- 1Research Group of Gastrointestinal Diseases, The Second Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 2Faculty of Health Science, Nord University, Campus Levanger, Levanger, Norway
- 3Division of Gastroenterology, Department of Internal Medicine, University Hospital of North Norway, Tromsø, Norway
Anti-tumor necrosis factor (TNF) biological therapy has generally been accepted as a standard therapeutic option in inflammatory bowel disease (IBD) patient who are refractory to steroids or immunomodulators. However, the primary and secondary nonresponse rates to anti-TNF bioagents in patients with IBD are high. To improve the response rate, anti-TNF bioagents must be offered to the appropriate IBD patients, and the withdrawal of anti-TNF bioagents needs to be done at the right time. In this context, reliable and reproducible biomarkers can provide important supportive information for clinicians to make correct decisions based on the patient’s individual situation. In this review, we summarized the current understanding of using mucosal TNF transcript (TNF) to improve the precision of anti-TNF biological therapy strategies in patients with ulcerative colitis (UC). Analysis of published literature showed that mucosal TNF could affect the precision of the early identification of candidates who will benefit from anti-TNF therapy prior to treatment, the assessment of response and mucosal healing, and the prediction of discontinuation of anti-TNF biological therapy and relapse after drug withdrawal. Challenges and limitations of using mucosal TNF as a biomarker in applying individualized anti-TNF biological therapy in patients with UC still remain and need to be further investigated.
Introduction
Ulcerative colitis (UC), that is one of main types of inflammatory bowel disease (IBD), is a group of chronic inflammatory disorders that mainly affect the colorectal tract. Although the exact etiology for UC has not been fully identified thus far, the currently most cited hypothesis proposes that a combination of environmental factors, genetic predisposition, and dysregulated immune response may significantly increase the risk for UC (1). Recent epidemiological evidence has highlighted an increasing incidence of IBD worldwide, and many developing countries previously thought of as traditionally low-incidence regions have been now reported to have a dramatically increasing rate of UC following the acceleration of industrialization and urbanization (2–7).
The traditional approaches for UC management rely on diverse medicines including aminosalicylates, corticosteroids and immunomodulators according to disease activity and severity. The clinical goals of treatments are to suppress inflammation, at best obtain mucosal healing, and finally improve the UC patient’s quality of life. However, being refractory to existing medicines and disease relapse have frequently been reported (8, 9). In the case of drug- refractory disease relapse, alternative second line therapeutic strategies are currently used (10, 11). Currently, neutralizing TNF monoclonal antibodies have been widely accepted as one of the standard strategies for the treatment of severe steroid or immunomodulator-refractory or -dependent IBD patients (12), which results in remarkably improved rates of disease remission and mucosal healing (12). Even so, not all IBD patients respond well to anti-TNF therapy (13); the primary response rate to the initial anti-TNF treatment is only ~60% and 20~30% of these responders will stop responding at some point during maintenance therapy (13–17). Furthermore, more than 30% of IBD patients in remission during the first year and over 60% of IBD patients in remission within 5 years will relapse after withdrawal of anti-TNF bioagents (13, 18, 19). Finally, escalating costs and several site effects are pertinent considerations with applications of anti-TNF biological therapy in patients with IBD (20–22). To reduce the incidence of primary and secondary nonresponse and improve its efficiency in patients with IBD, it is highly clinically and economically relevant to offer anti-TNF bioagents to IBD patients who will benefit from anti-TNF biological therapy given their individual condition (23–29).
Currently, the clinical outcomes of anti-TNF biological therapy in patients with UC are mainly defined based on validated endoscopic or histologic evaluations. However, to identify the responders and non-responders pretreatment and to predict relapse after remission, effective biomarkers can provide useful and critical information for understanding and analyzing disease activity, therapeutic response, and relapse of UC (30–34). As we know, colonoscopy is the “gold standard” tool for monitoring mucosal healing and is regularly performed in IBD patients with anti-TNF therapy. In addition, colonoscopy allows the collection of colorectal mucosal samples from the inflamed site of UC patients for histopathological evaluation of disease activity and changes, in conjunction with clinical indices, the response to medicines can be assessed and monitored (9, 35–38). As TNF plays an essential role in the pathogenesis of UC and one of the main working mechanisms for anti-TNF biologic agents in suppressing colorectal inflammation is to neutralize and decrease TNF levels in UC inflamed tissues (39); therefore, combined with histological and colonoscopy observations, TNF level changes in the inflamed mucosa could be considered an index of the efficacy of anti-TNF treatment in patients with UC (40, 41). Indeed, a growing body of evidence from various studies has examined and highlighted the potential of mucosal TNF transcript (TNF) as a biomarker of disease activity, the time of switching from ongoing conventional treatment to anti-TNF therapy and selecting candidates, the anti-TNF therapeutic response, and timepoints for drug withdrawal and relapse after anti-TNF discontinuation in patients with UC (42–48). Therefore, we conducted this review to provide an overview of the current understanding of mucosal TNF as a biomarker in the context of precision medicine algorithms in UC patents undergoing anti-TNF biological therapy.
Could Serum TNF Protein Levels Be Used as a Biomarker Candidate in UC Patients With Anti-TNF Biological Therapy?
Serological biomarkers have several advantages such as serum samples being more easily accessible, lower cost, more reproducible and more acceptable by patients than tissue biopsies (49, 50). However, the sensitivity of serological biomarkers is more easily influenced by systematic factors and sometimes lower than that of mucosal biomarkers in the reflection of inflammation and disease activity in patients with UC (23).
Regarding whether the serum TNF level could be used as a biomarker in the evaluation of disease activity in patients with UC. Owczarek et al. (51) reported that increased serum TNF levels correlated only with CD activity but not with the disease activity of UC. Avdagić et al. confirmed that serum TNF levels were not associated with disease activity in either CD or UC patients (52). However, Mateos et al. (53) recently measured the plasma concentration of TNF pretreatment in 30 active CD patients with infliximab (IFX) induction therapy and reported that increased TNFα levels were associated with an unfavorable response to IFX (53). Therefore, the reliability of a serological TNF level as a biomarker in predicting the anti-TNF response in patients with IBD is still unclear. Moreover, TNF intestinal mucosal levels were detectable in 100% of patients, while TNF serum levels were only detectable in 75% of patients (54). Therefore, the serum TNF level might not be an adequate biomarker for an assessment of disease activity in patients with UC (55, 56). One of the possible explanations is that serum TNF levels might be influenced by many factors in the body and not precisely reflect the degree of inflammation in the local mucosal environment in patients with IBD. Measuring TNF directly in the colorectal mucosa more accurately reflects the local environmental expression compared to serum levels in patients with UC (38).
Thus, the mucosal TNF level is a promising biomarker candidate and a potential tool for precision medicine in UC patients with anti-TNF therapy (37, 42, 43, 46, 47, 57, 58).
The Potential Role of the Mucosal TNF Level as a Biomarker in Anti-TNF Candidate Selection in Patients With UC
It is well acknowledged that a reliable and powerful biomarker is important to help clinicians make decisions regarding identifying the anti-TNF candidates and the timing of anti-TNF biologic agent withdrawal.
In terms of general mechanisms, the mechanism of anti-TNF biologic agents in treating IBD is to suppress inflammation via neutralizing and decreasing of TNF levels and to bind with TNF receptors in the inflamed mucosa and to induce mucosal cell apoptosis. Thus, the changed expression level of mucosal TNF may directly reflect therapeutic responses, e.g., suppression of inflammation degree and changes in disease activity to different medicines (58). Ishiguro reported that an enhanced mucosal TNF level was associated with the degree of inflammation in active UC specimens (59). By using an optimized q-PCR protocol for the precise quantification of TNF levels in endoscopic mucosal biopsies (60, 61), our group demonstrated that increased mucosal TNF (messenger RNA, mRNA) levels correlated with the UC disease activity index (UCDAI) score in newly diagnosed UC patients without treatment (58). Matsuda et al. quantified the mucosal expression levels of TNF-α, interleukin (IL)-6, IL-8, and IL-10 transcripts, and confirmed that the TNF level was significantly increased in the colonic mucosa in patients with steroid naïve UC (62). Furthermore, our validation data demonstrated that the baseline mucosal TNF level was a promising biomarker in patients with UC (23). In conjunction with histological inflammatory activity scores, mucosal TNF level could precisely predict the need for biological therapy within the first year after the diagnosis of disease in patients with UC (23). Therefore, mucosal TNF transcript level might hold a potential for anti-TNF candidate selection.
