
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol., 28 July 2022
Sec. Inflammation
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.901851
This article is part of the Research TopicNovel Insights into Inflammatory Roles of Mast Cells and BasophilsView all 31 articles
 Kanokvalai Kulthanan1
Kanokvalai Kulthanan1 Martin K. Church2,3
Martin K. Church2,3 Eva Maria Grekowitz2,3
Eva Maria Grekowitz2,3 Tomasz Hawro2,3,4
Tomasz Hawro2,3,4 Lea Alice Kiefer2,3
Lea Alice Kiefer2,3 Kanyalak Munprom1
Kanyalak Munprom1 Yanisorn Nanchaipruek1
Yanisorn Nanchaipruek1 Chuda Rujitharanawong1
Chuda Rujitharanawong1 Dorothea Terhorst-Molawi2,3
Dorothea Terhorst-Molawi2,3 Marcus Maurer2,3*
Marcus Maurer2,3*Background: Chronic inducible urticaria (CIndU) constitutes a group of nine different CIndUs in which pruritic wheals and/or angioedema occur after exposure to specific and definite triggers. Histamine released from activated and degranulating skin mast cells is held to play a key role in the pathogenesis of CIndU, but evidence to support this has, as of yet, not been reviewed systematically or in detail. We aim to characterize the role and relevance of histamine in CIndU.
Methods: We systematically searched 3 electronic databases (PubMed, Scopus, and Embase) for studies that reported increased serum or skin histamine concentration (direct evidence) or in vitro or ex vivo histamine release (indirect evidence) following trigger exposure.
Results: An initial total of 3,882 articles was narrowed down to 107 relevant studies of which 52 were in cold urticaria, 19 in cholinergic urticaria, 14 in heat urticaria, 10 in contact urticaria, 7 each in solar urticaria and vibratory angioedema, 4 each in symptomatic dermographism and aquagenic urticaria, and 3 in delayed pressure urticaria. The results of our review support that histamine has a key pathogenic role in the pathogenesis of all CIndUs, but it is not the sole mediator as evidenced by the often poor relationship between the level of histamine and severity of symptoms and the variable clinical efficacy of H1-antihistamines.
Conclusions: Histamine released from skin mast cells is a key driver of the development of signs and symptoms and a promising therapeutic target in CIndU.
Chronic inducible urticaria (CIndU) is a subgroup of chronic urticaria in which recurrent pruritic wheals and/or angioedema occur after exposure to specific and definite triggers (1). There are nine different CIndUs, with a wide spectrum of physical and chemical triggers. Examples of the former are exposure to cold and pressure in cold urticaria (ColdU) and delayed pressure urticaria (DPU), respectively. Examples of the latter are water in aquagenic urticaria (AquaU) and sweat in cholinergic urticaria (CholU). How these triggers cause the occurrence of wheals, angioedema, or both, in patients with CIndUs, is largely unclear (2, 3).
Histamine, released from activated and degranulating skin mast cells, is held to play a key role in the pathogenesis of CIndU. This is supported by several independent lines of evidence. First, the wheals that occur in patients with CIndU share many features of those induced by histamine skin prick testing or intracutaneous injection (4–6). Both occur within minutes of exposure, and they are, in most cases, pruritic and short lived, with resolution after minutes to a few hours (4, 5). Second, immunoglobulin E (IgE) autoantibodies appear to be involved, in some CIndUs, in the activation of skin mast cells (1, 7–10). IgE-mediated activation is a well characterized mechanism of mast cell histamine release and relevant in allergies and chronic spontaneous urticaria (9). Third, H1-antihistamines (AH1) protect many, but not all, patients with CIndU from the occurrence of signs and symptoms (11–15). In addition, there is also direct evidence, from in vivo studies, as well as indirect evidence, from in vitro studies, for histamine release from skin mast cells in CIndU, but this evidence has, as of yet, not been reviewed systematically or in detail.
Here, we review both in vivo and in vitro evidence for histamine release as a pathogenic driver in CIndU. Our aim is to better characterize the role and relevance of histamine, and, by proxy, those of its receptors and its only relevant source in human skin, mast cells, as mechanisms of the development of signs and symptoms and relevant treatment targets in CIndU.
Preferred Reporting Items for Systematic Reviews and Meta-Analysis were used in this study (16). We systematically searched 3 electronic databases (PubMed, Scopus, and Embase) for studies that reported direct and/or indirect evidence of histamine release in CIndU and were published before April 2021 (inclusion criteria). Increased serum or skin histamine concentration in patients with CIndU after provocation testing was defined as direct evidence (17), whereas other evidence for histamine release was classified as indirect (18). The advanced search option was used with the Mesh terms “histamine” AND each type of CIndU, for example, “cold urticaria” or “symptomatic dermographism”. All articles identified were screened by at least two independent reviewers, and their reference lists were searched for additional reports of relevance. Case reports, case series, randomized controlled trials (RCTs), cohort, case-control, and cross-sectional studies that reported evidence of histamine release in CIndU were included. Owing to the relatively limited number of studies of histamine release in CIndU, case reports and case series were included, aiming to collect data from available published evidence as much as possible.
From each report included in our review, we extracted the number of studied patients, how histamine release was assessed and what the outcome was, as well as the author information and year of publication. Our search identified 3,882 potentially relevant articles (429 from PubMed, 622 from Embase, and 2,831 from Scopus). After the exclusion of 976 duplicates, 2,906 studies were reviewed by title and abstract. Of these, 2617 articles were excluded because they did not fulfill our inclusion criteria. A total of 289 articles underwent full-text review. In the end, 107 studies, consisting of 1 RCT, 10 cohort studies, 9 case-control studies, 35 cross-sectional studies, 6 case series and 46 case reports, were included in our systematic review (Figure 1). Of these 107 studies, 52 were in ColdU, 19 in CholU, 14 in heat urticaria (HeatU), 10 in contact urticaria (ConU), 7 each in solar urticaria (SolU) and vibratory angioedema (VA), 4 each in symptomatic dermographism (SD) and AquaU, and 3 in DPU. Some articles reported multiple types of CIndU. All articles were in English. The quality and risk of bias assessment of included articles in systematic review was determined (Supplementary Table 1).
