- 1Beijing Key Laboratory of Hematopoietic Stem Cell Transplantation, National Clinical Research Center for Hematologic Disease, Collaborative Innovation Center of Hematology, Peking University Institute of Hematology, Peking University People’s Hospital, Beijing, China
- 2Peking-Tsinghua Centre for Life Sciences, Beijing, China
Secondary poor graft function (sPGF) increases the risk of life-threatening complications after hematopoietic stem cell transplantation (HSCT). The incidence, clinical outcomes, and risk factors of sPGF have not been elucidated in haploidentical (haplo-) HSCT for acquired aplastic anemia (AA) patients. We retrospectively reviewed 423 consecutive AA patients who underwent haplo-HSCT between January 2006 and December 2020 and report a 3-year cumulative incidence of 4.62% (95% confidence interval [CI]: 3.92%-10.23%) of sPGF. While no primary PGF occurred. The median time to sPGF was 121 days (range 30-626 days) after transplantation. To clarify the risk factors for sPGF, 17 sPGF cases and 382 without PGF were further analyzed. Compared to patients without PGF, the 2-year overall survival was significantly poorer for sPGF patients (67.7% vs 90.8%, p =.002). Twelve sPGF patients were alive until the last follow-up, and 7 achieved transfusion independency. The multivariable analyses revealed that later neutrophil engraftment (OR 2.819, p=.049) and a history of refractory cytomegalovirus viremia (OR=7.038, p=.002) post-transplantation were associated with sPGF. There was weak evidence that a history of grade 3-4 acute graft-versus-host disease increased the risk of sPGF (p=.063). We advocated better post-transplantation strategies to balance the risk of immunosuppression and viral reactivation for haplo-HSCT in AA patients.
1. Introduction
Survival after hematopoietic stem cell transplantation (HSCT) in acquired aplastic anemia (AA) has been remarkedly improved over the last three decades. Haploidentical (haplo-) HSCT provides easily available donors for patients with AA and guarantees a favorable engraftment rate (1–3). Stable hematopoietic recovery is the key point of successful HSCT for AA. However, hematologists have noticed that even if the patients achieved initial hematopoietic reconstitution and maintain complete donor-originated hematopoietic cells, they may develop intractable multilineage cytopenia afterwards (4, 5). This is defined as secondary poor graft function (sPGF), and it occurs in 5-27% of post-transplantation cases (6). Patients with sPGF lose their initial hematopoietic reconstitution, which leads to increased risks of severe infection, major bleeding events, and other life-threatening complications after transplantation. The therapeutic options for sPGF are limited, and the prognosis remains poor.
Studies have reported several risk factors for sPGF. Cytomegalovirus (CMV) reactivation and graft-versus-host disease (GvHD) are the two most recognized risk factors (6). Other potential risk factors include haplo-HSCT setting, recipient age, conditioning regimen, Epstein-Barr virus (EBV) infection, etc. (7–9) However, these conclusions were limited by heterogeneity in disease categories and transplantation settings. The clinical outcomes and risk factors of sPGF have not been elucidated in haplo-HSCT for AA patients. Based on the largest-scale AA cases receiving haplo-HSCT, we herein retrospectively analyzed the incidence, outcomes, and risk factors of sPGF.
2. Method
2.1 Study Population
In this study, we reviewed 423 consecutive AA patients who underwent haplo-HSCT as first HSCT between January 2006 and December 2020 at Peking University People’s Hospital (PKUPH). Written informed consent was obtained from each patient before transplantation. The study protocol followed the Declaration of Helsinki and was approved by the Ethics Review Committee of PKUPH. The cumulative incidence of PGF was estimated based on the whole cohort. Then, patients who developed graft failure after haplo-HSCT (primary graft failure n=2, and secondary graft failure n=3; 1.18% in total) or died of any cause within 28 days post-transplantation (n=19, 4.49%) were excluded from further analysis.
2.2 Transplantation Protocol
All patients received mixed graft infusion of granulocyte colony-stimulating factor (G-CSF) mobilized bone marrow (BM) and peripheral blood (PB) stem cells except for three cases (0.67%) in which only PB grafts were infused. The conditioning regimen for acquired AA patients included: (1) BuCy-ATG conditioning including busulfan (Bu, 3.2 mg/kg daily on days -8 and -7), cyclophosphamide (Cy, 50 mg/kg daily on days -5 to -2), and rabbit antithymocyte globulin (rATG, 2.5 mg/kg daily on days -5 to -2, from SangStat, France); and (2) the BuCylowFlu-ATG regimen consisting of Bu (0.8 mg/kg 4 times daily on days -8 and -7), Cy (25 mg/kg daily on days -5 to -2), Flu (30 mg/m2 daily on days -6 to -2), and rATG (2.5 mg/kg daily on days -5 to -2) (10, 11). The prophylaxis of GvHD was described elsewhere (10).
All patients received ganciclovir (GCV)-based preemptive therapy when CMV viremia was diagnosed. Foscarnet and immunoglobulin were administered if patients were intolerant to GCV or had an increase in CMV DNA copy after receiving full dose of GCV for 1 week. Refractory infections were treated with CMV-specific T cells at the discretion of physician. Once EBV viremia developed, a reduction in the dose of immunosuppressants would be taken for patients without or less than grade II aGvHD. Rituximab was applied to progressive EBV infection based on physician’s decision and EBV-associated post-transplant lymphoproliferative disease. For refractory CMV and EBV co-reactivation, CMV/EBV-specific T cells would be prepared and infused (12, 13).
2.3 Protocol for DSA Detection and Desensitization
The anti-human leukocyte antigen (HLA) antibody was routinely examined pre-transplantation. Detection of donor-specific antibody (DSA) was performed according to an established protocol. DSA-positive patients (2000 ≤ mean fluorescence intensity [MFI] < 10000) were given rituximab 3 days before graft infusion. If available, DSA-negative umbilical cord blood was also infused prior to infusion of allogeneic grafts (14, 15). No patients in this study had a DSA MFI of ≥ 10000.
2.4 Evaluation and Definitions
Poor graft function (PGF) was defined as sustained cytopenia of 2 or 3 lineages (neutrophil count <.5 × 109/L, hemoglobin < 70 g/L, and platelet count < 20 × 109/L) for over 2 weeks with full donor chimerism of > 95%, hypoplastic-aplastic BM, and absence of severe GvHD, active infection and drug toxicity. Primary PGF referred to PGF that failed to achieve initial engraftment, and sPGF was defined as a decrease of blood counts after prompt recovery (16, 17). Chimerism analysis was evaluated using PB at 1, 2, 3, 6, 12 months post-transplantation and at annual outpatient visits thereafter. The analysis of chimerism was also performed every time when the blood counts obviously fluctuated. Immune reconstitution within 30, 60, and 90 days after transplantation, including CD3+, CD4+, and CD19+ cells, was documented.
Neutrophil engraftment was defined as the first of 3 consecutive days when the absolute neutrophil count reached the level of >.5 × 109/L without G-CSF stimulation. Platelet engraftment was defined as the first of 7 consecutive days when the platelet count was > 20 × 109/L, independent of platelet infusion. Both acute GvHD (aGvHD) and chronic GvHD (cGvHD) were diagnosed and graded based on published criteria (18, 19). CMV and EBV DNAemia ≥ 1 × 103 genome copies/mL were considered positive using real-time quantitative PCR (12, 13). Refractory CMV reactivation was defined as growing CMV-DNA copies or viral load of the same level after at least 2 weeks of appropriately dosed antiviral therapy. Recurrent CMV reactivation was diagnosed when a patient who had previous evidence of CMV viremia and had no virus detected for at least 4 weeks during active surveillance developed a new CMV viremia (20, 21). Overall survival (OS) was defined as the time from the date of haplo-HSCT to death or the last follow-up.
