
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Immunol. , 27 May 2022
Sec. Inflammation
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.883239
This article is part of the Research Topic Cellular and Molecular Communication Networks within the Cutaneous Immune System View all 11 articles
Inflammation plays an active role during the wound healing process. There is a direct association between the extent of injury as well as inflammation and the amount of subsequent cutaneous scarring. Evidence to date demonstrates that high levels of inflammation are associated with excessive dermal scarring and formation of abnormal pathological scars such as keloids and hypertrophic scars. In view of the multiple important cell types being involved in the inflammatory process and their influence on the extent of scar formation, many scar therapies should aim to target these cells in order to control inflammation and by association help improve scar outcome. However, most current treatment strategies for the management of a newly formed skin scar often adopt a watch-and-wait approach prior to commencing targeted anti-inflammatory therapy. Moreover, most of these therapies have been evaluated in the remodelling phase of wound healing and the evaluation of anti-inflammatory treatments at earlier stages of healing have not been fully explored and remain limited. Taken together, in order to minimise the risk of developing a poor scar outcome, it is clear that adopting an early intervention prior to skin injury would be optimal, however, the concept of pre-emptively priming the skin prior to injury has not yet been thoroughly evaluated. Therefore, the aim of this review was to evaluate the available literature regarding scar therapies that aim to target inflammation which are commenced prior to when a scar is formed or immediately after injury, with a particular focus on the role of pre-emptive priming of skin prior to injury in order to control inflammation for the prevention of poor scarring outcome.
The wound healing process is highly complex and is comprised of a series of well-defined stages that include inflammation, proliferation, and remodelling/scar formation (1). After an initial injury, clotting occurs and invading microbes activate an inflammatory response that is spread by the local release of chemotactic factors (2). There are multiple key players in the inflammatory process including neutrophils, monocytes, and other immune cells that are recruited to the wound site to clear cell debris and infections and aid in the tissue repair response (1–3). The length of response and the degree vary which has an effect on the final outcome. Certainly, there are significant benefits of producing an inflammatory response, but there are also negative aspects which can lead to non-healing wounds and excessive scarring, including hypertrophic scar and keloid formation (4–6). Many studies have suggested a direct association between the extent of injury/inflammation and the amount of scarring and there has been evidence that high levels of inflammation are associated with excessive scarring or development of abnormal scars, whereas inflammation is significantly diminished in wounds that heal without scars (7–11).
A number of immune cells have been associated with scar formation, including mast cells, macrophages and neutrophils as well as many inflammatory mediators have been shown to influence scar formation (12, 13) (Figure 1). In particular, macrophages, T cells, and mast cells have all been shown to be increased in abnormal scar tissue in particular in keloid, although in varying degrees. In keloid tissue, macrophages have been shown to have upregulated M2-associated genes which are highly relevant to tissue repair and remodelling (12, 13). While macrophages are indeed important for tissue repair, an overabundance on the other hand can be detrimental (14, 15). Current research has shown that T cells have a complex role in regulating scar formation, mainly due to the diverse T cell subsets (16, 17). Coculture of keloid fibroblasts with a Treg-enriched condition demonstrated reduced collagen synthesis in comparison to keloid fibroblasts cocultured with a Treg deficient T cell population (18). Neutrophils are the earliest leukocytes to arrive on the site of injury and they have been identified as the key cells in preventing microbes spreading and have a role in fibrosis (19) but there is a lack of in vivo evidence of the role of neutrophils in scar formation. Mast cells, however, are important in hypersensitivity responses and allergic reactions and their link with scar formation has also been studied (8, 20–22). Observations of their numbers and activation status were positively correlated with the degree of scar formation (23, 24). They have been identified as being significantly increased in hypertrophic and keloid scars (8). Furthermore, our group has shown that not only is this a problem in excessive scarring but there are high numbers of mast cells in normal scarring compared to normal skin and this number is further increased after injury with levels not returning to normal even by week 8 post-injury (22).
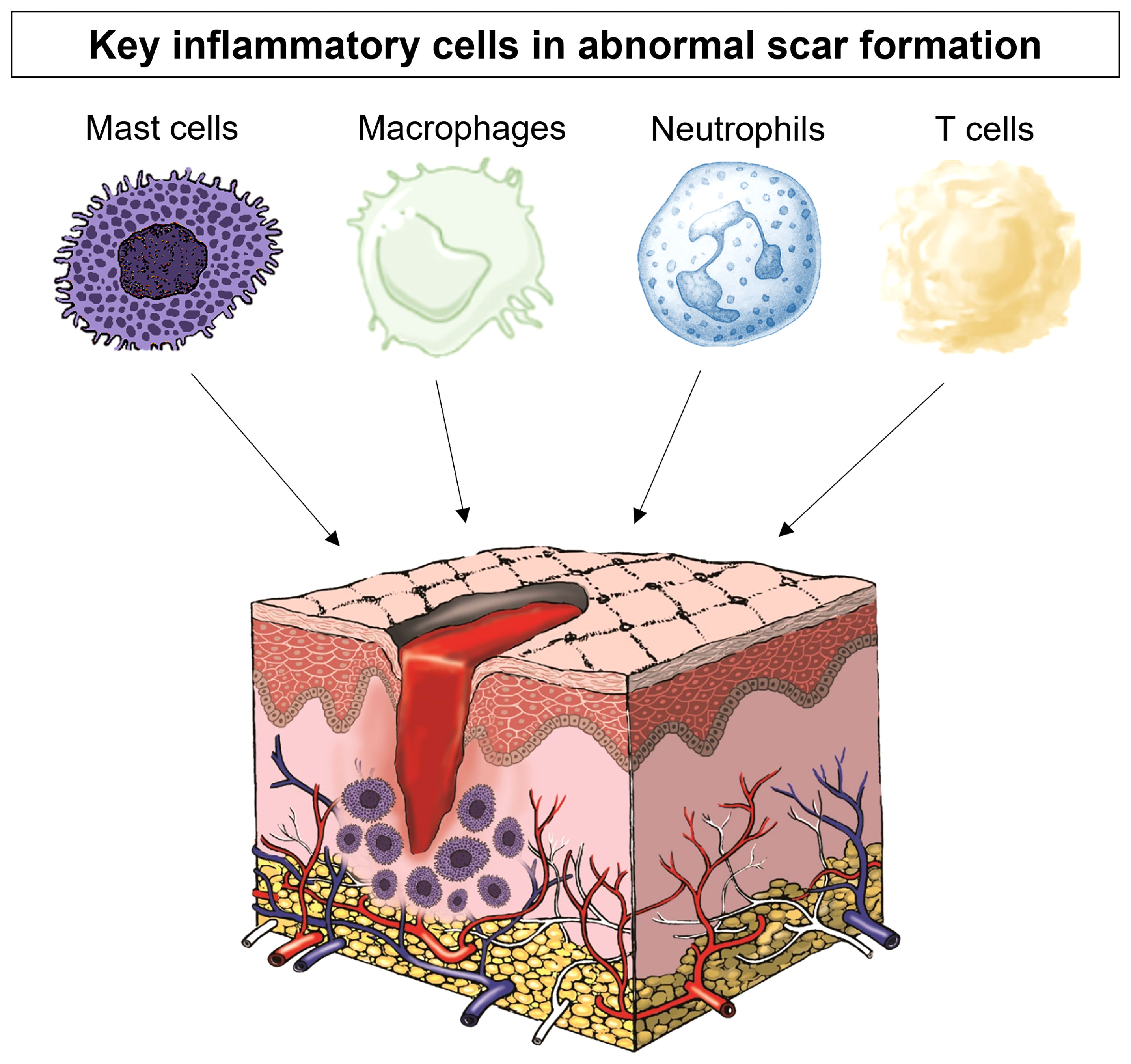
Figure 1 The key inflammatory cells in scar formation include mast cells, macrophages, neutrophils and T cells.
In view of these important cell types being involved in the inflammatory process and their influence on the extent of scar formation, many scar therapies aim to target these cells in order to quench inflammation to improve scar outcome (25–29). However, most of these therapies have been evaluated in the remodelling phase of wound healing mainly due to the scar being most evident at this stage and the amount of inflammation is most noticeable when correlated to scar severity. Conversely, the evaluation of anti-inflammatory treatments at earlier stages (30, 31) have been limited, because there has been the assumption that inflammation in the early phase of healing is necessary and beneficial for infection prevention and neovasculature development in wound healing. Most current treatment strategies for the management of a newly formed skin scar often adopt a watch-and-wait approach prior to commencing targeted therapy (32, 33). However, in order to minimise the risk of developing a poor scar outcome, taking an early intervention prior to skin injury is desirable. Priming is defined as a substance that prepares the skin for use. Priming the skin prior to an invasive intervention for achieving an optimal result has been studied (34–40). However, the concept of pre-emptively priming the skin prior to injury has not been thoroughly evaluated. Therefore, the aim of this review was to evaluate the available literature regarding scar therapies which aim to target inflammation which are commenced after a scar has formed or immediately after injury, and we will particularly focus on the role of pre-emptive priming of skin prior to injury in order to control inflammation for the prevention of poor scarring outcome (Figure 2).