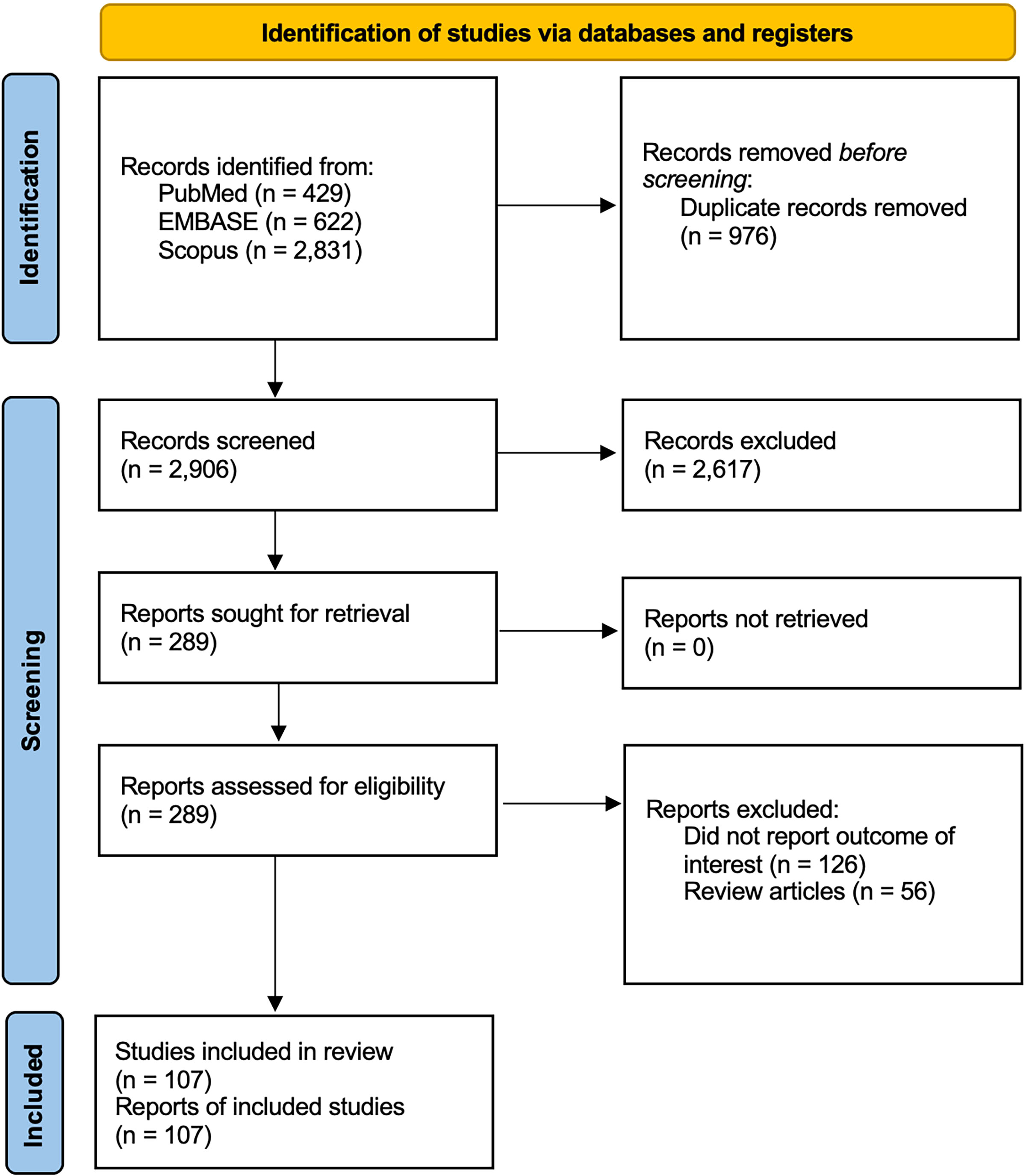
Figure 1 Flow diagram of the literature review in this systematic review. One hundred and seven articles met the inclusion criteria and were included in this systematic review (1 randomized controlled trial, 10 cohort studies, 9 case-control studies, 35 cross-sectional studies, 6 case series, and 46 case reports). There were 4 articles with SD, 52 articles with ColdU, 3 articles with DPU, 7 articles with SolU, 19 articles with CholU, 14 articles with HeatU, 7 articles with VA, 4 articles with AquaU, and 10 articles with ConU. Some of articles reported multiple chronic inducible urticaria cases. AquaU, aquagenic urticaria; CholU, cholinergic urticaria; ColdU, cold urticaria; ConU, contact urticaria; DPU, delayed pressure urticaria; HeatU, heat urticaria; SD, symptomatic dermographism; SolU, solar urticaria; VA, vibratory angioedema.
The rating system developed by de Croon et al. (19) was applied to categorized the levels of evidence for ‘association’ and ‘no association’ The Levels of evidence for “association” were categorized as strong: 3 studies available that find an association in the same direction or ≥ 4 studies available, of which > 66% find a significant association in the same direction and no more than 25% find an opposite association, weak: 2 studies available that find a significant association in the same direction or 3 studies available, of which two find a significant association in the same direction and the third study finds no significant association, no evidence: ≤ 1 study available, inconsistent: remaining cases. The levels of evidence for “no association” were defined as strong: > 4 studies are available, of which >85% find no significant association, weak: > four studies are available, of which >75% find no significant association.
Two studies provided direct evidence of an increase in blood histamine levels following provocation testing in symptomatic dermographism (SD). In the first, post-provocation blood histamine levels were increased in five of ten patients of whom two had markedly higher levels (20) (Supplementary Table 2). In the second, a patient with severe SD showed a marked increase in venous histamine from 18 ng/ml before scratching to a peak of 62 ng/ml two minutes after provocation. Blood histamine levels returned to baseline within 4 minutes (21).
In 1970, Greaves and Sondegaard reported direct evidence for histamine release in SD obtained by the use of fine bore needles to sample the skin in the provocation area of 8 patients and 16 controls (17). Histamine was detected in the basal perfusate of all 8 patients, probably due to whealing in response to needle insertion. None of the controls showed detectable basal histamine. In six of the 8 patients, stroking the skin produced further whealing accompanied by a further rise in skin histamine levels.
As for indirect evidence of histamine release in SD, a systematic review of RCTs and non-RCTs of treatment of SD revealed that first-generation H1-antihistamines (fgAH1) had variable efficacy and significant side effects, whereas second-generation H1-antihistamines (sgAH1), in all studies, were effective with a good safety profile and should be the first-line treatment (22).
That histamine is released in symptomatic patients with cold urticaria (ColdU) was first suggested by Bram Rose in 1941 who detected elevated blood histamine levels in these patients after cold challenge (20, 23) (Supplementary Table 3). This finding was confirmed by several subsequent studies, including the ones by Dunér et al. (24, 25), who found that blood histamine levels can also be elevated in healthy subjects after cold challenge, Spuzic et al. (26), who found plasma histamine to be elevated in 3 of 7 ColdU patients washed with cold water, and others (27–30).
Perhaps the most definitive experiments were performed by Kaplan et al. (18, 31), who showed, in six ColdU patients, that immersion of one hand in cold water caused a rise in plasma histamine of 10-36 ng/ml, peaking at 4 minutes. The results of these reports on blood histamine levels are supported by studies in which histamine skin levels were found to be increased in cold-induced wheals (3). Using skin microdialysis, Andersson et al. (32) showed ice cube-induced increases in skin histamine levels varying from 91 to 550 nM in three patients. Nuutinen et al. (33) reported that of six patients, two had high dialysate histamine levels (621 and 1,269 nM) while the others had lower levels (21 – 100 nM). Kring Tannert et al. (10) also detected, by skin microdialysis, histamine release following cold challenge, which was much reduced after tolerance induction.
In a study with the sgAH1 bilastine, post-provocation skin microdialysis was performed in 20 ColdU patients on three occasions, following treatment for one week each with placebo, bilastine 20, and bilastine 80 mg (12). While only group mean data were reported in the published paper, results for individual patients are presented here.
The results of microdialysis for each patient for the three occasions were remarkably consistent for each showing that the antihistamine, while significantly reducing the critical temperature threshold (CTT), had no effect on histamine release. Several conclusions may be made from the results shown in Figure 2. First, the increases in histamine release at the temperature at which wheals occurred were very variable, from 3 – 222 nM. Second, there is a significant (P = 0.003) relationship between dialysate histamine concentrations and CTTs suggesting that histamine is a major mediator. Third, the regression line intercepts the CTT line at 13°C indicating strongly that there is participation of another mediator in addition to histamine in producing the symptoms. Fourth, the patients with the highest histamine levels are in general less sensitive to 80 mg bilastine, the antihistamine used in this study.