2.5 Statistical Analysis
The last follow-up for all survivors was April 1st, 2021. All clinical data were analyzed using R software (version 3.6.3, https://www.r-project.org) and Prism 8 (GraphPad Software, La Jolla, CA). The continuous variables were summarized as median (range) for nonnormally distributed data and compared using the Mann-Whitney test, and the categorical variables were expressed as count and percentage and compared using the chi-square test or Fisher’s exact test. The cumulative incidence rate (CIR) of sPGF or engraftment was estimated with death as a competing event and performed using the “cmprsk” package. Virus reactivation and aGvHD that developed before sPGF were calculated. The CIR of GvHD and virus reactivation were also estimated competing with events including death and PGF. In addition, the Kaplan-Meier method was used to estimate survival curves. Univariable analysis was performed based on logistic regression models, and potential risk factors (p <.10) were further analyzed in multivariable analysis. The infused doses of CD34+ cells (stratified by median) and conditioning regimen (Flu-based vs noFlu) were included with interest in multivariable analysis regardless of their p-values. Before multivariable analysis, we examined the correlation and multicollinearity among potential risk factors. A multivariable logistic regression was used to determine the independent effect of the included factors. All statistical tests were 2-sided, and a p-value <.05 was considered statistically significant.
3. Result
3.1 Incidence and Characteristics of sPGF
In the whole cohort, no primary PGF and mixed chimerism were observed in our study. The 3-year CIR of sPGF was 4.62% (95% CI: 3.92%-10.23%). All patients with sPGF had neutropenia of <.5 × 109/L and thrombocytopenia of < 20 × 109/L with or without red blood cell transfusion dependence.
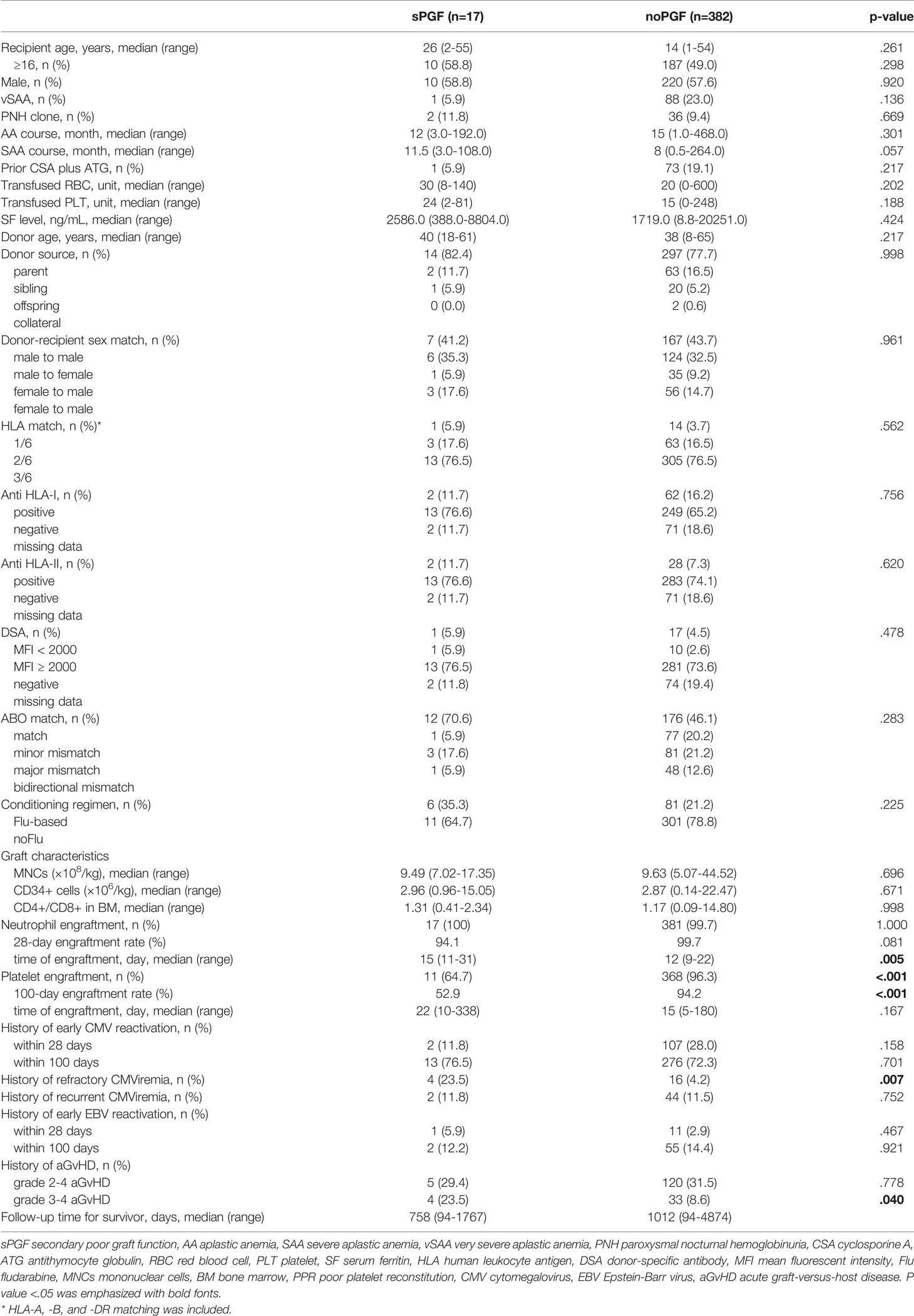
The characteristics of sPGF (n=17) and noPGF (n=382) patients are summarized in Table 1. The median time of sPGF was 120 (range 30-626) days post-transplantation. Compared to patients without PGF, patients with sPGF had marginally longer interval from disease onset to haplo-HSCT (p=.057). Except for the above parameters, patients with sPGF and those without PGF had equivalent recipient, donor, and graft characteristics.
3.2 Transplantation Outcomes and Complications Before sPGF
Except for one patient who died of thrombotic microangiopathy at day +34, all patients included in this study were confirmed to achieve neutrophil engraftment. The CIR of 28-day neutrophil engraftment for sPGF patients was marginally lower than that for patients without PGF (94.12% [95% CI: 88.82%-96.95%] vs 99.74% [95% CI: 99.64%-99.81%], p=.081, Figure 1A). The median time of neutrophil engraftment was delayed in the sPGF group (15 [range 11-31] vs 12 [range 9-22] days, p=.005, Table 1). Additionally, the CIR of 100-day platelet engraftment was lower in patients who subsequently developed sPGF (52.94% [95% CI: 28.89%-72.19%] vs 94.92% [95% CI: 94.11%-95.62%], p<.001, Figure 1B).
