- Centre for Immunology and Infection Control, School of Biomedical Sciences, Faculty of Health, Queensland University of Technology, Brisbane, QLD, Australia
CD4+ T cell responses to self-antigens are pivotal for immunological self-tolerance. Activation of Foxp3– T-conventional (T-conv) cells can precipitate autoimmune disease, whereas activation of Foxp3+ T-regulatory (T-reg) cells is essential to prevent autoimmune disease. This distinction indicates the importance of the thymus in controlling the differentiation of self-reactive CD4+ T cells. Thymocytes and thymic antigen-presenting cells (APC) depend on each other for normal maturation and differentiation. In this Hypothesis and Theory article, we propose this mutual dependence dictates which self-antigens induce T-reg cell development in the thymic medulla. We postulate self-reactive CD4+ CD8– thymocytes deliver signals that stabilize and amplify the presentation of their cognate self-antigen by APC in the thymic medulla, thereby seeding a niche for the development of T-reg cells specific for the same self-antigen. By limiting the number of antigen-specific CD4+ thymocytes in the medulla, thymocyte deletion in the cortex may impede the formation of medullary T-reg niches containing certain self-antigens. Susceptibility to autoimmune disease may arise from cortical deletion creating a “hole” in the self-antigen repertoire recognized by T-reg cells.
Introduction
Foxp3+ T-regulatory (T-reg) cells are an immunosuppressive lineage of T cells essential for immune tolerance (1). The development and function of T-reg cells depend on interactions between the T cell receptor (TCR) and peptide-major histocompatibility complex class II (pMHCII) antigens on the surface of other cells (2, 3). Some pMHCII self-antigens induce thymic lymphocytes (thymocytes) to upregulate Foxp3 (4–6); we refer to these pMHCII self-antigens as Tregitopes (T-reg epitopes). Some self-peptides fail to form a Tregitope because they cannot bind stably to the peptide-binding grooves of any MHCII alleles expressed in a given organism (7). In addition, the expression pattern of a self-peptide affects its capacity to form a Tregitope. Self-peptides not expressed in the thymus do not affect the development of responding thymocytes, whereas highly expressed self-peptides induce thymocyte deletion (8, 9). Highly expressed self-peptides induce thymocyte deletion because the high number of pMHCII complexes per APC, or high number of pMHCII+ APC, triggers persistent TCR signaling in thymocytes. Alternatively, highly expressed self-peptides may induce deletion because they are presented in the cortex to immature thymocytes that are more sensitive to deletion than mature thymocytes in the medulla (10, 11). Thus, according to current concepts, self-peptides with low or sparse presentation in the thymic medulla should form Tregitopes.
A self-peptide derived from the α3 chain of type IV collagen (α3) forms a Tregitope when presented by the MHCII molecule, human leucocyte antigen (HLA)-DR1, but not when presented by HLA-DR15 (4). HLA genotype would not be expected to affect α3 expression, which has been observed in the thymic medulla in a pattern suitable for Tregitope formation (12). Although HLA-DR1 and HLA-DR15 both present the α3 self-peptide to T cells, the peptide anchor residues are offset by one position so that the TCR “sees” different amino acids of the peptide when it is presented by HLA-DR1 versus HLA-DR15 (4). The distinct fates of α3-specific CD4+ T cells are of special interest because humans and mice expressing HLA-DR15 are susceptible to Goodpasture’s disease, also known as anti-glomerular basement membrane disease, characterized by pro-inflammatory T cell responses towards α3/DR15 (13, 14). However, co-expression of HLA-DR1 induces development of α3/DR1-specific T-reg cells and prevents Goodpasture’s disease in a manner that depends on T-reg cells (4).
To account for the distinct fates of CD4+ T cells specific for α3/DR1 versus α3/DR15, and other findings, here we propose an extension to current concepts of thymic T-reg cell development. We postulate the potential of medullary pMHCII self-antigens to form a Tregitope can be extinguished when a high percentage of cognate antigen-specific thymocytes are deleted by encountering the same or similar pMHCII self-antigens in the cortex. We suggest antigen-specific CD4+ CD8– (CD4 single-positive, CD4SP) thymocytes deliver signals that induce medullary thymic epithelial cells (mTEC) to “lock in” expression of their cognate self-antigen. Self-reactive CD4SP thymocytes may thereby generate a medullary niche for subsequent development of T-reg cells specific for the same self-antigen. Thus, antigen-specific T-reg niche size may be inversely related to the extent of cortical deletion of antigen-specific thymocyte populations. Implications of this extended model for the pathogenesis of organ-specific autoimmune diseases are discussed.
Impact of Thymocyte Deletion in the Cortex on T-reg Selection in the Medulla
Thymocyte deletion has been dissected based on the maturation stage and/or the intrathymic location of the thymocytes undergoing deletion (15). Most CD4+ CD8+ (double positive, DP) thymocytes are located in the cortex, whereas CD4SP thymocytes migrate between cortex and medulla, preferentially residing in the medulla (16, 17). In models in which deletion occurs at the DP stage, increased numbers of apoptotic cells are found in the cortex (18–20), whereas deletion at the CD4SP stage results in increased numbers of apoptotic cells in the medulla (18). Thus, it is plausible that deletion of DP thymocytes occurs in the cortex and deletion of CD4SP thymocytes occurs in the medulla. However, DP CD69+ thymocytes can enter the medulla in a CCR4-dependent mechanism (21). In mixed chimeras, Ccr4–/– thymocytes are overrepresented in all TCR-signalled thymocyte subsets starting at the DP CD69+ stage (21). Those findings indicate CCR4 is required for normal deletion and suggest this deletion may occur in DP thymocytes inside the medulla. Still, considering the high frequency of thymocytes that undergo deletion at the DP stage (22–24), the relatively mild effect of CCR4 deficiency on deletion (21, 25) suggests that a substantial amount of deletion at the DP stage is independent of CCR4. While the relative contributions of the cortex and medulla to thymocyte deletion at the DP stage remain unclear, for conceptual clarity, in this Hypothesis and Theory article we have assumed that deletion at the DP stage occurs predominantly in the cortex, and we refer to this process as cortical deletion. Cortical deletion is widely considered to be inconsequential to T-reg selection because a thymocyte deleted in the cortex cannot directly affect events taking place in the medulla. However, we postulate that cortical deletion can affect T-reg selection by creating variation in the number of antigen-specific CD4SP thymocytes in the medulla.
An antigen-specific CD4+ T cell is typically identified by the binding of its TCR to a given pMHCII tetramer (26). In a naïve C57BL/6 (B6) mouse, the number of self-antigen-specific CD4SP thymocytes varies by 100-fold depending on the peptide embedded in the MHCII tetramer (27). Most of this effect arises from variation in the proportion of antigen-specific thymocytes that undergo deletion (28). Deletion of antigen-specific thymocytes need not be triggered by the antigen itself. For example, the IgM:I-Ab-specific CD4SP thymocyte population in B6 mice is small (28). This population is ~ 8 times larger in mice that lack B cells–the only source of the IgM self-peptide–indicating IgM:I-Ab itself is required for the deletion of some IgM:I-Ab-specific thymocytes. However, the IgM:I-Ab-specific CD4SP thymocyte population is 450 times larger in mice with truly defective deletion due to MHCII expression being confined to cortical thymic epithelial cells, demonstrating that most IgM:I-Ab-specific thymocytes can be deleted by self-antigens other than IgM:I-Ab (28). Enumerating CD4+ T cells specific for a panel of foreign pMHCII antigens revealed the extent of such “deletion via TCR cross-reactivity” correlates with the number of self-peptides with the same or similar TCR-exposed amino acids (29). Deletion of thymocytes expressing a cross-reactive TCR (30) is indistinguishable from deletion of thymocytes specific for ubiquitous self-antigen (20). Both are initiated at the DP stage and the thymocytes never reach the CD4SP stage at which Foxp3 is upregulated in developing T-reg cells (31, 32). Cortical deletion prevents cross-reactive and ubiquitously self-reactive thymocytes from developing into T-reg cells.
The perinatal period is a critical time for immune tolerance (33). Perinatal T-reg cells are more effective than adult T-reg cells at preventing autoimmune disease provoked by Aire deficiency (34). The perinatal T-reg TCR repertoire is distinct from, and more diverse than, the adult T-reg TCR repertoire (34). The age-dependent change in T-reg selection is partly attributable to mTEC directly presenting a higher number of self-antigens in perinates than in adults (34). However, several findings suggest the extent of cortical deletion is also different in perinates and adults. The percentage of strongly TCR-signalled (Helios+) cells in the immature (CCR7– CD24+) thymocyte population increases with age (Figure 1). This effect, which was reported previously (35), suggests cortical deletion is smaller in magnitude during the perinatal period than in adult life. This may be due to MHCIIhigh CD8α+ DC being less frequent in the perinatal thymus than in the adult thymus (34). Although cortical thymic epithelial cells can induce strong TCR signaling in some thymocytes (20, 36, 37), BM-derived APC (BM-APC), including DC, are present in the cortex (38, 39) and are required for normal cortical deletion (20, 36, 37, 40). An age-dependent change in the fate of thymocytes specific for one natural self-antigen has been documented (6). In mice with transgenic expression of the TCRβ chain from the Yae62 TCR (Yae62β-tg) (41), peptidyl arginine deiminase type IV (Padi4):I-Ab is a Tregitope at 1-3 weeks after birth; however, Padi4:I-Ab-specific thymocytes are deleted at the DP stage or at the DP-CD4SP transition from 4 weeks after birth onwards (6). Analysis of Padi4–/– Yae62β-tg mice confirmed Padi4:I-Ab-specific thymocytes undergo T-reg development in perinates and deletion in adults in response to Padi4 itself, with Padi4 expression in BM-APC sufficient to induce deletion in adult mice (6). Thymocytes with the potential to develop into T-reg cells can be deleted instead of developing into T-reg cells if they encounter a related self-peptide, or the cognate self-peptide itself, at the DP stage in the thymic cortex.
