
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Immunol., 20 April 2022
Sec. Autoimmune and Autoinflammatory Disorders
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.894464
This article is part of the Research TopicMonoclonal Gammopathies of Clinical Significance: Clinical and Therapeutic ImplicationsView all 8 articles
Schnitzler syndrome is a rare adult-onset acquired autoinflammatory disorder typically characterized by chronic urticarial rash and immunoglobulin M (IgM) (rarely IgG) monoclonal gammopathy. Its clinical symptoms usually respond well to interleukin-1 blockade therapy, which, however, does not impact the underlying monoclonal gammopathy. Herein, we described a female patient who presented with urticarial rash, recurrent fevers, and fatigue for 7 years. Laboratory investigations revealed IgMκ monoclonal protein and MYD88 L265P mutation, but no lymphoplasmacytic lymphoma on bone marrow examination. She fulfilled the diagnosis of Schnitzler syndrome and was treated with the Bruton tyrosine kinase inhibitor ibrutinib in combination with prednisone. Her symptoms improved dramatically, and the level of IgMκ monoclonal protein also declined. She tolerated the treatment well. This case highlights the potential therapeutic role of Bruton tyrosine kinase inhibitors in Schnitzler syndrome.
Schnitzler syndrome is a rare late-onset autoinflammatory disorder characterized by urticarial rash and immunoglobulin M (IgM) (rarely IgG) monoclonal gammopathy. Patients with this non-inherited disorder usually have disease onset in their 50s, with clinical features typically including recurrent urticarial rash, fever, bone pain, lymphadenopathy, and elevated inflammatory markers (1). Its clinical manifestations and therapeutic response to interleukin-1 (IL-1) blockade mimic the cryopyrin-associated periodic syndromes, which are caused by gain-of-function mutations in the NLRP3 gene, a critical component of the NLRP3 inflammasome that is responsible for IL-1β production (1). Although IL-1 blockade therapy controls the symptoms well in most patients, it is not disease-modifying, so symptoms almost always relapse after discontinuation of the therapy. In addition, the underlying monoclonal gammopathy can progress while on treatment (2), which highlights the importance of exploring novel therapies. Bruton tyrosine kinase (BTK) inhibitors, such as ibrutinib, could target both the NLRP3 inflammasome and the underlying B-cell clone (3, 4). Herein, we report our successful experience of ibrutinib use in a patient with Schnitzler syndrome.
A 71-year-old Chinese woman was admitted to our hospital in 2021 due to recurrent urticarial rash with fever and fatigue. These symptoms first started 7 years ago, and initial workups revealed mild leukocytosis, elevated levels of C-reactive protein (CRP) (31.9 mg/L; normal range, 0–10 mg/L), and IgM (14.92 g/L; normal range, 0.4–2.3 g/L). Serum protein electrophoresis showed a monoclonal band, but immunofixation was not performed. Otherwise, the patient did not have eosinophilia, and autoimmune panels, including anti-nuclear antibodies, anti-neutrophil cytoplasmic antibodies, anti-phospholipid antibodies, and cryoglobulin, were negative. The patient was started on prednisone 30 mg daily, and the dose was gradually tapered to 20 mg daily. However, her clinical response was unremarkable. On admission, she had intermittent fevers up to 39°C, and urticarial rashes without angioedema involving both trunk and extremities (Figure 1A). She was anemic (hemoglobin, 8.2 g/dl) with elevated CRP (44.4 mg/L) and serum IgM (22.4 g/L), but normal IgG and IgA levels. Serum electrophoresis quantified a monoclonal protein of 10.34 g/L, and immunofixation demonstrated an IgMκ subtype. Bone marrow examination did not show lymphoplasmacytic lymphoma morphologically, but flow cytometry revealed an abnormal population of B cells positive for CD5, CD19, CD20, CD22, surface IgM, and cytoplasmic kappa. Genetic testing found MYD88 L265P mutation, but wild-type CXCR4. The imaging study did not show osteosclerosis. Her rashes were not typical for adult-onset Still’s disease, and her autoimmune studies were negative for other well-defined connective tissue diseases. Moreover, the bone marrow findings did not show evidence of multiple myeloma or Waldenstrom’s macroglobulinemia. Overall, this patient fulfilled the diagnosis of Schnitzler syndrome by meeting two obligate and two minor components of the Strasbourg criteria. Given difficult access to IL-1 blockade therapy and that the patient declined rituximab, she was started on ibrutinib 420 mg daily in combination with prednisone 25 mg daily for her debilitating symptoms. The urticarial rashes resolved completely (Figure 1B), and fatigue improved after 2 months of treatment. At the 12-month follow-up, her Schnitzler syndrome clinical activity score (a semiquantitative scale for rashes, pain, fever, and weight loss: 0, absent–rare; 1, moderate; 2, frequent–severe) decreased from 4 to 0 (5), and there was moderate improvement of her anemia (hemoglobin, 10.7 g/dl) and declines in the levels of monoclonal IgMκ (2.95 g/L) and serum IgM (7.33 g/L) (Figure 2). She tolerated the treatment well without infectious or cardiac complications, and her dose of prednisone was tapered to 15 mg daily.