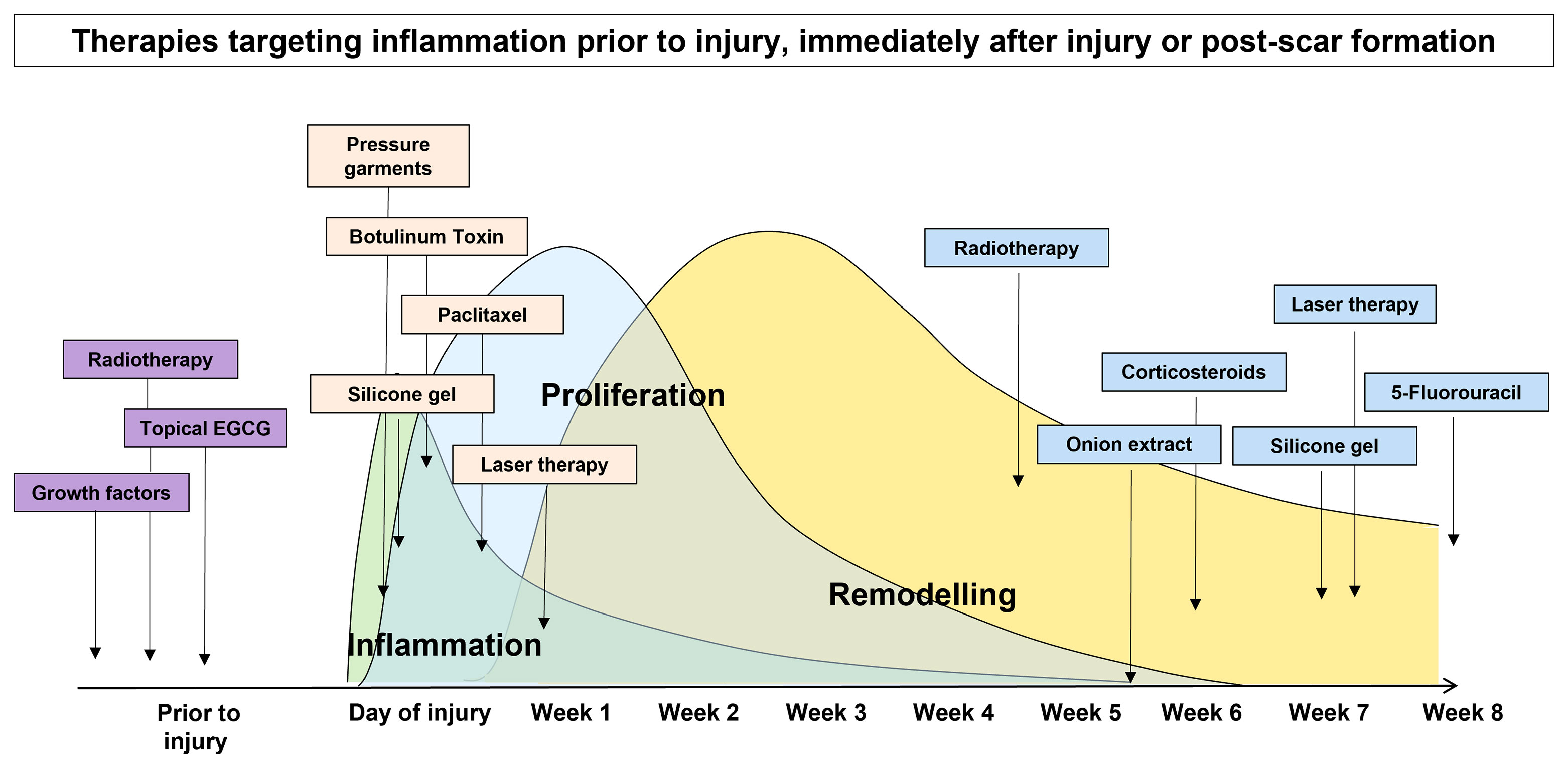
Figure 2 Scar therapies which aim to target inflammation pre-emptively prior to injury, immediately after injury and post-scar formation.
A number of anti-inflammatory treatments for skin scarring have been evaluated for existing scars including radiotherapy, compression (pressure therapy), laser and 5-fluorouracil therapy (40–43). These therapies have been thought to supress inflammation by inhibiting angiogenesis as inflammatory cells migrate through blood (5). A non-pharmacological treatment that has been used is onion extract ointment which is composed of phenolic compounds. This active ingredient can be converted to quercetin which is an anti-inflammatory derivative with its effects including mast cell stabilisation and anti-proliferative effects (44). This treatment has been used post-operatively to compare the efficacy of silicone gel containing onion extract and aloe vera to silicone gel sheets to prevent postoperative hypertrophic scars and keloids (45). Another study used onion extract on C-Section scars 4 weeks post-surgery and noted improved scar pigmentation and pliability (46).
There are a number of pharmacological treatments which have been used to target inflammation for scarring. Steroids are well known for their anti-inflammatory effect and are widely used for treatment of autoimmune diseases. They decrease inflammation by suppressing the activities of both myeloid and lymphoid cells (47). As increased inflammation promotes excessive scarring, steroid administration is one of the most commonly used treatments for keloid and hypertrophic scars (47). An anti-cancer drug named, Paclitaxel has been shown to regulate inflammatory responses and fibrosis. It has been found to inhibit the NF-kB pathway (48) and attenuate fibrosis by blocking STAT-3 signaling (49). This anti-inflammatory and anti-fibrosis effect has also been observed in animal models of keloid (50) and hypertrophic scars (51). In vitro studies have shown that the expression of IL-6 and TNF-a, as well as the production of a-SMA and collagen I, decreased in human keloid fibroblasts following paclitaxel administration (50).
Laser therapies have been used to target inflammation for existing scars. One study used a diode laser with an intralesional optical fibre delivery device in the treatment of hypertrophic and keloid scars and showed a significant reduction in pigmentation and blood perfusion levels (52). A PDL/Nd : YAG laser has also been used in the treatment of surgical scars and the short-term effects were evaluated by in vivo confocal microscopy and the long-term effects by clinical assessment of the scars appearance (53). The results demonstrated scar improvements with the laser treatment by showing lower numbers of vessels and decreased amount of collagen fibers.
A number of studies have aimed to explore the effects of early intervention of inflammation on scar formation with the outcomes varying by different approaches. Surgery, tapes, sheets, and pressure garments are considered useful in decreasing inflammation in the wound area by reducing the degree of tension at the wound edge and all have been used immediately during or after surgery/injury (54). Silicone gel is considered the first line of treatment for most scar management cases, including hypertrophic and keloid scars (55). This anti-scar activity could be partly attributed to the occlusive environment created by silicone gel dressings, and hydration is also believed to lead to the stabilisation of mast cells (16). This treatment is usually applied early once a scar has formed.
Laser therapies have been used both on existing scars and earlier in the healing process. In most studies, the recommended time frame for commencing scar treatment using fractional CO2 laser has remained relatively consistent at more than 2 months after surgery (56). However, recent studies have emphasised the importance of early treatment to reduce scar formation using fractional CO2 laser (56, 57). Resurfacing of scars using fractional CO2 laser with early interventional treatment has been shown to reduce scar formation (58–61). A meta-analysis has supported the efficacy of lasers in minimizing closed surgical scars when treated less than 1 month after surgery (62). There has also been reports on the use of intraoperative fractional CO2 laser treatment of wound edges, which significantly improved the appearance and texture of the scars (63). Erbium: YAG laser treatment, performed immediately after surgery, can also improve the appearance of a surgical scar (64). Thus, it appears that early treatment to the wound may lessen scar formation. Non-ablative fractional laser treatment has also been directly used on the wounded skin barrier immediately after punch biopsy collection, in order to improve scar appearance (65).
