- 1THERABEST Japan, Inc., Kobe, Japan
- 2Center for iPS Cell Research and Application (CiRA), Kyoto University, Kyoto, Japan
- 3Research and Development Center, THERABEST Co., Ltd., Seoul, South Korea
Natural killer (NK) cell immunotherapies for cancer can complement existing T cell therapies while benefiting from advancements already made in the immunotherapy field. For NK cell manufacturing, induced pluripotent stem cells (iPSCs) offer advantages including eliminating donor variation and providing an ideal platform for genome engineering. At the same time, extracellular vesicles (EVs) have become a major research interest, and purified NK cell extracellular vesicles (NKEVs) have been shown to reproduce the key functions of their parent NK cells. NKEVs have the potential to be developed into a standalone therapeutic with reduced complexity and immunogenicity compared to cell therapies. This review explores the role iPSC technology can play in both NK cell manufacturing and NKEV development.
Introduction
Natural killer (NK) cell adoptive cell transfer (ACT) is emerging as an important cancer immunotherapy. Despite engineered T cell therapies advancing through clinical trials to commercialization (1), some major challenges remain such as high rates of serious adverse side effects, production inefficiencies, and high costs for autologous treatment generation (2). Recent research has shown that NK cells can overcome these challenges to develop into an independent or complementary class of cancer immunotherapies (3–6). A complementary field benefiting from advances in NK cell development is that of NK cell extracellular vesicles (NKEVs), with purified NKEVs having proven to reproduce key functions of their parent NK cells (7).
These developments coincide with induced pluripotent stem cell (iPSC)-derived cell therapies reaching human clinical trials. A particular focus is on iPSC-derived cell products that can be given to patients allogeneically, reducing long-term risks that have slowed translation. iPSC-derived allogeneic cell therapies have the potential to create “off-the-shelf” products, allowing larger batches to be created, reducing costs, and increasing reproducibility. Together, these developments set the scene for iPSC technologies to offer advantages in the manufacture and translation of NK cell-based therapeutics.
NK Cells
NK cells are members of the innate lymphoid family, identified as CD56+CD3-, which provide frontline defense against infections and cancer, and clear damaged cells (8, 9). NK cells are typically classified into two main subpopulations: Cytotoxic CD56dimCD16+ (CD56dim) NK cells account for ~90% of the total NK population, and IFN-γ-producing immunoregulatory CD56brightCD16- (CD56bright) NK cells make up the remaining ~10%(10).
NK cells can discriminate normal from abnormal cells, a process called immune surveillance, via a repertoire of activating and inhibitory receptors (11–13). Direct binding to target cell ligands by a combination of natural cytotoxicity receptor (NCR) family members and NKG2D stimulate NK cell activation and the trafficking of constitutively expressed lytic granules to the site of cell contact and into the target cell (14–16) or the secretion of cytokines (5, 17). Alternatively, CD16 binding alone is sufficient to activate antibody-dependent cellular cytotoxicity (ADCC) (11).
To protect host cells, HLA class I molecules are selectively detected by NK cell inhibitory receptors such as NKG2A and killer immunoglobulin-like receptors (KIRs) (18). Other NK cell inhibitory receptors detect sialic acid, extracellular matrix components, and aminophospholipids, and the expression of immune checkpoints by NK cells, such as CTLA-4 and PD-1, can be stimulated by specific signaling environments (18, 19).
NK cells can be used allogeneically for ACT due to their ability to educate and establish “self-tolerance” to the host HLA class I environment (20). The major sources for NK cell ACT have been peripheral blood (PB-NK) and cord blood (CB-NK), as well as the immortalized line NK-92 (21, 22). Both PB-NK and CB-NK cells are derived from limited donor sources, introducing batch-to-batch variation. NK-92 cells possess anti-cancer potential (23, 24), and have shown efficacy in human clinical trials (25). However, they lack CD16 expression and require irradiation prior to transplantation to inactivate proliferation (26), which in turn impairs therapeutic properties (27, 28). iPSC-derived NK (iPSC-NK) cells have the potential to overcome these limitations while offering additional advantages (29).
Enhancing NK Cell Function
Over time, tumors develop immunosuppressive microenvironment features (30) (Figure 1A) such as altered expressions of receptors and ligands that activate or inhibit NK cells (18, 31, 32), the recruitment of immunomodulatory cells into the tumor mass (33), altered metabolism that results in lower oxygen and increased lactate (34, 35), and the production of inhibitory molecules including TGF-β, IL-10, PGE2, and immune checkpoint proteins such as PD-L1 (32, 36).
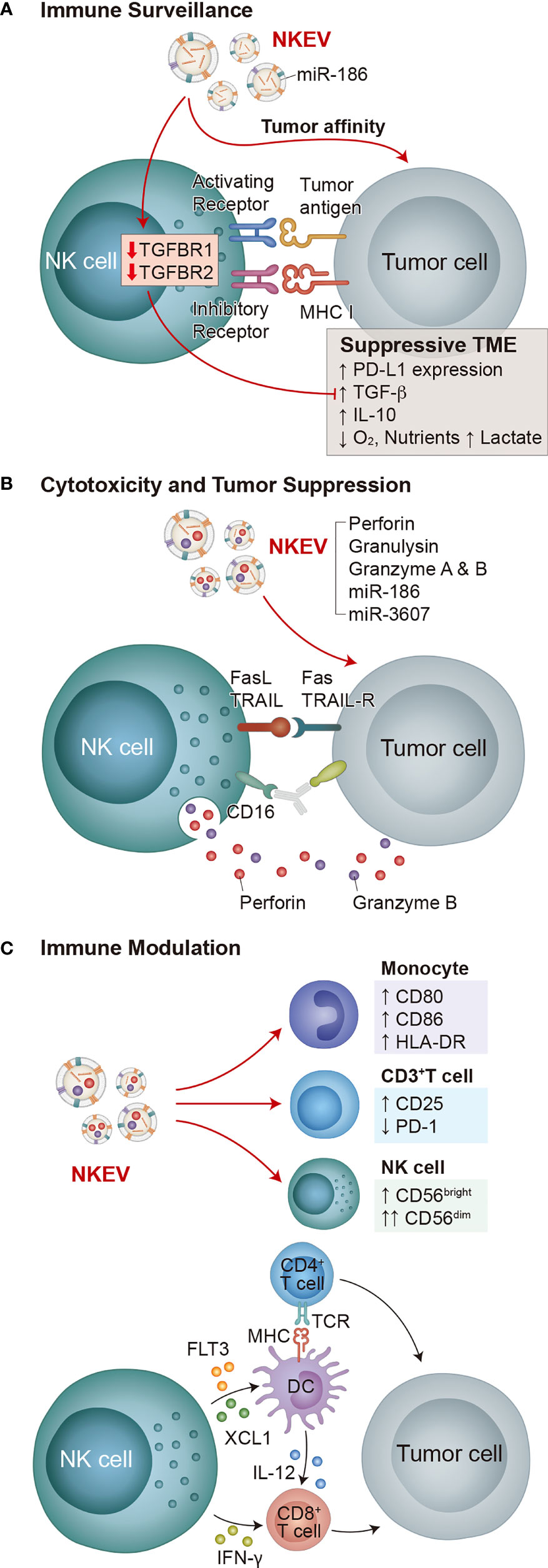
Figure 1 Functions of NK cells and NKEVs. The key functions of NK cells are immune surveillance, cytotoxicity and tumor suppression, and immune modulation, all of which involve both NK cells and NKEVs. (A) NK cell immune surveillance depends on the interaction of activating and inhibitory receptors with target cells. In suppressive TMEs, NK cells are inhibited by PD-L1 on tumor cells and tumor cell-secreted TGF-β and IL-10. Decreased oxygen concentration, increased lactate production, and decreased available nutrients also inhibit NK cells. NKEVs exhibit tumor affinity, and NKEV delivery of miR-186 to NK cells decreases TGFBR1/2 expression, fortifying them in suppressive TMEs. (B) Cytotoxicity and tumor suppression by NK cells depends on either CD16 regulated ADCC or degranulation of vesicles containing perforin and granzyme B in response to the combined activation of activating receptors. NKEVs directly deliver cytotoxic effector cargo of perforin, granulysin, granzyme A and B, as well as miRNAs miR-186 and miR-3607 to tumor cells. (C) NK cells produce immunomodulatory cytokines and chemokines in response to activation, directly activating CD8+ T cells, and stimulating dendritic cells to activate both CD8+ and CD4+ T cells, which subsequently attack tumors. Meanwhile, NKEVs increase CD80, CD86 and HLA-DR expression on monocytes, increase CD25 expression and decrease PD-1 expression on CD3+ T cells, and increase the total NK cell population and the CD56dim NK cell fraction.
Considering these immune evasion mechanisms, various strategies to enhance NK cell function have been developed (4, 37–39). Research into the optimal selection and dosing regimes of cytokines used for expansion and activation (39–42) has identified IL-15 as the preferred choice (43), while work on membrane bound IL-15 and IL-21 has also shown advantages (44–46). Cancers with decreased IL-15 expression correlate with decreased patient survival (47), which led to the development of IL-15 superagonists (48–50) and NK cell modifications that can overcome TGF-β-mediated inhibition of the IL-15 pathway (51, 52).
Another approach has been co-treatment with small molecule immunomodulatory drugs that activate NK cells and increase granzyme-B expression (53, 54) or that increase the expression of NK activating ligands on cancer cells (55, 56). Increased NK cell effects have also been achieved with the combined use of biologics, such as tumor-specific monoclonal antibodies (mAbs) that augment NK cell functions (57–63), or via bi-specific or tri-specific engagers that bind to tumor-specific antigens and NK cells to form immunological synapses (64–67).
Immune checkpoint blockade (ICB) deploying mAbs to block inhibitory pathways has revolutionized our approach to cancer treatment. PD-1/PD-L1 blockade has been shown to increase NK cell cytotoxicity against cancer cells (68–70), and combined treatment of lung cancer patients with allogeneic PB-NK cells and the ICB drug Pembrolizumab increased patient survival (71). Other ICB targets have been identified, and multiple mAbs targeting inhibitory NK cell pathways have reached human clinical trials (72).