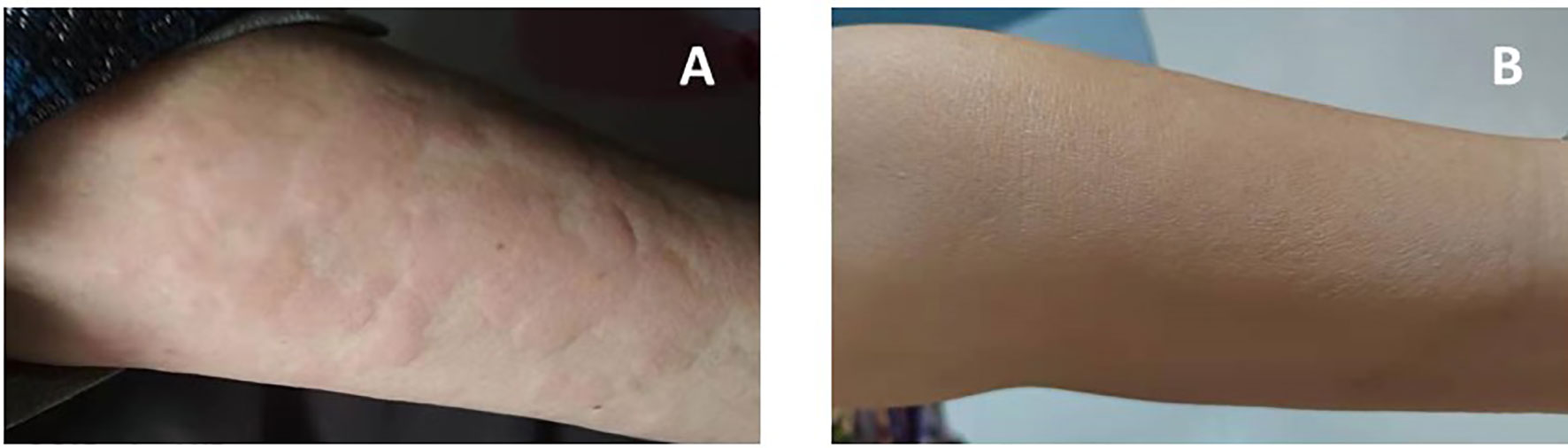
Figure 1 Resolution of urticarial rash after BTK inhibitor ibrutinib therapy [(A) pre-treatment; (B) post-treatment].
Schnitzler syndrome is an acquired autoinflammatory syndrome. In view of its striking similarity to cryopyrin-associated periodic syndromes, many studies have focused on the pathogenic role of the NLRP3 inflammasome pathway. Indeed, Schnitzler patients with active disease showed clearly higher serum levels of pro-inflammatory cytokines (i.e., IL-6 and IL-18) and the extracellular apoptosis-associated speck-like protein with caspase recruitment domain aggregates (6). Moreover, peripheral blood mononuclear cells from Schnitzler patients were found to be hypersensitive to lipopolysaccharide-stimulated IL-1 production (7). Dermal mast cells, neutrophils, and keratinocytes might also be involved in pro-inflammatory cytokine production. However, recent studies using next-generation sequencing have not revealed classic germline or somatic pathogenic mutations in NLRP3 and 32 autoinflammation-related genes in a large number of Schnitzler patients (6, 8). Although the mechanism remains obscure, these findings clearly indicate inflammasome activation in Schnitzler syndrome, and its pathogenic role is further corroborated by the therapeutic efficacy of IL-1 blockade. Anakinra and canakinumab control the symptoms and reduce inflammation successfully in most Schnitzler patients, but they are not disease-modifying, and treatment discontinuation almost always results in clinical relapse (2).
IgM monoclonal gammopathy is one of the hallmarks of Schnitzler syndrome according to the Strasbourg criteria. Besides IgM monoclonal gammopathy, genetic testing found an MYD88 L265P mutation in this case. MYD88 is at the crossroad of toll-like receptor (TLR), IL-1R, and B-cell receptor pathways and may participate in uncontrolled inflammation. The somatic MYD88 L265P mutation was found in peripheral blood samples of 9 out of 30 Schnitzler patients, a similar frequency of IgM monoclonal gammopathy of undetermined significance (MGUS) (8). In addition, its 10-year progression risk into lymphoproliferative disorders, most notably Waldenstrom’s macroglobulinemia, is also similar to IgM MGUS (9). These observations support the incorporation of Schnitzler syndrome into the spectrum of monoclonal gammopathy of clinical significance in which organ damages are related to monoclonal protein or other paraneoplastic mechanisms rather than to the proliferation of the underlying B-cell clone (10). However, conventional therapies, such as rituximab, against the underlying B-cell clone did not always result in organ improvement, which makes Schnitzler syndrome different from other typical monoclonal gammopathies of clinical significance. Moreover, IL-1 blockade did not affect the levels of monoclonal proteins, and IgM MGUS can progress while patients are receiving IL-1 blockade (11). Overall, the link between IgM monoclonal gammopathy and inflammasome activation in Schnitzler syndrome is still missing.