Botox which is the commercial name of botulinum toxin (BTX), a neurotoxin produced by clostridium botulinum has been shown to have anti-inflammatory effects on wound healing and scar formation (4, 66, 67). A meta-analysis evaluating intralesional injection of BTX-A versus corticosteroids and placebo in the treatment of hypertrophic scars and keloids showed that BTX-A was most effective (68). Another study compared the effects of inflammation intervention in earlier phases (on the day of operation) with that in later phase (2-week postoperatively) on scar formation after thyroidectomy (31). It has been suggested that by injecting BTX-A and its diffusion into the surrounding muscles at different times during the wound healing process this may have different effects on scar cosmesis by reducing the levels of inflammatory cytokines and mechanical tension on the wound edges (69–71). Although no difference was observed in scar size, early application of BTX-A achieved better scar appearance in relation to erythema, skin elasticity and patient satisfaction, compared to later application. However, the authors did not perform histopathologic assessments in order to evaluate the changes in inflammatory cell infiltration during specific phases of inflammation and structural changes of the skin.
Another study evaluated the effectiveness of intraoperative electro-abrasion for scar revision (72). This was a prospective, randomized, observer-blinded, split-scar study with 24 linear scar segments from patients undergoing Mohs micrographic surgery. After placement of dermal sutures, half of the wound was randomly treated with electro-abrasion whilst the other half was used as the control. Results showed improved scar topography but worsened erythema.
Several studies support the concept of pre-emptive priming of skin prior to cutaneous injury (34–39). For instance, research on the treatment of pigmented acne scars by ablative laser therapy advocates the use of priming agents to reduce wound healing time, decrease the risk of post-inflammatory hyperpigmentation and provide ultraviolet damage protection (34). Additionally, radiotherapy has been used as an adjuvant therapy for the treatment of keloid scarring both prior to extralesional excision and post-surgery and this has been shown to lead to lower recurrence rates (73).
Another option to target inflammation is by way of topical therapies which have been thought to be a viable therapeutic strategy for restricting scar tissue production and enhancing the cosmetic and functional clinical outcomes resulting from skin injury (74). Our group has conducted two double-blind randomised controlled clinical trials in humans to evaluate the concept of immediate versus delayed application of a topical formulation post-surgical wounding in an excisional punch-biopsy model (75) and the concept of pre-emptive priming of the wound site compared to immediate or delayed application (76). The objective was to deliver an active compound at the optimal time post-surgically induced injury, in order to maximise its impact and improve healing. The results from the first trial (75) demonstrated reduced mast cell numbers, scar thickness and angiogenesis plus increased hydration and elasticity when an anti-scarring topical formulation was applied immediately to the zone of injury and with delayed application of topical two-weeks post-wounding. The topical formulation used epigallocatechin-3-gallate (EGCG) as the active ingredient. EGCG is the most potent anti-inflammatory anti-oxidant and active component and the most extensively studied green tea catechin (75). Our group have previously evidenced the transdermal delivery uptake of EGCG by using high-performance liquid chromatography and demonstrated this penetrated to the deep dermal tissue and remained in the dermis (74). The mechanism of action of EGCG in wound healing and scarring remain unclear but a number of in vitro studies have been conducted to identify the mechanism of action of EGCG on mast cells, angiogenesis and scar shrinkage (77–79).
The findings from the first study provoked the hypothesis that even earlier application of an active such as EGCG could have beneficial effects on the outcome of scarring. Therefore, the second double-blind randomized placebo-controlled trial used various modes of the same anti-scarring topical formulation (containing EGCG) application utilising a full-thickness excisional surgical biopsy approach to identify whether pre-emptive priming pre-surgically induced injury had a greater impact on scarring outcome compared to immediate or delayed application (76). This demonstrated that the effects were further maximised by targeting the source of inflammation earlier. Based on our data, early intervention with the application of topical EGCG particularly 7 days prior to injury demonstrated greater reduction in mast cells (Figure 3) and angiogenesis (Figure 4), increases in elastin (Figure 5) and antioxidant levels and reductions in scar thickness (Figure 6) compared to the other modes of topical application. The reduction in mast cell numbers and subsequent lower levels of angiogenesis are beneficial as by targeting these cell types it is thought that this will contain inflammation to achieve a better scar outcome. Additionally, healing requires a strong angiogenic response but high angiogenesis also directly influences scar formation so partial inhibition of the angiogenic response could reduce scar formation or the potential for the development of abnormal skin scarring (78). Furthermore, animal studies have suggested that knocking out mast cells are not detrimental to acute wound healing and there have been a number of studies that have blocked mast cell activation to improve scar formation (80–82).
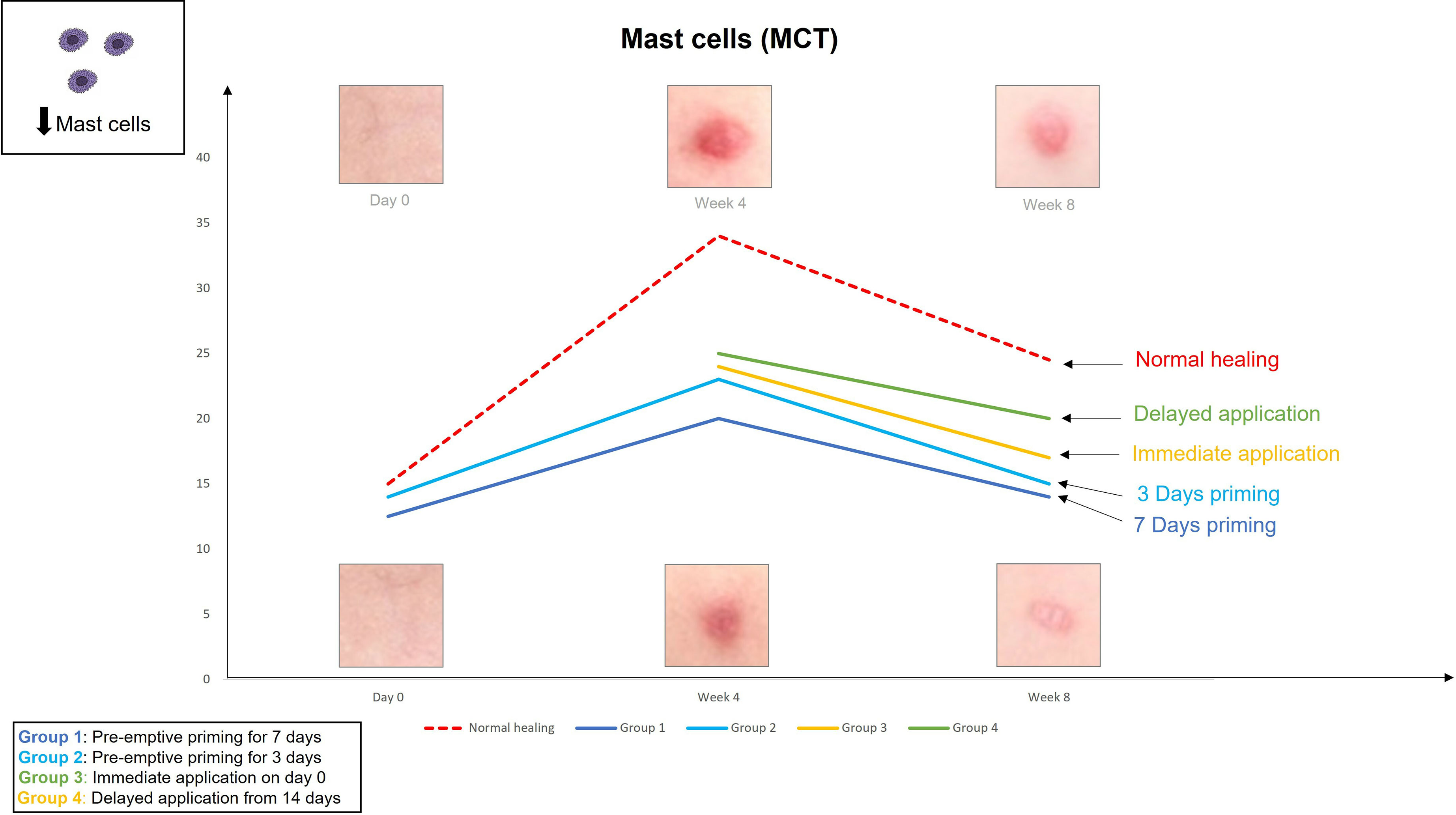
Figure 3 We evaluated the pre-emptive, immediate and delayed intervention of topical Epigallocatechin-3-gallate (EGCG) on skin scarring by conducting a randomsied double blind controlled trial on 40 healthy volunteers over 8 weeks. Here, we demonstrate graphical representations of the trends. Application of topical EGCG, particularly 7 days prior to injury demonstrated greater reductions in mast cells, compared to the other modes of topical application.