Finally, the growing importance of genetic strategies to enhance NK cell function (73), as discussed later, has brought iPSCs to the forefront of NK cell production (29).
Extracellular Vesicles
Extracellular vesicles (EVs), including endosome-derived exosomes (40-150 nm) and plasma membrane-derived microvesicles (50-1000 nm), are lipid nanoparticles secreted by most cell types that are involved in intercellular communication (74). Due to the difficulty determining the biogenesis pathway of individual vesicles, they are classified according to size or density, biochemical composition, or descriptions of conditions of the cell of origin (e.g. "NKEVs") (75).
In recent years EVs have become a major area of research interest. For mesenchymal stem cells (MSCs), it became clear that their immunomodulatory and regenerative functions primarily act through secretory paracrine pathways, including via EVs (76, 77). EVs have the potential to reproduce features of many parent cell therapies while potentially simplifying translational pipelines due to low immunogenicity and inability to replicate (78, 79). Furthermore, progress has been made in EV manufacturing and storage (80–84), critical areas for EV translation.
Mostly using MSC-EVs (85), interventional human clinical trials are active for dystrophic epidermolysis bullosa (NCT04173650), regeneration of macular holes (NCT03437759), acute ischemic stroke (NCT03384433), periodontitis (NCT04270006), craniofacial neuralgia (NCT04202783), inflammatory lung diseases (NCT04388982, NCT04276987), and neurodegenerative diseases (NCT04202770, NCT04388982). T-cell-derived EVs are being investigated for pneumonia (NCT04389385). EVs are also being investigated as liquid biopsy markers for diseases such as cancer (NCT04053855, NCT04523389, NCT04852653, NCT04529915, NCT03228277), diabetes (NCT03106246), neurodegeneration (NCT03944603), and panic disorder (NCT04029740).
Natural Killer Cell Extracellular Vesicles
Although NKEVs have yet to reach clinical trials (85), they have become a significant research focus (7). NKEVs are continuously produced by NK cells and are involved in key mechanisms of NK cell function including immune surveillance, cytotoxicity, and immune modulation (86–90) (Figure 1).
NKEVs express NK markers such as CD56, NKG2D, and cytotoxic effector proteins (e.g. perforin, granzymes A and B, granulysin, and FasL) (86, 87), as well as EV markers Rab5B, CD63, CD81, CD9, and TSG101 (87). Purified NKEVs are cytotoxic against diverse cancer cells (Figure 1B) including hematological cancers (Jurkat, K562, DAUDI) (87), neuroblastoma (CHLA-136) (91), breast carcinoma (MCF-7 (91), MDA-MB-231/F) (92)), ovarian cancer (A2780) (93), and melanoma (B16F10) (94). In mouse glioblastoma xenograft models NKEVs exhibit tumor affinity (92, 95) (Figure 1A).
As well as NK cell effector proteins, NKEVs carry miRNAs that have specific roles in cancer suppression. For example, Sun et al. showed that miR-3607, enriched in purified NKEVs, was required for NK cells to inhibit the malignant transformation of pancreatic cancer cells (Mia PaCa-2, PANC-1) by directly targeting IL-26, suppressing proliferation, migration, and invasion (96). Neviani et al. showed that miR-186 in NKEVs is partially responsible for their cytotoxic effect against neuroblastoma cells (CHLA-136, CHLA-255, and LAN-5) while fortifying other NK cells against the suppressive effect of TGF-β (97) (Figure 1A). NKEVs containing miR-207 have also been shown to reduce neuroinflammation (98).
NKEVs contain immunomodulatory proteins (88, 99, 100) and promote M1 macrophages in a mouse pseudomonas aeruginosa-induced lung injury model (101), reproducing immunomodulatory features of NK cells. Federici et al. reported that NKEVs stimulate CD25 expression on CD3+ T cells, HLA-DR and costimulatory molecule expression on monocytes, and increase the total NK cell population and the CD56dim NK cell fraction in vitro (88) (Figure 1C). Shoae-Hassani et al. showed that NK cells cocultured with neuroblastoma cells (SK-N-SH and CHLA-255) produce NKEVs that confer enhanced neuroblastoma cell cytotoxicity to fresh NK cells (102).
EVs may lack the signaling or metabolic pathways required to respond to inhibitory tumor microenvironment (TME) signals. Accordingly, some groups have shown experimentally that NKEVs retain tumor affinity, tumor suppressive, and immunomodulatory properties in simulated immunosuppressive TMEs using TGF-β, IL-10, and LPS (88, 97). The addition of NKEVs also reduced PD-1 expression on CD3+ T cells even in the presence of TGF-β and IL-10 (88).
Overall, mounting evidence suggests that NKEVs are an integral component of NK cell functions (39, 103), with purified NKEVs demonstrating therapeutic properties (7, 39).
Priming NK Cells for EV Production
NKEVs collected from IL-15-primed NK cells had increased concentration of cytotoxic effectors (92), improved cytolytic activity against cancer cells of glioblastoma, breast cancer, and thyroid cancer, showed improved tumor affinity, and inhibited glioblastoma growth in xenograft mice (92). In NK cells IL-15 regulates the small GTPase Rab27a (92), which was shown in MSCs to increase EV secretion by promoting maturation of endosomal multivesicular bodies (MVBs) containing exosomes (104). Zhu et al. showed similar effects in NK cells with IL-15 priming more than doubling particle number and EV-contained protein (92).
Hypoxic TMEs suppress NK immune surveillance via hypoxia-induced tumor cell shedding of MICA and MICB (105, 106), inhibit NK-mediated cell killing by reducing KIR expression (106), decrease intracellular perforin and granzyme B concentration (107), and reduce degranulation (106). Yet CD16 function is largely maintained, facilitating ADCC (106). To compound this, the NK cell response to the hypoxic TME actually assists blood vessel maturation (108). However, activity of the hypoxia-induced HIF-1α pathway promotes the infiltration of NK cells into tumors and the expression of granzyme B (108). Away from the TME, NK cells cultured in hypoxia for 48 hours produce larger yields of NKEVs with increased total protein, FasL, perforin, and granzyme B concentrations, increased cytotoxicity against breast (MCF-7) and ovarian (A2780) cancer cells in vitro, and increased inhibition of the migration and proliferation of these cancer cells (93). These results are similar to the effects of IL-15 priming, and the two approaches have been shown to be synergistic (109).
Harnessing these NK priming approaches and developing knowledge in this area, as well as more generally into the conditions that maximize NKEV yield and potency, may prove critical in NKEV manufacturing optimization, as seen for other EV sources.
iPSCs in NK Cell Manufacturing
iPSC-derived cell therapies are now featured in many clinical trials, including those using iPSC-NK cells (NCT04106167, NCT03841110) (110). The expansion potential of iPSCs eliminates the need for multiple donors, increasing cell product reproducibility, and epigenetic rejuvenation during iPSC reprogramming erases DNA modifications, producing cells that are biologically young (111–113). This has been shown to cause immune cells to exit exhausted states and adopt phenotypes effective at killing cancer cells (114).
For NK cell-based ACT, chemically defined differentiation protocols have been used to produce iPSC-NK cells with cytotoxicity and immunomodulatory function comparable to primary NK cells (29, 115–117). In cancer patients NK cells are known to undergo functional decline (118–120), similar to that observed in aged patients (121). This decline suggests iPSC-NK cell therapies have advantages, where biologically young, functional cells can replenish the diminished NK cell activity of older and sicker patients.
One of the key advantages of using iPSC technology for cell therapy is its suitability to genome engineering (122). A myriad of genetic NK cell enhancement strategies have been developed to improve targeting and homing to cancer cells, resist immunosuppressive TMEs, and increase cytotoxicity and persistence (73).
For example, deletion in iPSCs of CISH, which encodes the CIS protein, a negative regulator of IL-15, resulted in iPSC-NK cells with better metabolic fitness and increased IL-15 sensitivity (123). Another example is the addition to iPSCs of a cleavage resistant CD16 variant that resulted in enhanced iPSC-NK cells with superior ADCC compared to both unmodified iPSC-NK cells and primary NK cells, and caused comparatively more regression of hematopoietic malignancies and solid tumors when combined with a mAb treatment (124).
Chimeric antigen receptor (CAR)-NK cells, emerging as a key area of cancer immunotherapy development, are also better suited to using iPSC technology. Despite clinical approval of autologous CAR products, allogeneic products avoid patient cell morbidity due to aging or disease and the possible contamination of cancer cells (125). Using iPSC technology has allowed researchers to compare the effectiveness of CAR combinations (116), and two CAR iPSC-NK cell clinical trials are underway (NCT04245722 and jRCT2033200431).
In addition to genome engineering, iPSCs are compatible with synthetic biology. Tumor-derived TGF-β suppression of NK cell cytotoxicity (47) is ameliorated by knocking out TGF-β receptors (51). Intracellularly, TGF-β upregulates miR-27a-5p (126), which if inhibited also increases the cytotoxicity of NK cells (127). Intracellular targets like miR-27a-5p can be targeted by miRNA switches to enhance NK cell function in a context-dependent way. miRNA switches are synthetic mRNAs that can activate the expression of specific miRNAs or proteins in response to endogenous biomolecules (128). Moreover, miRNA switches can be designed to orthogonally, meaning multiple miRNA switches can be combined to tune NK cell cytotoxic and metabolic (129) responses to specific signaling environments. Employed in iPSC-NK cells, this approach could be used to engineer “intelligent” NK cells with programmed context-dependent functions.
iPSCs in NKEV Development
While research on NKEVs has increased, investigations into iPSC-NK cell-derived EVs (iPSC-NKEVs) remain unreported, raising the question of whether iPSC-NK cells also produce EVs (Figure 2). This may represent an important, currently underexplored therapeutic opportunity considering recent research from both NKEVs and EVs from other iPSC-derived cells (130, 131).