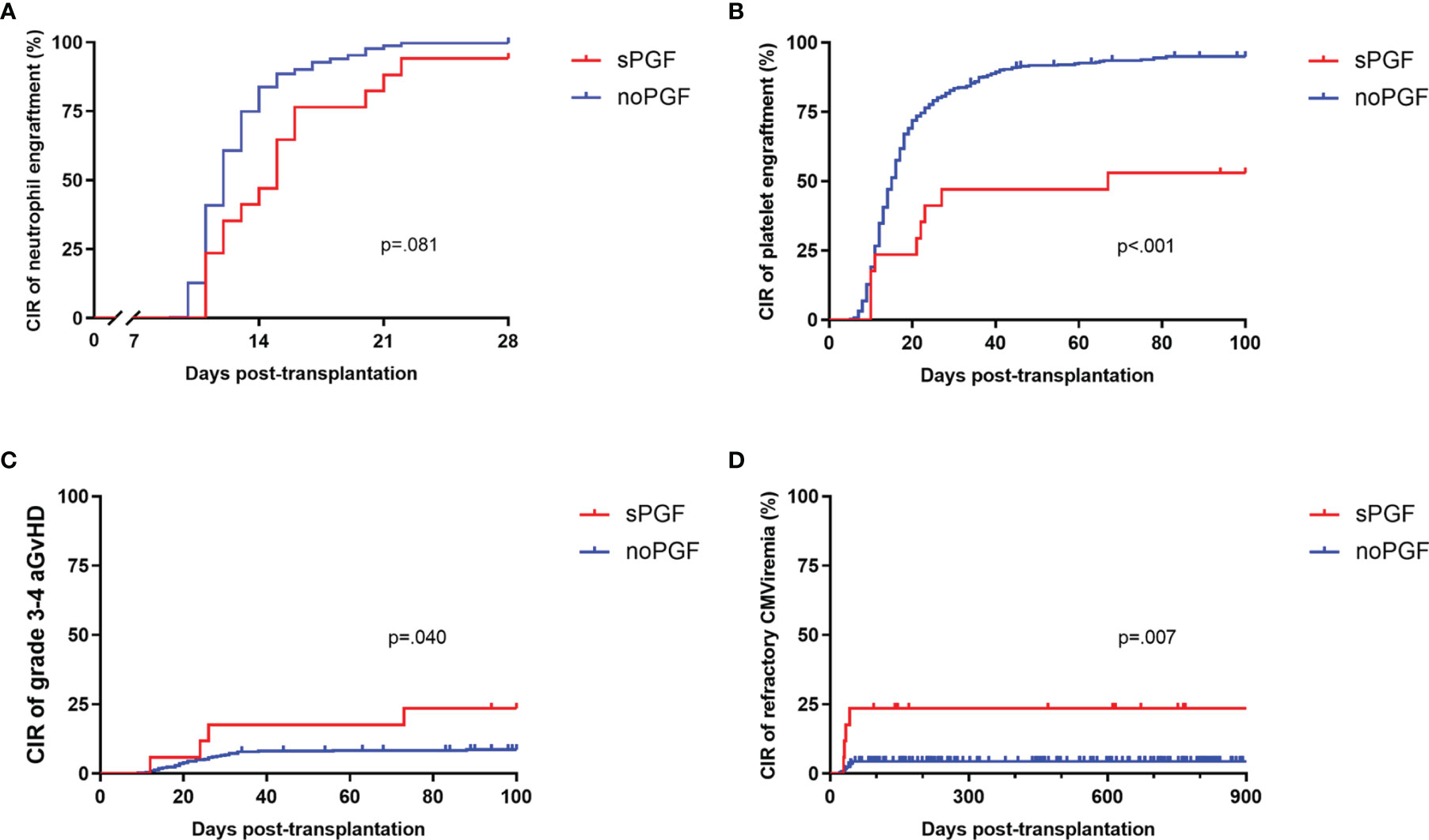
Figure 1 The CIR of transplantation outcomes and complications in sPGF patients and noPGF patients: (A) 28-day neutrophil engraftment, (B) 100-day platelet engraftment, (C) grade 3-4 aGvHD, (D) refractory CMV viremia. Patients were censored when they were diagnosed with sPGF.
Prior grade 3-4 aGvHD was also more likely to be observed among sPGF patients (p=.040, Figure 1C). The incidences of early CMV and EBV reactivation, either within 28 days (p=.158 and.467, respectively) or within 100 days (p=.701 and.921, respectively), were similar between groups. More patients with sPGF had a history of refractory CMV viremia (p=.007, Figure 1D).
T cell reconstitution before sPGF was comparable at day 30 and day 60. However, CD19+ B cell reconstitution was delayed in patients who developed sPGF later (p=.016, at day 30). At day 90, patients in the sPGF group had a trend toward poorer T cell reconstitution, although the data available was limited. In addition, lower levels of lymphocytes were observed at day 90. (Figure 2)
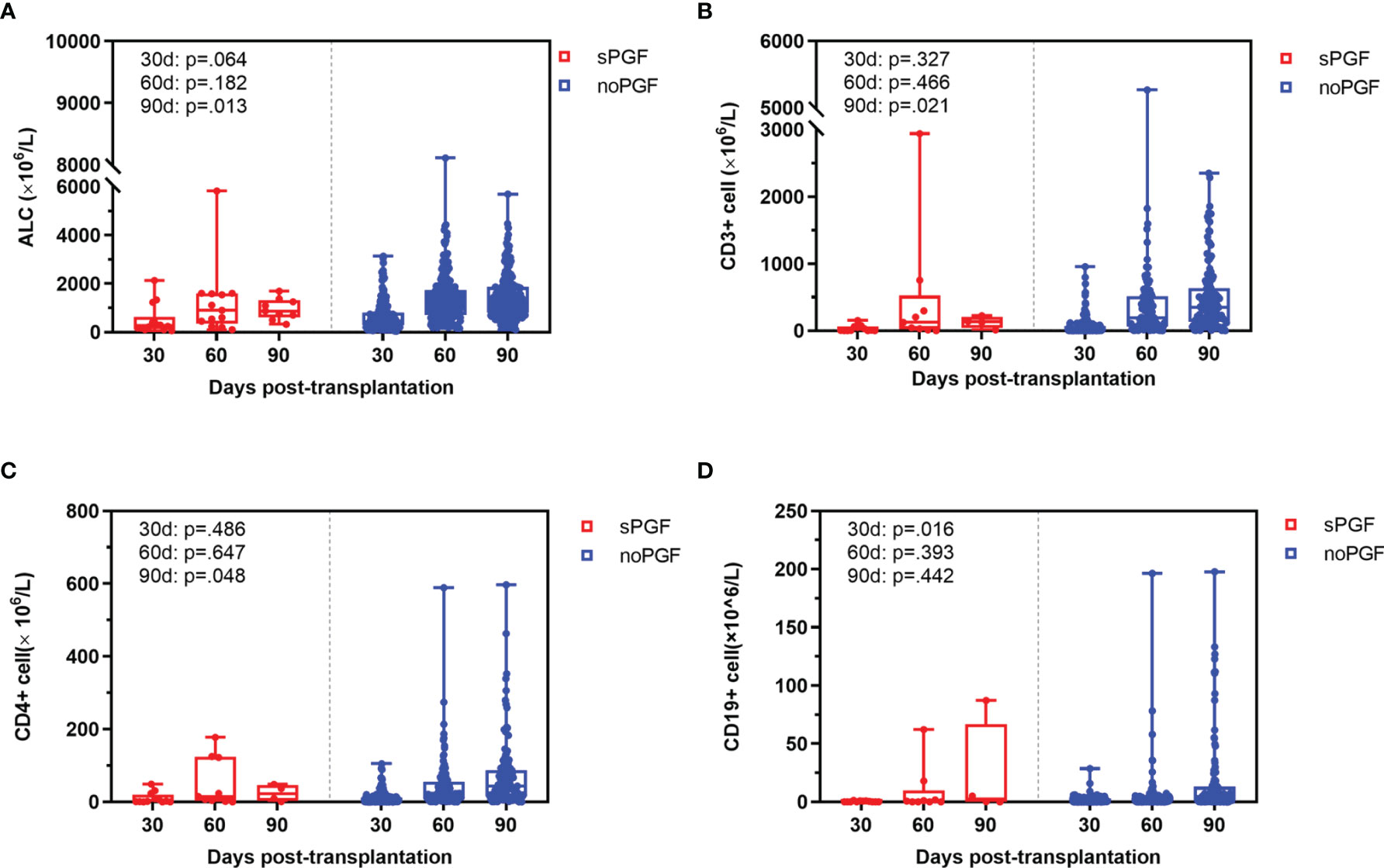
Figure 2 Immune reconstitution at day 30, 60, and 90 in sPGF patients and noPGF patients: (A) absolute lymphocyte count (ALC), (B) CD3+ T cell, (C) CD3+CD4+ T cell, (D) CD19+ B cell.