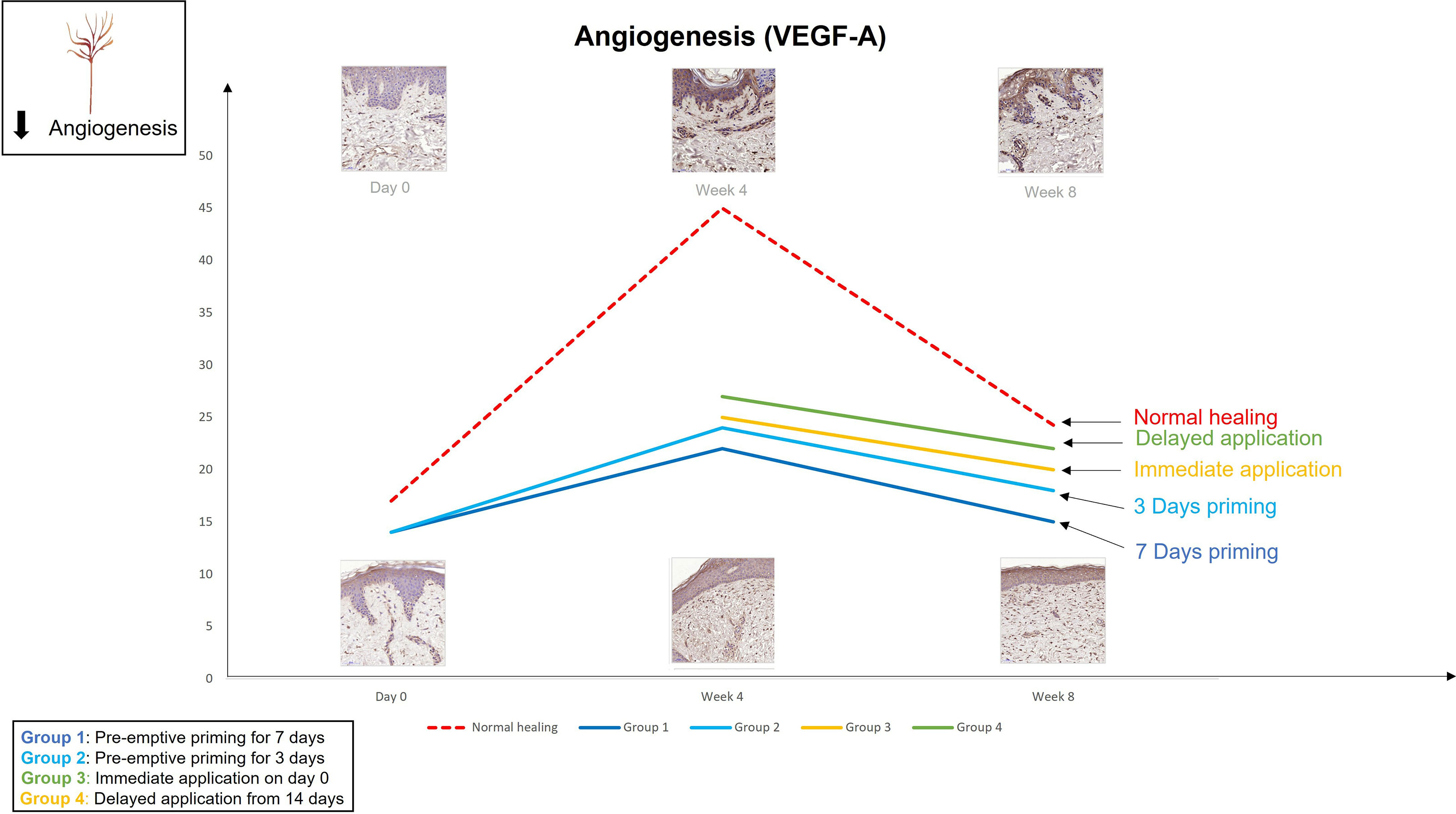
Figure 4 We evaluated the pre-emptive, immediate and delayed intervention of topical Epigallocatechin-3-gallate on skin scarring by conducting a randomsied double blind controlled trial on 40 healthy volunteers over 8 weeks. Graphical representation of the trends for angiogenesis (VEGFA) which demonstrates that pre-emptively priming the skin 7 days prior to injury shows greater reductions in VEGFA compared to all other modes of application.
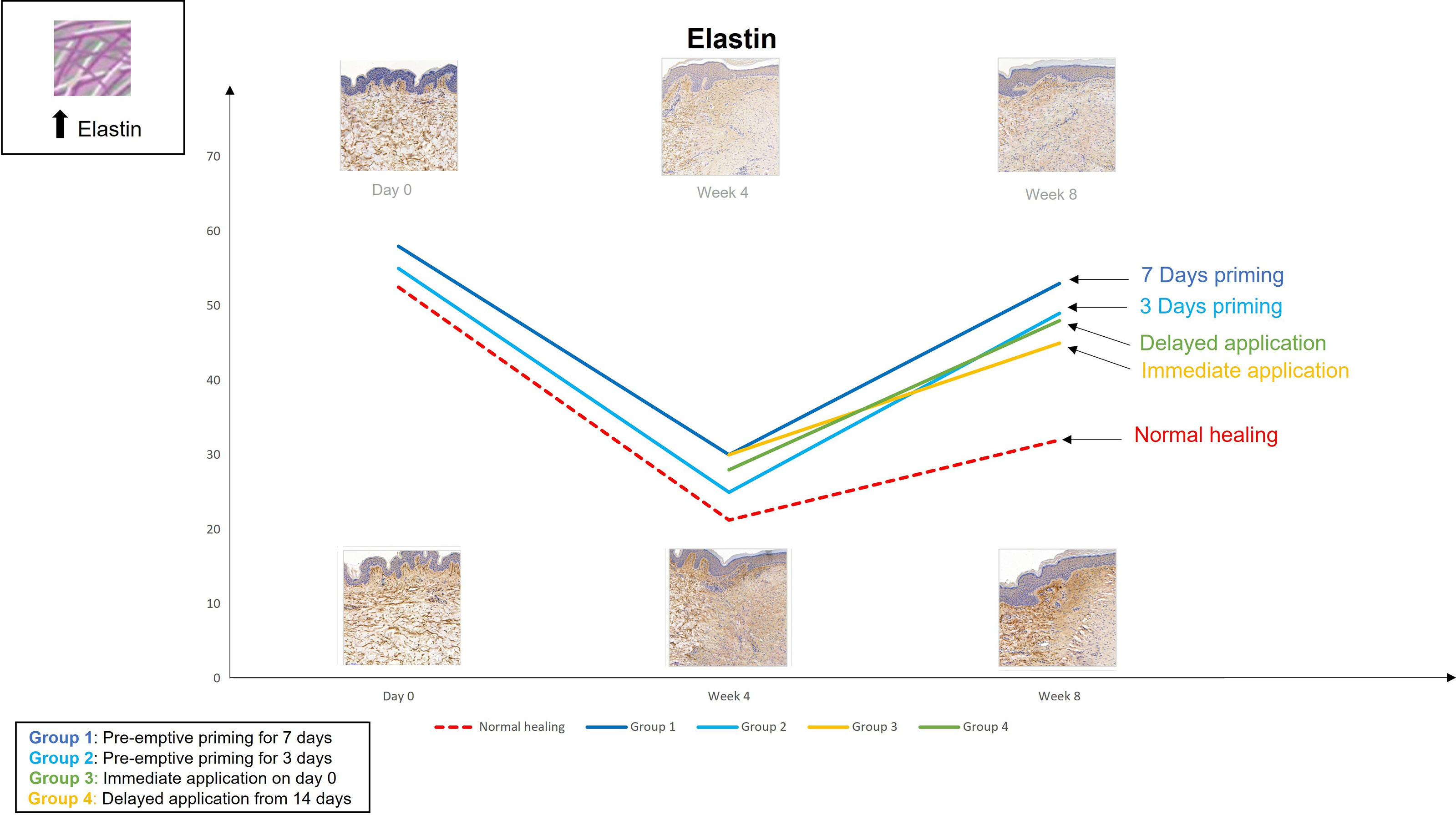
Figure 5 We evaluated the pre-emptive, immediate and delayed intervention of topical Epigallocatechin-3-gallate on skin scarring by conducting a randomsied double blind controlled trial on 40 healthy volunteers over 8 weeks. Graphical representation of the trends for elastin which demonstrates that pre-emptively priming the skin 7 days prior to injury shows greater increases in elastin compared to all other modes of application.