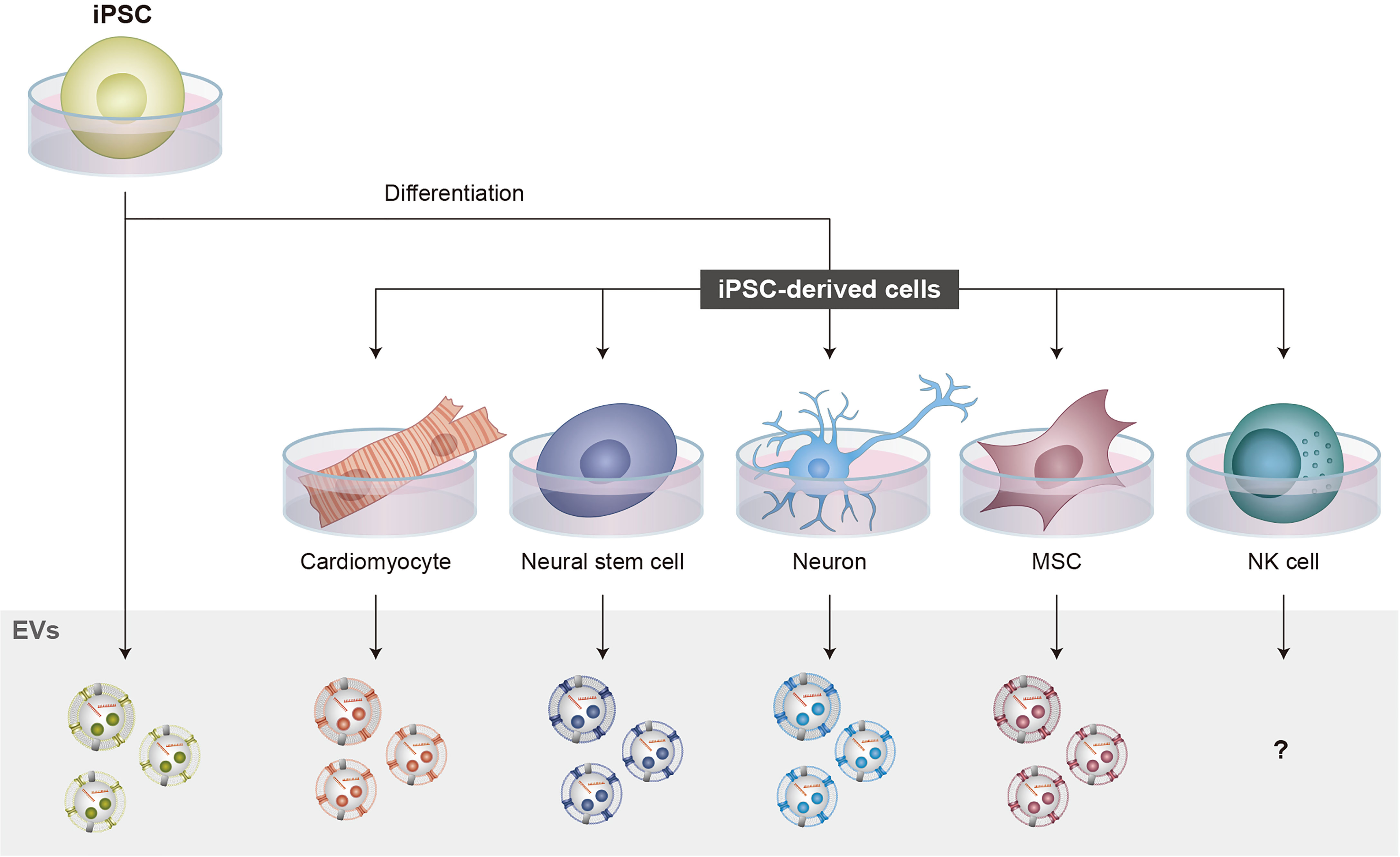
Figure 2 iPSCs in EV production. iPSCs can be differentiated into various cell types that have therapeutic potential. iPSC-NK cells have the advantages of increased expansion potential, the production of biologically young cells, and less donor variation compared to primary NK cells. For EV production, several iPSC-derived cells and iPSCs themselves have been shown to produce functional EVs. However, for NK cells, studies investigating iPSC-NKEVs have not been reported, raising the important question of whether iPSC-NK cells produce EVs. NKEVs can reproduce the functions of NK cell therapies while reducing the complexity and immunogenicity of the final therapeutic product, thus increasing safety. These features highlight how iPSC-NKEVs represent an important direction for NKEV research.
Therapeutic properties of iPSC-derived cardiomyocyte- (132–134), neuron- (135), neural stem cell- (136, 137), and MSC- (138–141) EVs, as well as iPSC-EVs (142–144), have already been demonstrated, as has the potential to improve performance for certain applications using bioengineering (145). Using iPSCs as a source for EV production may also help address EV manufacturing and translational challenges such as heterogeneity and scalability (146).
For iPSC-NK cells, Cichocki et al. have shown that they can reproduce key features of NK cells, including dose-dependent cytotoxicity against diverse cancer cells (lung carcinoma (A549), hepatocyte carcinoma (HepG2), ovarian adenocarcinoma (SKOV-3), myeloid leukemia (K562), and melanoma (SK-MEL2)), inflammatory cytokine production, in vivo immunomodulation (including activation and recruitment of circulating T cells), infiltration into solid tumor spheroids in vitro, and the ability to slow tumor progression in vivo (115). Other groups have reported functional iPSC-NK cells (116), and given the documented role of NKEVs in these NK cell processes, these results underline the importance of investigating iPSC-NK cells for EV production.
Similarly to the epigenetic rejuvenation discussed for iPSC-NK cells, Man et al. reported that epigenetic rejuvenation of osteoblast progenitors via histone deacetylase (HDAC) inhibition results in the production of EVs with enhanced function (147). Other studies have shown that, compared to older MSCs, young MSCs produce EVs with better therapeutic properties (148, 149) that are enriched in miRNAs and proteins involved in immunomodulation (148, 150, 151). Interestingly, studies directly comparing therapeutic potential have shown improved efficacy of iPSC-derived MSC-EVs compared to adult donor MSC-EVs in in vitro studies of wound healing (152) and in in vivo disease model studies of osteoarthritis (153). Together, these findings suggest that rejuvenated iPSC-derived cells may be a superior resource for EV manufacturing compared to other sources, although donor age prior to iPSC reprogramming does impact some EV properties (154).
Engineering EVs to increase potency and specificity has already shown promising results in other cell types, and the same principles may translate to NKEVs. Upregulated expression of miRNAs can increase the concentration of miRNAs in EVs, improving therapeutic performance (155–157). Clinical trials are in progress using modified EVs for drug delivery in pancreatic (NCT03608631), colon (NCT01294072), and lung cancer (NCT01159288) (85). While there are yet to be published reports of groups modulating the biochemical composition of NKEVs genetically, the principle of NKEV engineering has been established by Han et al., who used electroporation to load NKEVs with the chemotherapy drug paclitaxel, enhancing their ability to suppress the proliferation and induce the apoptosis of breast cancer cells (158). In MSCs, Böker et al. showed that overexpression of the EV tetraspanin CD9 resulted in increased exosome biogenesis (159), highlighting the role iPSC engineering can play in optimizing production efficiency as well as modulating EV composition.
Conclusion and Future Directions
While the EV industry has moved into a phase of production optimization and human clinical translation, NKEVs are at an earlier stage of development. On the one hand, this means that they can benefit from advancements in purification, storage, and scale-up technologies, but, on the other hand, key translational questions remain relatively unanswered. One question concerns the extent of NKEV heterogeneity, and how this relates to NK cell sub-populations and states. Another concerns the production efficiency of NKEVs, which depends on their potency and yield, and ultimately the number of particles required for effective therapeutic doses. For EVs from other cell types, appropriate doses have been determined (160), and production yields have been documented and linked to manufacturing processes (80, 161). For NK cells, Jong et al. reported that 1-3 x 109 activated NK cells cultured in the G-Rex100 culture system for 48 hours contained ~7 x 107 particles/ml, implying production yields of ~5-14 particles/cell/day (91). Other cell types have been reported to have much higher EV production yields (161). Indeed, Jong et al. compared their NKEV production data to HEK293 (~1841 particles/cell/day) and MSCs (~938 particles/cell/day) (91). Further reports on NKEV production efficiency and detailed investigation of effective therapeutic doses will provide important context to these early numbers. If it is confirmed that iPSC-NK cells secrete NKEVs, then the ability to expand iPSCs to vast numbers before differentiation could be exploited for iPSC-NKEV production.
For NK cell ACT, iPSCs offer advantages in key areas of manufacturing and translation, promising to provide a cell source for biologically young, “off-the-shelf”, and bioengineered enhanced iPSC-NK cells. With iPSCs already making an impact in the clinic, iPSC-NK cells can benefit from advances in manufacturing (162) and genome engineering strategies (163) to create iPSC-NK cells that have context-dependent functions and enhanced potency and specificity. For NKEVs, future work may soon confirm that their composition can be genetically controlled, and, similarly to enhanced NK cells, this could lead to the development of enhanced NKEVs with the potential to be purified as a stand-alone therapeutic or deployed as an addition to engineered iPSC-NK cells that can home to tumor sites and secrete enhanced NKEVs in situ.
Author Contributions
NBG, PK and SIK conceptualized the overall paper and NBG drafted the manuscript. NBG primarily researched and structured the EV sections, PK the iPSC-NK cell section, and SIK and DWH the NK cell biology and iPSC-NK sections. NBG and SIK conceptualized the figures. All authors contributed to reviewing and editing, and PK edited the English for the final submission. All authors contributed to the paper and approved the submitted version.
Funding
This work was supported by a 2020 Gibon Yeongu Program from the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology(2020R1F1A106727812).
Conflict of Interest
NBG is the CSO and a board member of THERABEST Japan, Inc. SIK is the CSO and a board member of THERABEST Co., Ltd and the co-CEO and a board member of THERABEST Japan, Inc. DWH is the CTO and a board member of THERABEST Co., Ltd.