3.3 Treatment and Outcomes of sPGF
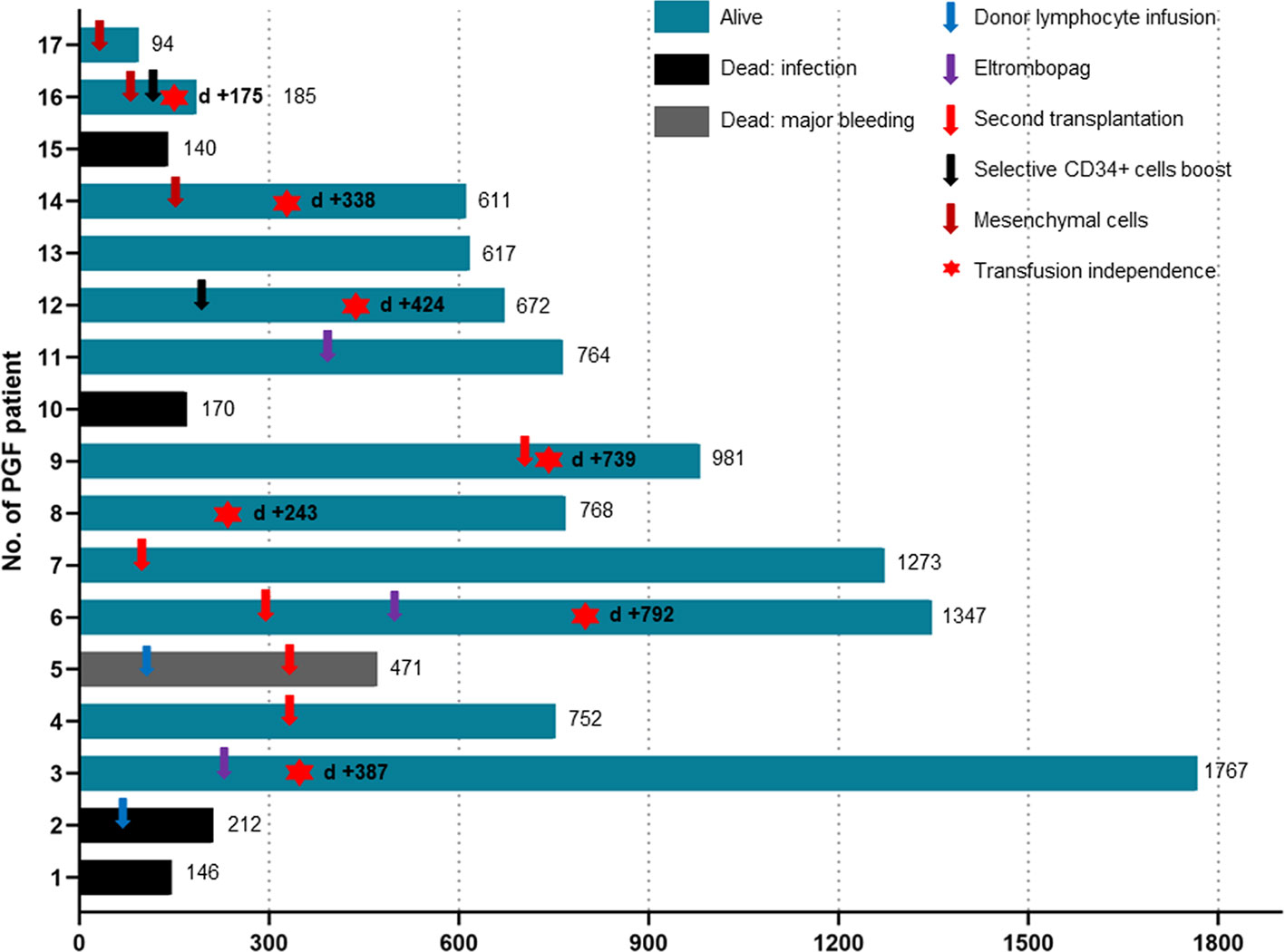
Compared to the noPGF group, the sPGF group experienced significantly poorer 2-year OS (67.71% [95% CI: 38.83%-85.15%] vs 91.46% [95% CI: 88.07%-93.93%], p =.002). Twelve sPGF patients were alive until the last follow-up, and 7 of them were transfusion independent. Infection was the leading cause of death (4/5) in sPGF group, and one died of intracranial hemorrhage.
All patients with sPGF received supportive treatment, G-CSF, blood transfusion, androgens, immunosuppression agents, etc. Only 1 patient had spontaneous hematopoietic recovery without further treatment. Among the other 4 patients who received only supportive treatment, 3 died of infection. Two patients additionally received eltrombopag. One of them became transfusion-independent and the other, although remained transfusion-dependent, had extended the transfusion interval.
Ten sPGF patients received salvage treatments in forms of cellular therapies (Figure 3). Two were infused with donor lymphocytes with no improvement in hematopoietic function. One of them died of infection, and the other died of intracranial hemorrhage following a second transplantation from the original donor. Upfront second transplantation was applied in another 4 cases. Three received grafts from their original donors and experienced prolonged isolated thrombocytopenia, one of whom discontinued blood transfusions after treatment with eltrombopag. The other one received second transplantation from another haploidentical donor (mother) and achieved sustained transfusion independence. Of the three sPGF patients who received mesenchymal cell infusions, only 1 patient achieved transfusion independence and the other 2 patients had no response. Notably, selective CD34+ cell boost successfully resulted in normal blood counts in 2 patients with sPGF.