To evaluate mucosal healing, colonoscopy is regularly performed during the anti-TNF therapy treatment period. In addition, biopsies taken by colonoscopy are critically important for histological evaluation of mucosal inflammation and disease activity changes in response to anti-TNF therapy. Therefore, the use of colonoscopic biopsy for the measurement of mucosal TNF is practicable and feasible in UC patients with anti-TNF therapy.
Validation of Mucosal TNF as a Reliable and Reproducible Biomarker in Patients With UC
To evaluate the reliability and power of a biomarker candidate, Siegel et al. have proposed that a new biomarker must be validated before it can be used in the clinical management of IBD patients (63, 64).
Following the validation principle of a potential biomarker candidate (65, 66), we conducted a two-step procedure to validate mucosal TNF as a biomarker in UC patients with anti-TNF biological therapy (23). In the first step, we compared the power of this biomarker candidate with commonly used biomarkers and clinical parameters i.e., fecal calprotectin, the UC disease activity index (UCDAI), the Mayo endoscopic score and the Robarts’ histopathology index (RHI) scores in predicting the severe clinical outcome in a calibration cohort with 66 UC patients. We found that the mucosal TNF had the best test performance with a sensitivity, specificity, and diagnostic odds ratio (DOR) compared with the above selected biomarker and clinical parameters. In the second step (validation test), the results demonstrated that the validated cutoff values of mucosal TNF with histological index (TNF ≥18,000 copies, RHI ≥ 9) showed a high reliability and specificity to predict a need for anti-TNF biological therapy within the first year of disease after diagnosis in a cohort with 89 UC patients (23).
The Role in Anti-TNF Candidate Selection in Patients With UC
In the context of precision medicine, medicines must be offered to patients who will benefit from or positively respond to medicines. To offer anti-TNF treatment to the right patients at the right time, a biomarker that can differentiate between anti-TNF-resistant and anti-TNF-sensitive IBD patients is needed (67). The strategy for clinicians for anti-TNF candidate selection in clinical practice mainly considers whether this patient is refractory to or dependent on steroids. However, such patient selection might include some unresponsive patients, and data have shown that only ~60% of IBD patients respond to the initial anti-TNF biological treatment (40, 68). A recent research has highlighted the potential for single-cell mapping tools to identify CD candidates for anti-TNF therapy (69), in which the GIMATS module that consisted a set of cellular and molecular parameters, such as IgG plasma cells, inflammatory mononuclear phagocytes, activated T cells, and stromal cells, at diagnosis could differentiate the responder and nonresponder to anti-TNF therapy in patients with CD prior to treatment (69). Other studies revealed that mucosal TNF levels might be a biomarker that can help clinicians identify appropriate patients and opportunities for anti-TNF therapy in patients with UC (37, 42, 43, 58). Therefore, we observed persistently high mucosal TNF levels in UC patients after treatment with steroids for a certain period, which might indicate a steroid-refraction/dependent phenotype and the need to consider switching to anti-TNF biological therapy (refer to Figure 1). On the other hand, we might prolong steroid use for a while in partial responders if the mucosal TNF level is nearly normalized after excluding high anti-drug antibody levels.
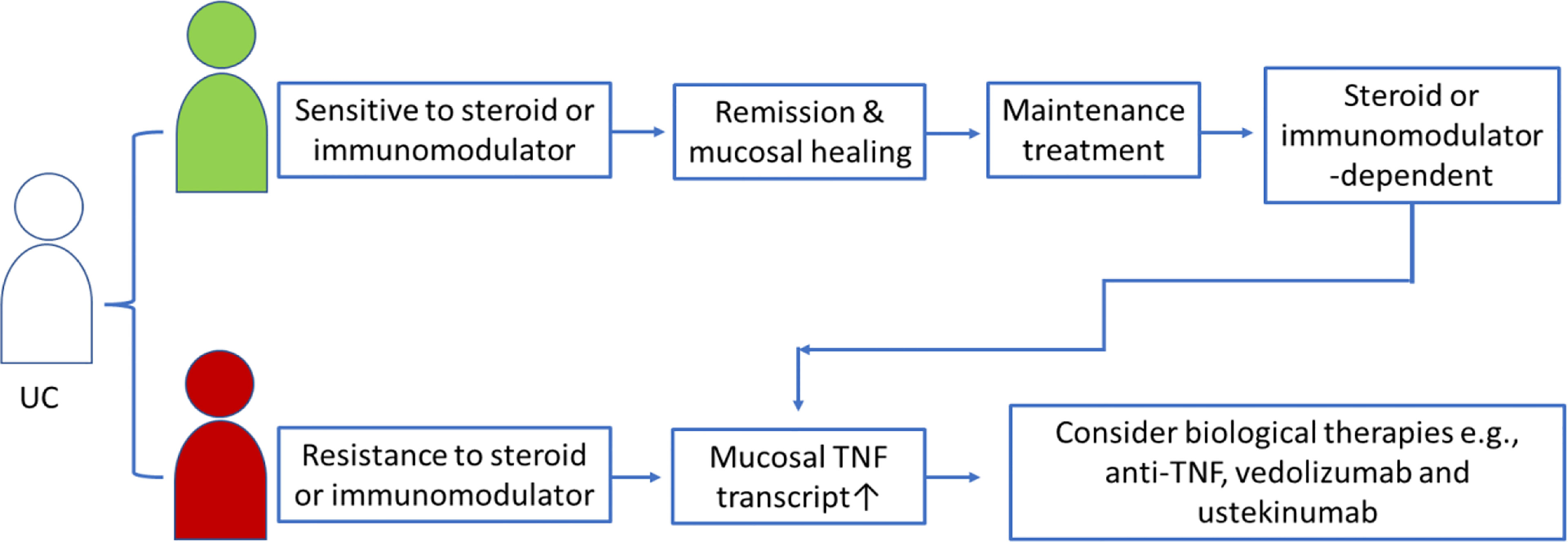
Figure 1 Schematic presentation of mucosal TNF in helping clinicians to select anti-TNF candidates in UC patients treated with steroid or immunomodulators.
Mucosal TNF Level Changes Predict Clinical Response to Anti-TNF Biological Therapy in Patients With UC
Matsuda et al. quantified the mucosal expression levels of TNF-α, interleukin (IL)-6, IL-8, and IL-10 transcripts, and confirmed that the TNF level was significantly increased in the colonic mucosa in patients with steroid naïve UC (62). Furthermore, our validation data demonstrated that the baseline mucosal TNF level was a promising biomarker in patients with UC (23). In conjunction with histological inflammatory activity scores, mucosal TNF level could precisely predict the need for biological therapy within the first year after the diagnosis of disease in patients with UC (23).
Studies have also suggested that changed mucosal TNF levels predict the clinical response to anti-TNF biologic agents and can be used for the evaluation of anti-TNF therapeutic efficacy. Tsukada et al. (70) have previously examined the mucosal cytokine transcript profile and its relation to disease activity in patients with UC. They reported that the TNF level was higher in inflamed mucosa of UC patients than in uninflamed controls. The expression level of mucosal TNF in patients with UC was remarkably decreased after prednisolone treatment (70). Raddatz et al. (71) quantitatively measured the mucosal levels of cytokine transcripts in 24 UC patients and 18 CD patients with glucocorticoid therapy. However, they found that the mucosal cytokine transcript levels were not associated with the response to a glucocorticoid therapy in either UC or CD (71). Such inconsistent results led to us to examine the potential role of mucosal TNF levels in the evaluation of anti-TNF therapeutic efficacy in UC patients treated with an anti-TNF bioagent (IFX). Indeed, our studies have shown that changed mucosal TNF levels might predict remission and mucosal healing rates in response to IFX in patients with UC (42). To investigate the correlation between the mucosal TNF transcript level prior to treatment and the response rate to IFX in patients with UC, we divided UC patients into 3 groups according to the cutoff values of pretreatment mucosal TNF values (high, medium and low mucosal TNF level groups) (42). We found that the rate of mucosal healing after IFX treatment in the 3 groups was very different (low vs. medium vs. high: 82% vs. 64% vs. 42%), although the UCDAI and endoscopic scores in the 3 groups before IFX treatment were comparable. Furthermore, mucosal TNF levels in nonremised UC patients were maintained at a higher level than those in remised patients (42). Our results indicated that pretreatment mucosal TNF values might have a predictive potential for the remission and mucosal healing rates in response to IFX therapy, and UC patients with a higher mucosal TNF value might need a longer period or higher dose of IFX after exclusion of high anti-drug antibody levels (42). Moreover, our recent observation data in UC patients with repeated intensified IFX induction therapy confirmed that normalization of mucosal TNF levels could predict long-term remission upon discontinuation of IFX (45). Other clinicians reported similar findings. Therefore, these findings provide new insights into the strengthening the use of mucosal TNF as a biomarker to optimize the precision of anti-TNF strategies in patients with UC. For example, to normalize the mucosal TNF level and achieve better efficacy of anti-TNF treatment, a patient with a higher TNF level might need a higher dose or longer duration of induction therapy with anti-TNF bioagents than patients with a lower TNF level.