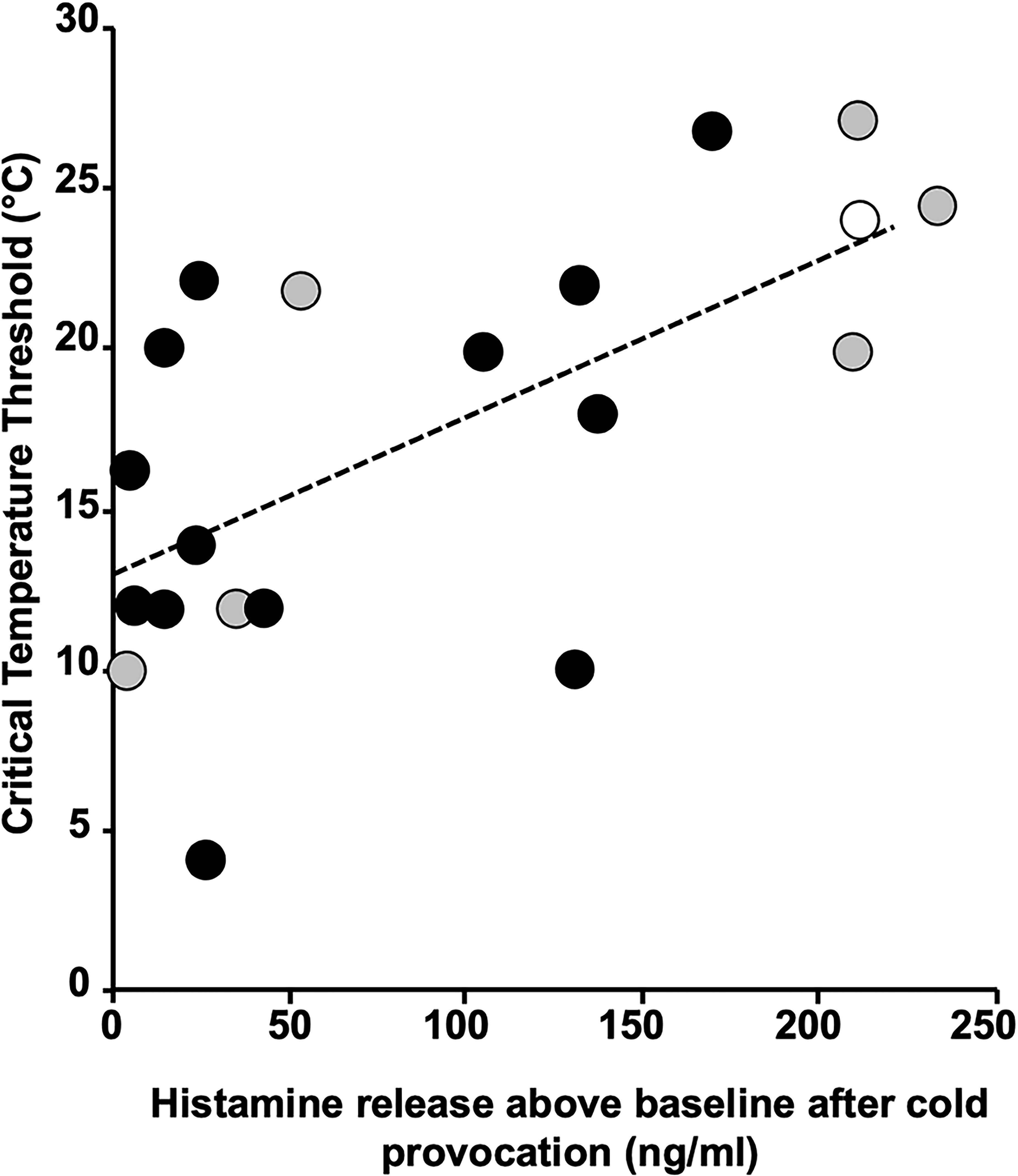
Figure 2 Regression plot of critical threshold temperatures against dialysate histamine concentrations. Patients are colour coded as to their responsiveness to 80 mg bilastine, green > 80% inhibition (responders), yellow 30–80% inhibition (partial responders) and red < 30% (nonresponders). The regression line is statistically significant (P = 0.003) and intercepts the critical threshold temperature axis at 13°C.
Studies that provided indirect evidence for histamine release in ColdU include those of Juhlin et al. (34), who reported that mast cells in biopsies of ColdU patients show signs of degranulation, and of Kaplan et al. (35), who demonstrated histamine release by ex vivo cooling and rewarming of skin biopsies from ColdU patients. One interesting feature of these studies is the wide variability of histamine release, ranging from 14 to 128 ng/ml (3).
Sigler et al. (36) showed that the fgAH1 cyproheptadine can prevent cold-induced hives, and a recent systematic review by Dressler et al. showed that sgAH1 were more effective than placebo in the treatment of patients with ColdU. Standard and high dose treatment were more effective than placebo in terms of the rates of patients who became “symptom free” (37).
Direct evidence of histamine release in DPU comes from 3 studies. Two of them measured histamine release in skin lesions of DPU patients. Kaplan et al. (3) assessed suction blister fluid obtained at 2-hour intervals from skin sites exposed to pressure and found histamine levels to be increased between 4-10 hours, with peak levels of approximately 30 ng/ml at 6-8 hours (Supplementary Table 4). Czarnetzki et al. (38) reported that mean histamine levels were elevated in wheals induced by pressure. They also reported indirect evidence that peripheral blood leukocytes had significantly increased intracellular histamine levels and increased histamine releasability.
Mijailović et al. (39) reported that pressure challenge on a patient’s forearm caused increased histamine levels in blood collected from the draining cubital vein 14 hours later as compared with the contralateral cubital vein and to baseline. Of note, 18 hours after challenge the patient developed systemic anaphylaxis and received adrenaline.
For indirect evidence, a systematic review of treatment of DPU showed that sgAH1 were effective in 3 RCTs. Combining a sgAH1 with montelukast (2 RCTs) or theophylline (1 non-RCT) was more effective than the sgAH1 alone. There are no studies on sgAH1 updosing in DPU (40).
Direct evidence for histamine release in solar urticaria (SolU) comes mostly from blood histamine assessment: Two patients with confirmed SolU were challenged and histamine was measured by Soter et al. (41) (Supplementary Table 5) Serum histamine rose from baseline of <0.1 ng/ml to peak levels of 7.0 and 37 ng/ml at 5 minutes. Levels returned to baseline by 20 minutes.
Hawk et al. studied histamine release in four SolU patients provoked by UVB (42). The results showed that two patients had increased histamine levels of 20 ng/ml and 8.5 ng/ml at 2 and 5 minutes after irradiation, respectively while the other two showed smaller increases. Additionally, electron microscopy of skin specimens taken after challenge showed numerous mast cells undergoing exocytotic changes, characteristically seen during histamine release, whereas in some cells no signs of degranulation were found.
Keahey et al. (6) measured venous blood histamine levels in three patients before and after UVA irradiation with various intervals up to 30 min compared with after induction of tolerance by repeated exposure to UVA. The results showed small and variable histamine release, which was reduced by tolerance induction. Watanabe et al. (43) reported increase in venous histamine in two SolU patients when wheals developed approximated 30 minutes after UV irradiation.
There is only one study, of Kaplan et al. (3), that performed histamine measurements in the skin. SolU was induced in one patient by ultraviolet (UV) light filtered through window glass for 30 seconds. Histamine levels in suction blisters induced over urticarial lesions and the contralateral non-urticarial skin were 127 ng/ml and 24 ng/ml, respectively.
As for indirect evidence, Baart de Faille et al. (44) reported that histological evaluation revealed higher numbers of mast cells in irradiated specimens, as well as appearance of degranulation 24 hours after irradiation, implying histamine release. Also, AH1 treatment is of benefit in 35-75% of SolU patients (45–47).
Histamine release in heat-induced urticaria (HeatU) was demonstrated by 14 studies, all of which have adopted an in vivo approach and showed a homogeneous picture with histamine rising to peak levels in venous blood draining the exposed site within minutes of exposure (14, 20, 48–57) (Supplementary Table 6). Most studies chose exposure temperatures of 40°C, going up to 45°C, with one exception of 56°C due to the high individual threshold of the examined patient (49). These results from venous blood are also supported by a study in which suction blister histamine peaked at 30 minutes after heat challenge, whereas it did not rise in healthy controls (15). Most of the blood studies did not have healthy controls who underwent the same procedures, but those who did showed no rise of histamine levels upon exposure to heat (50). Two studies reported that after successful treatment of HeatU patients with a desensitization protocol, patients remained symptom free and the previously seen rise of histamine could no longer be detected upon exposure to heat (50, 56).