PK declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Schultz L, Mackall C. Driving CAR T Cell Translation Forward. Sci Transl Med (2019) 11(481):eaaw2127. doi: 10.1126/scitranslmed.aaw2127
2. Goldenson BH, Hor P, Kaufman DS. iPSC-Derived Natural Killer Cell Therapies - Expansion and Targeting. Front Immunol (2022) 13. doi: 10.3389/fimmu.2022.841107
3. Wendel P, Reindl LM, Bexte T, Künnemeyer L, Särchen V, Albinger N, et al. Arming Immune Cells for Battle: A Brief Journey Through the Advancements of T and NK Cell Immunotherapy. Cancers (2021) 13(6):1481. doi: 10.3390/cancers13061481
4. Vogler M, Shanmugalingam S, Särchen V, Reindl LM, Grèze V, Buchinger L, et al. Unleashing the Power of NK Cells in Anticancer Immunotherapy. J Mol Med (2022) 100(3):337–49. doi: 10.1007/s00109-021-02120-z
5. Shimasaki N, Jain A, Campana D. NK Cells for Cancer Immunotherapy. Nat Rev Drug Discovery (2020) 19(3):200–18. doi: 10.1038/s41573-019-0052-1
6. Cheng M, Chen Y, Xiao W, Sun R, Tian Z. NK Cell-Based Immunotherapy for Malignant Diseases. Cell Mol Immunol (2013) 10(3):230–52. doi: 10.1038/cmi.2013.10
7. Wu F, Xie M, Hun M, She Z, Li C, Luo S, et al. Natural Killer Cell-Derived Extracellular Vesicles: Novel Players in Cancer Immunotherapy. Front Immunol (2021) 12. doi: 10.3389/fimmu.2021.658698
8. Kiessling R, Klein E, Pross H, Wigzell H. "Natural" Killer Cells in the Mouse. II. Cytotoxic Cells With Specificity for Mouse Moloney Leukemia Cells. Characteristics of the Killer Cell. Eur J Immunol (1975) 5(2):117–21. doi: 10.1002/eji.1830050209
9. Chan CJ, Smyth MJ, Martinet L. Molecular Mechanisms of Natural Killer Cell Activation in Response to Cellular Stress. Cell Death Differ (2014) 21(1):5–14. doi: 10.1038/cdd.2013.26
10. Cooper MA, Fehniger TA, Caligiuri MA. The Biology of Human Natural Killer-Cell Subsets. Trends Immunol (2001) 22(11):633–40. doi: 10.1016/S1471-4906(01)02060-9
11. Moretta L, Moretta A. Unravelling Natural Killer Cell Function: Triggering and Inhibitory Human NK Receptors. EMBO J (2004) 23(2):255–9. doi: 10.1038/sj.emboj.7600019
12. Waldhauer I, Steinle A. NK Cells and Cancer Immunosurveillance. Oncogene (2008) 27(45):5932–43. doi: 10.1038/onc.2008.267
13. Malmberg KJ, Carlsten M, Björklund A, Sohlberg E, Bryceson YT, Ljunggren HG. Natural Killer Cell-Mediated Immunosurveillance of Human Cancer. Semin Immunol (2017) 31:20–9. doi: 10.1016/j.smim.2017.08.002
14. Bryceson YT, Ljunggren HG, Long EO. Minimal Requirement for Induction of Natural Cytotoxicity and Intersection of Activation Signals by Inhibitory Receptors. Blood (2009) 114(13):2657–66. doi: 10.1182/blood-2009-01-201632
15. Bryceson YT, March ME, Ljunggren HG, Long EO. Synergy Among Receptors on Resting NK Cells for the Activation of Natural Cytotoxicity and Cytokine Secretion. Blood (2006) 107(1):159–66. doi: 10.1182/blood-2005-04-1351
16. Martínez-Lostao L, Anel A, Pardo J. How Do Cytotoxic Lymphocytes Kill Cancer Cells? Clin Cancer Res (2015) 21(22):5047–56. doi: 10.1158/1078-0432.CCR-15-0685
17. Abel AM, Yang C, Thakar MS, Malarkannan S. Natural Killer Cells: Development, Maturation, and Clinical Utilization. Front Immunol (2018) 9. doi: 10.3389/fimmu.2018.01869
18. Sivori S, Della Chiesa M, Carlomagno S, Quatrini L, Munari E, Vacca P, et al. Inhibitory Receptors and Checkpoints in Human NK Cells, Implications for the Immunotherapy of Cancer. Front Immunol (2020) 11. doi: 10.3389/fimmu.2020.02156
19. Russick J, Joubert PE, Gillard-Bocquet M, Torset C, Meylan M, Petitprez F, et al. Natural Killer Cells in the Human Lung Tumor Microenvironment Display Immune Inhibitory Functions. J Immunother Cancer (2020) 8(2):e001054. doi: 10.1136/jitc-2020-001054
20. Bachanova V, Miller JS. NK Cells in Therapy of Cancer. Crit Rev Oncog (2014) 19(0):133–41. doi: 10.1615/CritRevOncog.2014011091
21. Myers JA, Miller JS. Exploring the NK Cell Platform for Cancer Immunotherapy. Nat Rev Clin Oncol (2021) 18(2):85–100. doi: 10.1038/s41571-020-0426-7
22. Shankar K, Capitini CM, Saha K. Genome Engineering of Induced Pluripotent Stem Cells to Manufacture Natural Killer Cell Therapies. Stem Cell Res Ther (2020) 11(1):234. doi: 10.1186/s13287-020-01741-4
23. Zhang J, Zheng H, Diao Y. Natural Killer Cells and Current Applications of Chimeric Antigen Receptor-Modified NK-92 Cells in Tumor Immunotherapy. Int J Mol Sci (2019) 20(2):317. doi: 10.3390/ijms20020317
24. Klingemann H, Boissel L, Toneguzzo F. Natural Killer Cells for Immunotherapy - Advantages of the NK-92 Cell Line Over Blood NK Cells. Front Immunol (2016) 7:91. doi: 10.3389/fimmu.2016.00091
25. Williams BA, Law AD, Routy B, denHollander N, Gupta V, Wang XH, et al. A Phase I Trial of NK-92 Cells for Refractory Hematological Malignancies Relapsing After Autologous Hematopoietic Cell Transplantation Shows Safety and Evidence of Efficacy. Oncotarget (2017) 8(51):89256–68. doi: 10.18632/oncotarget.19204
26. Tam YK, Martinson JA, Doligosa K, Klingemann HG. Ex Vivo Expansion of the Highly Cytotoxic Human Natural Killer-92 Cell-Line Under Current Good Manufacturing Practice Conditions for Clinical Adoptive Cellular Immunotherapy. Cytotherapy (2003) 5(3):259–72. doi: 10.1002/eji.1830050209
27. Navarrete-Galvan L, Guglielmo M, Cruz Amaya J, Smith-Gagen J, Lombardi VC, Merica R, et al. Optimizing NK-92 Serial Killers: Gamma Irradiation, CD95/Fas-Ligation, and NK or LAK Attack Limit Cytotoxic Efficacy. J Transl Med (2022) 20(1):151. doi: 10.1186/s12967-022-03350-6
28. Walcher L, Kistenmacher AK, Sommer C, Böhlen S, Ziemann C, Dehmel S, et al. Low Energy Electron Irradiation Is a Potent Alternative to Gamma Irradiation for the Inactivation of (CAR-)NK-92 Cells in ATMP Manufacturing. Front Immunol (2021) 12. doi: 10.3389/fimmu.2021.684052
29. Karagiannis P, Kim SI. iPSC-Derived Natural Killer Cells for Cancer Immunotherapy. Mol Cells (2021) 44(8):541–8. doi: 10.14348/molcells.2021.0078
30. Kaweme NM, Zhou F. Optimizing NK Cell-Based Immunotherapy in Myeloid Leukemia: Abrogating an Immunosuppressive Microenvironment. Front Immunol (2021) 12:2348. doi: 10.3389/fimmu.2021.683381
31. Dianat-Moghadam H, Rokni M, Marofi F, Panahi Y, Yousefi M. Natural Killer Cell–Based Immunotherapy: From Transplantation Toward Targeting Cancer Stem Cells. J Cell Physiol (2019) 234(1):259–73. doi: 10.1002/jcp.26878
32. Groth A, Klöss S, von Strandmann EP, Koehl U, Koch J. Mechanisms of Tumor and Viral Immune Escape From Natural Killer Cell-Mediated Surveillance. J Innate Immun (2011) 3(4):344–54. doi: 10.1159/000327014
33. Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E, et al. Immune Evasion in Cancer: Mechanistic Basis and Therapeutic Strategies. Semin Cancer Biol (2015) 35:S185–98. doi: 10.1016/j.semcancer.2015.03.004
34. Cruz-Bermúdez A, Laza-Briviesca R, Casarrubios M, Sierra-Rodero B, Provencio M. The Role of Metabolism in Tumor Immune Evasion: Novel Approaches to Improve Immunotherapy. Biomedicines (2021) 9(4):361. doi: 10.3390/biomedicines9040361
35. Xia L, Oyang L, Lin J, Tan S, Han Y, Wu N, et al. The Cancer Metabolic Reprogramming and Immune Response. Mol Cancer (2021) 20(1):28. doi: 10.1186/s12943-021-01316-8
36. Park A, Lee Y, Kim MS, Kang YJ, Park YJ, Jung H, et al. Prostaglandin E2 Secreted by Thyroid Cancer Cells Contributes to Immune Escape Through the Suppression of Natural Killer (NK) Cell Cytotoxicity and NK Cell Differentiation. Front Immunol (2018) 9:1859. doi: 10.3389/fimmu.2018.01859
37. Reindl LM, Albinger N, Bexte T, Müller S, Hartmann J, Ullrich E. Immunotherapy With NK Cells: Recent Developments in Gene Modification Open Up New Avenues. OncoImmunology (2020) 9(1):1777651. doi: 10.1080/2162402X.2020.1777651