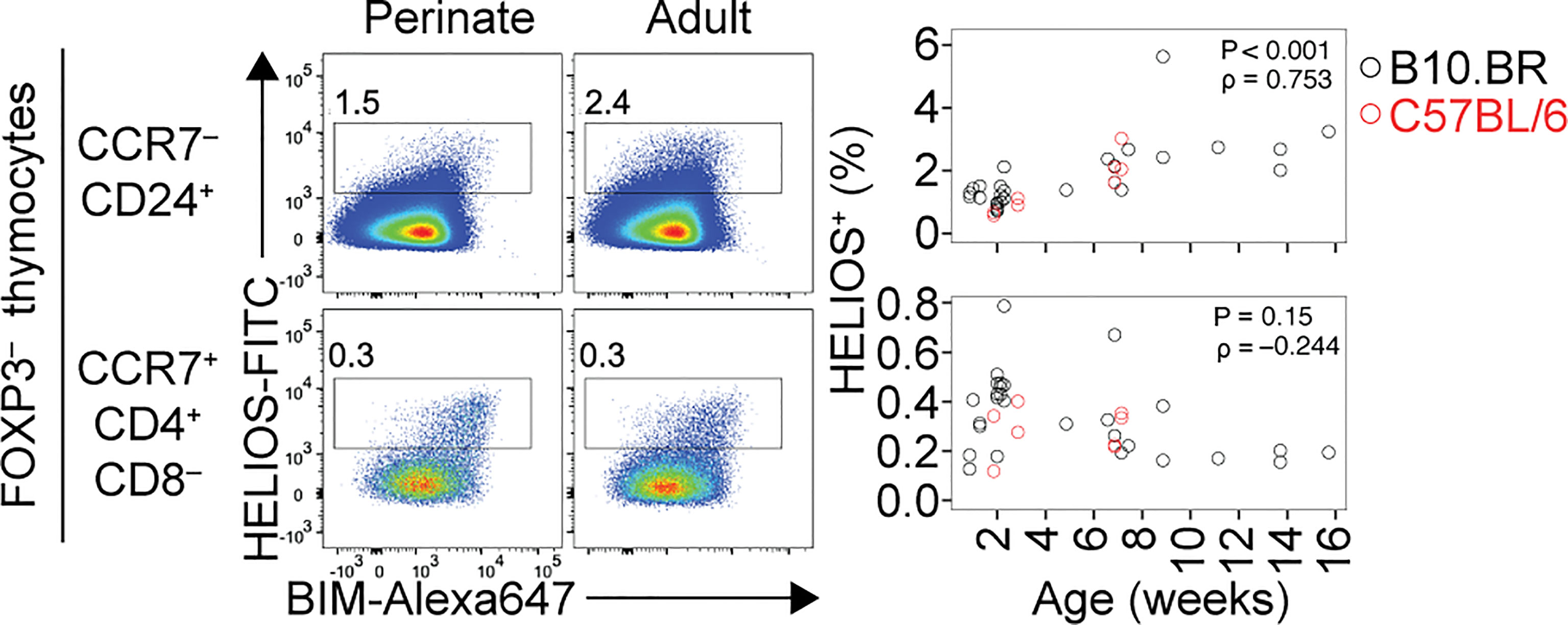
Figure 1 Age-dependent shift in cortical and medullary tolerance induction in the thymus. Flow cytometry plots (left) show HELIOS/BIM phenotypes of Foxp3– thymocytes, divided into CCR7– CD24+ (cortical) and CCR7+ CD4+ CD8– (medullary) populations. Note that HELIOS and BIM tend to be co-expressed. Within each population, numbers on the plots show the percentage of HELIOS+ cells among all thymocytes, with graphs (right) showing results for multiple mice of the indicated ages and strains. Cortical tolerance appears less prominent during perinatal life, potentially enabling a higher frequency of strongly self-reactive thymocytes to develop into T-reg cells in the medulla in perinates compared to adults. P and rho values were determined using Spearman’s test for correlation.
Events that occur within the DP stage in thymocytes that become T-reg cells have been puzzling to resolve. Commitment to the T-reg lineage within the DP stage was thought to be common, based on flow cytometry data indicating ~ 33% of Foxp3+ thymocytes in adult wild-type mice were DP cells (42). However, another study reported <10% of Foxp3+ thymocytes were DP cells including during the perinatal period (31). After rigorous exclusion of doublet events during flow cytometric analysis, <5% of Foxp3+ thymocytes had a DP phenotype (32). Accordingly, analysis of thymocytes that had incorporated a DNA label at the DP stage showed that Foxp3 upregulation predominantly occurs 4-8 days after label uptake, by which time the labelled cells have acquired a CCR7+ CD4SP phenotype (43) and moved to the medulla (44). Although we cannot exclude the possibility that commitment to the T-reg lineage can occur at the DP stage in the cortex, we favour the view that this usually occurs at the CCR7+ CD4SP stage when the thymocytes are in the medulla.
Current Concepts of Developmental Niches for T-reg Cells in the Thymic Medulla
T-reg cells that develop in the thymus are thought to encounter their cognate self-antigen for the first time in the medulla (45, 46). This is plausible because the intra-thymic expression of some self-antigens, including tissue-restricted antigens (TRA), is confined to mTEC (47). Two major mTEC subsets are distinguished by expression of CCL21 (48), a chemokine that attracts CCR7+ thymocytes to the medulla (49), or the nuclear protein, Aire, which is required for normal expression of thousands of TRA by mTEC (50–52). Another nuclear protein, Fezf2, which is required for a distinct program of TRA expression independent of Aire (53), is expressed by CCL21+ mTEC and Aire+ mTEC (54, 55). Self-antigens expressed by mTEC can be taken up and presented by BM-APC (56, 57) and the presentation of some self-antigens to thymocytes is completely dependent on this mechanism (58, 59). While the mTEC population collectively expresses almost all protein-coding genes, the expression of individual genes varies widely both at the level of transcript abundance and in the frequency of mTECs that express the transcript (52). Many self-antigens are thought to be presented to thymocytes in small and discrete foci, which form a “mosaic” of developmental niches for antigen-specific T-reg cells in the medulla (45, 60).
This “mosaic” of self-antigen expression is shaped by proliferation, differentiation, and maturation of mTEC. Proliferating mTEC, which express many chromatin-modifying factors and some TRA, give rise to cells that express Aire and a higher number of TRAs per mTEC (54, 61). Whether CCL21+ mTEC are precursors or progeny of proliferating mTEC remains unclear (35, 54, 61). The current concept is that an individual mTEC expresses different sets of self-antigens over its lifetime (61–63). In support of this “colinear differentiation” model (62), an individual mTEC can switch off expression of one self-antigen and switch on expression of another (62, 64). In addition, single-cell RNA sequencing identified sets of self-antigens that were co-expressed in multiple mTEC (61, 63, 65). In this model, the presence of cells spanning all mTEC subsets and all maturation stages is necessary and sufficient for the expression of a full “mosaic” of self-antigens in the medulla.
The thymic medulla is smaller in mice lacking CD4SP thymocytes compared to wild-type mice or mice lacking CD8SP thymocytes (66). Development of the mature mTEC population, defined by high expression of MHCII and the costimulatory molecule CD80, and comprising an Aire+ subset, requires cognate interactions between the TCR on CD4SP thymocytes and pMHCII on mTECs (67). CD4SP thymocytes express the ligands for RANK, CD40, and LTβR, which are cell-surface receptors necessary for mTEC maturation (66, 68, 69). Anti-RANK ligand (RANKL) antibody treatment and the absence of self-reactive CD4SP thymocytes both cause deficiency of Aire+ mTEC, whereas CCL21+ mTEC remain largely intact (54, 55). Notably, anti-RANKL antibodies diminish the frequency of proliferating cells in the mTEC population (54, 70), whereas the absence of self-reactive CD4SP thymocytes does not (55). It is possible that invariant NKT cells provide enough RANKL to support mTEC proliferation (71). However, normal transition from the proliferating stage to the Aire+ stage in mTEC development requires signals uniquely provided during cognate interactions with self-reactive CD4SP thymocytes (66). Similarly, normal development of mature thymic DCs requires cognate TCR-pMHCII interactions with CD4SP thymocytes (72).