Could the Mucosal TNF Predict the Anti-TNF Biological Therapy Discontinuation and Long-Term Remission After Drug Withdrawal?
Anti-TNF biologic therapy places a heavy economic burden on patients and the health system because anti-TNF biologic agents are very expensive and have several side effects (21, 72, 73). Clinically, patients receive an insufficient treatment and a high relapse rate if the end of anti-TNF treatment is too early; in contrast, overtreatment with anti-TNF bioagents may increase side effects and costs. Therefore, the cessation of anti-TNF biologic agents must be at the right time to reduce the relapse rate after anti-TNF withdrawal (74–76). However, no consensus has been currently reached regarding the timing of anti-TNF therapy discontinuation in patients with IBD. We have previously considered ceasing anti-TNF drugs when the mucosal TNF level becomes normalized (44). Our data showed that a normalized mucosal TNF transcript level indicated a higher rate of mucosal healing during the anti-TNF treatment period and a significantly long-term remission period after IFX withdrawal in patients with UC (44). Therefore, the mucosal TNF level might be a predicative biomarker for long-term remission in UC patients with mucosal healing after anti-TNF drug discontinuation. Taken together, monitoring mucosal TNF changes in patients with UC in remission under anti-TNF biological therapy might provide useful information to predict the possibility of long-term remission after drug withdrawal (refer to the summary in Figure 2).
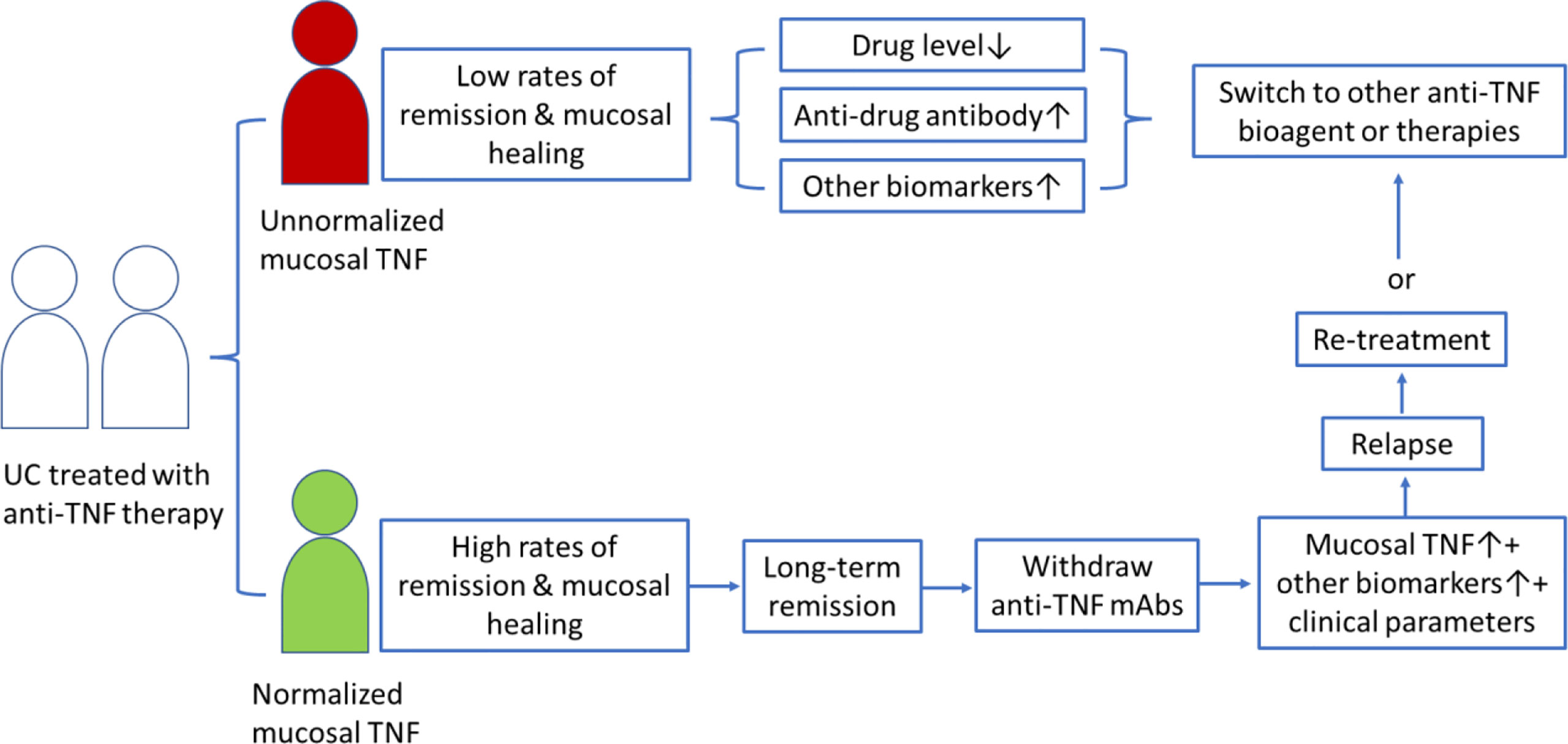
Figure 2 Schematic presentation of mucosal TNF in helping clinicians to discontinue anti-TNF therapy and predict relapse after drug withdrawal in patients with UC.
Could Mucosal TNF as a Biomarker in CD Patients With Anti-TNF Therapy?
Currently, supportive evidence for using mucosal TNF transcript as a biomarker candidate was largely from UC patients (refer to Table 2), only a limitative studies were performed in patients with CD.

Table 2 Summary of predictive significance of mucosal TNF transcript level as a biomarker candidate in UC patients with anti-TNF therapy.
For example, Schmidt et al. identified a close relationship between a decreased expression level of mucosal TNF and long-term remission in azathioprine or methotrexate-refractory CDs treated with infliximab (IFX) or cyclophosphamide therapies (41). Atreya et al. (77) showed that high numbers of mucosal membrane-bound TNF (mTNF) (mTNF) (+) immune cells could predict a significantly higher short-term response rate at week 12 compared to patients with low amounts of mTNF(+) cells (92% vs. 15%) in CD patents with anti-TNF therapy. Furthermore, Vatansever et al. reported that the mucosal TNF protein level before treatment determined by immunohistochemistry could predict a favorable response to anti-TNF biological therapy in 35 patients with CD (78). CD patients with high mucosal TNF protein levels at diagnosis might need earlier anti-TNF treatment (78). Schmidt et al. reported that mucosal TNF levels before IFX biological therapy could help to identify patients who will achieve a long-term remission in patients with CD (41). Our data confirmed that a higher mucosal TNF level in CD patients with mucosal healing could predict a high rate of relapse after anti-TNF drug withdrawal (47). However, the size of most of studies performed in CD patients with anti-TNF therapy were relatively small. Although this biomarker candidate has recently been validated in patients with UC (23), it is waiting to be done in a larger-scale cohort of CD patients.
Challenges and Limitations Regarding Mucosal TNF as a Biomarker Candidate in UC Patients With Anti-TNF Biological Therapy
Despite encouraging findings indicating that mucosal TNF levels could serve as a biomarker for individualized anti-TNF therapy in patients with IBD, a number of challenges remain.
For example, how to use biomarkers to identify patients who respond to anti-TNF therapy early has become a major challenge. However, to the best of our knowledge, no comparison studies of anti-TNF therapeutic efficacy between IBD patients with a normalized and high TNF levels are currently available. The prescription of anti-TNF therapy mainly depends on the disease activity defined by clinical symptoms and endoscopic observation. Thus, it is unclear whether an IBD patient with a normalized mucosal TNF level can benefit from anti-TNF biological therapy. As indicated by our studies, normalized mucosal TNF could predict a high mucosal healing response rate to anti-TNF therapy (42) and long remission time after anti-TNF biologic agent withdrawal (44, 47). To reach a higher rate of mucosal healing or remission, whether the dose or duration of anti-TNF bioagents in those partially mucosal healing patients with a high mucosal TNF level after excluding high anti-drug antibody levels should be increased remains to be investigated.