3.4 Risk Factors for sPGF
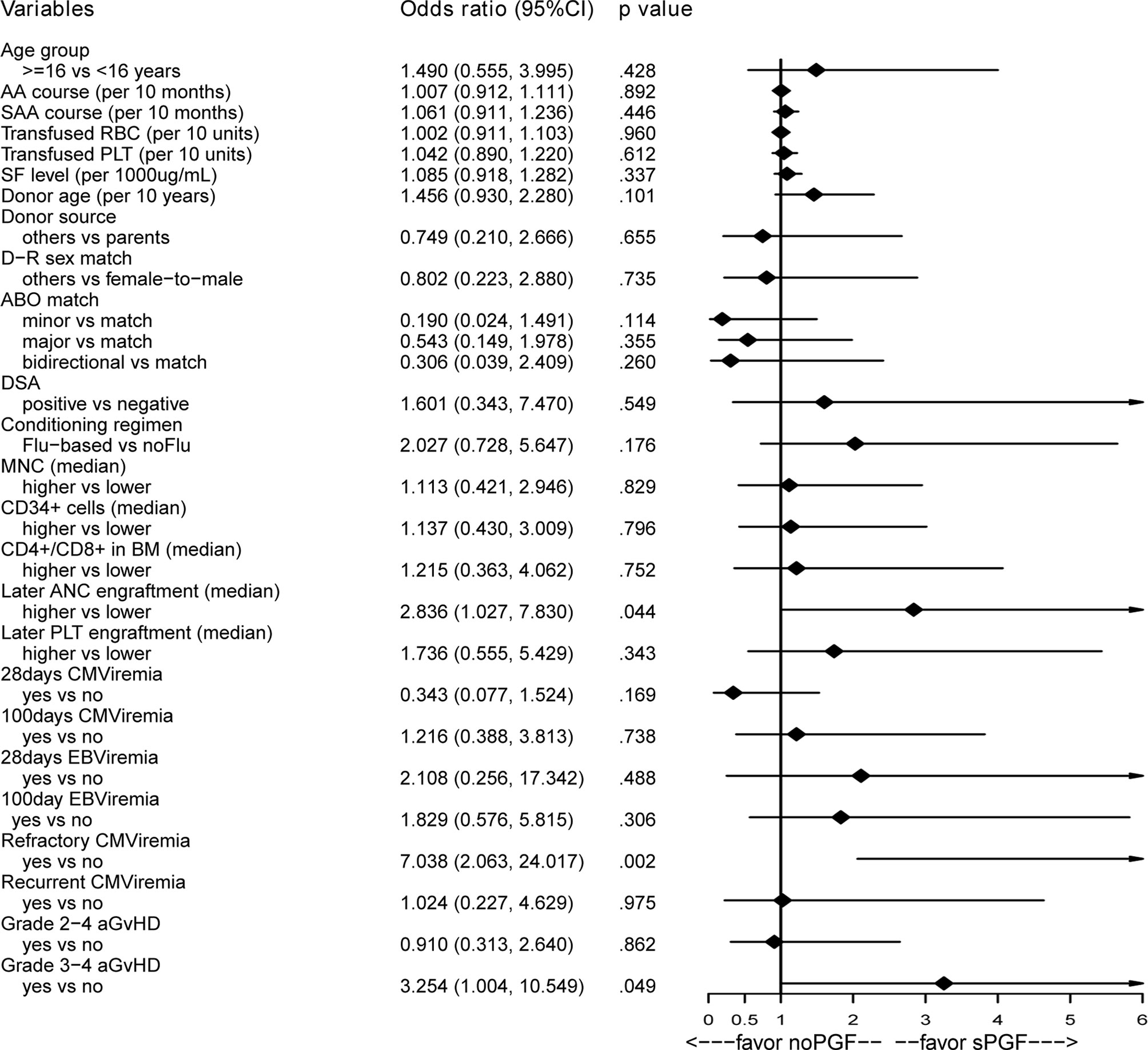
3.4.1 Pre- or Post-Transplantation Variables
As presented in Figure 4, no potential risk factor was found among the pre-transplantation variables of interest. Among the post-transplantation variables, we found that later neutrophil engraftment (OR 2.836, 95% CI [1.027, 7.830], p=.044), a history of refractory CMV viremia (OR 7.038, 95% CI [2.063, 24.017], p=.002), and a history of grade 3-4 aGvHD (OR 3.254, 95% CI [1.004, 10.549], p=.049) were associated with sPGF in the univariable analysis. No correlation was found among these variables (data not shown). When included in the multivariable analysis (Table 2: model 1), later neutrophil engraftment and a history of refractory CMV viremia were independent risk factors for sPGF.
3.4.2 Combined Analysis of Pre- and Post-Transplantation Variables
In the multivariable logistic regression model (Table 2: model 2), later neutrophil engraftment (OR 2.819, 95% CI [1.005, 7.909], p=.049) and refractory CMV viremia were independent risk factors for sPGF (OR 6.986, 95% CI [2.002, 24.379], p=.002). There was weak evidence that a history of grade 3-4 aGvHD was associated with sPGF (p=.063).
4. Discussion
PGF is a type of BM failure syndrome and leads to high morbidity and mortality post-transplantation. Based on the largest-scale AA cases that received haplo-HSCT, we revealed a CIR of 4.62% of sPGF at 3 years post-transplantation. OS was significantly decreased in sPGF patients. Later neutrophil engraftment and a history of refractory CMV viremia were the independent risk factors for sPGF.
Several studies on hematological malignancies suggested that haplo-HSCT can be associated with a greater risk of sPGF (9, 22). However, a previous report from our center demonstrated no association between haplo-HSCT setting and sPGF (23). In line with Liu et al. (24), our results reported an acceptable incidence of sPGF after haplo-HSCT for AA. In contrast, Japanese colleagues reported a higher incidence of sPGF of 15% in 49 pediatric AA patients, which included 3 transplantations from matched sibling donors (20.0%), 3 from unrelated donors (10.3%), and 1 from haploidentical donor (20%) (25). Similar to the study of Kako et al (8), Flu was suggested to be responsible for the increased incidence of sPGF, which was denied by our studies (11). The discrepancy in results can be explained by the difference in conditioning regimen instead of HSCT type, as Liu and we additionally applied 2 days of Bu to ensure successful engraftment and stable full donor chimerism in the haplo-setting (26). Of note, in line with prior reports, patients with high titers of DSA were successfully handled with rituximab desensitization (15, 27) and co-infusion with DSA-free cord blood (28), and as a result, none of the patient experienced primary PGF. Employing intensive conditioning regimens may be beneficial in maintaining stable donor-type chimerism and excellent hematopoietic recovery in AA patients undergoing HSCT. Further research should be conducted to clarify this hypothesis.
Widely proposed is the “seed, soil and climate” model for the pathophysiology of PGF (6, 29). Current literature suggests hematopoietic stem cells (seed) abnormalities have a causative role in PGF. In the present study, patients with sPGF or without PGF received grafts of similar dose and composition. Impressively, the dose of infused CD34+ cells was not associated with sPGF. However, we did observe distinct features of engraftment between the two groups. The univariable and multivariable analyses identified later neutrophil engraftment as an indicator of sPGF. Our results indicate that the development of sPGF is more likely the result of qualitative, rather than quantitative, abnormality of hematopoietic stem cells. Several studies demonstrated that no deficit was found in the cells’ capacity to repopulate the marrow when stored CD34+ cells from donors whose recipients developed PGF were xenografted to mice (30). Moreover, donor-derived CD34+ cells boost is an emerging therapeutic option with promising response rates in patients with sPGF, as presented in this report and others (31–33). Taken together with these findings, these data suggests that the deficits in “seed” are acquired after transplantation. Inducers or enhancers of allo-immunity (climate), such as CMV reactivation and GvHD, may amplify the intrinsic dysfunction of hematopoietic stem cells, ultimately leading to sPGF.