CD4SP thymocytes also contribute to thymic T-reg cell niches by producing IL-2 (73, 74). Consumption of this IL-2 prevents deletion of strongly TCR-signalled CD4SP thymocytes (75) and enables these T-reg precursors to upregulate Foxp3 expression (76, 77). CD4SP thymocytes are thus both inducers and “clients” of antigen-specific T-reg cell niches in the thymic medulla.
A Role for CD4SP Thymocytes in Generating the T-reg Niche Containing Their Cognate Self-Antigen?
To this picture we wish to add the hypothesis that strongly self-reactive CD4SP thymocytes foment the niche containing their cognate self-antigen. Sustained, repetitive, cognate interactions between CD4SP thymocytes and mTEC may be necessary for the survival of post-cycling Aire+ mTEC and may induce the mTEC to pause or arrest its “colinear differentiation” program. In other words, these interactions may “lock in” continued expression of those self-antigens that the mTEC is expressing at the time. Currently available data do not exclude this extended model. During the post-cycling Aire+ stage, different studies found the mean number of TRAs expressed per Aire+ mTEC remained constant (61) or increased by a factor of only two (54). Multiple mTEC that co-express sets of self-antigens may be “daughters of the same epithelial cell progenitor” (65).
Through this process, antigen-specific CD4SP thymocytes may seed a niche for the development of T-reg specific for the same self-antigen or another self-antigen in the same co-expression “module” (61). Generating a functional T-reg niche requires collaboration between CD4SP thymocytes because no single cell can fulfil all functions required of CD4SP thymocytes. These functions include: (i) to induce the post-cycling Aire+ mTEC to survive and “lock in” its current self-antigen expression profile; (ii) to induce the mTEC and local DC to upregulate antigen-presenting and costimulatory molecules; (iii) to produce IL-2; and (iv) to develop from a naïve CD4SP thymocyte into a T-reg precursor and then into a T-reg cell. At another level, there is inter-niche competition because the medullary volume limits the number of niches present at a given time. Success in this inter-niche competition may be proportional to the extent of intra-niche collaboration, which is in turn dictated by the size of the antigen-specific CD4SP thymocyte population in the medulla (Figure 2).
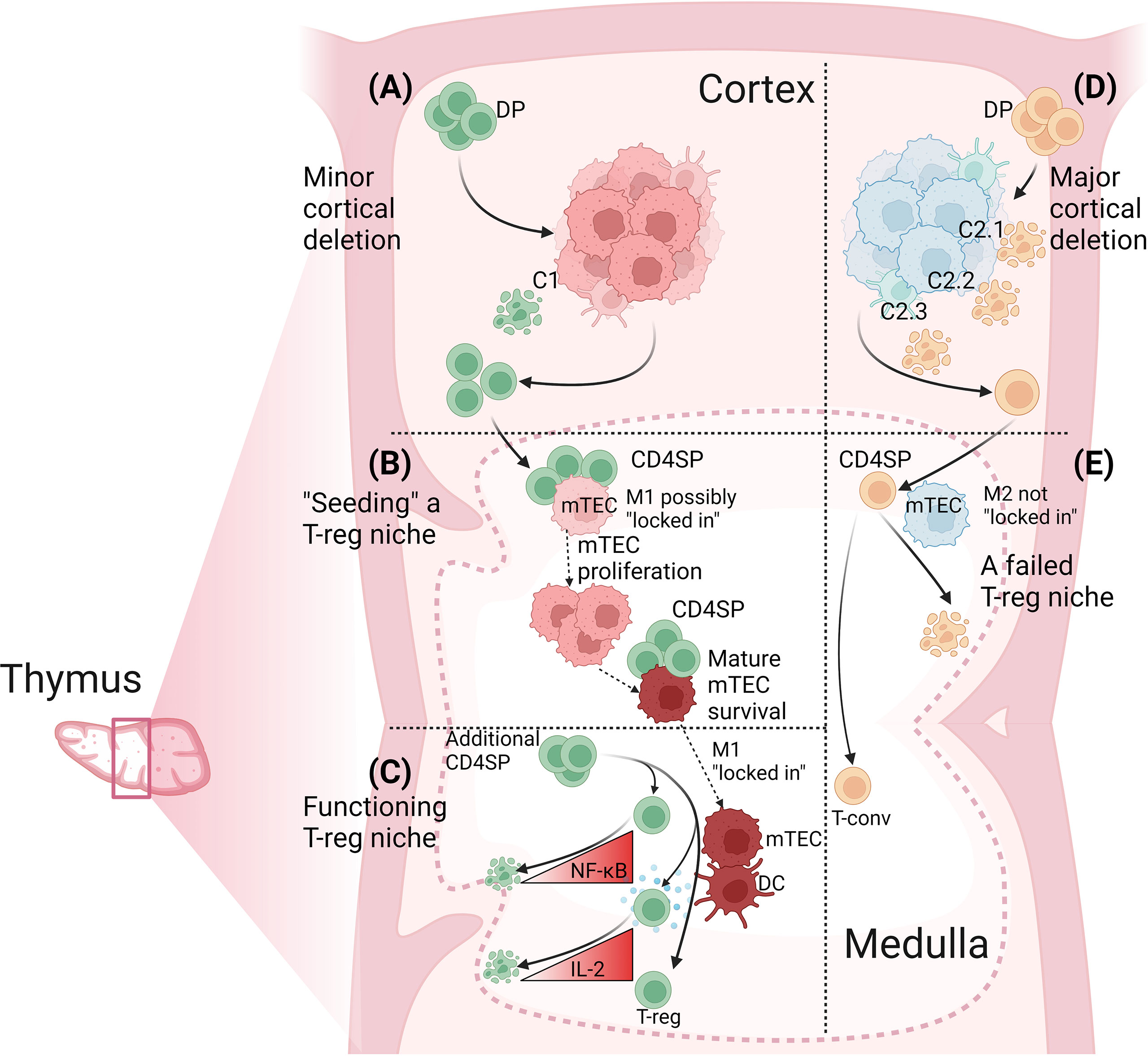
Figure 2 Determinants of antigen-specific T-reg niche size in the thymus. (A) The extent of cortical deletion is minor when the relatedness between cortical and medullary peptides is low. Out of four DP thymocytes specific for a medullary peptide, M1, one is deleted by a related cortical peptide, C1, and the other three progress to the CD4SP stage and migrate into the medulla. (B) Cognate CD4SP-mTEC interactions “seed” the T-reg niche. CD4SP thymocytes could mediate this effect by interacting with immature mTEC prior to the onset of proliferation and/or by promoting mTEC survival at the post-cycling stage through sustained engagement of RANK, CD40 and LTβR expressed on mTEC. The key outcome is that M1-specific CD4SP thymocytes induce the M1-presenting mTEC to “lock in” M1 expression. (C) A functioning T-reg niche. By now, the mature M1-expressing mTEC has high expression of MHCII, CD80, Aire and the protein from which the M1 peptide is derived. M1-specific CD4SP thymocytes also induce local DC to increase MHCII and CD80 expression. M1-specific CD4SP thymocytes also produce IL-2. However, most M1-specific CD4SP thymocytes undergo deletion due to insufficient NF-κB activation or insufficient IL-2 consumption. Rare M1-specific CD4SP thymocytes activate sufficient NF-κB and consume sufficient IL-2 to survive, upregulate Foxp3 and progress to the next stage of T-reg development. (D) The extent of cortical deletion is major when the relatedness between cortical and medullary peptides is high. Out of four DP thymocytes specific for medullary peptide, M2, three are deleted in the cortex by related self-peptides, C2.1, C2.2 and C2.3, and only one progresses to the CD4SP stage and migrates into the medulla. (E) A failed T-reg niche. The number of M2-specific CD4SP thymocytes is too low to provide inductive signals to the M2-expressing mTEC. The mTEC may switch off expression of M2 and switch on expression of different self-antigens. Activation of M2-specific CD4SP thymocytes is insufficient to induce T-reg development but may be sufficient to induce deletion of some cells. No M2-specific T-reg niche forms and the small M2-specific CD4SP population develops into T-conv cells. Figure created with BioRender.com.
This extended hypothesis can accommodate some unexplained findings. Two TCRs, called DO11 and N7, can facilitate T-reg development in mice expressing the neo-self-antigen, ovalbumin (OVA) (78). For these two TCRs, as had been observed in other models (2, 79), antigen-specific CD4SP thymocyte population size and Foxp3+ cell frequency were inversely related, consistent with T-reg development being constrained by OVA:I-Ad availability (78). Surprisingly, and in contrast to the inverse relationship, T-reg development failed when the CD4SP thymocyte populations expressing the DO11 or N7 TCR were very small (78). We suggest the OVA:I-Ad-specific population size had a lower limit, below which intra-clonal collaboration between the TCR-transgenic CD4SP thymocytes was insufficient to generate a niche for effective OVA:I-Ad-specific T-reg development.