This review focused only on a single biomarker (mucosal TNF) in patients with IBD in the context of precision medicine. Mucosal TNF level alone as a biomarker in precision anti-TNF biological therapy may have its shortcomings. The efficacy of anti-TNF therapy also depended on the formation of anti-drug antibodies, and a high level of anti-drug antibodies might significantly decrease the efficacy of anti-TNF bioagents. Yarur et al. (79) reported that inflamed tissue anti-TNF antibody levels combined with the endoscopic disease activity score could increase the predictive power of the response to IFX in patients with IBD. D’Haens et al. (80) analyzed and validated the significance of multiple (total 13) protein biomarkers, called the endoscopic healing index (EHI), in indicating mucosal damage and repair processes in 278 patients with CD. They reported that EHI showed a good correlation with the activity identified by endoscopy and a better performance than a single biomarker (for example, serum CRP) in reflecting the rate of mucosal healing (80).
Finally, clinical studies have shown that combination therapy with anti-TNF bioagents and immunomodulators is better than monotherapy for inducing remission or mucosal healing in patients who have failed to respond or lose response to their first anti-TNF bioagent (81–84). Currently, several novel biologic agents including ustekinumab (human monoclonal antibodies block IL-12 and IL-23), natalizumab (humanized monoclonal antibody against alpha-4 integrin) and vedolizumab (humanized monoclonal antibody against alpha-4-beta-7 integrin) have been developed for potential use in the management of IBD (85). Kopylov et al. reported that ustekinumab could be a candidate for effective therapy in CD patients who become unresponsive to anti-TNF biological therapy (86). Do the mucosal TNF level changes reflect clinical remission and mucosal healing in IBD patients treated with these novel biologic agents? Murate et al. (48) recently reported that a combination of high baseline serum TNF concentration with low simple endoscopic score predicts a high clinical response rate to ustekinumab treatment in patients with CD. More answers to these questions may improve the precision management of IBD with anti-TNF biological therapy.
Another limitation of this biomarker is the need for endoscopic biopsies, which does not allow for easy proactive monitoring of relapse during the remission period. Most UC patients with remission will not go to hospital to take colonoscopy examination until the release occurs and symptoms become obviously. There are a number of ways to measure TNF expressing cells in colorectal biopsies taken by colonoscopy, such as RNA transcript assay by in situ hybridization, TNF protein by immunohistochemistry. Intensive histological evidence suggested that TNF was widely expressed on many types of cells in the microenvironment of colorectal mucosa. Studies showed that TNF both at mRNA and protein levels were known to express in many types of cells, such as epithelial cells, Paneth cells, immune cells e.g., eosinophils, TH cells and macrophages, in the colorectal mucosa (37, 58, 87–92). In addition, TNF was expressed on intestinal lymph tissues and played a regulatory effect on the maintenance of microarchitecture and the local immune cell differentiation and function in the colorectal mucosa (93). Since the heterozygosity of TNF expressing cell source in the colorectal mucosa, the bulk TNF in biopsies taken from different sites of colorectal mucosa may be vary and does not have enough resolution for clinical use. For example, if biopsies sampling accidentally hit Prayer’s Patch, the massive amount of macrophages will result in extremely high levels of TNF transcript. Similar results may also be happen in biopsies with high densities of eosinophils and Paneth cells. Therefore, one of strategies that might avoid this sampling shortcoming is carefully to divide intestinal regions and established normal values according to different regions. To reduce the risk of sampling bias, the clinicians need to take multiple endoscopic biopsies from the same region.
Theoretically, protein synthesis is controlled by transcription. Previous studies have validated that mucosal cytokine/chemokine transcript profiles could reflect the degree of mucosal inflammation in patients with CD (94). However, comparison studies between mucosal TNF and proteins in evaluation of anti-TNF therapy in patients with IBD remain to be conducted. In addition, the anti-TNF therapeutic efficacy depends on several clinical factors such as age, disease severity, dose/duration, drug level and anti-drug level, rather than a single factor. Mucosal TNF changes reflect only suppressed degree of TNF in the colorectal mucosa after anti-TNF biological therapy. Clinical studies reported that anti-drug antibodies were developed in most UC patients with anti-TNF therapy, high levels of anti-drug antibody were associated with the low therapeutic efficacy in patients receiving anti-TNF therapy (95–100). Therefore. the level of anti-drug antibodies has been widely used as important biomarker for the evaluation of anti-TNF therapeutic efficacy and provided useful information to monitor response and confirm treatment decisions (99, 101, 102). Notably, anti-drug antibody combined with other biomarkers, for example drug level, could enhance the predictive significance in the evaluation of response to anti-TNF bioagents (103–106). UC patients with normalized mucosal transcript levels might possibly had a low anti-TNF efficacy, which made it difficult for clinicians to accurately determine whether or when the treatment decision should be shifted. In such case, the clinician needed to combine anti-drug antibody level with mucosal TNF transcript to evaluate the therapeutic response to anti-TNF treatment in patients with UC. It was time to consider to swift current anti-TNF therapeutic to another medicine when the increased level of anti-drug antibody level was observed in UC patients with anti-TNF therapy. Therefore, screening and grouping mucosal TNF with other biomarkers into a practice biomarker group for “anti-TNF efficacy analysis” use is necessary.
In our clinical practice, we have found that a few IBD patients with normalized mucosa TNF levels after withdrawal of anti-TNF biologic agents might still relapse. Considering that multiple factors, such as IL-33, participate in the complex regulatory network of intestinal inflammation in IBD (36), it is likely that the combination of mucosal TNF with other biomarkers and clinical parameters is needed.
Conclusion and Future Perspectives
Emerging evidence suggests that the changes in mucosal TNF level in IBD patients treated with anti-TNF bioagents provide translational information on the response to drugs, which is extremely important for precision anti-TNF therapy. Analysis of the current data suggested that mucosal TNF may potentially play a role in candidate selection for anti-TNF biological therapy, predicting remission and mucosal healing and helping predict drug withdrawal and relapse after withdrawal. Here, we summarized the clinical and translational role of mucosal TNF as a biomarker candidate for precision/personal treatment regimens in patients with IBD as shown in Table 1 and Figure 3, incorporating integrated analysis of current data.

Table 1 Summary of the role of mucosal TNF transcript in precision strategies of anti-TNF therapy in patients with UC.

Figure 3 Schematic summary of the role of mucosal TNF in precisive/personalized anti-TNF therapy in patients with UC, incorporating integrated analysis of current published data. Analysis of current data suggested that mucosal TNF might play the potential role in the candidate selection for anti-TNF biological therapy, predicating mucosal healing, helping drug withdraw and long-term remission after drug withdraw.
However, additional studies are still needed to resolve challenges and limitations in the comprehensive use of mucosal TNF levels as a biomarker in precision anti-TNF therapy. For example, should we measure mucosal TNF levels before we decide to prescribe anti-TNF biologic agents to an IBD patient? Should those patients with a high TNF level be given priority consideration for anti-TNF therapy? What is the effect of anti-TNF bioagents on IBD patients with a low or normal TNF level? Answering these questions might help clinicians precisely identify candidates who respond to anti-TNF therapy. Additionally, given the complexity of the cytokine network in the colorectal mucosa, a single cytokine biomarker may not be sufficient to predict an overall response to anti-TNF therapy in all IBD patients. This challenge might be overcome by developing combination strategies involving mucosal TNF and other biomarkers or clinical parameters (107). Therefore, the predictive power and value of such biomarker combinations must be further tested and validated in clinical practice.
Author Contributions
GC had the idea for this systematic review and performed the electronic search for literatures, GC and RG performed literature selection, data extraction and analysis. JF joined the data analysis and discussion. All the listed authors contributed to this manuscript in writing and final approvement.
Funding
This study was supported by the Medical Research Program, Northern Norway Regional Health Authority (Helse Nord RHF), Norway (Program No. SFP-922-10). The funder did not play any role in paper design, data collection, data analysis, interpretation, writing of the paper. We apologize for not being able to cite all the excellent studies and reviews due to space limitations.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CD, Crohn’s Disease; CDAI, Crohn’s Disease activity index; CRP, C-reactive protein; CT, cycle threshold; DOR, diagnostic odds-ratio; EHI, endoscopic healing index; ELISA, Enzyme-linked immunosorbent assay; FC, fecal calprotectin; IBD, Inflammatory bowel diseases; IFN-γ, Interferon-γ; IL, Interleukin; mRNA, Messenger RNA; q-PCR, Quantitative real-time PCR; RHI, The Robarts’ histopathology index; TNF, TNF transcript; TNF-α, Tumor necrosis factor-α; UC, Ulcerative colitis; USAI, Ulcerative colitis activity index.