As for indirect evidence, one study examined skin biopsies after heat provocation using electron microscopy, revealing features of mast cell degranulation (58). Also, approximately 60% of patients with HeatU respond to sgAH1, although only few patients achieve complete control (59).
Vibratory angioedema (VA) is divided into 2 subgroups, hereditary vibratory angioedema (HVA) and acquired vibratory angioedema (AVA), with histamine release being purported to be relevant for both subgroups.
Direct evidence of histamine release in HVA is reported in 3 studies. Metzger et al. (60) and Kaplan et al. (31) studied the same patient whose forearm was applied on a vortex mixer for 4 minutes on different occasions Supplementary Table 7). Venous histamine levels peaked at 22 and 53 ng/ml at 1-3 minutes and declined to baseline at 4-13 minutes. A similar study was reported by Boyden et al. (61), with serum histamine levels peaking at 90 and 130 nmol/l within 5 minutes after challenge in two patients.
Four in vivo investigations of histamine release provide direct evidence in AVA, using blood obtained before and after vibratory stimulation for 1-5 min at various intervals. Ting et al. (62) reported peak venous blood histamine of 24 ng/ml at 1 minute. Wener et al. (63) detected peak histamine of 18 ng/ml at 5 minutes. Keahey et al. (5) reported 2 patients having peak levels of serum histamine of 1,224 to 9,000 pg/ml at 5 minutes and a secondary rise at 3-4 hours. Zhao et al. (64) reported mean peak serum histamine levels of 9 ng/ml in 3 patients at 30 minutes after stimulation.
As for indirect evidence, three studies demonstrated mast cells in various stages of degranulation at provocation sites, as assessed by immunohistochemistry or electron microscopy (5, 61, 62). Also, VA patients can benefit from AH1 treatment (65).
The first report of direct evidence of histamine release in three patients with cholinergic urticaria (CholU) was by Kaplan et al. in 1975 (18) (Supplementary Table 8). Baseline venous blood histamine levels were ≤1 ng/ml. The first patient had mild symptoms and a peak histamine level of 3 ng/ml during exercise, which returned to baseline within 20 minutes. The second patient had more severe symptoms and a peak histamine level of 25 ng/ml during exercise, which was still elevated (4 ng/ml) at 35 minutes. The third patient had no itch accompanying the skin lesions and showed no detectable histamine release after exercise challenge. Variable elevations of venous blood histamine with exercise were reported in other studies (11, 14, 29, 31, 66–70).
Indirect evidence of histamine release was demonstrated in five recent in vitro studies including 84 patients using basophil histamine release tests (71–76). Although sgAH1 are used as first-line treatment for patients with CholU, many patients report only mild to moderate improvement. The addition of AH2 was effective in some patients with refractory cases of CholU (77). A systematic review by Dressler et al. showed that fgAH1 and sgAH1 are more effective than placebo, with increased doses resulting in higher efficacy compared with placebo (37).
Contact urticaria (ConU) may be divided into immunologic and non-immunologic ConU. Among 11 studies reporting direct evidence of histamine release in immunologic ConU, 20 cases were provoked by latex (78–80), and 1 each by limonium tataricum (81), cereal flour extract (82), rice (83), polyvinylpyrrolidone (84), and chlorhexidine (85) Supplementary Table 9). These urticariogenic substances all released histamine from patient leucocytes in vitro (78–83).
For in vivo results, serum histamine levels were assessed in 2 cases. The histamine levels of the patient with chlorhexidine allergy were measured before and after skin prick test and patch test. The results showed an increase of more than 3-fold and 2-fold after skin prick test and patch test, respectively (85).
In an in vivo study of nettle induced non-immunologic contact urticaria, histamine levels rose to 35 ± 240 nM at 15 minutes after challenge and declined rapidly (86).
For indirect evidence of ConU, sgAH1 are effective in controlling both the number and the duration of wheals in most patients with ConU had a good response and relieved the symptoms. In uncontrolled cases, updosing of antihistamines can be helpful (87).
In vivo histamine release in AquaU was demonstrated in two studies (67, 88) (Supplementary Table 10). Following skin provocation testing, increased plasma histamine levels were reported in 2 patients. Davis et al. reported peak plasma histamine of 2 ng/ml at 60 minutes after challenge, which returned to baseline within 2 hours (67). Sibbald et al. reported plasma histamine increase of up to 11 ng/ml after challenge (88). Acetone enhanced the plasma histamine increase in response to subsequent challenge, but it did not evoke wheals by itself (88).
As for indirect evidence, two studies demonstrated mast cell degranulation at sites of provocation in one patient each (88, 89). Also, an in vitro study of basophils from patients with AquaU, showed histamine release in response to challenge with all of 4 dilutions of human callus extract (90). FgAH1 and sgAH1 lead to benefit in most patients (91–93), and up-dosing can improve the response (94, 95).
Our systematic review demonstrates direct and indirect evidence of histamine release in all types of CIndUs and confirms the role of histamine in their pathogenesis. (Supplementary Tables 11, 12).
Comparisons of blood histamine levels before and after challenge was the most common in vivo approach and demonstrate the extent and kinetics of skin histamine release. The histamine detection methods used in these studies were various and varied over time. In earlier studies, use of guinea-pig ileum in a superfusion cascade system was frequently used (96, 97). In later studies more sensitive chemical methods were employed, including radioenzyme techniques (98–100) and competitive enzyme immunoassay (57, 101). Skin microdialysis was another in vivo method commonly used. Its advantages include that it does not require repeated blood sampling to assess the release of histamine in human skin (102).
Regarding in vitro methods, basophil tests were commonly performed to detect histamine release, e.g. by fluorometric assay (103–105), radioenzyme technique (98), enzymatic double-isotopic assay (106), enzyme-linked immunosorbent assay (107), and HPLC (72). Furthermore, demonstration of mast cell degranulation in skin biopsy by some studies provided indirect evidence that histamine might be involved in the development of signs and symptoms of CIndU. Because different studies used different methods with different sensitivity, the levels of histamine released are difficult to compare.
Although the exact pathogenesis of CIndU is still largely unclear (1), mast cell activation and degranulation with subsequent release of proinflammatory mediators are held to be critical and histamine is a main mediator. Other mediators and cytokines with pathogenic relevance in CIndU include tumor necrotic factor-α (108, 109), prostaglandin D2 (110–112), leukotriene (113), acetylcholine (114), platelet activating factor and interleukins (109, 115). However, the role of mediators other than histamine in each CIndU subtype remains ill defined, and their relevance should be explored in future studies. Treatment with AH1 in each CIndU can lead to various clinical responses ranging from non-response to complete response (12, 116). (Supplementary Table 11) Thus, it is likely that histamine is not of the same relevance in all CIndUs or in all patients with the same CIndU. Importantly, non-response to an AH1 does not rule out that histamine is a major CIndU driver, as histamine can also induce skin inflammation and itch via H4 receptors. H4 receptor antagonists should be assessed for their efficacy in the treatment of patients with CIndU (117). Also, further studies should explore and better define the spectrum and roles of mast cell mediators other than histamine that are involved in the induction of signs and symptoms of each CIndU. Finally, novel targeted therapies are needed and should be developed to improve the management of CIndUs (Supplementary Table 13).
This review supports a key pathogenic role of histamine in all types of CIndUs. However, some points are still unclear, for example, the trigger pathway for histamine release and the relationship between the level of histamine and severity of symptoms.