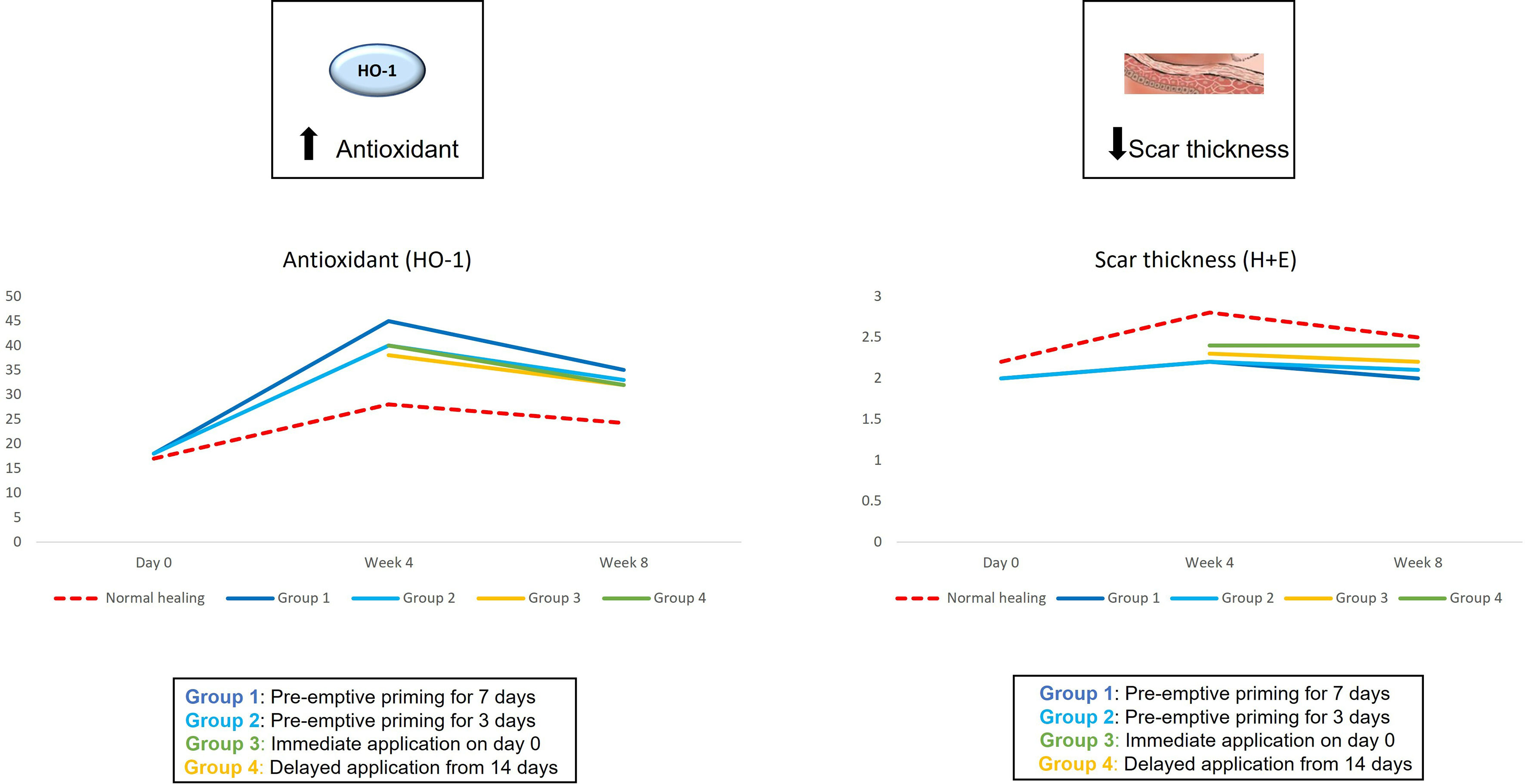
Figure 6 We evaluated the pre-emptive, immediate and delayed intervention of topical Epigallocatechin-3-gallate on skin scarring by conducting a randomsied double blind controlled trial on 40 healthy volunteers over 8 weeks. Graphical representation of the trends for antioxidant levels and skin thickness which demonstrated that pre-emptively priming the skin 7 days prior to injury shows greater reductions in skin thickness and greater increases in antioxidant levels compared to all other modes of application.
The potential therapeutic benefits of priming with proangiogenic factors and cells prior to surgery with a combination of proangiogenic growth factors for wound healing in normoglycemic and diabetic mice has been investigated (38, 83). Priming with a combination of VEGF, fibroblast growth factor (FGF) and PDGF has been shown to lead to more rapid closure times, higher vessel densities and better functional outcomes (38). In addition to proangiogenic growth factors, endothelial progenitor cells (EPCs) have presented a potential pre-treatment option for diabetic wounds (83). A murine study showed beneficial effects with pre-treatment by pro-angiogenic growth factors in the healing of diabetic incisional wounds (83). They demonstrated that priming with proangiogenic growth factors and EPCs enhanced incisional wound healing, as defined by a more rapid wound re-epithelialization, higher wound vascularization and higher tensile strength. In particular, the assessment of time-to-closure and functional outcome revealed an advantage for the groups primed with EPCs in comparison to the control animals. It is noted that while the 7 days pre-emptive treatment is feasible and beneficial for elective surgeries, this choice may not be available for traumatic injuries and emergency surgeries. Further research would be required to identify if application of this topical formulation for a short period of time, for example a number of hours prior to trauma surgery, could also be beneficial to the outcome of the scarring.
We cannot erase scarring, but we can modulate the course of healing, by reducing excessive inflammation, to achieve a better scar outcome. Normal wound healing requires inflammation and tissue remodelling mounting to appropriate degrees. Excessiveness of either of these two factors can lead to the development of keloid or hypertrophic scars. Particularly overabundance of inflammatory cells in the remodelling phase correlates with pathological scar formation, indicating the presence of a prolonged inflammatory phase overlapping with the later phases of healing. Inflammatory cell activation is vital for the prevention of infection in contaminated wounds particularly in the early stages of repair and can aid in cleaning the wound site and clearing debris (25, 26). Blocking the inflammatory pathway completely would be detrimental to this process therefore, instead of blocking, by reducing excessive inflammation this would allow infection prevention but not lead to abnormal scarring. However, blocking mast cell activation has been shown to not be detrimental to wound healing as there have been a number of studies that have blocked mast cells in order to improve scar formation (80–82). Furthermore, the concept of inducing altered inflammatory memory response in immune cells responding to injury or in tissue stem cells and epithelial cells has been studied. There have been suggestions that there are benefits in wound healing and tissue repair by priming these cell types to induce immune/inflammatory memory, characterized by epigenetic modifications, leading to increased responsiveness and more rapid inflammatory resolution upon secondary insult (84, 85). Further work could be carried out to elucidate the mechanisms involved in the therapeutic potential of inducing this inflammatory memory.
Early interventions targeting the source of inflammation have shown benefits in minimising scar formation. Many of the treatments used are general anti-inflammatory therapies, but more specific early interventions that target specific cell types such as reducing mast cells or supressing macrophage accumulation may be more optimal. Therefore, more research should be focussed on investigating the roles of the individual key players in inflammation and the effects of pre-emptive treatments to target these specific cell types to improve scar outcomes. By blocking certain pathways in the early phases of wound healing or prior to wounding this may have prophylactic effects for the prevention of abnormal skin scarring.
The studies involving human participants were reviewed and approved by University of Manchester Research Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
SU-D drafted the manuscript. AB developed the concept and edited the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to acknowledge the National Institute for Health Research Manchester Biomedical Research Centre (NIHR Manchester BRC) and Medical Research Council of South Africa for their support.