References
1. Digby-Bell JL, Atreya R, Monteleone G, Powell N. Interrogating Host Immunity to Predict Treatment Response in Inflammatory Bowel Disease. Nat Rev Gastroenterol Hepatol (2020) 17(1):9–20. doi: 10.1038/s41575-019-0228-5
2. Kaplan GG. The Global Burden of IBD: From 2015 to 2025. Nat Rev Gastroenterol Hepatol (2015) 12(12):720–7. doi: 10.1038/nrgastro.2015.150
3. Kaplan GG, Windsor JW. The Four Epidemiological Stages in the Global Evolution of Inflammatory Bowel Disease. Nat Rev Gastroenterol Hepatol (2021) 18(1):56–66. doi: 10.1038/s41575-020-00360-x
4. Cui G, Yuan A. A Systematic Review of Epidemiology and Risk Factors Associated With Chinese Inflammatory Bowel Disease. Front Med (Lausanne) (2018) 5:183. doi: 10.3389/fmed.2018.00183
5. Collaborators GBDIBD. The Global, Regional, and National Burden of Inflammatory Bowel Disease in 195 Countries and Territories, 1990-2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol (2020) 5(1):17–30. doi: 10.1016/S2468-1253(19)30333-4
6. Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. Worldwide Incidence and Prevalence of Inflammatory Bowel Disease in the 21st Century: A Systematic Review of Population-Based Studies. Lancet (2017) 390(10114):2769–78. doi: 10.1016/S0140-6736(17)32448-0
7. Cui G, Liu H, Xu G, Laugsand J-B, Pang Z. Exploring Links Between Industrialization, Urbanization, and Chinese Inflammatory Bowel Disease. Front Med (2021) 8:757025(2047). doi: 10.3389/fmed.2021.757025
8. Tremaine WJ. Refractory IBD: Medical Management. Neth J Med (1997) 50(2):S12–4. doi: 10.1016/s0300-2977(96)00065-4
9. Okuno H, Ogino H, Ihara E, Nishioka K, Iboshi Y, Chinen T, et al. Interleukin-1beta as a Predictor of Glucocorticoid Response in Ulcerative Colitis. Digestion (2021) 102(3):357–67. doi: 10.1159/000507435
10. Iborra M, García-Morales N, Rubio S, Bertoletti F, Calvo M, Taxonera C, et al. Real-Life Experience With 4 Years of Golimumab Persistence in Ulcerative Colitis Patients. Sci Rep (2020) 10(1):17774. doi: 10.1038/s41598-020-73577-0
11. Mahagna H, Ben-Horin S. Biologics’ Switching: New Insights Toward Establishing Practice Norms. United Eur Gastroenterol J (2019) 7(6):733–4. doi: 10.1177/2050640619851683
12. Olesen CM, Coskun M, Peyrin-Biroulet L, Nielsen OH. Mechanisms Behind Efficacy of Tumor Necrosis Factor Inhibitors in Inflammatory Bowel Diseases. Pharmacol Ther (2016) 159:110–9. doi: 10.1016/j.pharmthera.2016.01.001
13. Fine S, Papamichael K, Cheifetz AS. Etiology and Management of Lack or Loss of Response to Anti-Tumor Necrosis Factor Therapy in Patients With Inflammatory Bowel Disease. Gastroenterol Hepatol (N Y) (2019) 15(12):656–65.
14. Roda G, Jharap B, Neeraj N, Colombel JF. Loss of Response to Anti-TNFs: Definition, Epidemiology, and Management. Clin Transl Gastroenterol (2016) 7:e135. doi: 10.1038/ctg.2015.63
15. Allez M, Karmiris K, Louis E, Van Assche G, Ben-Horin S, Klein A, et al. Report of the ECCO Pathogenesis Workshop on Anti-TNF Therapy Failures in Inflammatory Bowel Diseases: Definitions, Frequency and Pharmacological Aspects. J Crohns Colitis (2010) 4(4):355–66. doi: 10.1016/j.crohns.2010.04.004
16. Papamichael K, Van Stappen T, Jairath V, Gecse K, Khanna R, D’Haens G, et al. Review Article: Pharmacological Aspects of Anti-TNF Biosimilars in Inflammatory Bowel Diseases. Aliment Pharmacol Ther (2015) 42(10):1158–69. doi: 10.1111/apt.13402
17. Iijima H, Kobayashi T, Nagasaka M, Shinzaki S, Kitamura K, Suzuki Y, et al. Management of Primary Nonresponders and Partial Responders to Tumor Necrosis Factor-Alpha Inhibitor Induction Therapy Among Patients With Crohn’s Disease. Inflammation Intest Dis (2020) 5(2):78–83. doi: 10.1159/000506337
18. Gisbert JP, Marin AC, Chaparro M. The Risk of Relapse After Anti-TNF Discontinuation in Inflammatory Bowel Disease: Systematic Review and Meta-Analysis. Am J Gastroenterol (2016) 111(5):632–47. doi: 10.1038/ajg.2016.54
19. Song JH, Kang EA, Park SK, Hong SN, Kim YS, Bang KB, et al. Long-Term Outcomes After the Discontinuation of Anti-Tumor Necrosis Factor-Alpha Therapy in Patients With Inflammatory Bowel Disease Under Clinical Remission: A Korean Association for the Study of Intestinal Disease Multicenter Study. Gut Liver (2021) 15(5):752–62. doi: 10.5009/gnl20233
20. Principi M, Labarile N, Bianchi FP, Contaldo A, Tafuri S, Ierardi E, et al. The Cost of Inflammatory Bowel Disease Management Matches With Clinical Course: A Single Outpatient Centre Analysis. Int J Environ Res Public Health (2020) 17(12):4549. doi: 10.3390/ijerph17124549
21. Norum J, Koldingsnes W, Aanes T, Antonsen MA, Florholmen J, Kondo M. The Economic Burden of TNFalpha Inhibitors and Other Biologic Treatments in Norway. Clinicoecon Outcomes Res (2011) 3:73–8. doi: 10.2147/CEOR.S15988
22. Jean L, Audrey M, Beauchemin C, Consortium O. Economic Evaluations of Treatments for Inflammatory Bowel Diseases: A Literature Review. Can J Gastroenterol Hepatol (2018) 2018:7439730. doi: 10.1155/2018/7439730
23. Florholmen JR, Johnsen KM, Meyer R, Olsen T, Moe OK, Tandberg P, et al. Discovery and Validation of Mucosal TNF Expression Combined With Histological Score - a Biomarker for Personalized Treatment in Ulcerative Colitis. BMC Gastroenterol (2020) 20(1):321. doi: 10.1186/s12876-020-01447-0
24. Atreya R, Neurath MF, Siegmund B. Personalizing Treatment in IBD: Hype or Reality in 2020? Can We Predict Response to Anti-TNF? Front Med (Lausanne) (2020) 7:517. doi: 10.3389/fmed.2020.00517
25. Noor NM, Verstockt B, Parkes M, Lee JC. Personalised Medicine in Crohn’s Disease. Lancet Gastroenterol Hepatol (2020) 5(1):80–92. doi: 10.1016/S2468-1253(19)30340-1
26. Buhl S, Kristina Borghede M, Brynskov J, Steenholdt C, Rasmussen M, Andrew Ainsworth M. Outcome of Continued Infliximab Therapy in Crohn’s Disease Patients With Response But Without Remission After One Year of Infliximab - a Retrospective Cohort Study. Scand J Gastroenterol (2018) 53(8):930–7. doi: 10.1080/00365521.2018.1481519
27. Singh S, George J, Boland BS, Vande Casteele N, Sandborn WJ. Primary Non-Response to Tumor Necrosis Factor Antagonists is Associated With Inferior Response to Second-Line Biologics in Patients With Inflammatory Bowel Diseases: A Systematic Review and Meta-Analysis. J Crohns Colitis (2018) 12(6):635–43. doi: 10.1093/ecco-jcc/jjy004
28. Marafini I, Monteleone G. Precision Medicine in Inflammatory Bowel Diseases. Front Pharmacol (2021) 12:653924. doi: 10.3389/fphar.2021.653924