Our systematic review identified direct and indirect evidence of histamine release by in vivo and/or in vitro analyses in all types of CIndUs. This should prompt further studies on the role of histamine receptors other than H1R, especially H4R in the pathogenesis of CIndUs.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
KK, MC, and MM contributed to conception and design of the study. KK, KM, and CR built the research strategy. MC, EG, TH, LK, KM, YN, CR, and DT-M performed data extraction. MC, EG, TH, LK, KM, YN, CR, and DT-M wrote the first draft of the manuscript. KK, MC, and MM reviewed the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.901851/full#supplementary-material
1. Maurer M, Fluhr JW, Khan DA. How to approach chronic inducible urticaria. J Allergy Clin Immunol Pract (2018) 6(4):1119–30. doi: 10.1016/j.jaip.2018.03.007
2. Church MK, Kolkhir P, Metz M, Maurer M. The role and relevance of mast cells in urticaria. Immunol Rev (2018) 282(1):232–47. doi: 10.1111/imr.12632
3. Kaplan AP, Horáková Z, Katz SI. Assessment of tissue fluid histamine levels in patients with urticaria. J Allergy Clin Immunol (1978) 61(6):350–4. doi: 10.1016/0091-6749(78)90113-6
4. Juhlin L. Cold urticaria with persistent weals. Br J Dermatol (1981) 104(6):705–7. doi: 10.1111/j.1365-2133.1981.tb00759.x
5. Keahey TM, Indrisano J, Lavker RM, Kaliner MA. Delayed vibratory angioedema: Insights into pathophysiologic mechanisms. J Allergy Clin Immunol (1987) 80(6):831–8. doi: 10.1016/s0091-6749(87)80273-7
6. Keahey TM, Lavker RM, Kaidbey KH, Atkins PC, Zweiman B. Studies on the mechanism of clinical tolerance in solar urticaria. Br J Dermatol (1984) 110(3):327–38. doi: 10.1111/j.1365-2133.1984.tb04639.x
7. Altrichter S, Peter H-J, Pisarevskaja D, Metz M, Martus P, Maurer M. Ige mediated autoallergy against thyroid peroxidase – a novel pathomechanism of chronic spontaneous urticaria? PloS One (2011) 6(4):e14794. doi: 10.1371/journal.pone.0014794
8. Gruber BL, Baeza ML, Marchese MJ, Agnello V, Kaplan AP. Prevalence and functional role of anti-ige autoantibodies in urticarial syndromes. J Invest Dermatol (1988) 90(2):213–7. doi: 10.1111/1523-1747.ep12462239
9. Kolkhir P, Church MK, Weller K, Metz M, Schmetzer O, Maurer M. Autoimmune chronic spontaneous urticaria: What we know and what we do not know. J Allergy Clin Immunol (2017) 139(6):1772–81.e1. doi: 10.1016/j.jaci.2016.08.050
10. Kring Tannert L, Stahl Skov P, Bjerremann Jensen L, Maurer M, Bindslev-Jensen C. Cold urticaria patients exhibit normal skin levels of functional mast cells and histamine after tolerance induction. Dermatology (2012) 224(2):101–5. doi: 10.1159/000336572
11. Kato T, Komatsu H, Tagami H. Exercise-induced urticaria and angioedema: Reports of two cases. J Dermatol (1997) 24(3):189–92. doi: 10.1111/j.1346-8138.1997.tb02770.x
12. Krause K, Spohr A, Zuberbier T, Church MK, Maurer M. Up-dosing with bilastine results in improved effectiveness in cold contact urticaria. Allergy (2013) 68(7):921–8. doi: 10.1111/all.12171
13. Kurtz AS, Kaplan AP. Regional expression of cold urticaria. J Allergy Clin Immunol (1990) 86(2):272–3. doi: 10.1016/s0091-6749(05)80076-4
14. McClean SP, Arreaza EE, Lett-Brown MA, Grant JA. Refractory cholinergic urticaria successfully treated with ketotifen. J Allergy Clin Immunol (1989) 83(4):738–41. doi: 10.1016/0091-6749(89)90008-0
15. Neittaanmäki H, Fräki JE. Combination of localized heat urticaria and cold urticaria. release of histamine in suction blisters and successful treatment of heat urticaria with doxepin. Clin Exp Dermatol (1988) 13(2):87–91. doi: 10.1111/j.1365-2230.1988.tb00665.x
16. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (Prisma-p) 2015 statement. Syst Rev (2015) 4(1):1. doi: 10.1186/2046-4053-4-1
17. Greaves MW, Sondergaard J. Urticaria pigmentosa and factitious urticaria. direct evidence for release of histamine and other smooth muscle-contracting agents in dermographic skin. Arch Dermatol (1970) 101(4):418–25. doi: 10.1001/archderm.101.4.418
18. Kaplan AP, Gray L, Shaff RE, Horakova Z, Beaven MA. In vivo studies of mediator release in cold urticaria and cholinergic urticaria. J Allergy Clin Immunol (1975) 55(6):394–402. doi: 10.1016/0091-6749(75)90078-0
19. de Croon EM, Sluiter JK, Nijssen TF, Dijkmans BA, Lankhorst GJ, Frings-Dresen MH. Predictive factors of work disability in rheumatoid arthritis: A systematic literature review. Ann Rheum Dis (2004) 63(11):1362–7. doi: 10.1136/ard.2003.020115
20. Rose B. Studies on blood histamine in cases of allergy: I. blood histamine during wheal formation. J Allergy (1941) 12(4):327–34. doi: 10.1016/S0021-8707(41)90260-5
21. Garafalo J, Kaplan AP. Histamine release and therapy of severe dermatographism. J Allergy Clin Immunol (1981) 68(2):103–5. doi: 10.1016/0091-6749(81)90166-4
22. Kulthanan K, Ungprasert P, Tuchinda P, Chularojanamontri L, Rujitharanawong C, Kiratiwongwan R, et al. Symptomatic dermographism: A systematic review of treatment options. J Allergy Clin Immunol Pract (2020) 8(9):3141–61. doi: 10.1016/j.jaip.2020.05.016
23. Rose B. Studies on blood histamine in patients with allergy. ii. alterations in the blood histamine in patients with allergic disease. J Clin Invest (1941) 20(4):419–28. doi: 10.1172/JCI101237
24. Duner H, Pernow B. Histamine in man under physiological and pathological conditions. Acta Med Scand (1960) 168:307–23. doi: 10.1111/j.0954-6820.1960.tb13452.x
25. Dunér H, Pernow B, Sterky G. The histamine concentration of the blood on exposure to cold and heat. Allergy (1960) 15(5):417–24. doi: 10.1111/j.1398-9995.1960.tb02869.x
26. Spuzic I, Ivkovic L. Histamine in plasma in the induced urticaria due to cold. Acta Allergol (1961) 16:228–31. doi: 10.1111/j.1398-9995.1961.tb02896.x