CMV inhibits hematopoiesis directly by infecting bone marrow or suppress hematopoiesis indirectly through the infection of stromal cells (34, 35). Previous studies (7, 23, 36) have identified CMV viremia as an independent risk factor for sPGF. It is reported that recipients undergoing haplo-HSCT have a higher incidence of CMV reactivation, as well as refractory CMV viremia (37, 38). Lv et al. recently revealed that CMV reactivation was the only hazard element for sPGF in haplo-HSCT (9). Nevertheless, one should note that refractory CMV viremia rather than early CMV reactivation increased the risk of sPGF in our study. Patients in the sPGF group had lower B cell levels in the first month after haplo-HSCT, which may explain their greater susceptibility to refractory CMV viremia (38). Our results indicated that the influence of CMV on the BM niche can be time-dependent and may be irreversible under sufficient viral load. Since the clinical course of CMV reactivation is often complicated with GvHD, administration of immunosuppressants and immune reconstitution, further study on the impact of clinical characteristics and kinetics of CMV on graft hematopoietic function is required. On the other hand, antiviral medications, including GCV and foscarnet, can exacerbate the suppression in hematopoiesis (22) and are major players in the development of sPGF. Given the fact that GCV was involved in all CMV-positive patients in this study, it was impossible to separate the influence of CMV itself and anti-CMV pharmacotherapy. Anyway, timely evaluation and initiation of CMV-specific cellular therapy may be of great help to avoid inhibition of hematopoiesis and provide better transplant outcomes (13, 39).
The occurrence of aGvHD has also been accepted as contributing to the development of sPGF (36, 40). In this study, we found a marginal association between grade 3-4 aGvHD and sPGF. In vivo studies corroborated that GvHD can lead to PGF via overactivated T cells and dysregulated cytokines (41, 42). Moreover, severe aGvHD requires intensive immunosuppression and thus always occurs in concert with prolonged viral reactivation. Limited by the number of cases, we were unable to analyze the effect of aGvHD and co-current CMV viremia on sPGF. Large-scale studies can help understand this process. Reducing the incidence of aGvHD is now one of the most important goals of unmanipulated haplo-HSCT for AA patients, but it is noteworthy that we need to develop better strategies to balance the risk of immunosuppression and viral reactivation. In the ATG-based modality of in vivo T cell depletion, the dose of ATG is positively related to delayed immune reconstitution and the risk of viral infection (37, 43, 44). Recent works provide a promising option by optimizing ATG dosing (2.5 mg/kg daily for 3 days) to reduce viral activation while maintaining sufficient GvHD prophylaxis in haplo-HSCT (45, 46). Similar phenomena were observed in haplo-HSCT using a combination of post-transplantation cyclophosphamide and low-dose ATG (47, 48). A lower dose of ATG (2.5 mg/kg) successfully reduces CMV reactivation without compromising the favorable effect of preventing GvHD (49). Studies should be conducted in larger cohorts to determine the optimal dose of ATG to reach maximum immunosuppression, minimum risk of severe infections and in the end the best survival.
In conclusion, sPGF can develop in 4.62% of AA patients after haplo-HSCT and significantly decreases survival. The independent hazard elements for sPGF were later neutrophil engraftment and a history of refractory CMV reactivation. Considering the limited number of sPGF cases in this report, our results warrant investigation in further studies.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Review Committee of Peking University People’s Hospital. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
LX and XH designed the study. The data analysis and manuscript development were led by FL and TH. All authors contributed to providing clinical data and approved the final version for submission.
Funding
This work was supported by the National Key Research and Development Program of China (No. 2017YFA0104500), Innovative Research Groups of the National Natural Science Foundation of China (No. 81621001), Key Program of the National Natural Science Foundation of China (No. 81530046), and National Natural Science Foundation of China (No. 82100227).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all staff at the Peking University Institute of Hematology.
References
1. Zhang XH, Chen J, Han MZ, Huang H, Jiang EL, Jiang M, et al. The Consensus From The Chinese Society of Hematology on Indications, Conditioning Regimens and Donor Selection for Allogeneic Hematopoietic Stem Cell Transplantation: 2021 Update. J Hematol Oncol (2021) 14:145. doi: 10.1186/s13045-021-01159-2
2. DeZern AE, Zahurak ML, Symons HJ, Cooke KR, Rosner GL, Gladstone DE, et al. Haploidentical BMT for Severe Aplastic Anemia With Intensive GVHD Prophylaxis Including Posttransplant Cyclophosphamide. Blood Adv (2020) 4:1770–9. doi: 10.1182/bloodadvances.2020001729
3. Xu L-P, Xu Z-L, Wang S-Q, Wu D-P, Gao S-J, Yang J-M, et al. Long-Term Follow-Up of Haploidentical Transplantation in Relapsed/Refractory Severe Aplastic Anemia: A Multicenter Prospective Study. Sci Bull (2022) 67:963–70. doi: 10.1016/j.scib.2022.01.024
4. Dominietto A, Raiola AM, van Lint MT, Lamparelli T, Gualandi F, Berisso G, et al. Factors Influencing Haematological Recovery After Allogeneic Haemopoietic Stem Cell Transplants: Graft-Versus-Host Disease, Donor Type, Cytomegalovirus Infections and Cell Dose. Br J Haematol (2001) 112:219–27. doi: 10.1046/j.1365-2141.2001.02468.x
5. Lee KH, Lee JH, Choi SJ, Lee JH, Kim S, Seol M, et al. Failure of Trilineage Blood Cell Reconstitution After Initial Neutrophil Engraftment in Patients Undergoing Allogeneic Hematopoietic Cell Transplantation - Frequency and Outcomes. Bone Marrow Transplant (2004) 33:729–34. doi: 10.1038/sj.bmt.1704428
6. Prabahran AA, Koldej R, Chee L, Ritchie DS. Clinical Features, Pathophysiology and Therapy of Poor Graft Function Post Allogeneic Stem Cell Transplantation. Blood Adv (2021) 6:1947–59. doi: 10.1182/bloodadvances.2021004537
7. Xiao Y, Song J, Jiang Z, Li Y, Gao Y, Xu W, et al. Risk-Factor Analysis of Poor Graft Function After Allogeneic Hematopoietic Stem Cell Transplantation. Int J Med Sci (2014) 11:652–7. doi: 10.7150/ijms.6337