38. Villalba M, Alexia C, Bellin-Robert A, Fayd’herbe de Maudave A, Gitenay D. Non-Genetically Improving the Natural Cytotoxicity of Natural Killer (NK) Cells. Front Immunol (2020) 10:3026. doi: 10.3389
39. Hu W, Wang G, Huang D, Sui M, Xu Y. Cancer Immunotherapy Based on Natural Killer Cells: Current Progress and New Opportunities. Front Immunol (2019) 10:1205. doi: 10.3389/fimmu.2019.01205
40. Felices M, Lenvik AJ, McElmurry R, Chu S, Hinderlie P, Bendzick L, et al. Continuous Treatment With IL-15 Exhausts Human NK Cells via a Metabolic Defect. JCI Insight (2018) 3(3):e96219. doi: 10.1172/jci.insight.96219
41. Wu Y, Tian Z, Wei H. Developmental and Functional Control of Natural Killer Cells by Cytokines. Front Immunol (2017) 8. doi: 10.3389/fimmu.2017.00930
42. Romee R, Rosario M, Berrien-Elliott MM, Wagner JA, Jewell BA, Schappe T, et al. Cytokine-Induced Memory-Like Natural Killer Cells Exhibit Enhanced Responses Against Myeloid Leukemia. Sci Transl Med (2016) 8(357):357ra123–357ra123. doi: 10.1126/scitranslmed.aaf2341
43. Miller JS, Lanier LL. Natural Killer Cells in Cancer Immunotherapy. Annu Rev Cancer Biol (2019) 3(1):77–103. doi: 10.1146/annurev-cancerbio-030518-055653
44. Imamura M, Shook D, Kamiya T, Shimasaki N, Chai SMH, Coustan-Smith E, et al. Autonomous Growth and Increased Cytotoxicity of Natural Killer Cells Expressing Membrane-Bound Interleukin-15. Blood (2014) 124(7):1081–8. doi: 10.1182/blood-2014-02-556837
45. Oyer JL, Pandey V, Igarashi RY, Somanchi SS, Zakari A, Solh M, et al. Natural Killer Cells Stimulated With PM21 Particles Expand and Biodistribute In Vivo: Clinical Implications for Cancer Treatment. Cytotherapy (2016) 18(5):653–63. doi: 10.1016/j.jcyt.2016.02.006
46. Denman CJ, Senyukov VV, Somanchi SS, Phatarpekar PV, Kopp LM, Johnson JL, et al. Membrane-Bound IL-21 Promotes Sustained Ex Vivo Proliferation of Human Natural Killer Cells. PloS One (2012) 7(1):e30264. doi: 10.1371/journal.pone.0030264
47. Mlecnik B, Bindea G, Angell HK, Sasso MS, Obenauf AC, Fredriksen T, et al. Functional Network Pipeline Reveals Genetic Determinants Associated With in Situ Lymphocyte Proliferation and Survival of Cancer Patients. Sci Transl Med (2014) 6(228):228ra37–228ra37. doi: 10.1126/scitranslmed.3007240
48. Wrangle JM, Velcheti V, Patel MR, Garrett-Mayer E, Hill EG, Ravenel JG, et al. ALT-803, an IL-15 Superagonist, in Combination With Nivolumab in Patients With Metastatic non-Small Cell Lung Cancer: A non-Randomised, Open-Label, Phase 1b Trial. Lancet Oncol (2018) 19(5):694–704. doi: 10.1016/S1470-2045(18)30148-7
49. Romee R, Cooley S, Berrien-Elliott MM, Westervelt P, Verneris MR, Wagner JE, et al. First-In-Human Phase 1 Clinical Study of the IL-15 Superagonist Complex ALT-803 to Treat Relapse After Transplantation. Blood (2018) 131(23):2515–27. doi: 10.1182/blood-2017-12-823757
50. Knudson KM, Hodge JW, Schlom J, Gameiro SR. Rationale for IL-15 Superagonists in Cancer Immunotherapy. Expert Opin Biol Ther (2020) 20(7):705–9. doi: 10.1080/14712598.2020.1738379
51. Yvon ES, Burga R, Powell A, Cruz CR, Fernandes R, Barese C, et al. Cord Blood Natural Killer Cells Expressing a Dominant Negative TGF-β Receptor: Implications for Adoptive Immunotherapy for Glioblastoma. Cytotherapy (2017) 19(3):408–18. doi: 10.1016/j.jcyt.2016.12.005
52. Wilson EB, El-Jawhari JJ, Neilson AL, Hall GD, Melcher AA, Meade JL, et al. Human Tumour Immune Evasion via TGF-β Blocks NK Cell Activation But Not Survival Allowing Therapeutic Restoration of Anti-Tumour Activity. PloS One (2011) 6(9):e22842. doi: 10.1371/journal.pone.0022842
53. Hideshima T, Ogiya D, Liu J, Harada T, Kurata K, Bae J, et al. Immunomodulatory Drugs Activate NK Cells via Both Zap-70 and Cereblon-Dependent Pathways. Leukemia (2021) 35(1):177–88. doi: 10.1038/s41375-020-0809-x
54. Hayashi T, Hideshima T, Akiyama M, Podar K, Yasui H, Raje N, et al. Molecular Mechanisms Whereby Immunomodulatory Drugs Activate Natural Killer Cells: Clinical Application. Br J Haematol (2005) 128(2):192–203. doi: 10.1111/j.1365-2141.2004.05286.x
55. Le Roy A, Prébet T, Castellano R, Goubard A, Riccardi F, Fauriat C, et al. Immunomodulatory Drugs Exert Anti-Leukemia Effects in Acute Myeloid Leukemia by Direct and Immunostimulatory Activities. Front Immunol (2018) 9. doi: 10.3389/fimmu.2018.00977
56. Fionda C, Abruzzese MP, Zingoni A, Cecere F, Vulpis E, Peruzzi G, et al. The IMiDs Targets IKZF-1/3 and IRF4 as Novel Negative Regulators of NK Cell-Activating Ligands Expression in Multiple Myeloma. Oncotarget (2015) 6(27):23609–30. doi: 10.18632/oncotarget.4603
57. Roda JM, Joshi T, Butchar JP, McAlees JW, Lehman A, Tridandapani S, et al. The Activation of Natural Killer Cell Effector Functions by Cetuximab-Coated, Epidermal Growth Factor Receptor Positive Tumor Cells is Enhanced by Cytokines. Clin Cancer Res Off J Am Assoc Cancer Res (2007) 13(21):6419–28. doi: 10.1158/1078-0432.CCR-07-0865
58. Beano A, Signorino E, Evangelista A, Brusa D, Mistrangelo M, Polimeni MA, et al. Correlation Between NK Function and Response to Trastuzumab in Metastatic Breast Cancer Patients. J Transl Med (2008) 6:25. doi: 10.1186/1479-5876-6-25
59. Wu L, Adams M, Carter T, Chen R, Muller G, Stirling D, et al. Lenalidomide Enhances Natural Killer Cell and Monocyte-Mediated Antibody-Dependent Cellular Cytotoxicity of Rituximab-Treated CD20+ Tumor Cells. Clin Cancer Res Off J Am Assoc Cancer Res (2008) 14(14):4650–7. doi: 10.1158/1078-0432.CCR-07-4405
60. Varchetta S, Gibelli N, Oliviero B, Nardini E, Gennari R, Gatti G, et al. Elements Related to Heterogeneity of Antibody-Dependent Cell Cytotoxicity in Patients Under Trastuzumab Therapy for Primary Operable Breast Cancer Overexpressing Her2. Cancer Res (2007) 67(24):11991–9. doi: 10.1158/0008-5472.CAN-07-2068
61. Koerner SP, André MC, Leibold JS, Kousis PC, Kübler A, Pal M, et al. An Fc-Optimized CD133 Antibody for Induction of NK Cell Reactivity Against Myeloid Leukemia. Leukemia (2017) 31(2):459–69. doi: 10.1038/leu.2016.194
62. Kim YM, Park JS, Kim SK, Jung KM, Hwang YS, Han M, et al. The Transgenic Chicken Derived Anti-CD20 Monoclonal Antibodies Exhibits Greater Anti-Cancer Therapeutic Potential With Enhanced Fc Effector Functions. Biomaterials (2018) 167:58–68. doi: 10.1016/j.biomaterials.2018.03.021
63. Ferrari de Andrade L, Tay RE, Pan D, Luoma AM, Ito Y, Badrinath S, et al. Antibody-Mediated Inhibition of MICA and MICB Shedding Promotes NK Cell–Driven Tumor Immunity. Science (2018) 359(6383):1537–42. doi: 10.1126/science.aao0505
64. Gauthier L, Morel A, Anceriz N, Rossi B, Blanchard-Alvarez A, Grondin G, et al. Multifunctional Natural Killer Cell Engagers Targeting NKp46 Trigger Protective Tumor Immunity. Cell (2019) 177(7):1701–1713.e16. doi: 10.1016/j.cell.2019.04.041
65. Felices M, Lenvik TR, Davis ZB, Miller JS, Vallera DA. Generation of BiKEs and TriKEs to Improve NK Cell-Mediated Targeting of Tumor Cells. Methods Mol Biol Clifton NJ (2016) 1441:333–46. doi: 10.1007/978-1-4939-3684-7_28
66. Kellner C, Bruenke J, Horner H, Schubert J, Schwenkert M, Mentz K, et al. Heterodimeric Bispecific Antibody-Derivatives Against CD19 and CD16 Induce Effective Antibody-Dependent Cellular Cytotoxicity Against B-Lymphoid Tumor Cells. Cancer Lett (2011) 303(2):128–39. doi: 10.1016/j.canlet.2011.01.020
67. Bruenke J, Barbin K, Kunert S, Lang P, Pfeiffer M, Stieglmaier K, et al. Effective Lysis of Lymphoma Cells With a Stabilised Bispecific Single-Chain Fv Antibody Against CD19 and Fcγriii (Cd16). Br J Haematol (2005) 130(2):218–28. doi: 10.1111/j.1365-2141.2005.05414.x