1. Eming SA, Martin P, Tomic-Canic M. Wound Repair and Regeneration: Mechanisms, Signaling, and Translation. Sci Transl Med (2014) 6:265sr6. doi: 10.1126/scitranslmed.3009337
2. Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound Repair and Regeneration. Nature (2008) 453:314–21. doi: 10.1038/nature07039
3. Martin P. Wound Healing—Aiming for Perfect Skin Regeneration. Science (1997) 276:75–81. doi: 10.1126/science.276.5309.75
4. Wang ZC, Zhao WY, Cao Y, Liu YQ, Sun Q, Shi P, et al. The Roles of Inflammation in Keloid and Hypertrophic Scars. Front Immunol (2020) 11:603187. doi: 10.3389/fimmu.2020.603187
5. Ogawa R. Keloid and Hypertrophic Scars Are the Result of Chronic Inflammation in the Reticular Dermis. Int J Mol Sci (2017) 1018(3):606. doi: 10.3390/ijms18030606
6. Wilgus TA. Inflammation as an Orchestrator of Cutaneous Scar Formation: A Review of the Literature. Plast Aesthet Res (2020) 7:54. doi: 10.20517/2347-9264.2020.150
7. Wulff BC, Pappa NK, Wilgus TA. Interleukin-33 Encourages Scar Formation in Murine Fetal Skin Wounds. Wound Repair Regen (2019) 27(1):19–28. doi: 10.1111/wrr.12687
8. Bagabir R, Byers RJ, Chaudhry IH, Müller W, Paus R, Bayat A. Site-Specific Immunophenotyping of Keloid Disease Demonstrates Immune Upregulation and the Presence of Lymphoid Aggregates. Br J Dermatol (2012) 167(5):1053–66. doi: 10.1111/j.1365-2133.2012.11190.x
9. Lee S, Kim SK, Park H, Lee YJ, Park SH, Lee KJ, et al. Contribution of Autophagy-Notch1-Mediated NLRP3 Inflammasome Activation to Chronic Inflammation and Fibrosis in Keloid Fibroblasts. Int J Mol Sci (2020) 21(21):8050. doi: 10.3390/ijms21218050
10. Fujita M, Yamamoto Y, Jiang JJ, Atsumi T, Tanaka Y, Ohki T, et al. NEDD4 Is Involved in Inflammation Development During Keloid Formation. J Invest Dermatol (2019) 139(2):333–41. doi: 10.1016/j.jid.2018.07.044
11. Liu XJ, Xu MJ, Fan ST, Wu Z, Li J, Yang XM, et al. Xiamenmycin Attenuates Hypertrophic Scars by Suppressing Local Inflammation and the Effects of Mechanical Stress. J Invest Dermatol (2013) 133(5):1351–60. doi: 10.1038/jid.2012.486
12. Shaker SA, Ayuob NN, Hajrah NH. Cell Talk: A Phenomenon Observed in the Keloid Scar by Immunohistochemical Study. Appl Immunohistochem Mol Morphol AIMM (2011) 19:153–9. doi: 10.1097/PAI.0b013e3181efa2ef
13. Gauglitz GG, Korting HC, Pavicic T, Ruzicka T, Jeschke MG. Hypertrophic Scarring and Keloids: Pathomechanisms and Current and Emerging Treatment Strategies. Mol Med (Cambridge Mass) (2011) 17:113–25. doi: 10.2119/molmed.2009.00153
14. Jin Q, Gui L, Niu F, Yu B, Lauda N, Liu J, et al. Macrophages in Keloid Are Potent at Promoting the Differentiation and Function of Regulatory T Cells. Exp Cell Res (2018) 362:472–6. doi: 10.1016/j.yexcr.2017.12.011
15. Mahdavian Delavary B, van der Veer WM, van Egmond M, Niessen FB, Beelen RH. Macrophages in Skin Injury and Repair. Immunobiology (2011) 216:753–62. doi: 10.1016/j.imbio.2011.01.001
16. Trace AP, Enos CW, Mantel A, Harvey VM. Keloids and Hypertrophic Scars: A Spectrum of Clinical Challenges. Am J Clin Dermatol (2016) 17:201–23. doi: 10.1007/s40257-016-0175-7
17. Chen Y, Jin Q, Fu X, Qiao J, Niu F. Connection Between T Regulatory Cell Enrichment and Collagen Deposition in Keloid. Exp Cell Res (2019) 383:111549. doi: 10.1016/j.yexcr.2019.111549
18. Murao N, Seino K, Hayashi T, Ikeda M, Funayama E, Furukawa H, et al. Tregenriched CD4+ T Cells Attenuate Collagen Synthesis in Keloid Fibroblasts. Exp Dermatol (2014) 23:266–71. doi: 10.1111/exd.12368
19. Chrysanthopoulou A, Mitroulis I, Apostolidou E, Arelaki S, Mikroulis D, Konstantinidis T, et al. Neutrophil Extracellular Traps Promote Differentiation and Function of Fibroblasts. J Pathol (2014) 233:294–307. doi: 10.1002/path.4359
20. Wilgus TA, Wulff BC. The Importance of Mast Cells in Dermal Scarring. Adv Wound Care (New Rochelle) (2014) 3(4):356–65. doi: 10.1089/wound.2013.0457
21. Wilgus TA, Ud-Din S, Bayat A. A Review of the Evidence for and Against a Role for Mast Cells in Cutaneous Scarring and Fibrosis. Int J Mol Sci (2020) 21(24):9673. doi: 10.3390/ijms21249673
22. Ud-Din S, Wilgus TA, Bayat A. Mast Cells in Skin Scarring: A Review of Animal and Human Research. Front Immunol (2020) 11:552205. doi: 10.3389/fimmu.2020.552205
23. Zhang Q, Kelly AP, Wang L, French SW, Tang X, Duong HS, et al. Green Tea Extract and (-)-Epigallocatechin-3-Gallate Inhibit Mast Cell-Stimulated Type I Collagen Expression in Keloid Fibroblasts via Blocking PI-3k/AkT Signaling Pathways. J Invest Dermatol (2006) 126:2607–13. doi: 10.1038/sj.jid.5700472
24. Wulff BC, Parent AE, Meleski MA, DiPietro LA, Schrementi ME, Wilgus TA. Mast Cells Contribute to Scar Formation During Fetal Wound Healing. J Invest Dermatol (2012) 132:458–65. doi: 10.1038/jid.2011.324
25. Herndon D, Capek KD, Ross E, Jay JW, Prasai A, Ayadi AE, et al. Reduced Postburn Hypertrophic Scarring and Improved Physical Recovery With Yearlong Administration of Oxandrolone and Propranolol. Ann Surg (2018) 268:431–41. doi: 10.1097/SLA.0000000000002926
26. Al-Nimer MS, Hameed HG, Mahmood MM. Antiproliferative Effects of Aspirin and Diclofenac Against the Growth of Cancer and Fibroblast Cells: In Vitro Comparative Study. Saudi Pharm J SPJ Off Publ Saudi Pharm Soc (2015) 23:483–6. doi: 10.1016/j.jsps.2015.01.002
27. Wang Y, He G, Tang H, Shi Y, Kang X, Lyu J, et al. Aspirin Inhibits Inflammation and Scar Formation in the Injury Tendon Healing Through Regulating JNK/STAT-3 Signalling Pathway. Cell Prolif (2019) 52:e12650. doi: 10.1111/cpr.12650
28. Ud-Din S, Bowring A, Derbyshire B, Morris J, Bayat A. Identification of Steroid Sensitive Responders Versus Non-Responders in the Treatment of Keloid Disease. Arch Dermatol Res (2013) 305(5):423–32. doi: 10.1007/s00403-013-1328-7
29. Tawfic SO, El-Tawdy A, Shalaby S, Foad A, Shaker O, Sayed SS, et al. Evaluation of Fractional CO2 Versus Long Pulsed Nd:YAG Lasers in Treatment of Hypertrophic Scars and Keloids: A Randomized Clinical Trial. Lasers Surg Med (2020) 52(10):959–65. doi: 10.1002/lsm.23249
30. Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Muller W, et al. Differential Roles of Macrophages in Diverse Phases of Skin Repair. J Immunol (Baltimore Md 1950) (2010), 184:3964–77. doi: 10.4049/jimmunol.0903356
31. An MK, Cho EB, Park EJ, Kim KH, Kim LS, Kim KJ. Appropriate Timing of Early Postoperative Botulinum Toxin Type A Injection for Thyroidectomy Scar Management: A Split-Scar Study. Plast Reconstr Surg (2019) 144(4):659e–68e. doi: 10.1097/PRS.0000000000006064
32. Ud-Din S, Bayat A. Strategic Management of Keloid Disease in Ethnic Skin: A Structured Approach Supported by the Emerging Literature. Br J Dermatol (2013) 169(Suppl. S3):71–81. doi: 10.1111/bjd.12588