As reviewed recently, TLRs, CD33, NLRP3, and BTK are all attractive therapeutic targets for diseases related to inflammasome activation (12). In addition, BTK has been validated as a therapeutic target in B-cell malignancy, most notably Waldenstrom’s macroglobulinemia and chronic lymphocytic leukemia (13). Of note is that two Schnitzler patients with prior exposure to IL-1 blockade therapy were treated successfully with the BTK inhibitor ibrutinib (Table 1) (14, 15), which may target both IgM monoclonal gammopathy and the NLRP3 inflammasome (4). These findings prompted our use of ibrutinib in the current case, and it did show dramatic clinical and biochemical responses. Compared to the two prior cases with concurrent hematologic malignancies, our patient only had IgM MGUS. These observations may highlight the pathogenic role of underlying B-cell clones in the development of Schnitzler syndrome and propose a possible universal therapeutic role of clone-directed therapy, such as BTK inhibitors. Whether patients respond differently according to the MYD88 mutational status remains unknown, and further studies are required.
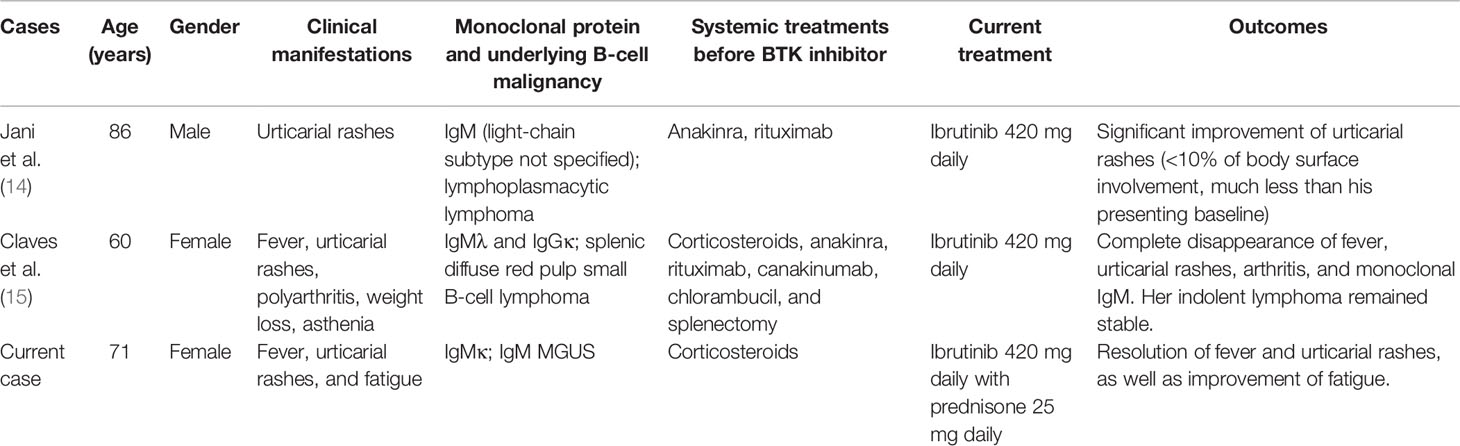
Table 1 Schnitzler syndrome patients treated with the Bruton tyrosine kinase (BTK) inhibitor ibrutinib.
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the participant for the publication of this case report.