68. Pesce S, Greppi M, Grossi F, Del Zotto G, Moretta L, Sivori S, et al. PD/1-PD-Ls Checkpoint: Insight on the Potential Role of NK Cells. Front Immunol (2019) 10. doi: 10.3389/fimmu.2019.01242
69. Hsu J, Hodgins JJ, Marathe M, Nicolai CJ, Bourgeois-Daigneault MC, Trevino TN, et al. Contribution of NK Cells to Immunotherapy Mediated by PD-1/PD-L1 Blockade. J Clin Invest (2018) 128(10):4654–68. doi: 10.1172/JCI99317
70. Juliá EP, Amante A, Pampena MB, Mordoh J, Levy EM. Avelumab, an IgG1 Anti-PD-L1 Immune Checkpoint Inhibitor, Triggers NK Cell-Mediated Cytotoxicity and Cytokine Production Against Triple Negative Breast Cancer Cells. Front Immunol (2018) 9. doi: 10.3389/fimmu.2018.02140
71. Lin M, Luo H, Liang S, Chen J, Liu A, Niu L, et al. Pembrolizumab Plus Allogeneic NK Cells in Advanced non–Small Cell Lung Cancer Patients. J Clin Invest (2020) 130(5):2560–9. doi: 10.1172/JCI132712
72. Poggi A, Zocchi MR. Natural Killer Cells and Immune-Checkpoint Inhibitor Therapy: Current Knowledge and New Challenges. Mol Ther - Oncolytics (2022) 24:26–42. doi: 10.1016/j.omto.2021.11.016
73. Mantesso S, Geerts D, Spanholtz J, Kučerová L. Genetic Engineering of Natural Killer Cells for Enhanced Antitumor Function. Front Immunol (2020) 11. doi: 10.3389/fimmu.2020.607131
74. Wiklander OPB, Brennan MÁ, Lötvall J, Breakefield XO, EL Andaloussi S. Advances in Therapeutic Applications of Extracellular Vesicles. Sci Transl Med (2019) 11(492):eaav8521. doi: 10.1126/scitranslmed.aav8521
75. Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J Extracell Vesicles (2018) 7(1):1535750. doi: 10.1080/20013078.2018.1535750
76. Lai RC, Yeo RWY, Lim SK. Mesenchymal Stem Cell Exosomes. Semin Cell Dev Biol (2015) 40:82–8. doi: 10.1016/j.semcdb.2015.03.001
77. Tan TT, Toh WS, Lai RC, Lim SK. Practical Considerations in Transforming MSC Therapy for Neurological Diseases From Cell to EV. Exp Neurol (2022) 349:113953. doi: 10.1016/j.expneurol.2021.113953
78. Kenjo E, Hozumi H, Makita Y, Iwabuchi KA, Fujimoto N, Matsumoto S, et al. Low Immunogenicity of LNP Allows Repeated Administrations of CRISPR-Cas9 mRNA Into Skeletal Muscle in Mice. Nat Commun (2021) 12(1):1–13. doi: 10.1038/s41467-021-26714-w
79. Lai RC, Arslan F, Lee MM, Sze NSK, Choo A, Chen TS, et al. Exosome Secreted by MSC Reduces Myocardial Ischemia/Reperfusion Injury. Stem Cell Res (2010) 4(3):214–22. doi: 10.1016/j.scr.2009.12.003
80. Paganini C, Palmiero UC, Pocsfalvi G, Touzet N, Bongiovanni A, Arosio P. Scalable Production and Isolation of Extracellular Vesicles: Available Sources and Lessons From Current Industrial Bioprocesses. Biotechnol J (2019) 14(10):1800528. doi: 10.1002/biot.201800528
81. Adlerz K, Patel D, Rowley J, Ng K, Ahsan T. Strategies for Scalable Manufacturing and Translation of MSC-Derived Extracellular Vesicles. Stem Cell Res (2020) 48:101978. doi: 10.1016/j.scr.2020.101978
82. Ng KS, Smith JA, McAteer MP, Mead BE, Ware J, Jackson FO, et al. Bioprocess Decision Support Tool for Scalable Manufacture of Extracellular Vesicles. Biotechnol Bioeng (2019) 116(2):307–19. doi: 10.1002/bit.26809
83. Marquez-Curtis LA, Janowska-Wieczorek A, McGann LE, Elliott JAW. Mesenchymal Stromal Cells Derived From Various Tissues: Biological, Clinical and Cryopreservation Aspects. Cryobiology (2015) 71(2):181–97. doi: 10.1016/j.cryobiol.2015.07.003
84. Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int J Nanomed (2020) 15:6917. doi: 10.2147/IJN.S264498
85. Herrmann IK, Wood MJA, Fuhrmann G. Extracellular Vesicles as a Next-Generation Drug Delivery Platform. Nat Nanotechnol (2021) 16(7):748–59. doi: 10.1038/s41565-021-00931-2
86. Wu CH, Li J, Li L, Sun J, Fabbri M, Wayne AS, et al. Extracellular Vesicles Derived From Natural Killer Cells Use Multiple Cytotoxic Proteins and Killing Mechanisms to Target Cancer Cells. J Extracell Vesicles (2019) 8(1):1588538. doi: 10.1080/20013078.2019.1588538
87. Lugini L, Cecchetti S, Huber V, Luciani F, Macchia G, Spadaro F, et al. Immune Surveillance Properties of Human NK Cell-Derived Exosomes. J Immunol (2012) 189(6):2833–42. doi: 10.4049/jimmunol.1101988
88. Federici C, Shahaj E, Cecchetti S, Camerini S, Casella M, Iessi E, et al. Natural-Killer-Derived Extracellular Vesicles: Immune Sensors and Interactors. Front Immunol (2020) 11:262. doi: 10.3389/fimmu.2020.00262
89. Vecchio FD, Martinez-Rodriguez V, Schukking M, Cocks A, Broseghini E, Fabbri M. Professional Killers: The Role of Extracellular Vesicles in the Reciprocal Interactions Between Natural Killer, CD8+ Cytotoxic T-Cells and Tumour Cells. J Extracell Vesicles (2021) 10(6):e12075. doi: 10.1002/jev2.12075
90. Li C, Donninger H, Eaton J, Yaddanapudi K. Regulatory Role of Immune Cell-Derived Extracellular Vesicles in Cancer: The Message Is in the Envelope. Front Immunol (2020) 11. doi: 10.3389/fimmu.2020.01525
91. Jong AY, Wu CH, Li J, Sun J, Fabbri M, Wayne AS, et al. Large-Scale Isolation and Cytotoxicity of Extracellular Vesicles Derived From Activated Human Natural Killer Cells. J Extracell Vesicles (2017) 6(1):1294368. doi: 10.1080/20013078.2017.1294368
92. Zhu L, Kalimuthu S, Oh JM, Gangadaran P, Baek SH, Jeong SY, et al. Enhancement of Antitumor Potency of Extracellular Vesicles Derived From Natural Killer Cells by IL-15 Priming. Biomaterials (2019) 190–191:38–50. doi: 10.1016/j.biomaterials.2018.10.034
93. Jiang Y, Jiang H, Wang K, Liu C, Man X, Fu Q. Hypoxia Enhances the Production and Antitumor Effect of Exosomes Derived From Natural Killer Cells. Ann Transl Med (2021) 9(6):473–3. doi: 10.21037/atm-21-347
94. Zhu L, Kalimuthu S, Gangadaran P, Oh JM, Lee HW, Baek SH, et al. Exosomes Derived From Natural Killer Cells Exert Therapeutic Effect in Melanoma. Theranostics (2017) 7(10):2732–45. doi: 10.7150/thno.18752
95. Wang G, Hu W, Chen H, Shou X, Ye T, Xu Y. Cocktail Strategy Based on NK Cell-Derived Exosomes and Their Biomimetic Nanoparticles for Dual Tumor Therapy. Cancers (2019) 11(10):1560. doi: 10.3390/cancers11101560
96. Sun H, Shi K, Qi K, Kong H, Zhang J, Dai S, et al. Natural Killer Cell-Derived Exosomal miR-3607-3p Inhibits Pancreatic Cancer Progression by Targeting IL-26. Front Immunol (2019) 10:2819. doi: 10.3389/fimmu.2019.02819
97. Neviani P, Wise PM, Murtadha M, Liu CW, Wu CH, Jong AY, et al. Natural Killer–Derived Exosomal miR-186 Inhibits Neuroblastoma Growth and Immune Escape Mechanisms. Cancer Res (2019) 79(6):1151–64. doi: 10.1158/0008-5472.CAN-18-0779
98. Li D, Wang Y, Jin X, Hu D, Xia C, Xu H, et al. NK Cell-Derived Exosomes Carry miR-207 and Alleviate Depression-Like Symptoms in Mice. J Neuroinflamm (2020) 17(1):1–19. doi: 10.1186/s12974-020-01787-4
99. Choi JW, Lim S, Kang JH, Hwang SH, Hwang KC, Kim SW, et al. Proteome Analysis of Human Natural Killer Cell Derived Extracellular Vesicles for Identification of Anticancer Effectors. Molecules (2020) 25(21):5216. doi: 10.3390/molecules25215216
100. Korenevskii AV, Milyutina Y, Zhdanova AA, Pyatygina KM, Sokolov DI, Sel’kov SA. Mass-Spectrometric Analysis of Proteome of Microvesicles Produced by NK-92 Natural Killer Cells. Bull Exp Biol Med (2018) 165(4):564–71. doi: 10.1007/s10517-018-4214-7
101. Jia R, Cui K, Li Z, Gao Y, Zhang B, Wang Z, et al. NK Cell-Derived Exosomes Improved Lung Injury in Mouse Model of Pseudomonas Aeruginosa Lung Infection. J Physiol Sci (2020) 70(1):50. doi: 10.1186/s12576-020-00776-9
102. Shoae-Hassani A, Hamidieh AA, Behfar M, Mohseni R, Mortazavi-Tabatabaei SA, Asgharzadeh S. NK Cell-Derived Exosomes From NK Cells Previously Exposed to Neuroblastoma Cells Augment the Antitumor Activity of Cytokine-Activated NK Cells. J Immunother (2017) 40(7):265–76. doi: 10.1097/CJI.0000000000000179