33. Bayat A, McGrouther DA, Ferguson MW. Skin scarring. BMJ (2003) 326(7380):88–92. doi: 10.1136/bmj.326.7380.88
34. Arsiwala SZ, Desai SR. Fractional Carbon Dioxide Laser: Optimizing Treatment Outcomes for Pigmented Atrophic Acne Scars in Skin of Color. J Cutan Aesthetic Surg (2019) 12:85–94. doi: 10.4103/JCAS.JCAS_171_18
35. Du F, Yu Y, Zhou Z, Wang L, Zheng S. Early Treatment Using Fractional CO2 Laser Before Skin Suture During Scar Revision Surgery in Asians. J Cosmet Laser Ther (2018) 20:102–5. doi: 10.1080/14764172.2017.1358452
36. Antoniou GA, Onwuka CC, Antoniou SA, Russell D. Meta-Analysis and Trial Sequential Analysis of Prophylactic Negative Pressure Therapy for Groin Wounds in Vascular Surgery. J Vasc Surg (2019) 70:1700–1710.e6. doi: 10.1016/j.jvs.2019.01.083
37. Huang H-P, Zhao W-J, Pu J, He F. Prophylactic Negative Pressure Wound Therapy for Surgical Site Infection in Obese Women Undergoing Cesarean Section: An Evidence Synthesis With Trial Sequential Analysis. J Matern Fetal Neonatal Med (2019) 25:1–8. doi: 10.1080/14767058.2019.1668924
38. Ackermann M, Pabst AM, Houdek JP, Ziebart T, Konerding MA. Priming With Proangiogenic Growth Factors and Endothelial Progenitor Cells Improves Revascularization in Linear Diabetic Wounds. Int J Mol Med (2014) 33:833–9. doi: 10.3892/ijmm.2014.1630
39. Anitha B. Prevention of Complications in Chemical Peeling. J Cutan Aesthetic Surg (2010) 3:186–8. doi: 10.4103/0974-2077.74500
40. Yang X, Shao Y, Yu W, Zhang X, Sun Y, Zhang L, et al. A Novel Radiotherapy Approach for Keloids With Intrabeam. BioMed Res Int (2019) 2019:4693528. doi: 10.1155/2019/4693528
41. DeBruler DM, Zbinden JC, Baumann ME, Blackstone BN, Malara MM, Bailey JK, et al. Early Cessation of Pressure Garment Therapy Results in Scar Contraction and Thickening. PloS One (2018) 13(6):e0197558. doi: 10.1371/journal.pone.0197558
42. Shi Y, Jiang W, Li W, Zhang W, Zou Y. Comparison of Fractionated Frequency-Doubled 1,064/532 Nm Picosecond Nd:YAG Lasers and Non-Ablative Fractional 1,540 Nm Er: Glass in the Treatment of Facial Atrophic Scars: A Randomized, Split-Face, Double-Blind Trial. Ann Transl Med (2021) 9(10):862. doi: 10.21037/atm-21-1715
43. Chen XE, Liu J, Bin Jameel AA, Valeska M, Zhang JA, Xu Y, et al. Combined Effects of Long-Pulsed Neodymium-Yttrium-Aluminum-Garnet Laser, Diprospan and 5-Fluorouracil in the Treatment of Keloid Scars. Exp Ther Med (2017) 13(6):3607–12. doi: 10.3892/etm.2017.4438
44. Saulis AS, Mogford JH, Mustoe TA. Effect of Mederma on Hypertrophic Scarring in the Rabbit Ear Model. Plast Reconstr Surg (2002) 110:177–83. doi: 10.1097/00006534-200207000-00029
45. Pangkanon W, Yenbutra P, Kamanamool N, Tannirandorn A, Udompataikul M. A Comparison of the Efficacy of Silicone Gel Containing Onion Extract and Aloe Vera to Silicone Gel Sheets to Prevent Postoperative Hypertrophic Scars and Keloids. J Cosmet Dermatol (2021) 20(4):1146–53. doi: 10.1111/jocd.13933
46. Conti V, Corbi G, Iannaccone T, Corrado B, Giugliano L, Lembo S, et al. Effectiveness and Tolerability of a Patch Containing Onion Extract and Allantoin for Cesarean Section Scars. Front Pharmacol (2020) 11:569514. doi: 10.3389/fphar.2020.569514
47. Rutkowski D, Syed F, Matthews LC, Ray DW, McGrouther DA, Watson RE, et al. An Abnormality in Glucocorticoid Receptor Expression Differentiates Steroid Responders From Nonresponders in Keloid Disease. Br J Dermatol (2015) 173(3):690–700. doi: 10.1111/bjd.13752
48. Zhang D, Li Y, Liu Y, Xiang X, Dong Z. Paclitaxel Ameliorates Lipopolysaccharide-Induced Kidney Injury by Binding Myeloid Differentiation Protein-2 to Block Toll-Like Receptor 4-Mediated Nuclear factor-kappaB Activation and Cytokine Production. J Pharmacol Exp Ther (2013) 345:69–75. doi: 10.1124/jpet.112.202481
49. Zhang L, Xu X, Yang R, Chen J, Wang S, Yang J, et al. Paclitaxel Attenuates Renal Interstitial Fibroblast Activation and Interstitial Fibrosis by Inhibiting STAT3 Signaling. Drug Design Dev Ther (2015) 9:2139–48. doi: 10.2147/DDDT.S81390
50. Wang M, Chen L, Huang W, Jin M, Wang Q, Gao Z, et al. Improving the Antikeloid Outcomes Through Liposomes Loading Paclitaxel-Cholesterol Complexes. Int J Nanomed (2019) 14:1385–400. doi: 10.2147/IJN.S195375
51. Huang L-P, Wang G-Q, Jia Z-S, Chen J-W, Wang G, Wang X-L. Paclitaxel Reduces Formation of Hypertrophic Scars in the Rabbit Ear Model. Ther Clin Risk Manag (2015) 11:1089–95. doi: 10.2147/TCRM.S82961
52. Li K, Nicoli F, Cui C, Xi WJ, Al-Mousawi A, Zhang Z, et al. Treatment of Hypertrophic Scars and Keloids Using an Intralesional 1470 Nm Bare-Fibre Diode Laser: A Novel Efficient Minimally-Invasive Technique. Sci Rep (2020) 10(1):21694. doi: 10.1038/s41598-020-78738-9
53. Vas K, Gaál M, Varga E, Kovács R, Bende B, Kocsis A, et al. Effects of the Combined PDL/Nd:YAG Laser on Surgical Scars: Vascularity and Collagen Changes Evaluated by In Vivo Confocal Microscopy. BioMed Res Int (2014) 2014:204532. doi: 10.1155/2014/204532
54. Ogawa R, Akaishi S, Kuribayashi S, Miyashita T. Keloids and Hypertrophic Scars Can Now Be Cured Completely: Recent Progress in Our Understanding of the Pathogenesis of Keloids and Hypertrophic Scars and the Most Promising Current Therapeutic Strategy. J Nippon Med School = Nippon Ika Daigaku zasshi (2016) 83:46–53. doi: 10.1272/jnms.83.46
55. Wang F, Li X, Wang X, Jiang X. Efficacy of Topical Silicone Gel in Scar Management: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Int Wound J (2020) 17(3):765–73. doi: 10.1111/iwj.13337
56. Lee SH, Zheng Z, Roh MR. Early Postoperative Treatment of Surgical Scars Using a Fractional Carbon Dioxide Laser: A Splitscar, Evaluator-Blinded Study. Dermatol Surg (2013) 2013-08-0139(8):1190–6. doi: 10.1111/dsu.12228
57. Jung JY, Jeong JJ, Roh HJ, Cho SH, Chung KY, Lee WJ, et al. Early Postoperative Treatment of Thyroidectomy Scars Using a Fractional Carbon Dioxide Laser. Dermatol Surg (2011) 37(2):217–23. doi: 10.1111/j.1524-4725.2010.01853.x
58. Bjorn M, Stausbol-Gron B, Braae OA, Hedelund L. Treatment of Acne Scars With Fractional CO2 Laser at 1-Month Versus 3-Month Intervals: An Intra-Individual Randomized Controlled Trial. Lasers Surg Med (2014) 46(2):89–93. doi: 10.1002/lsm.22165
59. Choi JE, Oh GN, Kim JY, Seo SH, Ahn HH, Kye YC. Ablative Fractional Laser Treatment for Hypertrophic Scars: Comparison Between Er: Yagand CO2 Fractional Lasers. J Dermatolog Treat (2014) 25(4):299–303. doi: 10.3109/09546634.2013.782090
60. Majid I, Imran S. Fractional CO2 Laser Resurfacing as Monotherapy in the Treatment of Atrophic Facial Acne Scars. J Cutan Aesthet Surg (2014) 7(2):87–92. doi: 10.4103/0974-2077.138326