8. Kako S, Yamazaki H, Ohashi K, Ozawa Y, Ota S, Kanda Y, et al. Mixed Chimerism and Secondary Graft Failure in Allogeneic Hematopoietic Stem Cell Transplantation for Aplastic Anemia. Biol Blood Marrow Transplant (2020) 26:445–50. doi: 10.1016/j.bbmt.2019.10.004
9. Lv WR, Zhou Y, Xu J, Fan ZP, Huang F, Xu N, et al. Haploidentical Donor Transplant is Associated With Secondary Poor Graft Function After Allogeneic Stem Cell Transplantation: A Single-Center Retrospective Study. Cancer Med (2021) 10:8497–506. doi: 10.1002/cam4.4353
10. Xu LP, Jin S, Wang SQ, Xia LH, Bai H, Gao SJ, et al. Upfront Haploidentical Transplant for Acquired Severe Aplastic Anemia: Registry-Based Comparison With Matched Related Transplant. J Hematol Oncol (2017) 10:25. doi: 10.1186/s13045-017-0398-y
11. Lin F, Zhang Y, Han T, Cheng Y, Mo X, Wang J, et al. A Modified Conditioning Regimen Based on Low-Dose Cyclophosphamide and Fludarabine for Haploidentical Hematopoietic Stem Cell Transplant in Severe Aplastic Anemia Patients at Risk of Severe Cardiotoxicity. Clin Transplant (2021) 36:e14514. doi: 10.1111/ctr.14514
12. Pei XY, Zhao XY, Chang YJ, Liu J, Xu LP, Wang Y, et al. Cytomegalovirus-Specific T-Cell Transfer for Refractory Cytomegalovirus Infection After Haploidentical Stem Cell Transplantation: The Quantitative and Qualitative Immune Recovery for Cytomegalovirus. J Infect Dis (2017) 216:945–56. doi: 10.1093/infdis/jix357
13. Zhao XY, Pei XY, Chang YJ, Yu XX, Xu LP, Wang Y, et al. First-Line Therapy With Donor-Derived Human Cytomegalovirus (HCMV)-Specific T Cells Reduces Persistent HCMV Infection by Promoting Antiviral Immunity After Allogenic Stem Cell Transplantation. Clin Infect Dis (2020) 70:1429–37. doi: 10.1093/cid/ciz368
14. Chang YJ, Zhao XY, Xu LP, Zhang XH, Wang Y, Han W, et al. Donor-Specific Anti-Human Leukocyte Antigen Antibodies Were Associated With Primary Graft Failure After Unmanipulated Haploidentical Blood and Marrow Transplantation: A Prospective Study With Randomly Assigned Training and Validation Sets. J Hematol Oncol (2015) 8:84. doi: 10.1186/s13045-015-0182-9
15. Chang YJ, Xu LP, Wang Y, Zhang XH, Chen H, Chen YH, et al. Rituximab for Desensitization During HLA-Mismatched Stem Cell Transplantation in Patients With a Positive Donor-Specific Anti-HLA Antibody. Bone Marrow Transplant (2020) 55:1326–36. doi: 10.1038/s41409-020-0928-z
16. Kharfan-Dabaja MA, Kumar A, Ayala E, Aljurf M, Nishihori T, Marsh R, et al. Standardizing Definitions of Hematopoietic Recovery, Graft Rejection, Graft Failure, Poor Graft Function, and Donor Chimerism in Allogeneic Hematopoietic Cell Transplantation: A Report on Behalf of the American Society for Transplantation and Cellular Therapy. Transplant Cell Ther (2021) 27:642–9. doi: 10.1016/j.jtct.2021.04.007
17. McLornan DP, Hernandez-Boluda JC, Czerw T, Cross N, Joachim Deeg H, Ditschkowski M, et al. Allogeneic Haematopoietic Cell Transplantation for Myelofibrosis: Proposed Definitions and Management Strategies for Graft Failure, Poor Graft Function and Relapse: Best Practice Recommendations of the EBMT Chronic Malignancies Working Party. Leukemia (2021) 35:2445–59. doi: 10.1038/s41375-021-01294-2
18. Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, et al. International, Multicenter Standardization of Acute Graft-Versus-Host Disease Clinical Data Collection: A Report From the Mount Sinai Acute GVHD International Consortium. Biol Blood Marrow Transplant (2016) 22:4–10. doi: 10.1016/j.bbmt.2015.09.001
19. Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-Versus-Host Disease: I. Diagnosis and Staging Working Group Report. Biol Blood Marrow Transplant (2005) 11:945–56. doi: 10.1016/j.bbmt.2005.09.004
20. Ljungman P, Boeckh M, Hirsch HH, Josephson F, Lundgren J, Nichols G, et al. Definitions of Cytomegalovirus Infection and Disease in Transplant Patients for Use in Clinical Trials. Clin Infect Dis (2017) 64:87–91. doi: 10.1093/cid/ciw668
21. Chemaly RF, Chou S, Einsele H, Griffiths P, Avery R, Razonable RR, et al. Definitions of Resistant and Refractory Cytomegalovirus Infection and Disease in Transplant Recipients for Use in Clinical Trials. Clin Infect Dis (2019) 68:1420–6. doi: 10.1093/cid/ciy696
22. Nakamae H, Storer B, Sandmaier BM, Maloney DG, Davis C, Corey L, et al. Cytopenias After Day 28 in Allogeneic Hematopoietic Cell Transplantation: Impact of Recipient/Donor Factors, Transplant Conditions and Myelotoxic Drugs. Haematologica (2011) 96:1838–45. doi: 10.3324/haematol.2011.044966
23. Sun YQ, Wang Y, Zhang XH, Xu LP, Liu KY, Yan CH, et al. Virus Reactivation and Low Dose of CD34+ Cell, Rather Than Haploidentical Transplantation, Were Associated With Secondary Poor Graft Function Within the First 100 Days After Allogeneic Stem Cell Transplantation. Ann Hematol (2019) 98:1877–83. doi: 10.1007/s00277-019-03715-w
24. Liu Z, Wu X, Wang S, Xia L, Xiao H, Li Y, et al. Co-Transplantation of Mesenchymal Stem Cells Makes Haploidentical HSCT a Potential Comparable Therapy With Matched Sibling Donor HSCT for Patients With Severe Aplastic Anemia. Ther Adv Hematol (2020) 11:2040620720965411. doi: 10.1177/2040620720965411
25. Hama A, Muramatsu H, Narita A, Nishikawa E, Kawashima N, Nishio N, et al. Risk Factors for Secondary Poor Graft Function After Bone Marrow Transplantation in Children With Acquired Aplastic Anemia. Pediatr Transplant (2020) 24:e13828. doi: 10.1111/petr.13828
26. Xu ZL, Cheng YF, Zhang YY, Mo XD, Han TT, Wang FR, et al. The Incidence, Clinical Outcome, and Protective Factors of Mixed Chimerism Following Hematopoietic Stem Cell Transplantation for Severe Aplastic Anemia. Clin Transplant (2021) 35:e14160. doi: 10.1111/ctr.14160
27. Hashem H, Rihani R, Shanap MA, Khattab E, Tbakhi A, Sultan I. Novel Conditioning Regimen for Upfront Haploidentical Hematopoietic Cell Transplantation in Children With Severe Aplastic Anemia and Donor-Specific Anti-HLA Antibodies. Bone Marrow Transplant (2022) 57:304–5. doi: 10.1038/s41409-021-01536-y
28. Liu H, Rich ES, Godley L, Odenike O, Joseph L, Marino S, et al. Reduced-Intensity Conditioning With Combined Haploidentical and Cord Blood Transplantation Results in Rapid Engraftment, Low GVHD, and Durable Remissions. Blood (2011) 118:6438–45. doi: 10.1182/blood-2011-08-372508
29. Kong Y. Poor Graft Function After Allogeneic Hematopoietic Stem Cell Transplantation-An Old Complication With New Insights(☆;). Semin Hematol (2019) 56:215–20. doi: 10.1053/j.seminhematol.2018.08.004
30. Kong Y, Song Y, Hu Y, Shi MM, Wang YT, Wang Y, et al. Increased Reactive Oxygen Species and Exhaustion of Quiescent CD34-Positive Bone Marrow Cells may Contribute to Poor Graft Function After Allotransplants. Oncotarget (2016) 7:30892–906. doi: 10.18632/oncotarget.8810