Our hypothesis also accommodates complementary findings based on CD4+ T cell responses to natural self-antigens. Although T-reg cell populations specific for myelin oligodendrocyte glycoprotein (MOG):I-Ab can be expanded in B6 mice immunized with MOG peptide (8, 80), MOG:I-Ab-specific T-reg cells are rare in naïve B6 mice (27, 81). A notably different phenotype is observed in B6.Kaa mice (81), which express a transgenic TCRβ chain repetitively found in MOG:I-Ab-specific T cells (82). Compared to naïve B6 mice, naïve B6.Kaa mice have 4 times more MOG:I-Ab-specific CD4+ T-conv cells and 16 times more MOG:I-Ab-specific CD4+ T-reg cells (81). Furthermore, proliferation and suppressive function of MOG:I-Ab-specific CD4+ T-reg cells were demonstrable in T-reg populations from naïve B6.Kaa mice but not from naïve B6 mice (81). Similar findings were made in an analogous study of CD4+ T cells specific for proteolipid protein (PLP):I-Ab (83). In the latter study, comparison of Plp1+/+ and Plp1–/– mice on the TCRβ-transgenic background provided the additional insight that PLP expression induced the post-thymic expansion of PLP:I-Ab-specific CD4+ T-reg and Foxp3– FR4+ CD73+ anergic (84) populations, but not the Foxp3– FR4– CD73– naïve T-conv cell population (83). In both studies (81, 83), enlarging the antigen-specific CD4SP thymocyte population via a TCRβ transgene expanded the antigen-specific T-reg cell niche.
A challenge to our hypothesis is that the MOG:I-Ab-specific CD4+ population in naïve B6 mice is relatively large, close to the top of the spectrum of foreign pMHCII-specific population sizes (29). Our hypothesis would predict the large MOG:I-Ab-specific CD4SP thymocyte population in B6 mice ought to establish a MOG:I-Ab-specific T-reg cell niche. To compare the intra-thymic expression of MOG with natural self-antigens known to form Tregitopes (4–6), we analyzed data from two studies that conducted RNA sequencing on mTEC samples (52, 63). In this panel of 20 self-antigens, both studies found MOG had the lowest expression in mTECs (Figure 3), suggesting low “basal” MOG expression in the thymic medulla might limit MOG:I-Ab-specific T-reg niche generation in B6 mice. An initial test of our hypothesis would be to compare the abundance of MOG transcripts in mTEC from B6 versus B6.Kaa mice. We predict the unusually large MOG:I-Ab-specific CD4SP thymocyte population in B6.Kaa mice would result in higher MOG transcription in the mTEC population. Our hypothesis would also predict the presence of B6.Kaa thymocytes should enhance T-reg development in co-resident wild-type thymocytes in mixed chimeras.
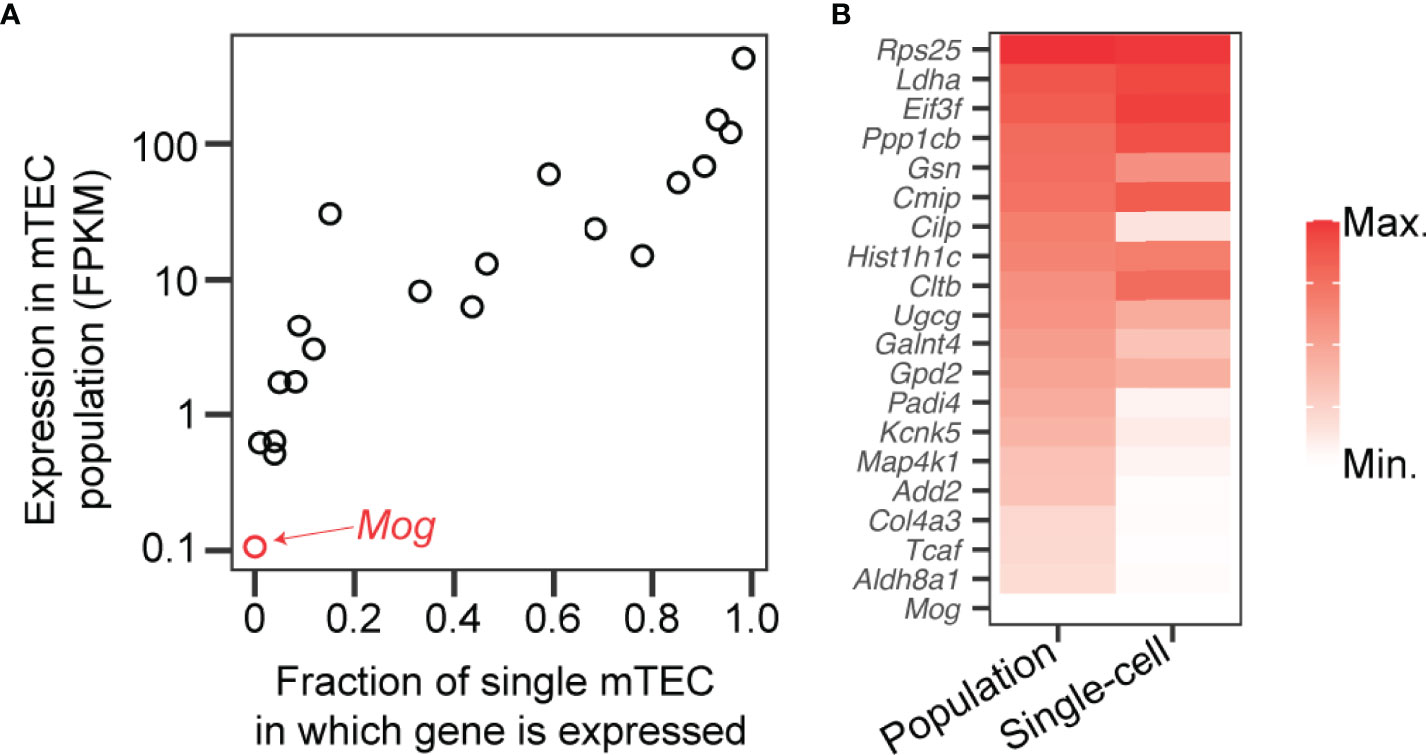
Figure 3 Low expression of Mog compared to self-antigens known to induce T-reg cell development in the thymus. (A) Transcripts encoding myelin oligodendrocyte glycoprotein (Mog) and 19 self-antigens known to induce thymic T-reg development (4–6) were measured by RNA sequencing of mTEC at the population level (y-axis) (52) and in 305 single cells (x-axis) (63). FPKM, fragments per kilobase of exon per million mapped fragments. (B) Transcription levels of the 20 genes represented in (A) in the mTEC population (left) and in single mTEC (right) with red shading according to the scales shown on the axes in (A).
Impact of Thymocyte Deletion in the Medulla on T-reg Selection
Outside the thymus, in a self-tolerant and functional immune system, T-reg cells are thought to outcompete T-conv cells for APCs that are presenting self-antigens, whereas the reverse would apply for APCs presenting foreign antigens (85). Theoretically, self-tolerance should be most robust if the thymus selected the most self-reactive thymocytes into the T-reg lineage, in order to maximize the difference in self-reactivity between T-reg and T-conv cells. However, this is not observed experimentally. Antigen-specific T-reg cells bind fewer pMHCII tetramer molecules per cell than some antigen-specific T-conv cells in mice lacking the self-antigen (8, 80). This suggests the self-antigen deletes those thymocytes that express the most self-reactive TCRs. Consistent with this conclusion, the half-lives and functional avidities of 10 Padi4:I-Ab-specific TCRs indicated the high, intermediate and low ranges of TCR self-reactivity induced deletion, (perinatal) T-reg cell development, and T-conv cell development, respectively (6). However, there is also compelling evidence that CD4SP thymocytes can undergo deletion as a result of their TCR self-reactivity being too low for T-reg development (86). Accordingly, in a panel of 4 PLP:I-Ab-specific TCRs, the TCRs with the highest and lowest functional avidity induced deletion, whereas the 2 TCRs with intermediate functional avidity induced T-reg development (83). The TCR self-reactivity most conducive to T-reg development would appear to be “sandwiched” between two ranges of TCR self-reactivity that induce deletion in the thymic medulla.
After CD4SP thymocytes initiate strong TCR signaling in the medulla, the thymocyte-intrinsic pathways required to prevent deletion change as the thymocyte matures. Canonical NF-κB activation prevents deletion within the Foxp3– T-reg precursor stage (22, 87–89), whereas IL-2 signaling prevents deletion at a later stage, close to the time of Foxp3 upregulation (75). DOCK8 inhibits deletion at both of these stages (90). For CD4SP thymocytes inside a medullary T-reg cell niche, survival requires signaling that is not required for deletion. Evidence that most of these cells are deleted (22, 75) suggests those cells that complete T-reg development are rigorously selected.