61. Nguyen JK, Weedon J, Jakus J, Heilman E, Isseroff RR, Siegel DM, et al. Dose-Ranging, Parallel Group, Split-Face, Single-Blind Phase II Study of Light Emitting Diode-Red Light (LED-RL) for Skin Scarring Prevention: Study Protocol for a Randomized Controlled Trial. Trials (2019) 20(1):432. doi: 10.1186/s13063-019-3546-6
62. Kent RA, Shupp J, Fernandez S, Prindeze N, DeKlotz CMC. Effectiveness of Early Laser Treatment in Surgical Scar Minimization: A Systematic Review and Meta-Analysis. Dermatol Surg (2020) 46(3):402–10. doi: 10.1097/DSS.0000000000001887
63. Ozog DM, Moy RL. A Randomized Split-Scar Study of Intraoperative Treatment of Surgical Wound Edges to Minimize Scarring. Arch Dermatol (2011) 147(9):1108–10. doi: 10.1001/archdermatol.2011.248
64. Kolli H, Moy RL. Prevention of Scarring With Intraoperative Erbium: YAG Laser Treatment. J Drugs Dermatol (2020) 19(11):1040–3. doi: 10.36849/JDD.2020.5244
65. Taudorf EH, Haedersdal M. Early non-Ablative Fractional Laser Improves the Appearance of Punch Biopsy Scars: A Clinical Report. J Eur Acad Dermatol Venereol (2016) 30(3):550–52. doi: 10.1111/jdv.12955
66. Prodromidou A, Frountzas M, Vlachos DE, Vlachos GD, Bakoyiannis I, Perrea D, et al. Botulinum Toxin for the Prevention and Healing of Wound Scars: A Systematic Review of the Literature. Plast Surg (Oakville Ont) (2015) 23:260–4. doi: 10.1177/229255031502300402
67. Lee BJ, Jeong JH, Wang SG, Lee JC, Goh EK, Kim HW. Effect of Botulinum Toxin Type a on a Rat Surgical Wound Model. Clin Exp Otorhinolaryngol (2009) 2:20–7. doi: 10.3342/ceo.2009.2.1.20
68. Bi M, Sun P, Li D, Dong Z, Chen Z. Intralesional Injection of Botulinum Toxin Type A Compared With Intralesional Injection of Corticosteroid for the Treatment of Hypertrophic Scar and Keloid: A Systematic Review and Meta- Analysis. Med Sci Monit Int Med J Exp Clin Res (2019) 25:2950–8. doi: 10.12659/MSM.916305
69. Kim YS, Lee HJ, Cho SH, Lee JD, Kim HS. Early Postoperative Treatment of Thyroidectomy Scars Using Botulinum Toxin: A Split-Scar, Double-Blind Randomized Controlled Trial. Wound Repair Regen (2014) 22:605–12. doi: 10.1111/wrr.12204
70. Zhibo X, Miaobo Z. Potential Therapeutical Effects of Botulinum Toxin Type A in Keloid Management. Med Hypotheses (2008) 71:623. doi: 10.1016/j.mehy.2008.04.018
71. Hsu TS, Dover JS, Arndt KA. Effect of Volume and Concentration on the Diffusion of Botulinum Exotoxin a. Arch Dermatol (2004) 140:1351–4. doi: 10.1001/archderm.140.11.1351
72. Kannan S, de Golian E, Lee N, Smith J, Brian Jiang SI. A Split-Scar Study Investigating the Effectiveness of Early Intervention With Electroabrasion on Improving the Cosmetic Appearance of Postsurgical Scars. Dermatol Surg (2020) 46(10):1300–5. doi: 10.1097/DSS.0000000000002324
73. Stahl S, Barnea Y, Weiss J, Amir A, Zaretski A, Leshem D, et al. Treatment of Earlobe Keloids by Extralesional Excision Combined With Preoperative and Postoperative "Sandwich" Radiotherapy. Plast Reconstr Surg (2010) 125(1):88–92. doi: 10.1097/PRS.0b013e3181c2a46e
74. Sidgwick GP, McGeorge D, Bayat A. Functional Testing of Topical Skin Formulations Using an Optimised Ex Vivo Skin Organ Culture Model. Arch Dermatol Res (2016) 308:297–308. doi: 10.1007/s00403-016-1645-8
75. Ud-Din S, Foden P, Mazhari M, Al-Habba S, Baguneid M, Bulfone-Paus S, et al. A Double-Blind, Randomized Trial Shows the Role of Zonal Priming and Direct Topical Application of Epigallocatechin-3-Gallate in the Modulation of Cutaneous Scarring in Human Skin. J Invest Dermatol (2019) 139(8):1680–90.e16. doi: 10.1016/j.jid.2019.01.030
76. Ud-Din S, Wilgus TA, McGeorge DD, Bayat A. Pre-Emptive Priming of Human Skin Improves Cutaneous Scarring and Is Superior to Immediate and Delayed Topical Anti-Scarring Treatment Post-Wounding: A Double-Blind Randomised Placebo-Controlled Clinical Trial. Pharmaceutics (2021) 13(4):510. doi: 10.3390/pharmaceutics13040510
77. Wang Z, Dabrosin C, Yin X, Fuster MM, Arreola A, Rathmell WK, et al. Broad Targeting of Angiogenesis for Cancer Prevention and Therapy. Semin Cancer Biol (2015) 35(Suppl):S224–43. doi: 10.1016/j.semcancer.2015.01.001
78. Singh BN, Shankar S, Srivastava RK. Green Tea Catechin, Epigallocatechin-3-Gallate (EGCG): Mechanisms, Perspectives and Clinical Applications. Biochem Pharmacol (2011) 82(12):1807–21. doi: 10.1016/j.bcp.2011.07.093
79. Syed F, Bagabir RA, Paus R, Bayat A. Ex Vivo Evaluation of Antifibrotic Compounds in Skin Scarring: EGCG and Silencing of PAI-1 Independently Inhibit Growth and Induce Keloid Shrinkage. Lab Invest (2013) 93(8):946–60. doi: 10.1038/labinvest.2013.82
80. Weitzmann A, Naumann R, Dudeck A, Zerjatke T, Gerbaulet A, Roers A. Mast Cells Occupy Stable Clonal Territories in Adult Steady-State Skin. J Invest Dermatol (2020) 140(12):2433–41.e5. doi: 10.1016/j.jid.2020.03.963
81. Artuc M, Hermes B, Steckelings UM, Grützkau A, Henz BM. Mast Cells and Their Mediators in Cutaneous Wound Healing–Active Participants or Innocent Bystanders? Exp Dermatol (1999) 8(1):1–16. doi: 10.1111/j.1600-0625.1999.tb00342.x
82. Voss M, Kotrba J, Gaffal E, Katsoulis-Dimitriou K, Dudeck A. Mast Cells in the Skin: Defenders of Integrity or Offenders in Inflammation? Int J Mol Sci (2021) 22(9):4589. doi: 10.3390/ijms22094589
83. Ackermann M, Wolloscheck T, Wellmann A, Li VW, Li WW, Konerding MA. Priming With a Combination of Proangiogenic Growth Factors Enhances Wound Healing in Streptozotocin-Induced Diabetes in Mice. Eur Surg Res (2011) 47(2):81–9. doi: 10.1159/000328143
84. Gonzales KAU, Polak L, Matos I, Tierney MT, Gola A, Wong E, et al. Stem Cells Expand Potency and Alter Tissue Fitness by Accumulating Diverse Epigenetic Memories. Science (2021) 374(6571):eabh2444. doi: 10.1126/science.abh2444
Keywords: skin scarring, wound healing, hypertrophic scars, keloid scars, inflammation, skin priming, pre-emptive skin priming
Citation: Ud-Din S and Bayat A (2022) Controlling Inflammation Pre-Emptively or at the Time of Cutaneous Injury Optimises Outcome of Skin Scarring. Front. Immunol. 13:883239. doi: 10.3389/fimmu.2022.883239
Received: 24 February 2022; Accepted: 21 April 2022;
Published: 27 May 2022.
Edited by:
Perenlei Enkhbaatar, University of Texas Medical Branch at Galveston, United StatesReviewed by:
Julia K. Bohannon, Vanderbilt University Medical Center, United StatesCopyright © 2022 Ud-Din and Bayat. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ardeshir Bayat, YXJkZXNoaXIuYmF5YXRAdWN0LmFjLnph; orcid.org/0000-0002-4116-6491
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.