31. Larocca A, Piaggio G, Podestà M, Pitto A, Bruno B, Di Grazia C, et al. Boost of CD34+-Selected Peripheral Blood Cells Without Further Conditioning in Patients With Poor Graft Function Following Allogeneic Stem Cell Transplantation. Haematologica (2006) 91:935–40. doi: 10.3324/%25x
32. Mainardi C, Ebinger M, Enkel S, Feuchtinger T, Teltschik HM, Eyrich M, et al. CD34(+) Selected Stem Cell Boosts can Improve Poor Graft Function After Paediatric Allogeneic Stem Cell Transplantation. Br J Haematol (2018) 180:90–9. doi: 10.1111/bjh.15012
33. Shahzad M, Siddiqui RS, Anwar I, Chaudhary SG, Ali T, Naseem M, et al. Outcomes With CD34-Selected Stem Cell Boost for Poor Graft Function After Allogeneic Hematopoietic Stem Cell Transplantation: A Systematic Review and Meta-Analysis. Transplant Cell Ther (2021) 27:877.e1–8. doi: 10.1016/j.jtct.2021.07.012
34. Randolph-Habecker J, Iwata M, Torok-Storb B. Cytomegalovirus Mediated Myelosuppression. J Clin Virol (2002) 25:51–6. doi: 10.1016/S1386-6532(02)00092-6
35. Capobianchi A, Iori AP, Micozzi A, Torelli GF, Testi AM, Girmenia C, et al. Cytomegalovirus in Bone Marrow Cells Correlates With Cytomegalovirus in Peripheral Blood Leukocytes. J Clin Microbiol (2014) 52:2183–5. doi: 10.1128/JCM.00702-14
36. Prabahran A, Koldej R, Chee L, Wong E, Ritchie D. Evaluation of Risk Factors for and Subsequent Mortality From Poor Graft Function (PGF) Post Allogeneic Stem Cell Transplantation. Leuk Lymph (2021) 62:1482–9. doi: 10.1080/10428194.2021.1872072
37. Xuan L, Huang F, Fan Z, Zhou H, Zhang X, Yu G, et al. Effects of Intensified Conditioning on Epstein-Barr Virus and Cytomegalovirus Infections in Allogeneic Hematopoietic Stem Cell Transplantation for Hematological Malignancies. J Hematol Oncol (2012) 5:46–6. doi: 10.1186/1756-8722-5-46
38. Shmueli E, Or R, Shapira MY, Resnick IB, Caplan O, Bdolah-Abram T, et al. High Rate of Cytomegalovirus Drug Resistance Among Patients Receiving Preemptive Antiviral Treatment After Haploidentical Stem Cell Transplantation. J Infect Dis (2014) 209:557–61. doi: 10.1093/infdis/jit475
39. Renzaho A, Podlech J, Kühnapfel B, Blaum F, Reddehase MJ, Lemmermann NAW. Cytomegalovirus-Associated Inhibition of Hematopoiesis Is Preventable by Cytoimmunotherapy With Antiviral CD8 T Cells. Front Cell Infect Microbiol (2020) 10:138–8. doi: 10.3389/fcimb.2020.00138
40. Reich-Slotky R, Al-Mulla N, Hafez R, Segovia-Gomez J, Goel R, Mayer S, et al. Poor Graft Function After T Cell-Depleted Allogeneic Hematopoietic Stem Cell Transplant. Leuk Lymph (2020) 61:2894–9. doi: 10.1080/10428194.2020.1789622
41. Masouridi-Levrat S, Simonetta F, Chalandon Y. Immunological Basis of Bone Marrow Failure After Allogeneic Hematopoietic Stem Cell Transplantation. Front Immunol (2016) 7:362–2. doi: 10.3389/fimmu.2016.00362
42. Kong Y, Wang YT, Cao XN, Song Y, Chen YH, Sun YQ, et al. Aberrant T Cell Responses in the Bone Marrow Microenvironment of Patients With Poor Graft Function After Allogeneic Hematopoietic Stem Cell Transplantation. J Trans Med (2017) 15:57. doi: 10.1186/s12967-017-1159-y
43. Duval M, Pédron B, Rohrlich P, Legrand F, Faye A, Lescoeur B, et al. Immune Reconstitution After Haematopoietic Transplantation With Two Different Doses of Pre-Graft Antithymocyte Globulin. Bone Marrow Transplant (2002) 30:421–6. doi: 10.1038/sj.bmt.1703680
44. Baron F, Mohty M, Blaise D, Socié G, Labopin M, Esteve J, et al. Anti-Thymocyte Globulin as Graft-Versus-Host Disease Prevention in the Setting of Allogeneic Peripheral Blood Stem Cell Transplantation: A Review From the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica (2017) 102:224–34. doi: 10.3324/haematol.2016.148510
45. Lin R, Wang Y, Huang F, Fan Z, Zhang S, Yang T, et al. Two Dose Levels of Rabbit Antithymocyte Globulin as Graft-Versus-Host Disease Prophylaxis in Haploidentical Stem Cell Transplantation: A Multicenter Randomized Study. BMC Med (2019) 17:156. doi: 10.1186/s12916-019-1393-7
46. Wang Y, Liu Q-F, Lin R, Yang T, Xu Y-J, Mo X-D, et al. Optimizing Antithymocyte Globulin Dosing in Haploidentical Hematopoietic Cell Transplantation: Long-Term Follow-Up of a Multicenter, Randomized Controlled Trial. Sci Bull (2021) 66:2498–505. doi: 10.1016/j.scib.2021.06.002
47. Stocker N, Gaugler B, Labopin M, Farge A, Ye Y, Ricard L, et al. High-Dose Post-Transplant Cyclophosphamide Impairs γδ T-Cell Reconstitution After Haploidentical Haematopoietic Stem Cell Transplantation Using Low-Dose Antithymocyte Globulin and Peripheral Blood Stem Cell Graft. Clin Transl Immunol (2020) 9:e1171–1. doi: 10.1002/cti2.1171
48. Anurathapan U, Hongeng S, Pakakasama S, Sirachainan N, Songdej D, Chuansumrit A, et al. Hematopoietic Stem Cell Transplantation for Homozygous β-Thalassemia and β-Thalassemia/Hemoglobin E Patients From Haploidentical Donors. Bone Marrow Transplant (2016) 51:813–8. doi: 10.1038/bmt.2016.7
49. Osumi T, Yoshimura S, Sako M, Uchiyama T, Ishikawa T, Kawai T, et al. Prospective Study of Allogeneic Hematopoietic Stem Cell Transplantation With Post-Transplantation Cyclophosphamide and Antithymocyte Globulin From HLA-Mismatched Related Donors for Nonmalignant Diseases. Biol Blood Marrow Transplant (2020) 26:e286–91. doi: 10.1016/j.bbmt.2020.08.008
Keywords: secondary poor graft function, acquired aplastic anemia, haploidentical hematopoietic stem cell transplantation, risk factors, cytomegalovirus (CMV), graft-versus- host disease
Citation: Lin F, Han T, Zhang Y, Cheng Y, Xu Z, Mo X, Wang F, Yan C, Sun Y, Wang J, Tang F, Han W, Chen Y, Wang Y, Zhang X, Liu K, Huang X and Xu L (2022) The Incidence, Outcomes, and Risk Factors of Secondary Poor Graft Function in Haploidentical Hematopoietic Stem Cell Transplantation for Acquired Aplastic Anemia. Front. Immunol. 13:896034. doi: 10.3389/fimmu.2022.896034
Received: 14 March 2022; Accepted: 19 April 2022;
Published: 09 May 2022.
Edited by:
Xi Zhang, Xinqiao Hospital, ChinaCopyright © 2022 Lin, Han, Zhang, Cheng, Xu, Mo, Wang, Yan, Sun, Wang, Tang, Han, Chen, Wang, Zhang, Liu, Huang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lanping Xu, bHB4dV8wNDE1QHNpbmEuY29t
†These authors have contributed equally to this work and share first authorship
 Fan Lin
Fan Lin Tingting Han
Tingting Han Yuanyuan Zhang1
Yuanyuan Zhang1 Zhengli Xu
Zhengli Xu Xiaodong Mo
Xiaodong Mo Yuqian Sun
Yuqian Sun Feifei Tang
Feifei Tang Yu Wang
Yu Wang Xiaohui Zhang
Xiaohui Zhang Xiaojun Huang
Xiaojun Huang Lanping Xu
Lanping Xu