Hashimoto M, Li W, Riley JL, Cheung J, et al. Role of PD-1 during effector CD8 T cell differentiation. Proc Natl Acad Sci USA (2018) 115(18):4749–54. doi: 10.1073/pnas.1718217115
285. Kalia V, Yuzefpolskiy Y, Vegaraju A, Xiao H, Baumann F, Jatav S, et al. Metabolic regulation by PD-1 signaling promotes long-lived quiescent CD8 T cell memory in mice. Sci Transl Med (2021) 13(615):eaba6006. doi: 10.1126/scitranslmed.aba6006
286. Chung K, Wallace J, Kim SY, Kalyanasundaram S, Andalman AS, Davidson TJ, et al. Structural and molecular interrogation of intact biological systems. Nature (2013) 497(7449):332–7. doi: 10.1038/nature12107
Keywords: stem-like T cells, niches, adoptive cell therapies, autoimmune disorders, immune checkpoint blockade
Citation: Yi L and Yang L (2022) Stem-like T cells and niches: Implications in human health and disease. Front. Immunol. 13:907172. doi: 10.3389/fimmu.2022.907172
Received: 29 March 2022; Accepted: 28 July 2022;
Published: 17 August 2022.
Edited by:
David L. Wiest, Fox Chase Cancer Center, United StatesReviewed by:
Dietmar Kappes, Fox Chase Cancer Center, United StatesCopyright © 2022 Yi and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Yang, eWwudHJhY3k3M0BnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.