29. Mao R, Chen M. Precision Medicine in IBD: Genes, Drugs, Bugs and Omics. Nat Rev Gastroenterol Hepatol (2022) 19(2):81–2. doi: 10.1038/s41575-021-00555-w
30. Vermeire S, Van Assche G, Rutgeerts P. Laboratory Markers in IBD: Useful, Magic, or Unnecessary Toys? Gut (2006) 55(3):426–31. doi: 10.1136/gut.2005.069476
31. Vande Casteele N, Herfarth H, Katz J, Falck-Ytter Y, Singh S. American Gastroenterological Association Institute Technical Review on the Role of Therapeutic Drug Monitoring in the Management of Inflammatory Bowel Diseases. Gastroenterology (2017) 153(3):835–57 e6. doi: 10.1053/j.gastro.2017.07.031
32. Gaujoux R, Starosvetsky E, Maimon N, Vallania F, Bar-Yoseph H, Pressman S, et al. Cell-Centred Meta-Analysis Reveals Baseline Predictors of Anti-TNFalpha non-Response in Biopsy and Blood of Patients With IBD. Gut (2019) 68(4):604–14. doi: 10.1136/gutjnl-2017-315494
33. Verstockt B, Verstockt S, Blevi H, Cleynen I, de Bruyn M, Van Assche G, et al. TREM-1, the Ideal Predictive Biomarker for Endoscopic Healing in Anti-TNF-Treated Crohn’s Disease Patients? Gut (2019) 68(8):1531–3. doi: 10.1136/gutjnl-2018-316845
34. Verstockt S, Verstockt B, Vermeire S. Oncostatin M as a New Diagnostic, Prognostic and Therapeutic Target in Inflammatory Bowel Disease (IBD). Expert Opin Ther Targets (2019) 23(11):943–54. doi: 10.1080/14728222.2019.1677608
35. Negreanu L, Voiosu T, State M, Voiosu A, Bengus A, Mateescu BR. Endoscopy in Inflammatory Bowel Disease: From Guidelines to Real Life. Therap Adv Gastroenterol (2019) 12:1756284819865153. doi: 10.1177/1756284819865153
36. Arkteg CB, Goll R, Gundersen MD, Anderssen E, Fenton C, Florholmen J. Mucosal Gene Transcription of Ulcerative Colitis in Endoscopic Remission. Scand J Gastroenterol (2020) 55(2):139–47. doi: 10.1080/00365521.2019.1710245
37. Rismo R, Olsen T, Cui G, Christiansen I, Florholmen J, Goll R. Mucosal Cytokine Gene Expression Profiles as Biomarkers of Response to Infliximab in Ulcerative Colitis. Scand J Gastroenterol (2012) 47(5):538–47. doi: 10.3109/00365521.2012.667146
38. Scaldaferri F, Correale C, Gasbarrini A, Danese S. Mucosal Biomarkers in Inflammatory Bowel Disease: Key Pathogenic Players or Disease Predictors? World J Gastroenterol (2010) 16(21):2616–25. doi: 10.3748/wjg.v16.i21.2616
39. Perše M, Unkovič A. The Role of TNF in the Pathogenesis of Inflammatory Bowel Disease. In: Leal RF, Torriani T, eds. Biological Therapy for Inflammatory Bowel Disease. London: IntechOpen; 2019 [cited 2022 May 09]. Available from: https://www.intechopen.com/chapters/65690. doi: 10.5772/intechopen.84375
40. Cui G, Fan Q, Li Z, Goll R, Florholmen J. Evaluation of Anti-TNF Therapeutic Response in Patients With Inflammatory Bowel Disease: Current and Novel Biomarkers. EBioMedicine (2021) 66:103329. doi: 10.1016/j.ebiom.2021.103329
41. Schmidt C, Giese T, Hermann E, Zeuzem S, Meuer SC, Stallmach A. Predictive Value of Mucosal TNF-Alpha Transcripts in Steroid-Refractory Crohn’s Disease Patients Receiving Intensive Immunosuppressive Therapy. Inflammation Bowel Dis (2007) 13(1):65–70. doi: 10.1002/ibd.20012
42. Olsen T, Goll R, Cui G, Christiansen I, Florholmen J. TNF-Alpha Gene Expression in Colorectal Mucosa as a Predictor of Remission After Induction Therapy With Infliximab in Ulcerative Colitis. Cytokine (2009) 46(2):222–7. doi: 10.1016/j.cyto.2009.02.001
43. Olsen T, Cui G, Goll R, Husebekk A, Florholmen J. Infliximab Therapy Decreases the Levels of TNF-Alpha and IFN-Gamma mRNA in Colonic Mucosa of Ulcerative Colitis. Scand J Gastroenterol (2009) 44(6):727–35. doi: 10.1080/00365520902803507
44. Olsen T, Rismo R, Gundersen MD, Paulssen EJ, Johnsen K, Kvamme JM, et al. Normalization of Mucosal Tumor Necrosis Factor-Alpha: A New Criterion for Discontinuing Infliximab Therapy in Ulcerative Colitis. Cytokine (2016) 79:90–5. doi: 10.1016/j.cyto.2015.12.021
45. Johnsen KM, Goll R, Hansen V, Olsen T, Rismo R, Heitmann R, et al. Repeated Intensified Infliximab Induction - Results From an 11-Year Prospective Study of Ulcerative Colitis Using a Novel Treatment Algorithm. Eur J Gastroenterol Hepatol (2017) 29(1):98–104. doi: 10.1097/MEG.0000000000000753
46. Rismo R, Olsen T, Ciu G, Paulssen EJ, Christiansen I, Florholmen J, et al. The Effect of Adalimumab for Induction of Endoscopic Healing and Normalization of Mucosal Cytokine Gene Expression in Crohn’s Disease. Scand J Gastroenterol (2012) 47(10):1200–10. doi: 10.3109/00365521.2012.711853
47. Rismo R, Olsen T, Cui G, Paulssen EJ, Christiansen I, Johnsen K, et al. Normalization of Mucosal Cytokine Gene Expression Levels Predicts Long-Term Remission After Discontinuation of Anti-TNF Therapy in Crohn’s Disease. Scand J Gastroenterol (2013) 48(3):311–9. doi: 10.3109/00365521.2012.758773
48. Murate K, Maeda K, Nakamura M, Sugiyama D, Wada H, Yamamura T, et al. Endoscopic Activity and Serum TNF-Alpha Level at Baseline Are Associated With Clinical Response to Ustekinumab in Crohn’s Disease Patients. Inflammation Bowel Dis (2020) 26(11):1669–81. doi: 10.1093/ibd/izaa086
49. van Haaften WT, Mortensen JH, Dige AK, Gronbaek H, Hvas CL, Bay-Jensen AC, et al. Serological Biomarkers of Tissue Turnover Identify Responders to Anti-TNF Therapy in Crohn’s Disease: A Pilot Study. Clin Transl Gastroenterol (2020) 11(9):e00217. doi: 10.14309/ctg.0000000000000217
50. Li L, Chen R, Zhang Y, Zhou G, Chen B, Zeng Z, et al. A Novel Model Based on Serum Biomarkers to Predict Primary Non-Response to Infliximab in Crohn’s Disease. Front Immunol (2021) 12:646673. doi: 10.3389/fimmu.2021.646673
51. Owczarek D, Cibor D, Glowacki MK, Ciesla A, Mach P. TNF-Alpha and Soluble Forms of TNF Receptors 1 and 2 in the Serum of Patients With Crohn’s Disease and Ulcerative Colitis. Pol Arch Med Wewn (2012) 122(12):616–23. doi: 10.20452/pamw.1537.
52. Avdagic N, Babic N, Seremet M, Delic-Sarac M, Drace Z, Denjalic A, et al. Tumor Necrosis Factor-Alpha Serum Level in Assessment of Disease Activity in Inflammatory Bowel Diseases. Med Glas (Zenica) (2013) 10(2):211–6.
53. Mateos B, Saez-Gonzalez E, Moret I, Hervas D, Iborra M, Cerrillo E, et al. TNF-Alpha, IL-7, and IL-13 Network Predicts Crohn’s Disease Response to Infliximab, as Assessed by Calprotectin Log Drop. Dig Dis (2021) 39(1):1–9. doi: 10.1159/000508069
54. Pecere S, Petito V, Amato A, Poscia A, Armuzzi A, Lopetuso LR, et al. Infliximab and Tumour Necrosis Factor Alpha Measurement on Intestinal Mucosa: A New Tool for Clinic? EMJ Gastroenterol (2016) 5(1):107.