CD4SP thymocytes can also develop into T-reg cells via a developmental pathway that includes a Foxp3+ CD25– T-reg precursor stage (31, 91). Compared to Foxp3– CD25+ T-reg precursors, Foxp3+ CD25– T-reg precursors take longer to develop, tend to have lower TCR self-reactivity and are less susceptible to deletion (92). This alternative pathway may be used by TCRs such as the OVA:I-Ad-specific TCR called R4 (78) and another TCR called G113 (2). The R4 and G113 TCRs still induce T-reg development when they are expressed by very few CD4SP thymocytes (2, 78), implying intra-clonal collaboration is unnecessary for these TCRs to support T-reg development. Unlike the DO11 and N7 TCRs, the R4 and G113 TCRs do not induce measurable deletion (2, 78), suggesting only TCRs that trigger deletion require the antigen-specific CD4SP thymocyte population size to exceed a lower limit in order to induce T-reg development. CD4SP thymocytes with a TCR self-reactivity too low to induce deletion would be expected to have a longer lifespan in the thymic medulla, which may increase their probability of finding a pre-existing, functional T-reg niche, and surviving long enough to upregulate Foxp3. Whether an antigen-specific CD4SP Foxp3– thymocyte is at risk of deletion or not, it can still contribute to the antigen-specific T-reg cell niche by providing inductive signals to APCs and by producing IL-2 (Figure 2).
The adult thymus also contains recirculating or thymus-resident T-reg cells (93), which may impact de novo thymic T-reg development. GK-transgenic mice, which have few peripheral CD4+ T cells and few non-nascent T-reg cells in the thymus due to transgenic expression of an anti-CD4 antibody, have a slightly higher frequency of Foxp3+ thymocytes than wild-type mice (94). Non-nascent T-reg cells may thus limit de novo thymic T-reg development by competing for limiting IL-2 (94, 95). However, de novo thymic T-reg development is not reduced in mice with enlarged non-nascent thymic T-reg cell populations (96, 97). As non-nascent T-reg cells express more Tnfsf11 and Cd40lg transcripts (which encode RANKL and CD40L, respectively) than nascent thymic T-reg cells (94), they may also positively affect the thymic T-reg niche by providing inductive signals to APC.
Discussion
Certain self-antigens reproducibly “select” CD4SP thymocytes to enter the T-reg lineage (4–6). We refer to these self-antigens as Tregitopes. Here, we postulate a mechanism that operates in the opposite direction, wherein CD4SP thymocytes “select” self-antigens to become Tregitopes. This hypothesis draws on evidence that the major T-reg-inducing APC subsets in the thymus, mTEC and DC, require cognate TCR-pMHCII-dependent interactions with CD4SP thymocytes in order to form mature populations (55, 66, 67, 72). We propose CD4SP thymocytes deliver signals that promote mature mTEC survival and “lock in” the set of self-antigens being expressed by the mTEC at the time. This endows a self-reactive CD4SP thymocyte with the ability to generate a medullary niche containing its cognate self-antigen, enabling subsequent development of T-reg cells specific for the same self-antigen. We propose the “mosaic” of antigen-specific T-reg niches in the thymic medulla (45, 60) is not predetermined but is shaped by the antigen specificities of CD4SP thymocytes in the medulla. Deletion creates variation in the number of CD4SP thymocytes specific for different self-antigens (27, 28). The size of the antigen-specific T-reg niche in the medulla may be inversely related to the extent of cortical deletion of antigen-specific thymocyte populations (Figure 2).
It is unclear why α3/DR1 is a Tregitope, whereas α3/DR15 is not (4). Although the thymus was not analyzed, peripheral CD4+ T cell populations in mice expressing these human HLA molecules contained a higher frequency of α3/DR1-specific cells than α3/DR15-specific cells (4). This difference may be due to greater cortical deletion of α3/DR15-specific thymocytes compared to α3/DR1-specific cells. If so, then this cortical deletion is unlikely to be triggered by α3/DR15 itself, as the α3 protein is sparsely expressed in the thymic medulla (12). Furthermore, α3/DR1 is a Tregitope, suggesting the α3 self-peptide is not displayed to cortical thymocytes. We infer that cortical deletion of α3/DR15-specific thymocytes is mediated by related self-peptides with similar TCR-exposed residues (29). An initial test of this hypothesis may involve enumerating antigen-specific thymocytes at distinct maturation stages, as described (6). Our hypothesis would predict the presence of DR1 ought to “lock in” α3 expression and augment selection of α3/DR15-specific T-reg cells. However, DR1 expression did not affect the α3/DR15-specific T-reg or T-conv cell frequency in DR1+ DR15+ mice compared to DR15+ mice (4). Differential affinity of the α3 peptide for DR1 versus DR15 may lead to differences in the quantity of the two pMHCII complexes. Alternatively, differences in the chemistry of the different TCR-exposed peptide residues may lead to differences in the TCR affinity distribution of CD4SP thymocytes specific for the two pMHCII complexes. These differences may bias T-reg development towards the α3/DR1 Tregitope despite co-expression of the two MHC alleles.
Associations between human autoimmune diseases and particular MHC alleles (98) indicate a role for TCR-peptide-MHC interactions in pathogenesis. The current paradigm is that autoimmune diseases are mediated by pro-inflammatory T-conv cells specific for self-peptides presented by disease-associated MHC alleles (99). Interestingly, compared to TCR-peptide-MHC interactions elicited by infection or immunization, some autoimmune interactions have unusual features, including atypical positioning of the TCR or the self-peptide, post-translational self-peptide modifications and self-peptide fusions (99). These findings shed light on the nature of inappropriate self-antigen recognition by T cells. However, they do not explain why only some people with disease-associated MHC alleles develop autoimmune disease. This implies the action of an additional predisposing factor, such as the absence of an antigen-specific T-reg cell population that would otherwise prevent autoimmune disease. The association between autoimmune diseases and particular MHC alleles may reflect the lack of an organ-specific self-peptide that can form a Tregitope when presented by the disease-associated MHCII allele. We refer to this as Tregitope deficiency.
Other genetic factors may combine with a disease-associated MHCII allele to avert or contribute to Tregitope deficiency. Co-expression of an MHCII allele that can form a Tregitope can avert Tregitope deficiency, as exemplified with HLA-DR1 in Goodpasture’s disease (4, 13). Tregitope sufficiency or deficiency may explain why pairs of HLA haplotypes are associated with a decreased or increased risk of autoimmune diseases beyond the additive contributions of each haplotype (100). In addition, Tregitope deficiency may require a high relatedness between (at least) two self-peptides, one presented in the cortex and the other in the medulla, a situation that may extinguish the potential of the medullary self-peptide to serve as a Tregitope. Hence, Tregitope deficiency would be expected in only a subset of individuals who inherit a disease-associated MHCII allele, providing an explanation for why most such individuals never develop autoimmune disease.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by The Animal Experimentation Ethics Committee of the Australian National University.
Author Contributions
CW and SD jointly prepared figures and wrote the paper. All authors contributed to the article and approved the submitted version.
Funding
CW and SD received support from the Queensland University of Technology. SD received support from the National Health and Medical Research Council (grant 1188589).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sakaguchi S, Mikami N, Wing JB, Tanaka A, Ichiyama K, Ohkura N. Regulatory T Cells and Human Disease. Annu Rev Immunol (2020) 38:541. doi: 10.1146/annurev-immunol-042718-041717
2. Bautista JL, Lio CW, Lathrop SK, Forbush K, Liang Y, Luo J, et al. Intraclonal Competition Limits the Fate Determination of Regulatory T Cells in the Thymus. Nat Immunol (2009) 10:610–17. doi: 10.1038/ni.1739
3. Levine AG, Arvey A, Jin W, Rudensky AY. Continuous Requirement for the TCR in Regulatory T Cell Function. Nat Immunol (2014) 15:1070–78. doi: 10.1038/ni.3004
4. Ooi JD, Petersen J, Tan YH, Huynh M, Willett ZJ, Ramarathinam SH, et al. Dominant Protection From HLA-Linked Autoimmunity by Antigen-Specific Regulatory T Cells. Nature (2017) 545:243–47. doi: 10.1038/nature22329
5. Leonard JD, Gilmore DC, Dileepan T, Nawrocka WI, Chao JL, Schoenbach MH, et al. Identification of Natural Regulatory T Cell Epitopes Reveals Convergence on a Dominant Autoantigen. Immunity (2017) 47:107–17. doi: 10.1016/j.immuni.2017.06.015
6. Stadinski BD, Blevins SJ, Spidale NA, Duke BR, Huseby PG, Stern LJ, et al. A Temporal Thymic Selection Switch and Ligand Binding Kinetics Constrain Neonatal Foxp3+ Treg Cell Development. Nat Immunol (2019) 20:1046–58. doi: 10.1038/s41590-019-0414-1
7. Ferrante A. For Many But Not for All: How the Conformational Flexibility of the Peptide/MHCII Complex Shapes Epitope Selection. Immunol Res (2013) 56:85–95. doi: 10.1007/s12026-012-8342-2
8. Malhotra D, Linehan JL, Dileepan T, Lee YJ, Purtha WE, Lu JV, et al. Tolerance is Established in Polyclonal CD4+ T Cells by Distinct Mechanisms, According to Self-Peptide Expression Patterns. Nat Immunol (2016) 17:187–95. doi: 10.1038/ni.3327
9. Legoux FP, Lim JB, Cauley AW, Dikiy S, Ertelt J, Mariani TJ, et al. CD4+ T Cell Tolerance to Tissue-Restricted Self Antigens Is Mediated by Antigen-Specific Regulatory T Cells Rather Than Deletion. Immunity (2015) 43:896–08. doi: 10.1016/j.immuni.2015.10.011
10. Ebert PJ, Ehrlich LI, Davis MM. Low Ligand Requirement for Deletion and Lack of Synapses in Positive Selection Enforce the Gauntlet of Thymic T Cell Maturation. Immunity (2008) 29:734–45. doi: 10.1016/j.immuni.2008.09.014
11. Peterson DA, DiPaolo RJ, Kanagawa O, Unanue ER. Cutting Edge: Negative Selection of Immature Thymocytes by a Few Peptide-MHC Complexes: Differential Sensitivity of Immature and Mature T Cells. J Immunol (1999) 162:3117–20.