103. Shaver KA, Croom-Perez TJ, Copik AJ. Natural Killer Cells: The Linchpin for Successful Cancer Immunotherapy. Front Immunol (2021) 12:679117. doi: 10.3389/fimmu.2021.679117
104. Wang J, Bonacquisti EE, Brown AD, Nguyen J. Boosting the Biogenesis and Secretion of Mesenchymal Stem Cell-Derived Exosomes. Cells (2020) 9(3):660. doi: 10.3390/cells9030660
105. Siemens DR, Hu N, Sheikhi AK, Chung E, Frederiksen LJ, Pross H, et al. Hypoxia Increases Tumor Cell Shedding of MHC Class I Chain-Related Molecule: Role of Nitric Oxide. Cancer Res (2008) 68(12):4746–53. doi: 10.1158/0008-5472.CAN-08-0054
106. Balsamo M, Manzini C, Pietra G, Raggi F, Blengio F, Mingari MC, et al. Hypoxia Downregulates the Expression of Activating Receptors Involved in NK-Cell-Mediated Target Cell Killing Without Affecting ADCC. Eur J Immunol (2013) 43(10):2756–64. doi: 10.1002/eji.201343448
107. Sarkar S, Germeraad WTV, Rouschop KMA, Steeghs EMP, van Gelder M, Bos GMJ, et al. Hypoxia Induced Impairment of NK Cell Cytotoxicity Against Multiple Myeloma Can Be Overcome by IL-2 Activation of the NK Cells. PloS One (2013) 8(5):e64835. doi: 10.1371/journal.pone.0064835
108. Krzywinska E, Kantari-Mimoun C, Kerdiles Y, Sobecki M, Isagawa T, Gotthardt D, et al. Loss of HIF-1α in Natural Killer Cells Inhibits Tumour Growth by Stimulating non-Productive Angiogenesis. Nat Commun (2017) 8(1):1–13. doi: 10.1038/s41467-017-01599-w
109. Velásquez SY, Killian D, Schulte J, Sticht C, Thiel M, Lindner HA. Short Term Hypoxia Synergizes With Interleukin 15 Priming in Driving Glycolytic Gene Transcription and Supports Human Natural Killer Cell Activities *. J Biol Chem (2016) 291(25):12960–77. doi: 10.1074/jbc.M116.721753
110. Yamanaka S. Pluripotent Stem Cell-Based Cell Therapy—Promise and Challenges. Cell Stem Cell (2020) 27(4):523–31. doi: 10.1016/j.stem.2020.09.014
111. Takahashi K, Yamanaka S. Induction of Pluripotent Stem Cells From Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell. (2006) 126(4):663–76. doi: 10.1016/j.cell.2006.07.024
112. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of Pluripotent Stem Cells From Adult Human Fibroblasts by Defined Factors. Cell. (2007) 131(5):861–72. doi: 10.1016/j.cell.2007.11.019
113. Maherali N, Sridharan R, Xie W, Utikal J, Eminli S, Arnold K, et al. Directly Reprogrammed Fibroblasts Show Global Epigenetic Remodeling and Widespread Tissue Contribution. Cell Stem Cell (2007) 1(1):55–70. doi: 10.1016/j.stem.2007.05.014
114. Karagiannis P, Iriguchi S, Kaneko S. Reprogramming Away From the Exhausted T Cell State. Semin Immunol (2016) 28(1):35–44. doi: 10.1016/j.smim.2015.10.007
115. Cichocki F, Bjordahl R, Gaidarova S, Mahmood S, Abujarour R, Wang H, et al. iPSC-Derived NK Cells Maintain High Cytotoxicity and Enhance In Vivo Tumor Control in Concert With T Cells and Anti–PD-1 Therapy. Sci Transl Med (2020) 12(568):eaaz5618. doi: 10.1126/scitranslmed.aaz5618
116. Li Y, Hermanson DL, Moriarity BS, Kaufman DS. Human iPSC-Derived Natural Killer Cells Engineered With Chimeric Antigen Receptors Enhance Anti-Tumor Activity. Cell Stem Cell (2018) 23(2):181–192.e5. doi: 10.1016/j.stem.2018.06.002
117. Lupo KB, Moon JI, Chambers AM, Matosevic S. Differentiation of Natural Killer Cells From Induced Pluripotent Stem Cells Under Defined, Serum- and Feeder-Free Conditions. Cytotherapy (2021) 23(10):939–52. doi: 10.1016/j.jcyt.2021.05.001
118. Cerwenka A, Lanier LL. Natural Killer Cell Memory in Infection, Inflammation and Cancer. Nat Rev Immunol (2016) 16(2):112–23. doi: 10.1038/nri.2015.9
119. Judge SJ, Murphy WJ, Canter RJ. Characterizing the Dysfunctional NK Cell: Assessing the Clinical Relevance of Exhaustion, Anergy, and Senescence. Front Cell Infect Microbiol (2020) 10:49. doi: 10.3389/fcimb.2020.00049
120. Rocca YS, Roberti MP, Arriaga JM, Amat M, Bruno L, Pampena MB, et al. Altered Phenotype in Peripheral Blood and Tumor-Associated NK Cells From Colorectal Cancer Patients. Innate Immun (2013) 19(1):76–85. doi: 10.1177/1753425912453187
121. Hazeldine J, Lord JM. The Impact of Ageing on Natural Killer Cell Function and Potential Consequences for Health in Older Adults. Ageing Res Rev (2013) 12(4):1069–78. doi: 10.1016/j.arr.2013.04.003
122. Woltjen K, Oceguera-Yanez F, Kagawa H, Kim SI. At the Conflux of Human Genome Engineering and Induced Pluripotency. In: Turksen K, editor. Genome Editing. Cham: Springer International Publishing (2016). p. 45–64.
123. Zhu H, Blum RH, Bernareggi D, Ask EH, Wu Z, Hoel HJ, et al. Metabolic Reprograming via Deletion of CISH in Human iPSC-Derived NK Cells Promotes In Vivo Persistence and Enhances Anti-Tumor Activity. Cell Stem Cell (2020) 27(2):224–237.e6. doi: 10.1016/j.stem.2020.05.008
124. Zhu H, Blum RH, Bjordahl R, Gaidarova S, Rogers P, Lee TT, et al. Pluripotent Stem Cell–Derived NK Cells With High-Affinity Noncleavable CD16a Mediate Improved Antitumor Activity. Blood (2020) 135(6):399–410. doi: 10.1182/blood.2019000621
125. Ruella M, Xu J, Barrett DM, Fraietta JA, Reich TJ, Ambrose DE, et al. Induction of Resistance to Chimeric Antigen Receptor T Cell Therapy by Transduction of a Single Leukemic B Cell. Nat Med (2018) 24(10):1499–503. doi: 10.1038/s41591-018-0201-9
126. Regis S, Caliendo F, Dondero A, Casu B, Romano F, Loiacono F, et al. TGF-β1 Downregulates the Expression of CX3CR1 by Inducing miR-27a-5p in Primary Human NK Cells. Front Immunol (2017) 25(8):868. doi: 10.3389/fimmu.2017.00868
127. Kim TD, Lee SU, Yun S, Sun HN, Lee SH, Kim JW, et al. Human microRNA-27a* Targets Prf1 and GzmB Expression to Regulate NK-Cell Cytotoxicity. Blood (2011) 118(20):5476–86. doi: 10.1182/blood-2011-04-347526
128. Fujita Y, Hirosawa M, Hayashi K, Hatani T, Yoshida Y, Yamamoto T, et al. A Versatile and Robust Cell Purification System With an RNA-Only Circuit Composed of microRNA-Responsive ON and OFF Switches. Sci Adv (2022) 8(1):eabj1793. doi: 10.1126/sciadv.abj1793
129. Cichocki F, Wu CY, Zhang B, Felices M, Tesi B, Tuininga K, et al. ARID5B Regulates Metabolic Programming in Human Adaptive NK Cells. J Exp Med (2018) 215(9):2379–95. doi: 10.1084/jem.20172168
130. Wang AYL. Human Induced Pluripotent Stem Cell-Derived Exosomes as a New Therapeutic Strategy for Various Diseases. Int J Mol Sci (2021) 22(4):1769. doi: 10.3390/ijms22041769
131. Taheri B, Soleimani M, Fekri Aval S, Esmaeili E, Bazi Z, Zarghami N. Induced Pluripotent Stem Cell-Derived Extracellular Vesicles: A Novel Approach for Cell-Free Regenerative Medicine. J Cell Physiol (2019) 234(6):8455–64. doi: 10.1002/jcp.27775
132. Santoso MR, Ikeda G, Tada Y, Jung JH, Vaskova E, Sierra RG, et al. Exosomes From Induced Pluripotent Stem Cell-Derived Cardiomyocytes Promote Autophagy for Myocardial Repair. J Am Heart Assoc (2020) 9(6):e014345. doi: 10.1161/JAHA.119.014345
133. Gao L, Wang L, Wei Y, Krishnamurthy P, Walcott GP, Menasché P, et al. Exosomes Secreted by hiPSC-Derived Cardiac Cells Improve Recovery From Myocardial Infarction in Swine. Sci Transl Med (2020) 12(561):eaay1318. doi: 10.1126/scitranslmed.aay1318
134. El Harane N, Kervadec A, Bellamy V, Pidial L, Neametalla HJ, Perier MC, et al. Acellular Therapeutic Approach for Heart Failure: In Vitro Production of Extracellular Vesicles From Human Cardiovascular Progenitors. Eur Heart J (2018) 39(20):1835–47. doi: 10.1093/eurheartj/ehy012
135. Hicks DA, Jones AC, Corbett NJ, Fisher K, Pickering-Brown SM, Ashe MP, et al. Extracellular Vesicles Isolated From Human Induced Pluripotent Stem Cell-Derived Neurons Contain a Transcriptional Network. Neurochem Res (2020) 45(7):1711–28. doi: 10.1007/s11064-020-03019-w
136. Upadhya R, Madhu LN, Attaluri S, Gitaí DLG, Pinson MR, Kodali M, et al. Extracellular Vesicles From Human iPSC-Derived Neural Stem Cells: miRNA and Protein Signatures, and Anti-Inflammatory and Neurogenic Properties. J Extracell Vesicles (2020) 9(1):1809064. doi: 10.1080/20013078.2020.1809064