55. Korolkova OY, Myers JN, Pellom ST, Wang L, M’Koma AE. Characterization of Serum Cytokine Profile in Predominantly Colonic Inflammatory Bowel Disease to Delineate Ulcerative and Crohn’s Colitides. Clin Med Insights Gastroenterol (2015) 8:29–44. doi: 10.4137/CGast.S20612
56. Beltran CJ, Candia E, Erranz B, Figueroa C, Gonzalez MJ, Quera R, et al. Peripheral Cytokine Profile in Chilean Patients With Crohn’s Disease and Ulcerative Colitis. Eur Cytokine Netw (2009) 20(1):33–8. doi: 10.1684/ecn.2009.0142
57. Olsen T, Florholmen J. Cytokine mRNA Expression in Steroid-Naive Patients With Ulcerative Colitis. Inflammation Bowel Dis (2010) 16(5):734. doi: 10.1002/ibd.21075
58. Olsen T, Goll R, Cui G, Husebekk A, Vonen B, Birketvedt GS, et al. Tissue Levels of Tumor Necrosis Factor-Alpha Correlates With Grade of Inflammation in Untreated Ulcerative Colitis. Scand J Gastroenterol (2007) 42(11):1312–20. doi: 10.1080/00365520701409035
59. Ishiguro Y. Mucosal Proinflammatory Cytokine Production Correlates With Endoscopic Activity of Ulcerative Colitis. J Gastroenterol (1999) 34(1):66–74. doi: 10.1007/s005350050218
60. Goll R, Olsen T, Cui G, Florholmen J. Evaluation of Absolute Quantitation by Nonlinear Regression in Probe-Based Real-Time PCR. BMC Bioinf (2006) 7:107. doi: 10.1186/1471-2105-7-107
61. Cui G, Olsen T, Christiansen I, Vonen B, Florholmen J, Goll R. Improvement of Real-Time Polymerase Chain Reaction for Quantifying TNF-Alpha mRNA Expression in Inflamed Colorectal Mucosa: An Approach to Optimize Procedures for Clinical Use. Scand J Clin Lab Invest (2006) 66(3):249–59. doi: 10.1080/00365510600590472
62. Matsuda R, Koide T, Tokoro C, Yamamoto T, Godai T, Morohashi T, et al. Quantitive Cytokine mRNA Expression Profiles in the Colonic Mucosa of Patients With Steroid Naive Ulcerative Colitis During Active and Quiescent Disease. Inflammation Bowel Dis (2009) 15(3):328–34. doi: 10.1002/ibd.20759
63. Siegel CA, Horton H, Siegel LS, Thompson KD, Mackenzie T, Stewart SK, et al. A Validated Web-Based Tool to Display Individualised Crohn’s Disease Predicted Outcomes Based on Clinical, Serologic and Genetic Variables. Aliment Pharmacol Ther (2016) 43(2):262–71. doi: 10.1111/apt.13460
64. Siegel CA. Refocusing IBD Patient Management: Personalized, Proactive, and Patient-Centered Care. Am J Gastroenterol (2018) 113(10):1440–3. doi: 10.1038/s41395-018-0246-x
65. Mosli MH, Feagan BG, Zou G, Sandborn WJ, D’Haens G, Khanna R, et al. Development and Validation of a Histological Index for UC. Gut (2017) 66(1):50–8. doi: 10.1136/gutjnl-2015-310393
66. Marchal-Bressenot A, Salleron J, Boulagnon-Rombi C, Bastien C, Cahn V, Cadiot G, et al. Development and Validation of the Nancy Histological Index for UC. Gut (2017) 66(1):43–9. doi: 10.1136/gutjnl-2015-310187
67. Gole B, Potocnik U. Pre-Treatment Biomarkers of Anti-Tumour Necrosis Factor Therapy Response in Crohn’s Disease-A Systematic Review and Gene Ontology Analysis. Cells (2019) 8(6):515. doi: 10.3390/cells8060515
68. Gisbert JP, Chaparro M. Predictors of Primary Response to Biologic Treatment [Anti-TNF, Vedolizumab, and Ustekinumab] in Patients With Inflammatory Bowel Disease: From Basic Science to Clinical Practice. J Crohns Colitis (2020) 14(5):694–709. doi: 10.1093/ecco-jcc/jjz195
69. Martin JC, Chang C, Boschetti G, Ungaro R, Giri M, Grout JA, et al. Single-Cell Analysis of Crohn’s Disease Lesions Identifies a Pathogenic Cellular Module Associated With Resistance to Anti-TNF Therapy. Cell (2019) 178(6):1493–508 e20. doi: 10.1016/j.cell.2019.08.008
70. Tsukada Y, Nakamura T, Iimura M, Iizuka BE, Hayashi N. Cytokine Profile in Colonic Mucosa of Ulcerative Colitis Correlates With Disease Activity and Response to Granulocytapheresis. Am J Gastroenterol (2002) 97(11):2820–8. doi: 10.1111/j.1572-0241.2002.07029.x
71. Raddatz D, Bockemuhl M, Ramadori G. Quantitative Measurement of Cytokine mRNA in Inflammatory Bowel Disease: Relation to Clinical and Endoscopic Activity and Outcome. Eur J Gastroenterol Hepatol (2005) 17(5):547–57. doi: 10.1097/00042737-200505000-00012
72. Dretzke J, Edlin R, Round J, Connock M, Hulme C, Czeczot J, et al. A Systematic Review and Economic Evaluation of the Use of Tumour Necrosis Factor-Alpha (TNF-Alpha) Inhibitors, Adalimumab and Infliximab, for Crohn’s Disease. Health Technol Assess (2011) 15(6):1–244. doi: 10.3310/hta15060
73. Lawton J, Achit H, Pouillon L, Boschetti E, Demore B, Matton T, et al. Cost-Of-Illness of Inflammatory Bowel Disease Patients Treated With Anti-Tumour Necrosis Factor: A French Large Single-Centre Experience. United Eur Gastroenterol J (2019) 7(7):908–13. doi: 10.1177/2050640619853448
74. Bortlik M, Duricova D, Machkova N, Hruba V, Lukas M, Mitrova K, et al. Discontinuation of Anti-Tumor Necrosis Factor Therapy in Inflammatory Bowel Disease Patients: A Prospective Observation. Scand J Gastroenterol (2016) 51(2):196–202. doi: 10.3109/00365521.2015.1079924
75. Casanova MJ, Chaparro M, Garcia-Sanchez V, Nantes O, Leo E, Rojas-Feria M, et al. Evolution After Anti-TNF Discontinuation in Patients With Inflammatory Bowel Disease: A Multicenter Long-Term Follow-Up Study. Am J Gastroenterol (2017) 112(1):120–31. doi: 10.1038/ajg.2016.569
76. Lee JM, Kim YJ, Lee KM, Yoon H, Lee BI, Kim DB, et al. Long-Term Clinical Outcome After Infliximab Discontinuation in Patients With Inflammatory Bowel Disease. Scand J Gastroenterol (2018) 53(10-11):1280–5. doi: 10.1080/00365521.2018.1524024
77. Atreya R, Neumann H, Neufert C, Waldner MJ, Billmeier U, Zopf Y, et al. In Vivo Imaging Using Fluorescent Antibodies to Tumor Necrosis Factor Predicts Therapeutic Response in Crohn’s Disease. Nat Med (2014) 20(3):313–8. doi: 10.1038/nm.3462
78. Vatansever A, Cekic C, Ekinci N, Yuksel ES, Avci A, Aslan F, et al. Effects of Mucosal TNF-Alpha Levels on Treatment Response in Crohn’s Disease Patients Receiving Anti-TNF Treatment. Hepatogastroenterology (2014) 61(136):2277–82.