12. Wong D, Phelps RG, Turner AN. The Goodpasture Antigen is Expressed in the Human Thymus. Kidney Int (2001) 60:1777–83. doi: 10.1046/j.1523-1755.2001.00014.x
13. Phelps RG, Rees AJ. The HLA Complex in Goodpasture's Disease: A Model for Analyzing Susceptibility to Autoimmunity. Kidney Int (1999) 56:1638–53. doi: 10.1046/j.1523-1755.1999.00720.x
14. Ooi JD, Chang J, O'Sullivan KM, Pedchenko V, Hudson BG, Vandenbark AA, et al. The HLA-DRB1*15:01-Restricted Goodpasture's T Cell Epitope Induces GN. J Am Soc Nephrol (2013) 24:419–31. doi: 10.1681/ASN.2012070705
15. Daley SR, Teh C, Hu DY, Strasser A, Gray DHD. Cell Death and Thymic Tolerance. Immunol Rev (2017) 277:9–20. doi: 10.1111/imr.12532
16. Ehrlich LI, Oh DY, Weissman IL, Lewis RS. Differential Contribution of Chemotaxis and Substrate Restriction to Segregation of Immature and Mature Thymocytes. Immunity (2009) 31:986–98. doi: 10.1016/j.immuni.2009.09.020
17. Halkias J, Melichar HJ, Taylor KT, Ross JO, Yen B, Cooper SB, et al. Opposing Chemokine Gradients Control Human Thymocyte Migration in Situ. J Clin Invest (2013) 123:2131–42. doi: 10.1172/JCI67175
18. Douek DC, Corley KT, Zal T, Mellor A, Dyson PJ, Altmann DM. Negative Selection by Endogenous Antigen and Superantigen Occurs at Multiple Thymic Sites. Int Immunol (1996) 8:1413–20. doi: 10.1093/intimm/8.9.1413
19. Liblau RS, Tisch R, Shokat K, Yang X, Dumont N, Goodnow CC, et al. Intravenous Injection of Soluble Antigen Induces Thymic and Peripheral T-Cells Apoptosis. Proc Natl Acad Sci USA (1996) 93:3031–36. doi: 10.1073/pnas.93.7.3031
20. McCaughtry TM, Baldwin TA, Wilken MS, Hogquist KA. Clonal Deletion of Thymocytes can Occur in the Cortex With No Involvement of the Medulla. J Exp Med (2008) 205:2575–84. doi: 10.1084/jem.20080866
21. Hu Z, Lancaster JN, Sasiponganan C, Ehrlich LI. CCR4 Promotes Medullary Entry and Thymocyte-Dendritic Cell Interactions Required for Central Tolerance. J Exp Med (2015) 212:1947–65. doi: 10.1084/jem.20150178
22. Daley SR, Hu DY, Goodnow CC. Helios Marks Strongly Autoreactive CD4+ T Cells in Two Major Waves of Thymic Deletion Distinguished by Induction of PD-1 or NF-κb. J Exp Med (2013) 210:269–85. doi: 10.1084/jem.20121458
23. Sinclair C, Bains I, Yates AJ, Seddon B. Asymmetric Thymocyte Death Underlies the CD4:CD8 T-Cell Ratio in the Adaptive Immune System. Proc Natl Acad Sci USA (2013) 110:E2905–14. doi: 10.1073/pnas.1304859110
24. Stritesky GL, Xing Y, Erickson JR, Kalekar LA, Wang X, Mueller DL, et al. Murine Thymic Selection Quantified Using a Unique Method to Capture Deleted T Cells. Proc Natl Acad Sci U S A (2013) 110:4679–84. doi: 10.1073/pnas.1217532110
25. Cowan JE, McCarthy NI, Parnell SM, White AJ, Bacon A, Serge A, et al. Differential Requirement for CCR4 and CCR7 During the Development of Innate and Adaptive αβt Cells in the Adult Thymus. J Immunol (2014) 193:1204–12. doi: 10.4049/jimmunol.1400993
26. Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, et al. Naive CD4+ T Cell Frequency Varies for Different Epitopes and Predicts Repertoire Diversity and Response Magnitude. Immunity (2007) 27:203–13. doi: 10.1016/j.immuni.2007.07.007
27. Watanabe M, Lu Y, Breen M, Hodes RJ. B7-CD28 Co-Stimulation Modulates Central Tolerance via Thymic Clonal Deletion and Treg Generation Through Distinct Mechanisms. Nat Commun (2020) 11:6264. doi: 10.1038/s41467-020-20070-x
28. Chu HH, Moon JJ, Kruse AC, Pepper M, Jenkins MK. Negative Selection and Peptide Chemistry Determine the Size of Naive Foreign Peptide-MHC Class II-Specific CD4+ T Cell Populations. J Immunol (2010) 185:4705–13. doi: 10.4049/jimmunol.1002276
29. Nelson RW, Beisang D, Tubo NJ, Dileepan T, Wiesner DL, Nielsen K, et al. T Cell Receptor Cross-Reactivity Between Similar Foreign and Self Peptides Influences Naive Cell Population Size and Autoimmunity. Immunity (2015) 42:95–107. doi: 10.1016/j.immuni.2014.12.022
30. McDonald BD, Bunker JJ, Erickson SA, Oh-Hora M, Bendelac A. Crossreactive αβ T Cell Receptors Are the Predominant Targets of Thymocyte Negative Selection. Immunity (2015) 43:859–69. doi: 10.1016/j.immuni.2015.09.009
31. Fontenot JD, Dooley JL, Farr AG, Rudensky AY. Developmental Regulation of Foxp3 Expression During Ontogeny. J Exp Med (2005) 202:901–6. doi: 10.1084/jem.20050784
32. Lee HM, Hsieh CS. Rare Development of Foxp3+ Thymocytes in the CD4+CD8+ Subset. J Immunol (2009) 183:2261–66. doi: 10.4049/jimmunol.0901304
33. Guerau-de-Arellano M, Martinic M, Benoist C, Mathis D. Neonatal Tolerance Revisited: A Perinatal Window for Aire Control of Autoimmunity. J Exp Med (2009) 206:1245–52. doi: 10.1084/jem.20090300
34. Yang S, Fujikado N, Kolodin D, Benoist C, Mathis D. Immune Tolerance. Regulatory T Cells Generated Early in Life Play a Distinct Role in Maintaining Self-Tolerance. Science (2015) 348:589–94. doi: 10.1126/science.aaa7017
35. Baran-Gale J, Morgan MD, Maio S, Dhalla F, Calvo-Asensio I, Deadman ME, et al. Ageing Compromises Mouse Thymus Function and Remodels Epithelial Cell Differentiation. Elife (2020) 9. doi: 10.7554/eLife.56221
36. Wirasinha RC, Chan A, Yap JY, Hu DY, Teh CE, Gray DHD, et al. Deletion of Self-Reactive CCR7- Thymocytes in the Absence of MHC Expression on Thymic Epithelial Cells. Cell Death Differ (2019) 26:2727–39. doi: 10.1038/s41418-019-0331-8
37. Kurd NS, Hoover A, Yoon J, Weist BM, Lutes L, Chan SW, et al. Factors That Influence the Thymic Selection of CD8αα Intraepithelial Lymphocytes. Mucosal Immunol (2021) 14:68–79. doi: 10.1038/s41385-020-0295-5
38. Raviola E, Karnovsky MJ. Evidence for a Blood-Thymus Barrier Using Electron-Opaque Tracers. J Exp Med (1972) 136:466–98. doi: 10.1084/jem.136.3.466
39. Ladi E, Schwickert TA, Chtanova T, Chen Y, Herzmark P, Yin X, et al. Thymocyte-Dendritic Cell Interactions Near Sources of CCR7 Ligands in the Thymic Cortex. J Immunol (2008) 181:7014–23. doi: 10.4049/jimmunol.181.10.7014
40. Melichar HJ, Ross JO, Herzmark P, Hogquist KA, Robey EA. Distinct Temporal Patterns of T Cell Receptor Signaling During Positive Versus Negative Selection in Situ. Sci Signal (2013) 6::ra92. doi: 10.1126/scisignal.2004400
41. Stadinski BD, Trenh P, Smith RL, Bautista B, Huseby PG, Li G, et al. A Role for Differential Variable Gene Pairing in Creating T Cell Receptors Specific for Unique Major Histocompatibility Ligands. Immunity (2011) 35:694–704. doi: 10.1016/j.immuni.2011.10.012
42. Liston A, Nutsch KM, Farr AG, Lund JM, Rasmussen JP, Koni PA, et al. Differentiation of Regulatory Foxp3+ T Cells in the Thymic Cortex. Proc Natl Acad Sci USA (2008) 105:11903–08. doi: 10.1073/pnas.0801506105
43. Hu DY, Yap JY, Wirasinha RC, Howard DR, Goodnow CC, Daley SR. A Timeline Demarcating Two Waves of Clonal Deletion and Foxp3 Upregulation During Thymocyte Development. Immunol Cell Biol (2016) 94:357–66. doi: 10.1038/icb.2015.95
44. Penit C. In Vivo Thymocyte Maturation. BUdR Labeling of Cycling Thymocytes and Phenotypic Analysis of Their Progeny Support the Single Lineage Model. J Immunol (1986) 137:2115–21.