27. Soter NA, Wasserman SI, Austen KF. Cold urticaria: Release into the circulation of histamine and eosinophil chemotactic factor of anaphylaxis during cold challenge. N Engl J Med (1976) 294(13):687–90. doi: 10.1056/nejm197603252941302
28. Bentley-Phillips CB, Black AK, Greaves MW. Induced tolerance in cold urticaria caused by cold-evoked histamine release. Lancet (1976) 2(7976):63–6. doi: 10.1016/s0140-6736(76)92285-6
29. Sigler RW, Levinson AI, Evans R 3rd, Horakova Z, Kaplan AP. Evaluation of a patient with cold and cholinergic urticaria. J Allergy Clin Immunol (1979) 63(1):35–8. doi: 10.1016/0091-6749(79)90159-3
30. Black AK, Eady RA, Greaves MW, Keahey TM, Sibbald G. Treatment of acquired cold urticaria by prednisone: Dissociation of histamine release and clinical improvement [Proceedings]. Br J Clin Pharmacol (1980) 9(1):116p–7p. doi: 10.1111/j.1365-2125.1980.tb04818.x
31. Kaplan AP, Beaven MA. In vivo studies of the pathogenesis of cold urticaria, cholinergic urticaria, and vibration-induced swelling. J Invest Dermatol (1976) 67(3):327–32. doi: 10.1111/1523-1747.ep12514352
32. Andersson T, Wårdell K, Anderson C. Human in vivo cutaneous microdialysis: Estimation of histamine release in cold urticaria. Acta Derm Venereol (1995) 75(5):343–7. doi: 10.2340/0001555575343347
33. Nuutinen P, Harvima IT, Ackermann L. Histamine, but not leukotriene C4, is an essential mediator in cold urticaria wheals. Acta Derm Venereol (2007) 87(1):9–13. doi: 10.2340/00015555-0185
34. Juhlin L, Shelley WB. Role of mast cell and basophil in cold urticaria with associated systemic reactions. Jama (1961) 177:371–7. doi: 10.1001/jama.1961.73040320001004
35. Kaplan AP, Garofalo J, Sigler R, Hauber T. Idiopathic cold urticaria: In vitro demonstration of histamine release upon challenge of skin biopsies. N Engl J Med (1981) 305(18):1074–7. doi: 10.1056/nejm198110293051808
36. Sigler RW, Evans R 3rd, Horakova Z, Ottesen E, Kaplan AP. The role of cyproheptadine in the treatment of cold urticaria. J Allergy Clin Immunol (1980) 65(4):309–12. doi: 10.1016/0091-6749(80)90161-x
37. Dressler C, Werner RN, Eisert L, Zuberbier T, Nast A, Maurer M. Chronic inducible urticaria: A systematic review of treatment options. J Allergy Clin Immunol (2018) 141(5):1726–34. doi: 10.1016/j.jaci.2018.01.031
38. Czarnetzki BM, Meentken J, Rosenbach T, Pokropp A. Clinical, pharmacological and immunological aspects of delayed pressure urticaria. Br J Dermatol (1984) 111(3):315–23. doi: 10.1111/j.1365-2133.1984.tb04729.x
39. Mijailović BB, Karadaglić DM, Ninković MP, Mladenović TM, Zecević RD, Pavlović MD. Bullous delayed pressure urticaria; pressure testing may produce a systemic reaction. Br J Dermatol (1997) 136(3):434–6. doi: 10.1111/j.1365-2133.1997.tb14962.x
40. Kulthanan K, Ungprasert P, Tuchinda P, Chularojanamontri L, Charoenpipatsin N, Maurer M. Delayed pressure urticaria: A systematic review of treatment options. J Allergy Clin Immunol Pract (2020) 8(6):2035–49.e5. doi: 10.1016/j.jaip.2020.03.004
41. Soter NA, Wasserman SI, Pathak MA, Parrish JA, Austen KF. Solar urticaria: Release of mast cell mediators into the circulation after experimental challenge. J Invest Dermatol (1979) 72(5):282.
42. Hawk JL, Eady RA, Challoner AV, Kobza-Black A, Keahey TM, Greaves MW. Elevated blood histamine levels and mast cell degranulation in solar urticaria. Br J Clin Pharmacol (1980) 9(2):183–6. doi: 10.1111/j.1365-2125.1980.tb05831.x
43. Watanabe M, Matsunaga Y, Katayama I. Solar urticaria: A consideration of the mechanism of inhibition spectra. Dermatology (1999) 198(3):252–5. doi: 10.1159/000018124
44. Baart de la Faille H, Rottier PB, Baart de la Faille-Kuyper EH. Solar urticaria. a case with possible increase of skin mast cells. Br J Dermatol (1975) 92(1):101–7. doi: 10.1159/000144432
45. Haylett AK, Koumaki D, Rhodes LE. Solar urticaria in 145 patients: Assessment of action spectra and impact on quality of life in adults and children. Photodermatol Photoimmunol Photomed (2018) 34(4):262–8. doi: 10.1111/phpp.12385
46. Beattie PE, Dawe RS, Ibbotson SH, Ferguson J. Characteristics and prognosis of idiopathic solar urticaria: A cohort of 87 cases. Arch Dermatol (2003) 139(9):1149–54 doi: 10.1001/archderm.139.9.1149
47. Du-Thanh A, Debu A, Lalheve P, Guillot B, Dereure O, Peyron JL. Solar urticaria: A time-extended retrospective series of 61 patients and review of literature. Eur J Dermatol (2013) 23(2):202–7. doi: 10.1684/ejd.2013.1933
48. Farnam J, Grant JA, Lett-Brown MA, Lord RA, Russell WL, Henry DP. Combined cold- and heat-induced cholinergic urticaria. J Allergy Clin Immunol (1986) 78(2):353–7. doi: 10.1016/s0091-6749(86)80089-6
49. Irwin RB, Lieberman P, Friedman MM, Kaliner M, Kaplan R, Bale G, et al. Mediator release in local heat urticaria: Protection with combined H1 and H2 antagonists. J Allergy Clin Immunol (1985) 76(1):35–9. doi: 10.1016/0091-6749(85)90801-2
50. Baba T, Nomura K, Hanada K, Hashimoto I. Immediate-type heat urticaria: Report of a case and study of plasma histamine release. Br J Dermatol (1998) 138(2):326–8. doi: 10.1046/j.1365-2133.1998.02084.x
51. Atkins PC, Zweiman B. Mediator release in local heat urticaria. J Allergy Clin Immunol (1981) 68(4):286–9. doi: 10.1016/0091-6749(81)90153-6
52. Grant JA, Findlay SR, Thueson DO, Fine DP, Krueger GG. Local heat Urticaria/Angioedema: Evidence for histamine release without complement activation. J Allergy Clin Immunol (1981) 67(1):75–7. doi: 10.1016/0091-6749(81)90049-x
53. Koro O, Dover JS, Francis DM, Kobza Black A, Kelly RW, Barr RM, et al. Release of prostaglandin D2 and histamine in a case of localized heat urticaria, and effect of treatments. Br J Dermatol (1986) 115(6):721–8. doi: 10.1111/j.1365-2133.1986.tb06654.x
54. Higgins EM, Friedmann PS. Clinical report and investigation of a patient with localized heat urticaria. Acta Derm Venereol (1991) 71(5):434–6.