YH and LL wrote the manuscript. YW, FY, XM, BW, and JL collected clinical data and generated results. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Kacar M, Pathak S, Savic S. Hereditary Systemic Autoinflammatory Diseases and Schnitzler's Syndrome. Rheumatol (Oxford) (2019) 58(Suppl 6):vi31–43. doi: 10.1093/rheumatology/kez448
2. de Koning HD, Schalkwijk J, van der Ven-Jongekrijg J, Stoffels M, van der Meer JW, Simon A. Sustained Efficacy of the Monoclonal Anti-Interleukin-1 Beta Antibody Canakinumab in a 9-Month Trial in Schnitzler's Syndrome. Ann Rheum Dis (2013) 72(10):1634–8. doi: 10.1136/annrheumdis-2012-202192
3. Liu X, Pichulik T, Wolz O-O, Dang TM, Stutz A, Dillen C, et al. Human NACHT, LRR, and PYD Domain-Containing Protein 3 (NLRP3) Inflammasome Activity Is Regulated by and Potentially Targetable Through Bruton Tyrosine Kinase. J Allergy Clin Immunol (2017) 140(4):1054–67.e10. doi: 10.1016/j.jaci.2017.01.017
4. Wang C. Killing Two Birds With One Stone: The Therapeutic Role of Ibrutinib in Schnitzler Syndrome. J Clin Immunol (2021) 41(7):1706–7. doi: 10.1007/s10875-021-01105-4
5. Darrieutort-Laffite C, Ansquer C, Aubert H, Kraeber-Bodéré F, Masseau A, Aggard C, et al. Rheumatic Involvement and Bone Scan Features in Schnitzler Syndrome: Initial and Follow-Up Data From a Single-Center Cohort of 25 Patients. Arthritis Res Ther (2020) 22(1):272. doi: 10.1186/s13075-020-02318-5
6. Rowczenio DM, Pathak S, Arostegui JI, Mensa-Vilaro A, Omoyinmi E, Brogan P, et al. Molecular Genetic Investigation, Clinical Features, and Response to Treatment in 21 Patients with Schnitzler Syndrome. Blood (2018) 131(9):974–81. doi: 10.1182/blood-2017-10-810366
7. Ryan JG, de Koning HD, Beck LA, Booty MG, Kastner DL, Simon A. IL-1 Blockade in Schnitzler Syndrome: Ex Vivo Findings Correlate With Clinical Remission. J Allergy Clin Immunol (2008) 121(1):260–2. doi: 10.1016/j.jaci.2007.09.021
8. Pathak S, Rowczenio DM, Owen RG, Doody GM, Newton DJ, Taylor C, et al. Exploratory Study of MYD88 L265P, Rare NLRP3 Variants, and Clonal Hematopoiesis Prevalence in Patients With Schnitzler Syndrome. Arthritis Rheumatol (2019) 71(12):2121–5. doi: 10.1002/art.41030
9. de Koning HD, Bodar EJ, van der Meer JW, Simon A, Schnitzler Syndrome Study Group. Schnitzler Syndrome: Beyond the Case Reports: Review and Follow-Up of 94 Patients With an Emphasis on Prognosis and Treatment. Semin Arthritis Rheumatol (2007) 37(3):137–48. doi: 10.1016/j.semarthrit.2007.04.001
10. Fermand JP, Bridoux F, Dispenzieri A, Jaccard A, Kyle RA, Leung N, et al. Monoclonal Gammopathy of Clinical Significance: A Novel Concept With Therapeutic Implications. Blood (2018) 132(14):1478–85. doi: 10.1182/blood-2018-04-839480
11. Takimoto-Ito R, Kambe N, Kogame T, Otsuka A, Nomura T, Izawa K, et al. Refractory Serum Immunoglobulin M Elevation During Anti-Interleukin (IL)-1- or IL-6-Targeted Treatment in Four Patients With Schnitzler Syndrome. J Dermatol (2021) 48(11):1789–92. doi: 10.1111/1346-8138.16124
12. Sallman DA, List A. The Central Role of Inflammatory Signaling in the Pathogenesis of Myelodysplastic Syndromes. Blood (2019) 133(10):1039–48. doi: 10.1182/blood-2018-10-844654
13. da Cunha-Bang C, Niemann CU. Targeting Bruton's Tyrosine Kinase Across B-Cell Malignancies. Drugs (2018) 78(16):1653–63. doi: 10.1007/s40265-018-1003-6
14. Jani P, Vissing MB, Ahmed S, Sluzevich JC, Aulakh S, Alegria V. Ibrutinib for the Management of Schnitzler Syndrome: A Novel Therapy for a Rare Condition. J Oncol Pract (2018) 14(6):387–8. doi: 10.1200/JOP.18.00050
Keywords: Schnitzler syndrome, interleukin-1, Bruton tyrosine kinase inhibitor, ibrutinib, case report
Citation: Huang Y, Wang Y, Yu F, Mao X, Wang B, Li J and Li L (2022) Case Report: Therapeutic Use of Ibrutinib in a Patient With Schnitzler Syndrome. Front. Immunol. 13:894464. doi: 10.3389/fimmu.2022.894464
Received: 11 March 2022; Accepted: 22 March 2022;
Published: 20 April 2022.
Edited by:
Chen Wang, USF Health, United StatesReviewed by:
Alexis Talbot, University of California, San Francisco, United StatesCopyright © 2022 Huang, Wang, Yu, Mao, Wang, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lihong Li, bGxoYTAzMDMyQGJ0Y2guZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.