45. Klein L, Robey EA, Hsieh CS. Central CD4+ T Cell Tolerance: Deletion Versus Regulatory T Cell Differentiation. Nat Rev Immunol (2019) 19:7–18. doi: 10.1038/s41577-018-0083-6
46. Savage PA, Klawon DEJ, Miller CH. Regulatory T Cell Development. Annu Rev Immunol (2020) 38:421–53. doi: 10.1146/annurev-immunol-100219-020937
47. Derbinski J, Schulte A, Kyewski B, Klein L. Promiscuous Gene Expression in Medullary Thymic Epithelial Cells Mirrors the Peripheral Self. Nat Immunol (2001) 2:1032–39. doi: 10.1038/ni723
48. Lkhagvasuren E, Sakata M, Ohigashi I, Takahama Y. Lymphotoxin β Receptor Regulates the Development of CCL21-Expressing Subset of Postnatal Medullary Thymic Epithelial Cells. J Immunol (2013) 190:5110–17. doi: 10.4049/jimmunol.1203203
49. Ueno T, Saito F, Gray DH, Kuse S, Hieshima K, Nakano H, et al. CCR7 Signals are Essential for Cortex-Medulla Migration of Developing Thymocytes. J Exp Med (2004) 200:493–05. doi: 10.1084/jem.20040643
50. Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, et al. Projection of an Immunological Self Shadow Within the Thymus by the Aire Protein. Science (2002) 298:1395–01. doi: 10.1126/science.1075958
51. Derbinski J, Gabler J, Brors B, Tierling S, Jonnakuty S, Hergenhahn M, et al. Promiscuous Gene Expression in Thymic Epithelial Cells is Regulated at Multiple Levels. J Exp Med (2005) 202:33–45. doi: 10.1084/jem.20050471
52. Sansom SN, Shikama-Dorn N, Zhanybekova S, Nusspaumer G, Macaulay IC, Deadman ME, et al. Population and Single-Cell Genomics Reveal the Aire Dependency, Relief From Polycomb Silencing, and Distribution of Self-Antigen Expression in Thymic Epithelia. Genome Res (2014) 24:1918–31. doi: 10.1101/gr.171645.113
53. Takaba H, Morishita Y, Tomofuji Y, Danks L, Nitta T, Komatsu N, et al. Fezf2 Orchestrates a Thymic Program of Self-Antigen Expression for Immune Tolerance. Cell (2015) 163:975–87. doi: 10.1016/j.cell.2015.10.013
54. Wells KL, Miller CN, Gschwind AR, Wei W, Phipps JD, Anderson MS, et al. Combined Transient Ablation and Single-Cell RNA-Sequencing Reveals the Development of Medullary Thymic Epithelial Cells. Elife (2020) 9. doi: 10.7554/eLife.60188
55. Lopes N, Boucherit N, Santamaria JC, Provin N, Charaix J, Ferrier P, et al. Thymocytes Trigger Self-Antigen-Controlling Pathways in Immature Medullary Thymic Epithelial Stages. Elife (2022) 11. doi: 10.7554/eLife.69982
56. Koble C, Kyewski B. The Thymic Medulla: A Unique Microenvironment for Intercellular Self-Antigen Transfer. J Exp Med (2009) 206:1505–13. doi: 10.1084/jem.20082449
57. Millet V, Naquet P, Guinamard RR. Intercellular MHC Transfer Between Thymic Epithelial and Dendritic Cells. Eur J Immunol (2008) 38:1257–63. doi: 10.1002/eji.200737982
58. Yap JY, Wirasinha RC, Chan A, Howard DR, Goodnow CC, Daley SR. Indirect Presentation in the Thymus Limits Naive and Regulatory T-Cell Differentiation by Promoting Deletion of Self-Reactive Thymocytes. Immunology (2018) 154:522–32. doi: 10.1111/imm.12904
59. Perry JS, Lio CW, Kau AL, Nutsch K, Yang Z, Gordon JI, et al. Distinct Contributions of Aire and Antigen-Presenting-Cell Subsets to the Generation of Self-Tolerance in the Thymus. Immunity (2014) 41:414–26. doi: 10.1016/j.immuni.2014.08.007
60. Klein L, Kyewski B, Allen PM, Hogquist KA. Positive and Negative Selection of the T Cell Repertoire: What Thymocytes See (and Don't See). Nat Rev Immunol (2014) 14:377–91. doi: 10.1038/nri3667
61. Dhalla F, Baran-Gale J, Maio S, Chappell L, Hollander GA, Ponting CP. Biologically Indeterminate Yet Ordered Promiscuous Gene Expression in Single Medullary Thymic Epithelial Cells. EMBO J (2020) 39:e101828. doi: 10.15252/embj.2019101828
62. Pinto S, Michel C, Schmidt-Glenewinkel H, Harder N, Rohr K, Wild S, et al. Overlapping Gene Coexpression Patterns in Human Medullary Thymic Epithelial Cells Generate Self-Antigen Diversity. Proc Natl Acad Sci USA (2013) 110:E3497–505. doi: 10.1073/pnas.1308311110
63. Brennecke P, Reyes A, Pinto S, Rattay K, Nguyen M, Kuchler R, et al. Single-Cell Transcriptome Analysis Reveals Coordinated Ectopic Gene-Expression Patterns in Medullary Thymic Epithelial Cells. Nat Immunol (2015) 16:933–41. doi: 10.1038/ni.3246
64. Tykocinski LO, Sinemus A, Rezavandy E, Weiland Y, Baddeley D, Cremer C, et al. Epigenetic Regulation of Promiscuous Gene Expression in Thymic Medullary Epithelial Cells. Proc Natl Acad Sci USA (2010) 107:19426–31. doi: 10.1073/pnas.1009265107
65. Meredith M, Zemmour D, Mathis D, Benoist C. Aire Controls Gene Expression in the Thymic Epithelium With Ordered Stochasticity. Nat Immunol (2015) 16:942–49. doi: 10.1038/ni.3247
66. Irla M, Guerri L, Guenot J, Serge A, Lantz O, Liston A, et al. Antigen Recognition by Autoreactive CD4+ Thymocytes Drives Homeostasis of the Thymic Medulla. PloS One (2012) 7:e52591. doi: 10.1371/journal.pone.0052591
67. Irla M, Hugues S, Gill J, Nitta T, Hikosaka Y, Williams IR, et al. Autoantigen-Specific Interactions With CD4+ Thymocytes Control Mature Medullary Thymic Epithelial Cell Cellularity. Immunity (2008) 29:451–63. doi: 10.1016/j.immuni.2008.08.007
68. Boehm T, Scheu S, Pfeffer K, Bleul CC. Thymic Medullary Epithelial Cell Differentiation, Thymocyte Emigration, and the Control of Autoimmunity Require Lympho-Epithelial Cross Talk via Ltβr. J Exp Med (2003) 198:757–69. doi: 10.1084/jem.20030794
69. Akiyama T, Shimo Y, Yanai H, Qin J, Ohshima D, Maruyama Y, et al. The Tumor Necrosis Factor Family Receptors RANK and CD40 Cooperatively Establish the Thymic Medullary Microenvironment and Self-Tolerance. Immunity (2008) 29:423–37. doi: 10.1016/j.immuni.2008.06.015
70. Metzger TC, Khan IS, Gardner JM, Mouchess ML, Johannes KP, Krawisz AK, et al. Lineage Tracing and Cell Ablation Identify a Post-Aire-Expressing Thymic Epithelial Cell Population. Cell Rep (2013) 5:166–79. doi: 10.1016/j.celrep.2013.08.038
71. White AJ, Jenkinson WE, Cowan JE, Parnell SM, Bacon A, Jones ND, et al. An Essential Role for Medullary Thymic Epithelial Cells During the Intrathymic Development of Invariant NKT Cells. J Immunol (2014) 192:2659–66. doi: 10.4049/jimmunol.1303057
72. Oh J, Wu N, Barczak AJ, Barbeau R, Erle DJ, Shin JS. CD40 Mediates Maturation of Thymic Dendritic Cells Driven by Self-Reactive CD4+ Thymocytes and Supports Development of Natural Regulatory T Cells. J Immunol (2018) 200:1399–412. doi: 10.4049/jimmunol.1700768
73. Owen DL, Mahmud SA, Vang KB, Kelly RM, Blazar BR, Smith KA, et al. Identification of Cellular Sources of IL-2 Needed for Regulatory T Cell Development and Homeostasis. J Immunol (2018) 200:3926–33. doi: 10.4049/jimmunol.1800097