55. Skrebova N, Takiwaki H, Miyaoka Y, Arase S. Localized heat urticaria: A clinical study using laser Doppler flowmetry. J Dermatol Sci (2001) 26(2):112–8. doi: 10.1016/s0923-1811(00)00162-6
56. Fukunaga A, Shimoura S, Fukunaga M, Ueda M, Nagai H, Bito T, et al. Localized heat urticaria in a patient is associated with a wealing response to heated autologous serum. Br J Dermatol (2002) 147(5):994–7. doi: 10.1046/j.1365-2133.2002.04952.x
57. Martín-Muñoz MF, Muñoz-Robles ML, González P, Martín-Esteban M. Immediate heat urticaria in a child. Br J Dermatol (2002) 147(4):813–5. doi: 10.1046/j.1365-2133.2002.49055.x
58. Koh YI, Choi IS, Lee SH, Lee JB, Park CH, Hong SN. Localized heat urticaria associated with mast cell and eosinophil degranulation. J Allergy Clin Immunol (2002) 109(4):714–5. doi: 10.1067/mai.2002.122462
59. Pezzolo E, Peroni A, Gisondi P, Girolomoni G. Heat urticaria: A revision of published cases with an update on classification and management. Br J Dermatol (2016) 175(3):473–8. doi: 10.1111/bjd.14543
60. Metzger WJ, Kaplan AP, Beaven MA, Irons JS, Patterson R. Hereditary vibratory angioedema: Confirmation of histamine release in a type of physical hypersensitivity. J Allergy Clin Immunol (1976) 57(6):605–8. doi: 10.1016/0091-6749(76)90012-9
61. Boyden SE, Desai A, Cruse G, Young ML, Bolan HC, Scott LM, et al. Vibratory urticaria associated with a missense variant in Adgre2. N Engl J Med (2016) 374(7):656–63. doi: 10.1056/NEJMoa1500611
62. Ting S, Reimann BE, Rauls DO, Mansfield LE. Nonfamilial, vibration-induced angioedema. J Allergy Clin Immunol (1983) 71(6):546–51. doi: 10.1016/0091-6749(83)90435-9
63. Wener MH, Metzger WJ, Simon RA. Occupationally acquired vibratory angioedema with secondary carpal tunnel syndrome. Ann Intern Med (1983) 98(1):44–6. doi: 10.7326/0003-4819-98-1-44
64. Zhao Z, Reimann S, Wang S, Wang Y, Zuberbier T. Ordinary vibratory angioedema is not generally associated with Adgre2 mutation. J Allergy Clin Immunol (2019) 143(3):1246–8.e4. doi: 10.1016/j.jaci.2018.10.049
65. Kulthanan K, Ungprasert P, Tapechum S, Rujitharanawong C, Kiratiwongwan R, Munprom K, et al. Vibratory angioedema subgroups, features, and treatment: Results of a systematic review. J Allergy Clin Immunol Pract (2021) 9(2):971–84. doi: 10.1016/j.jaip.2020.09.009
66. Soter NA, Wasserman SI, Austen KF, McFadden ER Jr. Release of mast-cell mediators and alterations in lung function in patients with cholinergic urticaria.N Engl J Med (1980) 302(11):604–8. doi: 10.1056/nejm198003133021104
67. Davis RS, Remigio LK, Schocket AL, Bock SA. Evaluation of a patient with both aquagenic and cholinergic urticaria. J Allergy Clin Immunol (1981) 68(6):479–83. doi: 10.1016/0091-6749(81)90202-5
68. Kaplan AP, Natbony SF, Tawil AP, Fruchter L, Foster M. Exercise-induced anaphylaxis as a manifestation of cholinergic urticaria. J Allergy Clin Immunol (1981) 68(4):319–24. doi: 10.1016/0091-6749(81)90158-5
69. Lawrence CM, Jorizzo JL, Kobza-Black A, Coutts A, Greaves MW. Cholinergic urticaria with associated angio-oedema. Br J Dermatol (1981) 105(5):543–50. doi: 10.1111/j.1365-2133.1981.tb00797.x
70. Lewis J, Lieberman P, Treadwell G, Erffmeyer J. Exercise-induced urticaria, angioedema, and anaphylactoid episodes. J Allergy Clin Immunol (1981) 68(6):432–7. doi: 10.1016/0091-6749(81)90197-4
71. Fukunaga A, Bito T, Tsuru K, Oohashi A, Yu X, Ichihashi M, et al. Responsiveness to autologous sweat and serum in cholinergic urticaria classifies its clinical subtypes. J Allergy Clin Immunol (2005) 116(2):397–402. doi: 10.1016/j.jaci.2005.05.024
72. Takahagi S, Tanaka T, Ishii K, Suzuki H, Kameyoshi Y, Shindo H, et al. Sweat antigen induces histamine release from basophils of patients with cholinergic urticaria associated with atopic diathesis. Br J Dermatol (2009) 160(2):426–8. doi: 10.1111/j.1365-2133.2008.08862.x
73. Washio K, Fukunaga A, Onodera M, Hatakeyama M, Taguchi K, Ogura K, et al. Clinical characteristics in cholinergic urticaria with palpebral angioedema: Report of 15 cases. J Dermatol Sci (2017) 85(2):135–7. doi: 10.1016/j.jdermsci.2016.11.001
74. Kim JE, Jung KH, Cho HH, Kang H, Park YM, Park HJ, et al. The significance of hypersensitivity to autologous sweat and serum in cholinergic urticaria: Cholinergic urticaria may have different subtypes. Int J Dermatol (2015) 54(7):771–7. doi: 10.1111/ijd.12549
75. Iijima S, Kojo K, Takayama N, Hiragun M, Kan T, Hide M. Case of cholinergic urticaria accompanied by anaphylaxis. J Dermatol (2017) 44(11):1291–4. doi: 10.1111/1346-8138.13951
76. Kozaru T, Fukunaga A, Taguchi K, Ogura K, Nagano T, Oka M, et al. Rapid desensitization with autologous sweat in cholinergic urticaria. Allergol Int (2011) 60(3):277–81. doi: 10.2332/allergolint.10-OA-0269
77. Fukunaga A, Washio K, Hatakeyama M, Oda Y, Ogura K, Horikawa T, et al. Cholinergic urticaria: Epidemiology, physiopathology, new categorization, and management. Clin Auton Res (2018) 28(1):103–13. doi: 10.1007/s10286-017-0418-6
78. Carrillo T, Cuevas M, Muñoz T, Hinojosa M, Moneo I. Contact urticaria and rhinitis from latex surgical gloves. Contact Dermatitis (1986) 15(2):69–72. doi: 10.1111/j.1600-0536.1986.tb01279.x
79. Turjanmaa K, Räsänen L, Lehto M, Mäkinen-Kiljunen S, Reunala T. Basophil histamine release and lymphocyte proliferation tests in latex contact urticaria. In vitro tests in latex contact urticaria. Allergy (1989) 44(3):181–6. doi: 10.1111/j.1398-9995.1989.tb02259.x
80. Fernández de Corres L, Moneo I, Muñoz D, Bernaola G, Fernández E, Audicana M, et al. Sensitization from chestnuts and bananas in patients with urticaria and anaphylaxis from contact with latex. Ann Allergy (1993) 70(1):35–9.
81. Quirce S, García-Figueroa B, Olaguíbel JM, Muro MD, Tabar AI. Occupational asthma and contact urticaria from dried flowers of limonium tataricum. Allergy (1993) 48(4):285–90. doi: 10.1111/j.1398-9995.1993.tb00730.x
82. Lezaun A, Igea JM, Quirce S, Cuevas M, Parra F, Alonso MD, et al. Asthma and contact urticaria caused by rice in a housewife. Allergy (1994) 49(2):92–5. doi: 10.1111/j.1398-9995.1994.tb00806.x
83. Yamakawa Y, Ohsuna H, Aihara M, Tsubaki K, Ikezawa Z. Contact urticaria from rice. Contact Dermatitis (2001) 44(2):91–3. doi: 10.1034/j.1600-0536.2001.440207.x
84. Adachi A, Fukunaga A, Hayashi K, Kunisada M, Horikawa T. Anaphylaxis to polyvinylpyrrolidone after vaginal application of povidone-iodine. Contact Dermatitis (2003) 48(3):133–6. doi: 10.1034/j.1600-0536.2003.00050.x
85. Nishioka K, Doi T, Katayama I. Histamine release in contact urticaria. Contact Dermatitis (1984) 11(3):191. doi: 10.1111/j.1600-0536.1984.tb00975.x
86. Taskila K, Saarinen JV, Harvima IT, Harvima RJ. Histamine and Ltc4 in stinging nettle-induced urticaria. Allergy (2000) 55(7):680–1. doi: 10.1034/j.1398-9995.2000.00635.x
87. Gimenez-Arnau AM, Maibach H. Contact urticaria. Immunol Allergy Clin North Am (2021) 41(3):467–80. doi: 10.1016/j.iac.2021.04.007