137. Li WY, Zhu QB, Jin LY, Yang Y, Xu XY, Hu XY. Exosomes Derived From Human Induced Pluripotent Stem Cell-Derived Neural Progenitor Cells Protect Neuronal Function Under Ischemic Conditions. Neural Regener Res (2021) 16(10):2064–70. doi: 10.4103/1673-5374.308665
138. Du Y, Li D, Han C, Wu H, Xu L, Zhang M, et al. Exosomes From Human-Induced Pluripotent Stem Cell–Derived Mesenchymal Stromal Cells (hiPSC-MSCs) Protect Liver Against Hepatic Ischemia/ Reperfusion Injury via Activating Sphingosine Kinase and Sphingosine-1-Phosphate Signaling Pathway. Cell Physiol Biochem (2017) 43(2):611–25. doi: 10.1159/000480533
139. Xia Y, Ling X, Hu G, Zhu Q, Zhang J, Li Q, et al. Small Extracellular Vesicles Secreted by Human iPSC-Derived MSC Enhance Angiogenesis Through Inhibiting STAT3-Dependent Autophagy in Ischemic Stroke. Stem Cell Res Ther (2020) 11(1):313. doi: 10.1186/s13287-020-01834-0
140. Gao R, Ye T, Zhu Z, Li Q, Zhang J, Yuan J, et al. Small Extracellular Vesicles From iPSC-Derived Mesenchymal Stem Cells Ameliorate Tendinopathy Pain by Inhibiting Mast Cell Activation. Nanomed (2022) 17(8):513–29. doi: 10.2217/nnm-2022-0036
141. Zhang J, Guan J, Niu X, Hu G, Guo S, Li Q, et al. Exosomes Released From Human Induced Pluripotent Stem Cells-Derived MSCs Facilitate Cutaneous Wound Healing by Promoting Collagen Synthesis and Angiogenesis. J Transl Med (2015) 13(1):49. doi: 10.1186/s12967-015-0417-0
142. Adamiak M, Cheng G, Bobis-Wozowicz S, Zhao L, Kedracka-Krok S, Samanta A, et al. Induced Pluripotent Stem Cell (iPSC)–Derived Extracellular Vesicles Are Safer and More Effective for Cardiac Repair Than iPSCs. Circ Res (2018) 122(2):296–309. doi: 10.1161/CIRCRESAHA.117.311769
143. Liu S, Mahairaki V, Bai H, Ding Z, Li J, Witwer KW, et al. Highly Purified Human Extracellular Vesicles Produced by Stem Cells Alleviate Aging Cellular Phenotypes of Senescent Human Cells. Stem Cells (2019) 37(6):779–90. doi: 10.1002/stem.2996
144. Karnas E, Sekuła-Stryjewska M, Kmiotek-Wasylewska K, Bobis-Wozowicz S, Ryszawy D, Sarna M, et al. Extracellular Vesicles From Human iPSCs Enhance Reconstitution Capacity of Cord Blood-Derived Hematopoietic Stem and Progenitor Cells. Leukemia (2021) 35(10):2964–77. doi: 10.1038/s41375-021-01325-y
145. de Abreu RC, Fernandes H, da Costa Martins PA, Sahoo S, Emanueli C, Ferreira L. Native and Bioengineered Extracellular Vesicles for Cardiovascular Therapeutics. Nat Rev Cardiol (2020) 17(11):685–97. doi: 10.1038/s41569-020-0389-5
146. Colao IL, Corteling R, Bracewell D, Wall I. Manufacturing Exosomes: A Promising Therapeutic Platform. Trends Mol Med (2018) 24(3):242–56. doi: 10.1016/j.molmed.2018.01.006
147. Man K, Brunet MY, Fernandez-Rhodes M, Williams S, Heaney LM, Gethings LA, et al. Epigenetic Reprogramming Enhances the Therapeutic Efficacy of Osteoblast-Derived Extracellular Vesicles to Promote Human Bone Marrow Stem Cell Osteogenic Differentiation. J Extracell Vesicles (2021) 10(9):e12118. doi: 10.1002/jev2.12118
148. Huang R, Qin C, Wang J, Hu Y, Zheng G, Qiu G, et al. Differential Effects of Extracellular Vesicles From Aging and Young Mesenchymal Stem Cells in Acute Lung Injury. Aging. (2019) 11(18):7996–8014. doi: 10.18632/aging.102314
149. Dorronsoro A, Santiago FE, Grassi D, Zhang T, Lai RC, McGowan SJ, et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles Reduce Senescence and Extend Health Span in Mouse Models of Aging. Aging Cell (2021) 20(4):e13337. doi: 10.1111/acel.13337
150. Kim H, Lee MJ, Bae EH, Ryu JS, Kaur G, Kim HJ, et al. Comprehensive Molecular Profiles of Functionally Effective MSC-Derived Extracellular Vesicles in Immunomodulation. Mol Ther (2020) 28(7):1628–44. doi: 10.1016/j.ymthe.2020.04.020
151. Fafián-Labora J, Lesende-Rodriguez I, Fernández-Pernas P, Sangiao-Alvarellos S, Monserrat L, Arntz OJ, et al. Effect of Age on Pro-Inflammatory miRNAs Contained in Mesenchymal Stem Cell-Derived Extracellular Vesicles. Sci Rep (2017) 7(1):1–12. doi: 10.1038/srep43923
152. Kim S, Lee SK, Kim H, Kim TM. Exosomes Secreted From Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Accelerate Skin Cell Proliferation. Int J Mol Sci (2018) 19(10):3119. doi: 10.3390/ijms19103119
153. Zhu Y, Wang Y, Zhao B, Niu X, Hu B, Li Q, et al. Comparison of Exosomes Secreted by Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells and Synovial Membrane-Derived Mesenchymal Stem Cells for the Treatment of Osteoarthritis. Stem Cell Res Ther (2017) 8(1):1–11. doi: 10.1186/s13287-017-0510-9
154. Skamagki M, Zhang C, Ross CA, Ananthanarayanan A, Liu Z, Mu Q, et al. RNA Exosome Complex-Mediated Control of Redox Status in Pluripotent Stem Cells. Stem Cell Rep (2017) 9(4):1053–61. doi: 10.1016/j.stemcr.2017.08.024
155. Xin H, Katakowski M, Wang F, Qian JY, Liu XS, Ali MM, et al. MicroRNA Cluster miR-17-92 Cluster in Exosomes Enhance Neuroplasticity and Functional Recovery After Stroke in Rats. Stroke (2017) 48(3):747–53. doi: 10.1161/STROKEAHA.116.015204
156. Xin H, Li Y, Liu Z, Wang X, Shang X, Cui Y, et al. MiR-133b Promotes Neural Plasticity and Functional Recovery After Treatment of Stroke With Multipotent Mesenchymal Stromal Cells in Rats via Transfer of Exosome-Enriched Extracellular Particles. Stem Cells Dayt Ohio (2013) 31(12):2737–46. doi: 10.1002/stem.1409
157. Shen H, Yao X, Li H, Li X, Zhang T, Sun Q, et al. Role of Exosomes Derived From miR-133b Modified MSCs in an Experimental Rat Model of Intracerebral Hemorrhage. J Mol Neurosci MN (2018) 64(3):421–30. doi: 10.1007/s12031-018-1041-2
158. Han D, Wang K, Zhang T, Gao GC, Xu H. Natural Killer Cell-Derived Exosome-Entrapped Paclitaxel can Enhance its Anti-Tumor Effect. Eur Rev Med Pharmacol Sci (2020) 24:5703–13. doi: 10.26355/eurrev_202005_21362
159. Böker KO, Lemus-Diaz N, Ferreira RR, Schiller L, Schneider S, Gruber J. The Impact of the CD9 Tetraspanin on Lentivirus Infectivity and Exosome Secretion. Mol Ther (2018) 26(2):634–47. doi: 10.1016/j.ymthe.2017.11.008
160. Kim S, Kim TM. Generation of Mesenchymal Stem-Like Cells for Producing Extracellular Vesicles. World J Stem Cells (2019) 11(5):270–80. doi: 10.4252/wjsc.v11.i5.270
161. Haraszti RA, Miller R, Stoppato M, Sere YY, Coles A, Didiot MC, et al. Exosomes Produced From 3D Cultures of MSCs by Tangential Flow Filtration Show Higher Yield and Improved Activity. Mol Ther (2018) 26(12):2838–47. doi: 10.1016/j.ymthe.2018.09.015
162. Tristan CA, Ormanoglu P, Slamecka J, Malley C, Chu PH, Jovanovic VM, et al. Robotic High-Throughput Biomanufacturing and Functional Differentiation of Human Pluripotent Stem Cells. Stem Cell Rep (2021) 16(12):3076–92. doi: 10.1016/j.stemcr.2021.11.004
Keywords: natural killer cells, extracellular vesicles, exosomes, induced pluripotent stem cells, manufacturing, genome engineering, immunotherapy, cancer
Citation: Boyd-Gibbins N, Karagiannis P, Hwang DW and Kim S-I (2022) iPSCs in NK Cell Manufacturing and NKEV Development. Front. Immunol. 13:890894. doi: 10.3389/fimmu.2022.890894
Received: 07 March 2022; Accepted: 03 June 2022;
Published: 08 July 2022.
Edited by:
Evelyn Ullrich, Goethe University Frankfurt, GermanyReviewed by:
Daniela Pende, San Martino Hospital (IRCCS), ItalyFrank M. Cichocki, University of Minnesota Twin Cities, United States
Copyright © 2022 Boyd-Gibbins, Karagiannis, Hwang and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shin-Il Kim, c2lraW1AdGhlcmFiZXN0LmNvLmty
 Nicholas Boyd-Gibbins
Nicholas Boyd-Gibbins Peter Karagiannis
Peter Karagiannis Do Won Hwang3
Do Won Hwang3 Shin-Il Kim
Shin-Il Kim