74. Hemmers S, Schizas M, Azizi E, Dikiy S, Zhong Y, Feng Y, et al. IL-2 Production by Self-Reactive CD4 Thymocytes Scales Regulatory T Cell Generation in the Thymus. J Exp Med (2019) 216:2466–78. doi: 10.1084/jem.20190993
75. Hu DY, Wirasinha RC, Goodnow CC, Daley SR. IL-2 Prevents Deletion of Developing T-Regulatory Cells in the Thymus. Cell Death Differ (2017) 24:1007–16. doi: 10.1038/cdd.2017.38
76. Lio CW, Hsieh CS. A Two-Step Process for Thymic Regulatory T Cell Development. Immunity (2008) 28:100–11. doi: 10.1016/j.immuni.2007.11.021
77. Burchill MA, Yang J, Vang KB, Moon JJ, Chu HH, Lio CW, et al. Linked T Cell Receptor and Cytokine Signaling Govern the Development of the Regulatory T Cell Repertoire. Immunity (2008) 28:112–21. doi: 10.1016/j.immuni.2007.11.022
78. Lee HM, Bautista JL, Scott-Browne J, Mohan JF, Hsieh CS. A Broad Range of Self-Reactivity Drives Thymic Regulatory T Cell Selection to Limit Responses to Self. Immunity (2012) 37:475–86. doi: 10.1016/j.immuni.2012.07.009
79. Leung MW, Shen S, Lafaille JJ. TCR-Dependent Differentiation of Thymic Foxp3+ Cells is Limited to Small Clonal Sizes. J Exp Med (2009) 206:2121–30. doi: 10.1084/jem.20091033
80. Lucca LE, Axisa PP, Aloulou M, Perals C, Ramadan A, Rufas P, et al. Myelin Oligodendrocyte Glycoprotein Induces Incomplete Tolerance of CD4+ T Cells Specific for Both a Myelin and a Neuronal Self-Antigen in Mice. Eur J Immunol (2016) 46:2247–59. doi: 10.1002/eji.201646416
81. Kieback E, Hilgenberg E, Stervbo U, Lampropoulou V, Shen P, Bunse M, et al. Thymus-Derived Regulatory T Cells Are Positively Selected on Natural Self-Antigen Through Cognate Interactions of High Functional Avidity. Immunity (2016) 44:1114–26. doi: 10.1016/j.immuni.2016.04.018
82. Fazilleau N, Delarasse C, Sweenie CH, Anderton SM, Fillatreau S, Lemonnier FA, et al. Persistence of Autoreactive Myelin Oligodendrocyte Glycoprotein (MOG)-Specific T Cell Repertoires in MOG-Expressing Mice. Eur J Immunol (2006) 36:533–43. doi: 10.1002/eji.200535021
83. Hassler T, Urmann E, Teschner S, Federle C, Dileepan T, Schober K, et al. Inventories of Naive and Tolerant Mouse CD4 T Cell Repertoires Reveal a Hierarchy of Deleted and Diverted T Cell Receptors. Proc Natl Acad Sci USA (2019) 116:18537–43. doi: 10.1073/pnas.1907615116
84. Kalekar LA, Schmiel SE, Nandiwada SL, Lam WY, Barsness LO, Zhang N, et al. CD4+ T Cell Anergy Prevents Autoimmunity and Generates Regulatory T Cell Precursors. Nat Immunol (2016) 17:304–14. doi: 10.1038/ni.3331
85. Waldmann H, Cobbold S. How do Monoclonal Antibodies Induce Tolerance? A Role for Infectious Tolerance? Annu Rev Immunol (1998) 16:619–44. doi: 10.1146/annurev.immunol.16.1.619
86. Picca CC, Simons DM, Oh S, Aitken M, Perng OA, Mergenthaler C, et al. CD4+ CD25+ Foxp3+ Regulatory T Cell Formation Requires More Specific Recognition of a Self-Peptide Than Thymocyte Deletion. Proc Natl Acad Sci (2011) 108:14890–95. doi: 10.1073/pnas.1103810108
87. Wirasinha RC, Davies AR, Srivastava M, Sheridan JM, Sng XYX, Delmonte OM, et al. Nfkb2 Variants Reveal a P100-Degradation Threshold That Defines Autoimmune Susceptibility. J Exp Med (2021) 218:18537-43. doi: 10.1084/jem.20200476
88. Zammit NW, Siggs OM, Gray PE, Horikawa K, Langley DB, Walters SN, et al. Denisovan, Modern Human and Mouse TNFAIP3 Alleles Tune A20 Phosphorylation and Immunity. Nat Immunol (2019) 20:1299. doi: 10.1038/s41590-019-0492-0
89. Fulford TS, Grumont R, Wirasinha RC, Ellis D, Barugahare A, Turner SJ, et al. C-Rel Employs Multiple Mechanisms to Promote the Thymic Development and Peripheral Function of Regulatory T Cells in Mice. Eur J Immunol (2021) 51:2006–26. doi: 10.1002/eji.202048900
90. Randall KL, Law HD, Ziolkowski AF, Wirasinha RC, Goodnow CC, Daley SR. DOCK8 Deficiency Diminishes Thymic T-Regulatory Cell Development But Not Thymic Deletion. Clin Transl Immunol (2021) 10:e1236. doi: 10.1002/cti2.1236
91. Tai X, Erman B, Alag A, Mu J, Kimura M, Katz G, et al. Foxp3 Transcription Factor is Proapoptotic and Lethal to Developing Regulatory T Cells Unless Counterbalanced by Cytokine Survival Signals. Immunity (2013) 38:1116–28. doi: 10.1016/j.immuni.2013.02.022
92. Owen DL, Mahmud SA, Sjaastad LE, Williams JB, Spanier JA, Simeonov DR, et al. Thymic Regulatory T Cells Arise via Two Distinct Developmental Programs. Nat Immunol (2019) 20:195–205. doi: 10.1038/s41590-018-0289-6
93. McCaughtry TM, Wilken MS, Hogquist KA. Thymic Emigration Revisited. J Exp Med (2007) 204:2513–20. doi: 10.1084/jem.20070601
94. Thiault N, Darrigues J, Adoue V, Gros M, Binet B, Perals C, et al. Peripheral Regulatory T Lymphocytes Recirculating to the Thymus Suppress the Development of Their Precursors. Nat Immunol (2015) 16:628–34. doi: 10.1038/ni.3150
95. Weist BM, Kurd N, Boussier J, Chan SW, Robey EA. Thymic Regulatory T Cell Niche Size is Dictated by Limiting IL-2 From Antigen-Bearing Dendritic Cells and Feedback Competition. Nat Immunol (2015) 16:635–41. doi: 10.1038/ni.3171
96. McCarthy NI, Cowan JE, Nakamura K, Bacon A, Baik S, White AJ, et al. Osteoprotegerin-Mediated Homeostasis of Rank+ Thymic Epithelial Cells Does Not Limit Foxp3+ Regulatory T Cell Development. J Immunol (2015) 195:2675–82. doi: 10.4049/jimmunol.1501226
97. Cowan JE, McCarthy NI, Anderson G. CCR7 Controls Thymus Recirculation, But Not Production and Emigration, of Foxp3+ T Cells. Cell Rep (2016) 14:1041–48. doi: 10.1016/j.celrep.2016.01.003
98. Invernizzi P, Gershwin ME. The Genetics of Human Autoimmune Disease. J Autoimmun (2009) 33:290. doi: 10.1016/j.jaut.2009.07.008
99. Dendrou CA, Petersen J, Rossjohn J, Fugger L. HLA Variation and Disease. Nat Rev Immunol (2018) 18:325–39. doi: 10.1038/nri.2017.143
Keywords: thymus, T-cell selection, T-cell tolerance, T-cell deletion, T-regulatory cells, self-antigen recognition, autoimmune disease
Citation: Wang C and Daley SR (2022) How Thymocyte Deletion in the Cortex May Curtail Antigen-Specific T-Regulatory Cell Development in the Medulla. Front. Immunol. 13:892498. doi: 10.3389/fimmu.2022.892498
Received: 09 March 2022; Accepted: 26 April 2022;
Published: 25 May 2022.
Edited by:
Joshua Daniel Ooi, Monash University, AustraliaReviewed by:
Troy Allen Baldwin, University of Alberta, CanadaMatthieu Giraud, Institut National de la Santé et de la Recherche Médicale (INSERM), France
Lauren Ehrlich, University of Texas at Austin, United States
Copyright © 2022 Wang and Daley. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stephen R. Daley, czUuZGFsZXlAcXV0LmVkdS5hdQ==
 Chenglong Wang
Chenglong Wang Stephen R. Daley
Stephen R. Daley