88. Sibbald RG, Black AK, Eady RA, James M, Greaves MW. Aquagenic urticaria: Evidence of cholinergic and histaminergic basis. Br J Dermatol (1981) 105(3):297–302. doi: 10.1111/j.1365-2133.1981.tb01289.x
89. Gimenez-Arnau A, Serra-Baldrich E, Camarasa JG. Chronic aquagenic urticaria. Acta Derm Venereol (1992) 72(5):389. doi: 10.1080/00015557238
90. Czarnetzki BM, Breetholt KH, Traupe H. Evidence that water acts as a carrier for an epidermal antigen in aquagenic urticaria. J Am Acad Dermatol (1986) 15(4 Pt 1):623–7. doi: 10.1016/s0190-9622(86)70215-6
91. Magerl M, Altrichter S, Borzova E, Giménez-Arnau A, Grattan CE, Lawlor F, et al. The definition, diagnostic testing, and management of chronic inducible urticarias - the Eaaci/Ga(2) Len/Edf/Unev consensus recommendations 2016 update and revision. Allergy (2016) 71(6):780–802. doi: 10.1111/all.12884
92. Fearfield LA, Gazzard B, Bunker CB. Aquagenic urticaria and human immunodeficiency virus infection: Treatment with stanozolol. Br J Dermatol (1997) 137(4):620–2. doi: 10.1111/j.1365-2133.1997.tb03798.x
93. Rorie A, Gierer S. A case of aquagenic urticaria successfully treated with omalizumab. J Allergy Clin Immunol Pract (2016) 4(3):547–8. doi: 10.1016/j.jaip.2015.12.017
94. Gallo R, Gonçalo M, Cinotti E, Cecchi F, Parodi A. Localized salt-dependent aquagenic urticaria: A subtype of aquagenic urticaria? Clin Exp Dermatol (2013) 38(7):754–7. doi: 10.1111/ced.12147
95. Kılıç M, Taşkın E, Tan M, Gözütok A. Association of cold urticaria with aquagenic urticaria in an adolescent girl: A case report. Asthma Allergy Immunol (2020) 18(2):105–9. doi: 10.21911/aai.529
96. Gaddum JH. The technique of superfusion. Br J Pharmacol Chemother (1953) 8(3):321–6. doi: 10.1111/j.1476-5381.1953.tb00801.x
97. Vane JR. The use of isolated organs for detecting active substances in the circulating blood. Br J Pharmacol Chemother (1964) 23(2):360–73. doi: 10.1111/j.1476-5381.1964.tb01592.x
98. Beaven MA, Jacobsen S, Horáková Z. Modification of the enzymatic isotopic assay of histamine and its application to measurement of histamine in tissues, serum and urine. Clin Chim Acta (1972) 37:91–103. doi: 10.1016/0009-8981(72)90419-6
99. Verburg KM, Bowsher RR, Henry DP. A new radioenzymatic assay for histamine using purified histamine n-methyltransferase. Life Sci (1983) 32(25):2855–67. doi: 10.1016/0024-3205(83)90322-3
100. Guilloux L, Hartmann D, Ville G. Enzymatic isotopic assay for human plasma histamine. Clin Chim Acta (1981) 116(3):269–75. doi: 10.1016/0009-8981(81)90046-2
101. Meyer J, Gorbach AM, Liu WM, Medic N, Young M, Nelson C, et al. Mast cell dependent vascular changes associated with an acute response to cold immersion in primary contact urticaria. PloS One (2013) 8(2):e56773. doi: 10.1371/journal.pone.0056773
102. Baumann KY, Church MK, Clough GF, Quist SR, Schmelz M, Skov PS, et al. Skin microdialysis: Methods, applications and future opportunities–an eaaci position paper. Clin Trans Allergy (2019) 9(1):24. doi: 10.1186/s13601-019-0262-y
103. May CD, Lyman M, Alberto R, Cheng J. Procedures for immunochemical study of histamine release from leukocytes with small volume of blood. J Allergy (1970) 46(1):12–20. doi: 10.1016/0021-8707(70)90056-0
104. Siraganian RP. An automated continuous-flow system for the extraction and fluorometric analysis of histamine. Anal Biochem (1974) 57(2):383–94. doi: 10.1016/0003-2697(74)90093-1
105. Oehling A, Ona J, Trento H, Sanz ML, Domínguez MA. The diagnostic value of the histamine release test in food allergy. Allergol Immunopathol (Madr) (1984) 12(6):439–48.
106. Beaven MA. Radiochemical assay procedures for drugs and transmitters. In: Iversen LL, Snyder SH, editors. Handbook of psychopharmacology, vol. 1 . New York: Plenum Press (1975). p. 253–90. Biochemical Principles and Techniques in Neuropharmacology.
107. Nishi H, Nishimura S, Higashiura M, Ikeya N, Ohta H, Tsuji T, et al. A new method for histamine release from purified peripheral blood basophils using monoclonal antibody-coated magnetic beads. J Immunol Methods (2000) 240(1-2):39–46. doi: 10.1016/s0022-1759(00)00169-1
108. Tillie-Leblond I, Gosset P, Janin A, Dalenne R, Joseph M, Wallaert B, et al. Tumor necrosis factor-alpha release during systemic reaction in cold urticaria. J Allergy Clin Immunol (1994) 93(2):501–9. doi: 10.1016/0091-6749(94)90360-3
109. Hermes B, Prochazka AK, Haas N, Jurgovsky K, Sticherling M, Henz BM. Upregulation of tnf-alpha and il-3 expression in lesional and uninvolved skin in different types of urticaria. J Allergy Clin Immunol (1999) 103(2 Pt 1):307–14. doi: 10.1016/s0091-6749(99)70506-3
110. Ormerod AD, Kobza Black A, Dawes J, Murdoch RD, Koro O, Barr RM, et al. Prostaglandin D2 and histamine release in cold urticaria unaccompanied by evidence of platelet activation. J Allergy Clin Immunol (1988) 82(4):586–9. doi: 10.1016/0091-6749(88)90968-2
111. Heavey DJ, Kobza-Black A, Barrow SE, Chappell CG, Greaves MW, Dollery CT. Prostaglandin D2 and histamine release in cold urticaria. J Allergy Clin Immunol (1986) 78(3 Pt 1):458–61. doi: 10.1016/0091-6749(86)90033-3
112. Grandel KE, Farr RS, Wanderer AA, Eisenstadt TC, Wasserman SI. Association of platelet-activating factor with primary acquired cold urticaria. N Engl J Med (1985) 313(7):405–9. doi: 10.1056/nejm198508153130702
113. Maltby NH, Ind PW, Causon RC, Fuller RW, Taylor GW. Leukotriene E4 release in cold urticaria. Clin Exp Allergy (1989) 19(1):33–6. doi: 10.1111/j.1365-2222.1989.tb02340.x
114. Kaplan AP. Exercise-induced hives. J Allergy Clin Immunol (1984) 73(5, Part 2):704–7. doi: 10.1016/0091-6749(84)90310-5
115. Lawlor F, Bird C, Camp RD, Barlow R, Barr RM, Kobza-Black A, et al. Increased interleukin 6, but reduced interleukin 1, in delayed pressure urticaria. Br J Dermatol (1993) 128(5):500–3. doi: 10.1111/j.1365-2133.1993.tb00225.x
116. Mellerowicz E, Weller K, Zuberbier T, Maurer M, Altrichter S. Real-life treatment of patients with cholinergic urticaria in German-speaking countries. J Dtsch Dermatol Ges (2019) 17(11):1141–7. doi: 10.1111/ddg.13979
Keywords: antihistamines, chronic inducible urticaria, histamine, mast cell, wheal
Citation: Kulthanan K, Church MK, Grekowitz EM, Hawro T, Kiefer LA, Munprom K, Nanchaipruek Y, Rujitharanawong C, Terhorst-Molawi D and Maurer M (2022) Evidence for histamine release in chronic inducible urticaria – A systematic review. Front. Immunol. 13:901851. doi: 10.3389/fimmu.2022.901851
Received: 22 March 2022; Accepted: 04 July 2022;
Published: 28 July 2022.
Edited by:
Satoshi Tanaka, Kyoto Pharmaceutical University, JapanReviewed by:
Chiara Cardamone, University of Salerno, ItalyCopyright © 2022 Kulthanan, Church, Grekowitz, Hawro, Kiefer, Munprom, Nanchaipruek, Rujitharanawong, Terhorst-Molawi and Maurer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marcus Maurer, bWFyY3VzLm1hdXJlckBjaGFyaXRlLmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.