79. Yarur AJ, Jain A, Sussman DA, Barkin JS, Quintero MA, Princen F, et al. The Association of Tissue Anti-TNF Drug Levels With Serological and Endoscopic Disease Activity in Inflammatory Bowel Disease: The ATLAS Study. Gut (2016) 65(2):249–55. doi: 10.1136/gutjnl-2014-308099
80. D’Haens G, Kelly O, Battat R, Silverberg MS, Laharie D, Louis E, et al. Development and Validation of a Test to Monitor Endoscopic Activity in Patients With Crohn’s Disease Based on Serum Levels of Proteins. Gastroenterology (2020) 158(3):515–26.e10. doi: 10.1053/j.gastro.2019.10.034
81. Matsumoto T, Motoya S, Watanabe K, Hisamatsu T, Nakase H, Yoshimura N, et al. Adalimumab Monotherapy and a Combination With Azathioprine for Crohn’s Disease: A Prospective, Randomized Trial. J Crohns Colitis (2016) 10(11):1259–66. doi: 10.1093/ecco-jcc/jjw152
82. Panaccione R, Ghosh S, Middleton S, Marquez JR, Scott BB, Flint L, et al. Combination Therapy With Infliximab and Azathioprine is Superior to Monotherapy With Either Agent in Ulcerative Colitis. Gastroenterology (2014) 146(2):392–400 e3. doi: 10.1053/j.gastro.2013.10.052
83. Colombel JF, Adedokun OJ, Gasink C, Gao LL, Cornillie FJ, D’Haens GR, et al. Combination Therapy With Infliximab and Azathioprine Improves Infliximab Pharmacokinetic Features and Efficacy: A Post Hoc Analysis. Clin Gastroenterol Hepatol (2019) 17(8):1525–32.e1. doi: 10.1016/j.cgh.2018.09.033
84. Din S, Cochrane CJ, Noble CL, Satsangi J, Arnott ID. Combination Therapy of Infliximab and Azathioprine Reduces Disease Progression in Crohn’s Disease. Inflammation Bowel Dis (2008) 14(1):143–5. doi: 10.1002/ibd.20260
85. O’Toole A, Moss AC. Optimizing Biologic Agents in Ulcerative Colitis and Crohn’s Disease. Curr Gastroenterol Rep (2015) 17(8):32. doi: 10.1007/s11894-015-0453-1
86. Kopylov U, Afif W, Cohen A, Bitton A, Wild G, Bessissow T, et al. Subcutaneous Ustekinumab for the Treatment of Anti-TNF Resistant Crohn’s Disease–the McGill Experience. J Crohns Colitis (2014) 8(11):1516–22. doi: 10.1016/j.crohns.2014.06.005
87. Hyams JS, Treem WR, Eddy E, Wyzga N, Moore RE. Tumor Necrosis Factor-Alpha is Not Elevated in Children With Inflammatory Bowel Disease. J Pediatr Gastroenterol Nutr (1991) 12(2):233–6. doi: 10.1097/00005176-199102000-00016
88. Murch SH, Braegger CP, Walker-Smith JA, MacDonald TT. Location of Tumour Necrosis Factor Alpha by Immunohistochemistry in Chronic Inflammatory Bowel Disease. Gut (1993) 34(12):1705–9. doi: 10.1136/gut.34.12.1705
89. Olsen T, Rismo R, Cui G, Goll R, Christiansen I, Florholmen J. TH1 and TH17 Interactions in Untreated Inflamed Mucosa of Inflammatory Bowel Disease, and Their Potential to Mediate the Inflammation. Cytokine (2011) 56(3):633–40. doi: 10.1016/j.cyto.2011.08.036
90. Cappello M, Keshav S, Prince C, Jewell DP, Gordon S. Detection of mRNAs for Macrophage Products in Inflammatory Bowel Disease by in Situ Hybridisation. Gut (1992) 33(9):1214–9. doi: 10.1136/gut.33.9.1214
91. Isaacs KL, Sartor RB, Haskill S. Cytokine Messenger RNA Profiles in Inflammatory Bowel Disease Mucosa Detected by Polymerase Chain Reaction Amplification. Gastroenterology (1992) 103(5):1587–95. doi: 10.1016/0016-5085(92)91182-4
92. Onizawa M, Nagaishi T, Kanai T, Nagano K, Oshima S, Nemoto Y, et al. Signaling Pathway via TNF-Alpha/NF-kappaB in Intestinal Epithelial Cells may be Directly Involved in Colitis-Associated Carcinogenesis. Am J Physiol Gastrointest Liver Physiol (2009) 296(4):G850–9. doi: 10.1152/ajpgi.00071.2008
93. Tumanov AV, Grivennikov SI, Kruglov AA, Shebzukhov YV, Koroleva EP, Piao Y, et al. Cellular Source and Molecular Form of TNF Specify its Distinct Functions in Organization of Secondary Lymphoid Organs. Blood (2010) 116(18):3456–64. doi: 10.1182/blood-2009-10-249177
94. Stallmach A, Giese T, Schmidt C, Ludwig B, Mueller-Molaian I, Meuer SC. Cytokine/chemokine Transcript Profiles Reflect Mucosal Inflammation in Crohn’s Disease. Int J Colorectal Dis (2004) 19(4):308–15. doi: 10.1007/s00384-003-0554-4
95. Kennedy NA, Heap GA, Green HD, Hamilton B, Bewshea C, Walker GJ, et al. Predictors of Anti-TNF Treatment Failure in Anti-TNF-Naive Patients With Active Luminal Crohn’s Disease: A Prospective, Multicentre, Cohort Study. Lancet Gastroenterol Hepatol (2019) 4(5):341–53. doi: 10.1016/S2468-1253(19)30012-3
96. Papamichael K, Cheifetz AS, Melmed GY, Irving PM, Vande Casteele N, Kozuch PL, et al. Appropriate Therapeutic Drug Monitoring of Biologic Agents for Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol (2019) 17(9):1655–68 e3. doi: 10.1016/j.cgh.2019.03.037
97. Sazonovs A, Kennedy NA, Moutsianas L, Heap GA, Rice DL, Reppell M, et al. HLA-DQA1*05 Carriage Associated With Development of Anti-Drug Antibodies to Infliximab and Adalimumab in Patients With Crohn’s Disease. Gastroenterology (2020) 158(1):189–99. doi: 10.1053/j.gastro.2019.09.041
98. Wang SL, Hauenstein S, Ohrmund L, Shringarpure R, Salbato J, Reddy R, et al. Monitoring of Adalimumab and Antibodies-to-Adalimumab Levels in Patient Serum by the Homogeneous Mobility Shift Assay. J Pharm BioMed Anal (2013) 78-79:39–44. doi: 10.1016/j.jpba.2013.01.031
99. Reinhold I, Blumel S, Schreiner J, Boyman O, Bogeholz J, Cheetham M, et al. Clinical Relevance of Anti-TNF Antibody Trough Levels and Anti-Drug Antibodies in Treating Inflammatory Bowel Disease Patients. Inflammation Intest Dis (2021) 6(1):38–47. doi: 10.1159/000511296
100. Pekala A, Filip R, Aebisher D. Anti-Drug Antibodies in Patients With Inflammatory Bowel Diseases Treated With Biosimilar Infliximab: A Prospective Cohort Study. J Clin Med (2021) 10(12):2653. doi: 10.3390/jcm10122653
101. Bendtzen K. Immunogenicity of Anti-TNF-Alpha Biotherapies: II. Clinical Relevance of Methods Used for Anti-Drug Antibody Detection. Front Immunol (2015) 6:109. doi: 10.3389/fimmu.2015.00109
102. Bendtzen K. Immunogenicity of Anti-TNF-Alpha Biotherapies: I. Individualized Medicine Based on Immunopharmacological Evidence. Front Immunol (2015) 6:152. doi: 10.3389/fimmu.2015.00152
103. Kharlamova N, Hermanrud C, Dunn N, Ryner M, Hambardzumyan K, Vivar Pomiano N, et al. Drug Tolerant Anti-Drug Antibody Assay for Infliximab Treatment in Clinical Practice Identifies Positive Cases Earlier. Front Immunol (2020) 11:1365. doi: 10.3389/fimmu.2020.01365
104. Moses J, Lambert-Jenkins K, Momotaz H, Sattar A, Debanne SM, Splawski J, et al. Time to Antibody Detection and Associated Factors for Presence of Anti-Drug Antibodies in Pediatric Inflammatory Bowel Disease Patients Treated With Anti-TNF Therapy. Eur J Gastroenterol Hepatol (2019) 31(10):1228–33. doi: 10.1097/MEG.0000000000001538
105. Lopez-Ibanez M, Marin-Jimenez I. [Drugs and Anti-Drug Antibody Levels in the Management of Patients With Inflammatory Bowel Disease]. Gastroenterol Hepatol (2016) 39(4):265–72. doi: 10.1016/j.gastrohep.2015.09.012
106. Grinman AB, de Souza M, Bouskela E, Carvalho ATP, de Souza HSP. Clinical and Laboratory Markers Associated With Anti-TNF-Alpha Trough Levels and Anti-Drug Antibodies in Patients With Inflammatory Bowel Diseases. Med (Baltimore) (2020) 99(10):e19359. doi: 10.1097/MD.0000000000019359
Keywords: mucosa, tumor necrosis factor, inflammatory bowel disease, anti-TNF therapy, biomarker
Citation: Cui G, Florholmen J and Goll R (2022) Could Mucosal TNF Transcript as a Biomarker Candidate Help Optimize Anti-TNF Biological Therapy in Patients With Ulcerative Colitis? Front. Immunol. 13:881112. doi: 10.3389/fimmu.2022.881112
Received: 22 February 2022; Accepted: 22 April 2022;
Published: 19 May 2022.
Edited by:
Jenny Gustafsson, University of Gothenburg, SwedenReviewed by:
Stamatia Papoutsopoulou, University of Thessaly, GreeceLing-shiang Felix Chuang, Icahn School of Medicine at Mount Sinai, United States
Copyright © 2022 Cui, Florholmen and Goll. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guanglin Cui, Z3VhbmdsaW4uY3VpQG5vcmQubm8=
 Guanglin Cui
Guanglin Cui Jon Florholmen3
Jon Florholmen3